Abstract
Objectives
Persistence of COVID-19 symptoms in nonhospitalized individuals beyond a few months has not been well characterized. In this longitudinal study from the Faroe Islands, we present prevalence of long COVID in mainly nonhospitalized patients who were followed up for up to 8 months.
Methods
All Faroese individuals with confirmed COVID-19 diagnosis from August to December 2020 were invited to participate in this study (n = 297). Demographic and clinical characteristics and self-reported symptoms were ascertained prospectively using a detailed questionnaire administered at repeated phone interviews.
Results
A total of 226 individuals participated at baseline (226/297, 76% participation rate), of whom 170 participants had more than 3 months follow-up. Of these, 39% (n = 67/170, 95% confidence interval [CI] 32-37%) reported persistent symptoms (median [range] 168 [93-231] days) after the acute phase and 8% (n = 14/170, 95% CI 5-13%) reported severe persistent symptoms. The most prevalent symptoms were fatigue (17%) and smell (17%) and taste (14%) dysfunction. Long COVID was more common in people reporting daily medication use (odds ratio 2.34, 95% CI 1.02-5.37).
Conclusion
Our results show that symptoms may take months to resolve, even among nonhospitalized individuals, with a mild illness in the acute phase. Continued monitoring for long COVID is needed to evaluate the added risk of a potential public health concern.
Keywords: COVID-19, Long COVID, Persisting symptoms, Longitudinal study, Faroe Islands
Introduction
Most patients recover after acute infection with SARS-CoV-2, but some experience persistent symptoms of COVID-19, even several months after infection; this is commonly referred to as long COVID. Long COVID has been defined by World Health Organization as a condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis (Soriano et al., 2022). The individuals who have long COVID report a wide range of symptoms, including fatigue, dyspnea, and cognitive dysfunction (Carfì et al., 2020; Nalbandian et al., 2021; Petersen et al., 2021). The occurrence and duration of long COVID is not fully elucidated, but studies show that attributable symptoms persist in these patients until 12 months after infection (Bellan et al., 2021; Fumagalli et al., 2022; Han et al., 2022; Kim et al., 2022; Pérez-González et al., 2022; Seeßle et al., 2022; Wynberg et al., 2021; Zhang et al., 2021). A systematic review and meta-analyses including 18 studies and 8591 individuals with a minimum of 1-year follow-up, reporting on pooled prevalence of specific post–COVID-19 symptoms (Han et al., 2022) found that fatigue/weakness, dyspnea, arthromyalgia, depression, anxiety, memory loss, concentration difficulties, and insomnia were the most prevalent symptoms at 1-year follow-up ranging from 12% to 28%. In this prospective follow-up study, we report the prevalence of long COVID up to 8 months after infection with SARS-CoV-2 in the Faroe Islands.
Methods
All individuals with COVID-19 confirmed by reverse transcription polymerase chain reaction testing of an oropharyngeal swab between August 5 and December 25, 2020, were invited to participate in this study. Disease onset, hospitalization, and symptoms during the acute phase were recorded during the acute phase by a task force consisting of medical doctors established in the Faroe Islands to monitor all Faroese individuals with COVID-19 (Kristiansen et al., 2021; Petersen et al., 2021). All diagnosed individals were asked permission to be contacted by the research team, with the purpose of assessing persistent symptoms of disease by phone interview.
At enrollment, after giving informed consent, the following data were recorded: self-assessed presence of COVID-19–related symptoms (prespecified list with 19 different symptoms) during the acute illness, rating the symptoms as mild, moderate, or severe. There were additional questions about education, employment, smoking habits, height, weight, selected chronic diseases, daily medication use, and self-assessed health. Subsequently, the same symptom-related questions were used when the participants were prospectively followed up with phone calls every second or third week for approximately 3 months after infection to evaluate any persistent symptoms. All follow-up phone calls were performed by doctors or a medical student. In addition to the regular follow-up phone calls, the subset of participants who were infected between August to October 31, 2020 (n = 140) was invited to a clinical examination (conducted from January 18 to March 24, 2021), where the same symptom-related questionnaire was used. Those affected in November and December 2020 were still followed by regular follow-up phone calls and were therefore not invited for the clinical examination. In some cases, it proved difficult to reach participants for follow-up, leading to a variable number of follow-up recordings (median 5 [range 1-6]) and duration between acute phase and last follow-up assessment (median 150 days [range 20-231 days]). Thus, a few of the participants have 1 to 3 follow-up calls. The last follow-up phone call included in this study were conducted May 15, 2021, and we used the latest follow-up for each participant. Parents answered on behalf of children below 15 years of age (0-15 years).
The Faroese Ethics Committee and Data Protection Agency have approved this study, and written informed consent was obtained from all participants.
Statistical analyses
Descriptive results are presented with mean and standard deviations for continuous variables and with number and percentages for categorical variables. We used the chi-square test and Kruskal-Wallis test to compare groups, such as individuals with and without persistent symptoms, age groups, and disease severity, as appropriate.
Univariable logistic regression was used to assess potential predictors of long COVID by including each variable separately in the models. The variables considered potential predictors were sex (female/male), age/age group (continuous/0-17, 18-34, 35-49, 50-66, 67+), smoking (ever/never), body mass index (BMI, ≤25, >25), self-reported medication use (yes/no) and chronic diseases (yes/no), days from acute illness to last follow-up (continuous), hospitalization (yes/no), and number of symptoms in the acute phase (continuous). The potential predictor variables included in the analysis were considered on the basis of previous knowledge of COVID-19 epidemiology and potential confounders (Han et al., 2022). Predictors with P-value <0.05 in univariable models were included in the multivariable model. All analyses were performed using SPSS version 25. P (two-tailed) < 0.05 was considered statistically significant.
Results
Of the 297 COVID-19 cases in the Faroes during the recruitment period, 226 participated at baseline (226/297, 76% participation rate). Of these, 170 had been followed up for a period longer than 3 months (median 168 days after onset) and were included in the analyses of long COVID (Table 1 and Supplemental Figure 1).
Table 1.
Self-reported demographic and clinical characteristics of individuals with COVID-19 from August to December 2020, with more than 3 months’ follow-up.
| Individuals participating at baseline (n = 226) | Individuals with more than 3 months of follow-up (n = 170) | Symptomatic individuals at last follow-up (n = 67) | Asymptomatic individuals at last follow-up (n = 103) | |
|---|---|---|---|---|
| Women, n (%) | 118 (52.2) | 93 (54.7) | 41 (61.2) | 52 (50.5) |
| Age (years), median (range) | 31.3 (0.3-82.6) | 35.4 (0.3-82.6) | 43.6 (9.7-78.4) | 31.1 (0.3-82.6) |
| Age groups, n (%) | ||||
| 0-17 | 31 (13.7) | 18 (10.6) | 5 (7.5) | 13 (12.6) |
| 18-34 | 90 (39.8) | 66 (38.8) | 22 (32.8) | 44 (42.7) |
| 35-49 | 46 (20.4) | 32 (18.8) | 13 (19.4) | 19 (18.4) |
| 50-66 | 40 (17.7) | 37 (21.8) | 17 (25.4) | 20 (19.4) |
| 67+ | 19 (8.4) | 17 (10.0) | 10 (14.9) | 7 (6.8) |
| Smoking statusa, n (%) | ||||
| Ever smoker | 91 (40.4) | 66 (38.8) | 34 (50.7) | 32 (31.1) |
| Never smoker | 134 (59.6) | 104 (61.2) | 34 (49.3) | 71 (68.9) |
| Self-reported daily medication useb, n (%) | 55 (25.2) | 46 (27.9) | 25 (38.5) | 21 (21.0) |
| Self-reported chronic diseasec, (%) | 72 (32.9) | 58 (35.2) | 28 (43.1) | 30 (30.0) |
| BMId, n (%) | ||||
| BMI ≤ 25 | 112 (67.9) | 81 (66.4) | 26 (56.9) | 52 (73.2) |
| BMI > 25 | 53 (32.1) | 41 (33.6) | 22 (43.1) | 19 (26.8) |
| Hospitalized during illness, n (%) | 6 (2.7) | 6 (3.5) | 5 (7.5) | 1 (1.0) |
| Number of symptoms at baselinee, median (range) | 7 (0-17) | 7 (0-17) | 7.5 (0-17) | 6 (0-17) |
| Number of follow-ups, n (%) | ||||
| 1 | 11 (4.9) | - | - | - |
| 2 | 8 (3.5) | 3 (1.8) | 1 (1.5) | 2 (1.9) |
| 3 | 10 (4.4) | 4 (2.4) | 1 (1.5) | 3 (2.9) |
| 4 | 79 (33.6) | 43 (25.3) | 17 (25.4) | 26 (25.2) |
| 5 | 117 (52.8) | 116 (68.2) | 47 (70.1) | 69 (67.0) |
| 6 | 4 (1.8) | 4 (2.4) | 1 (1.5) | 3 (2.9) |
| Follow-ups, median (range) | 5 (1-6) | 5 (2-6) | 5 (2-6) | 5 (2-6) |
| Days from onset to last follow-up, median (range) | 145.0 (20-231) | 167.5 (93-231) | 167 (95-229) | 168 (93-231) |
BMI, body mass index.
Missing data n = 1.
Missing data n = 8.
Missing data n = 7. Did a doctor ever tell you that you had: anxiety, asthma, osteoporosis, myocadiac infarct, carnitine transported defect, inflammatory bowel disease, cystic fibrosis, dementia, stroke, angina pectoris, heart insufficiency, hypertension, hypercholesterolemia, hyper or hypothyroidism, cancer, chronic obstructive pulmonary disease, arthritis, kidney disease, Parkinson's disease, psoriasis, type one and two diabetes and other.
Missing data n = 61.
Symptoms during acute phase were missing for 25 individuals.
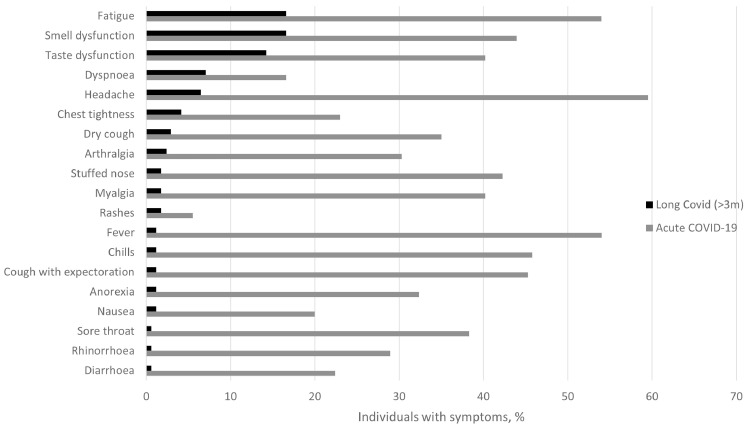
Long COVID, by our definition, was reported by 39% (n = 67/170, 95% confidence interval [CI] 32.0-47.2) of the 170 individuals with >3 months follow-up and 8% (n = 14/170, 95% CI 4.6-13.4) reported severe persistent symptoms, whereas 93% (n = 186/201, 95% CI 88.0-95.8) experienced symptoms during the acute phase (Table 2 ). The most commonly reported symptoms were fatigue (n = 28/170, 17%) and smell (n = 28/169, 17%) and taste (n = 24/169, 14%) dysfunction, whereas the most prevalent symptoms during the acute phase were headache (n = 119/200, 60%), fatigue (n = 108/200, 54%), and fever (n = 107/198, 54%) (Figure 1 ).
Table 2.
Presentation of symptoms during the acute phase and long COVID (>3 months after COVID-19) in Faroese patients with RT-PCR–confirmed COVID-19 from August to December 2020.
| Acute phasea | Long COVID (>3 m)b | |
|---|---|---|
| n = 201 | n = 170 | |
| Symptoms, n (%) | 186 (92.5) | 67 (39.4) |
| Mild symptoms, n (%)c | 178 (88.6) | 44 (25.9) |
| Moderate symptoms, n (%)c | 137 (68.2) | 39 (22.9) |
| Severe symptoms, n (%)c | 99 (49.3) | 14 (8.2) |
| Number of symptoms, n (%) | ||
| None | 15 (7.5) | 104 (61.2) |
| 1-2 | 24 (11.9) | 48 (28.2) |
| 3-5 | 39 (19.4) | 16 (9.4) |
| 6-8 | 53 (26.4) | 2 (1.2) |
| 9-12 | 56 (27.9) | 0 |
| 13+ | 14 (7.0) | 0 |
| Number of symptoms, mean (SD) | 6.7 (4.2) | 0.8 (1.3) |
| Mild symptoms | 3.4 (2.6) | 0.4 (0.8) |
| Moderate symptoms | 2.0 (2.1) | 0.3 (0.7) |
| Severe symptoms | 1.3 (1.8) | 0.1 (0.4) |
COVID-19, coronavirus disease 2019; RT-PCR, reverse transcription polymerase chain reaction.
Data on symptoms during the acute phase were missing for 25 individuals.
Median (range) days from onset to last follow-up: 167.5 (93-231).
Participants were asked to rate severity of any symptom separately, thus numbers in the column add up to more than total number of participants.
Figure 1.
Prevalence (%) of long COVID (>3 months after COVID-19) (black bar, n = 170) and symptoms during the acute phase (gray bars, n = 226) in Faroese patients with PCR-confirmed COVID-19 from August to December 2020. COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction.
The univariable logistic regression showed that long COVID was associated with age as a continuous variable (odds ratio [OR] 1.02, 95% CI 1.00-1.04), smoking status (OR 2.29, 95% CI 1.21-4.32), medication use (OR 2.34, 95% CI 1.16-4.43), and the number of symptoms in the acute phase (OR 1.11, 95% CI 1.02-1.21) (Table 3 ). In the multivariable analyses, medication use (p = 0.05) persisted significantly associated with long COVID, with similar estimates of association. The prevalence of symptoms at last follow-up was not significantly associated with age (c2 = 5.41, df = 4, P = 0.25) (Supplemental Figure 2), and mean symptoms and symptoms severity did not increase with increasing age (P > 0.05) (Supplemental Figures 3 and 4).
Table 3.
Univariable and multivariable binary logistic regression analysis of predictors of self-reported long COVID symptoms (one or more symptoms at last follow-upb).
| Univariable logistic regression |
Multivariable logistic regression |
||||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Adjusted odds ratioa (95% CI) | P-value | ||
| Sex | |||||
| Men | Ref | ||||
| Women | 1.55 (0.83-2.89) | 0.17 | |||
| Age | 1.02 (1.00-1.04) | 0.03 | 1.00 (0.98-1.02) | 0.82 | |
| Age groups | |||||
| 0-17 | Ref | ||||
| 18-34 | 1.3 (0.41-1.11) | 0.66 | |||
| 35-49 | 1.78 (0.51-6.21) | 0.37 | |||
| 50-66 | 2.21 (0.65-7.47) | 0.20 | |||
| 67+ | 3.71 (0.90-15.26) | 0.07 | |||
| Smoking status | |||||
| Never | Ref | Ref | |||
| Ever | 2.29 (1.21-4.32) | 0.01 | 1.74 (0.85-3.56) | 0.13 | |
| Self-reported chronic disease | |||||
| No | Ref | ||||
| Yes | 1.77 (0-92-3.39) | 0.09 | |||
| Hospitalized during illness | |||||
| No | Ref | ||||
| Yes | 8.23 (0.39-72.05) | 0.06 | |||
| Self-reported daily medication use | |||||
| No | Ref | Ref | |||
| Yes | 2.35 (1.18-4.70) | 0.02 | 2.34 (1.02-5.37) | 0.05 | |
| BMI groups | |||||
| ≤25 | Ref | ||||
| > 25 | 2.08 (0.97-4.46) | 0.06 | |||
| Days from onset to last follow-up | 1.00 (0.99-1.02) | 0.4 | |||
| Number of symptoms at baseline | 1.11 (1.02-1.21) | 0.02 | 1.08 (0.99-1.18) | 0.1 | |
BMI, body mass index; CI, confidence interval; Ref, reference.
Variables which give a P-value less than 0.05 in univariable analysis were added to the multivariable model.
Only including those with follow-up more than 3 months after acute illness.
Discussion
In this nationwide prospective, longitudinal COVID-19 cohort, with mainly nonhospitalized individuals of all ages, 39% reported at least one persistent symptom, and 8% reported severe persistent symptoms up to 8 months after infection. This proportion is comparable or slightly lower than in studies with up to 12 months follow-up, where they report persistent symptoms in 40 -77% of patients (Bellan et al., 2021; Boscolo-Rizzo et al., 2021; Fumagalli et al., 2022; Kim et al., 2022; Pérez-González et al., 2022; Seeßle et al., 2022; Wynberg et al., 2021; Zhang et al., 2021). We may have slightly overestimated the prevalence of long COVID because we do not have a control group to compare with, which would have enabled us to consider the potential effects that societal measures, including lockdown, might have inferred to the general population, regardless of COVID-19 status. The Faroe Islands were able to eliminate the infection, both after the first (March-May 2020) and the second (August-December) waves and have in general been successful during 2020, particularly in protecting the elderly and vulnerable groups from infection (Kristiansen et al., 2021; Strøm et al., 2021). This resulted in very low rates of hospitalizations and mortality due to COVID-19 in the acute phase and also resulted in an apparent low population prevalence of long COVID compared with other populations. Studies on long COVID that were based on hospitalized patients in general reported higher proportions of individuals with long COVID (Bellan et al., 2021; Fumagalli et al., 2022; Zhang et al., 2021). Furthermore, we were able to recruit young participants to our study to a greater extent than some of the previous studies, which may also have contributed to a relatively low prevalence of long COVID because persistent symptoms appear to be rarer in children than in adults (Behnood et al., 2022; Blomberg et al., 2021).
We found that fatigue and smell and taste dysfunction were the most prevalent symptoms in this study. The prevalence of fatigue is lower than that reported in a systematic review of 1-year follow-up studies on post–COVID-19 symptoms, including 18 reports, where they reported pooled prevalence of fatigue/weakness to be 28% (Han et al., 2022). In contrast, they found that the pooled prevalence of smell and taste disfunction was 6% (n = 9; 95% CI: 4-8; I2 = 94.1%) and 4% (n = 10; 95% CI: 3-6; I2 = 94.0%), respectively, which is lower than that in our study. However, a meta-analysis, which included 39 studies with more than 100 participants, reported taste and smell dysfunction in 14% and 15% of participants at 12 weeks or more after COVID-19, i.e. comparable to our results, whereas fatigue was reported in 25% (Michelen et al., 2021). Thus, these results may indicate that smell and taste dysfunction improve over time, whereas fatigue is more persistent.
We found that self-reported medication use was associated with risk for long COVID. However, we were not able to confirm female sex, number of symptoms or more severe symptoms in the acute phase, comorbidities, and older age as potential predictors of long COVID (Han et al., 2022; Michelen et al., 2021). However, all our results showed the same direction of association, and the lack of statistically significant association for the other tested predictors may be due to the relatively small sample size. Comparisons across studies are furthermore challenged by differing designs, settings, and follow-up time, which also may cause inconsistent findings.
The main strengths of this study include the nationwide cohort with a high participation rate and the participation of individuals of all ages, which we believe reduced the risk of selection bias. Furthermore, our prospective recording of symptoms by medical doctors have likely limited recall bias. Study limitations include the relatively small sample size, potential bias from self-reported symptoms, parents answering for their children, and lack of pre-COVID medical history. The lack of controls without COVID-19 might have led to a slight overestimation of the prevalence of long COVID. This study was performed on individuals who were infected in 2020, before the emergence of newer variants and thus, further studies are needed to gain insight into the prevalence and severity of long COVID in patients who were infected with variants of SARS-CoV-2 that emerged later.
Our results show that symptoms may take months to resolve, even among nonhospitalized individuals with mild to moderate illness in the acute phase. The health consequences of COVID-19 extend far beyond acute infection, with 8% of the participants reporting severe symptoms persisting beyond 3 months after infection onset. Further research is needed to identify risk factors and to precisely characterize long COVID, including the potential impacts of later variants and vaccination on long COVID and also to evaluate the added risk of a potential public health concern.
Conflict of interest
The authors have no competing interests to declare.
Acknowledgments
Funding source
The work was supported by the cooperation's p/f Krúnborg and Borgartún.
Ethical approval statement
Informed consent was obtained from all participants. The Faroese Ethics Committee and Data Protection Agency approved this study.
Authors’ contributions
Conceptualization: PW and MSP. Investigation: KDH, MED, BMF, and MFK. Formal Analysis: MSP. Writing the first draft: MSP. Review and editing: all authors. Funding acquisition: PW and MSP.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.06.031.
Appendix. Supplementary materials
References
- Behnood SA, Shafran R, Bennett SD, Zhang AXD, O'Mahoney LL, Stephenson TJ, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022;84:158–170. doi: 10.1016/j.jinf.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellan M, Baricich A, Patrucco F, Zeppegno P, Gramaglia C, Balbo PE, et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep. 2021;11:22666. doi: 10.1038/s41598-021-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P, Guida F, Polesel J, Marcuzzo AV, Capriotti V, D'Alessandro A, et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19) Int Forum Allergy Rhinol. 2021;11:1685–1688. doi: 10.1002/alr.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A, Bernabei R, Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli C, Zocchi C, Tassetti L, Silverii MV, Amato C, Livi L, et al. Factors associated with persistence of symptoms 1 year after COVID-19: a longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med. 2022;97:36–41. doi: 10.1016/j.ejim.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bitna-Ha Kim SW, Chang HH, Kwon KT, Bae S, et al. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect Dis. 2022;22:93. doi: 10.1186/s12879-022-07062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen MF, Heimustovu BH, Borg SÁ, Mohr TH, Gislason H, Møller LF, et al. Epidemiology and clinical course of first wave coronavirus disease cases, Faroe Islands. Emerg Infect Dis. 2021;27:749–758. doi: 10.3201/eid2703.202589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González A, Araújo-Ameijeiras A, Fernández-Villar A, Crespo M, Poveda E. Cohort COVID-19 of the Galicia Sur Health Research Institute, et al. Long COVID in hospitalized and non-hospitalized patients in a large cohort in Northwest Spain, a prospective cohort study. Sci Rep. 2022;12:3369. doi: 10.1038/s41598-022-07414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MS, Kristiansen MF, Hanusson KD, Danielsen ME, Steig BÁ, Gaini S, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2021;73:e4058–e4063. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeßle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients 1 year after coronavirus disease (COVID-19): a prospective cohort study. Clin Infect Dis. 2022;74:1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm M, Kristiansen MF, Christiansen DH, Weihe P, Petersen MS. Elimination of COVID-19 in the Faroe Islands: effectiveness of massive testing and intensive case and contact tracing. Lancet Reg Health Eur. 2021;1 doi: 10.1016/j.lanepe.2020.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynberg E, van Willigen HDG, Dijkstra M, Boyd A, Kootstra NA, van den Aardweg JG, et al. Evolution of COVID-19 symptoms during the first 12 months after illness onset. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab759. ciab759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang F, Shen Y, Zhang X, Cen Y, Wang B, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.