Abstract
The dissimilatory Fe(III)-reducing bacterium Geobacter sulfurreducens reduced and precipitated Tc(VII) by two mechanisms. Washed cell suspensions coupled the oxidation of hydrogen to enzymatic reduction of Tc(VII) to Tc(IV), leading to the precipitation of TcO2 at the periphery of the cell. An indirect, Fe(II)-mediated mechanism was also identified. Acetate, although not utilized efficiently as an electron donor for direct cell-mediated reduction of technetium, supported the reduction of Fe(III), and the Fe(II) formed was able to transfer electrons abiotically to Tc(VII). Tc(VII) reduction was comparatively inefficient via this indirect mechanism when soluble Fe(III) citrate was supplied to the cultures but was enhanced in the presence of solid Fe(III) oxide. The rate of Tc(VII) reduction was optimal, however, when Fe(III) oxide reduction was stimulated by the addition of the humic analog and electron shuttle anthaquinone-2,6-disulfonate, leading to the rapid formation of the Fe(II)-bearing mineral magnetite. Under these conditions, Tc(VII) was reduced and precipitated abiotically on the nanocrystals of biogenic magnetite as TcO2 and was removed from solution to concentrations below the limit of detection by scintillation counting. Cultures of Fe(III)-reducing bacteria enriched from radionuclide-contaminated sediment using Fe(III) oxide as an electron acceptor in the presence of 25 μM Tc(VII) contained a single Geobacter sp. detected by 16S ribosomal DNA analysis and were also able to reduce and precipitate the radionuclide via biogenic magnetite. Fe(III) reduction was stimulated in aquifer material, resulting in the formation of Fe(II)-containing minerals that were able to reduce and precipitate Tc(VII). These results suggest that Fe(III)-reducing bacteria may play an important role in immobilizing technetium in sediments via direct and indirect mechanisms.
Technetium-99, a fission product of uranium, is formed in kilogram quantities during nuclear reactions and has been released into the environment during weapons testing and the disposal of low- and intermediate-level wastes. As a result of these activities, 99Tc has been found in groundwaters at sites where nuclear wastes have been reprocessed or stored (32), and it remains a significant contaminant in effluents from nuclear fuel reprocessing plants currently in operation (28). Several factors make Tc contamination a matter of intense concern, principally the long half-life of 99Tc (2.13 × 105 years), its high environmental mobility as the stable pertechnetate anion (TcO4−), and subsequent uptake of pertechnetate into the food chain as an analog of sulfate (6). However, it is impractical to remove pertechnetate from contaminated groundwater using conventional adsorption and ion-exchange processes, because the anion is a weakly absorbing species present against a high background of competing electrolytes.
The redox chemistry of Tc is crucial in governing its mobility, and several recent studies have shown that 99Tc can be removed from aqueous solution via the reduction of pertechnetate to insoluble, low-valence forms. For example, the formation of Tc(IV) species (e.g., TcO2 · nH2O) should result in immobilization of the radionuclide in sediments (4). This can be achieved by abiotic mechanisms using zerovalent iron or Fe(II)-containing minerals under anoxic conditions (reference 9 and references therein). The latter group includes Fe(II) minerals in igneous rocks, which can reduce pertechnetate and lead to sorption on mineral surfaces (4). Magnetite has been shown to be a particularly efficient reductant for Tc(VII), with rates of reduction higher than those recorded for the Fe(II)-containing minerals hornblende and chlorite (8). Reduction of Tc(VII) by magnetite has also been enhanced electrochemically via anodic polarization of the mineral (9). Soluble ferrous iron can reduce Tc(VII), but the rate of Tc reduction may be too low to be of practical use in controlling technetium mobility (7).
Microbial metabolism may also significantly affect Tc speciation by indirect (chemical) and direct (enzymatic) mechanisms. Henrot (13) showed that the addition of sulfate-reducing bacteria to mixed cultures of anaerobically grown soil bacteria increased Tc removal by more than an order of magnitude. That author postulated that Tc removal was mediated by an indirect mechanism utilizing microbially generated H2S. This interpretation (i.e., metal sulfide formation) was also used to explain Tc accumulation by mixed cultures of anaerobic bacteria isolated from a marine sediment (37), but it was also proposed that Tc precipitation by oxygen-limited cultures of Moraxella and Planococcus spp. may have been catalyzed enzymatically.
Lloyd and Macaskie subsequently demonstrated direct enzymatic reduction of Tc(VII) by the Fe(III)-reducing bacteria Geobacter metallireducens and Shewanella putrefaciens (19). It seems that the ability to reduce Tc(VII) is widespread among bacteria (17), and later studies focused on the enteric bacterium Escherichia coli, which was shown to couple the oxidation of formate or hydrogen to the reduction of Tc(VII) (16). Tc(VII) reduction was catalyzed by the hydrogenase component of the formate hydrogenlyase complex. Similar hydrogen- and formate-dependent activities have been reported for the sulfate-reducing bacterium Desulfovibrio desulfuricans (20), and this organism has been immobilized in a flowthrough bioreactor and used to reduce and precipitate Tc from a contaminated solution containing a high background of nitrate (21, 22). Complete removal of the radionuclide was possible at a flow rate residence time of 2.1 h, compared to 62 or 19% removal at the same flow rate in a reactor containing the wild-type E. coli strain or an E. coli strain engineered to overexpress the formate hydrogenlyase complex, respectively (22).
Although the reduction and precipitation of Tc(VII) has been well studied in E. coli and the sulfate-reducing bacteria, comparatively little is known about the mechanisms of Tc(VII) reduction by the dissimilatory metal-reducing bacteria likely to predominate in sediments contaminated with metals and radionuclides. As recent studies have demonstrated that bacteria of the family Geobacteracea predominate in a range of sediments when dissimilatory metal [Fe(III)] reduction is stimulated (40), the primary aim of this study was to characterize the mechanisms by which a representative of this phylogenetic group (Geobacter sulfurreducens) can reduce Tc(VII). Two potentially important mechanisms were studied: (i) direct enzymatic reduction of Tc(VII) and (ii) indirect reduction via microbially generated Fe(II).
MATERIALS AND METHODS
Maintenance and growth of organism.
G. sulfurreducens (ATCC 51573) was obtained from our laboratory culture collection and was grown under strictly anaerobic conditions in modified freshwater medium as described previously (5). Sodium acetate (20 mM) and fumarate (40 mM) were supplied as the electron donor and electron acceptor, respectively. All manipulations were made under an atmosphere of N2-CO2 (80:20).
Metal reduction experiments.
Late-log-phase cultures were harvested by centrifugation (4,225 × g) and washed twice in bicarbonate buffer (NaHCO3; 30 mM, pH 7.1) under N2-CO2 (80:20) before use. Aliquots of the washed cell suspension (0.1 to 0.2 ml) were added, using a syringe fitted with a needle, to anaerobic tubes sealed with butyl rubber stoppers that contained 2 ml of bicarbonate buffer under N2-CO2 (80:20). The cell suspensions were incubated at 30°C. The following additions were made from anaerobic stock solutions as required: ammonium pertechnetate (250 μM) (Amersham Life Sciences, Arlington Heights, Ill.), poorly crystalline Fe(III) oxide (100 mM) (26), anthraquinone-2,6-disulfonate (AQDS) (50 μM), Fe(III) citrate (25 mM), sodium acetate (20 mM), and sodium formate (20 mM). Hydrogen mixed with CO2 (80:20 H2-CO2) was supplied as an electron donor in the headspace where noted.
Enrichment cultures.
Samples (0.5g) of sediment from the Shiprock aquifer, New Mexico, were added to 10 ml of freshwater medium (27) containing 10 mM acetate and 100 mol of poorly crystalline Fe(III) oxide per liter as the electron donor and acceptor, respectively. Tc(VII) was added to final concentrations of 2.5 μM, 25 μM, 250 μM, and 2.5 mM. Enrichment cultures were incubated at 20°C in anaerobic pressure tubes sealed with butyl rubber stoppers, under a headspace of N2-CO2, and transferred every 4 weeks.
Measurement of iron and technetium.
Total Tc in solution was assayed by autoradiography using a phosphorimager as described previously (19). Tc(VII) was also separated from reduced, nonmobile Tc using paper chromatography (19, 38), prior to autoradiography and quantification using a Storm 840 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) (19). In some experiments, very low concentrations of Tc were quantified using an LS 6500 Liquid Scintillation Analyzer (Beckman Instruments Inc., Fullerton, Calif.). Each sample (100 μl) was added to a glass scintillation vial with 10 ml of Ecolume scintillation fluid (ICN, Costa Mesa, Calif.). Disintegration counts per minute were recorded at between 20 and 250 keV for 20 min. HCl-soluble Fe(II) was measured after reaction with Ferrozine as described previously (26). Protein concentrations were measured with a bicinchoninic acid assay kit (Sigma) by the method of Smith et al. (39).
XAS.
X-ray absorption spectroscopy (XAS) data were collected at the ESRF beamline ID26 (11). The spectra were collected in the fluorescence detection mode using photodiodes as fluorescence detectors and as intensity monitors. No X-ray filter was used. A cryogenically cooled double-crystal fixed-exit Si-220 monochromator was used to generate a monochromatic beam. Harmonic rejection was achieved by using two Pt-coated mirrors.
Despite not being optimal for fluorescent detection, samples were kept in 1-mm-thick polypropylene containers due to safety constraints. Data were collected in Quick-EXAFS mode (41) in order to detect any possible sample modifications due to the X-ray beam.
16S rDNA analysis of enrichment cultures.
Bacteria in enrichment cultures were identified using 16S ribosomal DNA (rDNA) sequence analysis. Genomic DNA was extracted from 7-ml samples of enrichment cultures that had been mixed with 300 mM filter-sterilized oxalate, using a FastDNA SPIN Kit for Soil (Bio 101, Inc., Carlsbad, Calif.). A 5-μl portion of each extract was used as the template for amplification by PCR with the primers EUB 338F (the complement of EUB338 [2]) and 907R (14). PCR conditions were as described previously (33) with the addition of MgCl2 (1.5 mM) and dimethyl sulfoxide (5%, vol/vol). PCR mixtures (100 μl) were UV treated for 10 min, and 2.5 U of AmpliTaq (Perkin-Elmer Cetus, Norwalk, Conn.) was added to each reaction mixture, followed by the addition of template. A GeneAmp PCR System 2400 thermal cycler (Perkin-Elmer Cetus) was used for PCR amplification, with the following program: denaturation at 94°C for 60 s; followed by 30 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 7 min. PCR products were analyzed using agarose gel electrophoresis with ethidium bromide staining. Sufficient DNA for analysis using denaturing gradient gel electrophoresis was amplified using 1 μl of product as the template in a second round of PCR. The same protocol was used with the following exceptions: a 40-bp GC clamp (34) was added to the forward primer, no dimethyl sulfoxide was used in the reaction, and only 20 PCR cycles were used during amplification.
The resulting 16S rDNA amplicons were resolved using denaturing gradient gel electrophoresis with a 50 to 80% denaturing gradient in a 7% acrylamide gel (34). The gel was run for 16 h at 60 V and stained with ethidium bromide, and the resolved PCR products were visualized using UV transillumination. Bands containing the PCR products were excised, and the DNA was eluted by crushing with sterile pestles followed by suspension in 100 μl of 0.1 M Tris (pH 8.0) at 4.0°C overnight. The 16S rDNA from the bands was reamplified as described above without the addition of the GC clamp on the forward primer, and the PCR products were purified with a QIAquick PCR purification kit (Qiagen, Inc., Valencia, Calif.) prior to sequencing from position 338 of the 16S rRNA gene using Dye Deoxy Terminator Cycle Sequencing (Perkin-Elmer Cetus) and an ABI 377 automated sequencer (Applied Biosystems, Foster City, Calif.) at the University of Massachusetts Sequencing Facility.
The 16S rDNA sequences were checked for potential chimeras using the Ribosomal Database Project's CHECK_CHIMERA program (31). Sequences were analyzed using BLAST (National Center for Biotechnology Information) and SIMILARITY_RANK (Ribosomal Database Project) to find the most similar 16S rRNA sequences.
Electron microscopy.
Bacterial pellets were harvested using a microcentrifuge (12,000 × g), rinsed twice in distilled water, and air dried on carbon-coated copper grids prior to viewing. Thin sections were also prepared as follows. Bacterial pellets were fixed for 60 min in 2.5% (wt/vol) aqueous glutaraldehyde, washed once in distilled water, and then fixed for a further 60 min in 1% (wt/vol) aqueous osmium tetroxide. The cells were then dehydrated in progressively more concentrated ethanol solution (70, 90, 100, 100, and 100% [vol/vol] ethanol; 15 min for each step). Two 15-min washes in propylene preceded embedding in epoxy resin under vacuum for 20 min. The resin was then left to polymerize for 24 h at 60°C. Sections (100 to 150 nm thick) were cut from the resin block using a microtome and placed onto a carbon-coated copper grid prior to analyses. Sections and air-dried whole-cell preparations were viewed using a Jeol (Peabody, Mass.) 3010 300-kV transmission electron microscope fitted with a light-energy-dispersive X-ray spectrometer (Princeton Gamma-Tech, Princeton, N.J.).
RESULTS
Reduction of Tc(VII) by whole cells of G. sulfurreducens.
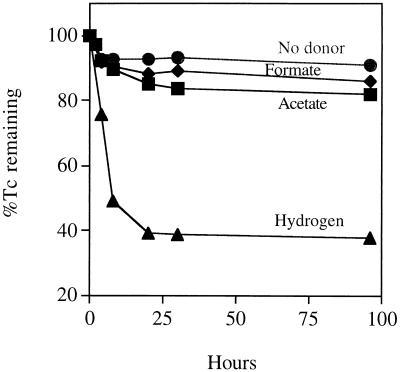
Washed cell suspensions of G. sulfurreducens coupled the oxidation of hydrogen to Tc(VII) reduction and precipitation. After 25 h approximately 60% of the total Tc (supplied at 250 μM) could be removed from solution by centrifugation (Fig. 1). These cultures contained a black precipitate that was cell associated and tentatively identified as reduced Tc (18, 20). Chromatographic separation of samples from these cultures, followed by visualization of different species of Tc using a phosphorimager, confirmed that approximately 60% of the Tc was reduced from the heptavalent oxidation state. Assuming that a protein concentration of 0.35 mg/ml equals 0.64 mg (dry weight) of biomass per ml (biomass contains 55% protein [dry weight] [35]), the specific rate of hydrogen-dependent Tc(VII) reduction was 33 nmol of Tc(VII) mg (dry weight) of biomass−1 h−1. Negligible Tc(VII) reduction was noted in the absence of added electron donor or when heat-treated cells (boiled for 5 min) were supplied with hydrogen. Although the cells used in these experiments were grown using acetate as the electron donor for fumarate reduction, Tc(VII) reduction was inefficient when acetate was supplied as an electron donor for Tc(VII) reduction. Formate was also a poor electron donor for Tc(VII) reduction by G. sulfurreducens, in contrast to the case for the closely related sulfate-reducing bacterium D. desulfuricans, which coupled formate oxidation to Tc(VII) reduction efficiently (21). This is consistent, however, with poor growth of G. sulfurreducens with formate as an electron donor for anaerobic respiration (5).
FIG. 1.
Tc(VII) reduction and removal from solution as an insoluble precipitate by washed cell suspensions of G. sulfurreducens. Acetate (20 mM), formate (20 mM), or hydrogen (80:20 H2-CO2 in the headspace) was supplied as an electron donor. Controls contained no added electron donor. Reduced insoluble Tc was removed from solution by centrifugation prior to analysis using a phosphorimager (19).
Localization and identification of Tc reduced by whole cells of G. sulfurreducens.
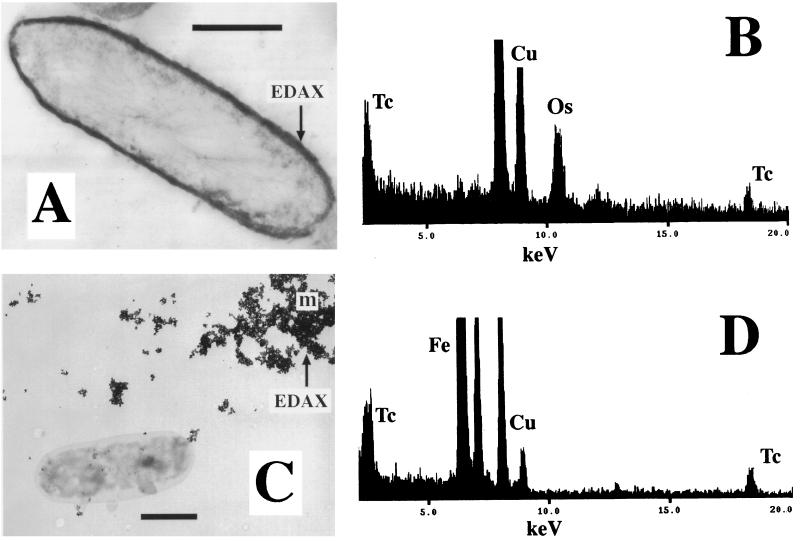
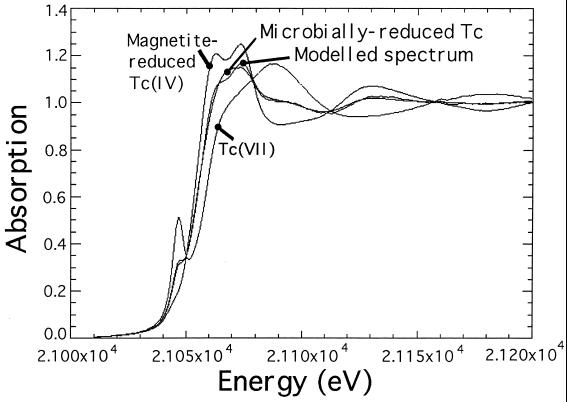
Cells of G. sulfurreducens, which had coupled hydrogen oxidation to enzymatic reduction and precipitation of Tc, were sectioned and viewed using transmission electron microscopy (TEM) (Fig. 2A). An electron-dense deposit was noted at the periphery of the cell and contained Tc as detected by energy-dispersive X-ray analysis (EDAX) (Fig. 2B), suggesting that Tc(VII) was reduced to an insoluble low-valence form at this site. Reduction of the radionuclide in these preparations was confirmed by analyzing the cultures directly using XAS (Fig. 3). The shift in the edge region of the spectrum, compared to that of the TcO4− spectrum, clearly demonstrated a change in oxidation state. The precipitate was identified as TcO2 by comparison with a reference spectrum (1); there was excellent agreement when the test spectra were modelled assuming that 60% of the Tc was reduced, leaving 40% (100 μM) Tc(VII) in solution in the resting cell cultures (as demonstrated by paper chromatography).
FIG. 2.
TEMs showing Tc-containing precipitates formed by G. sulfurreducens via direct hydrogen-dependent and indirect Fe(II)-mediated mechanisms. (A) TEM of thin sections of cells containing an electron-dense Tc precipitate formed by hydrogen-dependent reduction at the periphery of the cell. (B) EDAX spectrum from the electron-dense deposit at the cell periphery. (C) TEM of air-dried whole-cell preparations showing Tc-containing extracellular magnetite crystals (m). (D) EDAX spectrum from extracellular magnetite crystals. Bars, 0.5 μm.
FIG. 3.
XAS spectra of Tc(VII) (pertechnetate anion), Tc(IV) reduced via biogenic magnetite (in good agreement with the reference spectrum for TcO2 [1]), and Tc reduced via a direct hydrogen-dependent mechanism. A spectrum modelled by assuming 60% reduced Tc(IV) and 40% Tc(VII) is also included and is in good agreement with the spectrum obtained from the cultures containing Tc reduced by the direct, hydrogen-dependent mechanism [at a similar ratio of 60% Tc(IV) to 40% residual Tc(VII) as determined independently using a phosphorimager-based technique (19)].
Indirect reduction of Tc(VII) via microbially produced Fe(II).
Acetate is a suitable electron donor for Fe(III) reduction by G. sulfurreducens (5) but was not utilized for Tc(VII) reduction by this organism. This allowed for the development of a simple model system to test the hypothesis that microbially reduced iron can shuttle electrons to Tc(VII), facilitating Tc(VII) reduction and precipitation via an indirect mechanism. This hypothesis was tested by supplying washed cell suspensions of G. sulfurreducens with acetate and Fe(III). Tc(VII) was also added to the assay mix, and reduction of the radionuclide in these experiments was attributed to an indirect mechanism, via microbially produced Fe(II).
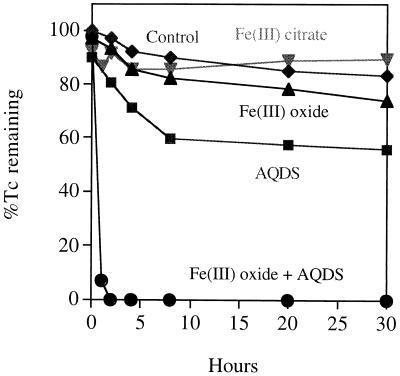
Fe(III) citrate when added at 25 mM did not, however, stimulate Tc precipitation (Fig. 4), despite the generation of up to 6 mM Fe(II) after 8 h of incubation [at an average rate of 1.2 μmol of Fe(II) produced mg (dry weight) of biomass−1 h−1]. Analysis by paper chromatography showed that although Tc precipitation was not efficient under these conditions, Fe(III) reduction was accompanied by Tc(VII) reduction [at a rate of 22.3 nmol of Tc(VII) mg (dry weight) of biomass−1 h−1], until only 62.5 μM (25%) Tc(VII) remained in solution after 30 h of incubation. It would seem that Tc, when reduced by soluble Fe(II), did not form a precipitate that could be removed by centrifugation. Insoluble Fe(III) oxide was reduced by G. sulfurreducens far more slowly than soluble Fe(III) citrate and at rates similar to those reported previously [50 nmol of Fe(II) produced mg (dry weight) of biomass−1 h−1 (15)]. Despite this low rate of Fe(III) oxide reduction, the rate of Tc precipitation was improved over that in the presence of soluble Fe(III) citrate (Fig. 4).
FIG. 4.
Tc(VII) reduction and precipitation via microbially produced Fe(II) and AQHDS. Washed cell suspensions of G. sulfurreducens were incubated with 250 μM Tc(VII), 20 mM acetate (electron donor), and either 25 mM Fe(III) citrate, 100 mM Fe(III) oxide, 50 μM mM AQDS, or 50 μM mM AQDS and 100 mM Fe(III) oxide. The control had no Fe(III) or AQDS. Reduced insoluble Tc was removed from solution by centrifugation prior to analysis using a phosphorimager (19).
As demonstrated previously (25), the reduction of Fe(III) oxides was enhanced dramatically by the addition of low concentrations (50 μM) of the soluble electron shuttle AQDS. G. sulfurreducens is able to couple the oxidation of acetate to the reduction of AQDS to AHQDS, which in turn can donate electrons to Fe(III) oxide, thus alleviating the need for direct contact between the cells and the solid-phase electron acceptor (25). As the regenerated AQDS is available for further rounds of electron shuttling, low concentrations of the electron shuttle can enhance the reduction of relatively high concentrations of Fe(III) oxide. In the presence of AQDS and Fe(III) oxide, the rate of Fe(II) reduction was increased to 4.6 μmol of Fe(II) produced mg (dry weight) of biomass−1 h−1, and after several hours a black magnetic mineral [Fe(II)-containing magnetite; Fe3O4] was noted in the cultures. Tc(VII) reduction and precipitation were both rapid and efficient under these conditions (Fig. 4), with complete removal of soluble Tc noted within 2 h. AQDS alone was also able to shuttle electrons to Tc(VII), but the rate and extent of Tc(VII) reduction and removal were far lower than those observed during AQDS-accelerated Fe(III) oxide reduction (Fig. 4). TEM and EDAX showed that the Tc was exclusively associated with fine-grain extracellular magnetite in the latter cultures (Fig. 2 C and D), and XAS studies confirmed that Tc was precipitated as TcO2 (Fig. 3). Unlike with the cultures containing enzymatically reduced Tc, which contained a mix of Tc(VII) and Tc(IV), the spectrum from the magnetite-containing culture was very similar to the spectrum reported for pure TcO2 (1), confirming that no oxidized Tc remained in the sample. It was concluded that Tc(VII) was reduced efficiently to Tc(IV) by the high local concentration of Fe(II) on the magnetite surface.
Tc(VII) reduction and precipitation by an enrichment culture of Fe(III)-reducing bacteria.
The cells of G. sulfurreducens used in all experiments described so far were pregrown in the absence of Tc(VII). However, Tc(VII) is an analog of sulfate (6) and may potentially interfere with the metabolism of sulfur by microorganisms. Therefore, to determine if Tc(VII) reduction by microbially produced Fe(II) is a valid mechanism, it was important to confirm that dissimilatory Fe(III)-reducing bacteria were able to grow and respire using Fe(III) as the electron acceptor with the imposed stress of added Tc(VII). Thus, enrichment cultures of Fe(III)-reducing bacteria were obtained from sediments taken from the Fe(III)-reducing zone of the Shiprock aquifer, New Mexico, both with and without added Tc(VII). These sediment samples were selected because previous studies had suggested that this site was contaminated with radionuclides (principally uranium from mining activities) and contained active communities of metal-reducing bacteria (K. T. Finneran, R. T. Anderson, and D. R. Lovley, unpublished data). Stable cultures of acetate-dependent Fe(III)-reducing bacteria were obtained when 25 μM Tc(VII) was added to the growth medium, although the rate of Fe(III) reduction was inhibited by approximately 50% in the presence of the radionuclide (Fig. 5 [data from the third transfer on selective medium are shown]). Addition of 250 μM or 2.5 mM Tc(VII) completely inhibited Fe(III) reduction, demonstrating toxicity of the radionuclide at high concentrations. Similar results were also obtained with pure cultures of G. sulfurreducens; growth was inhibited in fumarate-containing medium by these higher concentrations of Tc(VII), although there were no discernible effects on Fe(III) or Tc(VII) reduction by resting cells pregrown in the absence of radionuclide.
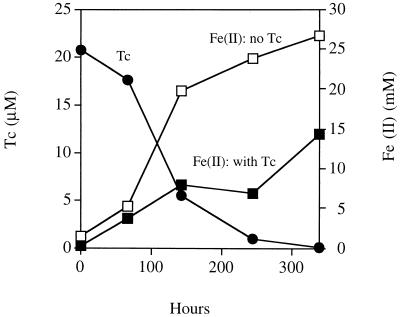
FIG. 5.
Tc(VII) reduction and precipitation by enrichment cultures of Fe(III)-reducing microorganisms. Fe(II) production was monitored in the presence and absence of 25 μM Tc(VII). Reduced insoluble Tc was removed from solution by centrifugation prior to analysis using a scintillation counter.
When 25 μM Tc(VII) was added to the enrichment cultures, Fe(III) reduction was accompanied by a gradual decline in the concentration of soluble Tc, and after 339 h of incubation, the cultures contained approximately 15 mM Fe(II) with no soluble Tc detected by scintillation counting. Again, the end product of Fe(III) oxide reduction in all enrichment cultures was magnetite. Given the high efficiency of Tc(VII) removal, it seems unlikely that the Tc(VII) was reduced enzymatically (see above), and it is more likely that the Tc was reduced by abiotic mechanisms in these experiments. Analysis of the enrichment cultures by XAS confirmed that all of the Tc was reduced and precipitated as Tc(IV) in these cultures. Molecular analysis of the enrichment cultures by PCR-based 16S rDNA sequence analysis detected a single Geobacter species, which was closely related to Geobacter akaganeitreducens (43) (96% homology; 404 of 420 bp).
Potential of microbially derived Fe(II) in aquifer sediments to reduce Tc(VII).
Recent studies have highlighted the need to corroborate results from studies demonstrating metal reduction in defined laboratory media with results from experiments using real aquifer materials (36). To assess whether microbially reduced Fe(II) in aquifer sediments can reduce Tc(VII), sediments from an uncontaminated region of the Bemidji aquifer (3, 40) were mixed with equal volumes of sterile water and challenged with Tc(VII) to a final concentration of 25 μmol liter−1 in the samples. Two sediment samples were used, one in which Fe(III) reduction had been stimulated by the addition of 10 mM acetate and an unamended control sample. Previous studies had demonstrated that the growth of Geobacter species was stimulated by the addition of acetate to these sediments (40), concomitant with the reduction of all of the Fe(III) (22 mM), under the conditions imposed (K. P. Nevin and D. R. Lovley, unpublished data). In comparison, the control samples contained only 2 mM Fe(II) and were rust colored, which is characteristic of Fe(III)-containing sediments. The acetate-amended samples were grey, which is characteristic of sediments containing Fe(II).
After incubation for 24 h at 20°C, sediment material was removed by centrifugation and the amount of Tc remaining in solution was measured using a scintillation counter. Negligible Tc removal was noted in the control samples (final concentration in the supernatant, 45.75 μM). Tc removal was far more efficient in the samples in which Fe(III) reduction had been stimulated; only 58 nM Tc remained, corresponding to removal of approximately 99.9% of the Tc(VII) added to the samples.
DISCUSSION
The mechanisms for Tc(VII) and Fe(III) reduction by G. sulfurreducens are distinct.
G. sulfurreducens reduced Tc(VII) via a mechanism that was distinct from that of Fe(III) reduction by the organism. Fe(III) reduction by G. sulfurreducens can be coupled to the oxidation of a variety of organic electron donors, including acetate (5), but in this study, Tc(VII) reduction by this organism had an exclusive requirement for hydrogen as the electron donor. Thus, it seems that Tc(VII) is reduced via a mechanism distinct from the cytochrome-mediated electron transport system reported to catalyze Fe(III) reduction in G. sulfurreducens (5, 10, 15, 30). Given the preference for hydrogen as an electron donor, it is possible that a hydrogenase may act directly as the Tc(VII) reductase in G. sulfurreducens, consistent with the role of hydrogenases in Tc(VII) reduction by E. coli (16) and metal reduction in other microorganisms (45). In keeping with a mechanism distinct from that of energy-conserving, acetate-dependent Fe(III) respiration, G. sulfurreducens was unable to couple the reduction of Tc(VII) to growth, although toxicity of the radionuclide at the higher concentrations used in this study could have prevented cell proliferation.
Direct hydrogen-dependent reduction of Tc(VII) by whole cells did not proceed to completion, with 40% of the 250 μM Tc(VII) added remaining in solution. This could also be explained by the relatively low affinity of whole-cell catalysts for Tc(VII), as noted in previous studies (e.g., the Km for Tc(VII) is 500 μM in whole cells of E. coli [22]). It should be noted that the efficiency of whole-cell-mediated Tc(VII) reduction could be enhanced for bioremediation applications by one of several means. First, once the protein catalyzing Tc(VII) reduction has been identified, it will be possible to identify its corresponding gene, opening the way to manipulation of the system at the genetic level. This work is ongoing in our laboratory. Alternatively, alteration of the local environment proximal to the biocatalyst may be achieved in an immobilized cell system, thus promoting accumulation of the radionuclide to higher concentrations, e.g., by use of a suitably charged support material. Also, enzymatic Tc(VII) reduction can be modeled by Michaelis-Menten kinetics (28), leading to the identification of the optimal flow residence time to meet legislative requirements during in situ or ex situ bioremediation.
TcO2 is the end product of microbial reduction of Tc(VII).
Although several previous studies have demonstrated that the products of microbial reduction of Tc(VII) are insoluble (16, 19, 21), the precipitates formed during this potentially important process remain poorly characterized. Indeed, several low-valence forms of Tc are possible, including Tc(VI), Tc(V), Tc(IV), and Tc(0) (29), and it is important, therefore, to identify the products accurately so that the long-term stability and environmental mobility of microbially reduced Tc following in situ bioremediation can be predicted. Knowledge of the stoichiometries of reactants for metal reduction is also required, so that bioremediation processes can be optimized, allowing precise delivery of the electron donor for metal reduction. Our results clearly identify insoluble TcO2 as the end product of Tc(VII) reduction by G. sulfurreducens. Moreover, the TcO2 formed by enzymatic reduction was precipitated at the periphery of the cell. This was in marked contrast to Tc(VII) reduced by membrane preparations of G. sulfurreducens enriched for hydrogenase activity, where Tc(VII) was reduced in the presence of hydrogen but did not form a precipitate that could be removed by centrifugation (J. R. Lloyd, unpublished data). Thus, it seems that additional factors such as the local environment of the Tc(VII) reductase in the cell may promote biomineralization of the Tc(IV).
High-efficiency Fe(II)-mediated mechanism for Tc(VII) reduction.
In addition to the hydrogen-dependent enzymatic activity, G. sulfurreducens was able to reduce Tc(VII) to Tc(IV) via an indirect, Fe(II)-mediated mechanism. Fe(II) formed via acetate-dependent reduction of soluble Fe(III) citrate was able to reduce Tc(VII) abiotically. This was in contrast to the conclusions of Cui and Ericksen (7), who noted that although the reduction of Tc(VII) by soluble Fe(II) is thermodynamically feasible, it is kinetically hindered and highly improbable. The conflicting results in our study could be explained if Fe(II) accumulates at the site of enzymatic reduction, giving a high local concentration of Fe(II), effectively forming a reactive compartment for enhanced Tc(VII) reduction. However, the rates of Tc(VII) reduction via soluble electron shuttles were lower than those by the direct, hydrogen-dependent mechanism described above, and the formation of insoluble Tc(IV) was hindered when soluble iron was used to shuttle electrons to Tc(VII). It was suspected that colloidal Tc(IV) was formed when soluble electron shuttles were used to reduce Tc(VII).
Ferric iron is present in most environments as insoluble Fe(III) oxide (23). A low rate of Fe(III) oxide reduction, consistent with previous studies, was noted and supported a low but significant rate of Fe(II)-mediated Tc(VII) reduction and precipitation. Addition of AQDS, a humic analog and soluble electron shuttle, increased the rate of Fe(III) reduction by approximately 2 orders of magnitude and led to the rapid formation of the magnetic mineral magnetite. Tc(VII) reduction was optimal under these conditions, with the end product of this indirect mechanism identified as TcO2. Indeed, no Tc was detected in solution after 2 h of incubation, with the limit of detection in this study (5 nM) being close to the maximum contaminant level set for drinking water by the U.S. Environmental Protection Agency (0.5 nM) (9). Several studies have found magnetite to be an efficient reductant for Tc(VII) (7–9), with surface-mediated reduction to Tc(IV) leading to the precipitation of TcO2 on the Fe3O4 mineral (12). Sorption of Tc(VII) to the mineral surface by ligand exchange mechanisms was previously identified as the rate-limiting step in Tc(VII) reduction by magnetite (8). The very rapid kinetics reported here suggest that the high surface area of the magnetite nanocrystals produced by Fe(III)-reducing bacteria (22, 42) may enhance sorption of Tc(VII) onto the magnetite, providing an efficient mechanism for the removal of Tc(VII) from contaminated groundwater. Finally, the magnetic properties of biogenic magnetite should not be overlooked and could provide a suitable sorbant for Tc(VII) and other high-valence metals [e.g., Cr(VI)] in a bioreactor, prior to removal using a magnetic separating device (as developed for the removal of magnetic metal sulfides from solution [44]).
Environmental relevance of Fe(II)-mediated Tc(VII) reduction.
An enrichment culture was obtained from radionuclide-contaminated sediments using selective medium containing insoluble Fe(III) oxide as the electron acceptor and 25 μM Tc(VII). A Geobacter sp. was the sole bacterial species detected in the enrichment culture by 16S rDNA analysis. In this culture, Tc(VII) reduction and precipitation were concomitant with Fe(III) reduction, and the very high efficiency of TcO2 deposition suggested that Tc(VII) reduction was driven by the indirect Fe(II)-mediated mechanism described above. These results suggest that Tc(VII) reduction via microbially generated Fe(II) is of potential environmental relevance. Additional evidence supporting this hypothesis was obtained in studies using aquifer sediments in which Fe(III) reduction had been stimulated by the addition of electron donor. Tc(VII) reduction and removal were also very efficient under these conditions. As oxidative desorption of TcO2 from Fe(II)-containing minerals into air-saturated groundwater has been shown to be very slow, probably due to competing reactions between oxygen and the surface of the Fe(II)-bearing solid (8), stimulation of Fe(III)-reducing communities in the subsurface may be a potentially useful approach to immobilize Tc contamination.
ACKNOWLEDGMENTS
This work was funded by the Department of Energy NABIR program by grants to J.R.L. (DE-FG02-99ER62867) and D.R.L. (DE-FG02-97ER62475). Use of the ID26 X-ray Absorption on Ultra Dilute Samples (XAUS) beamline was made possible through financial support from the European Synchrotron Radiation Facility. A grant from the National Science Foundation (NSF BBS 8714235) supported some of the electron microscopy facilities used in this study.
We acknowledge the technical assistance of Lucy Yin and Louis Raboin, and we thank Kelly Nevin and Kevin Finneran for technical advice and for supplying sediment samples.
REFERENCES
- 1.Allen P G, Siemering G S, Shuh D K, Bucher J J, Edelstein N M, Langton C A, Clark S B, Reich T, Denecke M A. Technetium speciation in cement waste forms determined by X-ray absorption fine structure spectroscopy. Radiochim Acta. 1997;76:77–86. [Google Scholar]
- 2.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R T, Rooney-Varga J, Gaw C V, Lovley D R. Anaerobic benzene oxidation in the Fe(III)-reduction zone of petroleum-contaminated aquifers. Environ Sci Technol. 1998;32:1222–1229. [Google Scholar]
- 4.Bondietti E A, Francis C W. Geologic migration potentials of technetium-99 and neptunium-237. Science. 1979;203:1337–1340. doi: 10.1126/science.203.4387.1337. [DOI] [PubMed] [Google Scholar]
- 5.Caccavo F, Jr, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cataldo D A, Garland T R, Wildung R E, Fellows R J. Comparative metabolic behaviour and interrelationships of Tc and S in soybean plants. Health Phys. 1989;57:281–288. doi: 10.1097/00004032-198908000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Cui D, Ericksen T E. Reduction of pertechnetate by ferrous iron in solution: influence of sorbed and precipitated Fe(II) Environ Sci Technol. 1996;30:2259–2262. [Google Scholar]
- 8.Cui D, Ericksen T E. Reduction of pertechnetate in solution by heterogeneous electron transfer from Fe(II)-containing geological material. Environ Sci Technol. 1996;30:2263–2269. [Google Scholar]
- 9.Farrell J, Bostick W D, Jarabek R J, Fiedor J N. Electrosorption and reduction by anodically polarized magnetite. Environ Sci Technol. 1999;33:1244–1249. [Google Scholar]
- 10.Gaspard S, Vazquez F, Holliger C. Localization and solubilization of the iron(III) reductase of Geobacter sulfurreducens. Appl Environ Microbiol. 1998;64:3188–3194. doi: 10.1128/aem.64.9.3188-3194.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauthier C, Solé V A, Signorato R, Goulon J, Moguiline E. The ESRF beamline ID26: X-ray absorption on ultra dilute sample. J Synchrotron Rad. 1999;6:164–166. doi: 10.1107/S0909049598016835. [DOI] [PubMed] [Google Scholar]
- 12.Haines R I, Owen R I, Vandergraff T T. Mineral interactions with technetium. Nucl J Can. 1987;1:32–37. [Google Scholar]
- 13.Henrot J. Bioaccumulation and chemical modification of Tc by soil bacteria. Health Phys. 1989;57:239–245. doi: 10.1097/00004032-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Lane D J, Pace B, Olsen G J, Stahl D A, Sogin M L, Pace N R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analysis. Proc Natl Acad Sci USA. 1985;82:6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd J R, Blunt-Harris E L, Lovley D R. The periplasmic 9.6-kilodalton c-type cytochrome of Geobacter sulfurreducens is not an electron shuttle to Fe(III) J Bacteriol. 1999;181:7647–7649. doi: 10.1128/jb.181.24.7647-7649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd J R, Cole J A, Macaskie L E. Reduction and removal of heptavalent technetium from solution by Escherichia coli. J Bacteriol. 1997;179:2014–2021. doi: 10.1128/jb.179.6.2014-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd J R, Macaskie L E. Bioremediation of radioactive metals. In: Lovley D R, editor. Environmental microbe-metal interactions. Washington, D.C.: ASM Press; 2000. pp. 277–327. [Google Scholar]
- 18.Lloyd J R, Macaskie L E. Microbially-mediated reduction and removal of technetium from solution. Res Microbiol. 1997;148:530–532. doi: 10.1016/S0923-2508(97)88358-1. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd J R, Macaskie L E. A novel phosphorimager-based technique for monitoring the microbial reduction of technetium. Appl Environ Microbiol. 1996;62:578–582. doi: 10.1128/aem.62.2.578-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd J R, Nolting H-F, Solé V A, Bosecker K, Macaskie L E. Technetium reduction and precipitation by sulphate-reducing bacteria. Geomicrobiol J. 1998;15:43–56. [Google Scholar]
- 21.Lloyd J R, Ridley J, Khizniak T, Lyalikova N N, Macaskie L E. Reduction of technetium by Desulfovibrio desulfuricans: biocatalyst characterization and use in a flowthrough bioreactor. Appl Environ Microbiol. 1999;65:2691–2696. doi: 10.1128/aem.65.6.2691-2696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd J R, Thomas G H, Finlay J A, Cole J A, Macaskie L E. Microbial reduction of technetium by Escherichia coli and Desulfovibrio desulfuricans: enhancement via the use of high activity strains and effect of process parameters. Biotechnol Bioeng. 1999;66:123–130. [PubMed] [Google Scholar]
- 23.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovley D R. Magnetite formation during microbial dissimilatory iron reduction. In: Frankel R B, Blakemore R P, editors. Iron biominerals. New York, N.Y: Plenum Press; 1990. pp. 151–166. [Google Scholar]
- 25.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 26.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovley D R, Phillips E R. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macaskie L E. The application of biotechnology to the treatment of wastes produced from nuclear fuel cycle: biodegradation and bioaccumulation as a means of treating radionuclide-containing streams. Crit Rev Biotechnol. 1991;11:41–112. doi: 10.3109/07388559109069183. [DOI] [PubMed] [Google Scholar]
- 29.Magee R J, Blutstein H. Technetium and rhenium. In: Bard A J, Parsons R, Jordan J, editors. Standard potentials in aqueous solution. New York, N.Y: Marcel Dekker, Inc.; 1985. pp. 439–451. [Google Scholar]
- 30.Magnuson T S, Hodges-Myerson A L, Lovley D R. Characterization of a membrane-bound NADH-dependent Fe(III) reductase from the dissimilatory Fe(III)-reducing bacterium Geobacter sulfurreducens. FEMS Microbiol Lett. 1999;185:205–211. doi: 10.1111/j.1574-6968.2000.tb09063.x. [DOI] [PubMed] [Google Scholar]
- 31.Maidak B L, Cole J R, Parker Jr C T, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough J, Hazen T C, Benson S M, Metting F B, Palmisano A C. Bioremediation of metals and radionuclides…what is it and how it works. Berkeley, Calif: Lawrence Berkeley National Laboratory; 1999. [Google Scholar]
- 33.Murray A E, Hollinbaugh J T, Orrego C. Phylogenetic compositions of bacterioplankton from two California estuaries compared by denaturing gradient gel electrophoresis of 16S rDNA fragments. Appl Environ Microbiol. 1996;62:2676–2680. doi: 10.1128/aem.62.7.2676-2680.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neidhardt F C, Ingraham J L, Schaechter M. Physiology of the bacterial cell. Sunderland, Mass: Sinauer Associates, Inc.; 1990. [Google Scholar]
- 36.Nevin K P, Lovley D R. Potential for nonenzymatic reduction of Fe(III) during microbial oxidation of organic matter coupled to Fe(III) reduction. Environ Sci Technol. 2000;34:2472–2478. [Google Scholar]
- 37.Pignolet L, Fonsny K, Capot F, Moureau Z. Role of various microorganisms on Tc behaviour in sediments. Health Phys. 1989;57:791–800. doi: 10.1097/00004032-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Shukla S K. Ion exchange paper chromatography of Tc(IV), Tc(V) and Tc(VII) in hydrochloric acid. J Chromatogr. 1966;21:92–97. doi: 10.1016/s0021-9673(01)91264-6. [DOI] [PubMed] [Google Scholar]
- 39.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E R, Goeke N M, Olsen B J, Kleck D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 40.Snoeyenbos-West O, Nevin K P, Anderson R T, Lovley D R. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb Ecol. 2000;39:153–167. doi: 10.1007/s002480000018. [DOI] [PubMed] [Google Scholar]
- 41.Solé V A, Gauthier C, Goulon J, Natali F. Undulator QEXAFS at the ESRF beamline ID26. J Synchrotron Rad. 1999;6:174–175. doi: 10.1107/S0909049598017531. [DOI] [PubMed] [Google Scholar]
- 42.Sparks N H C, Mann S, Bazylinski D A, Lovley D R, Jannasch H W, Frankel R B. Structure and morphology of magnetite formed by a marine magnetotactic bacterium and dissimilatory iron-reducing bacteria. Earth Planet Sci Lett. 1990;98:14–22. [Google Scholar]
- 43.von Wintzingerode F, Selent B, Hegemann W, Gobel U B. Phylogenetic analysis of an anaerobic trichlorobenzene-transforming microbial consortium. Appl Environ Microbiol. 1999;65:283–286. doi: 10.1128/aem.65.1.283-286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson J H P, Ellwood D C. Biomagnetic separation and extraction process for heavy metals from solution. Minerals Eng. 1994;7:1017–1028. [Google Scholar]
- 45.Yanke L J, Bryant R D, Laishley E J. Hydrogenase I of Clostridium pasteurianum functions as a novel selenite reductase. Anaerobe. 1995;1:61–67. doi: 10.1016/s1075-9964(95)80457-9. [DOI] [PubMed] [Google Scholar]