Abstract
In this work, we have used spores of Bacillus subtilis that specifically induce bioluminescence upon initiation of germination as a rapid, real-time monitor of the effects of preservative treatments on germination. Using this tool, we have demonstrated that the combination of mild acidity (pH 5.5 to 5.0), lactic acid (0.5%), and a pasteurization step (90°C for 5 min) results in enhanced inhibition of spore germination compared with the effects of the individual treatments alone. Inhibition by the combination treatment occurred as a result of both direct but reversible inhibition, entirely dependent on the physical presence of the preservative factors, and permanent, nonreversible damage to the l-alanine germination apparatus of the spore. However, we were able to restore germination of the preservative-damaged spores unable to germinate on l-alanine by supplementing the medium with the nonnutrient germinant calcium dipicolinic acid. The demonstration that simple combinations of preservative factors inhibit spore germination indicates that food preservation systems providing ambient stability could be designed which do not adhere to the strict limits set by commonly accepted processes and which are based on precise understanding of their inhibitory action.
Food manufacturers rely mainly on preservation by moist heat to produce ambient-stable products. These products are traditionally classified as low-acid foods, with pH values greater than 4.5, and medium- or high-acid foods, with pH values less than 4.5. The division is a reflection of the inability of spores of Clostridium botulinum and other mesophilic sporeformers to outgrow in foods with pH values of 4.5 or less (4). Thus, to ensure microbiological safety, low-acid foods are given severe heat treatments (121°C for 3 min or equivalent) which are designed to cause a 12-log reduction in the number of C. botulinum spores but which often result in foods with poor taste and organoleptic quality.
The target organisms for any low-acid preservation system providing ambient stability are endospore-forming bacteria of the genera Bacillus and Clostridium. The bacterial spore acts as a survival stage which is characterized by high resistance to heat and other adverse conditions typically used to kill vegetative cells (reviewed in reference 20). In foods, the spore itself does not represent a hazard. However, despite being metabolically dormant, the spore has a functional environmental sensory mechanism that can trigger germination under favorable conditions. Thus, the process of germination, outgrowth and proliferation, and/or toxin formation can result in spoilage and/or food poisoning.
Germination of Bacillus subtilis spores can be initiated upon exposure to two distinct types of nutrient stimuli: (i) l-alanine and (ii) a combination of l-asparagine, glucose, fructose, and KCl (AGFK) (28, 29). Subsequent work showed that metabolism of these nutrients did not occur during the process of germination (25) and that these molecules initiated germination by binding and activating specific receptors in the spore (30). Three homologous tricistronic operons, gerA, gerB, and gerK, have been proposed to encode germination receptor proteins because mutations in the genes that make up these operons result in germinant-specific defects in germination (16, 21). For example, spores with mutations in gerA failed to germinate in l-alanine, and mutations in gerB and gerK resulted in an inability to germinate in AGFK (16, 21). In addition, the first two proteins encoded by each operon are predicted to be hydrophobic integral membrane proteins, which is also consistent with the majority of characterized receptor molecules which are known to transmit environmental signals (10). Recently, Paidhungat and Setlow (24) confirmed that the gerB operon (and probably the gerA and gerK operons) encodes components of a spore germination receptor by isolating spores with gain-of-function mutations in the gerBA and gerBB genes that were able to germinate in the germination inhibitor d-alanine.
After germination is triggered, the spore becomes committed to germinate and loses many spore-specific properties in a sequential fashion, for example, heat resistance and refractility (reviewed in reference 13). The activation of a number of spore germination-specific cortex-lytic enzymes is an essential event in germination (1). In fact, inactivation of the gene encoding a germination-specific amidase in B. subtilis (sleB) results in spores that germinate more slowly than the wild type (22). Despite this, the signaling process that must be initiated after the nutrient germinant binds to the receptor complex and subsequently activates spore germination-specific cortex-lytic enzymes is not yet known. Any preservation system that effectively targets this germination mechanism has the potential to confer ambient stability on a manufactured food.
In practice, conditions for outgrowth in many foods is suboptimal due to the presence of a combination of factors, such as reduced pH, the presence of preservatives, and low water activity. Therefore, some heat-preserved foods, for example, canned meats (19) and tomatoes (31), are stabilized by heat processes using temperatures significantly lower than classic heat resistance data on spoilage organisms would indicate to be necessary. For example, Braithwaite and Perigo (4) and Bean (3) demonstrated that less severe heat processes were necessary to inactivate the spores of thermophilic bacilli at acidic pH values in combination with decreased water activity. Also, Banks et al. (2) measured inhibition of spore outgrowth from a range of heat-injured (65°C for 60 min) Bacillus species subsequently exposed to additional controlling factors (pH, organic acids, and preservatives). More recently, Oloyede and Scholefield (23) measured total loss of viability of Bacillus cereus at 85°C over 30 min in the presence of 0.4% (wt/vol) potassium sorbate with 6% (wt/vol) NaCl at pH 4.5. Importantly, none of these studies distinguished whether the inhibitory action of these combinations was on the mechanism of spore germination or on vegetative outgrowth and to what extent inhibition was due to spore injury or death. If milder, alternative methods of preservation are to be used that permit the manufacture of safe but higher-quality products, an essential part of any acceptance process will be a full understanding of their inhibitory action on bacterial spores.
Carmi et al. (8) were the first to introduce the genes for in vivo bioluminescence into Bacillus. Subsequently, Stewart et al. (26) proposed that bioluminescence could be used as an effective real-time monitor of the efficacy of inimical processes used to inactivate microorganisms. In this work, we have used a recombinant B. subtilis strain with a luxAB (originally from Vibrio harveyi) fusion inserted in front of the B. subtilis sspB gene promoter (15). The sspB gene codes for a spore core-associated small acid soluble protein, SASP-2, which binds to, and protects, the spore DNA (9). Hill et al. (15) were able to show that this system results in light emission only from germinating spores and that this emission occurs almost instantaneously with the start of germination, indicating that intact luciferase is packaged into the spore during sporulation. Thus, because this system results only in germination-dependent bioluminescence, it provides a sensitive real-time monitor of the germination and outgrowth process and how this is affected by preservation treatments (15).
In this study we demonstrated both recoverable and nonrecoverable damage to the l-alanine germination pathway of B. subtilis by using a combination of mild acidity (pH 5.5 to 5.0), lactic acid (0.5%), and a pasteurization temperature (90°C for 5 min).
MATERIALS AND METHODS
Organism and culture conditions.
Spores of B. subtilis(pSB357) (15, 17) were produced by growing a vegetative culture overnight at 30°C with shaking in heart infusion broth (Difco) with 100 μg of erythromycin (Sigma) ml−1 to approximately 109 cells ml−1. The culture was then spread onto heart infusion agar (Oxoid) supplemented with 100 μg of erythromycin ml−1. The plates were incubated at 30°C for 7 to 9 days, until at least 95% of the population consisted of phase-bright spores under a phase-contrast light microscope. Spores were then harvested by washing the heart infusion agar plates with cold, sterile, distilled water (4°C) to detach spores, followed by centrifugation at 4,000 × g for 20 min at 4°C. Subsequently spores were washed a further three times in cold, sterile, distilled water, pasteurized at 70°C for 30 min in a water bath, and then stored at −85°C until required.
Experimental procedure.
The aim of the study was to understand the inhibitory action of a combination preservation treatment on spores of B. subtilis (5). To understand the inhibitory action of the combination preservation system, we broke the system down into its four component parts and investigated the inhibitory effect of each of these. Thus, throughout this work we studied the inhibitory action of the following four treatments: (i) reduction of the pH from 7.0 to 4.0 (pH 7.0, 6.0, 5.5, 5.0, 4.8, 4.5, and 4.0, adjusted with HCl); (ii) reduction of the pH from 7.0 to 4.0 in the presence of 0.5% (wt/vol) lactic acid (Sigma); (iii) pasteurization at 90°C for 5 min with pH values reduced from 7.0 to 4.0; and (iv) the complete combination treatment, including pasteurization at 90°C for 5 min with pH values reduced from 7.0 to 4.0 in the presence of 0.5% (wt/vol) lactic acid.
Importantly, the effect on spore germination of the four treatments detailed above was measured in two ways. (i) Germination was measured under the actual preservative conditions listed above to measure the direct inhibitory effect of the preservative treatments on germination. (ii) Spores were exposed to the conditions listed above for 30 min followed by removal of the preservative treatments and measurement of germination under nonstress conditions. In this way we hoped to measure the degree of nonrecoverable damage or injury to the germination system induced upon exposure to the various preservative treatments.
Measurement of alanine-induced germination under actual preservative conditions.
Spores of B. subtilis(pSB357) (inoculum size, 3.0 × 109 per well) were treated in 50 mM potassium phosphate buffer at pH 4.0 to 7.0, with or without 0.5% lactic acid, for 5 min at 30 or 90°C (spores were incubated in an Eppendorf tube in a water bath). Then spores were immediately washed in 50 mM phosphate buffer at pH 4.0 to 7.0 and resuspended in nutrient broth (NB) (Oxoid) supplemented with 10 mM l-alanine at pH 4.0 to 7.0, with or without 0.5% lactic acid, and germination was measured at 30°C (see below). We chose to measure germination in NB with 10 mM l-alanine, as it is more representative of the constituents of a food than phosphate buffer is. Germination in NB without supplementation with l-alanine was negligible (data not shown).
Measurement of alanine-induced germination under optimal, nonstress conditions following exposure to preservative conditions.
Spores of B. subtilis(pSB357) (inoculum size, 3.0 × 109 per well) were pretreated in 50 mM phosphate buffer at pH 4.0 to 7.0, with or without 0.5% lactic acid, for 25 min at 30°C, followed by 5 min at 30 or 90°C (total time, 30 min). Following this, spores were immediately washed in 50 mM phosphate buffer at pH 4.0 to 7.0 and resuspended in NB supplemented with 10 mM l-alanine at pH 7.0, and germination was measured.
Measurement of germination by bioluminescence.
The production of germination-dependent bioluminescence from lux-containing spores has been shown to be a sensitive real-time monitor of the germination and outgrowth process (15). Germination-induced bioluminescence from spores of B. subtilis(pSB357) was determined using a Luminoskan luminometer (Labsystems, Basingstoke, Hampshire, United Kingdom). All measurements were carried out in a Microlite 1 luminescent assay microtiter plate (Dynatech Laboratories Inc., Chantilly, Va.). To measure germination, 158 μl of NB (Oxoid) supplemented with 10 mM l-alanine (Sigma) and 2 μl of 1% (vol/vol) dodecanol (Sigma) (required for the luciferase-catalyzed reaction to produce bioluminescence [15]), with or without lactic acid (0.5% [wt/vol]), was added to each well of a microtiter plate. Following this, 40 μl of treated spores (as described above), suspended in 50 mM potassium phosphate buffer, was added to start the reaction. Total bioluminescence was measured at 30°C over various time intervals which depended on the preservative conditions applied. For each sample point bioluminescence was measured over a 1.0-s period, and output was expressed as relative light units (RLU). For each test condition five replicate wells were assayed. In some cases germination was also measured by the decrease in optical density (600 nm) using a Philips PU 8630 spectrophotometer as described previously (27).
Recovery of germination in spores damaged by preservation treatments using CaDPA.
We studied whether germination could be recovered in spores damaged by exposure to the preservation treatments performed previously by the use of calcium dipicolinic acid (CaDPA; a nonnutrient germinant). Medium containing CaDPA has been extensively used for this purpose (6, 12).
NB containing CaDPA was made by dissolving DPA in 200 ml of sodium hydroxide (80 mM) to generate 40 mM NaDPA. Subsequently, NB was made using the 200-ml NaDPA solution, and then calcium chloride (44 mM) was added to the NB-NaDPA to form NB containing 40 mM CaDPA. CaCl2 was added immediately prior to the assay for bioluminescence because CaDPA formed a precipitate within 60 min which had no effect on the germination of spores (data not shown).
Spores of B. subtilis(pSB357) were pretreated in 50 mM phosphate buffer, pH 4.0 to 7.0, plus 0.5% lactic acid for 25 min at 30°C, followed by 5 min at 90°C. Then spores were immediately washed in NB supplemented with 10 mM l-alanine and 40 mM CaDPA, and germination-induced bioluminescence was assayed at pH 7.0.
RESULTS
Bioluminescence is a valid method to measure the effect of inhibitory treatments on alanine-induced spore germination.
A potential problem with using bioluminescence to measure the effect of inhibitory treatments on germination is that any effects observed may be due to indirect inhibition of the core-packaged luciferase enzyme itself and thus not the spore germination mechanism per se. To address this, we compared the inhibitory effect of reduced pH on germination measured by the classical method of drop in optical density with bioluminescence output.
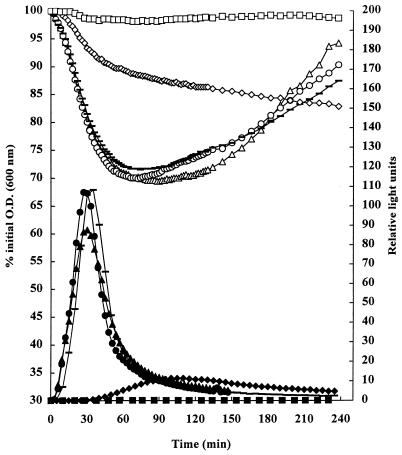
At all pH values tested there was good correlation between the two different methods of measuring germination (Fig. 1). In particular, at pH 4.5, where germination was significantly reduced, and pH 4.0, where germination was completely inhibited, the correlation between the two methods was very close. Thus, measuring germination by bioluminescence is a valid method to monitor the effects of inhibitory treatments on spore germination. Similar results were obtained with other inhibitory treatments, including the combination of pH reduction with lactic acid or a pasteurization step (results not shown).
FIG. 1.
Effect of decreasing pH on the germination of spores of B. subtilis(pSB357) at 30°C in NB plus 10 mM l-alanine, measured by loss of optical density (O.D.) (open symbols) and bioluminescence (expressed as RLU) (filled symbols). Germination measured by both methods was compared at pH 7.0 (▵ and ▴), 6.0 (○ and ●), 5.5 (-), 4.5 (◊ and ⧫), and 4.0 (□ and ■). Representative results of at least two experiments are shown.
Inhibition of alanine-induced spore germination during exposure to combinations of preservation treatments.
Alanine-induced germination of B. subtilis spores was measured under actual preservative conditions to measure the direct inhibitory effect of the preservative treatments on germination.
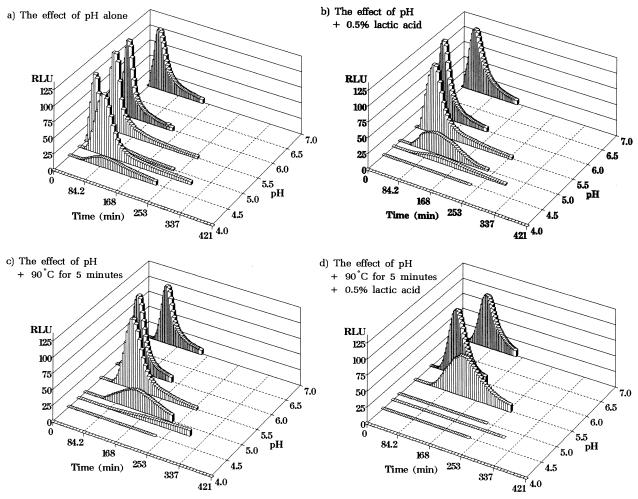
Reducing the medium pH from 7.0 to 5.0 had little inhibitory effect on germination (Fig. 2a). However, at pH 4.8 to 4.5, the rate and overall level of germination were considerably reduced, until at pH 4.0, germination-induced bioluminescence was not detectable over the duration of the experiment (Fig. 2a).
FIG. 2.
Effect of direct exposure to combinations of preservatives on germination of B. subtilis(pSB357) at 30°C. Germination was measured by bioluminescence and expressed as RLU. Germination was measured in NB plus 10 mM l-alanine at pH 7.0, 6.0, 5.5, 5.0, 4.8, 4.5, and 4.0 (a); pH 7.0 to 4.0 in the presence of 0.5% (wt/vol) lactic acid (b); pH 7.0 to 4.0 following heating at 90°C for 5 min at each pH value (c); and pH 7.0 to 4.0 in the presence of 0.5% (wt/vol) lactic acid following heating at 90°C for 5 min at each pH value with lactic acid (d). Each curve represents the mean of five separate experiments.
The presence of lactic acid (0.5% [wt/vol]), particularly at lower pH values closer to the pKa of the acid (3.73), resulted in greater inhibitory effects on germination than did pH reduction alone (Fig. 2b). For example, in the presence of lactic acid, germination-induced bioluminescence was severely inhibited at pH 5.0 and 4.8 and was undetectable at pH 4.5 (Fig. 2b).
Similar to the effect of lactic acid, the inclusion of a pasteurization step (90°C for 5 min) resulted in greater inhibition of germination than simply reducing the pH of the medium (Fig. 2c). Thus, after heating, germination-induced bioluminescence was severely inhibited at pH 5.0 and 4.8 and undetectable over the duration of the experiment at pH 4.5 (Fig. 2c). A unique feature of the addition of the heating step was the appearance of longer lag times before germination started. In particular, this was obvious at higher pH values, such as 6.0 and 5.5, where no inhibitory effect on germination was previously measured. In conclusion, either the presence of lactic acid or exposure to a pasteurization step raised the effective inhibitory pH of the preservation system by 0.5 of a pH unit. More significantly, with the combination of all three preservation treatments, the inhibitory effect on germination was even greater (Fig. 2d). In this case, bioluminescence was severely inhibited at pH 5.5 and undetectable for the duration of the experiment at pH 5.0 (Fig. 2c). Thus, the combination of mildly acidic pH, lactic acid, and a pasteurization step had an enhanced inhibitory effect on spore germination compared with the effect of the individual treatments alone.
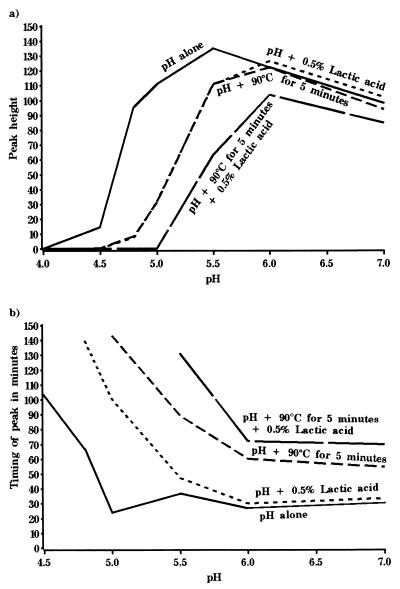
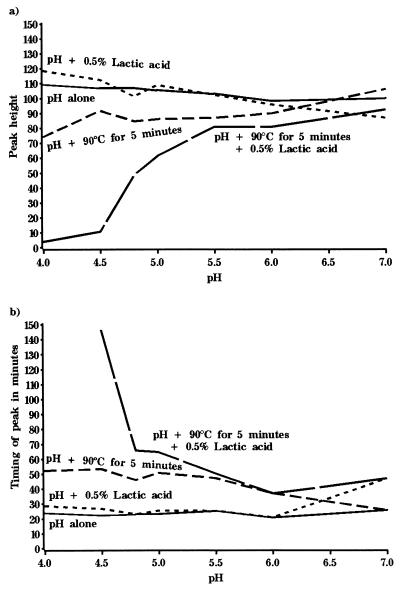
The effects of all four preservation treatments on spore germination can be summarized in different ways. For example, in a previous study (15) it was shown that the level of bioluminescence correlated exactly with the proportion of spores in the population that were recoverable on agar plates. Thus, Fig. 3a shows how the relationship between exposure pH and maximum bioluminescence, or peak height, changes during exposure to the four preservation regimens. Clearly, as the severity of the combination treatments is increased, the peak height, or number of spores germinating in the population, declines.
FIG. 3.
Effect of direct exposure to combinations of preservatives on germination of B. subtilis(pSB357) at 30°C in NB plus 10 mM l-alanine. The graph shows the relationship between exposure pH and germination during exposure to four combination preservation treatments, expressed as the maximum value of bioluminescence or peak height (expressed as RLU), and the time to reach maximum value of bioluminescence.
Alternatively, Fig. 3b shows the relationship between exposure pH and the time taken in minutes to reach maximum peak height during exposure to the four preservation treatments. We can hypothesize that this gives a measure of the time taken by the germinating population to reach the maximal germination rate. Thus, as increasingly inhibitory combinations are applied in conjunction with reduced pH, the time that spores take to reach the maximal germination rate increases.
Despite prior exposure to inhibitory combinations of preservation treatments, germination is largely reestablished under nonstress conditions.
After determining that combinations of preservation treatments inhibited germination, the following series of experiments was carried out to identify their inhibitory action. Spores were exposed to exactly the same combination of treatments as before, followed by their removal and the measurement of germination under nonstress conditions. In this way we hoped to measure the degree of nonrecoverable damage or injury to the germination system induced by prior exposure to the four preservative treatments.
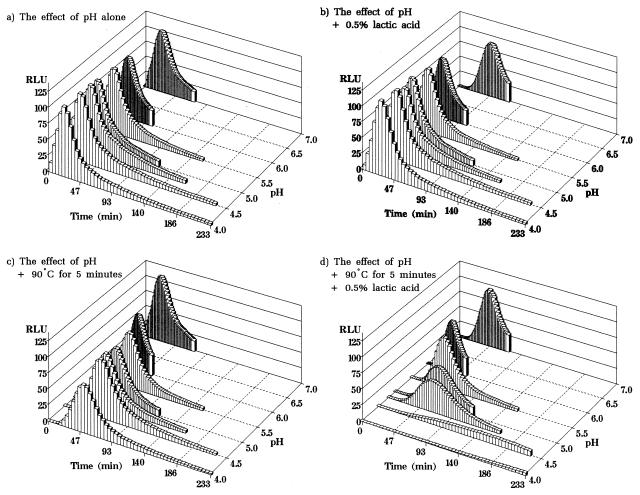
Thus, a 30-min prior exposure to decreasing pH (from 7.0 to 4.0) had no effect on the subsequent rate or overall level of germination of spores in NB plus 10 mM l-alanine, pH 7.0 (Fig. 4a). Similarly, exposure to decreasing pH in the presence of 0.5% lactic acid also had no effect on the subsequent germination of spores under nonstress conditions (Fig. 4b). Therefore, we can conclude that the complete inhibition of germination that occurred upon direct exposure to pH 4.0 alone (Fig. 2a) and pH 4.5 with lactic acid (Fig. 2b) was due to their physical presence and not due to spore death or permanent damage to the germination apparatus of the spore.
FIG. 4.
Effect of prior exposure to combinations of preservatives on the subsequent germination of B. subtilis(pSB357) under optimal conditions (NB plus 10 mM l-alanine, pH 7.0 at 30°C). Germination was measured by bioluminescence (and expressed as RLU) following 30 min of exposure to pH 7.0, 6.0, 5.5, 5.0, 4.8, 4.5, and 4.0 (a); 30 min of exposure to pH 7.0 to 4.0 in the presence of 0.5% (wt/vol) lactic acid (b); heating at 90°C for 5 min at pH 7.0 to 4.0 followed by an additional 25 min of incubation at each pH value (c); and heating at 90°C for 5 min at pH 7.0 to 4.0 in the presence of 0.5% (wt/vol) lactic acid followed by an additional 25 min of incubation at each pH value with lactic acid (d). Each curve represents the mean of five separate experiments.
Prior exposure to a combination of decreasing pH with a pasteurization step (90°C for 5 min) resulted in some inhibition of the rate and overall level of germination (Fig. 4c). However, prior exposure to the combination of all three preservation treatments resulted in significant inhibition of germination measured under nonstress conditions (Fig. 4d). This demonstration of apparently permanent damage to the spore germination mechanism was most obvious at pH 4.5 and 4.0 but was clearly dependent on the combination of all three factors. Therefore, the complete inhibition of germination that we observed as a consequence of direct exposure of spores to the full combination preservation treatment at pH 5.0 (Fig. 2d) could have resulted from a combination of spore death, direct physical inhibition of germination, or permanent damage to some unknown part of the alanine-inducible germination pathway.
The data can be summarized in the form of plots of maximum bioluminescence and time to reach the maximum peak height. Thus, Fig. 5a shows how the relationship between exposure pH and maximum bioluminescence, or peak height, changes during exposure to the four preservation treatments. Analyzed in this way, it is clear that only the combination of all three treatments results in any significant inhibitory effect on the numbers of spores germinating under nonstress conditions. Alternatively, Fig. 5b shows the relationship between exposure pH and the time taken in minutes to reach maximum bioluminescence after prior exposure to the four preservation treatments. As before, only the combination of all three treatments resulted in any significant increase in the time taken to reach the maximum rate of germination under nonstress conditions.
FIG. 5.
Effect of prior exposure to combinations of preservatives on the subsequent germination of B. subtilis(pSB357) under optimal conditions (NB plus 10 mM l-alanine, pH 7.0 at 30°C). The graph shows the relationship between exposure pH and germination after prior exposure to four combination preservation treatments expressed as the maximum value of bioluminescence or peak height (expressed as RLU), and the time to reach maximum value of bioluminescence.
In summary, the above-described experiments demonstrated that while some preservation treatments inhibited spore germination directly, once removed it was found that they did not result in any apparent permanent damage to the germination pathway. However, it was clear that as the severity of the prior treatment was increased, the proportion of the inhibitory effect due to permanent injury to the alanine pathway, or perhaps to the death of the spore, also increased.
Recovery of germination from preservative-damaged spores using CaDPA.
A manifestation of spore injury is that any supposed survivors of an inimical treatment require nonnutrient germination stimulants in order to be classed as viable by using classical microbiological techniques (14). Spores that cannot germinate are not necessarily dead, because the germination pathway could be irreversibly damaged. However, we can circumvent these pathways and induce germination by alternative means. Thus, in the following experiment we studied whether germination could be recovered under nonstress conditions in spores previously exposed to the combination of all three preservation treatments by the use of CaDPA (6, 12).
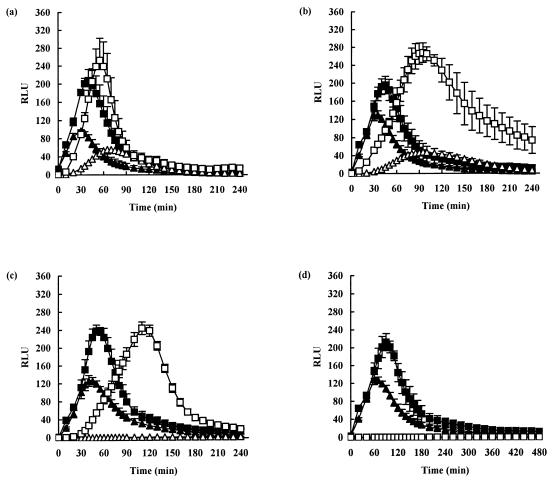
Comparison of germination under nonstress conditions of untreated spores and spores preexposed to the combination of 0.5% lactic acid and 90°C for 5 min at pH 7.0 is shown in Fig. 6a. Prior exposure to the preservation treatment resulted in only a minor inhibitory effect on germination at pH 7.0 compared with germination in the untreated spores. For example, the maximum peak height was reduced and the time taken to reach the maximum peak height was extended. Inclusion of CaDPA in the germination medium alone resulted in an approximately 100% increase in the number of spores germinating in the untreated sample (see Discussion).
FIG. 6.
Effect of CaDPA on the germination of untreated spores of B. subtilis(pSB357) and spores preexposed to a combination of preservatives. Germination was measured under optimal conditions (NB plus 10 mM l-alanine, pH 7.0, 30°C) with or without 40 mM CaDPA. (a) Spores were preexposed to 30 min at pH 7.0 (▴), 90°C for 5 min at pH 7.0 plus 0.5% (wt/vol) lactic acid (▵), 30 min at pH 7.0 (followed by supplementation with 40 mM CaDPA) (■), and 90°C for 5 min at pH 7.0 plus 0.5% (wt/vol) lactic acid (followed by supplementation with 40 mM CaDPA) (□). Experiments were repeated exactly as described above, except spores were preexposed to pH 4.8 (b) and pH 4.5 (c). (D) Spores were also exposed to the higher-temperature treatment of 100°C for 60 min at pH 6.0 with 0.5% (wt/vol) lactic acid instead of 90°C for 5 min before resuspension in NB plus 10 mM l-alanine, pH 7.0, with or without 40 mM CaDPA. The error bars indicate the standard deviations of the means of five independent experiments.
Significantly, treatment with the identical preservative combination as described above, followed by germination in the presence of CaDPA, resulted in very little evident inhibition. Similar results were obtained following pretreatment with the preservation combination at pH 4.8 (Fig. 6b). Even at pH 4.5, where germination was significantly inhibited, CaDPA was able to restore germination of the injured spores (Fig. 6c). This is consistent with the hypothesis that CaDPA must restore germination to these spores by a different route to the alanine pathway and that the inhibition of germination we observed on l-alanine was due not to spore death but to damage to the nutrient-germination pathway. To confirm this, we exposed spores to a more lethal preservation treatment of 100°C for 60 min at pH 6.0 with 0.5% lactic acid (Fig. 6d). Unsurprisingly, after this treatment no germination was detected even in the presence of CaDPA, presumably because in this case both germination pathways are inactivated or the spores are, in fact, dead.
DISCUSSION
Many studies have shown that spore outgrowth can be controlled by exploiting the inhibitory effect of combining low pH with subinhibitory levels of preservatives, organic acids, or salt and pasteurization steps (2, 23). Notably, none of these studies attempted to clarify what the inhibitory action of these combinations is on the spore and to what extent each component of the combination contributes to the inhibitory mechanism.
In this work, we have used spores of B. subtilis that specifically induce bioluminescence upon initiation of germination as a rapid, real-time monitor of germination (15). This system has a number of advantages over traditional methods of monitoring germination, including rapidity, fully automated data capture, and the need for only small quantities of spores because experiments can be carried out in the wells of microtiter plates. In particular, the latter allows the study of potentially hundreds of different conditions with many replicate experiments and is thus highly suitable for studying the effects of many different preservation conditions on germination.
Thus, using this tool we have demonstrated that the combination of mild acidity, lactic acid, and a pasteurization step results in enhanced inhibition of l-alanine-induced B. subtilis spore germination compared with the effect of the individual treatments. For example, using pH alone it is possible to inhibit germination at pH 4.0, but in the presence of heated spores or lactic acid, inhibition occurs at the higher pH of 4.5. Similarly, the combination of all three treatments results in inhibition at pH 5.0.
Additional experiments revealed that exposure to these treatments did not adversely affect subsequent germination under nonstress conditions. Thus, the inhibitory effect we observed was entirely dependent on spores being in the physical presence of these conditions, and exposure to low pH alone, or in the presence of lactic acid or a heating step, and had little permanent injurious effect on the l-alanine germination pathway. Only the combination of all three treatments resulted in some nonrecoverable damage to the l-alanine pathway. Thus, from these experiments, not only can we quantify the degree of inhibition that occurs as a result of exposure to each component of the preservation system, but also we can now determine which components are required for inhibition and which result in permanent injury.
It is well known that high temperatures cause protein unfolding because most proteins have melting temperatures below 100°C (11). Also, low pH alters the ionization state of amino acid side chains, thereby changing charge distributions and hydrogen bonding, which also alters protein conformation. Indeed, many proteins unfold at pH values of less than 5.0 (11). Thus, heating at a mildly acidic pH in the presence of organic acid could result in permanent damage or denaturation of a proteinaceous receptor-based germination system.
In this study, the measured levels of germination were low (approximately 30% optical density loss) because we did not include a heat activation step prior to initiating germination. Heat activation of the spore population was avoided to make the experiments more representative of events occurring during food preservation. However, it was clearly observed upon exposure of spores to CaDPA in the presence of l-alanine that the number of spores germinating in the population doubled. This can be explained by the fact that exposure to CaDPA results in optimal levels of recovery of spores to the same extent as if they had been heat activated (7), hence the large increase in germinated spores observed in this work. Germination induced by CaDPA is insensitive to l-alanine analogs (18). Thus, at least the initial stages of the germination pathway induced by CaDPA and l-alanine must involve different components. Therefore, the use of exogenous CaDPA is an effective route to restore germination to spores with an apparently damaged l-alanine germination pathway. In fact, we were able to almost fully restore germination to spores damaged by the combination preservative treatment (which would not germinate in l-alanine) by addition of CaDPA. This is an observation similar to that originally made by Edwards et al. (12) when studying ultrahigh-temperature-treated spores. The mechanism of germination induction by CaDPA is not known, but the compound clearly allowed damaged spores to germinate by some mechanism that did not require the use of the l-alanine germination pathway, which was inactivated.
Safe thermal processing of foods is based on the measured death kinetics of bacterial spores recovered on agar. Clearly, to recover and grow on agar a spore must have an intact germination apparatus. However, we have shown that by applying a different germination system, namely, CaDPA, we can restore germination to spores that would otherwise have been construed as nonviable. This prompts the question, can we actually clearly define when a spore is truly dead? In fact, the likelihood is that we cannot do this, because if we look only at the inability to germinate due to damage to the germination pathways, then one could argue that the spore could still be viable because we have not yet found the conditions where germination could be restored.
It could be argued that the finding of a combination preservation treatment that gives the potential for ambient stability without sterilization in vitro, in a broth-based system, with only one species of Bacillus has little direct relevance to what would actually occur in foods. However, preliminary studies with model foods and using other species of Bacillus have shown that the principal finding and mechanism of action described here are highly applicable to the design of high-quality ambient-stable foods (5).
Exploiting the germination-induced bioluminescence reporter system to study combination preservation systems will allow us to optimize existing treatments and study the effects of new treatments and combinations in order to develop milder preservation systems. The application of Lux-based methodology to other species of Bacillus is currently being evaluated. One advantage of this tool is that the data generated could be used to build comprehensive predictive models that precisely describe the boundaries of outgrowth such that the application of new preservation systems that confer ambient stability, without requiring sterilization, have the necessary data supporting their safe application.
In summary, we have identified a combination preservation treatment that inhibits bacterial spore germination. We have examined the inhibitory effects of the individual components of the treatment to understand and quantify the overall contribution of each component. In addition, we have described what the target and inhibitory action of the combination system are on the bacterial spore. The demonstration in this work that simple combinations of preservative factors inhibit spore germination indicates that preservation systems providing ambient stability could be designed which do not adhere to the strict limits set by commonly accepted processes and which are based on precise understanding of their inhibitory action.
ACKNOWLEDGMENTS
We acknowledge the contribution of the late Gordon Stewart, without whose ideas, enthusiasm, and support none of this work would have been possible. We also thank Kathy Debayle for carrying out the preliminary studies and Anne Moir and Simon Foster, University of Sheffield, for advice and helpful discussion.
REFERENCES
- 1.Atrih A, Zöllner P, Allmaier G, Foster S J. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks J G, Morgan S, Stringer M F. Inhibition of heated Bacillus spores by combinations of potassium sorbate, sodium benzoate, pH and organic acids. Lebensm-Wiss Technol. 1988;21:250–255. [Google Scholar]
- 3.Bean P G. Developments in heat treatment processes for shelf-stable products. In: Roberts T A, Skinner F A, editors. Food microbiology: advances and prospects. London, England: Academic Press; 1983. pp. 97–112. [Google Scholar]
- 4.Braithwaite P J, Perigo J A. The influence of pH, water activity and recovery temperature on the resistance and outgrowth of Bacillus spores. In: Barker A M, Gould G W, Wolf J, editors. Spore research 1971. London, England: Academic Press; 1971. pp. 289–302. [Google Scholar]
- 5.Brown M B, Cole M B, Goddard M R, McClure P J. Ambient stable food product. Australian patent 94300988, application 17065/95. 1999. [Google Scholar]
- 6.Busta F F, Adams D M. Identification of a germination system involved in the heat injury of Bacillus subtilis spores. Appl Microbiol. 1972;24:412–417. doi: 10.1128/am.24.3.412-417.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busta F F, Ordal Z J. Use of calcium dipicolinate for enumeration of total viable endospore populations without heat activation. Appl Microbiol. 1964;12:106–110. doi: 10.1128/am.12.2.106-110.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmi O A, Stewart G S A B, Ulitzur S, Kuhn J. Use of bacterial luciferase to establish a promoter probe vehicle capable of nondestructive real-time analysis of gene expression in Bacillus spp. J Bacteriol. 1987;169:2165–2170. doi: 10.1128/jb.169.5.2165-2170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors M J, Mason J M, Setlow P. Cloning and nucleotide sequencing of genes for three small, acid-soluble proteins from Bacillus subtilis spores. J Bacteriol. 1986;166:417–425. doi: 10.1128/jb.166.2.417-425.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corfe B M, Sammons R L, Smith D A, Mauel C. The gerB operon of the Bacillus subtilis 168 chromosome encodes a homologue of the gerA spore germination operon. Microbiology. 1994;140:471–478. doi: 10.1099/00221287-140-3-471. [DOI] [PubMed] [Google Scholar]
- 11.Creighton T E. Proteins: structures and molecular properties. 2nd ed. New York, N.Y: W. H. Freeman and Company; 1993. pp. 261–323. [Google Scholar]
- 12.Edwards J L, Jr, Busta F F, Speck M L. Heat injury of Bacillus subtilis spores at ultrahigh temperatures. Appl Microbiol. 1965;13:858–864. doi: 10.1128/am.13.6.858-864.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster S J, Johnstone K. The trigger mechanism of bacterial spore germination. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C.: American Society for Microbiology; 1989. pp. 89–108. [Google Scholar]
- 14.Gould G W. Injury and repair mechanisms in bacterial spores. In: Andrew M H E, Russell A D, editors. The revival of injured microbes. London, England: Academic Press; 1984. pp. 199–220. [PubMed] [Google Scholar]
- 15.Hill P J, Hall L, Vinicombe D A, Soper C J, Setlow P, Waites W M, Denyer S, Stewart G S A B. Bioluminescence and spores as biological indicators of inimical processes. Soc Appl Bacteriol Symp Ser. 1994;76:129S–134S. doi: 10.1111/j.1365-2672.1994.tb04364.x. [DOI] [PubMed] [Google Scholar]
- 16.Irie R, Okamoto T, Fujita Y. A germination mutant of Bacillus subtilis deficient in response to glucose. J Gen Appl Microbiol. 1982;28:345–354. [Google Scholar]
- 17.Jacobs M, Hill P J, Stewart G S A B. Highly bioluminescent Bacillus subtilis obtained through high-level expression of a luxAB fusion gene. Mol Gen Genet. 1991;230:251–256. doi: 10.1007/BF00290675. [DOI] [PubMed] [Google Scholar]
- 18.Keynan A, Halvorson H O. Calcium dipicolinic acid-induced germination of Bacillus cereus spores. J Bacteriol. 1962;83:100–105. doi: 10.1128/jb.83.1.100-105.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leistner L, Rodel W. Inhibition of micro-organisms in food by water activity. In: Skinner F A, Hugo W B, editors. Inhibition and inactivation of vegetative microorganisms. London, England: Academic Press; 1976. pp. 219–237. [Google Scholar]
- 20.Marquis T, Wurgler-Murphy S M, Saito H. Molecular mechanisms of resistance to heat and oxidative damage. J Appl Bacteriol. 1994;76:40S–48S. doi: 10.1111/j.1365-2672.1994.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 21.Moir A, Lafferty E, Smith D A. Genetic analysis of spore germination mutants of Bacillus subtilis: correlation of map location with phenotype. J Gen Microbiol. 1979;111:165–180. doi: 10.1099/00221287-111-1-165. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama R, Fukuoka H, Miyata S, Kudoh S, Hattori A, Kozuka S, Yasuda Y, Tochikubo K, Makino S. Expression of a germination-specific amidase, SleB, of bacilli in the forespore compartment of sporulating cells and its localization on the exterior side of the cortex in dormant spores. J Bacteriol. 1999;181:2373–2378. doi: 10.1128/jb.181.8.2373-2378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oloyede O B, Scholefield J. Inhibition of Bacillus spores by combinations of heat, potassium sorbate, NaCl and pH. World J Microbiol Biotechnol. 1994;10:579–582. doi: 10.1007/BF00367672. [DOI] [PubMed] [Google Scholar]
- 24.Paidhungat M, Setlow P. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J Bacteriol. 1999;181:3341–3350. doi: 10.1128/jb.181.11.3341-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott I R, Ellar D J. Metabolism and the triggering of germination of Bacillus megaterium, concentrations of amino acids, organic acids, adenine nucleotides and nicotinamide nucleotides during germination. Biochem J. 1978;174:627–634. doi: 10.1042/bj1740627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart G S A B, Smith A J, Denyer S P. Genetic engineering for bioluminescent bacteria. Food Sci Technol Today. 1989;3:19–22. [Google Scholar]
- 27.Venkatsabramanian P, Johnstone K. Biochemical analysis of the Bacillus subtilis 1604 spore germination response. J Gen Microbiol. 1989;135:2723–2733. doi: 10.1099/00221287-135-10-2723. [DOI] [PubMed] [Google Scholar]
- 28.Wax R, Freese E. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of l-alanine. J Bacteriol. 1968;95:433–438. doi: 10.1128/jb.95.2.433-438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wax R, Freese E, Cashel M. Separation of two functional roles of l-alanine in the initiation of Bacillus subtilis spore germination. J Bacteriol. 1967;94:522–529. doi: 10.1128/jb.94.3.522-529.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolgamott G D, Durham N N. Initiation of spore germination in Bacillus cereus, a proposed allosteric mechanism. Can J Microbiol. 1971;17:1043–1048. doi: 10.1139/m71-165. [DOI] [PubMed] [Google Scholar]
- 31.York G K, Heil J R, Marsh G L, Ansar A, Merson R L, Wolcott T, Leonard S. Thermobacteriology of canned whole peeled tomatoes. J Food Sci. 1975;40:764–769. [Google Scholar]