We recently conducted a systematic review and meta-regression of the duration of effectiveness of primary series COVID-19 vaccination against clinical outcomes before the predominance of the omicron (B.1.1.529) SARS-CoV-2 variant.1 Here we assess the duration of vaccine protection, after a primary vaccine series and after the first booster dose, against omicron, the current predominant variant, using the same methods.1
We systematically reviewed published and preprint literature from Dec 3, 2021, to April 21, 2022, by searching for studies assessing absolute vaccine effectiveness over time during an omicron-dominant period. We estimated the mean change in vaccine effectiveness from 1 month to 6 months after primary vaccine series completion and from 1 month to 4 months after booster vaccination, using random-effects meta-regression (appendix p 22).
Of 15 887 studies screened, 409 underwent full-text review, 18 of which met inclusion criteria (appendix pp 4–14). Seven vaccine effectiveness studies assessed primary series vaccination only, one assessed booster vaccination only, and ten assessed both, yielding 99 vaccine-specific evaluations over time since final dose (48 primary series, 51 booster).
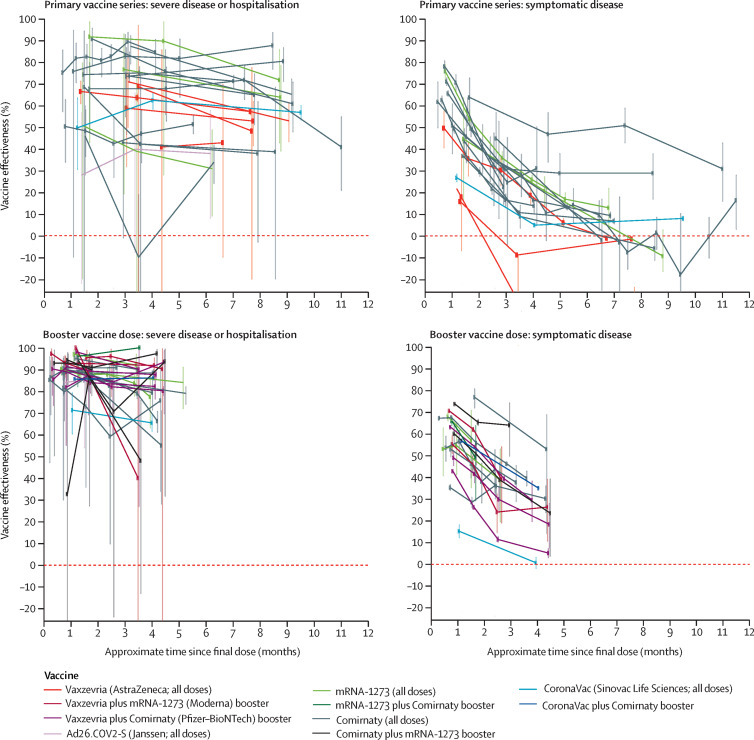
1 month after primary vaccine series completion, vaccine effectiveness against severe COVID-19 disease was lower for omicron (figure ) than for pre-omicron variants (reported previously1), but the mean decrease in vaccine effectiveness from 1 month to 6 months after the primary vaccine series was negligible (1·0 percentage point [95% CI –3·9 to 6·6] during omicron vs 10·0 percentage points [6·1 to 15·4] pre-omicron). Primary vaccine series effectiveness against symptomatic disease was also lower for omicron than pre-omicron variants, but unlike severe disease, vaccine effectiveness against omicron decreased more rapidly from 1 month to 6 months after primary vaccine series completion (47·6 percentage points [36·6 to 60·2] during omicron vs 24·9 percentage points [13·4 to 41·6] pre-omicron; figure).1 By 6 months after the primary vaccine series, little protection against symptomatic disease remained. Only five studies evaluated any omicron infection after the primary vaccine series, which showed a mean decline from 1 month to 6 months of 26·8 percentage points (16·5 to 38·6; appendix p 18).
Figure.
Duration of COVID-19 primary series and first booster dose vaccine effectiveness against the omicron variant
Mean percentage point decrease in vaccine effectiveness from 1 month to 6 months after vaccination from meta-regression (see appendix p 22).
1 month after booster vaccination, vaccine effectiveness against omicron was generally higher than after the primary vaccine series for all outcomes. As after the primary vaccine series, decreases in vaccine effectiveness after the booster vaccination were small for severe disease (5·3 percentage points [95% CI 2·4 to 8·7] from 1 month to 4 months after booster vaccination and 8·2 percentage points [3·7 to 14·3] projected out to 6 months after booster vaccination; appendix p 19). The mean decrease in vaccine effectiveness against symptomatic disease from 1 month to 4 months after booster vaccination was 24·3 percentage points (19·9 to 29·1), smaller than that observed after the primary vaccine series, and it was 28·5 percentage points (18·3 to 40·5) projected out to 6 months after booster vaccination. Only five vaccine-specific evaluations of effectiveness against any infection after booster vaccination were available, showing a 15·8 percentage point (–0·3 to 38·2) decrease from 1 month to 4 months (appendix p 18).
Limitations of our systematic review included potential biases in evaluating duration of vaccine effectiveness as described previously,1 scarce data for non-mRNA vaccines, and short follow-up after booster vaccination. Also, most studies of severe disease assessed vaccine effectiveness against hospitalisation. Given the high prevalence of the omicron variant, omicron infection might have been incidental rather than causal among some hospitalised people, which would have resulted in underestimated vaccine effectiveness against severe disease.2
Vaccine effectiveness of primary series COVID-19 vaccines against severe disease when the omicron variant was predominant was lower than that observed pre-omicron but showed little decline after vaccination. Booster vaccination increased vaccine effectiveness against omicron severe disease, which remained high 4 months after vaccination. Vaccine effectiveness against symptomatic disease decreased faster for omicron than pre-omicron variants, with protection from primary series vaccination nearly eroded by 4–6 months; protection after booster vaccination also decreased quickly, although less than after primary series vaccination.
MMH reports research grants from WHO, Coalition for Epidemic Preparedness Innovations (CEPI), Asian Development Bank (ADB), Bill & Melinda Gates Foundation, and Pfizer (all paid to the institution). AB and KKW report research grants from WHO and CEPI paid to the institution. EE reports stock ownership in GlaxoSmithKline. MDK reports research grants from WHO, CEPI, ADB, and Pfizer (all paid to the institution) and consultancy fees from Merck. All other authors declare no competing interests. DRF and MDK contributed equally. All data included were derived from publicly available documents cited in the references. Extracted data are available on request to the corresponding author. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Supplementary Material
References
- 1.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feikin DR, Abu-Raddad LJ, Andrews N, et al. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022;40:3516–3527. doi: 10.1016/j.vaccine.2022.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.