Abstract
Obesity is a disease with high potential for fatality. It perfectly fits the disease definition, as cancer does. This is because it damages body structure and functions, both mechanically and biologically, and alters physical, mental, and social health. In addition, it shares many common morbid characteristics with the most feared disease, cancer. For example, it is influenced by a sophisticated interaction between a person’s genetics, the environment, and an increasing number of other backgrounds. Furthermore, it displays abnormal cell growth and proliferation events, only limited to white fat, resulting in adipose tissue taking up an increasing amount of space within the body. This occurs through fat “metastases” and via altered signaling that further aggravates the pathology of obesity by inducing ubiquitous dishomeostasis. These metastases can be made graver by angiogenesis, which might boost diseased tissue growth. More common features with cancer include its progressive escalation through different levels of severity and its possibility of re-onset after recovery. Despite all these similarities with cancer, obesity is substantially less agitating for most people. Thus, the ideas proposed herein could have utility to sensitize the public opinion about the hard reality of obesity. This is increasingly needed, as the obesity pandemic has waged a fierce war against our bodies and society in general, while there is still doubt about whether it is a real disease or not. Hence, raising public consciousness to properly face health issues is crucial to improving our health instead of gaining weight unhealthily. It is obviously illogical to fight cancer extremely seriously on the one hand and to consider dying with obesity as self-inflicted on the other. In fact, obesity merits a top position among the most lethal diseases besides cancer.
Keywords: obesity, disease, cancer, white adipose tissue, cell growth, cell proliferation, metastasis, angiogenesis, recurrence, gut microbiome
1. Introduction
In our initial steps of perceiving life, we assimilate the names and categories of our surroundings, and we address them accordingly. Clearly, if we consider an apple to be a book, it will decompose after a few days on the bookshelf. Likewise, if we see a disease as a simple discomforting “dis-ease”, we might accelerate the extinction of a healthy humankind, especially if we do not identify a hazardous disease, such as obesity. Unfortunately, although obesity has been recognized as a disease by the World Health Organization (WHO) since 1948, followed by an increasing number of organizations worldwide, “globesity” has been undervalued, as Carol Condon emphasizes in the review paper rightly entitled “The fat bomb exploded but no one heard the bang” [1].
Erroneously, fatness is still considered a sign of wealth or beauty in some low-income countries, such as in some regions of the continent of one of us authors, Africa. Conversely, while being wealthier and having more access to health education, the populations of more industrialized countries still mostly show ambiguity regarding the idea that corpulence is a disease or a risk factor in many diseases. Globally, health insurance companies could have enhanced such unawareness through the refusal to recognize obesity as a disease. Moreover, the marketing strategies of potentially obesogenic food and beverage industries that neglect sensitization about the implications in obesity of their products could have magnified this unconsciousness [2]. Indeed, we encounter such products with attractive shapes, colors, and flavors everywhere, but they are never labeled with photos of things such as steatotic liver or atherosclerotic plaque, for example. In contrast, we commonly see photos of throat or lung cancer, or other smoking-related types of cancer, on cigarette packets, which have been shown to effectively alter public opinions about smoking [3,4]. In addition to all this, social media usually wrongly presents obesity as a mere issue of appearance, thus further boosting the general under-appreciation of this very serious clinical problem [5].
Obesity is a deadly global pandemic, as about 3.4 million obese patients and overweight individuals die yearly [6,7]. Additionally, it contributes to the onset, pervasiveness, severity, and fatality of other illnesses, such as osteoarthritis [8], non-alcoholic fatty liver disease [9], and obstructive sleep apnea [10]; it is also associated with the three leading causes of death, namely, heart disease [11], cancer [12] and—more recently—coronavirus disease [13], by affecting metabolism and different body systems, such as the immune system, and engendering a chronic low-grade inflammation that accelerates several other disorders [14,15]. Nonetheless, reluctance still exists regarding its labeling as a disease, with assertions that such recognition might render obese patients unwilling to make efforts to ameliorate their health [16]. However, such assumptions might be rather subjective, because they are not based on the inherent characteristics of what a disease is. Generally, many people are misled by believing that obesity solely adds extra X letters to the person’s clothing size, which could be exactly the same size as a robust person. Such a shallow view might blur the dangerous X letter that could be developing underneath, through the “metabolic syndrome X” [17], which could be gradually displacing tick marks from physiological towards pathological patterns, such as insulin resistance, dyslipidemia and hypertension [18]. On the other hand, this view also neglects the increasing realization that some types of moderate obesity (with a body mass index (BMI) around 30–32 kg/m2)—while being visually unpleasant for some—can be still devoid of health complications, whereas others—such as abdominal obesity (a condition that may also appear in individuals with 25 kg/m2 <BMI <30 kg/m2), which is still socially acceptable—can be accompanied by cardiometabolic risk as much as morbid obesity [19,20].
Being a contributor to multiple other sicknesses, including stroke, myocardial infarction, and cancer, could have led to the fallacious consideration of obesity as a mere risk factor, while it is one of the most dangerous illnesses. By contrast, there is a worldwide consensus that cancer is the most frightening of sicknesses [21]. Since obesity is a disease and a common cause of many other fatal illnesses, such misconception should be addressed urgently. Indeed, this is the objective of this piece of writing, which aims at depicting obesity morbidity and the numerous pathological attributes that it has in common with the worldwide number-one feared disease, cancer.
2. Obesity Is a Disease
It is widely agreed upon that cancer is a disease, and there is no rational reason that this does not apply to every other sickness. Obviously, we should not disregard obesity, which is itself a disease and contributes to the onset and progression of cancer and other lethal morbi [12]. Even though the definitions of disease and obesity and whether obesity is a morbus, are continually disputed [22,23], an international consensus based on mostly agreed-upon definitions should be established to circumvent such ambiguities. Certain claims support identifying obesity as a disease, as this might help destigmatize obese patients [23]. Although such stigmatization should undoubtedly be avoided, this should be achieved through proper education about the importance of respecting everyone, regardless of their status. Indeed, regardless of the positive outcomes that might result from labeling obesity as a disease, obesity’s morbus identity possesses objective merit based on solidly established definitions [24,25,26,27,28].
The Stedman’s Medical Dictionary introduces “disease” as a synonym for “illness”, “morbus”, and “sickness”, and as “1. An interruption, cessation, or disorder of a body, system, or organ structure or function. 2. A morbid entity ordinarily characterized by two or more of the following criteria: recognized etiologic agent(s), identifiable group of signs and symptoms, or consistent anatomic alterations”. Similarly, the Oxford Concise Medical Dictionary defines it as “a disorder with a specific cause (which may or may not be known) and recognizable signs and symptoms; any bodily abnormality or failure to function properly, except that resulting directly from physical injury (the latter, however, may open the way for disease)”. The WHO, on the other pan of the scales, presents obesity as an anomalous or excessive stockpile of body fat that poses a risk to the health [29], which is determined by a BMI that is greater than or equal to 30 kg/m2, a diagnosis criterion cutoff that could vary according to ethnic groups and other physiological statuses. For example, the BMI value can be healthily higher in the case of a highly muscular or a pregnant body.
By comparing the two concepts, obesity fits perfectly within the definition of “disease”. Indeed, patients with obesity witness a plethora of health disorders and present recognizable pathological manifestations. Mechanically, obesity imposes an excessive pressure on the bones and the joints besides potentially causing airway obstruction that can lead to obstructive sleep apnea [10]. Biologically, it can lead to osteoarthritis [30], and it presents with inflammatory and dysregulated adipokine levels secreted by dysfunctional adipocytes [31,32] in addition to the ectopic abnormal deposition of white adipose tissue (WAT) on vital organs, which seriously alters whole-organism homeostasis [33].
Although relying on the BMI criterion to diagnose obesity is usually controversial [34], as certain individuals can be metabolically healthy at a BMI > 30 kg/m2, such individuals still suffer from the symptoms of mechanical origin, with a few exceptions, such as individuals who present a high amount of muscle that supports the knees [35]. For example, sumo wrestlers are mostly metabolically healthy despite their extremely high BMI because of their intensive physical exercise that keeps them fit and fat, yet they suffer from obesity-induced mechanic disorders [36]. However, BMI through the sole measuring of the body’s height and weight cannot properly reflect the metabolic dysregulation and should be supported by waist girth, blood triglyceride levels, other measurements that can reflect abdominal (visceral) obesity, which is highly morbid [37].
In fact, patients with obesity may find sundry difficulties in accomplishing simple daily life activities, such as tying their shoelaces, which can affect their psychological health [38]. This is further worsened by social media’s deep influence through promoting an “ideal” unrealistic body shape image, which stigmatizes patients with obesity and erroneously makes them feel like incompetent humans [5] in addition to presenting deceptive advice about losing weight strategies [39]. Overall, obesity presents deteriorations in all levels of health, which is defined by the WHO as “a state of complete physical, mental and social well-being and not merely the absence of disease and infirmity” [40]. We can envision this through the difficulty we experience during snowy days when we find it difficult to put on our shoes after wearing a heavy winter jacket, not to mention the suffering of obesity patients. Considering the definitions of obesity, disease, and health, obesity is indisputably a disease.
3. The Obesity–Cancer Connection: Exemplification of Potential Disease Amplification
Obesity’s severity is further aggravated by its capacity to trigger, advance, or favor poor prognosis of, other illnesses through what we might call “obesity indirect downstream metastases”. We are suggesting this expression in reference to the ability of affected WAT alterations in obesity to “metastasize” by inducing or worsening health status with respect to other morbi in other organs. This can be potentially life-threatening, particularly in the case of cancer, which is per se metastatic.
The obesity–cancer causal connection has been extensively hypothesized, investigated, evidenced, and reviewed [41,42,43,44,45,46]. This has built a growing body of consensus about the association of these two pathologies, besides their broadly proven correlation, given the numerous epidemiological and clinical studies pointing out the significantly higher relative risk to develop cancer in obese versus non-obese populations [47,48]. Explorations about these connecting bridges have yielded promising answers, such as the recognition of fat mass and obesity-associated genes (e.g., FTO) as a common mechanistic basis for both cancer and obesity and the finding that obesity-associated dysmetabolism causes genotoxic stress in favor of cancer comorbidity [49,50,51]. This evidence adds to the results from many other in vitro [51,52,53], ex vivo [54,55], and in vivo studies [56,57,58,59] and has been reviewed and acknowledged by the International Agency for Research on Cancer, who announced that there is enough confident and unbiased evidence about the association of excess body weight with a reinforced cancer predisposition—in particular with regard to more than twelve types of cancer in various tissues/organ systems, such as blood, central nervous system, endometrium, esophagus, kidney, pancreas, liver, colon, postmenopausal breast, ovary, gallbladder, and thyroid gland—in agreement with the World Cancer Research Fund/American Institute for Cancer Research [58,60].
Importantly, not only are most WAT cell types potential tumor triggers and promoters, but mature adipocytes might further undergo dedifferentiation or reprogramming into cancer-associated adipocytes that boost tumor energy supply and progression [61,62]. Other obesity–cancer association mechanisms include obesity-induced chronic inflammation [63,64]; increased aromatase expression, circulating levels of estrogen, insulin, leptin and ceruloplasmin; and decreased amounts of adiponectin and sex-hormone-binding globulin [61,65,66,67,68,69,70,71,72,73]. Estrogen, for instance, which can be produced by WAT fibroblasts, is a tumoral growth and malignancy activator through stimulating the expression of crucial receptors, such as the progesterone receptor (PR) and adenosine A1 receptor (ADORA1) [74], besides enhancing cellular multiplication and inhibiting apoptosis [65,75,76].
The implication of genetics in cancer might be prominently exemplified through the critical BRCA1/2 tumor suppressor gene mutations, which, respectively, expose their holders to 60% and 30% higher risk of breast cancer [77,78,79] regardless of the woman’s menopausal status [67]. On the other hand, rare forms of obesity that are uniquely due to defects of genes encoding for, e.g., FTO, melanocortin 4 receptor or leptin, have also been identified [49,80,81,82]. Additionally, several studies have revealed the implication of obesity in strongly carcinogenic genomic instability [50,83]. This multi-branched and sophisticated obesity–cancer tie doubtlessly requires further interdisciplinary collaborative navigation across the genetic, epigenetic, and metabolic profiles of both diseases, and to be complemented also by deeper investigations into the implication of gut flora–host dishomeostasis [84,85,86,87].
4. Obesity and Cancer Common Features
4.1. Multifactorial Grounds and Deranged Metabolism
Both obesity and cancer are multifactorial, mainly caused by genetic predisposition, high-carbohydrate diet, and sedentary lifestyle, besides other etiologies that are being continually discovered and investigated, such as certain medications’ side effects or interactions [88,89], abnormal levels and impactful interactions of some sex hormones (such as estrogen and testosterone) [90,91,92,93], poor sleep habits [94], and stress-engendered pathways [95,96]. Although the genetic basis is best known as an origin of cancer, obesity also owes 40–70% of its interindividual variation to genetic grounds [97]. People should be sensitized to this so society can seriously act on the relatively more manageable factors, such as diet and physical exercise. However, this may not function for every person, as recent studies prove that certain individuals present an inherent state of baseline vulnerability towards obesity, which makes them non-respondent to weight-loss strategies such as low-energy diets [98] and further confirms the complexity of this disease.
Moreover, the predominance of the sedentary lifestyle during the current century, [99], promoted by cell phone (mobile) use, for example, has steered our attention away from our “cellular” health and how to stay “mobile”. This has intensified the positive energy balance and obesity levels within the population. Such a plurality of obesity origins should obviously trigger attentive caution. In fact, the term unifactorial disease is less and less utilized by scientists, as most morbi are rather classified into the multifactorial category. Even so, what is most featured in cancer and obesity, uncommonly to other morbi, is that they are also “multi-organ” diseases, where cancer cells as well as lipid depots might acquire a widespread existence throughout the organism. This multiplies the multifaceted challenging aspects of these diseases with regard to both their prevention and therapy.
It is also striking how deranged metabolism can hallmark both obesity and cancer. For example, high fructose consumption has been interrelated with tumoral growth and malignancy, as this sugar accelerates glycolysis, which fosters the Warburg effect through hastening aerobic fermentation and the production of lactate and uric acid, which are all in favor of cancer development and metastasis [100,101]. Interestingly, metabolic syndrome and obesity have also been suggested to be promoted by excessive fructose consumption [100,102,103]. As metabolism is a “cellular housekeeping process” that maintains whole-body functioning homeostasis, its disturbance in obesity and cancer is particularly intriguing because these morbi further disarrange cellular growth and proliferation.
4.2. Disordered Cell Growth and Proliferation
Obesity and cancer are both characterized by abnormal cellular growth and multiplication. The Dana–Farber comprehensive Cancer Treatment and Research Institution in Massachusetts justly defines cancer as “a disease in which cells, almost anywhere in the body, begin to divide uncontrollably. A tumor is when this uncontrolled growth occurs in solid tissue such as an organ, muscle, or bone. Tumors may spread to surrounding tissues through the blood and lymph systems. Cancer treatment aims to eradicate these abnormal cells, or to slow or stop them from spreading” [104]. Anomalous cell growth and proliferation are widely known fundamental aspects of cancer. Nonetheless, they are less perceived with respect to obesity pathways. One of the main physiological roles of WAT is fatty acid energy storage in the form of triglycerides in mature fat cells to prevent the circulation of these molecules throughout the bloodstream and their potential deposition in different ectopic locations. Additionally, at healthy adiposity levels, WAT plays its homeostatic physiological secretory roles to communicate with other organs—for example, by releasing the hormone leptin, which signals satiety to the brain, particularly the hypothalamus, as well as many other adipokines [105]. The physiological functions of WAT are preserved due to the healthy multiplication of preadipocytes; the progenitor cell reservoir that is ready to differentiate via adipogenesis into mature adipocytes that store lipids without a need for excessive cell growth or proliferation [106]. Nevertheless, obesity displays several alterations of these functions as mature fat cells undergo excessive hypertrophic growth, eventually resulting in cell death and triggering inflammation while preadipocytes proliferate excessively [107,108].
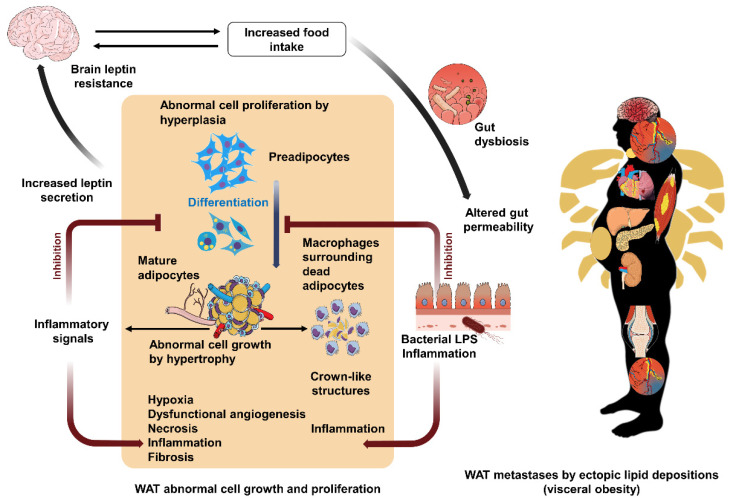
The WHO definition of obesity refers indirectly to these notions by defining it as the abnormal or excessive accumulation of WAT. This description indicates that there exists a balanced WAT amount in the absence of obesity [106]. Chronic, morbid positive energy balance alters WAT functionality, renders it insulin-resistant, and induces its immoderate secretion of leptin, resulting in a loss of sensitivity of the hypothalamus to this hormone in obesity [106]. This vicious circle further enhances food intake and lipid storage and aggravates the morbidity of adiposity. Indeed, in obesity, WAT is forced to highly multiply its number of preadipocytes in an endeavor to elevate the body’s ability to store lipids, especially when hypertrophic adipocytes reach their maximal capacity of growth, and die [106]. However, such proliferation further increases WAT mass via hyperplasia, which adds to that resulting from adipocyte hypertrophy [106]. This hyperproliferation can also be enhanced by obesity-induced gut dysbiosis that augments intestinal permeability and bacterial lipopolysaccharide (LPS) levels in the blood towards the WAT, where it can further increase this multiplication by inhibiting adipogenesis [109]. LPS, moreover, enhances WAT inflammation, which is already triggered by the crown-like structures formed by recruited immune cells, mainly macrophages, that surround dead hypertrophic adipocytes [109]. Such a chain of vicious circles promotes WAT morbid cellular growth and proliferation (Figure 1).
Figure 1.
Vicious circles of white adipose tissue (WAT) abnormal cell growth and proliferation, and fat mass metastases. In obesity, the brain becomes less sensitive to the satiety hormone leptin, and this favors feelings of hunger and increases food intake. This further boosts the positive energy balance, induces intestinal flora dysbiosis and augments gut permeability to the bacterial endotoxin lipopolysaccharide (LPS), which is highly inflammatory. LPS inhibits the differentiation of preadipocytes to mature fat cells. Thus, more preadipocytes are formed to generate new adipocytes to store the body’s excess of energy, which enhances obesity by hyperplasia. The inhibition of adipogenesis induces mature adipocytes to undergo extreme abnormal cell growth to pack lipids that surpass their maximum storage capacity through excessive hypertrophy. This further causes hypoxia, fibrosis, and eventually cell necrosis. Massive WAT hypertrophy might induce severe hypoxia that can trigger angiogenesis, which, however, generates fragile and dysfunctional capillaries that further boost tissue inflammation. The dead adipocytes attract immune cells—macrophages—to form inflammatory crown-like structures. The affected WAT secretes high leptin levels to the brain to reduce food intake; however, the brain becomes insensitive to leptin due to the ongoing processes of obesity. WAT in the obese organism further secrets inflammatory cytokines to promote the inflammatory reaction in a chain of vicious circles that devastates WAT structure and function. This leads to lipid storage in other organs, like skeletal muscles and vital organs such as the heart, the liver, the kidneys, and the pancreas. Such expansion of WAT localization through ectopic fat depositions that embody “metastases” alters these organs functionality and therefore whole-body homeostasis. In addition, accumulated free fatty acids in blood circulation may injure endothelial cells through provoking oxidative and inflammatory reactions, which might hasten atherosclerotic plaque rupture. This can cause venous or arterial thromboembolism, which can present fatal consequences when affecting vital organs, such as in pulmonary embolism, heart attack, and stroke. These biological alterations are accompanied by those starting from a mechanical origin, such as body weight pressure on the bones and joints or airway blockage, to induce osteoarthritis or obstructive sleep apnea, respectively. These crab (cancer)-leg-like projections of cell growth, cell proliferation, and fat ectopic depositions, and their impact on the whole body in obesity, justly present high similarity with cancer disease metastases. Created with BioRender.com and with MindtheGraph.com. Accessed on 24 March 2022.
4.3. Metastasis
Consulting the Oxford Concise Medical Dictionary, we find that metastasis is “the spread of a malignant tumour from its site of origin”. Equivalently, the medically inexpert Oxford Learner’s Dictionary defines it as “the development of tumours in different parts of the body resulting from cancer that has started in another part of the body”. By browsing through the PubMed search engine, again we realize that the word metastasis is always linked to tumors or cancer. Thus, metastasis is mostly considered to be solely associated with cancer both for the public and for medical experts. This feature of cancer is its most feared because it multiplies the number of organs that are affected by the disease and magnifies its deadly effect. The definition of cancer by the WHO inherently includes this notion as it introduces cancer as the following: “Cancer is a large group of diseases that can start in almost any organ or tissue of the body when abnormal cells grow uncontrollably, go beyond their usual boundaries to invade adjoining parts of the body and/or spread to other organs. The latter process is called metastasizing and is a major cause of death from cancer. A neoplasm and malignant tumour are other common names for cancer”.
These definitions show tight similarity with ectopic lipid deposition in obesity, where the WAT goes beyond its usual limits, which are mostly subcutaneous, to infiltrate other organs through the uncontrolled expansion of visceral WAT [110]— a depot that, like in cancer metastases, has been suggested to be functionally and metabolically different from healthy deposits [111,112]. When visceral WAT also becomes insufficient to store excess triglycerides, these end up being deposited not only in skeletal muscles, but also in the liver and other vital organs—such as the kidneys, pancreas, and heart—whose biological machinery is not suited to store lipids, and, therefore, eventually become dysfunctional to the point of complete organ failure [110,113]. In addition, the accumulated free fatty acids in the plasma may injure endothelial cells via triggering oxidative stress and inflammatory reactions, which may prompt atherosclerotic plaque rupture [114]. The migrating thrombus might cause venous or arterial vessel thromboembolism that can be lethal, such as in pulmonary embolism, myocardial infarction, and stroke [115]. Therefore, such fat metastases can lead to whole-body dishomeostasis (Figure 1). In summary, obesity, like cancer, is distinctive due to the danger of “metastases” when compared to other diseases that target mainly one organ. This concept can be extended even further if one considers that, although not necessarily via diffusion of fat in the body: (1) obesity is very often followed by type 2 diabetes (which is as a dysfunction of the pancreas), which in turn can lead to the failure of several peripheral organs and tissues [116], and (2) obesity is accompanied by neuroinflammation and alterations in synaptic plasticity, thus also affecting brain function [117,118].
4.4. Aggravation by Angiogenesis
Healthy forward and backward blood vascularization is key for the proper functioning of body tissues. Such a crucial input and output system is so necessary that capillary blood vessels can burgeon from the primary ones in response to tissue growth requirements through a process known as angiogenesis [119]. However, diseased tissues can also utilize such a sprouting strategy to grow, as best known in cancer [120]. Indeed, most research publications and therapeutical applications of angiostatic mechanisms are dedicated to anticancer treatment, for instance, through combining them with other interventions [121,122]. However, angiogenesis is implicated in many other common diseases, such as arthritis, polycystic ovary syndrome, asthma, and obesity [120].
In fact, WAT possesses rich blood vascularization that supports its multiple functions, and angiogenesis is one of its physiological growth mechanisms, besides hyperplasia and hypertrophy, which both can signal to enhance it [123,124]. In healthily growing WAT, angiogenesis is stimulated to compensate for the minor hypoxia resulting from mild WAT mass increase [125]. In contrast, the considerably expanded WAT size in obesity, through massive hypertrophy, results in serious tissue hypoxia, which the tissue vascularization cannot cope with [106]. In addition, this excessive hypoxia might lead to WAT fibrosis and inflammation and can eventually induce necrosis processes [106]. Furthermore, obesity-associated WAT inflammation drives and is enhanced by the growth of unhealthy capillaries, whose features are fragile structural remodeling and leakiness, that infiltrate the WAT with immune cells and boost its inflammation [126]. These pathological processes are exacerbated by the synergistic activation of inflamed WAT intrinsic immune cells with those that infiltrate the WAT, such as macrophages, which both release inflammatory secretions to further stimulate unhealthy capillary growth and add a further vicious circle to these tissue-diseasing processes [122,123,127,128].
Oncology research has shown that multiple angiogenic proteins and their receptors show increased expression triggered by cancer-related hypoxia and inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). One important such protein is the vascular endothelial growth factor (VEGF) [129,130]. Cancer-cell-secreted VEGF binds its receptor on the surface of endothelial cells to promote capillary growth that, in turn, nourishes cancer growth [129]. Likewise, WAT is richly vascularized with its inherent endothelial cells that communicate paracrinally with their “tissue-mate” fat cells, which can secrete similar proteins to those expressed in cancer—such as TNF-α, IL-6, and VEGF—and also antiangiogenic factors, such as endostatin, which—while balanced in healthy WAT—might indirectly or directly exacerbate this tissue hypoxia in obesity through inducing ineffective or repressed angiogenesis [124,131,132]. This might result in further exacerbation of the aforementioned tissue-damaging vicious circles that bring about hypoxia and inflammation. Additionally, aberrant angiogenesis impairs WAT communication and signal transmission to other organs and systems, which is a concrete endocrine role that WAT must play to maintain whole-body homeostasis [133].
However, targeting neovascularization to treat obesity is still in its very early stages compared to treating cancer [134]. For example, angiostatin is already undergoing clinical trials to treat human cancer [135], and endostatin is another antiangiogenic drug that is already an antineoplastic medication [136]. Obesity research might, therefore, follow and learn from the use of angiostatic statins against cancer.
The metastatic development of obesity and cancer presents a unique pathological networking strategy of disease-spreading throughout the body, as explained in the previous subsection. This networking escalation—by hijacking the functions of the body’s largest network, i.e., the vascular system, and burgeoning new, but unhealthy, blood vessels that nurture these diseases’ unhealthy growth, hypoxia, and inflammation—can directly further aggravate the progression of these disorders [120].
4.5. Progressive Development and Stages
One of the reasons why people may consider obesity as a less serious illness than cancer is that they compare advanced stages of cancer to “benign” obesity stages. In fact, both illnesses might advance progressively in the absence of proper diagnosis and intervention and may subtly develop until suddenly triggering stroke [137] or an advanced brain tumor [138], for instance. However, deaths that are caused by obesity are massively attributed to the consequent morbi and neglect the implication of obesity, such as in cardiovascular ischemic events or even cancer. On the contrary, cancer is always indicated as the death cause when it is involved. Obesity is globally classified into three categories based on BMI measurements, with respect to certain adaptations according to ethnicity and athlete or pregnant status, namely, obesity class I—BMI 30 kg/m2 to 34.9 kg/m2; obesity class II—BMI 35 kg/m2 to 39.9 kg/m2; and severe obesity (class III) for higher BMI values [139]. The more recent Edmonton Obesity Staging System (EOSS) ranges obesity stages from zero to four, in accordance with the occurrence of related comorbidities in addition to the overall health situation, where stage 4 is the severe, potentially end-stage [140] that might be properly labeled “malignant”, with reference to its seriously damaged health profile.
Likewise, tumor progression is classically divided into four classes considering its growth characteristics and propagation, namely, classes I (A, B, C), II, III, and finally class IV lesions marked with metastases [141]. The more updated tumor node metastasis (TNM) classification system based on recent advances in cancer research has been internationally recognized as the optimal criterion for cancer staging by involving tumor size and local growth (T), the magnitude of lymph node metastases (N), and the existence of distant metastases (M) [142]. Similar classifications might be suggested for obesity, to measure the extent of visceral deposits. In summary, both obesity and cancer present stages of progression and thus display categories of illness seriousness from “benign” to “malignant”. However, these descriptions are rarely used when referring to obesity. The phrase “malignant obesity” could properly warn of its peril.
4.6. Recurrence
The fear of cancer recurrence has been extremely heeded and scrutinized. This bestowed it with the 42-item Fear of Cancer Recurrence Inventory (FCRI) scale, which was developed to assess its severity [143]. Obesity can also present relapses, named weight cycling or yo-yo effect, which refers to unintentional and out of control weight regain and its maintenance [144]. However, when consulting the literature and obesity terminology, we fail to notice a scale that evaluates the fright of obesity. What we find is the 50-item Fat Phobia Scale, for example, which does not examine the fear of obesity recurrence, nor even of the obesity disease per se [145]. Instead, it estimates the feelings and judgments towards people suffering from obesity [145]. Even the updated versions of the Fat Phobia Scale did not add measurements of obese patients’ fear of their condition or its relapse, but rather were limited to shortening the scale form under the same old concept [145,146]. Obviously, a lot of work is needed to properly address obesity relapse. This can start by learning from cancer recurrence scales, such as by establishing one named Fear of Obesity Recurrence Inventory. This might concede and evaluate the fear of obesity relapse to better help approach the cases in which patients are frightened of becoming stuck in a recurrent obese status. Such endeavors could aid in the improvement of population awareness about such a potentially recurrent morbus that should be “feared” instead of the patients with obesity.
4.7. Multidimensional Complexity from the Gut Microbiome
An increasing body of evidence supports the existence of a sturdy link between the micro-organisms living in our gastrointestinal system and constituting, with their genes and their armamentarium of bioactive molecules, the gut microbiome, and their host health or disease status [147]. This association is specifically fateful in obesity and cancer [148,149] due to the synergistic combination of these diseases unique complex features, explained above, and the huge intricacy of the gut microbiota community that is still being deciphered [150]. Being both metastatic and chronic disorders, obesity and cancer can devastate body functions in terms of both the dimension of body area and that of time of progression. Their impact is mostly further complicated by their playing on a third, very sophisticated, dimension, i.e., that of the gut microflora. Several recent reports have highlighted that the gut microbiota do not merely play one major role in cancer and obesity pathology. In fact, they can play several roles, i.e.,: (i) by presenting potential transmissibility of the disease but also of a healthier status through therapeutic fecal microbiota transplantation [151,152]; (ii) by providing opportunities for nonconventional therapeutic strategies, such as probiotic, prebiotic, or symbiotic treatments [153,154]; and (iii) by acting as both an actor and biomarker of the host’s response to the treatment [155,156]. This tridimensional complexity that combines time, space, and gut microbiome is more perplexing if one considers the other dimensions that affect the gut microbiota ecosystem, such as genetics, epigenetics, and developmental and environmental/lifestyle factors [157,158].
5. Conclusions and Perspectives
Obesity is a disease by scientific definition and international organization recognition. It further shares a plethora of morbidity characteristics with the most feared illness worldwide, cancer. This is because (1) it has multiple causes, (2) it presents anomalous cell growth and proliferation, (3) it displays metastatic events, (4) it can unhealthily develop through angiogenesis, (5) it might advance progressively from a “benign” to a “malignant” stage, (6) it has a high risk of relapse, and (7) it can present multidimensional complexity from the gut microbiome. Moreover, obesity contributes to the onset and deleterious progress of several other serious morbi, including those of more than 13 types of cancer [12]. Thus, very strong similarities and connections exist with cancer. Finally, the development, progress, and response to therapy of both obesity and cancer are now considered to also be strongly influenced by the gut microbiome. Despite these facts, many people doubt the disease identity of obesity, which imposes the urgent need for more population sensitization about its dangers.
This can be achieved by correcting our obesity terminology to rectify our unconscious beliefs that can be deeply affected by our words [159,160,161]. We can encourage others to stop using the words “lose” and “gain” when describing body weight changes. Such words, besides employing the term deposit, when referring to obesity, may indirectly refer to our hidden desire to have extra weight and fat depots as we want extra money and its deposit. Has anyone had heard phrases like “lose” or “gain” a tumor? Similarly, the term “metastasis” could more suitably indicate the gravity of ectopic lipid deposition instead of the word “deposit”. Moreover, the word “yo-yo” should be replaced by the more serious designation “recurrence”, which seriously emphasizes that obesity is far from being a game. Likewise, the word “neoplasia”, which means new formation (from Greek), which is solely used to refer to tumoral growth, can be further utilized to appropriately describe ectopic fat establishment in visceral obesity, which indeed results from neo-fat-mass growth. These propositions can be applied in scientific publications and presentations and to refine official health organizations’ and associations’ definitions of obesity. To dispel any doubts, this serious pathology should be directly named “obesity morbus” to engrave this identity of it in our minds, attitudes, and actions. We hope that this multioriented vigilance weathervane will inspire optimized navigation through the perception of other diseases as well.
Author Contributions
Conceptualization, B.B.; writing—original draft preparation, B.B.; writing—review and editing, C.S. and V.D.M.; visualization, B.B.; supervision, C.S. and V.D.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Canada Research Excellence Chair in the Microbiome-Endocannabinoidome Axis in Metabolic Health (CERC-MEND), which is funded by the Tri-Agency of the Canadian Federal Government (The Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Social Sciences and Humanities Research Council of Canada (SSHRC)), to V.D.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Condon C. The fat bomb exploded but no one heard the bang. Eur. J. Cardiovasc. Nurs. 2006;5:99–101. doi: 10.1016/j.ejcnurse.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery K., Chester J., Nixon L., Levy L., Dorfman L. Big Data and the transformation of food and beverage marketing: Undermining efforts to reduce obesity? Crit. Public Health. 2019;29:110–117. doi: 10.1080/09581596.2017.1392483. [DOI] [Google Scholar]
- 3.Strong D.R., Pierce J.P., Pulvers K., Stone M.D., Villaseñor A., Pu M., Dimofte C.V., Leas E.C., Oratowski J., Brighton E., et al. Effect of Graphic Warning Labels on Cigarette Packs on US Smokers’ Cognitions and Smoking Behavior After 3 Months: A Randomized Clinical Trial. JAMA Netw. Open. 2021;4:e2121387. doi: 10.1001/jamanetworkopen.2021.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho Y.J., Thrasher J.F., Davis R., Kim S.-H., Hardin J., Popova L. Effective package warning label systems for communicating relative risks of cigarettes, heated tobacco products, and e-cigarettes: An experimental study with Korean adults. Int. J. Drug Policy. 2022;99:103468. doi: 10.1016/j.drugpo.2021.103468. [DOI] [PubMed] [Google Scholar]
- 5.Stanford F.C., Tauqeer Z., Kyle T.K. Media and Its Influence on Obesity. Curr. Obes. Rep. 2018;7:186–192. doi: 10.1007/s13679-018-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Lancet Gastroenterology & Hepatology Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021;6:411. doi: 10.1016/S2468-1253(21)00143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith K.B., Smith M.S. Obesity Statistics. Prim. Care Clin. Off. Pract. 2016;43:121–135. doi: 10.1016/j.pop.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni K., Karssiens T., Kumar V., Pandit H. Obesity and osteoarthritis. Maturitas. 2016;89:22–28. doi: 10.1016/j.maturitas.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Mariani S., Fiore D., Varone L., Basciani S., Persichetti A., Watanabe M., Saponara M., Spera G., Moretti C., Gnessi L. Obstructive sleep apnea and bone mineral density in obese patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2012;5:395–401. doi: 10.2147/DMSO.S37761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duflou J., Virmani R., Rabin I., Burke A., Farb A., Smialek J. Sudden death as a result of heart disease in morbid obesity. Am. Heart J. 1995;130:306–313. doi: 10.1016/0002-8703(95)90445-X. [DOI] [PubMed] [Google Scholar]
- 12.Friedenreich C.M., Ryder-Burbidge C., McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021;15:790–800. doi: 10.1002/1878-0261.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busetto L., Bettini S., Fabris R., Serra R., Dal Pra C., Maffei P., Rossato M., Fioretto P., Vettor R. Obesity and COVID-19: An Italian Snapshot. Obesity. 2020;28:1600–1605. doi: 10.1002/oby.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancková M., Betáková T. Pandemics of the 21st Century: The Risk Factor for Obese People. Viruses. 2021;14:25. doi: 10.3390/v14010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro A.M., Macedo-de la Concha L.E., Pantoja-Meléndez C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Médica Hosp. Gen. México. 2017;80:101–105. doi: 10.1016/j.hgmx.2016.06.011. [DOI] [Google Scholar]
- 16.Katz D.L. Perspective: Obesity is not a disease. Nature. 2014;508:S57. doi: 10.1038/508S57a. [DOI] [PubMed] [Google Scholar]
- 17.Cornier M.-A., Dabelea D., Hernandez T.L., Lindstrom R.C., Steig A.J., Stob N.R., Van Pelt R.E., Wang H., Eckel R.H. The Metabolic Syndrome. Endocr. Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
- 19.Parikh R.M., Joshi S.R., Menon P.S., Shah N.S. Index of central obesity—A novel parameter. Med. Hypotheses. 2007;68:1272–1275. doi: 10.1016/j.mehy.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 20.Sahakyan K.R., Somers V.K., Rodriguez-Escudero J.P., Hodge D.O., Carter R.E., Sochor O., Coutinho T., Jensen M.D., Roger V.L., Singh P. Normal-weight central obesity: Implications for total and cardiovascular mortality. Ann. Intern. Med. 2015;163:827–835. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrinten C., McGregor L.M., Heinrich M., von Wagner C., Waller J., Wardle J., Black G.B. What do people fear about cancer? A systematic review and meta-synthesis of cancer fears in the general population. Psychooncology. 2017;26:1070–1079. doi: 10.1002/pon.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scully J.L. What is a disease? EMBO Rep. 2004;5:650–653. doi: 10.1038/sj.embor.7400195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen H. Is Obesity A Disease or A Behavior Abnormality? Did the AMA Get It Right? MO Med. 2014;111:104–108. [PMC free article] [PubMed] [Google Scholar]
- 24.Jung R.T. Obesity as a disease. Br. Med. Bull. 1997;53:307–321. doi: 10.1093/oxfordjournals.bmb.a011615. [DOI] [PubMed] [Google Scholar]
- 25.Bray G.A., Kim K.K., Wilding J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017;18:715–723. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- 26.Tucker S., Bramante C., Conroy M., Fitch A., Gilden A., Wittleder S., Jay M. The Most Undertreated Chronic Disease: Addressing Obesity in Primary Care Settings. Curr. Obes. Rep. 2021;10:396–408. doi: 10.1007/s13679-021-00444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rippe J.M., Crossley S., Ringer R. Obesity as a Chronic Disease: Modern Medical and Lifestyle Management. J. Am. Diet. Assoc. 1998;98:S9–S15. doi: 10.1016/S0002-8223(98)00704-4. [DOI] [PubMed] [Google Scholar]
- 28.Fujioka K. Management of obesity as a chronic disease: Nonpharmacologic, pharmacologic, and surgical options. Obes. Res. 2002;10((Suppl. S2)):116S–123S. doi: 10.1038/oby.2002.204. [DOI] [PubMed] [Google Scholar]
- 29.Obesity. [(accessed on 22 March 2022)]. Available online: https://www.who.int/health-topics/obesity#tab=tab_1.
- 30.Berenbaum F., Eymard F., Houard X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013;25:114–118. doi: 10.1097/BOR.0b013e32835a9414. [DOI] [PubMed] [Google Scholar]
- 31.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skurk T., Alberti-Huber C., Herder C., Hauner H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 33.Kong Y., Zhang S., Wu R., Su X., Peng D., Zhao M., Su Y. New insights into different adipokines in linking the pathophysiology of obesity and psoriasis. Lipids Health Dis. 2019;18:171. doi: 10.1186/s12944-019-1115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothman K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008;32((Suppl. S3)):S56–S59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Ramirez D.C., van der Leeden M., van der Esch M., Roorda L.D., Verschueren S., van Dieën J., Lems W.F., Dekker J. Increased knee muscle strength is associated with decreased activity limitations in established knee osteoarthritis: Two-year follow-up study in the Amsterdam osteoarthritis cohort. J. Rehabil. Med. 2015;47:647–654. doi: 10.2340/16501977-1973. [DOI] [PubMed] [Google Scholar]
- 36.Nishizawa T., Akaoka I., Nishida Y., Kawaguchi Y., Hayashi E., Yoshimura T. Some factors related to obesity in the Japanese sumo wrestler. Am. J. Clin. Nutr. 1976;29:1167–1174. doi: 10.1093/ajcn/29.10.1167. [DOI] [PubMed] [Google Scholar]
- 37.de Cuevillas B., Alvarez-Alvarez I., Riezu-Boj J.I., Navas-Carretero S., Martinez J.A. The hypertriglyceridemic-waist phenotype as a valuable and integrative mirror of metabolic syndrome traits. Sci. Rep. 2021;11:21859. doi: 10.1038/s41598-021-01343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haga B.M., Furnes B., Dysvik E., Ueland V. Putting life on hold: Lived experiences of people with obesity. Scand J. Caring Sci. 2020;34:514–523. doi: 10.1111/scs.12756. [DOI] [PubMed] [Google Scholar]
- 39.Syed-Abdul S., Fernandez-Luque L., Jian W.S., Li Y.C., Crain S., Hsu M.H., Wang Y.C., Khandregzen D., Chuluunbaatar E., Nguyen P.A., et al. Misleading health-related information promoted through video-based social media: Anorexia on YouTube. J. Med. Internet Res. 2013;15:e30. doi: 10.2196/jmir.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nobile M. The who definition of health: A critical reading. Med. Law. 2014;33:33–40. [PubMed] [Google Scholar]
- 41.De Pergola G., Silvestris F. Obesity as a major risk factor for cancer. J. Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger N.A. Obesity and cancer pathogenesis. Ann. N. Y. Acad. Sci. 2014;1311:57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ungefroren H., Gieseler F., Lehnert H. Obesity and cancer. Internist. 2015;56:127–128, 130–136. doi: 10.1007/s00108-014-3536-4. [DOI] [PubMed] [Google Scholar]
- 44.The L. The link between cancer and obesity. Lancet. 2017;390:1716. doi: 10.1016/s0140-6736(17)32659-4. [DOI] [PubMed] [Google Scholar]
- 45.Ottaiano A., De Divitiis C., Capozzi M., Avallone A., Pisano C., Pignata S., Tafuto S. Obesity and Cancer: Biological Links and Treatment Implications. Curr. Cancer Drug Targets. 2018;18:231–238. doi: 10.2174/1568009617666170330125619. [DOI] [PubMed] [Google Scholar]
- 46.Allott E.H., Hursting S.D. Obesity and cancer: Mechanistic insights from transdisciplinary studies. Endocr. Relat. Cancer. 2015;22:R365–R386. doi: 10.1530/ERC-15-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutiérrez-Salmerón M., Chocarro-Calvo A., García-Martínez J.M., de la Vieja A., García-Jiménez C. Epidemiological bases and molecular mechanisms linking obesity, diabetes, and cancer. Endocrinol. Diabetes Nutr. 2017;64:109–117. doi: 10.1016/j.endinu.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez C.J., George A.S., Subrahmanyan N.A., Pappachan J.M. Epidemiological link between obesity, type 2 diabetes mellitus and cancer. World J. Methodol. 2021;11:23–45. doi: 10.5662/wjm.v11.i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X., Yang Y., Sun B.F., Zhao Y.L., Yang Y.G. FTO and obesity: Mechanisms of association. Curr. Diabetes Rep. 2014;14:486. doi: 10.1007/s11892-014-0486-0. [DOI] [PubMed] [Google Scholar]
- 50.Lan N., Lu Y., Zhang Y., Pu S., Xi H., Nie X., Liu J., Yuan W. FTO—A Common Genetic Basis for Obesity and Cancer. Front. Genet. 2020;11:559138. doi: 10.3389/fgene.2020.559138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavazos D.A., deGraffenried M.J., Apte S.A., Bowers L.W., Whelan K.A., deGraffenried L.A. Obesity promotes aerobic glycolysis in prostate cancer cells. Nutr. Cancer. 2014;66:1179–1186. doi: 10.1080/01635581.2014.951738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teslow E.A., Mitrea C., Bao B., Mohammad R.M., Polin L.A., Dyson G., Purrington K.S., Bollig-Fischer A. Obesity-induced MBD2_v2 expression promotes tumor-initiating triple-negative breast cancer stem cells. Mol. Oncol. 2019;13:894–908. doi: 10.1002/1878-0261.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ringel A.E., Drijvers J.M., Baker G.J., Catozzi A., García-Cañaveras J.C., Gassaway B.M., Miller B.C., Juneja V.R., Nguyen T.H., Joshi S., et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell. 2020;183:1848–1866.e1826. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asante E.C., Pallegar N.K., Hoffmann A.J., Viloria-Petit A.M., Christian S.L. Adipose Tissue from Lean and Obese Mice Induces a Mesenchymal to Epithelial Transition-Like Effect in Triple Negative Breast Cancers Cells Grown in 3-Dimensional Culture. Int. J. Mol. Sci. 2020;21:6439. doi: 10.3390/ijms21176439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salameh T.S., Le T.T., Nichols M.B., Bauer E., Cheng J., Camarillo I.G. An ex vivo co-culture model system to evaluate stromal-epithelial interactions in breast cancer. Int. J. Cancer. 2013;132:288–296. doi: 10.1002/ijc.27672. [DOI] [PubMed] [Google Scholar]
- 56.Kim W.G., Cheng S.Y. Mechanisms Linking Obesity and Thyroid Cancer Development and Progression in Mouse Models. Horm. Cancer. 2018;9:108–116. doi: 10.1007/s12672-017-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hursting S.D., Nunez N.P., Varticovski L., Vinson C. The obesity-cancer link: Lessons learned from a fatless mouse. Cancer Res. 2007;67:2391–2393. doi: 10.1158/0008-5472.CAN-06-4237. [DOI] [PubMed] [Google Scholar]
- 58.Avgerinos K.I., Spyrou N., Mantzoros C.S., Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Calle E.E., Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 60.Scully T., Ettela A., LeRoith D., Gallagher E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2020;10:615375. doi: 10.3389/fonc.2020.615375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieman K.M., Romero I.L., Van Houten B., Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snaebjornsson M.T., Janaki-Raman S., Schulze A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020;31:62–76. doi: 10.1016/j.cmet.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Kolb R., Sutterwala F.S., Zhang W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharm. 2016;29:77–89. doi: 10.1016/j.coph.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iyengar N.M., Gucalp A., Dannenberg A.J., Hudis C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cowey S., Hardy R.W. The metabolic syndrome: A high-risk state for cancer? Am. J. Pathol. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone T.W., McPherson M., Gail Darlington L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine. 2018;30:14–28. doi: 10.1016/j.ebiom.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iyengar N.M., Zhou X.K., Mendieta H., El-Hely O., Giri D.D., Winston L., Falcone D.J., Wang H., Meng L., Ha T., et al. Effects of obesity on breast aromatase expression and systemic metabo-inflammation in women with BRCA1 or BRCA2 mutations. NPJ Breast Cancer. 2021;7:18. doi: 10.1038/s41523-021-00226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, Inflammation, and Cancer. Ann. Rev. Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 69.Pu X., Chen D. Targeting Adipokines in Obesity-Related Tumors. Front. Oncol. 2021;11:685923. doi: 10.3389/fonc.2021.685923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ackerman G.E., Smith M.E., Mendelson C.R., MacDonald P.C., Simpson E.R. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J. Clin. Endocrinol. Metab. 1981;53:412–417. doi: 10.1210/jcem-53-2-412. [DOI] [PubMed] [Google Scholar]
- 71.Mair K.M., Gaw R., MacLean M.R. Obesity, estrogens and adipose tissue dysfunction—Implications for pulmonary arterial hypertension. Pulm. Circ. 2020;10:2045894020952019. doi: 10.1177/2045894020952023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker J.M., Al-Nakkash L., Herbst-Kralovetz M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 73.Nelson L.R., Bulun S.E. Estrogen production and action. J. Am. Acad. Derm. 2001;45:S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 74.Bulun S.E., Chen D., Moy I., Brooks D.C., Zhao H. Aromatase, breast cancer and obesity: A complex interaction. Trends Endocrinol. Metab. 2012;23:83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis-Wambi J.S., Jordan V.C. Estrogen regulation of apoptosis: How can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hopkins B.D., Goncalves M.D., Cantley L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016;34:4277–4283. doi: 10.1200/JCO.2016.67.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daniele A., Divella R., Pilato B., Tommasi S., Pasanisi P., Patruno M., Digennaro M., Minoia C., Dellino M., Pisconti S., et al. Can harmful lifestyle, obesity and weight changes increase the risk of breast cancer in BRCA 1 and BRCA 2 mutation carriers? A Mini review. Hered Cancer Clin. Pract. 2021;19:45. doi: 10.1186/s13053-021-00199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hawsawi Y.M., Al-Numair N.S., Sobahy T.M., Al-Ajmi A.M., Al-Harbi R.M., Baghdadi M.A., Oyouni A.A., Alamer O.M. The role of BRCA1/2 in hereditary and familial breast and ovarian cancers. Mol. Genet. Genom. Med. 2019;7:e879. doi: 10.1002/mgg3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sefton P. Testing for BRCA1/2 Mutations. JAMA. 2017;318:2054. doi: 10.1001/jama.2017.17280. [DOI] [PubMed] [Google Scholar]
- 80.Farooqi I.S., Keogh J.M., Yeo G.S., Lank E.J., Cheetham T., O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 81.Farooqi I.S., Yeo G.S., Keogh J.M., Aminian S., Jebb S.A., Butler G., Cheetham T., O’Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Investig. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fairbrother U., Kidd E., Malagamuwa T., Walley A. Genetics of Severe Obesity. Curr. Diabetes Rep. 2018;18:85. doi: 10.1007/s11892-018-1053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kompella P., Vasquez K.M. Obesity and cancer: A mechanistic overview of metabolic changes in obesity that impact genetic instability. Mol. Carcinog. 2019;58:1531–1550. doi: 10.1002/mc.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qin Y., Roberts J.D., Grimm S.A., Lih F.B., Deterding L.J., Li R., Chrysovergis K., Wade P.A. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018;19:7. doi: 10.1186/s13059-018-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mohr A.E., Jäger R., Carpenter K.C., Kerksick C.M., Purpura M., Townsend J.R., West N.P., Black K., Gleeson M., Pyne D.B., et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020;17:24. doi: 10.1186/s12970-020-00353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lynch B.M. Sedentary behavior and cancer: A systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol. Biomark. Prev. 2010;19:2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 87.Kushner R.F., Sorensen K.W. Lifestyle medicine: The future of chronic disease management. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20:389–395. doi: 10.1097/01.med.0000433056.76699.5d. [DOI] [PubMed] [Google Scholar]
- 88.Onitilo A.A., Engel J.M., Glurich I., Stankowski R.V., Williams G.M., Doi S.A. Diabetes and cancer II: Role of diabetes medications and influence of shared risk factors. Cancer Causes Control. 2012;23:991–1008. doi: 10.1007/s10552-012-9971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leslie W.S., Hankey C.R., Lean M.E.J. Weight gain as an adverse effect of some commonly prescribed drugs: A systematic review. QJM Int. J. Med. 2007;100:395–404. doi: 10.1093/qjmed/hcm044. [DOI] [PubMed] [Google Scholar]
- 90.Brown L.M., Clegg D.J. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kelly D.M., Jones T.H. Testosterone and obesity. Obes. Rev. 2015;16:581–606. doi: 10.1111/obr.12282. [DOI] [PubMed] [Google Scholar]
- 92.Zamani A.R.N., Avci Ç.B., Ahmadi M., Pouyafar A., Bagheri H.S., Fathi F., Heidarzadeh M., Rezaie J., Mirhosseini Y., Saberianpour S., et al. Estradiol modulated colorectal cancer stem cells bioactivity and interaction with endothelial cells. Life Sci. 2020;257:118078. doi: 10.1016/j.lfs.2020.118078. [DOI] [PubMed] [Google Scholar]
- 93.Yassin A., AlRumaihi K., Alzubaidi R., Alkadhi S., Al Ansari A. Testosterone, testosterone therapy and prostate cancer. Aging Male. 2019;22:219–227. doi: 10.1080/13685538.2018.1524456. [DOI] [PubMed] [Google Scholar]
- 94.St-Onge M.P. Sleep-obesity relation: Underlying mechanisms and consequences for treatment. Obes. Rev. 2017;18((Suppl. S1)):34–39. doi: 10.1111/obr.12499. [DOI] [PubMed] [Google Scholar]
- 95.Michopoulos V. Stress-induced alterations in estradiol sensitivity increase risk for obesity in women. Physiol. Behav. 2016;166:56–64. doi: 10.1016/j.physbeh.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iftikhar A., Islam M., Shepherd S., Jones S., Ellis I. Cancer and Stress: Does It Make a Difference to the Patient When These Two Challenges Collide? Cancers. 2021;13:163. doi: 10.3390/cancers13020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loos R.J. Recent progress in the genetics of common obesity. Br. J. Clin. Pharm. 2009;68:811–829. doi: 10.1111/j.1365-2125.2009.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tremblay A., Fogelholm M., Jalo E., Westerterp-Plantenga M.S., Adam T.C., Huttunen-Lenz M., Stratton G., Lam T., Handjieva-Darlenska T., Handjiev S., et al. What Is the Profile of Overweight Individuals Who Are Unsuccessful Responders to a Low-Energy Diet? A Preview Sub-study. Front. Nutr. 2021;8:707682. doi: 10.3389/fnut.2021.707682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arocha Rodulfo J.I. Sedentary lifestyle a disease from xxi century. Clin. Investig. Arter. 2019;31:233–240. doi: 10.1016/j.arteri.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 100.Pereira R.M., Botezelli J.D., da Cruz Rodrigues K.C., Mekary R.A., Cintra D.E., Pauli J.R., da Silva A.S.R., Ropelle E.R., de Moura L.P. Fructose Consumption in the Development of Obesity and the Effects of Different Protocols of Physical Exercise on the Hepatic Metabolism. Nutrients. 2017;9:405. doi: 10.3390/nu9040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakagawa T., Lanaspa M.A., Millan I.S., Fini M., Rivard C.J., Sanchez-Lozada L.G., Andres-Hernando A., Tolan D.R., Johnson R.J. Fructose contributes to the Warburg effect for cancer growth. Cancer Metab. 2020;8:16. doi: 10.1186/s40170-020-00222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tappy L., Lê K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 103.Mortera R.R., Bains Y., Gugliucci A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. (Landmark Ed.) 2019;24:186–211. doi: 10.2741/4713. [DOI] [PubMed] [Google Scholar]
- 104.Tumor Definition: What You Need to Know. [(accessed on 22 March 2022)]. Available online: https://blog.dana-farber.org/insight/2018/05/difference-cancer-tumor/#:~:text=Cancer%20is%20a%20disease%20in,the%20blood%20and%20lymph%20systems.
- 105.Fasshauer M., Blüher M. Adipokines in health and disease. Trends Pharm. Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 106.Ghaben A.L., Scherer P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019;20:242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 107.Jo J., Gavrilova O., Pack S., Jou W., Mullen S., Sumner A.E., Cushman S.W., Periwal V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Longo M., Zatterale F., Naderi J., Parrillo L., Formisano P., Raciti G.A., Beguinot F., Miele C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019;20:2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hersoug L.G., Møller P., Loft S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018;31:153–163. doi: 10.1017/S0954422417000269. [DOI] [PubMed] [Google Scholar]
- 110.Borén J., Taskinen M.R., Olofsson S.O., Levin M. Ectopic lipid storage and insulin resistance: A harmful relationship. J. Intern. Med. 2013;274:25–40. doi: 10.1111/joim.12071. [DOI] [PubMed] [Google Scholar]
- 111.Pascual G., Domínguez D., Benitah S.A. The contributions of cancer cell metabolism to metastasis. Dis. Models Mech. 2018;11:dmm032920. doi: 10.1242/dmm.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ibrahim M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 113.Opazo-Ríos L., Mas S., Marín-Royo G., Mezzano S., Gómez-Guerrero C., Moreno J.A., Egido J. Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities. Int. J. Mol. Sci. 2020;21:2632. doi: 10.3390/ijms21072632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang M.H., Cao Y.X., Wu L.G., Guo N., Hou B.J., Sun L.J., Guo Y.L., Wu N.Q., Dong Q., Li J.J. Association of plasma free fatty acids levels with the presence and severity of coronary and carotid atherosclerotic plaque in patients with type 2 diabetes mellitus. BMC Endocr. Disord. 2020;20:156. doi: 10.1186/s12902-020-00636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lowe G.D. Common risk factors for both arterial and venous thrombosis. Br. J. Haematol. 2008;140:488–495. doi: 10.1111/j.1365-2141.2007.06973.x. [DOI] [PubMed] [Google Scholar]
- 116.Daryabor G., Atashzar M.R., Kabelitz D., Meri S., Kalantar K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020;11:1582. doi: 10.3389/fimmu.2020.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valcarcel-Ares M.N., Tucsek Z., Kiss T., Giles C.B., Tarantini S., Yabluchanskiy A., Balasubramanian P., Gautam T., Galvan V., Ballabh P., et al. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J. Gerontol. Ser. A. 2019;74:290–298. doi: 10.1093/gerona/gly127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Z., Ge Q., Wu Y., Zhang J., Gu Q., Han J. Impairment of long-term memory by a short-term high-fat diet via hippocampal oxidative stress and alterations in synaptic plasticity. Neuroscience. 2020;424:24–33. doi: 10.1016/j.neuroscience.2019.10.050. [DOI] [PubMed] [Google Scholar]
- 119.Folkman J. Angiogenesis. In: Jaffe E.A., editor. Biology of Endothelial Cells. Springer; Boston, MA, USA: 1984. pp. 412–428. [DOI] [Google Scholar]
- 120.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 121.Hamming L.C., Slotman B.J., Verheul H.M.W., Thijssen V.L. The clinical application of angiostatic therapy in combination with radiotherapy: Past, present, future. Angiogenesis. 2017;20:217–232. doi: 10.1007/s10456-017-9546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weiss A., Bonvin D., Berndsen R.H., Scherrer E., Wong T.J., Dyson P.J., Griffioen A.W., Nowak-Sliwinska P. Angiostatic treatment prior to chemo- or photodynamic therapy improves anti-tumor efficacy. Sci. Rep. 2015;5:8990. doi: 10.1038/srep08990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hafidi M.E., Buelna-Chontal M., Sánchez-Muñoz F., Carbó R. Adipogenesis: A Necessary but Harmful Strategy. Int. J. Mol. Sci. 2019;20:3657. doi: 10.3390/ijms20153657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Herold J., Kalucka J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front. Physiol. 2021;11:1861. doi: 10.3389/fphys.2020.624903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crewe C., An Y.A., Scherer P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017;127:74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ribatti D., Crivellato E. Immune cells and angiogenesis. J. Cell Mol. Med. 2009;13:2822–2833. doi: 10.1111/j.1582-4934.2009.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sunderkötter C., Goebeler M., Schulze-Osthoff K., Bhardwaj R., Sorg C. Macrophage-derived angiogenesis factors. Pharmacol. Ther. 1991;51:195–216. doi: 10.1016/0163-7258(91)90077-Y. [DOI] [PubMed] [Google Scholar]
- 128.Catalan V., Gomez-Ambrosi J., Rodríguez A., Frühbeck G. Adipose tissue immunity and cancer. Front. Physiol. 2013;4:275. doi: 10.3389/fphys.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nishida N., Yano H., Nishida T., Kamura T., Kojiro M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aggarwal B.B., Shishodia S., Sandur S.K., Pandey M.K., Sethi G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 131.Nijhawans P., Behl T., Bhardwaj S. Angiogenesis in obesity. Biomed. Pharm. 2020;126:110103. doi: 10.1016/j.biopha.2020.110103. [DOI] [PubMed] [Google Scholar]
- 132.Muz B., de la Puente P., Azab F., Azab A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pogodziński D., Ostrowska L., Smarkusz-Zarzecka J., Zyśk B. Secretome of Adipose Tissue as the Key to Understanding the Endocrine Function of Adipose Tissue. Int. J. Mol. Sci. 2022;23:2309. doi: 10.3390/ijms23042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H., Chen Y., Lu X.A., Liu G., Fu Y., Luo Y. Endostatin Prevents Dietary-Induced Obesity by Inhibiting Adipogenesis and Angiogenesis. Diabetes. 2015;64:2442–2456. doi: 10.2337/db14-0528. [DOI] [PubMed] [Google Scholar]
- 135.Cao Y., Xue L. Seminars in Thrombosis and Hemostasis. Volume 30. Thieme Medical Publishers, Inc.; New York, NY, USA: 2004. Angiostatin; pp. 83–93. [DOI] [PubMed] [Google Scholar]
- 136.Zhang C., Deng W.-Y., Li N., Luo S.-X. Clinical observation and therapeutic evaluation of intravenous pump of recombinant human endostatin combined with TP regimen in treating patients with advanced ovarian cancer. Chronic Dis. Transl. Med. 2015;1:158–162. doi: 10.1016/j.cdtm.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Phillips L.H., Whisnant J.P., Reagan T.J. Sudden death from stroke. Stroke. 1977;8:392–395. doi: 10.1161/01.STR.8.3.392. [DOI] [PubMed] [Google Scholar]
- 138.Riezzo I., Zamparese R., Neri M., De Stefano F., Parente R., Pomara C., Turillazzi E., Ventura F., Fineschi V. Sudden, unexpected death due to glioblastoma: Report of three fatal cases and review of the literature. Diagn. Pathol. 2013;8:73. doi: 10.1186/1746-1596-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weir C.B., Jan A. StatPearls. StatPearls Publishing; Tampa Island, FL, USA: 2022. BMI Classification Percentile and Cut off Points. [PubMed] [Google Scholar]
- 140.Canning K.L., Brown R.E., Wharton S., Sharma A.M., Kuk J.L. Edmonton Obesity Staging System Prevalence and Association with Weight Loss in a Publicly Funded Referral-Based Obesity Clinic. J. Obes. 2015;2015:619734. doi: 10.1155/2015/619734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clark W.H. Tumour progression and the nature of cancer. Br. J. Cancer. 1991;64:631–644. doi: 10.1038/bjc.1991.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Telloni S.M. Tumor Staging and Grading: A Primer. Methods Mol. Biol. 2017;1606:1–17. doi: 10.1007/978-1-4939-6990-6_1. [DOI] [PubMed] [Google Scholar]
- 143.Smith A.B., Costa D., Galica J., Lebel S., Tauber N., van Helmondt S.J., Zachariae R. Spotlight on the Fear of Cancer Recurrence Inventory (FCRI) Psychol. Res. Behav. Manag. 2020;13:1257–1268. doi: 10.2147/PRBM.S231577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mehta T., Smith D.L., Jr., Muhammad J., Casazza K. Impact of weight cycling on risk of morbidity and mortality. Obes. Rev. 2014;15:870–881. doi: 10.1111/obr.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bacon J.G., Scheltema K.E., Robinson B.E. Fat phobia scale revisited: The short form. Int. J. Obes. 2001;25:252–257. doi: 10.1038/sj.ijo.0801537. [DOI] [PubMed] [Google Scholar]
- 146.Stein J., Luppa M., Ruzanska U., Sikorski C., König H.-H., Riedel-Heller S.G. Measuring Negative Attitudes towards Overweight and Obesity in the German Population—Psychometric Properties and Reference Values for the German Short Version of the Fat Phobia Scale (FPS) PLoS ONE. 2014;9:e114641. doi: 10.1371/journal.pone.0114641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 148.Lee C.J., Sears C.L., Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020;1461:37–52. doi: 10.1111/nyas.14107. [DOI] [PubMed] [Google Scholar]
- 149.Rajagopala S.V., Vashee S., Oldfield L.M., Suzuki Y., Venter J.C., Telenti A., Nelson K.E. The Human Microbiome and Cancer. Cancer Prev. Res. 2017;10:226–234. doi: 10.1158/1940-6207.CAPR-16-0249. [DOI] [PubMed] [Google Scholar]
- 150.Adak A., Khan M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019;76:473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen D., Wu J., Jin D., Wang B., Cao H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer. 2019;145:2021–2031. doi: 10.1002/ijc.32003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kang Y., Cai Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones. 2017;16:223–234. doi: 10.1007/BF03401517. [DOI] [PubMed] [Google Scholar]
- 153.Bultman S.J. Seminars in Oncology. Elsevier; Amsterdam, The Netherlands: 2016. The microbiome and its potential as a cancer preventive intervention; pp. 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Delzenne N.M., Neyrinck A.M., Cani P.D. Modulation of the gut microbiota by nutrients with prebiotic properties: Consequences for host health in the context of obesity and metabolic syndrome. Microb. Cell Factories. 2011;10:S10. doi: 10.1186/1475-2859-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang M., Fukui H., Eda H., Xu X., Kitayama Y., Hara K., Kodani M., Tomita T., Oshima T., Watari J., et al. Involvement of gut microbiota in association between GLP-1/GLP-1 receptor expression and gastrointestinal motility. Am. J. Physiol. Gastrointest Liver Physiol. 2017;312:G367–G373. doi: 10.1152/ajpgi.00232.2016. [DOI] [PubMed] [Google Scholar]
- 156.Thompson N.A., Stewart G.D., Welsh S.J., Doherty G.J., Robinson M.J., Neville B.A., Vervier K., Harris S.R., Adams D.J., Dalchau K., et al. The MITRE trial protocol: A study to evaluate the microbiome as a biomarker of efficacy and toxicity in cancer patients receiving immune checkpoint inhibitor therapy. BMC Cancer. 2022;22:99. doi: 10.1186/s12885-021-09156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cuevas-Sierra A., Ramos-Lopez O., Riezu-Boj J.I., Milagro F.I., Martinez J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019;10:S17–S30. doi: 10.1093/advances/nmy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miro-Blanch J., Yanes O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front. Genet. 2019;10:638. doi: 10.3389/fgene.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tabibnia G., Lieberman M.D., Craske M.G. The lasting effect of words on feelings: Words may facilitate exposure effects to threatening images. Emotion. 2008;8:307–317. doi: 10.1037/1528-3542.8.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Darlow B., Dowell A., Baxter G.D., Mathieson F., Perry M., Dean S. The enduring impact of what clinicians say to people with low back pain. Ann. Fam. Med. 2013;11:527–534. doi: 10.1370/afm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wood M.L. Naming the illness: The power of words. Fam. Med. 1991;23:534–538. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.