Abstract
Obstructive sleep apnea syndrome (OSAS) is one of the most common comorbidities in patients with Prader–Willi syndrome (PWS) and causes significant consequences. This observational study was conducted to investigate the progression of OSAS in pediatric patients with PWS, who had not undergone upper airway surgery, through a longitudinal follow-up of their annual polysomnography results. Annual body mass index (BMI), BMI z-score, sleep efficiency and stages, central apnea index (CAI), obstructive apnea–hypopnea index (OAHI), and oxygen saturation nadir values were longitudinally analyzed. At enrollment, of 22 patients (10 boys and 12 girls) aged 11.7 ± 3.9 years, 20 had OSAS. During the 4-year follow-up, only two patients had a spontaneous resolution of OSAS. The average BMI and BMI z-score increased gradually, but CAI and OAHI showed no significant differences. After statistical adjustment for sex, age, genotype, growth hormone use, and BMI z-score, OAHI was associated with the BMI z-score and deletion genotype. In conclusion, OSAS is common in patients with PWS, and rarely resolved spontaneously. Watchful waiting may not be the best OSAS management strategy. Weight maintenance and careful selection of surgical candidates are important for OSAS treatment in patients with PWS.
Keywords: Prader–Willi syndrome, obstructive sleep apnea syndrome, sleep-disordered breathing, polysomnography
1. Introduction
Prader–Willi syndrome (PWS) is a rare genetic disorder with an estimated prevalence of 1/10,000–1/30,000 individuals; the loss of function of the paternal chromosome 15q11-13 affects multiple systems [1]. Sleep-disordered breathing (SDB) is one of the most common comorbidities of PWS and causes significant consequences for patients with PWS [2,3]. SDB includes central apnea (CA) and obstructive sleep apnea (OSA). CA during sleep is a prominent feature in infants with PWS, and although it can gradually improve with maturation, it can also cause unexpected death [4,5,6]. Oxygen therapy may significantly reduce the CA index (CAI) in infants with PWS [5]. However, OSA frequently occurs in older children with PWS, and its risk factors include obesity and obesity-related narrowing of the upper airways, facial dysmorphism, increased viscosity of respiratory secretions, scoliosis, and hypotonia [2,3,7]. Furthermore, growth hormone (GH) therapy for PWS-related endocrinopathies may exacerbate obstructive sleep apnea syndrome (OSAS) [8,9].
Long-term OSAS may worsen patients’ behavior problems, cognitive deficits, and cardiovascular and endocrine disorders [10,11]. Treatment options include behavioral intervention, weight control, continuous positive airway pressure (CPAP), oral appliance therapy, and surgery such as adenotonsillectomy or other orthognathic surgeries [12,13]. Nevertheless, in patients with PWS, it is difficult to obtain complete resolution of sleep apnea through adenotonsillectomy alone. In some children with PWS, an increase in CA can even occur postoperatively [14,15]. Other orthognathic surgeries, including maxillomandibular advancement, are rarely performed in patients with PWS [16]. CPAP treatment improves sleep and quality of life in patients with severe OSAS, but side effects are frequently encountered [17]. Mask- or pressure-related discomfort, nasal congestion, headache, and bloating are common complaints among patients undergoing CPAP treatment [18]. Consequently, some patients refuse to undergo surgery or CPAP treatment after the diagnosis of OSAS. In the case of neurotypical children, some would have spontaneous resolution over time, and watchful waiting is an acceptable management for those children with mild or moderate OSAS [19]. However, there is a lack of data on the long-term progression of OSAS in patients with PWS that can help us determine an appropriate management strategy. Therefore, we conducted this study to evaluate the progression of OSAS using consecutive polysomnography (PSG) data obtained from pediatric patients with PWS in our institution.
2. Materials and Methods
2.1. Patients
In our institution, patients with PWS were annually screened for OSAS using PSG. We retrospectively reviewed their PSG reports from 2009 to 2017 and included 48 patients. All patients were diagnosed after one or more of the following tests: PWS-specific methylation-specific polymerase chain reaction, fluorescence in situ hybridization analysis, and microsatellite studies [20]. Among the 48 patients, 2 received CPAP treatment, 1 received adenotonsillectomy, and 23 had only 1- or 2-year longitudinal data. Therefore, 22 patients (84 PSG studies) who underwent at least 3 years of follow-up were included in this study. Among them, 18 patients were treated with GH, according to the protocol of de Lind van et al. [21]. This study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of Taipei Tzu Chi General Hospital (07-XD02-003, approved at 12 March 2018). Written informed consent was waived because the study was a retrospective data analysis.
2.2. Overnight PSG and Sleep Variables
The annual overnight PSG for all patients was conducted at Taipei Tzu Chi Hospital. The PSG study included an electroencephalogram (F3, F4, C3, C4, O1, O2), electrooculogram, chin and leg electromyogram, and electrocardiography (modified V2 lead). Respiration was measured using nasal thermistors and respiratory inductance plethysmography belts on the chest and upper abdominal walls. Oxyhemoglobin saturation was measured using pulse oximetry. Data in each sleep stage were manually rated according to the guidelines of the American Academy of Sleep Medicine on the basis of 30-s epochs [22]. OSAS and hypopnea were defined as a near absence (≥90%) or a baseline decrease (≥30%) in the amplitude of ventilation for at least two breaths as measured by nasal thermistors and associated with 4% oxygen desaturation. Baseline was defined as the mean amplitude of stable breathing and oxygenation in 2 min preceding the event onset (in individuals with a stable breathing pattern during sleep) or that of the three largest breaths in 2 min preceding the event onset (in individuals without a stable breathing pattern). CA was defined as an absence of nasal airflow for ≥10 s without respiratory effort or shorter events associated with arousal, desaturation (≥3%), or bradycardia (≤60 beats per min) [22]. CAI was defined as the mean number of central apneic episodes per hour of sleep. The obstructive apnea–hypopnea index (OAHI) indicated the mean number of obstructive hypopneic and apneic episodes per hour of sleep. OSAS categories were defined according to OAHI (no OSAS, OAHI < 1; mild OSAS, OAHI ≥1–<5; moderate OSAS, OAHI ≥5–<10; and severe OSAS, OAHI ≥ 10) [23].
2.3. Normalized Body Mass Index (BMI) Z-Score
To adjust for age and sex, we converted patients’ BMI values into z-scores using age- and sex-specific BMI norms for Taiwan [24]. Each z-score was calculated by subtracting a patient’s BMI from the mean BMI for the patient’s age group and dividing the difference by the standard deviation (SD) of BMI for that age group. Overweight was defined as a BMI z-score of >1, and obesity was defined as a BMI z-score of >2.
2.4. Statistical Analysis
Statistical analyses were conducted using SPSS version 19.0 for Windows (IBM, Chicago, IL, USA). Descriptive data are presented as the mean ± SD. Repeated measures analysis of variance (ANOVA) was used to compare the annual CAI, OAHI, BMI, and BMI z-scores of individual patients. The Mann–Whitney U test and Spearman’s correlation were used to evaluate the relationships between OAHI, BMI, and BMI z-scores in the first year of enrollment. Repeated measures linear regression (an exchangeable working within-subject correlation model by generalized estimating equation (GEE)) was used to compute the average rates of progression of OAHI in male and female patients with PWS after adjusting for age and BMI z-scores. The longitudinal progressions of OSAS severity in patients with PWS during the 4-year follow-up period were evaluated by entering the interaction terms (gender × PSG time) into the GEE models. The coefficients of the interaction terms reflected how the rate of progression of OAHI differed according to gender among the patients. Two-sided p values of <0.05 were considered to be statistically significant.
3. Results
The demographic data and summary of polysomnographic variables during the longitudinal 4-year follow-up of patients with PWS are shown in Table 1. In total, 10 boys and 12 girls with PWS were enrolled in this study, and they underwent three or four consecutive PSG studies annually. The mean age at enrollment was 11.7 ± 3.9 years (boys, 13.3 ± 4.2 years; girls, 10.4 ± 3.3 years; p = 0.146). In this cohort, 17 patients had a paternal deletion of 15q11-13, and 5 patients were of the nondeletion type. At enrollment, their mean BMI was 22.8 ± 4.5 kg/m2, and the mean BMI z-score was 1.7 ± 1.3. Of the 22 patients, 15 (8 boys and 7 girls) were overweight or obese. The BMI z-score of both sexes was similar (boys, 2.2 ± 1.4; girls, 1.3 ± 1.0; p = 0.187). Of the 22 patients, 18 were treated with GH. Adrenal function had been tested in 20 patients and the results were all normal, including 16 patients with a peak cortisol greater than 500 nmol/liter in the insulin hypoglycemia test, and 4 patients with basal cortisol levels more than 280 nmol/liter. All 22 patients had normal thyroid function on annual follow-up. Regarding the PSG parameters, the CAI of the first PSG was 0.3 ± 0.5 per hour. The OAHI at the first year of enrollment was 5.7 ± 6.4 per hour (boys, 3.7 ± 2.6, girls, 7.4 ± 8.2; p = 0.19). Of the 22 patients, 20 (90.9%) had OSAS at enrollment, including 8 (36.4%) patients with mild, 11 (50%) patients with moderate, and 1 (4.5%) patient with severe OSAS. We found no correlation between BMI and OAHI (p = 0.93, Spearman’s correlation) or between BMI z-score and OAHI (p = 0.48, Spearman’s correlation).
Table 1.
Demographic data and summary of the polysomnographic variables during longitudinal follow-up of patients with PWS for 4 years.
| 1st PSG | 2nd PSG | 3rd PSG | 4th PSG | |
|---|---|---|---|---|
| N | 22 | 22 | 22 | 18 |
| Age (years) | 11.7 ± 3.9 | 13.1 ± 4.1 | 14.4 ± 4.3 | 15.8 ± 4.5 |
| Female (%) | 12 (54.5) | 12 (54.5) | 12 (54.5) | 10 (55.6) |
| Del/non-Del | 17/5 | 17/5 | 17/5 | 15/3 |
| BMI | 22.8 ± 4.5 | 23.1 ± 4.1 | 23.8 ± 4.5 | 25.9 ± 6.4 |
| BMI z-score | 1.7 ± 1.3 | 1.6 ± 1.3 | 1.7 ± 1.5 | 2.3 ± 2.2 |
| GH Tx (%) | 18 (81.8) | 18 (81.8) | 18 (81.8) | 16 (88.9) |
| Total sleep time (m) | 414.6 ± 69.4 | 421.4 ± 52.2 | 393.0 ± 100.2 | 388.7 ± 73.6 |
| Sleep efficiency (%) | 87.4 ± 6.5 | 83.9 ± 8.3 | 79.8 ± 18.0 | 81.0 ± 12.2 |
| Awake (%) | 10.2 ± 6.1 | 12.5 ± 8.3 | 18.9 ± 16.7 | 17.6 ± 11.0 |
| Stage 1 (%) | 8.3 ± 4.2 | 7.6 ± 4.0 | 8.0 ± 5.5 | 9.5 ± 6.1 |
| Stage 2 (%) | 47.0 ± 11.8 | 45.1 ± 12.5 | 45.4 ± 16.5 | 46.6 ± 9.4 |
| SWS (%) | 23.2 ± 11.7 | 26.4 ± 11.6 | 24.6 ± 12.0 | 22.3 ± 10.3 |
| REM (%) | 20.4 ± 7.1 | 20.8 ± 5.9 | 21.9 ± 6.5 | 21.5 ± 4.8 |
| CAI (/h) | 0.3 ± 0.5 | 0.1 ± 0.2 | 0.0 ± 0.1 | 0.1 ± 0.1 |
| OAHI (/h) | 5.7 ± 6.4 | 6.5 ± 6.8 | 5.0 ± 3.5 | 6.4 ± 5.7 |
| Oxygen concentration nadir (%) | 83.1 ± 6.8 | 83.3 ± 6.5 | 82.8 ± 5.2 | 81.4 ± 9.8 |
| Arousal index (/h) | 12.5 ± 9.7 | 10.7 ± 7.6 | 8.0 ± 4.3 | 8.8 ± 6.1 |
| No OSAS (%) | 2 (9.1) | 1 (4.5) | 2 (9.1) | 1 (5.6) |
| Mild OSAS (%) | 8 (36.4) | 10 (45.5) | 10 (45.5) | 7 (38.9) |
| Moderate OSAS (%) | 11 (50) | 9 (40.9) | 8 (36.4) | 7 (38.9) |
| Severe OSAS (%) | 1 (4.5) | 2 (9.1) | 2 (9.1) | 3 (16.7) |
Values are expressed as the mean ± SD; Abbreviations: BMI, body mass index; CAI, central apnea index; Del, deletion; GH Tx, growth hormone treatment; OAHI, obstructive apnea–hypopnea index; OSAS, obstructive sleep apnea syndrome; PSG, polysomnography; PWS, Prader–Willi syndrome; REM, rapid eye movement; SWS, slow-wave sleep.
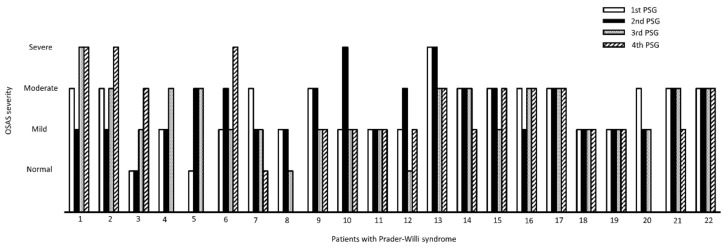
During the consecutive 4-year follow-up, the OSAS severity showed a constant distribution. The last PSG follow-up revealed that 5.6% of patients had no OSAS, 38.9% had mild OSAS, 38.9% had moderate OSAS, and 16.7% had severe OSAS (Table 1). The OSAS severity of the 22 patients during the 4-year follow-up is illustrated in Figure 1. Based on the data from the last follow-up PSG, one patient with moderate OSAS (patient 7 in Figure 1) and one with mild OSAS (patient 8 in Figure 1) had a normalized OAHI score, indicating that OSAS barely resolved spontaneously in patients with PWS. These two female patients were aged 8 and 20 years at enrollment, respectively. The BMI z-score decreased from 3.4 to 2.1 in patient 7 and from 1.5 to 1.1 in patient 8. By contrast, two patients were initially normal (patient 3 and patient 5 in Figure 1) and developed OSAS during the longitudinal follow-up. Patient 3 was an 18-year-old male patient whose OAHI was 0.7/h at enrollment. During the three-year follow-up, the OAHI increased to 11.3/h. He did not receive GH therapy, and his BMI z-score increased from 2.9 to 3.8 in three years. Patient 5 was an 8-year-old boy whose OAHI increased from 0.1 to 9.7/h in three years. Although he received GH therapy, his BMI z-score still increased from 3.1 to 4.3 during the longitudinal follow-up. Accordingly, we presumed that obesity is an important risk factor for OSAS in patients with PWS.
Figure 1.
The illustration of OSAS severity in patients of Prader–Willi syndrome. OSAS severity varied in some patients, but it rarely resolved spontaneously during 4-year longitudinal follow-up.
However, the overall CAI (F = 1.09, p = 0.34, repeated measures ANOVA) and OAHI (F = 0.60, p = 0.52, repeated measures ANOVA) of patients with PWS did not differ significantly across the study period, despite that there was an increase in the obesity of patients with PWS annually. The mean BMI increased from 22.8 to 25.9 kg/m2 (F = 9.84; p = 0.002, repeated measures ANOVA), and the mean BMI z-score increased from 1.7 to 2.3 (F = 4.42; p = 0.03, repeated measures ANOVA). Because weight gain is a well-known risk factor for OSAS aggravation [25], there must be factors other than obesity affecting the progression of OSAS in patients with PWS.
Therefore, we applied a GEE model that adjusted for the patients’ variables, including BMI z-score, age, sex, PSG time, genotype, GH treatment, BMI z-score × PSG time, and genotype × PSG time, to evaluate the risk factors for OSAS progression in the consecutive 4-year follow-up (Table 2). The linear regression revealed that OAHI correlated positively with the BMI z-score and negatively with the non-Del genotype, significantly so. The regression coefficients of the BMI z-score and non-Del genotype were 4.88 (Wald χ2 = 16.85, p < 0.001) and −3.73 (Wald χ2 = 4.09, p = 0.043), respectively (Table 2). In this GEE model, PSG time, BMI z-score × PSG time, and genotype × PSG time revealed no significant regression coefficients with OAHI, indicating that the time domain (PSG time) in this study had no effects on OAHI changes, which further validated the conclusion that OSAS in patients with PWS rarely resolved spontaneously over time.
Table 2.
Regression coefficients of OAHI in males and females with PWS according to the GEE model.
| Parameter | Estimate | Wald χ2 | p-Value |
|---|---|---|---|
| Intercept | −7.602 | 0.829 | 0.363 |
| Sex a | 2.294 | 0.282 | 0.595 |
| Age | 0.116 | 0.062 | 0.803 |
| BMI z-score | 4.883 | 16.852 | <0.001 ** |
| PSG Time | 0.259 | 0.101 | 0.751 |
| Genotype b | −3.728 | 4.086 | 0.043 * |
| GH Tx c | 4.886 | 1.511 | 0.219 |
| BMI z-score × PSG Time | −0.476 | 3.585 | 0.058 |
| Genotype × PSG Time | 0.478 | 0.158 | 0.691 |
a male = 0, females = 1; b deletion = 0, non-deletion = 1; c not using growth hormone = 0, using growth hormone = 1, * p < 0.05, ** p < 0.01. Abbreviations: BMI, body mass index; GEE, generalized estimating equation; GH Tx, growth hormone treatment; OAHI, obstructive apnea–hypopnea index; PWS, Prader–Willi syndrome; PSG, polysomnography.
4. Discussion
We investigated the progression of OSAS in patients with PWS who had not undergone adenotonsillectomy. The prevalence of OSAS was high in these patients, and only a few patients showed spontaneous resolution during the 4-year PSG follow-up. After multivariate linear analysis, obesity and deletion genotype were identified as the risk factors for high OAHI.
PWS is a multisystem disorder caused by the loss of function of multiple genes from the paternal chromosome 15q11-13. Affected infants present with marked hypotonia since birth, which causes feeding difficulties and failure to thrive. The majority of patients exhibit delayed motor and language milestones, as well as intellectual disability [26]. SDB, including CA and OSAS, was frequently observed in patients with PWS. Studies have reported that infants and toddlers with PWS had irregular breathing and frequent CA, which were possibly associated with necdin deficiency, a universal character of PWS, resulting in abnormal central chemoreceptor sensitivity [27,28]. As a toddler, an excessive appetite develops, and the patients gradually become obese. Furthermore, facial dysmorphism, including micrognathia and small oropharynx, sticky secretions, and hypotonia, have been identified as risk factors for OSAS in patients with PWS [2]. Consistent with previous studies, we also detected a high prevalence of OSAS in patients with PWS in our study (90.9% at the time of enrollment). To our knowledge, this is the first study to provide longitudinal PSG data of patients without surgical or CPAP intervention, which is important to understand the natural course of OSAS in patients with PWS. During the consecutive 4-year follow-up, only two patients (9.1%) showed spontaneous resolution of OSAS. In contrast, for neurotypical children, it has been reported that 65% of patients with mild or moderate OSAS and 26% of those with severe OSAS underwent spontaneous resolution of OSAS [19]. Our result indicated that watchful waiting is possibly not the best strategy to manage OSAS in patients with PWS, and it is necessary to consider aggressive interventions, including adenotonsillectomy, CPAP, and oral appliance therapy, earlier in this special group of patients.
SDB would result in multiple neurocognitive and cardiovascular consequences, including deficits in general intelligence, mental flexibility, working memory, and heart ventricular functions [12]. In patients with PWS, SDB even contributes to increased mortality [29]. Nevertheless, studies have reported that patients with PWS undergoing adenotonsillectomy, the first-line treatment for OSAS in neurotypical children [12], have increased risk of developing postoperative complications and residual OSA [30,31]. As adenotonsillectomy is effective in some patients with PWS and OSAS, careful selection of appropriate surgical candidates is crucial. Drug-induced sedation endoscopy (DISE) is considered as a valuable tool when selecting patients with OSAS for adenotonsillectomy [32]. We have earlier reported the DISE findings of nine patients with PWS and OSAS, wherein multilevel obstruction was found in six patients (66.7%), and patients with partial or complete anterior–posterior tongue base collapse had a significantly higher AHI [33]. These findings may partially explain the inadequate efficacy of adenotonsillectomy in these patients because the mere removal of adenoids and tonsils would not resolve a multilevel airway obstruction and suggests that a procedure such as DISE, which allows for a better evaluation of factors contributing to OSAS, provides a better method for OSAS management.
In our study, obesity and deletion genotype were the two risk factors for OSAS in patients with PWS. In neurotypical children, obesity is a proved risk factor for OSAS [34,35]. Compared with nonobese children with OSAS, obese children also have a higher risk of postoperative residual OSA [36], whereas weight reduction significantly improves OSAS in obese children or teenagers, indicating that tonsil enlargement is not the major origin of OSAS in obese patients [37]. In our study, OAHI at enrollment showed no positive correlation with the BMI or BMI z-score of each patient. However, during the longitudinal follow-up, patients with higher BMI z-scores showed a higher risk of developing OSAS. In patients with PWS, progressive obesity was found after 30 months of age [38], and GH treatment has been demonstrated to stabilize the BMI increment [38,39]. Although most of our study patients were treated with GH, progressive obesity was observed in some patients. Close monitoring of food intake and adequate exercise are important for weight maintenance in patients with PWS [40] and are beneficial in the control of OSAS. Although no patients with PWS in this study had hypothyroidism or adrenal insufficiency, from the literature, 4–24% of patients of PWS would be comorbid with hypothyroidism [41,42], and 4.8–60% of patients with adrenal insufficiency [43,44]. Either of these endocrine dysfunctions would affect metabolism and has a strong impact on weight gain and obesity. Since our data suggested that a higher BMI z-score during longitudinal follow-up was associated with a higher risk of OSAS, we recommended regular thyroid and adrenal function evaluation, and if necessary, hormone replacement therapy for controlling or even lowering BMI.
In our cohort, patients with the nondeletion genotype had a lower OAHI. Previous studies revealed several phenotype differences in the nondeletion genotype of PWS, including less hypopigmentation [45] and increased incidence of psychosis [46]; however, different severities of SDB in nondeletion PWS were never reported. Because only five patients with nondeletion PWS were included in our cohort, we cannot make a definite conclusion that we can have different strategies to treat SDB in patients with deletion or nondeletion PWS, and hence a larger-scale study is required.
This study has several limitations. First, our study had the inherent weakness of a retrospective design and a relatively small sample size. Craniofacial structures, such as tonsillar size, muscle tone, and allergic rhinitis, are important confounding factors for OSA [47], which were not evaluated in this study due to the retrospective design. Larger, prospectively controlled studies are warranted to further evaluate the progression of OSA in patients with PWS. Second, in our sleep center, we used a thermistor, not a nasal pressure transducer, to detect air flow. This might lead to an underestimation of the severity of OSA. Third, we lacked information regarding standardized OSAS symptom scores, which revealed a high discrepancy to OAHI changes [48]. Despite these limitations, to the best of our knowledge, this is the first study to explore the progression of OSAS for up to 4 years in patients with PWS. We have provided evidence that natural resolution of OSAS in these patients is rare and watchful waiting may not be the best strategy.
In conclusion, SDB is common in patients with PWS. Natural resolution of OSAS was rare during the longitudinal follow-up, which suggests that watchful waiting may not be the best strategy. Patients with PWS with obesity and those with the deletion genotype tended to have more significant OSAS. Weight maintenance and careful selection of surgical candidates are important for OSAS treatment in patients with PWS.
Author Contributions
Conceptualization, S.-B.W. and L.-P.T.; methodology, S.-B.W. and M.-C.Y.; validation, I.-S.T.; formal analysis, S.-B.W.; resources, S.-B.W., M.-C.Y., C.-C.L., W.-H.T. and L.-P.T.; data curation, S.-B.W.; writing—original draft preparation, S.-B.W. and L.-P.T.; writing—review and editing, S.-B.W. and L.-P.T.; project administration, L.-P.T.; funding acquisition, S.-B.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of Taipei Tzu Chi General Hospital (07-XD02-003).
Informed Consent Statement
Individual written informed consent was waived because of a retrospective data analysis.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, L.-P.T. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by a grant from Taipei Tzu Chi General Hospital, TCRD-TPE-110-23, to Shi-Bing Wong.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cassidy S.B., Schwartz S., Miller J.L., Driscoll D.J. Prader–Willi syndrome. Genet. Med. 2012;14:10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 2.Nixon G.M., Brouillette R.T. Sleep and breathing in Prader–Willi syndrome. Pediatr. Pulmonol. 2002;34:209–217. doi: 10.1002/ppul.10152. [DOI] [PubMed] [Google Scholar]
- 3.O’Donoghue F.J., Camfferman D., Kennedy J.D., Martin A.J., Couper T., Lack L.D., Lushington K., McEvoy R.D. Sleep-disordered breathing in Prader–Willi syndrome and its association with neurobehavioral abnormalities. J. Pediatr. 2005;147:823–829. doi: 10.1016/j.jpeds.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Nagai T., Obata K., Tonoki H., Temma S., Murakami N., Katada Y., Yoshino A., Sakazume S., Takahashi E., Sakuta R., et al. Cause of sudden, unexpected death of Prader–Willi syndrome patients with or without growth hormone treatment. Am. J. Med. Genet. A. 2005;136:45–48. doi: 10.1002/ajmg.a.30777. [DOI] [PubMed] [Google Scholar]
- 5.Urquhart D.S., Gulliver T., Williams G., Harris M.A., Nyunt O., Suresh S. Central sleep-disordered breathing and the effects of oxygen therapy in infants with Prader–Willi syndrome. Arch. Dis. Child. 2013;98:592–595. doi: 10.1136/archdischild-2012-303441. [DOI] [PubMed] [Google Scholar]
- 6.Khayat A., Narang I., Bin-Hasan S., Amin R., Al-Saleh S. Longitudinal evaluation of sleep disordered breathing in infants with Prader–Willi syndrome. Arch. Dis. Child. 2017;102:634–638. doi: 10.1136/archdischild-2016-311298. [DOI] [PubMed] [Google Scholar]
- 7.Sedky K., Bennett D.S., Pumariega A. Prader Willi syndrome and obstructive sleep apnea: Co-occurrence in the pediatric population. J. Clin. Sleep Med. 2014;10:403–409. doi: 10.5664/jcsm.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nixon G.M., Rodda C.P., Davey M.J. Longitudinal association between growth hormone therapy and obstructive sleep apnea in a child with Prader–Willi syndrome. J. Clin. Endocrinol. Metab. 2011;96:29–33. doi: 10.1210/jc.2010-1445. [DOI] [PubMed] [Google Scholar]
- 9.Al-Saleh S., Al-Naimi A., Hamilton J., Zweerink A., Iaboni A., Narang I. Longitudinal evaluation of sleep-disordered breathing in children with Prader–Willi syndrome during 2 years of growth hormone therapy. J. Pediatr. 2013;162:263–268.e1. doi: 10.1016/j.jpeds.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y.S., Guilleminault C., Li H.Y., Yang C.M., Wu Y.Y., Chen N.H. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: A treatment outcome study. Sleep Med. 2007;8:18–30. doi: 10.1016/j.sleep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Xanthopoulos M.S., Gallagher P.R., Berkowitz R.I., Radcliffe J., Bradford R., Marcus C.L. Neurobehavioral functioning in adolescents with and without obesity and obstructive sleep apnea. Sleep. 2015;38:401–410. doi: 10.5665/sleep.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus C.L., Brooks L.J., Ward S.D., Draper K.A., Gozal D., Halbower A.C., Jones J., Lehmann C., Schechter M.S., Sheldon S., et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 13.Camacho M., Certal V., Capasso R. Comprehensive review of surgeries for obstructive sleep apnea syndrome. Braz. J. Otorhinolaryngol. 2013;79:780–788. doi: 10.5935/1808-8694.20130139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMarcantonio M.A., Darrow D.H., Gyuricsko E., Derkay C.S. Obstructive sleep disorders in Prader–Willi syndrome: The role of surgery and growth hormone. Int. J. Pediatr. Otorhinolaryngol. 2010;74:1270–1272. doi: 10.1016/j.ijporl.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Meyer S.L., Splaingard M., Repaske D.R., Zipf W., Atkins J., Jatana K. OUtcomes of adenotonsillectomy in patients with Prader–Willi syndrome. Arch. Otolaryngol. Head Neck Surg. 2012;138:1047–1051. doi: 10.1001/2013.jamaoto.64. [DOI] [PubMed] [Google Scholar]
- 16.Xiao K.K., Tomur S., Beckerman R., Cassidy K., Lypka M. Orthognathic Correction in Prader-Willi Syndrome: Occlusion and Sleep Restored. Cleft Palate Craniofac. J. 2019;56:415–418. doi: 10.1177/1055665618775724. [DOI] [PubMed] [Google Scholar]
- 17.Kushida C.A., Nichols D.A., Holmes T.H., Quan S.F., Walsh J.K., Gottlieb D.J., Simon R.D., Guilleminault C., White D.P., Goodwin J.L., et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulander M., Johansson M.S., Ewaldh A.E., Svanborg E., Broström A. Side effects to continuous positive airway pressure treatment for obstructive sleep apnoea: Changes over time and association to adherence. Sleep Breath. 2014;18:799–807. doi: 10.1007/s11325-014-0945-5. [DOI] [PubMed] [Google Scholar]
- 19.Marcus C.L., Moore R.H., Rosen C.L., Giordani B., Garetz S.L., Taylor H.G., Mitchell R.B., Amin R., Katz E.S., Arens R., et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N. Engl. J. Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H.Y., Lin S.P., Yen J.L., Lee Y.J., Huang C.Y., Hung H.Y., Hsu C., Kao H., Chang J., Chiu N., et al. Prader–Willi syndrome in Taiwan. Pediatr. Int. 2007;49:375–379. doi: 10.1111/j.1442-200X.2007.02368.x. [DOI] [PubMed] [Google Scholar]
- 21.De Lind van Wijngaarden R.F.A., Siemensma E.P.C., Festen D.A.M., Otten B.J., van Mil E.G.A.H., Rotteveel J., Odink R.J.H., Bindels-de Heus G.C.B., Van Leeuwen M., Haring D.A.J.P., et al. Efficacy and safety of long-term continuous growth hormone treatment in children with Prader–Willi syndrome. J. Clin. Endocrinol. Metab. 2009;94:4205–4215. doi: 10.1210/jc.2009-0454. [DOI] [PubMed] [Google Scholar]
- 22.Iber C., American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; Westchester, NY, USA: 2007. [Google Scholar]
- 23.Sheldon S.H., Kryger M.H., Ferber R., Gozal D. Principles and Practice of Pediatric Sleep Medicine E-Book. Elsevier Health Sciences; Philadelphia, PA, USA: 2014. [Google Scholar]
- 24.Chen W., Chang M.-H. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr. Neonatol. 2010;51:69–79. doi: 10.1016/S1875-9572(10)60014-9. [DOI] [PubMed] [Google Scholar]
- 25.Peppard P.E., Young T., Palta M., Dempsey J., Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 26.Wang T.S., Tsai W.H., Tsai L.P., Wong S.B. Clinical characteristics and epilepsy in genomic imprinting disorders: Angelman syndrome and Prader–Willi syndrome. Tzu Chi Med. J. 2020;32:137–144. doi: 10.4103/tcmj.tcmj_103_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanella S., Barthelemy M., Muscatelli F., Hilaire G. Necdin gene, Respiratory disturbances and Prader–Willi syndrome. In: Poulin M., Wilson R.A., editors. Advances in Experimental Medicine & Biology. Volume 605. Integration in Respiratory Control; Springer; New York, NY, USA: 2008. pp. 159–164. [DOI] [PubMed] [Google Scholar]
- 28.Wu R.N., Hung W.C., Chen C.T., Tsai L.P., Lai W.S., Min M.Y., Wong S.B. Firing activity of locus coeruleus noradrenergic neurons decreases in necdin-deficient mice, an animal model of Prader–Willi syndrome. J. Neurodev. Disord. 2020;12:21. doi: 10.1186/s11689-020-09323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proffitt J., Osann K., McManus B., Kimonis V.E., Heinemann J., Butler M.G., Stevenson D.A., Gold J.-A. Contributing factors of mortality in Prader–Willi syndrome. Am. J. Med. Genet. A. 2019;179:196–205. doi: 10.1002/ajmg.a.60688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C.H., Hsu W.C., Ko J.Y., Yeh T.H., Lin M.T., Kang K.T. Adenotonsillectomy for the treatment of obstructive sleep apnea in children with Prader–Willi syndrome: A meta-analysis. Otolaryngol. Head Neck Surg. 2020;162:168–176. doi: 10.1177/0194599819893115. [DOI] [PubMed] [Google Scholar]
- 31.Clements A.C., Dai X., Walsh J.M., Sterni L.M., Prichett L., Boss E.F., Seal S.M., Ryan M.A. Outcomes of adenotonsillectomy for obstructive sleep apnea in Prader–Willi syndrome: Systematic review and meta-analysis. Laryngoscope. 2021;131:898–906. doi: 10.1002/lary.28922. [DOI] [PubMed] [Google Scholar]
- 32.Boudewyns A., Verhulst S., Maris M., Saldien V., Van de Heyning P. Drug-induced sedation endoscopy in pediatric obstructive sleep apnea syndrome. Sleep Med. 2014;15:1526–1531. doi: 10.1016/j.sleep.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Lan M.C., Hsu Y.B., Lan M.Y., Chiu T.J., Huang T.T., Wong S.B., Chen Y.C., Tsai L.P. Drug-induced sleep endoscopy in children with Prader–Willi syndrome. Sleep Breath. 2016;20:1029–1034. doi: 10.1007/s11325-016-1338-8. [DOI] [PubMed] [Google Scholar]
- 34.Alonso-Álvarez M.L., Cordero-Guevara J.A., Terán-Santos J., Gonzalez-Martinez M., Jurado-Luque M.J., Corral-Peñafiel J., Duran-Cantolla J., Kheirandish-Gozal L., Gozal D. Obstructive sleep apnea in obese community-dwelling children: The NANOS study. Sleep. 2014;37:943–949. doi: 10.5665/sleep.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su M.S., Zhang H.L., Cai X.H., Lin Y., Liu P.N., Zhang Y.B., Hu W.Z., Li C.C., Xiao Y.F. Obesity in children with different risk factors for obstructive sleep apnea: A community-based study. Eur. J. Pediatr. 2016;175:211–220. doi: 10.1007/s00431-015-2613-6. [DOI] [PubMed] [Google Scholar]
- 36.Alonso-Álvarez M.L., Terán-Santos J., Navazo-Egüia A.I., Martinez M.G., Jurado-Luque M.J., Corral-Peñafiel J., Duran-Cantolla J., Cordero-Guevara J.A., Kheirandish-Gozal L., Gozal D. Treatment outcomes of obstructive sleep apnoea in obese community-dwelling children: The NANOS study. Eur. Respir. J. 2015;46:717–727. doi: 10.1183/09031936.00013815. [DOI] [PubMed] [Google Scholar]
- 37.Verhulst S.L., Franckx H., Van Gaal L., De Backer W., Desager K. The effect of weight loss on sleep-disordered breathing in obese teenagers. Obesity. 2009;17:1178–1183. doi: 10.1038/oby.2008.673. [DOI] [PubMed] [Google Scholar]
- 38.Butler J.V., Whittington J.E., Holland A.J., McAllister C.J., Goldstone A.P. The transition between the phenotypes of Prader–Willi syndrome during infancy and early childhood. Dev. Med. Child. Neurol. 2010;52:e88–e93. doi: 10.1111/j.1469-8749.2009.03530.x. [DOI] [PubMed] [Google Scholar]
- 39.Bakker N.E., Kuppens R.J., Siemensma E.P.C., Tummers-de Lind van Wijngaarden R.F.A., Festen D.A.M., Bindels-de Heus G.C.B., Bocca G., Haring D.A.J.P., Hoorweg-Nijman J.J.G., Houdijk E.C.A.M., et al. Eight years of growth hormone treatment in children with Prader–Willi syndrome: Maintaining the positive effects. J. Clin. Endocrinol. Metab. 2013;98:4013–4022. doi: 10.1210/jc.2013-2012. [DOI] [PubMed] [Google Scholar]
- 40.Cassidy S.B., Driscoll D.J. Prader–Willi syndrome. Eur. J. Hum. Genet. 2009;17:3–13. doi: 10.1038/ejhg.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharkia M., Michaud S., Berthier M.T., Giguère Y., Stewart L., Deladoëy J., Deal C., Van Vliet G., Chanoine J.P. Thyroid function from birth to adolescence in Prader-Willi syndrome. J. Pediatr. 2013;163:800–805. doi: 10.1016/j.jpeds.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 42.Diene G., Mimoun E., Feigerlova E., Caula S., Molinas C., Grandjean H., Tauber M. Endocrine disorders in children with Prader-Willi syndrome--data from 142 children of the French database. Horm. Res. Paediatr. 2010;74:121–128. doi: 10.1159/000313377. [DOI] [PubMed] [Google Scholar]
- 43.Corrias A., Grugni G., Crinò A., Di Candia S., Chiabotto P., Cogliardi A., Chiumello G., De Medici C., Spera S., Gargantini L., et al. Assessment of central adrenal insufficiency in children and adolescents with Prader-Willi syndrome. Clin. Endocrinol. 2012;76:843–850. doi: 10.1111/j.1365-2265.2011.04313.x. [DOI] [PubMed] [Google Scholar]
- 44.De Lind van Wijngaarden R.F., Otten B.J., Festen D.A., Joosten K.F., de Jong F.H., Sweep F.C., Hokken-Koelega A. High prevalence of central adrenal insufficiency in patients with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2008;93:1649–1654. doi: 10.1210/jc.2007-2294. [DOI] [PubMed] [Google Scholar]
- 45.Gillessen-Kaesbach G., Robinson W., Lohmann D., Kaya-Westerloh S., Passarge E., Horsthemke B. Genotype-phenotype correlation in a series of 167 deletion and non-deletion patients with Prader–Willi syndrome. Hum. Genet. 1995;96:638–643. doi: 10.1007/BF00210291. [DOI] [PubMed] [Google Scholar]
- 46.Aman L.C.S., Manning K.E., Whittington J.E., Holland A.J. Mechanistic insights into the genetics of affective psychosis from Prader–Willi syndrome. Lancet Psychiatry. 2018;5:370–378. doi: 10.1016/S2215-0366(18)30009-9. [DOI] [PubMed] [Google Scholar]
- 47.Kaditis A.G., Alonso Alvarez M.L., Boudewyns A., Alexopoulos E.I., Ersu R., Joosten K., Larramona H., Miano S., Narang I., Trang H., et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2016;47:69–94. doi: 10.1183/13993003.00385-2015. [DOI] [PubMed] [Google Scholar]
- 48.Chervin R.D., Ellenberg S.S., Hou X., Marcus C.L., Garetz S.L., Katz E.S., Hodges E.K., Mitchell R.B., Jones D.T., Arens R., et al. PRognosis for spontaneous resolution of osa in children. Chest. 2015;148:1204–1213. doi: 10.1378/chest.14-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, L.-P.T. The data are not publicly available due to their containing information that could compromise the privacy of research participants.