Abstract
In this report we describe the development and evaluation of a fluorogenic PCR assay for the detection of pathogenic Yersinia enterocolitica. The assay targets the chromosomally encoded attachment and invasion gene, ail. Three primer-probe sets (TM1, TM2, and TM3) amplifying different, yet overlapping, regions of ail were examined for their specificity and sensitivity. All three primer-probe sets were able to detect between 0.25 and 0.5 pg of purified Y. enterocolitica DNA. TM1 identified all 26 Y. enterocolitica strains examined. TM3 was able to detect all strains except one, whereas TM2 was unable to detect 10 of the Y. enterocolitica strains tested. None of the primer-probe sets cross-reacted with any of the 21 non-Y. enterocolitica strains examined. When the TM1 set was utilized, the fluorogenic PCR assay was able to detect ≤4 Y. enterocolitica CFU/ml in pure culture and 10 Y. enterocolitica CFU/ml independent of the presence of 108 CFU of contaminating bacteria per ml. This set was also capable of detecting ≤1 CFU of Y. enterocolitica per g of ground pork or feces after a 24-h enrichment in a Yersinia selective broth.
Yersinia enterocolitica is a food-borne pathogen that has been estimated to cause 3,000 to 20,000 cases of human disease annually in the United States (14). Clinical manifestations typically include abdominal pain, fever, diarrhea, and nausea (5, 12). The disease can range from a self-limiting gastroenteritis to a potentially fatal septicemia (5). Human cases of yersiniosis have been attributed to consumption of contaminated milk, water, and tofu, as well as blood transfusions (5). Y. enterocolitica strains are found in both aquatic and animal reservoirs (5, 18). However, healthy swine are the only animals known to harbor human-pathogenic Y. enterocolitica (1). This bacterium is a fecal commensal of swine and is frequently isolated from tongues, tonsils, and pig carcasses (13). To accurately monitor the prevalence of Y. enterocolitica in animals and animal products, rapid, specific, and sensitive methods of identification and quantification are required. The information generated would be useful in identifying on-farm management and processing practices leading to Y. enterocolitica contamination. Modification of such practices would ultimately result in the reduction of Y. enterocolitica transmission from pork products to humans.
PCR is a powerful tool for the detection and identification of organisms, including pathogenic Y. enterocolitica, in animals and foods (2, 11, 14, 17, 19, 31). However, conventional PCR assays typically require lengthy enrichment and DNA extraction protocols, followed by PCR amplification and gel-based detection (4, 23). Newer PCR protocols no longer require gel-based detection but instead rely on cleavage of a fluorogenic probe for automated and specific detection of amplicons. The fluorogenic PCR assay is more specific and sensitive than conventional PCR for detecting pathogens (2, 7, 9, 10, 17, 29). In addition, the assays are performed in a 96-well format such that sampling can be automated, reducing sample-handling time and minimizing cross-contamination. Furthermore, the assay is quantitative, allowing for enumeration of the target present in a given sample.
The fluorogenic PCR assay utilizes the inherent 5′ → 3′ nuclease activity of Taq DNA polymerase to cleave an unextendable fluorescently labeled probe (consisting of an oligonucleotide with a 5′ reporter dye and a 3′ quencher dye) (24). During PCR, the fluorogenic probe anneals to the target DNA downstream of one of the primers and is cleaved during amplification by the 5′ nuclease activity of Taq DNA polymerase. Cleavage releases the fluorescent reporter from the probe and the attached quencher dye (which suppresses fluorescent emission of the reporter dye on the intact probe). Once released, the reporter emits its characteristic fluorescence. Consequently, an increase in reporter fluorescent emission indicates amplification of target DNA (24).
There are six biotypes of Y. enterocolitica; five of these, biotypes 1B, 2, 3, 4, and 5, are considered pathogenic in humans (5). Invariably, strains of these biotypes contain markers associated with virulence, such as the chromosomally coded ail, inv, yst, mfl, and irp genes and the pYV virulence plasmid (15). These are all well-characterized virulence determinants that have been shown to be required to cause disease in animal models (5). The sixth biotype, biotype 1A, is considered avirulent because most strains are noninvasive and devoid of the classical Y. enterocolitica virulence genes (5).
Expression of both plasmid and chromosomal genes is required for Y. enterocolitica virulence. The pYV plasmid of Y. enterocolitica carries many of the genes required for pathogenesis and appears to be an ideal target for identification of pathogenic strains using PCR (5). However, this plasmid has been shown to be difficult to maintain during laboratory culture, and therefore using it as a target in PCR would increase the chances of obtaining a false-negative result (21). Consequently, the pYV plasmid is not an ideal DNA target in a fluorogenic PCR assay.
One of the chromosomal genes required for Y. enterocolitica virulence is the attachment invasion locus (ail) gene (25). The ail gene product has been shown to be involved in attachment, invasion, and serum resistance (5). In addition, Harnett et al., Lambertz et al., and Miller et al. have shown that ail is associated only with disease-causing strains of Y. enterocolitica and not with avirulent biotype 1A strains (16, 23, 25). In contrast, Grant et al. state that ail sequences are detected, at low frequency, in biotype 1A strains, suggesting that ail may not be strictly confined to only pathogenic strains (15). However, in their report they also state that only 8 (4 clinical and 4 nonclinical) out of 111 biotype 1A strains examined hybridized with the ail probe and that 6 (2 clinical and 4 nonclinical) of these 8 strains displayed weak hybridization signals (15). In addition, the presence of these sequences did not correlate with an increase in attachment or invasion compared to positive controls or other 1A strains (15). Therefore, it appears that ail sequences are limited to only invasive and thus pathogenic strains of Y. enterocolitica. Accordingly, amplification of ail-specific sequences from an unknown sample would provide strong evidence of contamination with pathogenic Y. enterocolitica.
In this report we describe the development and evaluation of primers and probes used in a fluorogenic PCR assay amplifying regions of the ail locus and thus detecting pathogenic Y. enterocolitica. Evaluation of the assay included optimization of reaction conditions for each of three candidate primer-probe sets and detection of Y. enterocolitica in ground pork and feces.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains used in this study are listed in Tables 1 and 2. The strains listed in Table 1 were obtained from either the American Type Culture Collection (Manassas, Va.) or the Centers for Disease Control and Prevention (Atlanta, Ga.). Strain NADC 5571 is a Y. enterocolitica strain isolated from a yersiniosis case associated with contaminated chitterlings (Centers for Disease Control and Prevention). DNA from the bacterial strains listed in Table 2 was kindly provided by Vijay Sharma, National Animal Disease Center (NADC), Ames, Iowa. Yersinia strains were grown in either tryptic soy broth (TSB) (Difco, Detroit, Mich.) or ITC broth (30) overnight at 30°C with shaking. Yersinia selective agar (Oxoid, Basingstoke, Hampshire, England) supplemented with cefsulodin (15 μg/ml), Irgasan (4 μg/ml), and novobiocin (2.5 μg/ml) or tryptic soy agar (Difco) was used to enumerate bacteria.
TABLE 1.
Summary of Yersinia strains used in this study and comparison of the specificities of the three candidate primer-probe sets
| Strain
|
Serotype | Origina | Amplification with:
|
|||
|---|---|---|---|---|---|---|
| Species | NADC no. | TM1 | TM2 | TM3 | ||
| Y. bercovieri | 5230 | ATCC | − | − | − | |
| Y. enterocolitica | 5231 | ATCC | + | + | + | |
| Y. enterocolitica | 5232 | ATCC | + | − | + | |
| Y. enterocolitica | 5233 | ATCC | + | + | + | |
| Y. enterocolitica | 5234 | ATCC | + | + | + | |
| Y. enterocolitica | 5235 | ATCC | + | − | + | |
| Y. kristensenii | 5236 | ATCC | − | − | − | |
| Y. enterocolitica | 5237 | ATCC | + | + | − | |
| Y. enterocolitica | 5559 | O:4,32 | CDC | + | + | + |
| Y. enterocolitica | 5560 | O:8 | CDC | + | + | + |
| Y. enterocolitica | 5561 | O:9 | CDC | + | + | + |
| Y. enterocolitica | 5562 | O:18 | CDC | + | − | + |
| Y. enterocolitica | 5563 | O:20 | CDC | + | + | + |
| Y. enterocolitica | 5564 | O:21 | CDC | + | + | + |
| Y. enterocolitica | 5565 | O:13 | CDC | + | + | + |
| Y. enterocolitica | 5566 | O:5,27 | CDC | + | + | + |
| Y. enterocolitica | 5567 | O:1,2,3 | CDC | + | − | + |
| Y. enterocolitica | 5568 | O:2,3 | CDC | + | − | + |
| Y. enterocolitica | 5569 | O:3 | CDC | + | + | + |
| Y. enterocolitica | 5570 | O:3H | CDC | + | + | + |
| Y. enterocolitica | 5571 | CDC | + | − | + | |
| Y. enterocolitica | 5610 | ATCC | + | + | + | |
| Y. enterocolitica | 5611 | ATCC | + | + | + | |
| Y. aldovae | 5612 | ATCC | − | − | − | |
| Y. aldovae | 5613 | ATCC | − | − | − | |
| Y. aldovae | 5614 | ATCC | − | − | − | |
| Y. bercovieri | 5615 | ATCC | − | − | − | |
| Y. frederiksenii | 5616 | ATCC | − | − | − | |
| Y. frederiksenii | 5617 | ATCC | − | − | − | |
| Y. frederiksenii | 5618 | ATCC | − | − | − | |
| Y. intermedia | 5619 | ATCC | − | − | − | |
| Y. intermedia | 5620 | ATCC | − | − | − | |
| Y. intermedia | 5621 | ATCC | − | − | − | |
| Y. kristensenii | 5622 | ATCC | − | − | − | |
| Y. kristensenii | 5623 | ATCC | − | − | − | |
| Y. kristensenii | 5624 | ATCC | − | − | − | |
| Y. mollaretii | 5625 | ATCC | − | − | − | |
| Y. pseudotuberculosis | 8119 | ATCC | − | − | − | |
| Y. pseudotuberculosis | 8120 | ATCC | − | − | − | |
| Y. pseudotuberculosis | 8121 | ATCC | − | − | − | |
| Y. ruckeri | 8122 | ATCC | − | − | − | |
| Y. ruckeri | 8123 | ATCC | − | − | − | |
| Y. enterocolitica | 8177 | Swine | + | + | + | |
| Y. enterocolitica | 8178 | Swine | + | − | + | |
| Y. enterocolitica | 8179 | Swine | + | − | + | |
| Y. enterocolitica | 8180 | Swine | + | − | + | |
| Y. enterocolitica | 8181 | Swine | + | − | + | |
ATCC, American Type Culture Collection; CDC, Centers for Disease Control and Prevention.
TABLE 2.
Specificity of the TM1 set
| Species | Strain | Serotype | Origin | Amplification |
|---|---|---|---|---|
| Escherichia coli | 2409 | O157:H7 | Food | − |
| Escherichia coli | 431 | O101 | Pig, diarrhea | − |
| Escherichia coli | 912 | OX3:H11 | Pig, normal | − |
| Escherichia coli | 1477 | O149:H10 | Pig, diarrhea | − |
| Proteus vulgaris | NAa | NA | Unknown | − |
| Klebsiella pneumoniae | NA | NA | Unknown | − |
| Enterobacter cloacae | NA | NA | Unknown | − |
| Pseudomonas aeruginosa | NA | NA | Unknown | − |
| Salmonella enterica serovar typhimurium | NA | NA | Unknown | − |
| Listeria monocytogenes | NA | 4B | Human | − |
| Campylobacter jejuni | 5095 | NA | Pig feces, ATCCb | − |
| Campylobacter coli | 5096 | NA | Bovine feces, ATCC | − |
NA, not available.
ATCC, American Type Culture Collection.
Primers and probes.
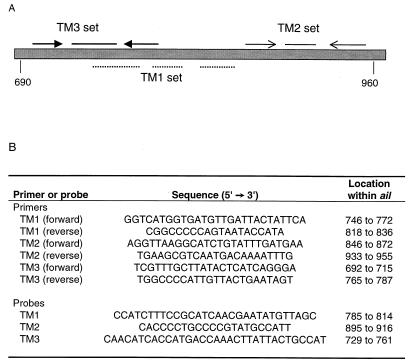
Three independent sets of primers and probes specific for the Y. enterocolitica ail gene were designed with Primer Express software (PE ABI Prism, Foster City, Calif.). Each of the primer-probe sets amplified different, overlapping regions of the ail gene (Fig. 1). Oligonucleotide primers and fluorescently labeled probes were synthesized by Integrated DNA Technologies (Coralville, Iowa), and each probe was labeled at the 5′ end with the fluorescent reporter dye 6-carboxy-fluorescein and at the 3′ end with the quencher dye 6-carboxy-tetramethyl-rhodamine.
FIG. 1.
Locations and nucleotide sequences of the oligonucleotide primers and fluorescently labeled probes used in the fluorogenic PCR assay. (A) Relative locations of the primers and probes used. (B) Ascribed base pair locations based on GenBank sequences (accession number M29945). Probes were conjugated at their 5′ and 3′ ends with the fluorescent dyes 6-carboxy-fluorescein and 6-carboxy-tetramethyl-rhodamine, respectively.
Fluorogenic 5′ nuclease PCR conditions.
PCR conditions were as follows. Each PCR mixture contained 3.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 0.2 mM, 1× GeneAmp PCR Gold Buffer (150 mM Tris-HCl [pH 8.0], 500 mM KCl) (PE Applied Biosystems, Foster City, Calif.), 1.25 U of AmpliTaq Gold DNA polymerase (PE Applied Biosystems), and 5 μl of DNA template in a total volume of 50 μl. Each of the primers was added at a concentration of 200 nM. The probe concentrations were as follows: TM1 probe, 25 nM; TM2 probe, 50 nM; and TM3 probe, 100 nM. The thermal cycling conditions were as follows: 95°C for 10 min and 35 cycles of 95°C for 15 s and 58°C for 1 min followed by an indefinite hold at 25°C.
Data analysis.
PCR were performed in a 96-well format with the PE ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems, Foster City, Calif.). Sequence Detector software, version 1.6.3 (PE Applied Biosystems), was used in the data analysis. This software analyzes the data and then determines a threshold. The threshold is defined as 10 times the standard deviation of the normalized fluorescent emission of the no-template control reactions. Unknown samples that cross the threshold are considered positive. According to PE Applied, Biosystems, a high confidence level is programmed into the analysis software such that if three no-template controls are included per microplate, any unknown sample that crosses the threshold has a 99% confidence level of being a true positive. The cycle at which a sample crosses the threshold is called the cycle threshold (Ct) and is defined as the cycle at which the fluorescence emission exceeds that of the no-template control.
DNA isolation.
Genomic DNA was isolated from pure bacterial cultures by a modified guanidine-silica particle extraction protocol (8). Briefly, 1 ml of bacterial culture was pelleted by centrifugation. The bacterial pellet was resuspended in 0.5 ml of diatom DNA binding solution (1% diatomaceous earth, 6 mM guanidine HCl), frozen at −70°C, and thawed at room temperature to lyse the cells. The samples were centrifuged for 1 min at 14,000 rpm to pellet the diatoms. The diatom pellet was washed with 95% ethanol, and the DNA was eluted by adding 50 μl of distilled H2O and then heating (65°C for 10 min). Some DNA preparations were ethanol precipitated and are referred to below as “clean” or “pure” preparations, whereas samples that were not ethanol precipitated are referred to as “crude.” Prepman reagent (PE Applied Biosystems, Foster City, Calif.) was used to extract bacterial DNA from ground pork and feces according to the manufacturer's suggestions. All DNA samples were stored at −20°C. DNA concentrations were determined spectrophotometrically at 260 nm.
Specificity assay.
To determine the specificity of the three candidate primer-probe sets, DNA from 47 Yersinia (Table 1) and 12 non-Yersinia (Table 2) strains were prepared and used as templates in PCR. Each Yersinia DNA sample was normalized to a concentration of 10 ng/μl and was present in reaction mixtures at a final concentration of 1 ng/μl. The DNA concentrations of the 12 non-Yersinia strain preparations were not determined. All results are the averages of two or more assays, and each sample was examined in duplicate. All standard deviations were within 10% of the mean.
Sensitivity assays.
To evaluate the sensitivity of the fluorogenic assay, the lower limit of purified DNA necessary to generate a fluorescent signal above background was determined. Y. enterocolitica NADC 5231 and NADC 5560 were used as standards. DNA samples were brought to a concentration of 0.1 μg/μl and then serially diluted 10-fold to 1 fg/μl, and 5 μl of each dilution was used per reaction mixture as the template.
The lowest number of Y. enterocolitica cells detectable was determined by growing strain NADC 5571 overnight in ITC broth and TSB at 30°C with shaking. Cultures were serially diluted 10-fold in the same media, and 1-ml aliquots were extracted with the Prepman reagent. DNA preparations were then examined by using the TM1 set. All results are the averages of two or more assays, and each sample was examined in duplicate. All standard deviations were within 10% of the mean. Background fluorescence was determined by using water as the template.
Spiked pork and feces samples.
Ground pork was purchased at a local grocery store and irradiated at the Iowa State University Irradiation Facility in Ames, Iowa. Fecal samples were obtained from disease-free swine housed at the NADC, Ames, Iowa. Five grams of each sample type (pork or feces) was spiked with 5 ml of 10-fold serial dilutions of Y. enterocolitica NADC 5571, followed by addition of 45 ml of ITC broth. The spiked samples were mixed and then incubated at room temperature without shaking for 0, 12, and 24 h. One-milliliter aliquots were removed from each spiked sample, and the DNA was extracted with the Prepman reagent. Unspiked pork and fecal samples were used to determine background fluorescence and were included as controls. All results are the averages of two or more assays, and each sample was examined in duplicate. All standard deviations were within 10% of the mean.
RESULTS
Assay optimization.
The fluorogenic assay was optimized for each candidate primer-probe set by testing a range of MgCl2, primer, and probe concentrations as well as the number of amplification cycles (data not shown). Optimal conditions were determined by comparing the Ct of each reaction under different conditions. The Ct is defined as the cycle at which reporter fluorescence can be detected above the background fluorescent emission. An optimal primer concentration of 200 nM was determined for each of the three candidate primer sets. However, the ideal probe concentrations differed, possibly indicating differences in probe annealing affinities or cleavage rates. For each primer-probe set the optimum cycle number was determined to be 35 and the optimum MgCl2 concentration was 3.5 mM.
Specificity.
Twenty-six Y. enterocolitica strains and nine Yersinia spp. (a total of 47 Yersinia strains) were examined (Table 1). Nine of the 11 Yersinia species were represented (Y. pestis and Y. rohdei were not included), as were all of the predominant disease-causing serogroups found in North America and Europe. Each of the candidate primer-probe sets was examined for its ability to positively identify Y. enterocolitica without cross-reacting with non-Y. enterocolitica spp. The TM1 set was the most specific, amplifying all the Y. enterocolitica strains and none of the non-Y. enterocolitica strains (Table 1). The TM2 set failed to amplify 10 Y. enterocolitica strains, whereas TM3 identified all Y. enterocolitica strains except strain NADC 5237 (Table 1). Despite their inability to detect all Y. enterocolitica strains, neither TM2 nor TM3 displayed any cross-reactivity with the non-Y. enterocolitica strains under optimal PCR conditions (Table 1). These data indicate that the possibility of obtaining false positives with any of the three candidate primer-probe sets in a fluorogenic PCR assay would be negligible.
Since the TM1 set displayed the highest specificity, it was used in all subsequent specificity experiments. Twelve non-Yersinia isolates were examined to determine if the TM1 set would amplify DNA of other bacteria that are commonly found in swine (Table 2). None of the 12 DNA samples tested were amplified by the TM1 set, indicating that this primer-probe set is specific for Y. enterocolitica (Table 2).
Sensitivity.
To assess the minimum amount of Y. enterocolitica DNA detectable by each of the candidate primer-probe sets, 10-fold serial dilutions of known concentrations of purified clean Y. enterocolitica DNA were examined. Each of the three sets could detect between 0.25 and 0.5 pg of purified Y. enterocolitica DNA. However, the sensitivity levels dropped approximately 1,000-fold when crude Y. enterocolitica DNA was assayed with any of the three primer-probe sets, indicating that there are residual fluorogenic PCR inhibitors in crude DNA preparations (data not shown).
The lowest number of Y. enterocolitica cells detectable by the fluorogenic assay using the TM1 set was determined. Strain NADC 5571 was grown overnight at 30°C in TSB and ITC broth and serially diluted 10-fold, and DNA was extracted from 1-ml aliquots with the Prepman reagent. Five microliters of each dilution was subjected to fluorogenic PCR. Several investigators have reported that ITC broth inhibits PCR presumably because of excess MgCl2. However, no statistically significant differences in assay sensitivities were observed between cells harvested from TSB and cells harvested from ITC broth. The TM1 set detected ≤4 CFU of Y. enterocolitica/ml from either TSB or ITC broth.
Most food and animal products typically harbor diverse bacterial populations, and therefore DNA extracted from such samples would contain a combination of different genomic DNA. Therefore, the ability of this assay to detect Y. enterocolitica in the presence of “contaminating” DNA was evaluated. Tenfold serial dilutions of Y. enterocolitica NADC 5571, ranging in concentration from 106 to 100 CFU/ml, were mixed with 108 Y. kristensenii NADC 5236 CFU/ml. DNA was extracted with the Prepman reagent and examined by the fluorogenic PCR assay performed with the TM1 set. The fluorogenic assay was able to detect 10 CFU of Y. enterocolitica/ml independent of the presence of 108 non-Y. enterocolitica CFU/ml.
Detection limits of the fluorogenic assay in pork and feces.
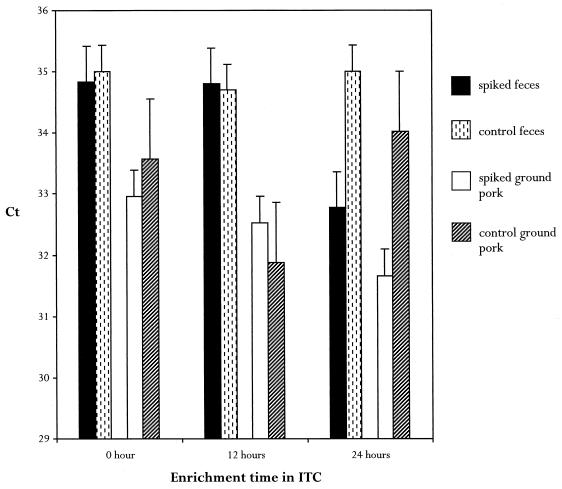
Since the fluorogenic assay would be used to screen animal tissues and meats, the sensitivity of the TM1 set for detecting Y. enterocolitica in swine feces and ground pork was determined. Feces and irradiated ground pork were spiked with 10-fold serial dilutions of known concentrations of Y. enterocolitica NADC 5571 and enriched in ITC broth. Aliquots were removed at selected intervals in order to determine the minimal amount of enrichment time required to detect the lowest numbers of cells. At zero time, the lower limit of detection in pork was 19 Y. enterocolitica CFU per 50-μl reaction mixture, whereas in feces it was 95 CFU per reaction mixture, which represented a fivefold difference. This may indicate that there are specific fluorogenic PCR inhibitors in feces that are not present in ground pork. After 24 h of enrichment, ≤1 CFU/g could be detected in both pork and feces, suggesting that after a short enrichment even extremely low numbers of Y. enterocolitica cells can be detected by this assay (Fig. 2).
FIG. 2.
Detection of 1 CFU of Y. enterocolitica per g of feces or irradiated ground pork after ITC broth enrichment. Five-gram portions of feces or irradiated ground pork were spiked with 1 CFU of Y. enterocolitica/g and enriched in ITC broth. At different times 1-ml aliquots were extracted, and 5 μl was used as the template in the fluorogenic PCR assay performed with the TM1 set. The Ct values were plotted versus enrichment times. Unspiked samples of feces and ground pork were extracted and used as templates to determine background fluorescence. Ct values of spiked samples that approximate Ct values of unspiked controls were considered negative.
DISCUSSION
The objective of this study was to design a sensitive and specific fluorogenic PCR assay to detect pathogenic Y. enterocolitica and to use the assay as a diagnostic tool to screen animals and meats. Of the three candidate primer-probe sets examined, the TM1 set was found to be the best suited for use in the assay.
The TM1 set was the most specific of the three sets examined, differentiating Y. enterocolitica from a broad spectrum of bacteria. TM1 amplified 26 Y. enterocolitica strains without cross-reacting with 12 non-Yersinia strains, some of which are found in swine, and 21 non-Y. enterocolitica Yersinia spp. Moreover, TM1 was able to detect 10 Y. enterocolitica CFU/ml in the presence of 108 nontarget bacteria per ml, illustrating the high level of specificity and sensitivity of this primer-probe set.
The TM1 set was able to detect Y. enterocolitica in pure culture at a concentration of ≤4 CFU/ml. Harnett et al. described a conventional multiplex PCR targeting the ail, yst, and virF genes that detects 5 to 10 Y. enterocolitica CFU/ml, thus demonstrating that conventional PCR can be as sensitive as fluorogenic PCR (16). However, many investigators who used conventional PCR assays have reported less sensitive detection, with the levels of detection typically in the range of 102 to 104 Y. enterocolitica CFU/ml (19, 26, 27, 31).
Currently, there are no reports describing the use of a fluorogenic PCR assay to identify pathogenic Y. enterocolitica; consequently, direct comparison of our assay with another assay is impossible. However, fluorogenic assays describing detection of other food-borne pathogens, such as Listeria, Salmonella, and Escherichia coli, have been reported. The detection limits for pure cultures of Salmonella and E. coli have been determined to be between 2 and 10 CFU per reaction mixture, and the detection limit for Listeria has been determined to be 50 CFU per reaction mixture (2, 9, 22, 28, 29, 32).
PCR inhibitors found in animal tissues and feces can have significant, adverse effects on the efficiency and sensitivity of PCR-based assays. Chen et al. reported detection of 2 Salmonella CFU per reaction mixture when a pure culture was analyzed (9). However, in order to achieve the same level of detection in ground beef or ground pork, overnight enrichment was required (9). We found that sensitivity decreased considerably when Y. enterocolitica was extracted from spiked ground pork and feces compared to samples extracted from pure cultures grown in ITC broth or TSB. However, sensitivity was restored when spiked ground pork and feces were enriched in ITC broth for 24 h. Since the presence of one culturable Y. enterocolitica cell must be detected in food or feces, it is necessary to include an enrichment step that increases the target cell number to detectable levels.
The assay also provides a more rapid means of accurately identifying pathogenic Y. enterocolitica than current methods provide and is conducive to simultaneous screening of a large number of samples. Traditional Y. enterocolitica identification protocols are both time-consuming and laborious (4, 23). They include 1 to 8 days of cold preenrichment plus a 48-h ITC broth enrichment followed by plating onto selective agar, PCR of suspect colonies, and finally gel-based detection of amplicons (4, 23). Use of the fluorogenic PCR assay would significantly reduce the amount of time required to identify Y. enterocolitica. Cold enrichment would be eliminated, enrichment in ITC broth would be for only 24 h, and neither plating nor gel-based detection would be required. This would decrease sample-processing time to approximately 2 days instead of more than 6 days.
Another advantage of the fluorogenic PCR assay is that it allows for quantification of the initial template. We have generated a standard curve for Y. enterocolitica using highly purified DNA samples. According to these standards, very low amounts of pure Y. enterocolitica DNA can be accurately and reproducibly detected. However, when this clean DNA was used as a standard in assays to quantify crude DNA prepared from food or animal samples, the quantitative accuracy was reduced. A standard curve for which crude DNA was used was then generated. However, it was concluded that the quality of DNA varied significantly between samples and that using the crude standard to evaluate the DNA concentration of an unknown sample was not accurate and consequently not quantitative. Nevertheless, crude standards could be used as a qualitative means of measuring the relative level of Y. enterocolitica contamination in animals or foods.
The fluorogenic assay has been compared to other detection methods in a field study. Various tissue samples from 300 market-weight hogs and 350 processed pork samples were examined by three methods: the fluorogenic PCR assay, conventional culturing techniques, and a multiplex PCR assay. Boyapalle et al. found that the fluorogenic Y. enterocolitica assay was three- to fourfold more sensitive than the multiplex PCR assay and ninefold more sensitive than the culturing method for detecting Y. enterocolitica in both hogs and processed pork samples (6). The results demonstrate that the fluorogenic assay is a useful tool for sensitive and accurate detection of Y. enterocolitica.
Some unexpected results worth noting were encountered while this assay was being developed. First, each of the primer-probe sets amplified DNA from different Y. enterocolitica strains (having the same DNA concentration per reaction mixture) with different efficiencies. Harnett et al. observed similar discrepancies while developing a multiplex PCR-based assay using primers derived from the same regions of the ail gene (16). Beer and Miller have shown that there are at least two ail gene variants between American and non-American Y. enterocolitica strains (3). Therefore, it is possible that the sequence of ail targeted in the fluorogenic PCR assay varies slightly in different Y. enterocolitica strains, especially strains isolated from different animal origins. This would interfere with primer and/or probe annealing and thus account for the inconsistencies in amplification and reporter fluorescence emission.
Second, when fluorogenic PCR mixtures containing TM1, TM2, or TM3 are allowed to amplify for more than 35 cycles, some of the non-Y. enterocolitica strains generate a positive signal. Y. pseudotuberculosis has been shown to contain a homologous ail locus which could explain this result (25). Unfortunately, there is insufficient sequence data for other Yersinia spp., and whether these species contain ail is unknown. However, the results obtained during development of this assay suggest that it is likely that Yersinia spp. other than Y. enterocolitica and Y. pseudotuberculosis harbor the ail gene or a version of this gene. Ibrahim et al. have shown that a similar situation occurs with the yst gene of Y. enterocolitica (19, 20). Originally it was reported that yst was specific for pathogenic strains of Y. enterocolitica, but it was later found that Y. kristensenii also harbors a variant form of the yst gene (19, 20).
In summary, the fluorogenic PCR assay described in this report appears to be a promising tool for rapid, sensitive, specific, and automated detection of Y. enterocolitica. In addition, the ability of the assay to accurately identify pathogenic strains of Y. enterocolitica in diverse sample types should benefit food producers, food processors, food safety regulatory agencies, and clinical microbiologists.
ACKNOWLEDGMENTS
We thank the Food Safety Consortium and the National Pork Producers' Council for partial support of this project.
We thank Vijay Sharma, USDA ARS NADC, Ames, Iowa, for kindly providing DNA from nine non-Yersinia bacteria and for critical review of the manuscript. We also thank Fred M. Tatum, USDA ARS NADC, Ames, Iowa, for critical review of the manuscript and Tom Stable, USDA ARS NADC, Ames, Iowa, for providing feces samples from disease-free pigs. Last, we thank Kathryn Becker, PE Applied Biosystems, Sequence Detection Systems, Foster City, Calif., for providing technical assistance.
REFERENCES
- 1.Andersen J K, Sorensen R, Glensbjerg M. Aspects of the epidemiology of Yersinia enterocolitica: a review. Int J Food Microbiol. 1991;13:231–238. doi: 10.1016/0168-1605(91)90007-c. [DOI] [PubMed] [Google Scholar]
- 2.Bassler H A, Flood S A, Livak K J, Mawmaro J, Knorr R, Batt C A. Use of a fluorogenic probe in a PCR-based assay for the detection of Listeria monocytogenes. Appl Environ Microbiol. 1995;61:3724–3728. doi: 10.1128/aem.61.10.3724-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer K B, Miller V L. Amino acid substitution in naturally occurring variants of Ail result in altered invasion activity. J Bacteriol. 1992;174:1360–1369. doi: 10.1128/jb.174.4.1360-1369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaduri S, Cottrell B, Pickard A R. Use of a single procedure for selective enrichment, isolation, and identification of plasmid-bearing virulent Yersinia enterocolitica of various serotypes from pork samples. Appl Environ Microbiol. 1997;63:1657–1660. doi: 10.1128/aem.63.5.1657-1660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottone E. Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev. 1997;10:257–276. doi: 10.1128/cmr.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyapalle S, Kanuganti S, Wesley I V, Reddy P G. Iowa State University Swine Research Report. Ames: Iowa State University; 1999. Comparison of a multiplex and 5′ nuclease PCR assays for the rapid detection of pathogenic Yersinia enterocolitica in swine and pork products; pp. 202–206. [Google Scholar]
- 7.Brandt M E, Padhye A A, Mayer L W, Holloway B P. Utility of random amplified polymorphic DNA PCR and TaqMan automated detection in molecular identification of Aspergillus fumigatus. J Clin Microbiol. 1998;36:2057–2062. doi: 10.1128/jcm.36.7.2057-2062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter M, Milton I. An inexpensive and simple method for DNA purifications on silica particles. Nucleic Acids Res. 1993;21:1044. doi: 10.1093/nar/21.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Yee A, Griffiths M, Larkin C, Yamashiro C T, Behari R, Paszko-Kolva C, Rahn K, de Grandis S A. The evaluation of a fluorogenic polymerase chain reaction assay for the detection of Salmonella species in food commodities. Int J Food Microbiol. 1997;35:239–250. doi: 10.1016/s0168-1605(97)01241-5. [DOI] [PubMed] [Google Scholar]
- 10.Desjardin L E, Chen Y, Perkins M D, Teixeira L, Cave M D, Eisenach K D. Comparison of the ABI 7700 system (TaqMan) and competitive PCR for quantification of IS6110 DNA in sputum during treatment of tuberculosis. J Clin Microbiol. 1998;36:1964–1968. doi: 10.1128/jcm.36.7.1964-1968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng P, Keasler S P, Hill W E. Direct identification of Yersinia enterocolitica in blood by polymerase chain reaction. Transfusion. 1992;32:850–854. doi: 10.1046/j.1537-2995.1992.32993110759.x. [DOI] [PubMed] [Google Scholar]
- 12.Feng P, Weagant S D. Yersinia. In: Hui Y H, Gorham J R, Murrell K D, Cliver D O, editors. Food borne diseases handbook: diseases caused by bacteria. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 427–460. [Google Scholar]
- 13.Funk J A, Trout H F, Isaacson R E, Fossler C P. Prevalence of pathogenic Yersinia enterocolitica in groups of swine at slaughter. J Food Prot. 1998;61:677–682. doi: 10.4315/0362-028x-61.6.677. [DOI] [PubMed] [Google Scholar]
- 14.Gibello A, Blanco M M, Moreno M A, Cutuli M T, Domenech A, Dominguez L, Fernandez-Garayzabal J F. Development of a PCR assay for detection of Yersinia ruckeri in tissues of inoculated and naturally infected trout. Appl Environ Microbiol. 1999;65:346–350. doi: 10.1128/aem.65.1.346-350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant T, Bennett-Wood V, Robins-Browne R M. Identification of virulence-associated characteristics in clinical isolates of Yersinia enterocolitica lacking classical virulence markers. Infect Immun. 1998;66:1113–1120. doi: 10.1128/iai.66.3.1113-1120.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harnett N, Lin Y P, Krishnan C. Detection of pathogenic Yersinia enterocolitica using the multiplex polymerase chain reaction. Epidemiol Infect. 1996;117:59–67. doi: 10.1017/s0950268800001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J A, Ezzell J, Hinnebusch B J, Shipley M, Henchal E A, Ibrahim M S. 5′ nuclease PCR assay to detect Yersinia pestis. J Clin Microbiol. 1998;36:2284–2288. doi: 10.1128/jcm.36.8.2284-2288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurvell B. Zoonotic Yersinia enterocolitica infection: host range, clinical manifestations, and transmission between animals and man. In: Bottone E J, editor. Yersinia enterocolitica. Boca Raton, Fla: CRC Press; 1981. pp. 145–159. [Google Scholar]
- 19.Ibrahim A, Liesack W, Griffiths M W, Robins-Browne R M. Development of a highly specific assay for rapid identification of pathogenic strains of Yersinia enterocolitica based on PCR amplification of the Yersinia heat-stable enterotoxin gene (yst) J Clin Microbiol. 1997;35:1636–1638. doi: 10.1128/jcm.35.6.1636-1638.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim A, Liesack W, Stackebrandt E. Polymerase chain reaction-gene probe detection system specific for pathogenic strains of Yersinia enterocolitica. J Clin Microbiol. 1992;30:1942–1947. doi: 10.1128/jcm.30.8.1942-1947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapperund G. Yersinia enterocolitica in food hygiene. Int J Food Microbiol. 1991;12:53–66. doi: 10.1016/0168-1605(91)90047-s. [DOI] [PubMed] [Google Scholar]
- 22.Kimura B, Kawasaki S, Fujii T, Kusunoki J, Itoh T, Flood S J A. Evaluation of Taqman PCR assay for detecting Salmonella in raw meat and shrimp. J Food Prot. 1999;62:329–335. doi: 10.4315/0362-028x-62.4.329. [DOI] [PubMed] [Google Scholar]
- 23.Lambertz S T, Ballagi-Pordany A, Nilsson A, Norberg P, Danielsson-Tham M-L. A comparison between a PCR method and a conventional culture method for detecting pathogenic Yersinia enterocolitica in food. J Appl Bacteriol. 1996;81:303–308. doi: 10.1111/j.1365-2672.1996.tb04332.x. [DOI] [PubMed] [Google Scholar]
- 24.Livak K J, Flood S J A, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 25.Miller V L, Farmer III J J, Hill W E, Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989;57:121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima H, Inoue M, Mori T, Itoh K-I, Arakawa E, Watanabe H. Detection and identification of Yersinia pseudotuberculosis and pathogenic Yersinia enterocolitica by an improved polymerase chain reaction method. J Clin Microbiol. 1992;30:2484–2486. doi: 10.1128/jcm.30.9.2484-2486.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson A, Lambertz S T, Stalhandske P, Norberg P, Danielsson-Tham M-L. Detection of Yersinia enterocolitica in food by PCR amplification. Lett Appl Microbiol. 1998;26:140–144. doi: 10.1046/j.1472-765x.1998.00296.x. [DOI] [PubMed] [Google Scholar]
- 28.Oberst R D, Hays M P, Bohra L K, Phebus R K, Yamashiro C T, Paszko-Kolva C, Flood S J A, Sargeant J M, Gillespie J R. PCR-based DNA amplification and presumptive detection of Escherichia coli O157:H7 with an internal fluorogenic probe and the 5′ nuclease (TaqMan) assay. Appl Environ Microbiol. 1998;64:3389–3396. doi: 10.1128/aem.64.9.3389-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma V K, Dean-Nystrom E A, Casey T A. Semi-automated fluorogenic PCR assays (TaqMan) for rapid detection of Escherichia coli O157:H7 and other shiga toxigenic E. coli. Mol Cell Probes. 1999;13:291–302. doi: 10.1006/mcpr.1999.0251. [DOI] [PubMed] [Google Scholar]
- 30.Wauters G, Goossens V, Janssens M, Vandepitte J. New enrichment method for isolation of pathogenic Yersinia enterocolitica serogroup O:3 from pork. Appl Environ Microbiol. 1988;54:851–854. doi: 10.1128/aem.54.4.851-854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weynants V, Jadot V, Denoel P A, Tibor A, Letesson J J. Detection of Yersinia enterocolitica serogroup O:3 by a PCR method. J Clin Microbiol. 1996;34:1224–1227. doi: 10.1128/jcm.34.5.1224-1227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witham P K, Yamashiro C T, Livak K J, Batt C A. A PCR-based assay for the detection of Escherichia coli shiga-like toxin genes in ground beef. Appl Environ Microbiol. 1996;62:1347–1353. doi: 10.1128/aem.62.4.1347-1353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]