Abstract
Lung cancer is the leading cause of cancer-related deaths. Surgery remains the best option to treat lung cancer when feasible. However, many cases are diagnosed beyond the initial stages. There has been tremendous progress in the treatment of lung cancer over the last few years. Studies have shown that biomarker-driven targeted therapies lead to better outcomes. Due to the technical difficulties and significant procedural risk associated with repeated tissue biopsies, analysis of tumor constituents circulating in the blood, such as circulating tumor DNA (ctDNA) and various proteins, is becoming more widely recognized as an alternative method of tumor sampling, i.e., liquid biopsy. Liquid biopsy is superior to tissue biopsy, as it is minimally invasive and easily repeatable. Given the recent data on changes in mutations as the disease progresses or responds to treatment, liquid biopsies can help monitor the changes and guide us in giving targeted drugs. Here we present a case of advanced NSCLC who was initially started on Alectinib based on positivity for ALK gene rearrangement found in the FISH study. At the time of progression, molecular profiling liquid biopsy was obtained, which revealed KRAS-p.G12C mutation. Thus, the patient’s therapy was later on changed to sotorasib after the FDA approved a KRAS-p.G12C mutation inhibitor.
Keywords: non-small-cell lung cancer (NSCLC), sotorasib, liquid biopsy, mutation
1. Introduction
Lung cancer is classified into small cell lung cancer (SCLC) and non-SCLC (NSCLC); NSCLC accounts for approximately 85% of all lung cancer cases [1]. Lung cancer remains the number one killer among cancers worldwide [2]. Smoking continues to be the most important risk factor for lung cancer, including secondhand smoke [3]. However, aggressive smoking cessation programs are not enough to prevent lung cancer. Biomarker-driven targeted therapies are required to improve clinical outcomes for patients meaningfully. Most NSCLC patients undergo systemic therapy, either being diagnosed at an already inoperable stage or experiencing disease relapse after surgery [4]. Next-generation sequencing (NGS) is being used to test for tumor mutations. The clinical applications of NGS will further increase as technology, bioinformatics, and resources improve to address the limitations and improve the quality of results.
The detection of EGFR, BRAF, and MET mutations and the analysis of ALK, ROS1, RET, and NTRK translocations have already been incorporated into NSCLC diagnostic guidelines, and their inhibitors are recommended whenever indicated. NSCLCs are also subjected to the analysis of PD-L1 protein expression in order to direct the use of immune checkpoint inhibitors.
2. Case Presentation
A 71-year-old gentleman was referred to the Oncology clinic for evaluation of a 2.5 cm left upper lobe lung nodule with a large left-sided pleural effusion observed at the academic hospital emergency department. Urgent thoracentesis was not performed, as the patient had no significant symptoms.
He had been in his usual state of health until the first week of July 2020, when he started to experience lower left-sided back pain, radiating to the left side and eventually radiating to the epigastric. The patient underwent extensive workup with gastroenterology, including esophagogastroduodenoscopy (EGD) and magnetic resonance imaging (MRI) abdomen, which were negative.
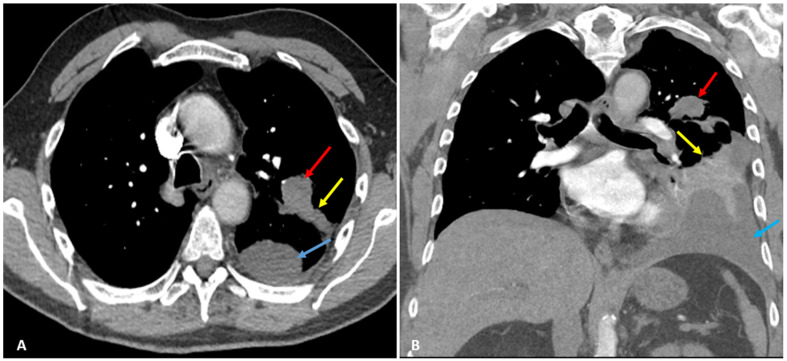
Later, he started to experience excruciating left lateral chest pain, radiating to his shoulder and neck, 10/10 in intensity, and worsened with deep inspiration, leading to shallow breathing and dyspnea. He returned to his primary physician, who ordered a chest X-ray (CXR) and computed tomography (CT) chest with intravenous (IV) contrast revealing left upper lobe peri-bronchial mass (red arrows) measuring 3 × 2 cm in diameter, distal obstructive atelectasis, and left pleural effusion (Figure 1).
Figure 1.
Axial (A) and coronal (B) CT scan with IV contrast shows left upper lobe peri-bronchial mass (red arrows) measuring approximately 3 × 2 cm in diameter. Distal obstructive atelectasis (yellow arrows) and left pleural effusion (blue arrows) are observed as well.
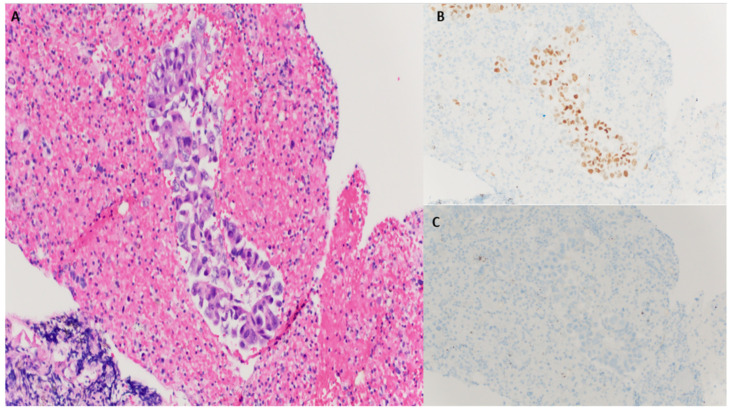
He was then sent to the emergency department to seek immediate medical attention. The patient reported a 13 lbs. (6 kg) intentional weight loss over the period of 2 months due to drastic changes in his diet in an effort to lose weight. A biopsy of the pulmonary nodule was performed. The histologic examination revealed irregular glandular clusters with hyperchromatic nuclei, nuclear pleomorphism, and abundant eosinophilic vacuolar cytoplasm. The tumor cells were strongly positive for transcription termination factor 1 (TTF1) and negative for P40. Based on morphology and immunohistochemical patterns, a diagnosis of adenocarcinoma was rendered (Figure 2). Genomic profiling and study timeline are described (Table 1).
Figure 2.
(A), H&E 20×, tumor cells forming incomplete lumina with hyperchromatic nuclei; (B) Strong nuclear staining of tumor cells with TTF1; (C) Tumor cells are negatively stained for P40.
Table 1.
Oncology history and treatment timeline.
| Pathologic Diagnosis and Molecular Profiling | Oncology Treatment | Treatment Duration |
|---|---|---|
| Tissue biopsy in August 2020 with ALK rearrangement | Alectinib | September 2020–November 2020 |
| Liquid biopsy in November 2020 with KRAS p.G12C mutation | Bevacizumab/atezolizumab/carboplatin/paclitaxel | November 2020–January 2021 |
| Bosutinib/pemetrexed via Phase 1 clinical trial | January 2021–July 2021 | |
| Sotorasib after FDA approval | July 2021–November 2021 |
Fluorescence in situ hybridization (FISH) study by using Vysis ALK break-apart probe showed 16% of cells were positive for an ALK rearrangement. PET scan showed a left upper lobe posterior apical lung adenocarcinoma mass 3.1 × 2.2 cm (stage T2a). The subcarinal left para-aortic and left hilar region were positive for metastatic disease (stage N2). Multiple left lung pleural metastases were also seen (stage M1a). He was diagnosed with clinical Stage IVA lung adenocarcinoma. Brain MRI was negative for extra or intracranial metastasis. He was started on Alectinib 600 mg PO BID (an ALK TKI) based on ALK rearrangement. The molecular profiling of the tumor cells revealed wild-type EGFR, KRAS/NRAS, and ROS1 rearrangement.
Interim PET scan after 2 months of ALK inhibitor therapy showed progression of the disease and new osseous bone metastases. Therapy was changed to Bevacizumab/Atezolizumab/Carboplatin/Paclitaxel s/p 4 cycles. A follow-up PET scan at that time showed a >20% increase and, thus, disease progression per review in the thoracic tumor board. At that time, liquid biopsy for molecular profiling revealed KRAS-p.G12C mutation and no ALK rearrangement. Liquid biopsy was obtained, and cell-free DNA was isolated from whole blood. Following DNA library preparation, next-generation sequencing (NGS) of specific gene regions was performed. In this particular case, we used a commercially available assay called guardant 360 for genomic profiling. The test detects single nucleotide variants in a targeted panel of 83 genes and selected copy number amplifications, fusions/rearrangements, and indels for a specific set of genes [5]. Yet, at that time, the therapy for such mutation was not available given the lack of FDA approval. On further disease progression, the patient was subsequently started on sotorasib in July 2021 after the FDA granted approval for KRAS-p.G12C targeted agent. The patient achieved a mixed response noted on restaging scans in September 2021. However, he continued to progress while on sotorasib and was eventually enrolled in hospice care after 4 months of sotorasib.
3. Discussion
Lung cancer is a molecularly heterogeneous disease, and insight into its biology is essential for developing effective therapies. The treatment of lung cancer has changed from the empirical use of chemotherapy to mutation-targeted therapies.
The rise of the personalized era in lung cancer prompted the evaluation of novel diagnostic tools to overcome some of the limitations of traditional tumor genotyping. The ability to obtain adequate tissue from the lung or metastatic sites may be limited due to the patient’s performance status or the risks associated with the procedures.
Liquid tumor biopsies have sparked a great deal of interest in the oncology community [6]. Liquid biopsy refers to a multitude of minimally invasive techniques that can allow real-time biomolecular characterization of the tumor through the analysis of human body fluids [7]. These somatic alterations can be determined using a variety of biomarkers, the most well studied and widely used of which are tests that analyze circulating tumor DNA (ctDNA).
While ctDNA analysis by liquid biopsy appears to be most well defined for the EGFR T790M mutation, they seem to be equally valid for other driver mutations such as ALK, ROS1, and NTRK, and the detection of resistance mutations for these driver mutations [8,9]. Analysis of ctDNA has been shown effective in detecting evidence of the T790M mutation with comparable accuracy to that of traditional tissue biopsy [10]. Liquid biopsy has also revealed KIF5B-RET fusions in patients who had previously tested negative for KIF5B-RET fusions in tissue samples [11].
The significance of liquid biopsy in identifying new mutations can determine a change of treatment. In a case report by Suppiah et al. [12], a patient’s therapy was changed to afatinib after an EGFR exon 19 deletion was identified by liquid biopsy, which was missed on a tissue biopsy. In another case report by Dietz et al. [13], rising allelic frequencies of the ALK fusion were detected by liquid biopsy, which led to a change in chemotherapy from crizotinib to ceritinib. Analysis of ctDNA for molecular characterization of acquired resistance was also shown in a case series by Bordi et al., in which ALK point mutations were identified in 5 of 20 NSCLC patients treated with crizotinib who showed disease progression. Following that, Bordi and the team reported that ALK and KRAS mutations are linked with acquired crizotinib resistance in ALK-positive NSCLC [14]. Thus, treatment decision-making is becoming even more individualized owing to liquid biopsy.
In the case presented here, the patient’s disease has been difficult to control with the current standard of care. He progressed after the 2nd line of therapy using platinum-based chemotherapy and taxane-based chemotherapy with or without antiangiogenic therapy. Pemetrexed was also utilized as the chemotherapy backbone on the treatment arm via clinical trial after he had progressed on 2nd line therapy. Then a change of therapy to sotorasib was promptly initiated after the KRASp.G12C mutation inhibitor was approved by the FDA in late May 2021.
The KRAS gene is the most frequently mutated oncogene in human cancers. It encodes a guanosine triphosphatase (GTPase) that cycles between active guanosine triphosphate (GTP)-bound and inactive guanosine diphosphate (GDP)-bound states to regulate signal transduction [15,16]. The KRAS p.G12C mutation occurs in approximately 13% of non-small cell lung cancers (NSCLCs) [17]. The glycine-to-cysteine mutation at position 12 favors the active form of the KRAS protein, resulting in a predominantly GTP-bound KRAS oncoprotein and enhanced proliferation and survival in tumor cells [18].
Sotorasib showed anticancer activity in patients with KRAS p.G12C-mutated advanced solid tumors in a phase 1 study, and particularly promising anticancer activity was observed in a subgroup of patients with non-small cell lung cancer (NSCLC) [19]. Sotorasib also showed clinical efficacy with reversible toxic effects, mainly of grade 1 or 2, in the phase 1 portion of the CodeBreaK100 trial [20].
In the NSCLC cohort of a phase 2 portion trial, an objective response was observed in 37.1% of patients, with a median duration of response of 11.1 months. The median progression-free survival was 6.8 months, and the median overall survival was 12.5 months. In addition, tumor shrinkage and disease control were observed in the majority of patients. These data provide further evidence in support of the clinical use of sotorasib in patients with KRAS p.G12C-mutated NSCLC [19].
For the future perspective, studies have shown that serial liquid biopsies of KRAS mutant NSCLC are correlated with clinical outcomes. The early assessment of NSCLC has the potential for monitoring outcomes in patients with NSCLC [21]. The study by Heitzer et al. evaluated the role of liquid biopsy in NSCLC. The results of their study suggested that liquid biopsy is helpful in cases when resistance to management is suspected, patients with discordant clinical history, and tumors with heterogeneity (intertumoral and intratumoral). They also suggested that liquid biopsy can help in situations when tumor locations are hard to biopsy and there is insufficient sampling on cytology/biopsy [22].
ALK fusion NSCLC is associated with heterogeneous clinical outcomes. A study by Wang et al. demonstrated the prognostic value of EML4-ALK fusion variants with the clinical outcomes in patients. The results of their study showed patients with variant 1 for EML4-ALK fusion are associated with equivalent overall survival (OS) and progression-free survival (PFS) with non-v1 variant patients. Patients with v3 and non-v3 had similar PFS. However, v3 had worse OS than non-v3 patients [23]. ALK/KRAS comutations are associated with resistance to ALK TKI. The outcomes with ALK and EGFR TKI are inferior in patients with either mutation alone [24]. Noordhof et al. demonstrated that KRAS mutation has no prognostic significance in treating patients with pembrolizumab when PD-L1 expression is >50% in stage IV lung adenocarcinoma. The survival was similar in patients with KRAS mutated versus KRAS wild-type in NSCLC when PD-L1 expression was >50% when pembrolizumab was used as first-line therapy. In selected patients with PD-L1 > 50%, KRAS mutations were more frequent in women in comparison to men [25].
4. Conclusions
The routine use of established liquid tumor biopsies in the management of non-small cell lung cancer should be considered in any case when the available ‘solid’ tissue does not allow for the important evaluation of the presence of a clinically validated ‘actionable’ molecular target. Our case study demonstrates the potential clinical utility of liquid biopsy for analyzing mechanisms of treatment failure and predicting future clinical outcomes and also mentions the newly approved drug sotorasib for KRAS p.G12C mutation.
Author Contributions
Conceptualization, S.S., T.L. and A.U.; literature search, S.S., T.L. and A.U.; writing—original draft preparation, S.S., T.L. and A.U.; writing—review and editing, I.A.E., S.K.K. and N.A.K.; supervision, N.A.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this case report.
Informed Consent Statement
Informed consent was obtained from the patient involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Navada S., Lai P., Schwartz A.G., Kalemkerian G.P. Temporal trends in small cell lung cancer: Analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J. Clin. Oncol. 2006;24((Suppl. S18)):7082. doi: 10.1200/jco.2006.24.18_suppl.7082. [DOI] [Google Scholar]
- 2.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M.J. Cancer Statistics, 2008. CA A Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Hackshaw A.K., Law M.R., Wald N.J. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997;315:980–988. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Cordero R., Devine W.P. Targeted Therapy and Checkpoint Immunotherapy in Lung Cancer. Surg. Pathol. Clin. 2020;13:17–33. doi: 10.1016/j.path.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Guardant360—Clinical Test—NIH Genetic Testing Registry (GTR)—NCBI. [(accessed on 6 June 2022)]; Available online: https://www.ncbi.nlm.nih.gov/gtr/tests/527948/methodology/
- 6.Alix-Panabières C., Pantel K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013;59:110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 7.Russo A., Perez D.D.M., Gunasekaran M., Scilla K., Lapidus R., Cooper B., Mehra R., Adamo V., Malapelle U., Rolfo C. Liquid biopsy tracking of lung tumor evolutions over time. Expert Rev. Mol. Diagn. 2019;19:1099–1108. doi: 10.1080/14737159.2020.1680287. [DOI] [PubMed] [Google Scholar]
- 8.Hochmair M.J., Buder A., Schwab S., Burghuber O.C., Prosch H., Hilbe W., Cseh A., Fritz R., Filipits M. Liquid-Biopsy-Based Identification of EGFR T790M Mutation-Mediated Resistance to Afatinib Treatment in Patients with Advanced EGFR Mutation-Positive NSCLC, and Subsequent Response to Osimertinib. Target. Oncol. 2019;14:75–83. doi: 10.1007/s11523-018-0612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito K., Suzuki Y., Saiki H., Sakaguchi T., Hayashi K., Nishii Y., Watanabe F., Hataji O. Utility of Liquid Biopsy by Improved PNA-LNA PCR Clamp Method for Detecting EGFR Mutation at Initial Diagnosis of Non-Small-Cell Lung Cancer: Observational Study of 190 Consecutive Cases in Clinical Practice. Clin. Lung Cancer. 2018;19:181–190. doi: 10.1016/j.cllc.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Passiglia F., Rizzo S., Di Maio M., Galvano A., Badalamenti G., Listì A., Gulotta L., Castiglia M., Fulfaro F., Bazan V., et al. Publisher Correction: The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: A systematic review and meta-analysis. Sci. Rep. 2018;8:17270. doi: 10.1038/s41598-018-35524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drilon A., Rekhtman N., Arcila M., Wang L., Ni A., Albano M., Van Voorthuysen M., Somwar R., Smith R.S., Montecalvo J., et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suppiah R., Gershenhorn B., Markman M. A Case Report Demonstrating the Potential Clinical Relevance of Liquid Tumor Biopsies in Lung Cancer. Case Rep. Oncol. 2016;9:714–717. doi: 10.1159/000450700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz S., Christopoulos P., Gu L., Volckmar A.L., Endris V., Yuan Z., Ogrodnik S.J., Zemojtel T., Heussel C.P., Schneider M.A., et al. Serial liquid biopsies for detection of treatment failure and profiling of resistance mechanisms in KLC1-ALK-rearranged lung cancer. Cold Spring Harb. Mol. Case Stud. 2019;5:a004630. doi: 10.1101/mcs.a004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordi P., Tiseo M., Rofi E., Petrini I., Restante G., Danesi R., Del Re M. Detection of ALK and KRAS Mutations in Circulating Tumor DNA of Patients With Advanced ALK-Positive NSCLC With Disease Progression During Crizotinib Treatment. Clin Lung Cancer. 2017;18:692–697. doi: 10.1016/j.cllc.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd F.A., Dancey J., Ramlau R., Mattson K., Gralla R., O’Rourke M., Levitan N., Gressot L., Vincent M., Burkes R., et al. Prospective Randomized Trial of Docetaxel Versus Best Supportive Care in Patients With Non–Small-Cell Lung Cancer Previously Treated With Platinum-Based Chemotherapy. J. Clin. Oncol. 2016;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 16.Simanshu D.K., Nissley D.V., McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostrem J.M., Peters U., Sos M.L., Wells J.A., Shokat K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skoulidis F., Li B.T., Dy G.K., Price T.J., Falchook G.S., Wolf J., Italiano A., Schuler M., Borghaei H., Barlesi F., et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong D.S., Fakih M.G., Strickler J.H., Desai J., Durm G.A., Shapiro G.I., Falchook G.S., Price T.J., Sacher A., Denlinger C.S., et al. KRAS G12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zulato E., Attili I., Pavan A., Nardo G., Del Bianco P., Boscolo Bragadin A., Verza M., Pasqualini L., Pasello G., Fassan M., et al. Early assessment of KRAS mutation in cfDNA correlates with risk of progression and death in advanced non-small-cell lung cancer. Br. J. Cancer. 2020;123:81–91. doi: 10.1038/s41416-020-0833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heitzer E., Van Den Broek D., Denis M.G., Hofman P., Hubank M., Mouliere F., Paz-Ares L., Schuuring E., Sültmann H., Vainer G., et al. Recommendations for a practical implementation of circulating tumor DNA mutation testing in metastatic non-small-cell lung cancer. ESMO Open. 2022;7:100399. doi: 10.1016/j.esmoop.2022.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S., Luo R., Shi Y., Han X. The impact of the ALK fusion variant on clinical outcomes in EML4-ALK patients with NSCLC: A systematic review and meta-analysis. Future Oncol. 2021;18:385–402. doi: 10.2217/fon-2021-0945. [DOI] [PubMed] [Google Scholar]
- 24.Schmid S., Gautschi O., Rothschild S., Mark M., Froesch P., Klingbiel D., Reichegger H., Jochum W., Diebold J., Früh M. Clinical outcome of ALK-positive non–small cell lung cancer (NSCLC) patients with De Novo EGFR or KRAS co-mutations receiving tyrosine kinase inhibitors (TKIs) J. Thorac. Oncol. 2017;12:681–688. doi: 10.1016/j.jtho.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Noordhof A.L., Damhuis R.A.M., Hendriks L.E.L., de Langen A.J., Timens W., Venmans B.J.W., van Geffen W.H. Prognostic impact of KRAS mutation status for patients with stage IV adenocarcinoma of the lung treated with first-line pembrolizumab monotherapy. Lung Cancer. 2021;155:163–169. doi: 10.1016/j.lungcan.2021.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.