Summary
Immune checkpoint blockade (ICB) therapy frequently induces immune related adverse events. To elucidate the underlying immunobiology, we performed a deep immune analysis of intestinal, colitis, and tumor tissue from ICB-treated patients with parallel studies in preclinical models. Expression of interleukin-6 (IL-6), neutrophil and chemotactic markers was higher in colitis than in normal intestinal tissue; T helper 17 (Th17) cells were more prevalent in immune-related enterocolitis (irEC) than T helper 1 (Th1). Anti-cytotoxic T-lymphocyte-associated antigen 4 (anti-CTLA-4) induced stronger Th17 memory in colitis than anti-program death 1 (anti-PD-1). In murine models, IL-6 blockade associated with improved tumor control and a higher density of CD4+/CD8+ effector T cells, with reduced Th17, macrophages, and myeloid cells. In an experimental autoimmune encephalomyelitis (EAE) model with tumors, combined IL-6 blockade and ICB enhanced tumor rejection while simultaneously mitigating EAE symptoms versus ICB alone. IL-6 blockade with ICB could de-couple autoimmunity from antitumor immunity.
Keywords: Immunity, Toxicity, Colitis, Arthritis, Immune checkpoint blockade, Interleukin-6, Melanoma, Th17 memory, TC1/TC17, Th1/Th17, EAE
Graphical Abstract

Introduction
Immune checkpoint blockade (ICB) is used to treat multiple cancers, however, durable remission rates with ICB monotherapy remain low, and several mechanisms of immune resistance have been identified(Kalbasi and Ribas, 2020). A multi-agent approach to overcome resistance is needed, but toxicity remains a major challenge. Combined treatment with anti-PD-1 and anti-CTLA-4 has a grade 3/4 immune-related adverse event (irAE) rate of 32-60%, often leading to treatment discontinuation(Wang et al., 2018). Therefore, mitigating irAEs without compromising anti-tumor immunity is a critical unmet need(Postow et al., 2018).
Indeed, anti-tumor immunity and irAEs have clinical commonalities, including higher response rates in patients experiencing irAEs and timing of onset from ICB initiation(Sznol et al., 2017). However, our understanding of the irAEs pathogenesis is limited; one proposed mechanism is generalized immune activation and induction of proinflammatory cytokines induced by ICB (Esfahani et al., 2020). Interleukin-6 (IL-6) is an essential cytokine for the differentiation of naive CD4+ T cells to Th17 cells and is known to play a role the in pathogenesis of several autoimmune diseases (Miossec et al., 2009), where agents that block this cytokine such as tocilizumab have been approved as effective treatments(Anderson and Rapoport, 2018; Ito et al., 2004; Ogata et al., 2014). Similarly, IL-6 has been associated with occurrence of psoriasiform dermatitis, pneumonitis, and arthritis in ICB-treated patients (Kowalski et al., 2021; Tanaka et al., 2017; Thanarajasingam U, 2019). Although one study linked low baseline serum IL-6 level with severe anti-CTLA-4–associated irAEs, it is important to highlight that inflamed tissues were not analyzed(Valpione et al., 2018). Therefore, further comprehensive studies of inflamed tissues and tumors are needed to identify the immunologic pathways involved in irAEs, which will allow for the development of therapies that can prevent or mitigate irAEs without hindering anti-tumor immunity.
To investigate the pathogenic mechanisms of irAEs, we examined immune-related enterocolitis (irEC) tissue as irEC is the most common serious complication from ICB that can lead to bowel perforation, sepsis, and death. We performed gene expression and multiplex immunohistochemistry (IHC) analyses of irEC tissue from ICB treated patients. We found that the IL-6–Th17 pathway could be a potential mediator of some irAEs, but these gene expression signatures were not seen in responding tumors. This observation provided us with the rationale to evaluate the effect of IL-6 blockade on autoimmunity and ICB therapeutic activity in murine models. Here we used i) the standard anti-CTLA-4 mouse model(van Elsas et al., 2001), and ii) the experimental autoimmune encephalomyelitis (EAE) mouse model (Robinson et al., 2014) that is biologically relevant for studying the contribution of T cells in development of irEC in humans(Anderson and Rapoport, 2018; Bamias et al., 2017; El-behi et al., 2010). To validate our findings, we then performed a single-center retrospective study of all cancer patients who received an FDA-approved ICB at the MD Anderson Cancer Center and evaluated the safety and efficacy of using IL-6 blockade for management of ICB induced irAEs.
Results
IL-6 mediated inflammation was observed in ICB induced irEC samples from patients with cancer.
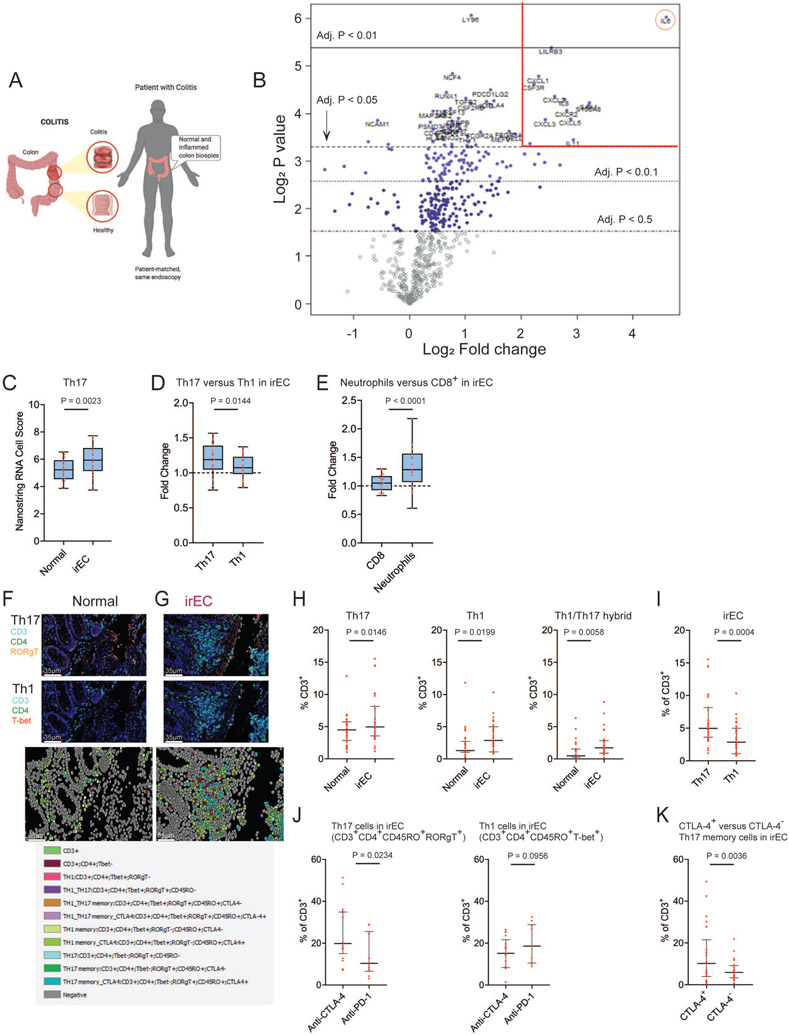
As an initial approach to understand the critical immune pathways of irEC, we used NanoString nCounter PanCancer Immune Profiling Panel (NanoPCIP) to comprehensively profile immune-relevant gene expression in human irEC tissue. Total RNA from patient-matched irEC and normal intestinal formalin-fixed paraffin-embedded tissue (Figure 1A, observation cohort, n = 12, Table S1) from ICB treated patients was profiled using NanoPCIP, and the fold change in gene expression was compared between irEC and normal tissue (Table S2). A total of 52 significantly upregulated genes were detected in the irEC tissue compared with normal tissue (Figure 1B), and IL6 had the highest fold-change in expression (fold change +24.1). The other significantly upregulated genes included acute phase reactants that are known to be induced by IL6 (i.e., IL11 and SAA1) and genes encoding neutrophil and monocyte chemotactic molecules (ie. CXCL2, CXCL3, CXCL5 and CXCL8) (Figure 1B).
Figure 1. Interleukin 6 mediated inflammation was observed in immune checkpoint blockade induced immune-related enterocolitis (irEC) samples from patients with cancer.
(A) Schematic diagram for sample collection for gene expression profiling and multiplex IHC analyses.
(B) Volcano plot of irEC compared with normal intestinal tissue. Significantly upregulated genes with log2 fold change >2 are shown inside the red lines. IL-6 log2 fold change (red circle).
(C-E) Box plots visualize estimate of abundance of immune cell subset populations using expression of characteristic genes. (C) Th17 cells within irEC compared with normal colon tissue (D) Th17 cells compared with Th1 cells in irEC. (E) Neutrophils compared with CD8+ cells in irEC. Data are presented as median and whiskers on the box plots extend minimum to maximum points (n=23, unpaired t test). The top and bottom lines of the box plots represent the interquartile range (IQR), the mid line represents the median, and the whiskers on the box plots represent minimum and maximum values.
(F,G) Example of multiplex IHC with cell type annotation and visualizations. (F) normal intestinal tissue (G) irEC tissue samples.
(H) Percentage of total T cells from multiplex IHC in normal intestinal tissue compared with irEC tissue samples. (I) Percentage of Th17 cells compared with Th1 cells in irEC. (J) Percentage of Th17 or Th1 memory cells in irEC induced by anti-CTLA-4 compared with anti-PD-1 monotherapy. (K) CTLA-4 expression among Th17 memory cells in irEC. Data are presented as median and IQR (n=27, unpaired t test). See also Figure S1, Table S1 and S2.
These data were validated in another ICB-treated cohort (validation cohort, n = 11, Table S1) using irEC and normal intestinal tissue obtained during the same colonoscopy. Twenty-nine of the 52 upregulated genes in the observation group were also upregulated in the validation cohort, and 18 of the top 20 upregulated genes (90%) were also upregulated in the validation cohort. The genes upregulated by 5-fold or greater in both cohorts are included in (Table S1).
To further characterize the immune mechanisms of irEC, we calculated cell scores based on the expression of genes previously shown to be characteristic of various immune cell subsets to estimate the abundance of these cell populations within irEC and normal intestinal tissue from all 23 patients (both cohorts, Table S2). Th17 cell gene expression (score) was significantly upregulated in irEC compared with normal intestinal tissue (Figure 1C), and Th17 cell score was significantly more upregulated than Th1 (Figure 1D). Neutrophils were also significantly more upregulated in irEC compared with CD8+ T cells (Figure 1E).
To investigate whether the increase in Th17 cell score is translated to the protein level, we performed multiplex immunohistochemistry to stain patient-matched normal intestinal and irEC tissue samples from 27 patients (Table S1). We stained tissue sections with CD3, CD4, RORγT, T-bet, CTLA-4, and CD45RO, known markers for T helper cell and memory subsets, to quantify infiltration of immune cells (Figures 1F-1K). Out of the total T cell compartment within each sample, percentage of Th17, Th1, and hybrid Th1/Th17 cells were all significantly higher in irEC compared to normal intestine, and Th17 cells were significantly higher in irEC than Th1 cells (Figures 1H and 1I).
Anti-CTLA-4 therapy induced irEC is similar to anti-PD-1 induced irEC except Th17 memory.
Among the 23 patients with irEC (observation and validation cohorts) with the NanoPCIP analysis, we evaluated the differences between irEC induced by anti-CTLA-4 based therapy (anti-CTLA-4, n=9; anti-CTLA-4 + anti-PD-1, n=5) and anti-PD-1 monotherapy (n=9). The gene epxression profiling was similar between these two groups. Fifteen of the top 20 significantly upregulated genes in anti-CTLA-4 induced irEC were significantly upregulated in anti-PD-1 induced irEC, which included IL-6, genes that are known to be induced by IL6 (i.e., IL11), and genes encoding neutrophil and monocyte chemotactic molecules (ie. S100A8, CXCL1, CXCL2, CXCL3, CXCL8) (Table S1). When specifically looking at IL-6, Th17 related cytokines, and neutrophil chemotactic molecule gene expression, there was no statistically significant difference in upregulation within anti-CTLA-4 and anti-PD1 induced irEC (Figure S1A). However, the NanoPCIP Th17 cell score was significantly higher in irEC in the anti-CTLA-4 group while not significantly upregulated in the anti-PD-1 monotherapy group (Figure S1B). Among 27 irEC patients with multiplex IHC analyzed samples, the only T cell subset significantly higher in irEC induced by anti-CTLA-4 based regimens (n=17) versus anti-PD-1 monotherapy (n=10) were Th17 memory cells (Figure 1J). Among the Th17 memory cells in all irEC samples, CTLA-4-positive were more prevalent than CTLA-4-negative Th17 memory cells (Figure 1K)
IL-6 inflammatory signature is higher in irEC than in responding tumor tissue from patients.
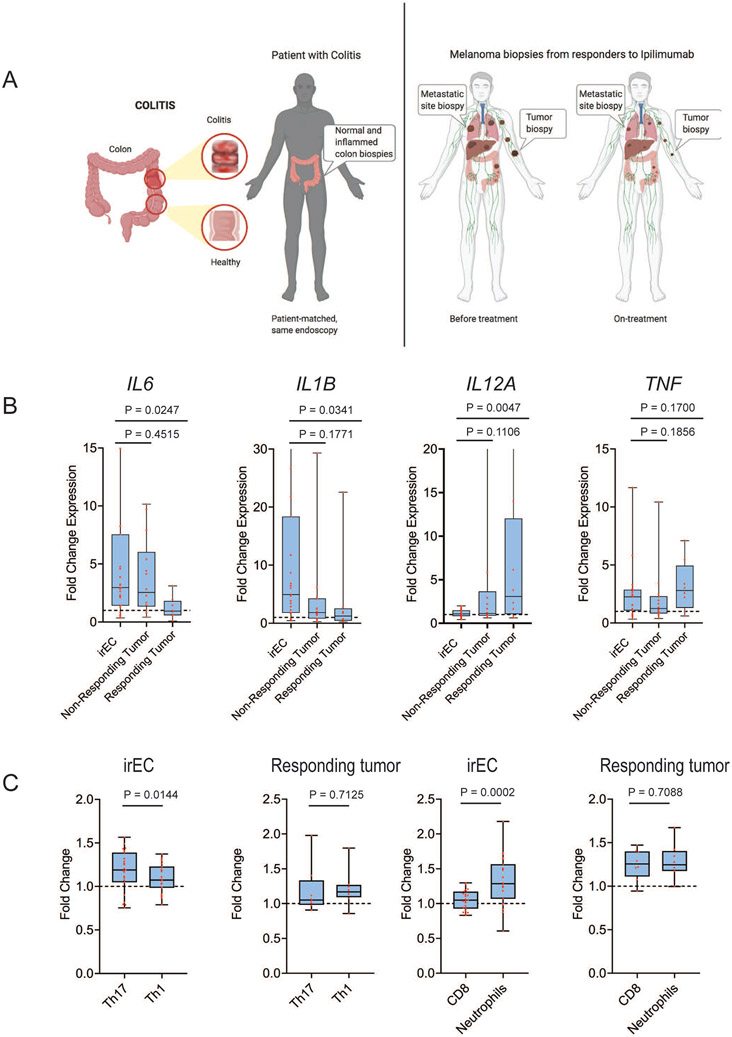
Next, to compare the immune signatures in irEC tissue with that of the tumor tissue, we performed gene expression profiling on melanoma tumor biopsies obtained at baseline and week 6 during treatment from ipilimumab responders (per RECIST v1.1, n=8) and non-responders (n=14) . As we could not obtain matched baseline and on-treatment tumor samples from the same cohort of patients with irEC, we used the same NanoPCIP panel to analyze tumor samples from a different cohort of melanoma patients treated in an ipilimumab (ipi)-based therapy (Figure 2A) We compared the average fold changes in the expression of each gene from our irEC NanoPCIP observation-cohort analysis (normal-intestine versus irEC) with the gene expression fold changes in the responding and non-responding tumors NanoPCIP analysis (baseline versus on-treatment tumors). The average fold changes from the irEC analysis were significantly different in 343 (out of 770 total) genes when compared to the responding tumor analysis (adjusted p < 0.05, Table S2) and in only 70 genes when compared to the non-responding tumor analyses (adjusted p < 0.05, Table S2). This suggests that more genes are differentially expressed in the irEC versus responding tumor. We also found among these 343 genes, those with the largest difference in log2 fold change between irEC and responding tumor were IL6 and genes that encode for neutrophil and monocyte chemotactic chemokines (Table 1). In contrast, the difference in log2 fold change of these genes between irEC and non-responding tumor was not significant.
Figure 2. Interleukin 6 inflammatory signature is higher in immune-related enterocolitis than in tumors responding to immune checkpoint blockade therapy.
(A) Schematic diagram for gene expression profiling: left, irEC samples and right, melanoma baseline and on-treatment tumor samples from patients treated with ICB. For melanoma, samples from a different cohort of melanoma patients treated with ipilimumab-based therapy were used. (B) Comparison of fold change of Th17- and Th1-differentiating cytokines and tumor necrosis factor alpha (TNF-A) in all patients from the irEC analysis and tumor response analysis. (C) Comparison of mean fold change of immune cell subset population in irEC or responding tumor. Data are presented as interquartile range and median and whiskers on the box plots extend minimum to maximum points (n=26, unpaired t test).
See also Table S2.
Table 1.
Fold change in top upregulated gene expression.
| Gene | irEC vs normal intestinal tissue |
Adjusted P | irEC vs responding tumor tissue |
Adjusted P | irEC vs nonresponding tumor tissue |
Adjusted P |
|---|---|---|---|---|---|---|
| IL6 | 24.1 | 0.0023 | 2.097 | 0.0002 | 0.221 | 1.0000 |
| CXCL2 | 6.04 | 0.0201 | 1.612 | 0.0129 | 0.051 | 1.0000 |
| CXCL3 | 5.4 | 0.0067 | 2.041 | 0.0209 | 0.055 | 1.0000 |
| CXCL1 | 4.94 | 0.0161 | 0.913 | 3.38 × 10−6 | 0.121 | 1.0000 |
When we compared the differential gene expression from all patients with irEC (observation and validation cohorts) to patients with responding tumors, multiple Th17 and Th1 differentiating cytokines were significantly differentially expressed (Figure 2B), while the fold change in their expression was not significantly different from what we observed in non-responding tumors (Figure 2B). Using the NanoString cell scoring techniqe described above, we found that Th17 cell score was higher than Th1, and neutrophils score was higher than CD8 T cell score in the irEC analysis, but this was not the case in the responding tumors (Figure 2C). Differential expression of TNF, which is a known target to mitigate irEC inflammation, was not significantly different from that of responding tumors (Figure 2B). In fact, TNF was one of only 11 genes among the 770 gene panel that was upregulated in both the irEC analysis and responding tumor analysis (Table S2).
Anti-CTLA-4 therapy increases IL-6 expression in mouse tumor supernatant.
Evidence from our patient RNA NanoPCIP analysis indicated that overexpression of the checkpoint-induced Th17-differentiating cytokine IL-6 may contribute to irEC through Th17 T cell–mediated inflammation. We therefore used C57BL/6 and Balb/c mouse strains to address a variety of scientific questions, such as whether the antitumor effect of anti-CTLA-4 therapy can be enhanced by IL-6 blockade and whether IL-6 blockade–enhanced ICB antitumor activity could also improve irAEs.
We first sought to determine whether our observation of increased IL-6 expression in irEC and nonresponding tumors (compared with tumors responding to ICB therapy) reflects a generalizable mechanism of tumor resistance to ICB therapy. To address this question, we analyzed the effects of anti-CTLA-4 therapy in mice with the aggressive B16.BL6 melanoma model. We randomized C57BL/6 mice into 2 groups (n = 5 per group) receiving subcutaneous GVAX (1 × 106 cells) and intraperitoneal anti-CTLA-4 (200 μg/mouse) on days 3, 6, and 9 after tumor injection or IgG control. We examined cytokine and chemokine expression in mice with B16.BL6 melanoma receiving standard anti-CTLA-4 therapy. Tumors were allowed to reach ~50 mm2 before mice were euthanized for tumor supernatant analysis by Luminex. This analysis detected significantly increased proinflammatory cytokine IL-6, IL-1β, and TNF-α levels in the tumor supernatant of mice receiving anti-CTLA-4 and GVAX compared with control mice (Figure S2).
IL-6 blockade enhances anti-CTLA-4 therapeutic activity in preclinical models.
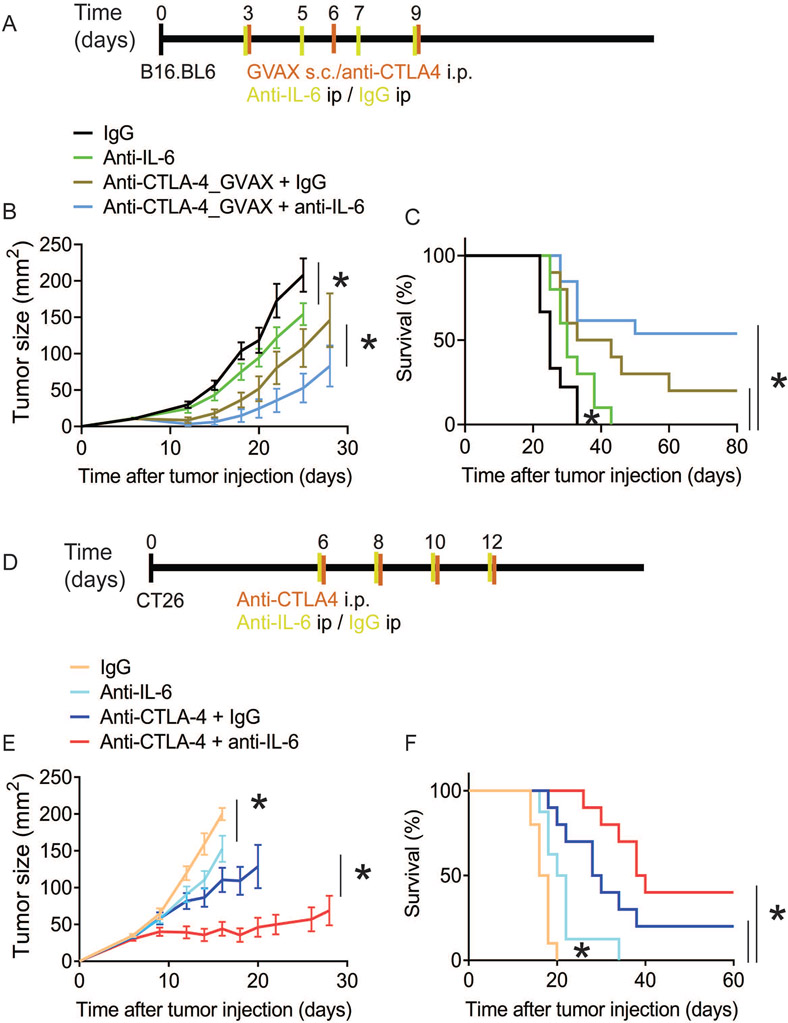
Given that anti-CTLA-4 therapy increases expression of a Th17-differentiating cytokine (IL-6) in the tumor microenvironment, we tested the impact of IL-6 blockade on anti-CTLA-4 therapeutic activity in B16.BL6 model and the more immunogenic CT26 colon carcinoma model. C57BL/6 mice were injected with B16.BL6 and then treated with anti-CTLA-4, IL-6 blockade, anti-CTLA-4 in combination with IL-6 blockade, or no treatment (control) (Figure 3A). Mice receiving IL-6 blockade showed reduced tumor burden compared with untreated mice, indicating that IL-6 blockade alone may have single-agent activity, although all mice eventually attained a tumor burden, with 0% cured (Figure 3B). No control mice, nor any mice receiving IL-6 blockade alone, were alive at 60 days, whereas 20% of mice receiving anti-CTLA-4 therapy and 58% of mice receiving anti-CTLA-4 therapy with IL-6 blockade were (Figure 3C).
Figure 3. Interleukin (IL)-6 blockade increases anti-CTLA-4 therapeutic efficacy.
(A-C) C57BL/6 mice were injected s.c.with B16.BL6 melanoma cells on day 0 and treated with GVAX and anti-CTLA-4 on days 3, 6, and 9, or anti-IL-6 or IgG on days 3, 5, 7, and 9. (A) Treatment scheme for B16.BL6 anti-CTLA-4 therapy in C57BL/6 mice. (B) Tumor size represented as average ± SEM, (n = 10, *P < 0.05, Multiple t test). (C) Kaplan-Meier survival curves (n=10, *P < 0.05, Log-rank test). (D-F) Balb/c mice were injected with CT26 colon carcinoma cells on day 0 and treated with anti-CTLA-4 , anti-IL-6, or IgG on days 6, 8, 10, and 12. (D) Treatment scheme for CT26 anti-CTLA-4 therapy in Balb/c mice. (E) Tumor size represented as average ± SEM (n = 10, *P < 0.05, Multiple t test). (F) Kaplan-Meier survival curves (n=10, *P < 0.05, Log-rank test).
See also Figure S2.
To extend and validate these findings, we treated Balb/c mice bearing CT26 murine colon cancer cells (Figure 3D). We found that the addition of IL-6 blockade to anti-CTLA-4 therapy resulted in significantly increased tumor shrinkage, with 32% of mice receiving anti-CTLA-4 alone cured compared with 48% of mice receiving IL-6 blockade and anti-CTLA-4 therapy (Figure 3E). Here also, no mice were cured with IL-6 blockade alone or with no treatment. However, IL-6 blockade showed improved tumor regression compared with no treatment, as indicated by reduced tumor burden and increased survival (Figures 3E and 3F), indicating possible IL-6 blockade single-agent therapeutic activity. This finding suggests that IL-6 blockade enhancement of anti-CTLA-4 therapeutic activity is not limited to or a peculiarity of the B16.BL6 tumor model or GVAX as a tumor vaccine antigen. Together, these results show a therapeutic benefit of IL-6 blockade as monotherapy and in combination with anti-CTLA-4 therapy.
IL-6 blockade increases anti-CTLA-4 therapy–induced tumor-infiltrating T cell localization in mice.
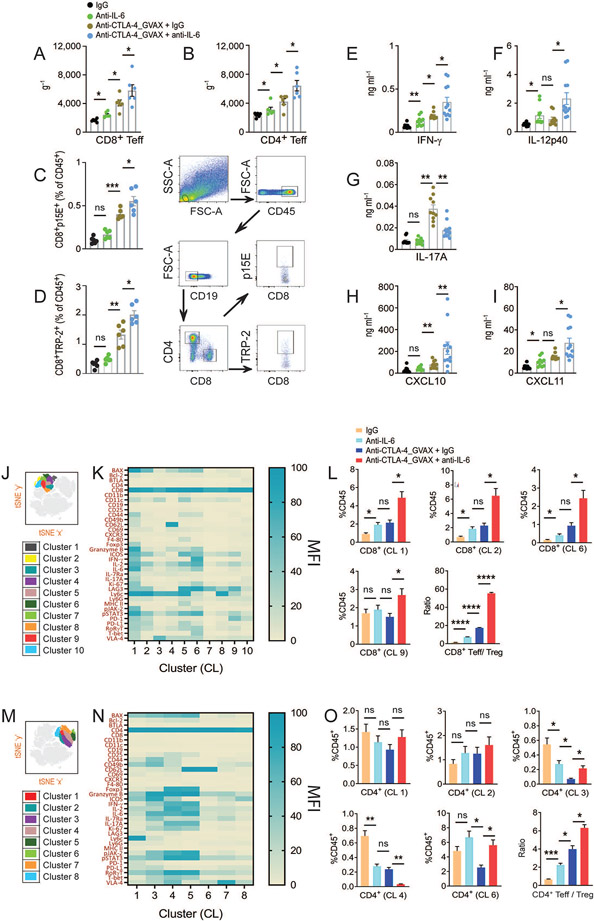
To gain insight into the mechanism by which IL-6 blockade, alone or in combination with anti-CTLA-4 therapy, increases antitumor response, we closely examined anti-CTLA-4 therapy–induced tumor-infiltrating T cell localization. We applied a previously used methodology to specifically detect all CD8+ and CD4+ effector T cells (Teff) on the basis of their CD44 and CD11a surface expression(Hailemichael et al., 2018; Masopust et al., 2007). We found that IL-6 blockade significantly increased anti-CTLA-4 therapy–induced CD44hi CD11ahi CD8+ and CD4+ Teff frequency in B16.BL6 (Figures 4A and 4B). The number of CD8+ Teff increased in tumors from mice that received IL-6 blockade alone compared with untreated mice (Figure 4A). In addition, and consistent with survival, the frequency of CD8+ Teff increased with specificity to B16 melanoma antigen TRP-2 and p15E, confirmed by pentamer staining (Figures 4C and 4D). Fourteen days following tumor injection, polyclonal CD4+ and CD8+ Teff exhibited an effector phenotype, as indicated by increased activation (PD-1, Tim-3, Lag-3, ICOS), survival (Bcl-2), and cytotoxic (granzyme B, IFN-γ, TNF-α, T-bet) marker expression (Figures S3A-H). Furthermore, consistent with the antitumor response, Th1 cytokines (IFN-γ, IL-12p40, IL-12p70, IL-2) and IFN-γ–induced chemokines C-X-C motif ligand 10 and 11 (CXCL10 and CXCL11) levels were increased, and the Th17 cytokine (IL-17A) level was decreased in tumors, suggesting that IL-6 blockade promotes a Th1-biased immune response (Figures 4E-I and S4).
Figure 4. Interleukin 6 blockade increases anti-CTLA-4 therapy–induced effector T cell localization.
(A-I) Mice were treated as in Fig. 3A and tumors were harvested on day 14 (A) Total number of polyclonal CD8+ Teff (B) Total number of polyclonal CD4+ Teff in B16.BL6 tumor were quantitated by flow cytometry and normalized to weight of the tumor ( g−1). (C,D) The number of (C) p15E-specific CD8+ T cells (D) TRP-2-specific CD8+ T cells detected by pentamer staining. Gating strategy for flow cytometry analysis shown to the right. (E-I) Cytokine and chemokine concentrations in supernatant from tumor site homogenates were analyzed by luminex multiplex assay 14 days after tumor injection. (E) IFN-γ (F) IL-12p40 (G) IL-17A (H) CXCL10 (I) CXCL11. (J-L) Profiling of CT26 tumor-infiltrating CD8+ Teffs by mass cytometry at day 14 in mice treated as in Fig. 3D. (J) t-SNE plots showing CD8+ T cell clusters. (K) Heatmap displaying the mean fluorescence intensity (MFI) of immune markers expressed on CD8+ T cell cluster, color coded with beryl green for lower expression and blue green for higher expression. (L) Bar graphs showing the relative frequency of representative CD8+ T cell cluster groups as a percentage of CD45+ leukocytes. (M-O) Profiling of CT26 tumor-infiltrating CD4+ Teffs (M) t-SNE plots showing CD4+ T cell clusters. (N) Heatmap displaying MFI of immune markers expressed on CD4+ T cell cluster, color coded with beryl green for lower expression and blue green for higher expression. (O) Bar graphs showing the relative frequency of representative of CD4+ T cell cluster groups as a percentage of CD45+ leukocytes. Data are represented as average ± SEM (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, One-way ANOVA).
See also Figures. S3 and S4.
We performed mass cytometry (CyTOF) to comprehensively profile tumor-infiltrating lymphocytes with a 40+ parameter CyTOF panel including a wide array of T cell lineage transcription factors (e.g., pSTAT3, FOXP3, T-bet, Bcl-2, RORγT, GATA3, GITR), activation and exhaustion markers (e.g., Ly6c, CD69, CD49d, ICOS, Lag-3, BTLA, PD-1), non–T cell lineage markers (e.g., CD11b, Ly6G, I-A/I-E MHC-II, CD19), and chemotaxis markers (e.g., CXCR3, CXCR2, CCR7). Using this approach, we analyzed tumor-infiltrating immune cells from CT26 colon cancer model treated with IgG, anti-IL-6, anti-CTLA-4 or combinatorial treatment. We used 27 surface and 16 intracellular markers to characterize tumor-infiltrating lymphoid and myeloid cells. We observed 10 CD8+ T cell clusters (Figures 4J and 4K). CD8+ Teff in cluster 1 and cluster 6 had a TC-1 cytotoxic phenotype with expression of IFN-γ and granzyme B activation markers (Lag-3, Ki-67, T-bet, pSTAT3, p-JAK-2). Teff in cluster 1 also maintained a TC-17 profile with co-expression of IL-17 and FOXP3 (Figures 4K and 4L). CD8+ Teffs in clusters 2 and 9 showed increased PD-1/Lag-3 and reduced IFN-γ/granzyme B expression, indicating an exhausted profile. Teff levels in all clusters except for cluster 9 were consistent with therapy-induced antitumor response (Figures 3D-3F). We identified three Th1/Th17-like CD4 clusters: cluster 1, exhausted CD4+ T cells (PD-1, Lag-3); cluster 2, Th1/Th17 hybrid cells (IFN-γ, IL-17, granzyme B, T-bet, pSTAT3); and cluster 6, featuring low effector cytokine expression but lacking the exhausted phenotype expression (Figures 4M and 4N). None of these three clusters corresponded with the antitumor response (Figures 3D-3F). In addition, we identified two CD4+ FOXP3+ regulatory T cell (Treg) subsets distinguished by ICOS and CD62L expression in clusters 3 and 4 (Figures 4N and 4O), with inverse association only between the ICOS− CD62L+ subset and antitumor response (Figures 3D-3F). Our analysis of CD8+ or CD4+ Teff and Treg tumor infiltrates indicated that IL-6 blockade increased the Teff:Treg ratio in both CD8 (Figure 4L) and CD4 compartments (Figure 4O).
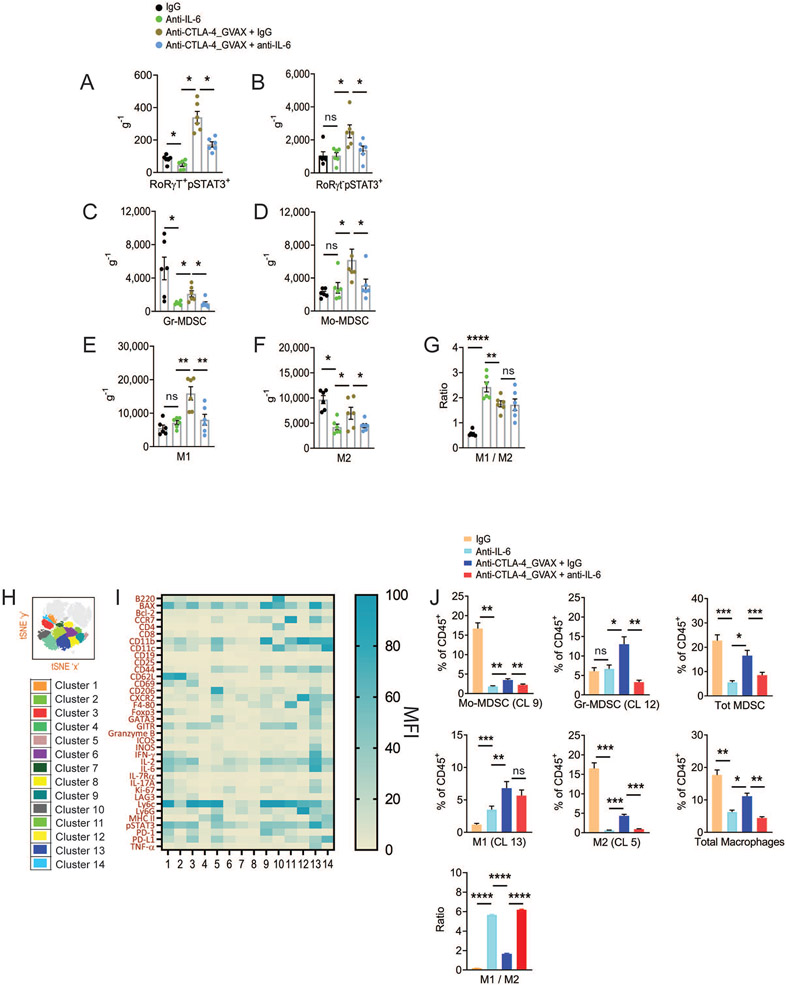
Because IL-6 with other cytokines controls naive CD4 T cell differentiation into Th17 cells, we sought to determine whether IL-6 inhibition reduces Th17 levels in B16.BL6 tumors. Our analysis of Th17 cells included the markers RORγT and pSTAT3. The IL-17+ Th17-like CD4 T cell level in tumors of mice receiving IL-6 blockade was significantly reduced (Figures 5A, 5B, and S4), in parallel with the observation that the key polymorphonuclear cell attractant chemokine CXCL1 was also reduced in tumors (Figure S4). As another mechanistic possibility by which IL-6 blockade could enhance anti-CTLA-4 therapeutic response, we explored whether a reduced CXCL1 level correlated with reduced myeloid-derived suppressor cells (MDSCs) in tumors. We found significantly reduced granulocytic MDSCs (Ly6Ghi Ly6clow MHC-II−) and monocytic MDSCs (Ly6Glow Ly6chi MHC-II−) in tumors from mice treated with IL-6 blockade alone or in combination with anti-CTLA-4 therapy (Figures 5C, 5D and S5A). In addition, we observed decreased tumor-associated type 1 (M1; Ly6Glow F4-80+ CD206− CCR2hi CCL5hi) and type 2 (M2; Ly6Glow F4-80+ CD206+ CCR2low CCL5low) macrophages with IL-6 blockade (Figure 5E-5G and S5A), which is consistent with a low concentration of macrophage attractant chemokine, CC-motif ligand 2 (CCL2; Figure S4). Closer examination of the macrophage phenotype revealed reduced CCL20 and CXCL9 expression in macrophages in mice receiving ICB therapy with IL-6 blockade (Data not shown).
Figure 5. Interleukin 6 blockade decreases macrophages and myeloid-derived suppressor cells (MDSCs) in tumor tissue.
(A-G) Mice were treated as in Fig. 4A. Tumors were harvested on day 14. Total number of (A) CD4+ RORγT+pSTAT3+ T cells, (B) CD4+RORγT−pSTAT3+ T cells, (C) Granulocytic-MDSCs, (D) Monocytic-MDSCs, (E) M1-like macrophages, (F) M2-like macrophages, and (G) M1 / M2 ratio in B16.BL6 tumor tissue quantitated by flow cytometry and normalized to weight of the tumor ( g−1). (H-I) Mice were treated as in Fig. 4J. (H) t-SNE plots showing clusters with the expression of the CD11b lineage marker. (I) Heatmap displaying MFI of immune markers expressed on CD1b+ myeloid cell cluster in CT26 tumor, color coded with beryl green for lower expression and blue green for higher expression. (J) Bar graphs showing the relative frequency of representative cluster groups as a percentage of CD45+ leukocytes are shown on the right. Data are represented as average ± SEM (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, One-way ANOVA).
See also Figure S5.
The above experiments examined myeloid cell infiltration in the context of murine melanoma. To better characterize the phenotype of therapy-induced CT26 tumor-infiltrating MDSCs and macrophages, we performed t-SNE analysis (Figures 5H and S5B). We identified 14 myeloid cell clusters (Figure 5I). Overall, we found that monocytic-MDSC–like cells (cluster 9) and granulocytic MDSCs (cluster 12) were reduced in tumors from mice receiving IL-6 blockade. Among macrophages, represented as M1-like (cluster 13) and M2-like (cluster 5), we observed a similar reduction in response to IL-6 blockade.
Taken together, our findings showed that IL-6 blockade and anti-CTLA-4 therapy promoted increased CD8+ Teff tumor infiltration, conversely reducing Tregs, Th17, MDSCs, and macrophages, resulting in increased CD8:Treg and CD8:MDSC ratios.
IL-6 blockade improves ICB–induced antitumor efficacy without exacerbating EAE.
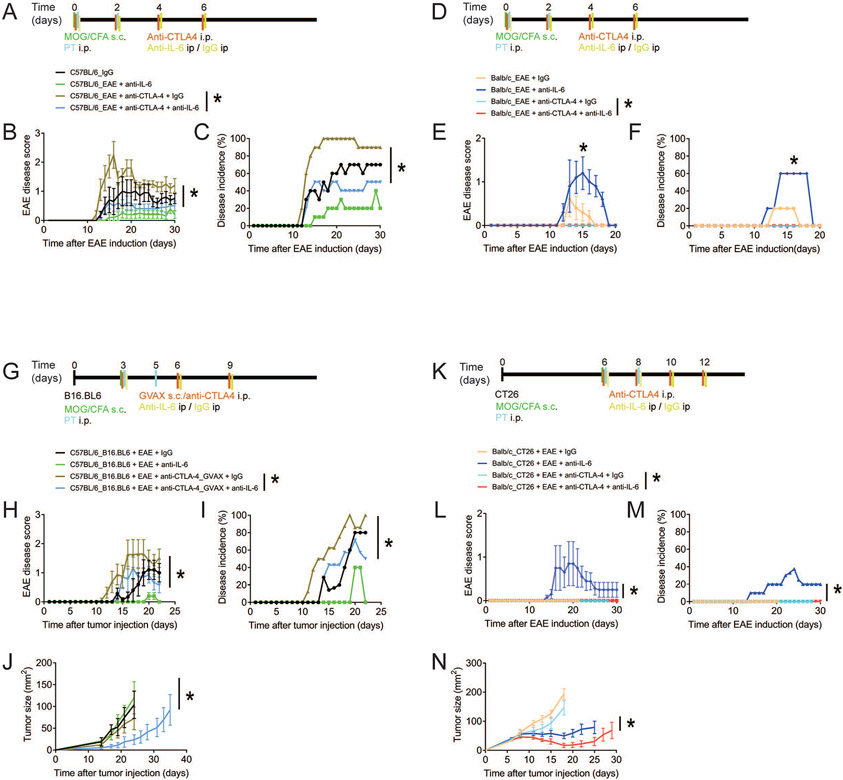
EAE was induced in mice to test whether anti-CTLA-4 therapy increases EAE severity and incidence and whether IL-6 blockade could ameliorate disease symptoms. EAE induction in specific-pathogen-free (SPF) mice is rare. For EAE induction, C57BL/6 mice were immunized with the autoantigen myelin-oligodendrocyte glycoprotein35–55 (MOG35–55) peptide in complete Freund adjuvant containing Mycobacterium tuberculosis along with intraperitoneal administration of pertussis toxin (Figures 6A and S6). We found that mice treated with anti-CTLA-4 showed significantly increased EAE disease severity, with the highest peak score at day 16 and a 5.3-, 29-, and 3.6-fold increase in disease score compared with mice receiving IL-6 blockade and anti-CTLA-4, IL-6 alone, and control, respectively (P < 0.05, Figure 6B). EAE disease incidence followed a similar pattern, with 10 of 10 mice (100%) treated with anti-CTLA-4 alone, 5 of 10 (50%) treated with IL-6 blockade and anti-CTLA-4, 2 of 10 (20%) treated with IL-6 blockade alone, and 7 of 10 (70%) control mice exhibiting paralysis (χ2 = 12.2, degrees of freedom = 3, P < 0.01; Figure 6C).
Figure 6. Interleukin 6 blockade improves anti-CTLA-4 therapeutic activity while not exacerbating autoimmunity.
Autoimmune encephalomyelitis (EAE) was induced in C57BL/6 mice (A-C) and Balb/c mice (D-F) by subcutaneous immunization with myelin-oligodendrocyte glycoprotein (MOG)35–55 peptide in complete Freund adjuvant (CFA) containing Mycobacterium tuberculosis and intraperitoneal administration of pertussis toxin (PT) on the day of immunization and 2 days later. (A) Treatment scheme for anti-CTLA-4 and anti-IL-6 therapy in C57BL/6 mice. (B) EAE disease score average ± SEM, n = 10, *P < 0.05, multiple t test). (C) EAE at the highest peak of disease incidence (*X2 = 12.2, degrees of freedom = 3, P < 0.01). (D) Treatment scheme for anti-CTLA-4 and anti-IL-6 therapy in Balb/c mice. (E) EAE disease score (average ± SEM, n = 10, *P < 0.05, Multiple t test). (F) EAE at the highest peak of disease incidence (*X2 = 15, degrees of freedom = 3, P < 0.01).
(G-J) EAE was induced as described in Figure 6A in C57BL/6 mice 3 days after mice were injected with B16.BL6 cells and treated with GVAX and anti-CTLA-4 on days 3, 6, and 9 or anti-IL-6 or IgG on days 3, 5, 7, and 9. (G) Treatment scheme for B16.BL/6 and EAE anti-CTLA-4 therapy. (H) EAE disease score average ± SEM, (n = 10, *P < 0.05 Multiple t test). (I) EAE at the highest peak of disease incidence (*X2 = 8.73, degrees of freedom = 3, P < 0.05). (J) Tumor size average ± SEM (n = 10, *P < 0.05 determined by Multiple t test).
(K-N) EAE was induced as described in Figure 6D in Balb/c mice 6 days after mice were injected with CT26 cells and treated with anti-CTLA-4, anti-IL-6, or IgG on days 6, 8, 10, and 12. (K) Treatment scheme for CT26 and EAE anti-CTLA-4 therapy. (L) EAE disease score (average ± SEM, n = 10, *P < 0.05 Multiple t test). (M) EAE at the highest peak of disease incidence (X2 = 12.9, degrees of freedom = 3, *P < 0.05). (N) Tumor size average ± SEM, (n = 10, *P < 0.05, Multiple t test).
See also Figures. S6, S7 and S8.
We then extended our observation in an EAE-resistant Balb/c model treated similarly to the C57BL/6 mice (Figures 6D-F and S6B). We found that mice receiving anti-CTLA-4 therapy had a 4-fold increase in EAE disease severity score at day 15 compared with control mice (P < 0.05; Figure 6E). Similarly, 6 of 10 mice (60%) treated with anti-CTLA-4 and 2 of 10 control mice (20%) exhibited paralysis, and these mice eventually recovered (χ2 = 15, degrees of freedom = 3, P < 0.01; Figure 6F). EAE symptoms were not observable in mice treated with IL-6 blockade and anti-CTLA-4 or IL-6 blockade alone (Figures 6E and 6F).
To test the possibility that IL-6 blockade would allow more effective immunotherapy of cancer without increasing EAE disease severity, we induced EAE in C57BL/6 mice 3 days after B16.BL6 tumor injection. Mice received anti-CTLA-4 therapy on days 3, 6, and 9 or anti-CTLA-4 therapy and IL-6 blockade on days 3, 5, 7, and 9 after tumor injection or control (Figures 6G and S6C). Mice receiving anti-CTLA-4 therapy had accelerated clinical signs of EAE compared with mice receiving IL-6 blockade and anti-CTLA-4, IL-6 blockade alone, or control (P < 0.05; Figures 6H and 6I). The combination of IL-6 blockade and anti-CTLA-4 therapy resulted in milder EAE clinical symptoms (χ2 = 8.73, degrees of freedom = 3, P < 0.05; Figure 6I) and better tumor control (P < 0.05; Figure 6J). In addition, we expanded on the therapeutic benefit of IL-6 blockade in mice receiving anti-PD-1 therapy (Figure S7A). We found that anti-PD-1 therapy exacerbated EAE symptoms and these symptoms ameliorated with increased tumor-free survival in mice treated with IL-6 blockade compared with control (P < 0.05; Figures S7B-S7E).
To validate these observations, we injected Balb/c mice with CT26 tumors and immunized them on day 6 with MOG35–55 peptide in complete Freund adjuvant, as well as administering the treatments as described above (Figures 6K and S8A). In contrast to C57BL/6 mice, only 2 of 10 Balb/c mice (20%) receiving anti-CTLA-4 therapy exhibited EAE clinical symptoms (χ2 = 12.9, degrees of freedom = 3, P < 0.01; Figures 6L and 6M). We also confirmed that mice with induced EAE treated with IL-6 blockade and anti-CTLA-4 showed significant tumor shrinkage compared with mice treated with anti-CTLA-4 alone, IL-6 blockade alone, or no treatment (P < 0.05; Figure 6N).
Collectively, our results indicate that IL-6 blockade abrogates ICB–induced EAE disease severity and promotes antitumor immunity.
IL-6 blockade could be an effective therapy for IrAEs without dampening the efficacy of ICB in cancer patients.
To validate our findings, we retrospectively reviewed our center experience using IL-6 blockade for irAE management. Of 13,735 ICB treated patients between January 2004 and March 2021, 31 melanoma patients who received tocilizumab or sarilumab were identified (Table S3). Their median age was 62 years (range 38-79), 58% were males, 61% received anti-PD-1 monotherapy. The indications for using IL-6 blockade were i) inflammatory arthritis (77%); ii) myositis overlap with myasthenia gravis and/or myocarditis (6.5%); iii) hepatitis (6.5%); and iv) 3% each with CNS vasculitis, encephalitis, and systemic sclerosis. Of note, 90% of these patients received first-line therapy with corticosteroids (up to 250 mg/day oral prednisone or equivalent), and 39% received disease modifying antirheumatic drugs (methotrexate, hydroxychloroquine, sulfasalazine, rituximab), however, none had irAEs improvement (resolution or change to ≤ grade 1 as per the Common Terminology Criteria for Adverse Events v5.0). The median time from irAEs onset till IL-6 blockade initiation was 3.7 months. We observed a 74% improvement of irAEs after a median of 2 months of IL-6 blockade initiation; the dose and frequency of administration varied widely but tocilizumab given as 162 mg subcutaneously every 1-2 weeks was the most frequently used. Of 19 evaluable patients with arthritis, the median clinical disease activity index (CDAI) at IL-6 blockade initiation was 24 (range, 6-60) suggesting high arthritis disease activity, and the use of IL-6 blockade decreased the median CDAI score to 3.5 (range, 0-29) within 2 months. The median CRP level at IL-6 blockade initiation was 43.04 mg/L (range, 1.5-300) and dropped to 1.2 mg/L (range, 0.15-14.7) within 10 weeks. Three patients (10%) discontinued IL-6 blockade because of adverse events (abdominal pain, tachycardia, and tinnitus), and another patient developed neutropenia after receiving sarilumab 200 mg subcutaneously every 2 weeks but she successfully tolerated treatment after reducing the dose to 150 mg. We evaluated the tumor response to ICB using the RECIST criteria before IL-6 blockade initiation or early on (within 8-12 weeks) therapy and the best overall response rate per last available follow-up (patients who progressed while on ICB and switched to another line of therapy before initiation of IL-6 blockade were excluded). Of 26 evaluable patients, the best overall response rate to ICB was 57.7% before IL-6 blockade initiation and 65.4% after therapy (P = 0.500) (Figure S8B). Thus, our clinical data support that targeting IL-6 can mitigate the irAEs without compromising the overall tumor response to ICB.
Discussion
Successful ICB antitumor activity is shown to be associated with higher frequency and more severe irAEs (Larkin et al., 2015). In addition to symptom burden and treatment-related fatalities, irAEs are a major barrier for developing multiagent diverse immunotherapy regimen needed to overcome heterogenous and treatment resistant tumor microenvironment(Kalbasi and Ribas, 2020; O'Donnell et al., 2019). Here, we provide an in depth understanding of the immunobiology of irAEs and possible treatment strategies to decouple tumor immunity from autoimmunity to improve outcomes of ICB. We conducted comprehensive clinical, translational, and preclinical analyses to understand irAE immunobiology. Here we found i) a contrasting immune signature in human irEC (IL-6 and Th17 cells more upregulated than IL-12/Th1) and responding tumor tissue (IL12 and Th1 gene signatures more upregulated) of ICB treated patients, but interestingly these differences were not seen between nonresponding tumors and irEC; ii) in mouse models, IL-6 blockade can mitigate autoimmunity while simultaneously enhance antitumor immunity with increased CD4+ and CD8+ T effector cells and decreased MDSCs and macrophages within the tumor microenvironment; iii) through retrospective analysis of a melanoma patient cohort experiencing ICB induced irAEs, IL-6 receptor blockade led to successful mitigation of the irAE symptoms without compromising ICB clinical efficacy. Building on these results, we hypothesize that IL-6 could be targeted to potentially mitigate autoimmunity while maintain and possible boost tumor immunity.
Some of the highest upregulated genes in our patients’ irEC tissue were IL-6, genes reported to be induced by IL-6, neutrophil/monocyte chemokines, as well as Th17-associated genes. The recruitment of neutrophils and other granulocytic cells through chemokines produced by Th17 cells has been shown to be associated with multiple autoimmune diseases(Miossec and Kolls, 2012). Although ICB was shown to increase serum Th17 cell levels(Dulos et al., 2012), and it was shown that elevated serum IL-17 coincides with active irEC in ipilimumab-treated patients(Callahan et al., 2011), there is a paucity of data linking Th17 cells directly to irAEs pathogenesis(Anderson and Rapoport, 2018). We identified Th17, Th1, and for the first time RORγT+ T-bet+ Th1/Th17 hybrid cells all significantly increased within the irEC tissue. Importantly, Th17 cells were significantly higher than Th1 cells within irEC. The pathogenicity of both Th17 and Th1/Th17 hybrid cells in autoimmunity and inflammatory diseases has been described in several studies(Abromson-Leeman et al., 2009; Fang et al., 2020; Gaublomme et al., 2015). A recent study through single-cell RNA sequencing of irEC demonstrated an upregulation of Th1 gene signatures compared to controls, as well as showed an increased expression of CCR6, IL23 receptor, IL4I1, and CXCR6(Luoma et al., 2020), suggesting that these cells could possibly be Th1/Th17 hybrid cells; also known as the ex-Th17 cells(Basdeo et al., 2017). These findings are consistent with a recent description of pathobiont-reactive Th17 cells of oral origin co-expressing RORγT and T-bet, and are associated with eliciting gut inflammation in commensal pathobiont-driven colitis model(Kitamoto et al., 2020).
We did not observe many differences in either gene expression profiling or multiplex IHC analyses when comparing irEC from patients receiving anti-CTLA-4 versus anti-PD-1. However, the Th17 cells score was upregulated in anti-CTLA-4 irEC but not in anti-PD-1 irEC. Interestingly, the only T cell subset that was significantly higher in anti-CTLA-4 irEC than anti-PD-1 from our multiplex IHC analysis was Th17 memory (CD3+CD4+RORγ+CD45RO+). It has been reported before that anti-CTLA-4 could induce a superior antitumor memory response than anti-PD-1, specifically facilitating CD4+ T cell memory(Wei et al., 2017). Our findings are novel in that superior CD4+ T cell memory induction by anti-CTLA-4 may also occur in inflamed tissues including the intestine and appear to be Th17 dominant. We also found that in both cohorts, more of these Th17 memory cells had high expression of CTLA-4. These findings are clinically relevant as reinduction of anti-CTLA-4 after occurrence of irEC typically results in recurrence of the same irAEs, even after long time since last treatment, and our group has reported that recurrence of irEC is more frequent with anti-CTLA-4 than with anti-PD-1(Abu-Sbeih et al., 2019). All together these data highlight the role of CTLA-4 expression on Th-17/memory cells, and it could suggest that the contraction of these memory cells is mediated, at least in part via CTLA-4.
By comparing immune-relevant gene expression levels from our irEC cohort and responding tumor (melanoma) tissue from patients receiving ipilimumab, we demonstrated potential mechanistic differences between autoimmunity and antitumor immunity. Since biopsies of both responding and nonresponding tumors were obtained 6 weeks after initiation of ICB, our findings revealed which pathways are essential in the early antitumor immune response. Genes associated with the IL-6–Th17 axis were more prominent within irEC tissue and non-responding tumors, while genes associated with the IL-12–Th1 axis were higher upregulated in responding tumors. These findings were further supported by our ICB induced irAEs clinical cohort, where we showed that blocking IL-6 with tocilizumab/sarilumab successfully mitigated the refractory irAEs and did not compromise the antitumor effect achieved by ICB. Our clinical data are in line with published data reporting successful management of irAEs-pneumonitis, cerebritis and other irAEs in cancer patients treated with nivolumab(Stroud et al., 2019). All together, these data provide substantial evidence that IL-6 possibly plays a pivotal role in the biology of irAEs but not in the immune-biology of a responding tumor.
To elucidate the mechanism of how IL-6 blockade would affect ICB activity in tumor bearing mouse models with autoimmune diseases, we utilized the EAE mouse model which we believe shares similar biology to irAEs and can be exacerbated by ICB(Hurwitz et al., 2002). We found that blocking IL-6 in both melanoma and colon cancer mouse models simultaneously augmented the antitumor activity of anti-CTLA-4 or anti-PD-1 therapy and concomitantly led to clinically meaningful amelioration of the EAE clinical severity and disease incidence. In the tumor tissue analysis, we also found that the IL-6 blockade induced a significant increase in both CD8+ and CD4+ effector cells including antigen specific CD8 cells, which provide strong evidence that IL-6 blockade did not interfere with the development of antigen specific/tumor immune responses. Our CyTOF and tumor supernatant cytokine analyses demonstrated that IL-6 blockade may increase the conversion of proinflammatory IL-17–producing Tc17 cells into IL-17– and IFN-γ–producing Tc1/Tc17 hybrid cells in tumors, suggesting Tc1/Tc17 hybrid cells could potentially become more functionally polarized toward antitumor activity. In patient irEC tissue, compared with normal intestinal tissue, we did not find an increase in Tc1 (CD3+CD8+T-bet+), Tc17 (CD3+ CD8+RORγT+), or Tc1/Tc17 hybrid (CD3+CD8+RORγT+T-bet+) cells. Although IL-17–secreting Tc17 cells are crucial for the onset of autoimmunity(Mittrücker et al., 2014), Tc1/Tc17 hybrid cells with expression of granzyme B, perforin, and IFN-γ are capable of mounting strong antitumor activity(Arra et al., 2017; Tajima et al., 2011). Our findings are consistent with previous reports that IL-6 is a key cytokine required for mouse Th17 and Tc17 cell differentiation(Bettelli et al., 2006; Hamada et al., 2009). Since, IL-6 can directly promote tumor growth through several distinct mechanisms, including, but not limited to induction of epithelial–mesenchymal transition(Hunter and Jones, 2015; Wang et al., 2009; Yadav et al., 2011), we think that blocking it can also enhance antitumor response.
We understand that our study does not provide the entire immunologic landscape specific for irAEs, which can be seen as a limitation. However, our objective was studying the IL-6–Th17 pathway that is known to play a major role in pathogenesis of several autoimmune diseases, and by providing evidence from multiple tissue toxicities (colitis, arthritis, encephalitis) both in human and mouse models, we have shown that it also plays a role in irAE biology. We also recognize that IL-6/Th17 is not the only pathway, but other cytokines may be involved. For example, IL-4 plays a role in pathogenesis of two separate tissue autoimmunity; eczematous rash and bronchial asthma(Rathinam et al., 2019; Tameez Ud Din et al., 2020), which have also been reported as ICB-induced irAEs, and targeting it has been therapeutically approved for these two different indications. The role of IL-23 and TNF-α in autoimmunity involving colon, joints, and skin is also well documented. Since it has been reported that IL-23 promote MDSCs-mediated tumor resistance(Calcinotto et al., 2018), blocking it specifically the IL-23 p19 that is not shared with IL-12 p40 can suppress irAEs and potentially enhance antitumor immunity. While a recent study demonstrated that TNF-α could be targeted prophylactically to prevent irEC without affecting antitumor efficacy(Perez-Ruiz et al., 2019), our gene expression data in human irEC and responding tumor tissue suggests that TNF-α may be involved in both colon inflammation and antitumor immunity; this would suggest TNF-α is a less desirable target for inhibition, particularly if it is given early overlapping with ICB. Thus, we believe that targeting certain cytokine pathways can have therapeutic benefits involving several tissue toxicities. We can also argue that irAEs could be classified based on cytokine pathways rather than organ/tissue specificity.
Finally, building on our murine, translational, and clinical data, we planned an investigator initiated phase II clinical trial (NCT04940299) with longitudinal blood, tumor, and inflamed tissue biopsies, to assess the safety and efficacy of tocilizumab in combination with ipilimumab and nivolumab in patients with treatment-naive metastatic melanoma, non-small cell lung cancer, and urothelial carcinoma.
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Adi Diab (adiab@mdanderson.org).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The raw data produced by a NanoString nCounter analysis is reported in Table S2. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODELS AND SUBJECT DETAILS
Human samples
We used RNA gene expression data from tumor tissue obtained from patients enrolled on an ipilimumab-based investigator-initiated clinical trial (NCT02644967; Institutional Review Board protocol 2015-0530). Best overall response per RECIST v1.1 was collected for these patients. Permission from the principal investigator of this trial was granted to use these data.
Formalin-fixed paraffin-embedded normal intestinal and irEC tissue samples from patients who developed irEC while receiving ICB therapy were obtained from the Department of Pathology and Laboratory Medicine at MD Anderson. All tissues were collected according to policies of the institution’s institutional review board under an approved protocol (PA19-0427). One hundred thirty biopsies from 65 patients were collected. Three to four 8- to 10-μm formalin-fixed paraffin-embedded rolls were obtained for gene expression studies. Unstained slides from patient-matched irEC and normal intestinal mucosal samples were obtained from 10 patients for multiplex immunohistochemistry analysis (Table S1).
Mouse models
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center. Eight- to 12-week-old female C57BL/6 and Balb/c mice were purchased from Charles River Laboratory. The variabilities in average tumor burden or EAE disease symptoms among female or male mice were similar. Thus, subsequently female mice were used in experiments. Mice were maintained under specific pathogen free conditions and age-matched littermates were randomly assigned to treatment and control groups in all experiments.
Mouse cell lines
B16.BL6 and the GM-CSF producing B16.BL6-GVAX (GVAX) cell line were obtained from P. Sharma (MD Anderson Cancer Center). CT26 colon carcinoma cell line was obtained from American Type Culture Collection (ATCC). B16.BL6 melanoma, GVAX and CT26 cell lines were maintained in complete medium including RPMI 1640 with 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 μg/ml penicillin (Life Technologies, Carlsbad, CA). Cell lines were periodically tested for mycobacterium contamination. Two tumor models were evaluated in 2 different mouse strains: C57BL/6 strain for B16.BL6 and Balb/c strain for CT26. Irradiated GVAX cell lines were used as tumor vaccine in B16.BL6 tumor bearing mice.
METHOD DETAILS
Gene expression profiling
Total RNA was extracted and purified using the High Pure formalin-fixed paraffin-embedded tissue RNA Isolation Kit (Roche, Indianapolis, IN) according to the manufacturer protocol. RNA was quantified and its quality was assessed using the Infinite 200 PRO NanoQuant reader (Tecan, Mannedorf, Switzerland) according to the manufacturer protocol.
Expression profiling of RNA samples (Table S2) was performed on the nCounter FLEX Instrument using the NanoPCIP panel of 770 genes (NanoString Technologies, Seattle, WA). Briefly, purified RNA was quantitated using the Qubit System (Life Technologies, Carlsbad, CA) and quality-checked using the NanoDrop One (Thermo Scientific, Waltham, MA) and TapeStation 4200 (Agilent, Santa Clara, CA). Fifty nanograms of RNA was hybridized to gene-specific, fluorescent-labeled probes that were then purified on the nCounter Prep Station. The fluorescent-labeled products were then scanned on the nCounter Digital Analyzer.
Gene expression data were analyzed by nSolver 3.0 software (NanoString Technologies), providing differential expression of all 770 genes in the NanoPCIP panel and immune cell profiling. Using expression of genes previously shown to be characteristic of various immune cell subsets, we estimated the abundance of 17 different immune cell subsets.
Multiplex immunohistochemistry assays
The Tyramide Signal Amplification–Based Immunofluorescent Multiplex Assay (Bethyl Labs, Montgomery, TX) was used to stain patient-matched normal intestinal and irEC samples. The tissue sections (Data S1) were stained with antibodies against CD3 (A700-016 at 1:250 dilution; Bethyl Labs), CD8 (A700-044 at 1:250 dilution; Bethyl Labs), CD45 (A700-012 at 1:100 dilution; Bethyl Labs), FOXP3 (A700-034 at 1:250 dilution; Bethyl Labs), T-bet (A700-110 at 1:250 dilution; Bethyl Labs), and RORγT (ACI3208B at 1:25 dilution; BioGenex).
Whole-slide digital image acquisition was performed using the Vis TissueArray software (Visiopharm, Denmark). This entailed segregating the tissue samples into individual samples, and cell segmentation was performed using the AI Deep Learning module of the software, including using cytoplasmic markers to assist in identification of the cell boundary. Segmented cells were then identified in the phenotyping module using all lineage markers focusing on the CD3+ populations. In short, t-SNE clustering was performed to identify cells by grouping them by similar expression of the lineage markers. Output variables (measurable features) were calculated, including counts, areas, and location. These data were used to calculate population percentages and cells per area.
In vivo murine tumor experiments
Mice were shaved on the back and received 100 μl phosphate-buffered saline (subcutaneous, 2.5 × 104 B16.BL6 melanoma cells). Mice were treated (intradermal injection, posterior costal region) with 1 × 106 irradiated (160 Gy) B16.BL6–producing granulocyte-macrophage colony-stimulating factor (GVAX) together with 3× 200 μg (10 mg/kg), 100 μg (5 mg/kg), and 100 μg (5 mg/kg) anti-CTLA-4 (clone 9H10, Bioxcell) at the time points indicated. Balb/c mice received similar treatment, except for GVAX. Mice were administered 200 μg (10 mg/kg) anti-IL-6 (clone MP5-2053, Bioxcell) or IgG1 isotype (control) intraperitoneally at the time points as indicated.
In vivo EAE induction
C57BL/6 or Balb/c mice were injected subcutaneously at the base of the tail with 100 μl emulsion consisting of 1.5 mg/ml myelin-oligodendrocyte glycoprotein35–55 peptide (CPC Scientific) in complete Freund adjuvant containing 10 mg/ml Mycobacterium tuberculosis (Chondrex), accompanied by an intraperitoneal injection of 100 μl (5 μg/ml) pertussis toxin (Thermo Fisher Scientific) on the day of immunization and 2 days later (Bittner et al., 2014). Animals were monitored daily for distress, and clinical signs of EAE were scored as follows: 0, no signs of disease; 1, loss of tone in the tail; 2, hind limb paresis; 3, hind limb paralysis; 4, tetraplegia; 5, moribund.
Tissue preparation and flow staining
Mice were euthanized by CO2 inhalation. Peripheral blood mononuclear cells were collected by tail bleed. On day 14, tumors, spleen or lymph nodes (LNs) were harvested and stored in cold phosphate-buffered saline (Life Technologies). To determine the absolute number of a given cell subset per tumor, we adjusted cell counts quantitated by flow cytometry to absolute weight of tumor. Single-cell suspensions from tissue samples were prepared in phosphate-buffered saline with 10% fetal calf serum and 2Mm EDTA (Sigma-Aldrich, St. Louis, MO) by mashing tissue against the surface of a 40-μm cell strainer using the plunger of a 3-ml syringe (BD biosciences, CA). Lymphocytes from tumor tissue were enriched on a Ficoll gradient (Histopaque 1119; Sigma-Aldrich) and red blood cells were removed from spleen samples using a hypotonic lysis buffer (StemCell Technologies, MA). Blood from mice was drawn using the tail snipping method and red blood cells were lysed using ACK lysis buffer (Thermo Fisher Scientific, MA) to get a peripheral blood mononuclear cell suspension.
Cells harvested from these tissues or in vitro culture were washed with stain media (PBS containing 2% FBS). 2×106 cells were stained for flow cytometry analyses. All fluorochrome-conjugated antibodies were used at 1:200 dilution unless otherwise indicated. Cells were washed again and resuspended in 200 μl of stain media prior to collection and analysis on a BD Fortessa flow cytometer. For intracellular stains, cells were fixed and permeabilized with the Foxp3/Transcription Factor Staining buffer set (Thermo Fisher Scientific) after surface marker staining. Intracellular antibodies were stained in permeabilization buffer for 30 minutes at 4° C. All antibodies used for flow cytometer analyses are listed in Key Resource Table.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse Ly-6C (clone HK1.4) | BioLegend | Cat# 128002; RRID:AB_1134214 |
| Anti-mouse CD4 (clone RM4-5) | BioLegend | Cat# 100506; RRID:AB_312709 |
| Anti-mouse CD11b (clone M1/70) | BioLegend | Cat# 101249; RRID:AB_2562797 |
| Anti-mouse Ly-6G (clone 1A8) | DVS-Fluidigm | Cat# 3141008B; RRID:AB_2814678 |
| Anti-mouse CD11c (clone N418) | DVS-Fluidigm | Cat# 3142003B; RRID:AB_2814737 |
| Anti-mouse CD183 (clone CXCR3-173) | BioLegend | Cat# 126502; RRID:AB_1027635 |
| Anti-mouse IL-2 (clone JES6-5H4) | DVS-Fluidigm | Cat# 3144002B |
| Anti-mouse CD69 (clone H1.2F3) | DVS-Fluidigm | Cat# 3145005B; RRID:AB_2895115 |
| Anti-mouse CD8a (clone 53-6.7) | BioLegend | Cat# 100702; RRID:AB_312741 |
| Anti-mouse CD223, LAG-3 (C9B7W) | BioLegend | Cat# 125202; RRID:AB_961187 |
| Anti-mouse CD19 (clone 4D5) | BioLegend | Cat# 115502; RRID:AB_313637 |
| Anti-mouse CD25 (clone 3C7) | DVS-Fluidigm | Cat# 3150002B; RRID:AB_2687835 |
| Anti-mouse p-Stat3 (Tyr705) (clone M9C6) | Cell Signaling Tech. | Cat# 4113S; RRID:AB_2198588 |
| Anti-mouse CD49d (clone MFR4.B) | BioLegend | Cat# 103701; RRID:AB_313042 |
| Anti-mouse CD274, PD-L1 (clone 10F.9G2) | BioLegend | Cat# 124337; RRID:AB_2783069 |
| Anti-mouse CD272, BTLA (clone 6F7) | DVS-Fluidigm | Cat# 3156028B |
| Anti-mouse Foxp3 (clone FJK-16s) | DVS-Fluidigm | Cat# 3158003A; RRID:AB_2814740 |
| Anti-mouse F4/80 (clone BM8) | DVS-Fluidigm | Cat# 3159009B; RRID:AB_2811238 |
| Anti-mouse CD44 (clone IM7) | BioLegend | Cat# 103002; RRID:AB_312953 |
| Anti-mouse T-bet (clone 4B10) | DVS-Fluidigm | Cat# 3161014B; RRID:AB_2858233 |
| Anti-mouse Bax (clone 5B7) | BioLegend | Cat# 633702; RRID:AB_2064657 |
| Anti-mouse CD62L (clone MEL-14) | DVS-Fluidigm | Cat# 3164003B; RRID:AB_2885021 |
| Anti-mouse IFNg (clone XMG1.2) | DVS-Fluidigm | Cat# 3165003B |
| Anti-mouse Bcl-2 (clone BCL/10C4) | BioLegend | Cat# 633502; RRID:AB_961014 |
| Anti-mouse IL-6 (clone MP5-20F3) | DVS-Fluidigm | Cat# 3167003B |
| Anti-mouse RORgt (clone 600214) | DVS-Fluidigm | Cat# 3168018B; RRID:AB_2687860 |
| Anti-mouse p-Jak2 (clone C80C3) | Cell Signaling Tech. | Cat# 3776; RRID:AB_2617123 |
| Anti-mouse CD49b, Integrin α2 (clone HMα2) | DVS-Fluidigm | Cat# 3170008B; RRID:AB_2814741 |
| Anti-mouse CD279, PD-1 (clone 29F.1A12) | BioLegend | Cat# 135202; RRID:AB_1877121 |
| Anti-mouse Ki67 (clone B56) | DVS-Fluidigm | Cat# 3172024B; RRID:AB_2858243 |
| Anti-mouse Granzyme B (clone GB11) | DVS-Fluidigm | Cat# 3173006B; RRID:AB_2811095 |
| Anti-mouse IL-17A (clone TC11-18H10.1) | BioLegend | Cat# 506935; RRID:AB_2562850 |
| Anti-mouse CD127, IL-7Ra (clone A7R34) | DVS-Fluidigm | Cat# 135029; RRID:AB_2563716 |
| Anti-mouse CD278, ICOS (clone 7E.17G9) | eBioscience | Cat# 14-9942-85; RRID:AB_468633 |
| Anti-mouse I-A/I-E, MHC-II (clone M5/114.15.2) | DVS-Fluidigm | Cat# 3209006B; RRID:AB_2885025 |
| Anti-mouse CD278, ICOS (clone C398.4A) | Biolegend | Cat# 313502; RRID:AB_416326 |
| Anti-mouse CD357, GITR (clone DTA1) | DVS-Fluidigm | Cat# 3143019B |
| Anti-mouse GATA3 (clone TWAJ) | eBioscience | Cat# 14-9966-82; RRID:AB_1210519 |
| Anti-mouse Ly-6G (clone 1A8) | Biolegend | Cat# 127637; RRID:AB_2563784 |
| Anti-mouse CXCR2 (clone SA044G4) | Biolegend | Cat# 149302; RRID:AB_2565277 |
| Anti-mouse CD197, CCR7 (clone SA044G4) | eBioscience | Cat# 16-1971-85; RRID:AB_494123 |
| Anti-mouse CD69 (clone H1.2F3) | Biolegend | Cat# 104533; RRID:AB_2563760 |
| Anti-mouse CD357, GITR (clone CXNFT) | DVS-Fluidigm | Cat# 3161011B |
| Anti-mouse TNFa (clone MP6-XT22) | DVS-Fluidigm | Cat# 3162002B; RRID:AB_2801437 |
| Anti-mouse Ki67 (clone B56) | BD Biosciences | Cat# 556003; RRID:AB_396287 |
| Anti-mouse CD206, MMR (clone C068C2) | DVS-Fluidigm | Cat# 3169021B; RRID:AB_2832249 |
| Anti-mouse CD127, IL-7Ra (clone A7R34) | Biolegend | Cat# 135029; RRID:AB_2563716 |
| Anti-mouse B220, CD45R (clone RA3-6B2) | Biolegend | Cat# 103202; RRID:AB_312987 |
| Anti-mouse CTLA-4 mouse IgG (clone 9H10) | BioXcell | Cat# BP0131; RRID:AB_10950184 |
| Anti-mouse PD-1 mouse IgG2A, K (clone RMP1-14) | BioXcell | Cat# BP0146; RRID:AB_10949053 |
| Anti-mouse IL-6 IgG1 (clone MP5-20F3) | BioXcell | Cat# BE0046; RRID:AB_1107709 |
| Mouse IgG isotype (clone N/A) | BioXcell | Cat# BE0087; RRID:AB_1107782 |
| Rat IgG2a, κ isotype (clone RMP1-14) | BioXcell | Cat# BP0146; RRID:AB_10949053 |
| Rat IgG1 isotype (clone HPRN) | BioXcell | Cat# BE0088; RRID:AB_1107775 |
| Anti-mouse CD11a-PE (clone M17/4) | eBioscience | Cat# 12-0111-82 |
| Anti-mouse TNFa–BV650 (clone MP6-XT22) | BD Biosciences | CAT# 563943; RRID:AB_465544 |
| Anti-mouse CD11c-PE-Cy7 (clone N418) | Biolegend | Cat# 117318; RRID:AB_493568 |
| Anti-mouse CD11c-BV421 (clone N418) | Biolegend | Cat# 117329; RRID:AB_10897814 |
| Anti-mouse CD11b-AF700 (clone M1/70) | eBiosciences | Cat# 56-0112-82; RRID:AB_657585 |
| Anti-mouse Ly6G-BUV395 (clone 1A8) | BD Biosciences | Cat# 563978; RRID:AB_2716852 |
| Anti-mouse CD19-PE Cy5 (clone MB19-1) | Biolegend | Cat# 115510; RRID:AB_313645 |
| Anti-mouse granzyme B-FITC (clone NGZB) | eBiosciences | Cat# 11-8898-80; RRID:AB_10732989 |
| Anti-mouse granzyme B-Efluor 660 (clone NGZB) | eBioscience | Cat# 50-8898-82; RRID:AB_11219679 |
| Anti-mouse CCR7-PE Cy7 (clone 4B12) | Biolegend | Cat# 120124; RRID:AB_2616688 |
| Anti-mouse CCR6-BV605 (clone 29-2L17) | Biolegend | Cat# 129819; RRID:AB_2562513 |
| Anti-mouse Foxp3-APC (clone FJK-16s) | Invitrogen | Cat# 17-5773-82; RRID:AB_469457 |
| Anti-mouse Ki-67-PE Cy7 (clone 16A8) | Biolegend | Cat# 652426; RRID:AB_2632694 |
| Anti-mouse Ki-67-PE (clone 16A8) | Biolegend | Cat# 652403; RRID:AB_2561524 |
| Anti-mouse CD45-BV421 (clone 30-F11) | Biolegend | Cat# 103133; RRID:AB_2562559 |
| Anti-mouse CD45-APC Cy7 (clone 30-F11) | Biolegend | Cat# 103116; RRID:AB_312981 |
| Anti-mouse CD45-AF700 (clone 30-F11) | Biolegend | Cat# 103128; RRID:AB_493715 |
| Anti-mouse CD44-FITC (clone IM7) | Biolegend | Cat# 103022; RRID:AB_493685 |
| Anti-mouse CD44-PE-Cy7 (clone IM7) | Biolegend | Cat# 103029; RRID:AB_830786 |
| Anti-mouse CD4-BV785 (clone GK1.5) | Biolegend | Cat# 100453; RRID:AB_2565843 |
| Anti-mouse F4/80-APC (clone BM8) | Biolegend | Cat# 123116; RRID:AB_893481 |
| Anti-mouse F4/80-BV421 (clone BM8) | Biolegend | Cat# 123132; RRID:AB_11203717 |
| Anti-mouse PD-L1-PE Cy7 (clone 10F.9G2) | Biolegend | Cat# 124314; RRID:AB_10643573 |
| Anti-mouse Bcl-2-Alexa Fluor 647 (clone BCL/10C4) | Biolegend | Cat# 633510; RRID:AB_2274702 |
| Anti-mouse Lag3-BV650 (clone C9B7W) | Biolegend | Cat# 125227; RRID:AB_2687209 |
| Anti-mouse PD-1-APC Cy7 (clone 29F.1A12) | Biolegend | Cat# 135224; RRID:AB_2563523 |
| Anti-mouse Tim-3-PE (clone B8.2C12) | Biolegend | Cat# 134003; RRID:AB_1626181 |
| Anti-mouse ICOS-FITC (clone C398.4A) | Biolegend | Cat# 313506; RRID:AB_416330 |
| Anti-mouse CXCR3-APC (clone CXCR3-173) | Biolegend | Cat# 126511; RRID:AB_1088994 |
| Anti-mouse Ly6c-BV605 (clone AL-21) | BD Biosciences | Cat# 563011; RRID:AB_2737949 |
| Anti-mouse Ly6c-APC (clone AL-21) | BD Biosciences | Cat# 560595; RRID:AB_1727554 |
| Anti-mouse CD25-FITC (clone 3C7) | Biolegend | Cat# 102005; RRID:AB_312854 |
| Anti-mouse Pstat3-Percp-Cy5.5 (clone 4/P-Stat3) | BD Biosciences | Cat# 560114; RRID:AB_1645335 |
| Anti-mouse IL-17A-APC Cy7 (clone TC11-18H10.1) | Biolegend | Cat# 506940; RRID:AB_2565781 |
| Anti-mouse T-bet-BV421 (clone 4B10) | Biolegend | Cat# 644815; RRID:AB_10896427 |
| Anti-mouse T-bet-PE (clone 4B10) | BD Biosciences | Cat# 561265; RRID:AB_10565980 |
| Anti-mouse CD206-AF647 (clone C068C2) | Biolegend | Cat# 141712; RRID:AB_10900420 |
| Anti-mouse CD103-PE (clone 2E7) | eBioscience | Cat# 12-1031-81; RRID:AB_465798 |
| Anti-mouse CCR2-BV510 (clone SA203G11) | Biolegend | Cat# 150617; RRID:AB_2721535 |
| Anti-mouse CXCL9-AF 647 (clone MIG-2F5.5) | Biolegend | Cat# 515606; RRID:AB_1877135 |
| Anti-mouse CCL5-PE (clone 2E9/CCL5) | Biolegend | Cat# 149104; RRID:AB_2564406 |
| Anti-mouse CCL20-AF 700 (clone 114906) | R&D | Cat# IC760N |
| Anti-mouse RORgt-BV650 (clone Q31-378) | Fisher Scientific | Cat# BDB564722 |
| Anti-mouse CD8 - BV711 (clone 53-6.7) | Biolegend | Cat# 100747; RRID:AB_11219594 |
| Anti-human CD3 (clone BL-298-5D12) | Fortis Life Science | Cat# A700-016; RRID:AB_2891817 |
| Anti-human CD8 (clone BLR044F) | Fortis Life Science | Cat# A700-044; RRID:AB_2891843 |
| Anti-human CD4 (clone EPR6855) | Abcam | Cat# AB133616; RRID:AB_2750883 |
| Anti-human T-bet (clone BLR11OH) | Fortis Life Science | Cat# A700-110; RRID:AB_2891907 |
| Anti-human CD45 RO (clone UCH LI) | Cell Signaling | Cat# 55618; RRID:AB_2799491 |
| Anti-human RORγT (clone 6F3.1) | Millipore Sigma | Cat# MABF81; RRID:AB_11205416 |
| Anti-human CTLA-4 (clone CAL49) | Abcam | Cat# AB237712; RRID:AB_2905652 |
| Bacterial and virus strains | ||
| N/A | ||
| Biological samples | ||
| Human FFPE | UT MD Anderson Cancer Center | IRB # PA19-0427 |
| Mouse tumor samples | UT MD Anderson Cancer Center | IACUC # 00000771 |
| Chemicals, peptides, and recombinant proteins | ||
| MOG35-55 (MEVGWYRSPFSRVVHLYRNGK) | CPC Scientific | N/A |
| Pertussis toxin | Thermo Fisher Scientific | Cat# PHZ1174 |
| Freund's Adjuvant, complete | Chondrex | Cat# 7027 |
| Cell-ID Intercalator-Ir | Fludigm | Cat# 201192A |
| Cell-ID™ Cisplatin | Fludigm | Cat# 201064 |
| RPMI 1640 | Corning | Cat# 10-040-CV |
| Pennicilin Streptomycin | Corning | Cat# 30-002-CI |
| 0.05% Trypsin, 0.53nM EDTA | Thermo Fisher | Cat# 25-052-CI |
| Histopaque | Sigma-Aldrich | Cat# 11191 |
| Phosphare Buffered Saline | Corning | Cat# 21-040-CV |
| ACK Lysing Buffer | Gibco | Cat# A10492-01 |
| Fetal Bovine Serum | Gemni Bio-Products | Cat# 110-106 |
| Tyramide Signal Amplification | Fortis Life Science | www.fortislife.com |
| Foxp3/ Transcription factor staining buffer set | eBioscience | Cat # 00-5523-00 |
| Maxpar Fix and perm buffer | Fludigm | Cat #201067 |
| H-2Kb - KSPWFTTL pentamer | PROIMMUNE | Cat # F828-2A-D-828 |
| H-2Kb - SVYDFFVWL pentamer | PROIMMUNE | Cat # F185-2A-D-185 |
| Deposited data | ||
| NanoString gene expression data | This paper | Table S2 |
| Experimental models: Cell lines | ||
| B16.BL6 | UT MD Anderson Cancer Center | N/A |
| B16-GM-CSF | UT MD Anderson Cancer Center | N/A |
| CT26 | ATCC | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6 | Charles River Laboratory | https://www.criver.com |
| Mouse: Balb/c | Charles River Laboratory | https://www.criver.com |
| Oligonucleotides | ||
| N/A | ||
| Recombinant DNA | ||
| N/A | ||
| Software and algorithms | ||
| Helios software 6.5.358 | Fludigm | https://www.fludigm.com/software |
| Helios CyTOF mass cytometer | Fludigm | https://www.fludigm.com/software |
| Vis TissueArray Softwae (v2020.05) | VisioPharm | www.visiopharm.com |
| nSolver 3.0 | NanoString Technologies | https://nanostring.com |
| LSRFORTESSA X-20 | BD Biosciences | www.bdbiosciences.com |
| FlowJo 10.7.1 | FlowJo LLC | N/A |
| Prism 8.0 | GraphPad | N/A |
| Luminex 200 | Millipore Sigma | N/A |
Pentamer staining
H-2Kb–restricted TRP-2 (SVYDFFVWL) and p15E (KSPWFTTL) pentamer purchased from Peptides International. 2x106 single cell suspension prepared from tumor or spleen harvested on day 14 were allocated per staining condition. Cells were washed in staining media and were resuspended in 50ul staining media. 10 μl of Kb/TRP2 or Kb/p15E pentamer was added to the tube containing 50 μl of freshly isolated T cells, mixed gently, and incubated for 15 minutes at room temperature, in the dark. Cells were washed 2 times with staining buffer, cells were stained with anti-CD19, anti-CD8 and anti-CD45. After 2 washes with staining buffer, samples were analyzed using LSRFortessa X-20.
Luminex assay
On day 14 after tumor injection, tumor were surgically removed from euthanized mice. Tumor weight measurements were performed. Tumors were homogenized in 1 ml cold PBS using a glass homogenizer. The homogenates were transferred to 1.5-ml Eppendorf tubes and centrifuged at 13,000 g for 10 minutes at 4°C, and the supernatant was stored at −80°C. The cytokines and chemokines in the supernatant were measured analyzed by Luminex (Millipore) according to the manufacturer’s instructions. Fluorescence signal was measured on a Luminex 200 system, and data were analyzed using Prism software. Final cytokine and chemokine readouts were normalized by tumor weight.
CyTOF
Metal-conjugated antibodies used for CyTOF analyses are presented in Key resource table. The CyTOF assay was performed according to the manufacturer’s instruction. Briefly, 3 × 106 cells were stained with 5mM Cell-ID Cisplatin (Fluidigm, San Francisco, CA) for 5 minutes and quenched with MaxPal Cell Staining Buffer (Fluidigm) using 5 times the volume of the cell suspension. After centrifugation, cell suspensions (50 μl) were incubated with 5 μl of human Fc-receptor blocking solution (Biolegend, San Diego, CA) for 10 minutes and 50 μl of pre-mixed antibody cocktail for 30 minutes. After washing, cells were incubated with 1 ml of cell intercalation solution (125nM MaxPal Intercalator-Ir into 1 ml MaxPal Fix and Pem Buffer, Fluidigm) overnight at 4°C. Cells were centrifuged with MaxPal Water and pelleted. The pelleted cells were suspended with EQ Calibration Beads (Fluidigm) and cell events were acquired by a Helios CyTOF mass cytometer (Fluidigm).
QUANTIFICATION AND STATISTICAL ANALYSIS
Expression profiling of RNA samples was evaluated by log2 fold changes. Differences in the averages of log2 fold changes between irEC and normal intestinal tissue were evaluated using 2-sample t tests. Differences in the averages of log2 fold changes between baseline and on-treatment tumor tissue were evaluated using 2-sample t tests; separate analyses were performed for responders (patients with best overall response per RECIST v1.1 of partial response or better) and non-responders (patients with best overall response per RECIST v1.1 of stable disease or progressive disease). In addition, log2 fold changes between irEC and normal intestinal tissue were compared with log2 fold changes between baseline and on-treatment tumors using 2-sample t tests. P values were corrected using the adjusted false discovery rate determined by the Benjamin-Yekutieli method.
Differences in averages of log2 cell type score fold changes between irEC and normal intestinal tissue were evaluated using 2-sample t tests. Differences in the averages of log2 cell type score fold changes between baseline and on-treatment tumors were evaluated using 2-sample t tests. All statistical tests used a significance level of 0.05.
Multiplex immunohistochemistry studies of markers were summarized by averages and standard deviations. Within-group differences were assessed using the Wilcoxon signed-rank test, and the Wilcoxon rank-sum exact test was performed to test distribution differences between the two groups. Statistical tests used a significance level of 0.05.
Mouse and sample group sizes were n = 5, unless otherwise indicated. Data were analyzed using paired or unpaired t tests, multiple t test, One-way ANOVA where applicable, and differences were considered significant at P < 0.05. The χ2 test was used to analyze the difference in EAE disease incidence. All experiments were performed at least twice, with similar results. Mice were euthanized when tumor size reached ≥200 mm2. Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. All results are expressed as average ± SEM.
Supplementary Material
Table S1. Baseline patient characteristics for the observation and validation cohort (Related to Figure 1).
Table S2. Fold change comparison of each gene from irEC NanoPCIP. (Related to Figures 1 and 2).
Table S3. Characteristics of melanoma patients treated with interleukin-6 blockade for checkpoint inhibitor-induced adverse events (Related to Figure 6).
Acknowledgments
We thank Erica Goodoff, Senior Scientific Editor in the Research Medical Library at The University of Texas MD Anderson Cancer Center, for editing this article. We thank Sung-Nam Cho and Michael Spencer for technical support. This research was supported by Wilkes philanthropist fund. Y.H. is supported by SPORE Melanoma DRP fund, the UT MDACC. D.H.J. received the Conquer Cancer Young Investigator Award. N.A. is supported by NIH (K01AI163412). SE is supported by Foundation for the NIH (FNIH)-PACT. R.N. is supported by NIH (R01HL141966, R01HL143520). S.K. is supported by NIH K08AR079587. Flow Cytometry & Cellular Imaging Core Facility is supported by NIH through the UT MDACC CA016672.
Footnotes
Declaration of Interests
A.D. received Institution Research fund: Bristol Myers and Squibb, Merck, Pfizer , Nektar Therapeutics, Idera Pharmaceuticals, Apexigen and advisory board fees: Bristol Myers and Squibb, Nektar Therapeutics, Idera Pharmaceuticals, Iovance Therapeutics, Apexigen.
References
- Abromson-Leeman S, Bronson RT, and Dorf ME (2009). Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles. Journal of neuroimmunology 215, 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Sbeih H, Ali FS, Naqash AR, Owen DH, Patel S, Otterson GA, Kendra K, Ricciuti B, Chiari R, De Giglio A, et al. (2019). Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, 2738–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, and Rapoport BL (2018). Immune Dysregulation in Cancer Patients Undergoing Immune Checkpoint Inhibitor Treatment and Potential Predictive Strategies for Future Clinical Practice. Frontiers in oncology 8, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arra A, Lingel H, Kuropka B, Pick J, Schnoeder T, Fischer T, Freund C, Pierau M, and Brunner-Weinzierl MC (2017). The differentiation and plasticity of Tc17 cells are regulated by CTLA-4-mediated effects on STATs. Oncoimmunology 6, e1273300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamias G, Delladetsima I, Perdiki M, Siakavellas SI, Goukos D, Papatheodoridis GV, Daikos GL, and Gogas H (2017). Immunological Characteristics of Colitis Associated with Anti-CTLA-4 Antibody Therapy. Cancer investigation 35, 443–455. [DOI] [PubMed] [Google Scholar]
- Basdeo SA, Cluxton D, Sulaimani J, Moran B, Canavan M, Orr C, Veale DJ, Fearon U, and Fletcher JM (2017). Ex-Th17 (Nonclassical Th1) Cells Are Functionally Distinct from Classical Th1 and Th17 Cells and Are Not Constrained by Regulatory T Cells. Journal of immunology (Baltimore, Md : 1950) 198, 2249–2259. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, and Kuchroo VK (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238. [DOI] [PubMed] [Google Scholar]
- Bittner S, Afzali AM, Wiendl H, and Meuth SG (2014). Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, De Bernardis G, Losa M, Mirenda M, Pasquini E, et al. (2018). IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MK, Yang A, Tandon S, Xu Y, Subudhi SK, Roman RA, Heine AI, Pogoriler E, Kuk D, Panageas K, et al. (2011). Evaluation of serum IL-17 levels during ipilimumab therapy: Correlation with colitis. Journal of Clinical Oncology 29, 2505. [Google Scholar]
- Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P, Punt CJ, et al. (2012). PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. Journal of immunotherapy (Hagerstown, Md : 1997) 35, 169–178. [DOI] [PubMed] [Google Scholar]
- El-behi M, Rostami A, and Ciric B (2010). Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 5, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, Miller WH Jr., and Calabrese L (2020). Moving towards personalized treatments of immune-related adverse events. Nature reviews Clinical oncology 17, 504–515. [DOI] [PubMed] [Google Scholar]
- Fang S, Zhang S, Huang Y, Wu Y, Lu Y, Zhong S, Liu X, Wang Y, Li Y, Sun J, et al. (2020). Evidence for Associations Between Th1/Th17 "Hybrid" Phenotype and Altered Lipometabolism in Very Severe Graves Orbitopathy. J Clin Endocrinol Metab 105. [DOI] [PubMed] [Google Scholar]
- Gaublomme JT, Yosef N, Lee Y, Gertner RS, Yang LV, Wu C, Pandolfi PP, Mak T, Satija R, Shalek AK, et al. (2015). Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 163, 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailemichael Y, Woods A, Fu T, He Q, Nielsen MC, Hasan F, Roszik J, Xiao Z, Vianden C, Khong H, et al. (2018). Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. The Journal of clinical investigation 128, 1338–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, and Dutton RW (2009). Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. Journal of immunology (Baltimore, Md : 1950) 182, 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, and Jones SA (2015). IL-6 as a keystone cytokine in health and disease. Nat Immunol 16, 448–457. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Sullivan TJ, Sobel RA, and Allison JP (2002). Cytotoxic T lymphocyte antigen-4 (CTLA-4) limits the expansion of encephalitogenic T cells in experimental autoimmune encephalomyelitis (EAE)-resistant BALB/c mice. Proceedings of the National Academy of Sciences of the United States of America 99, 3013–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N, et al. (2004). A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 126, 989–996. [DOI] [PubMed] [Google Scholar]
- Kalbasi A, and Ribas A (2020). Tumour-intrinsic resistance to immune checkpoint blockade. Nature reviews Immunology 20, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG 3rd, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, et al. (2020). The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 182, 447–462.e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski B, Valaperti A, Bezel P, Steiner UC, Scholtze D, Wieser S, Vonow-Eisenring M, Widmer A, Kohler M, and Franzen D (2021). Analysis of cytokines in serum and bronchoalveolar lavage fluid in patients with immune-checkpoint inhibitor-associated pneumonitis: a cross-sectional case-control study. J Cancer Res Clin Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine 373, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, Bowling P, Hodi FS, Rahma O, Sullivan RJ, et al. (2020). Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 182, 655–671.e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Murali-Krishna K, and Ahmed R (2007). Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. Journal of virology 81, 2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, and Kolls JK (2012). Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 11, 763–776. [DOI] [PubMed] [Google Scholar]
- Miossec P, Korn T, and Kuchroo VK (2009). Interleukin-17 and Type 17 Helper T Cells. New England Journal of Medicine 361, 888–898. [DOI] [PubMed] [Google Scholar]
- Mittrücker HW, Visekruna A, and Huber M (2014). Heterogeneity in the differentiation and function of CD8+ T cells. Archivum immunologiae et therapiae experimentalis 62, 449–458. [DOI] [PubMed] [Google Scholar]
- O'Donnell JS, Teng MWL, and Smyth MJ (2019). Cancer immunoediting and resistance to T cell-based immunotherapy. Nature reviews Clinical oncology 16, 151–167. [DOI] [PubMed] [Google Scholar]
- Ogata A, Tanimura K, Sugimoto T, Inoue H, Urata Y, Matsubara T, Kondo M, Ueki Y, Iwahashi M, Tohma S, et al. (2014). Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis care & research 66, 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, de Andrea C, Rodriguez-Ruiz ME, Perez-Gracia JL, Marquez-Rodas I, et al. (2019). Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432. [DOI] [PubMed] [Google Scholar]
- Postow MA, Sidlow R, and Hellmann MD (2018). Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. The New England journal of medicine 378, 158–168. [DOI] [PubMed] [Google Scholar]
- Rathinam KK, Abraham JJ, and Vijayakumar TM (2019). Dupilumab in the Treatment of Moderate to Severe Asthma: An Evidence-Based Review. Current therapeutic research, clinical and experimental 91, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AP, Harp CT, Noronha A, and Miller SD (2014). The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handbook of clinical neurology 122, 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, Cherukuri S, Parent T, Hardin J, and Walker P (2019). Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners 25, 551–557. [DOI] [PubMed] [Google Scholar]
- Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, Lebbe C, Kirkwood JM, Schachter J, Daniels GA, et al. (2017). Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 35, 3815–3822. [DOI] [PubMed] [Google Scholar]
- Tajima M, Wakita D, Satoh T, Kitamura H, and Nishimura T (2011). IL-17/IFN-γ double producing CD8+ T (Tc17/IFN-γ) cells: a novel cytotoxic T-cell subset converted from Tc17 cells by IL-12. International immunology 23, 751–759. [DOI] [PubMed] [Google Scholar]
- Tameez Ud Din A, Malik I, Arshad D, and Tameez Ud Din A (2020). Dupilumab for Atopic Dermatitis: The Silver Bullet We Have Been Searching for? Cureus 12, e7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Okiyama N, Okune M, Ishitsuka Y, Watanabe R, Furuta J, Ohtsuka M, Otsuka A, Maruyama H, Fujisawa Y, et al. (2017). Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-alpha is a biomarker of nivolumab recativity. J Dermatol Sci 86, 71–73. [DOI] [PubMed] [Google Scholar]
- Thanarajasingam U, Z X, Zhou X, Jaquith J, Li Y, Zeng H (2019). Inflammatory Arthritis Induced by Immune Checkpoint Inhibitor Therapy: A Distinct Clinical Entity and Immunologic Phenotype [abstract] Arthritis Rheumatol 71 (suppl 10). [Google Scholar]
- Valpione S, Pasquali S, Campana LG, Piccin L, Mocellin S, Pigozzo J, and Chiarion-Sileni V (2018). Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. Journal of translational medicine 16, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, Overwijk WW, Restifo NP, Melief CJ, Offringa R, et al. (2001). Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. The Journal of experimental medicine 194, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. (2018). Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 4, 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, and Yu H (2009). IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. The Journal of experimental medicine 206, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, et al. (2017). Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 170, 1120–1133.e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Kumar B, Datta J, Teknos TN, and Kumar P (2011). IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res 9, 1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline patient characteristics for the observation and validation cohort (Related to Figure 1).
Table S2. Fold change comparison of each gene from irEC NanoPCIP. (Related to Figures 1 and 2).
Table S3. Characteristics of melanoma patients treated with interleukin-6 blockade for checkpoint inhibitor-induced adverse events (Related to Figure 6).
Data Availability Statement
The raw data produced by a NanoString nCounter analysis is reported in Table S2. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.