Abstract
Chronic lung diseases such as asthma, chronic obstructive pulmonary disease, lung cancer, and the recently emerged COVID-19, are a huge threat to human health, and among the leading causes of global morbidity and mortality every year. Despite availability of various conventional therapeutics, many patients remain poorly controlled and have a poor quality of life. Furthermore, the treatment and diagnosis of these diseases are becoming increasingly challenging. In the recent years, the application of nanomedicine has become increasingly popular as a novel strategy for diagnosis, treatment, prevention, as well as follow-up of chronic lung diseases. This is attributed to the ability of nanoscale drug carriers to achieve targeted delivery of therapeutic moieties with specificity to diseased site within the lung, thereby enhancing therapeutic outcomes of conventional therapies whilst minimizing the risks of adverse reactions. For this instance, monoolein is a polar lipid nanomaterial best known for its versatility, thermodynamic stability, biocompatibility, and biodegradability. As such, it is commonly employed in liquid crystalline systems for various drug delivery applications. In this review, we present the applications of monoolein as a novel nanomaterial-based strategy for targeted drug delivery with the potential to revolutionize therapeutic approaches in chronic lung diseases.
Keywords: Monoolein, Nanomaterial, Drug delivery, Lung disease, Nanomedicine, Therapeutics
Graphical abstract
1. Introduction
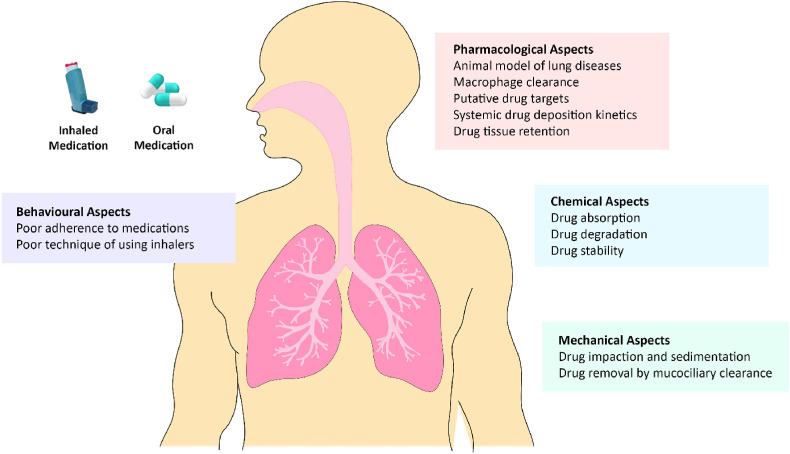
According to the World Health Organization (WHO), chronic lung diseases including asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and lung cancer are among the major contributors to the rising toll of morbidity, disability, and premature mortality around the world. Specifically, it has been reported that approximately 235 million individuals suffer from asthma and more than three million individuals succumb to COPD each year. Moreover, lung cancer, a major neoplasm, continues to be the leading cause of cancer-related premature deaths, with a higher rate of lung cancer deaths observed in women compared to men [1,2]. Researchers and health professionals have predicted that these figures will continue to increase over the coming years, posing huge burden on public health and socioeconomic growth, as well as raising the concerns that a huge population would be impacted by these diseases. At the same time, the burden brought upon by chronic lung diseases has also adversely impacted the livelihoods of individuals suffering from these diseases [[3], [4], [5], [6]]. Typically, pharmacotherapy is essential for the management of chronic lung diseases. Over the years, advancements in scientific research and medical technologies have led to the discovery and development of multiple synthetic drugs for treating these diseases. Nevertheless, most of these conventional therapeutic agents failed to effectively halt or reverse the progression of these diseases, and several patients remain poorly controlled with a poor quality of life [7,8]. Furthermore, certain therapies have resulted in the development of adverse reactions and side effects that may be potentially toxic, as well as poor delivery of therapeutic agents observed especially in naturally occurring plant-based moieties, which may hinder their therapeutic potential (Fig. 1 ) [[9], [10], [11]]. Thus, the need for researchers to develop novel innovative strategies for combating chronic lung diseases has become increasingly prominent.
Fig. 1.
Challenges involved in the development of therapeutics for chronic lung diseases.
Nanomedicine refers to the innovation of nanotechnology in the field of medicine. It is a multidisciplinary field of science involving material science, biology, chemistry, and physics that has gained rising popularity as the novel means for the diagnosis, treatment, prevention, and the follow-ups of various diseases [12,13]. Studies have shown that the application of nanomedicine in the management of human diseases has superior outcomes as compared to conventional therapeutic approaches [9,[13], [14], [15], [16]]. This can be attributed to the ability of nanocarriers to achieve targeted drug delivery to specific site within the body, as well as improved biodistribution, bioavailability, and enhanced solubility of hydrophobic agents. At the same time, nanocarriers can potentially reduce clearance and minimize premature degradation of encapsulated moieties that may be unstable in the biological environment, thereby enhancing overall therapeutic efficacy whilst avoiding off-target adverse reactions [9,11,15,17]. As such, the use of drug-loaded nanocarriers may be a feasible strategy for replacing conventional therapeutics in the treatment of chronic lung diseases, and it could contribute to the clinical translation of novel therapies that can effectively treat these diseases and improve patients’ quality of life. Generally, nanomedicine-based strategy employs colloid systems of sizes within the nanoscale range, in which drug-loaded nanocarriers can be engineered using a wide range of nanomaterials such as polymers, lipids, and other inorganic materials that can facilitate the loading and delivery of diverse chemical and biological moieties [9,18,19]. Monoolein is an example of lipid-based nanomaterial that is commonly utilized to prepare lyotropic cubic liquid crystalline drug delivery systems due to its physicochemical properties, including thermodynamic stability, non-toxicity, biocompatibility, biodegradability, as well as the ability to exhibit various phase behaviours in water [[20], [21], [22]]. In this review, special focus will be given to the applications of monoolein in advanced drug delivery systems for management of chronic lung diseases, explained by discussing some of the most recent studies in this field of research.
2. Overview of common chronic lung diseases
2.1. Asthma
Asthma is a common yet poorly understood chronic inflammatory disease of the respiratory system, in which the airways become swollen, narrow, and produce excessive mucus [23,24]. Generally, it is a disease that can manifest as episodic, where symptoms appear and resolve upon therapeutic intervention, or persistent, where symptoms are continuously present. The major clinical manifestations of asthma are shortness of breath, coughing, wheezing, and chest tightness. Some major risk factors of asthma include tobacco smoke, exposure to allergens such as pollens, moulds, dust mites, animal dander, and chemical fumes, as well as outdoor pollutants from biomass smoke, bushfire, and motor vehicles [25,26]. These triggers may vary from person to person and may be more pronounced under certain conditions, such as physical exercise, emotions and stress, consumption of alcohol, use of aspirin, beta blockers and non-steroidal anti-inflammatory drugs, as well as personal and family medical history [[27], [28], [29]].
Most cases of asthma are associated with airway sensitization by allergens, leading to a cascade of biological events that amplifies the chronic inflammatory responses. Typically, most asthmatics present type 2 inflammation that is commonly linked with pro-inflammatory cytokines such as interleukins (IL)-4, −5, and −14, as well as inflammatory cells such as eosinophils, basophils, mast cells, type 2 T helper (Th) lymphocytes, and immunoglobulin (Ig) E (IgE)-producing plasma cells [24,30]. Among these, IL-5 is the cytokine that plays a primary role in eosinophilic inflammation and asthma pathophysiology by modulating the recruitment, differentiation, growth, activation, and survival of eosinophils [31,32]. Besides, eosinophils also contribute to the production of transforming growth factor (TGF)-β which leads to airway fibrosis and remodelling, as well as IL-13 and cysteinyl leukotrienes which enhance goblet cells differentiation, leading to airway hyperresponsiveness (AHR) and mucus hypersecretion [33,34]. At the bronchial level, the damage brought upon by eosinophils accumulation is thought to be associated with their degranulation and subsequent release of toxic proteins, such as eosinophil peroxidase, eosinophil cationic protein, eosinophil-derived neurotoxin, and major basic protein, which synergistically leads to bronchoconstriction and the activation of basophils and mast cells that further produce prostaglandins (PG), histamine, and leukotrienes to amplify the ongoing inflammatory processes [35,36]. Moreover, IL-4 along with the stimulation of mast cells can promote IgE responses, resulting in the upregulation of multiple pro-inflammatory mediators that collectively induce smooth muscle contraction, vascular leakage, eventual airway narrowing and AHR that constitute the hallmarks of asthmatic patients [32,37].
Pharmacotherapy is essential in the management of asthma, and it aims to reduce frequent exacerbations, preserve lung functions, and minimize adverse reactions. Currently, the mainstay of asthma therapeutics are inhaled corticosteroids and long-acting β2-adrenoceptor agonists [38]. However, therapeutic success of these agents can be influenced by the individual characteristic of each patient, such as compliance, ability to perceive worsening asthma control, and the technique of using asthmatic inhalers. Besides, in patients whose symptoms are poorly controlled, more than one type of therapeutic agents may be required, thereby increasing the risks of adverse reactions whilst decreasing patient compliance [11,39]. For example, prolonged use of corticosteroids could result in adrenal suppression, osteoporosis, dysphonia, candidiasis, myopathies, and metabolic disturbs that may affect patients' quality of life and reduces patients’ adherence to medications [40,41]. Furthermore, increasing chronicity and severity of the disease could lead to irreversible airway narrowing, which can greatly diminish the effectiveness of bronchodilators in relieving asthmatic exacerbations. Tolerance and resistance may also be developed with prolonged high-dose administration of bronchodilators in severe asthmatic patients [42,43]. Therefore, the management of severe asthmatic exacerbations is extremely challenging and most conventional therapeutics are unable to manage the disease.
2.2. Chronic obstructive pulmonary disease
Apart from asthma, COPD is another chronic inflammatory disease of the respiratory system that represents a group of progressive and irreversible pulmonary conditions including small airway deterioration, chronic bronchitis, and emphysema [44,45]. Cigarette smoking is known as the major risk factor of COPD. Upon inhalation of cigarette smoke, more than 4500 different substances including heavy metals, toxins, carcinogens, and mutagens will be deposited along the respiratory tract from the upper airways to the deeper alveoli region. As a result, the balance between oxidants and antioxidants within the respiratory system will be distorted, further leading to mucus hypersecretion, deactivation of anti-proteases, as well as the upregulation of gene expressions that code for various inflammatory mediators. These could ultimately result in bronchial cell apoptosis and the destruction of local respiratory tissues [46,47]. Generally, the early symptoms presented by COPD patients are exertional dyspnoea while the three essential features of COPD being chronic cough, worsening episodes of dyspnoea, and increased production of sputum [48]. Although COPD is often regarded as a progressive disease with a high mortality rate, it is often preventable given that most COPD cases are induced by cigarette smoking [9,49].
Typically, COPD is characterized by chronic inflammation with an elevated number of CD8+ lymphocytes, macrophages, and neutrophils. The pathogenesis of COPD usually involves both arms of the immune system, namely, the innate and adaptive immune responses linked through dendritic cell activation, and it is initiated by a switch from self-limiting inflammatory response to a chronic persistent inflammatory response [50,51]. Inhaled cigarette smoke and other pollutants can directly upregulate pattern recognition receptors such as Toll-like receptors and purinergic receptors. At the same time, pattern recognition can also be initiated by damage-associated molecular patterns that are released by dying-autophagic, apoptotic, and necrotic cells [50,52]. Besides, these noxious gases can also damage airway epithelial cells by downregulating vascular endothelial growth factor (VEGF) and hepatocyte growth factor, which lead to the apoptosis of alveolar cells that is observed in the emphysema COPD phenotype. The damaged alveolar epithelial cells further release TGF-β that facilitates the upregulation of connective tissue growth factor, leading to collagen deposition, lung fibrosis, and airway remodelling [47,52]. The inflammatory response can also be propagated by chemotactic factors which attract inflammatory cells to the injured regions within the lung. Namely, chemokine ligand (CCL) 2 (CCL2) acts on chemokine receptor 2 (CCR2) to attract monocytes; C-X-C motif ligand (CXCL)-1 and CXCL-8 act on CCR2 to attract neutrophils and monocytes; whereas CXCL-9, -10, and −11 act on C-X-C motif receptor 3 (CXCR3) to attract Th1 cells and cytotoxic T cells [47,50,52]. These attracted inflammatory cells then release proteases such as matrix metalloproteinase (MMP)-9 at the injured site, leading to elastin degradation and emphysema, as well as switching of the immune system to a Th17 response for the promotion of chronic inflammatory processes [50,53].
Like asthma, pharmacotherapy in COPD aims to improve symptoms and reduce chronic exacerbations. Corticosteroids, long-acting β2-agonists, bronchodilators, and anticholinergics are the agents that are commonly utilized in the management of COPD. Nevertheless, these agents are unable to modify the course of the disease, whereby complete recovery is currently only possible with lung transplantation. Apart from that, these agents are also associated with undesirable adverse reactions that may further affect patients’ quality of life [8,9]. For example, myocardial ischemia and electrolyte disturbance are associated with long-acting β2-agonists, whereas acute glaucoma and supraventricular tachycardias are associated with anticholinergics [54]. Besides, it has been reported that COPD patients respond poorly to corticosteroids even at higher doses. Therefore, resistance to corticosteroids in COPD patients is thought to be attributed to reduced histone deacetylase expression in macrophages, which then impairs the ability to suppress production of CXCL-8, MMP-9, and tumour necrosis factor (TNF)-α. Despite that, corticosteroids are still commonly utilized due to the lack of effective therapies, leading to other adverse effects such as osteoporosis and cardiovascular events [[54], [55], [56]]. Furthermore, such abnormal macrophage activity also impairs the normal phagocytic processes, which could lead to chronic bacterial colonization of the airways and increases the risk of pneumonia in COPD patients [56]. Hence, there is an increasing need to develop novel alternatives to conventional COPD therapeutics to address the growing morbidity and mortality brough upon by this disease.
2.3. Idiopathic pulmonary fibrosis
IPF is a chronic and progressive disease of the respiratory system that is characterized by a dysregulated accumulation of fibrotic tissue in the lungs parenchyma, and the disease is often associated with a poor prognosis with significant morbidity. Dyspnoea is the most reported symptom by IPF patients, in which studies have demonstrated correlation between the severity of dyspnoea and the quality of life as well as the prognosis of IPF patients [57,58]. Generally, the risk factors of IPF include genetic predisposition, cigarette smoking, exposure to environmental pollutants, viral infection, chronic aspiration, and medication use. Nevertheless, none of these risk factors could adequately justify the progressive nature of IPF or the extensive remodelling and increased incidence of fibrosis with advancing age of patients [58].
Repair of damaged tissues is a fundamental biological mechanism of the human body critical for survival that enables the replacement of damaged or dead cells after an injury in an orderly manner. Fibrogenesis is part of the wound healing process that is initiated in response to tissue injury, leading to the ideal restoration of homeostasis [59]. In normal physiological conditions, such wound healing process upon initiation by epithelial injury, releases inflammatory mediators including IL-25 and -33, as well as thymic stromal lymphopoietin that facilitate the propagation of pro-fibrotic Th2 responses. The process is followed by the activation of platelets and enhanced vessel permeability for the recruitment of leucocytes. These inflammatory cells further release IL-4 and -13 which induce the development of a pro-fibrotic macrophage population that upregulates TGF-β, contributing to the recruitment and activation of fibroblasts, as well as their subsequent differentiation into myofibroblasts. Lastly, these myofibroblasts produce components of the extracellular matrix (ECM) to facilitate the wound healing process, resulting in eventual restoration of normal tissue and its structural integrity [59,60]. However, during the development of IPF-associated fibrosis, the wound healing process can be dysregulated in one or more stages, which contributes to an irreversible and undesirable deposition of scar tissue that is characterized by hypersecretion of ECM components including interstitial collagens, fibronectin, hyaluronic acid, and proteoglycans. Ultimately, the architecture of the lung is remodelled with the development of a permanent fibrotic scar, producing symptoms such as persistent cough, shortness of breath, and chest tightness [59,61].
Despite numerous studies that are performed over the years, efforts in identifying an effective therapy for the management of IPF remains elusive, with lung transplantation as the sole option that is effective in treating IPF. Therefore, IPF remains as a lung disease that is challenging to treat, thereby posing a significant burden to public health and patients’ quality of life [62,63]. Recently, there are a few anti-fibrotic agents that have been developed and approved as the potential disease-modifying therapeutics for IPF, namely, nintedanib and pirfenidone. However, the prolonged use of these therapeutic agents is associated with adverse reactions [64,65]. For instance, nintedanib has been found to cause acute nausea, diarrhoea, and modified liver enzyme activity, whereas pirfenidone has been found to cause various dermatological, gastrointestinal, and neurological side effects, as well as photosensitivity [[64], [65], [66]]. These may contribute to poor compliance to medications and increased discontinuation rates in IPF patients. As such, these agents are far from ideal therapies to prevent disease progression, mortality rate and reversing the pathophysiological process of the disease [11,67]. Thus, efforts to identify novel IPF therapeutics with greater efficacy and improved safety profiles are highly essential.
2.4. Lung cancer
Lung cancer refers to the development of tumours due to dysregulated cell growth in the lung parenchyma or bronchi [68]. Currently, there are two different types of lung cancer, namely, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with the latter accounting for most of the lung cancer cases throughout the world [69]. The greatest risk factor for lung cancer is exposure to cigarette smoke, whereby the rising prevalence of lung cancer can be largely attributed to growing smokers’ population globally. Other risk factors for lung cancer include genetic susceptibility, air pollution, occupational exposure, and poor diet [70,71].
Genetic damage and mutations that affect normal cell regulation processes are primarily the cause of lung cancer, where genetic alterations usually promote the upregulation of oncogenes and downregulation of tumour suppressor genes. Examples of driver mutation genes that have been established to be associated with NSCLC include B-raf proto-oncogene, epidermal growth factor receptor gene, echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase fusion oncogene, c-ROS oncogene 1, and p53 gene [72,73]. In addition, chronic inflammation has also been shown to increase the risk of tumorigenesis and induce the advancement of tumour from invasion to metastasis [68,[74], [75], [76]]. As oncogenes and tumour suppressor genes are implicated in tumorigenesis, they too play a role in inflammation by regulating inflammatory mediators and the induction of neovascularisation. Therefore, the same genetic alterations that result in tumorigenesis also aid in creating a favourable microenvironment for the development of tumours and promoting immune escape by reducing anti-tumour activity [68]. As described earlier, cigarette smoke contains mutagens and irritants that could lead to pulmonary inflammation and induce repeated insults to the lung parenchyma, producing a mutagenic environment [74]. As such, the persistent chronic inflammation present in COPD also increases the risk of developing malignant tissue within the lungs as it has been shown that low grade emphysema is a risk factor for lung cancer [74,77].
The goals in lung cancer treatment are to improve patients' quality of life, symptoms palliation, and to prolong survival, whereby the initiation and choice of therapeutic regimen often depends on the type, staging, and spread of the tumour cells, as well as the underlying health status of the patient [78]. Nevertheless, there is still no definite cure for lung cancer to-date, and all therapeutic options can only prolong the survival rates of patients [78,79]. Current conventional cancer chemotherapies are also associated with various long-term adverse reactions that could further decrease patients’ quality of life. For example, doxorubicin has been linked with weight loss, alveolar haemorrhage and reduction in pulmonary function; gemcitabine has been linked with pulmonary oedema and connective tissue formation; whereas 5-fluorouracil has been linked with weight loss and dose-dependent glossitis [80]. In addition, these chemotherapies often demonstrate a low long-term response rate as they lack specificity towards tumour tissues, which leads to wide systematic exposure with limited quantity of drugs being delivered to lung tumour, as well as undesirable diffusion to other non-tumour tissues, thereby resulting in high off-target toxicity, systemic side effects, and reduced therapeutic outcome [81,82]. Hence, there is a need to develop novel and safer chemotherapies with minimized systemic exposure and increased tumour targeting to improve therapeutic window and subsequent clinical success in lung cancer patients.
2.5. COVID-19
COVID-19 is a novel, highly contagious infectious disease of the respiratory system caused by severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) 2 (SARS-CoV-2). It first emerged in December 2019 in a cluster of patients presenting with pneumonia of unknown cause in the city of Wuhan, People's Republic of China [83]. This respiratory virus has demonstrated a significantly greater lethality compared to the earlier emerging outbreaks of respiratory viruses, including SARS-CoV in 2003 and Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV) in 2012 [84]. The early typical symptoms of COVID-19 include fever, dry couth, and shortness of breath. However, the clinical features of the disease may be highly variable, ranging from asymptomatic patients to severe forms of respiratory failure and widespread inflammatory dysregulated host response that could lead to multi-organ failure, thromboembolism, and death. COVID-19 can infect people of all ages, with older people and people with underlying comorbidities at a greater risk of developing severe disease. Cancer patients and those receiving immunosuppressants, as well as pregnant women are also thought to be at a greater risk of developing severe disease when infected with SARS-CoV-2 [[84], [85], [86]]. As of November 2021, SARS-CoV-2 has infected more than 259 million individuals and it has claimed more than 5 million lives globally [87].
As the ongoing outbreak of COVID-19 has posed an alarming and extraordinary threat to public health, researchers have rushed to understand the pathophysiology of the disease to pave the way for the development of medical interventions with the aim to contain the spread of the virus. Like all viral infection, receptor recognition is the major step for infecting host cells and is crucial for viral infectivity and pathogenesis [88]. Researchers have confirmed that SARS-CoV-2 depends upon angiotensin-converting enzyme 2 (ACE2) receptor to enter host cells. Upon binding of the virus to ACE2 receptor, type 2 transmembrane serine protease (TMPRSS2) that is present in the host cell facilitates viral uptake via ACE2 cleavage and priming of the SARS-CoV-2 spike (S) protein, thereby mediating coronavirus entry into the host cell through the endocytic route [84,88,89]. Therefore, it can be assumed that a higher risk of viral infection can be correlated with an upregulated ACE2 expression or a high-level co-expression of ACE2 and TMPRSS2 proteins in SARS-CoV-2 targeted cells [90]. In humans, extensive ACE2 and TMPRSS2 expression is shown in epithelial tissues of the heart, kidney, gastrointestinal and respiratory tract. Particularly, SARS-CoV-2 entry factors are highly expressed in the upper respiratory tract, nasal epithelial cells, bronchi, and lower airways [[88], [89], [90]]. These suggest that even without the presence of any underlying comorbidities, most vital human organs can potentially be susceptible to SARS-CoV-2 infection [90]. In the later stages of infection where viral replication accelerates, compromised epithelial-endothelial integrity contributes to the infectivity of SARS-CoV-2 on pulmonary capillary endothelial cells, thereby augmenting the inflammatory response and inducing the rapid influx of neutrophils and monocytes. Collectively, these result in lung injury, dysfunctional alveolar-capillary oxygen transmission, and impaired oxygen diffusion capacity that are the characteristic manifestations of COVID-19 [83,84,91].
Vaccines are the first responders on the COVID-19 scene, in which there have been substantial progress in the development of vaccines against COVID-19 at an unprecedented speed, which can also be attributed to the previous experiences gained by researchers from the vaccine development path against SARS and MERS [92,93]. As of November 3, 2021, eight types of vaccines have been listed for emergency use against COVID-19 by the WHO. These include the vaccines produced by Pfizer-BioNTech (Comirnaty®), AstraZeneca-Oxford (Vaxzevria®), Moderna (Spikevax®), Sinovac (Coronavac®), Janssen, Sinopharm, Serum Institute of India (Covishield™), and Bharat Biotech (Covaxin®) [94,95]. However, the emergence of various SARS-CoV-2 variants of concern (VOC), such as B.1.617.2 (Delta), P.1 (Gamma), B.1.351 (Beta), and B.1.1.7 (Alpha) has perturbed the initial optimism that COVID-19 vaccines may provide a long-term solution to the COVID-19 pandemic. Several studies have revealed that most vaccines produced a lower neutralizing response against these VOCs, thereby offering limited protection against mild to moderate COVID-19 in regions where SARS-CoV-2 VOCs are prevalent [[96], [97], [98], [99]]. For example, one study has reported that the Delta variant, which has four mutations in its S protein, had reduced susceptibility to vaccine sera especially in individuals who have only received one out of two doses of the Comirnaty® and Vaxzevria® vaccines [100]. Such findings also imply that these variants may spread more rapidly due to increased immune evasion potential arising from diverse mutations involving the receptor-binding domain of the SARS-CoV-2 S protein [[96], [97], [98], [99], [100]]. Furthermore, the most recently discovered SARS-CoV-2 variant, B.1.1.529 (Omicron), which was designated as a VOC by the WHO on November 26, 2021, was found to possess numerous mutations on its S protein which suggest an increased risk of reinfection with this variant in contrast to other VOCs [101]. Recently, oral antivirals developed by Pfizer (paxlovid) and Merck & Co. (molnupiravir) are also set to make their mark as the United States Food and Drug Administration (FDA) is considering granting authorizations for the use of these two oral antivirals in the treatment of COVID-19, whereby clinical studies have demonstrated that both paxlovid and molnupiravir halt COVID-19 hospitalizations and deaths in patients treated soon after their initial infection [93,[102], [103], [104]]. Nonetheless, given that the emergence and rapid transmission of SARS-CoV-2 and its VOCs have sparked great concerns on impending cases surge and negative public health outcome, accelerating the pace of vaccination and continued development of next-generation vaccines and/or therapeutic agents using the latest technologies are crucial to address the growing risk of COVID-19 and to modify the course of the pandemic.
3. Application of nanomedicine in chronic lung diseases
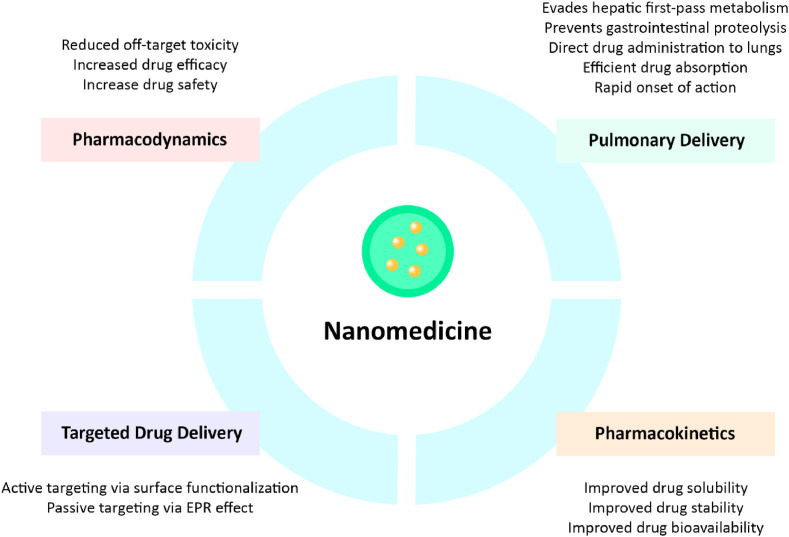
Chronic lung diseases result in impaired work productivity, limitations in the activities of daily life, as well as poor quality of life. As such, these diseases impose a remarkable financial and emotional burden on both patients and their families. It is predicted that by 2030, approximately 20% of global mortalities would be respiratory related [105,106]. Despite availability of various therapeutic agents, these conventional therapeutics have been insufficient in curing or slowing the progression of these diseases [17,105]. Therefore, there is a significant global unmet need for researchers to develop novel classes of safe and effective therapies that could enhance therapeutic efficacy by facilitating targeted delivery of therapeutic moieties to specific site within the lung. Application of nanomedicine offers a unique opportunity and a whole new vista of enhanced drug delivery for chronic lung diseases such as asthma, COPD, IPF, and lung cancer, as well as emerging infectious diseases such as influenza and COVID-19 (Fig. 2 ).
Fig. 2.
Potential benefits of nanomedicine-based strategy in managing chronic lung diseases.
3.1. Improvement in pharmacokinetic properties
Over the years, various chemical and biological agents, as well as immunomodulatory agents have been developed as therapeutics for the management of chronic lung diseases [[107], [108], [109]]. Besides, given the significant roles of chronic inflammation and oxidative stress in the pathogenesis of most chronic lung diseases, many plants and herbs have also been shown to be beneficial as they possess compounds that are rich in antioxidative and anti-inflammatory activities [5,[110], [111], [112]]. For example, resveratrol in grapes and red wine, garlic, as well as curcumin were found to exhibit anti-tumour effects by the inhibition of cyclooxygenase (COX) enzymes or VEGF, and by targeting pro-inflammatory signalling pathways including nuclear factor kappa B (NF-κB), mitogen activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) [76]. Despite that, these agents are often unable to achieve their maximal therapeutic potential due to several pharmacokinetic drawbacks, which include low solubility, rapid clearance, and poor in-vivo stability. Thus, the application of nanomedicine has been proposed as a feasible strategy to overcome these drawbacks and to improve the therapeutic outcomes of these potential agents in the management of chronic lung diseases [113,114].
In terms of solubility, the delivery of therapeutic moieties using nanocarriers appeared as a potential strategy for enhancing the bioavailability and therapeutic efficacy of poorly water-soluble compounds, as the use of nanocarriers can facilitate the delivery of therapeutic moieties to their physiological target whilst preserving their integrity and bioactivity [115]. For this instance, therapeutic moieties can be either conjugated, complexed, encapsulated, entrapped, adsorbed, and/or attached to the nanocarriers [116]. Studies have proven that once a therapeutic moiety is encapsulated within a nanocarrier, its pharmacokinetic properties, biodistribution, and in-vivo behaviour will be solely dictated by the physicochemical properties of the nanocarrier rather than the therapeutic moiety itself [117]. Apart from that, given the submicron size of nanocarriers, there will be a remarkable increase in surface area and the surface to volume ratio, which can be positively exploited to enhance dissolution rate and offer great opportunities for modulating the behaviour of nanocarriers [[118], [119], [120]].
Apart from that, the utilization of nanocarriers have been proven advantageous to optimise the circulation half-life of therapeutic moieties. This is due to the ability of these nanocarriers to achieve a sustained and controlled drug release profile, which could subsequently prolong therapeutic action and enhance therapeutic efficacy [121,122]. Besides, nanocarriers that exhibit a sustained release pattern can often improve patients’ compliance as reduction in required dosage and frequency of administration can be achieved. This is attributed to the ability of nanocarriers to maintain drug circulation time greater than the biological half-life of the drug, as well as generation of a steady-state and fixed therapeutic action due to persistent biological concentration of the drug within the therapeutic window [122,123]. As the half-life and bio-circulation of a compound is greatly influenced by its release from a nanocarrier, modification to the structure and composition of a nanocarrier and its method of preparation can produce varying effects on the overall therapeutic action. Other factors that can influence the release of therapeutic moieties from nanocarriers include pH, temperature, desorption of adsorbed or surface-bound drugs, diffusion of drugs through the nanoparticle matrix, as well as the swelling and erosion of nanoparticle matrix [121,124].
On the other hand, biologics offer the benefits of high specificity and potency as compared to other small molecule drugs in the treatment of diseases. However, the structural complexity of biologics has made formulation and intracellular delivery challenging [125]. In terms of chronic lung diseases, siRNA is an example of biologics that can be utilized to “silence” specific genes responsible for the expression of pro-inflammatory factors to modify the molecular mechanisms underlying the pathogenesis of these diseases [126]. Nevertheless, biologics are often highly unstable in-vivo. For example, environmental triggers such as temperature and moisture could lead to the loss of bioactivity, while chemical and enzymatic degradation can also occur both during storage and in the physiological environment [127]. In addition, although intranasal delivery of siRNAs is generally preferred due to reduced invasiveness and systemic toxicities, as well as increased specificity for the respiratory system, biological barriers such as the respiratory cilia and mucus layer have hindered their effective delivery [128]. Thus, the utilization of nanocarriers can potentially improve the delivery of biologics while enhancing their half-life and retention in the systemic circulation to facilitate their absorption, protect these sensitive cargoes from degradation in hostile biological environments and enhancing therapeutic outcomes [129,130].
3.2. Pulmonary drug delivery
Generally, pulmonary delivery via inhalation is the preferred route of delivering therapeutic agents in the management of chronic lung diseases, as therapeutics moieties can be directly delivered into the lungs with preferential deposition and retention within diseased regions. In contrast to other routes of administration, pulmonary inhalation presented various advantages in the treatment and management of these diseases [131,132]. Namely, drug delivery via pulmonary inhalation offers direct administration to the lungs, resulting in high drug concentrations within the diseased tissues with low systemic concentrations. Besides, the pulmonary route also evades hepatic first-pass metabolism and gastrointestinal proteolysis. This is further associated with high pulmonary efficacy and improved drug bioavailability within the lung, which can greatly minimize the risks of adverse effects as a lower dosage is needed to produce therapeutic effects [[132], [133], [134]]. Apart from improving the biodistribution of therapeutic agents for site-specific delivery, pulmonary inhalation also provides a large alveolar surface area, dense capillary network, and a thin biological barrier that contributes to efficient drug absorption and rapid onset of action [134,135]. In a nutshell, an efficient inhaled drug delivery system is typically designed to achieve high retention in the lung and does not penetrate the systemic circulation to protect other body parts from undesirable effects [135].
3.3. Targeted drug delivery
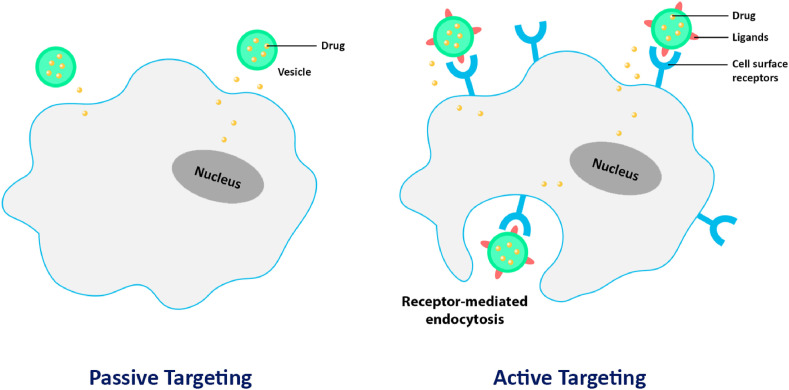
As discussed, targeted delivery is essential to retain therapeutic moieties within their desired site of action whilst limiting their accumulation in healthy tissues and organs. Such a goal can be achieved by both passive and active targeting of therapeutic agents (Fig. 3 ) [136]. The strategy of passive targeting allows drug-loaded nanocarriers to accumulate at targeted sites by exploiting specific pathophysiological characteristics of the diseased cells and tissues, as well as their surrounding microenvironment. Particularly, passive targeting can be achieved via the enhanced permeability and retention (EPR) effect, and it is highly useful in the treatment of lung cancer, whereby particles with sizes between 10 and 1000 nm can preferentially accumulate within tumour tissues instead of normal tissues [137,138]. This is due to the larger endothelial gap within the vasculature of angiogenic tumour as compared to those of normal vasculature, as tumour cells are generally non-responsive towards cell signalling processes involved in regulating vasculogenesis. Therefore, it allows the entry of drug-loaded nanocarriers into the tumour tissues through their leaky vasculature and abnormally wide pores. In addition, tumour tissues have limited lymphatic drainage that reduces molecule clearance, thereby resulting in higher drug retention [[137], [138], [139]]. Thus, nanocarriers can be utilized to deliver therapeutic moieties to targeted sites via the EPR effect, allowing for a high retention of these agents within diseased tissues whilst minimizing off-target adverse effects, leading to more effective therapy.
Fig. 3.
Targeted drug delivery via nanocarriers can be achieved by passive and active targeting mechanisms.
On the other hand, active targeting refers to the strategy in which the surface of nanocarriers is functionalized with targeting ligands such as proteins, antibodies, aptamers, peptides, carbohydrates, and glycoproteins that present great affinity to their cellular binding partners such as cell surface receptors, tumour antigens, and tumour vasculature [136]. Ideally, active targeting allows the specific localization of drug-loaded nanocarriers to diseased cells instead of healthy cells, which could also reduce non-specific interactions between the nanocarrier and cell plasma membrane [139,140]. Therefore, an active targeting strategy can be employed along with EPR-based passive targeting strategy to further enhance the deposition and retention of therapeutic moieties within the diseased site. Apart from targeting ligands, hydrophilic molecules such as poly (ethylene glycol) (PEG) can also be functionalized to the surface of nanocarriers to extend their bio-circulation time by avoiding recognition and clearance by the reticuloendothelial system [141].
4. Monoolein as a novel nanomaterial for drug delivery
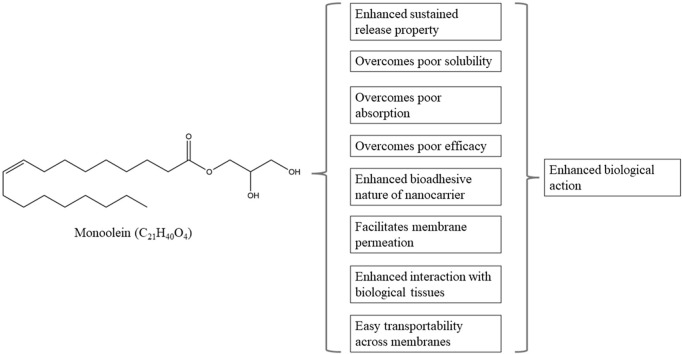
As mentioned earlier, the application of nanomedicine for the management of chronic lung diseases offers multiple advantages over conventional therapeutic approaches. Typically, the nanocarriers or drug delivery systems that are used to safely transport therapeutic moieties are designed to enhance chemical stability and aqueous solubility of active agents, improve pharmacological activity, and to minimize adverse effects. In the recent years, various drug delivery systems have been developed and have shown great potential in facilitating the loaded agents to achieve their desired therapeutic effects [142,143]. Among which, liquid crystalline nanoparticles have attracted the attention of many researchers as a promising nanocarrier for different agents and route of administration. It is a type of drug delivery system that is self-assembled from polar amphiphilic lipids in the presence of excess water. As they are present in an intermediate state between an ordered crystal and disordered liquid, they combine the merits of both fluidic and particulate delivery systems, which include sustained release profile, improved colloidal stability, bio-adhesiveness and flexible structure [144,145]. Besides, the amphiphilic nature of liquid crystalline nanoparticles enables them to carry both hydrophilic and lipophilic drugs, in which hydrophilic drugs are usually encapsulated in the core while lipophilic drugs will be loaded within the shell structure of the nanoparticles. Liquid crystalline nanoparticles also provide greater encapsulation potential as compared to other drug delivery systems as they typically contain a greater proportion of lipids that results in a higher surface area [146,147]. In addition, these nanoparticles do not degrade through enzymatic reactions, thereby preserving the loaded moieties from oxidation or hydrolysis, resulting in increased bioavailability with greater cellular uptake at the target site [148].
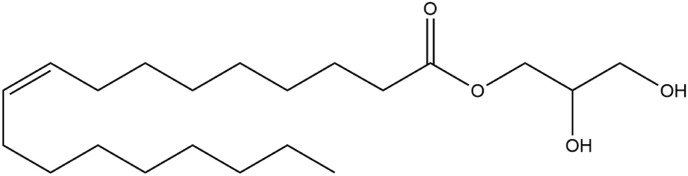
Many types of liquid crystal-forming lipids have been utilized for the preparation of liquid crystalline nanoparticles. For instance, glyceryl monooleate, or monoolein, is often regarded as a special lipid that has attracted much attention as a nanomaterial for developing liquid crystalline nanoparticles, attributed to its ability to self-assemble in water and to form a wide range of thermodynamically stable and well-defined liquid crystal structures, along with its unique properties including biocompatibility and biodegradability [22,145]. In terms of its structure, it is composed of a hydrocarbon chain that is attached to a glycerol backbone via an ester bond (Fig. 4 ). Generally, the two hydroxyl groups of the glycerol backbone confer polar characteristics to this part of the molecule, thereby enabling the formation of hydrogen bonds with water in aqueous solutions. Conversely, the C18 hydrocarbon chain which features a cis double bond at the 9,10 position is strongly hydrophobic. As such, monoolein is rendered as an amphiphilic molecule [144]. Monoolein can be produced via direct esterification of fatty acids, primarily oleic acid and glycerol, or via transesterification of refined vegetable oils, such as sunflower oil or erucic canola oil. It is commercially available in two different forms, namely, a mixed glyceride form that is simply referred to as monoolein, or distilled monoolein that is preferred for pharmaceutical applications due to its high purity [144,149].
Fig. 4.
The chemical structure of monoolein (C21H40O4).
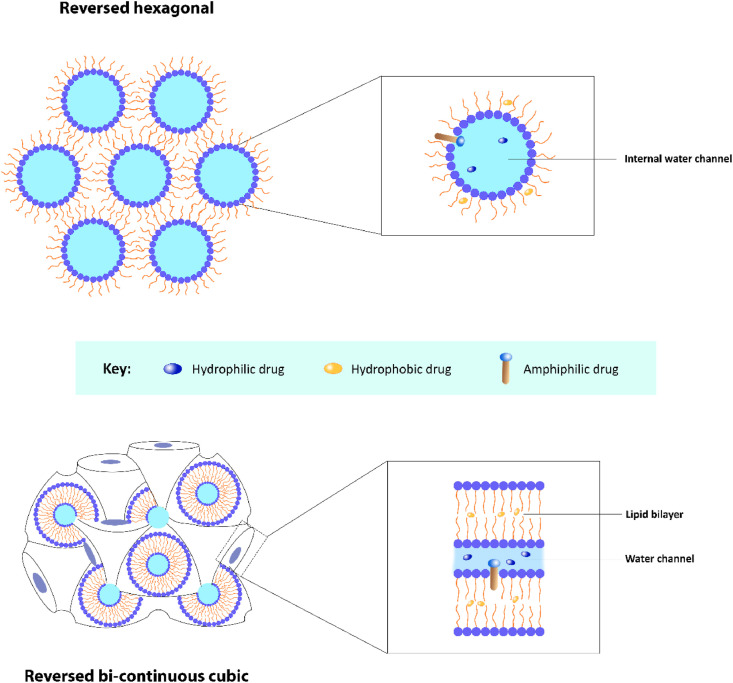
Lyotropic liquid crystals are formed by the self-assembly of amphiphilic molecules in solvent at a relative concentration and given temperature. Depending on the water to amphiphile ratio, spontaneous phase transitions can occur, resulting in various lyotropic phases. Such phases offer promising prospect for encapsulating a plethora of target molecules with varying sizes and polarities due to their unique internal structures of different phases. Besides, the drug release profile in different lyotropic phases is also distinguishable due to varying diffusion coefficients. In drug delivery applications, the most commonly occurring lyotropic phases are the reversed bi-continuous cubic and reversed hexagonal phases, and they have been shown to control and sustain the release of encapsulated molecules (Fig. 5 ) [150,151]. Upon further dispersion of these lyotropic phases in the presence of a stabilising compound, commonly triblock copolymer poloxamer 407, kinetically stable colloidal nanoparticles can be formed [152]. As for monoolein, given that it is a lipid that exhibits a wide range of temperature and composition sensitive behaviour, it has an extensive and intriguing structural repertoire, in which lamellar, reversed hexagonal, sponge, and cubic phases are among the phases monoolein can exhibit [153]. Specifically, the cubic phase in monoolein is highly attractive for drug delivery applications as it exhibits mechanical stiffness, and it is stable under both room and physiological environment. Other appealing properties include its bio-adhesive property, sustained release pattern, and the ability to shield sensitive cargoes from premature degradation. It is important to note that the cubic phase possess a structural organization that is identical to that of bio-membranes [149,150,153]. Cubosomes are the corresponding liquid crystalline nanoparticles that are formed upon dispersion of the cubic lyotropic phase [20,152]. In contrast to other lipid-based nanocarriers such as liposomes, cubosomes possess a greater breaking resistance as well as a greater ratio of bilayer area to particle volume. Over the years, studies have already proven cubosomes to be an excellent drug delivery system that can be utilized for various applications. For example, cubosomes can be conjugated with specific ligands, such as folic acid, to target tumour tissue with an overexpression of folate receptors which can increase the efficiency of drug delivery. Besides, co-delivery of multiple compounds is also possible with cubosomes. Therefore, when loaded with both therapeutic and imaging agents, they can be beneficial for personalised theranostic nanomedicine, which are particularly useful for the management of cancer [20,150].
Fig. 5.
Structures of the reversed hexagonal and reversed bi-continuous cubic phases of lyotropic liquid crystals.
5. Monoolein as drug delivery systems in chronic lung diseases
Over the years, there have been multiple studies that evaluated the effectiveness and potential of monoolein-based drug delivery systems in the management of chronic lung diseases (Table 1 ). Quercetin is a natural flavonoid compound found in fruits and vegetables that has a wide range of biological actions, including anti-inflammatory and antioxidative properties that are useful for treating chronic lung diseases. However, its practical use is often limited by its poor solubility and low absorption, thereby requiring interventions to overcome these liabilities [154]. One study by Yong et al. has investigated the anti-inflammatory activity of quercetin-loaded monoolein liquid crystalline nanoparticles in lipopolysaccharide (LPS)-stimulated human primary bronchial epithelial cell line. It was demonstrated that the monoolein nanocarrier remarkably suppressed the production of IL-1β, IL-6, and IL-8. Notably, there was a significant enhancement of the anti-inflammatory activity of quercetin when encapsulated within the nanocarrier as compared to free quercetin, with an efficacy comparable to those of fluticasone with respect to the downregulation of IL-1β and IL-6. Such findings can be attributed to the bio-adhesive nature of the nanocarrier, which facilitated membrane permeation to increase interaction with the bronchial epithelial cells. As the lipid bilayer of the monoolein nanocarrier resembles epithelial cell membrane, the nanocarrier can be easily transported across the membrane to yield greater drug absorption. Interestingly, a noticeable reduction in pro-inflammatory cytokines was also reported with the use of unloaded nanocarriers. This indicated that the component of the nanocarrier, namely, monoolein, may have presented synergistic anti-inflammatory activity with quercetin via the NF-κB and MAPK pathways [155]. Naringenin is also a flavonoid that has known biological activity against chronic lung diseases, but the presence of a large hydrophobic ring in its structure has prevented its clinical application due to poor solubility and bioavailability. To improve its pharmacological property, Wadhwa et al. loaded naringenin into monoolein-based liquid crystalline nanoparticles and evaluated their anti-inflammatory activity against LPS-stimulated human airway epithelium-derived basal cells. The nanoformulation exhibited a sustained release profile and effectively downregulated the expressions of IL-1β, IL-6, IL-8, and TNF-α, with an efficacy comparable to those of fluticasone propionate. While suppression of these pro-inflammatory cytokines can ameliorate chronic inflammatory processes in the airway, it can also exert a beneficial effect by mitigating the risk of developing lung cancer, or by slowing down its progression, given that chronic inflammation is a risk factor in driving tumorigenesis. This is consistent with the findings of the present study which showed that the nanoformulation when tested in A549 cell line, significantly inhibited cell proliferation and migration, as well as attenuated colony formation and induced cell apoptosis [156]. Apart from that, Paudel et al. in a study formulated berberine-loaded monoolein-based liquid crystalline nanoparticles and evaluated their potential as a novel therapeutic approach for inflammatory lung diseases caused by cigarette smoking. Berberine is an isoquinoline alkaloid commonly isolated from herbs in the Papaveraceae and Ranunculaceae families, in which its anti-inflammatory potential has been documented in multiple scientific studies. Similarly, the nanoformulation exhibited a sustained release profile, thereby enabling a steady state bioavailability of berberine for uptake by broncho-epithelial cells (16HBE) and macrophages (RAW264.7). The researchers showed that the nanoformulation had potent anti-inflammatory activity, justified by inhibition of the expressions of inflammatory mediators TNF-α, IL-1β and IL-6 in 16HBE cells, as well as the expression of TNF-α and production of nitric oxide in RAW264.7 cells. Moreover, the mRNA level of COX-2 was remarkably suppressed by the nanoformulation in 16HBE cells. Genes related to oxidative stress, such as Gpx2 in 16HBE cells and Nqo1 in RAW264.7 cells were also downregulated. Notably, these biological activities were more potent in contrast to free berberine even at a low dose, indicating that the application of monoolein-based nanovehicle can improve the physiochemical characteristics of berberine to exert a greater pharmacological action [157]. Taken together, these findings suggest that monoolein can potentially be utilized for the fabrication of drug delivery systems in the treatment of chronic lung diseases, such as asthma and COPD.
Table 1.
Summary of studies that demonstrated the potential of monoolein-based drug delivery systems in managing chronic lung diseases.
| Encapsulated drug(s) | Concentration/Dose | Type of study | Key findings | Reference |
|---|---|---|---|---|
| Quercetin | Not specified | In-vitro: LPS-stimulated human primary bronchial epithelial cell line (BCi-NS1.1) |
|
[155] |
| Naringenin | 0.5 mg/mL | In-vitro: LPS-induced human primary bronchial epithelial cell line (BCi-NS1.1); human lung epithelial carcinoma cell line (A549) |
|
[156] |
| Berberine | 1, 2.5, and 5 μM | In-vitro: Cigarette smoke extract-stimulated human bronchial epithelial cell line (16HBE) and macrophage cell line (RAW264.7) |
|
[157] |
| Bamboo shavings (Bambusae Caulis in Taeniam) (BCT) | 60 mg/mL | In-vitro: Carcinogenic fine dust-stimulated RAW 264.7 cells |
|
[158] |
| Rutin | 3, 4, and 5 μM | In-vitro: LPS-induced human primary bronchial epithelial cell line (BEAS-2B) |
|
[159] |
| Ascorbyl palmitate and alpha-tocopherol | Not specified | Preliminary study |
|
[160] |
| Curcumin | 5, 10, 15, and 20 μM | In-vitro: Human colon cancer cell line (HCT116) |
|
[161] |
| Berberine | 5 μM | In-vitro: Human adenocarcinomic alveolar basal epithelial cell line (A549) |
|
[162] |
| Berberine | 0.5, 1, 2.5, and 5 μM | In-vitro: Human lung epithelial cancer cell line (A549) |
|
[163] |
| Elesclomol | 0.1–100 nM | In-vitro: Human lung cancer cell line (A549) |
|
[164] |
| Pemetrexed and resveratrol | In-vitro: 10, 20, and 30 μg/mL | In-vitro: Human lung carcinoma cell line (A549) |
|
[165] |
| In-vivo: 0.94 mg/kg | In-vivo: Male BALB/c mice induced with lung cancer via intraperitoneal injection of chemical carcinogen and urethane |
|
||
| Etoposide | In-vitro: 1–100 μg/mL | In-vitro: Human breast carcinoma cell line (MCF-7) |
|
[166] |
| In-vivo: 2 mg Rh–B/kg (Drug not used, Rh–B used as dye for fluorescence imaging) | In-vivo: Female BALB/c mice induced with breast carcinoma via subcutaneous inoculation of MCF-7 cells |
|
||
| Brucea javanica oil and doxorubicin | 3.12, 6.25, 12.5, 25, and 50 μg/mL | In-vitro: Human breast cancer cell line (MCF-7) |
|
[168] |
| Pemetrexed and resveratrol | In-vitro: 0.468–30 μg/mL | In-vitro: Human lung cancer cell line (A549) |
|
[169] |
| In-vivo: 0.342 mg/kg | In-vivo: Male BALB/c mice induced with lung cancer via intraperitoneal injection of urethane |
|
||
| Docetaxel | 0.8 μM | In-vitro: Human adenocarcinoma cell line (HeLa) |
|
[170] |
Apart from anti-inflammatory effects, monoolein-based drug delivery systems have also been proven to enhance antioxidative effects of therapeutic agents that could be beneficial in the management of chronic lung diseases. Bambusae Caulis in Taeniam (BCT) extract was loaded in monoolein cubosomes by Park and Kim and the in-vitro antioxidative efficacy of the formulation on fine dust-stimulated RAW 264.7 cells was evaluated. It was found that the nanoformulation was more effective than free BCT to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical and the intracellular reactive oxygen species (ROS) of stimulated RAW 264.7 cells in a dose-dependent manner. The radical scavenging efficiency (RSE) of the nanoformulation can be comparable to those of ascorbic acid, a well-known antioxidant, in which the RSE was 58.1% at the concentration of 0.048 mg/mL as compared to the RSE of 56% in ascorbic acid at the concentration of 0.1 mg/mL. This indicated that the nanoformulation demonstrated similar potency as ascorbic acid in scavenging DPPH free radical with concentrations <50% of ascorbic acid used in the experiments. Similarly, at the concentration of 0.48 μg/mL, the nanoformulation demonstrated an intracellular ROS scavenging efficiency of about 31.7%, which was similar to that of Trolox solution, also a known antioxidative agent, at the concentration of 250 μM. Such findings were attributed to increased cellular uptake of the nanoformulation via the endocytosis mechanism, as the major component of the cubosomes, monoolein, is amphiphilic and miscible with phospholipids, the primary component of cellular membrane [158]. Paudel et al. have also prepared rutin-loaded monoolein-based liquid crystalline nanoparticles and investigated their antioxidant effects against LPS-induced oxidative damage in human bronchial epithelial cell line. Rutin is a potent bioflavonoid with well-established anti-inflammatory and antioxidative properties, but its applications are limited due to its poor solubility and rapid metabolism. Upon encapsulation of rutin into monoolein-based liquid crystalline nanoparticles, the researchers have showed that the nanoformulation at low doses of 3, 4, and 5 μM remarkably suppressed nitric oxide and other ROS that were generated following LPS stimulation, thereby mitigating oxidative stress and cell apoptosis, and ultimately protected bronchial cells against chronic airway inflammation [159]. Moonolein-based nanocarriers have also been proven to preserve the therapeutic activity of loaded drugs. One study by Sguizzato et al. had utilized monoolein aqueous dispersions as a delivery system for the antioxidant molecules alpha-tocopherol and ascorbyl palmitate. The researchers reported that the utilization of monoolein aqueous dispersions allowed a better control in the release of loaded drugs in contrast to the corresponding free drugs solution. In addition, the morphological aspect of the nanoformulation, specifically the presence of cubic structures within monoolein aqueous dispersions had enabled greater retention of the drugs for a longer period as compared to free drugs solution. The antioxidant potential of the drugs was also retained after encapsulation, indicating that monoolein aqueous dispersions can be a promising nanocarrier to sustain their pharmacological activity for mitigating oxidative stress in diseases such as COPD and lung cancer [160]. In short, all these findings from various studies have supported the use of monoolein-based nanocarriers to enhance biological activity of therapeutic agents, holding great promise as a novel therapeutic strategy in the management of chronic lung diseases.
There are several other studies that have also investigated the ability of monoolein-based drug delivery systems to enhance anticancer activity. Like most natural compounds, despite the promising anticancer potential, therapeutic application of curcumin has been limited due to its poor solubility, low bioavailability, and chemical fragility. Baskaran et al. encapsulated curcumin into monoolein-based liquid crystalline nanoparticles and evaluated the anticancer activity of the nanoformulation on human colon cancer cell line. It was found that the stability of curcumin within the nanoformulation was greatly enhanced with approximately 75% of curcumin survived after 45 days under 40 °C. A sustained release profile was also achieved over 15 days, which could contribute to a steady state bioavailability of curcumin to exert its biological action. As a result, the nanoformulation also remarkably enhanced cellular uptake of curcumin, as shown by fluorescence-activating cell sorting results which revealed 99.1% fluorescence gating for 5 μM curcumin nanoformulation in contrast to 1.36% for the same concentration of free curcumin. Consistent with these findings, cell cycle studies have demonstrated induction of apoptosis when the cells were treated with the nanoformulation, whereas there were no changes in cell cycle or any noticeable cell death when the cells were treated with free curcumin. Thus, these findings indicate that monoolein-based nanocarriers may offer an opportunity to overcome the hurdles with respect to stability, bioavailability, and cellular uptake of curcumin in treatment of various cancers, including lung cancer [161]. Apart from curcumin, berberine also possesses notable anticancer properties in addition to its anti-inflammatory properties. Mehta et al. in their study had investigated the inhibitory potential of berberine-loaded monoolein-based liquid crystalline nanoparticles against cancer progression using A549 adenocarcinomic human alveolar basal epithelial cell line. The results showed that the nanoformulation greatly suppressed the protein expressions of CCL-20, CXCL-8, and heme oxygenase (HO)-1 at the dose of 5 μM. Given the roles of CCL-20, CXCL-8 and HO-1 in tumour cell proliferation and migration, these findings suggest that the nanoformulation can inhibit the progression of cancer, which could represent as a novel therapeutic strategy for the management of lung cancer [162]. Likewise, Paudel et al. in a recent study developed berberine-loaded monoolein-based liquid crystalline nanoparticles and evaluated their antiproliferative and antimigratory effects in A549 cell line. A sustained release behaviour was exhibited by the nanoformulation, leading to remarkable suppression in cell proliferation, inhibition of colony formation, inhibition of tumour cell invasion and migration via the downregulation of proteins involved in epithelial-mesenchymal transition, such as zinc finger protein SNAI1, p27, and vimentin [163]. The anticancer potential of drug-loaded monoolein-based nanocarriers was also demonstrated in a study by Faria et al. Elesclomol, a poorly water-soluble anticancer drug which induces mitochondria cytotoxicity by raising ROS up to unsustainable levels, was encapsulated into monoolein-based cubosomes and their therapeutic effects were evaluated on A549 lung cancer cell line. It was found that the nanoparticles were taken up by tumour cells with specific accumulation at proximity of the mitochondria network, supporting the feasibility of the nanoformulation to deliver drugs to the inside of tumour cells. Generally, the induction of oxidative stress in the mitochondria is achieved through the chelation of elesclomol with copper (II) in the plasma, forming a elesclomol-copper complex in which copper (II) is then reduced to copper(I) in the mitochondria, generating ROS. For this instance, the study reported that loading of monoolein-based cubosomes with preformed elesclomol-copper complex can significantly enhance the cytotoxicity of the nanoformulation. Moreover, at an equivalent concentration, encapsulated elesclomol-copper complex had a superior anticancer efficacy in contrast to free elesclomol-copper complex, which is corroborated by a lower concentration of elesclomol required to reduce call viability by half (EC50 value) in A549 tumour cells [164]. Monoolein-based nanocarriers have also been used to deliver combinatorial chemotherapeutic and herbal drugs to lung cancer cells. Abdelaziz et al. had co-loaded pemetrexed and resveratrol into monoolein-based lyotropic liquid crystalline nanoparticles and evaluated their anticancer efficacy. In A549 lung carcinoma cells, the nanoformulation displayed a biphasic release pattern with an initial burst release followed by sustained release for up to 24 h. The nanoformulation also manifested superior cytotoxicity profile against lung cancer cells, justified by an IC50 value of 4.0628 μg/mL in contrast to the IC50 values of 7.94 μg/mL, 8.43 μg/mL and 4.51 μg/mL in free pemetrexed, free resveratrol and free pemetrexed/resveratrol, respectively. Besides, the nanoformulation demonstrated an enhanced cellular uptake over 24 h in a time-dependent manner, which can be attributed to the bio-adhesive nature of monoolein. In-vivo studies performed on lung cancer bearing mice also demonstrated tumour growth inhibition by the nanoformulation via suppression of angiogenesis and promotion of apoptosis. Notably, the synergistic combination of pemetrexed and resveratrol had greatly improved treatment outcomes and safety profile as hepatotoxicity and nephrotoxicity that are commonly associated with pemetrexed were alleviated. Furthermore, the nanoformulation allowed for a reduction in the dosage of pemetrexed whilst maintaining their capability in killing cancerous cells [165]. To sum up, these studies have clearly demonstrated the use of monoolein-based nanocarriers as a potential strategy to improve therapeutic outcomes in lung cancer.
As discussed earlier, the versatility of monoolein-based drug delivery systems has also allowed surface modification of the nanocarrier to achieve active targeting, which is specifically useful for therapeutic applications in cancer to reduce systemic side effects and improve treatment outcomes. In a study, Tian et al. developed monoolein cubosomes functionalized with poloxamer 407-folic acid (P407-FA) and evaluated their targeting ability and therapeutic effects on human breast carcinoma cell lines (MCF-7) and MCF-7 breast cancer-bearing mice. The results showed that normal cubosomes without surface modifications can target tumour cells due to the EPR effect, however, P407-FA functionalized cubosomes had superior tumour targeting capability, which can be attributed to the interaction between P407-FA and folate receptors that are overexpressed on the surface of MCF-7 cells, thereby resulting in a high specific tumour uptake. This was consistent with the findings that there was a remarkable increase in cellular accumulation of rhodamine B (Rh–B) when P407-FA-conjugated Rh–B-loaded cubosomes are utilized as compared to unconjugated Rh–B-loaded cubosomes and free Rh–B. In-vivo study also demonstrated that the tumour fluorescence intensity in mice injected with P407-FA-conjugated cubosomes was significantly higher than that of unconjugated cubosomes between 6 and 24 h post-injection. As a result, folate-modified etoposide-loaded cubosomes was found to exhibit the greatest cytotoxicity and anti-proliferative activity with an IC50 value of 3.60 ± 0.68 μg/mL, followed by normal cubosomes with IC50 value of 6.73 ± 1.69 μg/mL, and free etoposide with IC50 value of 47.89 ± 12.60 μg/mL [166]. On the other hand, imparting pH-responsiveness on nanocarriers can be useful for targeted drug delivery in cancer as the hallmark of malignant tumour cells is an acidic microenvironment due to glycolysis in tumour cells, hypoxia, and insufficient blood perfusion. Such an acidic microenvironment is not present in normal cells as glycolysis is inhibited by the presence of sufficient oxygen [167]. For this instance, Li et al. had developed a pH-responsive monoolein-based liquid crystalline nanoparticles co-loaded with Brucea javanica oil and doxorubicin. It was found that the release of drugs at pH of 5.3 was remarkably faster, whereas the release of drugs at pH of 6.8 was more rapid than that at pH of 7.4. The pH-dependent drug release can be attributed to the presence of different structures of liquid crystalline nanoparticles under varying pH conditions, namely, the inverted hexagonal or cubic phases. The researchers reported that there was a phase transition from inverted hexagonal in simulated normal cells environment where pH is 7.4, to cubic in simulated tumour cells environment where pH is 6.8, and to emulsified microemulsion in simulated intracellular lysosome environment where pH is 5.3. As drug diffusion from lyotropic liquid crystalline nanocarriers relies on the topology and diameters of water channels of their internal structure, the network of large open channels in the bicontinuous cubic phase allowed faster drug release from that of hexagonally packed channels of smaller diameters. Therefore, such an effect can greatly facilitate drug delivery and to exert their pharmacological actions in the pathological state whilst minimizing cytotoxicity to healthy cells. This is also consistent with the findings on human breast carcinoma cells (MCF-7) in which cytotoxicity and anticancer apoptotic effects demonstrated by the nanoformulation was remarkably greater than the free drugs in all the studied concentration range (3.12, 6.25, 12.5, 25, and 50 μg/mL) with an evidently greater drug uptake by the tumour cells [168]. Although these studies were not directly performed on models of lung cancer, the fundamentals of customised engineering via surface or structural modification of monoolein-based nanocarriers for achieving tumour specific targeting can also be applied to improve anticancer effects and therapeutic outcomes in lung cancer. In another study, Abdelaziz et al. had also attempted to formulate pemetrexed and resveratrol co-loaded monoolein-based liquid crystalline nanoparticles surface functionalized with lactoferrin (LF) and chondroitin sulphate (CS). In A549 lung cancer cells, LF/CS-conjugated nanoparticles had the highest cytotoxicity with an IC50 value of 2.56 μg/mL, followed by unconjugated nanoparticles with IC50 value of 3.85 μg/mL, and free pemetrexed-resveratrol mixture with IC50 value of 4.12 μg/mL. Such findings were also consistent with cellular uptake study in which there was a significant enhancement in cellular uptake of LF/CS-conjugated nanoparticles as compared with unconjugated nanoparticles and free dye. Besides, in-vivo study on tumour-bearing mice has showed that the lungs harvested from mice treated with LF/CS-conjugated nanoparticles had normal physiological characteristics with significantly diminished malignant surface lesions, as well as a remarkable reduction in lesion numbers and lung adenomatous foci. The tumour inhibitory effect of the nanoformulation was also characterized by the low level of VEGF-1 and higher level of active caspase-3 in contrast to the free drug mixture. In addition, in-vivo imaging of lung cancer-bearing mice revealed significantly greater fluorescence when treated with LF/CS-conjugated nanoparticles especially in the center and throughout the lungs without any luminescence in other regions of the body, indicating specific localization and uptake of the nanoformulation. Collectively, these findings affirmed the potential of LF/CS-conjugated monoolein-based nanoparticles in improving therapeutic outcomes in lung cancer [169]. Apart from that, Meli et al. engineered docetaxel-loaded monoolein-based cubosomes conjugated with the imaging probe rhodamine and the cancer cell targeting ligand folate for theranostic applications. The researchers documented that the nanoformulation was successfully utilized to image living cells on HeLa cell line and exerted significant cytotoxicity against these tumour cells with more than one order of magnitude greater than the free drug. Such findings indicate that monoolein-based cubosomes can be a promising candidate as nanocarriers for hydrophobic drugs with both therapeutic and diagnostic capabilities in the management of cancers, including lung cancer [170].
6. Conclusion and future directions
Chronic lung diseases are a global public health concern as they are associated with poor clinical outcomes and have resulted in high rates of morbidity and mortality. Although several agents have been developed against these diseases, most of the therapeutic agents only target the symptoms and do not reverse the progression of chronic lung diseases. Some of these agents are also associated with adverse reactions that can significantly affect the quality of life of suffering individuals. Besides, the clinical application of most herbal and plant-based compounds with proven biological activities against the pathogenesis of chronic lung diseases is also limited due to their poor pharmacokinetic properties. Collectively, the lack of effective management strategies has greatly contributed to a rise in the prevalence of chronic lung diseases, as well as increasing clinical and socioeconomic burden at an alarming rate. Therefore, there is a pressing need for the development of novel therapeutics that could address the shortcomings of current conventional therapeutics. Nanomedicine offers a versatile approach in managing these diseases as the use of advanced drug delivery systems can contribute to targeted drug delivery to specific sites within the body whilst improving the bioavailability of therapeutic agents, thereby leading to an enhanced therapeutic outcome. Monoolein is an amphiphilic lipid that is commonly employed to prepare cubosome liquid crystalline nanoparticles for drug delivery applications in various diseases. In targeting chronic lung diseases, monoolein-based drug delivery systems have also proven their potential as safe and effective alternatives to conventional therapeutics in multiple studies. Nevertheless, most of these studies are primarily pre-clinical evaluations focusing on cell lines or animal models of chronic lung diseases. Moreover, most studies were only performed on inflammatory and oxidative lung diseases such as asthma, COPD, and lung cancer while the efficacy of monoolein-based nanocarriers in the infectious respiratory diseases is yet to be demonstrated. Thus, this area of research should be further explored, to demonstrate whether the positive findings on inflammatory and oxidative lung diseases could potentially be extrapolated to produce novel monoolein-based nanotherapeutics for treatment of infectious respiratory diseases, such as pulmonary tuberculosis, influenza, and the ongoing COVID-19 pandemic. Clinical studies should be carried out vigorously prior to their clinical translation as the findings obtained from human studies may deviate from those of cell lines or animal models, due to the presence of a complex biological environment in the human body.
Authors' contributions
All authors contributed to the study's conception and design. Conceptualised by DKC, MG and KD; Data curation was performed by SKS, SW, PP and DK; Resources were organised by SW, APK, GG, GK, and MH; Writing – original draft by YC, DKC and APK; Writing – review & editing were done by MG, BGGO, JA, KD and DKC; All the authors have read and approved the final version of the manuscript.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chronic Respiratory Diseases, World Health Organization. (n.d.). https://www.who.int/health-topics/chronic-respiratory-diseases (accessed November 23, 2021).
- 2.Barta J.A., Powell C.A., Wisnivesky J.P. Global epidemiology of lung cancer. Annals of Global Health. 2019;85 doi: 10.5334/AOGH.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burney P. Chronic respiratory disease – the acceptable epidemic? Clin. Med. 2017;17:29. doi: 10.7861/CLINMEDICINE.17-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatipoglu U. Chronic obstructive pulmonary disease: more than meets the eye. Ann. Thorac. Med. 2018;13:1. doi: 10.4103/ATM.ATM_193_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan Y., Ng S.W., Dua K., Chellappan D.K. Targeting Cellular Signalling Pathways in Lung Diseases. Springer; Singapore: 2021. Plant-based chemical moieties for targeting chronic respiratory diseases; pp. 741–781. [DOI] [Google Scholar]
- 6.Labaki W.W., Han M.L.K. Chronic respiratory diseases: a global view. Lancet Respir. Med. 2020;8:531–533. doi: 10.1016/S2213-2600(20)30157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin B.F., Chiang B.L., Ma Y., Lin J.Y., Chen M.L. 2015. Traditional Herbal Medicine and Allergic Asthma, Evidence-Based Complementary and Alternative Medicine. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santana F.P.R., Pinheiro N.M., Mernak M.I.B., Righetti R.F., Martins M.A., Lago J.H.G., Lopes F., Tibério I.F.L.C., Prado C.M. Evidences of herbal medicine-derived natural products effects in inflammatory lung diseases. Mediat. Inflamm. 2016 doi: 10.1155/2016/2348968. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong W., Zhang X., Zeng Y., Lin D., Wu J. Recent applications and strategies in nanotechnology for lung diseases. Nano Res. 2021;14:2067–2089. doi: 10.1007/S12274-020-3180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan Y., Raju Allam V.S.R., Paudel K.R., Singh S.K., Gulati M., Dhanasekaran M., Gupta P.K., Jha N.K., Devkota H.P., Gupta G., Hansbro P.M., Oliver B.G.G., Chellappan D.K., Dua K. Nutraceuticals: unlocking newer paradigms in the mitigation of inflammatory lung diseases. Crit. Rev. Food Sci. Nutr. 2021 doi: 10.1080/10408398.2021.1986467. [DOI] [PubMed] [Google Scholar]
- 11.Yhee J.Y., Im J., Nho R.S. Advanced therapeutic strategies for chronic lung disease using nanoparticle-based drug delivery. J. Clin. Med. 2016;5:82. doi: 10.3390/JCM5090082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan Y., Ng S.W., Mehta M., Anand K., Kumar Singh S., Gupta G., Chellappan D.K., Dua K. Advanced drug delivery systems can assist in managing influenza virus infection: a hypothesis. Med. Hypotheses. 2020;144 doi: 10.1016/J.MEHY.2020.110298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doroudian M., MacLoughlin R., MacLoughlin R., MacLoughlin R., Poynton F., Prina-Mello A., Prina-Mello A., Prina-Mello A., Donnelly S.C. Nanotechnology based therapeutics for lung disease. Thorax. 2019;74:965–976. doi: 10.1136/THORAXJNL-2019-213037. [DOI] [PubMed] [Google Scholar]
- 14.Germain M., Caputo F., Metcalfe S., Tosi G., Spring K., Åslund A.K.O., Pottier A., Schiffelers R., Ceccaldi A., Schmid R. Delivering the power of nanomedicine to patients today. J. Contr. Release. 2020;326:164. doi: 10.1016/J.JCONREL.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17:20. doi: 10.1038/NRC.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahadori M., Mohammadi F. Nanomedicine for respiratory diseases. Tanaffos. 2012;11:18. [PMC free article] [PubMed] [Google Scholar]
- 17.Chan Y., Ng S.W., Liew H.S., Pua L.J.W., Soon L., Lim J.S., Dua K., Chellappan D.K. Medicinal Plants for Lung Diseases. Springer; Singapore: 2021. Introduction to chronic respiratory diseases: a pressing need for novel therapeutic approaches; pp. 47–84. [DOI] [Google Scholar]
- 18.Chan Y., Wu X.H., Chieng B.W., Ibrahim N.A., Then Y.Y. Superhydrophobic nanocoatings as intervention against biofilm-associated bacterial infections. Nanomaterials. 2021;11:1046. doi: 10.3390/NANO11041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/S12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cytryniak A., Nazaruk E., Bilewicz R., Górzyńska E., Żelechowska-Matysiak K., Walczak R., Mames A., Bilewicz A., Majkowska-Pilip A. Lipidic cubic-phase nanoparticles (cubosomes) loaded with doxorubicin and labeled with 177Lu as a potential tool for combined chemo and internal radiotherapy for cancers. Nanomaterials. 2020;10:1–14. doi: 10.3390/NANO10112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barriga H.M.G., Holme M.N., Stevens M.M. Cubosomes; the next generation of smart lipid nanoparticles? Angew. Chem. 2019;58:2958. doi: 10.1002/ANIE.201804067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagliardi A., Cosco D., Udongo B.P., Dini L., Viglietto G., Paolino D. Design and characterization of glyceryl monooleate-nanostructures containing doxorubicin hydrochloride. Pharmaceutics. 2020;12:1017. doi: 10.3390/PHARMACEUTICS12111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busse P.J., McDonald V.M., Wisnivesky J.P., Gibson P.G. Asthma across the ages: adults. J. Allergy Clin. Immunol. Pract. 2020;8:1828–1838. doi: 10.1016/J.JAIP.2020.03.044. [DOI] [PubMed] [Google Scholar]
- 24.Mims J.W. Asthma: definitions and pathophysiology. International Forum of Allergy & Rhinology. 2015;5:S2–S6. doi: 10.1002/ALR.21609. [DOI] [PubMed] [Google Scholar]
- 25.Papi A., Blasi F., Canonica G.W., Morandi L., Richeldi L., Rossi A. Treatment strategies for asthma: reshaping the concept of asthma management, Allergy, Asthma, and Clinical Immunology. Official Journal of the Canadian Society of Allergy and Clinical Immunology. 2020;16:75. doi: 10.1186/S13223-020-00472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gautier C., Charpin D. Environmental triggers and avoidance in the management of asthma. J. Asthma Allergy. 2017;10:47–56. doi: 10.2147/JAA.S121276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo J.R., Peters S.P., Busse W.W. Asthma exacerbations: pathogenesis, prevention, and treatment, the journal of allergy and clinical immunology. Practice. 2017;5:918. doi: 10.1016/J.JAIP.2017.05.001. in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dharmage S.C., Perret J.L., Custovic A. Epidemiology of asthma in children and adults. Frontiers in Pediatrics. 2019;7:246. doi: 10.3389/FPED.2019.00246/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oland A.A., Booster G.D., Bender B.G. Psychological and lifestyle risk factors for asthma exacerbations and morbidity in children. World Allergy Organization Journal. 2017;10:35. doi: 10.1186/S40413-017-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bush A. Pathophysiological mechanisms of asthma. Frontiers in Pediatrics. 2019;7:68. doi: 10.3389/FPED.2019.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelaia C., Paoletti G., Puggioni F., Racca F., Pelaia G., Canonica G.W., Heffler E. Interleukin-5 in the pathophysiology of severe asthma. Front. Physiol. 2019;10:1514. doi: 10.3389/FPHYS.2019.01514/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuruvilla M.E., Lee F.E.H., Lee G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin. Rev. Allergy Immunol. 2019;56:219–233. doi: 10.1007/S12016-018-8712-1/FIGURES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBrien C.N., Menzies-Gow A. The biology of eosinophils and their role in asthma. Front. Med. 2017;4:93. doi: 10.3389/FMED.2017.00093/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagome K., Nagata M. Involvement and possible role of eosinophils in asthma exacerbation. Front. Immunol. 2018;9:2220. doi: 10.3389/FIMMU.2018.02220/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matucci A., Vultaggio A., Maggi E., Kasujee I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir. Res. 2018;19:1–10. doi: 10.1186/S12931-018-0813-0/FIGURES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakakos A., Loukides S., Bakakos P. Severe eosinophilic asthma. J. Clin. Med. 2019;8:1375. doi: 10.3390/JCM8091375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holgate S.T. Mechanisms of asthma and implications for its prevention and treatment: a personal journey, allergy. Asthma & Immunology Research. 2013;5:343–347. doi: 10.4168/AAIR.2013.5.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke R., Lundy F.T., McGarvey L. Herbal treatment in asthma and COPD – current evidence. Clinical Phytoscience. 2015;1:1–7. doi: 10.1186/S40816-015-0005-0. [DOI] [Google Scholar]
- 39.Gross N.J., Barnes P.J. New therapies for asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017;195:159–166. doi: 10.1164/RCCM.201610-2074PP/SUPPL_FILE/DISCLOSURES.PDF. [DOI] [PubMed] [Google Scholar]
- 40.Zoratti E.M., O'Connor G.T. New therapeutic strategies for asthma. JAMA. 2020;323:517–518. doi: 10.1001/JAMA.2019.19985. [DOI] [PubMed] [Google Scholar]
- 41.Amaral-Machado L., Oliveira W.N., Moreira-Oliveira S.S., Pereira D.T., Alencar É.N., Tsapis N., Egito E.S.T. Use of natural products in asthma treatment, evidence-based complementary and alternative medicine. 2020. 2020. [DOI] [PMC free article] [PubMed]
- 42.Yim R.P., Koumbourlis A.C. Tolerance & resistance to β₂-agonist bronchodilators. Paediatr. Respir. Rev. 2013;14:195–198. doi: 10.1016/J.PRRV.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Williams D.M., Rubin B.K. Clinical pharmacology of bronchodilator medications. Respir. Care. 2018;63:641–654. doi: 10.4187/RESPCARE.06051. [DOI] [PubMed] [Google Scholar]
- 44.Shukla S.D., Swaroop Vanka K., Chavelier A., Shastri M.D., Tambuwala M.M., Bakshi H.A., Pabreja K., Mahmood M.Q., O'Toole R.F. Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems. Academic Press; 2020. Chronic respiratory diseases: an introduction and need for novel drug delivery approaches; pp. 1–31. [DOI] [Google Scholar]
- 45.O'Reilly S. Chronic obstructive pulmonary disease. Am. J. Lifestyle Med. 2016;11:296–302. doi: 10.1177/1559827616656593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lugg S.T., Scott A., Parekh D., Naidu B., Thickett D.R. Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease. Thorax. 2021 doi: 10.1136/THORAXJNL-2020-216296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hikichi M., Mizumura K., Maruoka S., Gon Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019;1:S2129–S2140. doi: 10.21037/JTD.2019.10.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015;4:e26. doi: 10.1186/S40169-015-0068-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quaderi S.A., Hurst J.R. The unmet global burden of COPD. Global Health, Epidemiology and Genomics. 2018;3:1–3. doi: 10.1017/GHEG.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aghasafari P., George U., Pidaparti R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2018;68:59–74. doi: 10.1007/S00011-018-1191-2. [DOI] [PubMed] [Google Scholar]
- 51.Suissa S., Dell'Aniello S., Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi: 10.1136/THORAXJNL-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnes P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017;131:1541–1558. doi: 10.1042/CS20160487. [DOI] [PubMed] [Google Scholar]
- 53.Lane N., Robins R.A., Corne J., Fairclough L. Regulation in chronic obstructive pulmonary disease: the role of regulatory T-cells and Th17 cells. Clin. Sci. 2010;119:75–86. doi: 10.1042/CS20100033. [DOI] [PubMed] [Google Scholar]
- 54.Ram A., Balachandar S., Vijayananth P., Singh V.P. Medicinal plants useful for treating chronic obstructive pulmonary disease (COPD): current status and future perspectives. Fitoterapia. 2011;82:141–151. doi: 10.1016/J.FITOTE.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Barnes P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013;131:636–645. doi: 10.1016/J.JACI.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 56.Boyce J.A., Finkelman F., Shearer W.T. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016;138:16–27. doi: 10.1016/J.JACI.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Sgalla G., Iovene B., Calvello M., Ori M., Varone F., Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir. Res. 2018;19 doi: 10.1186/S12931-018-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura Y., Suda T. Idiopathic pulmonary fibrosis: diagnosis and clinical manifestations, clinical medicine insights. Circulatory, Respiratory and Pulmonary Medicine. 2015;9:163. doi: 10.4137/CCRPM.S39897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011;208:1339–1350. doi: 10.1084/JEM.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hadjicharalambous M.R., Lindsay M.A. Idiopathic pulmonary fibrosis: pathogenesis and the emerging role of long non-coding RNAs. Int. J. Mol. Sci. 2020;21:524. doi: 10.3390/IJMS21020524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolters P.J., Collard H.R., Jones K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annual Review of Pathology. 2014;9:157. doi: 10.1146/ANNUREV-PATHOL-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heukels P., Moor C.C., von der Thüsen J.H., Wijsenbeek M.S., Kool M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019;147:79–91. doi: 10.1016/J.RMED.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 63.Guo J., Li B., Wu W., Wang Z., Wang F., Guo T. Chinese herbal medicines compared with N-Acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review of randomized controlled trials. Evid. base Compl. Alternative Med. 2019:2019. doi: 10.1155/2019/5170638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaw J., Marshall T., Morris H., Hayton C., Chaudhuri N. Idiopathic pulmonary fibrosis: a holistic approach to disease management in the antifibrotic age. J. Thorac. Dis. 2017;9:4700–4707. doi: 10.21037/JTD.2017.10.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mojiri-Forushani H., Hemmati A.A., Dehghani M.A., Malayeri A.R., Pour H.H. Effects of herbal extracts and compounds and pharmacological agents on pulmonary fibrosis in animal models: a review. Journal of Integrative Medicine. 2017;15:433–441. doi: 10.1016/S2095-4964(17)60363-7. [DOI] [PubMed] [Google Scholar]
- 66.Gulati S., Luckhardt T.R. Updated evaluation of the safety, efficacy and tolerability of pirfenidone in the treatment of idiopathic pulmonary fibrosis. Drug Healthc. Patient Saf. 2020;12:85. doi: 10.2147/DHPS.S224007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durham A.L., Caramori G., Chung K.F., Adcock I.M. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl. Res. 2016;167:192–203. doi: 10.1016/J.TRSL.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conway E.M., Pikor L.A., Kung S.H.Y., Hamilton M.J., Lam S., Lam W.L., Bennewith K.L. Macrophages, inflammation, and lung cancer. Am. J. Respir. Crit. Care Med. 2016;193:116–130. doi: 10.1164/rccm.201508-1545CI. [DOI] [PubMed] [Google Scholar]
- 69.Thomas K.W., Gould M.K. UpToDate; 2020. Overview of the Initial Evaluation, Diagnosis, and Staging of Patients with Suspected Lung Cancer.https://www.uptodate.com/contents/overview-of-the-initial-evaluation-diagnosis-and-staging-of-patients-with-suspected-lung-cancer accessed. [Google Scholar]
- 70.Dougall A.L. Cambridge Handbook of Psychology, Health and Medicine. StatPearls Publishing; 2007. Cancer: lung; pp. 605–606. [DOI] [Google Scholar]
- 71.Malhotra J., Malvezzi M., Negri E., la Vecchia C., Boffetta P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016;48:889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 72.Herbst R.S., Heymach J.v., Lippman S.M. Lung cancer. N. Engl. J. Med. 2009;359:1367–1380. doi: 10.1056/NEJMRA0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller Y.E. Pathogenesis of lung cancer: 100 Year report. Am. J. Respir. Cell Mol. Biol. 2005;33:216. doi: 10.1165/RCMB.2005-0158OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Callaghan D.S., O'Donnell D., O'Connell F., O'Byrne K.J. The role of inflammation in the pathogenesis of non-small cell lung cancer. J. Thorac. Oncol. 2010;5:2024–2036. doi: 10.1097/JTO.0b013e3181f387e4. [DOI] [PubMed] [Google Scholar]
- 75.Hua X., Chen J., Wu Y., Sha J., Han S., Zhu X. Prognostic role of the advanced lung cancer inflammation index in cancer patients: a meta-analysis. World J. Surg. Oncol. 2019;17:1–9. doi: 10.1186/s12957-019-1725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zappavigna S., Cossu A.M., Grimaldi A., Bocchetti M., Ferraro G.A., Nicoletti G.F., Filosa R., Caraglia M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamp D.W., Shacter E., Weitzman S.A. Chronic inflammation and cancer: the role of the mitochondria. Oncology. 2011;25 [PubMed] [Google Scholar]
- 78.Lemjabbar-Alaoui H., Hassan O.U.I., Yang Y.W., Buchanan P. Lung cancer: biology and treatment options. Biochim. Biophys. Acta. 2015;1856:189. doi: 10.1016/J.BBCAN.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen R., Manochakian R., James L., Azzouqa A.G., Shi H., Zhang Y., Zhao Y., Zhou K., Lou Y. Emerging therapeutic agents for advanced non-small cell lung cancer. J. Hematol. Oncol. 2020;13 doi: 10.1186/S13045-020-00881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sardeli C., Zarogoulidis P., Kosmidis C., Amaniti A., Katsaounis A., Giannakidis D., Koulouris C., Hohenforst-Schmidt W., Huang H., Bai C., Michalopoulos N., Tsakiridis K., Romanidis K., Oikonomou P., Mponiou K., Vagionas A., Goganau A.M., Kesisoglou I., Sapalidis K. Inhaled chemotherapy adverse effects: mechanisms and protection methods. Lung Cancer Management. 2019;8:LMT19. doi: 10.2217/LMT-2019-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.García-Fernández C., Fornaguera C., Borrós S. Nanomedicine in non-small cell lung cancer: from conventional treatments to immunotherapy. Cancers. 2020;12:1–26. doi: 10.3390/CANCERS12061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad J., Akhter S., Rizwanullah M., Amin S., Rahman M., Ahmad M.Z., Rizvi M.A., Kamal M.A., Ahmad F.J. Nanotechnology-based inhalation treatments for lung cancer: state of the art. Nanotechnol. Sci. Appl. 2015;8:55. doi: 10.2147/NSA.S49052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/JAMA.2020.12839. [DOI] [PubMed] [Google Scholar]
- 84.Azer S.A. COVID-19: pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes and New Infections. 2020;37 doi: 10.1016/J.NMNI.2020.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gattinoni L., Gattarello S., Steinberg I., Busana M., Palermo P., Lazzari S., Romitti F., Quintel M., Meissner K., Marini J.J., Chiumello D., Camporota L. COVID-19 pneumonia: pathophysiology and management. Eur. Respir. Rev. 2021;30 doi: 10.1183/16000617.0138-2021. 210138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodriguez-Guerra M., Jadhav P., Vittorio T.J. Current treatment in COVID-19 disease: a rapid review. Drugs Context (US) 2021:10. doi: 10.7573/DIC.2020-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.WHO Coronavirus (COVID-19) Dashboard, World Health Organization. (n.d.). https://covid19.who.int/(accessed November 27, 2021).
- 88.Chatterjee S.K., Saha S., Munoz M.N.M. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front. Mol. Biosci. 2020;7:196. doi: 10.3389/FMOLB.2020.00196/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trougakos I.P., Stamatelopoulos K., Terpos E., Tsitsilonis O.E., Aivalioti E., Paraskevis D., Kastritis E., Pavlakis G.N., Dimopoulos M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021;28:1–18. doi: 10.1186/S12929-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. 2021;97:312–320. doi: 10.1136/POSTGRADMEDJ-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chan Y., Ng S.W., Singh S.K., Gulati M., Gupta G., Chaudhary S.K., Hing G.B., Collet T., MacLoughlin R., Löbenberg R., Oliver B.G., Chellappan D.K., Dua K. Revolutionizing polymer-based nanoparticle-linked vaccines for targeting respiratory viruses: a perspective. Life Sci. 2021;280 doi: 10.1016/J.LFS.2021.119744. 119744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cully M. A tale of two antiviral targets — and the COVID-19 drugs that bind them. Nat. Rev. Drug Discov. 2021 doi: 10.1038/D41573-021-00202-8. [DOI] [PubMed] [Google Scholar]
- 94.Vaccines/Covid-19 vaccine EUL issued, world health organization. https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued (n.d.) accessed.
- 95.WHO Issues Emergency Use Listing for Eighth COVID-19 Vaccine. World Health Organization; 2021. https://www.who.int/news/item/03-11-2021-who-issues-emergency-use-listing-for-eighth-covid-19-vaccine (accessed November 27, 2021) [Google Scholar]
- 96.Krause P.R., Fleming T.R., Longini I.M., Peto R., Briand S., Heymann D.L., Beral V., Snape M.D., Rees H., Ropero A.-M., Balicer R.D., Cramer J.P., Muñoz-Fontela C., Gruber M., Gaspar R., Singh J.A., Subbarao K., van Kerkhove M.D., Swaminathan S., Ryan M.J., Henao-Restrepo A.-M. SARS-CoV-2 variants and vaccines. N. Engl. J. Med. 2021;385:179–186. doi: 10.1056/NEJMSR2105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., Prot M., Gallais F., Gantner P., Velay A., le Guen J., Kassis-Chikhani N., Edriss D., Belec L., Seve A., Courtellemont L., Péré H., Hocqueloux L., Fafi-Kremer S., Prazuck T., Mouquet H., Bruel T., Simon-Lorière E., Rey F.A., Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/S41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 98.Bian L., Gao F., Zhang J., He Q., Mao Q., Xu M., Liang Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expet Rev. Vaccine. 2021;20:1. doi: 10.1080/14760584.2021.1903879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mlcochova P., Kemp S., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., Datir R., Collier D.A., Albecka A., Singh S., Pandey R., Brown J., Zhou J., Goonawardane N., Mishra S., Whittaker C., Mellan T., Marwal R., Datta M., Sengupta S., Ponnusamy K., Radhakrishnan V.S., Abdullahi A., Charles O., Chattopadhyay P., Devi P., Caputo D., Peacock T., Wattal D.C., Goel N., Satwik A., Vaishya R., Agarwal M., Chauhan H., Dikid T., Gogia H., Lall H., Verma K., Dhar M.S., Singh M.K., Soni N., Meena N., Madan P., Singh P., Sharma R., Sharma R., Kabra S., Kumar S., Kumari S., Sharma U., Chaudhary U., Sivasubbu S., Scaria V., Wattal C., Oberoi J.K., Raveendran R., Datta S., Das S., Maitra A., Chinnaswamy S., Biswas N.K., Parida A., Raghav S.K., Prasad P., Sarin A., Mayor S., Ramakrishnan U., Palakodeti D., Seshasayee A.S.N., Thangaraj K., Bashyam M.D., Dalal A., Bhat M., Shouche Y., Pillai A., Abraham P., Atul P.V., Cherian S.S., Desai A.S., Pattabiraman C., Manjunatha M.v., Mani R.S., Udupi G.A., Nandicoori V., Bharadwaj K., Tallapaka, Sowpati D.T., Kawabata R., Morizako N., Sadamasu K., Asakura H., Nagashima M., Yoshimura K., Ito J., Kimura I., Uriu K., Kosugi Y., Suganami M., Oide A., Yokoyama M., Chiba M., Saito A., Butlertanaka E.P., Tanaka Y.L., Ikeda T., Motozono C., Nasser H., Shimizu R., Yuan Y., Kitazato K., Hasebe H., Nakagawa S., Wu J., Takahashi M., Fukuhara T., Shimizu K., Tsushima K., Kubo H., Shirakawa K., Kazuma Y., Nomura R., Horisawa Y., Takaori-Kondo A., Tokunaga K., Ozono S., Baker S., Dougan G., Hess C., Kingston N., Lehner P.J., Lyons P.A., Matheson N.J., Owehand W.H., Saunders C., Summers C., Thaventhiran J.E.D., Toshner M., Weekes M.P., Maxwell P., Shaw A., Bucke A., Calder J., Canna L., Domingo J., Elmer A., Fuller S., Harris J., Hewitt S., Kennet J., Jose S., Kourampa J., Meadows A., O'Brien C., Price J., Publico C., Rastall R., Ribeiro C., Rowlands J., Ruffolo V., Tordesillas H., Bullman B., Dunmore B.J., Fawke S., Gräf S., Hodgson J., Huang C., Hunter K., Jones E., Legchenko E., Matara C., Martin J., Mescia F., O'Donnell C., Pointon L., Pond N., Shih J., Sutcliffe R., Tilly T., Treacy C., Tong Z., Wood J., Wylot M., Bergamaschi L., Betancourt A., Bower G., Cossetti C., de Sa A., Epping M., Fawke S., Gleadall N., Grenfell R., Hinch A., Huhn O., Jackson S., Jarvis I., Krishna B., Lewis D., Marsden J., Nice F., Okecha G., Omarjee O., Perera M., Potts M., Richoz N., Romashova V., Yarkoni N.S., Sharma R., Stefanucci L., Stephens J., Strezlecki M., Turner L., de Bie E.M.D.D., Bunclark K., Josipovic M., Mackay M., Michael A., Rossi S., Selvan M., Spencer S., Yong C., Allison J., Butcher H., Caputo D., Clapham-Riley D., Dewhurst E., Furlong A., Graves B., Gray J., Ivers T., Kasanicki M., le Gresley E., Linger R., Meloy S., Muldoon F., Ovington N., Papadia S., Phelan I., Stark H., Stirrups K.E., Townsend P., Walker N., Webster J., Scholtes I., Hein S., King R., Mavousian A., Lee J.H., Bassi J., Silacci-Fegni C., Saliba C., Pinto D., Irie T., Yoshida I., Hamilton W.L., Sato K., Bhatt S., Flaxman S., James L.C., Corti D., Piccoli L., Barclay W.S., Rakshit P., Agrawal A., Gupta R.K. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599 doi: 10.1038/S41586-021-03944-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K.E., Hopkins S., Chand M., Ramsay M. Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMOA2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. World Health Organization; 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern accessed. [Google Scholar]
- 102.Ledford H. COVID antiviral pills: what scientists still want to know. Nature. 2021;599:358–359. doi: 10.1038/D41586-021-03074-5. [DOI] [PubMed] [Google Scholar]
- 103.Mahase E. Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/BMJ.N2713. [DOI] [PubMed] [Google Scholar]
- 104.Lee C.-C., Hsieh C.-C., Ko W.-C. Molnupiravir—a novel oral anti-SARS-CoV-2 agent. Antibiotics. 2021;10:1294. doi: 10.3390/ANTIBIOTICS10111294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.da Silva A.L., Cruz F.F., Rocco P.R.M., Morales M.M. New perspectives in nanotherapeutics for chronic respiratory diseases. Biophysical Reviews. 2017;9:793. doi: 10.1007/S12551-017-0319-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doroudian M., O’ Neill A., mac Loughlin R., Prina-Mello A., Volkov Y., Donnelly S.C. Nanotechnology in pulmonary medicine. Curr. Opin. Pharmacol. 2021;56:85. doi: 10.1016/J.COPH.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Omlor A.J., Nguyen J., Bals R., Dinh Q.T. Nanotechnology in respiratory medicine. Respir. Res. 2015;16:1–9. doi: 10.1186/S12931-015-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stolzenburg L.R., Harris A. The role of microRNAs in chronic respiratory disease: recent insights. Biol. Chem. 2018;399:219. doi: 10.1515/HSZ-2017-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Passi M., Shahid S., Chockalingam S., Sundar I.K., Packirisamy G. Conventional and nanotechnology based approaches to combat chronic obstructive pulmonary disease: implications for chronic airway diseases. Int. J. Nanomed. 2020;15:3803. doi: 10.2147/IJN.S242516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mehta M., Sharma P., Kaur S., Dhanjal D.S., Singh B., Vyas M., Gupta G., Chellappan D.K., Nammi S., Singh T.G., Dua K., Satija S. Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems. 2020. Plant-based drug delivery systems in respiratory diseases; pp. 517–539. [DOI] [Google Scholar]
- 111.Afzal S., Ahmad H.I., Jabbar A., Tolba M.M., Abouzid S., Irm N., Zulfiqar F., Iqbal M.Z., Ahmad S., Aslam Z. BioMed Research International; 2021. Use of Medicinal Plants for Respiratory Diseases in Bahawalpur, Pakistan; p. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Alamgeer, Younis W., Asif H., Sharif A., Riaz H., Bukhari I.A., Assiri A.M. Traditional medicinal plants used for respiratory disorders in Pakistan: a review of the ethno-medicinal and pharmacological evidence. Chin. Med. 2018;13:1–29. doi: 10.1186/S13020-018-0204-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu Y., Liu H., Song L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: a review. J. Nanobiotechnol. 2020;18:1–25. doi: 10.1186/S12951-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garcia F. de M. Nanomedicine and therapy of lung diseases. Einstein (São Paulo). 2014;12:531–533. doi: 10.1590/S1679-45082014MD3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thilakarathna S.H., Rupasinghe H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5:3367–3387. doi: 10.3390/NU5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Santos A.C., Pereira I., Pereira-Silva M., Ferreira L., Caldas M., Magalhães M., Figueiras A., Ribeiro A.J., Veiga F. Nanocarriers for resveratrol delivery: impact on stability and solubility concerns. Trends Food Sci. Technol. 2019;91:483–497. doi: 10.1016/J.TIFS.2019.07.048. [DOI] [Google Scholar]
- 117.Wang J., Li Y., Nie G., Zhao Y. Precise design of nanomedicines: perspectives for cancer treatment. Natl. Sci. Rev. 2019;6:1107–1110. doi: 10.1093/NSR/NWZ012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Merisko-Liversidge E.M., Liversidge G.G. Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol. Pathol. 2008;36:43–48. doi: 10.1177/0192623307310946. [DOI] [PubMed] [Google Scholar]
- 119.Bilia A.R., Piazzini V., Risaliti L., Vanti G., Casamonti M., Wang M., Bergonzi M.C. Nanocarriers: a successful tool to increase solubility, stability and optimise bioefficacy of natural constituents. Curr. Med. Chem. 2018;26:4631–4656. doi: 10.2174/0929867325666181101110050. [DOI] [PubMed] [Google Scholar]
- 120.Kalepu S., Nekkanti V. Improved delivery of poorly soluble compounds using nanoparticle technology: a review. Drug Delivery and Translational Research. 2016;6:319–332. doi: 10.1007/S13346-016-0283-1. [DOI] [PubMed] [Google Scholar]
- 121.Lee J.H., Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015;125:75–84. doi: 10.1016/J.CES.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Emami J., ShetabBoushehri M.A., Varshosaz J., Eisaei A. Preparation and characterization of a sustained release buccoadhesive system for delivery of terbutaline sulfate. Research in Pharmaceutical Sciences. 2013;8:219. /pmc/articles/PMC3757587/ accessed. [PMC free article] [PubMed] [Google Scholar]
- 124.Rizvi S.A.A., Saleh A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharmaceut. J. 2018;26:64–70. doi: 10.1016/J.JSPS.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mitragotri S., Burke P.A., Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zoulikha M., Xiao Q., Boafo G.F., Sallam M.A., Chen Z., He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm. Sin. B. 2021 doi: 10.1016/J.APSB.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wahlich J., Desai A., Greco F., Hill K., Jones A.T., Mrsny R.J., Pasut G., Perrie Y., Seib F.P., Seymour L.W., Uchegbu I.F. Nanomedicines for the delivery of biologics. Pharmaceutics. 2019;11:210. doi: 10.3390/PHARMACEUTICS11050210. 11 (2019) 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCaskill J., Singhania R., Burgess M., Allavena R., Wu S., Blumenthal A., McMillan N.A. Efficient biodistribution and gene silencing in the lung epithelium via intravenous liposomal delivery of siRNA. Mol. Ther. Nucleic Acids. 2013;2:e96. doi: 10.1038/MTNA.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen J., Tang Y., Liu Y., Dou Y. Nucleic acid-based therapeutics for pulmonary diseases. AAPS PharmSciTech. 2018;19:3670–3680. doi: 10.1208/S12249-018-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim Y.-D., Park T.-E., Singh B., Maharjan S., Choi Y.-J., Choung P.-H., Arote R.B., Cho C.-S. Nanoparticle-mediated delivery of siRNA for effective lung cancer therapy. Nanomedicine. 2015;10:1165–1188. doi: 10.2217/NNM.14.214. [DOI] [PubMed] [Google Scholar]
- 131.Anderson C.F., Grimmett M.E., Domalewski C.J., Cui H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases, Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology. 2020;12:e1586. doi: 10.1002/WNAN.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Borghardt J.M., Kloft C., Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Can. Respir. J. J. Can. Thorac. Soc. 2018;2018 doi: 10.1155/2018/2732017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chenthamara D., Subramaniam S., Ramakrishnan S.G., Krishnaswamy S., Essa M.M., Lin F.-H., Qoronfleh M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019;23:1–29. doi: 10.1186/S40824-019-0166-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Iyer R., Hsia C., Nguyen K. Nano-therapeutics for the lung: state-of-the-art and future perspectives. Curr. Pharmaceut. Des. 2015;21:5233–5244. doi: 10.2174/1381612821666150923095742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuzmov A., Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J. Contr. Release. 2015;219:500–518. doi: 10.1016/J.JCONREL.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 136.Majumder J., Minko T. Targeted nanotherapeutics for respiratory diseases: cancer, fibrosis, and coronavirus. Advanced Therapeutics. 2021;4 doi: 10.1002/ADTP.202000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Golombek S.K., May J.N., Theek B., Appold L., Drude N., Kiessling F., Lammers T. Tumor targeting via EPR: strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018;130:17–38. doi: 10.1016/J.ADDR.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi Y., van der Meel R., Chen X., Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921–7924. doi: 10.7150/THNO.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosenblum D., Joshi N., Tao W., Karp J.M., Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9:1–12. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y., Cao J., Yuan Z. Strategies and challenges to improve the performance of tumor-associated active targeting. J. Mater. Chem. B. 2020;8:3959–3971. doi: 10.1039/D0TB00289E. [DOI] [PubMed] [Google Scholar]
- 141.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/J.ADDR.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tan Y.Y., Yap P.K., Xin Lim G.L., Mehta M., Chan Y., Ng S.W., Kapoor D.N., Negi P., Anand K., Singh S.K., Jha N.K., Lim L.C., Madheswaran T., Satija S., Gupta G., Dua K., Chellappan D.K. Perspectives and advancements in the design of nanomaterials for targeted cancer theranostics. Chem. Biol. Interact. 2020;329 doi: 10.1016/J.CBI.2020.109221. [DOI] [PubMed] [Google Scholar]
- 143.Li C., Wang J., Wang Y., Gao H., Wei G., Huang Y., Yu H., Gan Y., Wang Y., Mei L., Chen H., Hu H., Zhang Z., Jin Y. Recent progress in drug delivery. Acta Pharm. Sin. B. 2019;9:1145. doi: 10.1016/J.APSB.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kulkarni C. v, Wachter W., Iglesias-Salto G., Engelskirchen S., Ahualli S. Monoolein: a magic lipid? Phys. Chem. Chem. Phys. 2011;13:3004–3021. doi: 10.1039/c0cp01539c. [DOI] [PubMed] [Google Scholar]
- 145.Freag M.S., Elnaggar Y.S.R., Abdelmonsif D.A., Abdallah O.Y. Stealth, biocompatible monoolein-based lyotropic liquid crystalline nanoparticles for enhanced aloe-emodin delivery to breast cancer cells: in vitro and in vivo studies. Int. J. Nanomed. 2016;11:4799. doi: 10.2147/IJN.S111736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng N., Gao X., Hu Q., Song Q., Xia H., Liu Z., Gu G., Jiang M., Pang Z., Chen H., Chen J., Fang L. Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: cellular interaction and in vivo absorption. Int. J. Nanomed. 2012;7:3703–3718. doi: 10.2147/IJN.S32599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee D.R., Park J.S., Bae I.H., Lee Y., Kim B.M. Liquid crystal nanoparticle formulation as an oral drug delivery system for liver-specific distribution. Int. J. Nanomed. 2016;11:853–871. doi: 10.2147/IJN.S97000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chan Y., Mehta M., Paudel K.R., Madheswaran T., Panneerselvam J., Gupta G., Su Q.P., Hansbro P.M., Macloughlin R., Dua K., Chellappan D.K. Versatility of liquid crystalline nanoparticles in inflammatory lung diseases. Nanomedicine. 2021;16:1545–1548. doi: 10.2217/NNM-2021-0114. [DOI] [PubMed] [Google Scholar]
- 149.Ganem-Quintanar A., Quintanar-Guerrero D., Buri P. Monoolein: a review of the pharmaceutical applications. Drug Dev. Ind. Pharm. 2010;26:809–820. doi: 10.1081/DDC-100101304. [DOI] [PubMed] [Google Scholar]
- 150.Chen Y., Ma P., Gui S. Cubic and hexagonal liquid crystals as drug delivery systems. BioMed Res. Int. 2014:2014. doi: 10.1155/2014/815981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang Y., Gui S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Adv. 2018;8:6978–6987. doi: 10.1039/C7RA12008G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matougui N., Boge L., Groo A.C., Umerska A., Ringstad L., Bysell H., Saulnier P. Lipid-based nanoformulations for peptide delivery. Int. J. Pharm. 2016;502:80–97. doi: 10.1016/J.IJPHARM.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 153.Reese C.W., Strango Z.I., Dell Z.R., Tristram-Nagle S., Harper P.E. Structural insights into the cubic–hexagonal phase transition kinetics of monoolein modulated by sucrose solutions. Phys. Chem. Chem. Phys. 2015;17:9194–9204. doi: 10.1039/C5CP00175G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Riva A., Ronchi M., Petrangolini G., Bosisio S., Allegrini P. Improved oral absorption of quercetin from quercetin Phytosome®, a new delivery system based on Food grade lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019;44:169–177. doi: 10.1007/S13318-018-0517-3/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cherk Yong D.O., Saker S.R., Wadhwa R., Chellappan D.K., Madheswaran T., Panneerselvam J., Tambuwala M.M., Bakshi H.A., Kumar P., Pillay V., Gupta G., Oliver B.G., Wark P., Hsu A., Hansbro P.M., Dua K., Zeeshan F. Preparation, characterization and in-vitro efficacy of quercetin loaded liquid crystalline nanoparticles for the treatment of asthma. J. Drug Deliv. Sci. Technol. 2019;54 doi: 10.1016/J.JDDST.2019.101297. [DOI] [Google Scholar]
- 156.Wadhwa R., Paudel K.R., Chin L.H., Hon C.M., Madheswaran T., Gupta G., Panneerselvam J., Lakshmi T., Singh S.K., Gulati M., Dureja H., Hsu A., Mehta M., Anand K., Devkota H.P., Chellian J., Chellappan D.K., Hansbro P.M., Dua K. Anti-inflammatory and anticancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J. Food Biochem. 2021;45 doi: 10.1111/JFBC.13572. [DOI] [PubMed] [Google Scholar]
- 157.Paudel K.R., Panth N., Manandhar B., Singh S.K., Gupta G., Wich P.R., Nammi S., MacLoughlin R., Adams J., Warkiani M.E., Chellappan D.K., Oliver B.G., Hansbro P.M., Dua K. Attenuation of cigaratte-smoke-induced oxidative stress, senescence, and inflammation by berberine-loaded liquid crystalline nanoparticles: in vitro study in 16HBE and RAW264.7 cells. Antioxidants. 2022;11:873. doi: 10.3390/antiox11050873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Park S.H., Kim J.C. Monoolein cubosomes for enhancement of in vitro anti-oxidative efficacy of Bambusae Caulis in Taeniam extract toward carcinogenic fine dust-stimulated RAW 264.7 cells. Kor. J. Chem. Eng. 2019;36:1466–1473. doi: 10.1007/S11814-019-0333-8. [DOI] [Google Scholar]
- 159.Paudel K.R., Wadhwa R., Mehta M., Chellappan D.K., Hansbro P.M., Dua K. Rutin loaded liquid crystalline nanoparticles inhibit lipopolysaccharide induced oxidative stress and apoptosis in bronchial epithelial cells in vitro. Toxicol. Vitro. 2020;68 doi: 10.1016/J.TIV.2020.104961. [DOI] [PubMed] [Google Scholar]
- 160.Sguizzato M., Drechsler M., Baldisserotto A., Cortesi R., Esposito E. Drug Delivery and Translational Research. 2022. Antioxidant-containing monoolein aqueous dispersions: a preliminary study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baskaran R., Madheswaran T., Sundaramoorthy P., Kim H.M., Yoo B.K. Entrapment of curcumin into monoolein-based liquid crystalline nanoparticle dispersion for enhancement of stability and anticancer activity. Int. J. Nanomed. 2014;9:3119–3130. doi: 10.2147/IJN.S61823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mehta M., Malyla V., Paudel K.R., Chellappan D.K., Hansbro P.M., Oliver B.G., Dua K. Berberine loaded liquid crystalline nanostructure inhibits cancer progression in adenocarcinomic human alveolar basal epithelial cells in vitro. J. Food Biochem. 2021;45 doi: 10.1111/JFBC.13954. [DOI] [PubMed] [Google Scholar]
- 163.Paudel K.R., Mehta M., Hew Suet Yin G., Li Yen L., Malyla V., Patel V.K., Panneerselvam J., Madheswaran T., MacLoughlin R., Kumar Jha N., Kumar Gupta P., Kumar Singh S., Gupta G., Kumar P., Oliver B.G., Hansbro P.M., Kumar Chellappan D., Dua K. Berberine-loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Environ. Sci. Pollut. Control Ser. 2022 doi: 10.1007/s11356-022-19158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Faria A.R., Silvestre O.F., Maibohm C., Adão R.M.R., Silva B.F.B., Nieder J.B. Cubosome nanoparticles for enhanced delivery of mitochondria anticancer drug elesclomol and therapeutic monitoring via sub-cellular NAD(P)H multi-photon fluorescence lifetime imaging. Nano Res. 2019;12:991–998. doi: 10.1007/s12274-018-2231-5. [DOI] [Google Scholar]
- 165.Abdelaziz H.M., Elzoghby A.O., Helmy M.W., Samaha M.W., Fang J.Y., Freag M.S. Liquid crystalline assembly for potential combinatorial chemo–herbal drug delivery to lung cancer cells. Int. J. Nanomed. 2019;14:499. doi: 10.2147/IJN.S188335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tian Y., Li J., Zhu J., Zhu N., Zhang H., Liang L., Sun L. Folic acid-targeted etoposide cubosomes for theranostic application of cancer cell imaging and therapy. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2017;23:2426–2435. doi: 10.12659/MSM.904683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin B., Chen H., Liang D., Lin W., Qi X., Liu H., Deng X. Acidic pH and high-H2O2 dual tumor microenvironment-responsive nanocatalytic graphene oxide for cancer selective therapy and recognition. ACS Appl. Mater. Interfaces. 2019;11:11157–11166. doi: 10.1021/acsami.8b22487. [DOI] [PubMed] [Google Scholar]
- 168.Li Y., Angelova A., Hu F., Garamus V.M., Peng C., Li N., Liu J., Liu D., Zou A. pH responsiveness of hexosomes and cubosomes for combined delivery of Brucea javanica oil and doxorubicin. Langmuir. 2019;35:14532–14542. doi: 10.1021/acs.langmuir.9b02257. [DOI] [PubMed] [Google Scholar]
- 169.Abdelaziz H.M., Elzoghby A.O., Helmy M.W., Abdelfattah E.A., Fang J., Samaha M.W., Freag M.S. Inhalable lactoferrin/chondroitin-functionalized monoolein nanocomposites for localized lung cancer targeting. ACS Biomater. Sci. Eng. 2020;6:1030–1042. doi: 10.1021/acsbiomaterials.9b01639. [DOI] [PubMed] [Google Scholar]
- 170.Meli V., Caltagirone C., Falchi A.M., Hyde S.T., Lippolis V., Monduzzi M., Obiols-Rabasa M., Rosa A., Schmidt J., Talmon Y., Murgia S. Docetaxel-loaded fluorescent liquid-crystalline nanoparticles for cancer theranostics. Langmuir. 2015;31:9566–9575. doi: 10.1021/acs.langmuir.5b02101. [DOI] [PubMed] [Google Scholar]