I f it were not for the great variability among individuals, medicine might as well be a science and not an art.
—Sir William Osler, 1892
William Osler, generally considered the Father of Modern Medicine, commented that variability among individuals represented the main barrier to medical practice transitioning from an art to a science. Variability is intrinsically interconnected with aging. Even at birth, differences between individuals are already evident because of heterogeneity of genetic inheritance, together with varied environmental exposures, as well as maternal lifestyle and behaviors during fetal growth. After birth, the degree of diversity expands dramatically with aging because of complex factors both intrinsic and extrinsic to the individual. Since Osler’s time, average life expectancy has nearly doubled from 45 to 79 years and with the growing percentage of older persons in the population, the variability of older patients has also expanded challenging traditional medical approaches to dealing with their complexity. Evidence-based geriatrics has emerged as a solution to this problem. Today’s geriatricians define the uniqueness of older patients’ needs and the specific nature of clinical expertise required to care for them by relying on direct clinical experiences and a profound knowledge of the published literature.1,2
Real-world clinical care of geriatric patients is anything but predictable. Older patients may present with clinical manifestations, geriatric syndromes, comorbidities, frailty, social needs, susceptibility to treatment side effects, and personal priorities that greatly differ from those seen in younger age groups. Because of such complexity, and despite an important emphasis on universal health outcomes in geriatric medicine,3 it is extremely unlikely that in the course of a day a geriatrician would ever see two patients with exactly the same constellation of clinical problems requiring precisely the same solutions. Indeed, the challenges and rewards of geriatric practice lie in the ability to find “wrinkles” and patterns within such overwhelming complexity that can then be targeted for functional and quality of life improvements.2 A skilled geriatrician needs to possess a deep knowledge of internal medicine, yet must also recognize that such knowledge is often insufficient to address by itself the intricacy of interactions between varied geriatric syndromes, multimorbidities, frailty as well contributing behavioral and environmental factors.4–6
For those dedicated to the care of older patients, it can be surprising and even frustrating that the special needs of geriatric patients are often not recognized by colleagues from other medical disciplines, healthcare administrators, and payers. However, we must admit that part of the problem lies in a scientific literature in geriatrics and gerontology that is still rooted on cross-sectional comparisons between younger and older persons and randomized clinical trials that impose narrow definitions of “cases” and “controls” together with exclusion criteria that are incompatible with the extreme heterogeneity that characterizes older populations. Indeed, in our view, higher-level geriatric training must begin by decreasing the emphasis on “averages” and endorsing the idea that no two older adults are ever exactly alike. Interindividual variability of health and function independent of and expanding with chronological age is a pillar of modern gerontology that justifies the idea of individual biological aging rates. While clinical aspects of this heterogeneity are “obvious,” the evidence in support of increasing heterogeneity as a fundamental feature of aging still remains somewhat vague and general with little support from empirical evidence.7 Indeed, to this day, most published observational or interventional studies of aging do not report or discuss variability in their findings, instead focusing on average differences between groups.
In the context of the above considerations, the report by Nguyen et al8 begins to fill an important void by examining both between-age and within-age heterogeneity using a large and well-established cohort, the Canadian Longitudinal Study on Aging. A total of 34 health characteristics in eight domains (physical measures, vital signs, physiological measures, physical performance, function/disability, chronic conditions, frailty, and laboratory values) were evaluated in 30,097 community-dwelling adults from 45 to 86 years of age. Of the 34 health characteristics studied, 17 showed increased heterogeneity, eight showed decreased heterogeneity, and nine had no association with age. Although these novel findings need to be replicated using other cohorts, they raise two important questions, which we wish to discuss. First, why does heterogeneity involving many parameters increase with aging, while others show no association with age or even decline? Second, is there a path to incorporating these emerging principles into future research studies aimed at improving the care of older adults?
At least theoretically, the increase in heterogeneity, which is seen with many aspects of aging, is not surprising. Aging can be conceptualized as a dynamic accumulation of molecular and cellular damage that is continuously counteracted by resilience strategies that have been evolutionarily selected for their ability to maintain homeostasis and function in the face of varied stressors.9 This dynamic process is expressed across many biological and physiological domains, each one attempting to maintain a system within a reference range of acceptable function. Over time, unrepaired damage accumulates and physiological measures spread outside of homeostatic boundaries and become more heterogeneous. Such increased heterogeneity may or may not then be evidenced in terms of basal variables under homeostatic control. However, hidden heterogeneity can be uncovered by a stressor, as seen when measuring, for example, orthostatic as opposed to merely sitting blood pressures. Therefore, aggregate deviations from homeostatic boundaries across physiological systems can determine heterogeneity of health at the organismal level.
However, not all measures collected in an epidemiological study are under tight homeostatic control, and those that are not may not show increased heterogeneity with aging. For example, fasting glucose is tightly controlled by a quite sophisticated homeostatic system, and, as expected, the heterogeneity of fasting glucose in people who do not self-report diabetes increases with aging (Figure 1A, data from the Baltimore Longitudinal Study of Aging). In contrast, height is established after puberty and is highly variable between individuals. While mechanical factors do influence bone mass, height is not subject to ongoing homeostatic regulation and demonstrates a near-universal decline with aging because of a combination of postural and intervertebral changes. However, the rate of decline is steeper in those, mostly men, who are taller to start with, therefore leading to a regression to the mean and a compression of heterogeneity.10
Figure 1.
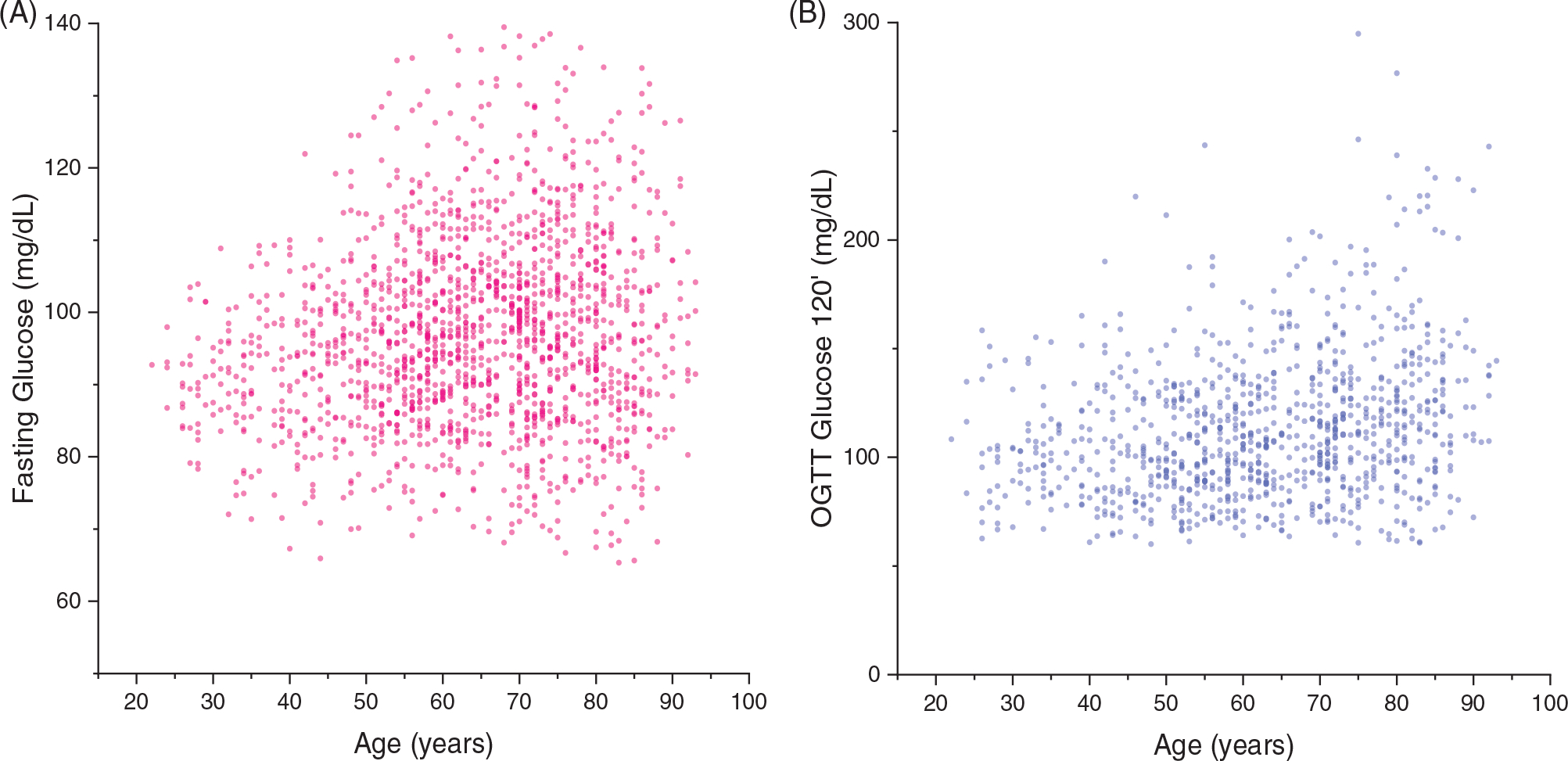
Evidence of increased heterogeneity in glucose handling with aging. Heterogeneity in both fasting glucose in people who do not self-report diabetes (A) and 2-hour glucose after an oral glucose tolerance test among those with fasting glucose less than 100 mg/dL (B) increases substantially with aging (unpublished data from the Baltimore Longitudinal Study of Aging).
It is also important to consider that deviations from the homeostatic equilibrium connected with deteriorations of health and varied comorbidities may confound associations with aging as a result of selective mortality and/or bias in study subject enrollment and retention. This problem is particularly relevant when analyzing cross-sectional studies. For example, a comparison of knee-extension isokinetic strength across age groups shows substantial shrinking of heterogeneity in the oldest age group, probably as a result of a floor effect, whereby those individuals with the lowest strength die or fail to participate for different reasons.11
In view of the above considerations, the inclusion of measures of heterogeneity in research studies will only be useful if clear definitions are developed and validated that can then lead to an enhanced understanding and ultimately improved clinical care paradigms. Nevertheless, we feel that even at this very early stage much can be learned. Indeed, dimensions and biomarkers that show progressive expansion of heterogeneity with aging followed by a decline of heterogeneity at oldest ages when most of expected mortality occurs may represent optimum measures since they indicate both a breaking of the homeostatic equilibrium as well as relevance for health and independence.
As noted, a degree of hidden heterogeneity in these dimensions due to shrinking resilience capacity may only be revealed by challenges involving daily life events. For example, among participants in the Baltimore Longitudinal Study of Aging who had a fasting glucose of less than 100 mg/dL, heterogeneity of a 2-hour glucose after an oral glucose tolerance test increased substantially with aging (Figure 1B). Although it is difficult to generalize from one example, it is possible that patterns of heterogeneity, which change with aging, may help in selecting parameters to be used as biomarkers of aging biology that are relevant for health. The importance of studying variability to better identify biomarkers of aging has been recently underlined in the context of plasma proteins whereby varied proteomic biomarkers undergo patterns of undulating waves of changes at different ages.12
There are also aspects of heterogeneity that may indicate healthy aging. For example, variability in the technical execution of a volitional movement declines with aging and negatively affects function and risk of falls, perhaps because of a reduced ability to respond to unexpected situations due to a lack of flexibility motor control.13 Similarly, there is some evidence that in spite of the accumulation of memory-like immune cells, naïve T cells decline and the heterogeneity of the human CD4+ and CD8+ T-cell receptor repertoire shrinks with aging, as does older adults’ capacity to respond to novel or altered pathogens.14 Again, these are examples of “good” heterogeneity of mechanisms that are not modulated by effective homeostatic mechanisms to maintain them within “normal boundaries.” Finally, there is strong evidence that the underlying physiology maintaining normal physical and cognitive function are redundant and similar functions can be accomplished with different strategies. For example, recent data demonstrate that muscle walking performance in men is affected by peak muscle strength while in women neurological control is most critical.15 Clearly, heterogeneity in these dimensions with aging will have different effects in the two sexes.15
Finally, an interesting aspect that the authors touch upon in their discussion, and one that is particularly important from a translational perspective, is how their research may influence our approach to the calculation of “normative values.” From a clinical perspective, we believe that the “normal” status reflects “extreme health” at any stage of life and that changes in parameters or their heterogeneity that occur with aging should not be used to modify “normal values.” However, as the authors point out, a description of the parameters that change with aging is helpful in understanding individual needs, helping to define and allocate resources, and individualized treatment approaches.
Viewed from the above perspective, development of deeper insights into aging-related heterogeneity can offer remarkable opportunities at enhancing functional outcomes and independence in older adults through improved targeting involving clinical approaches and interventions.7,16 Unlike Precision Medicine, which has been mostly guided by heterogeneity involving inherited genetic factors, the concept of Precision Gerontology encompasses a more holistic approach reflective of the multifactorial complexity of aging.7,16 To that end, heterogeneity of aging may reflect variability involving underlying risk factors, mechanisms, and treatment effects, offering opportunities for improved targeting of shared risk factors, shared mechanisms, and population subsets. Thus, the study of heterogeneity with aging represents important research that opens a new chapter of aging research. These and other novel approaches will be needed as we seek to achieve the ultimate dream of all geriatricians and gerontologists—making a widespread “compression of morbidity” a reality whereby all people follow the same very healthy trajectory until literally moments before dying, and therefore all heterogeneity involving functional declines disappears while our individual personal uniqueness of course remains.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Sponsor’s Role: The sponsor had no role in the writing or approving the text.
Footnotes
Conflict of Interest: The authors have no conflicts.
Contributor Information
Luigi Ferrucci, Intramural Research Program, National Institute on Aging, Baltimore, Maryland.
George A. Kuchel, UConn Center on Aging, UConn Health, Farmington, Connecticut.
REFERENCES
- 1.Warshaw GA, Bragg EJ, Fried LP, Hall WJ. Which patients benefit the most from a geriatrician’s care? Consensus among directors of geriatrics academic programs. J Am Geriatr Soc. 2008;56:1796–1801. [DOI] [PubMed] [Google Scholar]
- 2.Parks SM, Harper GM, Fernandez H, Sauvigne K, Leipzig RM. American Geriatrics Society/Association of Directors of Geriatric Academic Programs curricular milestones for graduating geriatric fellows. J Am Geriatr Soc. 2014;62:930–935. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, McAvay G, Chang SS, et al. Effect of chronic disease-related symptoms and impairments on universal health outcomes in older adults. J Am Geriatr Soc. 2011;59:1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierman AS, Tinetti ME. Precision medicine to precision care: managing multimorbidity. Lancet. 2016;388:2721–2723. [DOI] [PubMed] [Google Scholar]
- 6.Yan S, Seng BJJ, Kwan YH, et al. Identifying heterogeneous health profiles of primary care utilizers and their differential healthcare utilization and mortality—a retrospective cohort study. BMC Fam Pract. 2019;20:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry LC, Ferrucci L, Fortinsky R, Haynes L, Kuchel GA, Robison J. Heterogeneity of Aging: Implications for Team Care and Team Science. Washington, DC: Gerontological Society of America; 2020. [Google Scholar]
- 8.Nguyen QD, Moodie EM, Forget MF, Desmarais P, Keezer MR, Wolfson C. Health heterogeneity in older adults: exploration in the Canadian longitudinal study on aging. J Am Geriatr Soc. 2021;69:678–687. [DOI] [PubMed] [Google Scholar]
- 9.Hadley EC, Kuchel GA, Newman AB, Workshop Speakers and Participants. Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72:980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorkin JD, Muller DC, Andres R. Longitudinal change in height of men and women: implications for interpretation of the body mass index: the Baltimore longitudinal study of aging. Am J Epidemiol. 1999;150:969–977. [DOI] [PubMed] [Google Scholar]
- 11.Moore AZ, Caturegli G, Metter EJ, et al. Difference in muscle quality over the adult life span and biological correlates in the Baltimore longitudinal study of aging. J Am Geriatr Soc. 2014;62:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehallier B, Gate D, Schaum N, et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson C, Perkin OJ, McGuigan MP, Stokes KA. The effect of age on technique variability and outcome variability during a leg press. PLoS One. 2016;11:e0163764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolich-Zugich J The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19:10–19. [DOI] [PubMed] [Google Scholar]
- 15.Osawa Y, Studenski SA, Ferrucci L. Knee extension rate of velocity development affects walking performance differently in men and women. Exp Gerontol. 2018;112:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuchel GA. Function begets function and resilience in old age: is precision gerontology possible? J Am Geriatr Soc. 2017;65:1141–1144. [DOI] [PubMed] [Google Scholar]


