Abstract
Introduction
The COVID-19 vaccine is essential to reduce the global impact of the pandemic. Understanding its acceptance is key to Nigeria’s national COVID-19 control strategies.
Methods
Between the 6th and 22nd of January 2021, we conducted a non-probability convenience sampling of 3076 respondents using online and in-person interviews to assess the prevalence and predictors of the COVID-19 vaccine acceptance in Nigeria.
Findings
Of the 3076 recruited participants, 74.7% (n = 2300/3076) had tertiary education. The median age group was 30–39 years (35.1%, n = 1097/3076) whereas 31% (n = 952/3076) of all respondents had a monthly income<30,000 Naira (65 USD). The survey results indicated that a wide range of the respondents were in government employment (34.1%, n = 1050/3076). The majority of our study participants (92.2%, n = 2835/3076) believe that COVID-19 is real and not a hoax. Only 27.9% (n = 858/3076) of the study participants have been tested for COVID-19 and 17.8 % (n = 152/858) of the tested respondents were COVID-19 positive by PCR. Half (50.7%; n = 1560/3076) of the study participants were willing to take the vaccine once available. The majority of the respondents (81.1%, n = 2496/3076) were not willing to pay for the vaccine. Only 15.9% (n = 483/3076) of the respondents rated the government’s handling of the pandemic above average. The potential acceptance of the COVID-19 vaccine was significantly affected by the age and the monthly income of the respondents. Respondents older than 60 years old (OR: 3.02, 95% CI: 1.69,5.41; p < 0.001) and those that earn between 250,000–500,000 Naira monthly (OR: 1.38; 95% CI: 1.11,1.70; p < 0.001) were more likely to accept the COVID-19 vaccine respectively. In addition, the respondents’ perception of the existence of the disease (OR: 1.45; 95% CI: 0.99,2.18; p > 0.05), the need for a COVID-19 vaccine (OR: 16; 95% CI: 11.63,22.10; p < 0.001), the willingness to pay (OR: 1.68; 95% CI: 1.39,2.01; p < 0.001) and the rating of the government handling of the pandemic (OR: 2.25; 95% CI: 1.57,3.23; p < 0.001) were critical to the acceptance of the COVID-19 vaccine.
Interpretation
With 50.7% vaccine acceptance, Nigeria’s public health policymakers must prioritize and develop strategies that will effectively increase COVID-19 vaccine acceptance across the country with emphasis on trust, transparency and strong leadership.
Keywords: COVID-19, Vaccine Acceptance, Nigeria
1. Introduction
The 2019 coronavirus disease (COVID-19) has caused a serious global public health crisis. It has affected>535 million people and caused over 6.31 million deaths in 224 countries.[1], [2] Africa has recorded 11.7 million COVID-19 cases and 253,573 COVID-19-associated mortality.[3] Nigeria, the most populous African nation with over 214 million inhabitants [4] has recorded 256,254 COVID-19 cases and 3,144 deaths as of June 12th, 2022. [5].
To curb the spread of the severe acute respiratory syndrome coronavirus −2 (SARS-CoV-2), several interventions have been instituted globally. Some of these non-pharmaceutical interventions (banning international flights, use of face masks, frequent handwashing, social distancing, and in certain cases movement restrictions through full lockdowns [6]) have been effective in reducing the global spread of SARS-CoV-2. [7], [8] However, the need for pharmaceutical interventions such as vaccines cannot be over-emphasized and it is essential to attain herd immunity against SARS-CoV-2. [9] Vaccination is a simple, safe and effective way of boosting the host immunity by introducing a modified antigen (vaccine) before the host comes in contact with the wild-type pathogen. However, the concern and rise of vaccine hesitancy are growing worldwide and pose a serious threat to progress against vaccine-preventable diseases.[10], [11].
Several misconceptions concerning the existence of the COVID-19 disease and the accelerated pace of the COVID-19 vaccine development could be barriers to the acceptance of the COVID-19 vaccine in many African settings.[12] Hence, public health agencies and non-governmental organizations must adopt effective health communication strategies aimed at persuading the public to accept the COVID-19 vaccine.[13].
Of the over 230 COVID-19 vaccine candidates, only a few have passed phase III clinical trials and have been authorized for emergency use in several countries.[14] Nigeria received the first batch of the COVID-19 vaccine (Oxford-AstraZeneca) in March 2021 and a second batch (Moderna) in July 2021. [15] The anticipated arrival of the vaccine necessitates the urgent understanding of the acceptability of a COVID-19 vaccine so that public health officials and policymakers can tailor their health communication strategy to effectively vaccinate sufficient Nigerians to attain herd immunity against the SARS-CoV-2.
The COVID-19 vaccine acceptance is generally context-specific.[16], [17] Hence, we hypothesize it could vary with cultural beliefs, the geopolitical region, and other sociodemographic variables in Nigeria. Although several studies had assessed COVID-19 vaccine acceptance in Nigeria, [18], [19], [20], [21], [22] this study adds to the body of literature and provides further evidence-based information to guide public health decision-makers.
2. Materials and methods
2.1. Ethical approval
The ethical clearance of this study was obtained from the Kwara State Ministry of Education and Human Capital Development, Ilorin, Nigeria (Reference number: DE/PRIM/11/069).
2.2. Study design, study participants, and sampling
This study was designed as a cross-sectional survey of adult respondents (≥18 years) from the six geo-political regions of Nigeria. The survey was conducted between the 6th and the 22nd of January 2021. Study participants were recruited via two main mechanisms: 1. Online social media platforms (n = 1924) such as Facebook and WhatsApp with the aid of personal and professional networks (administered in English language only). 2. To accommodate respondents who do not understand English, we conducted one on one interviews (n = 1152) by verbally translating the survey instrument to the local dialect that was necessary for some of the regions (especially in the Northern part of Nigeria). Written informed consent was sought from all respondents as participation in the survey was without prejudice and voluntary as specified in the World Medical Association Declaration of Helsinki Ethical principles.[23].
2.3. Survey methodology
The required sample size was computed using Epi-Info V.7.0 (CDC, Atlanta, USA). At a 95% confidence interval and a vaccine acceptance rate of 51%,[24] a total of 384 study participants were required from each geopolitical zone of Nigeria (n = 6). Hence, at least 2,304 (384 × 6) respondents were required for this study. Furthermore, we added a 25% (n = 576) non-response rate to the total required participants for this study. However, the total number of participants included in this study was 3,076 respondents.
2.4. Questionnaire design
A structured questionnaire was developed and administered via google forms (online respondents) and paper surveys (one on one interviews). To assess the content validity, clarity, ease of response, scope, and face validity of the questions, three independent reviewers were selected to validate the questionnaire. Furthermore, to check for technical glitches, the aptness of the survey tool, and typographical errors, a pre-test survey on 10 volunteers was performed. Responses obtained were not incorporated in the final analysis of the data. The demographic characteristics of the respondents (age, gender, education, income, occupation, and geopolitical region) were determined in the first part (Section A) of the questionnaire. Section B focused on the acceptance of the COVID-19 vaccine (Supplementary file 1).
2.5. Data analysis
The data were summarized using Microsoft excel and subjected to further statistical analysis using Statistical Product and Service Solutions (SPSS) software, v.26. Qualitative data were presented as frequencies and proportions whereas quantitative data were presented as mean and standard deviation. A univariable logistic regression analysis was conducted to assess the association between the socio-demographic variables (age, gender, education, net monthly income, occupation, and geopolitical region) and the outcome variable (COVID-19 vaccine acceptance). Variables with a p-value ≤ 0.05 were selected for multivariable logistic regression analysis using the logit function and α at 0.25. The multivariable logistic regression analysis was performed and the variables with a p-value ≤ 0.05 were retained in the final model. The odds ratio (OR) and its 95% confidence interval (CI) of the variables associated with the outcome variables were derived from the final multivariable logistic regression model.
3. Results
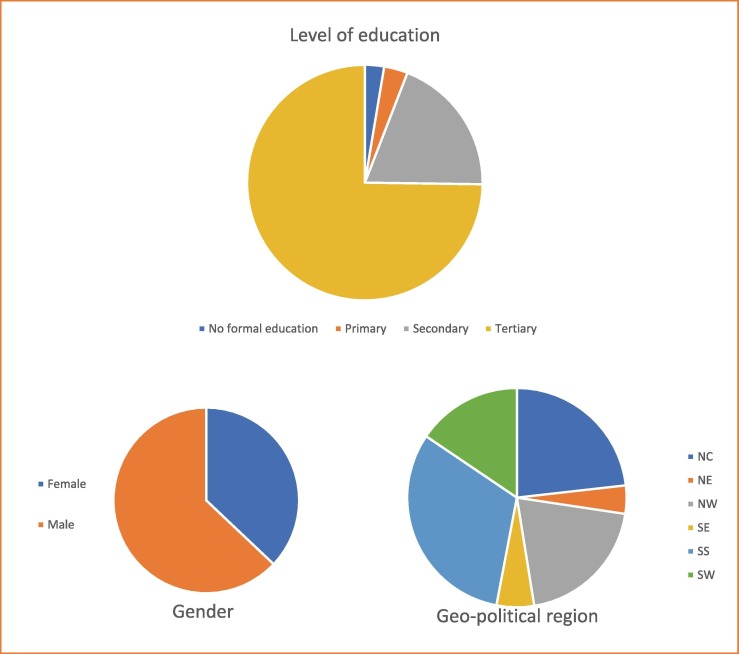
A total of 3076 respondents were included in this survey by online social media platforms (62.5%, n = 1,924/3,076) and one-on-one interviews (37.5%, n = 1,152/3,076) (Fig. 1 ). The online survey was attempted by 2,171 persons but only 1,924 of them gave consent and were the only ones included in the final analysis (response rate – 88.6%). The one-on one-interviews were administered in two randomly chosen states in the North-West (n = 384), North-Central (n = 384), and South-South (n = 384) geopolitical regions of Nigeria. Most of the respondents (74.7%, n = 2300/3076) had tertiary education (Fig. 1). The mean age of the respondents was 39 ± 10.9 years, the interquartile range was 16 years, and 31% (n = 952/3076) of all respondents had a monthly income of<30,000 Naira (US $59).[25] The survey results indicated that a wide range of the respondents were in government employment (34.1%, n = 1050/3076) (Table 1 ).
Fig. 1.
Respondent demographics showing Gender of respondents; Level of education of respondents and the geo-political region of respondents within Nigeria.
Table 1.
Description of the study participants (n = 3076).
| Variables | Frequency (%) |
|---|---|
| Age (years) | |
| <20 | 178 (5.8) |
| 20–29 | 808 (26.3) |
| 30–39 | 1097 (35.7) |
| 40–49 | 651 (21.1) |
| 50–59 | 224 (7.3) |
| >60 | 188 (6.1) |
| Occupation | |
| Government worker | 1050 (34.1) |
| Private company | 621 (20.2) |
| Self-employed | 646 (21) |
| Student | 407 (13.2) |
| Unemployed | 352 (11.4) |
| Monthly income (Naira) | |
| <30,000 | 952 (31) |
| 30,000–60,000 | 659 (21.4) |
| 60,000–100,000 | 592 (19.2) |
| 100,000–250,000 | 594 (19.3) |
| 250,000–500,000 | 206 (6.7) |
| >500,000 | 73 (2.4) |
3.1. COVID-19 vaccine acceptance in Nigeria
The majority of our study participants (92.2%, n = 2835/3076) believe that COVID-19 is real and not a hoax. However, adherence to the laid down non-pharmaceutical guidelines, especially the use of face masks was sub-optimal (45.4%, n = 1397/3076). Only 27.9% (n = 858/3076) of the study participants have been tested for COVID-19 (Table 2 ). Only 17.8 % (n = 152/858) of the tested respondents were positive. Most of our study participants (80.2%, n = 2488/3076) believed that the world needs a COVID-19 vaccine to hasten the attainment of herd immunity and curb the further spread of the virus and its associated mortality. However, only 50.7% (n = 1560/3076) of the study participants were willing to take the vaccine once available. Most of our study respondents (70.9%, n = 2180/3076) feel that the vaccine should not be mandated for the citizens. The majority of the respondents (81.1%, n = 2496/3076) were not willing to pay for the vaccine and opined that the vaccine has to be completely free. Only 15.9% (n = 483/3076) of the respondents rated the government’s handling of the pandemic above average.
Table 2.
COVID-19 vaccine acceptance in Nigeria (n = 3076).
| Variable | Frequency (%) |
|---|---|
| 1. Do you think COVID-19 is real? | |
| No | 241 (7.8) |
| Yes | 2835 (92.2) |
| 2. Adherence to non-pharmaceutical interventions | |
| a. Regular handwashing | 2659 (86.4) |
| b. Regular use of nose masks | 1397 (45.4) |
| c. Social distancing | 2272 (73.8) |
| d. Avoid crowded places | 2054 (66.7) |
| 3. Have you been tested for the COVID-19 virus? | |
| No | 2218 (72.1) |
| Yes | 858 (27.9) |
| *3b. If yes, was it positive? (n = 858) | |
| No | 706 (82.2) |
| Yes | 152 (17.8) |
| 4. Do you think we need a COVID-19 vaccine? | |
| No | 588 (19.1) |
| Yes | 2488 (80.2) |
| 5. Are you willing to take the COVID-19 vaccine if available? | |
| No | 1516 (49.3) |
| Yes | 1560 (50.7) |
| 6. Do you think the COVID-19 vaccine should be mandated on all Nigerian citizens? | |
| Maybe | 534 (17.4) |
| No | 2180 (70.9) |
| Yes | 362 (11.7) |
| 7. How much are you willing to pay for the COVID-19 vaccine (Naira)? | |
| a. It has to be completely free | 2496 (81.1) |
| b. < 1000 | 269 (8.7) |
| c. 1000–10,000 | 194 (6.3) |
| d. > 10,000 | 27 (0.9) |
| e. I will take the vaccine no matter the cost | 90 (2.9) |
| 8. On a scale of 5, rate the government’s handling of the COVID-19 pandemic. | |
| 1 (Poor) | 686 (22.3) |
| 2 (Fair) | 875 (28.4) |
| 3 (Average) | 1032 (33.6) |
| 4 (Good) | 284 (9.2) |
| 5 (Excellent) | 199 (6.7) |
3.2. Factors associated with COVID-19 vaccine acceptance in Nigeria
The potential acceptance of the COVID-19 vaccine was significantly affected by the age and monthly income of the respondents. Respondents older than 60 (OR: 3.02, 95% CI: 1.69,5.41; p < 0.001) and those that earn between 250,000–500,000 Naira monthly (OR: 1.38; 95% CI: 1.11,1.70; p < 0.001) were more likely to accept the COVID-19 vaccine respectively. Similarly, the potential of COVID-19 vaccine acceptance was affected by the perception of respondents. Respondents’ perception of the existence of the disease, the need for a COVID-19 vaccine, the willingness to pay, and the rating of the government's handling of the pandemic are critical to the acceptance of the vaccine in Nigeria. Respondents who believed in the existence of the coronavirus disease were likely to accept the vaccine (OR: 1.45; 95% CI: 0.99,2.18; p > 0.05) and those who perceived the need for the development of a COVID-19 vaccine were 16× (95% CI: 11.63,22.10; p < 0.001) more likely to accept the vaccine (Table 3 ). Those who were satisfied with the government’s handling of the pandemic were 2.25 × (95% CI: 1.57,3.23; p < 0.001) more likely to accept the vaccine. Respondents who were willing to pay for the vaccine were 1.68× (95% CI: 1.39,2.01; p < 0.001) more likely to accept the vaccine (result not shown).
Table 3.
Univariable and multivariable logistic regression analysis of the factors associated with the COVID-19 vaccine acceptance in Nigeria.
| Variable | Referent | Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 Vaccine acceptance | Odds Ratio (95% CI) | X2 | DF | p-value | Adjusted Odds Ratio (95% CI) | X2 | DF | p-value | ||
| Age (years) | <20 | 20–29 30–39 40–49 50–59 >60 |
1.38 (0.99,1.92)1.83 (1.32,2.53)1.75 (1.25,2.46) 1.79 (1.20,2.67)1.94 (1.21,3.11) |
23.2 | 5 | 0.001 | 1.30(0.90,1.90)1.67 (1.14,2.44)1.70 (1.13,2.57)2.05 (1.28,3.28)3.02 (1.69,5.41) |
20.97 | 5 | 0.001 |
| Gender | Female | Male | 1.16 (0.99,1.34) | 3.81 | 1 | 0.051 | – | – | – | – |
| Occupation | Government employee | Private company Self employed Student Unemployed |
0.61(0.50, 0.75) 0.90 (0.74,1.1)0.57 (0.45,0.72)0.73 (0.57,0.93) |
38.51 | 4 | 0.001 | 0.75 (0.59,0.95) 1.23 (0.96,1.56)0.89 (0.65,1.19)0.76 (0.58,1.00) |
19.01 | 4 | 0.001 |
| Level of education | No formal education | Primary Secondary Tertiary |
0.78 (0.44,1.41)0.78 (0.49,1.24) 0.95 (0.61,1.48) |
5.27 | 3 | 0.153 | – | – | – | – |
| Monthly income (Naira) | <30,000 | 30,000–60,000 60,000–100,000 100,000–250,000 250,000–500,000 >500,000 |
1.49 (1.22,1.82)1.27 (1.04,1.56)1.34 (1.09,1.65)1.41 (1.05,1.92)0.62 (0.38,1.02) |
26.8 | 5 | 0.001 | 1.21 (0.96,1.52)1.29 (0.93,1.78)1.38 (1.11,1.70)1.14 (0.92,1.43)0.65 (0.38,1.08) |
15.09 | 5 | 0.01 |
| Geopolitical zone | North Central | North East North West South East South South South West |
0.61 (0.36,1.01)0.92 (0.74,1.14)1.04 (0.72,1.51)1.08 (0.89,1.30)1.05 (0.83,1.32) |
7.1 | 5 | 0.213 | – | – | – | – |
| Reality of COVID-19 | No | Yes | 4.24 (3.09,5.83) | 96.53 | 1 | 0.001 | 1.45 (0.99,2.18) | 3.63 | 1 | 0.057 |
| Tested for COVID-19 | No | Yes | 1.22 (1.04,1.43) | 6.17 | 1 | 0.013 | 0.76 (0.54, 1.08) | 2.33 | 1 | 0.13 |
| Need for a COVID-19 vaccine | No | Yes | 17.01(12.56,23.04) | 592.7 | 1 | 0.001 | 16.03 (11.63,22.10) | 460.07 | 1 | 0.001 |
| Willingness to pay for COVID-19 vaccine, if available | Free vaccine | <1,000 1,000–10,000 >10,000 At any cost |
1.27 (0.99,1.63)2.03 (1.49,2.75)3.06 (1.29,7.25)2.25 (1.43,3.52) |
41.19 | 4 | 0.001 | 1.10 (0.84,1.46)1.53 (1.10,2.11)1.92 (0.80,4.62)1.50 (0.91,2.48) |
11.1 | 4 | 0.025 |
| Rating government handling of pandemic | 1 (poor) | 2 (fair)3 (average)4 (good)5 (excellent) |
1.28 (1.05,1.57) 1.97 (1.62,2.40) 2.00 (1.51,2.65) 2.68 (1.93,3.72) | 73.5 | 4 | 0.001 | 1.32 (1.05,1.65) 1.61 (1.30,1.99) 1.68 (1.23,2.28) 2.25 (1.57,3.23) | 17.06 | 4 | 0.001 |
4. Discussion
Vaccines have been one of the most effective public health interventions that have greatly reduced morbidity and associated mortality of infectious diseases.[26], [27].
Although non-pharmaceutical COVID-19 interventions were found to reduce the burden of COVID-19, a better public health outcome is likely if mass COVID-19 vaccination was integrated with existing non-pharmaceutical interventions.[28] Our findings showed that adherence to the non-pharmaceutical interventions among our study participants was poor. For instance, during the in-person interviews, only 29% (n = 334/1152) of the respondents were wearing a mask.
The COVID-19 vaccine acceptance rate in this study (50.7%) was similar to the report of Alice Tobin et al., [19] and Chiedozie et al., [24] in which only 50.2% and 51.1% of Nigerians were willing to take the COVID-19 vaccine respectively. However, the vaccine acceptance rate was lower than the 76% COVID-19 vaccine acceptance rate among Nigerians reported by the African Center for Disease Control (Africa CDC) in its’ 15-country COVID-19 vaccine perception study in 2021, [22] 65.2% reported by Lazarus et al.,[13] and 68% reported in the Geopoll vaccine hesitancy survey reports.[29], Significantly higher COVID-19 vaccine uptake has been reported in South Africa,[13] Indonesia,[30] Ecuador,[31] China,[32], [33] and most European countries.[13], [34], [35], [36], [37] However, lower potential COVID-19 vaccine acceptance has been reported in the general population in Jordan and Kuwait,[38] and amongst nurses in Hongkong mainly due to vaccine efficacy and safety concerns. [39].
Public trust is a critical component of any successful COVID-19 vaccination acceptance program.[40] Our findings showed that the COVID-19 vaccine acceptance in Nigeria was significantly influenced by the perception of respondents especially their trust in government. This is similar to the reports of Al-Mohaithef & Padhi [41] and Lazarus et al.,[13] who reported that trust in government significantly increased the likelihood of COVID-19 vaccine acceptance and compliance with other COVID-19 control guidelines in Saudi Arabia and the United State of America respectively. Respondents who rated the governments’ handling of the pandemic were 2.25 × more likely to accept the vaccine (Table 3).
Our findings confirmed that older respondents were more likely to accept the vaccine. This is consistent with the report of the Africa CDC 15-country vaccine perception study,[22] Lazarus et al.,[13] and Malik et al.[42] Self-employed respondents and those with a monthly income greater than the national minimum monthly salary of 30,000 Naira were more likely to receive the vaccine when compared to other occupations.
Our finding revealed that sociodemographic factors of gender, level of education, and the geopolitical region of the respondents did not affect the potential COVID-19 vaccine acceptance in Nigeria (Table 3). There were no significant differences in vaccine acceptance across the diverse ethnic and cultural groups across the six geopolitical zones of Nigeria. Respondents from Nigerian COVID-19 epicenters (Lagos and FCT) did not have an increased likelihood of accepting the vaccine compared to those from other Nigerian states (Table S1).
Similarly, we detected that neither the study participants who had been tested for COVID-19 (n = 858/3076) nor those who tested positive (n = 152/858) were any likely to accept the COVID-19 vaccine than the general population (Table 2). A wide range of our study participants (70.9%, n = 2180/3076) did not agree with making the COVID-19 vaccine mandatory for all Nigerians.
Our findings revealed that few socio-demographic factors and public perception of the pandemic were the main predictors of the COVID-19 vaccine uptake in Nigeria. In the univariate logistic regression analysis, respondents that believed that the COVID-19 is real and those who perceived the need for a COVID-19 vaccine were 4.24 × and 17 × more likely to accept the COVID-19 vaccine respectively.
It is imperative that Nigeria immediately improves its public health communication and vaccine literacy strategies to boost the confidence of the general public and increase the COVID-19 vaccine uptake. Several studies have highlighted strategies for increasing public trust and vaccine acceptance. These include addressing community-specific concerns while being sensitive to traditional and religious beliefs.[43], [44], [45] Increasing the COVID-19 vaccine uptake in Nigeria will require a multisectoral collaboration of important stakeholders such as traditional rulers and public figures to demonstrate leadership with clear, accurate, transparent, and evidence-based policy in addition to encouraging the public towards accepting the vaccine to attain community immunization (herd immunity).
The limitations of this study included the potential biases of our data collection methods and convenience sampling of one-on-one respondents. In addition, the binary outcome variable could have resulted in a lower resolution of assessment of COVID-19 vaccine hesitancy in Nigeria. Despite these, we feel that we have collected valuable insights through a combination of online and in-person interviews, from diverse respondents.
Authors' contributions.
AIA, MO, and AAT were involved in planning the study and data collection. AIA, OO, and AO drafted the initial manuscript. VOA, MOR, AAT, SO, AO, OEF, AJ, OAO, and NE reviewed the manuscript. All authors approved the final submission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
-
•
We acknowledge the dedication of Gomina Musa Muhammad, Abdulrahim Ibrahim, and Jimoh Yaqub to data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.06.050.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. COVID19.who.int. 2021 [cited 24 January 2021]. Available from: https://COVID19.who.int/?gclid=Cj0KCQiA0rSABhDlARIsAJtjfCd1LcjQvFusy9-jFT7kFBKXZSo3nXoeY-jbF7T3sO4aW8gSNbjlyOIaAtvnEALw_wcB.
- 2.COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet]. Johns Hopkins Coronavirus Resource Center. 2021 [cited 24 January 2021]. Available from: https://coronavirus.jhu.edu/map.html.
- 3.Africa CDC - COVID-19 Daily Updates [Internet]. Africa CDC. 2021 [cited 24 January 2021]. Available from: https://africacdc.org/COVID-19/
- 4.Population, total - Nigeria | Data [Internet]. Data.worldbank.org. 2021 [cited 24 January 2021]. Available from: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=NG
- 5.Nigeria Center for Disease Control (NCDC). COVID-19 Situation Report:Weekly Epidemiological Report 14. Available from: https://COVID19.ncdc.gov.ng.
- 6.Flaxman S., Mishra S., Gandy A., Unwin H., Mellan T., Coupland H., et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584(7820):257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 7.Vijayaraghavan P., Sriramkumar S. Non-Pharmaceutical Interventions are measures to control coronavirus disease-2019 (COVID-19) transmission in India. Coronaviruses. 2020;01 [Google Scholar]
- 8.Lai S., Ruktanonchai N., Zhou L., Prosper O., Luo W., Floyd J., et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020;585(7825):410–413. doi: 10.1038/s41586-020-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19 [Internet]. Who.int. 2021 [cited 24 January 2021]. Available from: https://www.who.int/news-room/q-a-detail/herd-immunity-lockdowns-and-COVID-19
- 10.Larson H.J., Jarrett C., Eckersberger E., Smith D.M.D., Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 11.Phadke, V.K.; Bednarczyk, R.A; Salmon, D.A.; Omer, S.B. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis. JAMA. 315(11):1149–1158. [DOI] [PMC free article] [PubMed]
- 12.Fadda M., Albanese E., Suggs L.S. When a COVID-19 vaccine is ready, will we all be ready for it? Int J Public Health. 2020;65:711–712. doi: 10.1007/s00038-020-01404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2020 doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines [Internet]. Who.int. 2021 [cited 23 January 2021]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-COVID-19-candidate-vaccines
- 15.Africa News. Nigeria receives four million COVID vaccine doses from the US | Africanews [Internet]. Africanews. 2021 [cited 18 August 2021]. Available from: https://www.africanews.com/2021/08/01/nigeria-receives-four-million-COVID-vacine-doses-from-the-us//.
- 16.De Figueiredo A., Simas C., Karafillakis E., Paterson P., Larson H. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. The Lancet. 2020;396(10255):898–908. doi: 10.1016/S0140-6736(20)31558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peretti-Watel P., Ward J., Schulz W., Verger P., Larson H. Vaccine Hesitancy: clarifying a theoretical framework for an ambiguous notion. PLoS. Currents. 2015 doi: 10.1371/currents.outbreaks.6844c80ff9f5b273f34c91f71b7fc289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solís Arce J., Warren S., Meriggi N., Scacco A., McMurry N., Voors M., et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–1394. doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alice Tobin E., Okonofua M., Adeke A., Obi A. Willingness to accept a COVID-19 vaccine in nigeria: a population-based cross-sectional study. Central African J Public Health. 2021;7(2):53. [Google Scholar]
- 20.Adejumo O., Ogundele O., Madubuko C., Oluwafemi R., Okoye O., Okonkwo K., et al. Perceptions of the COVID-19 vaccine and willingness to receive vaccination among health workers in Nigeria. Osong Public Health and Res Perspectives. 2021;12(4):236–243. doi: 10.24171/j.phrp.2021.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olu-Abiodun O., Abiodun O., Okafor N. COVID-19 vaccination in Nigeria: a rapid review of vaccine acceptance rate and the associated factors. PLoS ONE. 2022;17(5) doi: 10.1371/journal.pone.0267691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Africa CDC. COVID-19 Vaccine Perceptions: A 15-country study. [2021]. Available at: https://africacdc.org/download/COVID-19-vaccine-perceptions-a-15-country-study/. Accessed 28 February 2022.
- 23.Wma World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 24.Chiedozie A.P., Chukwuebuka O.J., Chidimma C.F., Onyinyechi O.V., Chijioke A.K., Chibuzor O.S., et al. Willingness to Accept a potential COVID-19 vaccine in nigeria. Am J Med Sci Med. 2021;9(1):1–5. doi: 10.12691/ajmsm-9-1-1. [DOI] [Google Scholar]
- 25.NgnRates. Track & Analyse Naira Exchange Rates [Internet]. Ngnrates.com. 2021 [cited 19 August 2021]. Available from: https://www.ngnrates.com/.
- 26.Palamenghi L., Barello S., Boccia S., Graffigna G. Mistrust in biomedical research and vaccine hesitancy: the forefront challenge in the battle against COVID-19 in Italy. Eur J Epidemiol. 2020;35:785–788. doi: 10.1007/s10654-020-00675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison A., Wu J.W. Vaccine confidence in the time of COVID-19. Eur J Epidemiol. 2020;35:325–330. doi: 10.1007/s10654-020-00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betti, M.; Bragazzi, N.L.; Heffernan, J.; Kong, J.; Raad, A. Integrated Vaccination and Non-Pharmaceutical Interventions based Strategies in Ontario, Canada, as a Case Study: a Mathematical Modeling Study. 2021. MedRxiv preprint. 10.1101/2021.01.06.21249272. [DOI] [PMC free article] [PubMed]
- 29.Lansell S. Vaccine hesitancy and COVID-19 vaccine acceptance in sub-saharan africa - geopoll [Internet] GeoPoll. 2021 [cited 22 January 2021]. Available from: [Google Scholar]
- 30.Harapan H., Wagner A.L., Yufika A., Winardi W., Anwar S., Gan A.K., et al. Acceptance of a COVID-19 vaccine in southeast asia: a cross-sectional study in indonesia. Front Public Health. 2020;8:381. doi: 10.3389/fpubh.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarasty O., Carpio C.E., Hudson D., Guerrero-Ochoa P.A., Borja I. The demand for a COVID-19 vaccine in ecuador. Vaccine. 2020;38:8090–8098. doi: 10.1016/j.vaccine.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Jing R., Lai X., Zhang H., Lyu Y., Knoll M.D., et al. Acceptance of COVID-19 vaccination during the COVID- 19 pandemic in china. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Y., Hu Z., Zhao Q., Alias H., Danaee M., Wong L.P. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell S., Clarke R., Mounier-Jack S., Walker J.L., Paterson P. Parents' and guardians' views on the acceptability of a future COVID-19 vaccine: A multi-methods study in England. Vaccine. 2020;38:7789–7798. doi: 10.1016/j.vaccine.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman D., Loe B.S., Chadwick A., Vaccari C., Waite F., Rosebrock L., et al. COVID-19 vaccine hesitancy in the UK: the oxford coronavirus explanations, attitudes, and narratives survey (OCEANS) II. Psychol Med. 2020:1–34. doi: 10.1017/S0033291720005188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barello S., Nania T., Dellafiore F., Graffigna G., Caruso R. ‘Vaccine hesitancy’among university students in Italy during the COVID-19 pandemic. Eur J Epidemiol. 2020;35:781–783. doi: 10.1007/s10654-020-00670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagneux-Brunon A., Detoc M., Bruel S., Tardy B., Rozaire O., Frappe P., et al. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross sectional survey. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallam M., Dababseh D., Eid H., Al-Mahzoum K., Al-Haidar A., Taim D., et al. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: a study in Jordan and Kuwait among other Arab countries. Vaccines. 2021 Jan;9(1):42. doi: 10.3390/vaccines9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K., Wong E.L.Y., Ho K.F., Cheung A.W.L., Chan E.Y.Y., Yeoh E.K., et al. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: a cross-sectional survey. Vaccine. 2020;38:7049–7056. doi: 10.1016/j.vaccine.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozawa S., Stack M. Public trust and vaccine acceptance-international perspectives. Hum Vaccines & Immunother. 2013;9(8):1774–1778. doi: 10.4161/hv.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Mohaithef, M.; Padhi, B.K. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: A web based national survey. J. Multidiscip. Healthc. 2020:13 1657–1663. [DOI] [PMC free article] [PubMed]
- 42.Malik A., McFadden S., Elharake J., Omer S. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biasio L.R. Vaccine hesitancy and health literacy. Hum Vaccines Immunother. 2017;13:701–702. doi: 10.1080/21645515.2016.1243633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomson A., Vallée-Tourangeau G., Suggs L.S. Strategies to increase vaccine acceptance and uptake: from behavioral insights to context-specific, culturally-appropriate, evidence-based communications and interventions. Vaccine. 2018;36:6457–6458. doi: 10.1016/j.vaccine.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 45.European Centre for Disease Prevention and Control. Catalogue of interventions addressing vaccine hesitancy. 2017. Available at: https://www.ecdc.europa.eu/ sites/ portal/files/documents/ Catalogue-interventions- vaccine-hesitancy.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.