Abstract
Cancer stem cells (CSCs) are a subset of highly tumorigenic cells in tumors. They have enhanced self-renewal properties, are usually chemo-radioresistant, and can promote tumor recurrence and metastasis. They can recruit macrophages into the tumor microenvironment and differentiate them into tumor-associated macrophages (TAMs). TAMs maintain CSC stemness and construct niches that are favorable for CSC survival. However, how CSCs and TAMs interact is not completely understood. An understanding on these mechanisms can provide additional targeting strategies for eliminating CSCs. In this review, we comprehensively summarize the reported mechanisms of crosstalk between CSCs and TAMs and update the related signaling pathways involved in tumor progression. In addition, we discuss potential therapies targeting CSC–TAM interaction, including targeting macrophage recruitment and polarization by CSCs and inhibiting the TAM-induced promotion of CSC stemness. This review also provides the perspective on the major challenge for developing potential therapeutic strategies to overcome CSC-TAM crosstalk.
Keywords: cancer stem cells, tumor-associated macrophages, tumor microenvironment, cancer immunotherapy
1. Introduction
Cancer stem cells (CSCs), also called tumor initiation cells, are specialized tumor cells that promote tumor initiation and progression [1]. The existence of CSCs was first confirmed in 1994 when Dick et al. grouped acute myeloid leukemia (AML) cells according to the expression of cell surface markers; when AML with CD34+CD38− surface markers were transplanted into severe combined immunodeficiency (SCID) mice, numerous progenitor cell colonies were formed. However, non-CD34+CD38− AML cells had limited proliferative capacity. Therefore, the CD34+CD38− population was considered to be CSCs [2]. Since then, CSCs have been reported in other cancers: breast cancer (CSC markers: CD44, CD24, and ALDH-1) [3,4], colon cancer (CSC markers: CD24, CD44, CD133, and LGR5) [5,6,7], and melanoma (CSC markers: CD34 and ABCB5) [8,9]. CSCs are characterized by their self-renewal and differentiation potential that contribute to chemo-radioresistant and tumor initiation, which primarily account for the failure of current anticancer therapies in CSC-containing cancers [4]. In addition, CSC stemness is maintained mainly through the CSC niche, which is conducive to CSC survival, thereby making it difficult for drugs or other therapeutic methods to completely kill them, which leads to tumor recurrence [10]. Therefore, the eradication of CSCs is considered a promising method for improving cancer survival rates and even for curing cancer.
The CSC niche is a part of the tumor microenvironment (TME) and is similar to the normal adult stem cell niche that regulates the biological activity of stem cells in the form of cell–cell contacts and secreted factors [11,12]. The TME comprises fibroblasts, immune cells, endothelial cells, perivascular cells, extracellular matrix (ECM) components, and cytokine networks, among which tumor-associated macrophages (TAMs) are the most abundant immune cells in the TME [11]. TAMs can be recruited by CSCs and participate in TME formation; hence, they are beneficial to CSC survival [13]. Some studies have reported using CSCs derived from human or murine cholangiocarcinoma, hepatocellular carcinoma (HCC), or glioblastoma (GBM) cells; under in vitro spheroid culture, many factors regulate monocytes/macrophages in the supernatant, including CC-motif chemokine ligand (CCL) 2, CCL5, colony-stimulating factor (CSF)-1, interleukin (IL)-13, transforming growth factor (TGF)-β, and periosteum proteins (POSTN). These cytokines recruit circulating monocytes as well as surrounding tissue macrophage precursors into the TME and polarize them into TAMs [14,15,16]. After recruiting macrophages into the TME, CSCs can avoid phagocytosis by macrophages through protective mechanisms [17]. In clinical studies, CD47 expression has been reported to be upregulated in CSCs of HCC [18], pancreatic ductal adenocarcinoma [19], and lung cancer [20]. CD47 interacts with signal regulatory protein α (SIRP-α), which is expressed on TAMs, to prevent CSCs from being phagocytosed. Macrophages recruited into the TME can be divided into classically activated M1 macrophages and alternatively activated M2 macrophages [21]. M1 macrophages are induced and activated by interferon (IFN)-γ and lipopolysaccharides. Activated M1 macrophages typically express CD40 and CD86 and secrete higher levels of proinflammatory factors than M2 macrophages, such as IL-1α, tumor necrosis factor (TNF), and IL-12, which mainly exert an antitumor immune response and directly kill tumor cells in the early stage of a tumor [22]. Therefore, injecting M1 macrophages into the tumor tissue may be a potential therapeutic strategy for cancer [23], but the specific mechanism of action remains unclear. Activated M2 macrophages, however, play a role opposite to that of M1 macrophages—promoting ECM remodeling and tumor growth and metastasis [24]. M2 macrophages support CSC signatures, and consequently, they are also called M2 TAMs [25]. They are induced by IL-4 and IL-13, often with CD163 and CD206 as markers [26]. M2 macrophages can secrete soluble mediators, such as platelet-derived growth factor (PDGF), vascular epidermal growth factor (VEGF), and cytokines, such as IL-6, IL-10, and TNF-α [15,27,28]. These soluble mediators and cytokines can maintain the stemness characteristics of tumor cells by regulating the nuclear factor-κB (NF-κB), signal transducer and activator of transcription (STAT)3, AKT, and Hippo pathways [14,27,29,30,31,32]. These findings highlight the importance of molecular crosstalk between CSCs and macrophages in tumor progression; targeting these molecular mechanisms may provide more effective immunotherapies.
The CSC niche provides favorable conditions for drug resistance, tumor recurrence, and metastasis. TAMs, as the main immune cells in the TME, also play a crucial role in cancer malignancy. In this review, we summarize the potential mechanisms of interaction between CSCs and TAMs and discuss the current molecular targeting studies thereof. An overview of these emerging insights can provide new directions for antitumor strategies.
2. Secretory Molecules of CSCs Induce Macrophage Recruitment and Tumor-Promoting Characteristics of the TME
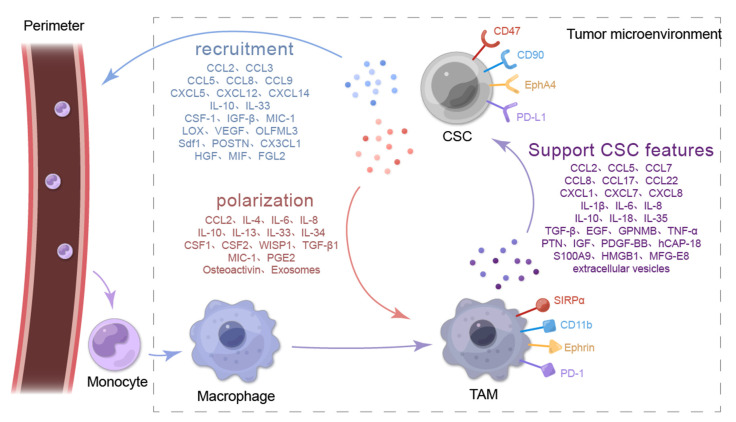
The functions of CSCs, such as immune escape, therapy resistance, metastasis, and maintenance of stemness, are largely dependent on the secretion of soluble factors (chemokines, interleukins, growth factors, or secreted proteins) and extracellular vesicles [33,34,35]. Specifically, CSCs signal by secreting soluble factors to recruit peripheral macrophages to the TME to become part of the tumor niche and by inducing macrophage transformation into TAMs [36]. A comprehensive understanding of the underlying mechanisms of macrophage recruitment and polarization by CSCs is beneficial for determining how to interrupt the mutual support pathway between CSCs and TAMs. Figure 1 presents the molecular interaction mechanisms between TAMs and CSCs.
Figure 1.
Molecular crosstalk between TAMs and CSCs. CSCs promote macrophage recruitment by secreting CCL2, CCL3, CCL5, CCL8, CCL9, CXCL5, CXCL12, CXCL14, IL-10, IL-33, CSF-1, IGF-β, MIC-1, LOX, VEGF, OLFML3, Sdf1, POSTN, CX3CL1, HGF, MIF, and FGL2. CSCs promote macrophage polarization by secreting CCL2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-33, IL-34, CSF1, CSF2, WISP1, TGF-β1, MIC-1, PGE2, Osteoactivin, and exosomes. TAMs produce factors to support CSC-like features, including CCL2, CCL5, CCL7, CCL8, CCL17, CCL22, CXCL1, CXCL7, CXCL8, IL-1β, IL-6, IL-8, IL-10, IL-18, IL-35, TGF-β, EGF, GPNMB, TNF-α, PTN, IGF, PDGF-BB, hCAP-18, S100A9, HMGB1, MFG-E8, and extracellular vesicles.
2.1. CSCs Recruit Macrophages into the TME
Many cytokines secreted by tumor cells can recruit macrophages to the TME, but some of them are exclusively produced by CSCs: CCL2, CCL3, CCL5, CXC motif chemokine ligand 12 (CXCL12), olfactomedin-like 3 (OLFML3), stromal-cell-derived factor-1b (Sdf1b), IL-6, IL-33, and CSF-1 [37,38,39,40,41,42,43,44,45] (Figure 1). CSCs contribute to the infiltration of macrophages or microglia to promote their own metastatic spread [38,39,43,46]. Here, we summarize the cytokines and soluble protein molecules secreted for macrophage recruitment by CSCs in different tumor types (Table 1).
Table 1.
CSCs secrete cytokines and other soluble protein molecules to recruit macrophages.
| Chemokines Produced by CSCs | Chemokine Receptor | Type of Cancer | Associated Signaling Pathway/Mechanism of Chemokine Secretion | Reference |
|---|---|---|---|---|
| CCL2 1 | CCR2 | Glioblastoma | IFNγ | [47] |
| Breast cancer | β-Catenin | [48] | ||
| Bladder cancer | Not shown | [39] | ||
| Hepatocellular carcinoma | Hippo | [43] | ||
| CCL3 | CCR1/CCR5 | Bladder cancer | Not shown | [49,50] |
| Leukemia | Not shown | [51] | ||
| CCL5 | CCR5, CCR1, CCR2 | Glioblastoma | PI3K 2/AKT | [52,53] |
| Optic glioma | Not shown | [54] | ||
| Hepatocellular carcinoma | Not shown | [55] | ||
| CCL8 | CCR2 | Bladder cancer | Not shown | [56] |
| Cutaneous squamous cell carcinoma | Not shown | [57] | ||
| CCL9 | CCR1 | Non-small cell lung cancer | Not shown | [58] |
| CXCL5 3 | CXCR2 | Hepatocellular carcinoma | Sox9 | [59] |
| CXCL12 | CXCR4 | Leukemia | Not shown | [42] |
| Colon cancer | NF-κB 4 | [60] | ||
| CXCL14 | Not shown | Lung cancer | Not shown | [61] |
| IL-10 | IL-10R | Ovarian cancer | WNT | [62] |
| IL-33 | ST2 | Squamous cell carcinoma | NRF2 | [44] |
| CSF1 5 | CSF1R | Hepatocellular carcinoma | Hippo | [43] |
| Non-small cell lung cancer | IRF5 | [63] | ||
| Glioblastoma | STAT3 6 | [64] | ||
| TGF-β 7 | Not shown | Glioblastoma | STAT3 | [64] |
| MIC-1 8 | Not shown | Glioblastoma | Not shown | [64] |
| Pancreatic cancer | Not shown | [65] | ||
| LOX 9 | Not shown | Pancreatic cancer | Not shown | [66] |
| VEGF 10 | VEGFR | Colorectal cancer | Six1/MAPK 11 & PI3K/AKT/mTOR | [67,68] |
| OLFML3 12 | Not shown | Glioblastoma | CLOCK-BMAL1 | [40] |
| Sdf1 13 | Cxcr4b | Brain tumor | AKT1 | [41] |
| POSTN 14 | Integrinαvβ₃ | Glioblastoma | Not shown | [69] |
| CX3CL1 | CX3CR1 | Testicular germ cell tumors | Not shown | [70] |
| HGF 15 | Not shown | Glioblastoma | HGF-MET axis | [71] |
| MIF | CXCR4 | Glioblastoma | Not shown | [72] |
| FGL2 | Not shown | Glioblastoma | Not shown | [73] |
1 C-C-motif chemokine ligand 2; 2 Phosphoinositide 3-kinase; 3 CXC motif chemokine ligand 5; 4 Nuclear factor-kappa B; 5 Colony-stimulating factor-1; 6 Signal transducer and activator of transcription (STAT)3; 7 Transforming growth factor β; 8 Macrophage inhibitory cytokine-1; 9 Lipoxygenase; 10 Vascular epidermal growth factor; 11 Mitogen-activated protein kinase; 12 Olfactomedin like 3; 13 Stromal-cell-derived factor-1b; 14 Periostin; 15 Hepatocyte growth factor.
2.1.1. CSCs Recruit Macrophages through the CC Chemokine Family and the CXC Chemokine Subfamily
The CC chemokine family and the CXC chemokine subfamily are involved in macrophage recruitment in tumors (or CSCs) [74]. CCL2, a CC chemokine, is secreted by CSCs of different tumor types to promote macrophage infiltration. CCL2 overexpression is associated with poor patient prognosis in various tumor types [75] (Table 1). In studies on GBM [38], prostate cancer [76], and bladder cancer [39], CCL2 expression was significantly increased in CSCs compared with non-CSCs, and the macrophage count in the TME was also increased. Another study showed that the blockade of CCL2 binding to its receptor CCR2 on macrophages or depletion of CCL2 decreased monocyte recruitment and prolonged tumor survival in mice [43]. Thus, CCL2 may play a vital role in macrophage recruitment. Studies have attempted to uncover why CCL2 is upregulated in CSCs. In a study of liver CSCs, CCL2 expression was associated with Yes-associated protein, which acts as a transcriptional coactivator and can upregulate CCL2 expression [43]. Furthermore, the upregulation of CCL2 is due to the increase of the stemness-related transcription factor TWIST, and the increased CCL2 secretion promotes the infiltration of M2 macrophages in lung cancer [77]. GBM stem cells (GSCs) have stable epigenetic changes that initiate a myeloid-dependent transcriptional program that expresses myeloid-specific master transcription factors such as interferon regulatory factor (IRF)1 and IRF8 [47]. Together, these findings reveal that CSCs may induce macrophage recruitment by secreting CCL2; however, the underlying mechanism remains unclear.
CCL3, also known as macrophage inflammatory protein-1α, is a ligand for the receptors CCR1 and CCR5 (Figure 1) and promotes macrophage recruitment [78]. CCL3 is secreted in CD14+ bladder CSCs rather than CD14− cancer cells, and macrophage infiltration is also increased in the TME [49]. However, another study determined that CCL3 recruits macrophages to the TME to exert anticancer activities [79]. These results indicate that CSCs can secrete CCL3 to recruit macrophages with antitumor properties in the TME.
Other chemokine members also participate in macrophage recruitment. In a study on GBM, CCL5 expression was significantly higher in GSCs compared with low-grade gliomas and noncancerous tissues, whereas GSCs did not express CCR5 (Figure 1). GSCs may secrete CCL5, which binds to microglial CCR5 and causes microglial infiltration [52,53]. Microglia are known as the macrophages of the nervous system, and their biological properties are similar to those of peripheral macrophages [80]. Notably, CCL5 is produced exclusively by CSCs, highlighting their unique role in macrophage recruitment. Furthermore, the ability to recruit microglia through CCL5 is enhanced when PTEN or FGFR1 is mutated [53]. By contrast, a study reported that CCL8 secreted by CSCs can recruit M1 macrophages and increase the number of CD16 and CD80 macrophages in the TME [57]. However, the study did not explain why CSCs recruited M1 macrophages, which are thought to have antitumor properties. In fact, tumor stromal mesenchymal stem cells (MSCs) in the TME are also involved in macrophage recruitment, and MSCs highly express various chemokines, especially CCL2, which increase the recruitment of monocytes/macrophages to the TME [81]. In conclusion, accumulating evidence indicates that the CC family of chemokines secreted by the CSCs of different cancer types are involved in macrophage recruitment.
Chemokines in the CXC family secreted by CSCs are also involved in macrophage recruitment. CXCL5 expression is higher in CD44+ CSCs compared with non-CSCs [82]. CXCL5 acts as a key downstream mediator of Sox9, which is a biomarker of liver CSCs. Sox9 can bind to the promoter of CXCL5 to induce CXCL5 secretion, and a high CXCL5 level enhances macrophage recruitment in HCC [59]. Furthermore, the CXCL12 level has a significant positive correlation with macrophage infiltration in tumors [60]. CD133+ GCSC can also secrete CXCL12, which binds to CXCR4 (CXCL12 receptor) on macrophages to recruit macrophages to the TME [83]. Similar results were also observed in breast cancer [84]. These data suggest that both the CC and CXC chemokine families secreted by CSCs play a crucial role in macrophage recruitment. Targeting these chemokines may inhibit macrophage recruitment by CSCs, thereby hindering CSC niche formation.
2.1.2. CSCs Recruit Macrophages by Secreting IL-33
CSCs recruit and induce myeloid cells to differentiate into TAMs by releasing IL-33 [85] (Figure 1). IL-33 combined with ST2 on TAMs stimulates TAMs to secrete TGF-β. TGF-β can regulate CSCs through a negative feedback mechanism, activate NRF2-mediated antioxidant responses, and release IL-33, thus promoting macrophage recruitment [44]. This suggests that macrophage recruitment by CSCs is an interactive process. Interruption of the IL-33–TGF-β signaling loop provides a potential therapeutic option for preventing CSC niche formation.
2.1.3. CSCs Recruit Macrophages through Other Types of Secreted Proteins
In addition to chemokines and ILs, CSCs can also secrete various other soluble cellular proteins to promote macrophage recruitment into the TME, such as CSF-1 [86] (Figure 1). Yamashina et al. reported that CSCs stimulated by interferon regulatory factor 5 (IRF5) could activate the IRF5/CSF-1 pathway in non-small-cell lung cancer (NSCLC), thereby increasing CSF-1 secretion and causing an increase in the number of M2 macrophages with CSF-1 receptors [63]. However, GSCs can produce CSF-1 and cytokines, such as TGF-β1 and macrophage inhibitory cytokine (MIC)-1, to recruit surrounding microglia [64]. These results suggest that CSCs promote macrophage recruitment by secreting these cytokines, but what drives CSCs to secrete these soluble cytokines is unclear. Targeting these cytokines may effectively block macrophage recruitment into the TME and prevent macrophages from participating in CSC niche formation.
CSCs can also promote macrophage recruitment by secreting other soluble proteins, including lipoxygenase (LOX), VEGF-A, OLFML3, IRF, Sdf1, and POSTN (Figure 1). In a mouse model of pancreatic cancer, Janakiram et al. demonstrated that LOX can also promote macrophage recruitment [66]. In a model of natural killer T cell loss, stimulation of 5-LOX expression in CSCs resulted in a synchronous increase in the number of M2-type macrophages, suggesting that LOX may be involved in macrophage recruitment [66]. In GBM, binding of the circadian regulator CLOCK and its heterodimeric partner BMAL1 upregulates the transcription of OLFML3, which acts as a chemokine and simultaneously recruits immunosuppressive microglia to the TME to enhance CSC stemness [40]. In the early stage of brain tumors, GSCs, marked by Sox2, were found to promote macrophage/microglia infiltration through the Sdf1b–CXCr4b signaling pathway, and in vivo confocal imaging revealed that macrophages/microglia and CSCs have highly dynamic interactions and do not lead to the phagocytosis of CSCs [41]. In GBM, CSCs secrete more POSTN than non-CSCs to recruit macrophages, and neutralization of POSTN can significantly reduce TAM density [87]. POSTN binds to integrin αvβ₃ receptors on macrophages to increase their recruitment, which can be inhibited by blocking this signaling with the RGD peptide [69]. These results reveal that CSCs promote macrophage recruitment by secreting several soluble proteins. Targeting these pathways can be a new strategy for cancer treatment.
2.2. CSCs Promote Macrophage Polarization in the TME
When macrophages infiltrate the TME, CSC-secreted cytokines can induce the polarization of macrophages into M2 TAMs. These cytokines include CC chemokines, the IL family, prostaglandin E2 (PGE2), WNT1-inducible signaling pathway protein 1 (WISP1), and exosomes, some of which are also involved in macrophage recruitment (Figure 1). Table 2 summarizes the CSC-secreted cytokines and soluble protein molecules that promote macrophage polarization in different tumor types.
Table 2.
CSCs secreted molecules to induce the polarization of macrophages.
| Chemokines Produced by CSCs | Chemokine Receptor | Type of Cancer | Associated Signaling Pathway/Mechanism of Cytokine Secretion | Reference |
|---|---|---|---|---|
| CCL2 | CCR2 | Breast cancer | β-catenin/CCL2 axis | [48] |
| IL-4 | IL-4R | Breast cancer | ERK1/2 1 | [88] |
| IL-6 | IL-6R | Glioblastoma | TLR4 | [89] |
| Triple negative breast cancer | HLF/MCT-1 | [90,91] | ||
| IL-8 | IL-8R | Ovarian cancer | STAT3 | [92] |
| IL-10 | IL-10R | Ovarian cancer | PPARγ 2/NF-κB | [93] |
| IL-13 | IL-13R | Cholangiocarcinoma | Not shown | [94] |
| IL-34 | IL-34R | Cholangiocarcinoma | Not shown | [94] |
| IL-33 | ST-2 | Squamous cell carcinoma | NRF2 | [44] |
| CSF1 | CSF1R | Glioblastoma | Not shown | [64] |
| Non-small cell lung cancer | Oct4 | [95] | ||
| Breast cancer | Not shown | [96] | ||
| CSF2 | CSFR2 | Triple negative breast cancer | NF-κB | [97] |
| Pancreatic cancer | Not shown | [98] | ||
| WISP1 | Integrinα6β1 | Glioblastoma | WNT/β-catenin | [99] |
| TGF-β | TGFBR | Glioblastoma | Not shown | [64] |
| Pancreatic cancer | Not shown | [98] | ||
| Hepatocellular carcinoma | TLR4 | [100] | ||
| MIC-1 | Not shown | Glioblastoma | Not shown | [64] |
| PGE2 3 | EP2 | Ovarian cancer | COX-2 4/PGE2 | [101] |
| Glioblastoma | ARS2 5/MAGL | [102] | ||
| Osteoactivin | Not shown | Cholangiocarcinoma | Not shown | [94] |
| Exosomes | Not shown | Pancreatic cancer | Not shown | [103,104] |
| Glioblastoma | Not shown | [105] | ||
| Esophageal squamous cell carcinoma | Not shown | [106] |
1 Extracellular signal-regulated protein kinases 1 and 2; 2 Peroxisome proliferator-activated receptor-γ; 3 Prostaglandin E2; 4 Cyclo-oxygenase 2; 5 Arsenite-resistance protein 2.
2.2.1. CSCs Induce Macrophage Polarization by Secreting CC Chemokines
Some chemokines secreted by CSCs, including CCL2 and CCL5, can also promote macrophage polarization while recruiting macrophages. For example, breast CSCs (BCSCs) activate the β-catenin pathway to regulate CCL2 expression and promote macrophage polarization to the M2 type [48] (Table 2). In addition, CSCs promote macrophage polarization through the β-catenin/CCL2 axis. However, macrophages secrete CCL2 to maintain the characteristics of BCSCs [48]. Zhuang et al. reported that CCL5 can transform M0 macrophages (inactivated macrophages) into M2 TAMs when cocultured with hepatoma cells; the result is the expression of high levels of IL-10, IL-12, and TNF-α [55]. However, the mechanism by which activated TAMs secrete cytokines via the CCL5–CCR5 pathway remains unclear, thus necessitating further research.
2.2.2. CSCs Promote Macrophage Polarization by Secreting ILs
To date, most studies support the idea that CSCs induce macrophage polarization in the TME by secreting ILs, including IL-6, IL-8, IL-10, IL-13, and IL-34 (Table 2). Weng et al. reported that MCT-1-overexpressing triple-negative breast cancer (TNBC) cells secrete IL-6, which activates monocytes/macrophages to an M2 TAM-like phenotype via the JAK2/STAT3 pathway and that this pathway can be inhibited after neutralization with IL-6 antibodies [90,91]. In liver cancer, M2 polarization of macrophages is also regulated via the IL-6/STAT3 pathway, the inhibition of which causes macrophages to be polarized to M1 [107]. In addition, CSCs may secrete IL-8 to promote macrophage polarization. IL-8 levels are increased when ovarian CSCs (OCSCs) are cocultured with macrophages [92]. OCSC can promote M2 polarization of macrophages via the IL-8/STAT3 signaling pathway. In addition, IL-10 is highly expressed in OCSCs and induces macrophage polarization toward M2 TAMs by activating the PPARγ/NF-κB pathway [93]. These findings suggest that CSCs can induce macrophage polarization in the TME by secreting ILs, but the specific underlying pathways remain unclear.
2.2.3. CSCs Promote Macrophage Polarization through Other Cytokines and Soluble Protein Molecules
The cytokine CSF secreted by CSCs, which promotes macrophage recruitment, can also cause macrophage polarization. In pancreatic cancer, TGF-β1 and CSF-2 secreted by pancreatic CSCs (Pa-CSCs) were reported to be positively correlated with the levels of M2 TAM-related molecules. In addition, the use of TGF-β1 or CSF-2 inhibitors repressed the polarization of M2 TAMs, indicating that Pa-CSCs can promote macrophage polarization to M2 TAMs through TGF-β1 and CSF-2 [98]. High CSF-2 levels can be secreted by TNBC cells in an NF-κB-dependent manner to promote macrophage polarization into M2 macrophages [97]. CSF-1 can also promote macrophage polarization in a glioma model [64]. M1 macrophages promote lung cancer to express octamer 4 (Oct4) to upregulate CSF-1 gene expression, and CSF-1 stimulates the activation of M2 macrophages [95]. Notably, M1 macrophages induce CSF-1 gene expression in CSCs, thereby stimulating M2 TAM activation and extending our understanding of TAMs.
CSCs can also secrete prostaglandin F2 (PGF2), WISP1, and other soluble protein molecules to promote the activation of macrophages in the TME. In GSCs, PGF2, produced via the ARS2–MAGL signaling pathway, can induce the transformation of macrophages to M2 TAMs [102]. In addition, OCSCs secrete PGE2 via the COX-2/PGE2 pathway, which activates Janus kinase (JAK) signaling in M2 macrophages and significantly increases the secretion of IL-10 and CD206+ on M2 macrophages [101]. CSCs may activate the related pathway of M2 macrophage polarization by secreting PGF2. Furthermore, GSCs induce the secretion of WISP1 via the WNT pathway, supporting M2 polarization of macrophages in a paracrine manner. In addition, inhibition of WNT/β-catenin-WISP1 signaling can significantly reduce macrophage polarization [99]. To summarize, CSCs can transform macrophages into TAMs by secreting soluble proteins.
The direct contact of some proteins on CSCs and TAMs can also promote macrophage polarization. CSCs can bind phosphatidylserine to apoptotic cell membranes by binding to receptor tyrosine kinase family protein molecules (Tyro3, Axl, and MerTK) on TAMs using bridging ligand Gas6 and protein S, and post-efferocytosis, macrophages are polarized to an M2-like phenotype [108]. This finding suggests that CSCs are involved in macrophage transformation into TAMs by secreting these proteins in different tumor types. More studies on macrophage transformation into TAMs are warranted to reveal the immunosuppressive mechanisms in malignant tumors, and these pathways will provide reliable ideas for future tumor therapy.
2.2.4. CSCs Polarize Macrophages by Secreting Exosomes
CSCs can also induce macrophage transformation into TAMs by producing exosomes in the TME [103,104]. GSC-derived exosomes can enter monocytes to reconstitute the actin cytoskeleton, thereby inducing their transformation into M2 TAMs [105]. The same result was also observed in esophageal squamous cell carcinoma [106]. In conclusion, CSCs may stimulate macrophage polarization by secreting exosomes and releasing miRNAs; this may provide a direction for the development of new biomarkers.
3. TAMs Enhance the Stemness Characteristics of Tumor Cells
The supporting role of TAMs in CSCs is crucial. After polarization, TAMs actively participate in CSC niche formation and play a crucial role in maintaining the self-renewal capacity of CSCs and tumor initiation. Notably, cytokines produced by TAMs can enable “differentiated” cancer cells to regain the characteristics of CSCs and maintain CSC stemness in tumors. This process of regaining the characteristics of CSCs transpires mainly through epithelial–mesenchymal transition (EMT) [109]. Table 3 summarizes the TAMs that support CSC stemness by secreting cytokines, soluble proteins, and exosomes in different tumor types.
Table 3.
TAMs secreted molecules to enhance the properties of CSCs.
| TAM Type | Factor of TAM Production | Type of Cancer | Associated Signaling Pathway/Mechanism of TAMs on CSCs | Reference |
|---|---|---|---|---|
| M2 | CCL2 | Breast cancer | β-catenin | [48] |
| CCL5 | Prostate cancer | β-catenin/STAT3 | [110] | |
| Glioblastoma | Not shown | [52] | ||
| CCL7 | Ovarian cancer | CCR3/MM9 1 | [111] | |
| CCL8 | Glioblastoma | CCR1/CCR5/ERK1/2 | [112] | |
| Gastric cancer | JAK1/STAT3 | [113] | ||
| CCL17 | Hepatocellular carcinoma | WNT/β-catenin | [114] | |
| CCL22 | Hepatocellular carcinoma | Not shown | [115] | |
| CXCL1 | Breast cancer | Not shown | [116] | |
| CXCL7 | Glioblastoma | Not shown | [73] | |
| CXCL8 | Breast cancer | CXCR2 | [117] | |
| IL-1β | Glioblastoma | p38 MAPK | [118] | |
| IL-6 | Glioblastoma | Not shown | [89] | |
| Ovarian cancer | WNT | [62] | ||
| Hepatocellular carcinoma | STAT3 | [119] | ||
| Breast cancer | STAT3 | [120] | ||
| IL-8 | Ovarian cancer | JAK2/STAT3 | [92] | |
| IL-10 | Ovarian cancer | WNT | [62] | |
| Non-small cell lung cancer | JAK1/STAT1/NF-κB/Notch1 | [121] | ||
| IL-18 | Gastric cancer | Not shown | [122] | |
| IL-35 | Breast cancer | Not shown | [123] | |
| TGF-β | Hepatocellular carcinoma | EMT | [124] | |
| Pancreatic cancer | TGF-β1/smad2/3 axis | [125] | ||
| Squamous cell carcinoma | NRF2 | [44] | ||
| Lung cancer | Not shown | [126] | ||
| Breast cancer | EMT | [127] | ||
| TNF-α | Clear cell renal cell carcinoma | NF-κB | [128] | |
| Chordoma | PI3K/AKT | [129] | ||
| EGF 1 | Breast cancer | EGFR/STAT3/Sox2 | [130] | |
| GPNMB 2 | Breast cancer | PI3K/AKT/mTOR & β-catenin/MAPKs/AMPK/Src | [131] | |
| PTN 3 | Lymphoma | PTPRZ1/β-catenin | [132] | |
| Glioblastoma | PTN-PTPRZ1 | [133] | ||
| IGF 4 | Thyroid cancer | PI3K/AKT/mTOR | [134] | |
| PDGF-BB 5 | Breast cancer | Not shown | [135] | |
| hCAP-18 | Pancreatic ductal adenocarcinoma | Not shown | [136] | |
| S100A9 6 | Hepatocellular carcinoma | NF-κB | [137] | |
| HMGB1 7 | Non-small cell lung cancer | NF-κB | [138] | |
| MFG-E8 8 | Colorectal cancer | STAT3/Sonic Hedgehog | [139] | |
| extracellular vesicles | Pancreatic cancer | KLF3 | [140] | |
| M1 | IL-6 | Oral squamous cell carcinoma | JAK/STAT3 | [141] |
| IL-12 | Glioblastoma | Not shown | [142] | |
| TNF-α | Lung cancer | Not shown | [143] | |
| Oral cancer | Not shown | [144] |
1 Epidermal growth factor; 2 Glycoprotein nonmetastatic B; 3 Pleiotrophin; 4 Inslin-like growth factor; 5 Platelet-derived growth factor-BB; 6 Matrix metalloproteinase 9; 7 High mobility group box 1; 8 Milk-fat globule-epidermal growth factor-VIII.
3.1. CC and CXC Chemokines Are Secreted by TAMs to Maintain Tumor Cell Stemness Characteristics
Notably, the current study shows that TAMs maintain the characteristics of CSCs by secreting CC chemokines, such as CCL2, CCL5, CCL7, CCL8, and CCL17 (Table 3) (Figure 1). As described in Section 2.2.1, β-catenin secretion by BCSCs directly regulates CCL2 expression, promoting macrophage recruitment and TAM polarization. TAMs also express CCL2 and promote the maintenance of BCSC properties [48]. Similarly, CCL5, a chemokine that induces the recruitment and activation of macrophages, has also been found to be secreted by TAMs to promote CSC properties. In GBM, TAMs secrete CCL5, which enhances GSC stemness and tumor invasion via the CCL5–CCR5 pathway [52]. CCL5 activates β-catenin/STAT3 signaling to promote the self-renewal and tumor metastasis of prostate CSCs (PCSCs), and its knockdown significantly inhibits the metastasis of prostate cancer and the self-renewal capacity of PCSCs [110]. TAMs secrete CCL7, which binds to CCR3 to activate MMP9 expression in ovarian cancer cells, significantly increasing their invasiveness [111]. In addition, TAMs secrete large amounts of CCL8 in the TME, binding to receptors CCR1 and CCR5 on glioma cells, which activates ERK1/2 and can induce the aggressive and stemness characteristics of GBM [112]. However, TAM-derived CCL-17, CCL-22, TGF-β, IL-10, arginase 1, and galectin-3 can significantly reduce tumor growth and metastatic sites, but the specific mechanism remains unclear [145]. In summary, TAMs can secrete CC chemokines to support the growth of CSCs and targeting these chemokines may effectively block the positive feedback loop-like association between CSCs and TAMs.
TAMs also secrete CXC chemokines (CXCL1 and CXCL7) that support CSC-like characteristics (Table 3). M2 TAMs secrete CXCL1 in breast cancer and promote tumor metastasis. The use of CXCL1 promoter inhibitors reduced the migration of breast cancer cells and the number of BCSCs, suggesting that TAMs may support BCSC survival by secreting CXCL1 [116]. In addition, CXCL7 secretion was significantly positively correlated with higher numbers of TAMs and the enhanced stemness of GBM. FGL2 produced by GBM induces macrophage recruitment and promotes macrophages to exhibit TAM-like properties. Activated TAMs secrete CXCL7 via the CD16/SYK/PI3K/HIF1α pathway, thereby enhancing the stem-like characteristics of GBM cells [73]. In conclusion, TAMs secrete CC and CXC chemokines to maintain CSC stemness. Therefore, blocking the secretion of these chemokines can block CSC–TAM interactions, thus effectively inhibiting tumor growth and metastasis.
3.2. TAMs Support the Stemness of Tumor Cells by Secreting ILs
TAMs can also promote the self-renewal and metastasis of CSCs by secreting ILs. IL-6, a common IL factor, plays a vital role in promoting tumorigenesis. Activation of the TLR4 signaling pathway on microglia promotes the secretion of IL-6, and IL-6 can support GSC in regulating tumor growth [89]. In human HCC samples, the same results were observed. TAMs activated STAT3 to produce IL-6 and induced an increase in the number of CD44+ hepatoma cells [119]. Another study reported that M2 TAMs could also secrete IL-6 through the WNT ligand WNT5B, inducing the enrichment of CSCs in ovarian cancer [62]. Moreover, CSC-derived WNT ligands can drive the activation of M2 macrophages. Thus, a positive feedback loop is formed between CSCs and M2 TAMs through WNTs. In addition, M1 macrophages can secrete IL-6. In oral squamous cell carcinoma cells, M1 TAMs can promote EMT and induce the CSC phenotype by upregulating the expression of MME and MMP14. However, M1 TAMs secrete IL-6, increasing CSC stemness by activating the JAK/STAT3 pathway [141]. These findings indicate that TAMs secrete IL-6 through the TLR4 signaling pathway and STAT3 pathway and that IL-6 promotes the chemoresistance and invasiveness of CSCs by activating the JAK/STAT3 pathway and WNT pathway in CSCs, forming a CSC–TAM–CSC positive feedback loop.
TAMs can also secrete IL-10, IL-8, IL-12, and IL-35, which can promote the stemness characteristics of tumor cells (Figure 1). In NSCLC, IL-10 is derived from TAMs via the JAK1/STAT1/NF-κB/Notch1 signaling pathway and induces CSC-like properties in NSCLC cells. Blocking IL-10/JAK1 signaling significantly inhibits TAM-mediated CSC-related genes, such as Sox2, Oct4, and Nanog [121]. This pathway may provide a new potential therapeutic target for the treatment of NSCLC. In addition, IL-8 secreted by TAMs in ovarian cancer via the STAT3 pathway can also increase the stemness characteristics of OCSC [146]. Notably, in GBM, GSCs express higher amounts of active spin cleavage-like products (ALPs) than non-GSCs. ALPs promote secretion of IL-12 by M1 TAMs, which can enhance the activity of GSCs and drive the formation of the GSC niche [142]. In addition, the expression of RON signaling in TAMs induces the secretion of IL-35, which supports the self-renewal and metastasis of CSCs; however, the specific mechanism remains under investigation [123]. Together, these findings reveal that TAMs secrete ILs to promote the expression of CSC genes in various cancer models. In addition, M1 macrophages can secrete IL-6 and IL-12, inducing the expression of CSC genes. Studies continue to find more IL factors secreted by TAMs, and they play a crucial role in the maintenance of CSC stemness, but further research is required to clarify the precise role of TAMs in the maintenance of CSC stemness.
3.3. Other Cytokines and Soluble Protein Molecules Secreted by TAMs to Promote the Stemness of Tumor Cells
TAMs can also directly support tumor stemness by secreting cytokines, such as TGF-β1, FGF, and PGE2 (Figure 1). TAMs secrete more TGF-β1 than macrophages of other phenotypes, and TGF-β1 activates EMT and acquires CSC-like properties [124]. Furthermore, FGF expression in macrophages significantly increases the stemness of GBM cells. FGF1, the most abundant FGF in the FGF family, was upregulated in TAMs cocultured with GBM and enhanced the stemness characteristics of GMB [147]. In addition, in a breast cancer model, TAMs were noted to establish a paracrine EGFR/STAT3/Sox2 signaling pathway and increase Sox2, Oct4, Nanog, AbcG2, and Sca-1 gene expression [130]. TAMs can also secrete another cytokine, PGE2. Overexpression of IL-33 by colon cancer cells induces macrophage recruitment and stimulates them to produce PGE2, thereby supporting the stemness of colon cancer cells [148]. These findings show that TAMs are crucial in supporting CSCs and targeting the cytokines that facilitate this process can block tumor cells from acquiring or maintaining stemness characteristics.
TAMs can also support CSCs by secreting soluble protein molecules, such as GPNMB, TNF-α, pleiotrophin (PTN), growth factor-BB, immunomodulatory cationic antimicrobial peptide 18/LL-37 (hCAP-18/LL-37), and S100A9. GPNMB is a glycoprotein that is highly expressed in macrophages and microglia and promotes inflammation. However, in mouse tumor models, M2 TAMs preferentially express soluble GPNMB and bind to the CD44 receptor on tumor cells to trigger CSC proliferation [131]. In addition, in clear-cell renal cell carcinoma (ccRCC), TAMs secrete TNF-α, which may upregulate CD44 expression through NF-κB signaling and enhance CSC stemness [128]. PTN is also a soluble protein molecule secreted by TAMs, which is significantly positively correlated with the expression of PTPRZ1 (PTN receptor) in lymphoma. PTN induces the generation of lymphoma stem cells via the β-catenin pathway [132]. In addition, GSCs can upregulate the expression of PTPRZ1 and activate PTPRZ1 signaling by binding to PTN secreted by M2 TAMs to maintain GSC stemness [133]. Together, these results indicate that PTN secreted by TAMs plays a crucial role in the maintenance of CSC stemness. A recent study revealed that TAMs can also secrete IGF-1 and IGF-2 to promote PI3K/AKT/mTOR signaling and increase the stemness characteristics of thyroid cancer [134]. In pancreatic ductal adenocarcinoma, TAMs secrete hCAP-18/LL-37, creating a paracrine niche beneficial to CSCs [136]. S100A9, a secreted protein associated with the TME, is significantly upregulated in TAMs [149]. It activates the NF-κB signaling pathway in a Ca2+-dependent manner to enhance the stem cell-like properties of hepatoma cells. Simultaneously, treatment of tumor cells with S100A9 significantly increased CCL2 expression, recruited and polarized more macrophages, and formed a CSC–TAM positive feedback loop [137]. These findings suggest that TAMs produce abundant protein molecules in the TME, and these abundant proteins promote the generation and maintenance of CSCs by activating CSC-related pathways.
3.4. TAMs Promote Tumor Cell Stemness through Surface Protein Molecules
TAMs can also directly bind to CSCs to support CSC stemness by expressing corresponding protein molecules on the cell surface, including CD90, EphA4, and SIRP1α. In breast cancer, EMT programmatically upregulates the expression of CD90 and EphA4. CD90 directly binds to receptors on TAMs to mediate physical effects and activate the NF-κB pathway in CSCs and induce the production of cytokines to maintain stemness [21] (Figure 1). In addition, CD47 expression was observed to be elevated in CD133+ lung cancer cells. CD47 also binds to SIRP1α on macrophages, thus inhibiting phagocytosis [20] and supporting immune escape. In conclusion, the surface protein molecules expressed on the surface of TAM-CSC, after sufficient contact, play a significant role in immunosuppression; these surface molecules can be used as immune checkpoints to improve the efficacy of immunotherapy.
3.5. TAMs Promote Tumor Cell Stemness by Secreting Extracellular Vesicles
TAM-derived extracellular vesicles can also promote tumor invasion. The expression of microRNA-21-5p is upregulated in M2 macrophage-derived extracellular vesicles and combines with KLF3 to induce Pa-CSC differentiation [140]. Accordingly, nanocarriers inspired by M2 macrophage microvesicles can penetrate tumor tissue to improve the accessibility of CSCs [150]. Therefore, the use of extracellular vesicles can produce an effective tumor treatment effect.
4. Therapeutic Strategies Targeting CSCs and TAMs
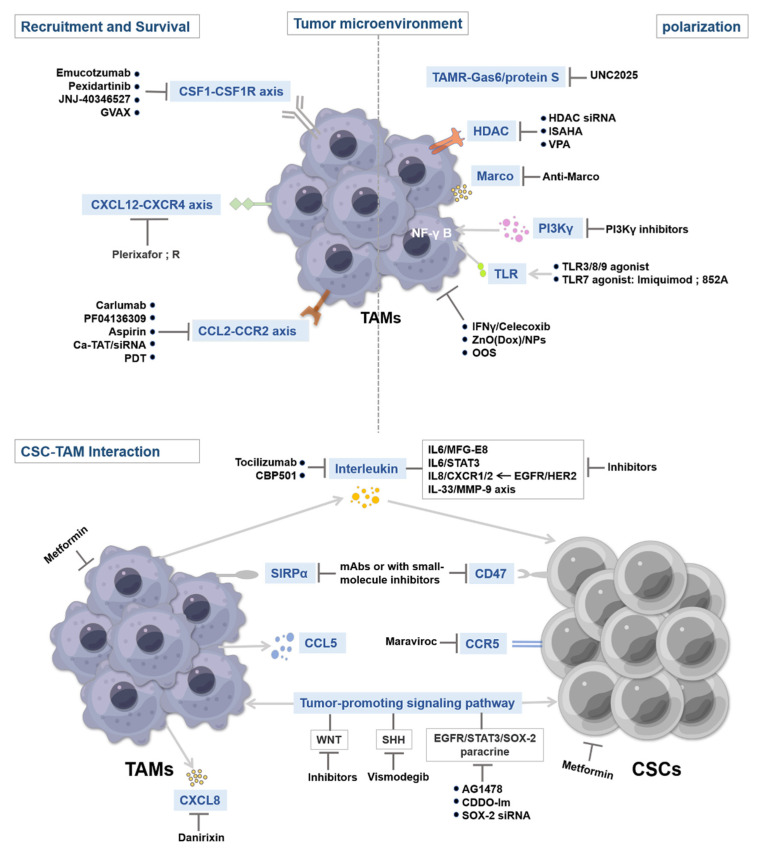
Macrophages constitute the bulk of immune cells. They have tumor-promoting and immunosuppressive roles in cancer [151,152]. For example, the number of TAMs in human tumors is positively correlated with tumor grade and negatively correlated with patient survival in cancers such as GBM, lymphoma, breast, and pancreatic cancers [153,154,155,156,157]. However, when TAMs lose immunosuppressive signals or undergo repolarization, survival is increased in mice and in patients with different types of cancer [158]. In recent tumor treatment studies, macrophage therapy has received great attention due to the contribution of macrophages to CSC growth, metastasis, and promotion of the CSC niche. Several common immunotherapeutic strategies include depleting monocytes to prevent TAM production and recruitment, inducing macrophage polarization toward the M1 phenotype and promoting phagocytosis, targeting TAMs supporting the CSC niche, and suppressing CSC occurrence and metastasis [159]. These strategies are now being tested to enhance antitumor immunity, in conjunction with either conventional chemo-radiotherapy or T cell–mediated immunotherapy [159]. We discuss some potential pathways that can translate into therapeutic strategies that interfere with the interaction between TAMs and CSCs, inhibiting TAM-mediated immunosuppression. Figure 2 presents several key targets that regulate CSC and TAM properties during tumor progression. In addition, we also list the relevant agents targeting TAM in ongoing clinical trials (Table 4).
Figure 2.
Targeting strategies of CSCs and TAMs in cancer therapy. Key pathways regulating CSC and TAM properties during tumorigenesis and progression have been identified, including TAM survival, recruitment, polarization, and phagocytosis and maintenance of CSC stemness. Targeting these pathways can help regulate CSC properties and TAMs and interfere with the interaction between them, thereby inhibiting the development of CSCs and providing more effective therapeutic strategies.
Table 4.
Drugs targeting the TAM-related pathways in clinical trials.
| Targets | Drugs | Cancer Type | Trial Phase | ClinicalTrials.Gov ID |
|---|---|---|---|---|
| CSF-1/CSF-1R | CM082 | Small cell lung cancer | II | NCT03904719 |
| ABSK021 | Advanced solid tumor | I | NCT04192344 | |
| AMB-051 | Tenosynovial giant cell tumor | II | NCT04731675 | |
| SNDX-6532 | Unresectable intrahepatic cholangiocarcinoma | II | NCT04301778 | |
| Chiauranib | Triple-negative breast cancer | II | NCT05336721 | |
| Small cell lung cancer | III | NCT04830813 | ||
| NMS-03592088 | Acute myeloid leukemia Chronic myelomonocytic leukemia |
I/II | NCT03922100 | |
| Pexidartinib | Tenosynovial giant cell tumor | III | NCT04488822 | |
| Q702 | Advanced solid cancer | I | NCT04648254 | |
| Surufatinib | Neuroendocrine tumor Non-hematologic malignancy |
I/II | NCT05077384 | |
| Hepatocellular carcinoma | II | NCT05171439 | ||
| Refractory metastatic digestive system carcinoma Primary peritoneal cancer |
II | NCT05030246 | ||
| TPX-0022 | Advanced metastatic solid tumor |
I/II | NCT03993873 | |
| Vorolanib | Refractory thoracic tumor | I/II | NCT03583086 | |
| Metastatic renal cell cancer | III | NCT03095040 | ||
| CCL2/CCR2 | BMS-813160 | Locally advanced pancreatic ductal Adenocarcinoma Pancreatic ductal adenocarcinoma |
I/II | NCT03767582 |
| CCL5/CCR5 | Maraviroc | Colorectal cancer metastatic | I | NCT04721301 |
| Leronlimab | Solid tumor | II | NCT04504942 | |
| POL6326 | Advanced breast cancer | I/II | NCT04826016 | |
| Metastatic breast cancer | III | NCT03786094 | ||
| Motixafortide | Pancreatic cancer | II | NCT04543071 | |
| CD40/CD40L | ABBV-368 | Advanced solid tumors | I | NCT04196283 |
| Triple-negative breast cancer Non small cell lung cancer Metastatic solid tumor |
I | NCT03893955 | ||
| ABBV-927 | Advanced solid tumor Triple-negative breast cancer Non-small cell lung cancer Metastatic solid tumor |
I | NCT03893955 | |
| Pancreatic cancer | II | NCT04807972 | ||
| CDX-1140 | Pancreatic cancer | II | NCT04536077 | |
| Solid tumor Diffuse large B-cell lymphoma Mantle cell lymphoma |
I | NCT03329950 | ||
| Ovarian clear cell adenocarcinoma | II | NCT05231122 | ||
| Melanoma | I/II | NCT04364230 | ||
| Metastatic triple negative breast cancer | I | NCT05029999 | ||
| Malignant epithelial neoplasm | I | NCT04520711 | ||
| GEN-1042 | Malignant solid tumor Non-small cell lung cancer Colorectal cancer Melanoma Head and Neck squamous cell carcinoma Pancreatic ductal adenocarcinoma |
I/II | NCT04083599 | |
| Mitazalimab | Metastatic pancreatic ductal adenocarcinoma | I/II | NCT04888312 | |
| NG-350A | Epithelial tumor Metastatic cancer |
I | NCT05165433 | |
| Selicrelumab | Colorectal cancer | I/II | NCT03555149 | |
| Triple-negative breast cancer | I/II | NCT03424005 | ||
| Pancreatic adenocarcinoma | I/II | NCT03193190 | ||
| YH-003 | Advanced solid tumor | I/II | NCT04481009 | |
| Melanoma Pancreatic ductal adenocarcinoma |
II | NCT05031494 | ||
| SL-172154 | Ovarian cancer Fallopian tube cancer Primary peritoneal carcinoma |
I | NCT04406623 | |
| Cutaneous squamous cell carcinoma Head and Neck squamous cell carcinoma |
I | NCT04502888 | ||
| Acute myeloid leukemia | I | NCT05275439 | ||
| BDB-001 | Solid tumor | II | NCT04819373 | |
| Pancreatic cancer Virus-associated tumor Non-small cell lung cancer Melanoma Bladder cancer Triple-negative breast cancer |
II | NCT03915678 | ||
| BDC-1001 | HER2 positive solid tumor | I/II | NCT04278144 | |
| BNT-411 | Extensive-stage small cell lung cancer | I/II | NCT04101357 | |
| DSP-0509 | Neoplasm | I/II | NCT03416335 | |
| Imiquimod | Metastatic melanoma | I | NCT03276832 | |
| Oral cancer | I | NCT04883645 | ||
| Squamous cell carcinoma | I | NCT03370406 | ||
| Resiquimod | Locally advanced solid tumor Metastatic solid tumor |
I/II | NCT04799054 | |
| Anaplastic astrocytoma | II | NCT01204684 | ||
| SBT-6050 | HER2 positive solid tumor | I | NCT04460456 | |
| TLR9 | AST008 | Advanced or Metastatic merkel cell carcinoma | I/II | NCT03684785 |
| CMP-001 | Head and Neck squamous cell carcinoma | II | NCT04633278 | |
| Metastatic melanoma | II | NCT04698187 | ||
| Merkel cell carcinoma | II | NCT04916002 | ||
| Malignant colorectal neoplasm | I | NCT03507699 | ||
| Advanced malignancy Non-small cell lung cancer Ovarian cancer Urothelial cancer Solid tumor |
III | NCT05059522 | ||
| Tilsotolimod | Advanced solid tumor | I | NCT04196283 | |
| Malignant melanoma | II | NCT04126876 | ||
| SD-101 | Metastatic uveal melanoma in the Liver | I | NCT04935229 | |
| Hepatocellular carcinoma Intrahepatic cholangiocarcinoma |
I/II | NCT05220722 | ||
| B-Cell Non-Hodgkin lymphoma | I | NCT03410901 | ||
| Metastatic pancreatic adenocarcinoma | I | NCT04050085 | ||
| TLR3 | Poly-ICLC | Hepatocellular carcinoma | I | NCT05281926 |
| Glioblastoma multiforme | I/II | NCT03665545 | ||
| Rintatolimod | Hematopoietic and lymphoid cell neoplasm | I/II | NCT04379518 | |
| Prostate adenocarcinoma | II | NCT03899987 | ||
| PI3Kγ signal pathway | Copanlisib | Non-small cell lung cancer | I | NCT04895579 |
| Diffuse large B-cell lymphoma | II | NCT04433182 | ||
| Refractory/Recurrent primary central system lymphoma | I/II | NCT03581942 | ||
| Follicular lymphoma Endometrial cancer |
II | NCT04750941 | ||
| Mantle cell lymphoma | I/II | NCT03877055 | ||
| Head and Neck squamous cell carcinoma Hepatocellular carcinoma |
I | NCT03735628 | ||
| Duvelisib | Non-Hodgkin lymphoma | I | NCT05065866 | |
| Unresectable melanoma | I/II | NCT04688658 | ||
| Recurrent diffuse large B-Cell lymphoma | I | NCT04890236 | ||
| Eganelisib | Bladder cancer Urothelial carcinoma |
II | NCT03980041 | |
| Non-small cell lung cancer Melanoma Head and Neck squamous cell cancer Triple-negative breast cancer Adrenocortical carcinoma Mesothelioma High-circulating myeloid-derived suppressor cells |
I | NCT02637531 | ||
| Breast cancer Renal cell carcinoma |
II | NCT03961698 | ||
| Gedatolisib | HER2-positive breast cancer Metastatic breast cancer |
II | NCT03698383 | |
| Triple-negative breast cancer | I/II | NCT03911973 | ||
| Squamous cell lung cancer Solid tumor Head and Neck cancer Pancreatic cancer |
I | NCT03065062 | ||
| Tenalisib | Locally advanced breast cancer Metastatic breast cancer |
II | NCT05021900 | |
| CD47/SIRPα pathway | ALX-148 | Microsatellite stable metastatic colorectal cancer | II | NCT05167409 |
| Aggressive B-Cell Non-Hodgkin lymphoma | I/II | NCT05025800 | ||
| Head and Neck squamous cell carcinoma | II | NCT04675294 | ||
| Acute myeloid leukemia | I/II | NCT04755244 | ||
| Gastroesophageal junction adenocarcinoma | II/III | NCT05002127 | ||
| Non-Hodgkin lymphoma | I | NCT03013218 | ||
| AO-176 | Multiple myeloma | I/II | NCT04445701 | |
| Solid tumor | I/II | NCT03834948 | ||
| HX-009 | Advanced solid tumor | II | NCT04886271 | |
| Relapsed/Refractory lymphoma | I/II | NCT05189093 | ||
| IBI-188 | Acute myeloid leukemia | I/II | NCT04485052 | |
| Lung adenocarcinoma Osteosarcoma |
I | NCT04861948 | ||
| IBI-322 | Advanced malignant tumor Lymphoma |
I | NCT04338659 | |
| Non-small cell lung cancer | II | NCT05296278 | ||
| Hematologic malignancy | I | NCT04795128 | ||
| Advanced malignancy | I | NCT04328831 | ||
| Myeloid tumor | I | NCT05148442 | ||
| IMC-002 | Advanced cancer | I | NCT05276310 | |
| Solid tumor Lymphoma |
I | NCT04306224 | ||
| IMM-01 | Acute myeloid leukemia | I/II | NCT05140811 | |
| IMM-0306 | B-cell non-Hodgkin’s lymphoma | I | NCT04746131 | |
| Lemzoparlimab | Acute myeloid leukemia Myelodysplastic syndrome |
I | NCT04912063 | |
| Multiple myeloma | I | NCT04895410 | ||
| Magrolimab | Malignant brain tumor | I | NCT05169944 | |
| Recurrent neuroblastoma | I | NCT04751383 | ||
| Solid tumor | II | NCT04827576 | ||
| Recurrent acute myeloid leukemia | I/II | NCT04435691 | ||
| Metastatic colorectal cancer | II | NCT05330429 | ||
| Multiple myeloma | II | NCT04892446 | ||
| Head and neck squamous cell carcinoma | II | NCT04854499 | ||
| Metastatic colorectal cancer | II | NCT05330429 | ||
| Multiple myeloma | II | NCT04892446 | ||
| Urothelial carcinoma Bladder cancer |
I/II | NCT03869190 | ||
| RRx-001 | Central nervous system neoplasm | I | NCT04525014 | |
| Small cell lung carcinoma | III | NCT03699956 | ||
| Small cell carcinoma Non-small cell lung carcinoma Neuroendocrine tumor Ovarian epithelial cancer |
II | NCT02489903 | ||
| TTI-622 | Multiple myeloma | I | NCT05139225 | |
| Fallopian tube cancer Primary peritoneal carcinoma |
I/II | NCT05261490 | ||
| Acute myeloid leukemia Diffuse large B-Cell lymphoma |
I | NCT03530683 | ||
| STING pathway | SNX-281 | Advanced solid tumor Advanced lymphoma |
I | NCT04609579 |
| BMS-986301 | Advanced solid cancer | I | NCT03956680 | |
| GSK-3745417 | Neoplasm | I | NCT03843359 | |
| E−7766 | Lymphoma Advanced solid tumor |
I | NCT04144140 | |
| SYNB-1891 | Metastatic solid neoplasm lymphoma |
I | NCT04167137 | |
| TAK-676 | Non-small cell lung carcinoma Triple-negative breast neoplasm Head and Neck squamous cell carcinoma |
I | NCT04879849 | |
| Solid neoplasm | I | NCT04420884 | ||
| MK-2118 | Lymphoma | I | NCT03249792 | |
| IMSA101 | Solid tumor | I/II | NCT04020185 | |
| CDK-002 | Advanced solid tumor | I/II | NCT04592484 | |
| MK-1454 | Head and neck squamous cell carcinoma | II | NCT04220866 | |
| NOX66 | Late-stage prostate cancer | I | NCT03307629 | |
| Metastatic soft-tissue sarcoma | I | NCT05100628 | ||
| Metastatic castration-resistant prostate cancer | I/II | NCT04957290 | ||
| SB-11285 | Melanoma Head and neck squamous cell carcinoma |
I | NCT04096638 |
4.1. Targeting Tumor-Associated Macrophage Recruitment and Survival
TAMs mainly originate from monocyte precursor cells in the circulation system. One strategy to deplete TAMs is to block the replenishment of TAMs through monocyte recruitment or by targeting M2 macrophage markers to specifically clear TAMs from the TME (Figure 2).
4.1.1. CSF1–CSF1R Inhibitors
Blocking the CSF1–CSF1R signaling pathway can inhibit macrophage recruitment to the CSC niche (Figure 2). The CSF1–CSF1R axis can promote TAM differentiation, survival, and recruitment [160]. Blockade of this signaling pathway can greatly reduce the number of TAMs or repolarize M2 macrophages to M1 macrophages in the TME [161,162,163]. Studies have reported that blockade of the CSF1–CSF1R axis improved the effects of immunotherapy [164,165]. Emactuzumab, a monoclonal antibody to CSF1, blocks the activation of this signaling pathway by inhibiting CSF1–CSF1R dimerization. Emactuzumab treatment can reduce the number of F4/80+ TAMs in CSC niches and increase the ratio of CD8+/CD4+ T cells, thereby improving antitumor immune function. Emactuzumab combined with paclitaxel can significantly reduce the number of CSF1R+ TAMs and effectively inhibit the development of advanced solid tumor CSCs [166]. Pexidartinib, an inhibitor of tyrosine kinases on CSF1R, can inhibit the activation of TAMs to block their tumor-promoting effects, thereby reducing the CSC count in the TME. Pexidartinib has been approved by the FDA as an alternative to surgery for tenosynovial giant cell tumor [167,168,169]. The use of monoclonal antibodies or small-molecule inhibitors that target the CSF1–CSF1R signaling pathway can successfully reduce the number of TAMs in the CSC niche and increase the number of infiltrating T cells, thereby enhancing antitumor immunity (Figure 2) [160].
In addition, the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene-transduced vaccine (GVAX), a cell-based tumor vaccine transduced by the GM-CSF gene, can be used to block the CSF2–CSF2R signaling pathway, which inhibits TAM activation [170]. Following GVAX inoculation, sustained local release of GM-CSF significantly increased levels of M1 macrophages, dendritic cell populations, and CD8+ T cells, exerting antitumor immunity effects [170]. In addition, the IL-2 vaccine can also induce specific antitumor immunity in mice, and the combination of GVAX and IL-2 vaccine was noted to have better antitumor effects than the single treatment [171]. These results suggest that GVAX can induce strong tumor immunity and can be used in tumor immunotherapy.
4.1.2. CCL2–CCR2 Inhibitors
The migration and recruitment of monocytes from bone marrow to the CSC niche occurs mainly via the CCL2–CCR2 signaling pathway. Therefore, targeting the CCL2–CCR2 axis effectively inhibits the number of invasive TAMs in the CSC niche [172,173] and synergistically improves the efficacy of chemo-radiotherapy and immunotherapy (Figure 2) [174]. TAM activity can also be inhibited by monoclonal antibodies or small-molecule inhibitors targeting CCL2 or CCR2. Carlumab and PF04136309 play a key role in targeting the CCL2–CCR2 signaling pathway. Carlumab is a monoclonal antibody with a high binding ability to CCL2, which can reduce the number of CD68+ TAMs in the CSC niche, increase the phagocytic ability of macrophages, and effectively control the occurrence and metastasis of CSCs [175,176]. PF04136309, a CCR2 antagonist, inhibits the metastatic infiltration of CCR2+ macrophages and reduces the proportion of TAMs in the CSC niche [177]. In addition, in esophageal cancer cells, aspirin can reduce the recruitment of M2 macrophages, the expression of CSC marker genes CD90 and Nanog, and the ability of CSCs to form spheroids by inhibiting CCL2 expression on TAMs [178].
The combined application of siRNA and drugs to block the CCL2/CCR2–STAT3 axis can inhibit the activity of TAMs in the CSC niche, thus enhancing the phagocytic activity of macrophages and inhibiting tumor growth and metastasis [179]. In addition, siRNA complexed with TAT cell-penetrating peptide (Ca-TAT) can silence genes more effectively than traditional antibody neutralization [75].
Low-dose photodynamic therapy can significantly inhibit CCL2 secretion in MSCs, possibly limiting the infiltration of precancerous macrophages [180]. However, whether it can inhibit CSC secretion of CCL2 remains to be further investigated.
4.1.3. CXCL12–CXCR4 Inhibitors
The CXCL12–CXCR4 signaling pathway is associated with macrophage recruitment, and its blockade in combination with traditional therapy has shown antitumor efficacy (Figure 2) [181]. Some small-molecule antagonists against the CXCL12–CXCR4 axis can be used for antitumor therapy, such as small peptide CXCR4 antagonists, nonpeptide CXCR4 antagonists, anti-CXCR4 antibodies, modified CXCL12 agonists, and antagonists [182,183]. Plerixafor is a CXCR4 antagonist that inhibits CXCL12–CXCR4-mediated chemotaxis and reduces macrophage recruitment in CSC niches. CXCR4 is highly expressed in most CSCs, and the combination of nonpeptide CXCR4 antagonists with etoposide and cisplatin reduces CSC proliferation and inhibits tumor growth [184]. In addition, highly diffused CD133+ CXCR4+ cells (metastasis promoter cells, MICs) can trigger distant metastasis and resist conventional chemotherapy. A new CXCR4 inhibitory peptide can prevent the expression of CD73, CD38, and IL-10 by MICs, thus inhibiting macrophage transformation into TAMs and rescuing the antitumor cytotoxic effects of T cells [185].
However, these target molecules for TAM-targeted therapy may exist in cell populations other than TAMs. For example, CCR2 and CXCR4 are also expressed on lymphocytes [186,187,188]. Simultaneous targeting of these molecules may lead to altered functions of these immune cells as well as various other complications; therefore, future studies should attempt to identify more specific targets.
4.2. Targeting TAM Activation
Another strategy is to reprogram macrophages to repolarize toward the M1 antitumor phenotype from the M2 tumor-promoting phenotype and enhance macrophage phagocytosis to produce antitumor effects (Figure 2).
4.2.1. TAMR Inhibitors
TAMR, a collective term for Tyro3, Axl, and MerTK, is a group of tyrosine kinase receptors that are ubiquitously expressed on CSCs and various immune cells. Axl and MERTK are overexpressed in CSCs, which can promote M2 polarization of macrophages [189,190,191]. The interaction of TAMR with ligands Gas6 and protein S can stimulate M2 polarization, promote the production of immunosuppressive cytokines, prevent the activation of immune T cells, limit the inflammatory response, and favor CSC survival [192]. Therefore, blocking the TAMR signaling pathway may effectively inhibit the activities of TAMs and CSCs (Figure 2). Researchers have discovered that UNC2025, a small-molecule inhibitor of MERTK, can inhibit macrophage transformation into TAMs, increase the phagocytosis of M1 macrophages, and reduce the CSC count [193].
4.2.2. Anti-MARCO Antibody
MARCO, also known as SCARA2, is a macrophage receptor with a collagenous structure that drives the transformation of macrophages into TAMs. Macrophages expressing MARCO represent a subset of macrophages with a protumor and anti-inflammatory immunosuppressive phenotype [194,195]. Therefore, targeting MARCO may effectively inhibit TAM activation (Figure 2). In breast and colon cancer and melanoma models, the use of anti-MARCO antibodies can reprogram TAMs toward the M1 phenotype, reduce the proportion of TAMs in the CSC niche, increase tumor immunogenicity, and improve the efficacy of antitumor immunotherapy [194,196].
4.2.3. Toll-Like Receptors and Stimulator of Interferon Gene Agonists
Toll-like receptors (TLRs) on macrophages can trigger TAM repolarization. TLRs can bind to some endogenous molecules, participate in antivirus and antitumor regulation by activating NF-κB, and produce various immune-stimulating cytokines, including type I IFN, which increase the expression of MHC-I, promote the repolarization of TAMs to the M1 phenotype, and enhance the specific recognition of CSCs by the immune system [197,198]. Encouraging results have been obtained within the TLR3, TLR7, TLR8, and TLR9 agonists in mouse tumor models. Among these, the TLR7 agonists imiquimod and 852A have shown potential antitumor ability and durable therapeutic effects in preclinical trials [199]. Another key factor regulating innate immunity is the stimulator of interferon genes (STING), which also leads to the production of immune-stimulating cytokines [200]. Activation of TLRs and STING can induce good antitumor effects in the body [201,202,203]. On the basis of these results, TLR and STING agonists are being used in CSC treatment trials in combination with other chemotherapy and immunotherapies (Figure 2) [202,203].
4.2.4. Others
PI3Kγ signaling can promote the immunosuppressive activity of TAMs. In melanoma, lung, and pancreatic cancer cell models, the blockade of PI3Kγ expression in TAMs activates NF-κB, which promotes the immunostimulatory transcriptional program, rescues CD8+ T cell cytotoxicity, and promotes the regression of CSCs and prolongs survival in mouse models in combination with immune checkpoint inhibitor therapy [151,158].
TAMs can express histone deacetylases (HDACs), and HDAC inhibitors can perform epigenetic reprogramming of macrophages, repolarizing them toward the M1 phenotype, and induce T cell toxicity [204]. Inhibition of HDAC2 expression in TAMs through siRNA knockdown or by using pharmacological inhibitors (ISAHA and VPA) can induce TAM repolarization, activate the antitumor phenotype of macrophages, help T-lymphocyte responses, and increase the sensitivity of CSCs to chemotherapy and immune checkpoint inhibition [204]. Furthermore, the histone methyltransferase EZH2 utilizes a methylation modification to inhibit miR-454-3p and promotes the m6A modification of PTEN to polarize macrophages toward the M2 phenotype. Of note, combined treatment with EZH2 inhibitors and PD-1 inhibitors restored the cytotoxicity of macrophages and blocked the progression of CSCs [205,206,207,208,209,210,211,212].
IFN-γ is a potent antiviral bioactive substance, and celecoxib is a nonsteroidal anti-inflammatory drug. In a study, their combination decreased the M2/M1 macrophage ratio in the CSC niche reduced the expression of MMP-2, MMP-9, and VEGF of TAMs; and inhibited microangiogenesis in the CSC niche [213]. Thus, IFN-γ and celecoxib may inhibit CSC development by regulating the ratio of M2/M1 macrophages in the TME.
Folic acid oral solution (OOS) has anti-inflammatory, antioxidant, and antitumor effects and can inhibit M2 macrophages in vitro and in vivo without side effects. In addition, OOS can target plastic CSCs as well as the TME that supports CSCs [214]. Therefore, OOS, which can counteract the hypoxic state and inhibit the activity of TAMs and CSCs in the TME, can be a candidate for adjuvant therapy in malignant tumors.
ZnO/NPs loaded with the chemotherapeutic drug doxorubicin (Dox) are multifunctional and multitargeted nanodrug carriers. ZnO(Dox)/NPs can promote Dox-induced polarization of macrophages to an M1 phenotype in vivo and increase the sensitivity of CSCs to doxorubicin treatment. ZnO(Dox)/NPs can also effectively downregulate CD44, a key surface marker of CSCs, reduce CSC stemness, and inhibit CSC development [215].
4.3. Targeting CSC–TAM Interaction
The molecules that mediate CSC–TAM interaction are important for maintaining CSC stemness. Accordingly, specifically interfering with this interaction and disrupting the plasticity of CSCs and TAMs in the TME may lead to more effective antitumor responses (Figure 2) [19,216].
4.3.1. Blockade of the SIRP-α–CD47 Pathway
SIRP-α can inhibit the phagocytosis of CSCs by TAMs and promote tumor immune evasion (Figure 2) [217]. Therefore, blocking the CD47–SIRP-α pathway can interfere with CSC–TAM interaction [218,219]. Several studies are currently underway to test the efficacy of monoclonal antibodies and small-molecule inhibitors targeting SIRP-α or CD47.
CD47 deletion may reduce the stemness properties of glioma CSCs. Anti-CD47 antibody therapy increased the phagocytic activity of macrophages to glioma stem cells, decreased cancer stemness, and resulted in a significant inhibition of tumor growth [219]. Subsequently, the combination of CD47 blockers and the antimicrotubule drug cabazitaxel promoted programmed cell clearance (PRCR) in TNBC cells as well as the activation of NF-κB and generation of immune-stimulating cell factors, which promoted macrophage polarization to the M1 phenotype and inhibited the activity and self-renewal of CSCs [220]. Accumulating evidence suggests that targeting CD47 may be a safe, effective, and promising treatment strategy for CSCs. Similarly, anti-SIRP-α antibodies can increase macrophage-mediated phagocytosis and T-cell-mediated cytotoxicity to CSCs and reduce the resistance of CSCs to antiangiogenic therapy [221,222]. Notably, blocking the SIRP-α-CD47 signaling pathway in combination with chemoradiotherapy can further improve the antitumor therapeutic effect [19]. Moreover, targeting E3-ligase-mediated CD47 degradation to boost anticancer immunity also has been discussed [223]. The above findings suggest that blocking the SIRP-α-CD47 signaling pathway with monoclonal antibodies or small-molecule inhibitors can effectively block CSC–TAM interaction, thereby inhibiting the progression of CSCs and reducing the tumor burden in vivo.
4.3.2. Targeting IL-Mediated Signaling Pathways
Cytokines such as IL-6 and IL-10 are essential for maintaining CSC stemness and promoting TAM activation, and their inhibitors have shown potent antitumor activity [224]. IL-6 secreted by TAMs can promote the proliferation and development of CSCs, and IL-6 levels correlate with the specific phenotype of CSCs. CD44+ HCC cell lines exhibit CSC activity in vitro; however, tocilizumab, a monoclonal antibody to the IL-6 receptor, can inhibit IL-6 function and downregulate TAM-mediated CD44+ cell activity, reducing the stemness of CD44+ cells [119]. TAMs can activate the Sonic Hedgehog (SHH) and STAT3 signaling pathways in CSCs by releasing milk fat globule-EGF factor 8 (MFG-E8), which plays a vital role in regulating the activity of CSCs and enhances the resistance to antitumor drugs [225,226]. IL-6 can increase the tumorigenic activity of CSCs by enhancing MFG-E8-induced activity, and the combined blockade of IL-6 and MFG-E8 can significantly reduce the number of primary tumor-derived CSCs, whereas blockade of IL-6 alone or MFG-E8 has only a partial antitumor effect. Furthermore, the inhibition of the MFG-E8-activated SHH and STAT3 pathways significantly increased cisplatin (CDDP)-induced apoptosis of CSCs [139]. In addition, blocking the IL-6/STAT3 signaling pathway has also shown some antitumor activity, with STAT3 involved in the recruitment and polarization of TAMs [64,227]. Anti-IL-6 or anti-IL-6R antibodies or small-molecule inhibitors targeting STAT3 can inhibit the activity of CSCs and M2 polarization of macrophages, thus inhibiting the progression of CSCs [39,91]. STAT3 knockdown by siRNA prevented tumor cell proliferation and blocked CSC-mediated immunosuppression [228,229].
A study reported an antitumor drug candidate, CBP501, which inhibits the lipopolysaccharide-induced secretion of TNF-α, IL-6, and IL-10 from TAMs. Coculture of CBP501 with lipopolysaccharide-treated TAMs inhibited the formation of tumor spheroid cells. In addition, CBP501 can also inhibit the expression of CSC marker ABCG2 by inhibiting the interaction between VCAM-1+ cancer cells and VLA-4+ macrophages [143].
IL-8 and CXCR1/2 are a pair of homologous receptors. IL-8 enhances the tumor-promoting activity of TAMs and promotes the progression of CSCs by helping the interaction between CSC and TAM. The IL-8/CXCR1/2 signaling pathway is partially mediated by the EGFR/HER2 signaling pathway. Therefore, the simultaneous application of CXCR1/2 inhibitors and HER2 inhibitors in cancer therapy can inhibit the tumor-promoting activity of TAMs, inhibit the proliferation and migration of CSCs, and improve the overall survival rate of patients with cancer [230,231].
IL-33 can upregulate the expression of MMP-9 in TAMs. MMP-9 can modify activated receptors on NK cells, CD8+ T cells, CD4+ T cells, as well as inhibit the expression of antigen-presenting molecule MHC-I on the surface of CSCs, which interferes with the interdependence between CSCs and TAMs [232]. These findings suggest that IL-33-induced TAMs can significantly reduce the toxic effects of immune cells on CSCs through MMP-9 blockade [233,234]. Therefore, the researchers reasoned that the antitumor effect of immune cells could be enhanced by inhibiting the proteolytic activity of MMPs, namely by targeting the IL-33–TAMs–MMP-9 axis-mediated immune tolerance of CSCs; this represents a new therapeutic modality against CSCs.
4.3.3. Others
Targeting promoting-cancer pathways, such as WNT, Notch, and Hedgehog, can help disrupt the interdependence between CSCs and TAMs [235]. During CSC–TAM interaction, activated WNT signaling initiated by TAMs can promote the chemoresistance and invasive phenotypes of CSCs [62], suggesting that targeting the WNT pathway may inhibit the activity of CSCs and TAMs.
The EGFR/STAT3/Sox2 paracrine signaling pathway, which mediates CSC–TAM interaction, can promote the expression of the CSC marker gene Sox2, thus enhancing CSC stemness, and increase the ability of drug excretion and chemotherapy resistance in vivo [130]. Therefore, knockdown of Sox2 in CSCs by siRNA can inhibit the TAM-mediated stemness of CSCs, thereby inhibiting tumor progression [130]. In addition, targeting EGFR and STAT3 by small-molecule inhibitors AG1478 and CDDO-Im, respectively, can also effectively block this signaling pathway [130]. Other pathway inhibitors, such as Vismodegib, an SHH pathway inhibitor, can be used to treat metastatic or advanced localized basal cell carcinoma [236]. In addition, imatinib is a tyrosine kinase inhibitor that can inhibit STAT6 phosphorylation and nuclear translocation, thereby promoting macrophage-induced phagocytosis and reducing CSC growth and migration [237]. Notably, these signaling pathways also act on normal tissue cells. Therefore, to minimize adverse effects, researchers should find therapeutic pathways that specifically target CSCs or TAMs, which is undoubtedly a major challenge.
One study demonstrated that inhibition of CSC-specific POSTN and its related pathways or the GBM derivative CCL5 disrupted CSC–TAM interaction in mouse models of GBM and prostate cancer [69,110]. Maraviroc is a CCR5 antagonist that significantly inhibits the proliferation, colony formation, and migration of CCR5+ CSCs [52].
Chemokine ligand 8 (CXCL8) is a key chemokine secreted by TAMs that can mediate CSC–TAM interaction. CXCL8 significantly increased the migration, invasion, and EMT events of breast cancer cells, as well as the self-renewal of BCSCs [117]. Although danirixin is a selective antagonist of CXC chemokine receptor 2 (CXCR2), in vivo analysis confirmed that it can also inhibit the activity of CXCL8, thus inhibiting the self-renewal and metastasis properties of BCSCs [238,239]. These results suggest that danirixin treatment significantly abrogates TAMs/CXCL8-mediated tumor-promoting efficacy.
Metformin is a traditional drug commonly used to treat type 2 diabetes. It can significantly reduce the proportion of ALDH+CD133+ CSCs in patients with ovarian cancer and can decrease HCC stemness [240,241]. In addition, metformin may also increase macrophage phagocytosis by reprogramming the functional activity of macrophages [242], thereby affecting the interaction of TAMs with CSCs, reducing CSC levels, and inhibiting tumor growth.
Survivin, an antiapoptotic protein, is present in human adipose tissue-derived stem cells (HASCs) and is a diagnostic marker of tumor recurrence. One study reported that HASCs isolated from obese patients had significantly elevated survivin expression; at the same time, the antiapoptotic capacity of HASCs and the activity of soluble proinflammatory cytokines (such as IL-1β) secreted by M1 macrophages were also improved [243]. Therefore, targeting survivin may present a new approach to cancer treatment.
Despite the attractive therapeutic strategies targeting CSCs, they lack efficient and specific CSC targets. Furthermore, due to their high plasticity, CSCs may become reestablished after the treatment-induced loss of stemness. Similarly, some TAM-targeting strategies, such as antagonizing CSF1R, also have only a weak antitumor effect, which may be due to some altered pathways in the TME leading to unknown therapy resistance mechanisms of TAMs.
5. Summary and Perspective
CSCs represent a small portion of the cancer cells in a tumor, but they have very powerful self-renewal properties, are often resistant to chemotherapy and radiation therapy, and promote tumor recurrence and metastasis. This article reviewed the molecular mechanism of how CSC–TAM interaction maintains tumor progression. This bidirectional effect is manifested at several levels, including the role of CSCs in promoting the recruitment and polarization of TAMs and the role of TAMs in promoting CSC stemness and CSC niche formation. We also described potential therapeutic strategies, including various monoclonal antibodies, small-molecule inhibitors, agonists, and antagonists, that involve targeting (1) tumor-associated macrophage recruitment and survival, (2) tumor-associated macrophage activation, and (3) cytokines and chemokines involved in CSC–TAM interaction. However, most treatments are still in preclinical studies. A major challenge is the high plasticity of CSCs and the lack of efficient and specific therapeutic targets. In addition, macrophages play various important roles in the immune system, and excessive depletion of these cells can seriously affect immune function. To address these issues, new therapeutic strategies are required to target markers of TAMs and CSCs efficiently and specifically to precisely target these tumor immunosuppressive cell populations. Furthermore, current knowledge of TAMs and CSCs relies heavily on tumor engraftment analysis in syngeneic and xenograft models; these models do not exhibit the exact same TME as the primary tumor, nor do they fully mimic the interaction between human CSCs and TAMs. Therefore, developing a mouse model with a human-like immune system that can support the growth of human primary tumors will elucidate the relationship between TAMs and CSCs.
In conclusion, the interaction between TAMs and CSCs during tumor progression constitutes a positive feedback loop and exerts structural maintenance and protective effects on the CSC niche. Although many therapeutic strategies have been proposed to target TAMs and CSCs, the results remain unsatisfactory. Further elucidation of the mechanisms and roles of CSC–TAM interaction in the progression of CSCs and using TAM-specific and CSC-specific targeted immunotherapy to treat CSCs are warranted to develop more effective therapies with minimal adverse effects.
Acknowledgments
Due to the limited space, we apologize for any publications that may be omitted from the references.
Author Contributions
The draft was written and edited by S.L., G.Y. and P.Y.; N.C. and X.C. reviewed and edited the manuscript; W.-H.Y. and X.Y. wrote the manuscript and supervised the entire work. All authors agree to be responsible for the publication. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the following: Guangzhou key medical discipline construction project fund; the National Natural Science Foundation of China (81872138 and 82172789); China Medical University YingTsai Young Scholar Award (CMU108-YTY-04).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou H.M., Zhang J.G., Zhang X., Li Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct. Target. Ther. 2021;6:62. doi: 10.1038/s41392-020-00430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapidot T., Sirard C., Vormoor J., Murdoch B., Hoang T., Caceres-Cortes J., Minden M., Paterson B., Caligiuri M.A., Dick J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Abdoli Shadbad M., Hosseinkhani N., Asadzadeh Z., Derakhshani A., Karim Ahangar N., Hemmat N., Lotfinejad P., Brunetti O., Silvestris N., Baradaran B. A Systematic Review to Clarify the Prognostic Values of CD44 and CD44(+)CD24(-) Phenotype in Triple-Negative Breast Cancer Patients: Lessons Learned and the Road Ahead. Front. Oncol. 2021;11:689839. doi: 10.3389/fonc.2021.689839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng X., Liu C., Yao J., Wan H., Wan G., Li Y., Chen N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol. Res. 2021;163:105320. doi: 10.1016/j.phrs.2020.105320. [DOI] [PubMed] [Google Scholar]
- 5.Kemper K., Prasetyanti P.R., De Lau W., Rodermond H., Clevers H., Medema J.P. Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells. 2012;30:2378–2386. doi: 10.1002/stem.1233. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z., Tang Y., Xie L., Huang A., Xue C., Gu Z., Wang K., Zong S. The Prognostic and Clinical Value of CD44 in Colorectal Cancer: A Meta-Analysis. Front. Oncol. 2019;9:309. doi: 10.3389/fonc.2019.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J.L., Oshi M., Endo I., Takabe K. Clinical relevance of stem cell surface markers CD133, CD24, and CD44 in colorectal cancer. Am. J. Cancer Res. 2021;11:5141–5154. [PMC free article] [PubMed] [Google Scholar]
- 8.Chong C., Müller M., Pak H., Harnett D., Huber F., Grun D., Leleu M., Auger A., Arnaud M., Stevenson B.J., et al. Integrated proteogenomic deep sequencing and analytics accurately identify non-canonical peptides in tumor immunopeptidomes. Nat. Commun. 2020;11:1293. doi: 10.1038/s41467-020-14968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rambow F., Marine J.C., Goding C.R. Melanoma plasticity and phenotypic diversity: Therapeutic barriers and opportunities. Genes Dev. 2019;33:1295–1318. doi: 10.1101/gad.329771.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prieto-Vila M., Takahashi R.U., Usuba W., Kohama I., Ochiya T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017;18:2574. doi: 10.3390/ijms18122574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plaks V., Kong N., Werb Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B., Ye P., Chen Y., Liu T., Cha J.H., Yan X., Yang W.H. Involvement of the Estrogen and Progesterone Axis in Cancer Stemness: Elucidating Molecular Mechanisms and Clinical Significance. Front. Oncol. 2020;10:1657. doi: 10.3389/fonc.2020.01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Haq A.T., Tseng H.Y., Chen L.M., Wang C.C., Hsu H.L. Targeting prooxidant MnSOD effect inhibits triple-negative breast cancer (TNBC) progression and M2 macrophage functions under the oncogenic stress. Cell Death Dis. 2022;13:49. doi: 10.1038/s41419-021-04486-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y., Yu Y., Wang X., Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song W., Mazzieri R., Yang T., Gobe G.C. Translational Significance for Tumor Metastasis of Tumor-Associated Macrophages and Epithelial-Mesenchymal Transition. Front. Immunol. 2017;8:1106. doi: 10.3389/fimmu.2017.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J., Liu Z., Sun S., Xie J., Cao L., Lv P., Nie S., Zhang B., Xie B., Peng S., et al. Tumor-associated macrophages recruited by periostin in intrahepatic cholangiocarcinoma stem cells. Oncol. Lett. 2018;15:8681–8686. doi: 10.3892/ol.2018.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H., Lu H., Xiang L., Bullen J.W., Zhang C., Samanta D., Gilkes D.M., He J., Semenza G.L. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc. Natl. Acad. Sci. USA. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castelli G., Pelosi E., Testa U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers. 2017;9:127. doi: 10.3390/cancers9090127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cioffi M., Trabulo S., Hidalgo M., Costello E., Greenhalf W., Erkan M., Kleeff J., Sainz B., Jr., Heeschen C. Inhibition of CD47 Effectively Targets Pancreatic Cancer Stem Cells via Dual Mechanisms. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015;21:2325–2337. doi: 10.1158/1078-0432.CCR-14-1399. [DOI] [PubMed] [Google Scholar]
- 20.Liu L., Zhang L., Yang L., Li H., Li R., Yu J., Yang L., Wei F., Yan C., Sun Q., et al. Anti-CD47 Antibody as a Targeted Therapeutic Agent for Human Lung Cancer and Cancer Stem Cells. Front. Immunol. 2017;8:404. doi: 10.3389/fimmu.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H., Clauser K.R., Tam W.L., Fröse J., Ye X., Eaton E.N., Reinhardt F., Donnenberg V.S., Bhargava R., Carr S.A., et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2014;16:1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orecchioni M., Ghosheh Y., Pramod A.B., Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019;10:1084. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerra A.D., Yeung O.W.H., Qi X., Kao W.J., Man K. The Anti-Tumor Effects of M1 Macrophage-Loaded Poly (ethylene glycol) and Gelatin-Based Hydrogels on Hepatocellular Carcinoma. Theranostics. 2017;7:3732–3744. doi: 10.7150/thno.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nusblat L.M., Carroll M.J., Roth C.M. Crosstalk between M2 macrophages and glioma stem cells. Cell. Oncol. 2017;40:471–482. doi: 10.1007/s13402-017-0337-5. [DOI] [PubMed] [Google Scholar]
- 25.Shao X.J., Xiang S.F., Chen Y.Q., Zhang N., Cao J., Zhu H., Yang B., Zhou Q., Ying M.D., He Q.J. Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharmacol. Sin. 2019;40:1343–1350. doi: 10.1038/s41401-019-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito A., Kagawa S., Sakamoto S., Kuwada K., Kajioka H., Yoshimoto M., Kikuchi S., Kuroda S., Yoshida R., Tazawa H., et al. Extracellular vesicles shed from gastric cancer mediate protumor macrophage differentiation. BMC Cancer. 2021;21:102. doi: 10.1186/s12885-021-07816-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedraza-Brindis E.J., Sánchez-Reyes K., Hernández-Flores G., Bravo-Cuellar A., Jave-Suárez L.F., Aguilar-Lemarroy A., Gómez-Lomelí P., López-López B.A., Ortiz-Lazareno P.C. Culture supernatants of cervical cancer cells induce an M2 phenotypic profile in THP-1 macrophages. Cell. Immunol. 2016;310:42–52. doi: 10.1016/j.cellimm.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Reyes K., Pedraza-Brindis E.J., Hernández-Flores G., Bravo-Cuellar A., López-López B.A., Rosas-González V.C., Ortiz-Lazareno P.C. The supernatant of cervical carcinoma cells lines induces a decrease in phosphorylation of STAT-1 and NF-κB transcription factors associated with changes in profiles of cytokines and growth factors in macrophages derived from U937 cells. Innate Immun. 2019;25:344–355. doi: 10.1177/1753425919848841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D., Wang X., Si M., Yang J., Sun S., Wu H., Cui S., Qu X., Yu X. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett. 2020;474:36–52. doi: 10.1016/j.canlet.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Gok Yavuz B., Gunaydin G., Gedik M.E., Kosemehmetoglu K., Karakoc D., Ozgur F., Guc D. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1(+) TAMs. Sci. Rep. 2019;9:3172. doi: 10.1038/s41598-019-39553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalcante R.S., Ishikawa U., Silva E.S., Silva-Júnior A.A., Araújo A.A., Cruz L.J., Chan A.B., de Araújo Júnior R.F. STAT3/NF-κB signalling disruption in M2 tumour-associated macrophages is a major target of PLGA nanocarriers/PD-L1 antibody immunomodulatory therapy in breast cancer. Br. J. Pharmacol. 2021;178:2284–2304. doi: 10.1111/bph.15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouzounova M., Lee E., Piranlioglu R., El Andaloussi A., Kolhe R., Demirci M.F., Marasco D., Asm I., Chadli A., Hassan K.A., et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017;8:14979. doi: 10.1038/ncomms14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clara J.A., Monge C., Yang Y., Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—A clinical update. Nat. Rev. Clin. Oncol. 2020;17:204–232. doi: 10.1038/s41571-019-0293-2. [DOI] [PubMed] [Google Scholar]
- 34.Wang J., Zheng Y., Zhao M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2016;7:533. doi: 10.3389/fphar.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H.H., Wang Y.N., Yang W.H., Xia W., Wei Y., Chan L.C., Wang Y.H., Jiang Z., Xu S., Yao J., et al. Human ribonuclease 1 serves as a secretory ligand of ephrin A4 receptor and induces breast tumor initiation. Nat. Commun. 2021;12:2788. doi: 10.1038/s41467-021-23075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng H., Wang Z., Fu L., Xu T. Macrophage Polarization in the Development and Progression of Ovarian Cancers: An Overview. Front. Oncol. 2019;9:421. doi: 10.3389/fonc.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen P., Hsu W.H., Han J., Xia Y., DePinho R.A. Cancer Stemness Meets Immunity: From Mechanism to Therapy. Cell Rep. 2021;34:108597. doi: 10.1016/j.celrep.2020.108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei J., Chen P., Gupta P., Ott M., Zamler D., Kassab C., Bhat K.P., Curran M.A., de Groot J.F., Heimberger A.B. Immune biology of glioma-associated macrophages and microglia: Functional and therapeutic implications. Neuro-Oncol. 2020;22:180–194. doi: 10.1093/neuonc/noz212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobatake K., Ikeda K.I., Nakata Y., Yamasaki N., Ueda T., Kanai A., Sentani K., Sera Y., Hayashi T., Koizumi M., et al. Kdm6a Deficiency Activates Inflammatory Pathways, Promotes M2 Macrophage Polarization, and Causes Bladder Cancer in Cooperation with p53 Dysfunction. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020;26:2065–2079. doi: 10.1158/1078-0432.CCR-19-2230. [DOI] [PubMed] [Google Scholar]
- 40.Chen P., Hsu W.H., Chang A., Tan Z., Lan Z., Zhou A., Spring D.J., Lang F.F., Wang Y.A., DePinho R.A. Circadian Regulator CLOCK Recruits Immune-Suppressive Microglia into the GBM Tumor Microenvironment. Cancer Discov. 2020;10:371–381. doi: 10.1158/2159-8290.CD-19-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chia K., Mazzolini J., Mione M., Sieger D. Tumor initiating cells induce Cxcr4-mediated infiltration of pro-tumoral macrophages into the brain. eLife. 2018;7:e31918. doi: 10.7554/eLife.31918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roversi F.M., Bueno M.L.P., Pericole F.V., Saad S.T.O. Hematopoietic Cell Kinase (HCK) Is a Player of the Crosstalk between Hematopoietic Cells and Bone Marrow Niche through CXCL12/CXCR4 Axis. Front. Cell Dev. Biol. 2021;9:634044. doi: 10.3389/fcell.2021.634044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo X., Zhao Y., Yan H., Yang Y., Shen S., Dai X., Ji X., Ji F., Gong X.G., Li L., et al. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 2017;31:247–259. doi: 10.1101/gad.294348.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniguchi S., Elhance A., Van Duzer A., Kumar S., Leitenberger J.J., Oshimori N. Tumor-initiating cells establish an IL-33-TGF-β niche signaling loop to promote cancer progression. Science. 2020;369:eaay1813. doi: 10.1126/science.aay1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathria P., Louis T.L., Varner J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019;40:310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., Kaiser E.A., Snyder L.A., Pollard J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gangoso E., Southgate B., Bradley L., Rus S., Galvez-Cancino F., McGivern N., Güç E., Kapourani C.A., Byron A., Ferguson K.M., et al. Glioblastomas acquire myeloid-affiliated transcriptional programs via epigenetic immunoediting to elicit immune evasion. Cell. 2021;184:2454–2470.e2426. doi: 10.1016/j.cell.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F., Li P., Liu S., Yang M., Zeng S., Deng J., Chen D., Yi Y., Liu H. β-Catenin-CCL2 feedback loop mediates crosstalk between cancer cells and macrophages that regulates breast cancer stem cells. Oncogene. 2021;40:5854–5865. doi: 10.1038/s41388-021-01986-0. [DOI] [PubMed] [Google Scholar]
- 49.Cheah M.T., Chen J.Y., Sahoo D., Contreras-Trujillo H., Volkmer A.K., Scheeren F.A., Volkmer J.P., Weissman I.L. CD14-expressing cancer cells establish the inflammatory and proliferative tumor microenvironment in bladder cancer. Proc. Natl. Acad. Sci. USA. 2015;112:4725–4730. doi: 10.1073/pnas.1424795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korbecki J., Grochans S., Gutowska I., Barczak K., Baranowska-Bosiacka I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020;21:7619. doi: 10.3390/ijms21207619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baba T., Mukaida N. Role of macrophage inflammatory protein (MIP)-1α/CCL3 in leukemogenesis. Mol. Cell. Oncol. 2014;1:e29899. doi: 10.4161/mco.29899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novak M., Koprivnikar Krajnc M., Hrastar B., Breznik B., Majc B., Mlinar M., Rotter A., Porčnik A., Mlakar J., Stare K., et al. CCR5-Mediated Signaling Is Involved in Invasion of Glioblastoma Cells in Its Microenvironment. Int. J. Mol. Sci. 2020;21:4199. doi: 10.3390/ijms21124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kranjc M.K., Novak M., Pestell R.G., Lah T.T. Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol. Oncol. 2019;53:397–406. doi: 10.2478/raon-2019-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo X., Pan Y., Gutmann D.H. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell-specific chemokine recruitment of T cells and microglia. Neuro-Oncol. 2019;21:1250–1262. doi: 10.1093/neuonc/noz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhuang Y., Zhao X., Yuan B., Zeng Z., Chen Y. Blocking the CCL5-CCR5 Axis Using Maraviroc Promotes M1 Polarization of Macrophages Cocultured with Irradiated Hepatoma Cells. J. Hepatocell. Carcinoma. 2021;8:599–611. doi: 10.2147/JHC.S300165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Chen X., Li D., Yang Z., Bai Y., Hu S., Liu Z., Gu J., Zhang X. Identification of prognostic and therapeutic value of CC chemokines in Urothelial bladder cancer: Evidence from comprehensive bioinformatic analysis. BMC Urol. 2021;21:173. doi: 10.1186/s12894-021-00938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji J., Wang P., Zhou Q., Zhu L., Zhang H., Zhang Y., Zheng Z., Bhatta A.K., Zhang G., Wang X. CCL8 enhances sensitivity of cutaneous squamous cell carcinoma to photodynamic therapy by recruiting M1 macrophages. Photodiagn. Photodyn. Ther. 2019;26:235–243. doi: 10.1016/j.pdpdt.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Kortlever R.M., Sodir N.M., Wilson C.H., Burkhart D.L., Pellegrinet L., Brown Swigart L., Littlewood T.D., Evan G.I. Myc Cooperates with Ras by Programming Inflammation and Immune Suppression. Cell. 2017;171:1301–1315.e1314. doi: 10.1016/j.cell.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren Z., Chen Y., Shi L., Shao F., Sun Y., Ge J., Zhang J., Zang Y. Sox9/CXCL5 axis facilitates tumour cell growth and invasion in hepatocellular carcinoma. FEBS J. 2022 doi: 10.1111/febs.16357. [DOI] [PubMed] [Google Scholar]
- 60.Jiang X., Cao G., Gao G., Wang W., Zhao J., Gao C. Triptolide decreases tumor-associated macrophages infiltration and M2 polarization to remodel colon cancer immune microenvironment via inhibiting tumor-derived CXCL12. J. Cell. Physiol. 2021;236:193–204. doi: 10.1002/jcp.29833. [DOI] [PubMed] [Google Scholar]
- 61.Takiguchi S., Korenaga N., Inoue K., Sugi E., Kataoka Y., Matsusue K., Futagami K., Li Y.J., Kukita T., Teramoto N., et al. Involvement of CXCL14 in osteolytic bone metastasis from lung cancer. Int. J. Oncol. 2014;44:1316–1324. doi: 10.3892/ijo.2014.2293. [DOI] [PubMed] [Google Scholar]
- 62.Raghavan S., Mehta P., Xie Y., Lei Y.L., Mehta G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J. Immunother. Cancer. 2019;7:190. doi: 10.1186/s40425-019-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamashina T., Baghdadi M., Yoneda A., Kinoshita I., Suzu S., Dosaka-Akita H., Jinushi M. Cancer stem-like cells derived from chemoresistant tumors have a unique capacity to prime tumorigenic myeloid cells. Cancer Res. 2014;74:2698–2709. doi: 10.1158/0008-5472.CAN-13-2169. [DOI] [PubMed] [Google Scholar]
- 64.Wu A., Wei J., Kong L.Y., Wang Y., Priebe W., Qiao W., Sawaya R., Heimberger A.B. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-Oncol. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jelski W., Mroczko B. Biochemical diagnostics of pancreatic cancer—Present and future. Clin. Chim. Acta Int. J. Clin. Chem. 2019;498:47–51. doi: 10.1016/j.cca.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 66.Janakiram N.B., Mohammed A., Bryant T., Ritchie R., Stratton N., Jackson L., Lightfoot S., Benbrook D.M., Asch A.S., Lang M.L., et al. Loss of natural killer T cells promotes pancreatic cancer in LSL-Kras(G12D/+) mice. Immunology. 2017;152:36–51. doi: 10.1111/imm.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan X., Zhang Z., Yao H., Shen L. Tim-4 promotes the growth of colorectal cancer by activating angiogenesis and recruiting tumor-associated macrophages via the PI3K/AKT/mTOR signaling pathway. Cancer Lett. 2018;436:119–128. doi: 10.1016/j.canlet.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 68.Xu H., Zhang Y., Peña M.M., Pirisi L., Creek K.E. Six1 promotes colorectal cancer growth and metastasis by stimulating angiogenesis and recruiting tumor-associated macrophages. Carcinogenesis. 2017;38:281–292. doi: 10.1093/carcin/bgw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou W., Ke S.Q., Huang Z., Flavahan W., Fang X., Paul J., Wu L., Sloan A.E., McLendon R.E., Li X., et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015;17:170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Batool A., Wang Y.Q., Hao X.X., Chen S.R., Liu Y.X. A miR-125b/CSF1-CX3CL1/tumor-associated macrophage recruitment axis controls testicular germ cell tumor growth. Cell Death Dis. 2018;9:962. doi: 10.1038/s41419-018-1021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sa J.K., Kim S.H., Lee J.K., Cho H.J., Shin Y.J., Shin H., Koo H., Kim D., Lee M., Kang W., et al. Identification of genomic and molecular traits that present therapeutic vulnerability to HGF-targeted therapy in glioblastoma. Neuro-Oncol. 2019;21:222–233. doi: 10.1093/neuonc/noy105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otvos B., Silver D.J., Mulkearns-Hubert E.E., Alvarado A.G., Turaga S.M., Sorensen M.D., Rayman P., Flavahan W.A., Hale J.S., Stoltz K., et al. Cancer Stem Cell-Secreted Macrophage Migration Inhibitory Factor Stimulates Myeloid Derived Suppressor Cell Function and Facilitates Glioblastoma Immune Evasion. Stem Cells. 2016;34:2026–2039. doi: 10.1002/stem.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan J., Zhao Q., Wang J., Tian X., Wang J., Xia X., Ott M., Rao G., Heimberger A.B., Li S. FGL2-wired macrophages secrete CXCL7 to regulate the stem-like functionality of glioma cells. Cancer Lett. 2021;506:83–94. doi: 10.1016/j.canlet.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bauer D., Mazzio E., Soliman K.F.A. Whole Transcriptomic Analysis of Apigenin on TNFα Immuno-activated MDA-MB-231 Breast Cancer Cells. Cancer Genom. Proteom. 2019;16:421–431. doi: 10.21873/cgp.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fang W.B., Yao M., Brummer G., Acevedo D., Alhakamy N., Berkland C., Cheng N. Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment. Oncotarget. 2016;7:49349–49367. doi: 10.18632/oncotarget.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J., Patel L., Pienta K.J. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang J., Wang X., Wang Y., Li S., Wang X. Krüppel like factor 6 splice variant 1 (KLF6-SV1) overexpression recruits macrophages to participate in lung cancer metastasis by up-regulating TWIST1. Cancer Biol. Ther. 2019;20:680–691. doi: 10.1080/15384047.2018.1550570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aldinucci D., Lorenzon D., Cattaruzza L., Pinto A., Gloghini A., Carbone A., Colombatti A. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: Involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int. J. Cancer. 2008;122:769–776. doi: 10.1002/ijc.23119. [DOI] [PubMed] [Google Scholar]
- 79.Kodama T., Koma Y.I., Arai N., Kido A., Urakawa N., Nishio M., Shigeoka M., Yokozaki H. CCL3-CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab. Investig. J. Tech. Methods Pathol. 2020;100:1140–1157. doi: 10.1038/s41374-020-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutmann D.H., Kettenmann H. Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology. Neuron. 2019;104:442–449. doi: 10.1016/j.neuron.2019.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi H., Sun Y., Ruan H., Ji C., Zhang J., Wu P., Li L., Huang C., Jia Y., Zhang X., et al. 3,3’-Diindolylmethane Promotes Gastric Cancer Progression via β-TrCP-Mediated NF-κB Activation in Gastric Cancer-Derived MSCs. Front. Oncol. 2021;11:603533. doi: 10.3389/fonc.2021.603533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Özcan Y., Çağlar F., Celik S., Demir A.B., Erçetin A.P., Altun Z., Aktas S. The role of cancer stem cells in immunotherapy for bladder cancer: An in vitro study. Urol. Oncol. 2020;38:476–487. doi: 10.1016/j.urolonc.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 83.Pavon L.F., Sibov T.T., de Souza A.V., da Cruz E.F., Malheiros S.M.F., Cabral F.R., de Souza J.G., Boufleur P., de Oliveira D.M., de Toledo S.R.C., et al. Tropism of mesenchymal stem cell toward CD133(+) stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res. Ther. 2018;9:310. doi: 10.1186/s13287-018-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wani N., Nasser M.W., Ahirwar D.K., Zhao H., Miao Z., Shilo K., Ganju R.K. C-X-C motif chemokine 12/C-X-C chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment. Breast Cancer Res. BCR. 2014;16:R54. doi: 10.1186/bcr3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Boeck A., Ahn B.Y., D’Mello C., Lun X., Menon S.V., Alshehri M.M., Szulzewsky F., Shen Y., Khan L., Dang N.H., et al. Glioma-derived IL-33 orchestrates an inflammatory brain tumor microenvironment that accelerates glioma progression. Nat. Commun. 2020;11:4997. doi: 10.1038/s41467-020-18569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu M., Sun X., Shi S. MORC2 Enhances Tumor Growth by Promoting Angiogenesis and Tumor-Associated Macrophage Recruitment via Wnt/β-Catenin in Lung Cancer. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;51:1679–1694. doi: 10.1159/000495673. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y., Li X., Zhang Y., Wang H., Rong X., Peng J., He L., Peng Y. An miR-340-5p-macrophage feedback loop modulates the progression and tumor microenvironment of glioblastoma multiforme. Oncogene. 2019;38:7399–7415. doi: 10.1038/s41388-019-0952-x. [DOI] [PubMed] [Google Scholar]
- 88.Hu J., Jo M., Eastman B.M., Gilder A.S., Bui J.D., Gonias S.L. uPAR induces expression of transforming growth factor β and interleukin-4 in cancer cells to promote tumor-permissive conditioning of macrophages. Am. J. Pathol. 2014;184:3384–3393. doi: 10.1016/j.ajpath.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dzaye O., Hu F., Derkow K., Haage V., Euskirchen P., Harms C., Lehnardt S., Synowitz M., Wolf S.A., Kettenmann H. Glioma Stem Cells but Not Bulk Glioma Cells Upregulate IL-6 Secretion in Microglia/Brain Macrophages via Toll-like Receptor 4 Signaling. J. Neuropathol. Exp. Neurol. 2016;75:429–440. doi: 10.1093/jnen/nlw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li H., Yang P., Wang J., Zhang J., Ma Q., Jiang Y., Wu Y., Han T., Xiang D. HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J. Hematol. Oncol. 2022;15:2. doi: 10.1186/s13045-021-01223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weng Y.S., Tseng H.Y., Chen Y.A., Shen P.C., Al Haq A.T., Chen L.M., Tung Y.C., Hsu H.L. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer. 2019;18:42. doi: 10.1186/s12943-019-0988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ning Y., Cui Y., Li X., Cao X., Chen A., Xu C., Cao J., Luo X. Co-culture of ovarian cancer stem-like cells with macrophages induced SKOV3 cells stemness via IL-8/STAT3 signaling. Biomed. Pharmacother. 2018;103:262–271. doi: 10.1016/j.biopha.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 93.Deng X., Zhang P., Liang T., Deng S., Chen X., Zhu L. Ovarian cancer stem cells induce the M2 polarization of macrophages through the PPARγ and NF-κB pathways. Int. J. Mol. Med. 2015;36:449–454. doi: 10.3892/ijmm.2015.2230. [DOI] [PubMed] [Google Scholar]
- 94.Raggi C., Correnti M., Sica A., Andersen J.B., Cardinale V., Alvaro D., Chiorino G., Forti E., Glaser S., Alpini G., et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J. Hepatol. 2017;66:102–115. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu C.S., Shiau A.L., Su B.H., Hsu T.S., Wang C.T., Su Y.C., Tsai M.S., Feng Y.H., Tseng Y.L., Yen Y.T., et al. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony-stimulating factor in lung cancer. J. Hematol. Oncol. 2020;13:62. doi: 10.1186/s13045-020-00887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang M., Liu Z.Z., Aoshima K., Cai W.L., Sun H., Xu T., Zhang Y., An Y., Chen J.F., Chan L.H., et al. CECR2 drives breast cancer metastasis by promoting NF-κB signaling and macrophage-mediated immune suppression. Sci. Transl. Med. 2022;14:eabf5473. doi: 10.1126/scitranslmed.abf5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sami E., Paul B.T., Koziol J.A., ElShamy W.M. The Immunosuppressive Microenvironment in BRCA1-IRIS-Overexpressing TNBC Tumors Is Induced by Bidirectional Interaction with Tumor-Associated Macrophages. Cancer Res. 2020;80:1102–1117. doi: 10.1158/0008-5472.CAN-19-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Q., Wu H., Li Y., Zhang R., Kleeff J., Zhang X., Cui M., Liu J., Li T., Gao J., et al. Combined blockade of TGf-β1 and GM-CSF improves chemotherapeutic effects for pancreatic cancer by modulating tumor microenvironment. Cancer Immunol. Immunother. CII. 2020;69:1477–1492. doi: 10.1007/s00262-020-02542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tao W., Chu C., Zhou W., Huang Z., Zhai K., Fang X., Huang Q., Zhang A., Wang X., Yu X., et al. Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat. Commun. 2020;11:3015. doi: 10.1038/s41467-020-16827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsukamoto H., Mishra L., Machida K. Alcohol, TLR4-TGF-β antagonism, and liver cancer. Hepatol. Int. 2014;8((Suppl. S2)):408–412. doi: 10.1007/s12072-013-9489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Q., Cai D.J., Li B. Ovarian cancer stem-like cells elicit the polarization of M2 macrophages. Mol. Med. Rep. 2015;11:4685–4693. doi: 10.3892/mmr.2015.3323. [DOI] [PubMed] [Google Scholar]
- 102.Yin J., Kim S.S., Choi E., Oh Y.T., Lin W., Kim T.H., Sa J.K., Hong J.H., Park S.H., Kwon H.J., et al. ARS2/MAGL signaling in glioblastoma stem cells promotes self-renewal and M2-like polarization of tumor-associated macrophages. Nat. Commun. 2020;11:2978. doi: 10.1038/s41467-020-16789-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heiler S., Wang Z., Zöller M. Pancreatic cancer stem cell markers and exosomes—The incentive push. World J. Gastroenterol. 2016;22:5971–6007. doi: 10.3748/wjg.v22.i26.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T., Liu B., Su L., Qiu Z. Correction: Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer Res. 2020;80:922. doi: 10.1158/0008-5472.CAN-19-3872. [DOI] [PubMed] [Google Scholar]
- 105.Gabrusiewicz K., Li X., Wei J., Hashimoto Y., Marisetty A.L., Ott M., Wang F., Hawke D., Yu J., Healy L.M., et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology. 2018;7:e1412909. doi: 10.1080/2162402X.2017.1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li B., Song T.N., Wang F.R., Yin C., Li Z., Lin J.P., Meng Y.Q., Feng H.M., Jing T. Tumor-derived exosomal HMGB1 promotes esophageal squamous cell carcinoma progression through inducing PD1(+) TAM expansion. Oncogenesis. 2019;8:17. doi: 10.1038/s41389-019-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yin Z., Ma T., Lin Y., Lu X., Zhang C., Chen S., Jian Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J. Cell. Biochem. 2018;119:9419–9432. doi: 10.1002/jcb.27259. [DOI] [PubMed] [Google Scholar]
- 108.Myers K.V., Amend S.R., Pienta K.J. Targeting Tyro3, Axl and MerTK (TAM receptors): Implications for macrophages in the tumor microenvironment. Mol. Cancer. 2019;18:94. doi: 10.1186/s12943-019-1022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gomez K.E., Wu F., Keysar S.B., Morton J.J., Miller B., Chimed T.S., Le P.N., Nieto C., Chowdhury F.N., Tyagi A., et al. Cancer Cell CD44 Mediates Macrophage/Monocyte-Driven Regulation of Head and Neck Cancer Stem Cells. Cancer Res. 2020;80:4185–4198. doi: 10.1158/0008-5472.CAN-20-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang R., Wang S., Wang N., Zheng Y., Zhou J., Yang B., Wang X., Zhang J., Guo L., Wang S., et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020;11:234. doi: 10.1038/s41419-020-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jeong M., Wang Y.Y., Choi J.Y., Lim M.C., Choi J.H. CC Chemokine Ligand 7 Derived from Cancer-Stimulated Macrophages Promotes Ovarian Cancer Cell Invasion. Cancers. 2021;13:2745. doi: 10.3390/cancers13112745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang X., Chen L., Dang W.Q., Cao M.F., Xiao J.F., Lv S.Q., Jiang W.J., Yao X.H., Lu H.M., Miao J.Y., et al. CCL8 secreted by tumor-associated macrophages promotes invasion and stemness of glioblastoma cells via ERK1/2 signaling. Lab. Investig. J. Tech. Methods Pathol. 2020;100:619–629. doi: 10.1038/s41374-019-0345-3. [DOI] [PubMed] [Google Scholar]
- 113.He Z., Chen D., Wu J., Sui C., Deng X., Zhang P., Chen Z., Liu D., Yu J., Shi J., et al. Yes associated protein 1 promotes resistance to 5-fluorouracil in gastric cancer by regulating GLUT3-dependent glycometabolism reprogramming of tumor-associated macrophages. Arch. Biochem. Biophys. 2021;702:108838. doi: 10.1016/j.abb.2021.108838. [DOI] [PubMed] [Google Scholar]
- 114.Zhu F., Li X., Chen S., Zeng Q., Zhao Y., Luo F. Tumor-associated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Med. Oncol. 2016;33:17. doi: 10.1007/s12032-016-0729-9. [DOI] [PubMed] [Google Scholar]
- 115.Yeung O.W., Lo C.M., Ling C.C., Qi X., Geng W., Li C.X., Ng K.T., Forbes S.J., Guan X.Y., Poon R.T., et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J. Hepatol. 2015;62:607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 116.Wang S., Wang N., Huang X., Yang B., Zheng Y., Zhang J., Wang X., Lin Y., Wang Z. Baohuoside i suppresses breast cancer metastasis by downregulating the tumor-associated macrophages/C-X-C motif chemokine ligand 1 pathway. Phytomed. Int. J. Phytother. Phytopharmacol. 2020;78:153331. doi: 10.1016/j.phymed.2020.153331. [DOI] [PubMed] [Google Scholar]
- 117.Nie G., Cao X., Mao Y., Lv Z., Lv M., Wang Y., Wang H., Liu C. Tumor-associated macrophages-mediated CXCL8 infiltration enhances breast cancer metastasis: Suppression by Danirixin. Int. Immunopharmacol. 2021;95:107153. doi: 10.1016/j.intimp.2020.107153. [DOI] [PubMed] [Google Scholar]
- 118.Feng X., Szulzewsky F., Yerevanian A., Chen Z., Heinzmann D., Rasmussen R.D., Alvarez-Garcia V., Kim Y., Wang B., Tamagno I., et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6:15077–15094. doi: 10.18632/oncotarget.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wan S., Zhao E., Kryczek I., Vatan L., Sadovskaya A., Ludema G., Simeone D.M., Zou W., Welling T.H. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou N., Zhang Y., Zhang X., Lei Z., Hu R., Li H., Mao Y., Wang X., Irwin D.M., Niu G., et al. Exposure of tumor-associated macrophages to apoptotic MCF-7 cells promotes breast cancer growth and metastasis. Int. J. Mol. Sci. 2015;16:11966–11982. doi: 10.3390/ijms160611966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang L., Dong Y., Li Y., Wang D., Liu S., Wang D., Gao Q., Ji S., Chen X., Lei Q., et al. IL-10 derived from M2 macrophage promotes cancer stemness via JAK1/STAT1/NF-κB/Notch1 pathway in non-small cell lung cancer. Int. J. Cancer. 2019;145:1099–1110. doi: 10.1002/ijc.32151. [DOI] [PubMed] [Google Scholar]
- 122.Shen Z., Seppänen H., Vainionpää S., Ye Y., Wang S., Mustonen H., Puolakkainen P. IL10, IL11, IL18 are differently expressed in CD14+ TAMs and play different role in regulating the invasion of gastric cancer cells under hypoxia. Cytokine. 2012;59:352–357. doi: 10.1016/j.cyto.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 123.Ruiz-Torres S.J., Bourn J.R., Benight N.M., Hunt B.G., Lester C., Waltz S.E. Macrophage-mediated RON signaling supports breast cancer growth and progression through modulation of IL-35. Oncogene. 2022;41:321–333. doi: 10.1038/s41388-021-02091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fan Q.M., Jing Y.Y., Yu G.F., Kou X.R., Ye F., Gao L., Li R., Zhao Q.D., Yang Y., Lu Z.H., et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 125.Zhang B., Ye H., Ren X., Zheng S., Zhou Q., Chen C., Lin Q., Li G., Wei L., Fu Z., et al. Macrophage-expressed CD51 promotes cancer stem cell properties via the TGF-β1/smad2/3 axis in pancreatic cancer. Cancer Lett. 2019;459:204–215. doi: 10.1016/j.canlet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X., Zhu M., Hong Z., Chen C. Co-culturing polarized M2 Thp-1-derived macrophages enhance stemness of lung adenocarcinoma A549 cells. Ann. Transl. Med. 2021;9:709. doi: 10.21037/atm-21-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Q., Hodge J., Wang J., Wang Y., Wang L., Singh U., Li Y., Yao Y., Wang D., Ai W., et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics. 2020;10:8365–8381. doi: 10.7150/thno.45395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma C., Komohara Y., Ohnishi K., Shimoji T., Kuwahara N., Sakumura Y., Matsuishi K., Fujiwara Y., Motoshima T., Takahashi W., et al. Infiltration of tumor-associated macrophages is involved in CD44 expression in clear cell renal cell carcinoma. Cancer Sci. 2016;107:700–707. doi: 10.1111/cas.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gulluoglu S., Tuysuz E.C., Sahin M., Yaltirik C.K., Kuskucu A., Ozkan F., Dalan A.B., Sahin F., Ture U., Bayrak O.F. The role of TNF-α in chordoma progression and inflammatory pathways. Cell. Oncol. 2019;42:663–677. doi: 10.1007/s13402-019-00454-y. [DOI] [PubMed] [Google Scholar]
- 130.Yang J., Liao D., Chen C., Liu Y., Chuang T.H., Xiang R., Markowitz D., Reisfeld R.A., Luo Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248–258. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 131.Liguori M., Digifico E., Vacchini A., Avigni R., Colombo F.S., Borroni E.M., Farina F.M., Milanesi S., Castagna A., Mannarino L., et al. The soluble glycoprotein NMB (GPNMB) produced by macrophages induces cancer stemness and metastasis via CD44 and IL-33. Cell. Mol. Immunol. 2021;18:711–722. doi: 10.1038/s41423-020-0501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wei X., Yang S., Pu X., He S., Yang Z., Sheng X., Meng X., Chen X., Jin L., Chen W., et al. Tumor-associated macrophages increase the proportion of cancer stem cells in lymphoma by secreting pleiotrophin. Am. J. Transl. Res. 2019;11:6393–6402. [PMC free article] [PubMed] [Google Scholar]
- 133.Shi Y., Ping Y.F., Zhou W., He Z.C., Chen C., Bian B.S., Zhang L., Chen L., Lan X., Zhang X.C., et al. Tumour-associated macrophages secrete pleiotrophin to promote PTPRZ1 signalling in glioblastoma stem cells for tumour growth. Nat. Commun. 2017;8:15080. doi: 10.1038/ncomms15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lv J., Liu C., Chen F.K., Feng Z.P., Jia L., Liu P.J., Yang Z.X., Hou F., Deng Z.Y. M2-like tumour-associated macrophage-secreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol. Med. Rep. 2021;24:1–10. doi: 10.3892/mmr.2021.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okuda H., Kobayashi A., Xia B., Watabe M., Pai S.K., Hirota S., Xing F., Liu W., Pandey P.R., Fukuda K., et al. Hyaluronan synthase HAS2 promotes tumor progression in bone by stimulating the interaction of breast cancer stem-like cells with macrophages and stromal cells. Cancer Res. 2012;72:537–547. doi: 10.1158/0008-5472.CAN-11-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sainz B., Jr., Alcala S., Garcia E., Sanchez-Ripoll Y., Azevedo M.M., Cioffi M., Tatari M., Miranda-Lorenzo I., Hidalgo M., Gomez-Lopez G., et al. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut. 2015;64:1921–1935. doi: 10.1136/gutjnl-2014-308935. [DOI] [PubMed] [Google Scholar]
- 137.Wei R., Zhu W.W., Yu G.Y., Wang X., Gao C., Zhou X., Lin Z.F., Shao W.Q., Wang S.H., Lu M., et al. S100 calcium-binding protein A9 from tumor-associated macrophage enhances cancer stem cell-like properties of hepatocellular carcinoma. Int. J. Cancer. 2021;148:1233–1244. doi: 10.1002/ijc.33371. [DOI] [PubMed] [Google Scholar]
- 138.Xu H.Z., Li T.F., Wang C., Ma Y., Liu Y., Zheng M.Y., Liu Z.J., Chen J.B., Li K., Sun S.K., et al. Synergy of nanodiamond-doxorubicin conjugates and PD-L1 blockade effectively turns tumor-associated macrophages against tumor cells. J. Nanobiotechnol. 2021;19:268. doi: 10.1186/s12951-021-01017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jinushi M., Chiba S., Yoshiyama H., Masutomi K., Kinoshita I., Dosaka-Akita H., Yagita H., Takaoka A., Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. USA. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chang J., Li H., Zhu Z., Mei P., Hu W., Xiong X., Tao J. microRNA-21-5p from M2 macrophage-derived extracellular vesicles promotes the differentiation and activity of pancreatic cancer stem cells by mediating KLF3. Cell Biol. Toxicol. 2021 doi: 10.1007/s10565-021-09597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.You Y., Tian Z., Du Z., Wu K., Xu G., Dai M., Wang Y., Xiao M. M1-like tumor-associated macrophages cascade a mesenchymal/stem-like phenotype of oral squamous cell carcinoma via the IL6/Stat3/THBS1 feedback loop. J. Exp. Clin. Cancer Res. CR. 2022;41:10. doi: 10.1186/s13046-021-02222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tabu K., Liu W., Kosaku A., Terashima K., Murota Y., Aimaitijiang A., Nobuhisa I., Hide T., Taga T. Glioma stem cell (GSC)-derived autoschizis-like products confer GSC niche properties involving M1-like tumor-associated macrophages. Stem Cells. 2020;38:921–935. doi: 10.1002/stem.3193. [DOI] [PubMed] [Google Scholar]
- 143.Mine N., Yamamoto S., Saito N., Sato T., Sakakibara K., Kufe D.W., VonHoff D.D., Kawabe T. CBP501 suppresses macrophage induced cancer stem cell like features and metastases. Oncotarget. 2017;8:64015–64031. doi: 10.18632/oncotarget.19292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pradhan R., Chatterjee S., Hembram K.C., Sethy C., Mandal M., Kundu C.N. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J. Nutr. Biochem. 2021;92:108624. doi: 10.1016/j.jnutbio.2021.108624. [DOI] [PubMed] [Google Scholar]
- 145.Sawa-Wejksza K., Kandefer-Szerszeń M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch. Immunol. Ther. Exp. 2018;66:97–111. doi: 10.1007/s00005-017-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ning Y., Feng W., Cao X., Ren K., Quan M., Chen A., Xu C., Qiu Y., Cao J., Li X., et al. Genistein inhibits stemness of SKOV3 cells induced by macrophages co-cultured with ovarian cancer stem-like cells through IL-8/STAT3 axis. J. Exp. Clin. Cancer Res. CR. 2019;38:19. doi: 10.1186/s13046-018-1010-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147.Hide T., Komohara Y., Miyasato Y., Nakamura H., Makino K., Takeya M., Kuratsu J.I., Mukasa A., Yano S. Oligodendrocyte Progenitor Cells and Macrophages/Microglia Produce Glioma Stem Cell Niches at the Tumor Border. EBioMedicine. 2018;30:94–104. doi: 10.1016/j.ebiom.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fang M., Li Y., Huang K., Qi S., Zhang J., Zgodzinski W., Majewski M., Wallner G., Gozdz S., Macek P., et al. IL33 Promotes Colon Cancer Cell Stemness via JNK Activation and Macrophage Recruitment. Cancer Res. 2017;77:2735–2745. doi: 10.1158/0008-5472.CAN-16-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu L., Liu Y., Kong X., Wu R., Peng Q., Zhang Y., Zhou L., Duan L. Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF-κB/S100A9 Cascade. Front. Immunol. 2021;12:658681. doi: 10.3389/fimmu.2021.658681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y., Gong X., Li J., Wang H., Xu X., Wu Y., Wang J., Wang S., Li Y., Zhang Z. M2 macrophage microvesicle-inspired nanovehicles improve accessibility to cancer cells and cancer stem cells in tumors. J. Nanobiotechnol. 2021;19:397. doi: 10.1186/s12951-021-01143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kaneda M.M., Cappello P., Nguyen A.V., Ralainirina N., Hardamon C.R., Foubert P., Schmid M.C., Sun P., Mose E., Bouvet M., et al. Macrophage PI3Kγ Drives Pancreatic Ductal Adenocarcinoma Progression. Cancer Discov. 2016;6:870–885. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schmid M.C., Khan S.Q., Kaneda M.M., Pathria P., Shepard R., Louis T.L., Anand S., Woo G., Leem C., Faridi M.H., et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018;9:5379. doi: 10.1038/s41467-018-07387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Atanasov G., Pötner C., Aust G., Schierle K., Dietel C., Benzing C., Krenzien F., Bartels M., Eichfeld U., Schmelzle M., et al. TIE2-expressing monocytes and M2-polarized macrophages impact survival and correlate with angiogenesis in adenocarcinoma of the pancreas. Oncotarget. 2018;9:29715–29726. doi: 10.18632/oncotarget.25690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang W.J., Wang X.H., Gao S.T., Chen C., Xu X.Y., Sun Q., Zhou Z.H., Wu G.Z., Yu Q., Xu G., et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J. Surg. Res. 2018;222:93–101. doi: 10.1016/j.jss.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 155.Sørensen M.D., Dahlrot R.H., Boldt H.B., Hansen S., Kristensen B.W. Tumour-associated microglia/macrophages predict poor prognosis in high-grade gliomas and correlate with an aggressive tumour subtype. Neuropathol. Appl. Neurobiol. 2018;44:185–206. doi: 10.1111/nan.12428. [DOI] [PubMed] [Google Scholar]
- 156.Wang H., Li P., Wang L., Xia Z., Huang H., Lu Y., Li Z. High numbers of CD68+ tumor-associated macrophages correlate with poor prognosis in extranodal NK/T-cell lymphoma, nasal type. Ann. Hematol. 2015;94:1535–1544. doi: 10.1007/s00277-015-2401-4. [DOI] [PubMed] [Google Scholar]
- 157.Zhang Y., Cheng S., Zhang M., Zhen L., Pang D., Zhang Q., Li Z. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS ONE. 2013;8:e76147. doi: 10.1371/journal.pone.0076147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kaneda M.M., Messer K.S., Ralainirina N., Li H., Leem C.J., Gorjestani S., Woo G., Nguyen A.V., Figueiredo C.C., Foubert P., et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539:437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.DeNardo D.G., Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cannarile M.A., Weisser M., Jacob W., Jegg A.M., Ries C.H., Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pyonteck S.M., Akkari L., Schuhmacher A.J., Bowman R.L., Sevenich L., Quail D.F., Olson O.C., Quick M.L., Huse J.T., Teijeiro V., et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhu Y., Knolhoff B.L., Meyer M.A., Nywening T.M., West B.L., Luo J., Wang-Gillam A., Goedegebuure S.P., Linehan D.C., DeNardo D.G. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Q., Lu Y., Li R., Jiang Y., Zheng Y., Qian J., Bi E., Zheng C., Hou J., Wang S., et al. Therapeutic effects of CSF1R-blocking antibodies in multiple myeloma. Leukemia. 2018;32:176–183. doi: 10.1038/leu.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ruffell B., Chang-Strachan D., Chan V., Rosenbusch A., Ho C.M., Pryer N., Daniel D., Hwang E.S., Rugo H.S., Coussens L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V., Rey-Giraud F., Pradel L.P., Feuerhake F., Klaman I., et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 167.Tap W.D., Gelderblom H., Palmerini E., Desai J., Bauer S., Blay J.Y., Alcindor T., Ganjoo K., Martín-Broto J., Ryan C.W., et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): A randomised phase 3 trial. Lancet. 2019;394:478–487. doi: 10.1016/S0140-6736(19)30764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yan D., Kowal J., Akkari L., Schuhmacher A.J., Huse J.T., West B.L., Joyce J.A. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene. 2017;36:6049–6058. doi: 10.1038/onc.2017.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Coniglio S.J., Eugenin E., Dobrenis K., Stanley E.R., West B.L., Symons M.H., Segall J.E. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol. Med. 2012;18:519–527. doi: 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sakamoto C., Kohara H., Inoue H., Narusawa M., Ogawa Y., Hirose-Yotsuya L., Miyamoto S., Matsumura Y., Yamada K., Takahashi A., et al. Therapeutic vaccination based on side population cells transduced by the granulocyte-macrophage colony-stimulating factor gene elicits potent antitumor immunity. Cancer Gene Ther. 2017;24:165–174. doi: 10.1038/cgt.2016.80. [DOI] [PubMed] [Google Scholar]
- 171.Wang C.Y., Hua R., Liu L., Zhan X., Chen S., Quan S., Chu Q.J., Zhu Y.T. Immunotherapy against metastatic bladder cancer by combined administration of granulocyte macrophage-colony stimulating factor and interleukin-2 surface modified MB49 bladder cancer stem cells vaccine. Cancer Med. 2017;6:689–697. doi: 10.1002/cam4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lim S.Y., Yuzhalin A.E., Gordon-Weeks A.N., Muschel R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697–28710. doi: 10.18632/oncotarget.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sainz B., Jr., Carron E., Vallespinós M., Machado H.L. Cancer Stem Cells and Macrophages: Implications in Tumor Biology and Therapeutic Strategies. Med. Inflamm. 2016;2016:9012369. doi: 10.1155/2016/9012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kalbasi A., Komar C., Tooker G.M., Liu M., Lee J.W., Gladney W.L., Ben-Josef E., Beatty G.L. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:137–148. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Loberg R.D., Ying C., Craig M., Yan L., Snyder L.A., Pienta K.J. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Loberg R.D., Ying C., Craig M., Day L.L., Sargent E., Neeley C., Wojno K., Snyder L.A., Yan L., Pienta K.J. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 177.Sanford D.E., Belt B.A., Panni R.Z., Mayer A., Deshpande A.D., Carpenter D., Mitchem J.B., Plambeck-Suess S.M., Worley L.A., Goetz B.D., et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: A role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang D., Yue D.L., Wang D., Chen X.F., Yin X.Y., Wang Y.P., Yang L., Zhang Y. [Aspirin inhibits cell stemness of esophageal cancer by downregulation of chemokine CCL2] Zhonghua Zhong Liu Za Zhi [Chin. J. Oncol.] 2018;40:744–749. doi: 10.3760/cma.j.issn.0253-3766.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 179.Izumi K., Fang L.Y., Mizokami A., Namiki M., Li L., Lin W.J., Chang C. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol. Med. 2013;5:1383–1401. doi: 10.1002/emmm.201202367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Udartseva O.O., Zhidkova O.V., Ezdakova M.I., Ogneva I.V., Andreeva E.R., Buravkova L.B., Gollnick S.O. Low-dose photodynamic therapy promotes angiogenic potential and increases immunogenicity of human mesenchymal stromal cells. J. Photochem. Photobiol. B Biol. 2019;199:111596. doi: 10.1016/j.jphotobiol.2019.111596. [DOI] [PubMed] [Google Scholar]
- 181.Eckert F., Schilbach K., Klumpp L., Bardoscia L., Sezgin E.C., Schwab M., Zips D., Huber S.M. Potential Role of CXCR4 Targeting in the Context of Radiotherapy and Immunotherapy of Cancer. Front. Immunol. 2018;9:3018. doi: 10.3389/fimmu.2018.03018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Burger J.A., Peled A. CXCR4 antagonists: Targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 183.Grande F., Giancotti G., Ioele G., Occhiuzzi M.A., Garofalo A. An update on small molecules targeting CXCR4 as starting points for the development of anti-cancer therapeutics. Eur. J. Med. Chem. 2017;139:519–530. doi: 10.1016/j.ejmech.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 184.Taromi S., Kayser G., Catusse J., von Elverfeldt D., Reichardt W., Braun F., Weber W.A., Zeiser R., Burger M. CXCR4 antagonists suppress small cell lung cancer progression. Oncotarget. 2016;7:85185–85195. doi: 10.18632/oncotarget.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bertolini G., Cancila V., Milione M., Lo Russo G., Fortunato O., Zaffaroni N., Tortoreto M., Centonze G., Chiodoni C., Facchinetti F., et al. A novel CXCR4 antagonist counteracts paradoxical generation of cisplatin-induced pro-metastatic niches in lung cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2021;29:2963–2978. doi: 10.1016/j.ymthe.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hao Q., Vadgama J.V., Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun. Signal. 2020;18:82. doi: 10.1186/s12964-020-00589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Laparidou M., Schlickenrieder A., Thoma T., Lengyel K., Schusser B. Blocking of the CXCR4-CXCL12 Interaction Inhibits the Migration of Chicken B Cells into the Bursa of Fabricius. Front. Immunol. 2019;10:3057. doi: 10.3389/fimmu.2019.03057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Poh A.R., Ernst M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018;8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Linger R.M., Cohen R.A., Cummings C.T., Sather S., Migdall-Wilson J., Middleton D.H., Lu X., Barón A.E., Franklin W.A., Merrick D.T., et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2013;32:3420–3431. doi: 10.1038/onc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang G., Wang M., Zhao H., Cui W. Function of Axl receptor tyrosine kinase in non-small cell lung cancer. Oncol. Lett. 2018;15:2726–2734. doi: 10.3892/ol.2017.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xie S., Li Y., Li X., Wang L., Yang N., Wang Y., Wei H. Mer receptor tyrosine kinase is frequently overexpressed in human non-small cell lung cancer, confirming resistance to erlotinib. Oncotarget. 2015;6:9206–9219. doi: 10.18632/oncotarget.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schmidt T., Ben-Batalla I., Schultze A., Loges S. Macrophage-tumor crosstalk: Role of TAMR tyrosine kinase receptors and of their ligands. Cell. Mol. Life Sci. 2012;69:1391–1414. doi: 10.1007/s00018-011-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cummings C.T., Zhang W., Davies K.D., Kirkpatrick G.D., Zhang D., DeRyckere D., Wang X., Frye S.V., Earp H.S., Graham D.K. Small Molecule Inhibition of MERTK Is Efficacious in Non-Small Cell Lung Cancer Models Independent of Driver Oncogene Status. Mol. Cancer Ther. 2015;14:2014–2022. doi: 10.1158/1535-7163.MCT-15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Georgoudaki A.M., Prokopec K.E., Boura V.F., Hellqvist E., Sohn S., Östling J., Dahan R., Harris R.A., Rantalainen M., Klevebring D., et al. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Rep. 2016;15:2000–2011. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 195.La Fleur L., Boura V.F., Alexeyenko A., Berglund A., Pontén V., Mattsson J.S.M., Djureinovic D., Persson J., Brunnström H., Isaksson J., et al. Expression of scavenger receptor MARCO defines a targetable tumor-associated macrophage subset in non-small cell lung cancer. Int. J. Cancer. 2018;143:1741–1752. doi: 10.1002/ijc.31545. [DOI] [PubMed] [Google Scholar]
- 196.Wang H.W., Joyce J.A. Alternative activation of tumor-associated macrophages by IL-4: Priming for protumoral functions. Cell Cycle. 2010;9:4824–4835. doi: 10.4161/cc.9.24.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fitzgerald K.A., Kagan J.C. Toll-like Receptors and the Control of Immunity. Cell. 2020;180:1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McWhirter S.M., Jefferies C.A. Nucleic Acid Sensors as Therapeutic Targets for Human Disease. Immunity. 2020;53:78–97. doi: 10.1016/j.immuni.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 199.Adams S., Kozhaya L., Martiniuk F., Meng T.C., Chiriboga L., Liebes L., Hochman T., Shuman N., Axelrod D., Speyer J., et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:6748–6757. doi: 10.1158/1078-0432.CCR-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vanpouille-Box C., Hoffmann J.A., Galluzzi L. Pharmacological modulation of nucleic acid sensors—Therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 2019;18:845–867. doi: 10.1038/s41573-019-0043-2. [DOI] [PubMed] [Google Scholar]
- 202.Tallerico R., Garofalo C., Carbone E. A New Biological Feature of Natural Killer Cells: The Recognition of Solid Tumor-Derived Cancer Stem Cells. Front. Immunol. 2016;7:179. doi: 10.3389/fimmu.2016.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Donini C., Rotolo R., Proment A., Aglietta M., Sangiolo D., Leuci V. Cellular Immunotherapy Targeting Cancer Stem Cells: Preclinical Evidence and Clinical Perspective. Cells. 2021;10:543. doi: 10.3390/cells10030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guerriero J.L., Sotayo A., Ponichtera H.E., Castrillon J.A., Pourzia A.L., Schad S., Johnson S.F., Carrasco R.D., Lazo S., Bronson R.T., et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. 2017;543:428–432. doi: 10.1038/nature21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Neele A.E., de Winther M.P.J. Repressing the repressor: Ezh2 mediates macrophage activation. J. Exp. Med. 2018;215:1269–1271. doi: 10.1084/jem.20180479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Qi B., Yang C., Zhu Z., Chen H. EZH2-Inhibited MicroRNA-454-3p Promotes M2 Macrophage Polarization in Glioma. Front. Cell Dev. Biol. 2020;8:574940. doi: 10.3389/fcell.2020.574940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hamaidia M., Gazon H., Hoyos C., Hoffmann G.B., Louis R., Duysinx B., Willems L. Inhibition of EZH2 methyltransferase decreases immunoediting of mesothelioma cells by autologous macrophages through a PD-1-dependent mechanism. JCI Insight. 2019;4:e128474. doi: 10.1172/jci.insight.128474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Christofides A., Karantanos T., Bardhan K., Boussiotis V.A. Epigenetic regulation of cancer biology and anti-tumor immunity by EZH2. Oncotarget. 2016;7:85624–85640. doi: 10.18632/oncotarget.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yamagishi M., Uchimaru K. Targeting EZH2 in cancer therapy. Curr. Opin. Oncol. 2017;29:375–381. doi: 10.1097/CCO.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 210.Eich M.L., Athar M., Ferguson J.E., 3rd, Varambally S. EZH2-Targeted Therapies in Cancer: Hype or a Reality. Cancer Res. 2020;80:5449–5458. doi: 10.1158/0008-5472.CAN-20-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Straining R., Eighmy W. Tazemetostat: EZH2 Inhibitor. J. Adv. Pract. Oncol. 2022;13:158–163. doi: 10.6004/jadpro.2022.13.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Italiano A., Soria J.C., Toulmonde M., Michot J.M., Lucchesi C., Varga A., Coindre J.M., Blakemore S.J., Clawson A., Suttle B., et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: A first-in-human, open-label, phase 1 study. Lancet. Oncol. 2018;19:649–659. doi: 10.1016/S1470-2045(18)30145-1. [DOI] [PubMed] [Google Scholar]
- 213.Ren F., Fan M., Mei J., Wu Y., Liu C., Pu Q., You Z., Liu L. Interferon-γ and celecoxib inhibit lung-tumor growth through modulating M2/M1 macrophage ratio in the tumor microenvironment. Drug Des. Dev. Ther. 2014;8:1527–1538. doi: 10.2147/dddt.S66302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hernández-SanMiguel E., Gargini R., Cejalvo T., Segura-Collar B., Núñez-Hervada P., Hortigüela R., Sepúlveda-Sánchez J.M., Hernández-Laín A., Pérez-Núñez A., Sanz E., et al. Ocoxin Modulates Cancer Stem Cells and M2 Macrophage Polarization in Glioblastoma. Oxidative Med. Cell. Longev. 2019;2019:9719730. doi: 10.1155/2019/9719730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang J., Lee J.S., Kim D., Zhu L. Exploration of Zinc Oxide Nanoparticles as a Multitarget and Multifunctional Anticancer Nanomedicine. ACS Appl. Mater. Interfaces. 2017;9:39971–39984. doi: 10.1021/acsami.7b11219. [DOI] [PubMed] [Google Scholar]
- 216.Chan K.S., Espinosa I., Chao M., Wong D., Ailles L., Diehn M., Gill H., Presti J., Jr., Chang H.Y., van de Rijn M., et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., Wang J., Contreras-Trujillo H., Martin R., Cohen J.D., et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Grégoire H., Roncali L., Rousseau A., Chérel M., Delneste Y., Jeannin P., Hindré F., Garcion E. Targeting Tumor Associated Macrophages to Overcome Conventional Treatment Resistance in Glioblastoma. Front. Pharmacol. 2020;11:368. doi: 10.3389/fphar.2020.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yuan J., Shi X., Chen C., He H., Liu L., Wu J., Yan H. High expression of CD47 in triple negative breast cancer is associated with epithelial-mesenchymal transition and poor prognosis. Oncol. Lett. 2019;18:3249–3255. doi: 10.3892/ol.2019.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cao X., Li B., Chen J., Dang J., Chen S., Gunes E.G., Xu B., Tian L., Muend S., Raoof M., et al. Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer. J. Immunother. Cancer. 2021;9:e002022. doi: 10.1136/jitc-2020-002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang X., Wang Y., Fan J., Chen W., Luan J., Mei X., Wang S., Li Y., Ye L., Li S., et al. Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J. Immunother. Cancer. 2019;7:346. doi: 10.1186/s40425-019-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang X., Fan J., Wang S., Li Y., Wang Y., Li S., Luan J., Wang Z., Song P., Chen Q., et al. Targeting CD47 and Autophagy Elicited Enhanced Antitumor Effects in Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2017;5:363–375. doi: 10.1158/2326-6066.CIR-16-0398. [DOI] [PubMed] [Google Scholar]
- 223.Ye P., Chi X., Cha J.H., Luo S., Yang G., Yan X., Yang W.H. Potential of E3 Ubiquitin Ligases in Cancer Immunity: Opportunities and Challenges. Cells. 2021;10:3309. doi: 10.3390/cells10123309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chin A.R., Wang S.E. Cytokines driving breast cancer stemness. Mol. Cell. Endocrinol. 2014;382:598–602. doi: 10.1016/j.mce.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 225.Agliano A., Calvo A., Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin. Cancer Biol. 2017;44:25–42. doi: 10.1016/j.semcancer.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 226.Gao Y.Y., Tao T., Wu D., Zhuang Z., Lu Y., Wu L.Y., Liu G.J., Zhou Y., Zhang D.D., Wang H., et al. MFG-E8 attenuates inflammation in subarachnoid hemorrhage by driving microglial M2 polarization. Exp. Neurol. 2021;336:113532. doi: 10.1016/j.expneurol.2020.113532. [DOI] [PubMed] [Google Scholar]
- 227.Shimagaki T., Yoshio S., Kawai H., Sakamoto Y., Doi H., Matsuda M., Mori T., Osawa Y., Fukai M., Yoshida T., et al. Serum milk fat globule-EGF factor 8 (MFG-E8) as a diagnostic and prognostic biomarker in patients with hepatocellular carcinoma. Sci. Rep. 2019;9:15788. doi: 10.1038/s41598-019-52356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang H., Zhang W., Sun X., Dang R., Zhou R., Bai H., Ben J., Zhu X., Zhang Y., Yang Q., et al. Class A1 scavenger receptor modulates glioma progression by regulating M2-like tumor-associated macrophage polarization. Oncotarget. 2016;7:50099–50116. doi: 10.18632/oncotarget.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gao L., Li F., Dong B., Zhang J., Rao Y., Cong Y., Mao B., Chen X. Inhibition of STAT3 and ErbB2 suppresses tumor growth, enhances radiosensitivity, and induces mitochondria-dependent apoptosis in glioma cells. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:1223–1231. doi: 10.1016/j.ijrobp.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 230.Singh J.K., Simões B.M., Clarke R.B., Bundred N.J. Targeting IL-8 signalling to inhibit breast cancer stem cell activity. Expert Opin. Ther. Targets. 2013;17:1235–1241. doi: 10.1517/14728222.2013.835398. [DOI] [PubMed] [Google Scholar]
- 231.Singh J.K., Farnie G., Bundred N.J., Simões B.M., Shergill A., Landberg G., Howell S.J., Clarke R.B. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:643–656. doi: 10.1158/1078-0432.CCR-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Andersson P., Yang Y., Hosaka K., Zhang Y., Fischer C., Braun H., Liu S., Yu G., Liu S., Beyaert R., et al. Molecular mechanisms of IL-33-mediated stromal interactions in cancer metastasis. JCI Insight. 2018;3:e122375. doi: 10.1172/jci.insight.122375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mondal S., Adhikari N., Banerjee S., Amin S.A., Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020;194:112260. doi: 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- 234.Liang Y., Yang N., Pan G., Jin B., Wang S., Ji W. Elevated IL-33 promotes expression of MMP2 and MMP9 via activating STAT3 in alveolar macrophages during LPS-induced acute lung injury. Cell. Mol. Biol. Lett. 2018;23:52. doi: 10.1186/s11658-018-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Venkatesh V., Nataraj R., Thangaraj G.S., Karthikeyan M., Gnanasekaran A., Kaginelli S.B., Kuppanna G., Kallappa C.G., Basalingappa K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018;5:5. doi: 10.21037/sci.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sekulic A., Migden M.R., Oro A.E., Dirix L., Lewis K.D., Hainsworth J.D., Solomon J.A., Yoo S., Arron S.T., Friedlander P.A., et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yao Z., Zhang J., Zhang B., Liang G., Chen X., Yao F., Xu X., Wu H., He Q., Ding L., et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol. Res. 2018;133:121–131. doi: 10.1016/j.phrs.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 238.Keir H.R., Richardson H., Fillmore C., Shoemark A., Lazaar A.L., Miller B.E., Tal-Singer R., Chalmers J.D., Mohan D. CXCL-8-dependent and -independent neutrophil activation in COPD: Experiences from a pilot study of the CXCR2 antagonist danirixin. ERJ Open Res. 2020;6:00583-2020. doi: 10.1183/23120541.00583-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Busch-Petersen J., Carpenter D.C., Burman M., Foley J., Hunsberger G.E., Kilian D.J., Salmon M., Mayer R.J., Yonchuk J.G., Tal-Singer R. Danirixin: A Reversible and Selective Antagonist of the CXC Chemokine Receptor 2. J. Pharmacol. Exp. Ther. 2017;362:338–346. doi: 10.1124/jpet.117.240705. [DOI] [PubMed] [Google Scholar]
- 240.Brown J.R., Chan D.K., Shank J.J., Griffith K.A., Fan H., Szulawski R., Yang K., Reynolds R.K., Johnston C., McLean K., et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight. 2020;5:e133247. doi: 10.1172/jci.insight.133247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen B., Cha J.H., Yan M., Cao N., Ye P., Yan X., Yang W.H. ATXN7L3B promotes hepatocellular carcinoma stemness and is downregulated by metformin. Biochem. Biophys. Res. Commun. 2021;573:1–8. doi: 10.1016/j.bbrc.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 242.Wang S., Lin Y., Xiong X., Wang L., Guo Y., Chen Y., Chen S., Wang G., Lin P., Chen H., et al. Low-Dose Metformin Reprograms the Tumor Immune Microenvironment in Human Esophageal Cancer: Results of a Phase II Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020;26:4921–4932. doi: 10.1158/1078-0432.CCR-20-0113. [DOI] [PubMed] [Google Scholar]
- 243.Ejarque M., Ceperuelo-Mallafré V., Serena C., Pachón G., Núñez-Álvarez Y., Terrón-Puig M., Calvo E., Núñez-Roa C., Oliva-Olivera W., Tinahones F.J., et al. Survivin, a key player in cancer progression, increases in obesity and protects adipose tissue stem cells from apoptosis. Cell Death Dis. 2017;8:e2802. doi: 10.1038/cddis.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]