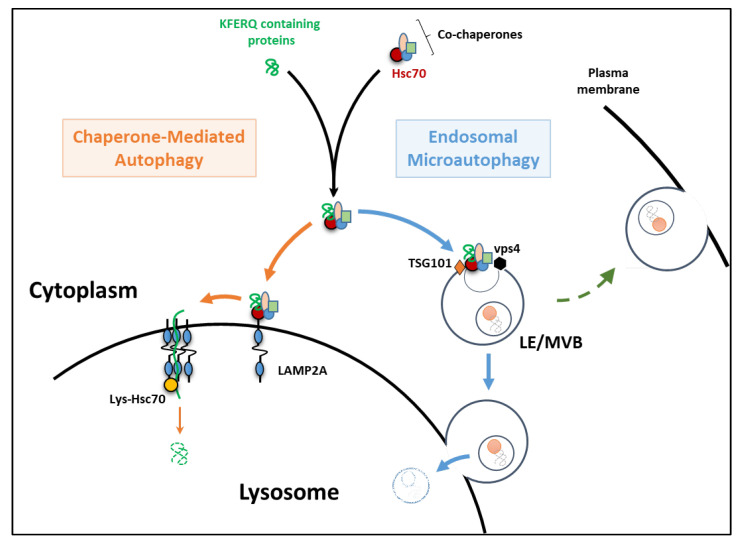
Figure 1.
Chaperone-mediated autophagy (CMA) and endosomal microautophagy (eMI) as described in mammals. The first step (dark arrows) of recognition of the KFERQ motif-containing proteins by HSC70 and its co-chaperones is shared by both pathways. During CMA (orange arrows), the substrate-chaperone complex is then directed to the lysosomal membrane through specific binding to the cytosolic tail of the lysosomal-associated membrane protein 2A (LAMP2A). LAMP2A then organizes into a multimeric complex that allows substrates to translocate across the lysosomal membrane and reach the lumen for degradation by lysosomal hydrolases. During eMI (blue arrows), the substrate-chaperone complex is transported to late endosomes/multivesicular bodies (LE/MVB) by direct binding of HSC70 to phosphatidylserine residues on the LE/MVB membrane. Members of the ESCRT machinery (including VPS4 and TSG101) will then mediate the internalization of the substrate into intraluminal vesicles. eMI substrates may then be degraded within the LE/MVB itself or upon their fusion with a lysosome. Alternatively, eMI could mediate extracellular protein releases due to the ability of LE/MVB to fuse with the plasma membrane (green dashed arrow).