Abstract
To describe the phenomena of bacterial adhesion to intestinal cells and the competition for adhesion between bacteria, mathematical equations based on a simple dissociation process involving a finite number of bacterial receptors on intestinal cell surface were developed. The equations allow the estimation of the maximum number of Lactobacillus sp. and Escherichia coli cells that can adhere to Caco-2 cells and intestinal mucus; they also characterize the affinity of the bacteria to Caco-2 cells and intestinal and fecal mucus and the theoretical adhesion ratio of two bacteria present in a mixed suspension. The competition for adhesion between Lactobacillus rhamnosus GG and E. coli TG1 appeared to follow the proposed kinetics, whereas the competition between Lactobacillus casei Shirota and E. coli TG1 may involve multiple adhesion sites or a soluble factor in the culture medium of the former. The displacement of the adhered Lactobacillus by E. coli TG1 seemed to be a rapid process, whereas the displacement of E. coli TG1 by the Lactobacillus took more than an hour.
Probiotics are viable bacterial cell preparations or components of bacterial cells that have beneficial effects on the health and well being of the host (9, 17). Many of the probiotic bacteria are lactic acid bacteria and are useful in the treatment of dysfunctions with disturb intestinal microflora and abnormal gut permeability (10). Successful probiotic bacteria are usually able to colonize the intestine, at least temporarily, by adhering to the intestinal mucosa (1, 11, 19, 20). Studies have also suggested that adhesive probiotic bacteria could prevent the attachment of pathogens, such as coliform bacteria and clostridia, and stimulate their removal from the infected intestinal tract (1, 11, 19, 20).
Laboratory models using human intestinal cell lines such as Caco-2 (2, 5, 8, 15, 22) and intestinal mucus (13) have been developed to study the adhesion of probiotic lactic acid bacteria and their competitive exclusion of pathogenic bacteria. In this study, a quantitative approach is proposed for the design of experiments and interpretation of data in laboratory studies using cell line and mucus models. This approach provides a better insight to the mechanism of competition between probiotic bacteria and pathogens, and thus allows development of more efficient probiotic products.
MATERIALS AND METHODS
Bacterial strains.
Two commercial probiotic strains were used: Lactobacillus casei Shirota obtained from Yakult Singapore Pty., Ltd., and Lactobacillus rhamnosus GG (ATCC 53103) obtained from the National Collection of Industrial and Marine Bacteria Ltd. (Aberdeen, Scotland). Both bacterial strains have clinically demonstrated probiotic properties (9). The bacteria were cultured in MRS broth (BBL Cockeysville, Md.) at 37°C with 5% CO2 for 18 h before the study. Escherichia coli TG1 (Gibson, 1984) was obtained from C. K. Lim (of the Microbiology Department) and it was grown in Luria-Bertani broth (BBL) at 37°C for 18 h before the study. E. coli TG1 was chosen for this bacterium has a maximum adhesion to Caco-2 cells which falls between that of L. casei Shirota and that of L. rhamnosus GG. For the mucus assay (see below) (methyl,1′,2′-3H) thymidin was added to the media at a concentration of 10 μl ml−1 (117 Ci mmol−1) to radiolabel the bacteria.
Intestinal cell culture.
The Caco-2 cell culture (7) was used in the adhesion assay. This human colon adenocarcinoma cell line was obtained from the American Type Culture Centre (Manassas, Va.). The cells were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM) (GIBCO-BRL), containing 25 mM glucose, 20% (vol/vol) heated inactivated fetal calf serum (GIBCO-BRL), and 1% nonessential amino acids (GIBCO-BRL). The cells were grown at 37°C in 5% CO2. For the adhesion assay, monolayers of Caco-2 cells were prepared in two-chamber slides (Lab-Tek chamber slide; Nunc Inc.) by inoculating 2.8 × 105 viable cells into 2 ml of culture medium. The medium was replaced every two days.
Intestinal mucus.
Intestinal mucus was isolated from feces of healthy adult volunteers as described earlier (13). In short, fecal extracts were prepared by homogenizing feces in phosphate-buffered saline (PBS) (pH 7.2) containing protease inhibitors and sodium azide and centrifuging the suspension at 15,000 × g. The mucus was isolated from the clear fecal extract by dual ethanol precipitation. The crude mucus was further purified by size exclusion chromatography.
Human ileostomy glycoproteins were a generous gift from J. G. H. Ruseler-van Embden of the Erasmus University, Rotterdam, The Netherlands.
Adhesion assay. (i) On Caco-2 cells.
Fifteen-day-postconfluent Caco-2 monolayers were washed twice with 1 ml of sterile PBS before the adhesion assay. One ml of the test bacteria at concentrations between 1 × 105 and 4 × 108 CFU ml−1 were added to 1 ml of complete Caco-2 medium. This suspension (2 ml) was added to each chamber of the two-chamber slide and incubated at 37°C, in a 5% CO2–95% air atmosphere, with gentle rocking. After incubation for 60 min, the monolayers were washed twice with sterile PBS (pH 7.2), fixed with methanol, Gram stained, and examined microscopically. Visual counting of adhered cells was adopted in this study, for it allows the differentiation of the gram-positive Lactobacillus and gram-negative E. coli. Each adherence assay was conducted in triplicate by two students (Y.Y.L. and W.L.T.), and the number of adherent bacteria was counted on about 1,000 Caco-2 cells, in 60 randomly selected microscopic fields. To stimulate the physiological pH condition of the gastrointestinal tract, all experiments were done at pH 7.
In the study of the competition for adhesion on Caco-2 cells, Lactobacillus and E. coli were added simultaneously or sequentially to the Caco-2 cultures before counting. In the latter case, free cells of the first bacterium were removed by washing with PBS (pH 7.2) before the second bacterium was added. The lactic acid bacteria have the tendency to form chains and aggregates. It was necessary to disperse the chains and aggregates of the bacterial cells before the adhesion study, to ensure that cells observed under the microscope in the adhesion assay were cells adhered to Caco-2 and ileosotomy glycoprotein surfaces (13).
(ii) On immobilized mucus and ileostomy glycoproteins.
The study of adhesion of the microorganisms on mucus and ileostomy glycoproteins was performed as described earlier (13). In short, human intestinal mucus or human ileostomy glycoprotein was passively immobilized in microtiter plate wells. Bacteria were allowed to bind to the mucus or ileostomy glycoproteins at concentrations between 4.4 × 106 and 4.1 × 108 CFU · ml−1. The radioactivity was assessed by liquid scintillation. The relation between the measured radioactivity and the number of bacteria was determined by plate counting.
Theory.
In many studies on the adhesion of bacterial cells to intestinal epithelial cells, when the number of bacterial cells adhered to intestinal cells is plotted against the concentration of bacterial culture, a section of a rectangular hyperbola is obtained, as shown in Fig. 1. Such a relationship implies a process of simple dissociation. That is,
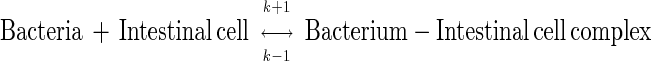 |
where k + 1 and k − 1 represent the dissociation constants for the reaction. The process is similar to the reaction between a substrate and an enzyme that forms a substrate-enzyme complex, without the formation of a product.
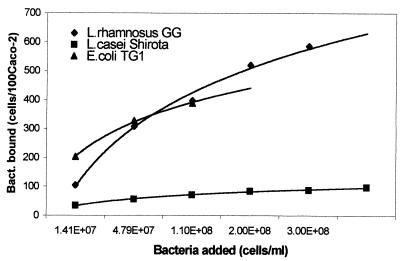
FIG. 1.
Adhesion of L. rhamnosus GG, L. casei Shirota, and E. coli TG1 to human intestinal cell line Caco-2, presented as the number of bacteria bound per 100 Caco-2 cells versus the concentration of bacteria added (CFU per milliliter).
The relationship is based on the assumption that the interaction between the bacterial cells and the intestinal cells or mucus remains in equilibrium. This condition should be achieved if the bacterial cells do not penetrate the intestinal cells.
It is also assumed that the concentration of the bacterial culture remained essentially unchanged throughout the study, so that the concentration of the bacterial culture can be considered equal to the initial bacterial concentration. This condition is usually achieved when the total number of bacterial cells is much greater than the number of bacterial cells adhering to the intestinal cells. This is usually the case in most of the adhesion studies, where the concentration of the bacterial cells added is in the range of 105 to 108 per ml, whereas that of the intestinal cell culture (e.g., Caco-2 cells) is about 102 per ml and the number of bacterial cells adhered to the Caco-2 cells is fewer than 10 per cell.
In the equation described above, if x is the concentration of the bacterial culture added, e is the intestinal epithelial cell or mucus concentration, and ex is the concentration of the bacterium-intestinal cell-mucus complex, then the concentration of free bacterial cells will be (x − ex).
Because the process is in equilibrium, the dissociation constant for the process (kx) can be defined as kx = (k − 1)/(k + 1) = (x − ex)x/ex. This equation can be rearranged to give an expression for the concentration of the bacterium-intestinal cell-mucus complex, ex = e · x/(kx + x). When x is very much larger than kx, ex approaches e. The maximum value of ex obtained when the intestinal cells or mucus is saturated with bacteria as em, which may be written as
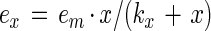 |
1 |
When x is equal to kx, ex is equal to em/2; thus, the value of kx could be experimentally obtained from the value of x, which gives half the maximum ex (i.e., em/2).
Equation 1 can be rearranged to give a linear relationship.
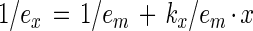 |
2 |
Hence, a plot of 1/ex against 1/x will give a straight line, in which the intercept on the ordinate gives a value of 1/em, and that on the abscissa gives a value of −1/kx.
In the case where two types of bacteria are present in the system and they compete for the same receptors or adhesion sites (through steric hindrance of cells in close vicinity), the competition for adhesion of each of the bacterial types is determined by the affinity of the bacteria to the intestinal cells or the intestinal mucus (kx) and the concentration of the bacterial culture (x). Thus, the ratio of ex for bacterium 1 and bacterium 2 can be described as
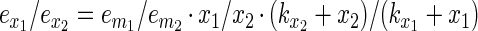 |
3 |
Statistics.
Differences between treatments were examined for the level of significance by Student's t test after analysis of variance.
RESULTS
Adhesion of bacteria to Caco-2 cells.
When the concentration of adhered bacterial cells (cells per 100 Caco-2 cells) was plotted against the concentration of bacterial cells added, a hyperbolic relation was observed for all three of the bacteria tested (Fig. 1).
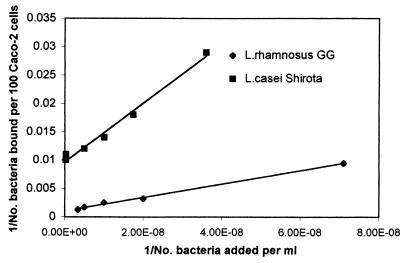
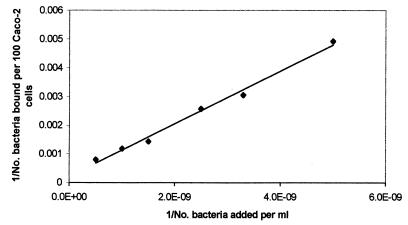
The plots of the reciprocal of adhered cell concentration versus the reciprocal of the concentration of cells added, for L. casei Shirota and L. rhamnosus GG are given in Fig. 2, and that for E. coli is given in Fig. 3. In all the cases a linear relationship was observed. It follows from equation 2 that the intercept on the ordinate gives the value of the reciprocal of the maximum number of bacterial cells adhered to 100 Caco-2 cells (em). The intercept on the abscissa is −1/kx, where kx is the dissociation constant for the adhesion process. Thus, the values of em and kx for the three bacteria were calculated, and they are summarized in Table 1.
FIG. 2.
Double-reciprocal representation of the adhesion of L. rhamnosus GG and L. casei Shirota to human intestinal cell line Caco-2. The lines indicate the linear fit according to the least-squares method.
FIG. 3.
Double-reciprocal representation of the adhesion of E. coli TG1 to human intestinal cell line Caco-2. The lines indicate the linear fit according to the least-squares method.
TABLE 1.
Maximum number of adhered bacterial cells on 100 Caco-2 cells and dissociation constant of the adhesion process for various strainsa
| Strain | em (cells/100 Caco-2 cells) | kx (cells/ml) |
|---|---|---|
| L. casei Shirota | 137.9 | 8.62 × 107 |
| L. rhamnosus GG | 1,612.9 | 2.08 × 108 |
| E. coli TG1 | 500 | 4.76 × 108 |
As shown in Table 1, among the three bacteria studied, the maximum number of L. rhamnosus GG cells that can adhere to Caco-2 cells is about 10 times that of the L. casei Shirota, whereas the maximum adhesion number of E. coli TG1 is between those of the two lactobacilli. E. coli TG1 was chosen for this study as it would allow us to understand the competition of an enterobacterium with a lactic acid bacterium which has a higher adhesion capacity (i.e., L. rhamnosus GG) and with one whose adhesion capacity is lower (i.e., L. casei Shirota).
L. casei Shirota's having the lowest kx implies that it has a higher affinity for adhesion to Caco-2 cells than do L. rhamnosus GG and E. coli TG1; i.e., adhered L. casei Shirota dissociates less easily than the other two bacteria.
Competition between Lactobacillus and E. coli for adhesion.
In this study, various concentrations of a Lactobacillus strain (2 × 108 to 6 × 108 cells ml−1) were mixed with an equal volume of E. coli (2 × 108 cells ml−1) and then added onto the Caco-2 cells. The final concentration of the respective bacterial strains is thus half of the original concentration. The Gram-stained Lactobacillus and E. coli adhered on Caco-2 cells could be easily differentiated and counted microscopically. The observed concentrations of the adhered Lactobacillus and E. coli and the predicted ratio of the two bacteria based on equation 3 are given in Tables 2 and 3.
TABLE 2.
Adhesion of bacteria to Caco-2 cells in mixed bacterial suspensiona
| L. rhamnosus added (cells/ml) | Total no. of cells counted
|
Observed eE ± SD | Observed eL ± SD | Observed eE + eL | Observed eE/eL | Predicted eE/eL | ||
|---|---|---|---|---|---|---|---|---|
| Adhered L. rhamnosus | Adhered E. coli | Caco-2 | ||||||
| 1 × 108 | 3,289 | 420 | 995 | 42.21 ± 1.32 | 330.55 ± 45.40 | 372.76 | 0.128 | 0.166 |
| 2 × 108 | 3,801 | 281 | 954 | 29.45 ± 19.36 | 398.43 ± 46.64 | 427.88 | 0.074 | 0.071 |
| 3 × 108 | 3,443 | 130 | 981 | 13.25 ± 8.85 | 350.97 ± 19.08 | 364.22 | 0.038 | 0.041 |
eE and eL number of E. coli TG1 and L. rhamnosus cells adhered to 100 Caco-2 cells, respectively.
TABLE 3.
Adhesion of bacteria to Caco-2 cells in mixed bacterial suspensiona
| L. casei added (cells/ml) | Total no. of cells counted
|
Observed eE ± SD | Observed eL ± SD | Observed eE + eL | Observed eE/eL | Predicted eE/eL | ||
|---|---|---|---|---|---|---|---|---|
| Adhered L. casei | Adhered E. coli | Caco-2 | ||||||
| 1 × 108 | 1,084 | 147 | 1,183 | 12.4 ± 3.82 | 91.63 ± 10.68 | 104.03 | 0.135 | 1.169 |
| 2 × 108 | 736 | 68 | 1,071 | 6.35 ± 0.35 | 68.72 ± 7.07 | 75.07 | 0.092 | 1.536 |
| 3 × 108 | 581 | 36 | 783 | 4.60 ± 1.91 | 74.20 ± 5.41 | 78.8 | 0.062 | 1.806 |
eE and eL, number of E. coli TG1 and L. casei Shirota cells adhered to 100 Caco-2 cells, respectively.
In the case of L. rhamnosus GG, the predicted ratio of E. coli and lactobacilli counted on Caco-2 cells (eE/eL) is comparable to the observed values (Table 2). In the case of L. casei Shirota, the predicted values of eE/eL are 1.169 to 1.806 for the three concentrations of Lactobacillus added. A value of >1 indicates that the Lactobacillus has been excluded by E. coli for adhesion to Caco-2 cells. The observed values of eE/eL are 8 to 23 times lower than the predicted values, ranging from 0.062 to 0.135.
Exclusion of E. coli by adhered lactobacilli.
In the study, the lactobacilli (108 cells ml−1) were allowed to adhere to Caco-2 cells. Nonadhered Lactobacillus cells were removed by PBS, and then the E. coli TG1 (108 cells ml−1) was added and incubated with Caco-2 cells for 1 h. The respective adhesion number of the two bacteria was counted and is shown in Table 4.
TABLE 4.
Results of the exclusion study in which Lactobacillus cells were allowed to adhere to Caco-2 cells before E. coli was addeda
| Strain | Total no. of cells counted
|
Observed eE ± SD | Observed eL ± SD | Observed eE + eL | Observed eE/eL | ||
|---|---|---|---|---|---|---|---|
| Adhered Lactobacillus | Adhered E. coli | Caco-2 | |||||
| L. rhamnosus GG | 484 | 1,246 | 776 | 160.56 ± 14.14 | 62.36 ± 11.10 | 222.92 | 2.57 |
| L. casei Shirota | 458 | 1,038 | 849 | 122.26 ± 15.66 | 53.95 ± 2.82 | 176.21 | 2.27 |
eE and eL, number of E. coli and Lactobacillus cells adhered to 100 Caco-2 cells, respectively.
In the case of L. rhamnosus GG, the concentration of the E. coli TG1 counted (160.56 ± 14.14 cells/100 Caco-2 cells) was not statistically different (P > 0.05) compared with E. coli when it was added alone and incubated with Caco-2 cells (169.16 ± 15.63 cells/100 Caco-2 cells), whereas in the case of L. casei Shirota, the concentration of the E. coli TG1 counted (122.26 ± 15.66 cells/100 Caco-2 cells) was significantly lower (P < 0.05) than that of E. coli (169.16 ± 15.63 cells/100 Caco-2 cells). However, in both of the studies involving L. rhamnosus GG (62.36 ± 11.10 cells/100 Caco-2 cells) and L. casei Shirota (53.95 ± 2.82 cells/100 Caco-2 cells), the counts of the lactobacilli were much lower than those when L. rhamnosus (397.7 ± 53.2 cells/100 Caco-2 cells) and L. casei (78.94 ± 9.60) alone were incubated with Caco-2 cells. The values of eE/eL were greater than 1; i.e., there were more E. coli cells than lactobacilli.
Displacement of adhered E. coli TG1 by lactobacilli.
In the study, the E. coli TG1 (108 cells ml−1) was allowed to adhere on Caco-2 cells. Nonadhered E. coli cells were removed by PBS, and then the lactobacilli (108 cells ml−1) was added and incubated with Caco-2 cells for 1 h. The adhesion numbers of the two bacteria were counted and are shown in Table 5.
TABLE 5.
Maximum number of adhered bacterial cells to human cells and dissociation constant for various strainsa
| Specimen and strain | ex (cells/well) | kx (cells/well) |
|---|---|---|
| Intestinal mucus | ||
| L. casei Shirota (low concn) | 5 × 104 | 6.10 × 106 |
| L. casei Shirota (high concn) | 2 × 105 | 9.47 × 107 |
| L. rhamnosus GG | 2 × 107 | 8.28 × 1010 |
| E. coli TG1 | 1.4 × 106 | 2.26 × 108 |
| Iliostomy glycoprotein | ||
| L. casei Shirota (low concn) | 1.96 × 105 | 2.24 × 106 |
| L. casei Shirota (high concn) | 1 × 106 | 1.14 × 108 |
| L. rhamnosus GG | 2 × 107 | 6.13 × 108 |
| E. coli TG1 | 5 × 106 | 1.85 × 108 |
In both cases, the concentrations of the E. coli TG1 counted are statistically lower (P < 0.05) than those when the E. coli alone was incubated with Caco-2 cells (169.16 ± 15.63 cells/100 Caco-2 cells). However, the values of eE/eL were greater than 1; i.e., there were more E. coli cells than lactobacilli.
Adhesion of bacteria to immobilized intestinal mucus and ileostomy glycoproteins.
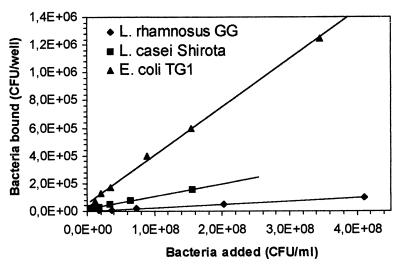
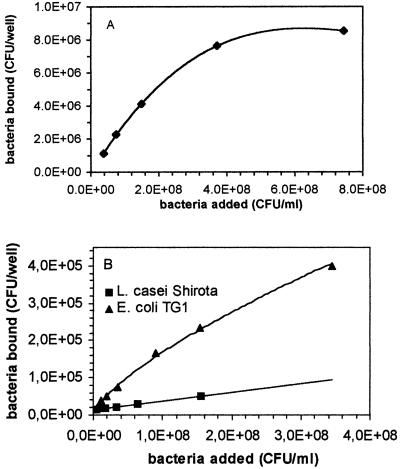
When the number of bacterial cells adhered to intestinal mucus or human ileostomy glycoproteins per well (0.1-cm2 wells) was plotted against the number of bacteria added per well, a near-linear to hyperbolic relationship was observed for all three of the bacteria tested (Fig. 4 and 5).
FIG. 4.
Adhesion of L. rhamnosus GG, L. casei Shirota, and E. coli TG1 to immobilized human intestinal mucus presented as the number of bacteria bound per microtiter plate well (surface area, 0.1 cm2) versus the concentration of bacteria added.
FIG. 5.
Adhesion of L. rhamnosus GG (A) and L. casei Shirota and E. coli TG1 (B) to immobilized human ileostomy glycoproteins presented as the number of bacteria bound per microtiter plate well (surface area, 0.1 cm2) versus the concentration of bacteria added.
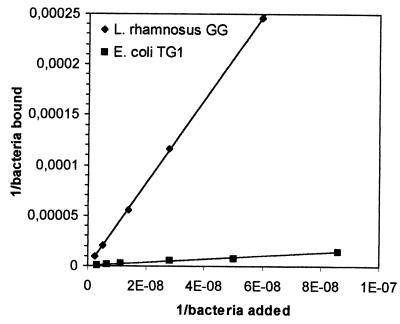
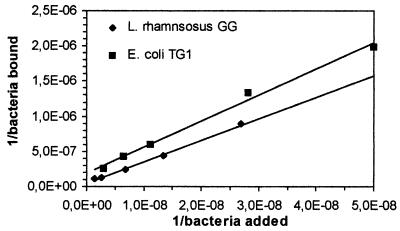
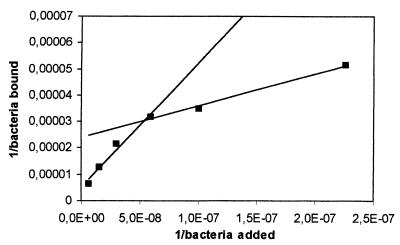
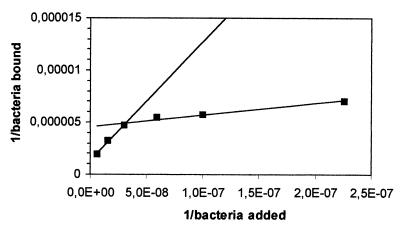
Double-reciprocal plots of adhered L. rhamnosus GG and E. coli TG1 against the concentration of bacteria added are presented in Fig. 6 and 7. A linear relation was observed for both plots. A double-reciprocal plot of adhered L. casei Shirota is shown in Fig. 8 and 9. This plot appears to be composed of two linear parts with a break at around 1.7 × 107 CFU ml−1 for the intestinal mucus and 9.3 × 107 CFU ml−1 for the human ileostomy glycoprotein. The calculated em and kx values for the bacteria are shown in Table 5. For L. casei Shirota, the maximum number of bacterial cells that can adhere to intestinal mucus at low cell concentrations (5 × 104 cells well−1) was four times lower than that measured at high cell concentrations (2 × 105 cells well−1), while the maximum number of bacteria that can adhere to ileostomy glycoprotein at low cell concentrations (1.96 × 105 cells well−1) was 5.1 times lower than that at high cell concentrations (106 cells well−1). However, its adhesion affinities on intestinal mucus and ileostomy glycoproteins were higher at low cell concentrations.
FIG. 6.
Double-reciprocal representation of the adhesion of L. rhamnosus GG and E. coli TG1 to immobilized human intestinal mucus. The lines indicate the linear fit according to the least-squares method.
FIG. 7.
Double-reciprocal representation of the adhesion of L. rhamnosus GG and E. coli TG1 to immobilized human ileostomy glycoproteins. The lines indicate the linear fit according to the least-squares method.
FIG. 8.
Double-reciprocal representation of the adhesion of L. casei Shirota to immobilized human intestinal mucus. The lines indicate the linear fit according to the least-squares method.
FIG. 9.
Double-reciprocal representation of the adhesion of L. casei Shirota to immobilized human ileostomy glycoproteins. The lines indicate the linear fit according to the least-squares method.
DISCUSSION
Our studies indicate that the direct microscopic counting and radioactive label counting as measures of bacteria adhesion gave comparable results. The microscopic method was adopted in the studies for competition for adhesion, for it allows the differentiation of the different bacterial types on the Caco-2 cell surface. The present study demonstrated that the adhesion process of bacteria on Caco-2 cells follows the mathematical relationship (equation 2) developed for a simple dissociation process involving a finite number of adhesion sites or receptors on Caco-2 cells (Fig. 2 and 3). Thus, this allows the maximum number of adhesion sites (em) to be estimated for each bacterial strain and the adhesion affinity of respective bacteria for Caco-2 cells to be estimated.
Among the bacteria studied, L. rhamnosus GG has the highest adhesion at saturation cell concentration, which is about 10 times higher than that for L. casei Shirota and three times higher than that for E. coli TG1 (Table 1). Thus, the calculations suggest that if L. casei Shirota and E. coli TG1 are competing for the same adhesion sites or surface receptors on Caco-2 cells, L. casei Shirota would not be able to prevent the adhesion of E. coli.
L. casei Shirota, on the other hand, has a higher affinity (lower dissociation constant [kx]) than L. rhamnosus and E. coli. That is, L. casei Shirota adheres more readily to and dissociates less easily from Caco-2 cells.
A similar approach, applying the principles of Michaelis-Menten enzyme kinetics to the study of E. coli adhesion to intestinal cell monolayers, was proposed (6). It was concluded that the adhesion of E. coli involved an initial reversible binding step that was followed by an irreversible step. In the present study and others (4, 11, 19, 20), we had observed competition between adhered cells and free cells for adhesion sites on intestinal cells. The discrepancy between the former and the latter studies may be due to the use of undifferentiated cells in the former study.
In the studies, each of the lactobacilli was mixed with an equal number of the E. coli TG1 cells, and the mixed culture suspension was incubated with Caco-2 cells. If the lactobacilli and E. coli were competing for the same adhesion sites or surface receptors, the ratio of adhered E. coli (eE) to lactobacilli (eL) would be expected to be determined by their em, cell concentration added (x), and kx as described by equation 3. This was the case when the L. rhamnosus GG and the E. coli TG1 were competing for adhesion on Caco-2 cells (Table 2). The predicted eE/eL values were close to the observed values obtained experimentally. The results of this study suggest that to achieve the lowest eE/eL, the concentration of L. rhamnosus should approach the saturation concentration, i.e., >100 × kx or >2.08 × 1010 cells/ml. A concentration of 105 viable cells per ml of a probiotic product has been suggested as the therapeutic minimum for humans (16). Consumption of 106 to 1010 viable cells per day is necessary for a beneficial effect to develop (9, 16, 18). Equation 3 developed in this study suggests that the concentration of the probiotic bacterium is a critical parameter in determining eE/eL. It is, nevertheless, expected that a sufficient volume of a probiotic cell suspension must be consumed so that the probiotic cells can saturate the entire gastrointestinal (GI) tract, especially the lower part of the GI tract.
In the study of the competition between L. casei Shirota and E. coli TG1, calculations from equation 3 predicted that the E. coli would compete well with L. casei for adhesion (eE/eL > 1), because of the lower em of L. casei (137.9 cells/100 Caco-2 cells) compared with E. coli (500 cells/100 Caco-2 cells). Even if L. casei occupied all of the 137.9 adhesion sites, it could not prevent the E. coli from adhering to the balance of the 362.1 sites. The observed eE/eL ranged between 0.135 and 0.062 (Table 3); i.e., the Lactobacillus prevented the adhesion of E. coli to Caco-2 cells. It is likely that the interaction of L. casei and E. coli involved more than competition for the adhesion sites on Caco-2 cells.
In the exclusion study, it was observed that L. rhamnosus GG did not prevent the adhesion of E. coli to Caco-2 cells. This may be due to the relatively high dissociation constant of L. rhamnosus (2.08 × 108 cells ml−1), and any dissociated Lactobacillus cells were readily replaced by surrounding E. coli cells in the culture medium. The concentration of E. coli determined (122.26 ± 15.66 cells/100 Caco-2 cells) in the study involving L. casei was slightly, though statistically significantly (P < 0.05) lower than the concentration determined when E. coli was present alone (169.16 ± 15.63 cells/100 Caco-2 cells). The lower dissociation constant (8.62 × 107 cells ml−1) and possibly a soluble protein factor produced by L. casei Shirota may have hindered their displacement by E. coli cells. This observation is in agreement with studies of humans and animals, where Lactobacillus cells were found to be replaced gradually by enterobacteria after the intake of lactobacilli had stopped (11, 19). Commercially available probiotic organisms have not been reported to establish themselves permanently in the human GI tract. Our results suggest that the concentration of free (nonadhering) Lactobacillus in the GI contents needs to be maintained at a high level to prevent the adhered lactobacilli from being replaced by other bacteria; alternatively, the lactobacilli need to divide rapidly to maintain a high local cell concentration. The observation that adhered Lactobacillus cells in the GI tract were gradually replaced by enterobacteria suggests that the lactobacilli used were not able to grow sufficiently rapidly to establish permanent residence in the GI tract.
There is ample laboratory and clinical evidence to demonstrate that oral administration of lactobacilli could be used to treat GI bacterial infection (9). The efficacy of lactobacilli in displacing adhered E. coli on Caco-2 cells was investigated in the displacement study (Table 6). The process of displacing adhered E. coli with Lactobacillus appears to be slow. After 1 h of incubation with lactobacilli alone, less than half of the adhered E. coli cells were displaced. In the studies using fermented milk containing lactobacilli to treat diarrheal disorders in human patients (4, 12, 15), it was necessary that patients consume the fermented milk for 2 to 3 days, before significant improvement in clinical symptoms of the illness was observed. It is important to recognize the slow process of displacing E. coli with lactobacilli in the in vitro study, which could not simply be explained by dissociation phenomena.
TABLE 6.
Results of the displacement study in which E. coli TG1 cells were allowed to adhere to Caco-2 cells before lactobacilli were addeda
| Strain | Total no. of cells counted
|
Observed eE ± SD | Observed eL ± SD | Observed eE + eL | Observed eE/eL | ||
|---|---|---|---|---|---|---|---|
| Adhered Lactobacillus | Adhered E. coli | Caco-2 | |||||
| L. rhamnosus GG | 209 | 1,183 | 982 | 120.47 ± 15.56 | 98.30 ± 11.30 | 141.75 | 5.66 |
| L. casei Shirota | 158 | 984 | 1,001 | 21.28 ± 4.10 | 15.78 ± 7.07 | 114.08 | 6.23 |
See footnote a to Table 4 for explanation of eE and eL.
Although L. rhamnosus GG had the highest saturation cell concentration, its adhesion affinity to intestinal mucus and ileostomy glycoproteins was considerably lower than that of L. casei Shirota or E. coli TG1. The double-reciprocal plots of L. casei Shirota added versus bound, allowed two linear curve fits (Fig. 8 and 9). This may mean that two binding mechanisms are involved for this bacterium, one for a high bacterial concentration (lower affinity) and one for a lower concentration (higher affinity). A possible explanation is that multiple adhesion sites on the mucous layer are involved in the adhesion of an L. casei Shirota cell. At low bacterial concentrations, a bacterium adheres to a maximum number of sites on mucus, whereas at high bacterial concentrations, a minimum number of adhesion sites are involved. The data in Table 5 suggest that up to four adhesion sites (2 × 105/5 × 104 = 4) on intestinal mucus and five adhesion sites on ileostomy glycoproteins could be involved in the adhesion of L. casei Shirota on intestinal mucus. This explains the observation that at low bacterial concentrations the affinity for mucus was high, whereas at high bacterial concentrations the adhesion affinity was lower (Table 5). In either case, the adhesion affinity is much higher than those for the other two tested bacteria.
The quantitative approach developed in this study has proven useful in the understanding of the mechanism and kinetics of the adhesion process of bacteria on intestinal cells and mucus and the competition between different bacteria for adhesion. Our results may provide help in estimating the numbers of bacteria needed for future competitive exclusion studies.
ACKNOWLEDGMENTS
The skillful technical assistance of Satu Tölkkö is gratefully acknowledged.
REFERENCES
- 1.Benno Y, Mitsuoka T. Impact of Bifidobacterium longum on human fecal microflora. Microbiol Immun. 1992;36:683–694. doi: 10.1111/j.1348-0421.1992.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernet M-F, Brassart D, Neeser J-R, Servin A L. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol. 1993;59:4121–4128. doi: 10.1128/aem.59.12.4121-4128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernet M-F, Brassart D, Neeser J-R, Servin A L. Lactobacillus acidophilus LA1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudraa G, Touhami M, Pochart P, Soltana R, Mary J Y, Desjeux J F. Effect of feeding yogurt versus milk in children with persistent diarrhea. J Pediatr Gastroenterol Nutr. 1990;11:509–512. doi: 10.1097/00005176-199011000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Chauviere G, Coconnier M-H, Kerneis S, Fourniat J, Servin A L. Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J Gen Microbiol. 1992;138:1689–1696. doi: 10.1099/00221287-138-8-1689. [DOI] [PubMed] [Google Scholar]
- 6.Cohen P S, Elbein A D, Solf R, Mett H, Regos J, Menge E B, Vosbeck K. Michaelis-Menten kinetic analysis of Escherichia coli SS142 adhesion to intestine 407 monolayers. FEMS Microbiol Lett. 1981;12:99–103. [Google Scholar]
- 7.Fogh J, Fogh J M, Orfeo T. One hundred and twenty-seven cultured human tumour cell lines producing tumours in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 8.Greene J D, Klaenhammer T R. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl Environm Microbiol. 1994;60:4487–4494. doi: 10.1128/aem.60.12.4487-4494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y K, Nomoto K, Salminen S, Gorbach S L. Handbook of Probiotics. New York, N.Y: John Wiley & Sons; 1999. [Google Scholar]
- 10.Lee Y K, Salminen S. The coming of age of probiotics. Trends Food Sci Technol. 1995;6:241–244. [Google Scholar]
- 11.Lidbeck A, Gustafasson J-A, Nord C. Impact of Lactobacillus acidophilus supplements on the human oropharyngeal and intestinal microflora. Scand J Infect Dis. 1987;19:531–537. doi: 10.3109/00365548709032419. [DOI] [PubMed] [Google Scholar]
- 12.Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ouwehand A C, Niemi P, Salminen S J. The normal fecal microflora does not affect the adhesion of probiotic bacteria in vitro. FEMS Microbiol Lett. 1999;177:35–38. doi: 10.1111/j.1574-6968.1999.tb13710.x. [DOI] [PubMed] [Google Scholar]
- 14.Pinto M, Robine-Leon S, Appay M-D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarisation of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 15.Raza S, Graham S M, Allen S J, Sultana S, Cuevas L, Hart C A. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr Infect Dis J. 1995;14:107–111. doi: 10.1097/00006454-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Robinson R K, editor. Therapeutic properties of fermented milks. London, United Kingdom: Elsevier Applied Science; 1991. pp. 23–43. [Google Scholar]
- 17.Salminen S, Ouwehand A, Benno Y, Lee Y K. Probiotics: how should they be defined? Food Sci Technol. 1999;10:107–110. [Google Scholar]
- 18.Salminen S, Deighton M, Gorbach S. Lactic acid bacteria in health and disease. In: Salminen S, von Wright A, editors. Lactic acid bacteria. New York, N.Y: Marcel Dekker; 1993. pp. 199–225. [Google Scholar]
- 19.Saxelin M, Pessi T, Salminen S. Fecal recovery following oral administration of Lactobacillus strain GG (ATCC 53103) in gelatine capsules to healthy volunteerts. Int J Food Microbiol. 1995;25:199–203. doi: 10.1016/0168-1605(94)00091-j. [DOI] [PubMed] [Google Scholar]
- 20.Tamura N, Norimoto M, Yoshida K, Hirayama C, Nakai R. Alteration of fecal bacterial flora following oral administration of bifidobacterial preparation. Gastroenterol Jpn. 1983;18:17–24. doi: 10.1007/BF02774859. [DOI] [PubMed] [Google Scholar]
- 21.Tuomola E M, Salminen S J. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int J Food Microbiol. 1998;41:45–51. doi: 10.1016/s0168-1605(98)00033-6. [DOI] [PubMed] [Google Scholar]