Abstract
Simple Summary
The aim of this matched-pair study including patients with locally advanced head and neck squamous cell carcinoma (HNSCC) was to identify patients who are biologically at high risk for the development of loco–regional recurrences after surgery and postoperative radiotherapy (PORT) but at intermediate risk according to clinical risk factors, with the help of a novel predictive gene signature. These patients may benefit from treatment with postoperative radiochemotherapy (PORT-C). Based on 108 matched patient pairs treated with PORT and PORT-C, we identified a gene signature consisting of two metagenes. A significant association of the interaction between the risk classification by this signature and the type of treatment was observed for the endpoint loco–regional control (LRC), i.e., the 2-metagene signature was indicative for the type of treatment. The developed signature may thus help to identify high-risk patients currently treated with PORT, who may benefit from additional concurrent chemotherapy.
Abstract
(1) Background: Patients with locally advanced head and neck squamous cell carcinoma (HNSCC) who are biologically at high risk for the development of loco–regional recurrences after postoperative radiotherapy (PORT) but at intermediate risk according to clinical risk factors may benefit from additional concurrent chemotherapy. In this matched-pair study, we aimed to identify a corresponding predictive gene signature. (2) Methods: Gene expression analysis was performed on a multicenter retrospective cohort of 221 patients that were treated with postoperative radiochemotherapy (PORT-C) and 283 patients who were treated with PORT alone. Propensity score analysis was used to identify matched patient pairs from both cohorts. From differential gene expression analysis and Cox regression, a predictive gene signature was identified. (3) Results: 108 matched patient pairs were selected. We identified a 2-metagene signature that stratified patients into risk groups in both cohorts. The comparison of the high-risk patients between the two types of treatment showed higher loco–regional control (LRC) after treatment with PORT-C (p < 0.001), which was confirmed by a significant interaction term in Cox regression (p = 0.027), i.e., the 2-metagene signature was indicative for the type of treatment. (4) Conclusion: We have identified a novel gene signature that may be helpful to identify patients with high-risk HNSCC amongst those at intermediate clinical risk treated with PORT, who may benefit from additional concurrent chemotherapy.
Keywords: head and neck squamous cell carcinoma, gene signature, postoperative radiotherapy, postoperative radiochemotherapy, propensity score matching
1. Introduction
Patients with locally advanced head and neck squamous cell carcinoma (HNSCC) are currently treated with primary radiotherapy, surgery followed by postoperative radiotherapy (PORT), or postoperative radiochemotherapy (PORT-C), depending on the clinical characteristics of the tumour [1,2]. Several studies and randomized controlled trials have demonstrated the benefits of concurrent radiochemotherapy over radiotherapy alone in patients with locally advanced HNSCC, showing improved local recurrence and disease-free survival with a manageable increase in toxicity [3,4,5,6,7,8,9]. Brizel et al. showed that patients with high-risk HNSCC demonstrated a significant improvement in loco–regional control and disease-free survival when treated with concurrent postoperative radiochemotherapy [7]. Another study by Bernier et al. found that concurrent radiochemotherapy improved progression-free survival from 36% to 47% and overall survival from 40% to 53% in comparison to radiotherapy alone in patients with locally advanced HNSCC [10].
Postoperative radiotherapy is commonly applied in patients with intermediate risk factors, often characterized by large tumours and none or few positive lymph nodes without or very little (<1 mm) extracapsular extension [11,12,13,14,15,16]. Additional concurrent chemotherapy is usually indicated in case of further clinical risk factors such as ≥2 positive lymph nodes, extracapsular extension of lymph node metastases, microscopic disease after surgery (R1 resection), and UICC stages III-IV [7,10,17]. Still, treatment outcome after PORT is heterogeneous with a local recurrence rate after two years of around 38% [18], i.e., some patients may be judged to be clinically at intermediate risk but actually are at high risk for the development of a recurrence and may benefit from the addition of concurrent chemotherapy.
In the past decade, gene expression data have been used to identify prognostic and predictive gene signatures that predict recurrence and response to therapy [19,20,21,22,23,24,25]. For example, the 15-gene hypoxia-associated signature [20], which consists of upregulated genes under hypoxic conditions [26], proved to be a useful predictive biomarker for the selection of patients with HNSCC that benefit from hypoxic modification of primary radiotherapy with nimorazole. Similarly, a 22-gene signature based on TCGA data showed that patients with HNSCC classified as high-risk who received radiochemotherapy demonstrated improved overall survival, relapse-free survival, and loco–regional control compared with those patients that received radiotherapy alone [27]. However, this study did not account for differences in clinical characteristics between patients who received PORT and PORT-C, which may induce a selection bias.
Therefore, in the present study, we aimed to develop a novel predictive gene signature to identify a subgroup of patients treated with PORT who were clinically judged to be at intermediate risk but actually had a high risk for loco–regional failure and may benefit from additional concurrent chemotherapy. Based on whole-transcriptome data, we performed a propensity score matched analysis of two retrospective datasets of patients with locally advanced HNSCC treated with PORT and PORT-C.
2. Materials and Methods
2.1. Patient Data
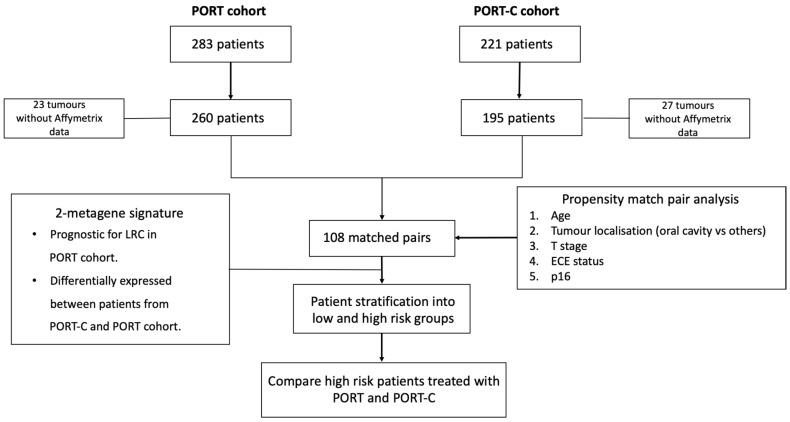
In this retrospective study, two cohorts with locally advanced HNSCC were included. In total, 221 patients were treated with PORT-C between 2004 and 2012 in 9 different institutions of the German Cancer Consortium—Radiation Oncology Group (DKTK-ROG) [28] and 283 patients were treated with PORT between 1999 and 2016 at the DKTK-ROG site in Dresden. All patients received surgery followed by postoperative radio(chemo)therapy and met the following inclusion criteria: histologically proven squamous cell carcinoma, curatively intended cisplatin-based PORT-C or PORT according to standard protocols covering the former tumour region and the neck nodes. Patients were excluded if whole-transcriptome data were not available, reducing the patient number to 195 in the PORT-C cohort and to 260 in the PORT cohort. Additional details on inclusion criteria, data collection, handling, and analyses of biomaterial have been described previously [28,29]. In this study, the 7th edition (2010) of the TNM classification has been used. The study design is illustrated in Figure 1.
Figure 1.
Study design.
The treating institution evaluated the disease status and first site of relapse. The radiotherapy treatment plan and radiological images of the recurrence (CT, MRI or PET-CT) for each loco–regional failure were reviewed by experienced radiation oncologists. FFPE blocks of the resected tumour specimens were collected centrally at the DKTK partner site in Dresden. Total RNA extraction was performed as described previously [28,29]. The CINtec Histology kit (Roche mtm laboratories AG, Basel, CH) was used to perform immunohistochemical staining of p16, according to the manufacturer’s instructions as described previously [28,29]. Tumours with intense p16 nuclear staining in at least 70% of the tumour cells were considered as p16 overexpressing. The ethical approval for the multicenter retrospective analyses of clinical and biological data were obtained by all the DKTK-ROG partner sites.
2.2. Microarray Analysis
The Human Transcriptome 2.0 Array (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to perform the whole transcriptome analysis as described previously [30]. Quality control was performed in Transcriptome Analysis Console (TAC) 4.0 (Applied Biosystems, Waltham, MA, USA) as per manufacturer’s instructions using the probe-level intensity files. The Signal Space Transformation in conjunction with the Robust Multiarray Average method (SST-RMA) were used to perform data normalization. Batch normalization was performed using ComBat method [31] to adjust for batch effects between the cohorts since the data were collected during different time intervals. For further analysis, coding genes were selected.
2.3. Clinical Endpoints and General Statistical Analysis
The primary endpoint was loco–regional control (LRC), which was calculated from the first day of radiotherapy to the date of event or censoring. Overall survival (OS) and freedom from distant metastases (DM) were the secondary endpoints. Survival curves were estimated using the Kaplan–Meier method and were compared using log-rank tests. To examine differences in continuous and categorical variables between the cohorts, Mann–Whitney-U tests and chi-squared (χ2) tests were used, respectively. To test the association of the genes and clinical features with the endpoints, univariable Cox regression was used. R Statistics version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) [32], Python (Python Software Foundation. Python Language Reference, version 3.7) and SPSS 25 software (IBM Corporation, Armonk, NY, USA) were used to perform the described statistical tests. Two-sided tests were performed and p-values < 0.05 were considered as statistically significant for all analyses.
2.4. Matched-Pair Analysis
The exposure of interest was whether patients received PORT or PORT-C (treatment status). Logistic regression was used to estimate a propensity score model, where the treatment status was regressed on the clinical variables of age (0: <57 years vs. 1: ≥57 years, based on median), T stage (1, 2 vs. 3, 4), tumour localization (oral cavity vs. others), extracapsular extension (ECE) status (0 vs. 1), and p16 overexpression (0 vs. 1). These parameters were significantly associated with LRC in at least one of the cohorts (Table S1). Pairs of patients treated with PORT and PORT-C were matched using the nearest method on the logit propensity score using different caliper widths. An optimal caliper width of 0.2 was chosen based on the standard mean square difference between the clinical parameters in PORT and PORT-C patients after propensity score matching and based on the number of matched patient pairs. The analysis was performed using the matchIt R package [33].
2.5. Statistical Framework to Identify Gene Signature and Perform Model Predictions
Before identifying the gene signature, the gene expression data of the PORT cohort were z-transformed to mean 0 and standard deviation of 1. Based on the obtained mean values and standard deviations, the corresponding gene expression data of the PORT-C cohort were transformed.
To identify a predictive gene signature, the following steps were carried out. (i) Univariable Cox regression analysis was performed on the PORT cohort to filter genes with high prognostic value for LRC. (ii) Differential gene expression (DGE) analysis was performed between the PORT-C cohort and the PORT cohort to identify genes that represent a differing response between both cohorts. The genes with a fold-change (FC) of ≥1.5 and with FDR corrected p-values of ≤0.05 in differential gene expression and univariable Cox regression were selected. To increase robustness of the signature, genes that were highly correlated in the PORT cohort (Spearman correlation coefficient r ≥ 0.8) were combined to create a new metagene, defined as the median expression of the contributing genes. Finally, the identified genes or metagenes were used to build a multivariable Cox model. The risk score of each patient was calculated as: ∑ coefficient of the feature in multivariable Cox model (βi) × value of the feature. An optimal risk score for patient stratification was calculated using the maximally selected rank statistics (maxstat) R package [34] based on 1000 bootstraps. The optimal risk score from the PORT cohort was used as a cut-off for stratifying patients into high-risk and low-risk groups in both the PORT and PORT-C cohort and thereby defines a corresponding gene classifier. Finally, the high-risk groups of both cohorts were compared by a log-rank test and an interaction term between treatment type and the expression of the gene signature was considered in Cox regression to test the predictive value of the signature. An experienced biostatistician (S.L) guided the statistical analyses.
3. Results
Patient data and clinical parameters of both cohorts before matching are summarized in Table S2. In the PORT cohort, 52.3% of the patients presented with oral cavity carcinomas, while this was the case for only 28.2% of patients in the PORT-C cohort (p < 0.001). A higher number of patients in the PORT-C cohort were associated to high T-stage (p = 0.038) and N-stage (p < 0.001). Patients in the PORT cohort had lower LRC than patients in the PORT-C cohort (p = 0.082), while OS (p = 0.24) and DM (p = 0.16) were similar (Figure S1).
After propensity score matching, 108 matched patient pairs were obtained. Standardized mean differences for the five clinical parameters were smaller than 0.1 (Table S3). Patient data and clinical parameters of the matched cohorts are summarized in Table 1. After matching, patients in the PORT cohort had lower LRC (p = 0.037) than patients in the PORT-C cohort, while OS (p = 0.12) and DM (p = 0.42) were similar (Figure S2).
Table 1.
Patient characteristics for the PORT and PORT-C cohorts (108 matched patient pairs). Significant p-values are marked in bold.
| Characteristics | PORT Cohort (1999–2016) | PORT-C Cohort (2004–2011) | p-Value | ||
|---|---|---|---|---|---|
| Median (Range) | Median (Range) | ||||
| Age (years) | 57.3 (39.0–84.3) | 57 (24–74) | 0.26 | ||
| Dose (Gy) | 60.0 (60–66) | 64.0 (56–68.4) | <0.001 | ||
| Number of pts | % | Number of pts | % | ||
| Age 0(<57)/1(≥57 years) |
51/57 | 47.2/52.8 | 53/55 | 49.1/50.9 | 0.79 |
| Gender Male/female |
90/18 | 88.3/11.7 | 88/20 | 81.5/18.5 | 0.72 |
| Tumour localization Oral cavity/Oropharynx/Hypopharynx/Larynx |
31/55/6/16 | 28.7/50.9/5.5/14.9 | 31/59/18/0 | 28.7/54.6/16.7/0 | 1.00 |
| Grading 1,2/3 |
51/57 | 47.2/52.8 | 45/63 | 41.7/58.3 | 0.10 |
| R status 0/1/missing |
100/8 | 92.6/7.4 | 59/49 | 54.6/45.4 | <0.001 |
| ECE status 0/1/missing |
85/23 | 78.7/21.3 | 85/23 | 78.7/21.3 | 1.00 |
| p16 overexpression 0/1 |
71/37 | 65.7/34.3 | 67/41 | 62.0/38.0 | 0.57 |
| T stage 1,2/3,4 |
74/34 | 68.5/31.5 | 69/39 | 63.9/36.1 | 0.47 |
| N stage 0,1/2,3 |
68/40 | 63.0/37.0 | 37/71 | 38.0/62.0 | <0.001 |
| Locoregional control | 25 | 23.1 | 14 | 13.0 | 0.037 a |
| Distant metastases | 19 | 17.6 | 15 | 13.9 | 0.42 a |
| Overall survival | 46 | 42.6 | 31 | 28.7 | 0.12 a |
a Log-rank test.
Seven genes, KRT6A, KRT6B, KRT6C, SPRR1A, SPRR1B, SPRR2A, and SPRR2C, that were differentially expressed between the matched cohorts and prognostic on the PORT cohort, were identified. Due to their high correlation (r > 0.80), two metagenes were formed based on KRT6A, KRT6B, and KRT6C and based on SPRR1A, SPRR1B, SPRR2A, and SPRR2C; finally defining the proposed predictive 2-metagene signature.
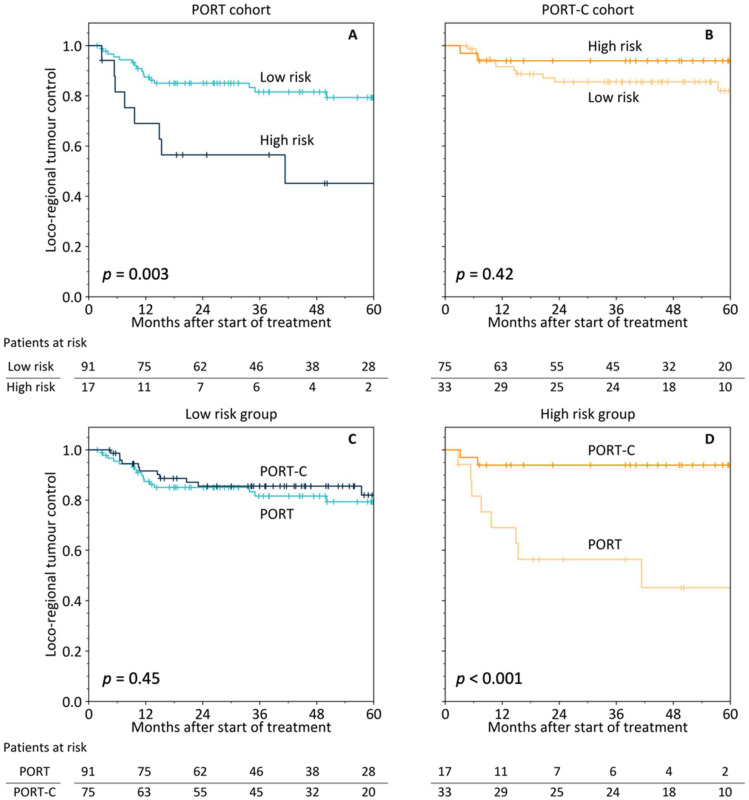
From the multivariable Cox regression model on the PORT cohort, an individual risk score (rs) was calculated for every patient: . Upregulation of both metagenes was related to reduced LRC. Patient stratification using the optimal risk score cut-off of 0.60 led to a significant difference in LRC for the PORT cohort (p = 0.003) but not for the PORT-C cohort (p = 0.42), Figure 2A,B. Comparing the patients classified to be at high risk between the two treatment types revealed a significant benefit of PORT-C (p < 0.001), while there was no difference between the patients classified to be at low risk (p = 0.45), Figure 2C,D. A multivariable model including the 2-metagene-classifier, treatment status, and their interaction term (gene classifier × treatment status) showed a statistically significant interaction term (p = 0.027, Table 2), i.e., the signature was predictive for the type of treatment. The interaction term remained significant when additional clinical characteristics were included in the model (p = 0.023, Table 2).
Figure 2.
Patient stratification by the 2-metagene signature for loco–regional tumour control (LRC) in the PORT (A) and the PORT-C cohort (B). Comparison of LRC between PORT and PORT-C for the low-risk (C) and high-risk (D) groups as defined by the 2-metagene signature.
Table 2.
Multivariable Cox regression of loco–regional tumour control for the 2-metagene signature, treatment, their interaction term, and relevant clinical parameters for the pooled matched dataset (n = 216). Significant p-values are marked in bold.
| Parameter | Coefficient (ß) | Loco–Regional Control HR (95 % CI) |
p-Value |
|---|---|---|---|
| 2-Gene signature | |||
| Gene classifier (high vs. low risk [b]) | 1.22 | 3.42 (1.47–7.97) | 0.004 |
| Treatment status (PORT-C vs. PORT [b]) | −0.30 | 0.74 (0.35–1.58) | 0.44 |
| Gene classifier × Treatment status | −1.73 | 0.18 (0.04–0.82) | 0.027 |
| 2-Gene signature and clinical parameters | |||
| Gene classifier (high vs. low risk [b]) | 1.19 | 3.29 (1.37–7.91) | 0.007 |
| Treatment status (PORT-C vs. PORT [b]) | −0.57 | 0.56 (0.24–1.33) | 0.19 |
| Gene classifier × Treatment status | −1.81 | 0.16 (0.03–0.78) | 0.023 |
| T stage (3, 4 vs. 1, 2 [b]) | 0.99 | 2.68 (1.33–5.40) | 0.005 |
| Tumour localization (oral cavity vs. others [b]) | 0.58 | 1.79 (0.88–3.64) | 0.11 |
| N stage (2, 3 vs. 0, 1 [b]) | 0.38 | 1.46 (0.65–3.27) | 0.36 |
| R status (1 vs. 0 [b]) | 0.40 | 1.49 (0.69–3.30) | 0.33 |
| ECE status (1 vs. 0 [b]) | 0.48 | 1.61 (0.66–3.92) | 0.29 |
| p16 overexpression (1 vs. 0 [b]) | −0.97 | 0.38 (0.15–0.94) | 0.037 |
[b] Baseline class.
Concerning the secondary endpoints, the 2-metagene signature was prognostic for OS in the PORT cohort but not for DM. Kaplan–Meier curves of patient groups stratified by the 2-metagene signature for OS and DM are presented in Figure S3 for both cohorts. The multivariable models including the 2-metagene-classifier, treatment status, and their interaction term (gene classifier × treatment status) did not show a significant interaction term. The results are presented in Tables S4 and S5, respectively.
4. Discussion
In this matched-pair study, we developed a novel 2-metagene signature based on differential gene expression analysis and Cox regression in order to identify HNSCC patients amongst those who are biologically at high risk for the development of loco–regional recurrences after postoperative radiotherapy (PORT) but clinically considered to be at intermediate risk. We showed that patients classified as high risk who were treated with PORT-C had significantly higher LRC compared to similar patients treated with PORT.
The genes KRT6A, KRT6B, and KRT6C encode a type II cytokeratin that is important in the formation of nail bed, filiform papillae, and the epithelial lining of oral mucosa and the esophagus. KRT6A gene silencing has been shown to suppress cell viability, invasion, and metastasis of nasopharyngeal carcinoma via the β-catenin/TCF pathway [35]. KRT6A is also overexpressed in lung adenocarcinoma and promotes lung cancer cell proliferation, migration, and colony formation ability via epithelial–mesenchymal transition and cancer stem cells transformation [36]. The overexpression of KRT6B has been shown to significantly suppress honokiol-induced human hepatoma cell apoptosis via notch signaling [37]. A 25-gene network signature model by Chang et al. was able to discriminate between two histological types of lung cancers, adenocarcinomas and squamous cell carcinomas, and 95% of the accuracy was explained by the interplay of KRT6A, KRT6B, and KRT6C, which were unique to squamous cells [38].
The SPRR genes encode a class of polypeptides (small proline rich proteins) that are involved in differentiation of keratinocytes, the primary cell type of the epidermis. SPRR1A is known to play a role in various types of cancer, such as diffuse large B-cell lymphomas [39], head and neck squamous cell carcinoma [40], and breast cancer [41]. The overexpression of SPRR1B has been shown to enhance the entry of cells in the G0 phase of the cell cycle [42]. SPRR1B is also known to be overexpressed in human oral squamous cell cancer stem-like cells and is related to their growth through activation of MAP kinase signal [43]. SPRR2A is overexpressed in lymph node metastases, along with an association to non-oropharyngeal location of the primary tumour and is an independent prognostic factor for regional disease recurrence after surgery and radiotherapy. It plays a dual role in invasion and therapeutic resistance in HNSCC, respectively through its downregulation and overexpression [44].
Studies on gene ontology revealed that the seven identified genes were enriched in biological processes such as keratinocyte differentiation, epithelial differentiation, and skin development. All genes and both metagenes showed positive coefficients in the univariable Cox model suggesting that overexpression was associated with worse prognosis in HNSCC, which is in line with the literature [35,36,37,38,39,40,41,42,43,44].
The clinical characteristics between the two considered patient cohorts are expected to differ, since patients with different clinical risk profiles were included. Significant differences were observed in age, radiation dose, tumour localization, R status, ECE status, T stage, and N stage between the PORT and PORT-C cohorts before matching (Table S2). After matching with five clinical characteristics, most clinical parameters were well aligned between the cohorts, except for dose, R status, and N stage. However, none of these parameters were significantly related to LRC (Table 3). Propensity score matching with additional clinical parameters led to too small patient numbers in the matched cohorts and was thus not considered.
Table 3.
Univariable Cox regression of loco–regional tumour control for clinical parameters and the identified two metagenes in the matched PORT and PORT-C cohorts (n = 108). Significant p-values are marked in bold.
| Parameter | PORT Cohort | PORT-C Cohort | ||||
|---|---|---|---|---|---|---|
| Coefficient (ß) | Loco–Regional Control HR (95 % CI) |
p-Value | Coefficient (ß) | Loco–Regional Control HR (95 % CI) |
p-Value | |
| Age (≥57 vs. <57 years [b]) | −0.75 | 0.47 (0.21–1.07) | 0.074 | −1.54 | 0.21 (0.06–0.78) | 0.019 |
| Gender (female vs. male [b]) | 0.11 | 1.12 (0.41–3.04) | 0.83 | 0.67 | 1.96 (0.61–6.26) | 0.26 |
| Tumour localization (oral cavity vs. others [b]) | 1.09 | 2.97 (1.34–6.60) | 0.007 | 0.55 | 1.73 (0.58–5.21) | 0.33 |
| T stage (3, 4 vs. 1, 2 [b]) | 1.13 | 3.10 (1.41–6.81) | 0.004 | 0.55 | 1.73 (0.60–5.03) | 0.31 |
| N stage (2, 3 vs. 0, 1 [b]) | 0.42 | 1.55 (0.69–3.38) | 0.30 | −0.01 | 0.99 (0.33–2.97) | 0.98 |
| Tumour grade (3 vs. 1, 2 [b]) | −0.33 | 0.72 (0.33–1.58) | 0.41 | −0.96 | 0.38 (0.11–1.37) | 0.14 |
| R status (1 vs. 0 [b]) | 0.95 | 2.58 (0.88–7.60) | 0.085 | 0.09 | 1.10 (0.38–31.8) | 0.87 |
| ECE status (1 vs. 0 [b]) | 0.83 | 2.29 (0.98–5.32) | 0.055 | 0.24 | 1.27 (0.35–4.62) | 0.72 |
| Dose (Gy) | 0.07 | 1.08 (0.93–1.25) | 0.32 | 0.07 | 1.08 (0.87–1.33) | 0.51 |
| p16 overexpression (1 vs. 0 [b]) | −1.20 | 0.30 (0.10–0.88) | 0.029 | −1.38 | 0.25 (0.06–1.13) | 0.071 |
| Metagene KRT6 | 0.59 | 1.80 (1.17–2.79) | 0.008 | 0.49 | 1.62 (0.87–3.02) | 0.13 |
| Metagene SPRR1 | 0.49 | 0.57 (0.34–0.98) | 0.004 | 0.21 | 1.23 (0.82–1.86) | 0.32 |
[b] Baseline class.
We showed that the 2-metagene signature may be used as a predictive biomarker to select HNSCC patients who are clinically considered at intermediate risk but may benefit from additional chemotherapy as a treatment intensification strategy. Further intensification of PORT-C was investigated in a phase II trial, where it was suggested that adding panitumumab, an antibody to EGFR, might be superior to PORT-C for high risk HPV-negative HNSCC patients [45]. In our PORT cohort, EGFR was found to be prognostic for LRC (p = 0.007), a high expression was related to worse outcome. In general, other prognostic biomarkers may also be considered for treatment intensification. Several genes or gene signatures have previously been identified [19,20,21,22,23,24,25]. On the PORT cohort, the potential stem cell marker SLC3A2 [19], the 6-gene signature associated to cell migration and invasion [46], the 12-gene immune signature [23], and the 15-gene hypoxia-associated signature [20] were prognostic for LRC. Therefore, the molecular pathways associated with these genes or gene signatures may contain potential targets for treatment intensification purposes.
There are several limitations to the study. First, this study is retrospective in nature, and although propensity score matching was performed, bias inevitably exists due to the exclusion of patients as no match could be found using the nearest method. After matching, from 195 patients treated with PORT-C and 260 treated with PORT, we were able to include 108 matched patient pairs, where the standard mean difference among the matched clinical features was smaller than 0.1 (Table S3). The obtained results need to be externally and prospectively validated. This is planned by using data from the prospective HNprädBio trial (NCT02059668, www.clinicaltrials.gov (accessed on 2 February 2022)) of the DKTK-ROG that will finish patient recruitment in 2022.
5. Conclusions
In conclusion, we identified a novel 2-metagene signature that may be used to identify high-risk HNSCC patients amongst those who are clinically at intermediate risk and, according to current guidelines, treated with PORT. These patients may benefit from treatment with additional concurrent chemotherapy. Independent prospective validation of this retrospective result is required before potential application in a clinical trial.
Acknowledgments
The authors wish to thank all of the tumour banks for providing material including the Tumour and Normal Tissue Bank (TNTB) of the National Center for Tumour Diseases Dresden (NCT/UCC) as well as the external pathologist Uwe Sturm (Dresden) for providing the respective FFPE tissue material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14123031/s1, Figure S1: Loco–regional tumour control (A), overall survival (B) and freedom from distant metastases (C) on the PORT and PORT-C cohort before propensity score matching; Figure S2: Loco–regional tumour control (A), overall survival (B) and freedom from distant metastases (C) on the PORT and PORT-C cohort after propensity score matching; Figure S3: Patient stratification by the 2-metagene signature for overall survival (OS) in the PORT (A) and the PORT-C cohort (B) and for freedom from distant metastases (DM) in the PORT (C) and the PORT-C cohort (D); Table S1: Univariable Cox regression of loco–regional tumour control for the clinical parameters in the PORT and PORT-C cohorts before propensity score matching.; Table S2: Patient characteristics for the PORT and PORT-C cohorts before propensity score matching; Table S3: Comparison of the clinical parameters between PORT and PORT-C patients in the original sample and in the propensity score matched sample. The standardized mean and the standardized mean difference between the cohorts are shown. Table S4: Multivariable Cox regression of overall survival for the 2-metagene signature and their interaction term with treatment type; Table S5: Multivariable Cox regression of freedom from distant metastases for the 2-metagene signature and their interaction term with treatment type.
Author Contributions
Conceptualization, A.L., S.L., M.B. and M.K. (Mechthild Krause); methodology, S.P. (Shivaprasad Patil), A.L. and S.L.; formal analysis, S.P. (Shivaprasad Patil) and S.L.; resources, A.L., H.H., M.G. (Marianne Grosser), F.L., V.G., M.K. (Max Kemper), A.N., D.H., I.T., V.B., M.G. (Maja Guberina), M.S., P.B., J.v.d.G., H.S., A.-L.G., A.A., J.D., U.G., C.B., S.P. (Steffi Pigorsch), S.E.C., S.B., D.Z., K.J., G.B.B., M.B. and M.K. (Mechthild Krause); writing—original draft preparation, S.P. (Shivaprasad Patil) and S.L.; writing—review and editing, S.P. (Shivaprasad Patil), S.L., A.L., H.H., M.G. (Marianne Grosser), F.L., V.G., M.K. (Max Kemper), A.N., D.H., I.T., V.B., M.G. (Maja Guberina), M.S., P.B., J.v.d.G., H.S., A.-L.G., A.A., J.D., U.G., C.B., S.P. (Steffi Pigorsch), S.E.C., S.B., D.Z., K.J., G.B.B., M.B. and M.K (Mechthild Krause); supervision, A.L., S.L., M.B. and M.K (Mechthild Krause). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Technische Universität Dresden, Germany (EK 397102014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in the study are available on request from the corresponding author. The data are not publicly available due to further ongoing analyses.
Conflicts of Interest
Michael Baumann, CEO and Scientific Chair of the German Cancer Research Center (DKFZ, Heidelberg) is responsible for collaborations with a large number of companies and institutions worldwide. In this capacity, he has signed contracts for research funding and/or collaborations, including commercial transfers, with industry and academia on behalf of his institute(s) and staff. He is a member of several supervisory boards, advisory boards, and boards of trustees. Michael Baumann confirms that there is no conflict of interest for this paper.
In the past 5 years, Dr. Mechthild Krause received funding for her research projects by IBA (2016), Merck KGaA (2014–2018 for preclinical study; 2018–2020 for clinical study), Medipan GmbH (2014–2018). Dr. Mechthild Krause and Dr. Annett Linge are involved in an ongoing publicly funded (German Federal Ministry of Education and Research) project with the companies Medipan (2019–2022), Attomol GmbH (2019–2022), GA Generic Assays GmbH (2019–2022), Gesellschaft für medizinische und wissenschaftliche genetische Analysen (2019–2022), Lipotype GmbH (2019–2022) and PolyAn GmbH (2019–2022). Dr. Krause and Dr. Linge confirm that, to the best of their knowledge, none of the above-mentioned funding sources were involved in the preparation of this paper.
The Department of Radiation Oncology Tübingen receives within the frame of research agreements financial and technical support as well as sponsoring for travels and scientific symposia from Elekta AB (Stockholm, Sweden), TheraPanacea (Paris, France), Philips GmbH (Best, The Netherlands); Dr. Sennewald Medizintechnik GmbH (München, Germany), PTW Freiburg (Germany).
Funding Statement
The study was partly funded by the German Cancer Consortium (DKTK). The DKTK is funded as one of the National German Health Centres by the Federal German Ministry of Education and Research (BMBF).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crews Q.E., Fletcher G.H. Comparative evaluation of the sequential use of radiation and surgery in primary tumors of the oral cavity, oropharynx, larynx, and hypopharynx. Am. J. Roentgenol. 1971;111:73–77. doi: 10.2214/ajr.111.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Vikram B., Farr H.W. Adjuvant Radiation Therapy in Locally Advanced Head and Neck Cancer. Cancer J. Clin. 1983;33:134–138. doi: 10.3322/canjclin.33.3.134. [DOI] [PubMed] [Google Scholar]
- 3.Adelstein D.J., Li Y., Adams G.L., Wagner H., Kish J.A., Ensley J.F., Schuller D.E., Forastiere A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Adelstein D.J., Kalish L.A., Adams G.L., Wagner H., Oken M.M., Remick S.C., Mansour E.G., Hoselow R.E. Concurrent radiation therapy and chemotherapy for locally unresectable squamous cell head and neck cancer: An Eastern Cooperative Oncology Group pilot study. J. Clin. Oncol. 1993;11:2136–2142. doi: 10.1200/JCO.1993.11.11.2136. [DOI] [PubMed] [Google Scholar]
- 5.Lavertu P., Saxton J.P., Secic M., Wood B.G., Wanamaker J.R., Eliachar I., Strome M., Larto M.A. Mature Results of a Phase III Randomized Trial Comparing Concurrent Chemoradiotherapy with Radiation Therapy Alone in Patients with Stage III and IV Squamous Cell Carcinoma of the Head and Neck. Cancer. 2000;88:876–883. doi: 10.1002/(sici)1097-0142(20000215)88:43.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Brizel D.M., Albers M.E., Fisher S.R., Scher R.L., Richtsmeier W.J., Hars V., George S.L., Huang A.T., Prosnitz L.R. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 1998;338:1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 7.Cooper J.S., Pajak T.F., Forastiere A.A., Jacobs J., Campbell B.H., Saxman S.B., Kish J.A., Kim H.E., Cmelak A.J., Rotman M., et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 8.Huguenin P., Beer K.T., Allal A., Rufibach K., Friedli C., Davis J.B., Pestalozzi B., Schmid S., Thöni A., Ozsahin M., et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J. Clin. Oncol. 2004;22:4613–4621. doi: 10.1200/JCO.2004.12.193. [DOI] [PubMed] [Google Scholar]
- 9.Cooper J.S., Guo M.D., Herskovic A., Macdonald J.S., Martenson J.A., Byhardt R., Russell A.H., Beitler J.J., Spencer S., Graham M.V., et al. Chemoradiotherapy of Locally Advanced. J. Am. Med. Assoc. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 10.Bernier J., Domenge C., Ozsahin M., Matuszewska K., Lefebvre J.L., Greiner R.H., Giralt J., Maingon P., Rolland F., Bolla M., et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. Cancer/Radiotherapie. 2005;9:203–204. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 11.Grégoire V., Lefebvre J.L., Licitra L., Felip E. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010;21:184–186. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 12.Machiels J.P., René Leemans C., Golusinski W., Grau C., Licitra L., Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1462–1475. doi: 10.1016/j.annonc.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Guideline Program Oncology: Laryngeal Carcinoma. [(accessed on 28 February 2022)]. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/larynxkarzinom/
- 14.Leitlinienprogramm Onkologie: Mundhoehlenkarzinom. [(accessed on 28 February 2022)]. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/mundhoehlenkarzinom/
- 15.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F., Westra W.H., Chung C.H., Jordan R.C., Lu C., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S.H., Xu W., Waldron J., Siu L., Shen X., Tong L., Ringash J., Bayley A., Kim J., Hope A., et al. Refining American joint committee on cancer/union for international cancer control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J. Clin. Oncol. 2015;33:836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 17.Bernier J., Cooper J.S., Pajak T.F., Van Glabbeke M., Bourhis J., Forastiere A., Ozsahin E.M., Jacobs J.R., Jassem J., Ang K., et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501) Head Neck J. Sci. Spec. Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 18.Cooper J.S., Pajak T.F., Forastiere A., Jacobs J., Fu K.K., Ang K.K., Laramore G.E., Al-sarraf M. Precisely defining high-risk operable head and neck tumors based on RTOG #85-03 and #88-24: Targets for postoperative radiochemotherapy? Head Neck J. Sci. Spec. Head Neck. 1998;20:588–594. doi: 10.1002/(sici)1097-0347(199810)20:7<588::aid-hed2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Linge A., Lohaus F., Löck S., Nowak A., Gudziol V., Valentini C., von Neubeck C., Jütz M., Tinhofer I., Budach V., et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation. Radiother. Oncol. 2016;121:364–373. doi: 10.1016/j.radonc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Toustrup K., Sørensen B.S., Nordsmark M., Busk M., Wiuf C., Alsner J., Overgaard J. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71:5923–5931. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- 21.Kim H.S., Kim S.C., Kim S.J., Park C.H., Jeung H.C., Kim Y.B., Ahn J.B., Chung H.C., Rha S.Y. Identification of a radiosensitivity signature using integrative metaanalysis of published microarray data for NCI-60 cancer cells. BMC Genomics. 2012;13:348. doi: 10.1186/1471-2164-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen S., Bai J., Wei Y., Wang G., Li Q., Zhang R., Duan W., Yang S., Du M., Zhao Y., et al. A seven-gene prognostic signature for rapid determination of head and neck squamous cell carcinoma survival. Oncol. Rep. 2017;38:3403–3411. doi: 10.3892/or.2017.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai S., Zhang P., Zhang J.C., Shen J., Xiang X., Yan Y.B., Xu Z.Q., Zhang J., Long L., Wang C., et al. A gene signature associated with prognosis and immune processes in head and neck squamous cell carcinoma. Head Neck. 2019;41:2581–2590. doi: 10.1002/hed.25731. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt S., Linge A., Zwanenburg A., Leger S., Lohaus F., Krenn C., Appold S., Gudziol V., Nowak A., Von Neubeck C.C., et al. Development and Validation of a Gene Signature for Patients with Head and Neck Carcinomas Treated by Postoperative Radio(chemo)therapy. Clin. Cancer Res. 2018;24:1364–1374. doi: 10.1158/1078-0432.CCR-17-2345. [DOI] [PubMed] [Google Scholar]
- 25.Chung C.H., Parker J.S., Ely K., Carter J., Yi Y., Murphy B.A., Ang K.K., El-Naggar A.K., Zanation A.M., Cmelak A.J., et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-κB Signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006;66:8210–8218. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen B.S., Toustrup K., Horsman M.R., Overgaard J., Alsner J. Identifying pH independent hypoxia induced genes in human squamous cell carcinomas in vitro. Acta Oncol. (Madr.) 2010;49:895–905. doi: 10.3109/02841861003614343. [DOI] [PubMed] [Google Scholar]
- 27.Chen J., Fu G., Chen Y., Zhu G., Wang Z. Gene-expression signature predicts survival benefit from postoperative chemoradiotherapy in head and neck squamous cell carcinoma. Oncol. Lett. 2018;16:2565–2578. doi: 10.3892/ol.2018.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohaus F., Linge A., Tinhofer I., Budach V., Gkika E., Stuschke M., Balermpas P., Rödel C., Avlar M., Grosu A.L., et al. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: Results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group. Radiother. Oncol. 2014;113:317–323. doi: 10.1016/j.radonc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Linge A., Löck S., Krenn C., Appold S., Lohaus F., Nowak A., Gudziol V., Baretton G.B., Buchholz F., Baumann M., et al. Independent validation of the prognostic value of cancer stem cell marker expression and hypoxia-induced gene expression for patients with locally advanced HNSCC after postoperative radiotherapy. Clin. Transl. Radiat. Oncol. 2016;1:19–26. doi: 10.1016/j.ctro.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt S., Linge A., Grosser M., Lohaus F., Gudziol V., Nowak A., Tinhofer I., Budach V., Sak A., Stuschke M., et al. Comparison of GeneChip, nCounter, and Real-Time PCR–Based Gene Expressions Predicting Locoregional Tumor Control after Primary and Postoperative Radiochemotherapy in Head and Neck Squamous Cell Carcinoma. J. Mol. Diagn. 2020;22:801–810. doi: 10.1016/j.jmoldx.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 32.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2017. [(accessed on 22 January 2022)]. Available online: https://www.R-project.org/
- 33.Ho D.E., King G., Stuart E.A., Imai K. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 34.Hothorn T., Lausen B. maxstat: Maximally Selected Rank Statistics. R News. 2002;2:3–5. [Google Scholar]
- 35.Chen C., Shan H. Keratin 6A gene silencing suppresses cell invasion and metastasis of nasopharyngeal carcinoma via the β-catenin cascade. Mol. Med. Rep. 2019;49:3477–3484. doi: 10.3892/mmr.2019.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang B., Zhang W., Zhang M., Wang X., Peng S., Zhang R. KRT6A Promotes EMT and Cancer Stem Cell Transformation in Lung Adenocarcinoma. Technol. Cancer Res. Treat. 2020;19:1–8. doi: 10.1177/1533033820921248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H., Huo M., Jia Y., Xu A. KRT6B, a key mediator of notch signaling in honokiol-induced human hepatoma cell apoptosis. Int. J. Clin. Exp. Med. 2015;8:16880–16889. [PMC free article] [PubMed] [Google Scholar]
- 38.Chang H., Dreyfuss J.M., Ramoni M.F. A Transcriptional Network Signature Characterizes Lung Cancer Subtypes. Cancer. 2011;117:353–360. doi: 10.1002/cncr.25592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H., Gao J., Zhao Z., Li M., Liu C. Clinical implications of SPRR1A expression in diffuse large B-cell lymphomas: A prospective, observational study. BMC Cancer. 2014;14:333. doi: 10.1186/1471-2407-14-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavón M.A., Arroyo-Solera I., León X., Téllez-Gabriel M., Virós D., Gallardo A., Céspedes M.V., Casanova I., Lopez-Pousa A., Barnadas A., et al. The combined use of EFS, GPX2, and SPRR1A expression could distinguish favorable from poor clinical outcome among epithelial-like head and neck carcinoma subtypes. Head Neck. 2019;41:1830–1845. doi: 10.1002/hed.25623. [DOI] [PubMed] [Google Scholar]
- 41.Chen G., Li G., Luo M., Wei X., Wang D., Zhang H., Zhao X., Chen B., Liu C. Clinical significance of SPRR1A expression in progesterone receptor-positive breast cancer. Tumor Biol. 2015;36:2601–2605. doi: 10.1007/s13277-014-2879-8. [DOI] [PubMed] [Google Scholar]
- 42.Tesfaigzi Y., Wright P.S., Belinsky S.A. SPRR1B overexpression enhances entry of cells into the G0 phase of the cell cycle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003;285:889–898. doi: 10.1152/ajplung.00065.2003. [DOI] [PubMed] [Google Scholar]
- 43.Michifuri Y., Hirohashi Y., Torigoe T., Miyazaki A., Fujino J., Tamura Y., Tsukahara T., Kanaseki T., Kobayashi J., Sasaki T., et al. Small proline-rich protein-1B is overexpressed in human oral squamous cell cancer stem-like cells and is related to their growth through activation of MAP kinase signal. Biochem. Biophys. Res. Commun. 2013;439:96–102. doi: 10.1016/j.bbrc.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Nisa L., Barras D., Medova M., Aebersold D.M., Medo M., Poliakova M., Koch J., Bojaxhiu B., Eliçin O., Dettmer M.S., et al. Comprehensive genomic profiling of patient-matched head and neck cancer cells: A preclinical pipeline for metastatic and recurrent disease. Mol. Cancer Res. 2018;16:1912–1926. doi: 10.1158/1541-7786.MCR-18-0056. [DOI] [PubMed] [Google Scholar]
- 45.Ferris R.L., Geiger J.L., Trivedi S., Schmitt N.C., Heron D.E., Johnson J.T., Kim S., Duvvuri U., Clump D.A., Bauman J.E., et al. Phase II trial of post-operative radiotherapy with concurrent cisplatin plus panitumumab in patients. Ann. Oncol. 2016;27:2257–2262. doi: 10.1093/annonc/mdw428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patil S., Linge A., Grosser M., Lohaus F., Gudziol V., Kemper M., Nowak A., Haim D., Tinhofer I., Budach V., et al. Development and validation of a 6-gene signature for the prognosis of loco-regional control in patients with HPV-negative locally advanced HNSCC treated by postoperative radio(chemo)therapy. Radiother. Oncol. 2022;171:91–100. doi: 10.1016/j.radonc.2022.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in the study are available on request from the corresponding author. The data are not publicly available due to further ongoing analyses.