Abstract
The promising outcomes of chimeric antigen receptor (CAR) T cell therapy in hematologic malignancies potentiates its capability in the fight against many cancers. Nevertheless, this immunotherapy modality needs significant improvements for the treatment of solid tumors. Researchers have incrementally identified limitations and constantly pursued better CAR designs. However, even if CAR T cells are armed with optimal killer functions, they must overcome and survive suppressive barriers imposed by the tumor microenvironment (TME). In this review, we will discuss in detail the important role of TME in CAR T cell trafficking and how the intrinsic barriers contribute to an immunosuppressive phenotype and cancer progression. It is of critical importance that preclinical models can closely recapitulate the in vivo TME to better predict CAR T activity. Animal models have contributed immensely to our understanding of human diseases, but the intensive care for the animals and unreliable representation of human biology suggest in vivo models cannot be the sole approach to CAR T cell therapy. On the other hand, in vitro models for CAR T cytotoxic assessment offer valuable insights to mechanistic studies at the single cell level, but they often lack in vivo complexities, inter-individual heterogeneity, or physiologically relevant spatial dimension. Understanding the advantages and limitations of preclinical models and their applications would enable more reliable prediction of better clinical outcomes.
Keywords: CAR T cells, solid tumors, T cell migration, trafficking, tumor microenvironment, immunotherapy, adoptive T cell therapy, 3D in vitro models
1. Introduction
Despite the great progress in understanding the biology of cancer and the discovery of new targeted therapies, cancer continues to be the second leading cause of mortality in the US with 1.8 million new cancer cases diagnosed in 2020 and over 600,000 cancer deaths in the United States [1]. The immune system is our body’s natural defense against predation which includes the development of cancer cells [2,3,4]. Unfortunately, cancer cells are often able to avoid immune destruction [5], leading to tumor formation. T cell immunotherapy has proven effective in restoring immune response against a plethora of malignancies [6] but remains limited by a dearth of antigenic targets. To overcome such obstacles, chimeric antigen receptor (CAR) T cells are designed to fuse the specificity of single-chain variable fragment regions from an antibody to the specific and cytolytic function of T cells, broadening the applicability of this new technology to surface antigen targets. Thus, CAR T cell therapy has emerged as a promising immuno-oncology approach that could enhance target recognition and cytotoxic function against antigen-specific cancer cells without the need for major histocompatibility complex and T cell receptor interactions. CAR T cells from patient-derived lymphocytes are genetically engineered to express an extracellular antibody fragment linked to modified intracellular co-stimulatory domains that induce activation upon engagement to the target antigen [7,8,9] and have led to almost 95% complete remission rates at 1 month post-infusion in patients with B cell leukemias and up to 40% in patients with B cell lymphomas [8]. However, the results have not been translated to solid tumors largely due to major challenges including on-target off-tumor toxicity, cytokine release syndrome, tumor heterogeneity, and poor CAR T trafficking into the immunosuppressive tumor microenvironment (TME).
In this review, we aim to discuss barriers to CAR T trafficking and locomotion within the immunosuppressive TME. The ability of CAR T cells to efficiently migrate to the tumor site, infiltrate suppressive barriers, and survive the harsh TME represents a crucial prerequisite for carrying out the anti-tumor function. To address this important challenge, preclinical models must closely recapitulate the spatial and temporal dynamics of a TME which imposes physical and biochemical barriers against CAR T trafficking and fitness. We will also discuss various preclinical models and platforms that have been employed to study CAR T infiltration and killing of solid tumors [10]. In particular, the complex TME of solid tumors highlights the need for three-dimensional (3D) in vitro models to better study cell–cell and cell–extracellular matrix (ECM) interactions. We discuss how CAR T cells can be engineered to eradicate antigen-specific cancer cells that are heterogeneously expressed, evolving, and shielded by layers of biochemical and physical barriers using novel 3D in vitro models and platforms [11,12,13].
2. CAR T Cell Trafficking and Infiltration
2.1. The Immunosuppressive Tumor Microenvironment and Physical Barriers Favorable to Resilient Tumor Growth
Circulating CAR T cells must gain sufficient traction and adhesion against receptors on the endothelial wall to initiate extravasation (Figure 1). However, secretion of angiogenic factors VEGF and bFGF during tumorigenesis leads to insufficient expression of adhesion molecules—such as intercellular adhesion molecule 1 (ICAM-1), 2 (ICAM-2), and vascular cell adhesion molecule 1 (VCAM-1)—on endothelial cells which prevents efficient T cell engagement [14]. Adhesion molecules expressed on both immune cells and endothelial walls modulate interactions necessary for transendothelial migration (TEM). Although mechanisms of TEM have been reported extensively, identification of a comprehensible approach to enhance the extravasation of T cells remains elusive. Instead, T cells have been engineered to target tumor-associated endothelial receptors such as VEGFR2 to disrupt tumor-supporting vasculature, enhance T cell trafficking, and deprive the tumor of nutrient and oxygen supplies [15,16]. This approach revealed the efficient killing of in vitro tumor spheroids [16] and prolonged survival in animal models [15,17,18].
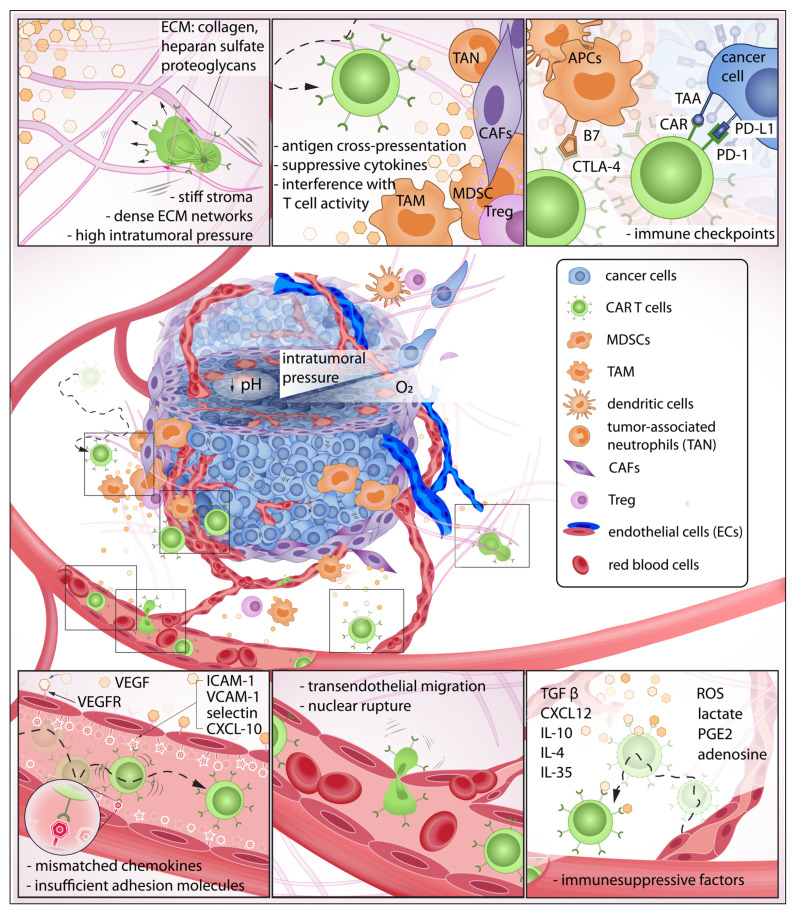
Figure 1.
The exhausting journey of CAR T cells in the TME: In this journey, CAR T cells must be able to detect chemokines at the tumor site, effectively roll and adhere to the blood vessel wall, initiate transendothelial migration, and invade the tumor stroma. Here, the immune cells must overcome various biochemical and physical barriers to then encounter pro-tumor cells that suppress CAR T cell activity. Some CAR T cells may eventually make contact with target cancer cells that express abundant immune checkpoints, further reducing anti-tumor function.
CAR T cells must traverse the endothelial junction and navigate through the tumor stroma that is inherently armed with abundant immunosuppressive factors to reach target cancer cells. The TME has long been identified as an active contributor to cancer progression [5]. In this neoplastic microenvironment, different cell types interact with each other and the surrounding ECM to orchestrate a constantly evolving habitat that is favorable to resilient tumor growth [19,20,21] and deleterious to immune function [22,23,24,25] (Figure 2). For instance, CAR T cells—upon successful infiltration of the TME—demonstrated a rapid loss of function [26]. Infiltrating CAR T isolated from xenograft tumors and cultured in vitro for 24 h regained killing ability superior to those freshly isolated [26]. This emphasizes the immunosuppressive influences of the TME that suppress CAR T anti-tumor activity. Furthermore, the stromal, immune, and cancer cells constantly remodel the TME in response to the surrounding biochemical and biophysical cues [19,27,28,29,30] (Figure 2). Unlike hematologic malignancies—which can be freely accessed by CAR T cells—solid tumors have a complex three-dimensional structure wherein malignant cells are more difficult to access [31,32,33,34]. One hallmark of tumor progression is the elevated stiffening of the ECM due to increased matrix crosslinking, collagen deposition, and fiber alignment which regulate cell migration, proliferation, and apoptosis via mechanotransduction [35,36,37,38,39]. The stiffening effect is often caused by collagen deposition and crosslinking, mediated by increased secretion of lysyl oxidase (LOX) [30,40] and overproduction of other ECM components such as heparan sulfate proteoglycans (HSPGs) [41,42]. Due to this physical constraint, primary tumor growth and cell migration within the TME are greatly dependent on matrix metalloproteinases (MMP) and heparinase enzymes to degrade and reorganize the crosslinked networks [43,44,45,46,47,48]. However, T cells do not often secrete enzymes to degrade ECM, but rather choose the path of least resistance [49] or follow contact guidance imposed by architectural features of the surrounding ECM [50,51]. Therefore, densely packed and oriented stromal fibers have been reported to impose significant challenges to T cell infiltration [52].
Figure 2.
Overview of the immunosuppressive tumor microenvironment (TME) and important contributors to tumor progression: Within the TME, there is a dynamic relationship between peritumoral and intratumoral components that include immune cells, cancer cells, and the stromal elements often exhibiting context-dependent functionality. Different components of the TME carry out pro- or anti-tumor functions, and such polarizations are caused by reciprocal interactions with physical and biochemical cues from the surroundings. The polarized functions are not distinctive but rather heterogenous contributing to an immunosuppressive TME [5,19,20,28,29,35,39,40,46,48,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75].
Tumor growth is critically dependent on angiogenesis [27,76,77] which is promoted in an MMP–dependent manner [43]. Given nutrient support from neovessel networks, the growing tumor continues to modify its microenvironment through elevation of growth-induced solid stress [78,79], interstitial fluid pressure [80,81], enzymatic secretion [40,43], and cellular contractility onto the ECM [68,71]. One example of ECM modification is the physical alignment of collagen fibers due to cell-induced strain stiffening [28,69,82] from cancer cells and their stromal neighbors such as cancer-associated fibroblasts (CAFs) [28,64,67]. This reorganization further stiffens the TME, suppresses immune activity and mobility [83,84], and promotes local invasion of cancer cells along the aligned fibers [55,73,83,85,86,87,88]. In addition, intratumoral pressure caused by growth-induced solid stress and interstitial fluid pressure obstruct delivery of therapeutic agents and trafficking of immune cells to the solid tumors [89]. Elevated interstitial fluid pressure—reported in most solid cancers [90]—is mainly caused by leaky blood vessels, impaired lymphatic transport, and hyaluronan swelling [72,91]. The fluid pressure contributes to an increasing growth-induced solid stress and presents a non-trivial barrier to immune infiltration and uptake of therapeutic agents [90,92]. An in vitro study demonstrated that an interstitial fluid pressure ≥ 1 KPa—simulated by hydrostatic pressure—is sufficient to physically hindered antigen-specific T cell infiltration into tumor site [93]. Furthermore, growth-induced solid stress (≥2 kPa) —while impeding proliferation of cancer cells [78,94] —can collapse blood vessels [72] and contribute to vasculature abnormalities and subsequent increase in interstitial pressure [71]. The reciprocal relationship further escalates total intratumoral pressure, exacerbates hypoxia, and increases pH and metabolic waste accumulation. TME determinants—such as hypoxia [95] and TGFβ [63]—stimulate the production of ECM [23,65,96,97] and LOX [98] to further reinforce the physical barriers that impede CAR T infiltration [33,99]. The feedback loop continually contributes an immunosuppressive TME responsible for tumor pathogenic traits, its progression [40,100,101,102], subtle microevolution [103,104,105,106,107], and metastasis [73,74,75,108,109,110,111]. To overcome these challenges, therapeutic agents for vascular normalization [112] and degradation of ECM components—such as hyaluronidases [113], collagens [114], and LOX inhibition [115]—have been developed to alleviate intratumoral pressure and improve perfusion transport of therapeutic agents and immune cell infiltration [116].
2.2. Immunosuppressive Chemokines: The Invisible Barrier against CAR T Infiltration
The recruitment of CAR T cells to the TME, upon systemic or regional administration, depends largely on efficient chemotactic migration. Without biochemical hints, the probability that CAR T cells localize and migrate to a solid tumor would be low. Within a TME, the local accumulation of suppressive chemokines and cytokines—such as CXCL12 [117,118], TGFβ [63,119], IL-10 [120,121,122], IL-4 [123], IL-35 [124,125] and other factors (e.g., reactive oxygen species (ROS), lactate [126], prostaglandin E2 (PGE2), and adenosine [127])—constitute a first major barrier of immune suppression. The ability to sense and migrate towards a tumor-specific site is a critical prerequisite to immune extravasation and infiltration; however, as a physiological protective mechanism, tumors produce insufficient chemoattractant ligands for T cells. Therefore, there have been significant efforts to increase CAR T chemotaxis and extravasation that endow the local delivery of favorable chemokines and cytokines (e.g., CXCL11 [128], RANTES, and IL-15 [129]); these efforts include the use of intratumorally delivered oncolytic virus to improve CAR T cell recruitment and anti-tumor activity with resulting better survival [128,129,130]. Alternatively, there have been promising strategies that employ CAR T cells as cytokine carriers to promote infiltration and maintenance of a healthy TILs population. CAR T cells—engineered to secrete proinflammatory cytokines, IL-12 [17,131], IL-18 [132], IL-7 [133], and CCL19 [134] —have been reported to augment autocrine stimulation and potentiate paracrine signaling advantageous for survival, persistence, and recruitment of endogenous immune cells.
To enhance locomotion in the TME, CAR T cells are engineered to express receptors specific to tumor-derived chemokines. This approach has shown efficient chemotactic migration and demonstrated remarkable tumor regression in both in vitro and in vivo models. T cells transduced with a retroviral vector to express CXCR2, a receptor of chemokine CXCL1, were able to enhance direct migration toward tumor-derived chemokine [135]. In another study, CCR2—a receptor of chemokine CCL2, co-expressed with a chimeric antigen receptor targeting tumor antigen GD2 [136] (expressed on neuroblastoma) and mesothelin [137] (expressed on malignant pleural mesotheliomas)—demonstrated a significant increase (more than 10-fold) in homing and anti-tumor activity. Our group, Jin et al., has developed CD70-directed CXCR1 or CXCR2-modified CAR T cells that can co-opt IL-8 with robust antitumor activity and long-lasting immunologic memory against glioblastoma, ovarian, and pancreatic cancer xenograft models [138]. This approach has received FDA IND approval soon to be evaluated in a first clinical trial in humans [138,139]. Furthermore, CAR T cells have been designed to co-express dominant-negative receptors of factors (e.g., TGFβ [140], PD-L1 [141]) to resist immune-inhibitory signals in the TME or co-express inverted cytokine receptor [123,142] to reverse suppressive cytokine functions and stimulate anti-tumor activity. In a preclinical study for breast cancer, CAR T cells targeting MUC1 co-expressed with an inverted cytokine receptor comprised of an IL-4 exodomain linked to an IL-7 endodomain reversed inhibitory signals from IL-4 present in the TME and demonstrated durable T cells infiltration and memory formation [143].
2.3. Pro-Tumor Stromal and Immune Cells Are Active Contributors to Immune Suppression
Once CAR T cells invade chemorepellent and physical barriers of the TME, they encounter stromal and immune cell populations that often exhibit context-dependent functionality. Cancer-associated fibroblasts (CAFs) are present in all solid tumors and represent a major component of the reactive tumor stroma integral to the development and maintenance of a complex TME [144]. CAFs are responsible for abundant production of ECM, crosslinking enzymes, and cytokines that fortify the tumor and constitute a complex and heterogenous barrier against immune attacks [145]. CAFs are major contributors to the production of immunosuppressive cytokines, such as TGFβ, which have also been shown to interfere with T cell migration and infiltration into tumors [63,146]. CAFs have been reported to cross-present antigen and kill CD8+T cells in an antigen-dependent manner via PD-L2 and FASL expression [147] and recruit other inhibitory immune cells to participate in suppressing anti-tumor function [61]. Direct immunotherapeutic attenuation of CAF activity shows promising improvement of T cell trafficking and infiltration [148]. Kakarla et al. developed CAR T cells targeting fibroblast activation protein (FAP), demonstrating a significant reduction in FAP-positive stromal cells and tumor growth in murine models. A combined application of FAP-CAR T and tumor-antigen CAR T cells resulted in anti-tumor activity superior to treatment with either CAR T alone [149]. Despite generally being known for pro-tumor function, the role of CAFs in tumor progression is not totally understood. Depletion of CAFs accelerated pancreatic ductal adenocarcinoma progression and reduced survival in transgenic mice models [150]. Although each cellular component of a TME exhibits a supportive role in tumor progression, their existing anti-tumor functions further complicate therapeutic strategies.
Tumor initiation and progression lead to inflammatory reactions that trigger recruitment and repair mechanisms by innate and adaptive immune response [151]. In the complex TME, cancer, stromal, and immune cell populations continuously evolve. The pro-tumor immune subsets within the TME generally comprise tumor-associated macrophages (TAM) [56,152], myeloid-derived suppressor cells (MDSCs), tumor-associated neutrophils (TAN), and regulatory T cells (Treg) [54,62,153]. Macrophages and dendritic cells (DCs) are important myeloid cells of the innate immune system. TAM often adopt an M2- macrophage phenotype [154] which is anti-inflammatory and pro-tumorigenic [155,156]. TAMs secrete CCL2, which recruits and activates additional TAMs. M2 TAMs have revealed pro-tumor functions and immune suppressive effects via secretion of epidermal and angiogenic factors, IL10 and TGFβ [157]. CAR T cells targeting M2-like TAMs in murine ovarian carcinoma, colon adenocarcinoma, and melanoma models reprogrammed the TME with enrichment of pro-inflammatory monocytes and activated CD8+T cells [158].
MDSCs, a heterogeneous population of immature myeloid cells, are recruited into the tumor by various chemokines CCL1, CCL2, CCL5, or CXCL5 [153,159]. MDSCs can be divided into two major subtypes: granulocytic and monocytic. Granulocytic MDSCs secrete reactive oxygen species (ROS), whereas monocytic MDSCs produce increased levels of nitric oxide derivatives resulting in decreased T cell immune responses [60,160]. A MUC1-directed CAR T cell approach concomitantly targeting tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TR2) expressed on MDSCs demonstrated enhanced antitumor tumor activity in breast cancers enriched with MDSCs and TME remodeling [161]. In addition, there is a growing body of data on the anti-antitumor immunity of TANs. The role of TANs seems to vary based on the type of solid tumors [162]. It has been described that TANs recruit MDSCs, TAMs, and Tregs to TME through secretion of IL-4, IL-10, arginine-1, and ROS which have inhibitory effects on cytotoxic T cells [162].
Tregs are cells born in the thymus and express CD4+ FoxP3. Tregs within the TME can act antagonistically to effector T cells by secreting a number of cytokines (e.g., IL10, IL35, TGFβ), upregulating cytotoxic T lymphocyte antigen 4 (CTLA4), and inhibiting CD80/CD86 co-stimulatory pathways, by competing for binding of IL-2 [163]. An agonist antibody specific against glucocorticoid-induced TNFR-related receptor (GITR) decreased Treg mediated immunosuppression which correlated with augmented anti-glioblastoma immune response [164]. Once in the TME, CAR T cells must overcome checkpoint inhibitory signals. Checkpoints (e.g., PD-L1, GITR) are physiological brakes that prevent autoimmunity [158,159,160]. However, cancer cells abuse such mechanisms to avoid immune surveillance, and the complex TME further supports cancer cells by employing multifactorial and reciprocal pathways advantageous to immune suppression. For instance, IFNγ release during CAR T cell activation and killing of tumor cells induces upregulation of immune checkpoint proteins (e.g., PD-L1) which attenuate the anti-tumor function of cytotoxic T cells [165]. On the other hand, cancer-associated cells can be reprogrammed to target cancer cells. Reinhard et al. revealed nanoparticles delivering RNA encoding for claudin 6—a developmental antigen—can transduce antigen presenting cells to express the immunological targets and enhance CAR T expansion and tumor regression in a mouse model [166].
2.4. Physical Confinement and Mechanical Properties of Cell Nucleus Modulate T Cell Locomotion
T cell locomotion within the TME is an important aspect of immuno-oncology and has been extensively studied [50,51,167,168]. T cells are capable of dynamically adapting to various modes of migration depending on ECM composition within the TME [50,168,169]. However, the mechanism behind this adaptation has not been clearly explained. A better understanding of T cell migration will help enhance CAR T trafficking to solid tumors. The modes of cell migration are often regulated by the density of binding sites on substrate [170], proteolytic activity [171], actomyosin contractility [50,172], microtubule stability [51], and geometrical and mechanical properties of the confined surroundings [51,53,167,169,173]. T cells are fast migrators, for they need rapid scanning abilities to carry out efficient immunological response. Two-photon microscopy imaging of intact mouse lymph nodes revealed that T cells can migrate at an average speed of 10 µm min−1 and peak at 25 µm min−1 [174]. Unlike cancer and stromal cells that actively secrete protease enzymes to degrade the ECM [59] and migrate via focal adhesion mediated attachment, T cells do not need mature focal adhesions and prefer to explore the TME via paths of least resistance [168,175,176]. In other words, leukocytes probe their surroundings and selectively squeeze through accessible pores without proteolytically breaking down ECM to create paths for migration. Although capable of integrin-dependent migration, T cells only form short-lived adhesion complexes to facilitate rapid detachment and engagement onto the next location [177]. The most common mode of T cell migration is amoeboid which employs coordinated membrane extrusion, cell contractility, and contact-induced traction in confined spaces to move forward [168,177]. Amoeboid migration is critically dependent on cortical contractility regulated via the Rho/ROCK pathway and myosin II activity [50]. It has been reported that inducing actomyosin contractility by increasing myosin II or activation of the Rho/ROCK pathway is sufficient to transform cell mode of migration into amoeboid [172,178,179]. Besides cell contractility, proper geometrical confinement is an essential determinant of amoeboid migration [169]. T cells protrude oscillatory leading-edge membrane blebs into narrow pores, probe for potential paths, and generate contraction-mediated retrograde actin flow beneath the cell membranes to transmit equal and opposite frictional forces onto the substrate to drive the cell forward [50,168].
The compliance of the cells can better facilitate constricted migration [55]. The deformability of a cell depends significantly on the mechanical property of its nucleus [180,181]. During transendothelial migration through tight spaces, CAR T may be subjected to nuclear rupture which could severely impact cell function [182]. Major determinants of nuclear stiffness are lamin A/C content and chromatin decondensation which are mechanically regulated by force transmission between cell–cell and cell–matrix interaction via cytoskeleton and LINC complex [70]. Lamin A is known to be responsible for the viscous portion of the nucleus which leads to plastic deformation post-migration, whereas lamin B is responsible for the elastic portion promoting shape recovery after deformation [183,184]. Although high lamin A expression impedes migration through small pores (<3 µm in dia.), it promotes cell survival against stress-induced apoptosis by upregulation of DNA damage repair protein, HSP90 [185,186].
Furthermore, cells are well-known mechanosensors [36,70,187,188,189,190,191] and can alter their mechanical properties and mode of migration according to the stiffness of local surroundings [51,55,57,58]. On a soft substrate, lamin A/C phosphorylation is increased; whereas on stiff substrate, myosin II-generated tension promotes dephosphorylation and stabilization of lamin A/C levels which in turn enhance nuclear stiffness [192]. Moreover, mechanotransduction is a key regulator in T cell activation [193,194] and cytotoxic activity [191]. In the context of T cell migration, the mechanical stiffness of the substrate is important for durotaxis and contact guidance-directed locomotion in 3D [51]. T cells apply traction forces (100 pN) via TCR to physically probe the rigidity of their surroundings [189] and migrate along with the aligned ECM fibers [195]. Although matrix optimal confinement and alignment could induce rapid T cell amoeboid migration, there is a limit to which cells are physically trapped and suppressed. For instance, high-density collagen deposition and crosslinking have been shown to suppress proliferation and anti-tumor activity of infiltrating CD8+ T cells in triple-negative breast cancer tissue explants [84]. Another study shows that a densely packed and oriented stromal fibers at the perivascular niche and around the tumor impose a significant challenge to T cell infiltration [52]. Recently, Caruana et al. developed CAR T cells expressing heparinase to degrade HSPGs and promote tumor infiltration. In xenografted mouse models, the strategy improved T cell capability to degrade ECM, infiltrate, and carry out antitumor activity [47].
3. Preclinical Models for Trafficking and Infiltration
In this section, preclinical approaches aimed to improve CAR T cell trafficking and survival are summarized. While most inhibitory barriers of the TME mentioned in the previous section have been considered and targeted, much work remains to be studied for better translation to clinical outcomes (Table 1).
Table 1.
Preclinical CAR T cell approaches to improve trafficking.
| Reference | Tumor | TME | Appproach | Outcome(s) |
|---|---|---|---|---|
| [196] | B16 melanoma | Vasculature | VEFGR-2 CAR T cells in combination with anti-VEFG-A ligand antibody |
Combination of anti-VEFG-A ligand to overcome competition enhances CAR T cell activity |
| [197] | Murine breast carcinoma | ECM | Engineered macrophages to HER2 CAR T cells and production of metalloproteases as byproduct of activation | In addition to controlling HER2+ tumors, this approach decreased collagen deposition and facilitated T cell infiltration |
| [47] | Stroma-rich solid tumors | ECM | CAR T cells expressing enzyme heparanase (HPSE) | The expression of HPSE improves CAR T cell infiltration through degradation of the ECM |
| [198] | Breast, colon, melanoma, fibrosarcoma |
ECM | Fibroblast activated protein targeted CAR T cells (FAP-CAR T cells) | Toxicity and off-target effects in bone marrow killed mouse models and therapy showed limited antitumor results |
| [148] | Carcinomas | ECM | FAP-CAR T cells | Decreased tumor density and reduced autochthonous pancreatic cancer growth |
| [149] | Non-small cell lung carcinoma |
ECM | FAP-CAR T cells | Reduction in FAP positive stroma and improved antitumor killing alone or when paired with anti-EpHA2 CAR T cells |
| [199] | Hepatocellular carcinoma (HCC) |
Migration | CXCR2-expressing CAR T cells | Improved T cell migration and accumulation at the tumor site compared to control |
| [200] | Solid tumors | Hypoxemia | CD19 and B cell maturation antigen (BCMA) CAR T cells at normoxic and hypoxic oxygen levels | Hypoxic conditions attenuated CAR T cell expansion, differentiation to CD8 T cells, and cytokine production |
| [201] | Solid tumor | Hypoxemia | CAR T cells were transcriptionally paired to hypoxia response elements (HREs) including hypoxia-inducible-1 factor-alpha (HIF1α) | Hypoxia-induced CAR T cells showed improved antitumor activity in hypoxia compared with normoxia |
| [166] | Solid tumor | Immune cells | Delivery of developmental antigen-encoding RNA via nanoparticles | RNA loaded nanoparticles enhanced expansion and activity of CAR T cells in claudin-expression solid tumors |
3.1. In Vivo Models
In vivo murine models have provided a great understanding of anti-tumor CAR T cell activity [139]. The preclinical murine models can be divided into two major groups: immunocompromised and immunocompetent models. Frequently utilized models encompass immunosuppressed, NSG (e.g., NOD SCID) mice where human tumor cells and adoptively transferred immune cells can survive and persist in the host. However, these immune-suppressed models lack autologous immune cells, thus providing unreliable representation of a TME [202]. To overcome such limitations, immunodeficient mice are transplanted with human CD34+ hematopoietic stem cells (HSCs) to reconstitute with human immune components. Ideally, autologous patient-derived tumor xenografts (humanized PDXs) can be implanted in those humanized murine models to closely recapitulate the interactions between the TME-associated stromal and immune components and immunotherapeutic interventions (e.g., CAR T cells) seen in human patients [139,203,204]. Given the low rate of tumor engraftment in autologous systems, there have been multiple approaches to improve tumor implantation such as using: (1) closely HLA matched HSCs, which might represent a potential confounder of allogeneic immunoreactivity; (2) expanded autologous-infiltrating T cells; or (3) engineering HSCs to secrete cytokines that favorably condition the host environment for immune cells [205,206,207,208].
In immunocompetent systems, the TME can be studied as transplantable models where murine tumor cell lines harbor multiple evolving mutations which does not follow the sequential mutational evolution of human cancers [202,209]. The emergence of genetically engineered mouse models (GEMMs) has gotten us closer to what actually happens in tumorigenesis, tumor progression, and metastasis in human patients. Particularly, advanced GEMMs with patient-relevant mutations are engineered to model clinically pertinent tumors that might behave differently to immunotherapies compared with their null counterparts (e.g., Trp53 hotspot mutations, RING-specific BRCA1 mutations) [210,211,212,213]. GEMM models allow for a more accurate mirroring of human tumor heterogeneity in an immunocompetent environment where immunotherapies (e.g., CAR T cells) can be studied more faithfully [214,215,216]. Using these models, CAR T cell migration can be evaluated by a variety of imaging techniques including reporter gene-transfected CAR T cells (e.g., luciferase), in vivo imaging using two-photon intravital microscopy techniques, and magnetic particle imaging (MPI) that leverages the use of nanoparticle cell tracers [138,139,217,218]. These immunocompetent murine models however are not perfect replicates of human immune biology.
There is increasing interest in preclinical large animal models, such canine models and oncopig models. In comparative oncology that studies animal tumor models that develop cancers spontaneously, pet dog models are gaining significant attraction as they are one step closer to real world cancers given not only clinical and molecular similarities but also patient heterogeneity. A number of trials using different immunotherapy approaches—including CAR T cells—are used to treat HER2+ osteosarcoma, B7-H3+ sarcomas, and gliomas [4,219]. However, the paucity of patient recruitment similarly seen in human trials might need more expeditious cancer models. Genetically engineered pigs (oncopigs or OCMs) represent an exciting approach to study large animal models with substantial anatomic, genomic, immunologic, and tumor micro niche similarities; patient heterogeneity; and, additionally, a cohort of oncopigs could potentially be readily available for correlative in vivo studies simultaneously carried out with human clinical trials [220]. The oncopigs can be genetically modified to express patient-relevant mutations, such as the model that harbors a Cre-recombinase induced expression of KRAS and TP53 mutations, that have been identified in many human cancers [221]. The immune profiling of pigs demonstrates the high correlation between their innate and adaptive immune systems as well as the intratumoral T cell milieu and the immune signature of human cancers [222,223,224,225,226]. Further characterization of the whole immune repertoire of tumors developed in oncopigs will allow better understanding of the role of TME and its crosstalk with CAR T cells for better optimization of adoptive T cell therapies. The goal of this review however will not focus on covering the novelties and advantages of genetically modified small and large animal cancer models in detail.
Despite cell trafficking being one of the major limitations of CAR T cell activity, most of the clinical trials focused largely on targeting single tumor-specific immune targets. We have identified relevant clinical trials proposed to improve CAR T cell trafficking by locoregional delivery of immune cells, genetically modified to express chemokine receptors (e.g., CCR4 and CXCR5) or to reprogram the TME. Locoregional delivery of CAR T cells is an attempt to secure direct delivery of CAR T cells bypassing peripheral blood circulation and perhaps the blood–brain barrier as in the case of brain tumors. Our group has taken advantage of IL-8 tumor secretion and genetically engineered CD70-directed CAR T cells to constitutively expressed IL-8 receptor to be tested in a first-in-human trial (NCT05353530) [138]. Furthermore, novel approaches are under investigation to positively reprogram the TME to enhance CAR T cell migration into tumor niche (Table 2) [227].
Table 2.
Human clinical trials with strategies to enhance CAR T trafficking.
| NCT | Title | Phase | Author(s) | Disease(s) | Approach | Status |
|---|---|---|---|---|---|---|
| NCT04185038 | Study of B7-H3-Specific CAR T Cell Locoregional Immunotherapy for Diffuse Intrinsic Pontine Glioma/Diffuse Midline Glioma and Recurrent or Refractory Pediatric Central Nervous System Tumors | I | Vitanza et al. | DIPG/recurrent pediatric CNS tumors | Locoregional delivery targeting B7-H3 |
Recruiting |
| NCT03696030 | HER2-CAR T Cells in Treating Patients with Recurrent Brain or Leptomeningeal Metastases | I | Pornow et al. |
CNS metastasis | Intraventricular delivery of HER2 CAR T cells for CNS metastasis from HER2+ tumors. |
Recruiting |
| NCT03638167 | EGFR806-specific CAR T Cell Locoregional Immunotherapy for EGFR-positive Recurrent or Refractory Pediatric CNS Tumors |
I | Gust et al. | EGFR-positive recurrent or refractory pediatric CNS tumors | Locoregional delivery targeting EGFR806 |
Recruiting |
| NCT03283631 | Intracerebral EGFR-vIII CAR-T Cells for Recurrent GBM (INTERCEPT) | I | Landi et al. | Recurrent GBM | Intracerebral EGFR-vIII CAR T cells | Terminated. Patient enrollment was halted. |
| NCT04153799 | Study of CXCR5 Modified EGFE Chimeric Antigen Receptor Autologous T cells in EGFR-Positive Patients with Advanced Non-Small Cell Lung Cancer | I | Zhang et al. | Advanced non-small-cell lung cancer | CXCR5 modified CAR T cells targeting EGFR | Recruiting |
| NCT03602157 | Study of CAR-T Cells Expressing CD30 and CCR4 for r/r CD30+ HL and CTCL | I | Grover et al. | Relapsed and recurrent Hodgkin lymphoma and cutaneous T cell lymphoma |
CCR4 modified CAR T cells targeting CD30 | Recruiting |
| NCT05081479 | A Study of N-Acetylcysteine (N-AC) in People Receiving CAR T cell Therapy for Lymphoma | I | Batlevi et al. | B cell lymphoma | Reduce tumor reactive oxygen species to condition lymphoma TME for CD19 CAR T cells | Recruiting |
| NCT04976218 | TGFβR-KO CAR-EGFR T Cells in Previously Treated Advanced EGFR-positive Solid Tumors | I | Han et al. | EGFR-positive solid tumors | CAR T cells resistant to TGFβ receptor |
Recruiting |
| NCT03740256 | Binary Oncolytic Adenovirus in Combination with HER2-Specific Autologous CAR VST, Advanced HER2 Positive Solid Tumors (VISTA) | I | Wang et al. | Advanced HER2+ solid tumors | Oncolytic adenovirus delivers PDL1 blocking mini antibody to enhance CAR T cell killing | Recruiting |
| NCT04381741 | CD19 CAR-T Expressing IL7 and CCL19 Combined with PD1 mAb for Relapsed or Refractory Diffuse Large B Cell Lymphoma (CICPD) | I | Qian and Liu et al. | Recurrent or relapsed diffuse large B cell lymphoma |
CAR T cells are potentiated with co-expression of IL-7 and CCL19 to better migrate into the TME and enhanced T cell fitness. This approach is also combined with PD1 blocking antibody. | Recruiting |
| NCT03545815 | Study of CRISPR-Cas9 Mediated PD-1 and TCR Gene-Knocked Out Mesothelin-Directed CAR-T Cells in Patients with Mesothelin Positive Multiple Solid Tumors | I | Han et al. | Mesothelin-directed CAR T cells | CAR T cells have PD1 TCR receptor knocked out to enhance their activity |
Recruiting |
| NCT02706405 | JCAR014 and Durvalumab in Treating Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma | 1b | Gauthier et al. |
B cell non-Hodgkin lymphoma | PDL1 blocking antibody to improved CD19 CAR T cells |
Terminated due to slow accrual |
| NCT03070327 | BCMA Targeted CAR T Cells with or without Lenalidomide for the Treatment of Multiple Myeloma |
I | Mailankody et al. | Multiple myeloma | Lenalidomide has been shown to inhibit regulatory T cells and activate CD8 T cells | Active, not recruiting |
To complement these models, we discuss three-dimensional in vitro models to evaluate cell–cell interaction dynamics, which include proliferation upon effector–target engagement, duration of interaction, tumor escape kinetics, exhaustion, and rate of killing. These technologies can potentially be considered for use as theranostic tools for biomarker identification, response to immunotherapies, and co-clinical trial models performed simultaneously to human clinical trials for treatment decision evaluation.
3.2. Three-Dimensional (3D) Supports and In Vitro Tumor Models
Fundamental research in biology has been trapped by the engineering blueprints of ubiquitous 2D infrastructure, where cells are constrained to grow in a monolayer as copies of their original biology in a plastic dish. In conventional T cell killing assays, CAR T cells are directly cocultured with cancer cells in a 2D monolayer. Therefore, the lack of 3D support and physical barriers in these models ignores the need for trafficking which is a crucial challenge of CAR T therapy for solid tumors. By doing so, T cell locomotion and cytotoxic function against cancer cells are erroneously simplified. Lack of success in early phase studies is often due to an absence of biological response correlates and the inaccurate representation of a TME in oversimplified in vitro models [228,229,230,231]. 3D in vitro models can better recapitulate a TME from distinct cell types, presence of surrounding ECM, cellular trafficking, and drug sensitivity [232]. Furthermore, cells cultured in 3D may alter gene expression, leading to distinct phenotypic traits [233,234] as compared to 2D monolayers. Sureban et al. demonstrated that depending on the clonogenic capacity, surface markers of colorectal cancer cell lines grown as spheres in 3D can be different than those grown on 2D monolayer, resulting in distinct CAR T mediated cytotoxicity and associated IFNγ release [235]. Due to advancements in biotechnologies, various 3D in vitro models and platforms have been invented and applied to investigate CAR T cell motility, antigen specificity, and killing efficacy [236,237,238,239]. In this section, we review existing 3D in vitro models for preclinical assessment of CAR T therapy. These models are comprised of spheroids [236], organoids [239], or patient-derived explants [240,241] which are cultured in liquid media [237,238], hydrogels [83,237,242,243,244], or microgel platforms [245,246,247]. The existing preclinical 3D models and their associated in vitro platforms are valuable tools to advance CAR T therapy for solid tumors; however, as models, they are not meant to be the perfect representation of in vivo conditions. Understanding the advantages and disadvantages of each model will help select optimal approaches for each application.
3.3. Common Three-Dimensional Support Materials for In Vitro Assays
Hydrogels have been empowered as materials of choice for 3D cell culture platforms [83,237,242,243,244]. Hydrogels can be classified into naturally derived, synthetic, and hybrid. Natural hydrogels include collagen [28,111,248], MatrigelTM [242,249], fibrin [250,251], and alginate, [101,252,253]. Alternatively, synthetic hydrogel counterparts are commonly comprised of polyacrylamide (PAAm) [254] and polyethylene glycol (PEG) [255]. The major advantages of natural hydrogels are their cytological compatibility and the presence of intrinsic cell adhesion molecules. Some natural hydrogels have tunable mechanical properties (e.g., collagen, fibrin) and allow closer recapitulation of different tissues [250,256]. However, modulating polymer concentration of natural hydrogels is often compromised by the expense of available cell adhesion density [244]. Alternatively, the mechanical properties of synthetic hydrogels simply require modification of monomer to crosslinker ratio or polymer contents independent of ligand density for cell adhesion [257,258,259]. Natural hydrogels often contain great batch-to-batch variability and ill-defined protein composition [243,260], making reproducibility and consistency challenging [243,261,262]. For PEG or PAAm synthetic hydrogels, the polymer content, mesh size, and elastic modulus can be chemically predetermined and synthesized with trivial variation between batches [259,263]. Furthermore, natural hydrogels are either particularly sensitive to protease enzymes (e.g., fibrin) or nondegradable (e.g., alginate), which present limitations for long-term cell culture studies [244]. On the other hand, PEG crosslinkers are either non- or MMP-degradable, offering a relevant ECM synthetic material for 3D cell culture studies—including migration, differentiation, angiogenesis, and organoid assembly [243,264,265].
The microgel platforms represent alternatives to hydrogels. Microgels have been increasingly applied in biomedical research. More comprehensive reviews of microgel platforms have been reported elsewhere [246,247]. In brief, microgels belong to a special class of materials that unites all three major classes of colloids: rigid particles, flexible macromolecules, and surfactants [245,246,247]. Microgels are aqueous and crosslinked macromolecular networks of hydrogel colloidal particles swollen by solvents. Like hydrogels, the monomer-to-crosslinker ratio dictates the elastic modulus and mesh size of the microgels, thereby determining their colloidal or macromolecule character [245,246]. External stimuli—such as temperature, ionic strength, pH, pressure, and light—could alter the mass exchange of the microgel with the surroundings thereby changing their volume, shape, and mechanical properties [246,247]. The properties of microgels can be optimized to specific applications in the synthesis process with proper selection of building blocks and reaction sequence [246]. Microgel platforms from different ensembles of (e.g., PEG [264], PAAm [266], hyaluronic acids [267], or gelatin methacryloyl (GelMA) microbeads [268]) have been valuable tools for drug delivery [269,270], wound healing [264], and mechanotransduction studies [271].
3.4. Tumor Spheroids
Tumor spheroid models are the simplest 3D in vitro models that can be generated by methods of cell aggregation (e.g., hanging drop [272], ultralow attachment plates [273]). These straightforward methods enable great control over sample size and the number of cells for each spheroid. The tumor spheroids can be cultured in liquid media or embedded in a hydrogel [274]. Platforms (e.g., hydrogels or microgels) that support cells in 3D can better recapitulate the physical and biochemical constraints to better study the trafficking of CAR T in solid tumors. Wallstable et al. applied a small intestinal submucosa and mucosa (SISmuc) hydrogel platform—a collagen matrix with an intact basement membrane derived from decellularized porcine jejunum—to demonstrate CAR T efficient migration into the ECM, infiltration into the tumor, and elimination of tumor cells [237]. In another study, Ando et al. developed a microfluidic platform encasing a GelMA to study the role of hypoxia on immune-tumor interactions, which revealed that CAR T cell-mediated killing is dependent on oxygen gradients [238]. Similarly, Grunewald et al. applied 3D bioprinting to construct 3D TMEs [275] allowing for detection and quantification of L1 cell adhesion molecule (L1CAM). CAR T infiltration in this bioprinted 3D (neuroblastoma) model showed activation superior to 2D assays [275]. These tumor spheroid models resemble solid TMEs, which is useful for investigations of immune-tumor interactions, tumor invasion, or drug resistance [276]. However, the spheroid models are formed using immortalized cell lines, making them less recapitulative of in vivo tumors than organoids and patient-derived explants.
3.5. Tumor Organoids
Organoids are alternative 3D in vitro models often cultured in a 3D hydrogel support platform. The organoids are cultured from embryonic, adult, or induced pluripotent stem cells [276,277]. Tumor organoids are generated from patient-derived tumor explants by means of tissue dissociation, enzyme digestion, collection of single cells, and embedment culture in hydrogels (MatrigelTM). The isolated cells are cultured in particular growth factors such as Wnt activators (Wnt3a and R-spondin), exogenous receptor tyrosine kinase ligands (epidermal and fibroblast growth factors), and bone morphogenetic proteins antagonist noggin, among others [239,276]. Not only can the organoids recapitulate original tissue histologically and genetically, but they also have the ability to self-organize, differentiate, and function in response to mechanical and chemical cues [276,277]. Organoids are widely cultured in 3D in various platforms such as static ECM-embedment culture [237], microfluidic devices [248], and 3D bioprinting [275]. In one study, co-culture of CAR-engineered natural killer cells (EPCAM-CAR NK-92) and patient-derived colon organoids on the Matrigel matrix demonstrated an increase in immune cell migration and cytotoxicity against cancer cells [239]. Although the tumor organoid models have gained popularity in basic cancer research, the initial tissue dissociation, enzyme digestion, potential clonal selection, and lengthy production time are major disadvantages of the models. The process of tissue digestion removes natural ECM structure and eliminates endogenous immune and stromal cell components. The subsequent culture of isolated cancer cells in MatrigelTM containing exogenous and ill-defined growth factors potentiates significant modification of the original tumor organization and cell types.
3.6. Tumor Explants
In 1985, Robert Hoffman et al. pioneered the histoculture studies of tumor explants. The study showed that different cell types within the cultured tissue slices can grow, organize, differentiate, and maintain in vivo properties [278]. Despite its great potential, the models have not received much attention for the last two decades. The difficulty to maintain these tissue explants ex vivo, coupled with the straightforward application of cellular spheroids and the increasing popularity of organoid models, may have overshadowed the application of tumor explants. In recent years, the tumor explant models have gained traction due to advancements in cell culture platforms [11,279] and identifications of novel media compositions. Unlike spheroid and organoid models, resected tumor explants are directly cultured in well-studied media or 3D supported (e.g., hydrogels, microgels) platforms without enzyme digestion and cells isolation process, leading to better preservation of heterogeneity of the original tumor [241,279,280]. In one study, Jacob et al. described a procedure of generation and biobanking of glioblastoma organoid (GBO)-like explants from ex vivo culture [240]. The GBO explants were cultured without tissue dissociation, cell selection, or Matrigel matrix [240]. The resected and minced microexplants were cultured in a specialized culture medium on an orbital shaker which resulted in organoid-like phenotypes with a proliferation rate similar to that of parent tumors [240]. The GBO can be passaged and cryopreserved efficiently allowing repeatability of experiments. Furthermore, the heterogeneity of the GBO model was maintained as demonstrated by the expression of endogenous mutant proteins, epidermal growth factor receptor variant 3 (EGFRvIII), commonly expressed in glioblastomas. Co-culture of EGFRvIII specific-CAR T with GBO samples demonstrated immune cell proliferation and activation with antigen-specific tumor cell killing [240]. The significant roles of the TME and its heterogeneity highlight the need for these complimentary 3D in vitro models and platforms to better evaluate cancer biology and immunological trafficking.
4. Conclusions
This review highlights the significant difficulties imposed by biochemical and physical barriers on CAR T trafficking and infiltration in the TME. Despite a better understanding of tumor biology and promising new treatment options, CAR T therapy remains largely untapped in solid tumors. The shortcoming of therapeutic success highlights the need for advanced preclinical models with the well-preserved complex TME and the associated immune suppressive components. The mechanistic studies of CAR T trafficking and interaction with solid cancers are difficult to carry out solely in animal models which are fundamentally different from human biology. Advanced 3D in vitro models provide a complementary approach to enable investigations at the single-cell level, thereby providing better insights to CAR T cell design, activity, and therapeutic applications.
Author Contributions
D.T.N.: Conceptualization, funding acquisition, methodology, visualization, writing—original draft, writing—review and editing. E.O.-R.: Methodology, visualization, project administration, writing—review and editing. R.L.: Project administration, resources. T.W., J.R., L.J. and H.T.: Resources, W.W.S.: Methodology, visualization. H.R.M.-G. and M.C. Resources, D.A.M., J.H., W.G.S. and E.J.S.: Funding acquisition, resources, supervision, P.C.: Conceptualization, funding acquisition, methodology, visualization, writing—original draft, writing—review and editing. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
D.T.N. is supported by the National Science Foundation Graduate Research Fellowship under grant no. DGE-1842473. E.J.S. is supported by NCI R37CA251978, Rally Foundation (P0216283), and a Bankhead Coley Research Grant (P0172137). P.C. is supported by a Live Like Bella Discovery grant (22L07), University of Florida Health Cancer Center / University of Florida and Florida State University Clinical and Translational Science Awards KL2TR001429 and UL1TR001427, and the Stop Children’s Cancer foundation (F023891).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Melenhorst J.J., Castillo P., Hanley P.J., Keller M.D., Krance R.A., Margolin J., Leen A.M., Heslop H.E., Barrett A.J., Rooney C.M., et al. Graft versus leukemia response without graft-versus-host disease elicited by adoptively transferred multivirus-specific T-cells. Mol. Ther. 2015;23:179–183. doi: 10.1038/mt.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo P., Wright K.E., Kontoyiannis D.P., Walsh T., Patel S., Chorvinsky E., Bose S., Hazrat Y., Omer B., Albert N., et al. A New Method for Reactivating and Expanding T Cells Specific for Rhizopus oryzae. Mol. Ther.-Methods Clin. Dev. 2018;9:305–312. doi: 10.1016/j.omtm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sayour E.J., Grippin A., De Leon G., Stover B., Rahman M., Karachi A., Wummer B., Moore G., Castillo-Caro P., Fredenburg K., et al. Personalized Tumor RNA Loaded Lipid-Nanoparticles Prime the Systemic and Intratumoral Milieu for Response to Cancer Immunotherapy. Nano Lett. 2018;18:6195–6206. doi: 10.1021/acs.nanolett.8b02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg S.A., Restifo N.P., Yang J.C., Morgan R.A., Dudley M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude S.L., Teachey D.T., Porter D.L., Grupp S.A. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F., et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W., Moore L., Ji P. Mouse models for cancer research. Chin. J. Cancer. 2007;30:149–152. doi: 10.5732/cjc.011.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Convery N., Gadegaard N. 30 Years of Microfluidics. Micro Nano Eng. 2019;2:76–91. doi: 10.1016/j.mne.2019.01.003. [DOI] [Google Scholar]
- 12.Park D., Son K., Hwang Y., Ko J., Lee Y., Doh J., Jeon N.L. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-ImpacT platform) Front. Immunol. 2019;10:1133. doi: 10.3389/fimmu.2019.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeseleer F., Eichholz K., Tareen S.U., Iwamoto N., Roederer M., Kirchhoff F., Park H., Okoye A.A., Corey L. Real-Time Killing Assays to Assess the Potency of a New Anti-Simian Immunodeficiency Virus Chimeric Antigen Receptor T Cell. AIDS Res. Hum. Retrovir. 2020;36:998–1009. doi: 10.1089/aid.2020.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellone M., Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front. Oncol. 2013;3:231. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinnasamy D., Yu Z., Theoret M.R., Zhao Y., Shrimali R R.K., Morgan R.A., Feldman S.A., Restifo N.P., Rosenberg S.A. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J. Clin. Investig. 2010;120:3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Englisch A., Altvater B., Kailayangiri S., Hartmann W., Rossig C. VEGFR2 as a target for CAR T cell therapy of Ewing sarcoma. Pediatr. Blood Cancer. 2020;67:e28313. doi: 10.1002/pbc.28313. [DOI] [PubMed] [Google Scholar]
- 17.Chinnasamy D., Yu Z., Kerkar S.P., Zhang L., Morgan R.A., Restifo N.P., Rosenberg S.A. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 2012;18:1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinnasamy D., Tran E., Yu Z., Morgan R.A., Restifo N.P., Rosenberg S.A. Simultaneous targeting of tumor antigens and the tumor vasculature using t lymphocyte transfer synergize to induce regression of established tumors in mice. Cancer Res. 2013;73:3371–3380. doi: 10.1158/0008-5472.CAN-12-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kai F.B., Drain A.P., Weaver V.M. The Extracellular Matrix Modulates the Metastatic Journey. Dev. Cell. 2019;49:332–346. doi: 10.1016/j.devcel.2019.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Napoli M., Flores E.R. Beware of thy neighbor: Senescent cancer cells feast on adjacent ells to persist. J. Cell Biol. 2019;218:3535–3536. doi: 10.1083/jcb.201910040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox T.R., Erler J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessenbrock K., Plaks V., Werb Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon E.K., Wang L.C., Dolfi D.V., Wilson C.B., Ranganathan R., Sun J., Kapoor V., Scholler J., Puré E., Milone M.C., et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin. Cancer Res. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustave I., Albini A. Contribution to Tumor Angiogenesis From innate immune Cells within the Tumor Microenvironment: Implications for immunotherapy. Front. Immunol. 2018;9:527. doi: 10.3389/fimmu.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Helvert S., Friedl P. Strain Stiffening of Fibrillar Collagen during Individual and Collective Cell Migration Identified by AFM Nanoindentation. ACS Appl. Mater. Interfaces. 2016;8:21946–21955. doi: 10.1021/acsami.6b01755. [DOI] [PubMed] [Google Scholar]
- 29.Ahmadzadeh H., Webster M.R., Behera R., Jimenez A.M., Wirtz D. Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proc. Natl. Acad. Sci. USA. 2017;114:E1617–E1626. doi: 10.1073/pnas.1617037114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butcher D.T., Alliston T., Weaver V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Aloia M.M., Zizzari I.G., Sacchetti B., Pierelli L., Alimandi M. CAR-T cells: The long and winding road to solid tumors review-article. Cell Death Dis. 2018;9:282. doi: 10.1038/s41419-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Li W., Huang K., Zhang Y., Kupfer G., Zhao Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018;11:22. doi: 10.1186/s13045-018-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yong C.S.M., Dardalhon V., Devaud C., Taylor N., Darcy P.K., Kershaw M.H. CAR T-cell therapy of solid tumors. Immunol. Cell Biol. 2017;95:356–363. doi: 10.1038/icb.2016.128. [DOI] [PubMed] [Google Scholar]
- 34.Sterner R.C., Sterner R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F.T., Csiszar K., Giaccia A., Weninger W., et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coste B., Xiao B., Santos J.S., Syeda R., Grandl J., Spencer K.S., Kim S.E., Schmidt M., Mathur J., Dubin A.E., et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin L.K., Xia Y., Discher D.E., Janmey P.A. Mechanotransduction in cancer. Curr. Opin. Chem. Eng. 2016;11:77–84. doi: 10.1016/j.coche.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feitelson M.A., Arzumanyan A., Kulathinal R.J., Blain S.W., Holcombe R.F., Mahajna J., Marino M., Martinez-Chantar M.L., Nawroth R., Sanchez-Garcia I., et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015;35:S25–S54. doi: 10.1016/j.semcancer.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei B., Zhou X., Liang C., Zheng X., Lei P., Fang J., Han X., Wang L., Qi C., Wei H. Human colorectal cancer progression correlates with LOX-induced ECM stiffening. Int. J. Biol. Sci. 2017;13:1450–1457. doi: 10.7150/ijbs.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su G., Meyer K., Nandini C.D., Qiao D., Salamat S., Friedl A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am. J. Pathol. 2006;168:2014–2026. doi: 10.2353/ajpath.2006.050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iozzo R.V., Sanderson R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bordeleau F., Mason B.N., Lollis E.M., Mazzola M., Zanotelli M.R., Somasegar S., Califano J.P., Montague C., LaValley D.J., Huynh J., et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. USA. 2017;114:492–497. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mcmahon M., Ye S., Pedrina J., Dlugolenski D., Stambas J. Extracellular Matrix Enzymes and Immune Cell Biology. Front. Mol. Biosci. 2021;8:703868. doi: 10.3389/fmolb.2021.703868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mondal S., Adhikari N., Banerjee S., Amin S.A., Jha T. Matrix metalloproteinase-9 ( MMP-9 ) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020;194:112260. doi: 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S., Das A., Barai A., Sen S. MMP Secretion Rate and Inter-invadopodia Spacing Collectively Govern Cancer Invasiveness. Biophysj. 2018;114:650–662. doi: 10.1016/j.bpj.2017.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caruana I., Savoldo B., Hoyos V., Weber G., Liu H., Kim E.S., Ittmann M.M., Marchetti D., Dotti G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 2015;21:524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jabłońska-Trypuć A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 49.Renkawitz J., Kopf A., Stopp J., de Vries I., Driscoll M.K., Merrin J., Hauschild R., Welf E.S., Danuser G., Fiolka R., et al. Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature. 2019;568:546–550. doi: 10.1038/s41586-019-1087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krummel M.F., Friedman R.S., Jacobelli J. Modes and mechanisms of T cell motility: Roles for confinement and Myosin-IIA. Curr. Opin. Cell Biol. 2014;30:9–16. doi: 10.1016/j.ceb.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabdanov E.D., Rodríguez-Merced N.J., Cartagena-Rivera A.X., Puram V.V., Callaway M.K., Ensminger E.A., Pomeroy E.J., Yamamoto K., Lahr W.S., Webber B.R., et al. Engineering T cells to enhance 3D migration through structurally and mechanically complex tumor microenvironments. Nat. Commun. 2021;12:1–17. doi: 10.1038/s41467-021-22985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salmon H., Franciszkiewicz K., Damotte D., Dieu-Nosjean M.C., Validire P., Trautmann A., Mami-Chouaib F., Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fanfone D., Wu Z., Mammi J., Berthenet K., Neves D., Weber K., Halaburkova A., Virard F., Bunel F., Jamard C., et al. Confined migration promotes cancer metastasis through resistance to anoikis and increased invasiveness. Elife. 2022;11:e73150. doi: 10.7554/eLife.73150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Cicco P., Ercolano G., Ianaro A. The New Era of Cancer Immunotherapy: Targeting Myeloid-Derived Suppressor Cells to Overcome Immune Evasion. Front. Immunol. 2020;11:1680. doi: 10.3389/fimmu.2020.01680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rianna C., Radmacher M., Kumar S., Discher D. Direct evidence that tumor cells soften when navigating confined spaces. Mol. Biol. Cell. 2020;31:1726–1734. doi: 10.1091/mbc.E19-10-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Condeelis J., Pollard J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Bufi N., Saitakis M., Dogniaux S., Buschinger O., Bohineust A., Richert A., Maurin M., Hivroz C., Asnacios A. Human primary immune cells exhibit distinct mechanical properties that are modified by inflammation. Biophys. J. 2015;108:2181–2190. doi: 10.1016/j.bpj.2015.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wullkopf L., West A.K.V., Leijnse N., Cox T.R., Madsen C.D., Oddershede L.B., Erler J.T. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol. Biol. Cell. 2018;29:2378–2385. doi: 10.1091/mbc.E18-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhar M., Lam J.N., Walser T., Dubinett S.M., Rettig M.B., Di Carlo D. Functional profiling of circulating tumor cells with an integrated vortex capture and single-cell protease activity assay. Proc. Natl. Acad. Sci. USA. 2018;115:9986–9991. doi: 10.1073/pnas.1803884115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiang H., Ramil C.P., Hai J., Zhang C., Wang H., Watkins A.A., Afshar R., Georgiev P., Sze M.A., Song X.S., et al. Cancer-associated fibroblasts promote immunosuppression by inducing ROS-generating monocytic MDSCs in lung squamous cell carcinoma. Cancer Immunol. Res. 2020;8:436–450. doi: 10.1158/2326-6066.CIR-19-0507. [DOI] [PubMed] [Google Scholar]
- 62.Tamura R., Tanaka T., Akasaki Y., Murayama Y., Yoshida K., Sasaki H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med. Oncol. 2020;37:2. doi: 10.1007/s12032-019-1329-2. [DOI] [PubMed] [Google Scholar]
- 63.Tauriello D.V.F., Palomo-Ponce S., Stork D., Berenguer-Llergo A., Badia-Ramentol J., Iglesias M., Sevillano M., Ibiza S., Cañellas A., Hernando-Momblona X., et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 64.Deleon-pennell K.Y., Barker T.H., Lindsey M.L. Fibroblasts: The arbiters of extracellular matrix remodeling. Matrix Biol. 2020;91–92:1–7. doi: 10.1016/j.matbio.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tracy L.E., Minasian R.A., Caterson E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care. 2016;5:119–136. doi: 10.1089/wound.2014.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., Ivanova Y., Hundal J., Arthur C.D., Krebber W.J., et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jang I., Beningo K.A. Integrins, CAFs and mechanical forces in the progression of cancer. Cancers. 2019;11:721. doi: 10.3390/cancers11050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A., Reinhart-King C.A., Margulies S.S., Dembo M., Boettiger D., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Kim J., Feng J., Jones C.A.R., Mao X., Sander L.M., Levine H., Sun B. Stress-induced plasticity of dynamic collagen networks. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-01011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swift J., Discher D.E. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J. Cell Sci. 2014;127:3005–3015. doi: 10.1242/jcs.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Northcott J.M., Dean I.S., Mouw J.K., Weaver V.M. Feeling Stress: The Mechanics of Cancer Progression and Aggression. Front. Cell Dev. Biol. 2018;6:17. doi: 10.3389/fcell.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stylianopoulos T., Martin J.D., Snuderl M., Mpekris F., Jain S.R., Jain R.K. Co-evolution of solid stress and interstitial fluid pressure in tumors during progression: Implications for vascular collapse. Cancer Res. 2013;73:3833–3841. doi: 10.1158/0008-5472.CAN-12-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goetz J.G., Minguet S., Navarro-Lérida I., Lazcano J.J., Samaniego R., Calvo E., Tello M., Osteso-Ibáñez T., Pellinen T., Echarri A., et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lugano R., Ramachandran M., Dimberg A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuazo-gaztelu I., Casanovas O. Unraveling the Role of Angiogenesis in Cancer ecosystems. Front. Oncol. 2018;8:248. doi: 10.3389/fonc.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forster J.C., Harriss-phillips W.M., Douglass M.J.J., Bezak E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia. 2017;5:21–32. doi: 10.2147/HP.S133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishida N., Yano H., Nishida T., Kamura T., Kojiro M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng G., Tse J., Jain R.K., Munn L.L. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS ONE. 2009;4:e4632. doi: 10.1371/journal.pone.0004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nia H.T., Liu H., Seano G., Datta M., Jones D., Rahbari N., Incio J., Chauhan V.P., Jung K., Martin J.D., et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 2016;1:0004. doi: 10.1038/s41551-016-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hofmann M., Guschel M., Bernd A., Bereiter-Hahn J., Kaufmann R., Tandi C., Helge W., Kippenberger S. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rofstad E.K., Galappathi K., Mathiesen B.S. Tumor Interstitial Fluid Pressure-A Link between Tumor Hypoxia, Microvascular Density, and Lymph Node Metastasis. Neoplasia. 2014;16:586–594. doi: 10.1016/j.neo.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rens E.G., Merks R.M.H. Cell Contractility Facilitates Alignment of Cells and Tissues to Static Uniaxial Stretch. Biophys. J. 2017;112:755–766. doi: 10.1016/j.bpj.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Provenzano P.P., Eliceiri K.W., Campbell J.M., Inman D.R., White J.G., Keely P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:1–15. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuczek D.E., Larsen A.M.H., Thorseth M.L., Carretta M., Kalvisa A., Siersbæk M.S., Simões A.M.C., Roslind A., Engelholm L.H., Noessner E., et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer. 2019;7:68. doi: 10.1186/s40425-019-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riching K.M., Cox B.L., Salick M.R., Pehlke C., Riching A.S., Ponik S.M., Bass B.R., Crone W.C., Jiang Y., Weaver A.M., et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2015;107:2546–2558. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gocheva V., Wang H.W., Gadea B.B., Shree T., Hunter K.E., Garfall A.L., Berman T., Joyce J.A. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Erdogan B., Ao M., White L.M., Means A.L., Brewer B.M., Yang L., Washington M.K., Shi C., Franco O.E., Weaver A.M., et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017;216:3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koorman T., Jansen K.A., Khalil A., Haughton P.D., Visser D., Rätze M.A.K., Haakma W.E., Sakalauskaitè G., van Diest P.J., de Rooij J., et al. Spatial collagen stiffening promotes collective breast cancer cell invasion by reinforcing extracellular matrix alignment. Oncogene. 2022 doi: 10.1038/s41388-022-02258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jain R.K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 2012;64:353–365. doi: 10.1016/j.addr.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heldin C.H., Rubin K., Pietras K., Östman A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 91.Provenzano P.P., Hingorani S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer. 2013;108:1–8. doi: 10.1038/bjc.2012.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Libutti S.K., Tamarkin L., Nilubol N. Targeting the invincible barrier for drug delivery in solid cancers: Interstitial fluid pressure. Oncotarget. 2018;9:35723–35725. doi: 10.18632/oncotarget.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen S.C., Wu P.C., Wang C.Y., Kuo P.L. Evaluation of cytotoxic T lymphocyte-mediated anticancer response against tumor interstitium-simulating physical barriers. Sci. Rep. 2020;10:13662. doi: 10.1038/s41598-020-70694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Helmlinger G., Netti P.A., Lichtenbeld H.C., Melder R.J., Jain R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 95.Chen A., Sceneay J., Gödde N., Kinwel T., Ham S., Thompson E.W., Humbert P.O., Möller A. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene. 2018;37:4214–4225. doi: 10.1038/s41388-018-0259-3. [DOI] [PubMed] [Google Scholar]
- 96.Lewis C., Murdoch C. Macrophage responses to hypoxia: Implications for tumor progression and anti-cancer therapies. Am. J. Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Winkler J., Werb Z., Metcalf K.J. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pickup M., Novitskiy S., Moses H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer. 2013;13:788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez M., Moon E.K. CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front. Immunol. 2019;10:1–21. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gargalionis A.N., Basdra E.K., Papavassiliou A.G. Mechanosignalling in tumour progression. J. Cell. Mol. Med. 2018;22:704–705. doi: 10.1111/jcmm.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chaudhuri O., Koshy S.T., Branco Da Cunha C., Shin J.W., Verbeke C.S., Allison K.H., Mooney D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 102.Lu P., Weaver V.M., Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crotti S., Piccoli M., Rizzolio F., Giordano A., Nitti D., Agostini M. Extracellular Matrix and Colorectal Cancer: How Surrounding Microenvironment Affects Cancer Cell Behavior? J. Cell. Physiol. 2017;232:967–975. doi: 10.1002/jcp.25658. [DOI] [PubMed] [Google Scholar]
- 104.Moriggi M., Giussani M., Torretta E., Capitanio D., Sandri M., Leone R., De Palma S., Vasso M., Vozzi G., Tagliabue E., et al. ECM Remodeling in Breast Cancer with Different Grade: Contribution of 2D-DIGE Proteomics. Proteomics. 2018;18:e1800278. doi: 10.1002/pmic.201800278. [DOI] [PubMed] [Google Scholar]
- 105.Ingber D.E. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ. Res. 2002;91:877–887. doi: 10.1161/01.RES.0000039537.73816.E5. [DOI] [PubMed] [Google Scholar]
- 106.Tuxhorn J.A., Mcalhany S.J., Dang T.D., Ayala G.E., Rowley D.R. Stromal Cells Promote Angiogenesis and Growth of Human Prostate Tumors in a Differential Reactive Stroma ( DRS ) Xenograft Model 1. Cancer Res. 2002;62:3298–3307. [PubMed] [Google Scholar]
- 107.Gupta M.K., Qin R. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. WJG. 2003;9:1144–1155. doi: 10.3748/wjg.v9.i6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Friedl P., Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 109.Kim J., Zheng Y., Alobaidi A.A., Nan H., Tian J., Jiao Y., Sun B. Collective ECM remodeling organizes 3D collective cancer invasion. arXiv. 20191903.03290 [Google Scholar]
- 110.Chaudhuri P.K., Low B.C., Lim C.T. Mechanobiology of Tumor Growth. Chem. Rev. 2018;118:6499–6515. doi: 10.1021/acs.chemrev.8b00042. [DOI] [PubMed] [Google Scholar]
- 111.Henke E., Nandigama R., Ergün S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang Y., Yuan J., Righi E., Kamoun W.S., Ancukiewicz M., Nezivar J., Santosuosso M., Martin J.D., Martin M.R., Vianello F., et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Whatcott C.J., Han H., Posner R.G., Hostetter G., Von Hoff D.D. Targeting the tumor microenvironment in cancer: Why hyaluronidase deserves a second look. Cancer Discov. 2011;1:291–296. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Venning F.A., Wullkopf L., Erler J.T. Targeting ECM disrupts cancer progression. Front. Oncol. 2015;5:224. doi: 10.3389/fonc.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barry-Hamilton V., Spangler R., Marshall D., McCauley S., Rodriguez H.M., Oyasu M., Mikels A., Vaysberg M., Ghermazien H., Wai C., et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 116.Lanitis E., Irving M., Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 2015;33:55–63. doi: 10.1016/j.coi.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feig C., Jones J.O., Kraman M., Wells R.J.B., Deonarine A., Chan D.S., Connell C.M., Roberts E.W., Zhao Q., Caballero O.L., et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zboralski D., Hoehlig K., Eulberg D., Frömming A., Vater A. Increasing tumor-infiltrating T cells through inhibition of CXCL12 with NOX-A12 synergizes with PD-1 blockade. Cancer Immunol. Res. 2017;5:950–956. doi: 10.1158/2326-6066.CIR-16-0303. [DOI] [PubMed] [Google Scholar]
- 119.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel E.E., Koeppen H., Astarita J.L., Cubas R., et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ito T., Hanabuchi S., Wang Y.H., Park W.R., Arima K., Bover L., Qin F.X.F., Gilliet M., Liu Y.J. Two Functional Subsets of FOXP3+ Regulatory T Cells in Human Thymus and Periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hombach A.A., Heiders J., Foppe M., Chmielewski M., Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sato T., Terai M., Tamura Y., Alexeev V., Mastrangelo M.J., Selvan S.R. Interleukin 10 in the tumor microenvironment: A target for anticancer immunotherapy. Immunol. Res. 2011;51:170–182. doi: 10.1007/s12026-011-8262-6. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y., Jiang H., Luo H., Sun Y., Shi B., Sun R., Li Z. An IL-4/21 Inverted Cytokine Receptor Improving CAR-T Cell Potency in Immunosuppressive Solid-Tumor Microenvironment. Front. Immunol. 2019;10:1691. doi: 10.3389/fimmu.2019.01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu K., Huang A., Nie J., Tan J., Xing S., Qu Y., Jiang K. IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment. Front. Immunol. 2021;12:683332. doi: 10.3389/fimmu.2021.683332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heim L., Kachler K., Siegmund R., Trufa D.I., Mittler S., Geppert C.I., Friedrich J., Rieker R.J., Sirbu H., Finotto S. Increased expression of the immunosuppressive interleukin-35 in patients with non-small cell lung cancer. Br. J. Cancer. 2019;120:903–912. doi: 10.1038/s41416-019-0444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morrot A., da Fonseca L.M., Salustiano E.J., Gentile L.B., Conde L., Filardy A.A., Franklim T.N., da Costa K.M., Freire-de-Lima C.G., Freire-de-Lima L. Metabolic symbiosis and immunomodulation: How tumor cell-derived lactate may disturb innate and adaptive immune responses. Front. Oncol. 2018;8:81. doi: 10.3389/fonc.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vigano S., Alatzoglou D., Irving M., Ménétrier-Caux C., Caux C., Romero P., Coukos G. Targeting adenosine in cancer immunotherapy to enhance T-Cell function. Front. Immunol. 2019;10:925. doi: 10.3389/fimmu.2019.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moon E.K., Wang L.C.S., Bekdache K., Lynn R.C., Lo A., Thorne S.H., Albelda S.M. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology. 2018;7:e1395997. doi: 10.1080/2162402X.2017.1395997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nishio N., Diaconu I., Liu H., Cerullo V., Caruana I., Hoyos V., Bouchier-Hayes L., Savoldo B., Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74:5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McGrath K., Dotti G. Combining oncolytic viruses with chimeric antigen receptor T cell therapy. Hum. Gene Ther. 2021;32:150–157. doi: 10.1089/hum.2020.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koneru M., Purdon T.J., Spriggs D., Koneru S., Brentjens R.J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu B., Ren J., Luo Y., Keith B., Young R.M., Scholler J., Zhao Y., June C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017;20:3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shum T., Omer B., Tashiro H., Kruse R.L., Wagner D.L., Parikh K., Yi Z., Sauer T., Liu D., Parihar R., et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7:1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adachi K., Kano Y., Nagai T., Okuyama N., Sakoda Y., Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018;36:346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 135.Kershaw M.H., Wang G., Westwood J.A., Pachynski R.K., Tiffany H.L., Marincola F.M., Wang E., Young H.A., Murphy P.M., Hwu P. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum. Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 136.Craddock J.A., Lu A., Bear A., Pule M., Brenner M.K., Rooney C.M., Foster A.E. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moon E.K., Carpenito C., Sun J., Wang L.C.S., Kapoor V., Predina J., Powell D.J., Riley J.L., June C.H., Albelda S.M. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jin L., Tao H., Karachi A., Long Y., Hou A.Y., Na M., Dyson K.A., Grippin A.J., Deleyrolle L.P., Zhang W., et al. CXCR1- or CXCR2-modi fi ed CAR T cells co-opt IL- 8 for maximal antitumor ef fi cacy in solid tumors. Nat. Commun. 2019;10:4016. doi: 10.1038/s41467-019-11869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jin L., Ge H., Long Y., Yang C., Chang Y.E., Mu L., Sayour E.J., De Leon G., Wang Q.J., Yang J.C., et al. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro. Oncol. 2018;20:55–65. doi: 10.1093/neuonc/nox116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang L., Yu Z., Muranski P., Palmer D.C., Restifo N.P., Rosenberg S.A., Morgan R.A. Inhibition of TGF-β signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther. 2013;20:575–580. doi: 10.1038/gt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen N., Morello A., Tano Z., Adusumilli P.S. CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology. 2017;6:e1273302. doi: 10.1080/2162402X.2016.1273302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mohammed S., Sukumaran S., Bajgain P., Watanabe N., Heslop H.E., Rooney C.M., Brenner M.K., Fisher W.E., Leen A.M., Vera J.F. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Mol. Ther. 2017;25:249–258. doi: 10.1016/j.ymthe.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bajgain P., Tawinwung S., D’Elia L., Sukumaran S., Watanabe N., Hoyos V., Lulla P., Brenner M.K., Leen A.M., Vera J.F. CAR T cell therapy for breast cancer: Harnessing the tumor milieu to drive T cell activation. J. Immunother. Cancer. 2018;6:1–13. doi: 10.1186/s40425-018-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu T., Han C., Wang S., Fang P., Ma Z., Xu L., Yin R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019;12:86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu T., Zhou L., Li D., Andl T., Zhang Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front. Cell Dev. Biol. 2019;7:60. doi: 10.3389/fcell.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harryvan T.J., Verdegaal E.M.E., Hardwick J.C.H., Hawinkels L.J.A.C., van der Burg S.H. Targeting of the cancer-associated fibroblast—t-cell axis in solid malignancies. J. Clin. Med. 2019;8:1989. doi: 10.3390/jcm8111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lakins M.A., Ghorani E., Munir H., Martins C.P., Shields J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 + T Cells to protect tumour cells. Nat. Commun. 2018;9:948. doi: 10.1038/s41467-018-03347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lo A., Wang L.C.S., Scholler J., Monslow J., Avery D., Newick K., O’Brien S., Evans R.A., Bajor D.J., Clendenin C., et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kakarla S., Chow K.K.H., Mata M., Shaffer D.R., Song X.T., Wu M.F., Liu H., Wang L.L., Rowley D.R., Pfizenmaier K., et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 2013;21:1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Özdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R., Laklai H., Sugimoto H., Kahlert C., Novitskiy S.V., et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Law A.M.K., Valdes-mora F., Gallego-ortega D. Myeloid-Derived Suppressor Cells as a Therapeutic. Cells. 2020;27:561. doi: 10.3390/cells9030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pollard J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qian B.-Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sica A., Saccani A., Bottazzi B., Polentarutti N., Vecchi A., Van Damme J., Mantovani A. Autocrine Production of IL-10 Mediates Defective IL-12 Production and NF-κB Activation in Tumor-Associated Macrophages. J. Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 158.Rodriguez-Garcia A., Lynn R.C., Poussin M., Eiva M.A., Shaw L.C., O’Connor R.S., Minutolo N.G., Casado-Medrano V., Lopez G., Matsuyama T., et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat. Commun. 2021;12:877. doi: 10.1038/s41467-021-20893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang G., Lu X., Dey P., Deng P., Wu C.C., Jiang S., Fang Z., Zhao K., Konaparthi R., Hua S., et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alban T.J., Bayik D., Otvos B., Rabljenovic A., Leng L., Jia-Shiun L., Roversi G., Lauko A., Momin A.A., Mohammadi A.M., et al. Glioblastoma Myeloid-Derived Suppressor Cell Subsets Express Differential Macrophage Migration Inhibitory Factor Receptor Profiles That Can Be Targeted to Reduce Immune Suppression. Front. Immunol. 2020;11:1191. doi: 10.3389/fimmu.2020.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nalawade S.A., Shafer P., Bajgain P., McKenna M.K., Ali A., Kelly L., Joubert J., Gottschalk S., Watanabe N., Leen A., et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J. Immunother. Cancer. 2021;9:e003237. doi: 10.1136/jitc-2021-003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shaul M.E., Fridlender Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019;16:601–620. doi: 10.1038/s41571-019-0222-4. [DOI] [PubMed] [Google Scholar]
- 163.Togashi Y., Shitara K., Nishikawa H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 164.Amoozgar Z., Kloepper J., Ren J., Tay R.E., Kazer S.W., Kiner E., Krishnan S., Posada J.M., Ghosh M., Mamessier E., et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat. Commun. 2021;12:2582. doi: 10.1038/s41467-021-22885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reinhard K., Rengstl B., Oehm P., Michel K., Billmeier A., Hayduk N., Klein O., Kuna K., Ouchan Y., Wöll S., et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367:446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 167.Sadjadi Z., Zhao R., Hoth M., Qu B., Rieger H. Migration of Cytotoxic T Lymphocytes in 3D Collagen Matrices. Biophys. J. 2020;119:2141–2152. doi: 10.1016/j.bpj.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wolf K., Müller R., Borgmann S., Bröcker E.B., Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 169.Jacobelli J., Friedman R.S., Conti M.A., Lennon-Dumenil A.M., Piel M., Sorensen C.M., Adelstein R.S., Krummel M.F. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat. Immunol. 2010;11:953–961. doi: 10.1038/ni.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kawauchi T. Cell adhesion and its endocytic regulation in cell migration during neural development and cancer metastasis. Int. J. Mol. Sci. 2012;13:4564–4590. doi: 10.3390/ijms13044564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Petrie R.J., Harlin H.M., Korsak L.I.T., Yamada K.M. Activating the nuclear piston mechanism of 3D migration in tumor cells. J. Cell Biol. 2017;216:93–100. doi: 10.1083/jcb.201605097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ruprecht V., Wieser S., Callan-Jones A., Smutny M., Morita H., Sako K., Barone V., Ritsch-Marte M., Sixt M., Voituriez R., et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell. 2015;160:673–685. doi: 10.1016/j.cell.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yamada K.M., Sixt M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019;20:738–752. doi: 10.1038/s41580-019-0172-9. [DOI] [PubMed] [Google Scholar]
- 174.Miller M.J., Wei S.H., Parker I., Cahalan M.D. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 175.Friedl P., Entschladen F., Conrad C., Niggemann B., Zänker K.S. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize β1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur. J. Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 176.Paluch E.K., Aspalter I.M., Sixt M. Focal Adhesion-Independent Cell Migration. Annu. Rev. Cell Dev. Biol. 2016;32:469–490. doi: 10.1146/annurev-cellbio-111315-125341. [DOI] [PubMed] [Google Scholar]
- 177.Lämmermann T., Bader B.L., Monkley S.J., Worbs T., Wedlich-Söldner R., Hirsch K., Keller M., Förster R., Critchley D.R., Fässler R., et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 178.Goudarzi M., Banisch T.U., Mobin M.B., Maghelli N., Tarbashevich K., Strate I., van den Berg J., Blaser H., Bandemer S., Paluch E., et al. Identification and Regulation of a Molecular Module for Bleb-Based Cell Motility. Dev. Cell. 2012;23:210–218. doi: 10.1016/j.devcel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 179.Wu S.K., Priya R. Spatio-temporal regulation of RhoGTPases signaling by myosin II. Front. Cell Dev. Biol. 2019;7:90. doi: 10.3389/fcell.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wolf K., te Lindert M., Krause M., Alexander S., te Riet J., Willis A.L., Hoffman R.M., Figdor C.G., Weiss S.J., Friedl P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lele T.P., Dickinson R.B., Gundersen G.G. Mechanical principles of nuclear shaping and positioning. J. Cell Biol. 2018;217:3330–3342. doi: 10.1083/jcb.201804052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xia Y., Pfeifer C.R., Discher D.E. Nuclear mechanics during and after constricted migration. Acta Mech. Sin. Xuebao. 2019;35:299–308. doi: 10.1007/s10409-018-00836-9. [DOI] [Google Scholar]
- 183.Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C.D.P., Pinter J., Pajerowski J.D., Spinler K.R., Shin J.W., Tewari M., et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Harada T., Swift J., Irianto J., Shin J.W., Spinler K.R., Athirasala A., Diegmiller R., Dingal P.C.D.P., Ivanovska I.L., Discher D.E. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 2014;204:669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stecklein S.R., Kumaraswamy E., Behbod F., Wang W., Chaguturu V., Harlan-Williams L.M., Jensen R.A. BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation. Proc. Natl. Acad. Sci. USA. 2012;109:13650–13655. doi: 10.1073/pnas.1203326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stephens A.D., Banigan E.J., Adam S.A., Goldman R.D., Marko J.F. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell. 2017;28:1984–1996. doi: 10.1091/mbc.e16-09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 188.Lambert L.H., Goebrecht G.K.E., De Leo S.E., O’Connor R.S., Nunez-Cruz S., De Li T., Yuan J., Milone M.C., Kam L.C. Improving T Cell Expansion with a Soft Touch. Nano Lett. 2017;17:821–826. doi: 10.1021/acs.nanolett.6b04071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bashoura K.T., Gondarenko A., Chen H., Shen K., Liu X., Huse M., Hone J.C., Kam L.C. CD28 and CD3 have complementary roles in T-cell traction forces. Proc. Natl. Acad. Sci. USA. 2014;111:2241–2246. doi: 10.1073/pnas.1315606111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim S.T., Takeuchi K., Sun Z.Y.J., Touma M., Castro C.E., Fahmy A., Lang M.J., Wagner G., Reinherz E.L. The αβ T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Basu R., Whitlock B.M., Husson J., Le Floc’h A., Jin W., Oyler-Yaniv A., Dotiwala F., Giannone G., Hivroz C., Biais N., et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell. 2016;165:100–110. doi: 10.1016/j.cell.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Buxboim A., Swift J., Irianto J., Spinler K.R., Dingal P.C.D.P., Athirasala A., Kao Y.R.C., Cho S., Harada T., Shin J.W., et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 2014;24:1909–1917. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Judokusumo E., Tabdanov E., Kumari S., Dustin M.L., Kam L.C. Mechanosensing in T lymphocyte activation. Biophys. J. 2012;102:L5–L7. doi: 10.1016/j.bpj.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jin W., Tamzalit F., Chaudhuri P.K., Black C.T., Huse M., Kam L.C. T cell activation and immune synapse organization respond to the microscale mechanics of structured surfaces. Proc. Natl. Acad. Sci. USA. 2019;116:19835–19840. doi: 10.1073/pnas.1906986116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pruitt H.C., Lewis D., Ciccaglione M., Connor S., Smith Q., Hickey J.W., Schneck J.P., Gerecht S. Collagen fiber structure guides 3D motility of cytotoxic T lymphocytes. Matrix Biol. 2020;85–86:147–159. doi: 10.1016/j.matbio.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lanitis E., Kosti P., Ronet C., Cribioli E., Rota G., Spill A., Reichenbach P., Zoete V., Dangaj Laniti D., Coukos G., et al. VEGFR-2 redirected CAR-T cells are functionally impaired by soluble VEGF-A competition for receptor binding. J. Immunother. Cancer. 2021;9:e002151. doi: 10.1136/jitc-2020-002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang W., Liu L., Su H.F., Liu Q., Shen J., Dai H., Zheng W., Lu Y., Zhang W., Bei Y., et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br. J. Cancer. 2019;121:837–845. doi: 10.1038/s41416-019-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tran E., Chinnasamy D., Yu Z., Morgan R.A., Lee C.C.R., Restifo N.P., Rosenberg S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013;210:1065–1068. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu G., Rui W., Zheng H., Huang D., Yu F., Zhang Y., Dong J., Zhao X., Lin X. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur. J. Immunol. 2020;50:712–724. doi: 10.1002/eji.201948457. [DOI] [PubMed] [Google Scholar]
- 200.Berahovich R., Liu X., Zhou H., Tsadik E., Xu S., Golubovskaya V., Wu L. Hypoxia selectively impairs CAR-T cells in vitro. Cancers. 2019;11:602. doi: 10.3390/cancers11050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liao Q., He H., Mao Y., Ding X., Zhang X., Xu J. Engineering T cells with hypoxia-inducible chimeric antigen receptor (HiCAR) for selective tumor killing. Biomark. Res. 2020;8:56. doi: 10.1186/s40364-020-00238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kersten K., Visser K.E., Miltenburg M.H., Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017;9:137–153. doi: 10.15252/emmm.201606857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wu Y., Yu X.Z. Modelling CAR-T therapy in humanized mice. EBioMedicine. 2019;40:25–26. doi: 10.1016/j.ebiom.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jin C.H., Xia J., Rafiq S., Huang X., Hu Z., Zhou X., Brentjens R.J., Yang Y.G. Modeling anti-CD19 CAR T cell therapy in humanized mice with human immunity and autologous leukemia. EBioMedicine. 2019;39:173–181. doi: 10.1016/j.ebiom.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Drake A.C., Chen Q., Chen J. Engineering humanized mice for improved hematopoietic reconstitution. Cell. Mol. Immunol. 2012;9:215–224. doi: 10.1038/cmi.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Holzapfel B.M., Wagner F., Thibaudeau L., Levesque J.P., Hutmacher D.W. Concise review: Humanized models of tumor immunology in the 21st century: Convergence of cancer research and tissue engineering. Stem Cells. 2015;33:1696–1704. doi: 10.1002/stem.1978. [DOI] [PubMed] [Google Scholar]
- 207.Wang M., Yao L.C., Cheng M., Cai D., Martinek J., Pan C.X., Shi W., Ma A.H., De Vere White R.W., Airhart S., et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018;32:1537–1549. doi: 10.1096/fj.201700740R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gitto S.B., Kim H., Rafail S., Omran D.K., Medvedev S., Kinose Y., Rodriguez-Garcia A., Flowers A.J., Xu H., Schwartz L.E., et al. An autologous humanized patient-derived-xenograft platform to evaluate immunotherapy in ovarian cancer. Gynecol. Oncol. 2020;156:222–232. doi: 10.1016/j.ygyno.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 209.Bareham B., Georgakopoulos N., Matas-Céspedes A., Curran M., Saeb-Parsy K. Modeling human tumor-immune environments in vivo for the preclinical assessment of immunotherapies. Cancer Immunol. Immunother. 2021;70:2737–2750. doi: 10.1007/s00262-021-02897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lang G.A., Iwakuma T., Suh Y.A., Liu G., Rao V.A., Parant J.M., Valentin-Vega Y.A., Terzian T., Caldwell L.C., Strong L.C., et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 211.Olive K.P., Tuveson D.A., Ruhe Z.C., Yin B., Willis N.A., Bronson R.T., Crowley D., Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 212.Drost R., Bouwman P., Rottenberg S., Boon U., Schut E., Klarenbeek S., Klijn C., van der Heijden I., van der Gulden H., Wientjens E., et al. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer Cell. 2011;20:797–809. doi: 10.1016/j.ccr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 213.Drost R., Dhillon K.K., Van Der Gulden H., Van Der Heijden I., Brandsma I., Cruz C., Chondronasiou D., Castroviejo-Bermejo M., Boon U., Schut E., et al. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J. Clin. Investig. 2016;126:2903–2918. doi: 10.1172/JCI70196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Willimsky G., Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 215.Garbe A.I., Vermeer B., Gamrekelashvili J., Von Wasielewski R., Greten F.R., Westendorf A.M., Buer J., Schmid R.M., Manns M.P., Korangy F., et al. Genetically induced pancreatic adenocarcinoma is highly immunogenic and causes spontaneous tumor-specific immune responses. Cancer Res. 2006;66:508–516. doi: 10.1158/0008-5472.CAN-05-2383. [DOI] [PubMed] [Google Scholar]
- 216.DuPage M., Cheung A.F., Mazumdar C., Winslow M.M., Bronson R., Schmidt L.M., Crowley D., Chen J., Jacks T. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rivera-Rodriguez A., Hoang-Minh L.B., Chiu-Lam A., Sarna N., Marrero-Morales L., Mitchell D.A., Rinaldi-Ramos C.M. Tracking adoptive t cell immunotherapy using magnetic particle imaging. Nanotheranostics. 2021;5:431–444. doi: 10.7150/ntno.55165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cazaux M., Grandjean C.L., Lemaître F., Garcia Z., Beck R.J., Milo I., Postat J., Beltman J.B., Cheadle E.J., Bousso P. Single-cell imaging of CAR T cell activity in vivo reveals extensive functional and anatomical heterogeneity. J. Exp. Med. 2019;216:1038–1049. doi: 10.1084/jem.20182375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang S., Black R.G., Kohli K., Hayes B.J., Miller C., Koehne A., Schroeder B.A., Abrams K., Schulte B.C., Alexiev B.A., et al. B7-H3 Specific CAR T Cells for the Naturally Occurring, Spontaneous Canine Sarcoma Model. Mol. Cancer Ther. 2022;21:999–1009. doi: 10.1158/1535-7163.MCT-21-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schachtschneider K.M., Schwind R.M., Newson J., Kinachtchouk N., Rizko M., Mendoza-Elias N., Grippo P., Principe D.R., Park A., Overgaard N.H., et al. The oncopig cancer model: An innovative large animal translational oncology platform. Front. Oncol. 2017;7:190. doi: 10.3389/fonc.2017.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schook L.B., Collares T.V., Hu W., Liang Y., Rodrigues F.M., Rund L.A., Schachtschneider K.M., Seixas F.K., Singh K., Wells K.D., et al. A genetic porcine model of cancer. PLoS ONE. 2015;10:e0128864. doi: 10.1371/journal.pone.0128864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Overgaard N.H., Frøsig T.M., Welner S., Rasmussen M., Ilsøe M., Sørensen M.R., Andersen M.H., Buus S., Jungersen G. Establishing the pig as a large animal model for vaccine development against human cancer. Front. Genet. 2015;6:286. doi: 10.3389/fgene.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mair K.H., Sedlak C., Käser T., Pasternak A., Levast B., Gerner W., Saalmüller A., Summerfield A., Gerdts V., Wilson H.L., et al. The porcine innate immune system: An update. Dev. Comp. Immunol. 2014;45:321–343. doi: 10.1016/j.dci.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Darfour-Oduro K.A., Megens H.J., Roca A.L., Groenen M.A.M., Schook L.B. Adaptive evolution of Toll-like receptors (TLRs) in the family Suidae. PLoS ONE. 2015;10:e0124069. doi: 10.1371/journal.pone.0124069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Darfour-Oduro K.A., Megens H.J., Roca A.L., Groenen M.A.M., Schook L.B. Evolutionary patterns of Toll-like receptor signaling pathway genes in the Suidae. BMC Evol. Biol. 2016;16:33. doi: 10.1186/s12862-016-0602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Darfour-Oduro K.A., Megens H.J., Roca A., Groenen M.A.M., Schook L.B. Evidence for adaptation of porcine Toll-like receptors. Immunogenetics. 2016;68:179–189. doi: 10.1007/s00251-015-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ogando-Rivas E., Castillo P., Jones N., Trivedi V., Drake J., Dechkovskaia A., Candelario K.M., Yang C., Mitchell D.A. Effects of immune checkpoint blockade on antigen-specific CD8+ T cells for use in adoptive cellular therapy. Microbiol. Immunol. 2022;66:201–211. doi: 10.1111/1348-0421.12967. [DOI] [PubMed] [Google Scholar]
- 228.Anton D., Burckel H., Josset E., Noel G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015;16:5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kaczmarczyk J.A., Roberts R.R., Luke B.T., Chan K.C., van Wagoner C.M., Felder R.A., Saul R.G., Simona C., Blonder J. Comparative microsomal proteomics of a model lung cancer cell line NCI-H23 reveals distinct differences between molecular profiles of 3D and 2D cultured cells. Oncotarget. 2021;12:2022–2038. doi: 10.18632/oncotarget.28072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tagle D.A. The NIH microphysiological systems program: Developing in vitro tools for safety and efficacy in drug development. Curr. Opin. Pharmacol. 2019;48:146–154. doi: 10.1016/j.coph.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 231.Zips D., Kunz-schughart L.A., Cordes N. EGFR / JIP-4 / JNK2 Signaling Attenuates Cetuximab- Mediated Radiosensitization of Squamous Cell Carcinoma. Cancer Res. 2021;73:297–307. doi: 10.1158/0008-5472.CAN-12-2021. [DOI] [PubMed] [Google Scholar]
- 232.Jensen C., Teng Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020;7:1–15. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Longati P., Jia X., Eimer J., Wagman A., Witt M.R., Rehnmark S., Verbeke C., Toftgård R., Löhr M., Heuchel R.L. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer. 2013;13:95. doi: 10.1186/1471-2407-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Enzerink A., Salmenperä P., Kankuri E., Vaheri A. Clustering of fibroblasts induces proinflammatory chemokine secretion promoting leukocyte migration. Mol. Immunol. 2009;46:1787–1795. doi: 10.1016/j.molimm.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 235.Sureban S.M., Berahovich R., Zhou H., Xu S., Wu L., Ding K., May R., Qu D., Bannerman-menson E., Golubovskaya V., et al. DCLK1 Monoclonal Antibody-Based CAR-T Cells as a Novel Treatment Strategy against Human Colorectal Cancers. Cancers. 2020;12:54. doi: 10.3390/cancers12010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dillard P., Köksal H., Inderberg E.M., Wälchli S. A spheroid killing assay by CAR T cells. J. Vis. Exp. 2018;2018:1–7. doi: 10.3791/58785. [DOI] [PubMed] [Google Scholar]
- 237.Wallstabe L., Göttlich C., Nelke L.C., Kühnemundt J., Schwarz T., Nerreter T., Einsele H., Walles H., Dandekar G., Nietzer S.L., et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight. 2019;4:1–14. doi: 10.1172/jci.insight.126345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ando Y., Siegler E.L., Ta H.P., Cinay G.E., Zhou H., Gorrell K.A., Au H., Jarvis B.M., Wang P., Shen K. Evaluating CAR-T Cell Therapy in a Hypoxic 3D Tumor Model. Adv. Healthc. Mater. 2019;8:1–15. doi: 10.1002/adhm.201900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schnalzger T.E., Groot M.H., Zhang C., Mosa M.H., Michels B.E., Röder J., Darvishi T., Wels W.S., Farin H.F. 3D model for CAR -mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38:e100928. doi: 10.15252/embj.2018100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jacob F., Ming G.-L., Song H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat. Protoc. 2020;15:4000–4033. doi: 10.1038/s41596-020-0402-9. [DOI] [PubMed] [Google Scholar]
- 241.Powley I.R., Patel M., Miles G., Pringle H., Howells L., Thomas A., Kettleborough C., Bryans J., Hammonds T., MacFarlane M., et al. Patient-derived explants (PDEs) as a powerful preclinical platform for anti-cancer drug and biomarker discovery. Br. J. Cancer. 2020;122:735–744. doi: 10.1038/s41416-019-0672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kleinman H.K., Martin G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 243.Aisenbrey E.A., Murphy W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020;5:539–551. doi: 10.1038/s41578-020-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mourran A., Wu Y., Gumerov R.A., Rudov A.A., Potemkin I.I., Pich A., Möller M. When Colloidal Particles Become Polymer Coils. Langmuir. 2016;32:723–730. doi: 10.1021/acs.langmuir.5b03931. [DOI] [PubMed] [Google Scholar]
- 246.Plamper F.A., Richtering W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017;50:131–140. doi: 10.1021/acs.accounts.6b00544. [DOI] [PubMed] [Google Scholar]
- 247.Agrawal G., Agrawal R. Stimuli-responsive microgels and microgel-based systems: Advances in the exploitation of microgel colloidal properties and their interfacial activity. Polymers. 2018;10:418. doi: 10.3390/polym10040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lee J., Kim S.E., Moon D., Doh J. A multilayered blood vessel/tumor tissue chip to investigate T cell infiltration into solid tumor tissues. Lab Chip. 2021;21:2142–2152. doi: 10.1039/D1LC00182E. [DOI] [PubMed] [Google Scholar]
- 249.Hughes C.S., Postovit L.M., Lajoie G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 250.Shin J.-W., Discher D.E. Soft gels select tumorigenic cells. Nat. Mater. 2012;11:662–663. doi: 10.1038/nmat3388. [DOI] [PubMed] [Google Scholar]
- 251.Janmey P.A., Winer J.P., Weisel J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee K.Y., Mooney D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vining K.H., Mooney D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pelham R.J., Wang Y.L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Burdick J.A., Anseth K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/S0142-9612(02)00176-X. [DOI] [PubMed] [Google Scholar]
- 256.Acerbi I., Cassereau L., Dean I., Shi Q., Au A., Park C., Chen Y.Y., Liphardt J., Hwang E.S., Weaver V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015;7:1120–1134. doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pedro D.I., Nguyen D.T., Trachsel L., Rosa J.G., Chu B., Eikenberry S., Sumerlin B.S., Sawyer W.G. Superficial Modulus, Water-Content, and Mesh-Size at Hydrogel Surfaces. Tribol. Lett. 2021;69:1–9. doi: 10.1007/s11249-021-01538-3. [DOI] [Google Scholar]
- 258.Appel E.A., del Barrio J., Loh X.J., Scherman O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012;41:6195–6214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 259.Lin C.C., Anseth K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kozlowski M.T., Crook C.J., Ku H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021;4:1387. doi: 10.1038/s42003-021-02910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vukicevic S., Kleinman H.K., Luyten F.P., Roberts A.B., Roche N.S., Reddi A.H. Identification of multiple active growth factors in basement membrane matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-Q. [DOI] [PubMed] [Google Scholar]
- 262.Talbot N.C., Caperna T.J. Proteome array identification of bioactive soluble proteins/peptides in Matrigel: Relevance to stem cell responses. Cytotechnology. 2015;67:873–883. doi: 10.1007/s10616-014-9727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tse J.R., Engler A.J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 2010:1–16. doi: 10.1002/0471143030.cb1016s47. [DOI] [PubMed] [Google Scholar]
- 264.Griffin D.R., Weaver W.M., Scumpia P., Di Carlo D., Segura T. Scaffolds Assembled From Annealed Building Blocks. Nat. Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lutolf M.P., Lauer-Fields J.L., Schmoekel H.G., Metters A.T., Weber F.E., Fields G.B., Hubbell J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Girardo S., Träber N., Wagner K., Cojoc G., Herold C., Goswami R., Schlüßler R., Abuhattum S., Taubenberger A., Reichel F., et al. Standardized microgel beads as elastic cell mechanical probes. J. Mater. Chem. B. 2018;6:6245–6261. doi: 10.1039/C8TB01421C. [DOI] [PubMed] [Google Scholar]
- 267.Sideris E., Griffin D.R., Ding Y., Li S., Weaver W.M., Di Carlo D., Hsiai T., Segura T. Particle Hydrogels Based on Hyaluronic Acid Building Blocks. ACS Biomater. Sci. Eng. 2016;2:2034–2041. doi: 10.1021/acsbiomaterials.6b00444. [DOI] [PubMed] [Google Scholar]
- 268.Sheikhi A., de Rutte J., Haghniaz R., Akouissi O., Sohrabi A., Di Carlo D., Khademhosseini A. Microfluidic-enabled bottom-up hydrogels from annealable naturally-derived protein microbeads. Biomaterials. 2019;192:560–568. doi: 10.1016/j.biomaterials.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Malmsten M., Bysell H., Hansson P. Biomacromolecules in microgels—Opportunities and challenges for drug delivery. Curr. Opin. Colloid Interface Sci. 2010;15:435–444. doi: 10.1016/j.cocis.2010.05.016. [DOI] [Google Scholar]
- 270.Oh J.K., Drumright R., Siegwart D.J., Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008;33:448–477. doi: 10.1016/j.progpolymsci.2008.01.002. [DOI] [Google Scholar]
- 271.Wagner K., Girardo S., Goswami R., Rosso G., Ulbricht E., Müller P., Soteriou D., Träber N., Guck J. Colloidal crystals of compliant microgel beads to study cell migration and mechanosensitivity in 3D. Soft Matter. 2019;15:9776–9787. doi: 10.1039/C9SM01226E. [DOI] [PubMed] [Google Scholar]
- 272.Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 2011;20:4–7. doi: 10.3791/2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Raghavan S., Mehta P., Horst E.N., Ward M.R., Rowley K.R., Mehta G. Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget. 2016;7:16948–16961. doi: 10.18632/oncotarget.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lin R.Z., Chang H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 275.Grunewald L., Lam T., Andersch L., Klaus A., Schwiebert S., Winkler A., Gauert A., Heeren-Hagemann A.I., Astrahantseff K., Klironomos F., et al. A Reproducible Bioprinted 3D Tumor Model Serves as a Preselection Tool for CAR T Cell Therapy Optimization. Front. Immunol. 2021;12:689697. doi: 10.3389/fimmu.2021.689697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gunti S., Hoke A.T.K., Vu K.P., London N.R. Organoid and spheroid tumor models: Techniques and applications. Cancers. 2021;13:874. doi: 10.3390/cancers13040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Clevers H., Tuveson D.A. Organoid models for cancer research. Annu. Rev. Cancer Biol. 2019;3:223–234. doi: 10.1146/annurev-cancerbio-030518-055702. [DOI] [Google Scholar]
- 278.Freeman A.E., Hoffman R.M. In vivo-like growth of human tumors in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:2694–2698. doi: 10.1073/pnas.83.8.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Nguyen D.T., Famiglietti J.E., Smolchek R.A., Dupee Z., Diodati N., Pedro D.I., Urueña J.M., Schaller M.A., Sawyer W.G. 3D In Vitro Platform for Cell and Explant Culture in Liquid-like Solids. Cells. 2022;11:967. doi: 10.3390/cells11060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.He C., Xu K., Zhu X., Dunphy P.S., Gudenas B., Lin W., Twarog N., Hover L.D., Kwon C.H., Kasper L.H., et al. Patient-derived models recapitulate heterogeneity of molecular signatures and drug response in pediatric high-grade glioma. Nat. Commun. 2021;12:4089. doi: 10.1038/s41467-021-24168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.