Abstract
Fabrication of anisotropic materials is highly desirable in designing biomaterials and tissue engineered constructs. Electrospinning has been broadly adopted due to its versatility in producing non-woven fibrous meshes with tunable fiber diameters (from 10 nanometers to 10 microns), microarchitectures, and construct geometries. A myriad of approaches have been utilized to control fiber alignment of electrospun materials to achieve complex microarchitectures, improve mechanical properties, and provide topographical cellular cues. This review provides a comparative analysis of the techniques developed to generate fiber alignment in electrospun materials. A description of the underlying mechanisms that drive fiber alignment, setup variations for each technique, and the resulting impact on the aligned microarchitecture is provided. A critical analysis of the advantages and limitations of each approach is provided to guide researchers in method selection. Finally, future perspectives of advanced electrospinning methodologies are discussed in terms of developing a scalable method with precise control of microarchitecture.
Keywords: Electrospinning, fiber alignment, anisotropy
Graphical Abstract

eTOC:
Aligned electrospinning methods provide researchers with new tools to achieve complex microarchitectures, improved mechanical properties, and topographical cellular cues. This review provides a comparative analysis of techniques to generate fiber alignment in electrospun materials with a discussion of advantages and limitations of each setup to guide method selection.
Introduction
Nanofiber and microfiber materials have grown in popularity in numerous fields including tissue engineering, bioprosthetics, drug delivery, and biosensors due to their unique properties.1 In particular, these fibrous materials provide large surface area-to-volume ratios, interconnected porosity, and diverse mechanical properties. Many methods can be used to synthesize nanofiber meshes including phase separation, template synthesis, drawing, and self-assembly.2, 3 However, these methods are time consuming, limited in fiber length, and may be unable to produce continuous fiber scaffolds.2, 4 Comparatively, electrospinning offers a distinct advantage over other methods as it allows for relatively rapid production of continuous fiber meshes, can be used with a broad range of materials, and is highly tunable. Polymers, ceramics, and composites have all been used in electrospinning.5 Ceramic nanofibers via electrospinning have been fabricated utilizing a composite solution with subsequent sintering, calcination, or chemical conversion.6 This material diversity makes electrospinning a well-suited method in the production of fibrous constructs for applications in biomedical engineering.
Electrospinning is an electrostatically driven micro- and nanofiber fabrication technique that enables the rapid and cost-effective fabrication of non-woven fibrous constructs.7–9 This fiber formation technique setup typically utilizes a syringe pump to dispense a polymer solution through a charged needle. Increasing the applied voltage on the needle allows for the electrostatic force to overcome the solution surface tension promoting the droplet to form a Taylor cone from which a jet erupts.4 As the jet travels through the air, it undergoes a bending instability resulting in jet stretching and the production of fibers with nano- to micrometer diameters that are deposited on an oppositely charged or grounded collector.10 The modulation of operational parameters, such as polymer type, viscosity, voltage, and distance to collector, allow for control over mesh properties, fiber morphology, and fiber diameter making electrospinning a highly versatile technique. The basic stationary setup of electrospinning limits the microarchitecture to random orientations.11
Control over fiber alignment is highly desirable to produce anisotropic meshes with increased complexity and performance. Due to the expanded properties and biological effects that microarchitecture and alignment methods can impart, it is highly desirable to precisely fabricate nanofibrous meshes with controlled anisotropy in numerous fields including tissue engineering,1 drug delivery,12 biosensors,13 piezoelectrics,14 and other biomaterial applications.15, 16 Significant advancements in the electrospinning process have allowed for control over mesh microarchitecture through alterations in the setup, Table 1. Fiber alignment is present in the extracellular matrix of numerous tissue structures including the heart valve,17 neural basement membrane,18 urinary bladder,19 and ligament/tendon.18 Replicating this anisotropy in synthetic materials has been shown to improve mechanical properties and direct cellular responses and migration due to the topographical cues.20–22 In addition to fiber alignment, induced polymer chain alignment and a corollary increase in crystallinity during the electrospinning process can enhance mechanical properties. As the fiber is drawn from the needle tip, shear stresses are applied to the polymer chains resulting in molecular alignment along the fiber axis.23 This polymer chain alignment and crystallinity can be further enhanced with alignment methods that increase the drawing force, such as the use of a rotating mandrel or centrifugal spinning.24 The fabrication of aligned fibers has also demonstrated utility in drug delivery by altering release performance.25 Compared to randomly oriented constructs, aligned fibers demonstrated a reduced burst release and improvements in sustained release compared to their randomly orientated counterparts.12 In sensor applications, recent work has utilized aligned fibers to facilitate the production of transistor based and piezoelectric sensors for biosensing applications.26, 27
Table 1:
A timeline for the evolution of electrospinning from its inception through current advancements with a focus on controlling anisotropy
| Year | Development |
|---|---|
| 1600 | William Gilbert describes the deformation of liquids due to an electrostatic force resulting in a cone28 |
| 1882 | Lord Rayleigh investigated the instability of electrically charged liquids and noted that when the electrical charge equilibrates the surface tension a liquid jet is ejected28 |
| 1902 | John Cooley obtains the first patent describing electrospinning29 |
| 1934-1944 | Anton Formals obtains patents for the production of fibers from electrospinning30–33 |
| 1964 | Description of the electrically induced cone formation by Sir Geoffrey Ingram Taylor forming the basis of the Taylor cone34 |
| 1971 | Imaging and description of the continuous bending instability by Baumgarten35 |
| 1977 | Electrospinning used to fabricate a biomaterial construct by Martin and Cockshott36 |
| 2003 | Gap electrospinning described by Li et al.37 |
| 2003 | Kameoka et al. utilize near-field electrospinning for the fabrication of aligned fibers38 |
| 2005 | Andrady et al. patent centrifugal electrospinning equipment39 |
| 2006 | Elmarco starts manufacturing electrospinning systems for industrial use |
| 2007 | Magnetic field-assisted electrospinning utilizing magnetic nanoparticles for aligned arrays described by Yang et al.40 |
| 2008 | Yang et al. produces aligned fiber constructs utilizing magnetic field-assisted electrospinning without magnetic nanoparticles41 |
| 2015 | Amsbio launches Mimetix® line that includes aligned Mimetix® Scaffolds |
The objective of this review is to provide a comparative analysis of the advances in electrospinning methods to control fiber alignment and generate anisotropic meshes. A description of the setup and underlying mechanism of alignment is provided for established alignment methods including gap electrospinning, rotational electrospinning, auxiliary electrode electrospinning, magnetic field-assisted electrospinning, centrifugal electrospinning, and post-drawing. Variations in setup and a critical analysis of each technique with advantages and disadvantages is then provided. We conclude with a discussion of the current challenges of electrospinning and future perspectives. Overall, this comparative analysis will provide researchers guidance on choice of technique for fabricating anisotropic electrospun constructs.
1. Rotating Collector
In the rotating collector setup, mechanical forces are used to draw the fiber as it is deposited on a spinning drum thereby inducing fiber alignment, Figure 1.42 At low speeds, the rotation of the mandrel is insufficient to influence the bending instability resulting in the deposition of random fibers. As the rotational velocity of the mandrel increases so does the mechanical drawing force with a corresponding increase in fiber alignment.43 The highest degree of alignment is obtained when the rotational velocity of the collector is balanced or slightly exceeds the ejection rate of the fiber.44 Researchers report the induction of fiber alignment occurring with rotational velocities of 3.0 m/s to 10.9 m/s;45 however, the specific velocity needed to produce the desired degree of alignment is highly dependent on the system parameters such as voltage and solution viscosity.46
Figure 1:
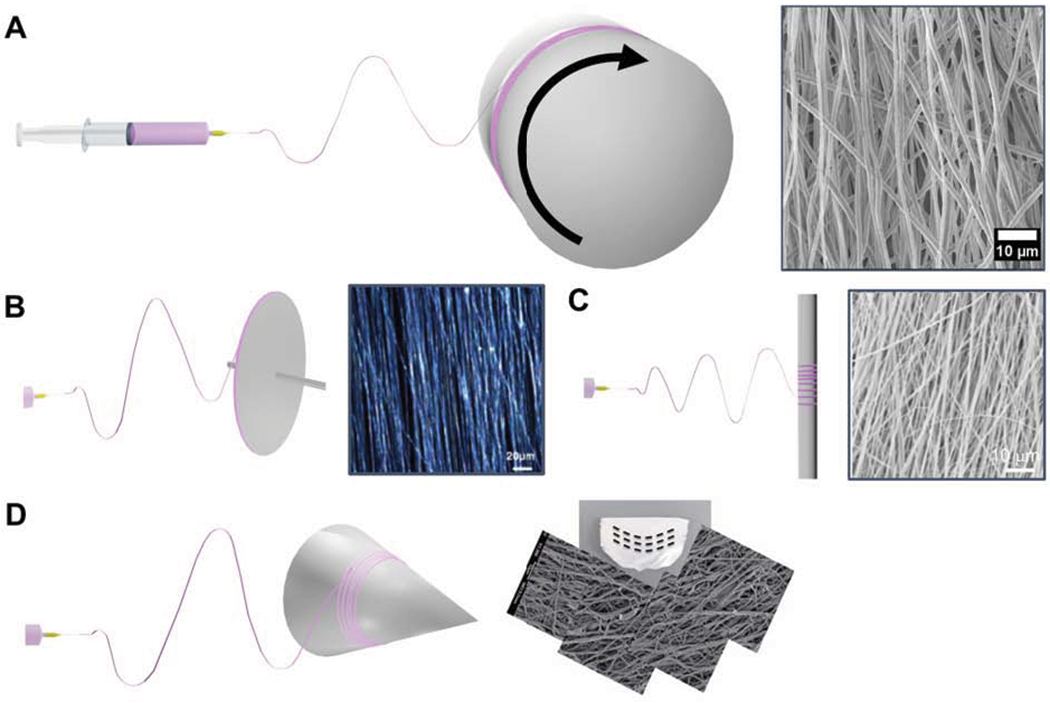
The use of a rotating collector during electrospinning to produce anisotropic constructs including mechanism and alternative setups. A) Rotational collector setup with the rotational velocity mechanically drawing the fiber resulting in fiber alignment. B) Disc collector for increasing the degree of aligned fibers. Reprinted with permission from Xu et al.36 Copyright 2004, Elsevier. C) Circumferential fiber alignment for conduits using rotating rods. Reprinted with permission from McClure et al.47 Copyright 2009, IOP Publishing. D) Conical mandrel for the fabrication of curvilinear fiber meshes with a change in the main orientation angle. Reprinted with permission from Hobson et al.48 Copyright 2015, Wiley Periodicals, Inc.
1.1. Rotating Collector Setup
A rotating collector permits variations of fiber alignment through alterations in the geometry and rotational speed of the collector, Figure 1. Courtney et al. demonstrated the impact of rotational velocity on the degree of alignment. In their study, a mandrel velocity of 2.0 m/s was required to initiate the induction of alignment demonstrating that a minimum velocity is required to fabricate anisotropic constructs with increasing rotational velocity resulting in improvements to the degree of alignment.16 The increase in fiber alignment was found to result in an increase in the anisotropic mechanical behaviors such as an increased modulus in the preferred direction.16 A rotating drum or mandrel is commonly used, although its basic form may not provide the highest degree of alignment of other methods such as gap electrospinning.49 In order to improve the alignment, researchers have utilized a rotating disc compared to a drum. The thin or knife edge of the collector results in a concentration of the electric field permitting the fabricating of highly aligned fibers; however, this severely limits the size of the mesh that can be fabricated.4, 36, 50 Further alterations in the collector’s geometry allows for the fabrication of aligned three-dimensional (3D) constructs and microarchitectural control. By utilizing a 4 mm diameter rotating rod, McClure et al. fabricated conduits for vascular grafting applications with circumferential fiber alignment by rotating the mandrel at speeds of 8000 revolutions per minute (RPM). By altering the fibers from a random to a circumferentially aligned orientation, the burst pressure of the vascular graft was significantly increased.47 Utilizing a conical mandrel, Hobson et al. was able to produce curvilinear fibers with a change in the main angle of alignment that approximated the native pulmonary valve. The curvilinear fiber alignment demonstrated a decrease in areas of localized strain compared to linearly aligned fibers that have been implicated in accelerating fatigue mechanisms.48
In addition, setup parameters such as flow rate and voltage may affect the degree of alignment when using a rotating mandrel setup. Dorati et al. investigated the impact of voltage and flow rate on alignment. 51 They reported that either low flow rates or high voltage caused a reduction in the degree of alignment; whereas, an increase in flow rate and decrease in voltage improved the degree of alignment. This indicates that there is a need to carefully balance setup parameters to achieve optimal alignment.51 These studies demonstrate the impact of collector geometry, rotational speed, and setup parameters in fabricating diverse fibrous structures toward modulating the degree of alignment and mechanical properties of the fabricated meshes.
1.2. Considerations for Utilizing Rotating Collectors
Utilizing a rotating collector allows for a tunable degree of fiber alignment with the degree of fiber alignment primarily controlled by the rotational velocity. This tunability over the degree of fiber alignment through rotational speed permits the fabrication of meshes with tunable topography and mechanical properties. For example, it has been shown that increasing the degree of fiber alignment of electrospun meshes can increase the modulus and yield stress in the preferred direction.15, 52 In addition to flat meshes, collection on a rotating drum also enables the fabrication of three-dimensional (3D) constructs such as conduits that can be used as vascular grafts. Lastly, mechanical alignment is one of the most conducive electrospinning methods for scaling toward the production of larger quantities. Simply by increasing the diameter and length of the mandrel or drum, the size of the mesh can be increased lending toward the commercialization of electrospun materials.4
Rotational alignment is limited by the degree of alignment obtainable compared to other methods and thus may not be suitable depending on the degree of alignment required.49 Although using a disc collector can increase the alignment significantly, a disc severely limits the size of the mesh that can be fabricated, potentially impacting its utility. The mechanical drawing force can also result in fiber elongation. Thus, fiber diameter has been found to decrease with an increase in the rotational velocity due to stretching of the jet and fibers with the increased rate of uptake.43, 53 Additionally, the rotational velocity needed to obtain highly aligned fibers can result in fiber necking and breakage.44 Therefore, careful consideration must be taken to ensure fiber morphology is not vastly altered during fabrication if varying rotational velocities are utilized.
The choice to utilize mechanical alignment should be based on mesh size, degree of alignment desired, and ability to modulate the angle of alignment. The developed method allows for a range of alignment based on the rotational velocity of the mandrel, with little additional equipment required. The method can aid in investigations where the control over the degree of anisotropy is necessary. However, the degree of alignment that is attainable has been noted to be less than other methods and thus may hinder the utility of the method where a high degree of alignment is desired.
2. Gap Electrospinning
Gap electrospinning was first described by Li et al. in 2003 and uses fiber collection across two parallel electrodes to achieve a high degree of fiber alignment, Figure 2.37 The alignment of fibers is driven by electrostatic forces that guide the polymer jet during fiber deposition on the collector.54 In its most basic form, electrospun fibers are collected across two parallel electrodes, either charged or grounded, that are separated by either a void or a substrate that acts as an insulator.55 Compared to a plate collector that has no preferential electric field line direction, parallel electrodes induce an alteration to the electric field where the field lines become pointed towards the electrodes.7, 56 This electric field alteration causes fibers to span the electrodes perpendicular to the electrode edge resulting in a high degree of fiber alignment.57
Figure 2:

The use of gap electrospinning to produce anisotropic constructs including mechanism and alternative setups. A) Parallel electrode setup used for gap electrospinning with visual representation of the impact on the electric field and resulting fiber alignment. B) Rotating wire drum for continuously aligned scaffolds. Reprinted with permission from Katta et al.58 Copyright 2004, American Chemical Society. C) Custom air gap wheel to permit polymer gradients along the direction of fiber alignment. Reprinted with permission from Kishan et al.59 Copyright 2017, Elsevier. D) Two-pole setup for longitudinally-aligned conduits. Reprinted with permission from Jha et al.60 Copyright 2011, Elsevier. E) Planar electrodes with gap for random to aligned fiber meshes. Reprinted with permission from Xie et al.61 Copyright 2010, Royal Society of Chemistry.
2.1. Gap Electrospinning Setups
Numerous alterations have been made to the basic setup to achieve variations of microarchitecture, Figure 2. Xie et al. utilized gap electrospinning in the fabrication of scaffolds for a bone tissue interface by sectioning the scaffold to include fibers that aligned across the gap and fibers that collected in a random orientation on the plate resulting in “aligned-to-random” gradient mesh.61 The transition allowed for a significant increase in both the modulus and ultimate tensile strength for the aligned section compared to the randomly oriented section that mimics the mechanical and microarchitectural transition at the tendon-to-bone insertion site.61 In addition to alignment gradients, the method permits the fabrication of 3D scaffolds with alignment in a non-circumferential orientation. Utilizing a two-pole setup, Jha et al. produced a 3D cylindrical scaffold with longitudinally aligned fibers. They noted that this method can produce a conduit intended to direct axon growth during nerve regeneration by bridging the gap between severed nerves in injuries such as crush injuries that make end-to-end anastomosis unfavorable.60 Gap spinning is not only amenable to fiber alignment and alignment gradients, but also macromolecular gradients along the length of alignment. Kishan et al. utilized a custom 3D printed wheel with wire struts combined with in-line blending to produce a mesh with fiber alignment in the direction of the macromolecular gradient intended for tendon-bone enthesis grafts.59 The polymer gradient from a biodegradable polyurethane (BPUR) 50 (50% hard segment) to BPUR 10 (10% hard segment) in the direction of alignment permitted a graded transition of mechanical properties.59 Although gap electrospinning permits the fabrication of highly aligned meshes and unique microarchitectural modulations, the method is limited in the size and thickness of the mesh. To increase the obtainable size of the electrospun mesh, Katta et al. used a wire drum collector to produce continuously aligned fibers thereby increasing the obtainable mesh size.58
Setup up parameters to increase the degree of alignment and gap size have also been explored to improve the capabilities of the method. Liu et al. demonstrated that increasing the gap distance between electrodes supports an increase in the horizontal component of the electric field thereby supporting an increase in the fiber alignment.57 At short distances, the horizontal component of the electric field may be too small to adequately drive the direction of the jet to span the gap in a highly oriented fashion. However, at increasingly large distances, fibers may be unable to span the gap or may break under their weight.62 Beachley and Wen determined that polycaprolactone (PCL) fibers less than 500 nm in diameter were able to span a gap up to 42.5 cm while fibers less than 1 μm were able to be collected at gap distances up to 50 cm.63 The diameter of electrospun fibers is dependent on parameters such as polymer concentration, voltage, and flow rate demonstrating the system parameter dependence on the obtainable gap size. 64–66 In addition, applied voltage has been shown to affect the degree of fiber alignment when the gap distance is maintained. Jalili et al. reported that the highest degree of alignment was observed at an applied voltage of 11 kV; whereas, voltages above and below this value demonstrated reduced alignment.67 A study by Gou et al. investigated the impact of flow rate on fiber alignment and reported that a flow rate of 50 μL/hr resulted in random fiber alignment but an increased flow rate of 100-250 μL/hr resulted in improved alignment.68 A subsequent increase to 300 μL/hr resulted in a decrease in alignment. These studies demonstrate that both flow rate and applied voltage must be optimized to achieve good fiber alignment in gap electrospinning.
2.2. Design Considerations in Gap Electrospinning
Gap electrospinning has notable advantages toward fabricating highly aligned electrospun materials. In its most basic setup, nearly no new additional equipment is needed beyond a standard electrospinning apparatus making it a cost-efficient method. In addition to the degree of alignment, diverse setup modifications enable alterations to the aligned microarchitecture to create unique alignment orientations (longitudinal verse circumferential), alignment gradients, and molecular gradients along the length of the mesh that limit other methods. These modifications provide gap electrospinning with distinct advantages over methods such as rotational, centrifugal, and post processing modifications.
There are several noteworthy limitations to the technique. As mentioned, the gap distance limits the size of the mesh that is obtainable. Although researchers have been able to obtain fibers that can span a 50 cm gap, the gap is typically limited to 10 cm or less. 5, 37 The technique is additionally limited by the achievable thickness of the mesh. Researchers have noted that residual charge builds with an increase in thickness of the meshes.57 Over time the increase in residual charge results in repulsion and a loss of fiber alignment as the mesh becomes thicker resulting in the deposition of increasingly random fiber orientations.57 Methods have been developed to overcome this limitation by layering fabricated meshes to create thicker constructs while maintaining alignment; however, it is often desirable to have a single continuous mesh.69 Lastly, fiber collection is noted to favor the electrodes resulting in reduced deposition in the aligned portion of the mesh.70 This electrode favoring can result in mesh non-uniformity if fibers are collected across multiple electrodes to form a larger mesh.
The choice to utilize gap electrospinning primarily relies on the desired size, thickness, and degree of alignment. The attainable fiber alignment is often higher than that of other methods, making it an attractive option where a high degree of orientation is desirable. However, the decision to utilize the technique ultimately needs to be grounded in the desired area and thickness over which constant alignment is necessitated as these are restricted in gap electrospinning.
3. Magnetic Field-Assisted Electrospinning
The production of highly aligned fibers via magnetic field-assisted electrospinning was first described in 2007 by Yang et al.40 The ability of polymer fibers to align in the presence of a magnetic field was observed even earlier for fibers suspended in solution.71 During magnetic field-assisted electrospinning, a magnetic field is applied to a conventional electrospinning setup using permanent magnets, Figure 3. The most common setup adheres two, nonconductive, permanent magnets onto a grounded flat plate collector.40, 41, 72–74 The magnetic field alters the whipping jet diameter due to the creation of a radial Lorenz force and increases jet stability.72, 75, 76 As the electrospinning jet approaches the collector, the jet aligns in response to the magnetic field gradient and results in the suspension of highly aligned fibers across the magnets on the collector.72 Investigation at the molecular level reveals the polymer chains are molecularly aligned as well.41
Figure 3:
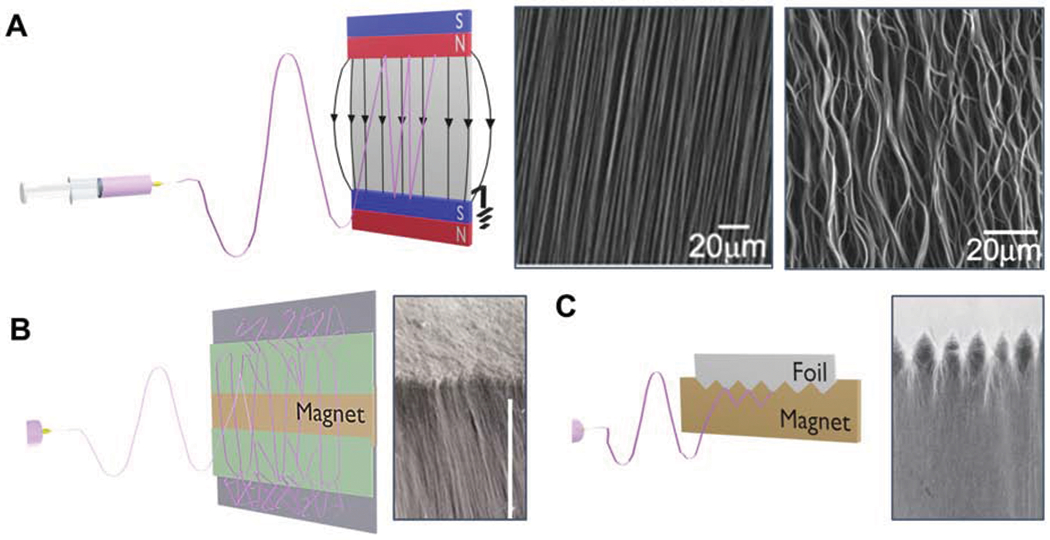
The use of magnetic electrospinning to produce anisotropic constructs with mechanism and alternative setups. A) Magnetic field-assisted electrospinning uses a conventional electrospinning setup with the addition of two parallel-positioned permanent magnets. An applied magnetic field results in highly aligned fiber deposition between the two magnets. Tortuosity may be introduced into aligned fibers by modulating the electrospinning flow rate. Reprinted with permission from Liu et al.72 Copyright 2010, Wiley-VCH GmbH, Weinheim. B) Alternate magnet configurations resulting in fiber alignment gradients from aligned to semi-aligned to random. Reprinted with permission from Tindell et al.77 Copyright 2020,Tindell et al. C) Interdigitated interfaces can be obtained through alterations in magnet configuration. Reprinted with permission from Tindell et al.77 Copyright 2020, Tindell et al.
3.1. Setups of Magnetic Field-Assisted Electrospinning
Magnetic field-assisted electrospun fiber properties are dependent on a wide range of manufacturing parameters, including: magnetic susceptibility of the electrospinning polymer, inclusion of magnetic nanoparticles in the electrospinning solution, magnetic field strength, magnet configuration, and others. Early studies by Yang et al. used magnetic field-assisted electrospinning to generate complex multilayered fiber structures by collecting and rotating the fibers during the electrospinning process40 The inclusion of magnetic nanoparticles, typically superparamagnetic Fe3O4 nanoparticles, has been observed to increase fiber alignment;73, 78 however, additives are not required to generate fiber alignment in response to a magnetic field.41, 72, 73, 79 Beyond the use of superparamagnetic nanoparticles, researchers have also added carbon nanotubes80 and silver nanowires81 to the electrospinning solution to create multi-functional composite fiber meshes. Several researchers have noted magnetic field-assisted electrospinning decreased fiber diameter compared to conventional electrospinning, especially without the use of magnetic nanoparticles.72, 73
A wide range of electrospinning parameters have also been explored to control fiber morphology and exhibit spatial control over fiber alignment. For example, Liu et al. used electrospinning polymer flow rate to produce straight and wavy fibers, where the fibers were wavier at higher flow rates, Figure 3A.72 Fiber alignment is also dependent on magnet geometry and configuration. Ajao et al. demonstrated this phenomenon using a cylindrical magnet, where fibers were highly aligned on top of the magnet and unaligned on the other sides.79 In more recent work, Tindell et al. demonstrated fine spatial control over fiber alignment using magnetic field-assisted electrospinning.77 Depending on the magnet configuration, different fiber gradients were generated, including: aligned to random, multi-directional, and other complex gradients, Figure 3B. Recent research demonstrated that this gradient of fiber alignment can also be used as topographical cellular cues to provide spatial control of cell alignment and elongation on these gradient fiber meshes. Furthermore, Tindell et al. demonstrated the ability to create a wavy interface between the aligned and random fiber regions, Figure 3C.77 These are important features common to musculoskeletal interfacial tissues and are responsible for reducing stress concentrations at the interface to two dissimilar materials.82
The alignment of fibers in magnetic field-assisted electrospinning can also be influenced by setup parameters. Mei et al. investigated the effects of voltage on alignment and noted that an increase in voltage resulted in reduced fiber alignment that was attributed to an increase in the jet instability.74 Additionally, the group noted that for magnetic nanoparticle doped solutions, an increase in the concentration of nanoparticles can be used to increase the alignment due to the increased magnetic field force.74 Similarly, as described earlier by Liu et al., the flow rate can impact morphology of the fibers resulting in wavy compared to straight fibers.72 These studies demonstrate the impact of setup parameters on the alignment of magnetic field-assisted electrospinning.
3.2. Considerations of Magnetic Field-Assisted Electrospinning
There are a number of advantages of using magnetic field-assisted electrospinning to generate aligned fiber meshes. The general setup requires minimal modification to a conventional electrospinning setup and is very low cost. Furthermore, magnets can be added in a modular fashion using a range of magnet types and configurations to spatially control fiber alignment and generate more complex structures. Magnetic field-assisted electrospinning is also compatible with a range of other electrospinning techniques (e.g. offset, in-line blending).
Several limitations to magnetic field-assisted electrospinning should be considered prior to using this technique. For magnetic field-assisted electrospinning using two parallel magnets, the aligned fibers are suspended in air between the two magnets. As a result, the fiber mesh area is limited as the fibers are unable to suspend in air over large distances. However, this is not an issue for other configurations where the fibers are collected directly on the magnet or another collector.77–79 Limited research has investigated the ability to fabricate meshes with varying thicknesses, where the mesh thickness is likely dependent on the magnetic field strength. Lastly, the ability of polymers to align in the presence of a magnetic field, especially without magnetic nanoparticles, is dependent on the material’s magnetic susceptibility.71,83 Polymers with low magnetic susceptibility may not align in the presence of a magnetic field or require the addition of magnetic nanoparticles to align.
More research is needed to elucidate the range of properties resulting from magnetic field-assisted electrospinning, including the available range of polymer selection, fiber mesh area, and fiber mesh thickness. Nonetheless, the ease and flexibility of using magnetic field-assisted electrospinning makes it a promising method for fabricating fiber meshes with spatially controlled fiber alignment.
4. Auxiliary Electrodes
Initial efforts performed by Deitzel et al. investigated the use of auxiliary electrodes to reduce the area of fiber deposition with later efforts in the field aimed at producing highly aligned electrospun meshes, Figure 4.84 During the electrospinning process, the jet undergoes a bending instability as it travels from the needle tip toward the collector due to repulsions from the charge carried by the jet.85, 86 The bending instability of the jet leads to the unpredictable deposition of fibers, thereby limiting the degree of fiber alignment obtainable in various electrospinning techniques.87, 88 As the bending instability is produced through charge repulsion, auxiliary electrodes have been employed to influence the charged jet to reduce and control the bending instability and the resulting jet path. This method is typically not a standalone method but rather an addition to methods such as rotational alignment and gap electrospinning to improve the degree of alignment and provide further control over the aligned microarchitecture.
Figure 4:

The use of auxiliary electrodes to improve fiber alignment and control over orientation during various electrospinning methods. A) Auxiliary copper ring setup and micrographs depicting a reduction in the bending instability. Reprinted with permission from Deitzel et al.84 Copyright 2001, Elsevier. B) Use of auxiliary electrodes to increase the degree of alignment in the circumferential direction of a rotating mandrel. Reprinted with permission from Arras et al.49 Copyright 2012, National Institute for Materials Science. C) Auxiliary electrodes parallel to a rotating collector to induce alignment perpendicular to the direction of rotation. Reprinted with permission from Arras et al.49 Copyright 2012, National Institute for Materials Science. D) Knife-edge bar electrodes behind the collector to focus the electric field and improve alignment. Reprinted with permission from Teo et al.89 Copyright 2005, IOP Publishing. E) Controlled orientation of aligned fibers through rotation of the auxiliary electrodes around the needle axis. Reprinted with permission from Grasl et al.90 Copyright 2013, American Institute of Physics.
4.1. Auxiliary Electrode Setups
Researchers have used a variety of setups with auxiliary electrodes to influence the electrospinning jet toward controlling the deposition area and orientation of the electrospun fibers.84, 91 Zhao et al. utilized a charged auxiliary copper ring to reduce the bending instability for improving the degree of alignment during gap electrospinning.92 A significant improvement in fiber alignment was reported with the auxiliary ring electrodes resulting in a degree of alignment above 70% as compared to ~45% with standard gap electrospinning. Importantly, it was also reported that the degree of alignment was able to be maintained above 35% after 60 minutes of spin time with the addition of the auxiliary ring electrodes as compared to less than 5% with the standard gap method.92 Similarly, combining auxiliary electrode plates with a rotating target has demonstrated an improvement in degree of fiber alignment compared to rotational spinning alone, Figure 4B 49, 93 Arras et al. demonstrated an increase from 70% of fibers aligned within 2° without the auxiliary electrodes to 90% with the electrodes.49 Following control of circumferential alignment, the group aimed to further the influence of the jet path to control the orientation of alignment on the rotating collector. By positioning the electrodes parallel to the rotating surface, the researchers were able to direct the deposition of fibers to produce fibers with alignment perpendicular to the direction of rotation, Figure 4C.49 This is a notable achievement given the difficulty in producing longitudinal fiber alignment on rotating collectors. Although typically used in between the spinneret and the collector, auxiliary electrodes can be placed behind the collector to concentrate the electric field.94 The fabrication of aligned fibers on small diameter rods is challenging due to the low linear velocity even at high RPMs. For example, Matsuda et al. found that even 3500 RPMs was insufficient to induce fiber alignment on a 3 mm mandrel95 due to the low linear velocity of 0.5 m/s.16 Teo et al. investigated the use of auxiliary electrodes behind the collector as a means to influence the collection of the electrospun fiber. It was noted that knife-edged bars behind the collector reduced the envelope size of the cone and resulted in an improvement in the degree of alignment, Figure 4D.89 Nezarati et al. utilized a similar method to fabricate polyurethane vascular grafts with circumferential fiber alignment. They found that by inducing circumferential alignment the burst pressure of the vascular graft increased.94 The use of auxiliary electrodes also permits the fabrication of more complex aligned microarchitectures. Grasl et al. demonstrated the utility of auxiliary electrodes to continuously produce aligned fibers of adjustable orientations with a rotating target by rotating the auxiliary electrodes and alternating voltages at 40 Hz during the electrospinning process, Figure 4E. The group noted that this offers an advantage as interruption in the electrospinning process may lead to layer delamination.90 Overall, the use of auxiliary electrodes has demonstrated the ability to influence the jet path in order to improve the degree of fiber alignment in base methods such as gap and rotational electrospinning.
The setup with regards to the auxiliary electrodes for obtaining highly aligned fibers also needs to be carefully considered. For electrodes between the needle and collector, Arras et al. noted that there is a maximum focusing ability of the auxiliary electrodes and once this is surpassed, a reduction in efficiency was observed due to repulsive forces.49 Zhao et al. demonstrated this effect by showing how the alignment initially increases as the voltage on the auxiliary electrode increases and then decreases after the maximum focusing voltage is exceeded.92 This demonstrates the impact of auxiliary electrode setup parameters on fiber alignment.
4.2. Considerations for Utilizing Auxiliary Electrodes
The ability to direct the electrospun jet using auxiliary electrodes has distinct advantages when combined with standalone electrospinning methods. Auxiliary electrodes have been shown to improve the degree of alignment obtainable through reducing the bending instability that is the primary source of random fiber deposition.49 The ability to apply external electrodes to a multitude of standalone methods furthers the utility of the technique. In addition to improving the degree of alignment, auxiliary electrodes have been investigated to overcome some of the limitations of standalone methods. As mentioned previously, the use of external electrodes combined with gap electrospinning was shown to improve the alignment through the thickness of the mesh overtime that is inherently lost due to the build-up of residual charge.96 Similarly, the use of external electrodes can allow for aligned microarchitectures that are not normally fabricable with the base method, such as longitudinally aligned fibers for a rotating mandrel and the fabrication of fibers with varying orientations of alignment on a continuously spun mesh. The ability to improve the degree of alignment and fabricate complex aligned microarchitectures provide auxiliary electrodes a unique advantage.
However, the use of auxiliary electrodes necessitates additional resources that can range from the addition of an electrode behind the collector to more advanced setups. Although more advanced setups allow for a high degree of control, they require additional equipment such as the ability to apply a controlled alternating current source to external electrodes.97 This increase in setup complexity and additional equipment may limit the accessibility of auxiliary electrode setups. Careful considerations need to be taken during the design of the setup, such as the applied voltage to the auxiliary electrodes, as these parameters can impact the resulting mesh.49 For example, auxiliary electrodes between the spinnerets and the collector that carry the same charge as the electrospun jet may result in reduction or prevention of deposition due to the electrostatic repulsion.49
The decision to apply auxiliary electrodes should be based on the desire to increase the degree of fiber alignment of a base method. Additionally, the ability to modulate the degree of orientation utilizing auxiliary electrodes furthers its utility. However, increasingly intricate setups and additional equipment may be required to take full advantage of the method.
5. Centrifugal Electrospinning
Centrifugal electrospinning is an approach capable of assembling aligned fiber arrays driven by electrostatic and centrifugal forces through the combination of electrospinning and Forcespinning™ technologies, Figure 5.98 This combinatory method was first reported by Liao et al. in 2010.99 The basic setup of centrifugal electrospinning includes a rotating spinneret that supplies a polymeric solution and a stationary collector, either grounded or charged, that circularly surrounds the rotating spinneret. In a typical experiment, the applied electrostatic force overcomes the surface tension of the droplets ejected from the spinneret towards the collector while the centrifugal force creates a radial force which induces fiber stretching and thinning during its flight to the collector.100–102 The centrifugal rotation provides the driving mechanism that promotes aligned deposition of fibers, acquiring moderate to high degree of alignment though operating the spinneret within the range of 2000 to 4000 RPM.98, 99, 102–104
Figure 5:

The use of centrifugal electrospinning to produce anisotropic constructs including mechanism and alternative setups. A) Basic setup of centrifugal electrospinning consisting of rotatory spinneret and stationary collector with representation of fiber formation stages and resulting fiber alignment. Reprinted with permission from Erickson et al.105 Copyright 2015, Elsevier. B) Centrifugal electrospinning using plate collectors for the improvement of alignment at lower RPM. C) Parallel wire-electrode setup for the improvement of alignment at lower RPM. D) Centrifugal electrospinning with a parallel configuration.
5.1. Centrifugal Electrospinning Setups
Modifications on centrifugal electrospinning setup are typically made to the collector with the aim to increase fiber orientation. Edmondson et al. successfully produced aligned fibers with piezoelectric properties using a parallel electrode collector consisting of grounded aluminum plates with a gap length of 1.57 cm operated at 200 RPM, Figure 5B.106 Width gap variations from 0.34 to 2.36 cm on the collector frame significantly influenced fiber alignment. Similar to gap electrospinning, there is a maximum gap width to produce highly oriented fibers, widths above this maximum results in loss of alignment due to the reduction of electric field strength that guides fiber deposition.106 In a similar setup, Erickson et al. reported a modified collector system composed of parallel wires with a 1.27 cm gap width and a rotational velocity of 108 RPM that produced highly aligned fibers with yields up to 75% compared to the plate method that had yields as low as 20%, Figure 5C.105 In general, parallel electrode collectors designed for centrifugal electrospinning allow for the production of moderate to highly aligned fibers operating at slower rotating speeds between 100 to 400 RPM in comparison to the standard collector of same diameter size, which requires 2000 to 4000 RPM to achieve comparable results. Wang et al. implemented a different collector setup consisting of multiple conductive iron wire rings disposed horizontally around the spinneret to fabricate highly aligned and multi-layer patterned meshes at a rotational speed of 70 RPM. In this study, the high degree of fiber orientation provided superior control over drug release rate.107
Other advanced settings have been performed to achieve a setup able to enhance the balance of centrifugal, electrostatic, and gravitational forces. Liu et al. proposed a parallel centrifugal electrospinning apparatus capable of producing a degree of alignment with >97% of fibers aligned within 5° at rotational speed of 420 RPM. The parallel configuration allows for the production of cross-aligned fiber meshes by performing electrospinning in two steps, Figure 5D. The production of patterned meshes is promoted when the direction of the first layer of aligned fibers is set perpendicular to the direction of needle rotation.108
In addition to the rotational speed, setup parameters such as voltage can impact the degree of fiber alignment. Liu et al. demonstrated that at a constant speed of 420 RPM, a voltage of 6 kV allowed for the highest degree of alignment; whereas, voltages of 4 kV and 8 kV displayed reduced fiber alignment.108 This result is similar to other methods and indicates that voltage plays an important role in achieving a high degree of alignment.
5.2. Design Considerations in Centrifugal Electrospinning
Centrifugal electrospinning has remarkable advantages, such as the facility to produce thicker meshes and large-scale fabrication107 without deterioration of the degree of alignment106 due to the combined alignment drivers provided by electrostatic and centrifugal force.109 The degree of alignment is tunable by controlling the speed of spinneret. In addition, the fiber length can be extended by expanding the diameter size of collector, which also increases the total length of mesh.110
Collectors composed of parallel electrodes are often recommended to achieve the highest possible degree of alignment. Nonetheless, beyond the production of aligned and cross-aligned arrays of fibers, the production of complex microarchitectures and 3D scaffolds through this method is limited by the limited tunability of collectors.107, 108 Moreover, this method combines not only high voltage, but also high rotating speed and temperature, which can present potential safety concerns.39 Furthermore, air fluctuations can affect the fiber alignment during the process, and a vacuum system is usually recommended to overcome this potential limitation.100, 111
The choice of utilizing centrifugal electrospinning relies on the intention to produce meshes with a high degree of alignment, greater thickness, or an increased production rate. However, the selection of centrifugal electrospinning requires consideration of the incorporation of additional equipment such as a parallel electrode collector to achieve the highest possible degree of orientation as well as a vacuum system.
6. Near-Field Electrospinning
Near-field electrospinning was first reported in 2003 by Kameoka et al. to produce highly aligned fibers at working distances less than 10 mm, Figure 6.38 This approach was formally named as near-field electrospinning by Sun et al. in 2006.112 In a standard electrospinning procedure, the jet exhibits a bending instability due to repulsive forces between the surface charges generating chaotic whipping that favors random fiber deposition.113 During the early stages of the fiber ejection from the Taylor cone, longitudinal stress due to the electric field force maintains jet stability and allows it to follow in straight path during a short distance prior to the onset of the instability.35, 38 By taking advantage of this early jet stability, near-field electrospinning induces direct writing of highly aligned and complex microarchitectures.
Figure 6:

Use of near-field electrospinning setups for the production of highly ordered microarchitectures. A) Near-field electrospinning with depiction of the straight jet segment. Reprinted with permission from Kameoka et al.38 Copyright 2003, IOP publishing. B) X-Y-Z platform for the fabrication of 2D and 3D aligned, curvilinear and cross-aligned microarchitectures. Reprinted with permission from Fuh et al.114 Copyright 2013, Springer Nature. C) Rotatory mandrel adapted to X-Y stage for continuously aligned fibers with control over winding angle for tubular structures. Reprinted with permission from Brown et al.115 Copyright 2012, Springer Nature.
6.1. Near-Field Electrospinning Setups
Architectural control in near-field electrospinning has largely focused on varying the collector design. The introduction of programmable X-Y-Z platforms has significantly expanded the ability to generate complex microarchitectures, Figure 6B. For example, Lee et al. utilized the translational stage to produce highly aligned and flexible piezoelectric fibers capable of producing sound-sensing elements that functioned without a power source.14 The ability to precisely fabricate fibrous constructs has significantly improved the utility of electrospinning for piezoelectric and sensor applications that demonstrate improved electrical, flexibility, and mechanical properties.116–118 In addition to highly aligned constructs, near-field electrospinning allows precise control of fiber placement to produce more complex anisotropic microarchitectures such as curvilinear and cross-aligned structures by programming stage movement.114 Di Camillio et al. fabricated cross-aligned fibers for applications in anisotropic light-emitting devices and photonic circuits.119 Fuh et al. utilized near-field electrospinning to investigate the effects of fiber density while maintaining a high degree of alignment.114 The ability to control fiber spatial distribution has been further applied to control pore size to improve cell culture applications.120 Near-field electrospinning additionally allows for precise layering of constructs that is not available in other methods. Park et al. fabricated precisely aligned layered constructs up to 80 fiber layers in thickness for use as 3D nanoelectrodes.121 Florczak et al. utilized melt electro-writing of complex 3D patterns to investigate electroactive responses. Cross-aligned microarchitectures increased the electroactive crystalline phase producing a piezoelectric response and increased its potential use in sensing applications.122
Additional modifications to the near-field setup allow the precise fabrication of 3D constructs with unique anisotropic features up to a centimeter in thickness. For example, Jungst et al. utilized near-field melt electrospinning using rotating cylindrical collectors coupled to a programmable stage. They were able to produce tubular scaffolds composed by aligned arrays of fibers through controlling rotational velocity and collector diameter. Moreover, adjusting the translating speed of X-Y programmable stage enables the production of different geometries induced by the winding angle.123 Brown et al. demonstrated that it is possible to control mechanical properties and porosity of the construct for its application in tissue engineering by altering the winding angles to produce aligned helical fibers using near-field melt electrospinning on a rotating mandrel, Figure 6C.115
The production of highly ordered constructs in near field electrospinning relies on careful tuning of setup parameters. Balancing fiber deposition and movement of the stage is of high priority in controlling fiber morphology. When the jet deposition exceeds that of the stage, instabilities can occur due to repulsions of the already deposited fibers leading to coils thereby reducing the alignment. However, at increased speeds, straight fibers can be fabricated.124 Additionally, the applied voltage can impact the fiber deposition. Bisht et al. noted that at voltages of 600 V looped fibers were observed due to the onset of bending in the polymer jet; whereas, an applied voltage of 300 V eliminated the bending instability and permitted the deposition of straight fibers.125 This demonstrates the impact of setup parameters on fiber morphology and alignment in near-near field electrospinning.
6.2. Design Considerations in Near-Field Electrospinning
The popularity of near-field electrospinning has considerably risen due to the development of small electronic devices and scaffolding biomaterials that take advantage of the enhanced architectural control of this methodology.119, 126, 127 Near-field electrospinning is an effective method to produce two-dimensional (2D) and 3D microarchitectures of highly aligned fibers and complex patterns with greater organization. In comparison to other techniques, fiber deposition control does not deteriorate with increased thickness as discussed with prior methods.121 Additionally, the reduced distance permits electrospinning at reduced voltages between 0.2 and12 kV,121, 128, 129 as compared to standard electrospinning setups that may reach 30 kV or greater.130
Although this method offers significant improvements in microarchitectural control, there are several notable limitations. The reduction of the working distance to microscale involves the reduction of the polymer flow rate, commonly <2 μL/min,38,131,132 compared to standard electrospinning setup that can be as high as 5 mL/h.133, 134 The reduction in flow rate significantly increases the fabrication time and reduces the method’s utility for large scale production.128 In order to achieve unique microarchitectures, this method requires specialized equipment such as a precise programmable stage in order to fabricate unique microarchitectures, which potentially reduces the accessibility of the method. Additionally, the elimination of the bending instability results in larger fiber diameters up to 3-6 μm135 compared to non-near-field methods that often have diameters less than 1 μm.59, 72, 128
The decision to select near-field electrospinning as a production method relies on the intention to achieve extremely accurate control of microarchitectures composed of highly aligned fibers or patterns. The ability to precisely fabricate layers and 3D constructs furthers the utility of this technique. However, the limited ability for large scale preparation needs to be considered if larger constructs are desired.
7. Post-drawing
Post-drawing as a method to convert random meshes into highly aligned constructs was first described by Zong et al. in 2003, Figure 7.136 This post-fabrication fiber alignment permits improved degree of orientation driven by combining stretching force and thermal annealing to convert poor fiber alignment into highly oriented meshes. The procedure is usually carried out on a tensile stretching apparatus customized with a heating chamber.137–141
Figure 7:

The use of post-drawing to produce anisotropic constructs and alternative setup. A) Adapted tensile stretching instrument for the post-drawing of fibers to increase alignment. Reprinted with permission from Ji et al.140 Copyright 2009, American Chemical Society. B) Automated track system that facilities the collection of aligned meshes by drawing the fibers during deposition stage. Reprinted with permission from Brennan et al.142 Copyright 2016, Elsevier.
7.1. Post-drawing Setups
Zong et al. obtained aligned poly(glycolide)-based fibers by post-drawing using a tensile stretching instrument that was customized with a heating chamber to stretch randomly oriented fibers at an extension ratio of 200% at 60 °C. The resulting mesh of highly oriented fibers induced anisotropic cell growth of cardiac myocytes.139 Ji et al. reported enhanced mechanical properties of polyacrylonitrile (PAN)-grafted multiwalled carbon nanotube composites through increasing fiber alignment. Post-drawn aligned fibers were produced by applying a tensile force of 1.25 N at 140 °C; aligned fibrous composites exhibited 200% increase in tensile strength and increased elastic modulus.140
Utilizing a manual drawing device, Afifi et al. increased the degree of alignment of poly(L-Lactide) electrospun fibers from 60% to 90%. Besides increased tensile strength and modulus, post-drawing decreased the fiber diameter size distribution.141 Hsu et al. proposed a stretching module composed of copper plates connected to a motorized reel to control speed. Through this post-drawing device, polystyrene (PS) and poly(methyl methacrylate) (PMMA) aligned arrays of fibers were obtaining from randomly oriented meshes. Processed PS and PMMA meshes presented significant improvement in degree of alignment, fiber diameter uniformity, and improved wettability as demonstrated by a reduction in the water contact angle that the researchers noted to indicate the surface properties and morphology of the fibers.143
Other advanced setups are able to increase fiber alignment by adapting the stretching apparatus.142, 144, 145 Brennan et al. implemented automated tracks to post-draw PCL fibers immediately after deposition. This device improved the degree of alignment by collecting fibers across the gap between parallel tracks, similar to gap electrospinning. Immediately after collection, the device individually elongates thousands of fibers before the complete evaporation of the solvent enhancing macromolecular alignment, improving anisotropy and mechanical properties, Figure 7B. In this study, the degree of alignment increased from 15% to 83% by increasing the draw ratio of the fibers. After post-drawing, meshes were still extensible up to 42% and showed increased toughness compared to meshes aligned through the traditional postdrawing (combining stretching and annealing).142 In a second study, the effectiveness of the automated track device was demonstrated using PAN as a model. This re-orientation process is capable of drawing individual fibers up to a ratio of 4 (from 4 cm to 16 cm), narrowing the average diameter from 708 to 289 nm, and improving mechanical properties.144 Conte et al. performed the improvement of alignment through a similar automated track system to improve orientation toward increase piezoelectric effect of poly(vinylidene fluoride-co-hexafluoropropylene). In this study, improved alignment showed significant impact on mechanical and electrical behavior as a consequence of enhanced anisotropy.145
7.2. Design Considerations in Post-Drawing
The post-drawing method has notable advantages given the improvement in fiber alignment. This method is a feasible alternative to achieve highly aligned fibers from randomly oriented meshes or to increase the degree of orientation. The ability to control the degree of alignment by regulating stretching force from 1.25 to 15 N under heating conditions allows for a significant increase in anisotropy and crystallinity.137, 138, 140 The method allows to gradually align curved fibers along the stretching direction, thereby, notable increases of mechanical properties are achieved.139–141 Post-drawing enables the reduction of diameter distribution improving uniformity of the treated fibers.137, 143 The automated tracks system has the additional advantage of reducing the individual stretching of thousands of fibers simultaneously, offering superior control in terms of uniform degree of alignment of the complete mesh.142
However, there are important disadvantages associated with the post-drawing treatment including the requirement of specialized equipment to increase the alignment of microarchitectures. Additionally, porosity of microarchitecture typically decreases about 20% after post-drawing. To overcome this limitation, biaxial stretching is often recommended.139 Although tensile strength is commonly increased, the ultimate elongation can be reduced. In addition, post-drawing can produce dissimilar degrees of alignment on different areas of the processed meshes with the degree of alignment higher in the middle of the mesh than at the upper and lower edges.143
The decision to select post-drawing to induce fiber alignment relies on the need to increase orientation, improve fiber uniformity and enhance mechanical properties. Post-drawing is an effective method to produce highly oriented fibers regardless of mesh thickness or size. However, side-effects of post-drawing can affect the microstructure by reducing porosity, producing coalescence of fibers, and resulting in spatial variations in alignment.
8. Quantification of Fiber Alignment
Methods to quantify mesh microarchitecture and anisotropy are needed to be able to rigorously compare between methodologies. Unfortunately, qualitative assessment is often the only information available for comparison across publications.146 Quantification methods of fiber morphology are critical to understanding the impact of anisotropic microarchitecture on material properties and cellular responses.
Alignment quantification is often performed utilizing 2D image analysis obtained by scanning electron microscopy. Current open access image analysis software that has been employed for quantification of alignment includes Directionality59, 147 and Orientation J148, 149 plugins for Image J. Fourier-based or Local Gradients methods can be used in Directionality to determine angular distributions.147, 150 The program provides the overall direction of alignment based on the distribution peak. Additionally, the plugin provides a dispersion and amount value. The dispersion is the standard deviation of the Gaussian fit that can be used to provide information on the angular distribution, and the amount value can provide information on the proportion of structures in a preferred direction.150 An alternative ImageJ plugin for characterizing anisotropy is Orientation J that utilizes a structure tensor method.150 Similar to Directionality, Orientation J provides a histogram for visual representation of fiber distribution. The plugin additionally provides a measure of the predominant direction of orientation and a measure of coherency. Coherency is a measure of the degree to which structures are oriented with an isotropic image returning a coherency of 0 for a completely isotropic image and a value of 1 for highly oriented structures.151 Although both Directionality and Orientation J provide a measure of the aligned structure, more in-depth characterization of the electrospun material structure may be needed. Features such as fiber density and fiber nodes that result in physical net points can also impact material properties. Quantification of these parameters permits more robust material mechanical modeling and elucidation of the role of microarchitecture in material properties.152 D’Amore et al. developed a custom Matlab algorithm that creates a skeletonized fiber network. The outputs provide information of the density of fiber intersections, connectivity distributions, and fiber orientation for a more robust structural characterization.152 Additional algorithms can provide alternatives to provide custom structural characterization relevant to a given application.
Although advanced characterization methods have also included fiber density, fiber intersection, and tortuosity, full mesh analysis is hindered by the limited depth of current 2D imaging methods. Topographical measurements of anisotropy may not be representative of the depth that may display microarchitectural heterogeneity. Depending on the intended use of the material, structural characterization through the depth may be needed in order to improve our understanding of the material properties. Recently, the use of nano- and microcomputed tomography has enabled 3D recreations of electrospun materials.153 These re-creations will allow for in-depth analysis of structural homogeneity throughout the construct depth that will lead to improvements in material modeling.
Summary and Future Perspectives
Electrospinning has rapidly grown in popularity over the last twenty years due to the unique properties of these fibrous materials and the versatility of the approach. Recent advancements in controlling fiber microarchitecture using the different setups described above have advanced the fabrication of anisotropic constructs. Although significant progress has been made, there remain several challenges that need to be addressed. First, reproducibility of fiber morphology and microarchitecture remains a concern during precise fabrication of materials. Reproducibility relies on accurately controlling not only the electrospinning parameters including concentration and electric field strength that have been shown to influence fiber diameter and morphology,11 but also control over the ambient environment. Temperature and humidity are often under reported parameters that are critical to the reproduction of electrospun meshes. Temperature has been shown to impact jet solidification and fiber diameter.154 Additionally, humidity can widely very by geographic location and time of the year. Humidity has shown to affect fiber morphology and diameter due to electrostatic discharge11 in addition to the degree of alignment.155, 156 Thus, the reporting and controlling of ambient parameters is just as vital to reproducible fiber morphology and control of anisotropy as setup parameters. Future research requires the reporting of these parameters and the adoption of climate-controlled systems, either custom or commercial,157 that will enable the control over the environment for wide spread reproducibility of results.
In addition, the ability to fabricate meshes of relevant dimensions limits electrospinning in some applications and is often further restricted when fabricating anisotropic constructs using the methods described above. Size limitations of both the area and thickness are key factors that need to be addressed in order to advance the utility of electrospinning. Rotational alignment is the most conducive to producing large scale meshes; however, the ability to obtain a high degree of alignment and produce more complex microarchitectures beyond circumferential alignment remains limited. Other methods that are able to produce a high degree of alignment, such as gap electrospinning, are limited in their ability to produce sizable materials due to loss of alignment over time57 and physical limitations of fibers.63 This loss of alignment over time due to charge repulsion is a continuing challenge that needs to be overcome in order to provide a microarchitecture homogenous material. Thus, continued research will aim to address the ability to fabricate large scale meshes with a high degree of fiber alignment and the ability to produce more complex microarchitectures that maintain fiber alignment through the thickness of the mesh.
The rate of production is also a limitation in scale up of many electrospinning methods. Electrospinning often utilizes flow rates that are lower than 5 mL/h 133, 134 with methods such as near-field that are able to produce highly controlled and ordered structures being further limited.38, 131, 132 In order to overcome throughput challenges, multiple-needle setups have been utilized. For example, commercial setups developed by Invenso inc. utilize 110 needles capable of producing electrospun mats with a production rate of 5 kg/day.124 Although the use of multiple spinnerets can improve the rate of production in electrospinning, multiple spinnerets are known to have numerous challenges including jet repulsion and lower process controllability.158 This can significantly impact control over precise fabrication of anisotropic meshes. Future advancements will need to address the limited rate of production either with reducing jet to jet repulsions or the ability to further control the electric field in order to maintain control over the process.
Overall, the development of electrospinning methods to generate anisotropic constructs with greater control over the fiber microarchitectures has expanded the utility of electrospinning. In selecting the appropriate aligned electrospinning method for a given application, researchers should consider the desired degree of alignment/complexity of anisotropic microarchitecture, thickness, and area of the mesh. Continued research is needed to provide techniques that are able to address each of these considerations, the aforementioned reproducibility, and scaling limitations to produce electrospinning techniques capable of fabricating large dimension constructs with improved microarchitecture control.
Progress and Potential.
Electrospinning is a widely employed technique for the fabrication of non-woven fibrous materials. Methods to induce electrospun fiber alignment provide researchers with new tools to guide cellular behavior, expand mechanical properties, and improve a variety of physical properties. This comparative analysis provides a detailed description of popular methods to generate fiber alignment and mesh anisotropy. A discussion of the advantages, disadvantages, and setup variations is provided for each method to guide researchers on method selection for a given application. Although significant progress has been made to advance electrospinning processes, there remains a need for continued improvements to address reproducibility, inhomogeneity of microarchitecture, and scalability of meshes in both size and production rate. Overall, the versatility and expanding control of microarchitecture has secured aligned electrospinning methods a growing role in advanced manufacturing of medical devices.
Highlights.
Fiber alignment methods in electrospinning expands material properties
Advances in method development provide improved microarchitectural control
Comparative method analysis provides selection criteria for researchers
Acknowledgments:
Funding was provided by the National Institutes of Health, grant number R01HL130436.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors declare no competing interests
References
- 1.Jin L, Xu Q, Li C, Huang J, Zhang Y, Wu D, Wang Z (2017) Engineering 3D aligned nanofibers for regulation of cell growth behavior. Macromol. Mater. Eng, 302:1600448. [Google Scholar]
- 2.Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites science and technology, 63:2223–53. [Google Scholar]
- 3.Zhang Y, Liu X, Zeng L, Zhang J, Zuo J, Zou J, Ding J, Chen X (2019) Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater, 29:1903279. [Google Scholar]
- 4.Alghoraibi I, Alomari S (2018) Different methods for nanofiber design and fabrication. In Handbook of Nanofibers, Barhoum A, Bechelany M, Makhlouf A, ed. (Springer International Publishing; ), pp. 1–46. [Google Scholar]
- 5.Teo WE, Ramakrishna S (2006) A review on electrospinning design and nanofibre assemblies. Nanotechnology, 17:R89. [DOI] [PubMed] [Google Scholar]
- 6.Li D, McCann JT, Xia Y, Marquez M (2006) Electrospinning: a simple and versatile technique for producing ceramic nanofibers and nanotubes. J. Am. Ceram. Soc, 89:1861–9. [Google Scholar]
- 7.Kishan AP, Cosgriff-Hernandez EM (2017) Recent advancements in electrospinning design for tissue engineering applications: a review. J. Biomed. Mater. Res. A, 105:2892–905. [DOI] [PubMed] [Google Scholar]
- 8.Rim NG, Shin CS, Shin H (2013) Current approaches to electrospun nanofibers for tissue engineering. Biomed. Mater, 8:014102. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimoto H, Shin Y, Terai H, Vacanti J (2003) A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials, 24:2077–82. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Meng N, Xin B (2018) Effects of jet path on electrospun polystyrene fibers. Polymers, 10:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nezarati RM, Eifert MB, Cosgriff-Hernandez E (2013) Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng., Part C, 19:810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eslamian M, Khorrami M, Yi N, Majd S, Abidian MR (2019) Electrospinning of highly aligned fibers for drug delivery applications. J. Mater. Chem. B, 7:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stafiniak A, Boratyήski B, Baranowska-Korczyc A, Szyszka A, Ramiączek-Krasowska M, Prażmowska J, Fronc K, Elbaum D, Paszkiewicz R, Ttaczata M (2011) A novel electrospun ZnO nanofibers biosensor fabrication. Sens. Actuators B Chem, 160:1413–8. [Google Scholar]
- 14.Lee TH, Chen CY, Tsai CY, Fuh YK (2018) Near-field electrospun piezoelectric fibers as sound-sensing elements. Polymers, 10:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W-J, Mauck RL, Cooper JA, Yuan X, Tuan RS (2007) Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J. Biomech, 40:1686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR (2006) Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials, 27:3631–8. [DOI] [PubMed] [Google Scholar]
- 17.Wells SM, Sacks MS (2002) Effects of fixation pressure on the biaxial mechanical behavior of porcine bioprosthetic heart valves with long-term cyclic loading. Biomaterials, 23:2389–99. [DOI] [PubMed] [Google Scholar]
- 18.Jain D, Mattiassi S, Goh EL, Yim EK (2020) Extracellular matrix and biomimetic engineering microenvironment for neuronal differentiation. Neural Regener. Res, 15:573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert TW, Wognum S, Joyce EM, Freytes DO, Sacks MS, Badylak SF (2008) Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterials, 29:4775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade RJ, Burdick JA (2012) Engineering ECM signals into biomaterials. Mater. Today, 15:45–49. [Google Scholar]
- 21.Cooper A, Bhattarai N, Zhang M (2011) Fabrication and cellular compatibility of aligned chitosan–PCL fibers for nerve tissue regeneration. Carbohydr. Polym, 85:149–56. [Google Scholar]
- 22.Bauer AL, Jackson TL, Jiang Y (2009) Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput Biol, 5:e1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ero-Phillips O, Jenkins M, Stamboulis A (2012) Tailoring crystallinity of electrospun plla fibres by control of electrospinning parameters. Polymers, 4:1331–48. [Google Scholar]
- 24.Baji A, Mai Y-W, Wong S-C, Abtahi M, Chen P (2010) Electrospinning of polymer nanofibers: effects on oriented morphology, structures and tensile properties. Compos. Sci. Technol, 70:703–18. [Google Scholar]
- 25.Feng X, Li J, Zhang X, Liu T, Ding J, Chen X (2019) Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. Journal of Controlled Release, 302:19–41. [DOI] [PubMed] [Google Scholar]
- 26.Sapountzi E, Braiek M, Chateaux J-F, Jaffrezic-Renault N, Lagarde F (2017) Recent advances in electrospun nanofiber interfaces for biosensing devices. Sensors, 17:1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C-M, Chou M-H, Zeng W-Y (2018) Piezoelectric response of aligned electrospun polyvinylidene fluoride/carbon nanotube nanofibrous membranes. Nanomaterials, 8:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LF Nascimento M, S Araujo E, R Cordeiro E, HP de Oliveira A, P de Oliveira H (2015) A literature investigation about electrospinning and nanofibers: historical trends, current status and future challenges. Recent Pat. Nanotechnol, 9:76–85. [DOI] [PubMed] [Google Scholar]
- 29.Tucker N, Stanger JJ, Staiger MP, Razzaq H, Hofman K (2012) The history of the science and technology of electrospinning from 1600 to 1995. J. Eng. Fibers Fabr, 7:155892501200702S10. [Google Scholar]
- 30.Anton F Process and apparatus for preparing artificial threads. Google Patents; 1934. [Google Scholar]
- 31.Anton F Production of artificial fibers. Google Patents; 1937. [Google Scholar]
- 32.Anton F Method and apparatus for spinning. Google Patents; 1939. [Google Scholar]
- 33.Anton F Method and apparatus for spinning. Google Patents; 1944. [Google Scholar]
- 34.Taylor GI (1964) Disintegration of water drops in an electric field. Proc. Math. Phys. Eng. Sci, 280:383–97. [Google Scholar]
- 35.Reneker DH, Yarin AL, Fong H, Koombhongse S (2000) Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys, 87:4531–47. [Google Scholar]
- 36.Xu C, Inai R, Kotaki M, Ramakrishna S (2004) Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials, 25:877–86. [DOI] [PubMed] [Google Scholar]
- 37.Cai X, Zhu P, Lu X, Liu Y, Lei T, Sun D (2017) Electrospinning of very long and highly aligned fibers. J. Mater. Sci, 52:14004–10. [Google Scholar]
- 38.Kameoka J, Orth R, Yang Y, Czaplewski D, Mathers R, Coates GW, Craighead H (2003) A scanning tip electrospinning source for deposition of oriented nanofibres. Nanotechnology, 14:1124. [Google Scholar]
- 39.Peng H, Liu Y, Ramakrishna S (2017) Recent development of centrifugal electrospinning. J. Appl. Polym. Sci, 134:44578. [Google Scholar]
- 40.Yang D, Lu B, Zhao Y, Jiang X (2007) Fabrication of aligned fibrous arrays by magnetic electrospinning. Adv. Mater, 19:3702–6. [Google Scholar]
- 41.Yang D, Zhang J, Zhang J, Nie J (2008) Aligned electrospun nanofibers induced by magnetic field. J. Appl. Polym. Sci, 110:3368–72. [Google Scholar]
- 42.Dotivala AC, Puthuveetil KP, Tang C (2019) Shear Force Fiber Spinning: Process Parameter and Polymer Solution Property Considerations. Polymers, 11:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas V, Jose MV, Chowdhury S, Sullivan JF, Dean DR, Vohra YK (2006) Mechano-morphological studies of aligned nanofibrous scaffolds of polycaprolactone fabricated by electrospinning. J. Biomater. Sci. Polym. Ed, 17:969–84. [DOI] [PubMed] [Google Scholar]
- 44.Kiselev P, Rosell-Llompart J (2012) Highly aligned electrospun nanofibers by elimination of the whipping motion. J. Appl. Polym. Sci, 125:2433–41. [Google Scholar]
- 45.Markatos D, Sarakinis A, Mavrilas D (2018) Tuning Fiber Alignment to Achieve Mechanical Anisotropy on Polymeric Electrospun Scaffolds for Cardiovascular Tissue Engineering. J Material Sci Eng, 7:2169–0022.1000466. [Google Scholar]
- 46.Tong HW, Wang M (2011) An investigation into the influence of electrospinning parameters on the diameter and alignment of poly (hydroxybutyrate-co-hydroxyvalerate) fibers. J. Appl. Polym. Sci, 120:1694–706. [Google Scholar]
- 47.McClure M, Sell S, Ayres C, Simpson D, Bowlin G (2009) Electrospinning-aligned and random polydioxanone–polycaprolactone–silk fibroin-blended scaffolds: geometry for a vascular matrix. Biomed. Mater, 4:055010. [DOI] [PubMed] [Google Scholar]
- 48.Hobson CM, Amoroso NJ, Amini R, Ungchusri E, Hong Y, D’Amore A, Sacks MS, Wagner WR (2015) Fabrication of elastomeric scaffolds with curvilinear fibrous structures for heart valve leaflet engineering. J. Biomed. Mater. Res. A, 103:3101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arras MM, Grasl C, Bergmeister H, Schima H (2012) Electrospinning of aligned fibers with adjustable orientation using auxiliary electrodes. Sci. Technol. Adv. Mater, 13:035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang HB, Mullins ME, Cregg JM, Hurtado A, Oudega M, Trombley MT, Gilbert RJ (2008) Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J. Neural Eng, 6:016001. [DOI] [PubMed] [Google Scholar]
- 51.Dorati R, Chiesa E, Pisani S, Genta I, Modena T, Bruni G, Brambilla CR, Benazzo M, Conti B (2020) The Effect of Process Parameters on Alignment of Tubular Electrospun Nanofibers for Tissue Regeneration Purposes. J. Drug Deliv. Sci. Technol:101781. [Google Scholar]
- 52.Yin Y, Xiong J (2017) Effect of the distribution of fiber orientation on the mechanical properties of silk fibroin/polycaprolactone nanofiber mats. J. Eng. Fibers Fabr, 12:155892501701200303. [Google Scholar]
- 53.El-hadi AM, Al-Jabri FY (2016) Influence of electrospinning parameters on fiber diameter and mechanical properties of poly (3-hydroxybutyrate)(PHB) and polyanilines (PANI) blends. Polymers, 8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C, Li Y, Wang P, Zhang H (2020) Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf, 19:479–502. [DOI] [PubMed] [Google Scholar]
- 55.Li D, Wang Y, Xia Y (2004) Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater, 16:361–6. [Google Scholar]
- 56.Sell S, McClure M, Ayres C, Simpson D, Bowlin G (2011) Preliminary investigation of airgap electrospun silk-fibroin-based structures for ligament analogue engineering. J. Biomater. Sci., Polym. Ed, 22:1253–73. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, Dzenis YA (2008) Analysis of the effects of the residual charge and gap size on electrospun nanofiber alignment in a gap method. Nanotechnology, 19:355307. [DOI] [PubMed] [Google Scholar]
- 58.Katta P, Alessandro M, Ramsier R, Chase G (2004) Continuous electrospinning of aligned polymer nanofibers onto a wire drum collector. Nano Lett., 4:2215–8. [Google Scholar]
- 59.Kishan AP, Robbins AB, Mohiuddin SF, Jiang M, Moreno MR, Cosgriff-Hernandez EM (2017) Fabrication of macromolecular gradients in aligned fiber scaffolds using a combination of in-line blending and air-gap electrospinning. Acta Biomater., 56:118–28. [DOI] [PubMed] [Google Scholar]
- 60.Jha BS, Colello RJ, Bowman JR, Sell SA, Lee KD, Bigbee JW, Bowlin GL, Chow WN, Mathern BE, Simpson DG (2011) Two pole air gap electrospinning: fabrication of highly aligned, three-dimensional scaffolds for nerve reconstruction. Acta Biomater., 7:203–15. [DOI] [PubMed] [Google Scholar]
- 61.Xie J, Li X, Lipner J, Manning CN, Schwartz AG, Thomopoulos S, Xia Y (2010) “Aligned-to-random” nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale, 2:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li D, Wang Y, Xia Y (2003) Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett., 3:1167–71. [Google Scholar]
- 63.Beachley V, Wen X (2009) Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C, 29:663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deitzel JM, Kleinmeyer J, Harris D, Tan NB (2001) The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer, 42:261–72. [Google Scholar]
- 65.Zargham S, Bazgir S, Tavakoli A, Rashidi AS, Damerchely R (2012) The effect of flow rate on morphology and deposition area of electrospun nylon 6 nanofiber. J. Eng. Fibers Fabr, 7:155892501200700414. [Google Scholar]
- 66.Lee JS, Choi KH, Ghim HD, Kim SS, Chun DH, Kim HY, Lyoo WS (2004) Role of molecular weight of atactic poly (vinyl alcohol)(PVA) in the structure and properties of PVA nanofabric prepared by electrospinning. J. Appl. Polym. Sci, 93:1638–46. [Google Scholar]
- 67.Jalili R, Morshed M, Ravandi SAH (2006) Fundamental parameters affecting electrospinning of PAN nanofibers as uniaxially aligned fibers. J. Appl. Polym. Sci, 101:4350–7. [Google Scholar]
- 68.Gou Y, Liu C, Lei T, Yang F, editors. Nanofiber alignment during electrospinning: effects of collector structures and governing parameters. 2014 International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO); 2014: IEEE. [Google Scholar]
- 69.Orr SB, Chainani A, Hippensteel KJ, Kishan A, Gilchrist C, Garrigues NW, Ruch DS, Guilak F, Little D (2015) Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater, 24:117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teo W-E, Inai R, Ramakrishna S (2011) Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mater, 12:012002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimura T (2003) Study on the effect of magnetic fields on polymeric materials and its application. Polym. J, 35:823–43. [Google Scholar]
- 72.Liu Y, Zhang X, Xia Y, Yang H (2010) Magnetic-field-assisted electrospinning of aligned straight and wavy polymeric nanofibers. Adv. Mater, 22:2454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li P, Liu C, Song Y, Niu X, Liu H, Fan Y (2013) Influence of Fe3O4 nanoparticles on the preparation of aligned PLGA electrospun fibers induced by magnetic field. J. Nanomater, 2013. [Google Scholar]
- 74.Mei L, Chen H, Shao Y, Wang J, Liu Y (2019) Highly aligned magnetic composite nanofibers fabricated by magnetic-field-assisted electrospinning PAN/FeCo solution. High Perform. Polym, 31:230–7. [Google Scholar]
- 75.Xu L, Wu Y, Nawaz Y (2011) Numerical study of magnetic electrospinning processes. Comput. Math. with Appl, 61:2116–9. [Google Scholar]
- 76.Bagheri H, Piri-Moghadam H, Rastegar S (2014) Magnetic and electric field assisted electrospun polyamide nanofibers for online μ-solid phase extraction and HPLC. RSC Adv, 4:52590–7. [Google Scholar]
- 77.Tindell RK, Busselle LP, Holloway JL (2020) Magnetic fields enable precise spatial control of electrospun fiber alignment for fabricating. ChemRxiv. [DOI] [PubMed] [Google Scholar]
- 78.Hu H, Jiang W, Lan F, Zeng X, Ma S, Wu Y, Gu Z (2013) Synergic effect of magnetic nanoparticles on the electrospun aligned superparamagnetic nanofibers as a potential tissue engineering scaffold. RSC Adv, 3:879–86. [Google Scholar]
- 79.Ajao JA, Abiona AA, Chigome S, Fasasi A, Osinkolu G, Maaza M (2010) Electric-magnetic field-induced aligned electrospun poly (ethylene oxide)(PEO) nanofibers. J. Mater. Sci, 45:2324–9. [Google Scholar]
- 80.Mei LY, Song P, Liu YQ (2015) Magnetic-field-assisted electrospinning highly aligned composite nanofibers containing well-aligned multiwalled carbon nanotubes. J. Appl. Polym. Sci, 132. [Google Scholar]
- 81.Zhang CL, Lv KP, Hu NY, Yu L, Ren XF, Liu SL, Yu SH (2012) Macroscopic-scale alignment of ultralong Ag nanowires in polymer nanofiber mat and their hierarchical structures by magnetic-field-assisted electrospinning. Small, 8:2936–40. [DOI] [PubMed] [Google Scholar]
- 82.Hu Y, Birman V, Deymier-Black A, Schwartz AG, Thomopoulos S, Genin GM (2015) Stochastic interdigitation as a toughening mechanism at the interface between tendon and bone. Biophys. J, 108:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garrido L (2010) Magnetic orientation of diamagnetic amorphous polymers. J. Polym. Sci., Part B: Polym. Phys, 48:1009–15. [Google Scholar]
- 84.Deitzel JM, Kleinmeyer JD, Hirvonen JK, Tan NB (2001) Controlled deposition of electrospun poly (ethylene oxide) fibers. Polymer, 42:8163–70. [Google Scholar]
- 85.Shariatpanahi SP, Bonn D, Ejtehadi MR, Zad AI (2016) Electrical bending instability in electrospinning visco-elastic solutions. J. Polym. Sci., Part B: Polym. Phys, 54:1036–42. [Google Scholar]
- 86.Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS (2005) Electrospinning of nanofibers. J. Appl. Polym. Sci, 96:557–69. [Google Scholar]
- 87.Park S, Park K, Yoon H, Son J, Min T, Kim G (2007) Apparatus for preparing electrospun nanofibers: designing an electrospinning process for nanofiber fabrication. Polym. Int, 56:1361–6. [Google Scholar]
- 88.Carnell LS, Siochi EJ, Wincheski RA, Holloway NM, Clark RL (2009) Electric field effects on fiber alignment using an auxiliary electrode during electrospinning. Scr. Mater, 60:359–61. [Google Scholar]
- 89.Teo W, Kotaki M, Mo X, Ramakrishna S (2005) Porous tubular structures with controlled fibre orientation using a modified electrospinning method. Nanotechnology, 16:918. [Google Scholar]
- 90.Grasl C, Arras MM, Stoiber M, Bergmeister H, Schima H (2013) Electrodynamic control of the nanofiber alignment during electrospinning. Appl. Phys. Lett, 102:053111. [Google Scholar]
- 91.Bellan LM, Craighead H (2006) Control of an electrospinning jet using electric focusing and jet-steering fields. J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.. Process., Meas., Phenom., 24:3179–83. [Google Scholar]
- 92.Zhao J, Liu H, Xu L (2016) Preparation and formation mechanism of highly aligned electrospun nanofibers using a modified parallel electrode method. Mater. Des, 90:1–6. [Google Scholar]
- 93.Lee H, Yoon H, Kim G (2009) Highly oriented electrospun polycaprolactone micro/nanofibers prepared by a field-controllable electrode and rotating collector. Appl. Phys. A, 97:559. [Google Scholar]
- 94.Nezarati RM, Eifert MB, Dempsey DK, Cosgriff-Hernandez E (2015) Electrospun vascular grafts with improved compliance matching to native vessels. J. Biomed. Mater. Res., Part B, 103:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsuda T, Ihara M, Inoguchi H, Kwon IK, Takamizawa K, Kidoaki S (2005) Mechano-active scaffold design of small-diameter artificial graft made of electrospun segmented polyurethane fabrics. J. Biomed. Mater. Res. A, 73:125–31. [DOI] [PubMed] [Google Scholar]
- 96.Collins G, Federici J, Imura Y, Catalani LH (2012) Charge generation, charge transport, and residual charge in the electrospinning of polymers: A review of issues and complications. J. Appl. Phys, 111:044701. [Google Scholar]
- 97.Balogh A, Cselkó R, Démuth B, Verreck G, Mensch J, Marosi G, Nagy ZK. (2015) Alternating current electrospinning for preparation of fibrous drug delivery systems. Int. J. Pharm, 495:75–80. [DOI] [PubMed] [Google Scholar]
- 98.Chang W-M, Wang C-C, Chen C-Y (2014) The combination of electrospinning and forcespinning: Effects on a viscoelastic jet and a single nanofiber. Chem. Eng. J, 244:540–51. [Google Scholar]
- 99.Liao C-C, Hou S-S, Wang C-C, Chen C-Y (2010) Electrospinning fabrication of partially crystalline bisphenol A polycarbonate nanofibers: the effects of molecular motion and conformation in solutions. Polymer, 51:2887–96. [Google Scholar]
- 100.Hashemi AR, Pishevar AR, Valipouri A, Părău EI (2018) Numerical and experimental study on the steady cone-jet mode of electro-centrifugal spinning. Phys. Fluids, 30:017103. [Google Scholar]
- 101.Hosseinian H, Valipouri A, Hosseini Ravandi SA, Alirezazadeh A (2019) Determining the effect of centrifugal and electrical forces on the jet behaviors, the nanofiber structure, and morphology. Polym. Adv. Technol, 30:941–50. [Google Scholar]
- 102.Khamforoush M, Asgari T (2015) A modified electro-centrifugal spinning method to enhance the production rate of highly aligned nanofiber. Nano, 10:1550016. [Google Scholar]
- 103.Kancheva M, Toncheva A, Manolova N, Rashkov I (2014) Advanced centrifugal electrospinning setup. Mater. Lett, 136:150–2. [Google Scholar]
- 104.Dabirian F, Ravandi SH, Pishevar A, Abuzade R (2011) A comparative study of jet formation and nanofiber alignment in electrospinning and electrocentrifugal spinning systems. J. Electrost, 69:540–6. [Google Scholar]
- 105.Erickson AE, Edmondson D, Chang F-C, Wood D, Gong A, Levengood SL, Zhang M (2015) High-throughput and high-yield fabrication of uniaxially-aligned chitosan-based nanofibers by centrifugal electrospinning. Carbohydr. Polym, 134:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edmondson D, Cooper A, Jana S, Wood D, Zhang M (2012) Centrifugal electrospinning of highly aligned polymer nanofibers over a large area. J. Mater. Chem, 22:18646–52. [Google Scholar]
- 107.Wang L, Chang M-W, Ahmad Z, Zheng H, Li J-S (2017) Mass and controlled fabrication of aligned PVP fibers for matrix type antibiotic drug delivery systems. Chem. Eng. J, 307:661–9. [Google Scholar]
- 108.Liu S-L, Long Y-Z, Zhang Z-H, Zhang H-D, Sun B, Zhang J-C, Han W-P (2013) Assembly of oriented ultrafine polymer fibers by centrifugal electrospinning. J. Nanomater, 2013. [Google Scholar]
- 109.Dabirian F, Hosseini Ravandi S, Pishevar A (2010) Investigation of parameters affecting PAN nanofiber production using electrical and centrifugal forces as a novel method. Curr. Nanosci, 6:545–52. [Google Scholar]
- 110.Khamforoush M, Asgari T, Hatami T, Dabirian F (2014) The influences of collector diameter, spinneret rotational speed, voltage, and polymer concentration on the degree of nanofibers alignment generated by electrocentrifugal spinning method: Modeling and optimization by response surface methodology. Korean J. Chem. Eng, 31:1695–706. [Google Scholar]
- 111.Valipouri A, Ravandi SH, Pishevar A (2013) A novel method for manufacturing nanofibers. Fibers Polym, 14:941–9. [Google Scholar]
- 112.Sun D, Chang C, Li S, Lin L (2006) Near-field electrospinning. Nano Lett, 6:839–42. [DOI] [PubMed] [Google Scholar]
- 113.Wang Z, Chen X, Zeng J, Liang F, Wu P, Wang H (2017) Controllable deposition distance of aligned pattern via dual-nozzle near-field electrospinning. AIP Adv., 7:035310. [Google Scholar]
- 114.Fuh YK, Chen SZ, He ZY (2013) Direct-write, highly aligned chitosan-poly (ethylene oxide) nanofiber patterns for cell morphology and spreading control. Nanoscale Res. Lett, 8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown TD, Slotosch A, Thibaudeau L, Taubenberger A, Loessner D, Vaquette C, Dalton PD, Hutmacher DW (2012) Design and fabrication of tubular scaffolds via direct writing in a melt electrospinning mode. Biointerphases, 7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Long Y-Z, Yu M, Sun B, Gu C-Z, Fan Z (2012) Recent advances in large-scale assembly of semiconducting inorganic nanowires and nanofibers for electronics, sensors and photovoltaics. Chem. Soc. Rev, 41:4560–80. [DOI] [PubMed] [Google Scholar]
- 117.Fuh Y-K, Chen S-Y, Ye J-C (2013) Massively parallel aligned microfibers-based harvester deposited via in situ, oriented poled near-field electrospinning. Appl. Phys. Lett, 103:033114. [Google Scholar]
- 118.Zhang Y, Ding J, Qi B, Tao W, Wang J, Zhao C, Peng H, Shi J (2019) Multifunctional fibers to shape future biomedical devices. Adv. Funct. Mater, 29:1902834. [Google Scholar]
- 119.Di Camillo D, Fasano V, Ruggieri F, Santucci S, Lozzi L, Camposeo A, Pisignano D (2013) Near-field electrospinning of light-emitting conjugated polymer nanofibers. Nanoscale, 5:11637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He F-L, Li D-W, He J, Liu Y-Y, Ahmad F, Liu Y-L, Deng X, Ye Y-J, Yin D-C (2018) A novel layer-structured scaffold with large pore sizes suitable for 3D cell culture prepared by near-field electrospinning. Mater. Sci. Eng. C, 86:18–27. [DOI] [PubMed] [Google Scholar]
- 121.Park Y-S, Kim J, Oh JM, Park S, Cho S, Ko H, Cho Y-K (2019) Near-Field Electrospinning for Three-Dimensional Stacked Nanoarchitectures with High Aspect Ratios. Nano Lett., 20:441–8. [DOI] [PubMed] [Google Scholar]
- 122.Florczak S, Lorson T, Zheng T, Mrlik M, Hutmacher DW, Higgins MJ, Luxenhofer R, Dalton PD (2019) Melt electrowriting of electroactive poly (vinylidene difluoride) fibers. Polym. Int, 68:735–45. [Google Scholar]
- 123.Jungst T, Muerza-Cascante ML, Brown TD, Standfest M, Hutmacher DW, Groll J, Dalton PD (2015) Melt electrospinning onto cylinders: effects of rotational velocity and collector diameter on morphology of tubular structures. Polym. Int, 64:1086–95. [Google Scholar]
- 124.Xue J, Wu T, Dai Y, Xia Y (2019) Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem. Rev, 119:5298–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bisht GS, Canton G, Mirsepassi A, Kulinsky L, Oh S, Dunn-Rankin D, Madou MJ (2011) Controlled continuous patterning of polymeric nanofibers on three-dimensional substrates using low-voltage near-field electrospinning. Nano Lett., 11:1831–7. [DOI] [PubMed] [Google Scholar]
- 126.Hu S, Li H, Su Z, Yan Y (2017) Parallel patterning of SiO2 wafer via near-field electrospinning of metallic salts and polymeric solution mixtures. Nanotechnology, 28:415301. [DOI] [PubMed] [Google Scholar]
- 127.Sugimoto R, Lee JH, Lee J-H, Jin H-E, Yoo SY, Lee S-W (2019) Bacteriophage nanofiber fabrication using near field electrospinning. RSC Adv., 9:39111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He X-X, Zheng J, Yu G-F, You M-H, Yu M, Ning X, Long Y-Z (2017) Near-field electrospinning: progress and applications. J. Phys. Chem. C, 121:8663–78. [Google Scholar]
- 129.Lei T, Lu X, Yang F (2015) Fabrication of various micro/nano structures by modified near-field electrospinning. AIP Adv., 5:041301. [Google Scholar]
- 130.Okutan N, Terzi P, Altay F (2014) Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids, 39:19–26. [Google Scholar]
- 131.King Iii WE, Gilespie Y, Gilbert K, Bowlin GL (2019) Near-field electrospinning of polydioxanone tissue regeneration templates. Engineering of Biomaterials, 22.30735893 [Google Scholar]
- 132.Zhao L, Xia Y, Hebibul R, Wang J, Zhou X, Hu Y, Li Z, Luo G, Zhao Y, Jiang Z (2018) Evaluation of width and width uniformity of near-field electrospinning printed micro and sub-micrometer lines based on optical image processing. J. Micromech. Microeng, 28:035010. [Google Scholar]
- 133.Gómez-Tejedor JA, Van Overberghe N, Rico P, Ribelles JLG (2011) Assessment of the parameters influencing the fiber characteristics of electrospun poly (ethyl methacrylate) membranes. Eur. Polym. J, 47:119–29. [Google Scholar]
- 134.Jose MV, Thomas V, Johnson KT, Dean DR, Nyairo E (2009) Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater, 5:305–15. [DOI] [PubMed] [Google Scholar]
- 135.Chang C, Limkrailassiri K, Lin L (2008) Continuous near-field electrospinning for large area deposition of orderly nanofiber patterns. Appl. Phys. Lett, 93:123111. [Google Scholar]
- 136.Zong X, Ran S, Fang D, Hsiao BS, Chu B (2003) Control of structure, morphology and property in electrospun poly (glycolide-co-lactide) non-woven membranes via post-draw treatments. Polymer, 44:4959–67. [Google Scholar]
- 137.He B, Tian L, Li J, Pan Z (2013) Effect of hot-stretching on morphology and mechanical properties of electrospun PMIA nanofibers. Fibers Polym., 14:405–8. [Google Scholar]
- 138.Wahab JA, Lee H, Wei K, Nagaishi T, Khatri Z, Behera BK, Kim K-B, Kim IS (2017) Post-electrospinning thermal treatments on poly (4-methyl-1-pentene) nanofiber membranes for improved mechanical properties. Polym. Bull, 74:5221–30. [Google Scholar]
- 139.Zong X, Bien H, Chung C-Y, Yin L, Fang D, Hsiao BS, Chu B, Entcheva E (2005) Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials, 26:5330–8. [DOI] [PubMed] [Google Scholar]
- 140.Ji J, Sui G, Yu Y, Liu Y, Lin Y, Du Z, Ryu S, Yang X (2009) Significant improvement of mechanical properties observed in highly aligned carbon-nanotube-reinforced nanofibers. J. Phys. Chem. C, 113:4779–85. [Google Scholar]
- 141.Afifi AM, Nakajima H, Yamane H, Kimura Y, Nakano S (2009) Fabrication of Aligned Poly (L-lactide) Fibers by Electrospinning and Drawing. Macromol. Mater. Eng, 294:658–65. [Google Scholar]
- 142.Brennan DA, Jao D, Siracusa MC, Wilkinson AR, Hu X, Beachley VZ (2016) Concurrent collection and post-drawing of individual electrospun polymer nanofibers to enhance macromolecular alignment and mechanical properties. Polymer, 103:243–50. [Google Scholar]
- 143.Hsu HH, Chiu YJ, Li JW, Tseng HF, Chang KC, Chen JT (2020) Alignment-Improved and Diameter-Reduced Electrospun Polymer Fibers via the Hot-Stretching Process. Macromol. Mater. Eng, 305:1900637. [Google Scholar]
- 144.Brennan DA, Shirvani K, Rhoads CD, Lofland SE, Beachley VZ (2019) Electrospinning and post-drawn processing effects on the molecular organization and mechanical properties of polyacrylonitrile (PAN) nanofibers. MRS Commun., 9:764–72. [Google Scholar]
- 145.Conte AA, Shirvani K, Hones H, Wildgoose A, Xue Y, Najjar R, Hu X, Xue W, Beachley VZ (2019) Effects of post-draw processing on the structure and functional properties of electrospun PVDF-HFP nanofibers. Polymer, 171:192–200. [Google Scholar]
- 146.Fee T, Downs C, Eberhardt A, Zhou Y, Berry J (2016) Image-based quantification of fiber alignment within electrospun tissue engineering scaffolds is related to mechanical anisotropy. J. Biomed. Mater. Res. A, 104:1680–6. [DOI] [PubMed] [Google Scholar]
- 147.Sensini A, Gotti C, Belcari J, Zucchelli A, Focarete ML, Gualandi C, Todaro I, Kao AP, Tozzi G, Cristofolini L (2019) Morphologically bioinspired hierarchical nylon 6, 6 electrospun assembly recreating the structure and performance of tendons and ligaments. Med. Eng. Phys, 71:79–90. [DOI] [PubMed] [Google Scholar]
- 148.Pisani S, Genta I, Dorati R, Kavatzikidou P, Angelaki D, Manousaki A, Karali K, Ranella A, Stratakis E, Conti B (2020) Biocompatible polymeric electrospun matrices: Micro–nanotopography effect on cell behavior. J. Appl. Polym. Sci:49223. [Google Scholar]
- 149.Vatankhah E, Semnani D, Prabhakaran MP, Tadayon M, Razavi S, Ramakrishna S (2014) Artificial neural network for modeling the elastic modulus of electrospun polycaprolactone/gelatin scaffolds. Acta Biomater., 10:709–21. [DOI] [PubMed] [Google Scholar]
- 150.Clemons T, Bradshaw M, Toshniwal P, Chaudhari N, Stevenson A, Lynch J, Fear M, Wood F, Iyer KS (2018) Coherency image analysis to quantify collagen architecture: implications in scar assessment. RSC Adv., 8:9661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rezakhaniha R, Agianniotis A, Schrauwen JTC, Griffa A, Sage D, Bouten C.v., Van De Vosse F, Unser M, Stergiopulos N (2012) Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol, 11:461–73. [DOI] [PubMed] [Google Scholar]
- 152.D’Amore A, Stella JA, Wagner WR, Sacks MS (2010) Characterization of the complete fiber network topology of planar fibrous tissues and scaffolds. Biomaterials, 31:5345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jervis R, Kok MD, Montagut J, Gostick JT, Brett DJ, Shearing PR (2018) X-ray Nano Computed Tomography of Electrospun Fibrous Mats as Flow Battery Electrodes. Energy Technol., 6:2488–500. [Google Scholar]
- 154.Islam MS, Ang BC, Andriyana A, Afifi AM (2019) A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci, 1:1248. [Google Scholar]
- 155.Barua B, Saha MC (2018) Influence of humidity, temperature, and annealing on microstructure and tensile properties of electrospun polyacrylonitrile nanofibers. Polym. Eng. Sci, 58:998–1009. [Google Scholar]
- 156.Putti M, Simonet M, Solberg R, Peters GW (2015) Electrospinning poly (ε-caprolactone) under controlled environmental conditions: Influence on fiber morphology and orientation. Polymer, 63:189–95. [Google Scholar]
- 157.Persano L, Camposeo A, Tekmen C, Pisignano D (2013) Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol. Mater. Eng, 298:504–20. [Google Scholar]
- 158.SalehHudin HS, Mohamad EN, Mahadi WNL, Muhammad Afifi A (2018) Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Processes, 33:479–98. [Google Scholar]


