Abstract
Introduction
Patients with cancer have been prioritized for vaccination against severe acute respiratory syndrome coronavirus 2. Nevertheless, there are limited data regarding the safety, efficacy, and risk of developing immune-related adverse events (irAEs) associated with mRNA vaccines in patients with lung cancer, especially those being actively treated with immune checkpoint inhibitors.
Methods
This multicenter observational study was conducted at nine hospitals in Japan. Patients with lung cancer (≥20 y) actively treated with immune checkpoint inhibitors between 4 weeks prefirst vaccination and 4 weeks postsecond vaccination were enrolled. The primary end point was the incidence of irAEs of any grade on the basis of an assumed incidence without vaccination rate of 35%. Immunogenicity was assessed by measuring anti–spike (S)-IgG antibody levels against severe acute respiratory syndrome coronavirus 2.
Results
A total of 126 patients with lung cancer (median age, 71 y; interquartile range, 65–74) were enrolled from May to November 2021 and followed up until December 2021. There were 26 patients (20.6%, 95% confidence interval: 13.9%–28.8%) and seven patients (5.6%, 95% confidence interval: 2.3%–11.1%) who developed irAEs of any grade pre- and postvaccination, respectively, which was lower than the predicted incidence without vaccination. None of the patients experienced exacerbation of preexisting irAE postvaccination. S-IgG antibodies were seroconverted in 96.7% and 100% of the patients with lung cancer and controls, respectively, but antibody levels were significantly lower in patients with lung cancer (p < 0.001).
Conclusions
Patients with lung cancer who were actively treated with ICIs were safely vaccinated without an increased incidence of irAEs; however, their vaccine immunogenicity was lower. This requires further evaluation.
Keywords: COVID-19, Immune-related adverse event, Immunoglobulin, SARS-CoV-2, Immune checkpoint inhibitor
Introduction
Since the end of 2019, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has become a public health and economic concern worldwide.1 To overcome this pandemic, various vaccines against SARS-CoV-2 have been developed and are being widely administered.2
Patients with cancer are at an increased risk for SARS-CoV-2 infection and severe COVID-19 because of the lowered immunity associated with anticancer drugs, radiation therapy, and cancer itself, including increased exposure owing to frequent visits to the hospital.3, 4, 5 In addition, patients with cancer are often elderly, and old age itself leads to declined immunocompetence.6 Hence, in many countries, patients with cancer are prioritized for vaccination against SARS-CoV-2. Nevertheless, there is less evidence regarding the safety and efficacy of vaccination in patients with cancer who are being actively treated. This is largely due to phase 3 trials of the vaccines, approved by the European Medicines Agency and the United States Food and Drug Administration, only including healthy individuals or those with stable chronic diseases and excluding patients with cancer being actively treated.7 , 8
Currently, immune checkpoint inhibitors (ICIs), such as anti–programmed cell death protein 1 (PD-1), anti–programmed death-ligand 1 (PD-L1), and anti–CTLA-4 antibodies, are widely used as single agents or in combination with other anticancer agents and radiation therapy for the treatment of various carcinomas, including lung cancer. Although these ICIs often exert dramatic antitumor effects, they can also be associated with side effects, referred to as immune-related adverse events (irAEs), which can cause damage to various organs through immune mechanisms, such as dermatitis, interstitial pneumonia, and abnormal hormone secretion associated with endocrine gland disorders.9 To avoid these side effects, caution is required in the use of ICIs in patients with autoimmune diseases.10 Similarly, clinicians are aware that the mRNA-based vaccines for SARS-CoV-2 may cause new-onset irAEs and aggravate existing irAEs.11 Vaccines against SARS-CoV-2 are already being widely administered to ICI-treated patients with lung cancer in response to the COVID-19 pandemic; thus, evaluation and validation of their safety and efficacy are urgently needed.
In this study, our primary objective was to investigate the incidence of all irAEs in patients with lung cancer treated with ICIs who had received an mRNA vaccine for SARS-CoV-2 in a real-world setting by comparing historical data from previous studies on ICIs. In addition, we investigated the mRNA vaccine immunogenicity between patients with lung cancer treated with ICIs and controls by comparing levels of anti–spike (S)-IgG antibodies against SARS-CoV-2.
Materials and Methods
Study Design and Setting
This multicenter observational study was carried out at nine different hospitals in Japan, which are as follows: Shonan Fujisawa Tokushukai Hospital, Yao Tokushukai General Hospital, Japanese Red Cross Kyoto Daini Hospital, Japanese Red Cross Kyoto Daiichi Hospital, University Hospital, Kyoto Prefectural University of Medicine, Saiseikai Suita Hospital, Izumi City General Hospital, Otsu City Hospital, and Uji Tokushukai Medical Center.
Participants
Patients were enrolled from May 2021 to November 2021 and followed up until December 2021. Patients who were at least 20 years of age were eligible for enrolment if they had cytologically or pathologically confirmed lung cancer of any histologic type; had locally advanced and unresectable, metastatic, or recurrent disease; and had been treated with the ICI antibodies anti–PD-1 (nivolumab or pembrolizumab) or anti–PD-L1 (durvalumab or atezolizumab) at least once between 4 weeks before the first dose of the SARS-CoV-2 vaccine and 4 weeks after the second dose of the vaccine. The vaccine BNT162b2 or mRNA-1273 was used for both doses. Patients who (1) had already received a COVID-19 vaccine, (2) were in the active phase of COVID-19, (3) did not wish to be vaccinated, or (4) did not provide informed consent, were excluded from the study.
To evaluate immunogenicity, we compared the S-IgG antibody titer between patients with lung cancer being actively treated with ICIs (n = 65) and control patients (n = 65) who visited the outpatient departments of respiratory medicine and rheumatology at the Shonan Fujisawa Tokushukai Hospital and did not receive anticancer drugs or immunosuppressive therapy in ongoing observational studies on the immunogenicity of SARS-CoV-2 vaccines. The control patients were 1:1 matched for age and sex to each patient with lung cancer being treated with ICIs for whom at least one surplus serum sample was available. For evaluating the immunogenicity, patients with previous COVID-19 infection or nucleocapsid (N)-IgG positivity at baseline, suggesting previous infection, were excluded.
End Points and Assessments
The primary end point was the incidence of irAEs of any grade. The irAEs were classified and graded by the attending physician according to the Management of Immune-Related Adverse Events in Patients with Immune Checkpoint Inhibitor Therapy by the American Society of Clinical Oncology and Clinical Practice Guidelines and Clinical Practice Guidelines and Common Terminology Criteria for Adverse Events (version 5.0).9
The secondary end points were immunogenicity of the mRNA vaccines, details of irAEs, and frequency of fever above 37.5°C within one week after each vaccination. When multiple irAEs occurred in the same patient, the largest grade was used; all multiple irAEs in the same patient, if any, were used for type classification. Immunogenicity was assessed to evaluate the change in the S-IgG antibody titer against SARS-CoV-2 over time after vaccination and was assessed by comparing S-IgG antibody titers between patients with lung cancer being treated with ICIs and control patients.
Study Variables: Sample Collection and Antibody Measurement
Surplus serum samples were collected from blood samples drawn by venipuncture during routine pharmacotherapy practices for lung cancer. When multiple samples were available from the same patient in the following period, the sample with a collection time closest to the specified date was used: 21 plus or minus 3 days, 35 plus or minus 7 days, 60 plus or minus 14 days, 90 plus or minus 14 days, 120 plus or minus 14 days, 150 plus or minus 14 days, and 180 plus or minus 14 days after the initial dose of vaccine. For baseline, the sample closest to the date of the first dose of vaccine was used from the prevaccination blood collection (−35 d allowed); otherwise, the earliest sampled blood, up to 7 days after the first dose of vaccine, was used if available. For the control group, blood samples were collected by venipuncture at the baseline (allowance, −35 d to day 0) and 21 plus or minus 2 days, 35 plus or minus 2 days, 90 plus or minus 7 days, and 180 plus or minus 14 days after the initial vaccine dose.
Antibodies were measured centrally at Shonan Fujisawa Tokushukai Hospital. Serum that could not be measured on the day of collection was stored below −80°C at other institutions and transported below −20°C for testing.
We measured antibodies against SARS-CoV-2 using SARS-CoV-2 IgG II Quant and ARCHITECT SARS-CoV-2 IgG (Abbott, Abbott Park, IL), a chemiluminescent microparticle immunoassay designed to detect IgG antibodies against the receptor-binding domain in the S and N proteins of SARS-CoV-2 in serum and plasma samples, respectively. The measurement range of anti–S-IgG was 6.8 to 80,000 arbitrary units (AU)/mL, and the manufacturer’s recommended positivity cutoff was 50 AU/mL (positive agreement, 99.4%; negative agreement, 99.6%).12 Recent studies evaluating the antibody level of anti–S-IgG found that more than or equal to 4160 AU/mL can be used as a surrogate measure of antibody neutralization. Titer estimates consistently corresponded to a 0.95 probability in the plaque reduction neutralization test.12 , 13
The results for anti–N-IgG are reported as an index (the chemiluminescent signal ratio between the samples and a calibrator, S/C), with a manufacturer-recommended positivity cutoff index value of 1.4 S/C.
Ethical Approval and Data Collection
This study was conducted in accordance with the principles of the Declaration of Helsinki. This study was reviewed and approved by the Tokushukai Group Ethics Committee (approval number TGE01700-008) and the institutional review boards or ethics committees of the participating facilities. The requirement for informed consent was waived because this was a retrospective analysis of anonymized patient data; however, all participants whose antibody titers were measured with their surplus serum samples provided written informed consent before participation. Patients were allowed to opt out of the research use of their data, and the related information was publicly available on the website of each hospital. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry on May 25, 2020 (identification UMIN000044316). Baseline characteristics and clinical information were collected from the patients’ electronic medical records.
Statistical Analysis
The appropriate sample size for this study was calculated as in subsequent discussion. According to previous reports, the incidence of irAEs of any grade with pembrolizumab was 29.2% and 27.8% in the pembrolizumab monotherapy arm in the KEYNOTE-024 and -042 studies, respectively, and 26.4% and 28.8% in the pembrolizumab combined with chemotherapy arm in the KEYNOTE-189 and -407 studies, respectively.14, 15, 16, 17 The incidence of irAEs of any grade with durvalumab was 24.2% in the PACIFIC study with durvalumab alone after concurrent chemoradiotherapy and 19.6% in the durvalumab combined with platinum and VP-16 arm in the CASPIAN study.18 , 19 For atezolizumab, the rates were 31.0% in the monotherapy (OAK trial) and 48% in combination with chemotherapy (pooled analyses of IMpower130, IMpower132, and IMpower150).20 , 21 In patients with advanced or recurrent NSCLC who received nivolumab monotherapy, irAEs of any grade were observed in 51% of the patients.22 The incidence of irAEs of any grade during influenza vaccination using various regimens, including ICI, varied between 20% and 50%.23, 24, 25, 26 On the basis of the average incidence of irAEs in the above-mentioned previous studies, we assumed that the incidence rate of irAEs in patients with lung cancer being treated with ICIs without SARS-CoV-2 vaccination would be 35%. Thus, a total of 67 cases were required to detect an increase of 15% or more in the incidence of irAEs on the basis of a one-sided 5% significance level, 80% power, and one-sample binomial test (normal approximation). Considering missing data and dropouts, we set the target number of cases to be at least 75. To secure more surplus serum specimens, because some facilities were unable to provide serum specimens, no disadvantage to patients could be assumed owing to an increase in the number of patients. Furthermore, recruitment continued to the maximum extent possible, even if the target number of cases was reached during the enrolment period.
Data analysis and visualization were performed using the R software (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are expressed as median with interquartile range (IQR). Mann-Whitney U test was used for nonparametric univariate between-group comparisons. The median antibody titer was calculated and evaluated using the Steel–Dwass method for comparisons among three or more groups. Categorical variables are presented as numbers (percentages) and compared using the chi-square test and Fisher’s exact test. Statistical significance was set at p value less than 0.05.
Results
Participants
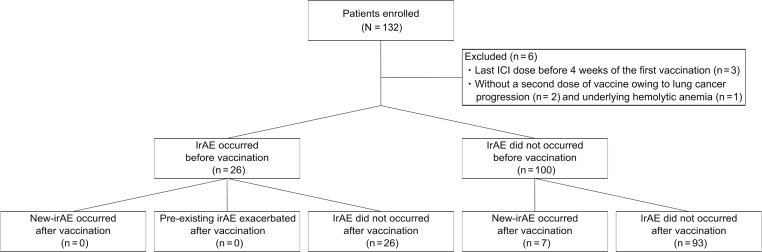
In total, 132 patients who were actively treated with ICIs for lung cancer were enrolled in this study at nine hospitals in Japan between May 1, 2021, and November 30, 2021. Six patients did not meet the criteria and were excluded: three patients had their last ICI dose before 4 weeks of the first vaccination, two patients did not receive a second dose of vaccine owing to lung cancer progression, and one patient could not receive a second dose of vaccine because of worsening underlying hemolytic anemia (Fig. 1 ). Therefore, the final analysis included 126 patients. The median observation time from the initiation of ICI treatment to the first dose of vaccine was 208.0 days (IQR: 80.5–391.5), and the median follow-up was 145.0 days (IQR: 116.2–164.0) after the first dose of vaccine at the data cutoff on December 2021. The median patient age was 71 years (IQR: 65–74 y), and 73.0% of the patients were male. The demographic and disease characteristics of the patients at baseline are listed in Table 1 . A total of 124 patients were vaccinated twice with 30 μg of BNT162b2 mRNA vaccine at a median interval of 21 days (IQR: 21–21 d), and two patients were vaccinated twice with 100 μg of mRNA-1273 vaccine at an interval of 32 days. The most frequently used ICI between 4 weeks before the first vaccination and 4 weeks after the second vaccination was pembrolizumab (n = 62, 49.2%), followed by atezolizumab (n = 28, 22.2%), and durvalumab (n = 21, 16.7%). In addition, 41.3% (n = 52) of the patients were on maintenance ICI monotherapy after chemotherapy or concurrent chemoradiation therapy, 29.4% (n = 37) were on a combination therapy of ICIs with cytotoxic chemotherapy, and 29.4% (n = 37) were on continuous immunotherapy. In addition, 37 patients were subjected to cytotoxic chemotherapy as immunochemotherapy from 4 weeks before the administration of the first SARS-CoV-2 vaccine dose to 4 weeks after the administration of the second dose, a period close to the vaccination.
Figure 1.
Patient flowchart.
Table 1.
Baseline Characteristics of Patients With Lung Cancer Being Treated With ICIs Who Received an mRNA Vaccine
| Variables | Taking ICIs |
|---|---|
| (N = 126) | |
| Sex, male; n (%) | 92 (73.0) |
| Age (y), median (IQR) | 71 (65–74) |
| Height (cm), median (IQR) | 165.0 (159.2–169.4) |
| Weight (kg), median (IQR) | 59.2 (51.9–68.4) |
| Body mass index, median (IQR) | 21.9 (19.5–24.9) |
| Past history of COVID-19, n (%) | 3 (2.4) |
| Vaccine, n (%) | |
| BNT162b2 | 124 (98.4) |
| mRNA-1273 | 2 (1.6) |
| Vaccination interval (d), median (IQR) | 21 (21–21) |
| Underlying autoimmune disease,a n (%) | 11 (8.7) |
| ECOG performance status, n (%) | |
| 0 | 63 (50.0) |
| 1 | 54 (42.9) |
| 2 | 9 (7.1) |
| ¾ | 0 (0) |
| Histologic subtype of lung cancer, n (%) | |
| Adenocarcinoma | 73 (57.9) |
| Squamous cell carcinoma | 38 (30.2) |
| Small cell carcinoma | 4 (3.2) |
| Others | 11 (8.7) |
| Clinical stage, n (%) | |
| Ⅲ | 32 (25.4) |
| Ⅳ | 73 (57.9) |
| Recurrent | 19 (15.1) |
| Others | 2 (1.6) |
| ICIs used during the doses of vaccine, including 4-wk prefirst vaccination and 4-wk postsecond vaccination | |
| Pembrolizumab, n (%) | 62 (49.2) |
| Immunotherapy (ICI monotherapy)/maintenance of ICI monotherapy after treatment including cytotoxic therapy/immunochemotherapy, n | 12/24/26 |
| Nivolumab, n (%) | 10 (7.9) |
| Immunotherapy (ICI monotherapy)/maintenance of ICI monotherapy after treatment including cytotoxic therapy/immunochemotherapy, n | 10/0/0 |
| Atezolizumab, n (%) | 28 (22.2) |
| Immunotherapy (ICI monotherapy)/maintenance of ICI monotherapy after treatment including cytotoxic therapy/immunochemotherapy, n | 11/8/9 |
| Durvalumab, n (%) | 21 (16.7) |
| Immunotherapy (ICI monotherapy)/maintenance of ICI monotherapy after treatment including cytotoxic therapy/immunochemotherapy, n | 0/20/1 |
| Nivolumab + ipilimumab, n (%) | 5 (4.0) |
| Immunotherapy (ICI monotherapy)/maintenance of ICI monotherapy after treatment including cytotoxic therapy/immunochemotherapy, n | 4/0/1 |
| Lines of treatment, median (IQR) | 1 (1–2) |
| Number of cycles of ICI received at first vaccine dose, median (IQR) | 8.5 (4.0–18.0) |
| ICI administration, n (%) | |
| Before first dose of vaccine | 122 (96.8) |
| Between two doses of vaccine | 3 (2.4) |
| After second dose of vaccine | 1 (0.8) |
| Interval between vaccine and ICI dose | |
| Immediately preceding ICI to first dose of vaccine (d), median (IQR) | 10.5 (6.0–17.0) |
| First dose of vaccine to ICI between two vaccine doses (d), median (IQR) | 10.0 (6.0–14.0) |
| ICI between two vaccine doses to second dose of vaccine (d), median (IQR) | 11.0 (6.0–15.0) |
| Second dose of vaccine to ICI just after the dose of vaccine (d), median (IQR) | 12.0 (7.0–17.3) |
| Observation period | |
| Time from the start of ICI treatment to the first dose of vaccine (d), median (IQR) | 208.0 (80.5–391.5) |
| Time from the first vaccine dose to end of observation (d), median (IQR) | 145.0 (116.2–164.0) |
COVID-19, coronavirus disease 2019; ICI, immune checkpoint inhibitor; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group.
Disease (number of patients): Hashimoto’s disease (5) psoriasis (1), polymyalgia rheumatica (1), slowly progressive insulin-dependent diabetes mellitus (1), idiopathic thrombocytopenic purpura (1), rheumatoid arthritis (1), and SAPHO syndrome (1).
Eight patients were lost to follow-up before the data cutoff on December 2021: five died of lung cancer, two were transferred to hospice, and one was homebound and had an interruption in hospital visits owing to the progression of lung cancer. None of the patients developed COVID-19 during the entire observation period.
Safety of mRNA Vaccine in Patients With Lung Cancer Treated With ICIs
Among all 126 participants, irAEs of any grade newly occurred in seven patients after vaccination (5.6%, 95% CI: 2.3%–11.1%;Figs. 1 and 2 ). Before the vaccination, 26 patients (20.6%, 95% CI: 13.9%–28.8%) had a new irAE (of any grade); none of these 26 patients developed a new irAE or exhibited exacerbation of a preexisting irAE after vaccination. During the entire observation period, irAEs of any grade newly occurred in a total of 33 patients (26.2%, 95% CI: 18.8%–34.8%). The percentage of patients who developed any grade of new-onset irAE was not higher than the expected value of 35% inferred from previously published literature.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26
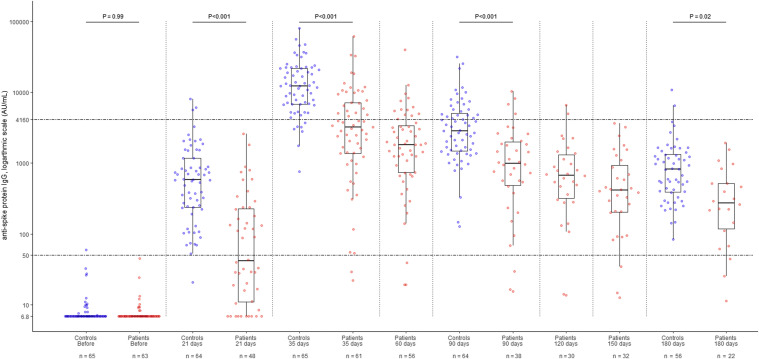
Figure 3.
Beeswarm and box plots revealing the temporal dynamic changes in the anti–spike (S) protein-specific IgG antibody levels of patients with lung cancer actively treated with ICIs and control patients who did not receive anticancer drugs or immunosuppressive therapy, before and after vaccination with the mRNA SARS-CoV-2 vaccine. Data for the 65 patients with lung cancer who were being actively treated with ICIs are illustrated with red dots and the age- and sex-matched control patient data are illustrated with blue dots; all patients received two doses of the BNT162b2 vaccine. Steel–Dwass test was used for comparing antibody titers in the same period between groups. Box plots display the median values with the IQR (lower and upper hinge) ±1.5-fold the IQR from the first and third quartiles (lower and upper whiskers, respectively). The lower dotted line represents 50 AU/mL, which was the manufacturer’s cutoff value; the upper dotted line represents 4160 AU/mL, a surrogate measure of antibody neutralization. AU, arbitrary unit; ICI, immune checkpoint inhibitor; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
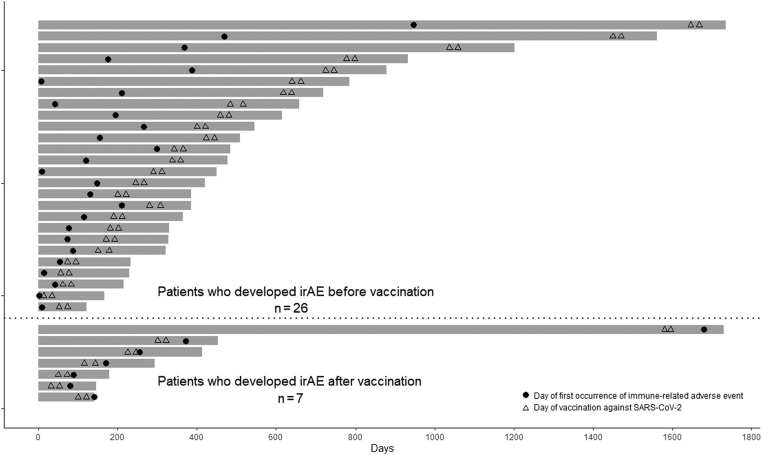
Figure 2.
Swimmer plot of patients who developed an irAE. The swimmer plot reveals the total observation period (d) from the start date of the ICI treatment regimen to the end date of observation. The dates of the two doses of mRNA vaccine against SARS-CoV-2 are indicated by triangles, and the date of onset of an irAE is indicated by a dot for each of the 26 patients who developed an irAE before vaccination and the seven patients who developed an irAE after vaccination. ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; mRNA, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The seven patients who developed a new irAE after the first vaccination, at a median of 47.0 days (IQR: 39.0–62.0) after first vaccination and a median of 170.0 days (IQR: 114.0–313.0) after the initiation of ICI treatment, were all vaccinated with BNT162b2. None of the patients developed irAEs between the first and second doses of vaccine, and all developed irAEs after the second dose. Regarding the grade (G) of irAEs, G1 to 2 irAEs occurred in six patients (4.8%) and G3 irAEs occurred in one patient (0.8%) (Table 2). G4 to G5 irAEs were not observed in any patient. The most common irAE was interstitial pneumonia, which occurred in three patients (2.4%), followed by endocrine disorders in two patients (1.6%), and gastrointestinal disorders in two patients (1.6%).
Table 2.
irAEs During the Entire Observation Period
| Variables | Taking ICIs (N = 126) |
||
|---|---|---|---|
| Prevaccination | Postvaccination | During the Entire Observation Period | |
| irAEs | |||
| Grade, n (%) | |||
| Any grade, | 26 (20.6) | 7 (5.6) | 33 (26.2) |
| Grades 1–2 | 21 (16.7) | 6 (4.8) | 27 (21.4) |
| Grade 3 | 5 (4.0) | 1 (0.8) | 6 (4.8) |
| Grades 4–5 | 0 (0) | 0 (0) | 0 (0) |
| Type, n (%) | |||
| Skin | 9 (7.1) | 0 (0) | 9 (7.1) |
| Gastrointestinal | 5 (4.0) | 2 (1.6) | 7 (5.6) |
| Hepatic | 0 (0) | 0 (0) | 0 (0) |
| Lung | 1 (0.8) | 3 (2.4) | 4 (3.2) |
| Endocrine | 14 (11.1) | 2 (1.6) | 16 (12.7) |
| Musculoskeletal/rheumatologic | 1 (0.8) | 0 (0) | 1 (0.8) |
| Renal | 1 (0.8) | 1 (0.8) | 2 (1.6) |
| Nervous system (neurologic) | 0 (0) | 0 (0) | 0 (0) |
| Hematologic | 0 (0) | 0 (0) | 0 (0) |
| Cardiovascular | 0 (0) | 0 (0) | 0 (0) |
| Ocular | 0 (0) | 0 (0) | 0 (0) |
| ICI treatment discontinuation due to irAEs | 2 (1.6) | 2 (1.6) | 4 (3.2) |
ICI, immune checkpoint inhibitor; irAE, immune-related adverse event.
Furthermore, 26 patients developed irAEs before the first vaccination, at a median of 119.5 days (IQR: 65.5–390.5) before the first vaccination and a median of 125.0 days (IQR: 44.8–210.0) after ICI treatment initiation. Of them, one patient received mRNA-1273, whereas all others received BNT162b2. G1 to 2 irAEs occurred in 21 patients (16.7%) and G3 irAEs occurred in five patients (4.0%). G4 to 5 irAEs were not observed in any patient. The most common irAE was endocrine abnormalities, which occurred in 14 patients (11.1%), followed by skin disorders in nine patients (7.1%).
There was no significant difference between the group of patients who developed new irAEs before vaccination and the group who developed new irAEs after vaccination in the time between the introduction of ICIs and the onset of irAEs (p = 0.21). Discontinuation of ICI treatment owing to irAEs was observed in four (3.2%) patients during the entire observation period; two developed irAEs before the first dose of the vaccine and the remaining two developed irAEs after the second dose.
A fever of 37.5°C or higher was observed in six (4.8%) and 33 patients (26.2%) with lung cancer within 1 week of the first and second doses, respectively. The median length of time of onset of fever was 2 days (IQR: 2–3) after the first dose and 1 day (IQR: 1–1) after the second dose. These incidences were not notably different from the data of a cohort study led by the Japanese Ministry of Health, Labour and Welfare during the early stages of BNT162b2 mRNA vaccine administration in Japan. In this case, 3% and 38% of the participants had a fever of 37.5°C or higher within 1 week after receiving the first and second vaccination, respectively.27 For fever, only follow-up or medication with acetaminophen or nonsteroidal anti-inflammatory drugs was prescribed; antimicrobial agents and systemic corticosteroids were not administered.
SARS-CoV-2 Antibody Titer Trends Over Time
We collected and tested 371 serum samples from 65 patients with lung cancer who were actively being treated with ICIs, had no history of COVID-19 (50 males, 76.9%; median age 71 y [IQR: 65.3–75.0]), and had sequential samples available. There were 21 duplicate samples collected from the same patient during the same period that were excluded from the analysis. The age- and sex-matched control group consisted of 65 patients (50 males, 76.9%; median age 70 y [IQR: 65.0–75.0]) who did not receive anticancer drugs or immunosuppressive therapy, from which we collected a serum sample for each time period. A total of 314 samples from control patients were tested for analysis. All 130 patients among both groups were vaccinated twice with BNT162b2, with a median interval of 21 days (IQR: 21–21).
The titer of S-IgG antibody in the lung cancer patient group peaked at day 35 (median, 35 d [IQR: 33–38] after the first dose of vaccine, which corresponded to a median of 14 d [IQR: 14–15] after the second dose of vaccine). The titer of S-IgG antibody in the control group also peaked at day 35 (median, 35 d [IQR: 35–36] after the first dose of vaccine, which corresponded to a median of 14 d [IQR: 14–15] after the second dose of vaccine; Fig. 3 ). On day 35, 59 of 61 (96.7%) patients with lung cancer treated with ICIs tested positive for S-IgG antibody according to the manufacturer’s cutoff, whereas 65 of 65 control patients (100%) tested positive (p = 0.23). Considering 4160 AU/mL as the surrogate marker for a sufficiently neutralizing response titer, antibody positivity was 24 of 61 (39.3%) and 58 of 65 (89.2%) in the patients with lung cancer and the controls, respectively, with significantly lower positivity in patients with lung cancer (p < 0.001). The median values of S-IgG were 3238.6 (IQR: 1370.7–7087.1) and 12,260.7 (IQR: 6795.1–21,791.9) AU/mL in the two groups, respectively, and were significantly lower in patients with lung cancer (p < 0.001). Furthermore, the S-IgG level was significantly lower in the lung cancer group than in the control group at 21 and 90 days (p < 0.001) after the first vaccination and at 180 days (p = 0.02) after the first vaccination.
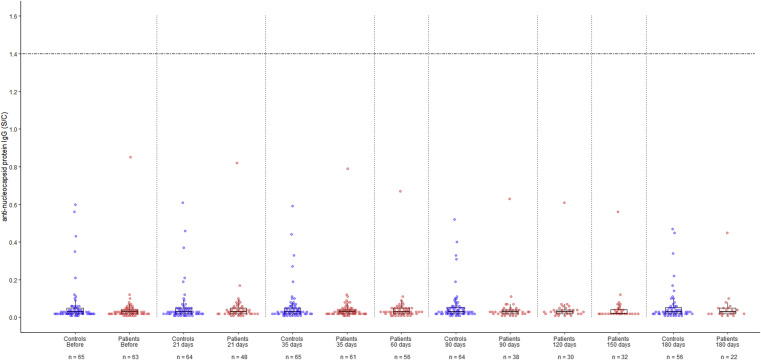
No seroconversion was observed in anti–N-IgG antibody on the basis of the manufacturer’s cutoff value, and the antibody titers did not change over time in either patients with lung cancer or controls (Fig. 4 ). This was consistent with the fact that none of the study participants had been infected with SARS-CoV-2 just before or after vaccination.
Figure 4.
Beeswarm and box plots revealing the temporal dynamic changes in the antinucleocapsid (N) protein IgG antibody levels of patients with lung cancer actively treated with ICIs and control patients, who did not receive anticancer drugs or immunosuppressive therapy, before and after vaccination with mRNA SARS-CoV-2 vaccine. Data for the 65 patients with lung cancer who were being actively treated with ICIs are illustrated with red dots and the data for 65 control patients are illustrated with blue dots; all patients received two doses of the BNT162b2 vaccine. Box plots display the median values with IQR (lower and upper hinges) ±1.5-fold the IQR from the first and third quartiles (lower and upper whiskers, respectively). The horizontal dotted line represents the manufacturer’s 1.4 S/C value (S/C: the chemiluminescent signal ratio between the samples and a calibrator). ICI, immune checkpoint inhibitor; IQR, interquartile range; mRNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The median antibody titers of S-IgG at 35 days after the first vaccination in the patients who received immunotherapy (ICI monotherapy), maintenance ICI monotherapy after treatment including cytotoxic therapy, and immunochemotherapy were 4833.0 (IQR: 2312.8–7146.1, n = 16), 2470.5 (IQR: 1084.2–5933.8, n = 29), and 3582.8 (IQR: 1413.6–10,916.6, n = 16), respectively, with no significant difference. Furthermore, no significant difference was observed between patients who received chemotherapy in a period close to vaccination and those who did not, with median S-IgG titers at 35 days after initial vaccination being 3582.8 (IQR: 1413.6–10,916.6, n = 16) and 3098.9 (IQR: 1235.1–6961.4, n = 45; p = 0.41), respectively. Similarly, there was no significant difference in the patients treated with anti–PD-1 and anti–PD-L1 inhibitors, and the median titer of S-IgG on day 35 after the first vaccination was 3402.6 (IQR: 1374.2–7547.0, n = 42) and 3098.9 (IQR: 1329.2–5303.6, n = 19; p = 0.60), respectively. The median S-IgG antibody titer at day 35 in the 21 patients who developed irAEs (17 before and four after vaccination) and the 40 patients who did not develop irAEs was 3945 (IQR: 1639.0–6961.0) and 2875.1 (IQR: 1197.4–7669.4), respectively, with no significant difference (p = 0.38).
Discussion
This report statistically confirms that mRNA vaccination against SARS-CoV-2 does not increase irAEs in patients with lung cancer being actively treated with ICIs in a real-world setting. This study is the first to report changes in antibody titers over time before and after vaccination in this specific group of patients and to compare immunogenicity after full vaccination in the same patient population with an age- and sex-matched control group. The results reveal that antibody titers in patients with lung cancer receiving ICIs were significantly lower than those in the matched control group during peak antibody titers. In addition, there was no significant difference in S-IgG antibody titer between patients with and without irAEs.
Several studies have reported on the safety of vaccines against SARS-CoV-2 in patients with cancer who are treated with ICIs in which irAEs were descriptively detailed.28, 29, 30, 31 The short-term safety of the BNT162b2 vaccine in patients receiving ICIs was evaluated in one study.28 In this case, 134 patients with cancer undergoing treatment with ICIs, including 66 patients with lung cancer, received two doses of the vaccine and were assessed by telephone for irAEs (median 19 d after the second dose of vaccination). The frequency of irAEs to the vaccine was not significantly different from that of the control group, and there were no new or worsening conditions of existing irAEs after vaccination.28 A small number of patients, however, reported the development of irAEs after vaccination in other studies. For example, of 59 patients with cancer being treated with ICIs who received a vaccine (BNT162b2 69.5%, mRNA-1273 25.4%, ChAdOx1-S 5.1%), including 16 patients with lung cancer, one patient (1.7%) experienced an irAE after receiving one dose of vaccine.29 Among patients with solid tumors who were vaccinated with mRNA-1273, 6 of 137 (4.4%) treated with ICI monotherapy and 7 of 163 (4.3%) treated with chemoimmunotherapy experienced irAEs 28 days after the second dose was administered.30 In addition, Chen et al.31 conducted a review of the U.S. Food and Drug Administration’s Vaccine Adverse Event Reporting System database and found that of 81 patients with cancer, including 22 patients with lung cancer, who received ICIs within 1 month of receiving a COVID-19 mRNA vaccine, nine had irAEs before the vaccination (all ≤ grade 2) and six had clinical presentations suggestive of irAEs after vaccination. This included two with possible flares of preexisting irAEs and four de novo irAEs.31
The fact that the incidence of irAEs did not increase in the present study after a relatively long duration (median 144 d after vaccination) compared with historical data is consistent with the above-mentioned information. In addition, the fact that there was no difference in the period between the introduction of ICI treatment and the occurrence of irAEs in the two groups of patients who developed irAEs before and after vaccination in this study can be regarded as collateral evidence that vaccination was not involved in the occurrence of irAEs.
A previous study revealed that after two doses of mRNA vaccine against SARS-CoV-2, patients treated with ICI-containing regimens (including monotherapy and immunochemotherapy) seroconverted 99% and 100% and healthy controls.30 Among them, 0.4% in the control group and 6.9% and 11.1% in the immunotherapy and immunochemotherapy groups, respectively, were suboptimal responders.30 The present study also revealed a high seroconversion rate of 98%. In contrary, the rate of suboptimal responders was higher and the antibody titer was predominantly lower in patients with lung cancer than in controls, which implies that patients with lung cancer on ICI therapy mount a subdued immune response. Recently, Mack et al.32 and Valanparambil et al.33 reported that patients with lung cancer have lower immunogenicity than healthy controls; furthermore, no significant difference was observed in immunogenicity among patients receiving different types of cancer treatment (targeted therapy, chemotherapy, immunotherapy, immunochemotherapy, and no treatment); the results of the present study are consistent with those reported in these studies. The reason for the low antibody titer in patients with lung cancer being treated with ICIs who participated in the present study is not clear, but it could be due to the ICI, cancer, low nutritional status owing to cancer, or treatment other than with ICIs (treatment with cytotoxic anticancer agents before vaccination, steroid use for antiemetics, or other palliative measures). In addition, Mack et al.32 reported an increase in serum antibody titer after the administration of the third booster vaccination dose in patients with lung cancer with undetectable antibody titer after two times vaccination with mRNA vaccine. In view of these findings, in patients with lung cancer, serial vaccination dosing and modified vaccine schedules, such as the number of doses and intervals, should be considered to obtain and maintain active immunity.
This study has some limitations. The first involves the comparison of the incidence of irAEs with historical data. Originally, we wanted to compare the incidence of irAEs in patients with lung cancer who were being treated with ICIs, with and without vaccination. Nevertheless, we were unable to determine the percentage of patients who were willing to be vaccinated, and we felt that it would be ethically difficult to intentionally create a group that would not be vaccinated at the time of COVID-19 pandemic. Second, the diagnosis and grading of irAEs were performed at each participating facility and not centrally reviewed, although the classification and grading were performed on the basis of the same standard at each facility. It is possible that the incidence of irAEs may have been underestimated compared with historical data from clinical trials. Third, the S-IgG level was used as a surrogate for vaccine efficacy. We could not measure neutralizing antibodies and cellular immunity owing to the complexity of the measurements required. Neutralizing antibodies and cellular immunity are potentially directly related to the susceptibility to infection. Finally, this study revealed a response to only one type of vaccine against SARS-CoV-2 (mRNA vaccines), although other vaccines have been launched.
In conclusion, patients with lung cancer who were actively treated with ICIs were safely vaccinated without an increased incidence of irAEs in a real-world setting; however, their immune response to vaccination was lower and might be less effective in preventing COVID-19. In the future, real-world vaccination efficacy in patients with lung cancer, who are actively being treated with ICIs, should be evaluated as they are a particularly vulnerable patient group.
CRediT Authorship Contribution Statement
Makoto Hibino: conceptualization, data curation, formal analysis, investigation, methodology, and writing of the original draft.
Kiyoaki Uryu: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Takayuki Takeda: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Yusuke Kunimatu: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Shinsuke Shiotsu: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Junji Uchino: Methodology and writing of the manuscript (review and editing).
Soichi Hirai: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Tadaaki Yamada: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Asuka Okada: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Yoshikazu Hasegawa: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Osamu Hiranuma: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Yusuke Chihara: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Riko Kamada: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Shunichi Tobe: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Kazunari Maeda: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Shigeto Horiuchi: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Tetsuri Kondo: Data curation, investigation, provision of resources, and writing of the manuscript (review and editing).
Koichi Takayama: Writing of the manuscript (review and editing).
Data Availability
All data relevant to the study are included in the article.
Acknowledgments
The authors thank the patients for their consent to participate; the clinical research coordinator and laboratory staff at our hospital for specimen collection and antibody measurement; Editage (www.editage.jp) for English language editing; Abbott Laboratories (IL), for providing antibody reagents against SARS-CoV-2 (it did not have any active role in the study); and all of our colleagues, friends, and families for their dedicated support during the COVID-19 pandemic. All authors met the authorship criteria and certify that they have participated sufficiently in the work to take public responsibility for the content.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Pak A., Adegboye O.A., Adekunle A.I., Rahman K.M., McBryde E.S., Eisen D.P. Economic consequences of the COVID-19 outbreak: the need for epidemic preparedness. Front Public Health. 2020;8:241. doi: 10.3389/fpubh.2020.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal Yatoo M., Hamid Z., Parray O.R., et al. COVID-19—recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Hum Vaccin Immunother. 2020;16:2891–2904. doi: 10.1080/21645515.2020.1788310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakouny Z., Hawley J.E., Choueiri T.K., et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivas P., Khaki A.R., Wise-Draper T.M., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartleson J.M., Radenkovic D., Covarrubias A.J., Furman D., Winer D.A., Verdin E. SARS-CoV-2, COVID-19 and the ageing immune system. Nat Aging. 2021;1:769–782. doi: 10.1038/s43587-021-00114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J.R., Lacchetti C., Schneider B.J., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coureau M., Meert A.P., Berghmans T., Grigoriu B. Efficacy and toxicity of immune -checkpoint inhibitors in patients with preexisting autoimmune disorders. Front Med (Lausanne) 2020;7:137. doi: 10.3389/fmed.2020.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desage A.L., Bouleftour W., Rivoirard R., et al. Vaccination and immune checkpoint inhibitors: does vaccination increase the risk of immune-related adverse events? a systematic review of literature. Am J Clin Oncol. 2021;44:109–113. doi: 10.1097/COC.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 12.Abbott Laboratories . Abbott Laboratories; Abbott Park, IL: 2020. Diagnostics Division. SARS-CoV-2 IgG II Quant Assay User Manual.https://s3.eu-west-2.amazonaws.com/s3.privatecoronavirustests.com/content/pages/Quantitative.pdf [Google Scholar]
- 13.Ebinger J.E., Fert-Bober J., Printsev I., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 15.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomized, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 16.Gadgeel S., Rodríguez-Abreu D., Speranza G., et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 17.Paz-Ares L., Vicente D., Tafreshi A., et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Antonia S.J., Villegas A., Daniel D., et al. Durvalumab after chemoradiotherapy in Stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 20.von Pawel J., Syrigos K., Mazieres J., et al. Association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: analyses from the phase III study OAK. Ann Oncol. 2017;28(suppl 5):v469. [Google Scholar]
- 21.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Pooled analyses of immune-related adverse events (irAEs) and efficacy from the phase 3 trials IMpower130, IMpower132, and IMpower150. J Clin Oncol. 2021;39(suppl 15) doi: 10.1001/jamaoncol.2022.7711. 9002–9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haratani K., Hayashi H., Chiba Y., et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong C.R., Park V.J., Cohen B., Postow M.A., Wolchok J.D., Kamboj M. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;70:193–199. doi: 10.1093/cid/ciz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Failing J.J., Ho T.P., Yadav S., et al. Safety of influenza vaccine in patients with cancer receiving pembrolizumab. JCO Oncol Pract. 2020;16:e573–e580. doi: 10.1200/JOP.19.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijn D.H., Groeneveld G.H., Vollaard A.M., et al. Influenza vaccination in patients with lung cancer receiving anti-programmed death receptor 1 immunotherapy does not induce immune-related adverse events. Eur J Cancer. 2018;104:182–187. doi: 10.1016/j.ejca.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Bayle A., Khettab M., Lucibello F., et al. Immunogenicity and safety of influenza vaccination in cancer patients receiving checkpoint inhibitors targeting PD-1 or PD-L1. Ann Oncol. 2020;31:959–961. doi: 10.1016/j.annonc.2020.03.290. [DOI] [PubMed] [Google Scholar]
- 27.Ministry of Health, Labour and Welfare Survey on the health status after vaccination against SARS-CoV-2. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine_pfizer.html
- 28.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terpos E., Zagouri F., Liontos M., et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J Hematol Oncol. 2021;14:86. doi: 10.1186/s13045-021-01099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oosting S.F., van der Veldt A.A.M., GeurtsvanKessel C.H., et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumors: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22:1681–1691. doi: 10.1016/S1470-2045(21)00574-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y.W., Tucker M.D., Beckermann K.E., Iams W.T., Rini B.I., Johnson D.B. COVID-19 mRNA vaccines and immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. 2021;155:291–293. doi: 10.1016/j.ejca.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack P.C., Gomez J.E., Rodilla A.M., et al. Longitudinal COVID-19-vaccination-induced antibody responses and Omicron neutralization in patients with lung cancer. Cancer Cell. 2022 June 13;40(6):P575–P577. doi: 10.1016/j.ccell.2022.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valanparambil R, Carlisle J, Linderman S, et al. Antibody response to SARS-CoV-2 mRNA vaccine in lung cancer patients: reactivity to vaccine antigen and variants of concern. Preprint. medRxiv. https://doi.org/10.1101/2022.01.03.22268599, Accessed May 17, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article.