Abstract
Atmospheric methane consumption by Maine forest soils was inhibited by additions of environmentally relevant levels of aluminum. Aluminum chloride was more inhibitory than nitrate or sulfate salts, but its effect was comparable to that of a chelated form of aluminum. Inhibition could be explained in part by the lower soil pH values which resulted from aluminum addition. However, significantly greater inhibition by aluminum than by mineral acids at equivalent soil pH values indicated that inhibition also resulted from direct effects of aluminum per se. The extent of inhibition by exogenous aluminum increased with increasing methane concentration for soils incubated in vitro. At methane concentrations of >10 ppm, inhibition could be observed when aluminum chloride was added at concentrations as low as 10 nmol g (fresh weight) of soil−1. These results suggest that widespread acidification of soils and aluminum mobilization due to acid precipitation may exacerbate inhibition of atmospheric methane consumption due to changes in other parameters and increase the contribution of methane to global warming.
A number of factors adversely affect atmospheric methane consumption by soils. Ammonium is one of the most important of these (6, 8, 13, 14, 20, 30, 31, 37, 49), but other factors include water stress (47), terpenes (3), salts (1, 21, 27, 32), and land use (22, 23, 25, 28). Soil pH has also been documented as a potentially important limiting factor, with both acidic (pH <4) and alkaline (pH >7) regimes inhibiting activity (2, 12, 22; J. Benstead and G. M. King, unpublished results). Although some evidence supports a role in methane consumption for acid-tolerant or moderately acidophilic methanotrophs in peats (12), the acid-tolerant peat isolates described to date have not been shown to consume atmospheric methane, and acid-tolerant methanotrophs have not been documented for soils.
In contrast to the impact of pH in peats, the effects of pH on methanotrophic activity in acidic soils may be compounded by solubilization of aluminosilicates, which constitute a major fraction of the mineral horizons where atmospheric methane consumption occurs most actively. Although the chemistry of aluminum is well understood (36) and its toxic effects on multicellular organisms are known in some detail (15, 17, 34–36, 52), the response of microbes to aluminum is not well documented (42).
Several studies have examined the effects of aluminum on cyanobacteria and fungi and documented a range of responses (10, 41–43). Physiological studies with bacteria have emphasized well-known strains, such as Escherichia coli, Staphylococcus aureus, Bacillus megaterium, and Pseudomonas fluorescens (4, 11, 19, 42, 44). A broader range of studies have focused on rhizobia and documented toxicity in cultures and in bacterium-legume symbioses (7, 8, 18, 24, 26, 33, 38, 39, 43, 50, 51). However, the effects of aluminum on microbes or microbial processes in a more general ecological context have not been adequately assessed.
Nonetheless, dissolved aluminum concentrations can reach toxic levels in soil solutions with pH values of <4.8 or >7.4. These values occur naturally in many soils and are increasingly common because of widespread acidification associated with acid precipitation (42). Acidification clearly mobilizes aluminum and has been associated with significant impacts on plant and animal populations (7, 16, 48). Whether microbial processes in soils are similarly affected is not certain.
We report here responses of atmospheric methane consumption in Maine forest soils to exogenous aluminum salts. At aluminum concentrations of >1 μmol g (fresh weight) (gfw) of soil−1, atmospheric methane consumption decreased as a consequence of direct effects of aluminum and indirect effects due to decreased soil pH. The effects of aluminum were comparable for several different forms (a citrate chelate and nitrate and sulfate salts) but were greatest for the chloride salt. At concentrations of <1 μmol gfw of soil−1, which did not affect soil pH significantly, aluminum had a dramatic effect on the kinetics of methane consumption: the maximum uptake velocity (Vmax) decreased markedly and there were somewhat smaller and more variable decreases in the apparent Km. In contrast, addition of ammonium decreased the Vmax and increased the apparent Km.
MATERIALS AND METHODS
The effects of aluminum salts on methane consumption were determined by using sieved (2-mm mesh) A-horizon soils from the depth of greatest methanotrophic activity, 6 to 10 cm, in a mixed conifer-hardwood forest at the Darling Marine Center. Various characteristics of the site have been described previously (1, 29–33, 46). For routine assays, 10-gfw soil samples were transferred to glass jars with a headspace of about 110 cm3. One-half-milliliter volumes of deionized water, stock solutions containing an aluminum salt (chloride, nitrate, or sulfate), or sulfuric acid were pipetted carefully onto the soil samples, each of which was mixed gently but thoroughly with a small spatula. The final concentrations of added aluminum ranged from <0.1 to 8 μmol of Al gfw of soil−1. Sulfuric acid or other mineral acids were added at concentrations based on the maximum proton production expected from aluminum hydrolysis (i.e., a ratio of 3 H+ to 1 Al3+). After aluminum stocks, acid, or deionized water was added, the jars were sealed with butyl rubber stoppers that did not release detectable levels of methane or other hydrocarbons. The initial methane concentrations in the jar headspaces ranged from the atmospheric concentration to 1%, with superatmospheric levels obtained by adding ultra-high-purity methane as needed. For these and all other assays, the soil water contents were 25 to 35% as determined by drying soils for 24 h at 105°C.
Methane uptake rates were determined by using time course measurements of headspace subsamples (0.3 cm3) removed from the jars with a needle and syringe for assay by flame ionization gas chromatography with a Shimadzu GC-14AM gas chromatograph as described by King and Adamsen (29). Detector responses were analyzed with an HP-3396 integrator (Hewlett-Packard, Inc.) and standardized with 3.16 ppm of methane in nitrogen (Maine OxyAcetylene, Inc.). For initial headspace methane concentrations of <10 ppm, methane uptake rate constants were estimated from a nonlinear regression analysis (Kaliedagraph; Adelbeck Software, Inc.) of exponential decreases over time; linear regressions were used for initial headspace concentrations of >10 ppm. All treatments were run in triplicate.
The effects of chelated aluminum compared with those of nonchelated aluminum were assessed by preparing stock solutions of aluminum chloride with sodium citrate, with concentration of the latter one-third of the aluminum concentration in accord with the stoichiometry of aluminum citrate complexes. Treatments consisted of adding aluminum chloride, aluminum citrate, or citrate to soil at final concentrations of either 0.1 or 1.6 μmol gfw of soil−1. Initial headspace methane concentrations of 1.8 and 64 ppm were used for each of the salt treatments. Uptake rates were assessed as described above in triplicate.
Kinetic parameters for methane uptake were determined after addition of 0, 0.01, 0.04, 0.1, 0.4, or 1.6 μmol of Al3+ (as the sulfate salt) gfw of soil−1. At each of these Al3+ concentrations, soils were incubated with headspace methane concentrations of 1.8, 8, 16, 32, 64, 120, and 240 ppm. Methane uptake rates were determined as before. Apparent half-saturation constants (Ks) and Vmax were estimated from nonlinear regression analysis by using a Michaelis-Menten model and Kaleidagraph software (Adelbeck Software). Similar assays were conducted by using ammonium chloride at final concentrations of 0 to 4 μmol gfw of soil−1 and methane headspace concentrations of 1.8, 5, 10, 20, 50, and 100 ppm.
The ability of added aluminum to desorb ammonium was measured by adding 0.5-ml volumes of aluminum sulfate or sulfuric acid stock solutions to triplicate 10-gfw soil samples, producing final added concentrations of 1 μmol of Al3+ or 3 μmol of H+ gfw soil−1. Ammonium was subsequently extracted by adding deionized water and centrifuging the slurries (32). Ammonium concentrations were assayed by a salicylate-hypochlorite colorimetric method described by Bower and Holm-Hansen (5).
RESULTS
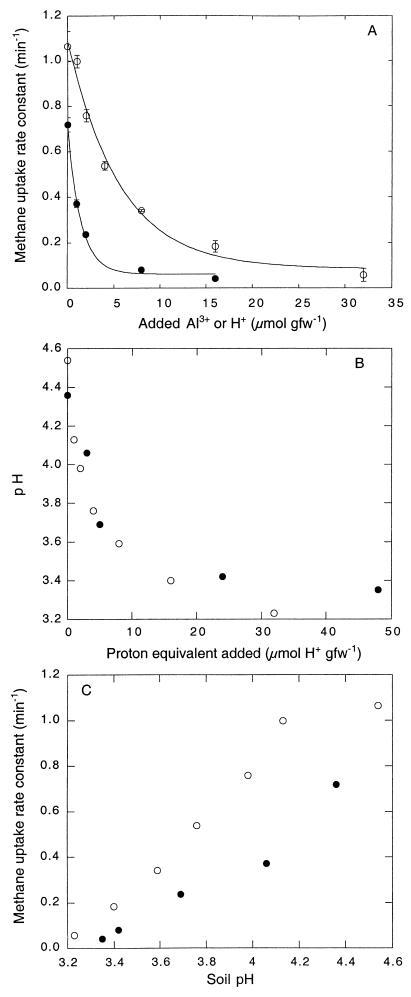
Atmospheric methane uptake rate constants decreased with increasing aluminum sulfate concentrations (Fig. 1). Compared to deionized water controls, the uptake rates were reduced by approximately 50 and 90% for additions of 1 and 8 μmol of Al gfw of soil−1, respectively. The uptake rate constants also decreased as a function of increasing sulfuric acid concentrations (Fig. 1A). The decreases in the uptake rate constants for the aluminum and sulfuric acid treatments were fit using nonlinear regression to a relationship of the following form (Fig. 1A): RC = RCfinal + (RCinit − RCfinal)e−kX, where RC is the uptake rate constant, RCinit is the initial uptake rate constant value, RCfinal is the final uptake rate constant value, k is a decay or inhibition constant, and X is the concentration of Al or H+ added to the soils. For this relationship, the inhibition constants for Al and H+ were 0.699 ± 0.066 and 0.177 ± 0.027, respectively.
FIG. 1.
(A) Atmospheric methane uptake rate constants for soils amended with various concentrations of aluminum sulfate (●) or sulfuric acid (○). Data are means of triplicate determinations ± 1 standard error. (B) Plot of soil pH versus amount of proton equivalent added for aluminum sulfate (●) or sulfuric acid (○). Note that the molar ratio of proton equivalents is 3:1 for aluminum additions. (C) Plot of methane uptake rate constants for aluminum sulfate (●) or sulfuric acid (○) versus soil pH.
Since the hydrolysis of Al(6H2O)3+ is accompanied by up to 3 H+ equivalents [i.e., Al(6H2O)3+ → Al(H2O)3 + 3H+], the estimates indicated that acidification itself might have accounted for about 76.1% of the observed inhibition, with other direct effects of Al accounting for the remainder (23.9%). However, the soil pH was lower (Fig. 1B) for sulfuric acid treatments than for aluminum treatments at comparable levels of proton addition (i.e., at a ratio of 3 H+ equivalents for sulfuric acid per mol of aluminum sulfate). Furthermore, the methane uptake values for soils at comparable pH values were lower for aluminum sulfate treatments than for sulfuric acid treatments (Fig. 1C). Thus, the level of inhibition directly attributable to aluminum was likely greater than that indicated by the preceding calculation.
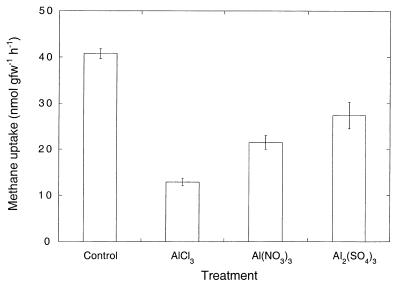
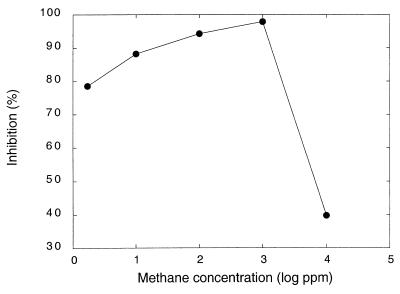
Aluminum chloride was significantly more inhibitory than the nitrate and sulfate salts at a concentration of 1 μmol of Al gfw of soil−1 for a methane concentration of 100 ppm; inhibition by aluminum nitrate and inhibition by aluminum sulfate did not differ statistically (Fig. 2). For each of the salts, the extent of inhibition was greater at 100 ppm of methane than at atmospheric methane concentrations (data not shown). The trend of increasing inhibition with increasing methane concentrations was confirmed by incubating soils containing aluminum chloride at 2 μmol gfw of soil−1 with headspace methane levels ranging from the atmospheric concentration to 10,000 ppm (Fig. 3). Compared to controls without aluminum, aluminum inhibition was greatest for 1,000 ppm of methane, and lower levels of inhibition occurred at methane concentrations below or above this level.
FIG. 2.
Methane uptake rates for various aluminum salt additions (1 μmol of Al3+ gfw−1). Soils were incubated with an initial headspace methane concentration of 100 ppm. Data are means of triplicate determinations ± 1 standard error.
FIG. 3.
Percent inhibition of methane consumption by AlCl3 (2 μmol of Al3+ gfw−1) for soils incubated with various methane concentrations (atmospheric concentration to 10,000 ppm). Percent inhibition was determined from the ratio of methane uptake for aluminum-treated soils to methane uptake for untreated soils at each methane concentration. Data are means of triplicate determinations ± 1 standard error.
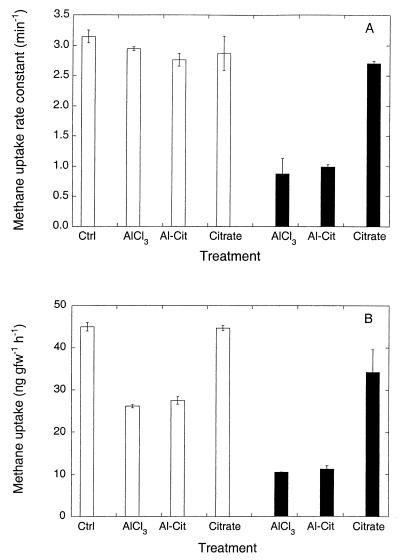
Addition of aluminum in a chelated form (as a citrate complex) did not affect the inhibition patterns. Neither aluminum chloride nor aluminum citrate at 0.1 μmol of Al gfw of soil−1 significantly decreased atmospheric methane consumption compared to a treatment with citrate alone or deionized water controls. However, both aluminum treatments, but not the citrate treatment, decreased methane uptake at headspace concentrations of 64 ppm (Fig. 4A). At concentrations of 1.6 μmol of Al gfw of soil−1, the effects of the chloride salt and the citrate complex were also comparable, but in this case inhibition was observed for both atmospheric methane and 64 ppm of methane, with greater inhibition for the latter (Fig. 4B).
FIG. 4.
(A) Methane uptake rate constants for soils incubated with atmospheric methane after addition of AlCl3, aluminum citrate, or citrate at a concentration of 0.1 μmol of Al3+ gfw−1 (open bars) or 1.6 μmol of Al3+ gfw−1 (solid bars). Note that the citrate concentrations are one-third those of aluminum. Data are means of triplicate determinations ± 1 standard error. (B) Same as panel A, but soils were incubated with an initial headspace methane concentration of 64 ppm. Ctrl, control; Al-Cit, aluminum citrate.
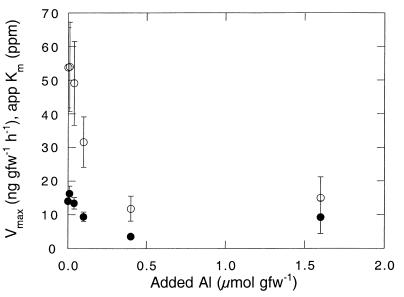
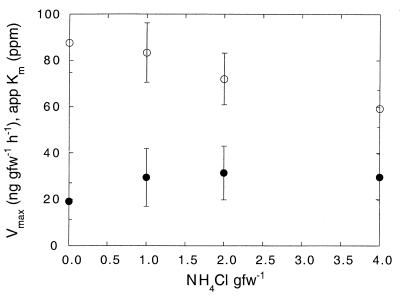
A kinetic analysis revealed consistently low apparent Km values (14.0 ± 0.5 ppm) for soils amended with only deionized water. Vmax values for these soils were 53.8 ± 12.0 ng of CH4 gfw of soil−1 h−1. Addition of aluminum chloride at concentrations ranging from 0.01 to 1.6 μmol of Al gfw of soil−1 tended to decrease the apparent Km, especially at the higher aluminum concentrations, although the changes were not statistically significant (P > 0.1) (Fig. 5). In contrast, Vmax was strongly depressed, with distinct inhibition apparent at 0.1 μmol Al of gfw of soil−1 (Fig. 5). Added ammonium at relatively high concentrations decreased Vmax, but little effect on Vmax was noted with ammonium at final concentrations of 0.1 to 1 μmol gfw of soil−1 (Fig. 6). Overall, the Vmax appeared to be more sensitive to exogenous aluminum than to ammonium. In contrast, ammonium at all concentrations increased the apparent Km by comparable amounts (Fig. 6).
FIG. 5.
Vmax (○) and apparent Km (app Km) (●) as a function of added aluminum chloride. Data are means of triplicate determinations ± 1 standard error.
FIG. 6.
Vmax (○) and apparent Km (app Km) (●) as a function of added NH4Cl. Data are means of triplicate determinations ± 1 standard error.
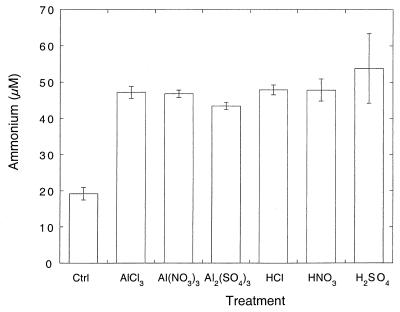
Aluminum salt or acid additions increased the ammonium concentrations in aqueous soil extracts approximately twofold compared to soils treated with only deionized water (Fig. 7). However, there were no significant differences among the aluminum or acid treatments (P > 0.1). The amounts of ammonium mobilized by aluminum or acid additions, about 25 to 35 nmol gfw of soil−1, represented <1% of the cation equivalents added to the soil but accounted for a large fraction (50 to 70%) of the ammonium typically found in 1 N KCl extracts of the soils.
FIG. 7.
Ammonium concentrations in aqueous extracts (1 ml of deionized water gfw−1) for soils incubated with 1 μmol of aluminum salts gfw−1 or 3 μmol of acids gfw−1. Data are means of triplicate determinations ± 1 standard error. Ctrl, control.
DISCUSSION
Exogenous aluminum inhibits atmospheric methane consumption by Maine forest soils (Fig. 1). At pH values typical of the soils used in this study (pH ≤4.5) and a water content of 30%, aluminum is soluble, with expected concentrations of approximately 3.3 mM for an addition of 1 μmol of Al gfw of soil−1. Since acidic soils often contain millimolar levels of dissolved aluminum (42), the amounts of aluminum added during this study and the responses to them are ecologically significant.
Although exogenous aluminum inhibits atmospheric methane consumption, the specific causes of inhibition are complex and include several factors. For example, exogenous aluminum can decrease methane uptake by decreasing the soil pH (Fig. 1A and B 5). However, since sulfuric acid inhibits activity less than equivalent amounts of aluminum sulfate inhibit activity and since aluminum is notably more inhibitory than sulfuric acid at a given soil pH (Fig. 1C), pH changes only partially account for inhibition by exogenous aluminum.
Based on the responses of several microbial taxa, including methanotrophs (P. Milligan and G. M. King, unpublished data), aluminum toxicity for methanotrophs in soils likely involves a variety of direct effects. These may include changes in membranes, disruption of enzyme activities, and decreased ATP synthesis (42). In addition, Gulledge and Schimel (21) have suggested that certain cations, e.g., K+, may decrease methanotrophic activity in soils by some general, but unspecified, mechanism that may apply to aluminum. However, the absence of significant Na+ or K+ inhibition in cultures, in contrast to distinct inhibition by aluminum and ammonium (32; Milligan and King, unpublished data), suggests that aluminum inhibition in soils arises from element-specific phenomena that cannot be readily controlled for or estimated by comparisons with other cations.
The effects of exogenous aluminum may depend in part on the form of aluminum added and on the anionic regime in a given soil solution since aluminum chloride appears to be more inhibitory than nitrate or sulfate salts (Fig. 2). Similar differences have been reported for chloride, nitrate, and sulfate salts of ammonium and other cations (21, 32). Gulledge and Schimel (21) have argued that differential anion effects for K+ and ammonium reflect an unspecified toxicity of chloride per se. However, since ammonium chloride salts are no more inhibitory than sulfate salts in pure cultures (32), differential sensitivity of soil methanotrophy to various anions may result from interactions between anions and cations that are expressed in soils but not in cultures. Such interactions include but are not limited to the effects of ion pairing on ion sorption and desorption and cell uptake (32). Regardless, aluminum chloride salts should not be avoided in future studies unless chloride is not an important component of the soil solution in the system being examined. For terrestrial systems affected by acid precipitation, nitrate and sulfate salts may be most appropriate; for systems with a maritime influence, a mixture of chloride and other salts may be required.
Addition of chelated aluminum rather than aluminum salts appears to have little impact on toxicity. This may indicate that soil methanotrophs are equally sensitive to dissolved inorganic aluminum species and low-molecular-weight complexed species. Alternatively, aluminum speciation and toxicity in Maine forest soils may be dominated by naturally occurring ligands in fulvic and humic acid fractions of the soil organic matter, the significance of which is not affected by exogenous citrate. Since the total soil organic matter content (about 5% or 2 mmol of C gfw of soil−1 [1]) vastly exceeds the amount of citrate added in this study (≤1 μmol gfw of soil−1), some redistribution of exogenous aluminum among humic and fulvic acids is expected. The extent to which this occurs and controls aluminum toxicity merits further attention.
Although citrate additions do not affect aluminum toxicity significantly in Maine forest soils, the extent of aluminum toxicity in general depends on the methane concentrations to which soils are exposed (Fig. 3 and 4). The nature of this dependency (that is, increasing inhibition with increasing methane concentrations followed by a decrease in toxicity at elevated levels [>1,000 ppm]) is similar to patterns observed for ammonium toxicity in soils and methanotroph cultures (30, 31, 45). However, the relationship between methane concentration and ammonium inhibition can be explained by competitive effects of ammonium at the level of methane monooxygenase and noncompetitive effects resulting from intracellular hydroxylamine and nitrite production (30, 31). No such explanation is obvious in the case of aluminum. Similarly, increasing inhibition with increasing methane concentrations has been reported for potassium salts in forest soils (21), but since no such trend occurs in methanotroph cultures (32), a physiological explanation for the soil results is uncertain.
For Maine forest soils, increased aluminum (and perhaps potassium) inhibition at elevated methane concentrations may be attributed in part to desorbed ammonium (Fig. 7), which elicits responses comparable to those observed for cultures (31). The extents of desorption observed in this study and previously indicate that cation additions can increase soil water ammonium concentrations by 0.5 to 1 mM, levels that clearly cause inhibition (1, 32, 45). Nonetheless, the mechanisms of increased ammonium and nonammonium salt toxicity warrant further study with soil and culture models since decreases in atmospheric methane consumption capacity represent positive feedback on methane accumulation and greenhouse warming (30).
Results of kinetic analyses indicate that soil methane consumption is especially sensitive to exogenous aluminum but that the inhibition patterns are complex. Decreases in Vmax (Fig. 5) presumably arise from direct effects of aluminum added at relatively low concentrations (0.01 to 0.4 μmol gfw of soil−1), since pH and the concentrations of ammonium and other cations change to only minor extents at these levels. The apparent Km also decreases with low levels of added aluminum, which contrasts with the increase in apparent Km (Fig. 6) observed for ammonium added at 0.3 μmol gfw of soil−1. However, at higher aluminum and ammonium concentrations (e.g., >0.4 and 1 μmol gfw of soil−1, respectively) changes in Vmax and apparent Km likely involve multiple phenomena, including processes previously documented in cultures (31) as well as interactions among various ionic species.
Due to the solubility of aluminum at pH values of <4.8 and the widespread acidification of soils resulting from anthropogenic disturbances, aluminum mobilization may play a significant and increasingly important role in the dynamics of soil-atmosphere methane exchanges. In particular, aluminum mobilization may further exacerbate inhibition of atmospheric methane consumption caused by changes in other parameters (e.g., ammonium, pH) and contribute to a greater global warming potential for methane.
ACKNOWLEDGMENTS
This work was supported in part by NSF award DEB 97-28363.
We thank K. Hardy for excellent technical support.
Footnotes
Contribution 358 from the Darling Marine Center.
REFERENCES
- 1.Adamsen A P S, King G M. Methane consumption in temperate and subarctic forest soils: rates, vertical zonation, and response to water and nitrogen. Appl Environ Microbiol. 1993;59:485–490. doi: 10.1128/aem.59.2.485-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral J A, Ren T, Knowles R. Atmospheric methane consumption by forest soils and extracted bacteria at different pH values. Appl Environ Microbiol. 1998;64:2397–2402. doi: 10.1128/aem.64.7.2397-2402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral J A, Ekins A, Richards S R, Knowles R. Effect of selected monoterpenes on methane oxidation, denitrification, and aerobic metabolism by bacteria in pure culture. Appl Environ Microbiol. 1998;64:520–525. doi: 10.1128/aem.64.2.520-525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appanna V D, St. Pierre M. Influence of phosphate on aluminum tolerance in Pseudomonas fluorescens. FEMS Microbiol Lett. 1994;124:327–322. [Google Scholar]
- 5.Bower C E, Holm-Hansen T. A salicylate-hypochlorite method for determining ammonia in seawater. Can J Fish Aquat Sci. 1980;37:794–798. [Google Scholar]
- 6.Bronson K F, Mosier A R. Suppression of methane oxidation in aerobic soil by nitrogen fertilizers, nitrification inhibitors, and urease inhibitors. Biol Fertil Soils. 1994;17:263–268. [Google Scholar]
- 7.Bruce R C, Warrell L A, Edwards D G, Bell L C. Effects of aluminum and calcium in the soil solution of acid soils on root elongation of Glycine max cv. Forest J Agric Res. 1988;38:319–338. [Google Scholar]
- 8.Carvalho M M, Edwards D G, Asher C J, Andrew C S. Aluminum toxicity, nodulation and growth of Stylosanthes species. Agron J. 1981;73:261–265. [Google Scholar]
- 9.Castro M S, Peterjohn W T, Mellilo J M, Steudler P A, Gholz H L, Lewis D. Effects of nitrogen fertilization on the fluxes of N2O, CH4, and CO2 from soils in a Florida slash pine plantation. Can J For Res. 1994;24:9–13. [Google Scholar]
- 10.Claesson A, Törnqvist L. The toxicity of aluminum to two acido-tolerant green algae. Water Res. 1988;22:977–983. [Google Scholar]
- 11.Davis W B, McCauley M J, Byers B R. Iron requirements and aluminum sensitivity of hydroxamic requiring strains of Bacillus megaterium. J Bacteriol. 1971;105:589–594. doi: 10.1128/jb.105.2.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dedysh S N, Panikov N S, Tiedje J M. Acidophilic methanotrophic communities from Sphagnum peat bogs. Appl Environ Microbiol. 1998;64:922–929. doi: 10.1128/aem.64.3.922-929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunfield P F, Knowles R. Kinetics of inhibition of methane oxidation by nitrate, nitrite, and ammonium in a humisol. Appl Environ Microbiol. 1995;61:3129–3135. doi: 10.1128/aem.61.8.3129-3135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunfield P F, Topp E, Archambault C, Knowles R. Effect of nitrogen fertilizers and moisture content on CH4 and N2O fluxes in a humisol: measurements in the field and intact soil cores. Biogeochemistry. 1995;29:199–222. [Google Scholar]
- 15.Exley C, Birchall D. The cellular toxicity of aluminum. J Theor Biol. 1992;159:83–98. doi: 10.1016/s0022-5193(05)80769-6. [DOI] [PubMed] [Google Scholar]
- 16.Gagen C J, Sharpe W E, Carline R F. Mortality of brook trout, mottled sculpins and slimy sculpins during acidic episodes. Trans Am Fish Soc. 1993;122:616–628. [Google Scholar]
- 17.Ganrot P O. Metabolism and possible health effects of aluminum. Environ Health Perspect. 1986;65:363–441. doi: 10.1289/ehp.8665363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham P H. Stress tolerance in Rhizobium and Bradyrhizobium and nodulation under adverse soil conditions. Can J Microbiol. 1992;38:474–484. [Google Scholar]
- 19.Guida L, Saidi Z, Huges M N, Poole R K. Aluminum toxicity and binding to Escherichia coli. Arch Microbiol. 1991;156:507–512. doi: 10.1007/BF00245400. [DOI] [PubMed] [Google Scholar]
- 20.Gulldege J A, Doyle A P, Schimel J P. Different NH4+ inhibition patterns of soil CH4 consumption: a result of distinct CH4-oxidizer populations across sites? Soil Biol Biochem. 1997;29:13–21. [Google Scholar]
- 21.Gulldege J A, Schimel J P. Low-concentration kinetics of atmospheric CH4 oxidation in soil and mechanism of NH4+ inhibition. Appl Environ Microbiol. 1998;64:4291–4298. doi: 10.1128/aem.64.11.4291-4298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hütsch B W, Webster C P, Powlson D S. Methane oxidation in soil as affected by land use, pH, and N fertilization. Soil Biol Biochem. 1994;26:1613–1622. [Google Scholar]
- 23.Hütsch B W, Russell P, Mengel K. CH4 oxidation in two temperate arable soils as affected by nitrate and ammonium application. Biol Fertil Soils. 1996;23:86–92. [Google Scholar]
- 24.Johnson A C, Wood M. DNA, a possible site of action of aluminum in Rhizobium spp. Appl Environ Microbiol. 1990;56:3629–3633. doi: 10.1128/aem.56.12.3629-3633.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller M E, Veldkamp E, Weitz A M, Reiners W A. Effect of pasture age on soil trace-gas emissions from a deforested area of Costa Rica. Nature (London) 1993;365:244–246. [Google Scholar]
- 26.Keyser H H, Munns D N. Tolerance of rhizobia to acidity, aluminum and phosphate. Soil Sci Soc Am J. 1979;43:519–523. [Google Scholar]
- 27.Kightley D, Nedwell D B, Cooper M. Capacity for methane oxidation in landfill cover soils measured in laboratory-scale soil microcosms. Appl Environ Microbiol. 1995;61:592–601. doi: 10.1128/aem.61.2.592-601.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King G M. Responses of atmospheric methane consumption by soils to global climate change. Glob Change Biol. 1997;3:351–362. [Google Scholar]
- 29.King G M, Adamsen A P S. Effects of temperature on methane consumption in a forest soil and in pure cultures of the methanotroph Methylomonas rubra. Appl Environ Microbiol. 1992;58:2758–2763. doi: 10.1128/aem.58.9.2758-2763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King G M, Schnell S. Enhanced ammonium inhibition of methane consumption in forest soils by increasing atmospheric methane. Nature. 1994;370:282–284. doi: 10.1128/aem.60.10.3514-3521.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King G M, Schnell S. Ammonium and nitrite inhibition of methane oxidation by Methylobacter albus BG8 and Methylosinus trichosporium OB3b at low methane concentrations. Appl Environ Microbiol. 1994;60:3508–3513. doi: 10.1128/aem.60.10.3508-3513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King G M, Schnell S. Effects of ammonium and non-ammonium salt additions on methane oxidation by Methylosinus trichosporium OB3b and Maine forest soils. Appl Environ Microbiol. 1998;64:253–257. doi: 10.1128/aem.64.1.253-257.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesueur D, Diem H G, Dianda M, LeRoux C. Selection of Bradyrhizobium strains and provenances of Acacia mangium and Faiderbia albida: relationship with their tolerance to acidity and aluminum. Plant Soil. 1993;149:159–166. [Google Scholar]
- 34.Macdonald T L, Martin R B. Aluminum ion in biological systems. Trends Biochem Sci. 1988;13:15–19. doi: 10.1016/0968-0004(88)90012-6. [DOI] [PubMed] [Google Scholar]
- 35.Madsen E L, Alexander M. Effects of chemical speciation on the mineralization of organic compounds by microorganisms. Appl Environ Microbiol. 1985;50:342–349. doi: 10.1128/aem.50.2.342-349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin R B. The chemistry of aluminum as related to biology and medicine. Clin Chem. 1986;32:1797–1806. [PubMed] [Google Scholar]
- 37.Mosier A R, Delgado J A, Keller M. Methane and nitrous oxide fluxes in an acid Oxisol in western Puerto Rico: effects of tillage, liming and fertilization. Soil Biol Biochem. 1998;30:2087–2093. [Google Scholar]
- 38.Munns D N. Acid soil tolerance in legumes and rhizobia. Adv Plant Nutr. 1986;2:63–91. [Google Scholar]
- 39.Murphy H E, Edwards D G, Asher C J. Effects of aluminum on nodulation and early growth of four tropical pasture legumes. Aust J Agric Res. 1984;32:663–673. [Google Scholar]
- 40.Parent L, Twiss M R, Campbell P G C. Influences of natural dissolved organic matter on the interaction of aluminum with the microalga Chlorella: a test of the free-ion model of trace metal toxicity. Environ Sci Technol. 1996;30:1713–1720. [Google Scholar]
- 41.Pettersson A, Hällbom L, Bergman B. Physiological and structural responses of the cyanobacterium Anabaena cylindrica to aluminum. Physiol Plant. 1985;63:153–158. [Google Scholar]
- 42.Pina G R, Cervantes C. Microbial interactions with aluminum. BioMetals. 1996;9:311–316. doi: 10.1007/BF00817932. [DOI] [PubMed] [Google Scholar]
- 43.Richardson A E, Simpson R J, Djordjevic M A, Rolfe B J. Expression of nodulation genes in Rhizobium leguminosarum bv. trifolii is affected by low pH and Ca and Al ions. Appl Environ Microbiol. 1988;54:2541–2548. doi: 10.1128/aem.54.10.2541-2548.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scharf R, Mamet R, Zimmels Y, Kimchie S, Schoenfeld N. Evidence for the interference of aluminum with bacterial porphyrin biosynthesis. BioMetals. 1994;7:135–141. doi: 10.1007/BF00140483. [DOI] [PubMed] [Google Scholar]
- 45.Schnell S, King G M. Mechanistic analysis of ammonium inhibition of atmospheric methane consumption in forest soils. Appl Environ Microbiol. 1994;60:3514–3521. doi: 10.1128/aem.60.10.3514-3521.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnell S, King G M. Stability of methane oxidation capacity to variations in methane and nutrient concentrations. FEMS Microbiol Ecol. 1995;17:285–294. [Google Scholar]
- 47.Schnell S, King G M. Responses of methanotrophic activity in soils and cultures to water stress. Appl Environ Microbiol. 1996;62:3203–3209. doi: 10.1128/aem.62.9.3203-3209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenson J A E, Svensson J E, Cronberg G. Changes and interactions in the pelagic community in acidified lakes in Sweden. Ambio. 1993;22:277–282. [Google Scholar]
- 49.Steudler P A, Bowden R D, Mellilo J M, Aber J D. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature (London) 1989;341:314–316. [Google Scholar]
- 50.Whelan A M, Alexander M. Effects of low pH and high Al, Mn and Fe levels on the survival of Rhizobium trifolii and the nodulation of subterranean clover. Plant Soil. 1986;92:363–371. [Google Scholar]
- 51.Wood M, Cooper J E, Bjourson A J. Response of Lotus rhizobia to acidity and aluminum in liquid culture and in soil. Plant Soil. 1988;197:227–231. [Google Scholar]
- 52.Yamamoto Y, Hachiya A, Hamada H, Matsumoto H. Phenylpropanoids as a protectant of aluminum toxicity in cultured tobacco cells. Plant Cell Physiol. 1998;39:950–957. [Google Scholar]