Abstract
Significance:
Ergothioneine (ET) is an unusual sulfur-containing amino acid derived from histidine, acquired predominantly from food. Its depletion is associated with deleterious consequences in response to stress stimuli in cell culture models, prompting us to classify it as a vitamin in 2010, which was later supported by in vivo studies. ET is obtained from a variety of foods and is taken up by a selective transporter. ET possesses antioxidant and anti-inflammatory properties that confer cytoprotection. ET crosses the blood–brain barrier and has been reported to have beneficial effects in the brain. In this study, we discuss the cytoprotective and neuroprotective properties of ET, which may be harnessed for combating neurodegeneration and decline during aging.
Recent Advances:
The designation of ET as a stress vitamin is gaining momentum, opening a new field of investigation involving small molecules that are essential for optimal physiological functioning and maintenance of health span.
Critical Issues:
Although ET was discovered more than a century ago, its physiological functions are still being elucidated, especially in the brain. As ET is present in most foods, toxicity associated with its deprivation has been difficult to assess.
Future Directions:
Using genetically engineered cells and mice, it may now be possible to elucidate roles of ET. This coupled with advances in genomics and metabolomics may lead to identification of ET function. As ET is a stable antioxidant with anti-inflammatory properties, whose levels decline during aging, supplementing ET in the diet or consuming an ET-rich diet may prove beneficial. Antioxid. Redox Signal. 36, 1306–1317.
Keywords: antioxidant, histidine thiol, neurodegeneration, Alzheimer's disease, Parkinson's disease, oxidative stress
Introduction
Ergothioneine (ET) is a sulfur-containing derivative of the amino acid, histidine. Discovered more than a century ago in 1909, physiological functions for this molecule are still being elucidated (126). Mammals cannot synthesize ET and acquire it predominantly from food. Its de2pletion is linked to impaired stress responses and toxicity, prompting us to designate it as a vitamin (102). This designation has gained recognition and momentum in the field, opening new avenues of investigation (4, 10). ET was first isolated from the ergot of rye, Claviceps purpurea (hence the name ergothioneine), and its structure was determined in 1911 (78). Mycobacteria, members of the genus Actinomycetales, and fungi were the first organisms reported to produce ET (37, 38). Later, ET was also discovered in cyanobacteria, methylobacteria, and other microbes (3, 75, 105). Unlike eukaryotes and Gram-negative bacteria, where glutathione (GSH) is the primary thiol, in mycobacteria, GSH is absent and small molecules such as ET and mycothiol (MSH) constitute the major thiol reserve. ET and MSH are utilized in the biosynthesis of lincomycin A, a sulfur-containing C8 sugar (lincosamide) antibiotic (137). The presence of ET in mammals was first identified in pig blood in independent studies, where it was identified as the substance that interfered with the detection of uric acid (11, 57).
Physicochemical Properties of ET
The molecular weight of ET is 229.3 Da. It is a colorless, and odorless compound that is readily soluble in water. Structurally, ET is a betaine of histidine thiol or 2-mercaptohistidine trimethylbetaine (Fig. 1). In 1911, Barger and Ewins showed that ET is the betaine of thiolhistidine. ET exhibits tautomerism and exists predominantly as a thione at physiological pH. Thus, several of its properties are different from thiol molecules. ET is relatively more stable and does not auto-oxidize at physiological pH and generates free radicals such as GSH (88). Unlike most thiols whose standard redox potential of the thiol–disulfide couple ranges from −0.2 to −0.32 V, the value for ET is −0.06 V. An important feature of ET is its thermostability (it does not decompose upon cooking), a feature desirable for its use in culinary preparations.
FIG. 1.
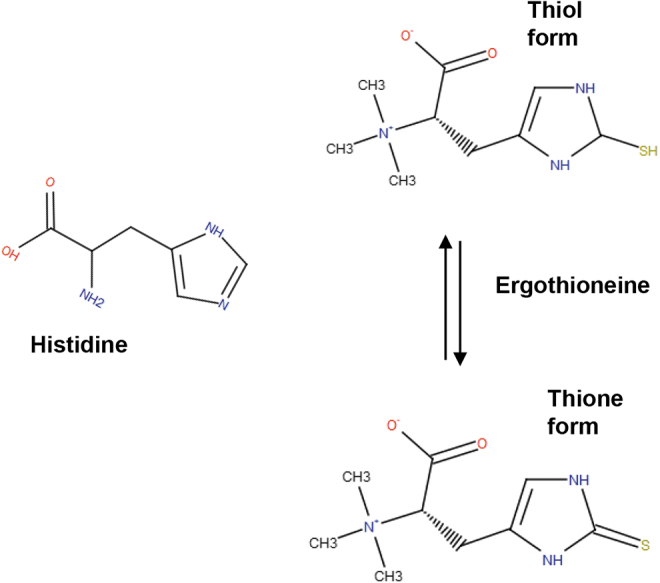
Thione–thiol tautomerism of ET. ET is an unusual sulfur-containing histidine derivative and is the betaine of 2-mercapto-L-histidine. Chemically, it is an N,N,N-trimethylhistidine with a sulfhydryl group linked at the C2 position of the imidazole ring. ET undergoes tautomerism and exists both in the thiol form and thione form, with the latter predominating at physiological pH. Due to this property, ET is resistant to auto-oxidation. ET, ergothioneine.
Another characteristic of ET, that contributes to its cytoprotective properties, is its capacity to absorb ultraviolet (UV) light. ET absorbs light in the UV range similar to DNA, with a molar extinction coefficient of 1.4 × 10−4 M−1·cm−1, λmax 257, indicating that ET acts as a physiological UV filter (16). In the caterpillar fungus, Cordyceps militaris, a mushroom harvested for its medicinal activities, irradiation with ultraviolet B (UVB) increased ET content (55). These studies also revealed that ET prevents DNA damage induced by UV irradiation in a dose-dependent manner (102). In addition to absorbing UV light, ET present in the Coprinus comatus extract protects UVB-induced DNA damage (halogenation) by inhibiting myeloperoxidase activity and scavenging halogenous species (7). The mammalian skin is particularly vulnerable to UV damage, which may induce sunburn, immunosuppression, skin aging, and carcinogenesis, in addition to other damage (26, 123). ET accumulates in skin cells and not only prevents oxidative damage but also facilitates DNA repair in UV-irradiated cells (80). For these reasons, ET has been included as an ingredient in several skin care products and cosmetics.
Biosynthesis of ET
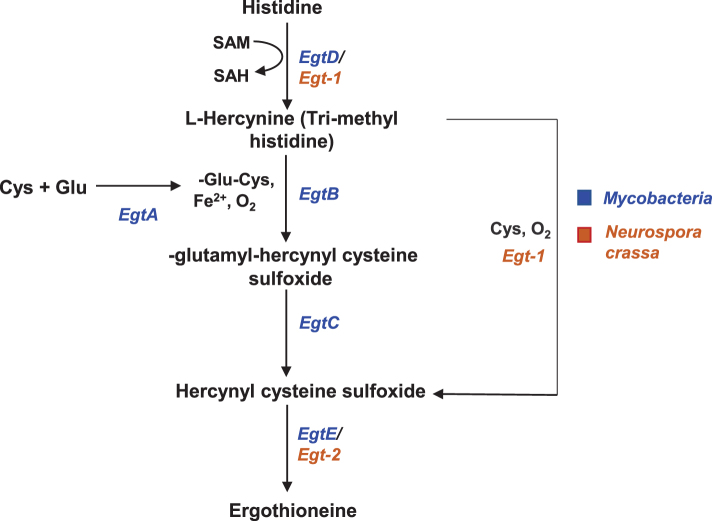
The biosynthetic pathway of ET involves a series of reactions involving histidine and cysteine (8, 86) (Fig. 2). The use of radioactive precursors showed that histidine was first converted to hercynine by addition of three methyl groups, followed by incorporation of sulfur from cysteine, to generate ET (49–51). The conversion of hercynine to ET involved a sulfoxide intermediate (61). More recently, the gene clusters involved in ET biosynthesis were identified in Mycobacteria and Neurospora crassa (54, 60, 112). The mycobacterial pathway involves EgtA-EgtE enzymes, while fungal biosynthesis involves Egt1-Egt2 enzymes. The key steps involve a nonheme iron enzyme-catalyzed oxidative C-S bond formation (EgtB/Egt1 catalysis) and a pyridoxal 5′-phosphate (PLP)-catalyzed C-S lyase (EgtE/Egt2) reaction, which culminates in the transfer of a sulfur atom from a cysteine to a histidine side chain. Although biosynthesis of ET was believed to require oxygen, recent studies show that the anaerobic bacterium, Chlorobium limicola, might produce ET via oxygen-independent reactions, which suggests that ET may have been present on the planet in an anoxic environment (15).
FIG. 2.
Biosynthesis of ET. The biosynthesis of ET utilizes histidine as a precursor and cysteine as the sulfur donor in both Mycobacteria and Neurospora crassa. The enzymes involved in ET biosynthesis (EgtA-E) in Mycobacteria are depicted in blue and those in N. crassa (Egt1-2) are depicted in brown.
Features of ET Meriting Its Classification as a Vitamin
In this section, the features that ET shares with vitamins are described. Vitamins are essential nutrients and constituents of a healthy diet, which cannot be synthesized by humans, but if so, only in suboptimal amounts (1). The concept of vitamins, although not originally termed so, originated in the 18th and 19th centuries when it was noted that nutritional deficiencies caused disease (113). A study by Frederick Hopkins in 1912 revealed that growth of young rats was retarded on a basal diet of protein, starch, cane sugar, lard, and minerals. Normal growth resumed when a small amount of milk was added to the diet. The as yet unknown components in milk, which support life, were reported to be present in astonishingly small amounts and called accessory factors (52). Later, Casimir Funk proposed the term “vitamine” for these factors in 1912 (36). Soon these unknown factors in foods became synonymous with both “vitamine” and “accessory food factors.” Most vitamins are associated with deficiency syndromes, which led to their discovery to begin with. For instance, deficiency of vitamin A causes night blindness, vitamin C causes scurvy, and vitamin D causes rickets (84). Although ET has not been afforded the status of a classical vitamin, its importance in the well-being of humans may have gone unnoticed as it is present in a wide variety of foodstuffs. No pathological syndrome of ET deficiency has been reported. However, lack of such reports may simply reflect the relative paucity of ET research as well as the difficulty of depleting ET. ET is obtained by mammals exclusively from their diet like many vitamins. ET is concentrated in cells and tissues that are frequently exposed to oxidative stress, such as blood, liver, eye lens, and seminal fluid, and its concentration approaches high micromolar or millimolar levels in some of these tissues (97, 115, 116, 121, 124).
Similar to vitamin C, ET is taken up by a very specific transporter (130). The avidity with which ET is retained in mammalian systems and toxicity associated with its depletion in response to stress and its dietary origin led us to propose that ET merits designation as a vitamin. With accumulating evidence, it appears that ET is a stress vitamin that comes into play during adverse conditions or under duress (102) (Table 1). Foods such as mushrooms are a rich source of ET, with certain species, including king oyster, enoki, and shiitake mushrooms, having higher levels. Interestingly, yellow oyster and porcini mushrooms have higher levels of ET compared with GSH, the major antioxidant in most species (65). Plants too obtain ET from the soil, presumably through fungi present in their vicinity. Other foodstuffs that have higher levels of ET include garlic and Brazilian and gingko nuts (33, 44, 65) (Fig. 3).
Table 1.
Stress Stimuli That Induce the Ergothioneine/Ergothioneine Transporter System
| Stress stimuli | System | References |
|---|---|---|
| UV-B irradiation | Increased ET content in caterpillar mushroom, Cordyceps militaris. | (55) |
| Inflammatory cytokines | IL-1β and TNF-α increased the expression of the ET transporter (ETT/OCTN1/SLC22A4) in the human fibroblast-like synoviocyte cell line, MH7A, derived from RA patients. | (77) |
| SDS | SDS increased the population of Lactobacillus reuteri, which produced ET, in the intestine of rats exposed to SDS. | (83) |
| Starvation | Under starvation conditions, ET levels increase in fission yeast, Schizosaccharomyces pombe. | (106) |
| High-cholesterol diet | Livers of guinea pigs fed a cholesterol-rich diet accumulated higher levels of ET. | (24) |
| Liver fibrosis inducing stress | Injection of the hepatotoxin, DMN, which induces liver fibrosis, increased expression of ETT/SLC22A4 and ET content in wild-type mice. | (125) |
| Metabolic acidosis | Metabolic acidosis induced by NH4Cl caused upregulation of ETT in the mouse kidney. | (41) |
| AIMD | Mice treated with a mixture of antibiotics (ampicillin, vancomycin, neomycin, metronidazole, and amphotericin B) displayed upregulation of ETT. | (136) |
| Ni2+ ion irradiation | The human EC line (EA.hy926) irradiated with accelerated nickel ions exhibited an increase in ETT. | (9) |
| Vaccination using a recombinant virus | Nonhuman primates injected with vaccines directed against VSV-EBOV exhibited upregulation of ETT/SLC22A4. | (87) |
| RUPP rat model | In the RUPP model of preeclampsia, ET upregulates antioxidant enzymes such as Nrf2, UCP1, PGC-1α, and SOD1 and SOD2. | (133) |
AIMD, antibiotic-induced microbiome depletion; DMN, dimethylnitrosamine; EC, endothelial cell; ET, ergothioneine; ETT, ergothioneine transporter; IL, interleukin; Nrf2, nuclear factor [erythroid-derived 2]-like 2; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; RA, rheumatoid arthritis; RUPP, reduced uterine perfusion pressure; SDS, social defeat stress; SOD, superoxide dismutase; TNF-α, tumor necrosis factor α; UCP1, uncoupling protein 1; UV, ultraviolet; VSV-EBOV, vesicular stomatitis virus expressing the EBOV glycoprotein.
FIG. 3.
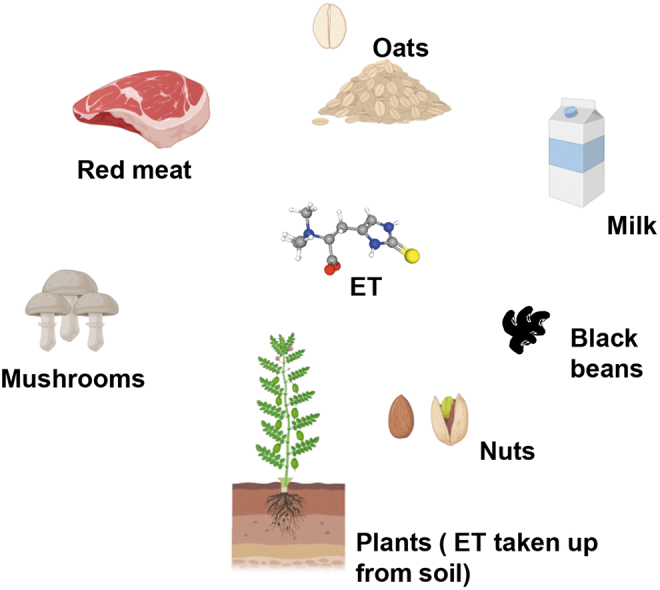
Dietary sources of ET. ET (depicted as a ball and stick model) is present in a variety of foodstuffs. It is enriched in mushrooms and fungi in the soil, which is taken up by plants. ET is also enriched in red meat, black beans, nuts, milk, and oats.
Similar to several vitamins, which have specific transporters for their uptake, ET is imported into cells by a specific transporter, the ergothioneine transporter (ETT/SLC22A4), in a sodium- and pH-dependent manner (42, 130). Knockdown of ETT decreases ET uptake in cell lines and mice, indicating that ETT is the major transporter for ET (67, 102). Additionally, a general evolutionary ancient genomic approach, which identifies genetic variants with frequency changes that are significantly greater over a given time period than expected under genetic drift alone, revealed ETT or SLC22A4 as one of the genes positively selected over evolutionary time, attesting to its importance (79). Differences in abundance of fungi in soil may also give rise to variations in the ET content of crops. It has been reported that excessive tillage of the soil can deplete ET levels in crops by disruption of mycelia of mycorrhizal fungi in symbiotic association with plants (10, 14, 100). A functional variant in the ETT is proposed to have provided protection against ET deficiency through increased absorption of this unusual amino acid in European agriculturalists (56). Although this allele was present at low frequencies in the early Neolithic populations, its enrichment only occurred within the last 4000 years (82). Functional variants of ETT such as L503F, which increase absorption of ET, have been linked to Crohn's disease and believed to be protective in nature (103).
Interestingly, ET levels increase during periods of starvation in both yeast and humans (106). Metabolomic studies have also confirmed these findings in the blood of humans, where ET is enriched (70). Additionally, levels of ET decrease as a function of aging in blood (17, 72, 121). In a study measuring age-related decline in gait speed, ET content was positively correlated with gait speed in middle-aged adults (96). ET levels also diminished twofold in the blood of sickle cell anemia patients and were associated with increased markers of oxidative stress (18). In a longitudinal study analyzing mortality and coronary artery disease (CAD), ET was identified as the metabolite most significantly associated with lower morbidity and mortality, being associated with a lower risk of CAD (117). This study also proposed ET as a biomarker for a healthy diet and low cardiometabolic risk. Consumption of an ET-based nutritional supplement has also been reported to improve joint range of motion and reduction of chronic pain (12). More recently, decrease in ET in the whole blood of human subjects has identified it as a potential marker of frailty (66). In the subsequent sections, the properties of ET that set it apart from other known antioxidant cytoprotectants and its role in neuroprotection are discussed.
ET as an Antioxidant
The fact that mammals do not synthesize ET, but import it via a specific evolutionarily conserved transporter, and retain it with high avidity suggests important physiological functions. ET accumulates in cells and tissues frequently exposed to oxidative stress, with concentrations approaching the millimolar range in blood, lens of the eye, liver, bone marrow, and seminal fluid (97, 115, 116, 120). One of the principal functions of ET is its antioxidant–cytoprotectant function (2, 44, 46, 102). High levels of ET are present in red blood cells, which also express the transporter. At the cellular level, ET has been reported to be present in the mitochondria, which produce reactive oxygen species during respiration (68). Thus, it is not surprising that its transporter has been localized to the mitochondria (73). ET mitigates deleterious effects of several free radicals, including reactive oxygen and reactive nitrogen species (Table 2). ET protects against the deleterious effects of hydroxyl radicals (•OH), peroxynitrite (ONOO−), hypochlorous acid (HOCl), and singlet oxygen 1O2 (2, 47). ET is a better scavenger of 1O2 than GSH (122). 1O2 is generated by photosensitizers activated by sunlight in the eye and skin, which can also affect red blood cells, where ET is enriched. Sunlight exposure causes generation of 1O2 from protoporphyrin IX, the iron-free precursor of heme, while the iron-bound form does not produce 1O2. The peroxidase activity of hemoglobin can also lead to 1O2 production (32, 92). Cell culture studies have revealed that ET protects against ONOO−-induced DNA damage (5). Knocking down ETT causes increased levels of oxidative damage, as reflected by increase in protein carbonylation, lipid peroxidation, and DNA damage (102). Knocking out this transporter in Caenorhabditis elegans leads to increased oxidative stress and decrease in longevity (23). Similarly, knocking out the transporter in zebrafish, Danio rerio, results in increased oxidation of DNA, as revealed by accumulation of 8-oxoguanine (104). ET scavenges 1O2 more efficiently than GSH or ascorbate (99). In addition to these properties, ET can chelate divalent cations such as Cu2+, Zn2+, Ni2+, and Zn2+, and chelation of Cu2+ accounts for its ability to counteract Cu2+-mediated DNA damage (45, 91, 138).
Table 2.
Free Radical Scavenging/Neutralizing Activity of Ergothioneine
| Free radical and oxidants | System | References |
|---|---|---|
| ONOO− | Prevented peroxynitrite-dependent nitration of tyrosine and inactivation of α1-antiproteinase. | (6) |
| 1O2 | Prevented oxidation of BHMF. Scavenges singlet oxygen in vitro. | (31, 108) |
| O2•− | Prevented cell death induced by pyrogallol, a superoxide generator in HeLa cells. Scavenged superoxide and singlet oxygen in UV-irradiated human dermal fibroblasts. Reduced cytotoxicity of paraquat, a superoxide generating agent in ECs, and formed the hercynine and sulfonic acid derivative (ESO3H) in both cell-free systems when reacted with superoxide and also in ECs exposed to high glucose. | (98, 102, 114) |
| HOCl | ET protected α1-antiproteinase against inactivation by HOCl. | (132) |
| •OH | ET is a powerful scavenger of hydroxyl radicals and an inhibitor of iron or copper ion-dependent generation of •OH from hydrogen peroxide. | (2) |
O2, singlet oxygen; BHMF, 2,5-bis(hydroxymethyl) furan; HOCl, hypochlorous acid; O2•−, superoxide; •OH, hydroxyl radical; ONOO−, peroxynitrite.
ET and Inflammation
A link between ET and inflammation was observed in rheumatoid arthritis, where an SNP was found to be associated with the disease (129). In addition to these observations, expression of ETT was increased in response to the proinflammatory cytokine, tumor necrosis factor α (TNF-α), in inflamed joints. Moreover, mice lacking ETT exhibit increased susceptibility to inflammation after ischemia–reperfusion injury (67). The anti-inflammatory property of ET was evident in studies where both H2O2 and TNF-α mediated activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and release of interleukin 8 (IL-8) was prevented by ET (107). Furthermore, NF-κB has binding sites in the promoter of human ETT and regulates its expression, further supporting a role for ETT in modulating inflammatory processes (77).
ET Functions in the Nervous System
The presence of ET in the brain was observed as early as the sixties and was initially believed to be a neurotransmitter and identified as the cerebellar factor (28, 29). Later, it was shown that ET did not support neurotransmission and the cerebellar factor and ET had distinct properties, although they shared several similar features (13, 71). It is not surprising that ET is enriched in the cerebellum as its transporter is abundant in this tissue (134). ET is also present in other brain regions. The basal concentration of ET in the cortex of the brain has been reported to be ∼3.73 ± 0.59 ng/mg (124). Exogenous administration of ET revealed that ET is widely distributed in the brain in regions such as the cerebellum, striatum, medulla and pons, midbrain, hippocampus, hypothalamus, and cortex and the concentration correlates with the expression of its transporter, indicating its ability to cross the blood–brain barrier (94, 95).
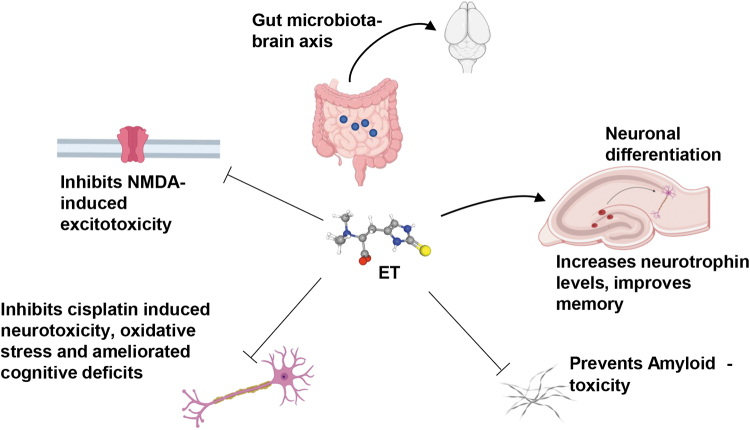
ET exerts potent neuroprotective effects in the brain (Fig. 4). In the brain, the transporter, ETT, is functionally present in neurons, but not in astrocytes (58, 95). ET protects neuronal cells against oxidative stress (5). ET also protected against neurotoxicity induced by the excitotoxin, N-methyl-d-aspartate (NMDA) and cisplatin in vivo (90, 118). The protective effect of ET was also observed against β-amyloid toxicity. Mice injected with β-amyloid developed learning and memory deficits, while those pretreated with ET were spared (135). In addition, ET prevented oxidative stress, as revealed by decreased lipid peroxidation and maintenance of the GSH/GSH disulfide ratio and superoxide dismutase (SOD). ET has also been shown to be protective against learning and memory impairment induced by d-galactose in mice (119). Other studies have reported a role for ET in neuronal differentiation (63, 95). The effects on neuronal differentiation are partly attributed to phosphorylation of p70 ribosomal protein S6 kinase 1 (S6K1), a component of the mTOR signaling pathway, at Thr389 and by activation of the neurotrophin receptor, Tropomyosin receptor kinase B (TrkB) signaling, by upregulation of the neurotrophin, NT5 (62). Recently, it has been reported that ET activates human carbonic anhydrase VII at nanomolar levels (89). Carbonic anhydrases have been linked to modulation of redox homeostasis in cells and thus activation of these enzymes could be beneficial in the treatment of conditions involving redox imbalance. Neurodegenerative diseases have been associated with elevated oxidative and nitrosative stress, and ET may be beneficial in decreasing damage caused by reactive oxygen and nitrogen species in these diseases. ET prevented cisplatin-induced neuronal injury in neuronal cultures as well as mice and enhanced cognition, likely through inhibition of oxidative stress and restoration of acetylcholinesterase (AChE) activity in neuronal cells (118). Metabolomic analysis of Parkinson's disease (PD), the second most common neurodegenerative disease after Alzheimer's disease (AD), revealed a significant decline in ET levels, suggesting a decreased ability in antioxidant defenses (48). In addition, decrease in ET levels has also been observed in vascular dementia and dementia (21, 127). A study of subjects who consumed mushrooms, a rich source of ET, revealed an inverse correlation between mild cognitive impairment and mushroom intake, which was independent of age, gender, or lifestyle habits such as smoking or alcohol consumption (35). Oral administration of ET also promoted memory in rodents, as measured by the novel object recognition test (93). In another study, ET was reported to mitigate the deleterious effects of 7-ketocholesterol (7KC), an oxidation product of cholesterol, in the human brain endothelial cell line (69). 7KC induced elevation in messenger RNA (mRNA) levels of proinflammatory cytokines, IL-1β, IL-6, IL-8, TNF-α, and cyclooxygenase-2 (COX2), and COX2 activity was decreased by ET. Increased 7KC levels have been observed in the AD brain and administering ET may afford neuroprotection (128). Indeed, ET counters neurotoxicity induced in the cell line, C. elegans, and mouse models of AD (22, 64, 135).
FIG. 4.
Effects of ET on brain function. ET (depicted as a ball and stick model) is a neuroprotective molecule affecting multiple aspects of brain function. ET promotes neuronal differentiation and increases neurotrophin levels in the brain. ET prevents neurotoxicity induced by the excitotoxin, NMDA, and cisplatin in vivo. ET also ameliorates learning and memory deficits induced by amyloid β in mice. ET produced by the gut microbiota such as Lactobacillus reuteri protects against stress-induced sleep disturbances and social defeat stress. NMDA, N-methyl-d-aspartate.
ET has also been implicated in behavioral responses to social stress. Oral delivery of ET significantly prevented major depressive disorder (MDD)-like social avoidance and sleep abnormalities in a social defeat stress (SDS) model in rats (83). Symptoms of MDD include lack of interest or pleasure and depressed mood in addition to sleep abnormalities, a psychiatric disorder affecting millions worldwide (25). SDS had effects on the gut microbiota as well. The study reported increases in fecal Lactobacillus reuteri in correlation with ET levels at around day 11, which continued for at least 1 month following SDS administration. Thus, ET may participate in the gut–brain axis via the microbiota that produce it.
An interesting aspect of neuroprotection mediated by ET has been observed in the parasitic interaction between a fungus, Ophiocordyceps kimflemingiae, and the carpenter ant, Camponotus castaneus (76). The fungal infection triggers neurobehavioral alterations in behavior of these ants, which then invade plants and bite into them, before being killed by the fungus. The metabolic profile of the ant's brain revealed an elevation of ET levels, which presumably prevents neurodegeneration and preserves brain function.
ET and Antiaging Effects
Blood ET levels have been found to decrease significantly beyond 60 years of age. The serum concentrations of ET showed an inverse correlation with age (121). Moreover, a subset of the population exhibiting mild cognitive impairment had significantly lower plasma ET levels compared with age-matched controls, indicating that ET deficiency could contribute to aging (19). ET was found to delay endothelial cell senescence caused by high glucose through a mechanism involving the histone deacetylases, sirtuin 1 (SIRT1) and sirtuin 6 (SIRT6) (30). Due to its cytoprotective properties and UV filtering capability, ET is one of the top ingredients used in antiaging creams (27). ET protects UV-irradiated human dermal fibroblasts by scavenging 1O2 and O2•− and reduces levels of inflammation (98). ETT is present on skin cells, allowing them to import ET and reduce levels of reactive oxygen species and DNA, protein, and lipid damage in keratinocytes subjected to solar-simulating UV oxidative stress (80). ET has been reported to protect ultaviolet A (UVA)-irradiated human dermal fibroblasts via inhibition of the activator protein-1 (AP-1) pathway and activation of nuclear factor [erythroid-derived 2]-like 2 (Nrf2)-mediated antioxidant genes (53). ET is also protective in the eye, and formation of cataract is associated with a decline in ET levels (116). Interestingly, levels of ET in the eye lens exceed that of GSH, unlike the scenario in other tissues.
Cardiovascular Benefits
Cardiovascular disease is responsible for a vast majority of deaths worldwide and there is a constant search for drugs that can improve cardiovascular function. Endothelial dysfunction is a major cause of cardiovascular disease with links to oxidative and nitrosative stress (34, 59). ET, with its proven in vitro antioxidant functions, has also been reported to be imported by endothelial cells and reduce markers of oxidative damage (74). ET prevents toxicity induced by mercury chloride and preserves acetylcholine-mediated relaxation, improves the ratio of reduced GSH to oxidized GSH and catalase levels, and reduces overall oxidative stress (40). ET elicits a concentration-dependent relaxation in endothelium-intact aortic rings, which is abrogated endothelial denudation or NO synthase inhibition (39). The study also describes protection against the Cu/Zn SOD inhibitor, diethyldithiocarbamate (DETCA), and hypoxanthine/xanthine oxidase-induced impairment in vasorelaxation, all of which involved decreases in superoxide production. In addition to these effects, ET also prevents the binding of monocytes to endothelial cells, an early event in cardiovascular dysfunction (81).
Concluding Remarks: Future Perspectives and Therapeutic Avenues
The body has evolved multiple mechanisms to counteract stress. Some of these defenses act constitutively, while others are inducible and act during stress. No single antioxidant can scavenge or neutralize the wide variety of reactive oxygen and nitrogen species single-handedly. Thus, the search is on for molecules that counter a wide variety of reactive oxygen and nitrogen species. Additionally, molecules that possess anti-inflammatory effects in addition to antioxidant scavenging roles would provide improved neuroprotection. ET is an unusual antioxidant, in that it is exceptionally stable and does not auto-oxidize at physiological pH and is not destroyed upon heating. ET is water soluble and neutralizes several reactive oxygen and nitrogen species, including •OH, O2•−, ONOO−, HOCl, and 1O2. Accumulating evidence suggests that ET is endowed with cytoprotective signaling functions in addition to its antioxidant and anti-inflammatory role in cells. It has also been posited that ET is an adaptive antioxidant, with cells deliberately accumulating ET in times of stress (43). Thus, ET is a stress metabolite that is obtained via specific transport, implying that regulation of the transporter is part of an adaptive stress response. Accumulation of ET has been observed in infarcted mouse hearts, diabetes, and preeclampsia (109, 110, 131). Similarly, metabolomic analysis of mice repeatedly injected with metamphetamine, a drug of abuse, led to increase in ET levels in the brain (85). These observations, in conjunction with the fact that ET has not been associated with any toxic or adverse effects, support its use in therapies against a wide range of diseases and conditions, ranging from cardiovascular diseases to aging and neurodegeneration. ET is a rare antioxidant–cytoprotectant capable of crossing the blood–brain barrier, a feature that is necessary to treat neurodegenerative disorders where oxidative stress plays a central role in disease progression (111). It is present in mitochondria, which is a feature that can be harnessed in therapies for disorders involving mitochondrial dysfunction such as PD, where this molecule is significantly depleted (48). Another avenue of exploration could be its anti-inflammatory potential to develop a new series of nonsteroidal anti-inflammatory drugs. Because of its antioxidant and anti-inflammatory properties, the use of ET as a therapeutic in the treatment of COVID-19 patients has been proposed (20). A feature of COVID-19 is dysregulated redox balance, which is also observed in patients exhibiting chronic fatigue (COVID-19 long haulers) long after the infection was cleared. Presumably, ET may be beneficial in this aspect of the disease as well (101). Future studies that elucidate its precise mechanism of action in signal transduction cascades could pave the way for development of novel strategies to combat aging and disease. In summary, ET may afford a more stable mode of cytoprotection. It is not metabolized to any significant extent in mammalian tissues, the half-life of dietary ET being ∼1 month. Its existence as a tautomer of thiol and thione forms confers resistance to auto-oxidation, distinguishing it from other common thiols. These properties suggest a role for ET as a bulwark, a final defense for cells against oxidative damage. Evidence that ET is a physiological antioxidant raises the question of its status in biology. Despite its high concentration and ubiquitous presence, mammalian ET is mostly derived from dietary sources. The existence of ETT establishes ET as an important normal body constituent, and in this regard, ET fits the definition of a vitamin.
Abbreviations Used
- 1O2
singlet oxygen
- 7KC
7-ketocholesterol
- AD
Alzheimer's disease
- AIMD
antibiotic-induced microbiome depletion
- BHMF
2,5-bis(hydroxymethyl) furan
- CAD
coronary artery disease
- COX2
cyclooxygenase-2
- DMN
dimethylnitrosamine
- EC
endothelial cell
- ET
ergothioneine
- ETT
ergothioneine transporter
- GSH
glutathione
- HOCl
hypochlorous acid
- IL
interleukin
- MDD
major depressive disorder
- MSH
mycothiol
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA
N-methyl-D-aspartate
- Nrf2
nuclear factor [erythroid-derived 2]-like 2
- O2•−
superoxide
- •OH
hydroxyl radical
- ONOO−
peroxynitrite
- PD
Parkinson's disease
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- RA
rheumatoid arthritis
- RUPP
reduced uterine perfusion pressure
- SDS
social defeat stress
- SIRT
sirtuin
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor α
- UCP1
uncoupling protein 1
- UV
ultraviolet
- VSV-EBOV
vesicular stomatitis virus expressing the EBOV glycoprotein
Author Disclosure Statement
The author declares no conflicts of interest.
Funding Information
This work was supported by the American Heart Association/Paul Allen Frontiers Group Project 19PABH134580006 (to B.D.P. and associates) and NIH 1R21AG073684-01 (to B.D.P).
References
- 1. Vitamins. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 2012. Accessed June 10, 2021. [PubMed] [Google Scholar]
- 2. Akanmu D, Cecchini R, Aruoma OI, and Halliwell B. The antioxidant action of ergothioneine. Arch Biochem Biophys 288: 10–16, 1991. [DOI] [PubMed] [Google Scholar]
- 3. Alamgir KM, Masuda S, Fujitani Y, Fukuda F, and Tani A. Production of ergothioneine by Methylobacterium species. Front Microbiol 6: 1185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ames BN. Prolonging healthy aging: longevity vitamins and proteins. Proc Natl Acad Sci U S A 115: 10836–10844, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aruoma OI, Spencer JP, and Mahmood N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food Chem Toxicol 37: 1043–1053, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Aruoma OI, Whiteman M, England TG, and Halliwell B. Antioxidant action of ergothioneine: assessment of its ability to scavenge peroxynitrite. Biochem Biophys Res Commun 231: 389–391, 1997. [DOI] [PubMed] [Google Scholar]
- 7. Asahi T, Wu X, Shimoda H, Hisaka S, Harada E, Kanno T, Nakamura Y, Kato Y, and Osawa T. A mushroom-derived amino acid, ergothioneine, is a potential inhibitor of inflammation-related DNA halogenation. Biosci Biotechnol Biochem 80: 313–317, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Askari A and Melville DB. The reaction sequence in ergothioneine biosynthesis: hercynine as an intermediate. J Biol Chem 237: 1615–1618, 1962. [PubMed] [Google Scholar]
- 9. Beck M, Rombouts C, Moreels M, Aerts A, Quintens R, Tabury K, Michaux A, Janssen A, Neefs M, Ernst E, Dieriks B, Lee R, De Vos WH, Lambert C, Van Oostveldt P, and Baatout S. Modulation of gene expression in endothelial cells in response to high LET nickel ion irradiation. Int J Mol Med 34: 1124–1132, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Beelman RB, Kalaras MD, Phillips AT, and Richie JP Jr. Is ergothioneine a ‘longevity vitamin’ limited in the American diet? J Nutr Sci 9: e52, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benedict SR, Newton EB, and Behre JA. A new sulfur-containing compound (thiasine) in the blood. J Biol Chem 67: 267–277, 1926. [Google Scholar]
- 12. Benson KF, Ager DM, Landes B, Aruoma OI, and Jensen GS. Improvement of joint range of motion (ROM) and reduction of chronic pain after consumption of an ergothioneine-containing nutritional supplement. Prev Med 54(Suppl): S83–S89, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Briggs I. Ergothioneine in the central nervous system. J Neurochem 19: 27–35, 1972. [DOI] [PubMed] [Google Scholar]
- 14. Brito I, Goss MJ, de Carvalho M, Chatagnier O, and van Tuinen D. Impact of tillage system on arbuscular mycorrhiza fungal communities in the soil under Mediterranean conditions. Soil Till Res 121: 63–67, 2012. [Google Scholar]
- 15. Burn R, Misson L, Meury M, and Seebeck FP. Anaerobic origin of ergothioneine. Angew Chem Int Ed Engl 56: 12508–12511, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Carlsson J, Kierstan MP, and Brocklehurst K. Reactions of L-ergothioneine and some other aminothiones with 2,2′-and 4,4′-dipyridyl disulphides and of L-ergothioneine with iodoacetamide. 2-Mercaptoimidazoles, 2- and 4-thiopyridones, thiourea and thioacetamide as highly reactive neutral sulphur nucleophils. Biochem J 139: 221–235, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaleckis R, Murakami I, Takada J, Kondoh H, and Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci U S A 113: 4252–4259, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaves NA, Alegria TGP, Dantas LS, Netto LES, Miyamoto S, Bonini Domingos CR, and da Silva DGH. Impaired antioxidant capacity causes a disruption of metabolic homeostasis in sickle erythrocytes. Free Radic Biol Med 141: 34–46, 2019. [DOI] [PubMed] [Google Scholar]
- 19. Cheah IK, Feng L, Tang RMY, Lim KHC, and Halliwell B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem Biophys Res Commun 478: 162–167, 2016. [DOI] [PubMed] [Google Scholar]
- 20. Cheah IK and Halliwell B. Could ergothioneine aid in the treatment of coronavirus patients? Antioxidants (Basel) 9: 595, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheah IK and Halliwell B. Ergothioneine, recent developments. Redox Biol: 42: 101868, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheah IK, Ng LT, Ng LF, Lam VY, Gruber J, Huang CYW, Goh FQ, Lim KHC, and Halliwell B. Inhibition of amyloid-induced toxicity by ergothioneine in a transgenic Caenorhabditis elegans model. FEBS Lett 593: 2139–2150, 2019. [DOI] [PubMed] [Google Scholar]
- 23. Cheah IK, Ong RL, Gruber J, Yew TS, Ng LF, Chen CB, and Halliwell B. Knockout of a putative ergothioneine transporter in Caenorhabditis elegans decreases lifespan and increases susceptibility to oxidative damage. Free Radic Res 47: 1036–1045, 2013. [DOI] [PubMed] [Google Scholar]
- 24. Cheah IK, Tang R, Ye P, Yew TS, Lim KH, and Halliwell B. Liver ergothioneine accumulation in a guinea pig model of non-alcoholic fatty liver disease. A possible mechanism of defence? Free Radic Res 50: 14–25, 2016. [DOI] [PubMed] [Google Scholar]
- 25. Chirita AL, Gheorman V, Bondari D, and Rogoveanu I. Current understanding of the neurobiology of major depressive disorder. Rom J Morphol Embryol 56: 651–658, 2015. [PubMed] [Google Scholar]
- 26. Christensen L, Suggs A, and Baron E. Ultraviolet photobiology in dermatology. Adv Exp Med Biol 996: 89–104, 2017. [DOI] [PubMed] [Google Scholar]
- 27. Cronin H and Draelos ZD. Top 10 botanical ingredients in 2010 anti-aging creams. J Cosmet Dermatol 9: 218–225, 2010. [DOI] [PubMed] [Google Scholar]
- 28. Crossland J, Mitchell J, and Woodruff GN. The presence of ergothioneine in the central nervous system and its probable identity with the cerebellar factor. J Physiol 182: 427–438, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crossland J, Woodruff GN, and Mitchell JF. Identity of the cerebellar factor. Nature 203: 1388–1389, 1964. [DOI] [PubMed] [Google Scholar]
- 30. D'Onofrio N, Servillo L, Giovane A, Casale R, Vitiello M, Marfella R, Paolisso G, and Balestrieri ML. Ergothioneine oxidation in the protection against high-glucose induced endothelial senescence: involvement of SIRT1 and SIRT6. Free Radic Biol Med 96: 211–222, 2016. [DOI] [PubMed] [Google Scholar]
- 31. Dahl TA, Midden WR, and Hartman PE. Some prevalent biomolecules as defenses against singlet oxygen damage. Photochem Photobiol 47: 357–362, 1988. [DOI] [PubMed] [Google Scholar]
- 32. Everse J, Johnson MC, and Marini MA. Peroxidative activities of hemoglobin and hemoglobin derivatives. Methods Enzymol 231: 547–561, 1994. [DOI] [PubMed] [Google Scholar]
- 33. Ey J, Schomig E, and Taubert D. Dietary sources and antioxidant effects of ergothioneine. J Agric Food Chem 55: 6466–6474, 2007. [DOI] [PubMed] [Google Scholar]
- 34. Feletou M and Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 291: H985–H1002, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Feng L, Cheah IK, Ng MM, Li J, Chan SM, Lim SL, Mahendran R, Kua EH, and Halliwell B. The association between mushroom consumption and mild cognitive impairment: a community-based cross-sectional study in Singapore. J Alzheimers Dis 68: 197–203, 2019. [DOI] [PubMed] [Google Scholar]
- 36. Funk C. The journal of State Medicine. Volume XX: 341–368, 1912. The etiology of the deficiency diseases, Beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra. Nutr Rev 33: 176–177, 1975. [DOI] [PubMed] [Google Scholar]
- 37. Genghof DS. Biosynthesis of ergothioneine and hercynine by fungi and Actinomycetales. J Bacteriol 103: 475–478, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Genghof DS and Vandamme O. Biosynthesis of ergothioneine and hercynine by mycobacteria. J Bacteriol 87: 852–862, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gokce G and Arun MZ. Ergothioneine produces relaxation in isolated rat aorta by inactivating superoxide anion. Eur Rev Med Pharmacol Sci 18: 3339–3345, 2014. [PubMed] [Google Scholar]
- 40. Gokce G, Arun MZ, and Ertuna E. Ergothioneine prevents endothelial dysfunction induced by mercury chloride. Exp Ther Med 15: 4697–4702, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gottier Nwafor J, Nowik M, Anzai N, Endou H, and Wagner CA. Metabolic acidosis alters expression of Slc22 transporters in mouse kidney. Kidney Blood Press Res 45: 263–274, 2020. [DOI] [PubMed] [Google Scholar]
- 42. Grundemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, and Schomig E. Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A 102: 5256–5261, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Halliwell B, Cheah IK, and Drum CL. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem Biophys Res Commun 470: 245–250, 2016. [DOI] [PubMed] [Google Scholar]
- 44. Halliwell B, Cheah IK, and Tang RMY. Ergothioneine—a diet-derived antioxidant with therapeutic potential. FEBS Lett 592: 3357–3366, 2018. [DOI] [PubMed] [Google Scholar]
- 45. Hanlon DP. Interaction of ergothioneine with metal ions and metalloenzymes. J Med Chem 14: 1084–1087, 1971. [DOI] [PubMed] [Google Scholar]
- 46. Hartman PE. Ergothioneine as antioxidant. Methods Enzymol 186: 310–318, 1990. [DOI] [PubMed] [Google Scholar]
- 47. Hartman PE, Hartman Z, and Ault KT. Scavenging of singlet molecular oxygen by imidazole compounds: high and sustained activities of carboxy terminal histidine dipeptides and exceptional activity of imidazole-4-acetic acid. Photochem Photobiol 51: 59–66, 1990. [DOI] [PubMed] [Google Scholar]
- 48. Hatano T, Saiki S, Okuzumi A, Mohney RP, and Hattori N. Identification of novel biomarkers for Parkinson's disease by metabolomic technologies. J Neurol Neurosurg Psychiatry 87: 295–301, 2016. [DOI] [PubMed] [Google Scholar]
- 49. Heath H, Rimington C, Glover T, Mann T, and Leone E. Studies using radioactive sulphur on ergothioneine formation in the pig. Biochem J 54: 606–611, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heath H and Wildy J. The biosynthesis of ergothioneine and histidine by Claviceps purpurea. I. The incorporation of [2–14C]acetate. Biochem J 64: 612–620, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heath H and Wildy J. Biosynthesis of ergothioneine. Nature 179: 196–197, 1957. [DOI] [PubMed] [Google Scholar]
- 52. Hopkins FG. Feeding experiments illustrating the importance of accessory factors in normal dietaries. J Physiol 44: 425–460, 1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hseu YC, Vudhya Gowrisankar Y, Chen XZ, Yang YC, and Yang HL. The antiaging activity of ergothioneine in UVA-irradiated human dermal fibroblasts via the inhibition of the AP-1 pathway and the activation of Nrf2-mediated antioxidant genes. Oxid Med Cell Longev 2020: 2576823, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu W, Song H, Sae Her A, Bak DW, Naowarojna N, Elliott SJ, Qin L, Chen X, and Liu P. Bioinformatic and biochemical characterizations of C-S bond formation and cleavage enzymes in the fungus Neurospora crassa ergothioneine biosynthetic pathway. Org Lett 16: 5382–5385, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang SJ, Lin CP, Mau JL, Li YS, and Tsai SY. Effect of UV-B irradiation on physiologically active substance content and antioxidant properties of the medicinal caterpillar fungus Cordyceps militaris (Ascomycetes). Int J Med Mushrooms 17: 241–253, 2015. [DOI] [PubMed] [Google Scholar]
- 56. Huff CD, Witherspoon DJ, Zhang Y, Gatenbee C, Denson LA, Kugathasan S, Hakonarson H, Whiting A, Davis CT, Wu W, Xing J, Watkins WS, Bamshad MJ, Bradfield JP, Bulayeva K, Simonson TS, Jorde LB, and Guthery SL. Crohn's disease and genetic hitchhiking at IBD5. Mol Biol Evol 29: 101–111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hunter G and Eagles BA. The isolation from blood of a hitherto unknown substance, and its bearing on present methods for the estimation of uric acid. J. Biol. Chem 65: 623–642, 1925. [Google Scholar]
- 58. Inazu M, Takeda H, Maehara K, Miyashita K, Tomoda A, and Matsumiya T. Functional expression of the organic cation/carnitine transporter 2 in rat astrocytes. J Neurochem 97: 424–434, 2006. [DOI] [PubMed] [Google Scholar]
- 59. Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, and Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 100: 1–19, 2018. [DOI] [PubMed] [Google Scholar]
- 60. Irani S, Naowarojna N, Tang Y, Kathuria KR, Wang S, Dhembi A, Lee N, Yan W, Lyu H, Costello CE, Liu P, and Zhang YJ. Snapshots of C-S cleavage in Egt2 reveals substrate specificity and reaction mechanism. Cell Chem Biol 25: 519.e4–529.e4, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ishikawa Y, Israel SE, and Melville DB. Participation of an intermediate sulfoxide in the enzymatic thiolation of the imidazole ring of hercynine to form ergothioneine. J Biol Chem 249: 4420–4427, 1974. [PubMed] [Google Scholar]
- 62. Ishimoto T, Masuo Y, Kato Y, and Nakamichi N. Ergothioneine-induced neuronal differentiation is mediated through activation of S6K1 and neurotrophin 4/5-TrkB signaling in murine neural stem cells. Cell Signal 53: 269–280, 2019. [DOI] [PubMed] [Google Scholar]
- 63. Ishimoto T, Nakamichi N, Hosotani H, Masuo Y, Sugiura T, and Kato Y. Organic cation transporter-mediated ergothioneine uptake in mouse neural progenitor cells suppresses proliferation and promotes differentiation into neurons. PLoS One 9: e89434, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jang JH, Aruoma OI, Jen LS, Chung HY, and Surh YJ. Ergothioneine rescues PC12 cells from beta-amyloid-induced apoptotic death. Free Radic Biol Med 36: 288–299, 2004. [DOI] [PubMed] [Google Scholar]
- 65. Kalaras MD, Richie JP, Calcagnotto A, and Beelman RB. Mushrooms: a rich source of the antioxidants ergothioneine and glutathione. Food Chem 233: 429–433, 2017. [DOI] [PubMed] [Google Scholar]
- 66. Kameda M, Teruya T, Yanagida M, and Kondoh H. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc Natl Acad Sci U S A 117: 9483–9489, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kato Y, Kubo Y, Iwata D, Kato S, Sudo T, Sugiura T, Kagaya T, Wakayama T, Hirayama A, Sugimoto M, Sugihara K, Kaneko S, Soga T, Asano M, Tomita M, Matsui T, Wada M, and Tsuji A. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm Res 27: 832–840, 2010. [DOI] [PubMed] [Google Scholar]
- 68. Kawano H, Otani M, Takeyama K, Kawai Y, Mayumi T, and Hama T. Studies on ergothioneine. VI. Distribution and fluctuations of ergothioneine in rats. Chem Pharm Bull (Tokyo) 30: 1760–1765, 1982. [DOI] [PubMed] [Google Scholar]
- 69. Koh SS, Ooi SC, Lui NM, Qiong C, Ho LT, Cheah IK, Halliwell B, Herr DR, and Ong WY. Effect of ergothioneine on 7-ketocholesterol-induced endothelial injury. Neuromolecular Med 23: 184–198, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kondoh H, Teruya T, and Yanagida M. Metabolomics of human fasting: new insights about old questions. Open Biol 10: 200176, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Krnjevic K, Randic M, and Straughan DW. Ergoyhioneine and central neurones. Nature 205: 603–604, 1965. [DOI] [PubMed] [Google Scholar]
- 72. Kumosani TA. L-ergothioneine level in red blood cells of healthy human males in the Western province of Saudi Arabia. Exp Mol Med 33: 20–22, 2001. [DOI] [PubMed] [Google Scholar]
- 73. Lamhonwah AM and Tein I. Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria. Biochem Biophys Res Commun 345: 1315–1325, 2006. [DOI] [PubMed] [Google Scholar]
- 74. Li RW, Yang C, Sit AS, Kwan YW, Lee SM, Hoi MP, Chan SW, Hausman M, Vanhoutte PM, and Leung GP. Uptake and protective effects of ergothioneine in human endothelial cells. J Pharmacol Exp Ther 350: 691–700, 2014. [DOI] [PubMed] [Google Scholar]
- 75. Liao C and Seebeck FP. Convergent evolution of ergothioneine biosynthesis in cyanobacteria. Chembiochem 18: 2115–2118, 2017. [DOI] [PubMed] [Google Scholar]
- 76. Loreto RG and Hughes DP. The metabolic alteration and apparent preservation of the zombie ant brain. J Insect Physiol 118: 103918, 2019. [DOI] [PubMed] [Google Scholar]
- 77. Maeda T, Hirayama M, Kobayashi D, Miyazawa K, and Tamai I. Mechanism of the regulation of organic cation/carnitine transporter 1 (SLC22A4) by rheumatoid arthritis-associated transcriptional factor RUNX1 and inflammatory cytokines. Drug Metab Dispos 35: 394–401, 2007. [DOI] [PubMed] [Google Scholar]
- 78. Mann T and Leone E. Studies on the metabolism of semen. VIII. Ergothioneine as a normal constituent of boar seminal plasma; purification and crystallization; site of formation and function. Biochem J 53: 140–148, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Marciniak S and Perry GH. Harnessing ancient genomes to study the history of human adaptation. Nat Rev Genet 18: 659–674, 2017. [DOI] [PubMed] [Google Scholar]
- 80. Markova NG, Karaman-Jurukovska N, Dong KK, Damaghi N, Smiles KA, and Yarosh DB. Skin cells and tissue are capable of using L-ergothioneine as an integral component of their antioxidant defense system. Free Radic Biol Med 46: 1168–1176, 2009. [DOI] [PubMed] [Google Scholar]
- 81. Martin KR. The bioactive agent ergothioneine, a key component of dietary mushrooms, inhibits monocyte binding to endothelial cells characteristic of early cardiovascular disease. J Med Food 13: 1340–1346, 2010. [DOI] [PubMed] [Google Scholar]
- 82. Mathieson I, Lazaridis I, Rohland N, Mallick S, Patterson N, Roodenberg SA, Harney E, Stewardson K, Fernandes D, Novak M, Sirak K, Gamba C, Jones ER, Llamas B, Dryomov S, Pickrell J, Arsuaga JL, de Castro JM, Carbonell E, Gerritsen F, Khokhlov A, Kuznetsov P, Lozano M, Meller H, Mochalov O, Moiseyev V, Guerra MA, Roodenberg J, Verges JM, Krause J, Cooper A, Alt KW, Brown D, Anthony D, Lalueza-Fox C, Haak W, Pinhasi R, and Reich D. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528: 499–503, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Matsuda Y, Ozawa N, Shinozaki T, Wakabayashi KI, Suzuki K, Kawano Y, Ohtsu I, and Tatebayashi Y. Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl Psychiatry 10: 170, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maxfield L and Crane JS. Vitamin C Deficiency. Treasure Island, FL: StatPearls, 2020. [Google Scholar]
- 85. McClay JL, Adkins DE, Vunck SA, Batman AM, Vann RE, Clark SL, Beardsley PM, and van den Oord EJ. Large-scale neurochemical metabolomics analysis identifies multiple compounds associated with methamphetamine exposure. Metabolomics 9: 392–402, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Melville DB, Eich S, and Ludwig ML. The biosynthesis of ergothioneine. J Biol Chem 224: 871–877, 1957. [PubMed] [Google Scholar]
- 87. Menicucci AR, Jankeel A, Feldmann H, Marzi A, and Messaoudi I. Antiviral innate responses induced by VSV-EBOV vaccination contribute to rapid protection. mBio 10: e00597-19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Misra HP. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem 249: 2151–2155, 1974. [PubMed] [Google Scholar]
- 89. Mollica A, Macedonio G, Stefanucci A, Carradori S, Akdemir A, Angeli A, and Supuran CT. Five- and six-membered nitrogen-containing compounds as selective carbonic anhydrase activators. Molecules 22: 2178, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moncaster JA, Walsh DT, Gentleman SM, Jen LS, and Aruoma OI. Ergothioneine treatment protects neurons against N-methyl-D-aspartate excitotoxicity in an in vivo rat retinal model. Neurosci Lett 328: 55–59, 2002. [DOI] [PubMed] [Google Scholar]
- 91. Motohashi N, Mori I, and Sugiura Y. Complexing of copper ion by ergothioneine. Chem Pharm Bull (Tokyo) 24: 2364–2368, 1976. [DOI] [PubMed] [Google Scholar]
- 92. Nagababu E and Rifkind JM. Reaction of hydrogen peroxide with ferrylhemoglobin: superoxide production and heme degradation. Biochemistry 39: 12503–12511, 2000. [DOI] [PubMed] [Google Scholar]
- 93. Nakamichi N, Nakao S, Nishiyama M, Takeda Y, Ishimoto T, Masuo Y, Matsumoto S, Suzuki M, and Kato Y. Oral administration of the food derived hydrophilic antioxidant ergothioneine enhances object recognition memory in mice. Curr Mol Pharmacol 14: 220–233, 2020. [DOI] [PubMed] [Google Scholar]
- 94. Nakamichi N, Nakayama K, Ishimoto T, Masuo Y, Wakayama T, Sekiguchi H, Sutoh K, Usumi K, Iseki S, and Kato Y. Food-derived hydrophilic antioxidant ergothioneine is distributed to the brain and exerts antidepressant effect in mice. Brain Behav 6: e00477, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nakamichi N, Taguchi T, Hosotani H, Wakayama T, Shimizu T, Sugiura T, Iseki S, and Kato Y. Functional expression of carnitine/organic cation transporter OCTN1 in mouse brain neurons: possible involvement in neuronal differentiation. Neurochem Int 61: 1121–1132, 2012. [DOI] [PubMed] [Google Scholar]
- 96. Nierenberg JL, He J, Li C, Gu X, Shi M, Razavi AC, Mi X, Li S, Bazzano LA, Anderson AH, He H, Chen W, Guralnik JM, Kinchen JM, and Kelly TN. Serum metabolites associate with physical performance among middle-aged adults: evidence from the Bogalusa Heart Study. Aging (Albany NY) 12: 11914–11941, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nikodemus D, Lazic D, Bach M, Bauer T, Pfeiffer C, Wiltzer L, Lain E, Schomig E, and Grundemann D. Paramount levels of ergothioneine transporter SLC22A4 mRNA in boar seminal vesicles and cross-species analysis of ergothioneine and glutathione in seminal plasma. J Physiol Pharmacol 62: 411–419, 2011. [PubMed] [Google Scholar]
- 98. Obayashi K, Kurihara K, Okano Y, Masaki H, and Yarosh DB. L-Ergothioneine scavenges superoxide and singlet oxygen and suppresses TNF-alpha and MMP-1 expression in UV-irradiated human dermal fibroblasts. J Cosmet Sci 56: 17–27, 2005. [PubMed] [Google Scholar]
- 99. Oumari M, Goldfuss B, Stoffels C, Schmalz HG, and Grundemann D. Regeneration of ergothioneine after reaction with singlet oxygen. Free Radic Biol Med 134: 498–504, 2019. [DOI] [PubMed] [Google Scholar]
- 100. Park EJ, Lee WY, Kim ST, Ahn JK, and Bae EK. Ergothioneine accumulation in a medicinal plant Gastrodia elata. J Med Plants Res 4: 1141–1147, 2010. [Google Scholar]
- 101. Paul BD, Lemle MD, Komaroff AL, and Snyder SH. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci U S A 118: e2024358118, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Paul BD and Snyder SH. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ 17: 1134–1140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Peltekova VD, Wintle RF, Rubin LA, Amos CI, Huang Q, Gu X, Newman B, Van Oene M, Cescon D, Greenberg G, Griffiths AM, St George-Hyslop PH, and Siminovitch KA. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet 36: 471–475, 2004. [DOI] [PubMed] [Google Scholar]
- 104. Pfeiffer C, Bach M, Bauer T, Campos da Ponte J, Schomig E, and Grundemann D. Knockout of the ergothioneine transporter ETT in zebrafish results in increased 8-oxoguanine levels. Free Radic Biol Med 83: 178–185, 2015. [DOI] [PubMed] [Google Scholar]
- 105. Pfeiffer C, Bauer T, Surek B, Schomig E, and Grundemann D. Cyanobacteria produce high levels of ergothioneine. Food Chem 129: 1766–1769, 2011. [Google Scholar]
- 106. Pluskal T, Hayashi T, Saitoh S, Fujisawa A, and Yanagida M. Specific biomarkers for stochastic division patterns and starvation-induced quiescence under limited glucose levels in fission yeast. FEBS J 278: 1299–1315, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rahman I, Gilmour PS, Jimenez LA, Biswas SK, Antonicelli F, and Aruoma OI. Ergothioneine inhibits oxidative stress- and TNF-alpha-induced NF-kappa B activation and interleukin-8 release in alveolar epithelial cells. Biochem Biophys Res Commun 302: 860–864, 2003. [DOI] [PubMed] [Google Scholar]
- 108. Rougee M, Bensasson RV, Land EJ, and Pariente R. Deactivation of singlet molecular oxygen by thiols and related compounds, possible protectors against skin photosensitivity. Photochem Photobiol 47: 485–489, 1988. [DOI] [PubMed] [Google Scholar]
- 109. Salt HB. The ergothioneine content of the blood in health and disease. Biochem J 25: 1712–1719, 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, Prabhu SD, Bhatnagar A, Jones SP, and Hill BG. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 7: 634–642, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sbodio JI, Snyder SH, and Paul BD. Regulators of the transsulfuration pathway. Br J Pharmacol 176: 583–593, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Seebeck FP. In vitro reconstitution of Mycobacterial ergothioneine biosynthesis. J Am Chem Soc 132: 6632–6633, 2010. [DOI] [PubMed] [Google Scholar]
- 113. Semba RD. The discovery of the vitamins. Int J Vitam Nutr Res 82: 310–315, 2012. [DOI] [PubMed] [Google Scholar]
- 114. Servillo L, D'Onofrio N, Casale R, Cautela D, Giovane A, Castaldo D, and Balestrieri ML. Ergothioneine products derived by superoxide oxidation in endothelial cells exposed to high-glucose. Free Radic Biol Med 108: 8–18, 2017. [DOI] [PubMed] [Google Scholar]
- 115. Shires TK, Brummel MC, Pulido JS, and Stegink LD. Ergothioneine distribution in bovine and porcine ocular tissues. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 117: 117–120, 1997. [DOI] [PubMed] [Google Scholar]
- 116. Shukla Y, Kulshrestha OP, and Khuteta KP. Ergothioneine content in normal and senile human cataractous lenses. Indian J Med Res 73: 472–473, 1981. [PubMed] [Google Scholar]
- 117. Smith E, Ottosson F, Hellstrand S, Ericson U, Orho-Melander M, Fernandez C, and Melander O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 106: 691–697, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Song TY, Chen CL, Liao JW, Ou HC, and Tsai MS. Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem Toxicol 48: 3492–3499, 2010. [DOI] [PubMed] [Google Scholar]
- 119. Song TY, Lin HC, Chen CL, Wu JH, Liao JW, and Hu ML. Ergothioneine and melatonin attenuate oxidative stress and protect against learning and memory deficits in C57BL/6J mice treated with D-galactose. Free Radic Res 48: 1049–1060, 2014. [DOI] [PubMed] [Google Scholar]
- 120. Sotgia S, Taras A, Zinellu A, Cherchi R, Mangoni AA, Carru C, and Bogliolo L. Hercynine, ergothioneine and redox state in Stallion's seminal plasma. Antioxidants (Basel) 9: 855, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sotgia S, Zinellu A, Mangoni AA, Pintus G, Attia J, Carru C, and McEvoy M. Clinical and biochemical correlates of serum L-ergothioneine concentrations in community-dwelling middle-aged and older adults. PLoS One 9: e84918, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Stoffels C, Oumari M, Perrou A, Termath A, Schlundt W, Schmalz HG, Schafer M, Wewer V, Metzger S, Schomig E, and Grundemann D. Ergothioneine stands out from hercynine in the reaction with singlet oxygen: resistance to glutathione and TRIS in the generation of specific products indicates high reactivity. Free Radic Biol Med 113: 385–394, 2017. [DOI] [PubMed] [Google Scholar]
- 123. Svobodova A, Walterova D, and Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 150: 25–38, 2006. [DOI] [PubMed] [Google Scholar]
- 124. Tang RMY, Cheah IK, Yew TSK, and Halliwell B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci Rep 8: 1601, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tang Y, Masuo Y, Sakai Y, Wakayama T, Sugiura T, Harada R, Futatsugi A, Komura T, Nakamichi N, Sekiguchi H, Sutoh K, Usumi K, Iseki S, Kaneko S, and Kato Y. Localization of xenobiotic transporter OCTN1/SLC22A4 in hepatic stellate cells and its protective role in liver fibrosis. J Pharm Sci 105: 1779–1789, 2016. [DOI] [PubMed] [Google Scholar]
- 126. Tanret C. Sur une base nouvelle retiree du seigle ergote, l'ergothioneine. Compt Rende 49: 22–224, 1909. [Google Scholar]
- 127. Teruya T, Chen YJ, Kondoh H, Fukuji Y, and Yanagida M. Whole-blood metabolomics of dementia patients reveal classes of disease-linked metabolites. Proc Natl Acad Sci U S A 118: e2022857118, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Testa G, Staurenghi E, Zerbinati C, Gargiulo S, Iuliano L, Giaccone G, Fanto F, Poli G, Leonarduzzi G, and Gamba P. Changes in brain oxysterols at different stages of Alzheimer's disease: their involvement in neuroinflammation. Redox Biol 10: 24–33, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, Suzuki M, Nagasaki M, Ohtsuki M, Ono M, Furukawa H, Nagashima M, Yoshino S, Mabuchi A, Sekine A, Saito S, Takahashi A, Tsunoda T, Nakamura Y, and Yamamoto K. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet 35: 341–348, 2003. [DOI] [PubMed] [Google Scholar]
- 130. Tschirka J, Kreisor M, Betz J, and Grundemann D. Substrate selectivity check of the ergothioneine transporter. Drug Metab Dispos 46: 779–785, 2018. [DOI] [PubMed] [Google Scholar]
- 131. Turner E, Brewster JA, Simpson NA, Walker JJ, and Fisher J. Imidazole-based erythrocyte markers of oxidative stress in preeclampsia—an NMR investigation. Reprod Sci 16: 1040–1051, 2009. [DOI] [PubMed] [Google Scholar]
- 132. Whiteman M and Halliwell B. Thiols and disulphides can aggravate peroxynitrite-dependent inactivation of alpha1-antiproteinase. FEBS Lett 414: 497–500, 1997. [DOI] [PubMed] [Google Scholar]
- 133. Williamson RD, McCarthy FP, Manna S, Groarke E, Kell DB, Kenny LC, and McCarthy CM. L-(+)-ergothioneine significantly improves the clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 75: 561–568, 2020. [DOI] [PubMed] [Google Scholar]
- 134. Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, and Ganapathy V. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta 1466: 315–327, 2000. [DOI] [PubMed] [Google Scholar]
- 135. Yang NC, Lin HC, Wu JH, Ou HC, Chai YC, Tseng CY, Liao JW, and Song TY. Ergothioneine protects against neuronal injury induced by beta-amyloid in mice. Food Chem Toxicol 50: 3902–3911, 2012. [DOI] [PubMed] [Google Scholar]
- 136. Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, and Panda S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun 9: 2872, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhao Q, Wang M, Xu D, Zhang Q, and Liu W. Metabolic coupling of two small-molecule thiols programs the biosynthesis of lincomycin A. Nature 518: 115–119, 2015. [DOI] [PubMed] [Google Scholar]
- 138. Zhu BZ, Mao L, Fan RM, Zhu JG, Zhang YN, Wang J, Kalyanaraman B, and Frei B. Ergothioneine prevents copper-induced oxidative damage to DNA and protein by forming a redox-inactive ergothioneine-copper complex. Chem Res Toxicol 24: 30–34, 2011. [DOI] [PubMed] [Google Scholar]