Abstract
Objective:
To evaluate the long-term clinical outcomes of children with RHD in Uganda, and determine characteristics that predict adverse outcomes.
Methods:
This retrospective cohort study evaluated the risk of death in Ugandan children with clinical RHD from 2010-2018; enrolling 5-18 year-olds from an existing registry. Demographic data and clinical data (baseline complications, RHD severity, cardiac interventions) were collected. The primary outcome was survival. Univariable and multivariable hazard ratios were obtained from Cox proportional hazards regression. Survival probabilities were developed using Kaplan Meier curves; log-rank tests compared survival based on cardiac interventions, disease severity, and time of enrollment.
Results:
612 cases met inclusion criteria; median age 12.8 years (IQR5.3), 37% were male. Thirty-one percent(187/612) died during the study period; median time to death 7.8 months (IQR18.3). In univariable analysis, older age (HR1.26, 95%CI=1.0,1.58), presence of baseline complications (HR2.06, 95%CI=1.53,2.78), and severe RHD (HR5.21, 95%CI=2.15,12.65) were associated with mortality. Cardiac intervention was associated with a lower risk of mortality (HR0.06, 95%CI=0.02,0.24). In multivariable models, baseline complications (HR1.78, 95%CI=1.31,2.41), severe RHD (HR4.58, 95%CI=1.87,11.23), and having an intervention (HR0.05, 95%CI=0.01,0.21) remained statistically significant. Kaplan Meier survival curves demonstrated >25% mortality in the first 30 months, with significant differences in mortality based on intervention status and severity of disease.
Conclusions:
The mortality rate of children with clinical RHD in Uganda exceeds 30%, over an 8-year timeframe, despite in-country access to cardiac interventions. Children at highest risk were those with cardiac complications at baseline and severe RHD.
Keywords: Heart valve disease, Global Health, Echocardiography, Cardiac Surgical Procedures, Epidemiology
Introduction
Rheumatic heart disease (RHD) continues to affect children and young adults at very high rates, and disproportionately in low-resource regions of the world.1 In 2019, the worldwide prevalence of RHD was estimated to be over 40 million cases, with 306,000 RHD-related deaths.2 The Global Rheumatic Heart Disease Registry (REMEDY) trial, a multicenter international study, evaluated RHD patients of all ages from 12 African countries, plus India and Yemen, and found that very few patients were offered any type of intervention.3 Specifically, only 11% of patients were operated on in low-income countries.3 Mortality at 2-year follow up in patients from low income countries was 20.8%.4
Through echocardiographic screening, prior studies have demonstrated high prevalence rates of RHD, 2.5-3%, in the general population in Uganda.5-7 A country-wide RHD registry was developed in Uganda in 2010 to improve the care and follow up of children and adults with RHD.8 In addition, there have been important advances in access to care and interventions in the past 10 years. Compared to many other low-income countries without any interventional services, Uganda has both an active cardiac catheterization laboratory (cath lab) and cardiac surgery program.9,10 Despite these advances, limited resources continue to inhibit the ability to provide necessary and life-saving procedures to all RHD patients in need. In a prior prospective cohort study in Uganda, subjects with a median age of 30 years were followed for 1 year, and found a nearly 18% 1-year mortality rate, along with very high rates of morbidity, with 35% developing heart failure, and 63.7% developing atrial fibrillation (afib).11
Many studies have focused on short-term outcomes and primarily focused on adults with RHD, but there remains a paucity of data on the outcomes of children with RHD in endemic regions. This study evaluated the long-term clinical outcomes of children with RHD in Uganda, and helps determine which characteristics put patients most at risk for adverse outcomes.
Methods
Study Design
This retrospective cohort study evaluated the risk of death in Ugandan children with clinical RHD. Children were enrolled from an existing patient registry from March 1st, 2010 through December 31st, 2018. The Uganda National RHD Registry, managed by the Uganda Heart Institute (UHI) at Mulago Hospital, was developed to improve clinical care and epidemiological surveillance of RHD in Uganda. In 2013, the registry was converted to an online REDCap12 platform hosted at University Hospitals Cleveland Medical Center. Patients are from four regional sites (Kampala [since 2011], Lubowa [2013], Mbarara [2014], and Gulu [2015]) who either present with clinical manifestations of RHD or have sub-clinical RHD by echocardiographic screening. Clinical RHD is defined as those who have symptoms secondary to cardiac disease, with subsequent confirmation on echocardiogram by expert cardiologist. Latent/sub-clinical RHD is defined as those without clinical symptoms, diagnosed by echocardiographic screening. This study evaluated children with clinical RHD; outcomes for children with latent RHD have been previously published.13
Any child under 18 years of age at time of registry enrollment with clinical RHD was considered for inclusion into the study. Subjects were excluded for the following criteria: 1) enrollment diagnosis of latent or sub-clinical RHD, 2) enrollment diagnosis of acute rheumatic fever, 3) enrollment into the registry after the study end-date (December 31st, 2018), 4) failure of follow up after the baseline evaluation, or 5) missing core data (i.e., age, diagnosis). Semiannual follow-up was recommended for patients with clinical RHD and annual follow-up was recommened for those with latent RHD. Any patient with a lapse in follow-up greater than one year was actively recalled for an annual visit, as is standard practice at the UHI for all patients.
Data collection
Demographic data (age, gender, primary clinical site, and distance to nearest health unit) and clinical data (baseline complications, disease severity, and history of cardiac intervention(s)) were collected. Cardiac-related complications included congestive heart failure (CHF), stroke/transient ischemic attack (TIA), atrial fibrillation (afib), pulmonary embolus (PE)/deep vein thrombosis (DVT), and endocarditis. RHD disease severity was defined as mild, moderate, or severe by expert cardiologists using criteria for valvular heart disease.14 With mixed valve disease, severity was based on the highest level of valvar pathology. Any amount of mitral stenosis was included in the severe disease category.15 Severe stenosis or regurgitation of any other valve qualified as severe valve disease. Length of follow up was calculated from the time of enrollment to September 1, 2019. Cardiac interventions included percutaneous valvulplasty by catheterization and cardiac surgeries (valve repair or replacement). The primary outcome evaluated was survival. Risk of death was further evaluated based on history of a cardiac intervention (before or after registry enrollment), disease severity, and by year of enrollment (before and after 2015).
Statistical analysis
Continuous variables were reported as medians with interquartile ranges (IQR) and categorical variables reported as frequencies and percentages. Hazard ratios were obtained from Cox proportional hazards regression. Univariable models included a single predictor and site as a stratification variable to allow for different baseline hazards to be calculated for each site. Multivariable models included stratification on site and age, gender, distance to nearest health unit, complications at baseline, disease severity, and intervention as model covariates. Restricted cubic spline terms were included to model potential non-linear associations for continuous variables. Spline terms were retained only for distance to the nearest health center with three knots placed at the 10th, 50th, and 90th percentiles based on the Akaike information criterion. Hazard ratios are reported in tabular form for a contrast to the nearest health center of 1 versus 5 km (Supplement Figure I). The assumption of proportional hazards was examined by plots of scaled Schoenfeld residuals against time. Values for distance to the nearest health center and disease severity were missing for 10.0% and 2.1% of participants, respectively. Missing values for distance and disease severity were multiply imputed using flexible additive regression and predictive mean matching as implemented by the Hmisc::aregImpute (version 5.1.3.1) function. Imputation models included all variables contained in the analysis model, as well as congestive heart failure at baseline, history of stroke/TIA, history of afib, history of PE/DVT, history of endocarditis, prescription of antibiotics, any complication over follow-up, duration of follow-up, and date of enrollment as auxiliary variables. The default settings were utilized other than setting the number of imputations to n=50. Hazard ratios were obtained by pooling estimates over each of the 50 imputed datasets using the Hmisc::fit.mult.impute function. Survival probabilities were also examined using Kaplan Meier curves over the 8-year study timeframe. Log-rank tests were used to test for differences in survival distribution among those with and without cardiac interventions, enrolled in the registry before and after January 1, 2015, and by disease severity. P-values less than 0.05 (two-sided) were considered statistically significant. Analyses were conducted using R version 3.6.0.
Ethics
The study was approved by the Institutional Review Boards of Makerere University School of Medicine (Kampala, Uganda), the Uganda National Council for Science and Technology, and University Hospitals Cleveland Medical Center (Cleveland, OH), (IRB 03-13-42). All participants >18 years of age provided written informed consent. Written parental consent and participant assent were obtained for those <18 years of age. Neither participants nor the public were involved in the research process.
Results
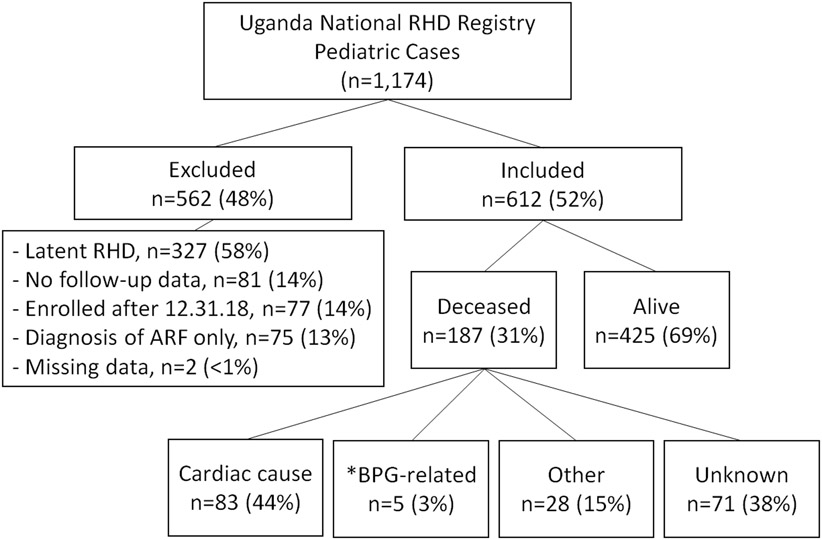
A total of 1,174 pediatric cases were reviewed and 612 (52%) met inclusion criteria (Figure I). Of the 562 subjects removed due to exclusion criteria (Figure I), the majority were excluded for a diagnosis of latent RHD (58%) and 14% had no follow up data. The median age at enrollment into the registry was 12.8 years (IQR 5.3) and 229 (37%) were male. The majority (456/612, 74.5%) were from Kampala, the most populated of all 5 sites, and where the largest hospital systems exist (Table I). The median distance from a health unit was 3 km (IQR 4). Nearly one third (179/610, 29%) of those enrolled had at least one cardiac complication at baseline, the majority of which were CHF in 169 cases (28%), followed by afib in 14 cases (2.3%). Diagnoses of stroke/TIA, PE/DVT, or endocarditis occurred in 1% or less of the cases (Table I). The majority (433/610, or 71%) had no cardiac complications at baseline. Interventions were performed in 73, or 12%, of the 612 cases, of which 68 (93%) had cardiac surgery and 5 (7%) had undergone transcatheter interventions. Only 11 of these procedures (8 surgeries and 3 transcatheter interventions) were performed in Uganda. Of 68 cardiac surgeries, 16 (24%) were valve repair surgeries, 32 (47%) were valve replacement surgeries, and 20 (29%) were a combination of both (Table I).
Figure I.
Study participant flow diagram
Table I.
Demographic and clinical characteristics
| All (n=612) |
Alive (n=425) |
Deceased (n=187) |
|
|---|---|---|---|
| Age, median (IQR) | 12.8 (5.27) | 12.6 (5.3) | 13.3 (4.2) |
| Gender | |||
| Male, n (%) | 229 (37.4%) | 157 (37%) | 72 (39%) |
| Female, n (%) | 383 (62.6%) | 268 (63%) | 115 (61%) |
| Primary Clinic Site, n (%) | |||
| Kampala | 456 (74.5%) | 302 (71.1%) | 154 (82.4%) |
| Gulu | 54 (8.8%) | 48 (11.3%) | 6 (3.2%) |
| Lira | 47 (7.7%) | 33 (7.8%) | 14 (7.5%) |
| Mbarara | 45 (7.3%) | 32 (7.5%) | 13 (7%) |
| Lubowa | 10 (1.6%) | 10 (2.4%) | 0 (0%) |
| Distance to nearest health unit in km, median (IQR) | (*n=551) 3 (4) |
(*n=382) 2.8 (4) |
(*n=169) 3 (4.5) |
| Complications at time of enrollment, n (%) | (*n=610) | (*n=423) | (n=187) |
| None | 433 (71%) | 325 (76.8%) | 108 (57.8%) |
| CHF | 169 (27.7%) | 92 (21.7%) | 77 (41.1%) |
| Stroke/TIA | 5 (0.8%) | 5 (1.2%) | 0 (0%) |
| Afib | 14 (2.3%) | 9 (2.1%) | 5 (2.7%) |
| PE or DVT | 1 (0.2%) | 1 (0.2%) | 0 (0%) |
| Endocarditis | 7 (1.1%) | 4 (0.9%) | 3 (1.6%) |
| Any complication | 179 (29.3%) | 100 (23.6%) | 79 (42.2%) |
| Cardiac interventions | (*n=601) | (*n=417) | (*n=184) |
| Any intervention | 73 (12.1%) | 71 (17%) | 2 (1.1%) |
| Percutaneous | 5 (0.8%) | 5 (1.2%) | 0 (0%) |
| valvuloplasty | 68 (11.3%) | 66 (15.8%) | 2 (1.1%) |
| Cardiac surgery | 16 (2.7%) | 15 (3.6%) | 1 (0.5%) |
| Valve repair | 32 (5.3%) | 31 (7.4%) | 1 (0.5%) |
| Valve replacement | 20 (3.3%) | 20 (4.8%) | 0 (0%) |
| Valve repair & replacement |
Number of cases is listed when data was missing from that particular variable
Abbreviations: IQR, Interquartile range; KM, Kilometer; CHF, Congestive heart failure; TIA, Transient ischemic attack; Afib, Atrial fibrillation; PE, Pulmonary embolism; DVT, Deep vein thrombosis
At the end of the study period (12/31/2018), 187 (31%) were found to be deceased, and 425 (69%) were alive. From the time of enrollment into the registry until 12/31/2018, the median follow-up time for living cases was 50.3 months (IQR 43.3), and the median time to death among those that died was 7.8 months (IQR 18.3). Of the 187 deaths, 140 (75%) died in the first 2 years. Nearly half (77/187, 41.2%) of all deaths were from CHF and another 7.5% (14/187) were cardiac-related, described as cardiac arrest, cardio-pulmonary arrest, or cardiogenic shock (Figure I). Five (5/187, 2.7%) died in the immediate period (<1 hour) following a Benzathine Penicillin G injection, all of whom had severe heart disease. Ten deaths (10/187, 5.3%) were due to unrelated causes, such as septicemia, malaria/severe anemia, bleeding, and respiratory issues. The remaining 81 deaths (81/187, 43%) were from unknown causes. Of the 73 children who underwent an interventional procedure, 71 (97%) children were alive at the time of data analysis and 2 (3%) were deceased.
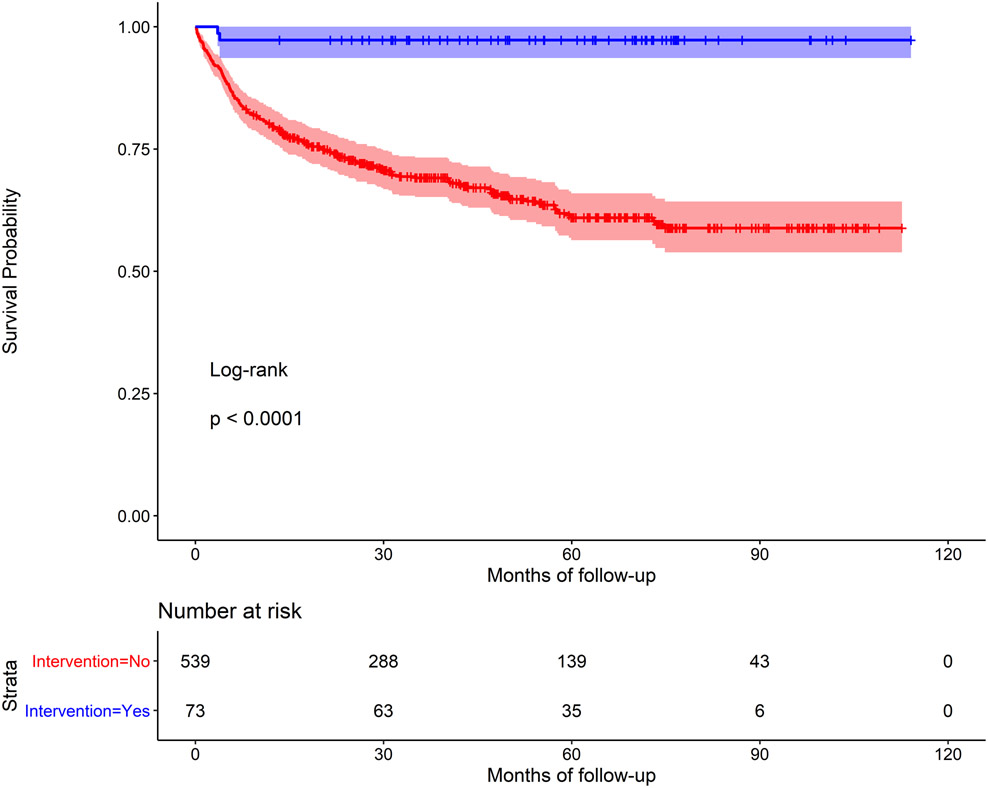
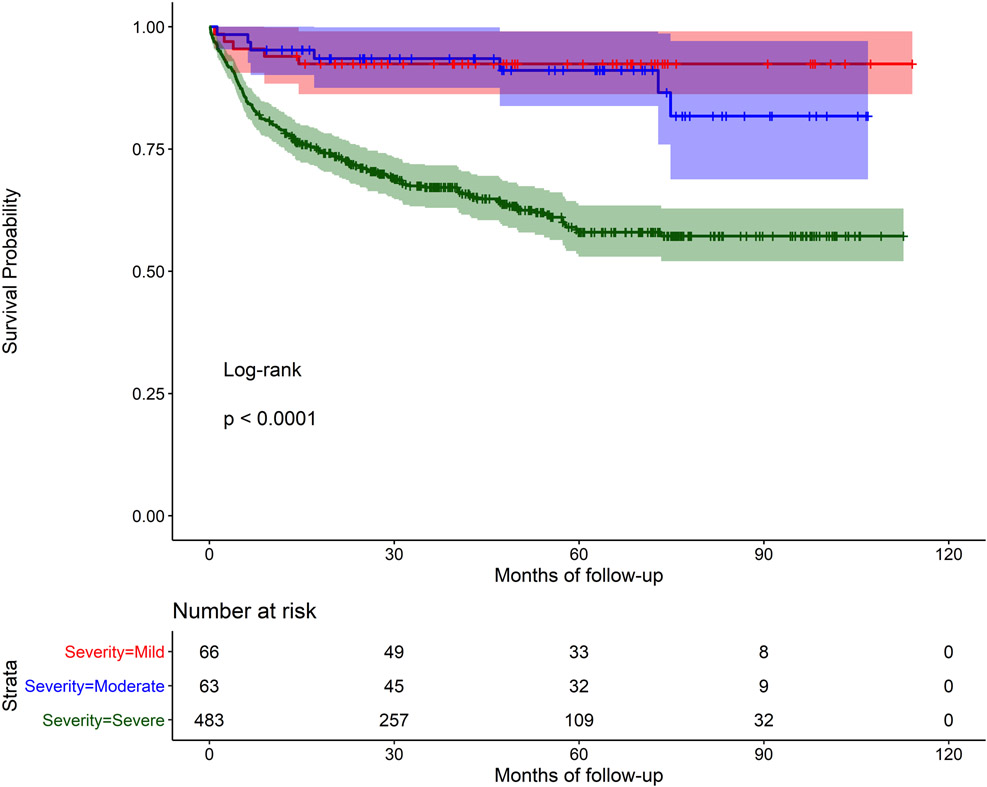
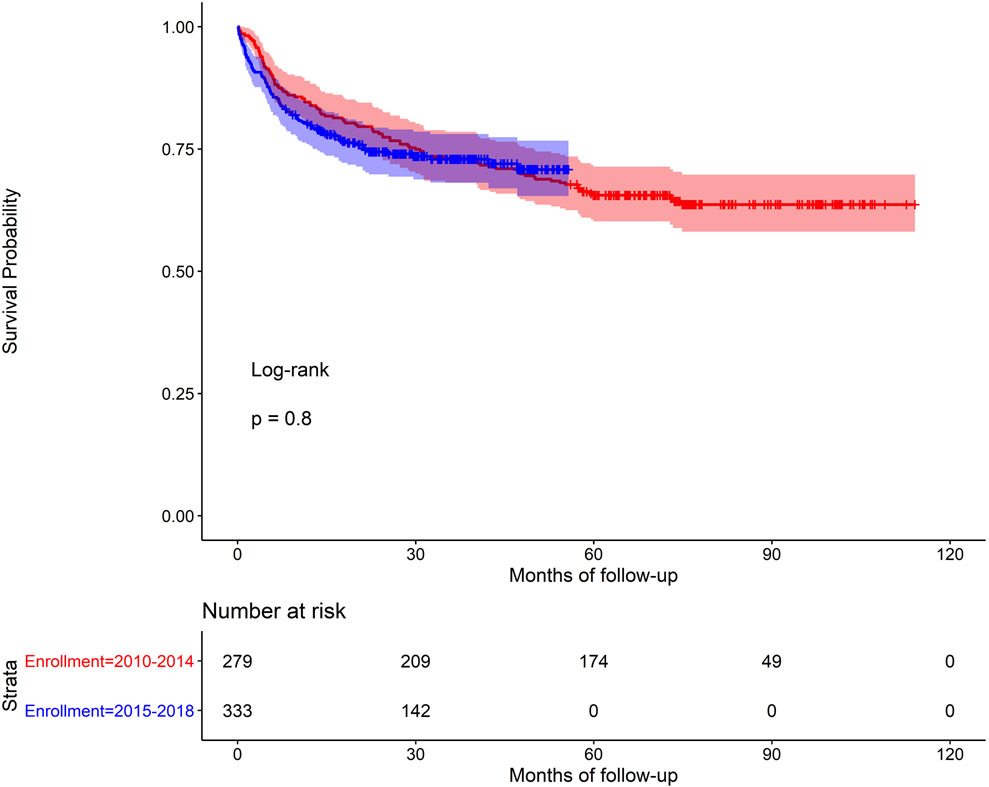
In univariable analysis, older age (HR 1.26 95% CI=1.0, 1.58), the presence of baseline cardiac-related complications (HR 2.06, 95% CI=1.53, 2.78), and moderate/severe RHD at baseline (HR 5.21, 95% CI = 2.15, 12.65) were all associated with greater risk of mortality. History of cardiac intervention was associated with a lower risk of mortality compared to those without an intervention (HR 0.06, 95% CI=0.02, 0.24) (Table II). Mortality was not associated with the distance to the nearest health unit (χ2 = 0.87, d.f. = 2, p = 0.65; Supplement Figure I). In multivariable models (stratified by site; model covariates: age, gender, distance to nearest health unit, complications at baseline, disease severity, and intervention as model covariates), the presence of complications at baseline (HR 1.78, 95% CI=1.31, 2.41), severe RHD at baseline (HR 4.58, 95% CI=1.87, 11.23), and history of cardiac intervention (HR 0.05, 95% CI=0.01, 0.21) remained statistically significant. Age was no longer significant. History of cardiac intervention remained significant after controlling for severity of disease. Kaplan Meier survival curves demonstrated very poor survival rates, especially among those with no cardiac intervention (Figure IIa). Log-rank tests were statistically significant for intervention vs. no intervention (p<0.001, Figure IIa) and severe RHD vs. mild RHD (p<0.001, Figure IIc), but not for period effects (before 2015 vs. after 2015; p=0.8, Figure IIb).
Table II.
Risk of death associated with demographic and clinical characteristics
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Reference/Risk | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age at enrollment (yrs) | 9.7 yrs/15.0 yrs | 1.26 (1.00; 1.58) | 0.048 | 1.24 (0.99; 1.55) | 0.066 |
| Gender | Female/Male | 1.06 (0.79; 1.42) | 0.720 | 1.10 (0.81; 1.49) | 0.540 |
| Distance nearest health unit (km) | 1 km/5 km | 1.16 (0.84; 1.61) | 0.647 | 1.09 (0.79; 1.51) | 0.864 |
| Baseline complications | No/Yes | 2.06 (1.53; 2.78) | <0.001 | 1.78 (1.31; 2.41) | <0.001 |
| Disease severity | Mild/moderate | 1.15 (0.36; 3.74) | <0.001 | 0.92 (0.28; 3.00) | <0.001 |
| Severe | 5.21 (2.15; 12.65) | 4.58 (1.87; 11.23) | |||
| Intervention | No/Yes | 0.06 (0.02; 0.24) | <0.001 | 0.05 (0.01; 0.21) | <0.001 |
Abbreviations: HR, Hazard Ratio; CI, confidence interval; yrs, Years; KM, Kilometer
HRs and 95% CIs obtained from Cox proportional hazards regression. Interquartile range (25th vs. 75th percentile) HR is shown for continuous covariates. P-value for the Wald Chi-squared test for the model parameters(s).
Figure IIa.
Kaplan Meier survival curve for children with RHD, with and without a prior cardiac intervention
Figure IIc.
Kaplan Meier survival curve for children with RHD, with mild, moderate or severe disease at baseline
Figure IIb.
Kaplan Meier survival curve for children with RHD, early (2010-2014) verses late (2015-2018) enrollment periods
Discussion
This study is the first to evaluate outcomes of children with clinical RHD and demonstrate that the risk of death in children with clinical RHD in Uganda is exceedingly high. Nearly one third (31%) of all cases died during the 8-year study period, and the majority of deaths (75%) occurred in the first 2 years after enrollment into the registry. Not surprisingly, the risk of death is much higher in those with clinical RHD than those with latent, non-clinical, RHD. In prior studies evaluating RHD outcomes, few deaths were reported in latent cases and lower rates were reported in clinical cases. In a study out of Fiji, only one death (1.4%) occurred in the latent RHD group compared to 9 (12.9%) deaths in the clinical RHD group.16 A study in Uganda in 2017, using the same registry, evaluated the outcomes of children with latent RHD, with a particular focus on echocardiographic progression of disease, and found that 2 children (0.9%), both with moderate/severe disease, died during the follow-up period.13
In the REMEDY follow-up study, which included RHD cases of all ages, 16.9% died within 2 years,4 which is considerably lower than the rate detected in our study. This may in part be because the REMEDY trial included cases from 25 centers (14 countries) with variable income levels and access to cardiac care. However, when broken down by income levels, the REMEDY trial still showed a mortality rate of only 20.8% in the low-income countries, compared to 12.5% in upper-middle-income countries.4 While it has generally been assumed that rates of death were lower in the younger population, our data show the opposite; the risk of death in children with RHD may be just as high, if not higher.
Similar to the REMEDY trial, our data show that those older in age, with severe disease at baseline, and complications at baseline were more likely to die during follow up. In our multivariable model, individuals with severe RHD had a 5 times greater risk of dying compared to those with mild disease. The REMEDY trial also found that afib and male gender were associated with increased risk of death. Our study found no association with gender. Afib is more common in older ages, and was present one fifth of patients enrolled in the REMEDY trial.4 The rate of afib was very low (2.3%) in our population, likely related to younger age, and therefore was not evaluated as an independent predictor of death. While the cause of death was not documented or determined in nearly half of cases, the majority of those with a documented cause, died of cardiac-related causes. In addition, 5 children died in the immediate period (<1 hour) following a Benzathine Penicillin G injection, all of whom had severe heart disease. Recent reports of similar deaths have raised concern that patients with severe valvular disease are at risk for sudden-death after BPG injection due to hemodynamic compromise, and are not related to anaphylaxis, as previously thought.17
For those who died, the median time from enrollment into the registry to death was 7.8 months (IQR 18.3), demonstrating that many children died very soon after their initial presentation for care. On the other hand, history of cardiac intervention was protective for survival. The decision to perform an operation or interventional cardiac catheterization is dependent on a combination of factors: disease severity, risk/benefit analysis, location of patient, and financial considerations. This is complicated even further by the fact that children in Uganda commonly have procedures performed in other countries, as was true for this cohort, with only 11 of the 73 interventions being performed in Uganda. It is reasonable to assume that children who had very severe disease at the time of enrollment may have been considered too high risk for surgical intervention, which may have led to selection bias. However, in multivariable analysis, history of an intervention remained significant, even after controlling for other variables, including disease severity. Cardiac interventions clearly have an impact on survival in children with RHD, and further highlights the need for developing comprehensive cardiac surgery and referral programs in regions with endemic RHD for both children and adult patients.18 This is particularly true in the lowest socioeconomic regions, like Uganda, where we demonstrated that even close access to rural health units do not protect children with RHD from poor outcomes.
Using publicly available data from the Global Burden of Disease Study, recent estimates of RHD mortality in Uganda were 331 deaths per year in 2017.19 Our data showed that 140 children with RHD died in the first 2 years, equating to about 70 deaths per year. If we assume that there are 2-3 times the number of RHD cases in the adult population, as suggested by the ages of those enrolled in the REMEDY trial,3 and many more undiagnosed children throughout the country, then the number of deaths per year may in fact significantly exceed current estimates. The outcomes of children with RHD is crucial to better understanding the worldwide burden of RHD. This data helps inform future RHD research, and highlights the need fro prevention and treatment programs in low-resource settings. While intervention is possible, it is not readily available for most children in endemic RHD regions, and therefore, prevention strategies are crucial to protect children.
There are limitations to this study. Similar to other low-income countries, data is transcribed from paper to the online registry. Uganda has poor vital registration data, potentially leading to inaccurate birth dates. Only clinical RHD cases were included in this study. Many symptomatic children never present to care, and therefore a subset of cases may have been mislabeled as sub-clinical cases, and excluded. The number of cases lost to follow-up was significant (14%) and survival rates may have been impacted by this missing data. The registry does not allow for formal analysis of the gap between those who need interevention and those who receive it. The majority of children in this study came from the central, highly-populated region around Kampala, Uganda. Therefore, results may not be generalizable to other RHD-endemic regions. Lastly, this study was unable to evaluate the risk of morbidity over time, as this data was missing for more than one third of all cases. Similarly, the data on antibiotic prophylaxis, including adherence was missing for a large suset and could not be analyzed. This prevents our study from evaluating the risk of non-fatal outcomes and evaluating the impact of prophylactic antibiotics on the risk of death.
Conclusions
Among pediatric patients with RHD in Uganda, there are extremely high mortality rates. Children most at risk had complications at baseline, severe valvar disease, and had no access to an intervention. This study is the first to evaluate clinical outcomes of children with RHD in an endemic region, highlights the greater than expected mortality rates in these children, and identifies factors that place children at increased risk for mortality. In regions with limited resources, the ability to risk-stratify and triage appropriate care to high-risk children is critical.
Supplementary Material
Supplement Figure I. Hazard ratio for death according to distance to nearest health unit
Key Questions:
What is already known about this subject?
Rheumatic heart disease (RHD) disproportionately affects children and young adults at staggeringly high rates in low and middle income countries worldwide. Prior studies have primarily evaluated outcomes of adults with RHD and identified risk factors that predict worse outcomes.
What does this study add?
This is the first study to evaluate long-term outcomes of children with clinical RHD, using a large cohort in an endemic setting.
How might this impact on clinical practice?
While RHD is a completely preventable disease, mortality rates in children continue to be high. There are multiple identifiable risk factors that increase mortality, which can be utilized in resource-limited regions to risk-stratify and triage appropriate care to high-risk children.
Sources of Funding:
This work was funded by the American Heart Association Children’s Strategically Focused Research Network Grant #17SFRN33670607.
The Uganda National Rheumatic Heart Disease Registry is supported in part by the National Institutes of Health (UL1 RR024989) and by the Medtronic Foundation.
Dr Okello is supported by the Developing Excellence in Leadership, Training, and Science Africa Initiative (grant number DEL-15-011) to Training Health Researchers into Vocational Excellence in East Africa-2.
Footnotes
Disclosures:
None
Contributor Information
Meghan Zimmerman, Dartmouth Hitchcock Medical Center, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA.
Samalie Kitooleko, Uganda Heart Institute, Mulago National Referral Hospital, Makerere University, Kampala, Uganda.
Emmy Okello, Uganda Heart Institute, Mulago National Referral Hospital, Makerere University, Kampala, Uganda.
Nicholas J. Ollberding, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati OH, USA.
Pranava Sinha, Children’s National Hospital, George Washington University School of Medicine, Washington D.C., USA.
Tom Mwambu, Uganda Heart Institute, Mulago National Referral Hospital, Makerere University, Kampala, Uganda.
Craig A. Sable, Children’s National Hospital, George Washington University School of Medicine, Washington D.C., USA.
Andrea Beaton, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati OH, USA.
Chris Longenecker, University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH, USA.
Peter Lwabi, Uganda Heart Institute, Mulago National Referral Hospital, Makerere University, Kampala, Uganda.
References
- 1.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990-2015. N Engl J Med. 2017;377(8):713–722. doi: 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zühlke L, Engel ME, Karthikeyan G, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: The Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015. doi: 10.1093/eurheartj/ehu449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zühlke L, Karthikeyan G, Engel ME, et al. Clinical Outcomes in 3343 Children and Adults with Rheumatic Heart Disease from 14 Low-and Middle-Income Countries: Two-Year Follow-Up of the Global Rheumatic Heart Disease Registry (the REMEDY Study). Circulation. 2016. doi: 10.1161/CIRCULATIONAHA.116.024769 [DOI] [PubMed] [Google Scholar]
- 5.Ploutz M, Lu JC, Scheel J, et al. Handheld echocardiographic screening for rheumatic heart disease by non-experts. Heart. 2016;102(1):35–39. doi: 10.1136/heartjnl-2015-308236 [DOI] [PubMed] [Google Scholar]
- 6.Beaton A, Okello E, Lwabi P, Mondo C, McCarter R, Sable C. Echocardiography screening for rheumatic heart disease in ugandan schoolchildren. Circulation. 2012. doi: 10.1161/CIRCULATIONAHA.112.092312 [DOI] [PubMed] [Google Scholar]
- 7.Scheel A, Ssinabulya I, Aliku T, et al. Community study to uncover the full spectrum of rheumatic heart disease in Uganda. Heart. 2019. doi: 10.1136/heartjnl-2018-313171 [DOI] [PubMed] [Google Scholar]
- 8.Okello E, Longenecker CT, Scheel A, et al. Impact of regionalisation of a national rheumatic heart disease registry: The Ugandan experience. Heart Asia. 2018. doi: 10.1136/heartasia-2017-010981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rwebembera J, Aliku T, Kayima J, et al. Starting and Operating a Public Cardiac Catheterization Laboratory in a Low Resource Setting: The Eight-Year Story of the Uganda Heart Institute Catheter Laboratory. Glob Heart. 2021. doi: 10.5334/gh.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aliku TO, Lubega S, Namuyonga J, et al. Pediatric cardiovascular care in Uganda: Current status, challenges, and opportunities for the future. Ann Pediatr Cardiol. 2017. doi: 10.4103/0974-2069.197069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okello E, Longenecker CT, Beaton A, Kamya MR, Lwabi P. Rheumatic heart disease in Uganda: Predictors of morbidity and mortality one year after presentation. BMC Cardiovasc Disord. 2017. doi: 10.1186/s12872-016-0451-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaton A, Aliku T, Dewyer A, et al. Latent Rheumatic Heart Disease: Identifying the Children at Highest Risk of Unfavorable Outcome. Circulation. 2017. doi: 10.1161/CIRCULATIONAHA.117.029936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 15.Beaton A, Aliku T, Dewyer A, et al. Latent Rheumatic Heart Disease: Identifying the Children at Highest Risk of Unfavorable Outcome. Circulation. 2017. doi: 10.1161/CIRCULATIONAHA.117.029936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelman D, Mataika RL, Ah. Kee M, et al. Clinical outcomes for young people with screening-detected and clinically-diagnosed rheumatic heart disease in Fiji. Int J Cardiol. 2017. doi: 10.1016/j.ijcard.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 17.Marantelli S, Hand R, Carapetis J, Beaton A, Wyber R. Severe adverse events following benzathine penicillin G injection for rheumatic heart disease prophylaxis: cardiac compromise more likely than anaphylaxis. Heart Asia. 2019. doi: 10.1136/heartasia-2019-011191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilla P, Yacoub M, Zühlke L, et al. Global Unmet Needs in Cardiac Surgery. Glob Heart. 2018. doi: 10.1016/j.gheart.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 19.Institute of Health Metrics and Evaluation. GBD Results Tool. http://ghdx.healthdata.org/gbd-results-tool. Published2017. Accessed June 22, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure I. Hazard ratio for death according to distance to nearest health unit