Abstract
Simple Summary
Electrochemotherapy and irreversible electroporation are primarily used for treating patients with cutaneous and subcutaneous tumors and pancreatic cancer, respectively. Increasing numbers of studies have shown that the treatments may elicit an immune response in addition to eliminating the tumor cells. The purpose of this review is to give an in-depth introduction to the electroporation-induced immune response and the local and peripheral immune systems, and to describe the various studies investigating the combination of electroporation and immunotherapy. The review may help guide and inspire the design of future clinical trials investigating the potential synergy of electroporation and immunotherapy in cancer treatment.
Abstract
The discovery of electroporation in 1968 has led to the development of electrochemotherapy (ECT) and irreversible electroporation (IRE). ECT and IRE have been established as treatments of cutaneous and subcutaneous tumors and locally advanced pancreatic cancer, respectively. Interestingly, the treatment modalities have been shown to elicit immunogenic cell death, which in turn can induce an immune response towards the tumor cells. With the dawn of the immunotherapy era, the potential of combining ECT and IRE with immunotherapy has led to the launch of numerous studies. Data from the first clinical trials are promising, and new combination regimes might change the way we treat tumors characterized by low immunogenicity and high levels of immunosuppression, such as melanoma and pancreatic cancer. In this review we will give an introduction to ECT and IRE and discuss the impact on the immune system. Additionally, we will present the results of clinical and preclinical trials, investigating the combination of electroporation modalities and immunotherapy.
Keywords: electrochemotherapy, irreversible electroporation, immunotherapy, immune response, abscopal effect
1. Introduction
In 1968, the first experiments with electroporation were conducted and showed that electric fields increased cell membrane permeability [1]. In 1982, electroporation was demonstrated as an efficient method for transferring DNA into cells [2], thus increasing the uptake of DNA to alter the properties of the cells, which was termed gene electrotransfer (GET). Later in the 1980s, experiments combining electroporation with chemotherapy, i.e., electrochemotherapy (ECT), paved the way for the first clinical trial in 1993 in head and neck squamous cell carcinomas [3]. The use of irreversible electroporation (IRE) was patented in 2003, followed by the first clinical trial in 2010 in prostate cancer [4]. To this day, the major use of electroporation in medicine is within cancer treatment.
The Abscopal Effect
In 1953, R. H. Mole first coined the term “abscopal”, meaning “away from target”, after documenting the remission of tumors located outside the radiation field in patients with metastatic disease [5]. Since then, almost 50 case reports have been published on the abscopal effect due to radiotherapy alone within various cancers, including melanoma, renal cell carcinoma, and breast cancer [6,7]. The median time to progression was 6 months at the site of the abscopal effect, while potential improvements in survival were not reported. A number of patients showed no recurrence for up to 10 years after achieving abscopal effects with complete response (CR). The abscopal effect is caused by an immune response against tumors that have not been treated directly with, e.g., radiotherapy. However, the rarity of the abscopal effect underlines the difficulties needed to be overcome in order to elicit a significant immune response. Five key events have been linked to effective priming of the T cells: (I) release of tumor-associated antigens (TAAs), (II) release of damage-associated molecular patterns (DAMPs), (III) uptake and processing of TAAs by antigen-presenting cells (APCs), (IV) antigen presentation by APCs to naïve T cells, and finally (V) activation and proliferation of cancer-specific CD8+ T cells [7,8]. In recent years, following the introduction of immune checkpoint inhibitors (ICIs) such as anti-programmed cell death receptor/ligand 1 (anti-PD-1/PD-L1) and anti-cytotoxic T lymphocyte antigen 4 (anti-CTLA-4), novel immunotherapy drugs for intratumoral administration have been investigated. These include stimulator of interferon gene (STING) agonists and Toll-like receptor (TLR) agonists, which can be combined with systemic immunostimulatory drugs. To provide an effective immune response and boost the low abscopal effect rates [7], the focus is emerging on combining electroporation modalities with immunotherapy [9,10,11,12]. In this review, we introduce the concepts of ECT and IRE and the interplay between these treatment modalities—the local and systemic immune responses and cancer cells. Finally, we address the current and future perspectives of combining electroporation with immunotherapy.
2. Electroporation
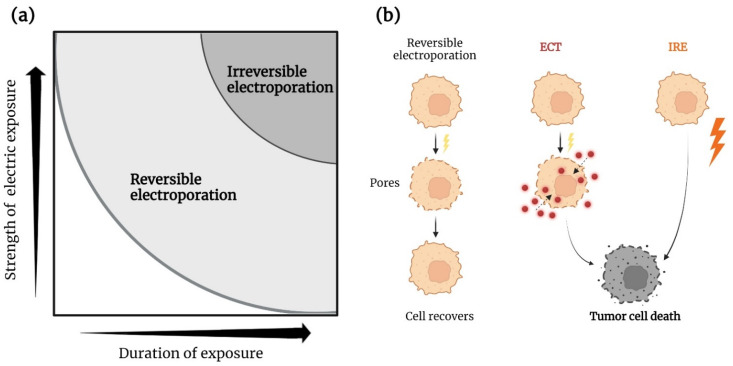
Electroporation causes the plasma cell membrane to become permeable to otherwise impermeable molecules due to the exposure of an external electrical current. Electroporation is either reversible or irreversible depending on the duration and the voltage of the electrical pulses (Figure 1). The electric field generated by reversible electroporation temporarily increases the permeability of the cell membrane by the formation of nanopores in the lipid bilayer. This is achieved by applying a series of around eight electrical pulses with a duration of 100 μsec and an amplitude of 100–1000 volts. These parameters depend on a number of factors including the type of electrodes and tumor [12,13,14]. Reversible electroporation in itself only temporarily disrupts cell homeostasis, leaving the cell to fully recover after exposure. This formation of nanopores makes the modality optimal for transferring otherwise insoluble drugs and small molecules into cells [15].
Figure 1.
(a) The strength and duration of electrical stimulation determines the cellular outcome. (b) The addition of agents such as chemotherapy can prevent cancer cells from recovery and lead to cell death. ECT, electrochemotherapy; IRE, irreversible electroporation. Created with BioRender.com (accessed on 7 April 2022).
IRE is executed by a large number of pulses ranging from 80 to 100 and up to 3000 volts [16]. Due to the longer duration of exposure and higher amplitude, the treated cells cannot regain homeostasis and undergo cell death. The cell death is non-selective and occurs through both apoptosis and necrosis by irreversible disruption of the membrane and the transfer of ATP and electrolytes [17].
The type of cell death initiated by electroporation is important for its ability to elicit immunogenic cell death (ICD) [18]. ICD is associated with the release of DAMPs, most importantly ATP, high mobility group box 1 (HMGB1) protein, and calreticulin [19]. ATP acts as a “homing” signal, attracting and activating dendritic cells (DCs) [20]; HMGB1 binds to TLR4 and enhances the processing and presentation of TAAs by DCs [21]; and calreticulin acts as an “eat me” signal for phagocytes [22]. Apoptosis is generally considered a non-immunogenic form of cell death; however, caspases can up- or downregulate the release of DAMPs [23], leading to immunogenicity [24]. Necrosis causes the release of higher levels of DAMPs than does apoptosis and is considered an immunogenic type of cell death [18]. Surpassing apoptosis and necrosis in eliciting ICD is necroptosis, which is a form of programmed necrosis independent of caspase activity, which more efficiently activates and primes CD8+ T cells against tumor cells [25]. Finally, pyroptosis, a highly inflammatory type of programmed cell death as opposed to apoptosis, is associated with a marked release of DAMPs [18,26]. The properties of the way electroporation initiates cell death are also key, as both the amount and preservation of released TAAs impact the generation of tumor antigen-specific CD8+ T cells. IRE has been shown to produce a greater release and preservation of proteins compared to thermal therapy (e.g., radiofrequency and microwave), which was correlated to T cell activation and proliferation [8]. Thus, the quality of both ICD and the release of TAAs are linked to the abscopal effect.
In recent years, a technology similar to IRE called nanosecond pulsed electric fields has attracted attention. As the name implies, the range of the impulses is within the nanosecond range, which might potentially improve the pulse energy control and reduce the muscle contractions related to the IRE treatment [27]. However, due to limited use of the technique and sparse literature available, this will not be further discussed in this review [28]. Finally, it has been proposed to use adjuvant calcium and thereby either lower the electric field thresholds of IRE to reduce potential thermal damage or to induce cell death of the reversibly electroporated cells in the periphery of the tumors [29].
2.1. Electrochemotherapy
Bleomycin and cisplatin are the most used chemotherapy drugs in combination with reversible electroporation. Both drugs possess two central properties; they both diffuse poorly across the cell membrane and are highly cytotoxic inside cells, making them optimal for ECT. Bleomycin and cisplatin mainly exert their cytotoxicity by damaging nuclear DNA via forming double-stranded DNA crosslinks and inducing DNA strand breaks, respectively [30]. Administering bleomycin with reversible electroporation decreases the inhibitory concentration several hundred fold [31]. It potentiates the cytotoxicity of bleomycin up to 5000 fold and that of cisplatin up to 12 fold [13]. Additionally, due to the low dosage of the drugs, fewer adverse reactions are seen [32].
In Europe alone, ECT as a monotherapy is being used in 140 cancer centers treating numerous types of cancers, including liver and pancreatic tumors [33,34,35]; melanoma, Kaposi sarcoma; and breast, renal cell, and basal cell carcinoma [36], though it is mainly being used for treating cutaneous and subcutaneous tumors in a palliative setting [37,38]. The procedure has been introduced into European clinical guidelines in melanoma for inoperable skin metastases or primary tumors of the limbs [39,40] and in primary squamous cell carcinoma for inoperable, locally advanced lesions [41]. Further, the Italian Society of Orthopedics and Traumatology has included the procedure in guidelines for unresectable bone metastases of the sacrum [42].
2.2. Irreversible Electroporation
IRE is primarily used for treating solid tumors that are unresectable and where thermal ablation or radiotherapy are contra-indicated due to the close proximity of vital structures such as large blood vessels. Due to its mainly non-thermal properties, IRE preserves structures such as blood vessels and biliary tracts by sparing connective tissue and the integrity of the surrounding healthy tissue. The safety and efficacy of IRE have been investigated in several cancers [16], with the most promising overall survival (OS) and recurrence results in pancreatic [43,44], liver [45], and prostate cancer [46,47]. In particular, locally advanced pancreatic cancer represents the most immediate and biggest perspectives of IRE [48]. However, despite the numerous phase I and II trials, larger prospective registries and randomized controlled trials (RCT) directly comparing IRE with standard of care treatment are warranted before IRE can become a widely used cancer treatment modality.
3. Modulation of the Immune System
3.1. The Interplay between Cancer and Immune Cells
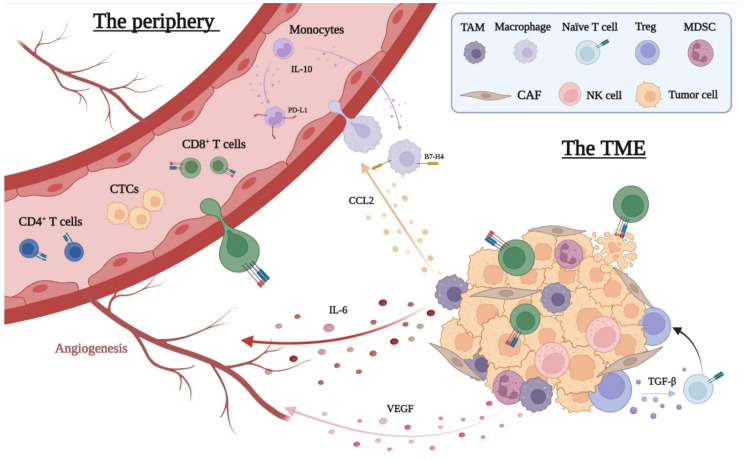
The immune system’s recognition and elimination of malignant cells is of major importance in the development and progression of cancer [49], and its key role in cancer biology and therapy is becoming increasingly more evident [50,51]. Immune evasion, a well-described hallmark of cancer, has gained great research interest during the past decade, and the increasing use of immunotherapy for certain types of solid cancers, such as non-small cell lung cancer, melanoma, breast cancer, and colorectal cancer [52], has made this even more evident. However, despite the development of novel immunotherapy modalities, such as ICIs and adaptive cell transfer for different types of cancer, as well as the continuous research in the field of immuno-oncology, most solid cancers are less responsive or resistant to immunotherapy [53,54]. In continuation, patients with the same type of cancer may respond dissimilarly to identical therapies [54]. The response variations to immunotherapy across cancers and between patients with the same cancer emphasizes the complexity of tumor biology. The constitution of the local tumor microenvironment (TME) as well as the responsiveness of peripheral immune cells may shift the balance towards either immune elimination or immune evasion of cancer cells [55]. This mechanism is very complex and involves the changing interplay between cancer cells, immune cells, and the physical properties of the TME. Here, we will describe immune responses related to cancer cells located in the primary tumor site and in the periphery (Figure 2).
Figure 2.
The interplay between the peripheral immune system and the tumor microenvironment (TME). CAF, cancer-associated fibroblast; CCL, CC chemokine ligand; CTC, circulating tumor cell; IL, interleukin; MDSC, myeloid-derived suppressor cell; PD-L1, programmed death-ligand 1; TAM, tumor-associated macrophage; Treg, regulatory T cell; VEGF, vascular endothelial growth factor. Created with BioRender.com (accessed on 7 April 2022).
3.2. The Local Immune Response to Cancer
When immune cells, especially lymphocytes but also natural killer cells (NK cells), recognize the presence of malignant cells, they can infiltrate the tumor site to eliminate cancer cells through various killing mechanisms. The immune cells that are primarily involved in this process are the CD8+ T cells and the CD4+ T cells. Both cell types are central components of the adaptive immune system and are activated by APCs of the innate immune system. On the other hand, NK cells of the innate immune system do not depend on activation by APCs to elicit cancer-killing capabilities through cell lysis and the secretion of cytokines [56]. The activation of CD8+ and CD4+ T cells is highly dependent on signals from the APCs, for which the direct cell-to-cell contact is essential. Activated APCs take up antigens or epitopes and present them to T cells, thereby activating adaptive responses and T cell infiltration. Thus, cancer immunosurveillance is highly dependent on both adaptive and innate immune responses and the interplay between them [57].
Abnormal innate and adaptive immune responses contribute to the development of malignant tumors. Failed immune responses aid in the selection of cancer cell clones that are less likely to undergo immune destruction [58]. Selection pressure and a tumor promoting TME ensure continuous growth and survival advantage. The TME consists of various cell types and signaling molecules that influence cancer progression as well as responses to anti-neoplastic treatments. High levels of CD8+ T cells at the tumor site have been correlated with improved survival outcomes in various cancers, including colorectal, breast, and pancreatic cancer [59,60,61]. On the other hand, high levels of the immunosuppressive FoxP3+ regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSC) at the tumor site have been associated with worse prognosis in cancer [62]. Normally, Tregs ensure immune tolerance towards the body’s own cells and prevent the development of autoimmune diseases. The immune responses are continuously calibrated through the secretion of pro- and anti-inflammatory mediators such as interleukins and chemokines. Ultimately, a balance is maintained between the elimination of abnormal cells and the survival of healthy cells under normal circumstances. Through tumor secreted factors and tumor secreted exosomes, cancer cells manage to tip this balance towards a higher infiltration of Tregs and thereby towards an immunosuppressive TME. Together with other immune cell subsets, such as the tumor-associated macrophages (TAMs), the Tregs can compose an immune-evasive TME to enhance the survival and proliferation of cancer cells. Tumor secreted factors and tumor secreted exosomes factors cause an expansion of MDSC, known to possess powerful immunosuppressive properties and are attracted to the TME by chemokine gradients. Tregs, MDSC, and TAMs are known to secrete interleukin 10 (IL-10), transforming growth-factor β (TGF-β), vascular endothelial growth factor, and prostaglandins, leading to immune evasion, neo-angiogenesis, cancer cell proliferation, migration, and survival [63,64]. Chemokines such as CC chemokine ligand 2 (CCL2), CCL5, and vascular endothelial growth factor can recruit TAMs to the tumor-site, thus contributing to the maintenance of an immunosuppressive TME [65]. Likewise, CCL5 can attract Tregs to the tumor-site, whereas high levels of IL-10 and TGF-β in the TME stimulate the differentiation of naïve T cells into Tregs. Thus, the anti-inflammatory signals from the TME can reinforce the Tregs and TAMs, whereas the Tregs and TAMs simultaneously increase the levels of anti-inflammatory signals, resulting in a chain reaction of immunosuppression [66]. Furthermore, TGF-β can activate fibroblasts, especially cancer-associated fibroblasts in the TME, thereby increasing the production of collagen and ultimately desmoplasia. The desmoplastic reaction is the dense extracellular matrix within the tumor that creates a physical barrier for infiltrating immune cells. The activity and efficacy of cytotoxic immune cells are reliant on the direct contact between cancer cells and the lymphocytes [67]. Without this direct cell-to-cell contact, the CD8+ T cells have no chance of eliminating cancer cells, and the response to immunotherapy such as ICIs is considerably reduced or absent [54]. However, even when T cells are able to infiltrate the tumor site, their survival and ability to expand can be a serious challenge. The TME is often dominated by hypoxia, acidity, and low nutrient levels, which impair the expansion and survival of immune cells. Thus, the various components of the TME and the interplay between immune cells and stromal cells highly affect the resilience of cancer cells.
3.3. The Peripheral Immune Response to Cancer
DAMPs can be released from stressed, damaged, or dying cells and are recognized among others by APCs such as DCs, macrophages, and neutrophils through pattern-recognition receptors, including TLRs. This recognition typically occurs in the peripheral tissue. Once APCs have been activated by innate immune responses such as DAMPs, they can migrate to secondary lymphoid tissue to present and activate lymphocytes against the danger perceived. Thus, the binding of DAMPs to pattern recognition receptors initiates an inflammatory response that ultimately can activate adaptive immune responses involving the T and B lymphocytes [68].
In cancer, DAMPs may be released in response to high cell turnover, cellular stress, or anti-neoplastic treatments. Released DAMPs from tumor cells can bind to the pattern-recognition receptors on the APCs, thereby stimulating the APCs to present endocytosed antigens to CD8+ and CD4+ T cells through major histocompatibility complexes (MHCs). Once presented with an antigen and activated by APCs, the T cells may proliferate and release further cytokines. By following chemokine gradients and homing receptors, e.g., CD103 for the intestines and cutaneous lymphocyte antigens for the skin, they migrate towards not only the target site but also potential metastatic sites.
Important pro-inflammatory cytokines are IL-2 and IL-15. IL-2 promotes the expansion and activation of NK cells, CD4+, and CD8+ T cells, while IL-15 activates DCs, stimulates the proliferation of T cells, and enhances the development of NK cells, cells which are all involved in the cancer immuno-surveillance [69]. Activated NK cells and T cells can also release interferon γ (IFN-γ), which is important for the activation of macrophages. IFN-γ also upregulates the expression of MHC-II molecules that are central for the activation of specialized adaptive immune responses.
In summary, the activation of the adaptive immune system against cancer cells, through APCs of the innate immune system, is highly affected by the presence of DAMPs. Once the adaptive immune system has been warned against cancer cells, activated T cells can induce further specialized immune responses and recruit more immune cells through the release of pro-inflammatory cytokines. However, in cancer, the balance between pro- and anti-inflammatory signals is tipped towards anti-inflammation and immunosuppression, mainly due to the properties of stromal cells in the TME. Tumor-secreted factors and tumor-secreted exosomes affect the systemic immune responses to cancer, whereas the systemic immune responses may change the constitution of the TME. Ideally, multimodal treatment approaches targeting both the local and systemic changes related to tumorigenesis and dissemination could be promising.
3.4. ECT and the Immune System
Preclinical studies of immunocompetent versus immunodeficient mice have established that the efficacy of ECT depends on the competency of the immune system [70,71]. ECT induces ICD through the liberation of ATP, HMGB1, and calreticulin [71], which in turn might increase the tumor infiltration of several immune cells, including CD8+ T cells and NK cells [72,73,74]. The number of tumor-infiltrating NK cells increased up to four fold following ECT in both preclinical and clinical studies [72,74,75]. In addition, ECT has been shown to preserve large blood vessels [76,77] and to enhance the release of TAAs [74]. ECT can induce an anti-tumor immune response and has been shown to repress distant tumor growth of a non-treated lesion in a murine model of colorectal cancer [75] and to induce systemic responses in murine models [73], thus eliciting abscopal effects, findings that have not yet been replicated in clinical studies [78,79] (Table 1).
Table 1.
Summary of ECT studies investigating the effects on the immune system.
| Species | Authors | Interventions (Type, n) | Cancer Types | Key Findings |
|---|---|---|---|---|
| Human | Gasljevic et al., 2017 [77] | ECT (bleomycin, 7) | Colorectal cancer | ECT induced coagulation necrosis. The majority of vessels >5 mm in diameter remained functional. |
| Bigi et al., 2016 [74] | ECT (bleomycin, 2) | Cutaneous melanoma | High prevalence of tumor-infiltrating CD8+ T cells and foci of NK cells 3 h to 1 month after ECT. Apoptotic cell death was followed by necrosis 48–72 h after ECT. | |
| Gerlini et al., 2013 [79] | ECT (bleomycin, 9) | Metastatic melanoma | ECT promoted Langerhans cell migration from the tumor to draining lymph nodes and DC recruitment to the tumor. Further, DCs found in low number before ECT greatly increased at day 7 to 14. | |
| Mouse | Tremble et al., 2019 [75] | ECT (cisplatin) | Colorectal cancer | ECT increased tumor infiltration of macrophages, neutrophils, B, NK, natural killer T cells, and DCs. Further, it decreased tumor growth of both treated and distal non-treated tumors. |
| Ursic et al., 2018 [72] | ECT (cisplatin/oxaliplatin) | Melanoma | ECT induced a 4-fold increase in tumor infiltration of NK cells and CD8+ T cells. | |
| Calvet et al., 2014 [71] | ECT (bleomycin) | Colon cancer | ECT induced ICD through the liberation of ATP and HMGB1 and the translocation of calreticulin to the cell surface. Seven out of 8 immunocompetent mice were disease-free 24 days after ECT treatment, whereas all immunodeficient mice presented PD. |
|
| Markelc et al., 2013 [80] | ECT (bleomycin) | Colorectal cancer | ECT induced a complete stop of the tumor blood vessels for up to 24 h. No damage to peritumoral normal blood vessels. | |
| Roux et al., 2008 [73] | ECT (bleomycin) | Sarcoma | ECT induced recruitment of tumor-infiltrating DCs and CD8+ T cells after 48–96 h, while the presence of CD4+ T cells remained stable. | |
| Torrero et al., 2006 [81] | ECT (bleomycin) | Breast cancer | ECT induced inhibition of angiogenesis in tumors but did not increase CD8+ T cell activity. | |
| Mekid et al., 2003 [82] | ECT (bleomycin) | Sarcoma | ECT increased the tumor infiltration of lymphocytes after 25, 50, and 75 h, in particular in the vicinity of apoptotic cells. | |
| Sersa et al., 1997 [70] | ECT (cisplatin) | Sarcoma | The tumor growth delay in immunocompetent mice was twice as long as in immunodeficient mice. Further, a high percentage of tumor cures was achieved in immunocompetent mice but none in immunodeficient mice. Of the mice cured after ECT, 75% rejected the tumor challenge, while none of the control mice did. |
|
| Cell | Fernandes et al., 2019 [83] | ECT (bleomycin/cisplatin/oxaliplatin) | Pancreatic cancer | ECT led to necroptosis. |
| Ali et al., 2018 [84] | ECT (bleomycin/cisplatin/oxaliplatin) | Pancreatic cancer | The ECT treatments induced changes in stemness inducing factors related to cancer stem cells. |
DC, dendritic cell; ECT, electrochemotherapy; ICD, immunogenic cell death; NK cells, natural killer cells; PD, progressive disease.
3.5. IRE and its Effects on the Immune System
IRE induces an ICD by the release of DAMPs from tumor cell apoptosis [85,86,87,88]; however, more inflammatory types of cell death, such as necrosis, necroptosis, and pyroptosis, are also linked to ICD [89]. These different findings may in part be explained by differences in the cell-death induction between cell types [18] and the presence of more than one type of cell death [90,91], while it may also be due to differences or limitations in the way cell death is assessed [92]. Nonetheless, several properties of IRE do increase the likelihood of activating the immune system against cancer. First, preservation of the larger vessels and increased microvessel density allow the APCs to infiltrate the treated lesion, carry tumor antigens to draining lymph nodes, and activate the adaptive immune system and the subsequent immune infiltration [93,94,95]. Second, the release of large quantities of tumor-associated antigens can be taken up by APCs. These antigens are highly preserved compared to heat-based therapies (radiofrequency and microwave) [8]. Third, the release of DAMPs including ATP, HMGB1, HSP70, and calreticulin from tumor cells are vital for inducing ICD [87,88]. In addition, HMGB1 released from IRE-treated tumor cells have been shown to reprogram TAMs from immune-suppressive (M2) to immune-promoting (M1) phenotypes [88], thereby increasing the M1/M2 ratio in the TME and the periphery [96]. Fourth, immune suppression may be counteracted through lower-level Tregs and MDSCs, both peripherally and in the TME [96,97]. Finally, modulation of the tumor stroma may increase the infiltration of immune cells by increasing the microvessel density and decreasing the rigidity of the extracellular matrix [95]. These properties, as well as the abscopal effects [98], can be exploited in the preclinical setting of micrometastatic or metastatic disease, as studies indicate a significant immune response, including enhanced immune memory [95,99]. Investigations of immunocompetent and immunodeficient mice have shown that a responsive immune system is vital for the optimal efficacy of IRE [100], although a murine sarcoma model did not reveal any tumor-infiltrating CD4+ or CD8+ T lymphocytes within six hours after IRE [101]. IRE-treated tumors have shown increased infiltration of CD30-positive cells [98], which indicate the presence of lymphocytes after primary allo-antigenic stimulation [102] and undifferentiated pluripotent stem cells [103]. This indicate an enhanced immune response via TAA activation of T cells or recruitment of stem cells by residual tumor cells. However, until further studies elucidate these mechanisms, the impact of this finding remains unknown. The key findings of the effects of IRE on the immune system are summarized in Table 2.
Table 2.
Summary of IRE studies investigating the effects on the immune system.
| Species | Authors | Interventions (n) | Cancer Types | Key Findings |
|---|---|---|---|---|
| Human | Guo et al., 2021 [112] | IRE (11) | Hepatocellular carcinoma | The peripheral neutrophils and monocytes increased by day 1 after IRE and returned to baseline at day 7, while CD4+ T cells decreased by day 1 followed by an increase in the next days. CD8+ T cells remained unchanged. Treg cells decreased from day 3 to 14 followed by an increase at one month. |
| He et al., 2019 [104] | IRE (34) | Locally advanced pancreatic cancer | The peripheral CD4+ T cells, CD8+ T cells, and NK cells decreased by day 3 after IRE followed by an increase at day 7, while a reverse trend was shown for Treg cells. IL-6 and IL-10 levels increased at day 3 after IRE followed by a decrease at day 7. IL-2 increased from day 3 to day 7. Concentrations of IFN-γ and TNF did not significantly change. Increased numbers of CD4+ T cells, CD8+ T cells, and NK cells or decreased Treg cells were associated with longer OS. |
|
| Pandit et al., 2019 [97] | IRE/pancreatectomy (11/4) | Locally advanced pancreatic cancer | The peripheral Treg populations increased day 1 to 3 and decreased from day 3 to 5 in the IRE group compared to increases on day 1 to 3 as well as increases on day 3 to 5 in the pancreatectomy group. | |
| Scheffer et al., 2019 [113] | IRE (10) | Locally advanced pancreatic cancer | Pre- and post-IRE peripheral levels of CD4+ and CD8+ T cells did not change. At 2 weeks following IRE, a decrease in total Tregs was observed, as well as in aTregs and in resting Tregs, accompanied by a transient increase in both peripheral CD4+PD-1+ and CD8+PD-1+ T cell numbers. | |
| Beitel-White et al., 2019 [114] | IRE (8) | Pancreatic cancer (stage III) | An increase in current change during IRE treatment was associated with decreases in Treg populations 24 h after IRE. Changes in current above 20A induced decreased Treg populations. Further, a trend was shown towards increased survival for the group of patients with a >2% decrease in Treg cells. | |
| Swine | Fujimori et al., 2021 [115] | IRE/microwave ablation | Normal lung | Fifty percent of blood vessels and collagen were intact 2 days after IRE compared to 0% after microwave ablation. Further, the number of CD3+ T cells increased more after IRE than after microwave ablation. |
| Rabbit | Lee et al., 2012 [98] | IRE | Hepatocellular carcinoma | Examinations of non-IRE treated organs, e.g., the lungs, showed no metastases in the IRE group, while all 15 rabbits in the control group had lung metastases. IRE-treated tumors showed increased levels of CD30-positive cells, mainly in the zone between viable and dead tumor. |
| Mouse | Dai et al., 2021 [99] | IRE | Hepatocellular carcinoma | IRE increased the percentage of IFN-γ+ CD8+ T cells in splenocytes and increased tumor infiltration of CD8+ T cells. On day 7, reductions of both peripheral and intratumoral Treg cells and PD-1+ T cells were shown. Mice rejected the tumor re-challenge with hepatocellular carcinoma cells following IRE. |
| He et al., 2020 [95] | IRE | Pancreatic cancer | IRE resulted in longer survival and more proliferating CD8+ T cells in the tumor and spleen. Both memory and effector CD8+ T cells were increased in the tumor and the tumor-draining lymph node regions. The viable region showed increased microvessel density and softening of the extracellular matrix. Mice that were re-challenged with pancreatic cancer cells after IRE rejected the tumor challenge. |
|
| Chen et al., 2017 [116] | IRE | Hepatocellular carcinoma | IRE induced a change in the T helper 1/T helper 2 cell ratio towards T helper 1 dominance, an increase in macrophage tumor infiltration, and an increase in IFN-γ and IL-2 compared to controls. | |
| White et al., 2018 [117] | IRE or cryoablation | Pancreatic cancer | IRE induced a higher number of tumor-infiltrating T cells and macrophages at 12 and 24 h after treatment. | |
| Bulvik et al., 2016 [94] | IRE/radiofrequency ablation (82/82) | Normal liver Hepatocellular carcinoma |
The tumor infiltration of neutrophils and macrophages was increased in both groups; however, it was greater in the radiofrequency ablation group. In the IRE group, the infiltration of the neutrophils and macrophages extended along the preserved vessels within the ablation zone. At 72 h, persistent vessels in the ablation zone were seen for IRE-treated mice only. IL-6 levels peaked after 6 h, 3 and 10 times higher than controls (radiofrequency ablation and IRE, respectively). By 24 h, no elevations were seen. Radiofrequency ablation of the liver slowed the growth of an untreated tumor, while IRE resulted in greater reduction in tumor growth. Three days after treatment, the number of CD3+ cells was elevated in the untreated tumor in both groups. |
|
| Neal et al., 2013 [100] | IRE | Renal carcinoma | IRE-treated immunocompetent mice showed robust T-cell infiltration at the zone between viable and dead tumors. Further, IRE-treated immunocompetent mice showed a greater treatment response than did immunodeficient mice. | |
| José et al., 2012 [93] | IRE | Pancreatic cancer | IRE was not found to activate apoptotic cell death measured by caspase-3 positive cells in the tumors. The vascular architecture of the tumor was disrupted from day 1 after IRE and onward. | |
| Al-sakere et al., 2007 [101] | IRE | Sarcoma | No tumor infiltration of CD4+ or CD8+ T lymphocytes, macrophages, APCs, dendritic cells were observed 2 and 6 h after IRE. | |
| Li et al., 2012 [105] | IRE/sham surgery/resection/control (28/28/28/28) | Osteosarcoma | IRE and resection increased the percentages of the peripheral CD3+ and CD4+ cells, as well as the CD4+/CD8+ ratio 7 days after treatment. A more rapid and prolonged increase was seen in the IRE group. IRE and resection caused decreases in IL-10 from day 3 to 21. The percentage of INF-γ-positive splenocytes was higher in the IRE group. | |
| Rat | He et al., 2021 [88] | IRE | Pancreatic cancer | IRE caused increased levels of HMGB1, HSP70, and calreticulin. Seven days after IRE, higher frequencies of M1 macrophages in the tumor and a regional lymph node were seen compared to controls, while a decrease in M2 macrophages was seen in the tumor. |
| Cell | He et al., 2021 [88] | IRE | Pancreatic cancer | HMGB1 were shown to induce M1 macrophage polarization via receptor of advanced glycation end-product. Further, HMGB1 could enhance the phagocytosis of dying tumor cells by macrophages. |
| Shao et al., 2019 [8] | IRE/thermal therapy/cryosurgery | Melanoma | IRE caused the greatest protein release, second lowest denaturation rate of the released protein (30%), the most TLR2 (a measure of the relative antigen content of the released protein) release, and the strongest T cell response. | |
| Zhao et al., 2019 [87] | IRE/radiotherapy | Pancreatic cancer Melanoma |
IRE increased the ATP and HMGB1 levels by 11 and 13 fold, respectively, compared to radiotherapy, which did not cause the release of ATP and HMGB1. IRE: Cells increased the expression of makers for DC activation/maturation by 51–72%, compared to non-IRE treated cells. Radiotherapy: Cells did not increase the expression of makers for DC activation/maturation, compared to non-radiotherapy treated cells. IRE increased the ATP and HMGB1 levels by 8 and 9 fold, respectively. |
|
| Goswami et al., 2017 [118] | IRE/thermal shock/chemical poration | Triple negative breast cancer | IRE caused upregulation of IL-6 and TNF, while thymic stromal lymphopoietin was down-regulated. Cancer cells treated with thermal shock or chemical poration showed no down-regulation of thymic stromal lymphopoietin. |
APC, antigen-presenting cell; aTreg, activated Tregs; DC, dendritic cell; IL, interleukin; IRE, irreversible electroporation; Treg, regulatory T cell; TRP2, Toll-like receptor 2.
The cytokine levels of IL-2, IL-6, and IL-10 have been shown to be elevated up to 7 days after IRE [94,104], while IL-2 and IL-10 have been shown to decrease 3–21 days after IRE [105]. IL-2 is associated with tumor inhibiting properties by the modulation of lymphocyte proliferation and function [106,107]. IL-6 is associated with increased angiogenesis and subsequent tumorigeneses via different pathways, in particular the Janus kinase-signal transducer and activator of transcription 3 pathway [108]. Finally, IL-10 may play an immunosuppressive role in the TME via increased expression of B7-H4 on macrophages and PD-L1 on monocytes [109,110,111]. It is not clear if the combined effect of the cytokine levels are tumor promoting or not. This may depend on the dose relationship, time-dependent changes in levels, and the interplay with other cytokines and transcription factors [109,111].
4. The Synergy of Electroporation and Immunotherapy
4.1. Immunotherapy
Immunotherapy is the broad term for therapies that augment anti-cancer immune responses. The most prominent immunotherapies include ICIs, cytokines, and cell transfers. ICIs target inhibitory receptors located on T cells, such as CTLA-4 and PD-1, as well as their ligands on tumors cells, e.g., anti-PD-L1 and anti-CTLA-4. ICIs prevent T cell exhaustion and apoptosis, thereby enhancing cytotoxicity [52]. ICIs have changed the cancer treatment landscape in a number of cancer types since the first drug was approved by the FDA in 2011 [119]. However, the remarkable efficacy seen in some cancers has been limited to subgroups of tumors. They have been characterized by high expression of PD-1/PD-L1, a high mutational burden, or by a deficient mismatch repair system, together commonly known as immunogenically “hot” tumors. “Cold” tumors on the contrary respond poorly to ICIs and are characterized by being sparsely infiltrated or devoid of immune cells [52].
The most prominent cytokine in immunotherapy is IL-2, a T cell proliferation factor which may also enhance the cytotoxicity of NK cells [106]. Today, IL-2 is no longer the most widely used immunotherapy drug, in part due to the potential life-threatening adverse reactions when used in high doses [120] as well as the approval of novel immunotherapies including ICIs. NK cell transfer therapy (NK cell transfer) is one of the cell transfer therapies that has been known for the longest. It utilizes the inherent tumor killing capabilities of the NK cells, which are independent of MHC molecule presentation [121]. NK cells do not require HLA matching, and promising early results have been reported using allogenic NK cell transfer for the treatment of acute myeloid leukemia [120]; however, indications are limited. Today there are several FDA-approved T cell transfer therapies. However, chimeric antigen receptor T cell therapies are limited due to manufacturing processes and cost and treatment-related toxicity.
4.2. ECT and the Synergy with Immunotherapy
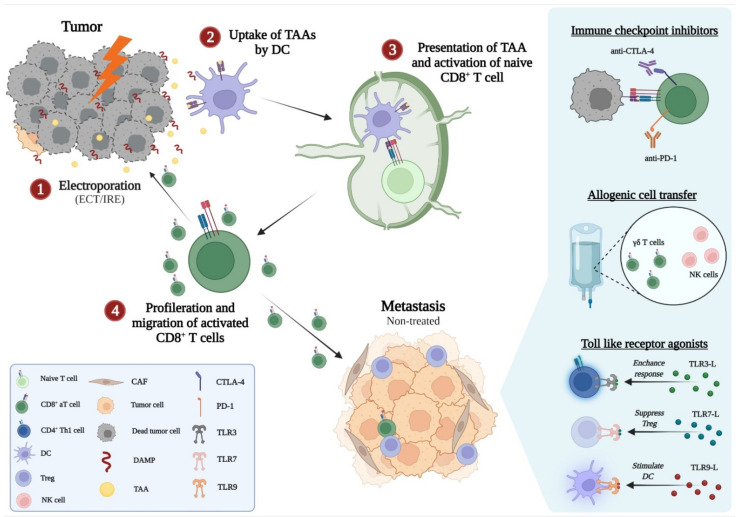
In 2003, the first study investigating ECT in combination with immunotherapy was published [122]. Since then, several small-scale studies investigating different types of immunotherapy in combination with ECT have been conducted. The clinical trials have been focused on patients with advanced or metastatic melanoma, combining ECT with either anti-PD-1, anti-CTLA-4, INF-α, or low-dose IL-2. Figure 3 shows how ECT or IRE in combination with immunotherapy may induce a local immune response and an abscopal effect to increase the local and systemic tumor responses. In 2016, the largest prospective study in patients with advanced malignant melanoma was published [123]. It found significantly increased OS among the patients that received both local therapy (ECT, radiotherapy, or stereotactic radiation) + ipilimumab (anti-CTLA-4) compared to ipilimumab alone (median OS of 93 weeks vs. 42 weeks), though it should be noted that only 4 in 45 patients received ECT treatment. Recently, the largest retrospective study to date included 130 patients with metastatic melanoma [124]. Data from the European InspECT group was combined with Slovenian registry data. Local tumor response rates were significantly higher in the group of patients that received either ECT or ECT + pembrolizumab (anti-PD-1) compared to pembrolizumab alone. Systemic tumor response rates were comparable between ECT and ECT + pembrolizumab; however, the time to progression was doubled among the patients that received ECT + pembrolizumab (8 and 17 months, respectively, p < 0.05). Survival data showed 2-year overall survival (OS) of 43% and 70% in the ECT and ECT + pembrolizumab groups, respectively. The chance of survival was significantly higher in the ECT + pembrolizumab group, when data were adjusted for previous systemic therapies. No serious adverse events were reported in the treatment groups. A retrospective study by Heppt et al. [125] of 33 patients with metastatic melanoma showed 15 months median OS among the five patients that received ECT + anti-PD-1, while the median OS in the ECT + ipilimumab group was not reached. The local and systemic objective response rates were comparable to those in the study by Campana et al. [124].
Figure 3.
Model of how electroporation may induce an immune response and elicit an abscopal effect when combined with immunotherapy. aT cell, activated T cell; CAF, cancer-associated fibroblast; CTLA-4, cytotoxic T lymphocyte antigen 4; DAMP, damage-associated molecular pattern; DC, dendritic cell; ECT, electrochemotherapy; IRE, irreversible electroporation; NK cell, natural killer cell; PD-1, programmed death receptor 1; PD-L1, programmed death-ligand 1; TAA, tumor-associated antigen; TLR, Toll-like receptor; TLR3-L, TLR3 ligand; Treg, regulatory T cell. Created with BioRender.com (accessed on 7 April 2022).
IFN-α administered as a post-surgical adjuvant before ECT treatment was investigated in a small retrospective study in five patients with recurrent melanoma [126]. Two patients received low-dose IFN-α, while three patients received high-dose IFN-α. The time interval between the end of IFN-α treatment and ECT treatment ranged from 7 months to 12 years, and all patients presented with metastatic disease at the time of ECT treatment. Three patients with 1–23 metastatic lesions achieved CR 4 weeks after ECT, one patient achieved CR in >85% of 80 lesions, and one patient achieved 100% PR in five lesions. No adverse events were reported. These findings indicate that IFN-α and subsequent ECT could be a safe and effective treatment combination, though the study had many limitations, including a retrospective design and a small heterogeneous group of patients both regarding IFN-α dose and treatment interval.
In preclinical studies, ECT has been investigated in combination with IL-12 GET, showing improved CR rates compared to ECT alone [81,127,128,129]. Interestingly, a multi-arm study found the added efficacy of IL-12 to be the greatest in the poorly immunogenic melanoma model (0% CR vs. 38%, alone and combined treatment, respectively) [127]. The immune status pre-treatment was evaluated by CD4+ and CD8+ T cell tumor infiltration and expression of MHC-1 and PD-L1. Promising preclinical results of ECT in combination with either inducible T-cell co-stimulator activating antibody [130], TNF-α [131,132], or CpG oligodeoxynucleotides [73] have yet to enter clinical trials, so conclusions cannot yet be drawn. Antibodies targeting the inducible T-cell co-stimulator receptor located on T cells are novel and result in the activation and expansion of anti-tumor T cells. In summary, clinical trials of ECT and ICI have shown promising results with systemic responses indicating abscopal effects in melanoma patients, although larger RCTs are warranted (Table 3).
Table 3.
Summary of ECT + immunotherapy studies.
| Species | Authors | Interventions (n) | Cancer Types | Key Findings |
|---|---|---|---|---|
| Human | Campana et al., 2021 [124] | ECT (bleomycin)/pembrolizumab/ECT + pembrolizumab (41/44/45) ** | Metastatic melanoma | Local response: ECT/pembrolizumab: 44%/32 CR, 37%/7% PR ECT + pembrolizumab: 49% CR, 29% PR Systemic response: Pembrolizumab: 21% CR, 4% PR ECT + pembrolizumab: 11% CR, 13% PR Two-year OS: Pembrolizumab: 43% ECT + pembrolizumab: 70% |
| Quaresmini et al., 2021 [133] | ECT (bleomycin) + nivolumab (1) * | Metastatic melanoma | Durable CR (>1 year) | |
| Karaca et al., 2018 [134] | ECT (bleomycin) + nivolumab (1) * | Metastatic melanoma | Durable CR (>1 year) locally and systemic | |
| Hribernok et al., 2016 [126] | ECT (bleomycin/cisplatin) + INF-α (5) ** | Advanced melanoma | Three patients with CR (1–23 lesions), 1 patient with CR of >85% of lesions (80 lesions), 1 patient with PR (5 lesions) | |
| Theurich et al., 2016 [123] | (ECT/radiotherapy) + ipilimumab/ipilimumab (45/82) *** | Advanced melanoma | Local response: Ipilimumab: 0% CR, 18% PR Ipilimumab + (ECT/radiotherapy): 7% CR, 31% PR Median OS: Ipilimumab: 42 weeks Ipilimumab + (ECT/radiotherapy): 93 weeks (hazard ratio 0.46) |
|
| Heppt et al., 2016 [125] | ECT (bleomycin) + ICI (ipilimumab/pembrolizumab/nivolumab, 33) ** | Metastatic melanoma | Local response: 15% CR, 52% PR Systemic response: 6% CR, 16% PR Median progression free survival: 2.5 months; median OS: not reached |
|
| Mozzillo et al., 2015 [135] | ECT (bleomycin) + ipilimumab (15) ** | Metastatic melanoma | Local response: 27% CR, 40% PR Systemic response: 0% CR, 33% PR One-year OS: 86% At week 10 and 12, a decrease in the absolute Treg number was seen in responders compared to no responders |
|
| Brizio et al., 2015 [136] | ECT (bleomycin) + ipilimumab (1) * | Metastatic melanoma | ECT: Multiple liver and adrenal glands metastases after 3 ECT treatments ECT + ipilimumab: Durable CR (1 year) locally and systemically |
|
| Andersen et al., 2003 [122] | ECT (bleomycin) + IL-2 (6) *** | Metastatic melanoma | ECT + IL-2 induced a partial remission Antitumor cytotoxic T lymphocyte responses declined following IL-2 therapy |
|
| Dog | Salvadori et al., 2017 [137] | ECT (cisplatin) + IL-12 GET | Mast cell tumor | Sixty-four percent CR Increased tumor infiltration of T lymphocytes at 4 weeks |
| Rabbit | Ramirez et al., 1998 [138] | ECT (bleomycin) + IL-2 secreting cells | Hepatocellular carcinoma | Median survival: Controls: 50 days (average number of metastases of 27) ECT: 82 days (average number of metastases of 18) ECT + IL-2: 80 days (average number of metastases of 3) |
| Mouse | Ursic et al., 2021 [127] | ECT (cisplatin/oxaliplatin/bleomycin) + IL-12 GET | Colorectal cancer Breast cancer Melanoma |
ECT (cisplatin/oxaliplatin/bleomycin): 83%/83%/50% CR ECT + IL-12: 100%/100%/50% CR ECT: 50%/33%/17% CR ECT + IL-12: 67%/83%/33% CR ECT: 0%/0%/0% CR ECT + IL-12: 38%/0%/0% CR |
| Tremble et al., 2018 [130] | ECT (cisplatin) + inducible T-cell co-stimulator | Colorectal cancer Metastatic Lewis Lung Carcinoma |
Median survival: Control/ECT: 12/24 days ECT + inducible T-cell co-stimulator: 80 days ECT + inducible T-cell co-stimulator reduced the tumor growth of secondary non-treated tumors and increased the survival One hundred-day survival of 33% compared to 0% in monotherapy groups |
|
| Cemazar et al., 2015 [131] | ECT (cisplatin) + TNF-α | Fibrosarcoma | Control/ECT: 0% CR ECT + TNF-α: 36% CR |
|
| Sedlar et al., 2012 [128] | ECT (cisplatin) + IL-12 GET | Fibrosarcoma | Control/ECT: 0%/17% CR ECT + IL-12: 60% CR |
|
| Roux et al., 2008 [73] | ECT (bleomycin) + CpG oligodeoxynucleotides | Fibrosarcoma Melanoma |
ECT: Recruitment of tumor-infiltrating CD8+ cells 48–96 h after ECT; CD4+ cells remained stable Forty-three percent CR in treated tumors, 0% CR in non-treated tumors ECT + CpG: 100% CR in treated tumors, 57% CR in non-treated tumors ECT + CpG: Superior efficacy in reducing tumor volume compared to ECT, both in treated and non-treated tumors; induced a functional and specific activation of T cells both regionally (draining lymph node) and peripherally |
|
| Torrero et al., 2006 [81] | ECT (bleomycin) + IL-12 GET | Breast cancer | Median survival: Control/ECT: 34/46 days ECT + IL-12: 60 days |
|
| Kishida et al., 2003 [129] | ECT (bleomycin) + IL-12 GET | Melanoma | Median survival: Control/ECT: 18/37 days ECT + IL-12: 62 days In a metastatic model, ECT + IL-12 reduced the number of metastatic foci and increased the survival compared to monotherapy |
|
| Sersa et al., 1997 [132] | ECT (bleomycin) + TNF-α | Fibrosarcoma | Median survival: Control/ECT: 24/33 days. 0% CR ECT + TNF-α: 50 days. 33% CR |
|
| Mir et al., 1995 [139] | ECT (bleomycin) + IL-2 secreting cells | Fibrosarcoma | ECT: 60% CR ECT + IL-2: 100% CR Fifty percent CR in non-treated tumors; increased infiltration of CD4+ and CD8+ T cells in both treated and non-treated tumors |
* case report; ** retrospective study; *** prospective study; CR, complete response; ECT, electrochemotherapy; GET, gene electrotransfer; ICI, immune checkpoint inhibitor; IL, interleukin; OS, overall survival; PR, partial response; TNF-α, tumor necrosis factor α.
4.3. IRE Plus Immunotherapy
In recent years, several clinical studies have investigated IRE in combination with either ICIs or immune cell transfer therapy (Table 4). These studies have primarily been conducted on patients with locally advanced pancreatic cancer, likely due to IRE as monotherapy already being indicated in this patient population. One prospective trial of 10 patients with locally advanced pancreatic cancer treated with IRE + anti-PD-1 showed a median OS of 18 months [140]. This contrasts with a retrospective study of IRE + anti-PD-1, which showed a median OS of 44 months in locally advanced pancreatic cancer [141]. Small sample sizes and different study designs make the studies difficult to compare; however, the retrospective study did show a superior OS in the IRE + anti-PD-1 group versus IRE alone (median OS 44 months vs. 23 months, respectively). In addition, CD4+ and CD8+ T cell numbers increased in the IRE + anti-PD-1 group. Finally, the levels of IL-4, IL-6, TNF, and IFN-γ increased more in the combination group than in the IRE monotherapy group [141], indicating a potential survival and molecular benefit when combining IRE with anti-PD-1. The combination of IRE and allogenic NK cell transfer in locally advanced pancreatic cancer showed significantly improved response rates compared to IRE alone in an RCT [142]. However, the response rates did not translate into significantly improved survival. Higher numbers of CD4+ and CD8+ T cells, NK cells, and B cells were also seen in the IRE + NK cell transfer group compared to IRE alone. A prospective trial showed significantly improved survival data in the same patient population of IRE + NK cell transfer compared to IRE alone (median OS 13.2 months and 11.4 months, respectively) [143]. Thus, evidence indicates added efficacy when transferring NK cells in combination with the IRE treatment, though the gain may be minor. An RCT investigating the use of IRE and allogenic Vγ9Vδ2 T cell transfer showed more promising results in locally advanced pancreatic cancer [144]. Significantly increased survival was seen in the IRE + allogenic γδ T cell transfer group versus IRE alone (median OS 14.5 months vs. 11.0 months, respectively). Vγ9Vδ2 T cells are the major subset of peripheral γδ T cells, which share central properties with both T and NK cells. In contrast to αβ T cells (CD4+, CD8+ T cells), γδ T cells recognize and kill transformed cells by endogenous tumor-derived pyrophosphates. A mechanism independent of the major histocompatibility complex class molecules was displayed by APCs and target cells [145]. In addition, γδ T cells express activating NK receptors, e.g., NKp30, NKp44, and NKG2D, that bind to stress-inducible molecules frequently expressed by cancer cells [146]. Thus, γδ T cells have two independent recognition systems to sense tumor cells and to initiate cytotoxicity and cytokine production. Moreover, patients receiving multiple transfers of γδ T cells had significantly longer median OS compared to patients that only received a single course (17 months vs. 13.5 months, respectively). In addition, trials of adoptive cell transfer therapy in combination with IRE found no significantly added safety issues when adding cell therapy to IRE, which further support continued research in the combinatory regimens [143,144]. γδ T cell therapy does seem superior to NK cells, though the RCTs of the two cell therapies would bring vital information for future studies when deciding the most optimal cell therapy to combine with IRE in locally advanced pancreatic cancer.
Table 4.
Summary of IRE + immunotherapy studies.
| Species | Authors | Interventions (n, Study Design) | Cancer Types | Key Findings |
|---|---|---|---|---|
| Human | He et al., 2021 [141] | IRE/IRE + toripalimab (70/15) ** | Locally advanced pancreatic cancer | Median OS: 1, 2, and 3 year OS rates: IRE: 23.4 months: 91%, 45%, and 12%. IRE + toripalimab: 44.3 months: 100%, 100%, and 33.3%. Increased CD4+ and CD8+ T cells, while CD8+ Treg cells decreased compared to IRE. Further the levels of IL-4, IL-6, TNF, and IFN-γ increased markedly more than in the IRE group. |
| Pan et al., 2020 [142] | IRE/IRE + allogenic NK cell transfer (46/46) **** | Locally advanced pancreatic cancer | Median OS: Response rates: IRE: 11.8 months: 15% CR, 41% PR. IRE + NK cells: 12.4 months: 30% CR, 41% PR. Increased CD4+, CD8+ T cells, NK cells, and B cells compared to IRE alone. Further, the levels of IL-2, TNF-β, and IFN-γ increased markedly more than in the IRE group. |
|
| Lin et al., 2020 [144] | IRE/IRE + allogenic γδ T cell transfer (32/30) **** | Locally advanced pancreatic cancer | Median OS: IRE: 11.0 months. IRE + T cells: 14.5 months. Twenty-five incidences of grade 3/4 adverse events equally distributed in both groups. |
|
| O’Neill et al., 2020 [140] | IRE + nivolumab (10) *** | Locally advanced pancreatic cancer | Median OS: 18 months; 1 year OS: 67%. Adverse events ≥ grade 3: 70% of patients. By day 90, T effector memory cells were increased two fold from baseline. |
|
| Yang et al., 2019 [147] | IRE/IRE + allogenic NK cell transfer (22/18) **** | Unresectable Intrahepatic cholangiocarcinoma/hepatocellular carcinoma | Median OS: Response rates: IRE: 17.9 months: 5% CR, 64% PR IRE + NK cells: 23.2 months: 17% CR, 72% PR Higher lymphocyte count and IL-2, TNF-β, IFN-γ levels post-treatment compared to IRE alone. No serious adverse events. |
|
| Alnaggar et al., 2018 [148] | IRE/IRE + allogenic NK cell transfer (20/20) ** | Metastatic hepatocellular carcinoma | Median OS: IRE: 8.9 months. IRE + NK cells: 10.1 months. Lower number of circulating tumor cells in the IRE + NK cell group at 7 and 30 days after treatment. No serious adverse events and no differences in lymphocyte subsets between the two groups after treatment. |
|
| Lin et al., 2017 [143] | IRE/IRE + allogenic NK cell transfer (39/32) *** | Pancreatic cancer (stage III/IV) | Median OS: IRE: 11.4 months (stage III), 8.7 months (stage IV). IRE + NK cells: 13.2 months (stage III), 9.8 months (stage IV). No serious adverse events. |
|
| Lin et al., 2017 [155] | IRE/IRE + allogenic NK cell transfer (19/20) *** | Metastatic pancreatic cancer | IRE: 16% CR, 47% PR IRE + NK cells: 30% CR, 50% PR |
|
| Mouse | Burbach et al., 2021 [149] | IRE + anti-CTLA-4 + anti-PD-1 | Prostate cancer | IRE/anti-CTLA-4: 0%/15% CR. IRE + anti-CTLA-4: 46% CR Increased number of CD8+ T cells both locally and systemically compared to IRE or anti-CTLA-4. IRE + anti-CTLA-4, and subsequent anti-PD-1: Sustained tumor regression after CR. |
| Shi et al., 2021 [150] | IRE + anti-PD-L1 | Hepatocellular carcinoma | IRE + anti-PD-L1-induced necrosis, T cell and inflammatory cell infiltration in both treated and non-treated tumors. | |
| Babikr et al., 2021 [96] | IRE + anti-PD-L1 + TLR3 + TLR9 agonists | Lymphoma Breast cancer |
IRE: 0% CR. IRE + TLR3 + TLR9: Superior primary tumor growth inhibition and CD8+ T cell response compared to IRE and IRE + anti-PD-1. IRE + anti-PD-1 + TLR3 + TLR9: 100% CR of treated and non-treated tumors. Increased the tumor infiltration of CD8+ and CD4+ T cells and the CD8+ T cell response compared to IRE alone. Induced a M1/M2 macrophage balance towards the anti-tumor M1 and reduced Tregs and MDSCs. IRE + anti-PD-1 + TLR3 + TLR9: 100% CR of treated tumors. |
|
| Zhang et al., 2021 [156] | IRE + anti-OX40 | Pancreatic cancer Metastatic pancreatic cancer |
Median survival: Control/anti-OX40/IRE: 22/24/51 days. IRE + anti-OX40: 80% were alive at 120 days (median survival not reached). Increased tumor infiltration of CD8+ T cells and decreased MDSCs, as well as higher levels of IFN-γ and TNF-α compared to IRE alone. Secondary, non-treated tumor, median survival: Control/anti-OX40/IRE: 21/21/31 days, respectively. IRE + anti-OX40: 44 days. |
|
| Sun et al., 2021 [90] | IRE + M1 oncolytic virus | Pancreatic cancer | Median survival: Control/M1 virus/IRE: 31/34/46 days. IRE + M1: 58 days. Increased tumor infiltration of CD4+ and CD8+ T cells. |
|
| Yang et al., 2021 [157] | IRE + DC vaccine | Pancreatic cancer | Median survival: Control/IRE/DC vaccine: 35/44/49 days. IRE + anti-PD-1: 77 days. Twice as high mean number of tumor-infiltrating CD8+ T cells compared to IRE alone. |
|
| Lasarte-Cia et al., 2021 [152] | IRE + STING agonist | Melanoma Hepatocellular carcinoma |
Control/IRE/STING: 0% CR. IRE + STING: 13% CR. Control/IRE/STING: 0%/17%/20% CR. IRE + STING: 67% CR. |
|
| Go et al., 2020 [153] | IRE + STING agonist | Lewis lung carcinoma | IRE + STING: Reduced the tumor volume, induced a M1/M2 macrophage balance towards the anti-tumor M1 phenotype, and increased the tumor infiltration of CD8+ and CD4+ T cells compared to IRE or STING alone. | |
| Yu et al., 2020 [158] | IRE + indoleamine 2,3-dioxygenase inhibitor loaded electric pulse responsive iron-oxide-nanocube clusters | Prostate cancer | Combination treatment induced higher calreticulin tumor exposure, increased frequency of tumor-infiltrating CD3+ T cells, and higher CD8+ T cell-to-Tregs ratio compared to IRE alone. Further, it reduced the tumor growth of both treated and non-treated tumors more than IRE alone. | |
| Narayanan et al., 2019 [151] | IRE + TLR7 agonist/anti-PD-1 | Pancreatic cancer | IRE: 20–35% CR in immunocompetent mice; 0% CR in immunodeficient mice. Generated tumor antigen-specific T cell responses. IRE + TLR7/anti-PD-1 were not superior to IRE alone in survival and tumor growth reduction. |
|
| Zhao et al., 2019 [87] | IRE + anti-PD-1 + anti-CTLA-4/radiotherapy + anti-PD-1 | Pancreatic cancer Melanoma |
Median survival: Control/anti-PD-1/IRE: 6/8/12 days. Radiotherapy + anti-PD-1: 30 days; 0% were alive at 120 days. IRE + anti-PD-1: 32 days; 36% were alive at 120 days. IRE + anti-PD-1 + anti-CTLA-4: 41 days. However, not significantly different from IRE + anti-PD-1, and weight loss suggested considerable toxicity. Median survival: Control/anti-PD-1/IRE: 5/6/8 days. IRE + anti-PD-1: 23 days |
|
| Vivas et al., 2019 [159] | IRE + polyinosinic-polycytidylic acid and poly-L-lysine | Hepatocellular carcinoma | Control/IRE/polyinosinic-polycytidylic acid and poly-L-lysine: 0%/27%/30% CR. IRE + polyinosinic-polycytidylic acid and poly-L-lysine: 71% CR. |
|
| Pasquet et al., 2019 [160] | IRE + IL-12 GET | Melanoma | Control/IRE/IL-12 GET: 0% CR. IRE + IL-12 GET: 42% CR. |
** retrospective study; *** prospective study; **** RCT; anti-CTLA-4, cytotoxic T-lymphocyte-associated antigen 4 inhibitor; anti-PD-1, programmed death-1 receptor inhibitor; CR, complete response; DC, dendritic cell; GET, gene electrotransfer; IFN, interferon; IL, interleukin; IRE, irreversible electroporation; MDSC, myeloid-derived suppressor cell; NK, natural killer; OS, overall survival; STING, stimulator of interferon genes; TLR, Toll-like receptor; TNF, tumor necrosis factor; Treg, regulatory T cell.
The combination of IRE and allogenic NK cell transfer has also been investigated in primary liver cancer (intrahepatic cholangiocarcinoma and hepatocellular carcinoma). An RCT of 40 patients yielded median OS values of 23.2 months and 17.9 months in IRE + NK cell transfer and IRE-treated patients, respectively (p < 0.05) [147]. Moreover, a retrospective study of 40 patients with metastatic hepatocellular carcinoma found IRE + NK cell transfer to yield significantly longer survival [148]. Both treatments had benign safety profiles, as no serious adverse events were reported in either group [147,148]. Thus, combining IRE with immunotherapy may have elicited an abscopal effect, resulting in improved survival.
Preclinical studies have investigated a wide range of immunotherapies administered in various combinations with IRE, including anti-CTLA-4 [149], anti-PD-1 [87,150], TLR agonists [96,151], M1 oncolytic virus [90], and STING agonist [152,153]. Studies have been focused mainly on pancreatic cancer and to a lesser extent hepatocellular carcinoma, melanoma, prostate cancer, and breast cancer. IRE + anti-CTLA-4 and anti-PD-1 have shown CR rates of 46% in prostate cancer along with increased numbers of CD8+ T cells specific for the prostate tumor antigen, both locally and peripherally [149]. Further, IRE + anti-PD-L1 + TLR3 and TLR9 agonist have shown CR rates of 100% in both lymphoma and breast cancer models. Moreover, this combination regimen induced tumor infiltration of CD8+ and CD4+ T cells and increased the ratio of the anti-tumor M1 macrophages, while reducing both the number of Tregs cells and MDSCs [96]. IRE + STING agonist showed limited efficacy in melanoma [152] and great efficacy in hepatocellular carcinoma [152]. Further, in a Lewis lung carcinoma model, IRE + STING increased the tumor infiltration of CD8+ and CD4+ T cells and induced a shift in the M1/M2 macrophage ratio towards the anti-tumor M1 phenotype [153]. Among the combinations investigated in preclinical studies, the combination of IRE and anti-PD-L1, and the TLR agonist look the most promising, and an explorative study is underway investigating the combination treatment in metastatic pancreatic cancer [154].
5. Perspectives
5.1. Ongoing Trials
One trial is registered on clinicaltrials.gov investigating ECT + pembrolizumab in unresectable melanoma, which aims to include 53 patients (NCT03448666). Several trials investigating IRE in combination with immunotherapy are ongoing. Three trials will include 10 to 18 patients with locally advanced pancreatic cancer or metastatic pancreatic cancer treated with IRE + nivolumab (NCT03080974 and NCT04212026) or pembrolizumab (NCT04835402). One trial will investigate nivolumab, IRE + nivolumab, and IRE + nivolumab + TLR9 agonist in 18 patients with metastatic pancreatic cancer (NCT04612530) [154]. Finally, one trial will investigate IRE + nivolumab in 43 patients with advanced hepatocellular carcinoma (NCT03630640).
5.2. Intertumoral Heterogeneity
Increasing numbers of studies are investigating ECT or IRE in combination with immunotherapy to enhance the local immune response and induce an abscopal effect, which depends on the genetic composition of the tumor. Differences in the genetic landscape (intratumoral heterogeneity) caused by constant selection pressure that leads to the survival and growth of the most resilient clones have been known for decades. Thus, intermetastatic heterogeneity, including the composition of the TME, exists among different metastatic lesions in the same patients as well as heterogeneity between the primary tumor and metastases [161,162]. This may be associated with the low concordance of T cell tumor infiltration between primary tumor and matched metastases seen in colorectal cancer [163]. Although typical driver gene mutations are shared by all lesions, a patient with metastatic cancer will have other critical tumor mutations that are not shared [164,165]. Employing multiple-site electroporation can help to ensure (I) generating TAAs specific for all lesions, (II) releasing a sufficient amount of TAAs, and (III) releasing enough DAMPs. This could help overcome the inherent intertumor and intermetastatic heterogeneity and elicit a pronounced immune response [166]. This approach is already commonly used in ECT plus immunotherapy studies, as ECT can readily be used to treat multiple cutaneous and subcutaneous metastatic lesions [125,135]. In contrast, IRE is mainly used to treat single lesions, in part due to numerous studies investing IRE plus immunotherapy in locally advanced pancreatic cancer. Moving forward, we propose designing studies that aim at treating two or more lesions simultaneously, if feasible. Combining electroporation modalities may also be an option to investigate a two-target approach.
6. Conclusions
ECT and IRE are becoming implemented in an increasing number of disease areas as larger clinical trials are being published. They have been shown to illicit ICD and have been linked to the abscopal effect. Moreover, preclinical studies investigating the use of ECT and IRE in combination with various immunotherapy regimes have shown promising efficacy in melanoma, pancreatic cancer, and hepatocellular carcinoma, which has led to the launch of several clinical studies. The most promising focus area is ECT + ICIs in patients with metastatic melanoma and IRE + ICIs or adoptive cell transfer using allogenic γδ T cells in patients with locally advanced pancreatic cancer and hepatocellular carcinoma. In the coming years, novel immunotherapy therapies such as IL-12 GET and TLR agonists, not only in combinations with electroporation modalities, but potentially also in triple regimens including ICIs should be investigated. Finally, meticulous patient selection for different combinatory regimens must be highly prioritized in order to optimize personalized treatment options.
Abbreviations
| anti-CTLA-4 | cytotoxic T-lymphocyte-associated antigen 4 inhibitor |
| anti-PD-1 | programmed death-1 receptor inhibitor |
| anti-PD-L1 | programmed death-1 receptor ligand inhibitor |
| APC | antigen-presenting cell |
| aTreg | activated Treg |
| CAF | cancer-associated fibroblast |
| CCL | CC chemokine ligand |
| CR | complete response |
| DAMP | danger-associated molecular pattern |
| DC | dendritic cell |
| ECT | electrochemotherapy |
| GET | gene electrotransfer |
| HMGB1 | high mobility group box 1 |
| ICD | immunogenic cell death |
| ICI | immune checkpoint inhibitor |
| IFN | interferon |
| IL | interleukin |
| M | macrophage |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility complex |
| NK cell | natural killer cell |
| NK cell transfer | NK cell transfer therapy |
| OS | overall survival |
| PD | progressive disease |
| PR | partial response |
| RCT | randomized controlled trial |
| STING | stimulator of interferon genes |
| TAA | tumor-associated antigen |
| TAM | tumor-associated macrophage |
| TGF | transforming growth-factor |
| TLR | toll-like receptor |
| TME | tumor microenvironment |
| TNF | tumor necrosis factor |
| Treg | regulatory T cell |
Author Contributions
Conceptualization, T.F.J., A.O., H.R., C.N. and I.G.; writing—original draft, T.F.J. and A.O.; writing—review and editing, T.F.J., A.O., H.R., C.N. and I.G.; visualization, T.F.J. and A.O.; supervision, H.R., C.N. and I.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sale A.J., Hamilton W.A. Effects of High Electric Fields on Micro-Organisms: III. Lysis of Erythrocytes and Protoplasts. Biochim. Biophys. Acta. 1968;163:37–43. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- 2.Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P.H. Gene Transfer into Mouse Lyoma Cells by Electroporation in High Electric Fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belehradek M., Domenge C., Luboinski B., Orlowski S., Belehradek J.J., Mir L.M. Electrochemotherapy, a New Antitumor Treatment. First Clinical Phase I-II Trial. Cancer. 1993;72:3694–3700. doi: 10.1002/1097-0142(19931215)72:12<3694::AID-CNCR2820721222>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Onik G., Rubinsky B. Irreversible Electroporation. Springer; Berlin/Heidelberg, Germany: 2010. Irreversible Electroporation: First Patient Experience Focal Therapy of Prostate Cancer; pp. 235–247. [DOI] [Google Scholar]
- 5.Mole R.H. Whole Body Irradiation—Radiobiology or Medicine? Br. J. Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 6.Abuodeh Y., Venkat P., Kim S. Systematic Review of Case Reports on the Abscopal Effect. Curr. Probl. Cancer. 2021;40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Ngwa W., Irabor O.C., Schoenfeld J.D., Hesser J., Demaria S., Formenti S.C. Using Immunotherapy to Boost the Abscopal Effect. Nat. Rev. Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao Q., Flanagan S.O., Lam T., Roy P., Pelaez F., Burbach B.J., Azarin S.M., Shimizu Y., Bischof J.C. Engineering T Cell Response to Cancer Antigens by Choice of Focal Therapeutic Conditions. Int. J. Hyperth. 2019;36:130–138. doi: 10.1080/02656736.2018.1539253. [DOI] [PubMed] [Google Scholar]
- 9.Calvet C.Y., Mir L.M. The Promising Alliance of Anti-Cancer Electrochemotherapy with Immunotherapy. Cancer Metastasis Rev. 2016;35:165–177. doi: 10.1007/s10555-016-9615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goggins C.A., Khachemoune A. The Use of Electrochemotherapy in Combination with Immunotherapy in the Treatment of Metastatic Melanoma: A Focused Review. Int. J. Dermatol. 2019;58:865–870. doi: 10.1111/ijd.14314. [DOI] [PubMed] [Google Scholar]
- 11.Rai Z.L., Feakins R., Pallett L.J., Manas D., Davidson B.R. Irreversible Electroporation (IRE) in Locally Advanced Pancreatic Cancer: A Review of Current Clinical Outcomes, Mechanism of Action and Opportunities for Synergistic Therapy. J. Clin. Med. 2021;10:1609. doi: 10.3390/jcm10081609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geboers B., Scheffer H.J., Graybill P.M., Ruarus A.H., Nieuwenhuizen S., Puijk R.S., Tol P.M., Davalos R.V., Rubinsky B., Gruijl T.D., et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology. 2020;295:254–272. doi: 10.1148/radiol.2020192190. [DOI] [PubMed] [Google Scholar]
- 13.Miklavčič D., Mali B., Kos B., Heller R., Serša G. Electrochemotherapy: From the Drawing Board into Medical Practice. Biomed. Eng. Online. 2014;13:29. doi: 10.1186/1475-925X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sersa G., Miklavcic D., Cemazar M., Rudolf Z., Pucihar G., Snoj M. Electrochemotherapy in Treatment of Tumours. Eur. J. Surg. Oncol. 2008;34:232–240. doi: 10.1016/j.ejso.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Yarmush M.L., Golberg A., Serša G., Kotnik T., Miklavčič D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622. [DOI] [PubMed] [Google Scholar]
- 16.Aycock K.N., Davalos R.V. Irreversible Electroporation: Background, Theory, and Review of Recent Developments in Clinical Oncology. Bioelectricity. 2019;1:214–234. doi: 10.1089/bioe.2019.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gissel H., Lee R.C., Gehl J. Clinical Aspects of Electroporation. Springer; Berlin, Germany: 2011. Electroporation and Cellular Physiology; pp. 9–17. [DOI] [Google Scholar]
- 18.Brock R.M., Beitel-white N., Davalos R.V., Allen I.C., Allen I.C. Starting a Fire Without Flame: The Induction of Cell Death and Inflammation in Electroporation-Based Tumor Ablation Strategies. Front. Oncol. 2020;10:1235. doi: 10.3389/fonc.2020.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 20.Aymeric L., Apetoh L., Ghiringhelli F., Tesniere A., Martins I. Tumor Cell Death and ATP Release Prime Dendritic Cells and Efficient Anticancer Immunity. Cancer Res. 2010;70:855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 21.Kepp O., Tesniere A., Zitvogel L., Kroemer G. The Immunogenicity of Tumor Cell Death. Curr. Opin. Oncol. 2009;21:71–76. doi: 10.1097/CCO.0b013e32831bc375. [DOI] [PubMed] [Google Scholar]
- 22.Gardai S.J., Mcphillips K.A., Frasch S.C., Janssen W.J., Starefeldt A., Murphy-ullrich J.E., Bratton D.L., Oldenborg P., Michalak M., Henson P.M., et al. Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through Trans-Activation of LRP on the Phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casares N., Pequignot M.O., Tesniere A., Ghiringhelli F., Roux S., Chaput N., Schmitt E., Hamai A., Hervas-stubbs S., Obeid M., et al. Caspase-Dependent Immunogenicity of Doxorubicin-Induced Tumor Cell Death. J. Exp. Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guss K.A., Nelson C.E., Hudson A., Kraus M.E., Carroll S.B., Barreira R., Reis C., Green D.R. RIPK1 and NF-KB Signaling in Dying Cells Determines Cross-Priming of CD8+ T Cells. Science. 2015;350:1720–1723. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh M.P., Duncan B., Larabee S., Krauss A., Davis J.P.E., Cui Y., Kim S.Y., Guimond M., Bachovchin W., Fry T.J. Val-BoroPro Accelerates T Cell Priming via Modulation of Dendritic Cell Trafficking Resulting in Complete Regression of Established Murine Tumors. PLoS ONE. 2013;8:e58860. doi: 10.1371/journal.pone.0058860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arena C.B., Davalos R.V. Advances in Therapeutic Electroporation to Mitigate Muscle Contractions. Membr. Sci. Technol. 2012;2:1000e102. doi: 10.4172/2155-9589.1000e102. [DOI] [Google Scholar]
- 28.Zhao J., Chen S., Zhu L., Zhang L., Liu J., Xu D., Tian G., Jiang T. Antitumor Effect and Immune Response of Nanosecond Pulsed Electric Fields in Pancreatic Cancer. Front. Oncol. 2020;10:621092. doi: 10.3389/fonc.2020.621092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasson E.M., Ivey J.W., Verbridge S.S., Davalos R.V., Tech V., Building S.E., Tech V. The Feasibility of Enhancing Susceptibility of Glioblastoma Cells to IRE Using a Calcium Adjuvant. Ann. Biomed. Eng. 2017;45:2535–2547. doi: 10.1007/s10439-017-1905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escoffre J.-M., Rols M.-P. Electrochemotherapy: Progress and Prospects. Curr. Pharm. Des. 2012;18:3406–3415. doi: 10.2174/138161212801227087. [DOI] [PubMed] [Google Scholar]
- 31.Poddevin B., Orlowski S., Belehradek J.J., Mir L.M. Very High Cytotoxicity of Bleomycin Introduced into the Cytosol of Cells in Culture. Biochem. Pharmacol. 1991;42:67–75. doi: 10.1016/0006-2952(91)90394-K. [DOI] [PubMed] [Google Scholar]
- 32.Mir L.M., Gehl J., Sersa G., Collins C.G., Garbay J.R., Billard V., Geertsen P.F., Rudolf Z., O’Sullivan G.C., Marty M. Standard Operating Procedures of the Electrochemotherapy: Instructions for the Use of Bleomycin or Cisplatin Administered Either Systemically or Locally and Electric Pulses Delivered by the CliniporatorTM by Means of Invasive or Non-Invasive Electrodes. Eur. J. Cancer Suppl. 2006;4:14–25. doi: 10.1016/j.ejcsup.2006.08.003. [DOI] [Google Scholar]
- 33.Tarantino L., Busto G., Nasto A., Fristachi R., Cacace L., Talamo M., Accardo C., Bortone S., Gallo P., Tarantino P., et al. Percutaneous Electrochemotherapy in the Treatment of Portal Vein Tumor Thrombosis at Hepatic Hilum in Patients with Hepatocellular Carcinoma in Cirrhosis: A Feasibility Study. World J. Gastroenterol. 2017;23:906–918. doi: 10.3748/wjg.v23.i5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edhemovic I., Brecelj E., Cemazar M., Boc N., Trotovsek B., Djokic M., Dezman R., Ivanecz A., Potrc S., Bosnjak M., et al. Intraoperative Electrochemotherapy of Colorectal Liver Metastases: A Prospective Phase II Study. Eur. J. Surg. Oncol. 2020;46:1628–1633. doi: 10.1016/j.ejso.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 35.Izzo F., Granata V., Fusco R., D’alessio V., Petrillo A., Lastoria S., Piccirillo M., Albino V., Belli A., Tafuto S., et al. Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated with Electrochemotherapy. J. Clin. Med. 2021;10:1305. doi: 10.3390/jcm10061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sersa G. The State-of-the-Art of Electrochemotherapy before the ESOPE Study; Advantages and Clinical Uses. Eur. J. Cancer Suppl. 2006;4:52–59. doi: 10.1016/j.ejcsup.2006.08.007. [DOI] [Google Scholar]
- 37.Clover A.J.P., de Terlizzi F., Bertino G., Curatolo P., Odili J., Campana L.G., Kunte C., Muir T., Brizio M., Sersa G., et al. Electrochemotherapy in the Treatment of Cutaneous Malignancy: Outcomes and Subgroup Analysis from the Cumulative Results from the Pan-European International Network for Sharing Practice in Electrochemotherapy Database for 2482 Lesions in 987 Patients (2008–2019) Eur. J. Cancer. 2020;138:30–40. doi: 10.1016/j.ejca.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Plaschke C.C., Bertino G., McCaul J.A., Grau J.J., de Bree R., Sersa G., Occhini A., Groselj A., Langdon C., Heuveling D.A., et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) Project: Results from the Treatment of Mucosal Cancers. Eur. J. Cancer. 2017;87:172–181. doi: 10.1016/j.ejca.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Garbe C., Peris K., Hauschild A., Saiag P., Middleton M., Bastholt L., Grob J.-J., Malvehy J., Newton-Bishop J., Stratigos A.J., et al. Diagnosis and Treatment of Melanoma. European Consensus-Based Interdisciplinary Guideline—Update 2016. Eur. J. Cancer. 2016;63:201–217. doi: 10.1016/j.ejca.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Michielin O., van Akkooi A., Lorigan P., Ascierto P., Dummer R., Robert C., Arance A., Blank C., Sileni V.C., Donia M., et al. ESMO Consensus Conference Recommendations on the Management of Locoregional Melanoma: Under the Auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020;31:1449–1461. doi: 10.1016/j.annonc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Stratigos A., Garbe C., Lebbe C., Malvehy J., del Marmol V., Pehamberger H., Peris K., Becker J.C., Zalaudek I., Saiag P., et al. Diagnosis and Treatment of Invasive Squamous Cell Carcinoma of the Skin: European Consensus-Based Interdisciplinary Guideline. Eur. J. Cancer. 2015;51:1989–2007. doi: 10.1016/j.ejca.2015.06.110. [DOI] [PubMed] [Google Scholar]
- 42.Capanna R., Piccioli A., Gasbarrini A., Di Martino A., Biagini R., Denaro V., Ghermandi R., Girolami M., Spinelli M.S., Zoccali C., et al. Algoritmo Terapeutico per Il Trattamento Delle Metastasi Del Sacro. Raccomandazioni Del Gruppo Di Studio SIOT Sulle Metastasi Ossee. G. Ital. Ortop. Traumatol. 2016;42:242–250. [Google Scholar]
- 43.Rudno-Rudzińska J., Kielan W., Guziński M., Płochocki M., Antończyk A., Kulbacka J. New Therapeutic Strategy: Personalization of Pancreatic Cancer Treatment-Irreversible Electroporation (IRE), Electrochemotherapy (ECT) and Calcium Electroporation (CaEP)—A Pilot Preclinical Study. Surg. Oncol. 2021;38:101634. doi: 10.1016/j.suronc.2021.101634. [DOI] [PubMed] [Google Scholar]
- 44.Ruarus A.H., Vroomen L.G.P.H., Geboers B., van Veldhuisen E., Puijk R.S., Nieuwenhuizen S., Besselink M.G., Zonderhuis B.M., Kazemier G., de Gruijl T.D., et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology. 2020;294:212–220. doi: 10.1148/radiol.2019191109. [DOI] [PubMed] [Google Scholar]
- 45.Meijerink M.R., Ruarus A.H., Vroomen L.G.P.H., Puijk R.S., Geboers B., Nieuwenhuizen S., van den Bemd B.A.T., Nielsen K., de Vries J.J.J., van Lienden K.P., et al. Irreversible Electroporation to Treat Unresectable Colorectal Liver Metastases (COLDFIRE-2): A Phase II, Two-Center, Single-Arm Clinical Trial. Radiology. 2021;299:470–480. doi: 10.1148/radiol.2021203089. [DOI] [PubMed] [Google Scholar]
- 46.van den Bos W., Scheltema M.J., Siriwardana A.R., Kalsbeek A.M.F., Thompson J.E., Ting F., Böhm M., Haynes A.M., Shnier R., Delprado W., et al. Focal Irreversible Electroporation as Primary Treatment for Localized Prostate Cancer. BJU Int. 2018;121:716–724. doi: 10.1111/bju.13983. [DOI] [PubMed] [Google Scholar]
- 47.Guenther E., Klein N., Zapf S., Weil S., Schlosser C., Rubinsky B., Stehling M.K. Prostate Cancer Treatment with Irreversible Electroporation (IRE): Safety, Efficacy and Clinical Experience in 471 Treatments. PLoS ONE. 2019;14:e0215093. doi: 10.1371/journal.pone.0215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He C., Wang J., Sun S., Zhang Y., Lin X., Lao X., Cui B. Irreversible Electroporation versus Radiotherapy after Induction Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer: A Propensity Score Analysis. BMC Cancer. 2019;19:394. doi: 10.1186/s12885-019-5607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Barone A., Hazarika M., Theoret M.R., Mishra-Kalyani P., Chen H., He K., Sridhara R., Subramaniam S., Pfuma E., Wang Y., et al. FDA Approval Summary: Pembrolizumab for the Treatment of Patients with Unresectable or Metastatic Melanoma. Clin. Cancer Res. 2017;23:5661–5665. doi: 10.1158/1078-0432.CCR-16-0664. [DOI] [PubMed] [Google Scholar]
- 51.Dong Y., Sun Q., Zhang X. PD-1 and Its Ligands Are Important Immune Checkpoints in Cancer. Oncotarget. 2017;8:2171–2186. doi: 10.18632/oncotarget.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A. Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hegde P.S., Chen D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity. 2020;52:17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Galon J., Bruni D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 55.Galon J., Bruni D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity. 2020;52:55–81. doi: 10.1016/j.immuni.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 56.Chretien A., Le Roy A., Vey N., Prebet T., Blaise D., Fauriat C., Olive D. Cancer-Induced Alterations of NK-Mediated Target Recognition: Current and Investigational Pharmacological Strategies Aiming at Restoring NK-Mediated Anti-Tumor Activity. Front. Immunol. 2014;5:122. doi: 10.3389/fimmu.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hiam-Galvez K.J., Allen B.M., Spitzer M.H. Systemic Immunity in Cancer. Nat. Rev. Cancer. 2021;21:345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng M.W.L., Galon J., Fridman W.H., Smyth M.J. From Mice to Humans: Developments in Cancer Immunoediting. J. Clin. Invest. 2015;125:3338–3346. doi: 10.1172/JCI80004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Idos G.E., Kwok J., Bonthala N., Kysh L., Gruber S.B., Qu C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020;10:3360. doi: 10.1038/s41598-020-60255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orhan A., Vogelsang R.P., Andersen M.B., Madsen M.T., Hölmich E.R., Raskov H., Gögenur I. The Prognostic Value of Tumour-Infiltrating Lymphocytes in Pancreatic Cancer: A Systematic Review and Meta-Analysis. Eur. J. Cancer. 2020;132:71–84. doi: 10.1016/j.ejca.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Gao Z.-H., Li C.-X., Liu M., Jiang J.-Y. Predictive and Prognostic Role of Tumour-Infiltrating Lymphocytes in Breast Cancer Patients with Different Molecular Subtypes: A Meta-Analysis. BMC Cancer. 2020;20:1150. doi: 10.1186/s12885-020-07654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shang B., Liu Y., Jiang S.J., Liu Y. Prognostic Value of Tumor-Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frydrychowicz M., Boruczkowski M., Kolecka-Bednarczyk A., Dworacki G. The Dual Role of Treg in Cancer. Scand. J. Immunol. 2017;86:436–443. doi: 10.1111/sji.12615. [DOI] [PubMed] [Google Scholar]
- 64.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel E.E., Koeppen H., Astarita J.L., Cubas R., et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stamatovic S.M., Keep R.F., Mostarica-Stojkovic M., Andjelkovic A.V. CCL2 Regulates Angiogenesis via Activation of Ets-1 Transcription Factor. J. Immunol. 2006;177:2651–2661. doi: 10.4049/jimmunol.177.4.2651. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Q., Wu X., Wu Y., Wang X. Interaction between Treg Cells and Tumor-Associated Macrophages in the Tumor Microenvironment of Epithelial Ovarian Cancer. Oncol. Rep. 2016;36:3472–3478. doi: 10.3892/or.2016.5136. [DOI] [PubMed] [Google Scholar]
- 67.Raskov H., Orhan A., Gaggar S., Gögenur I. Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in Cancer and Cancer Immunotherapy. Front. Oncol. 2021;11:668731. doi: 10.3389/fonc.2021.668731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abu N., Rus Bakarurraini N.A.A., Nasir S.N. Extracellular Vesicles and DAMPs in Cancer: A Mini-Review. Front. Immunol. 2021;12:740548. doi: 10.3389/fimmu.2021.740548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaudino S.J., Kumar P. Cross-Talk between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019;10:360. doi: 10.3389/fimmu.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sersa G., MikIavcic D., Cemazar M., Jean Belehradek J., Jarm T., Mir L.M. Electrochemotherapy with CDDP on LPB Sarcoma: Comparison of the Anti-Tumor Effectiveness in Immunocompetent and Immunodeficient Mice. Bioelectrochem. Bioenerg. 1997;43:279–283. doi: 10.1016/S0302-4598(96)05194-X. [DOI] [Google Scholar]
- 71.Calvet C.Y., Famin D., André F.M., Mir L.M. Electrochemotherapy with Bleomycin Induces Hallmarks of Immunogenic Cell Death in Murine Colon Cancer Cells. Oncoimmunology. 2014;3:e28131. doi: 10.4161/onci.28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ursic K., Kos S., Kamensek U., Cemazar M., Scancar J., Bucek S., Kranjc S., Staresinic B., Sersa G. Comparable Effectiveness and Immunomodulatory Actions of Oxaliplatin and Cisplatin in Electrochemotherapy of Murine Melanoma. Bioelectrochemistry. 2018;119:161–171. doi: 10.1016/j.bioelechem.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 73.Roux S., Bernat C., François B.A., Opolon P., Carpentier A.F., Zitvogel L., Mir L.M., Robert C. Tumor Destruction Using Electrochemotherapy Followed by CpG Oligodeoxynucleotide Injection Induces Distant Tumor Responses. Cancer Immunol. Res. Immunother. 2008;57:1291–1300. doi: 10.1007/s00262-008-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bigi L., Galdo G., Cesinaro A.M., Vaschieri C., Marconi A., Fantini F. Electrochemotherapy Induces Apoptotic Death in Melanoma Metastases: A Histologic and Immunohistochemical Investigation. Clin. Cosmet. Investig. Dermatol. 2016;9:451–459. doi: 10.2147/CCID.S115984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tremble L.F., O’Brien M.A., Soden D.M., Forde P.F. Electrochemotherapy with Cisplatin Increases Survival and Induces Immunogenic Responses in Murine Models of Lung Cancer and Colorectal Cancer. Cancer Lett. 2019;442:475–482. doi: 10.1016/j.canlet.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 76.Zmuc J., Gasljevic G., Sersa G., Edhemovic I., Boc N., Seliskar A., Plavec T., Brloznik M., Milevoj N., Brecelj E., et al. Large Liver Blood Vessels and Bile Ducts Are Not Damaged by Electrochemotherapy with Bleomycin in Pigs. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-40395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gasljevic G., Edhemovic I., Cemazar M., Brecelj E., Gadzijev E.M., Music M.M., Sersa G. Histopathological Findings in Colorectal Liver Metastases after Electrochemotherapy. PLoS ONE. 2017;12:e0180709. doi: 10.1371/journal.pone.0180709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campana L.G., Mocellin S., Basso M., Puccetti O., De Salvo G.L., Chiarion-Sileni V., Vecchiato A., Corti L., Rossi C.R., Nitti D. Bleomycin-Based Electrochemotherapy: Clinical Outcome from a Single Institution’s Experience with 52 Patients. Ann. Surg. Oncol. 2009;16:191–199. doi: 10.1245/s10434-008-0204-8. [DOI] [PubMed] [Google Scholar]
- 79.Gerlini G., Sestini S. Dendritic Cells Recruitment in Melanoma Metastasis Treated by Electrochemotherapy. Clin. Exp. Metastasis. 2013;30:37–45. doi: 10.1007/s10585-012-9505-1. [DOI] [PubMed] [Google Scholar]
- 80.Markelc B., Sersa G., Cemazar M. Differential Mechanisms Associated with Vascular Disrupting Action of Electrochemotherapy: Intravital Microscopy on the Level of Single Normal and Tumor Blood Vessels. PLoS ONE. 2013;8:e59557. doi: 10.1371/journal.pone.0059557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torrero M.N., Henk W.G., Li S. Regression of High-Grade Malignancy in Mice by Bleomycin and Interleukin-12 Electrochemogenetherapy. Clin. Cancer Res. 2006;12:257–263. doi: 10.1158/1078-0432.CCR-05-1514. [DOI] [PubMed] [Google Scholar]
- 82.Mekid H., Tounekti O., Spatz A., Cemazar M., El Kebir F.Z., Mir L.M. In Vivo Evolution of Tumour Cells after the Generation of Double-Strand DNA Breaks. Br. J. Cancer. 2003;88:1763–1771. doi: 10.1038/sj.bjc.6600959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernandes P., Donovan T.R.O., Mckenna S.L., Forde P.F. Electrochemotherapy Causes Caspase-Independent Necrotic-Like Death in Pancreatic Cancer Cells. Cancers. 2019;11:1177. doi: 10.3390/cancers11081177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali M.S., Gill K.S., Saglio G., Cilloni D., Soden D.M., Forde P.F. Expressional Changes in Stemness Markers Post Electrochemotherapy in Pancreatic Cancer Cells. Bioelectrochemistry. 2018;122:84–92. doi: 10.1016/j.bioelechem.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Kim H.-B., Sung C.-K., Baik K.Y., Moon K.-W., Kim H.-S., Yi J.-H., Jung J.-H., Moon M.-H., Choi O.-K. Changes of Apoptosis in Tumor Tissues with Time after Irreversible Electroporation. Biochem. Biophys. Res. Commun. 2013;435:651–656. doi: 10.1016/j.bbrc.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 86.Long G., Bakos G., Shires P.K., Gritter L., Crissman J.W., Harris J.L., Clymer J.W. Histological and Finite Element Analysis of Cell Death Due to Irreversible Electroporation. Technol. Cancer Res. Treat. 2014;13:561–569. doi: 10.7785/tcrtexpress.2013.600253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao J., Wen X., Tian L., Li T., Xu C., Wen X., Melancon M.P., Gupta S., Shen B., Peng W., et al. Irreversible Electroporation Reverses Resistance to Immune Checkpoint Blockade in Pancreatic Cancer. Nat. Commun. 2019;10:899. doi: 10.1038/s41467-019-08782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He C., Sun S., Zhang Y., Xie F., Li S. The Role of Irreversible Electroporation in Promoting M1 Macrophage Polarization via Regulating the HMGB1-RAGE-MAPK Axis in Pancreatic Cancer. Oncoimmunology. 2021;10:1897295. doi: 10.1080/2162402X.2021.1897295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ichikawa M.L., Vu N.K., Nijagal A., Rubinsky B., Chang T.T. Neutrophils Are Important for the Development of pro - Reparative Macrophages after Irreversible Electroporation of the Liver in Mice. Sci. Rep. 2021;11:14986. doi: 10.1038/s41598-021-94016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun S., Liu Y., He C., Hu W., Liu W., Huang X., Wu J., Xie F., Chen C., Wang J., et al. Combining NanoKnife with M1 Oncolytic Virus Enhances Anticancer Activity in Pancreatic Cancer. Cancer Lett. 2021;502:9–24. doi: 10.1016/j.canlet.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 91.Ringel-scaia V.M., Beitel-white N., Lorenzo M.F., Brock R.M., Huie K.E., Coutermarsh-ott S., Eden K., Mcdaniel D.K., Verbridge S.S., Rossmeisl J.H., et al. High-Frequency Irreversible Electroporation Is an Effective Tumor Ablation Strategy That Induces Immunologic Cell Death and Promotes Systemic Anti-Tumor Immunity. EBioMedicine. 2019;44:112–125. doi: 10.1016/j.ebiom.2019.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo Y., Zhang Y., Klein R., Nijm G.M., Alan V., Omary R.A., Yang G., Andrew C. Liver-Directed Irreversible Electroporation Therapy: Longitudinal Efficacy Studies in a Rat Model of Hepatocellular Carcinoma. Cancer Res. 2011;70:1555. doi: 10.1158/0008-5472.CAN-09-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.José A., Sobrevals L., Ivorra A., Fillat C. Irreversible Electroporation Shows Efficacy against Pancreatic Carcinoma without Systemic Toxicity in Mouse Models. Cancer Lett. 2012;317:16–23. doi: 10.1016/j.canlet.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 94.Bulvik B.E., Ahmed M., Andriyanov A.V., Goldberg S.N. Irreversible Electroporation versus Radiofrequency Ablation: A Comparison of Local and Systemic. Radiology. 2016;280:413–424. doi: 10.1148/radiol.2015151166. [DOI] [PubMed] [Google Scholar]
- 95.He C., Lin X., Zhang Y., Lin X., Li S. T-Cell Activation and Immune Memory Enhancement Induced by Irreversible Electroporation in Pancreatic Cancer. Clin. Transl. Med. 2020;10:e39. doi: 10.1002/ctm2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Babikr F., Wan J., Xu A., Wu Z., Ahmed S., Freywald A., Chibbar R., Wu Y., Moser M., Groot G., et al. Distinct Roles but Cooperative Effect of TLR3/9 Agonists and PD-1 Blockade in Concerting the Immunotolerant Microinvironment of Irreversible Electroporation-Ablated Tumors. Nat. Cellluar Mol. Immunol. 2021;18:2632–2647. doi: 10.1038/s41423-021-00796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pandit H., Hong Y.K., Li Y., Rostas J., Pulliam Z. Evaluating the Regulatory Immunomodulation Effect of Irreversible Electroporation (IRE) in Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2019;26:800–806. doi: 10.1245/s10434-018-07144-3. [DOI] [PubMed] [Google Scholar]
- 98.Lee E.W., Wong D., Tafti B.A., Prieto V., Totonchy M., Hilton J., Dry S., Cho S., Loh C.T. Irreversible Electroporation in Eradication of Rabbit VX2 Liver Tumor. JVIR. 2012;23:833–840. doi: 10.1016/j.jvir.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 99.Dai Z., Wang Z., Lei K., Liao J., Peng Z., Lin M., Liang P., Yu J., Peng S., Chen S., et al. Irreversible Electroporation Induces CD8 + T Cell Immune Response against Post-Ablation Hepatocellular Carcinoma Growth. Cancer Lett. 2021;503:1–10. doi: 10.1016/j.canlet.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Neal R.E., Jr J.H.R., Robertson J.L., Arena C.B., Davis E.M., Singh R.N., Stallings J., Davalos R.V. Improved Local and Systemic Anti-Tumor Efficacy for Irreversible Electroporation in Immunocompetent versus Immunodeficient Mice. PLoS ONE. 2013;8:e64559. doi: 10.1371/journal.pone.0064559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Al-Sakere B., Bernat C., André F., Connault E., Opolon P., Davalos R.V., Mir L.M. A Study of the Immunological Response to Tumor Ablation with Irreversible Electroporation. Technol. Cancer Res. Treat. 2007;6:301–306. doi: 10.1177/153303460700600406. [DOI] [PubMed] [Google Scholar]
- 102.Martinez O.M., Villanueva J., Abtahi S., Beatty P.R., Esquivel C.O., Krams S.M. CD30 Expression Identifies a Functional Alloreactive Human T-Lymphocyte Subset. Transplantation. 1998;65:1240–1247. doi: 10.1097/00007890-199805150-00016. [DOI] [PubMed] [Google Scholar]
- 103.Mateizel I., Spits C., Verloes A., Mertzanidou A., Liebaers I., Sermon K. Characterization of CD30 Expression in Human Embryonic Stem Cell Lines Cultured in Serum-Free Media and Passaged Mechanically. Hum. Reprod. 2009;24:2477–2489. doi: 10.1093/humrep/dep234. [DOI] [PubMed] [Google Scholar]
- 104.He C., Wang J., Sun S., Zhang Y., Li S. Immunomodulatory Effect after Irreversible Electroporation in Patients with Locally Advanced Pancreatic Cancer. J. Oncol. 2019;2019:9346017. doi: 10.1155/2019/9346017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li X., Xu K., Li W., Qiu X., Ma B., Fan Q., Li Z. Immunologic Response to Tumor Ablation with Irreversible Electroporation. PLoS ONE. 2012;7:e48749. doi: 10.1371/journal.pone.0048749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Phillips J.H., Gemlo B.T., Myers W.W., Rayner A.A., Lanier L.L. In Vivo and in Vitro Activation of Natural Killer Cells in Advanced Cancer Patients Undergoing Combined Recombinant Interleukin-2 and LAK Cell Therapy. J. Clin. Oncol. 1987;5:1933–1941. doi: 10.1200/JCO.1987.5.12.1933. [DOI] [PubMed] [Google Scholar]
- 107.Wrangle J.M., Patterson A., Johnson C.B., Neitzke D.J., Mehrotra S., Denlinger C.E., Paulos C.M., Li Z., Cole D.J., Rubinstein M.P. IL-2 and beyond in Cancer Immunotherapy. J. Interf. Cytokine Res. 2018;38:45–68. doi: 10.1089/jir.2017.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taniguchi K., Karin M. IL-6 and Related Cytokines as the Critical Lynchpins between Inflammation and Cancer. Semin. Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 109.Kryczek I., Zou L., Rodriguez P., Zhu G., Wei S., Mottram P., Brumlik M., Cheng P., Curiel T., Myers L., et al. B7-H4 Expression Identifi Es a Novel Suppressive Macrophage Population in Human Ovarian Carcinoma. J. Exp. Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang J.Y., Wang W.P. B7-H4, a Promising Target for Immunotherapy. Cell. Immunol. 2019;347:104008. doi: 10.1016/j.cellimm.2019.104008. [DOI] [PubMed] [Google Scholar]
- 111.Kuang D., Zhao Q., Peng C., Xu J., Zhang J., Wu C., Zheng L. Activated Monocytes in Peritumoral Stroma of Hepatocellular Carcinoma Foster Immune Privilege and Disease Progression through PD-L1. J. Exp. Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo X., Du F., Liu Q., Guo Y., Wang Q., Huang W., Wang Z. Immunological Effect of Irreversible Electroporation on Hepatocellular Carcinoma. BMC Cancer. 2021;21:443. doi: 10.1186/s12885-021-08176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scheffer H.J., Stam A.G.M., Geboers B., Vroomen L.G.P.H., Ruarus A., de Bruijn B., van den Tol M.P., Kazemier G., Meijerink M.R., de Gruijl T.D. Irreversible Electroporation of Locally Advanced Pancreatic Cancer Transiently Alleviates Immune Suppression and Creates a Window for Antitumor T Cell Activation. Oncoimmunology. 2019;8:1652532. doi: 10.1080/2162402X.2019.1652532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martin R.C.G., Li Y., Brock R.M., Allen I.C., Davalos R.V. Real-Time Prediction of Patient Immune Cell Modulation during Irreversible Electroporation Therapy. Sci. Reports. 2019;9:17739. doi: 10.1038/s41598-019-53974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fujimori M., Kimura Y., Ueshima E., Dupuy D.E., Adusumilli P.S., Solomon S.B., Srimathveeravalli G. Lung Ablation with Irreversible Electroporation Promotes Immune Cell Infiltration by Sparing Extracellular Matrix Proteins and Vasculature: Implications for Immunotherapy. Bioelectricity. 2021;3:204–214. doi: 10.1089/bioe.2021.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen X., Ren Z., Yin S., Xu Y., Guo D., Xie H., Zhou L., Wu L., Jiang J., Li H., et al. The Local Liver Ablation with Pulsed Electric Field Stimulate Systemic Immune Reaction against Hepatocellular Carcinoma (HCC) with Time-Dependent Cytokine Profile. Cytokine. 2017;93:44–50. doi: 10.1016/j.cyto.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 117.White S.B., Zhang Z., Chen J., Gogineni V.R., Larson A.C. Early Immunologic Response of Irreversible Electroporation versus Cryoablation in a Rodent Model of Pancreatic Cancer. J. Vasc. Interv. Radiol. 2018;29:1764–1769. doi: 10.1016/j.jvir.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 118.Goswami I., Coutermarsh-Ott S., Morrison R.G., Allen I., Davalos R.V., Verbridge S.S., Bickford L.R. Irreversible Electroporation Inhibits Pro-Cancer Inflammatory Signaling in Triple Negative Breast Cancer Cells. Bioelectrochemistry. 2017;113:42–50. doi: 10.1016/j.bioelechem.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cameron F., Whiteside G., Perry C. Ipilimumab. Drugs. 2011;71:1093–1104. doi: 10.2165/11594010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 120.Myers J.A., Miller J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams R., Cooley S., Bachanova V., Waldmann T.A., Blazar B.R., Weisdorf D.J., Miller J.S., Verneris M.R. Role of Recipient CD8+ T Cell Exhaustion in the Rejection of Adoptively Transferred Haploidentical NK Cells. Blood. 2016;128:503. doi: 10.1182/blood.V128.22.503.503. [DOI] [Google Scholar]
- 122.Andersen M.H., Gehl J., Reker S., Pedersen L., Becker J.C., Geertsen P., Thor Straten P. Dynamic Changes of Specific T Cell Responses to Melanoma Correlate with IL-2 Administration. Semin. Cancer Biol. 2003;13:449–459. doi: 10.1016/j.semcancer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 123.Theurich S., Rothschild S.I., Hoffmann M., Fabri M., Sommer A., Garcia-Marquez M., Thelen M., Schill C., Merki R., Schmid T., et al. Local Tumor Treatment in Combination with Systemic Ipilimumab Immunotherapy Prolongs Overall Survival in Patients with Advanced Malignant Melanoma. Cancer Immunol. Res. 2016;4:744–754. doi: 10.1158/2326-6066.CIR-15-0156. [DOI] [PubMed] [Google Scholar]
- 124.Campana L.G., Peric B., Mascherini M., Spina R., Kunte C., Kis E., Rozsa P., Quaglino P., Jones R.P., Clover A.J.P., et al. Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry. Cancers. 2021;13:4289. doi: 10.3390/cancers13174289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heppt M.V., Eigentler T.K., Kähler K.C., Herbst R.A., Göppner D., Gambichler T., Ulrich J., Dippel E. Immune Checkpoint Blockade with Concurrent Electrochemotherapy in Advanced Melanoma: A Retrospective Multicenter Analysis. Cancer Immunol. Immunother. 2016;65:951–959. doi: 10.1007/s00262-016-1856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hribernik A., Cemazar M., Sersa G., Bosnjak M., Snoj M. Effectiveness of Electrochemotherapy after IFN-α Adjuvant Therapy of Melanoma Patients. Radiol. Oncol. 2016;50:21–27. doi: 10.1515/raon-2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ursic K., Kos S., Kamensek U., Cemazar M., Miceska S., Markelc B., Bucek S., Staresinic B., Kloboves V., Heller R., et al. Potentiation of Electrochemotherapy Effectiveness by Immunostimulation with IL-12 Gene Electrotransfer in Mice Is Dependent on Tumor Immune Status. J. Control. Release. 2021;332:623–635. doi: 10.1016/j.jconrel.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 128.Sedlar A., Dolinsek T., Markelc B., Prosen L., Kranjc S., Bosnjak M., Blagus T., Cemazar M., Sersa G. Potentiation of Electrochemotherapy by Intramuscular IL-12 Gene Electrotransfer in Murine Sarcoma and Carcinoma with Different Immunogenicity. Radiol. Oncol. 2012;46:302–311. doi: 10.2478/v10019-012-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kishida T., Asada H., Itokawa Y., Yasutomi K., Shin-ya M. Electrochemo-Gene Therapy of Cancer: Intratumoral Delivery of Interleukin-12 Gene and Bleomycin Synergistically Induced Therapeutic Immunity and Suppressed Subcutaneous and Metastatic Melanomas in Mice. Mol. Ther. 2003;8:739–745. doi: 10.1016/j.ymthe.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Tremble L.F., O’Brien M.A., Forde P.F., Soden D.M. ICOS Activation in Combination with Electrochemotherapy Generates Effective Anti-Cancer Immunological Responses in Murine Models of Primary, Secondary and Metastatic Disease. Cancer Lett. 2018;420:109–115. doi: 10.1016/j.canlet.2018.01.081. [DOI] [PubMed] [Google Scholar]
- 131.Cemazar M., Todorovic V., Scancar J., Lampreht U., Stimac M., Kamensek U., Kranjc S., Coer A., Sersa G. Adjuvant TNF-α Therapy to Electrochemotherapy with Intravenous Cisplatin in Murine Sarcoma Exerts Synergistic Antitumor Effectiveness. Radiol. Oncol. 2015;49:32–40. doi: 10.1515/raon-2015-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sersa G., Cemazar M., Menart V. Anti-Tumor Effectiveness of Electrochemotherapy with Bleomycin Is Increased by TNF-a on SA-l Tumors in Mice. Cancer Lett. 1997;116:85–92. doi: 10.1016/S0304-3835(97)00170-5. [DOI] [PubMed] [Google Scholar]
- 133.Quaresmini D., Di Lauro A., Fucci L., Strippoli S., De Risi I., Sciacovelli A.M., Albano A., Achille G., Montepara M., Russo S., et al. Electrochemotherapy as a Trigger to Overcome Primary Resistance to Anti-PD-1 Treatment: A Case Report of Melanoma of the Scalp. Front. Oncol. 2021;11:742666. doi: 10.3389/fonc.2021.742666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karaca B., Yayla G., Erdem M., Gürler T. Electrochemotherapy with Anti-PD-1 Treatment Induced Durable Complete Response in Heavily Pretreated Metastatic Melanoma Patient. Anticancer. Drugs. 2018;29:190–196. doi: 10.1097/CAD.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 135.Mozzillo N., Simeone E., Benedetto L., Curvietto M., Giannarelli D., Gentilcore G., Camerlingo R., Capone M., Madonna G., Festino L., et al. Assessing a Novel Immuno-Oncology-Based Combination Therapy: Ipilimumab plus Electrochemotherapy. Oncoimmunology. 2015;4:e1008842. doi: 10.1080/2162402X.2015.1008842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brizio M., Fava P., Astrua C., Cavaliere G., Savoia P. Complete Regression of Melanoma Skin Metastases after Electrochemotherapy plus Ipilimumab Treatment: An Unusual Clinical Presentation. Eur. J. Dermatol. 2015;25:271–272. doi: 10.1684/ejd.2015.2522. [DOI] [PubMed] [Google Scholar]
- 137.Salvadori C., Svara T., Rocchigiani G., Millanta F., Pavlin D., Cemazar M., Tratar U.L., Sersa G., Tozon N., Poli A. Effects of Electrochemotherapy with Cisplatin and Peritumoral IL-12 Gene Electrotransfer on Canine Mast Cell Tumors: A Histopathologic and Immunohistochemical Study. Radiol. Oncol. 2017;51:286–294. doi: 10.1515/raon-2017-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ramirez L.H., Orlowski S., An D., Bindoula G., Dzodic R., Ardouin P., Bognel C., Belehradek J., Munck J.N., Mir L.M. Electrochemotherapy on Liver Tumours in Rabbits. Br. J. Cancer. 1998;77:2104–2111. doi: 10.1038/bjc.1998.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mir L.M., Roth C., Stéphane O., Françoise Q.C., Fradelizi D., Belehradek J., Kourilsky P. Systemic Antitumor Effects of Electrochemotherapy Combined with Histoincompatible Cells Secreting Interleukin-2. J. Immunother. 1995;17:30–38. doi: 10.1097/00002371-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 140.O’Neill C., Hayat T., Hamm J., Healey M., Zheng Q., Li Y., Martin R.C.G. A Phase 1b Trial of Concurrent Immunotherapy and Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Adenocarcinoma. Surgery. 2020;168:610–616. doi: 10.1016/j.surg.2020.04.057. [DOI] [PubMed] [Google Scholar]
- 141.He C., Sun S., Zhang Y., Li S. Irreversible Electroporation Plus Anti-PD-1 Antibody versus Irreversible Electroporation Alone for Patients with Locally Advanced Pancreatic Cancer. J. Inflamm. Res. 2021;14:4795–4807. doi: 10.2147/JIR.S331023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan Q., Hu C., Fan Y., Wang Y., Li R., Hu X. Efficacy of Irreversible Electroporation Ablation Combined with Natural Killer Cells in Treating Locally Advanced Pancreatic Cancer. JBUON. 2020;25:1643–1649. [PubMed] [Google Scholar]
- 143.Lin M., Alnaggar M., Liang S., Wang X., Liang Y., Zhang M., Chen J., Niu L., Xu K. An Important Discovery on Combination of Irreversible Electroporation and Allogeneic Natural Killer Cell Immunotherapy for Unresectable Pancreatic Cancer. Oncotarget. 2017;8:101795–101807. doi: 10.18632/oncotarget.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin M., Zhang X., Liang S., Luo H., Alnaggar M., Liu A., Yin Z., Chen J., Niu L., Jiang Y. Irreversible Electroporation plus Allogenic Vγ9Vδ2 T Cells Enhances Antitumor Effect for Locally Advanced Pancreatic Cancer Patients. Nat. Signal Transduct. Target. Ther. 2020;5:215. doi: 10.1038/s41392-020-00260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu Y., Liu T., Li J., Mai F., Li J., Chen Y., Jing Y., Dong X., Lin L., He J., et al. Selenium Nanoparticles as New Strategy to Potentiate Γδ T Cell Anti-Tumor Cytotoxicity through Upregulation of Tubulin-α Acetylation. Biomaterials. 2019;222:119397. doi: 10.1016/j.biomaterials.2019.119397. [DOI] [PubMed] [Google Scholar]
- 146.Kabelitz D., Serrano R., Kouakanou L., Peters C., Kalyan S. Cancer Immunotherapy with Γδ T Cells: Many Paths Ahead of Us. Cell. Mol. Immunol. 2020;17:925–939. doi: 10.1038/s41423-020-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang Y., Qin Z., Du D., Wu Y., Qiu S., Mu F., Xu K., Chen J. Safety and Short-Term Efficacy of Irreversible Electroporation and Allogenic Natural Killer Cell Immunotherapy Combination in the Treatment of Patients with Unresectable Primary Liver Cancer. Cardiovasc. Intervent. Radiol. 2019;42:48–59. doi: 10.1007/s00270-018-2069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alnaggar M., Lin M., Mesmare A., Liang S., Qaid A., Xu K., Chen J., Niu L., Yin Z. Allogenic Natural Killer Cell Immunotherapy Combined with Irreversible Electroporation for Stage IV Hepatocellular Carcinoma: Survival Outcome. Cell. Physiol. Biochem. 2018;48:1882–1893. doi: 10.1159/000492509. [DOI] [PubMed] [Google Scholar]
- 149.Burbach B.J., Flanagan S.D.O., Shao Q., Young K.M., Slaughter J.R., Rollins M.R., Street T.J.L., Granger V.E., Beura L.K., Azarin S.M., et al. Irreversible Electroporation Augments Checkpoint Immunotherapy in Prostate Cancer and Promotes Tumor Antigen-Specific Tissue-Resident Memory CD8+ T Cells. Nat. Commun. 2021;12:3862. doi: 10.1038/s41467-021-24132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shi X., Neill C.O., Wang X., Chen Y., Yu Y., Tan M., Lv G., Li Y., Martin R.C. Irreversible Electroporation Enhances Immunotherapeutic Effect in the Off-Target Tumor in a Murine Model of Orthotopic HCC. Am. J. Cancer Res. 2021;11:3304–3319. [PMC free article] [PubMed] [Google Scholar]
- 151.Narayanan J.S.S., Ray P., Hayashi T., Whisenant T.C., Vicente D., Carson D.A., Miller A.M., Schoenberger S.P., White R.R. Irreversible Electroporation Combined With Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol. Res. 2019;7:1714–1726. doi: 10.1158/2326-6066.CIR-19-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lasarte-Cia A., Lozano T., Cano D., Martín-otal C., Navarro F., Gorraiz M., Casares N., Vivas I., Lasarte J.J. Intratumoral STING Agonist Injection Combined with Irreversible Electroporation Delays Tumor Growth in a Model of Hepatocarcinoma. Biomed Res. Int. 2021;2021:8852233. doi: 10.1155/2021/8852233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Go E., Yang H., Chon H.J., Yang D., Ryu W. Combination of Irreversible Electroporation and STING Agonist for Effective Cancer Immunotherapy. Cancers. 2020;12:3123. doi: 10.3390/cancers12113123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Geboers B., Timmer F.E.F., Ruarus A.H., Pouw J.E.E., Schouten E.A.C., Bakker J., Puijk R.S., Nieuwenhuizen S., Dijkstra M., Tol M.P., et al. Irreversible Electroporation and Nivolumab Combined with Intratumoral Administration of a Toll-Like Receptor Ligand, as a Means of In Vivo Vaccination for Metastatic Pancreatic Ductal Adenocarcinoma (PANFIRE-III). A Phase-I Study Protocol. Cancers. 2021;13:3902. doi: 10.3390/cancers13153902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin M., Liang S., Wang X., Liang Y., Zhang M., Chen J., Niu L., Xu K. Short-Term Clinical Efficacy of Percutaneous Irreversible Electroporation Combined with Allogeneic Natural Killer Cell for Treating Metastatic Pancreatic Cancer. Immunol. Lett. 2017;186:20–27. doi: 10.1016/j.imlet.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Q., Guo X., Zhou Y., Wang Q., Liu Q., Wu Z., Ding X. OX40 Agonist Combined with Irreversible Electroporation Synergistically Eradicates Established Tumors and Drives Systemic Antitumor Immune Response in a Syngeneic Pancreatic Cancer Model. Am. J. Cancer Res. 2021;11:2782–2801. [PMC free article] [PubMed] [Google Scholar]
- 157.Yang J., Eresen A., Shangguan J., Ma Q., Yaghmai V. Irreversible Electroporation Ablation Overcomes Tumor-Associated Immunosuppression to Improve the Efficacy of DC Vaccination in a Mice Model of Pancreatic Cancer. Oncoimmunology. 2021;10:1875638. doi: 10.1080/2162402X.2021.1875638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yu B., Zhang W., Kwak K., Choi H., Kim D.H. Electric Pulse Responsive Magnetic Nanoclusters Loaded with Indoleamine 2,3-Dioxygenase Inhibitor for Synergistic Immuno-Ablation Cancer Therapy. ACS Appl. Mater. Interfaces. 2020;12:54415–54425. doi: 10.1021/acsami.0c15679. [DOI] [PubMed] [Google Scholar]
- 159.Vivas I., Iribarren K., Lozano T., Cano D., Lasarte-cia A., Chocarro S., Gorraiz M., Sarobe P., Herv S., Bilbao I., et al. Therapeutic Effect of Irreversible Electroporation in Combination with Poly-ICLC Adjuvant in Preclinical Models of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2019;30:1098–1105. doi: 10.1016/j.jvir.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 160.Pasquet L., Bellard E., Chabot S., Markelc B., Rols M., Teissie J., Golzio M. Pre-Clinical Investigation of the Synergy Effect of Interleukin-12 Gene-Electro-Transfer during Partially Irreversible Electropermeabilization against Melanoma. J. Immunother. Cancer. 2019;7:161. doi: 10.1186/s40425-019-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Kinzler K.W. Cancer Genome Landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Østrup O., Dagenborg V.J., Rødland E.A., Skarpeteig V., Silwal-Pandit L., Grzyb K., Berstad A.E., Fretland Å.A., Mælandsmo G.M., Børresen-Dale A.L., et al. Molecular Signatures Reflecting Microenvironmental Metabolism and Chemotherapy-Induced Immunogenic Cell Death in Colorectal Liver Metastases. Oncotarget. 2017;8:76290–76304. doi: 10.18632/oncotarget.19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dagenborg V.J., Marshall S.E., Grzyb K., Fretland Å.A., Lund-Iversen M., Mælandsmo G.M., Ree A.H., Edwin B., Yaqub S., Flatmark K. Low Concordance Between T-Cell Densities in Matched Primary Tumors and Liver Metastases in Microsatellite Stable Colorectal Cancer. Front. Oncol. 2021;11:671629. doi: 10.3389/fonc.2021.671629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yachida S., Jones S., Bozic I., Antal T., Leary R., Kamiyama M., Hruban R.H., Eshleman J.R., Nowak M.A., Velculescu V.E., et al. Distant Metastasis Occurs Late during the Genetic Evolution of Pancreatic Cancer. Nature. 2011;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Campbell P.J., Yachida S., Mudie L.J., Stephens P.J., Erin D., Stebbings L.A., Morsberger L.A., Latimer C., Mclaren S., Lin M., et al. The Patterns and Dynamics of Genomic Instability in Metastatic Pancreatic Cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brooks E.D., Chang J.Y. Time to Abandon Single-Site Irradiation for Inducing Abscopal Effects. Nat. Rev. Clin. Oncol. 2019;16:123–135. doi: 10.1038/s41571-018-0119-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.