Abstract
Simple Summary
The aim of this study is to describe the prevalence of diabetes mellitus (DM) among patients with the diagnosis of pancreatic ductal adenocarcinoma (PDAC), analyse the association between the occurrence of DM and clinicopathological factors, and detect variables influencing overall survival. Diabetes mellitus is prevalent among patients with pancreatic cancer. In our study, patients with diabetes mellitus receiving palliative chemotherapy had significantly higher median OS than those without. Among variables influencing survival, TNM stage, nodal involvement, tumour site, levels of CEA and CRP were confirmed.
Abstract
Background: pancreatic ductal adenocarcinoma (PDAC) is the seventh leading cause of cancer-related deaths with increasing incidence and link to the onset of diabetes mellitus (DM). The aim of this study is to describe the prevalence of DM among patients with the diagnosis of PDAC, analyse the association between the occurrence of DM and clinicopathological factors, and detect variables influencing overall survival. Methods: a retrospective analysis of medical records was performed. The patients were divided into non-DM (n = 101) and DM (n = 74) groups. Statistical analysis with the usage of appropriate tests was conducted. Results: Patients in the groups of DM and NODM had significantly longer median OS than the non-DM group. Nodal involvement, tumour location, level of CEA, CRP and CRP/lymphocytes ratio were significantly associated with OS among patients with any type of DM. Neutropenia was less frequently observed in the DM group. Conclusions: DM is prevalent among patients with pancreatic cancer. In our study, patients with DM receiving palliative chemotherapy had significantly higher median OS than those without DM. The increased comprehension of the mechanisms of the relationship between DM and pancreatic cancer needs further research, which might provide avenues for the development of novel preventive and therapeutic strategies.
Keywords: pancreatic cancer, diabetes mellitus, oncology
1. Introduction
Pancreatic cancer (PC) is the seventh leading cause of cancer-related deaths worldwide. Unlike other malignancies, incidence continues to increase, with a slight improvement in survival rates [1,2]. Specifically, pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic malignancy, representing over 90% of the pancreatic lesions [3]. Complete surgical resection provides the only chance for a cure; however, only 20% of patients are diagnosed with resectable disease. Additionally, 80% of surgically resected PDACs experience recurrence within five years of the resection [4]. PC patients’ overall 5-year survival rate is <5% [5]. Poor prognosis is associated with several factors, encompassing diagnosis at an advanced stage, early distant metastases, remarkable resistance to most conventional treatment options and a dense tumour microenvironment [3]. Identifying risk factors might lead to the earlier detection of pancreatic cancer and a more favourable prognosis. One potentially significant risk factor for this malignancy is diabetes mellitus (DM) [6]. Type 2 diabetes mellitus (T2DM) is the most prevalent form of diabetes, estimated for approximately 90% of diabetic patients. Hyperglyceamia results from resistance to insulin action combined with inadequate insulin secretion [7]. Clinical and experimental analysis revealed that pancreatic cancer is frequently linked to the onset of DM [8]. Studies confirm that the highest risk for PDAC is observed within the first two years after diabetes diagnosis [9]. Moreover, surgical procedures, both Whipple and distal resection, might lead to new-onset diabetes mellitus (NODM); however, the exact risk of this complication is unknown [10]. NODM is defined as a disease caused by the loss or destruction of the pancreatic endocrine parenchyma [7].
Despite the close relationship between DM and PDAC, little is known about the exact prevalence and impact of T2DM and NODM on clinical outcomes in PDAC [11]. Available information concerning this subject is limited and inconsistent. Various studies suggested that DM did not significantly affect overall survival (OS), whereas others found that DM significantly reduced survival [12].
Our study aims to describe the prevalence of diabetes mellitus among patients diagnosed with PDAC, analyse the association between the occurrence of DM and clinicopathological factors and detect variables influencing overall survival (OS).
2. Materials and Methods
2.1. Patients and Data Collection
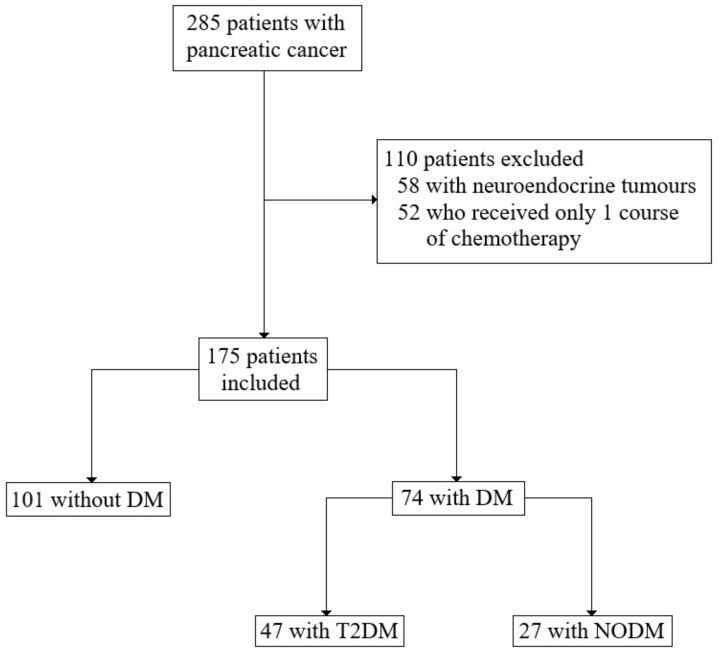
We retrospectively analysed the medical history of 285 patients with the diagnosis of pancreatic cancer [C25 according to the International Statistical Classification of Diseases and Related Health Problems (ICD-10)], who were treated in the Department of Oncology and Haematology and Department of Gastroenterological Surgery and Transplantation at the Central Clinical Hospital (CSK) of the Ministry of Interior and Administration (MSWiA) in Warsaw, Poland between February 2012 and March 2021. After excluding 52 patients with neuroendocrine tumours and 58 patients who received only one course of chemotherapy, 175 patients were included in the study for the analysis (Figure 1). The analysed medical data encompassed sex, age, ECOG, other diseases (diabetes, hypertension, immunological and malignancies), pathological variables (tumour site, tumour size, histological grading, nodal involvement, tumour stage, neuro- and angioinvasion and resection margin), treatment data (type of the operation, vascular reconstruction, postoperative complications, adjuvant and palliative chemotherapy with side effects), laboratory findings, survival and progression time.
Figure 1.
Summary of study design with inclusion and exclusion criteria.
2.2. Study Design
The patients were divided into two following groups: patients without diabetes mellitus (non-DM) (n = 101) and patients with DM (n = 74). The DM group was further sub-divided into T2DM and NODM. Diabetes mellitus was diagnosed according to the below criteria:
-
(i)
Two consecutive fasting glucose levels ≥140 mg/dL (7.8 mmol/L);
-
(ii)
Random plasma glucose ≥200 mg/dL (11.1 mmol/L) in patients with classic symptoms of hyperglycaemia or hyperglycaemic crisis; or
-
(iii)
2-h plasma glucose ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT).
Tumour staging was performed according to the American Joint Cancer Committee (AJCC) Staging Manual, 8th edition. Deaths were identified by reviewing the medical records. Recurrence was detected by abdominal and chest computed tomography (CT) during the follow-up period. The study focused on the DM group—the non-DM group was enrolled for the comparison concerning clinicopathological variables.
2.3. Statistical Analysis
Statistical analysis was conducted with the usage of IBM SPSS Statistics 27. All analysed variables are presented as mean and standard deviation or frequency with percentages. Differences in categorical variables were assessed as appropriate by either Chi-square or Fisher-exact test. The Student’s test and one-way analysis of variance were used to compare continuous variables. Survival (presented as median value) was calculated from the time of diagnosis of pancreatic cancer to the time of death from any cause. Alive patients were censored at their last follow-up. Survival was estimated using the Kaplan–Meier method and compared using the log-rank test. The Cox proportional hazards model was used to determine the prognostic factors in the univariate analysis of survival rates. The prognostic factors detected in univariate analyses as statistically significant were analysed further with multivariate Cox regression. The analysis was performed using the backward method based on the Wald statistic. In each step of this method, one prognostic factor with the weakest association with survival was excluded. The multivariate analyses allowed for indicating the strongest prognostic factor. A p-value of ≤0.05 (two-sided) was regarded as statistically significant in all analyses.
2.4. Ethics Approval and Consent to Participate
The work described in this article has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) on medical research involving human subjects, which is the ethical principles defined in the Farmington Consensus of 1997. The study was acknowledged by the Bioethics Committee of the Medical University of Warsaw (AKBE/144/2022).
3. Results
Seventy-four patients with both DM and PDAC were enrolled in the analysis. Forty-seven of them were diagnosed with type 2 diabetes, while 27 developed NODM after the surgery (which accounted for 21.2% of all operated patients in the study). The majority of patients with DM were men (54.1%) with ECOG 1 status (71.6%). The mean age of the patients was 64.3 [standard deviation (SD) 8.2, range: 40–87]. Most of the patients were diagnosed with PDAC in the head of the pancreas (77.0%), in the IIB stage (29.7%), and with grading 2 (57.2%). Nodal involvement was confirmed in 33.9% of cases. Neuroinvasion was confirmed in 87.5% of the analysed samples, while angioinvasion in 69%.
Concerning other diseases, 9 patients were diagnosed with autoimmune disease (8—hypothyroidism, 1—rheumatoid arthritis), 45 with hypertension, and 8 with other cancers, encompassing prostate, breast, ovarian and colorectal malignancies. Forty-eight patients received adjuvant chemotherapy, and 74.5% of them developed adverse effects, among which neutropenia was the most common one. Thirty-one patients (64.6%) in the adjuvant group experienced disease progression and further received palliative therapy. Twenty-six patients received palliative chemotherapy from the beginning. In total, 57 patients eventually received palliative treatment, and 71.9% of them developed adverse effects, among which neutropenia was the most common one. Concerning T2DM treatment, 22 patients received metformin, 23 insulin, 1 empagliflozin, and 1 sulphonylurea.
Statistical analysis comparing the non-DM group with other studied groups is presented in Table 1. Hypertension was more often diagnosed among the DM group (p < 0.024) and T2DM group (p < 0.011) than in the non-DM group. In the laboratory findings, patients with DM had a lower CRP/lymphocytes (CLR) ratio before the first course of the chemotherapy (p < 0.050). Neutropenia as the side effect of adjuvant chemotherapy was more frequently observed in the non-DM group than in the DM group (p < 0.034) and NODM group (p < 0.013). On the other hand, neutropenia as the side effect of palliative chemotherapy was more frequently observed in the non-DM group than in the DM 2 group (p < 0.020). There were no significant differences in the pathological variables.
Table 1.
Characteristics of the three study groups and comparison to the non-DM group.
| Variable | Non-DM (n = 101) | DM (n = 74) | T2DM (n = 47) | NODM (n = 27) | |||
|---|---|---|---|---|---|---|---|
| M ± SD/n (%)/ MD (95% CI) |
M ± SD/n (%)/ MD (95% CI) |
p * | M ± SD/n (%)/ MD (95% CI) |
p ** | M ± SD/n (%)/ MD (95% CI) |
p *** | |
| Gender (male) | 47 (46.5%) | 40 (54.1%) | 0.326 | 27 (57.4%) | 0.216 | 13 (48.1%) | 0.881 |
| Age (years) | 63.41 ± 10.68 | 64.30 ± 8.23 | 0.550 | 65.66 ± 7.99 | 0.200 | 61.93 ± 8.26 | 0.506 |
| ECOG (0/1/2) | 6/75/17 | 6/54/14 | 0.927 | 5/35/7 | 0.893 | 1/20/6 | 0.441 |
| History of other cancers | 9 (8.9%) | 8 (11.1%) | 0.632 | 7 (15.2%) | 0.255 | 1 (3.8%) | 0.686 |
| Autoimmune disease | 11 (10.9%) | 9 | 0.936 | 7 | 0.717 | 2 | 1.000 |
| Hypertension | 44 (43.6%) | 45 (60.8%) | 0.024 | 31 (66.0%) | 0.011 | 14 (51.9%) | 0.442 |
| Adverse effects—adjuvant chemotherapy | 44 (83.0%) | 35 (74.5%) | 0.295 | 24 (82.8%) | 1.000 | 11 (61.1%) | 0.099 |
| Neurological | 4 (7.5%) | 2 (4.3%) | 0.681 | 2 (6.9%) | 1.000 | 0 | 0.566 |
| Neutropenia | 38 (71.7%) | 24 (51.1%) | 0.034 | 17 (58.6%) | 0.228 | 7 (38.9%) | 0.013 |
| Hepatological | 2 (3.8%) | 4 (8.5%) | 0.416 | 2 (6.9%) | 0.612 | 2 (11.1%) | 0.265 |
| Adverse effects—palliative chemotherapy | 67 (83.8%) | 41 (71.9%) | 0.095 | 24 (68.6%) | 0.065 | 17 (77.3%) | 0.531 |
| Neurological | 14(17.5%) | 7 (12.3%) | 0.403 | 4 (11.4%) | 0.410 | 3 (13.6%) | 1.000 |
| Neutropenia | 44 (55.0%) | 22 (38.6%) | 0.058 | 11 (31.4%) | 0.020 | 11 (50%) | 0.677 |
| Hepatological | 5 (6.3%) | 6 (10.5%) | 0.525 | 5 (14.3%) | 0.170 | 1 (4.5%) | 1.000 |
| Operative complications | 5 (7.0%) | 3 (5.6%) | 1.000 | 2 (6.3%) | 1.000 | 1 (4.5%) | 1.000 |
| Neuroinvasion | 43 (79.6%) | 35 (87.5%) | 0.315 | 24 (88.9%) | 0.365 | 11 (84.6%) | 1.000 |
| Angioinvasion | 46 (85.2%) | 29 (69.0%) | 0.058 | 21 (72.4%) | 0.160 | 8 (61.5%) | 0.110 |
| Grading (1/2/3) | 11/51/14 | 9/39/12 | 0.964 | 5/27/7 | 0.965 | 4/12/5 | 0.632 |
| T (1/2/3/4) | 2/15/51/5 | 1/15/34/2 | 0.689 | 1/11/19/2 | 0.519 | 0/4/15/0 | 0.825 |
| N (0/1/2) | 13/43/16 | 17/25/11 | 0.181 | 9/17/6 | 0.508 | 8/8/5 | 0.121 |
| M (0/1) | 56/45 | 52/22 | 0.046 | 32/15 | 0.145 | 20/7 | 0.080 |
| TNM Stage (IA/IB/IIA/IIB/III/IV) | 1/3/5/31/12/45 | 1/6/6/22/10/21 | 0.268 | 1/5/2/15/5/15 | 0.354 | 0/1/4/7/5/6 | 0.156 |
| Localisation | 77/9/7/3/2/3 | 57/4/5/0/4/4 | 0.475 | 33/3/5/0/3/3 | 0.414 | 24/1/0/0/1/1 | 0.579 |
| Metastases | 29/4/30/3/9 | 25/6/11/3/3 | 0.131 | 16/4/7/2/2 | 0.219 | 9/2/4/1/1 | 0.440 |
| CEA > 5 ng/mL | 22 (32.8%) | 19 (36.5%) | 0.673 | 15 (40.5%) | 0.432 | 4 (26.7%) | 0.765 |
| CA19-9 > 37 IU/mL | 51 (56.7%) | 38 (58.6%) | 0.824 | 26 (57.8%) | 0.902 | 12 (60.0%) | 0.785 |
| CRP/lymphocytes ratio > 1.8 | 37 (66.1%) | 21 (46.7%) | 0.050 | 17 (50.0%) | 0.131 | 4 (36.4%) | 0.092 |
| Lymphocytes > 1 × 103/µL | 93 (92.1%) | 66 (91.7%) | 0.922 | 42 (89.4%) | 0.552 | 24 (96.0%) | 0.687 |
| Haemoglobin > 12 g/dL | 63 (63.6%) | 51 (70.8%) | 0.324 | 33 (70.2%) | 0.434 | 18 (72.0%) | 0.432 |
| Platelets > 400 × 103/µL | 18 (17.8%) | 11 (15.3%) | 0.659 | 6 (12.8%) | 0.437 | 5 (20.0%) | 0.777 |
| CRP > 5 mg/L | 28 (59.0%) | 18 (40.0%) | 0.316 | 15 (44.1%) | 0.588 | 3 (27.3%) | 0.167 |
| OS | 18 (15.7–20.3) | 22 (18.4–26.6) | 0.050 | 20 (15.9–24.1) | 0.462 | 23 (16.0–30.0) | 0.017 |
| DFS | 12 (9.0–15.0) | 9 (7.7–10.3) | 0.533 | 9 (4.9–13.1) | 0.706 | 7 (4.9–9.1) | 0.580 |
| PFS | 5 (3.9–6.1) | 6 (4.34–7.6) | 0.206 | 6 (4.6–7.4) | 0.389 | 7 (4.7–9.3) | 0.230 |
Abbreviations: Non-DM—group without diabetes mellitus, DM—diabetes mellitus, T2DM—type 2 diabetes mellitus, NODM—new-onset diabetes mellitus, M—mean, SD—standard deviation, n—number, MD—median, 95% CI—95% Confidence Interval, OS—overall survival, DFS—disease-free survival and PFS—progression-free survival. p * non-DM vs. DM, p ** non-DM vs. T2DM and p *** non-DM vs. NODM. Bolded value – value statistically significant.
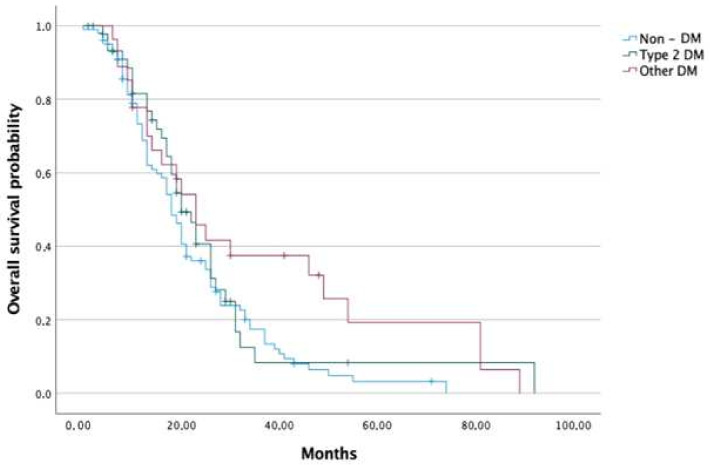
Median OS was 18, 22 20, and 23 months in the non-DM, DM, T2DM and NODM groups, respectively. Patients in the groups of DM and NODM had significantly higher median OS than the non-DM group (p < 0.050 and 0.017, respectively). Analysed groups were further sub-divided into a group receiving adjuvant chemotherapy and a group receiving palliative chemotherapy (presented with advanced disease at the time of diagnosis). Regarding adjuvant chemotherapy, no difference in survival between non-DM and DM groups was detected; nevertheless, the NODM group had a significantly higher median OS than the non-DM group (26.5 months vs. 20.5 months, p < 0.028). In terms of palliative chemotherapy, patients with DM had significantly higher median OS than those without DM (18 months vs. 13 months, p < 0.034) (Figure 2 and Figure 3).
Figure 2.
Overall survival of pancreatic cancer patients in DM and non-DM groups.
Figure 3.
Overall survival of pancreatic cancer patients in the non-DM, T2DM and NODM groups.
No significant differences concerning DFS and PFS were confirmed (Table 1).
In the univariate analysis for survival in all DM patients, nodal involvement (N2 stage) (p = 0.020), tumour location (p = 0.050), level of CEA (p = 0.019), CRP (p < 0.001) and CLR (p = 0.001) were significantly associated with OS (Table 2). These prognostic factors were analysed further in multivariate Cox regression using the backward method based on Wald statistics. The results are depicted in Table 3. Out of five prognostic factors analysed, the CRP level was the last excluded, meaning it was the strongest predictor of survival in the DM group.
Table 2.
Univariate analyses of prognostic factors for overall survival—DM group.
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Age (years) | 0.986 (0.952–1.021) | 0.424 |
| Gender | - | - |
| Female | Ref | - |
| Male | 1.194 (0.690–2.063) | 0.526 |
| Autoimmune disease | 0.660 (0.260–1.676) | 0.382 |
| Hypertension | 0.643 (0.372–1.112) | 0.114 |
| T | - | - |
| T1 | Ref | - |
| T2 | 0.336 (0.041–2.729) | 0.308 |
| T3 | 0.254 (0.032–1.996) | 0.192 |
| T4 | 0.456 (0.040–5.222) | 0.528 |
| N | - | - |
| N0 | Ref | - |
| N1 | 1.692 (0.768–3.730) | 0.192 |
| N2 | 3.163 (1.200–8.338) | 0.020 |
| M | - | - |
| M0 | Ref | - |
| M1 | 1.524 (0.852–2.725) | 0.156 |
| TNM stage | - | - |
| IA | Ref | - |
| IB | 0.310 (0.033–2.862) | 0.301 |
| IIA | 0.149 (0.015–1.465) | 0.103 |
| IIB | 0.302 (0.039–2.359) | 0.254 |
| III | 0.551 (0.067–4.513) | 0.579 |
| IV | 0.450 (0.058–3.450) | 0.449 |
| Angioinvasion | 1.193 (0.525–2.712) | 0.674 |
| Neuroinvasion | 0.563 (0.192–1.653) | 0.296 |
| R | - | 0.081 |
| R0 | Ref | |
| R1 | 1.789 (0.930–3.442) | |
| Grading | - | - |
| G1 | Ref | - |
| G2 | 1.691 (0.659–4.339) | 0.275 |
| G3 | 2.155 (0.711–6.531) | 0.175 |
| Tumour site | - | - |
| Head | Ref | - |
| Other | 1.809 (0.999–3.277) | 0.050 |
| Operation type | - | - |
| Whipple | Ref | - |
| Other | 1.367 (0.688–2.717) | 0.372 |
| Vascular reconstruction | 1.242 (0.543–2.842) | 0.608 |
| Adverse effects—adjuvant chemotherapy | 1.521 (0.650–3.557) | 0.333 |
| Neutropenia | 1.673 (0.792–3.535) | 0.178 |
| Hepatological | 1.113 (0.263–4.722) | 0.883 |
| Adverse effects—palliative chemotherapy | 1.025 (0.544–1.931) | 0.939 |
| Neutropenia | 0.651 (0.356–1.191) | 0.163 |
| Hepatological | 1.182 (0.418–3.345) | 0.751 |
| Neurological | 0.490 (0.173–1.386) | 0.179 |
| History of other cancers | 1.907 (0.838–4.337) | 0.124 |
| CEA | 1.004 (1.001–1.007) | 0.019 |
| CA 19-9 | 1.000 (0.999–1.002) | 0.059 |
| Lymphocytes | 0.956 (0.844–1.082) | 0.475 |
| CRP/lymphocytes ratio | 1.032 (1.013–1.053) | 0.001 |
| Haemoglobin | 0.954 (0.720–1.263) | 0.741 |
| Platelets | 1.001 (0.999–1.003) | 0.249 |
| Calcium | 0.446 (0.051–3.899) | 0.465 |
| CRP | 1.017 (1.008–1.025) | <0.001 |
Abbreviations: Ref–reference. Bolded value–value statistically significant.
Table 3.
Multivariate analysis of prognostic factors for overall survival—DM group.
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Step 1 | ||
| N | - | - |
| N0 | Ref | - |
| N1 | 1.180 (0.182–7.667) | 0.862 |
| N2 | 1.239 (0.126–12.214) | 0.854 |
| Tumour site | - | - |
| Head | Ref | - |
| Other | 1.149 (0.226–5.833) | 0.867 |
| CEA | 1.006 (0.983–1.030) | 0.620 |
| CRP/lymphocytes ratio | 0.984 (0.900–1.075) | 0.718 |
| CRP | 1.027 (0.965–1.092) | 0.401 |
| Step 2 | ||
| Tumour site | - | - |
| Head | Ref | - |
| Other | 1.030 (0.352–3.011) | 0.957 |
| CEA | 1.006 (0.984–1.028) | 0.621 |
| CRP/lymphocytes ratio | 0.988 (0.917–1.065) | 0.756 |
| CRP | 1.024 (0.971–1.079) | 0.381 |
| Step 3 | ||
| CEA | 1.005 (0.984–1.028) | 0.623 |
| CRP/lymphocytes ratio | 0.988 (0.917–1.065) | 0.988 |
| CRP | 1.024 (0.972–1.079) | 0.369 |
| Step 4 | ||
| CEA | 1.005 (0.984–1.027) | 0.633 |
| CRP | 1.016 (0.997–1.035) | 0.095 |
| Step 5 | ||
| CRP | 1.017 (1.008–1.025) | <0.001 |
Abbreviations: Ref–reference. Bolded value–value statistically significant.
In NODM group, only the TNM stage was independent prognostic factor for survival (p < 0.015, HR 1.774 95% CI 1.116–2.822) (Table 4). The multivariate analysis was not performed in this case because there was only one statistically significant prognostic factor.
Table 4.
Univariate analyses of prognostic factors for overall survival—NODM group.
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Age | 0.563 (0.229–1.383) | 0.210 |
| Gender | - | 0.187 |
| Female | Ref | |
| Male | 0.517 (0.194–1.377) | |
| Hypertension | 0.418 (0.164–1.069) | 0.069 |
| Nodal involvement | 3.142 (0.808–12.216) | 0.098 |
| TNM stage (IA/IB/IIA/IIB/III/IV) | 1.774 (1.116–2.822) | 0.015 |
| R | - | 0.081 |
| R0 | Ref | |
| R1 | 1.287 (0.443–3.737) |
Abbreviations: Ref–reference. Bolded value–value statistically significant.
In T2DM group, nodal involvement (N2 stage) (p = 0.023), CRP level (p < 0.001) and CLR (p = 0.004) were significantly associated with OS (Table 5). The results of subsequent multivariate analysis are depicted in Table 6. Out of three prognostic factors analysed, the CRP level again was the last one excluded, meaning it was the strongest predictor of survival in the T2DM group.
Table 5.
Univariate analyses of prognostic factors for overall survival—T2DM group.
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Age (years) | 0.978 (0.926–1.033) | 0.426 |
| Gender | - | - |
| Female | Ref | - |
| Male | 0.898 (0.639–1.263) | 0.536 |
| Autoimmune disease | 0.962 (0.334–2.771) | 0.942 |
| Hypertension | 0.732 (0.358–1.500) | 0.393 |
| T | - | - |
| T1 | Ref | - |
| T2 | 0.268 (0.030–2.383) | 0.238 |
| T3 | 0.196 (0.025–1.702) | 0.139 |
| T4 | 0.358 (0.030–4.325) | 0.419 |
| N | - | - |
| N0 | Ref | - |
| N1 | 1.383 (0.465–4.111) | 0.560 |
| N2 | 5.342 (1.264–22.564) | 0.023 |
| M | - | - |
| M0 | Ref | - |
| M1 | 1.524 (0.852–2.725) | 0.156 |
| TNM stage | - | - |
| IA | Ref | - |
| IB | 0.179 (0.017–1.900) | 0.154 |
| IIB | 0.215 (0.025–1.827) | 0.159 |
| III | 0.734 (0.079–6.829) | 0.786 |
| IV | 0.255 (0.029–2.172) | 0.211 |
| Angioinvasion | 2.023 (0.670–6.109) | 0.212 |
| Neuroinvasion | 0.865 (0.194–3.863) | 0.849 |
| R | - | 0.064 |
| R0 | Ref | |
| R1 | 2.185 (0.956–4.996) | |
| Grading | - | - |
| G1 | Ref | - |
| G2 | 2.111 (0.494–9.014) | 0.313 |
| G3 | 1.433 (0.259–7.920) | 0.680 |
| Tumour site | - | - |
| Head | Ref | - |
| Other | 1.705 (0.841–3.454) | 0.139 |
| Operation type | - | - |
| Whipple | Ref | - |
| Other | 1.307 (0.567–3.184) | 0.555 |
| Vascular reconstruction | 0.877 (0.295–2.606) | 0.814 |
| DM treatment | - | - |
| Insulin | Ref | - |
| Metformin | 0.726 (0.363–1.451) | 0.364 |
| Adverse effects—adjuvant chemotherapy | 0.833 (0.237–2.934) | 0.776 |
| Neutropenia | 1.055 (0.376–2.957) | 0.919 |
| Adverse effects—palliative chemotherapy | 0.745 (0.336–1.650) | 0.468 |
| Neutropenia | 0.541 (0.215–1.259) | 0.191 |
| Hepatological | 0.763 (0.228–2.556) | 0.662 |
| Neurological | 0.224 (0.030–1.669) | 0.144 |
| History of other cancers | 1.748 (0.695–4.396) | 0.235 |
| CEA | 1.012 (0.978–1.048) | 0.493 |
| Lymphocytes | 0.964 (0.840–1.107) | 0.607 |
| CRP/lymphocytes ratio | 1.013 (1.004–1.022) | 0.004 |
| Haemoglobin | 0.900 (0.639–1.263) | 0.536 |
| Calcium | 0.738 (0.050–10.854) | 0.825 |
| CRP | 1.018 (1.007–1.028) | <0.001 |
Abbreviations: Ref–reference. Bolded value–value statistically significant.
Table 6.
Multivariate analyses of prognostic factors for overall survival—T2DM group.
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Step 1 | ||
| N | - | - |
| N0 | Ref | - |
| N1 | 0.620 (0.169–2.280 | 0.472 |
| N2 | 2.116 (0.390–11.471) | 0.385 |
| CRP/lymphocytes ratio | 0.998 (0.904–1.102) | 0.971 |
| CRP | 1.017 (0.950–1.089) | 0.629 |
| Step 2 | ||
| N | - | - |
| N0 | Ref | - |
| N1 | 0.616 (0.177–2.139) | 0.445 |
| N2 | 2.097 (0.416–10.562) | 0.370 |
| CRP | 1.016 (0.995–1.037) | 0.134 |
| Step 3 | ||
| CRP | 1.018 (1.007–1.028) | <0.001 |
Abbreviations: Ref–reference. Bolded value–value statistically significant.
4. Discussion
Our study focused on various aspects to characterize patients with both DM and PC. We investigated not only oncological variables but also pathological and surgical ones. In the analysis, we incorporated data about chemotherapy schemes with side effects and also DM medicaments. Pancreatic cancer and DM are bidirectionally associated—DM is proved to be both the cause and consequence of PDAC [13]. In the analysed sample, 42.3% of patients developed DM—type 2 before the PDAC diagnosis or new-onset DM after the surgery. These results are in line with previous ones, in which the prevalence of DM among PDAC patients is estimated to reach 40–65% [11]. Except for PDAC, the prevalence of diabetes in other malignancies is similar to that of healthy controls [14]. Various mechanisms are responsible for the strong correlation between DM and PDAC. Clinical studies proved that the β cell response (measured by response to an oral glucose load, hyperglycaemic clamp or glucagon stimulation) is impaired in patients with PDAC [14]. Insulin released by β cells might indirectly promote carcinogenesis via acting on the growth hormone/insulin-like growth factor (GH/IGF) axis and increasing levels of free and bioactive insulin-like growth factor-1 (IGF-1) [15]. Both insulin and redundant IGF-1 reveal trophic effects on insulin receptors on acinar cells and IGF-I receptors present in any nearby cells, including transformed ones. As a result, insulin and IGF-1 promote their survival and proliferation, thus possibly contributing to the observed increased risk of developing PDAC [16]. Moreover, inadequate glycaemic control leads to producing increased levels of advanced glycation end products (AGE). AGE activate their receptors, which bind certain other ligands (inflammatory cytokines and S100 family) that are implicated in inflammation and PDAC progression [17].
Our study revealed no significant differences in the pathological variables between DM and non-DM groups. In the study by Hank et al. [18] (2019) conducted on patients undergoing pancreatic resection, diabetic patients had significantly larger tumours, increased lymph-node involvement and higher rates of perineural and lympho-vascular invasion. Chu et al. [19] (2010) proved tumour size as the only pathological variable differentiating DM and non-DM groups. Ben et al. [20] (2012) detected that the percentage of cases with neural invasion in those with DM was significantly higher than those without DM.
Oncological patients with pre-existing diabetes have 50% higher post-operative mortality rates, probably due to greater inflammation risk [19,21]. On the other hand, consistently with previous findings, in this study, DM was not associated with a higher rate of post-surgical complications [18,22,23]. However, Chu et al. [22] (2010) suggested that the role of PDAC-associated DM as a risk factor for postresection pancreatic fistula should be further explored, as in their study DM patients had a significantly higher likelihood of developing fistulas. Pancreatic cancer related-DM improves after the resection of tumour mass despite surgical removal of a variable amount of pancreatic tissue, which supports the hypothesis that pancreatic carcinoma cells induce DM themselves [6].
Reports concerning the exact impact of DM on the overall survival of patients with PC are ambiguous. Our study revealed that patients with DM had significantly higher median OS than those without DM. After subdividing patients into those receiving adjuvant or palliative chemotherapy, DM positively impacted survival only among patients with advanced disease. However, DM treatment with neither insulin nor metformin served as a prognostic factor for OS. Numerous studies suggested that DM is associated with reduced OS [6,18,20,24,25,26]. On the contrary, other studies proved that DM does not affect pancreatic cancer OS or even improve survival [27,28,29,30,31]. The meta-analysis conducted by Mao et al. [12] (2015) suggested the negative effect of DM on survival primarily among patients with resectable tumours but not in those with late-stage disease. Chu et al. [19] proved that compared with non-DM, NODM was independently associated with survival reduction after PDAC resection; nevertheless, a reduction in patients with longstanding DM versus those without DM turned out to be no longer statistically significant. Choi et al. [32] (2016) revealed that individuals with advanced PC and DM tend to survive longer than those without DM. They linked this phenomenon to metformin usage, which conferred a prognostic benefit. Moreover, in the paper introduced during the 2013 ASCO Annual Meeting I, it was suggested that in patients with advanced tumours, recent-onset DM and metformin treatment are positive prognostic indicators associated with longer OS [33]. Various preclinical studies demonstrated a positive impact of metformin on PC through increasing the chemosensitivity of PC cells to gemcitabine [34], presenting an anti-tumour effect in combination with liraglutide [35] synergistically with pitavastatin activating apoptosis and autophagy in PC cells [36], or finally inhibiting tumour growth, and prolonging the overall survival in mice model [37]. Concerning insulin, the recent study by Pretta et al. [38] (2021) revealed that insulin-treated patients compared with non-DM had a significantly increased survival in the multivariate analysis. The role of insulin in carcinogenesis is disputed. According to preclinical and clinical trials, it is generally considered to promote tumour growth; however, there is no unanimity, as Pircher et al. [39] (2018) proved that insulin might suppress the activation of mTOR and inhibit tumour growth.
Several studies reported that tumour sites influenced survival. Our study aligns with these findings and confirms that patients with PDAC located in the head have a worse prognosis. The various analysis also suggested that pancreatic body and tail locations were independent indicators for better survival [40,41]. It might be linked to the pancreatic head’s more complex lymphatic drainage system and is more frequently associated with higher nodal involvement [42,43]. Moreover, it could be correlated with intervention differences. The resection of distal pancreatic tumours is safer and more feasible [44]. Body or tail location does not lead to malignant biliary obstruction and thus is not associated with potential biliary drainage and further complications delaying appropriate surgery [45]. Contrastingly, a few studies suggested better outcomes among patients with head tumours. The authors explained it with an additional period resulting from an earlier diagnosis rather than tumour biology or differences in interventions [41].
Neuropathy is a common adverse effect associated with gemcitabine-nab-paclitaxel and mFOLFIRINOX chemotherapy schemes [46,47]. On the other hand, DM is the most common cause of autonomic neuropathy; nevertheless, the pathophysiology of diabetic neuropathy is not fully understood, and its aetiology seems to be multifactorial [48]. Interestingly, our study revealed that patients with DM are not more likely to develop neuropathy as a side effect of palliative chemotherapy. To our best knowledge, this is the first analysis comparing the prevalence of neuropathy as a chemotherapy’s side effect between patients with and without DM. Some studies confirmed only a significantly higher prevalence of perineural invasion among patients with DM [19,49]. Jian et al. [50] (2020) analysed genes associated with PDAC and involved in diabetic neuropathy progression. They selected matrix metalloproteinase-9 (MMP9) as a prognostic marker for pancreatic cancer and possible adjustment to the treatment of diabetes, pancreatic cancer and associated neuropathy.
The risk of PDAC declines with the increasing duration of DM [15]. This phenomenon might be linked to possible lifestyle changes and selected glucose-lowering medication usage. Various epidemiologic studies confirmed the association of metformin use with a reduced incidence of PDAC among patients with DM; however, the published reports seem to not be universally consistent [51]. Its anticancer effect might be associated with direct actions on transformed pancreatic cells and systemic actions. Metformin inhibits synthesis of mitochondrial adenosine triphosphate (ATP), leading to insufficient energy production. As a result, it increases cellular adenosine monophosphate/adenosine triphosphate (AMP/ATP) and adenosine diphosphate/adenosine triphosphate (ADP/ATP) ratios and activates AMP-activated protein kinase (AMPK). Stimulated AMPK inhibits the synthesis of macromolecules essential for further cell growth and inhibits the mechanistic target-of-rapamycin complex (mTORc), responsible for activating various cellular pathways [52,53]. Lower blood glucose leads to decreased mitogenic insulin secretion and further attenuates cell division [54]. In the studies conducted on genetic mice models, metformin inhibited cancer initiation, suppressed chronic pancreatitis-induced tumorigenesis and presented promising therapeutic effect in PDAC [37,55,56].
Various studies confirm that cancer progression is stimulated by systemic and local inflammatory reactions [57]. The immune system might suppress tumour development or progression by destroying cells with mutations; however, it can also promote pancreatic cancer progression by establishing favourable conditions for immunosuppression and further metastasis [58]. In terms of pancreatic cancer, one of the widely described inflammation-based parameters is CRP/albumin ratio (CAR). CAR was established as a strong prognostic and predictive factor in resectable and advanced tumours [59,60,61]. Lately, Fan et al. [62] (2020) have suggested elevated CLR as an independent risk factor for poor OS among PDAC patients. Another study by Strijker et al. [63] (2021) detected that CRP combined with CA19-9, albumin and LDH had a prognostic value, which was at least similar to that of ECOG performance status. The level of CLR is suspected to reflect the state of equilibrium between the systemic inflammatory and immunologic response. In our study, both higher CLR and higher CRP levels alone were significantly associated with worse OS among all patients with DM. Our multivariate analysis suggested CRP level as the strongest predictor of survival. CRP was previously claimed to associate with survival and cachexia among patients with PDAC [64,65]. Although some analyses also suggest that a reduced number of circulating white blood cells might present prognostic implications in PDAC patients, in our study, leukopenia alone revealed no correlation with survival [66].
Our analysis revealed that among patients with PDAC, those with DM are significantly more likely to develop hypertension, which is consistent with known risk factors for type II DM. Epidemiological studies have proven that metabolic syndrome and its components (hypertension, insulin resistance, central obesity, decreased levels of high-density lipoproteins (HDL) cholesterol and elevated triglyceride levels) may independently or in combination increase the risk of many types of cancer, including PDAC [67,68]. Moreover, some studies suggest that obesity significantly reduces OS among PDAC patients [23]. Our study did not include BMI in the analysis to compare. Xia et al. [69] (2020) suggested a potential joint effect of CRP and metabolic syndrome in pancreas tumorigenesis. The disruption of the inflammatory system is involved in the development of pancreatic cancer, and metabolic syndrome is related to pancreatic cancer risk, with diabetes being the critical component [70]. Elevated CRP was proven to be positively associated with the presence of the metabolic syndrome.
This study meets with some limitations. Firstly, it was a single-centre study. The juxtaposition of results obtained in the different clinical centres would present a broader view of the discussed subject. Secondly, it is a retrospective study and thus may present some potential selection bias. The duration of DM was not directly reported due to the encountered lack in the past medical history. Nonetheless, we firmly believe that outcomes acquired in our centre are a meaningful puzzle piece in the knowledge concerning the relationship between diabetes mellitus and pancreatic cancer.
5. Conclusions
Diabetes mellitus is prevalent among patients with pancreatic cancer. The results concerning DM impact on PDAC patients’ survival are contradictory; however, in our study, patients with DM receiving palliative chemotherapy had significantly higher median OS than those without DM. Regarding adjuvant chemotherapy, no difference in survival between non-DM and DM groups was detected; nevertheless, we have observed that the NODM patients had a significantly longer median OS than the non-DM patients. Among variables influencing survival, TNM stage, nodal involvement, tumour site, levels of CEA and CRP were confirmed, with CRP level being the strongest one. As an adverse effect of chemotherapy, neutropenia affects patients with DM less often. The increased comprehension of the mechanisms of the relationship between DM and pancreatic cancer needs further research, which might provide avenues for developing novel preventive and therapeutic strategies.
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| PC | Pancreatic cancer |
| DM | Diabetes mellitus |
| NODM | New-onset diabetes mellitus |
| T2DM | Type 2 DM |
| OS | Overall survival |
| ICD-10 | The International Statistical Classification of Diseases and Related Health Problems |
| CSK | Central Clinical Hospital |
| MSWiA | Ministry of Interior and Administration |
| ECOG | Eastern Cooperative Oncology Group performance status |
| non-DM | Group without diabetes mellitus |
| OGTT | Oral glucose tolerance test |
| AJCC | American Joint Cancer Committee |
| CT | Computed tomography |
| SD | Standard deviation |
| HR | Hazard ratio |
| CRP | C reactive protein |
| CLR | CRP/lymphocytes ratio |
| M | Mean |
| n | Number |
| MD | Median |
| 95% CI | 95% Confidence Interval |
| DFS | Disease-free survival |
| PFS | Progression-free survival |
| CEA | Carcino-embryonic antigen |
| CA19-9 | Carbohydrate antigen 19-9 |
| TNM | TNM Classification of Malignant Tumors |
| T | Tumour size |
| N | Lymph nodal involvement |
| M | Distant metastasis |
| R | Resection margin |
| GH/IGF | Growth hormone/insulin-like growth factor |
| IGF-1 | Insulin-like growth factor-1 |
| AGE | Advanced glycation end products |
| ASCO | American Society of Clinical Oncology |
| mTOR | The mammalian target of rapamycin |
| mFOLFIRINOX | A chemotherapy regimen: FOL—folinic acid |
| F | Fluorouracil (5-FU) |
| IRIN | Irinotecan |
| OX | Oxaliplatin |
| MMP9 | Matrix metalloproteinase-9 |
| AMP/ATP | Adenosine monophosphate/adenosine triphosphate |
| ADP/ATP | Adenosine diphosphate/adenosine triphosphate |
| AMPK | AMP-activated protein kinase |
| CAR | CRP/albumin ratio |
| HDL | High-density lipoproteins |
Author Contributions
Conceptualization, A.B.-K., M.F., D.K. and A.D.; Data curation, M.F., D.K. and A.D.; Formal analysis, A.M., E.W., A.C. and A.D.; Funding acquisition, A.B.-K., A.D., A.C. and T.B.; Investigation, A.B.-K., M.F., D.K. and A.D.; Methodology, M.F., A.M., E.W., A.C. and T.B.; Supervision, A.B.-K. and A.D.; Validation, A.B.-K., M.D., A.N.-G. and A.D.; Writing—original draft, A.B.-K., M.F., D.K., A.M., E.W. and A.D.; Writing—review and editing, A.B.-K., M.F., M.D., A.N.-G., A.C., T.B. and A.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and acknowledged by the Bioethics Committee of the Medical University of Warsaw (AKBE/144/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Medical University of Warsaw, grant number PW/Z/1/1/20(1).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khalaf N., El-Serag H.B., Abrams H.R., Thrift A.P. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin. Gastroenterol. Hepatol. 2021;19:876–884. doi: 10.1016/j.cgh.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., et al. Pancreatic cancer. Nat. Rev. Dis. Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 4.Wangjam T., Zhang Z., Zhou X.C., Lyer L., Faisal F., Soares K.C., Fishman E., Hruban R.H., Herman J.M., Laheru D., et al. Resected pancreatic ductal adenocarcinomas with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget. 2015;6:36903–36910. doi: 10.18632/oncotarget.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidalgo M. Pancreatic cancer. N. Engl. J. Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 6.Khadka R., Tian W., Hao X., Koirala R. Risk factor, early diagnosis and overall survival on outcome of association between pancreatic cancer and diabetes mellitus: Changes and advances, a review. Int. J. Surg. 2018;52:342–346. doi: 10.1016/j.ijsu.2018.02.058. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37((Suppl. S1)):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 8.Gallo M., Adinolfi V., Morviducci L., Acquati S., Tuveri E., Ferrari P., Zatelli M.C., Faggiano A., Argentiero A., Natalicchio A., et al. Early prediction of pancreatic cancer from new-onset diabetes: An Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Società Italiana Endocrinologia (SIE)/Società Italiana Farmacologia (SIF) multidisciplinary consensus position paper. ESMO Open. 2021;6:100155. doi: 10.1016/j.esmoop.2021.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menini S., Iacobini C., Vitale M., Pesce C., Pugliese G. Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress. Cancers. 2021;13:313. doi: 10.3390/cancers13020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholten L., Mungroop T.H., Haijtink S.A.L., Issa Y., van Rijssen L.B., Koerkamp B.G., van Eijck C.H., Busch O.R., DeVries J.H., Besselink M.G. New-onset diabetes after pancreatoduodenectomy: A systematic review and meta-analysis. Surgery. 2018;164:6–16. doi: 10.1016/j.surg.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Lee W., Yoon Y.S., Han H.S., Cho J.Y., Choi Y., Jang J.Y., Choi H. Prognostic relevance of preoperative diabetes mellitus and the degree of hyperglycemia on the outcomes of resected pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2016;113:203–208. doi: 10.1002/jso.24133. [DOI] [PubMed] [Google Scholar]
- 12.Mao Y., Tao M., Jia X., Xu H., Chen K., Tang H., Li D. Effect of Diabetes Mellitus on Survival in Patients with Pancreatic Cancer: A Systematic Review and Meta-analysis. Sci. Rep. 2015;5:17102. doi: 10.1038/srep17102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Cao G., Ma Q., Liu H., Li W., Han L. The bidirectional interation between pancreatic cancer and diabetes. World J. Surg. Oncol. 2012;10:171. doi: 10.1186/1477-7819-10-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera C.J., Falasca M., Chari S.T., Greenfield J.R., Xu Z., Pirola R.C., Wilson J.S., Apte M.V. Role of Pancreatic Stellate Cell-Derived Exosomes in Pancreatic Cancer-Related Diabetes: A Novel Hypothesis. Cancers. 2021;13:5224. doi: 10.3390/cancers13205224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzato M., Turati F., Rosato V., La Vecchia C. Exploring the link between diabetes and pancreatic cancer. Expert. Rev. Anticancer Ther. 2019;19:681–687. doi: 10.1080/14737140.2019.1642109. [DOI] [PubMed] [Google Scholar]
- 16.Andersen D.K., Korc M., Petersen G.M., Eibl G., Li D., Rickels M.R., Chari S.T., Abbruzzese J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes. 2017;66:1103–1110. doi: 10.2337/db16-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leclerc E., Vetter S.W. The role of S100 proteins and their receptor RAGE in pancreatic cancer. Biochim. Biophys. Acta. 2015;1852:2706–2711. doi: 10.1016/j.bbadis.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hank T., Sandini M., Qadan M., Weniger M., Ciprani D., Li A., Ferrone C.R., Warshaw A.L., Lillemoe K.D., Fernández-Del Castillo C. Diabetes mellitus is associated with unfavorable pathologic features, increased postoperative mortality, and worse long-term survival in resected pancreatic cancer. Pancreatology. 2020;20:125–131. doi: 10.1016/j.pan.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Chu C.K., Mazo A.E., Goodman M., Egnatashvili V., Sarmiento J.M., Staley C.A., Galloway J.R., Adsay N.V., Jacobs S., Kooby D.A. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann. Surg. Oncol. 2010;17:502–513. doi: 10.1245/s10434-009-0789-6. [DOI] [PubMed] [Google Scholar]
- 20.Ben Q., Xu M., Jiang Y., Yuan Y., Wang K., Fang J., Li Z. Clinical profiles and long-term outcomes of patients with pancreatic ductal adenocarcinoma and diabetes mellitus. Diabetes Metab. Res. Rev. 2012;28:169–176. doi: 10.1002/dmrr.1284. [DOI] [PubMed] [Google Scholar]
- 21.Barone B.B., Yeh H.C., Snyder C.F., Peairs K.S., Stein K.B., Derr R.L., Wolff A.C., Brancati F.L. Postoperative mortality in cancer patients with preexisting diabetes: Systematic review and meta-analysis. Diabetes Care. 2010;33:931–939. doi: 10.2337/dc09-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu C.K., Mazo A.E., Sarmiento J.M., Staley C.A., Adsay N.V., Umpierrez G.E., Kooby D.A. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J. Am. Coll. Surg. 2010;210:463–473. doi: 10.1016/j.jamcollsurg.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Li D., Mao Y., Chang P., Liu C., Hassan M.M., Yeung S.J., Abbruzzese J.L. Impacts of new-onset and long-term diabetes on clinical outcome of pancreatic cancer. Am. J. Cancer Res. 2015;5:3260–3269. [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan C., Rubinson D.A., Qian Z.R., Wu C., Kraft P., Bao Y., Ogino S., Ng K., Clancy T.E., Swanson R.S., et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J. Clin. Oncol. 2015;33:29–35. doi: 10.1200/JCO.2014.57.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen H., Zhan M., Wang W., Yang D., Wang J. Impact of diabetes mellitus on the survival of pancreatic cancer: A meta-analysis. Onco. Targets Ther. 2016;9:1679–1688. doi: 10.2147/ott.S95744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein M.R., Mascitelli L., Pezzetta F. Obesity and survival among patients with pancreatic cancer. JAMA. 2009;302:1752. doi: 10.1001/jama.2009.1510. author reply 1752–1753. [DOI] [PubMed] [Google Scholar]
- 27.Karlin N.J., Amin S.B., Kosiorek H.E., Buras M.R., Verona P.M., Cook C.B. Survival and glycemic control outcomes among patients with coexisting pancreatic cancer and diabetes mellitus. Future Sci. OA. 2018;4:Fso291. doi: 10.4155/fsoa-2017-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng C.M., Wang H.H., Wang W.L., Lee C.T., Tai C.M., Tseng C.H., Chen C.C., Tsai Y.N., Sun M.S., Hsu Y.C. Prognostic Impact of Diabetes Mellitus on Overall Survival in A Nationwide Population-Based Cohort of Patients with Pancreatic Cancer. Endocr. Pract. 2020;26:707–713. doi: 10.4158/EP-2019-0565. [DOI] [PubMed] [Google Scholar]
- 29.Olson S.H., Chou J.F., Ludwig E., O’Reilly E., Allen P.J., Jarnagin W.R., Bayuga S., Simon J., Gonen M., Reisacher W.R., et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int. J. Cancer. 2010;127:2412–2419. doi: 10.1002/ijc.25240. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno S., Nakai Y., Isayama H., Takahara N., Miyabayashi K., Yamamoto K., Kawakubo K., Mohri D., Kogure H., Sasaki T., et al. Diabetes is a useful diagnostic clue to improve the prognosis of pancreatic cancer. Pancreatology. 2013;13:285–289. doi: 10.1016/j.pan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Barone B.B., Yeh H.C., Snyder C.F., Peairs K.S., Stein K.B., Derr R.L., Wolff A.C., Brancati F.L. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi Y., Kim T.Y., Oh D.Y., Lee K.H., Han S.W., Im S.A., Kim T.Y., Bang Y.J. The Impact of Diabetes Mellitus and Metformin Treatment on Survival of Patients with Advanced Pancreatic Cancer Undergoing Chemotherapy. Cancer Res. Treat. 2016;48:171–179. doi: 10.4143/crt.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh D.-Y., Choi Y., Kim T.-Y., Lee K.-H., Han S.-W., Im S.-A., Kim T.-Y., Bang Y.-J. The impact of diabetes mellitus and metformin on survival of patients with advanced pancreatic cancer receiving chemotherapy. J. Clin. Oncol. 2013;31:4044. doi: 10.1200/jco.2013.31.15_suppl.4044. [DOI] [Google Scholar]
- 34.Gu Y., Zhang B., Gu G., Yang X., Qian Z. Metformin Increases the Chemosensitivity of Pancreatic Cancer Cells to Gemcitabine by Reversing EMT Through Regulation DNA Methylation of miR-663. Onco. Targets Ther. 2020;13:10417–10429. doi: 10.2147/OTT.S261570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu R., Yang J., Wei R., Ke J., Tian Q., Yu F., Liu J., Zhang J., Hong T. Synergistic anti-tumor effects of liraglutide with metformin on pancreatic cancer cells. PLoS ONE. 2018;13:e0198938. doi: 10.1371/journal.pone.0198938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y.H., Huang Y.C., Yang S.F., Yen H.H., Tsai H.D., Hsieh M.C., Hsiao Y.H. Pitavastatin and metformin synergistically activate apoptosis and autophagy in pancreatic cancer cells. Environ. Toxicol. 2021;36:1491–1503. doi: 10.1002/tox.23146. [DOI] [PubMed] [Google Scholar]
- 37.Chen K., Qian W., Jiang Z., Cheng L., Li J., Sun L., Zhou C., Gao L., Lei M., Yan B., et al. Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol. Cancer. 2017;16:131. doi: 10.1186/s12943-017-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pretta A., Ziranu P., Puzzoni M., Lai E., Orsi G., Liscia N., Molinaro E., Mariani S., Riggi L., Rovesti G., et al. Retrospective survival analysis in patients with metastatic pancreatic ductal adenocarcinoma with insulin-treated type 2 diabetes mellitus. Tumori. 2021;107:550–555. doi: 10.1177/0300891620976945. [DOI] [PubMed] [Google Scholar]
- 39.Pircher A., Zieher M., Eigentler A., Pichler R., Schäfer G., Fritz J., Puhr M., Steiner E., Horninger W., Klocker H., et al. Antidiabetic drugs influence molecular mechanisms in prostate cancer. Cancer Biol. Ther. 2018;19:1153–1161. doi: 10.1080/15384047.2018.1491490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winer L.K., Dhar V.K., Wima K., Morris M.C., Lee T.C., Shah S.A., Ahmad S.A., Patel S.H. The Impact of Tumor Location on Resection and Survival for Pancreatic Ductal Adenocarcinoma. J. Surg. Res. 2019;239:60–66. doi: 10.1016/j.jss.2019.01.061. [DOI] [PubMed] [Google Scholar]
- 41.Meng Z., Cao M., Zhang Y., Liu Z., Wu S., Wu H. Tumor location as an indicator of survival in T1 resectable pancreatic ductal adenocarcinoma: A propensity score-matched analysis. BMC Gastroenterol. 2019;19:59. doi: 10.1186/s12876-019-0975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sohn T.A., Yeo C.J., Cameron J.L., Koniaris L., Kaushal S., Abrams R.A., Sauter P.K., Coleman J., Hruban R.H., Lillemoe K.D. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J. Gastrointest. Surg. 2000;4:567–579. doi: 10.1016/S1091-255X(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 43.Tol J.A., Gouma D.J., Bassi C., Dervenis C., Montorsi M., Adham M., Andrén-Sandberg A., Asbun H.J., Bockhorn M., Büchler M.W., et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156:591–600. doi: 10.1016/j.surg.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roch A.M., Singh H., Turner A.P., Ceppa E.P., House M.G., Zyromski N.J., Nakeeb A., Schmidt C.M. Extended distal pancreatectomy for pancreatic adenocarcinoma with splenic vein thrombosis and/or adjacent organ invasion. Am. J. Surg. 2015;209:564–569. doi: 10.1016/j.amjsurg.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 45.van der Gaag N.A., Rauws E.A., van Eijck C.H., Bruno M.J., van der Harst E., Kubben F.J., Gerritsen J.J., Greve J.W., Gerhards M.F., de Hingh I.H., et al. Preoperative biliary drainage for cancer of the head of the pancreas. N. Engl. J. Med. 2010;362:129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 46.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang B., Zhou F., Hong J., Ng D.M., Yang T., Zhou X., Jin J., Zhou F., Chen P., Xu Y. The role of FOLFIRINOX in metastatic pancreatic cancer: A meta-analysis. World J. Surg. Oncol. 2021;19:182. doi: 10.1186/s12957-021-02291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freeman R. Diabetic autonomic neuropathy. Handb. Clin. Neurol. 2014;126:63–79. doi: 10.1016/b978-0-444-53480-4.00006-0. [DOI] [PubMed] [Google Scholar]
- 49.Sahin I.H., Shama M.A., Tanaka M., Abbruzzese J.L., Curley S.A., Hassan M., Li D. Association of diabetes and perineural invasion in pancreatic cancer. Cancer Med. 2012;1:357–362. doi: 10.1002/cam4.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jian L., Yang G. Identification of Key Genes Involved in Diabetic Peripheral Neuropathy Progression and Associated with Pancreatic Cancer. Diabetes Metab. Syndr. Obes. 2020;13:463–476. doi: 10.2147/DMSO.S235011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eibl G., Rozengurt E. Metformin: Review of epidemiology and mechanisms of action in pancreatic cancer. Cancer Metastasis Rev. 2021;40:865–878. doi: 10.1007/s10555-021-09977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardie D.G., Ross F.A., Hawley S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broadhurst P.J., Hart A.R. Metformin as an Adjunctive Therapy for Pancreatic Cancer: A Review of the Literature on Its Potential Therapeutic Use. Dig. Dis. Sci. 2018;63:2840–2852. doi: 10.1007/s10620-018-5233-y. [DOI] [PubMed] [Google Scholar]
- 54.De Souza A., Khawaja K.I., Masud F., Saif M.W. Metformin and pancreatic cancer: Is there a role? Cancer Chemother. Pharmacol. 2016;77:235–242. doi: 10.1007/s00280-015-2948-8. [DOI] [PubMed] [Google Scholar]
- 55.Duan W., Chen K., Jiang Z., Chen X., Sun L., Li J., Lei J., Xu Q., Ma J., Li X., et al. Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett. 2017;385:225–233. doi: 10.1016/j.canlet.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Ren D., Qin G., Zhao J., Sun Y., Zhang B., Li D., Wang B., Jin X., Wu H. Metformin activates the STING/IRF3/IFN-β pathway by inhibiting AKT phosphorylation in pancreatic cancer. Am. J. Cancer Res. 2020;10:2851–2864. [PMC free article] [PubMed] [Google Scholar]
- 57.Elinav E., Nowarski R., Thaiss C.A., Hu B., Jin C., Flavell R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 58.Ren B., Cui M., Yang G., Wang H., Feng M., You L., Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol. Cancer. 2018;17:108. doi: 10.1186/s12943-018-0858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arakawa Y., Miyazaki K., Yoshikawa M., Yamada S., Saito Y., Ikemoto T., Imura S., Morine Y., Shimada M. Value of the CRP-albumin ratio in patients with resectable pancreatic cancer. J. Med. Investig. 2021;68:244–255. doi: 10.2152/jmi.68.244. [DOI] [PubMed] [Google Scholar]
- 60.Fan Z., Fan K., Gong Y., Huang Q., Yang C., Cheng H., Jin K., Ni Q., Yu X., Luo G., et al. The CRP/Albumin Ratio Predicts Survival And Monitors Chemotherapeutic Effectiveness In Patients With Advanced Pancreatic Cancer. Cancer Manag. Res. 2019;11:8781–8788. doi: 10.2147/CMAR.S211363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z., Jin K., Guo M., Long J., Liu L., Liu C., Xu J., Ni Q., Luo G., Yu X. Prognostic Value of the CRP/Alb Ratio, a Novel Inflammation-Based Score in Pancreatic Cancer. Ann. Surg. Oncol. 2017;24:561–568. doi: 10.1245/s10434-016-5579-3. [DOI] [PubMed] [Google Scholar]
- 62.Fan Z., Luo G., Gong Y., Xu H., Qian Y., Deng S., Huang Q., Yang C., Cheng H., Jin K., et al. Prognostic Value of the C-Reactive Protein/Lymphocyte Ratio in Pancreatic Cancer. Ann. Surg. Oncol. 2020;27:4017–4025. doi: 10.1245/s10434-020-08301-3. [DOI] [PubMed] [Google Scholar]
- 63.Strijker M., van Veldhuisen E., van der Geest L.G., Busch O.R., Bijlsma M.F., Haj Mohammad N., Homs M.Y., van Hooft J.E., Verheij J., de Vos-Geelen J., et al. Readily available biomarkers predict poor survival in metastatic pancreatic cancer. Biomarkers. 2021;26:325–334. doi: 10.1080/1354750X.2021.1893814. [DOI] [PubMed] [Google Scholar]
- 64.Moses A.G., Maingay J., Sangster K., Fearon K.C., Ross J.A. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: Relationship to acute phase response and survival. Oncol. Rep. 2009;21:1091–1095. doi: 10.3892/or_00000328. [DOI] [PubMed] [Google Scholar]
- 65.Haas M., Heinemann V., Kullmann F., Laubender R.P., Klose C., Bruns C.J., Holdenrieder S., Modest D.P., Schulz C., Boeck S. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: Results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J. Cancer Res. Clin. Oncol. 2013;139:681–689. doi: 10.1007/s00432-012-1371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Padoan A., Plebani M., Basso D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019;20:676. doi: 10.3390/ijms20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 68.You L., Ning L., Xie W., Lang J., Huang Y. Metabolic syndrome and pancreatic cancer risk: A systematic review and meta-analysis. Oncotarget. 2018:5. doi: 10.18632/oncotarget.24209. [DOI] [Google Scholar]
- 69.Xia B., He Q., Pan Y., Gao F., Liu A., Tang Y., Chong C., Teoh A.Y.B., Li F., He Y., et al. Metabolic syndrome and risk of pancreatic cancer: A population-based prospective cohort study. Int. J. Cancer. 2020;147:3384–3393. doi: 10.1002/ijc.33172. [DOI] [PubMed] [Google Scholar]
- 70.Rosato V., Tavani A., Bosetti C., Pelucchi C., Talamini R., Polesel J., Serraino D., Negri E., La Vecchia C. Metabolic syndrome and pancreatic cancer risk: A case-control study in Italy and meta-analysis. Metabolism. 2011;60:1372–1378. doi: 10.1016/j.metabol.2011.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.