Abstract
The antibiotic resistance patterns of fecal streptococci and fecal coliforms isolated from domestic wastewater and animal feces were determined using a battery of antibiotics (amoxicillin, ampicillin, cephalothin, chlortetracycline, oxytetracycline, tetracycline, erythromycin, streptomycin, and vancomycin) at four concentrations each. The sources of animal feces included wild birds, cattle, chickens, dogs, pigs, and raccoons. Antibiotic resistance patterns of fecal streptococci and fecal coliforms from known sources were grouped into two separate databases, and discriminant analysis of these patterns was used to establish the relationship between the antibiotic resistance patterns and the bacterial source. The fecal streptococcus and fecal coliform databases classified isolates from known sources with similar accuracies. The average rate of correct classification for the fecal streptococcus database was 62.3%, and that for the fecal coliform database was 63.9%. The sources of fecal streptococci and fecal coliforms isolated from surface waters were identified by discriminant analysis of their antibiotic resistance patterns. Both databases identified the source of indicator bacteria isolated from surface waters directly impacted by septic tank discharges as human. At sample sites selected for relatively low anthropogenic impact, the dominant sources of indicator bacteria were identified as various animals. The antibiotic resistance analysis technique promises to be a useful tool in assessing sources of fecal contamination in subtropical waters, such as those in Florida.
Fecal coliform bacteria are the most commonly used indicators of fecal pollution in water and food. They inhabit the gastrointestinal tracts of all warm-blooded and some cold-blooded animals (1, 8) and therefore provide no information about the specific source of fecal contamination. This information is important because (i) fecal material from sources such as humans and cattle can be regarded as “high risk” due to the possible presence of human pathogens and (ii) identification of the fecal source is necessary if management plans for prevention of further contamination are to be developed. For these reasons, an indicator that is source discriminant would be useful to investigators and regulators of water quality.
A second group of bacteria, the fecal streptococci, has been proposed for use as a water quality indicator (1). Fecal streptococci are gram-positive, catalase-negative cocci that cleave esculin and are not inhibited by bile salts. They are classified as group D streptococci by antiserum reactivity. The enterococci, which were formerly classified as fecal streptococci, came to be recognized as a useful water quality indicator (1) and are now classified in the genus Enterococcus (16). They can be differentiated from the larger fecal streptococcus group by their ability to grow at 10 and 45°C, at pH 9.6, and in medium with 6.5% NaCl (9).
Findings from several studies have suggested that the enterococci may be better indicator organisms than fecal coliforms. Enterococcus spp. may survive longer in marine environments than fecal coliforms (3), and their survival rate through wastewater treatment processes is higher than that of fecal coliforms (18). Their numbers in marine and fresh recreational waters correlate with the risk of human pathogens and disease (3, 4, 12), while those of fecal coliforms do not. Like fecal coliforms, enterococci are found in the feces of all warm-blooded animals and therefore share the drawback of nonspecificity with the fecal coliforms.
Resistance to multiple classes of antibiotics is not uncommon in enterococci and fecal coliforms isolated from animals and humans. The selective pressure imposed on the commensal gastrointestinal flora of animals and humans by antibiotic use results in patterns of antibiotic resistance that reflect to some extent the microflora's exposure to antibiotics. It has been proposed that antibiotic resistance patterns (ARPs) of Escherichia coli (13, 19) and fecal streptococci (7, 23, 24) can be used as phenotypic “fingerprints” to determine the source of fecal pollution in natural waters or food.
Discriminant analysis is a multivariate statistical technique that can be used to classify subjects in categories based on a series of test variables (10, 22). The correct classification rate for isolates from each known source can be used to evaluate the predictive capabilities of databases used for discriminant analysis. The rate of correct classification is calculated by self-crossing the database and is the percentage of isolates from a source that are actually classified by discriminant analysis in the correct source category. As a form of data reduction, discriminant analysis relies on the computation of “derived variables” from n isolates and p variables of k groupings (sources) to distinctly separate the sources (6).
The work presented here describes the application of antibiotic resistance analysis (ARA) (24) using discriminant analysis as a tool to differentiate between animal and human fecal isolates in subtropical surface waters of Florida. The ARPs of fecal streptococci and fecal coliforms from known animal sources and from human-dominated sources (domestic wastewater) were determined in order to create separate databases (fecal streptococcus and fecal coliform) to which ARPs of isolates from surface waters could be compared and categorized by probable source. The ability of the fecal streptococcus and fecal coliform databases to identify a dominant source of contamination in surface waters was assessed by sampling at sites impacted by septic tank effluent, which were contrasted with sites receiving relatively slight anthropogenic impacts.
MATERIALS AND METHODS
Sample collection.
All fecal and wastewater samples were collected in Florida. During each sampling event, feces from multiple animals of the same source category were collected and combined into a single sample. Each farm site was sampled on two to four separate occasions. Wastewater samples (designated “human”) were obtained from sources where the majority of isolates would be of human origin, i.e., influent from wastewater treatment plants that receive input primarily from residential sources (Jacksonville) and from wastewater lift stations in residential neighborhoods (Tampa). Chicken and cattle (dairy and beef) feces were each collected from at least five different farms within a 30-mile radius of Jacksonville. Chicken feces were collected inside poultry barns. Cattle feces were obtained from fields (beef), in which case multiple fresh, widely dispersed piles were sampled, or from central solid-waste collection points in dairy barns. Pig feces were obtained from an auction site in Lake City, Florida, that receives animals from Florida and Georgia. Dog fecal samples were obtained from healthy animals (not undergoing antibiotic therapy) at the Jacksonville and Clearwater Florida Humane Societies.
Because wild-animal feces were more difficult to obtain than those of domestic animals, the number of isolates in each wild-animal category was small, ranging from 83 fecal coliform isolates from rabbits to 274 fecal coliform isolates from birds. There were 125 fecal streptococcus isolates from birds and 212 fecal streptococcus isolates from raccoons. The numbers of isolates from individual wild sources were low compared to those in the domestic-animal and human categories; therefore, isolates from various wild-animal sources were grouped together for some analyses to form a “wild” category, which consisted of fecal coliform isolates from birds, raccoons, rabbits, and ducks and fecal streptococcus isolates from birds and raccoons. Bird feces were obtained from several natural rookeries located on the University of North Florida campus and at Matanzas inlet (south of St. Augustine, Florida) and containing a mix of wood storks, night herons, and egrets. Raccoon feces were obtained from trapped animals, from a State Park campground, and from a congregation site near a restaurant dumpster. Rabbit and duck feces were collected from piles on the ground.
Sample processing and isolation of indicator bacteria.
The samples were transported to the laboratory on ice and were processed within 8 h of collection. One gram of fecal material or 1 ml of wastewater was placed into 100 ml of 0.5% saline buffer, pH 7.0. The samples were further diluted, depending on the source of the sample. Dilutions were filtered with a 0.45-μm-pore-size membrane filter. Fecal coliforms were incubated at 44.5°C in mFC broth (Difco) for 18 to 24 h. Individual blue colonies from membrane filters containing 30 to 70 colonies were transferred with sterile toothpicks into the wells of a 96-well microtiter plate containing 0.2 ml of EC broth (Difco)/well. Microtiter plates were incubated at 37°C for 18 to 24 h. Fecal streptococci were incubated at 37°C in Enterococcosel broth (BBL) for 24 h. Individual colonies were transferred with sterile toothpicks to microtiter plate wells containing Enterococcosel broth. The microtiter plates were incubated at 37°C for 24 h. The ARPs of isolates in wells that turned dark brown, indicating esculin hydrolysis, were included in the database.
Vancomycin-resistant fecal streptococcus isolates were subjected to further biochemical testing in order to determine their placement in the genus Enterococcus. Those isolates that were catalase negative, grew in brain heart infusion broth at 44.5°C and in brain heart infusion broth with 6.5% NaCl at 37°C, and were esculin positive on bile esculin agar were tentatively identified as Enterococcus spp. (1). Membership in the genus Enterococcus was further confirmed by positive pyrrolidonyl arylamidase and leucine arylamidase tests (5).
Determination of ARP.
The ARP of each fecal streptococcus isolate comprised 36 observations (nine antibiotics at four concentrations each). Vancomycin resistance is an intrinsic characteristic of the fecal coliforms (2); therefore, their ARPs comprised 32 observations. A 48-prong replicator (Sigma) was used to transfer isolates onto 100-mm-diameter tryptic soy agar plates amended with the following concentrations of antibiotics: ampicillin, 10, 15, 30, and 50 μg/ml; amoxicillin, 5, 10, 15, and 20 μg/ml; cephalothin, 10, 15, 20, and 25 μg/ml; chlortetracycline, oxytetracycline, and streptomycin, 20, 40, 60, and 80 μg/ml; tetracycline and erythromycin, 10, 15, 30, and 50 μg/ml; and vancomycin, 5, 10, 15, and 30 μg/ml (fecal streptococci only). The ARPs of isolates that failed to grow on the last tryptic soy agar plate were discarded. The plates were incubated at 37°C for 18 to 20 h. The colonies were recorded as positive if there was growth or negative if there was no growth. Antibiotic concentrations were chosen to coincide with those used by B. A. Wiggins at James Madison University so that the data could eventually be compared (23).
Statistical analysis of isolates from known sources.
Discriminant analysis was performed essentially as described by Wiggins (23). The pattern of each isolate was entered into a spreadsheet (Excel). Discriminant analysis using SAS software (version 6.12 for Windows; SAS Institute, Inc.) was used to classify the isolates by source. The table generated by the DISCRIM procedure displays the number and percent of isolates from each known source that are classified in each source category. The correct classification rates were calculated by using one set of ARPs both to establish the classification rule and as test subjects (10). The number of isolates from a given source that are placed in the correct source category by discriminant analysis is termed the rate of correct classification. The average rate of correct classification (ARCC) for the database is obtained by averaging the correct classification percentages for all sources (23). The holdout method of cross validation, in which isolates from known sources are randomly removed from the data set and treated as test subjects, was used as a more rigorous test of the predictive power of the databases (10). For the holdout analysis, approximately 25% of isolates from each source were used as subjects, i.e., of 1,420 fecal coliform isolates from cattle, 355 were held out for the cross validation.
The statistical significance of the discriminant analysis results was validated by an F statistic given by the equation (p. 339 of reference 6) F = [n1n2(n1 + n2 − p − 1)/(n1 + n2(n1 + n2 − 2)p]D2, where n is the number of isolates in each group or source (k), p is the number of response variables, and D2 is the distance squared between the two groups being compared. An F value was computed for each possible pairing of groups and compared to the critical value of the F distribution using df1 = p and df2 = n1 + n2 − p − 1 (6).
Comparisons of correct classification rates between the fecal streptococcus and the fecal coliform databases were assessed by likelihood ratio tests. To determine whether the databases were adversely affected by sample inequality, Spearman's ranked correlation was used to compare the rates of correct classification of isolates from each source to factors such as their respective source sample sizes and number of sampling events. The chi-square test was used to determine significant differences in the percentages of antibiotic-resistant isolates obtained from different sources and to compare correct classification rates between databases.
Analysis of isolates from surface waters.
Natural water samples were collected approximately 0.5 m below the surface in sterile bottles. The samples were transported to the laboratory on ice and were processed within 8 h of collection. Appropriate sample volumes were filtered. Generally, the ARPs of 48 to 96 isolates per site were analyzed, but occasionally fewer isolates were obtained from samples. Discriminant analysis was used to assign each isolate to a source category based on the comparison of its ARP to those of isolates from known sources.
RESULTS
Antibiotic resistance in bacterial isolates from animals and wastewater.
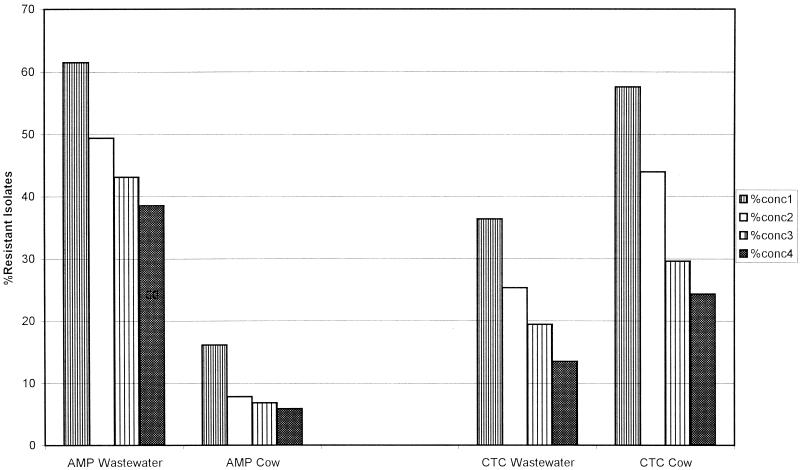
Fecal streptococci and fecal coliforms displayed distinctive ARPs depending on their sources, i.e., animal feces or wastewater dominated by human waste sources (human). For example, a significantly greater percentage (P < 0.01) of fecal coliform isolates from human sources than of their counterparts from cattle feces were resistant to ampicillin (Fig. 1). A significantly higher percentage (P < 0.01) of fecal coliform isolates from cattle than of fecal coliforms from human sources were resistant to chlortetracycline (Fig. 1). Fecal coliform isolates from human sources were more frequently resistant to ampicillin, amoxicillin, and cephalothin than were animal isolates (data not shown) (P < 0.01). Fecal coliform isolates from animal feces were more likely to be resistant to tetracycline and its derivatives (oxytetracycline and chlortetracycline), erythromycin, and streptomycin than isolates from human sources (P < 0.01).
FIG. 1.
Comparison of percentages of ampicillin-resistant (AMP) and chlortetracycline-resistant (CTC) fecal coliform isolates from human (Wastewater) and cattle (Cow) sources. The concentrations (conc) of ampicillin were as follows: conc1, 10 μg/ml; conc2, 15 μg/ml; conc3, 30 μg/ml; and conc4, 50 μg/ml. The concentrations of chlortetracycline were as follows: conc1, 20 μg/ml; conc2, 40 μg/ml; conc3, 60 μg/ml; and conc4, 80 μg/ml.
Antibiotic resistance in fecal streptococcus isolates followed a similar pattern, except that erythromycin resistance was more prevalent in isolates from humans than in animal isolates (P < 0.01), and there was no significant difference in the percentage of cephalothin-resistant isolates. Vancomycin-resistant (Vanr) fecal streptococci were more prevalent in animal feces than in wastewater (P < 0.01). Further biochemical characterization of the Vanr isolates was conducted in order to determine the genus, as Enterococcus spp. which possess the transferable genetic elements for high-level vancomycin resistance are extremely rare or nonexistent in animal populations in the United States (17). All of the isolates from animal feces proved to be members of intrinsically resistant genera, such as Pediococcus or Leuconostoc, or members of the genus Enterococcus, which frequently display low-level, intrinsic resistance to vancomycin (5), while greater than 80% of the wastewater isolates were confirmed as Enterococcus.
Discriminant analysis: testing the classification accuracy of ARPs.
The ARPs of fecal isolates obtained from known sources were used to create two separate databases, one composed of fecal streptococcus patterns and one composed of fecal coliform patterns. The first step in assessing the classification accuracy of the databases was to determine the rate of correct classification of isolates from known sources. The analysis can be set up so that source animals are treated as separate categories, i.e., dog, horse, and cow, or their ARPs can be pooled in one category, “animals,” while human isolates are included in a second category.
When nonhuman sources were pooled so that each isolate was classified as animal or human, the correct classification rate for human fecal streptococcus isolates was 75.5%, while that for animal fecal streptococcus isolates was 72.4% (Table 1). It is important to note that when animal sources are pooled for discriminant analysis, different equations are generated than when each animal source is maintained as a separate category. In the case of a source, such as human, that remains constant over the different data treatments, the number of correctly classified isolates for that source will not be the same from one scheme of source grouping to the next (compare Table 1 and Table 2). Similarly, the number of animals correctly classified in each separate category (dog, cow, or “wild”) cannot simply be added to determine the number correctly classified in the pooled analysis.
TABLE 1.
Classification of known fecal coliform and fecal streptococcus isolates by animal versus human source
| Fecal source | No. (%) of database isolates assigned to each source category
|
|||||
|---|---|---|---|---|---|---|
| Fecal coliforms
|
Fecal streptococci
|
|||||
| Human | Animal | Total | Human | Animal | Total | |
| Human | 1,232 (69.3) | 545 (30.7) | 1,777 | 1,248 (75.5) | 405 (24.5) | 1,653 |
| Animal | 944 (21.6) | 3,423 (78.4) | 4,367 | 819 (27.6) | 2,147 (72.4) | 2,966 |
TABLE 2.
Classification of known fecal streptococcus isolates by source based on ARPs
| Fecal source (n) | No. (%) of database isolates assigned to each source category
|
|||||
|---|---|---|---|---|---|---|
| Human | Chicken | Cow | Dog | Pig | Wild | |
| Human (1,653) | 1,000 (60.5) | 159 (9.6) | 134 (8.1) | 157 (9.5) | 81 (4.9) | 122 (7.4) |
| Chicken (844) | 171 (20.3) | 290 (34.4) | 61 (7.2) | 24 (2.8) | 35 (4.2) | 263 (31.1) |
| Cow (1,112) | 234 (21.0) | 79 (7.1) | 495 (44.5) | 132 (11.9) | 40 (3.6) | 132 (11.9) |
| Dog (153) | 4 (2.6) | 6 (3.9) | 9 (5.9) | 116 (75.8) | 0 (0) | 18 (11.8) |
| Pig (520) | 22 (4.2) | 7 (1.4) | 14 (2.7) | 0 (0) | 462 (88.9) | 15 (2.8) |
| Wild (337) | 35 (10.3) | 17 (5.1) | 17 (5.1) | 12 (3.6) | 22 (6.5) | 234 (69.4) |
When each animal source of fecal streptococci was analyzed as a separate category, the correct classification rate for human isolates was 60.5% (Table 2) and the ARCC for the six source categories was 62.3%. The probability of an isolate falling into one of six categories by chance alone is 16.7%; therefore, the database categorized isolates much more accurately than would be predicted if classification were a random process.
Fecal coliform isolates from humans were correctly classified at a rate of 69.3%, and pooled animal isolates were correctly classified at a rate of 78.4% (Table 1). When fecal coliforms from animal sources were analyzed as separate source categories (Table 3), human isolates were correctly classified at a rate of 54.2%, with an ARCC of 63.9%.
TABLE 3.
Classification of known fecal coliform isolates by source based on ARPs
| Fecal source (n) | No. (%) of database isolates assigned to each source category
|
|||||
|---|---|---|---|---|---|---|
| Human | Chicken | Cow | Dog | Pig | Wild | |
| Human (1,777) | 961 (54.2) | 171 (9.6) | 198 (11.1) | 159 (8.9) | 101 (5.7) | 187 (10.5) |
| Chicken (1,238) | 169 (13.7) | 711 (57.4) | 108 (8.7) | 55 (4.5) | 149 (12.0) | 46 (3.7) |
| Cow (1,420) | 189 (13.3) | 219 (15.4) | 774 (54.5) | 55 (3.9) | 154 (10.9) | 29 (2.0) |
| Dog (288) | 1 (0.3) | 0 (0) | 2 (0.7) | 273 (94.8) | 2 (0.7) | 10 (3.5) |
| Pig (784) | 73 (9.3) | 88 (11.2) | 48 (6.1) | 4 (0.5) | 568 (72.5) | 3 (0.4) |
| Wild (637) | 148 (23.2) | 14 (2.2) | 99 (15.5) | 46 (7.2) | 8 (1.3) | 322 (50.6) |
To further test the predictive accuracy of the fecal streptococcus and fecal coliform databases, a holdout cross validation was performed for each source, in which isolates were randomly removed from the database and subsequently analyzed. The rates of correct classification for hold-out isolates were not significantly different from those for all isolates in any source category, indicating that the databases are capable of identifying the sources of isolates whose ARPs are not part of the database.
The ability of the data (ARPs) in each category to discriminate between ARPs in all other categories was validated by the F statistic for both databases, as all F values exceeded the critical value for F(df1,df2,0.05)(P < 0.001) (6). The fecal coliform database had a significantly greater ARCC than the fecal streptococcus database (χ2 = 43.1; df = 1; P < 0.001). This result varied for some sources, i.e., fecal coliform isolates from cattle were classified correctly at a higher rate than those of fecal streptococci (χ2 = 15.04; df = 1; P < 0.001). Conversely, fecal streptococcus isolates from humans were correctly classified at a higher rate than those from fecal coliform isolates (χ2 = 18.53; df = 1; P < 0.001).
Spearman's ranked correlation using the percentage of correctly classified isolates versus the corresponding number of sampling events resulted in a significant negative correlation (rs = −0.8125; P < 0.05) between sampling events and the percentage of correctly classified isolates for the fecal coliform database but not for the fecal streptococcus database (rs = −0.6071; P > 0.05). The databases exhibited a significant negative correlation between the percentage of correctly classified isolates and the number of isolates per source category (rs = −0.7143 and rs = −0.8929 for the fecal coliform and fecal streptococcus databases, respectively; P < 0.05 for both).
Field tests of the predictive capabilities of the databases.
Surface waters receiving effluent from malfunctioning onsite wastewater treatment and disposal systems (OSTDS) were sampled in order to evaluate the predictive use of the databases in a field setting. In July 1999, effluent from a defective OSTDS at a restaurant was sampled at the curb of the parking lot and at the adjacent water-filled ditch. Standing water in a pasture downslope and approximately 30 m from the drainfield mound was also sampled. No animals were in the pasture, and no animal feces were evident within several hundred meters of the water. Both the fecal coliform and the fecal streptococcus databases identified the source of the majority of the isolates as human (Table 4) in all samples.
TABLE 4.
Identification of isolate source from samples collected at failing septic system
| Indicator | Source | No. (%) of database isolates assigned to each source category
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Human | Bird | Chicken | Cow | Dog | Pig | Raccoon | Total | ||
| Fecal streptococcus | Curb | 10 (66.7) | 0 (0) | 4 (26.6) | 0 (0) | 0 (0) | 0 (0) | 1 (6.7) | 15 (100) |
| Ditch | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | |
| Pasture | 26 (76.5) | 1 (2.9) | 0 (0) | 0 (0) | 5 (14.7) | 0 (0) | 2 (5.9) | 34 (100) | |
| Total | 38 (74.5) | 1 (2.0) | 4 (7.8) | 0 (0) | 5 (9.8) | 0 (0) | 3 (5.9) | 51 (100) | |
| Fecal coliform | Curb | 40 (88.9) | 3 (6.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (4.4) | 45 (100) |
| Ditch | 36 (87.8) | 4 (9.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.4) | 41 (100) | |
| Pasture | 5 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (100) | |
| Total | 81 (89.0) | 7 (7.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (3.3) | 91 (100) | |
Six weeks after the system had been repaired, the area was sampled again. At that time there was no visible effluent at the curb or standing water in the pasture, and cattle occupied the pasture. Feces from several cow stools, water in the ditch, and water in a storm drain across the street were sampled. The sources of most of the fecal streptococcus and fecal coliform isolates were identified as nonhuman (Table 5). The predicted dominant sources of fecal streptococci in the water samples were bird, chicken, cow, and dog. The dominant sources of fecal coliform isolates in the water samples were identified as cow and raccoon. The levels of human isolates identified by both databases had fallen below the level that would be expected by chance classification (14.3%) after the OSTDS was repaired.
TABLE 5.
Identification of isolate source from samples collected at repaired septic system
| Indicator | Source | No. (%) of database isolates assigned to each source category
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Human | Bird | Chicken | Cow | Dog | Pig | Raccoon | Total | ||
| Fecal streptococcus | Ditch | 3 (3.1) | 6 (6.3) | 20 (20.8) | 59 (61.5) | 6 (6.3) | 1 (1.0) | 1 (1.0) | 96 (100) |
| Storm drain | 0 (0) | 24 (25.5) | 20 (21.3) | 17 (18.1) | 27 (28.7) | 4 (4.3) | 2 (2.1) | 94 (100) | |
| Cow | 0 (0) | 1 (2.2) | 8 (18.2) | 35 (79.6) | 0 (0) | 0 (0) | 0 (0) | 44 (100) | |
| Total | 3 (1.3) | 31 (13.2) | 48 (20.5) | 111 (47.5) | 33 (14.1) | 5 (2.1) | 3 (1.3) | 234 (100) | |
| Fecal coliform | Ditch | 7 (14.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 41 (85.4) | 48 (100) |
| Storm drain | 3 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 45 (93.7) | 48 (100) | |
| Cow | 5 (5.3) | 1 (1.1) | 1 (1.1) | 72 (75.8) | 15 (15.8) | 0 (0) | 1 (1.1) | 95 (100) | |
| Total | 15 (7.9) | 1 (0.5) | 1 (0.5) | 72 (37.7) | 15 (7.9) | 0 (0) | 87 (45.5) | 191 (100) | |
Greater than 75% of fecal streptococcus and fecal coliform isolates from cow feces collected in the pasture were placed in the correct source category by discriminant analysis (Table 5). The values shown in Table 5 were calculated before the cow isolates from that site were added to the database; therefore, the analysis can be considered a cross validation. When the ARPs of the fecal streptococci from cattle were added to the database but were treated as a separate category (“BCow”), 97.7% of the isolates were classified as BCow, demonstrating the tight clustering of ARPs of bacterial isolates from a discrete source. When the BCow ARPs were added to the cow source category in the database, 84.1% of all cow isolates were correctly classified as cow compared to 79.6% for the original (cross-validation) analysis.
A similar experiment was conducted at a sports bar with a failing OSTDS. In this case, the effluent had been discharging from the drainfield within 48 h of sampling; however, no drainage was evident at the time of sampling. A pool of standing water and a creek approximately 30 m from the mound were sampled. The sources of 20 of 24 fecal streptococcus isolates (83.3%) and 41 of 48 fecal coliform isolates (85.4%) from the standing water were identified as human. Inputs to the creek were more varied, as would be expected for a flowing body of water. The dominant sources of fecal streptococcus isolates were cow (33.3%) and human (38.9%). The major sources of fecal coliform isolates in the creek were chicken (32.4%) and human (62.2%).
Samples were also collected at relatively nonimpacted sites: at an abandoned sand quarry and at an isolated creek at Anastasia State Park in Florida and at the mouth of the Matanzas River located in the Guana-Tolomato-Matanzas (GTM) National Estuarine Research Reserve. The GTM sites are interconnected canals which receive tidal flushing. At the time of sampling, a large bird rookery was populated by wood storks, egrets, and night herons. A single-family dwelling with an OSTDS is located approximately 100 m from the shore. The dominant sources of fecal coliform isolates in each of these relatively nonimpacted sites were identified as nonhuman (Table 6). In total, 151 of 197 isolates (76.6%) at the relatively nonimpacted sites were identified as wild-animal isolates.
TABLE 6.
Identification of fecal coliform source from samples collected in Anastasia State Park (ASP) and Matanzas Inlet (GTM)
| Source | No. (%) of database isolates assigned to each source category
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human | Bird | Chicken | Cow | Dog | Duck | Pig | Rabbit | Raccoon | Total | |
| ASP quarry | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 3 (6.2) | 1 (2.1) | 0 (0) | 1 (2.1) | 42 (87.5) | 48 (100) |
| ASP creek | 1 (2.5) | 37 (92.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.0) | 40 (100) |
| GTM canal 1 | 10 (21.7) | 20 (43.5) | 0 (0) | 5 (10.9) | 0 (0) | 0 (0) | 0 (0) | 9 (19.6) | 2 (4.3) | 46 (100) |
| GTM canal 2 | 9 (14.3) | 0 (0) | 0 (0) | 16 (25.4) | 1 (1.6) | 0 (0) | 0 (0) | 15 (23.8) | 22 (34.9) | 63 (100) |
DISCUSSION
The ARPs of the commensal fecal flora of mammals and birds are influenced by many factors, including the presence of intrinsically antibiotic-resistant bacteria and the fact that the antibiotics ingested by the host animal select for the survival and growth of drug-resistant strains. Most classes of antibiotics are approved for both human and animal use, and this practice almost certainly contributes to shared patterns of antibiotic resistance in the fecal flora of certain animals and humans. Although there are demonstrable differences in the prevalences of antibiotic resistance among isolates from different host species, there are enough similarities so that previous efforts (11, 13) to identify the source of indicator bacteria by antibiotic resistance characteristics were not very useful in the field. The use of several concentrations of each antibiotic rather than one concentration to establish the ARP coupled with statistical treatment of the data by discriminant analysis provides the predictive power necessary to provide useful information about the sources of isolates from surface waters (23).
The comparison of ARA results using fecal streptococcus and fecal coliform ARPs revealed that the accuracies of the two databases were similar, as demonstrated by their correct classification rates for human isolates and their ARCCs. The slightly superior correct classification rates for human isolates by the fecal streptococcus database may be due to the number of antibiotics used for the analyses. Fecal streptococcus ARPs were established using nine antibiotics, while only eight antibiotics were used for fecal coliforms because gram-negative bacteria are not susceptible to vancomycin (2). The highest correct classification rates in previous studies were obtained by using a subset of the antibiotics tested for analysis (7, 23). In this study, however, omission of any of the antibiotic resistance data resulted in lower correct classification rates for both databases.
Regardless of how well constructed a database is, in the event of polytomous categorization of isolates from unknown sources (about the same number of isolates assigned to each category), it may be difficult to determine the dominant source of contamination. The prediction of a small percentage of isolates from a particular source may also be difficult to interpret, as in the example of the repaired septic system (Table 5), where 10 of 96 (10.4%) fecal coliform isolates from the ditch and storm drain were placed in the human category. It will generally be unclear whether such assignments are a result of misclassification or truly represent a small proportion of isolates from that source. We suggest that an expected frequency of misclassification for each source will be helpful in clarifying the significance of similar results. For example, in the human category in the fecal coliform database, the rate of correct classification was 54.2%. However, the frequency at which all other isolates were classified in the human category was 9.4% (580 of 6,144). Therefore, in a sample containing fecal coliforms from many sources but none from humans, one could expect this fecal coliform database to classify about 9.4% of the isolates as human. By using the expected frequency of misclassification as a standard, it is clear that the 10 predicted human isolates from the ditch and storm drain at the repaired OSTDS could all be the result of misclassification of isolates from other sources.
The correct classification rates of isolates from known sources obtained in this study (Florida) are lower than those for Wiggins' original Virginia study (23). However, that database was composed of bacteria isolated from sources within a limited geographical area, and the sample sizes were relatively small (134 to 285 isolates per source). As Spearman's ranked correlation test demonstrated in this study, smaller sample sizes and fewer sampling sites per source result in higher correct classification rates, a result of the relative homogeneity of the ARPs of isolates from individual animal populations. A second study by Wiggins et al. in which samples were less homogeneous (more sampling sites) and sample sizes were larger resulted in correct classification rates comparable to those shown here (24).
A study of fecal streptococcus ARPs by Hagedorn et al. in Montgomery County, Va., resulted in higher correct classification rates than those obtained in Florida (7). The sources of isolates designated human in the two studies probably contributed to the differences in correct classification rates. In the Florida study, human isolates were obtained from domestic wastewater, which provides a cross-section of human ARPs and thus high variability in ARPs. In the Montgomery County study, human isolates were from OSTDS at individual homes, providing lower variability and a sample that is not likely to be representative of a large human population. This type of sample yields higher correct classification rates precisely because ARP variability is lower.
The sampling constraint applies to animal sources as well. While ARPs tend to form a tight cluster within one population, patterns of isolates from different populations of a given source are more heterogeneous (e.g., the BCow example in this study). Cattle feces for the Florida study were obtained from seven different farms over a three-county area in an attempt to broaden the population sampled. Fecal samples from cattle and chickens were obtained at only two farms in the Montgomery County study (7); however, the turnover and import rates of animals at these farms are high (C. Hagedorn, personal communication). The tight clustering of ARPs of isolates from one location could be advantageous in some types of studies, i.e., differentiation of septic tank and cattle farm inputs in a particular tributary watershed.
Water quality managers who need to identify the source(s) of fecal pollution in a watershed are generally primarily interested in discriminating between animal and human contamination and secondarily interested in determining the major source(s) of animal contamination. ARA and other bacterial-source-tracking methods, such as ribotyping, are relatively novel, and none achieve perfect discrimination between different sources, yet it seems reasonable to expect that a useful technique would classify greater than 50% of isolates correctly when there are five or more possible source categories. In our experience, managers in regulatory agencies find 60 to 70% correct classification rates very useful. When designing a sampling strategy, it should be noted that the best strategy for identifying human versus nonhuman sources may vary depending upon whether one is attempting to establish a broadly representative database that can be used over a broad geographic range or a more limited database that might be used to determine the major source of fecal pollution in a discrete area, such as a specific creek.
Both the fecal streptococcus and fecal coliform databases were able to identify a dominant human or nonhuman source of pollution, yet after the repair of the restaurant septic tank, the specific sources identified by each database were not identical. One possible explanation for this difference is that the two groups of indicator organisms have different survival or inactivation rates (time required to become nonculturable under the specified isolation conditions) in natural waters (20). The ARPs may reflect a relatively long-term history of contamination for the more persistent group and a “snapshot” of the most immediate source(s) of contamination for the less persistent group. A second possible explanation of such discrepancies is the fact that feces from different animals characteristically contain different ratios of fecal coliforms and fecal streptococci (20). Further investigation of these questions will help determine which of the indicator organisms is more useful for ARA. It is possible that in some situations the use of both fecal coliform and fecal streptococcus ARPs will be preferable to the use of a single group.
As patterns of antibiotic use change, so do bacterial patterns of antibiotic resistance. In the United States, the emergence of fluoroquinolone-resistant Campylobacter jejuni in chickens is linked to the approval of fluoroquinolone use in poultry in 1995 (21). Withdrawal of antibiotic pressure can result in decreased prevalence of antibiotic resistance, as was the case when antibiotic use was terminated in swine herds (14, 15). Because the selective pressure of antibiotic treatment on the commensal microflora of animals is an important determinant of the prevalence of antibiotic resistance in a population (25), the databases that are developed for discriminant analysis will require periodic updating.
A major expense in this type of analysis lies in building the database. Currently, it is not known if ARPs of isolates from one geographic location can be used to predict the source of isolates from another. Keys to building broadly applicable databases will be the choice of antibiotics used and the variability of animal husbandry practices in different regions of the country. A detailed study of the feasibility of cross application between the two Virginia databases and the Florida database is planned, which should begin to address the question of how useful antibiotic resistance patterns from one geographic area are in predicting the source of fecal contamination in a different area.
ACKNOWLEDGMENTS
This work was supported by grants from the Jacksonville Environmental Protection Board and the Jacksonville, Fla., chapter of the Association for Women in Science.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C.: American Public Health Association, Inc.; 1995. [Google Scholar]
- 2.Beers M H, Berkow R, editors. The Merck manual of diagnosis and therapy. Whitehouse Station, N.J: Merck & Co.; 1997. [Google Scholar]
- 3.Cabelli V J. Health effects criteria for marine recreational waters. U.S. Environmental Protection Agency publication no. EPA 600/1-80-031. U.S. Washington, D.C.: Environmental Protection Agency; 1980. [Google Scholar]
- 4.Dufour A P. Health effects criteria for fresh recreational waters. U.S. Environmental Protection Agency publication no. EPA-600/1-84-004. U.S. Washington, D.C.: Environmental Protection Agency; 1984. [Google Scholar]
- 5.Facklam R, Pigott N, Franklin R, Elliott J. Evaluation of three disk tests for identification of enterococci, leuconostocs, and pediococci. J Clin Microbiol. 1995;33:885–887. doi: 10.1128/jcm.33.4.885-887.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardiner W P. Statistics for the biosciences. London, United Kingdom: Prentice Hall; 1997. [Google Scholar]
- 7.Hagedorn C, Robinson S L, Filtz J R, Grubbs S M, Angier T A, Beneau R B. Determining sources of fecal pollution in a rural Virginia watershed with antibiotic resistance patterns in fecal streptococci. Appl Environ Microbiol. 1999;65:5522–5531. doi: 10.1128/aem.65.12.5522-5531.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harwood V J, Butler J, Parrish D, Wagner V. Isolation of fecal coliform bacteria from the diamondback terrapin (Malaclemys terrapin centrata) Appl Environ Microbiol. 1999;65:865–867. doi: 10.1128/aem.65.2.865-867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt J G, editor. Bergey's manual of determinative bacteriology. 9th ed. Baltimore, Md: Williams & Wilkins; 1994. [Google Scholar]
- 10.Huberty C J. Applied discriminant analysis. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 11.Kaspar C W, Burgess J L, Knight I T, Colwell R R. Antibiotic resistance indexing of Escherichia coli to identify sources of fecal contamination in water. Can J Microbiol. 1990;36:891–894. doi: 10.1139/m90-154. [DOI] [PubMed] [Google Scholar]
- 12.Kay D, Fleisher J M, Salmon R L, Jones F, Wyer M D, Godfree A F, Zelenauch-Jacquotte Z, Shore R. Predicting likelihood of gastroenteritis from sea bathing: results from randomized exposure. Lancet. 1994;344:905–909. doi: 10.1016/s0140-6736(94)92267-5. [DOI] [PubMed] [Google Scholar]
- 13.Krumperman P H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol. 1983;46:165–170. doi: 10.1128/aem.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langlois B E, Cromwell G L, Stahly T S, Dawson K A, Hays V W. Antibiotic resistance of fecal coliforms after long-term withdrawal of therapeutic and subtherapeutic antibiotic use in a swine herd. Appl Environ Microbiol. 1983;46:1433–1434. doi: 10.1128/aem.46.6.1433-1434.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langlois B E, Dawson K A, Leak I, Aaron D K. Antimicrobial resistance of fecal coliforms from pigs in a herd not exposed to antimicrobial agents for 126 months. Vet Microbiol. 1988;18:147–153. doi: 10.1016/0378-1135(88)90060-0. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig W, Seewaldt E, Kilpper-Balz R, Schleifer K H, Magrum L, Woese C R, Fox G E, Stackebrandt E. The phylogenetic position of Streptococcus and Enterococcus. J Gen Microbiol. 1985;131:543–551. doi: 10.1099/00221287-131-3-543. [DOI] [PubMed] [Google Scholar]
- 17.McDonald C L, Kuehnert M J, Tenover F C, Jarvis W R. Vancomycin-resistant enterococci outside the health-care setting: prevalence, sources, and public health implications. Emerg Infect Dis. 1997;3:311–317. doi: 10.3201/eid0303.970307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miescier J J, Cabelli V J. Enterococci and other microbial indicators in municipal wastewater effluents. J Water Pollut Control Fed. 1982;54:1599–1606. [Google Scholar]
- 19.Parveen S, Murphree R L, Edmiston L, Kaspar C W, Portier K M, Tamplin M L. Association of multiple-antibiotic-resistance profiles with point and nonpoint sources of Escherichia coli in Apalachicola Bay. Appl Environ Microbiol. 1997;63:2607–2612. doi: 10.1128/aem.63.7.2607-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinton L W, Donnison A M, Hastie C M. Faecal streptococci as faecal pollution indicators: a review. Part II. Sanitary significance, survival and use. N Z J Mar Freshwater Res. 1993;27:117–137. [Google Scholar]
- 21.Smith K E, Besser J M, Hedberg C W, Leano F T, Bender J B, Wicklund J H, Johnson B P, Moore K A, Osterholm M T. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992–1998. N Engl J Med. 1999;340:1525–1532. doi: 10.1056/NEJM199905203402001. [DOI] [PubMed] [Google Scholar]
- 22.Stevens J. Applied multivariate statistics for the social sciences. Hillsdale, N.J: Lawrence Erlbaum Assoc.; 1996. Discriminant analysis; pp. 261–268. [Google Scholar]
- 23.Wiggins B A. Discriminant analysis of antibiotic resistance patterns in fecal streptococci, a method to differentiate human and animal sources of fecal pollution in natural waters. Appl Environ Microbiol. 1996;62:3997–4002. doi: 10.1128/aem.62.11.3997-4002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiggins B A, Andrews R W, Conway R A, Corr C L, Dobratz E J, Dougherty D P, Eppard J R, Knupp S R, Limjoco M C, Mettenburg J M, Rinehardt J M, Sonsino J, Torrijos R L, Zimmerman M E. Use of antibiotic resistance analysis to identify nonpoint sources of fecal pollution. Appl Environ Microbiol. 1999;65:3483–3486. doi: 10.1128/aem.65.8.3483-3486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witte W. Impact of antibiotic use in animal feeding on resistance of bacterial pathogens in humans. Ciba Found Symp. 1997;207:61–75. doi: 10.1002/9780470515358.ch5. [DOI] [PubMed] [Google Scholar]