Abstract
Understanding the immune response to dengue virus (DENV) is essential for developing a dengue vaccine that is protective against all 4 DENV serotypes. We evaluated the immune response after vaccination (live attenuated tetravalent dengue vaccine TV005 or trivalent admixture) and after challenge with DEN2Δ30 (Tonga/74) to better understand the importance of homotypic immunity in vaccine protection. Significant increases in IP-10 expression were observed following receipt of either the trivalent or tetravalent vaccine. After challenge, a large increase in IP-10 expression was observed in the placebo and trivalent admixture groups but not in the tetravalent vaccine group. MCP-1, IL-1RA, and MIP-1β exhibited a similar pattern as IP-10. These results demonstrate protective effects of trivalent and tetravalent vaccines against DENV and suggest that the tetravalent vaccine has a better protective effect compared with the trivalent admixture. We also explored the postvaccination and postchallenge immune response differences between Black and White participants. White participants responded to vaccine differently than Black participants; Black participants receiving trivalent and tetravalent vaccines responded strongly and White participants responded only transiently in trivalent group. In response to challenge, White participants elicited a stronger response than Black participants. These results may explain why White participants may have a more vigorous DENV immune response than Black participants, as reported in literature.
Keywords: Immunology, Infectious disease
Keywords: Innate immunity
Introduction
Dengue, the most prevalent arthropod borne viral disease, is transmitted to humans by Aedes sp. mosquitoes. According to a WHO report, about 3.9 billion people worldwide are at risk of dengue infection, and there has been an unprecedented increase in the incidence of dengue, with outbreaks of rising frequency and magnitude in the last 50 years (1). Dengue virus (DENV) infection and illness are caused by 4 different serotypes (DENV1–DENV4) that cross-react immunologically. Each of the serotypes is independently capable of causing a broad spectrum of disease, ranging from asymptomatic or mild illness to severe disease, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS).
Development and use of a safe and effective dengue vaccine could greatly reduce global dengue burden. Among all dengue vaccine candidates, Dengvaxia is the only vaccine that has been licensed in some countries (2). However, its overall efficacy is still limited by the serotype, age, serostatus at vaccination, and region (3). Additionally, Dengvaxia is recommended only for DENV-seropositive individuals 9 years of age and older. Therefore, a more universally effective vaccine is needed. Currently, several vaccine clinical trials are ongoing (4). Of these vaccine trials, the live attenuated tetravalent vaccine candidate TV003/TV005 from the NIH and DENVax from Takeda are in phase III trials (2). Compared with Dengvaxia, TV003/TV005 induced greater infectivity of the individual vaccine-component viruses, resulting in a more balanced immune response (3). TV003 was found to have complete protection against DENV2 viremia following challenge with the recombinant DENV2 challenge virus rDEN2Δ30 (5).
The immune response plays a key role in both protection and pathogenesis of dengue, and vaccine candidates must simultaneously protect against all 4 serotypes to avoid the induction of partial immunity to one or more DENV serotypes, as this could lead to antibody-dependent enhancement infection upon subsequent DENV infection. Therefore, it is critical for those in dengue vaccine development to have a thorough understanding of how the innate immune responses to the vaccine shape and influence the adaptive immune response. Viral replication and antibody responses to NIH DENV vaccine strains have been studied within rhesus macaques and humans, but the innate immune response induced by those vaccine candidates at early stages of vaccination have yet to be well characterized (6, 7).
The overall goal of our research is to characterize immune profiles following vaccination and challenge in a DENV human challenge model. Two randomized, double-blind, placebo-controlled trials, CIR299 and CIR300, were designed to assess the protective efficacy of two dengue vaccine admixtures against DENV2 virus and to evaluate the role of homotypic versus heterotypic antibody in that protection. Participants in the CIR299 trial received the live attenuated tetravalent dengue vaccine admixture TV005 (rDEN1Δ30, rDEN2/4Δ30, rDEN3Δ30/31, rDEN4Δ30, n = 18) or placebo (n = 18), while participants in the CIR300 trial received an attenuated trivalent admixture (rDEN1Δ30, rDEN3Δ30/31, rDEN4Δ30, n = 15) or placebo (CIR-300; n = 6). The only difference between the 2 admixtures is the presence of DENV2 prM and E in the tetravalent admixture. All participants in both trials were challenged with DEN2Δ30 (Tonga/74) on day 180 after enrollment. We hypothesized that immune profiling studies could reveal a more balanced immune response in vaccine recipients, especially in the tetravalent dengue vaccines, as compared with controls.
Historically, there have been reports of racial differences being associated with the response to DENV infection and disease severity. The development of severe dengue and DHF/DSS in a population is dependent on various risk factors, and race has been demonstrated to play a significant role in morbidity and clinical presentation of dengue disease (8). Epidemiological studies of Cuban epidemics have shown that Black participants had a lower morbidity and mortality compared with White participants (8, 9). Therefore, we also explored the differences of postvaccination and postchallenge responses in Black and White participants.
Results
Study cohorts.
Fifty-seven healthy volunteers from the 2 clinical trials (CIR299 and CIR300) were included in the study (18 tetravalent admixture recipients, 15 trivalent admixture recipients, and 24 placebo recipients). Participants from each clinical trial were randomized to receive either vaccine (tetravalent in CIR299, trivalent in CIR300) or placebo on day 0. All participants were challenged with DENV2 Tonga/74 virus on day 180. Cytokine levels at baseline (day 0), after vaccination (day 8 and day 21), at the day of challenge (day 180), and after challenge (day 188 and day 201) were measured to evaluate the immune responses of participants after vaccination and DENV challenge. A schematic illustration of the trial design is shown in Figure 1.
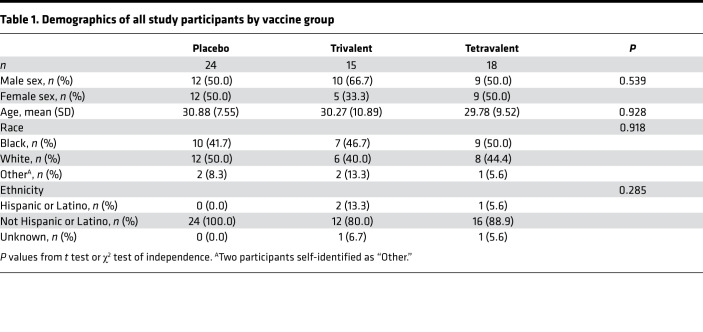
Participants were on average around 30 years old; there was a comparable number of male and female participants (54.5% male). Twenty-six participants (45.6%) self-identified as Black and 26 participants self-identified as White (45.6%), and the majority (91.2%) of participants are not Hispanic or Latino. Statistically significant differences in age, sex, race, and ethnicity were not found between the 3 vaccine groups (Table 1).
Table 1. Demographics of all study participants by vaccine group.
Cytokine levels are differentially expressed after vaccination and after challenge.
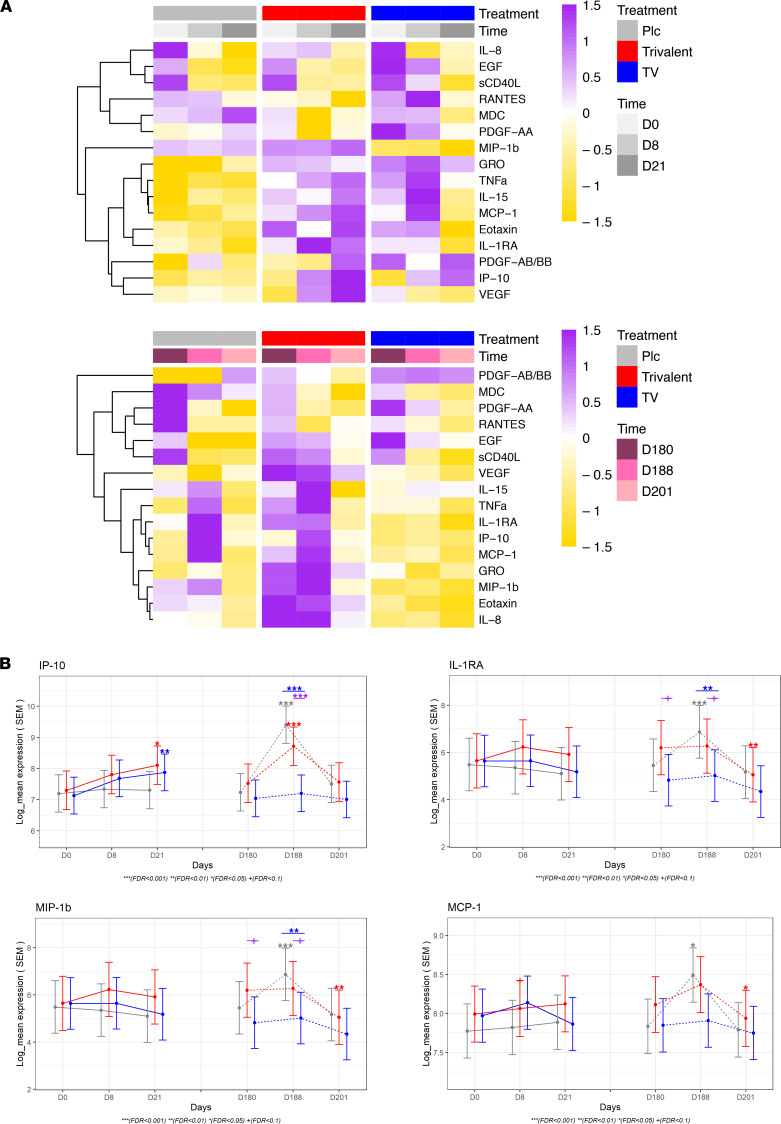
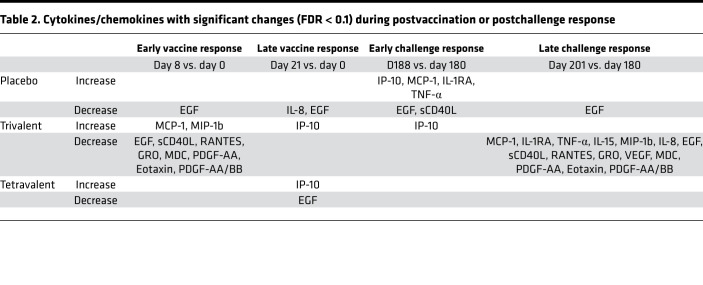
To understand the acute immune profiles after vaccination (day 8 and day 21 vs. day 0) and after challenge (day 188 and day 201 vs. day 180), we assessed the changes of the cytokine levels across time and between vaccine groups in all participants (n = 57) (Figure 2A and Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.157811DS1). Both vaccine groups induced overall proinflammatory cytokines. Significant increases in IP-10 expression were observed following vaccination with either the trivalent or tetravalent vaccine on day 21 compared with day 0. MDC, PDGF-AA, RANTES, and sCD40L levels decreased on day 8 (vs. day 0) in trivalent group but not in other groups (Table 2). Compared with day 0, EGF decreased on day 8 in the trivalent and placebo groups and decreased in the tetravalent and placebo groups on day 21. In regards to antibodies of different serotypes before challenge, no DENV2-specific antibodies were found in the trivalent admixture group, while antibodies specific to all 4 serotypes were found in the tetravalent vaccine group, and no antibodies were found in the placebo group.
Figure 2. Cytokine levels are differentially expressed after vaccination and after challenge.
(A) Heatmap showing the average expression of proteins with significant changes (FDR < 0.05) of cytokine levels over time (day 8 vs. day 0, day 21 vs. day 0, day 188 vs. day 0, day 201 vs. day 0, day 188 vs. day 180, day 201 vs. day 180) or significant differences between group (tetravalent vs. placebo, trivalent vs. placebo). Dendrograms were from hierarchical clustering applied to proteins (y axis). The color of the heatmap in each row is based on the Z score values calculated by centering and scaling the data by SD using the formula (X – m)/SD, where X denotes individual values, and m represents the mean of the row. Plc, placebo; TV, tetravalent. (B) Plots showing estimated least-squares mean and SEM for IP-10, IL-1RA, MIP-1b, and MCP-1. Symbols right above the CI bars represent significance of the changes from day 8 or day 21 vs. day 0 as well as day 188 or day 201 vs. day 180 for each vaccine group (placebo, gray; trivalent admixture, red; and tetravalent vaccine, blue). Mixed-effects model in the limma framework was used, with age, sex, and race as fixed effects covariates as well as interaction time by vaccine group and a random intercept for each participant. Symbols on the top represent significance of the comparison between intervention groups (trivalent vs. placebo [purple] and tetravalent vs. placebo [blue]). The adjusted P value is shown as +FDR < 0.1, *FDR < 0.05, **FDR < 0.01, ***FDR < 0.001.
Table 2. Cytokines/chemokines with significant changes (FDR < 0.1) during postvaccination or postchallenge response.
Following receipt of the challenge virus on day 180, a large increase in IP-10 expression was observed on day 188 in the placebo (fold change [FCH] = 4.5) and trivalent groups (FCH = 2.3) but not in the tetravalent group (FCH = 1.1). MCP-1, IL-1RA, and MIP-1b exhibited a similar pattern as IP-10 (Figure 2B). Compared with day 180, EGF decreased in the placebo and trivalent groups on day 201; sCD40L, RANTES, GRO (CXCL1), VEGF, MDC, PDGF-AA, eotaxin, and PDGF-AA/BB also decreases in the trivalent group on day 201 (Table 2).
Racial differences in cytokine expressions at baseline, after vaccination, and after challenge.
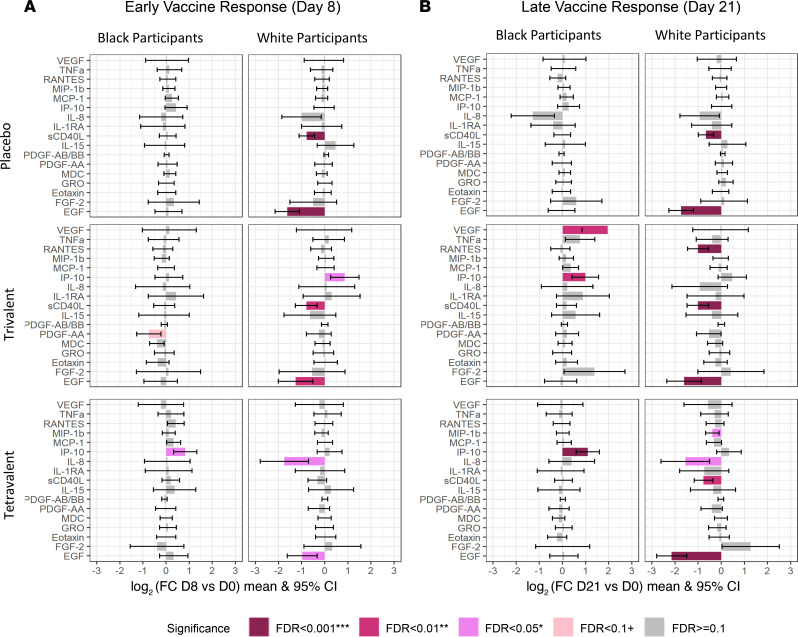
Because epidemiology studies have found that White race is a risk factor for more severe dengue (9–12) relative to that in individuals self-identified as Black, we explored the differences in immune response after vaccination and after challenge in all vaccine groups between those who identified as Black (n = 26) and White participants (n = 26). To better understand the immune responses, we first examined the racial differences of prevaccination baseline cytokine levels at day 0 and prechallenge baseline cytokine levels at day 180 (Figure 3 and Supplemental Table 2). On day 0, Black participants exhibited higher levels of GRO, VEGF, and MDC levels and lower levels MCP-1 and IP-10 than White participants. To assess if there were racial differences in terms of immune response after vaccination and challenges, we compared the changes after vaccination in the 3 vaccine groups between White and Black volunteers as well as the immune responses after challenge (Figure 4).
Figure 3. Racial differences in cytokine expression are differentially expressed at baseline and postchallenge baseline.
Bar plots showing differences (95% CI) in cytokine expression at baseline between White and Black participants. Mixed-effects model in the limma framework was used, with age and sex as fixed effects covariates as well as interaction time by race and by vaccine group and a random intercept for each participant. The adjusted P value is shown as FDR ≥ 0.01 (gray), FDR < 0.01 (light pink), and FDR < 0.001 (dark red). FCH, fold change; M, mean.
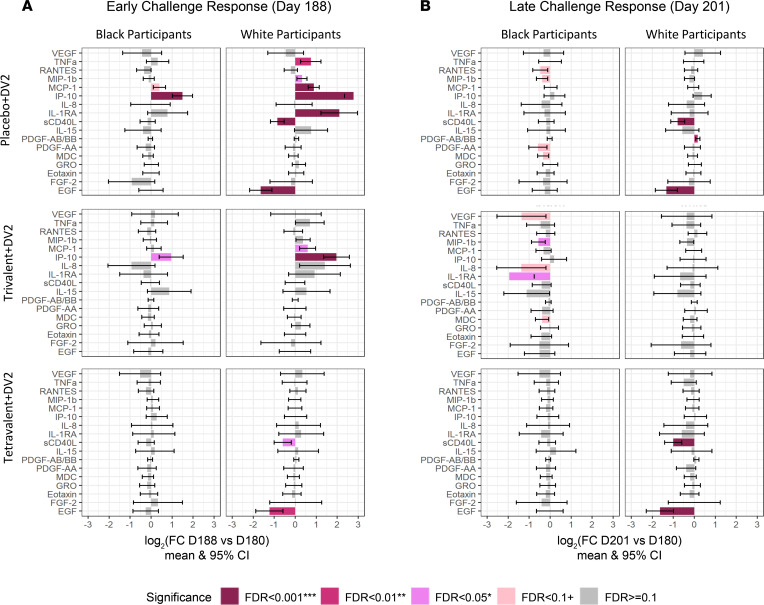
Figure 4. Bar plots showing cytokine response after vaccination.
(A) Early vaccine response: day 8 vs. day 0. (B) Later vaccine response: day 21 vs. day 0. Mixed-effects model in the limma framework was used, with age and sex as fixed effects covariates as well as interaction time by race and by vaccine group and a random intercept for each participant. The adjusted P value is shown as FDR ≥ 0.01 (gray), FDR < 0.01 (light pink), FDR < 0.05 (pink), FDR < 0.01 (dark pink), and FDR < 0.001 (dark red).
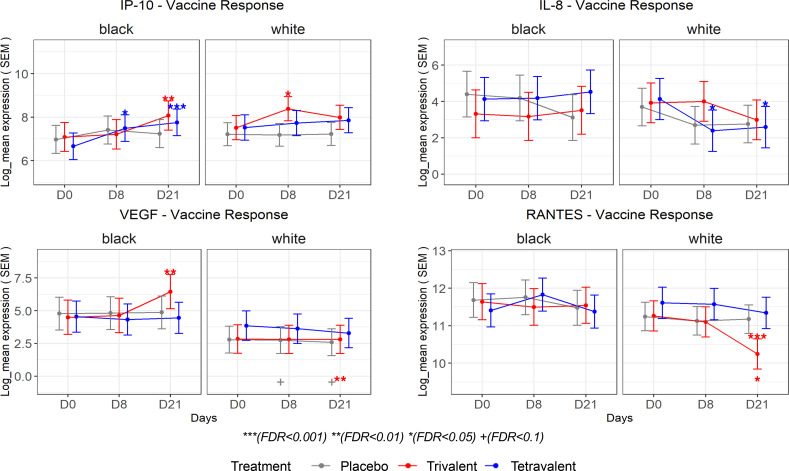
In terms of response to vaccination, there was a strong decrease in EGF in all vaccine groups, but this is only observed among White participants (Figure 4). Black participants showed a stronger increase in IP-10 levels after vaccination when compared with White participants, with significantly increased levels at day 8 after tetravalent vaccine. Levels of IP-10 were only transiently elevated at day 8 after trivalent admixture vaccine in White participants. VEGF levels among White participants were unchanged in all vaccine groups and remained significantly lower than those of Black participants. However, VEGF levels increased among Black participants as a delayed response to the trivalent admixture vaccine (Figure 5). On the other hand, no change was observed in IL-8 and RANTES levels among Black participants, while in White participants, tetravalent vaccine induced an early decrease in IL-8 and trivalent admixture decreased RANTES levels at day 21 (Figure 5).
Figure 5. Estimated least-squares mean and SEM by race are shown for selected cytokines.
Symbols above the CI bars represent significance of the changes from day 8 or day 21 vs. day 0 as well as day 188 or day 201 vs. day 180. Symbols on the top represent significance of the comparison between intervention groups (trivalent vs. placebo [purple] and tetravalent vs. placebo [blue]). Symbols below the CI bar on the bottom represent significance of differences between White participants and Black participants. Mixed-effects model in the limma framework was used, with age and sex as fixed effects covariates as well as interaction time by race and by vaccine group and a random intercept for each participant. The adjusted P value is shown as +FDR < 0.1, *FDR < 0.05, **FDR < 0.01, ***FDR < 0.001.
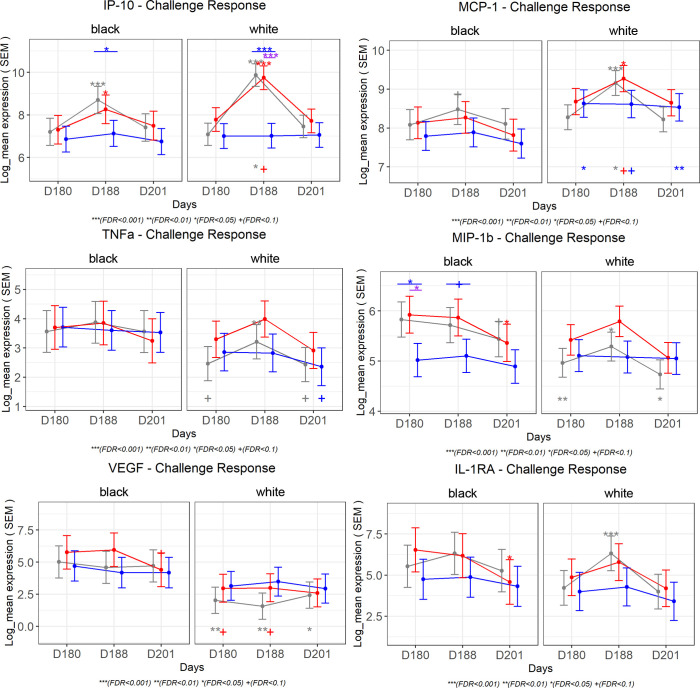
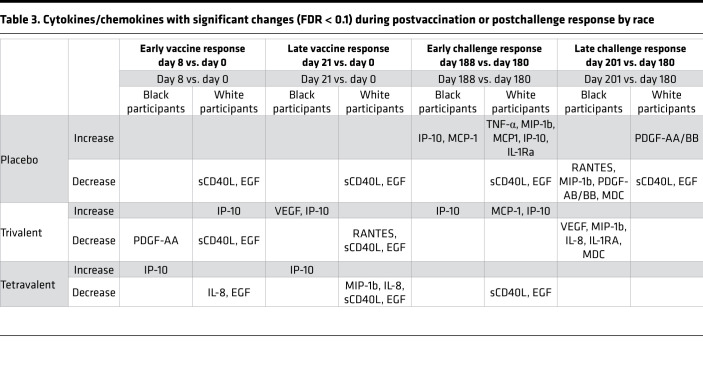
Important differences were observed in the immune response to DENV2 challenge among the 2 racial groups (Figure 6). White participants showed stronger early changes in many cytokine levels as a result of DENV2 challenge. The magnitude of the response was greater among unvaccinated participants, followed by those in the trivalent admixture group and the tetravalent vaccine group. The early increases (day 188 vs. day 180) in IP-10 levels observed in the placebo and trivalent groups after DENV2 challenge were attenuated among Black participants. White participants showed increases in IP-10 levels 2.5 times higher than those of Black participants in the placebo group and 1.5 times higher than those in the trivalent group (Figure 7). Among tetravalent vaccinees, IP-10 response was not observed following DENV2 challenge in either racial group. MCP-1, IL-1RA, and TNF-α followed a similar pattern as IP-10. MIP-1b levels were higher at day 180, and these decreased significantly on day 201 in response to DENV2 challenge in Black participants that were unvaccinated or received trivalent admixture, but no challenge response was observed among White participants that received the same vaccines or in participants of any race that received tetravalent vaccine. VEGF remained consistently higher in the trivalent and placebo groups in Black participants compared with that in White participants, but a significant challenge response was not observed for VEGF in either racial group (Figure 7). A summary of the cytokines with statistically significant changes after vaccination and after challenge among Black and White participants is presented in Table 3.
Figure 6. Bar plots showing cytokine response after challenge.
(A) Early challenge response: day 188 vs. day 180. (B) Later challenge response: day 201 vs. day 180. Mixed-effects model in the limma framework was used, with age and sex as fixed effects covariates as well as interaction time by race and by vaccine group and a random intercept for each participant. The adjusted P value is shown as FDR ≥ 0.01 (gray), FDR < 0.01 (light pink), FDR < 0.05 (pink), FDR < 0.01 (dark pink), and FDR < 0.001 (dark red).
Figure 7. Estimated least-squares mean and SEM by race are shown for selected cytokines.
Symbols above the CI bars represent significance of the changes from day 8 or day 21 vs. day 0 as well as day 188 or day 201 vs. day 180. Symbols on the top represent significance of the comparison between intervention groups (trivalent vs. placebo [purple] and tetravalent vs. placebo [blue]). Symbols below the CI bar on the bottom represent significance of differences between White participants and Black participants. Mixed-effects model in the limma framework was used, with age and sex as fixed effects covariates as well as interaction time by race and by vaccine group and a random intercept for each participant. The adjusted P value is shown as +FDR < 0.1, *FDR < 0.05, **FDR < 0.01, ***FDR < 0.001.
Table 3. Cytokines/chemokines with significant changes (FDR < 0.1) during postvaccination or postchallenge response by race.
To further investigate if the racial effects observed in this cohort were due to differences in social economic status, we decided to use household income as a proxy for socioeconomic status. We requested IRB approval from all participating institutions to have access to the participant zip codes and link them to median household incomes using the public database American Community Survey. No significant differences in median household income were observed between the 2 racial groups in our study participants. However, we explored the effect of socioeconomic status in the observed racial differences by including household income as a covariate in our model. As a sensitivity analysis, racial differences were estimated with and without income adjustment and also excluding participants with linked median household income of less than $45,000. Differences at day 0 or day 180 were the same with or without income adjusted (both correlations between with and without income adjustment for day 0 and day 180 = 0.99). The differences in vaccine response and challenge response between Black and White participants remained the same with income adjustment, indicating the observed racial differences are not explained by the income.
Vaccine protection and cytokine expression.
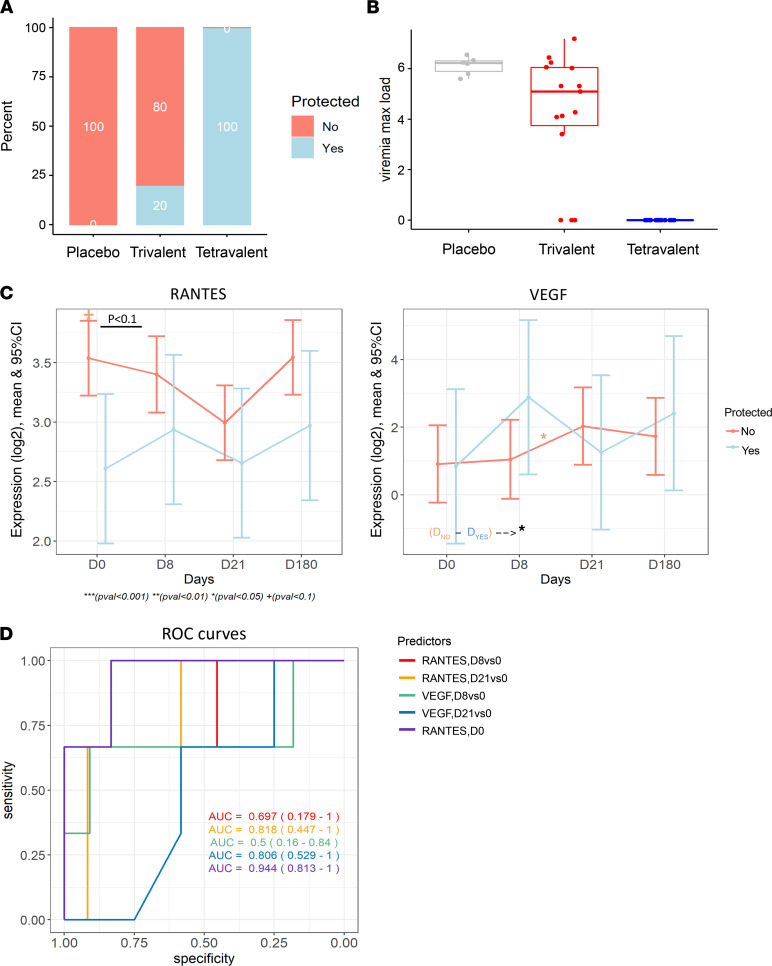
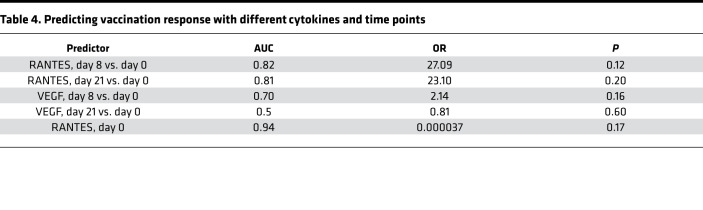
We further explored the immune profiles of individuals that were protected against DENV challenge following vaccination. While all participants that received the tetravalent vaccine were protected against viremia, as detected by PCR every 2 days from day 184 to day 196 and on days 201 and 208, only 20% of the participants in the trivalent group (n = 3) were protected (Figure 8A). Of the 3 participants that responded to trivalent admixture, none of them were Black; 1 of these participants self-identified as White and 2 participants self-identified as “other.” As for adverse events, none of the participants (0%) developed rash, myalgias, or retro-orbital pain after challenge, while among participants without protection, 33.3% (n = 4) of developed rash, 41.7% (n = 5) developed myalgias, and 25% (n = 3) developed retro-orbital pain after challenge. No statistically significant differences in any serotype-specific antibodies were found between responders and nonresponders in the trivalent group at day 180 or day 208. The maximum viremia load after challenge across 3 groups followed a similar pattern; it is shown as percentage protected again viremia (Figure 8B). To identify markers associated with vaccine protection, we compared the changes cytokine levels before DENV2 challenge (day 0 to day 180) between protected and unprotected participants vaccinated with the trivalent admixture. We identified that baseline levels of RANTES, as well as the change in VEGF levels from day 8 to day 21 after vaccination, were significantly different between groups (P < 0.05, FDR > 0.05) (Figure 8C) and, hence, potentially associated with vaccine protection against viremia. We further explored whether either baseline levels of RANTES or postvaccination responses in RANTES and VEGF were associated with vaccine protection against DENV (with or without viremia) using a logistic regression model. Our results indicated that baseline levels of RANTES may be predictive of vaccine protection against viremia in the trivalent admixture group (AUC = 0.94, P >0.05) (Figure 8D and Table 4). Participants with RANTES levels below 12.96 (equivalent to 7968 pg/mL) at baseline will be more likely to be protected against viremia, while participants with higher RANTES levels will likely not be protected against viremia.
Figure 8. Cytokine expression and vaccination response.
(A) Bar plots showing the percentage of nonresponders and responders to vaccines in each vaccine group. (B) Box plots showing the maximum viremia load after challenge in each vaccine group. (C) Line plots showing the mean expression of 2 cytokines, RANTES and VEGF, over time from day 0 to day 180. There was suggestive evidence (P < 0.05) that RANTES at day 0 and change of VEGF from day 8 to day 21 were associated with response to vaccination over time. Linear model was used, with age, sex, and race as covariates. (D) Receiver operating characteristics (ROC) curves for predicting vaccination response with different cytokines and time points. Cytokines and time points used as predictors are indicated. AUC (95% CI) achieved by each predictor is also shown. Linear model was used, with age, sex, and race as covariates.
Table 4. Predicting vaccination response with different cytokines and time points.
Discussion
In this study, we comprehensively analyzed the immune profiles of samples from volunteers who received placebo, trivalent, or tetravalent vaccination, followed by the DENV challenge. Our primary goal was to evaluate the role of heterotypic protection in a challenge model, as well as the effect of homotypic antibodies, on the infectivity of the DENV2 virus challenge. The only difference between the trivalent and tetravalent admixtures was the presence of prM and E proteins of DENV2 NGC exchanged for those of DENV4 in the rDEN4Δ30 genome (rDEN2/4Δ30). In challenge-response immunological profiles, we identified key markers of infection (IP-10, MCP-1, IL-1RA, and MIP-1β) that illustrate that the tetravalent vaccine had better protective effects against the rDEN2Δ30 challenge virus than the trivalent admixture. The differences in postchallenge immune response, between trivalent and tetravalent admixtures, indicate that the presence of DENV2 serotype–specific antibodies affects the ability of the challenge virus to induce postchallenge immune responses.
The tetravalent vaccine offers a higher level of protection against rDEN2Δ30 challenge virus compared with the trivalent admixture, as indicated by lower expression of IP-10, MCP-1, IL-1RA, and MIP-1β in viral challenge response profiles as well as a lower percentage of patients with viremia across each group. This finding illustrates that the rDEN2/4Δ30 component of the vaccine is crucial for the protection DENV2 virus and, thus, indicates that homotypic antibodies against DENV2 are much more protective than the heterotypic antibodies induced by the rDEN1Δ30, rDEN3Δ30/31, and rDEN4Δ30 vaccine components (5). Dengue vaccines must induce durable protection against all 4 DENV serotypes to mitigate the risk for more severe disease years following vaccination, as was observed for Dengvaxia (13). Dengvaxia did not induce a balanced homotypic antibody response, which likely contributed to this outcome (14).
Our results indicate that IP-10, MCP-1, IL-1RA, and MIP-1β were key markers, indicating that the tetravalent vaccination offers a better protection against challenge virus compared with the trivalent admixture. These markers have been previously found to be associated with robust antiviral immune responses. Elevated levels of IP-10 levels in vitro have been shown to directly affect DENV replication by inhibiting viral binding to target cells in vitro (15). Additionally, anti–IP-10–treated mice were found to show reduced NK cell infiltration after DENV infection, suggesting that IP-10 mediates the recruitment of activated NK cells (15). MIP-1β, a chemoattractant for NK cells, was associated with dengue disease severity in 59 patients with dengue in Brazil (16). In patients with acute DENV infection, levels of IP-10, MCP-1, and MIP-1β were correlated with the absolute number and frequency of CD14+CD16+ monocytes (17). MCP-1 is a highly expressed chemokine in plasma of patients with DHF/DSS. It was found that MCP-1 expression was induced in DENV2-infected monocytes and lymphocytes, and it may increase endothelial permeability (18). IL-1RA could alleviate the damage, including vascular leakage and tissue injury, produced by IL-1β during dengue disease in vivo and in vitro (19).
Based on the viremia analysis, all participants were protected by tetravalent vaccines and baseline RANTES levels were found to have the best classification capability for vaccination protection against viremia in the trivalent admixture group. It is possible that the baseline RANTES level makes the protective capacity of cross-reactive immunity more effective. In a study with a dengue-predisposed population from Guwahati, India, it was suggested that expression of both RANTES and CCR5 has prognostic value in DENV infections through altered immunomodulation from the RANTES-CCR5 axis (20). Although the finding with RANTES was not statistically significant, we think that this is due to limited sample size; only 3 responders were available in the trivalent group, and our exploratory results could provide potential insights for future studies.
Racial differences associated with response to DENV infection and disease severity have been reported (8, 9). Although income and ethnic backgrounds are correlated and income has been associated with dengue independently, Blanton et al. found that the both self-reported Afro-Brazilian ethnicity and genetic African ancestry were protective for DHF after adjustment for income (11). We believe our cohort is suitable to examine the racial differences in dengue responses. The cohort participants were specifically recruited to be multiracial and evenly divided by race. Therefore, we had a good proportion of participants of African descent and participants self-identifying as White. This would be an appropriate cohort in which to identify racial differences because it is well-balanced in terms of White and Black participants; even with mild dengue disease, we could study if there were racial differences in infection.
Racial differences in baseline MCP-1 and GRO observed here have been previously reported in the population-based NHLBI Heart Study as well as in patients with hepatitis C and patients in the Dallas Heart Study, respectively (21–23), indicating intrinsic differences in these cytokine levels between Black and White participants. However, we found consistently lower VEGF in White participants compared with Black participants, whereas another study, based on Emory Cardiovascular Biobank, did not observe this difference (24). Given that baseline RANTES has the best predictive ability for protection by the trivalent admixture in our study and the protective effects for Black participants found in the above literature, we would expect that baseline RANTES is higher in White participants. However, baseline RANTES differences between Black and White participants were not found.
The differences in the immune responses to vaccination and challenge between Black and White participants may contribute to racial differences in disease progression and clinical manifestations of DENV infection. Previous studies examining 4 different admixtures of a live attenuated tetravalent dengue vaccine found that Black vaccinees were less likely to have infectious vaccine virus recovered from the blood and were less likely to develop a tetravalent antibody response compared with vaccinees who are not Black (25). In our current study, IP-10 continuously increased in Black participants until 3 weeks after tetravalent vaccination, whereas it increased only transiently in White volunteers. Viral replication is reduced in the presence of increased IP-10 induction. We found higher IP-10 induction in Black tetravalent vaccine recipients, perhaps explaining why Durbin et al. (25) found reduced vaccine viremia in Black participants after vaccination. Islam et al. suggest that DENV has a competitive edge of infecting the monocytes and initiating infection pathogenesis under a RANTES-deficient condition (20). Lower RANTES levels were found in White participants receiving trivalent admixture compared with Black recipients, contributing to a possible advantageous environment for DENV infection, which also potentially explains the a higher frequency of viremia in White volunteers following vaccination observed in Durbin et al. (25). IL-8 was reported to be higher in patients with severe dengue compared with those with mild disease, and IL-8 levels gradually decreased in patients with mild disease as they recovered but not in those with severe disease (26). We observed a gradual decrease in IL-8 levels in tetravalent vaccine recipients that may indicate a gradual recovery from tetravalent vaccine stimulation. VEGF, a potent permeability enhancing cytokine, was higher in patients with severe dengue as compared with patients with nonsevere dengue with and without warning signs (27). We would expect to see an increase in VEGF in White trivalent admixture recipients, but only a late increase in VEGF was found in Black trivalent admixture recipients. sCD40L and EGF decreased in all vaccine and placebo recipients. We hypothesize that this could be an injury response or response to components in the placebo.
Antibody-dependent enhancement assays of DENV2 virus replication have shown that there is a striking increase in virus replication in cells of White participants in the presence of DENV antibodies, while replication was not observed in cells of Black participants even without DENV antibodies (28). In another study of 80 Cubans infected with 4 DENV serotypes, White participants show stronger and highly cross-reactive DENV-specific memory CD4+ T lymphocyte proliferation and interferon-γ release compared with Black participants (12). In our cohort, unvaccinated White volunteers or those vaccinated with the trivalent admixture experienced a significant increase in IP-10 and MCP-1 after DENV challenge, compared with Black volunteers. Both IP-10 and MCP-1 are inducible by interferon-γ (29–31), and the greater release of interferon-γ in White participants reported by Sierra et al. may contribute to the greater challenge response for IP-10 and MCP-1 in White participants seen in our study. sCD40L and EGF were found to be lower in sever dengue cases compared with nonsevere dengue cases (32, 33), which is consistent with the sCD40L and EGF decrease we expect in unvaccinated White participants. But it is unclear why both cytokines also decreased in White recipients of tetravalent vaccine.
One strength of this study is that we comprehensively characterized and compared the immune profiles after vaccination and after challenge among participants at multiple time points. In addition, the DENV human challenge model offers insights in the early assessment of efficacy for vaccine candidates before initiation of large efficacy trials. One limitation of our study is that only specific time points were used, so it is possible that transient changes in some cytokines or chemokines were not captured. Another limitation is that we used publicly available median household income at a specific region as a proxy for social economic status for the participants, where other components of social economic status were not represented. It would be important to collect this information for future studies. Although limited by sample size, we explored the racial differences in immune profiles and examined the chemokines and cytokines that could be predictive of vaccine protection. This study was composed of healthy volunteers without underlying health conditions; however, we are unsure of volunteer nutrition status and other lifestyle factors and cannot therefore fully address the role of racial differences in vaccine protection.
In conclusion, our comprehensive immune profiling of 57 patients after vaccination and after challenge revealed that tetravalent vaccine offers better protection against DENV compared with a trivalent admixture lacking a DENV2 vaccine component, indicating a crucial role of the rDEN2/4Δ30 component to vaccine protection. Consistent with published reports (21–23), we observed differences in chemokine and cytokine levels at baseline between Black and White participants. Additionally, we found that White participants have elevated immune responses to vaccination and challenge virus compared with Black participants. We believe that our findings provide an overall perspective on the immunological characteristics of dengue vaccines and DENV challenge after vaccination and contribute to new hypothesis generation for future research to further elucidate the mechanisms of dengue pathogenesis and vaccine development in humans.
Methods
Study design of the clinical trial.
Healthy adult male and nonpregnant female volunteers 18–50 years of age were enrolled in Baltimore, Maryland, and Burlington, Vermont. The clinical trials registered at clinicaltrials.gov (NCT02317900 and NCT02433652). Volunteers were enrolled if they met the following eligibility criteria: normal findings during physical examination; no history of receipt of any flavivirus vaccine, licensed or experimental; negative for serum neutralizing antibodies against all DENV serotypes, yellow fever virus, West Nile virus, and St. Louis encephalitis virus; negative for hepatitis B and C; negative for HIV; normal blood hematology; and serum chemistry. Female volunteers were required to have a negative urine or serum pregnancy test at screening and on vaccination day and to agree to use contraception. Blood samples for PBMC isolation, and protein expression were collected at 0, 8, and 21 days after vaccination and after challenge. Volunteers were assessed for adverse events, and serum collection for virus isolation was performed approximately every other day for the first 16 days after vaccination and after challenge. There were not any clinical or laboratory abnormalities in vaccine recipients in CIR299. In CIR300, there were 4 trivalent vaccine recipients with rash. For CIR299, none of the TV005 or placebo recipients developed a fever after challenge. 100% of placebo recipients developed a rash, 28.6% developed retro-orbital pain, and 28.6% developed myalgias. These were the only adverse events that occurred more frequently in placebo recipients after challenge than vaccine recipients (without multiple comparisons). In CIR300, 83.3% of placebo recipients developed rash after challenge compared with 26.7% of trivalent recipients. 100% of placebo recipients were viremic after challenge (culture and PCR positive) compared with 80% (PCR) and 27% (culture) of trivalent recipients. None of the volunteers developed a fever secondary to challenge virus.
Vaccine (TV005, trivalent) and challenge virus (rDEN2Δ30).
TV005 is an admixture composed of 4 DENV strains attenuated by a 30-nucleotide deletion in the 3′ UTR (rDEN1Δ30 and rDEN4Δ30), one 30-nucleotide and one-31 nucleotide deletion in the 3′ UTR (rDEN3Δ30/31), or by replacing the prM and E of rDEN4Δ30 with those of DENV2 NGC (rDEN2/4Δ30). The trivalent admixture is composed of the DENV1, DENV3, and DENV4 vaccine components rDEN1Δ30, rDEN3Δ30/31, and rDEN4Δ30. The only difference between trivalent and tetravalent admixtures is the presence of prM and E proteins of DENV2 NGC (New Guinea C strain) exchanged for DENV4 in the rDEN4Δ30 genome (rDEN2/4Δ30). The challenge strain rDEN2Δ30 is a recombinant virus derived from DENV2 Tonga/74 WT virus, a different genotype than DEN2 NGC. Although it contains a 30-nucleotide deletion in the 3′ UTR, the virus was not attenuated in preclinical animal models and induced a viremia 10-fold to 100-fold higher than rDEN2/4Δ30 in human clinical trials.
Challenge viremia determination.
Serum samples collected approximately every other day at follow-up visits through day 16 after challenge (study days 180–196) were tested for the presence of infectious virus by tissue culture and for the presence of DENV RNA by RT-PCR. Viral RNA was extracted from 400 μL serum using the EZ1 Advanced XL system and EZ1 virus mini kit v2.0 (Qiagen). Following extraction, 7 μL extracted RNA was transcribed to cDNA using the Veriti 96-well thermal cycler (Applied Biosystems) and the SuperScript III first-strand synthesis system (Life Technologies) standard protocol and reagents. PCR master mix was prepared using TaqMan Fast Universal PCR Master Mix, DEN-2 Tonga/74–specific forward primer and reverse primers (D2Tonga208F, CTGCAGGGACGAGGACCACT; D2Tonga275R, GGGATTGTTAGGAAACGAAGGA; D2Tonga229P, AAATTGTTCATGGCCCTGGTGGCA), and a TaqMan FAM5′/3′ BHQ1 probe. 20 μL master mix was mixed with 5 μL cDENA template, and the reaction was carried out using the QuantStudio 6 Flex instrument. Standard curves were generated from plasmid DNA to quantify the genome equivalents.
Multiplex ELISA.
Cytokines and chemokines were measured using a multiplex ELISA-based assay (Luminex). Samples were randomized across the plates based on the vaccine cohorts, keeping all samples from a participant together. Assays were run in sample duplicates in a 96-well microtiter plate using 25 μL serum/well from each participant and multiplex cytokine panels (Multiplex Human Cytokine/Chemokine Panel, Millipore Corp.). Forty-one analytes (cytokines, chemokines, and growth factors) were measured using a Luminex-200 system and the XMap Platform (Luminex Corporation). Acquired mean fluorescence intensity data were analyzed and calculated using Beadview software. The minimum detectable concentration was calculated using Milliplex Analyst 5.1. The lower and upper detection limits for these assays are 3.0 pg/mL and 15,000 pg/mL, respectively. Quality controls were included in all plates, and any analytes with a bead count of less than 50 were not used for analysis.
Statistics.
R (https://www.r-project.org/) version 4.0.2 and available packages were used for all analyses. Luminex data were analyzed as log2 of the protein concentration obtained from the standard curve interpolation. Analytes were eliminated if the expression level value (pg/mL) was under LOD for more than 50% of the samples. The batch effect for Luminex was visually ascertained using principal component analysis, and the percentage of variance explained by the batch was estimated by principal variation component analysis. Luminex data were adjusted for each batch using a linear model with age, sex, and time as factors. Protein expression was modeled using linear mixed-effect models.
Expression profiles were modeled using mixed-effects models in the limma framework to account for the paired structure of the data. Limma uses an empirical Bayes moderation of the standard errors toward the prior transcript variances, which was fitted using an intensity-dependent trend. Models included age, sex, and race as fixed effects covariates as well as interaction time by vaccine group and a random intercept for each participant. As literature has shown that race is a risk factor for dengue, an exploratory analysis adding a time by race and by vaccine group interaction as a fixed effect was also conducted, with age and sex as covariates (8, 9). In all models, hypotheses of interest were tested via contrasts, and the P values from the moderated (paired) 2-tailed t test were adjusted for multiple hypotheses using the Benjamini-Hochberg (FDR-controlling) procedure. FDR values of less than 0.05 were considered significant.
We also conducted an exploratory analysis investigating the association between postchallenge response (viremia) and cytokine expression levels at baseline and postvaccination response using linear models, and we identified cytokines predictive of postvaccination response with a logistic regression. Both models considered age, sex, and race as covariates. Examination of postvaccination response in regards to viremia was not possible due to insufficient power.
Study approval.
The clinical trials (Clinicaltrials.gov NCT02317900 and NCT02433652) were conducted under FDA investigational new drug application 1575. They were approved by the Institutional Review Board and the Institutional Biosafety committee at each institution (Icahn School of Medicine at Mount Sinai; John Hopkins University, Baltimore, Maryland, USA; and University of Vermont, Burlington, Vermont, USA) and were overseen by the Data Safety and Monitoring Board of the NIH.
Author contributions
AFS, APD, MSF, and SSW conceived the study. RH, LET, and MSF provided methodology. RH and MSF performed investigations. AFS, APD, MSF, and SSW acquired funding. AFS, APD, and MSF provided supervision. RH, JPS, and MSF wrote the original draft of the manuscript. LET, SKS, AFS, APD, MSF, and SSW reviewed and edited the manuscript.
Supplementary Material
Figure 1. Schematic diagram of the clinical trial design.
Acknowledgments
This study was funded by National Institute of Allergy and Infectious Diseases grant U19AI118610, as part of the National Institute of Allergy and Infectious Diseases–funded Human Immune Project Consortia. The previously conducted CIR299 and CIR300 dengue vaccine human trials were supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, NIH (contract no. HHSN272200900010C). We acknowledge the help of Dabeiba Bernal, Irene Ramos-Lopez, and Tarrell Malloy for coordinating calls and sample shipping for the study.
Version 1. 05/05/2022
In-Press Preview
Version 2. 06/08/2022
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2022, Hou et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2022;7(11):e157811.https://doi.org/10.1172/jci.insight.157811.
Contributor Information
Ruixue Hou, Email: ruixue.hou@mountsinai.org.
Lewis E. Tomalin, Email: lewis.tomalin@mountsinai.org.
Jessica Pintado Silva, Email: jessica.pintadosilva@icahn.mssm.edu.
Seunghee Kim-Schulze, Email: seunghee.kim-schulze@mssm.edu.
Stephen S. Whitehead, Email: swhitehead@niaid.nih.gov.
Ana Fernandez-Sesma, Email: ana.sesma@mssm.edu.
Anna P. Durbin, Email: adurbin1@jhu.edu.
Mayte Suárez-Fariñas, Email: Mayte.SuarezFarinas@mssm.edu.
References
- 1.World Health Organization. Dengue vaccines: WHO position paper — September 2018. Wkly Epidemiol Rec. 2018;93(36):457–476. [Google Scholar]
- 2.Prompetchara E, et al. Dengue vaccine: global development update. Asian Pac J Allergy Immunol. 2019;10(3):178–185. doi: 10.12932/AP-100518-0309. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead SS. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYDTM vaccine? Expert Rev Vaccines. 2016;15(4):509–517. doi: 10.1586/14760584.2016.1115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng S-Q, et al. A review on dengue vaccine development. Vaccines (Basel) 2020;8(1):63. doi: 10.3390/vaccines8010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkpatrick BD, et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med. 2016;8(330):330ra36–330ra36. doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 6.Lindow JC, et al. Primary vaccination with low dose live dengue 1 virus generates a proinflammatory, multifunctional T cell response in humans. PLoS Negl Trop Dis. 2012;6(7):e1742. doi: 10.1371/journal.pntd.0001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaney JE, Jr, et al. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol. 2005;79(9):5516–5528. doi: 10.1128/JVI.79.9.5516-5528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouri GP, et al. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull World Health Organ. 1989;67(4):375–380. [PMC free article] [PubMed] [Google Scholar]
- 9.Sierra BDLC, et al. Race: a risk factor for dengue hemorrhagic fever. Arch Virol. 2007;152(3):533–542. doi: 10.1007/s00705-006-0869-x. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso IM, et al. Dengue: clinical forms and risk groups in a high incidence city in the southeastern region of Brazil. Rev Soc Bras Med Trop. 2011;44(4):430–435. doi: 10.1590/S0037-86822011005000044. [DOI] [PubMed] [Google Scholar]
- 11.Blanton RE, et al. Genetic ancestry and income are associated with dengue hemorrhagic fever in a highly admixed population. Eur J Hum Genet. 2008;16(6):762–765. doi: 10.1038/ejhg.2008.4. [DOI] [PubMed] [Google Scholar]
- 12.Sierra BDLC, et al. Ethnicity and difference in dengue virus-specific memory T cell responses in Cuban individuals. Viral Immunol. 2006;19(4):662–668. doi: 10.1089/vim.2006.19.662. [DOI] [PubMed] [Google Scholar]
- 13.Hadinegoro SR, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373(13):1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 14.Henein S, et al. Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naive and dengue-exposed individuals. J Infect Dis. 2017;215(3):351–358. doi: 10.1093/infdis/jiw576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J-P, et al. Dengue virus induces expression of CXC chemokine ligand 10/IFN-gamma-inducible protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J Immunol. 2006;177(5):3185–3192. doi: 10.4049/jimmunol.177.5.3185. [DOI] [PubMed] [Google Scholar]
- 16.Bozza FA, et al. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis. 2008;8:86. doi: 10.1186/1471-2334-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwissa M, et al. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell Host Microbe. 2014;16(1):115–127. doi: 10.1016/j.chom.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y-R, et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol. 2006;87(12):3623–3630. doi: 10.1099/vir.0.82093-0. [DOI] [PubMed] [Google Scholar]
- 19.Pan P, et al. Dengue virus infection activates interleukin-1β to induce tissue injury and vascular leakage. Front Microbiol. 2019;10:2637. doi: 10.3389/fmicb.2019.02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam M, et al. Significance of RANTES-CCR5 axis and linked downstream immunomodulation in dengue pathogenesis: a study from Guwahati, India. J Med Virol. 2019;91(12):2066–2073. doi: 10.1002/jmv.25561. [DOI] [PubMed] [Google Scholar]
- 21.Tang W, et al. Association of sICAM-1 and MCP-1 with coronary artery calcification in families enriched for coronary heart disease or hypertension: the NHLBI Family Heart Study. BMC Cardiovasc Disord. 2007;7:730. doi: 10.1186/1471-2261-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo L, et al. Associations of four circulating chemokines with multiple atherosclerosis phenotypes in a large population-based sample: results from the Dallas Heart Study. J Interferon Cytokine Res. 2010;30(5):339–347. doi: 10.1089/jir.2009.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson S, et al. Plasma levels of growth-related oncogene (CXCL 1-3) associated with fibrosis and platelet counts in HCV-infected patients. Aliment Pharmacol Ther. 2015;42(9):1111–1121. doi: 10.1111/apt.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samman Tahhan A, et al. Circulating progenitor cells and racial differences. Circ Res. 2018;123(4):467–476. doi: 10.1161/CIRCRESAHA.118.313282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durbin AP, et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis. 2013;207(6):957–965. doi: 10.1093/infdis/jis936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, et al. Slow resolution of inflammation in severe adult dengue patients. BMC Infect Dis. 2016;16(1):291. doi: 10.1186/s12879-016-1596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thakur P, et al. Elevated levels of vascular endothelial growth factor in adults with severe dengue infection. Virusdisease. 2016;27(1):48–54. doi: 10.1007/s13337-015-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morier L, et al. Antibody-dependent enhancement of dengue 2 virus in people of white descent in Cuba. Lancet. 1987;329(8540):1028–1029. doi: 10.1016/s0140-6736(87)92289-6. [DOI] [PubMed] [Google Scholar]
- 29.Grandaliano G, et al. Gamma interferon stimulates monocyte chemotactic protein (MCP-1) in human mesangial cells. J Lab Clin Med. 1994;123(2):282–289. [PubMed] [Google Scholar]
- 30.Valente AJ, et al. A complex element regulates IFN-gamma-stimulated monocyte chemoattractant protein-1 gene transcription. J Immunol. 1998;161(7):3719–3728. [PubMed] [Google Scholar]
- 31.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166(4):1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harenberg A, et al. Cytokine profile of children hospitalized with virologically-confirmed dengue during two phase III vaccine efficacy trials. PLoS Negl Trop Dis. 2016;10(7):e0004830. doi: 10.1371/journal.pntd.0004830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singla M, et al. Immune response to dengue virus infection in pediatric patients in New Delhi, India—association of viremia, inflammatory mediators and monocytes with disease severity. PLoS Negl Trop Dis. 2016;10(3):e0004497. doi: 10.1371/journal.pntd.0004497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.