Abstract
With continuously increasing living standards and health requirements of consumers, meat quality is becoming an important consideration while buying meat products. To date, no genome-wide association study (GWAS) for copy number variants (CNVs) and single nucleotide polymorphisms (SNPs) has been conducted to reveal the genetic effects on meat quality in ducks. This study analyzed the phenotypic correlation and heritability of fat, water, collagen, and protein content of duck breast muscle. To identify the candidate variants for meat quality, we performed a GWAS using 273 ducks from an F2 population. The results of the SNP GWAS showed that the BARHL2, COPS7B, and CCDC50 genes were associated with fat content; BLM, WDR76, and EOMES with water content; CAMTA1, FGD5, GRM7, and RAPGEF5 with collagen production; and RIMS2, HNRNPU, and SPTBN1 with protein content. Additionally, 3, 7, 1, and 3 CNVs were associated with fat, water, collagen, and protein content, respectively, in duck breast muscle. The genes identified in this study can serve as markers for meat quality. Furthermore, our findings may help devise effective breeding plans and selection strategies to improve meat quality.
Keywords: duck, meat quality, genome-wide associate study, single nucleotide polymorphism, copy number variants
1. Introduction
Over the last several decades, meat consumption has increased substantially worldwide. Customers’ expectations of meat safety and quality are increasing [1,2,3,4]. Meat quality is a complex trait that has several defining aspects, such as collagen, water, protein, and fat content, which play critical roles in consumer acceptance and product pricing [5,6,7]. Several studies on the meat quality of ducks, especially the fat, protein, collagen, and water content of breast muscle, have been conducted [8,9]. Numerous studies have shown that the fat content of meat is positively correlated with the tenderness of the meat, and increased fat content may reduce the physical strength of adipose tissue and connective tissue, resulting in improved meat sensory quality [10]. Fat is an important flavor precursor, and adipose tissue undergoes an oxidation reaction when heated to produce aromatic substances, which are the main source of duck meat flavor. The tenderizing ability of muscle depends on the interaction between muscle protein molecules. It is generally believed that muscles with high collagen content have high tenderness, and muscles with low collagen content have low tenderness. The main factor that affects muscle tenderness is thermally dissolved collagen. Meat quality, similar to other traits, is co-regulated by several factors related to environmental conditions and genetic background [11,12,13]. However, almost all meat quality traits have been shown to be potentially heritable [14,15]. The measurement of meat quality requires a lot of time and money, and so the improvement of breeding efficiency is lower. Therefore, using genomic variation (such as SNP and CNV, etc.) to predict the breeding value of meat quality is a promising strategy to improve meat quality. However, it is unclear which genome-wide SNPs and CNVs significantly affect duck meat quality.
The availability of genome-wide single nucleotide polymorphism (SNP) and copy number variant (CNV) panels has made genomic prediction possible for a broad range of livestock [16,17]. In the past several years, genome-wide association studies (GWAS) have identified an increasing number of potential markers associated with phenotypes of interest, including weight, plumage color [18,19,20,21], disease [22,23,24], and other economically important traits [25,26], thereby contributing to an extensive understanding of traits biology and providing a list of positional candidate genes for quantitative traits in poultry and livestock. Meanwhile, the GWAS has also revealed candidate sites and genes for meat quality, which is a critical economic trait for livestock and poultry species. In addition, functional analyses combined with GWASs have identified that a cluster of interacting genes and pathways are co-regulated with meat quality traits [27]. Previous studies have reported that ryanodine receptor 1 (RYR1), phosphorylase kinase catalytic subunit γ 1 (PHKG1), protein kinase AMP-activated non-catalytic subunit γ 3 (PRKAG3), melanocortin 4 receptor (MC4R), and insulin-like growth factor 2 (IGF2) are associated with meat quality in livestock [28,29,30,31]. Recently, epigenetics has also identified polymorphisms in seven epigenetic-related genes associated with meat quality in beef populations [32]. In addition, TYRO3 protein tyrosine kinase (TYRO3), microsomal Glutathione S-Transferase 1 (MGST1), kinesin family member 2A (KIF2A), and nucleoside-Triphosphatase cancer-related (NTPCR) were shown to be related to the intramuscular fat content in the breast muscle (IMFBr). Furthermore, the expression of the other genes examined (collagen type XII α 1 chain (COL12A1), vacuolar protein sorting 4 homolog B (VPS4B), BR serine/threonine kinase 2 (BRSK2), and forkhead box C1 (FOXC1)) was significantly lower in chickens with high abdominal fat weight (AbFW) and as a percentage of eviscerated weight (AbFP), but the magnitude of the difference was less [33].
In the present study, to identify the candidate loci affecting meat quality, based on fat, protein, collagen, and water content in breast muscle, 273 duck offspring were used for GWAS analysis. The results will allow farmers to gain insights into the underlying biological processes and identify potential genes and markers that might be utilized in breeding programs to increase the meat quality of ducks.
2. Materials and Methods
2.1. Ethics Statement
All blood samples were collected and meat quality traits were measured strictly in accordance with the guidelines proposed by the China Council on Animal Care and Ministry of Agriculture of the People’s Republic of China. The study was approved by the Institutional Animal Care and Use Committee and the School of Animal Experiments Ethics Committee (license number: SYXK (Su) IACUC 2012-0029), Yangzhou University.
2.2. Breeding Experiments or Breeding for Sample Size
The experimental population consisted of 273 F2 segregating ducks. To construct the F2 segregating population, the F1 generation was produced from orthogonal crosses between the Chinese Crested (CC) ducks and Cherry Valley (CV) ducks. In the orthogonal experiment, 86 CC and 13 CV ducks were randomly selected and divided into 7 families. The number of offspring in the F1 generation exceeded 500 individuals. Moreover, there were no ducks with crest traits in the F1 generation. The ratio of male to female ducks was consistent. The F2 generation was produced from the natural mating of F1 hybrids, and mating was internally limited to orthogonal experiments. In building the families, we considered and complied with the following principles: (1) the male-to-female ratio was 1:3, (2) males and females in the same family were not from the same nest, and (3) female ducks within a family were not half-siblings. The F2 generation was composed of almost 2000 ducks that displayed segregation of various genetic characteristics, including meat quality-related traits. After the ducklings hatched, they were weighed on a weekly basis. Three weeks after hatching, all members of the F2 generation were moved from the duckling house to a designed individual shed and raised to the age of six weeks. We performed a slaughter experiment with more than 800 ducks and measured a series of traits, including meat quality. In all families, we found that the color trait followed the recessive inheritance of Mendel’s law of segregation. To identify candidate loci for meat quality-related phenotypes, we randomly selected 21 ducks per lineage (273 in total) from 800 individuals that had been measured for a series of traits for genome resequencing.
2.3. Meat Quality Trait Detection and Correlation Analysis
All meat quality measurements were taken on the left side of the carcasses. To increase the accuracy of meat quality detection results, we first removed the fascia from the breast meat and cut it into small pieces. Thereafter, we put the small piece of meat into a high-speed universal crusher to make a puree. Finally, FoodScan™ (FOSS, Hillerød, Denmark) was used to measure the crude protein content, crude fat content, water content, and collagen content [34]. To determine the correlation of all meat qualities, the R/corrplot package was used for the phenotypic correlation visualization [35].
2.4. Variant Calling and Genotyping
For GWAS analysis, 273 samples were aligned to the genome of mallard (assembly number: GCA_008746955.1) using BWA (settings: mem-t 4-k 32-M-R) [36]. The sample alignment rates were 96–98%. The average coverage depth for the reference genome (excluding the N region) was between 9.34–15.74X, and the 4X base coverage (≥4) was greater than 82.64%. Variant calling was performed for all samples using the Genome Analysis Toolkit (GATK) v 3.7, with the UnifiedGenotyper method [37]. The SNPs were filtered using the Perl script. After filtering, the GWAS sample retained 12.6 Mb of SNPs (filter conditions: only two alleles; single-sample quality = 5; single-sample depth = 5–75; total-sample quality = 20; total-sample depth = 273–1,000,000; maximum missing rate of individuals and site = 0.1; and a minor allele frequency (MAF) = 0.05).
2.5. CNV Calling
CNVnator merged the BAM files with a bin size of 200 bp [38]. After CNV calling, quality control was performed on the raw CNV data for each duck. The parameters of filtering were a p-value < 0.01 (pval1 calculated using t-test statistics), size > 1 kb, and q0 < 0.5. A p-value <0.01 indicated that the region between two calls was not the same CNV, and q0 was the fraction of mapped reads with zero quality. In addition, CNVs that overlapped with gaps or unplaced chromosomes (chrUn in the crested duck genome) were removed.
2.6. Population Structure and SNP and CNV Distribution
PLINK was used to identify the underlying population structure and principal component analysis (PCA) was performed within and across all 273 samples. R/ggplot2 was used for PCA visualization. The R/CMplot package was used to visualize SNP and CNV distributions on chromosomes [39].
2.7. Genome-Wide Association Analysis
Association analyses were conducted using a linear mixed model (LMM) to correct for the population structure and kinship matrix using EMMAX [40]. Briefly, the general mixed model used in this approach can be specified as: , where y represents an n × 1 vector of phenotypes, X is an n × q matrix of fixed effects, β is a q × 1 vector representing the coefficients of fixed effects, and Z is an n × t matrix relating the random effect to the phenotypes of interest.
Manhattan plots illustrating the GWAS results were produced using the R/qqman package in R [41]. The significance threshold (α) of the association of SNP and CNV markers with different traits was calculated using the Bonferroni correction.
3. Results
3.1. Meat Quality Trait Correlation Analysis
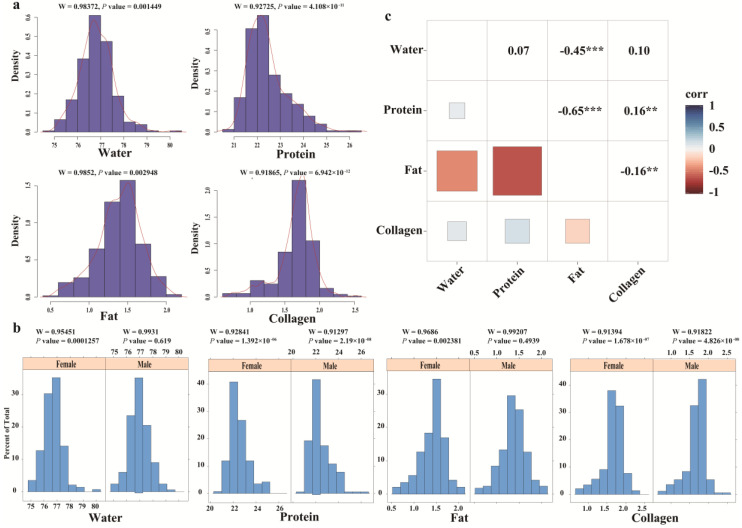
The descriptive statistics for all meat quality traits in the F2 population of ducks used in the present study are shown in Table 1. Based on descriptive statistics analysis, the maximum and minimum values of fat content were 26.42% and 20.52%, respectively. The coefficient of variation (CV) of protein (21%) was higher than that of collagen (16%). To identify candidate variants for all meat quality traits, we first performed a normal test on all meat quality traits. The results show that all meat quality traits are normally distributed (Figure 1a). Moreover, through the normal distribution test of the meat quality traits of male ducks and female ducks, it was found that they all followed a normal distribution (Figure 1b). Through the correlation between the traits, it was found that there was a very significant negative correlation between fat and protein and water, respectively (Figure 1c). In addition, the phenotypic heritability (h2) of water, fat, protein, and collagen content was 0.2454, 0.5310, 0.401, and 0.42, respectively.
Table 1.
Descriptive statistics of meat quality trait a.
| Trait | Mean (g) | SD (g) | CV | Min (g) | Max (g) |
|---|---|---|---|---|---|
| Water | 76.83 | 0.76 | 0.01 | 74.98 | 80.12 |
| Fat | 22.47 | 0.9 | 0.04 | 20.52 | 26.42 |
| Protein | 1.39 | 0.29 | 0.21 | 0.58 | 2.07 |
| Collagen | 1.65 | 0.27 | 0.16 | 0.73 | 2.52 |
a n = 273.
Figure 1.
The phenotype of meat quality statistics analysis. (a) Frequency distribution of the adjusted phenotypes of meat quality; (b) frequency distribution of the adjusted phenotypes of male and female meat quality; (c) pairwise Pearson correlation coefficients for the four meat quality traits; ‘**’ represents the p value less than 0.01 (p value ≤ 0.01); ‘***’ represents the p value less than 0.001 (p value ≤ 0.001).
3.2. SNP Disequilibrium and Population Structure
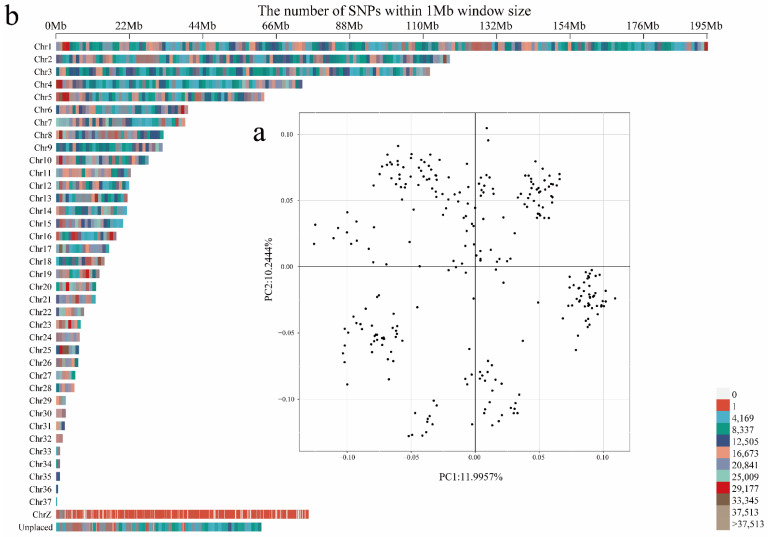
After SNP quality control, 12,661,915 SNPs were distributed in across 39 chromosomes. Chromosome 1 showed the highest number of SNPs, whereas chromosome 25 contained the fewest SNPs. The MAF for all SNPs was re-calculated after quality control; only a MAF of >5% was retained. The PCA of the population was used to investigate the genetic structure and relationship. The PCA results showed that the first three PCs explained 11.99%, 10.24%, and 6.11% of the total genetic variation, respectively (Figure 2a). The population was divided into six separate candidate clusters, demonstrating potential stratification in the reference population. The distribution of the SNP information within 1 Mb windows on different chromosomes is shown in Figure 2b.
Figure 2.
SNP distribution and PCA analysis of all samples of the present study. (a) PCA; (b) SNP distribution.
3.3. GWAS for Four Meat Quality Traits Based on SNPs
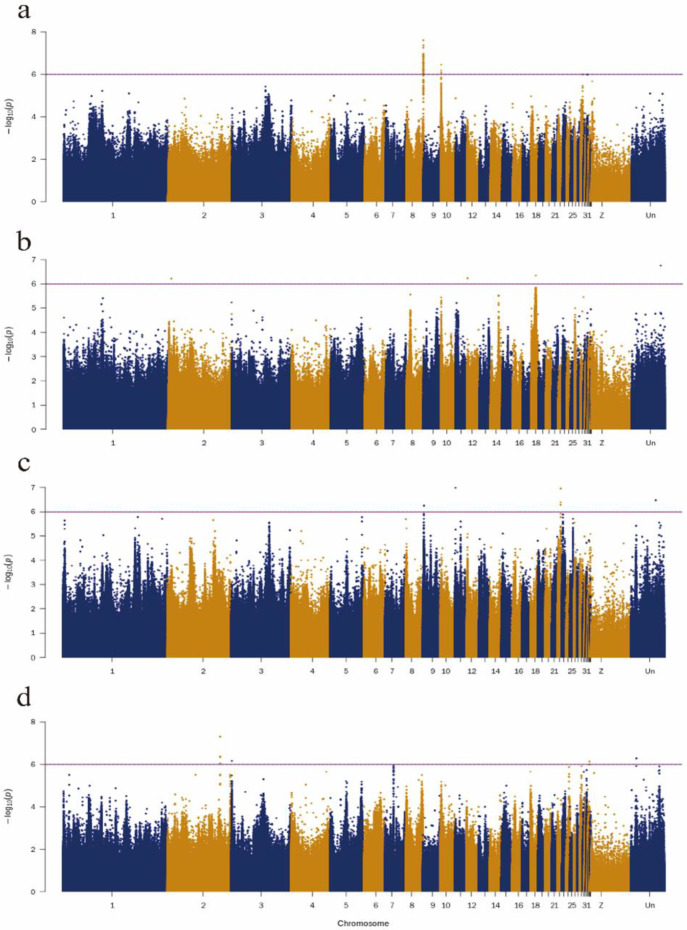
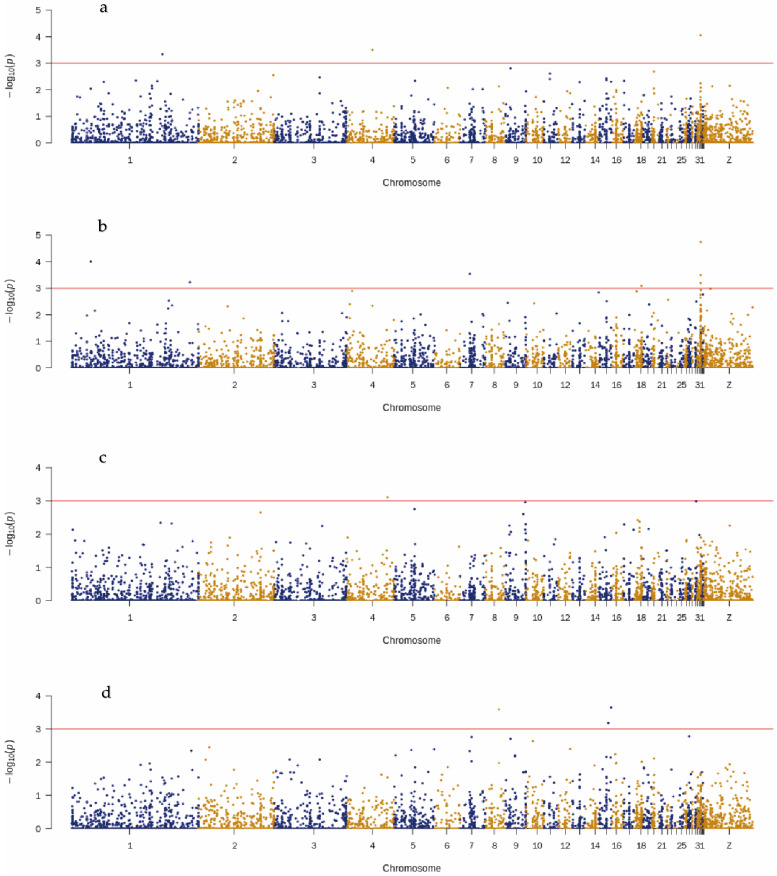
The efficient mixed-model association eXpedited (EMMAX) was used to identify candidate SNPs of four meat quality traits: fat, collagen, protein, and water content in the breast muscle of ducks. The results of GWAS showed that 41 SNPs located on chromosomes 8 and 10 were associated with breast muscle fat content (Figure 3a, Table S1). Nearest to the candidate SNPs for fat content, the following genes are located: barh like homeobox 2 (BARHL2, APL8), COP9 signalosome subunit 7b (COPS7B) and Coiled-Coil domain containing 50 (CCDC50, APL10). The three SNPs nearest to the BLM RecQ Like Helicase (BLM, APL2), WD repeat domain 76 (WDR76, APL8), and eomesodermin (EOMES) genes were associated with water content in breast muscle (Figure 3b, Table S1). Three SNPs located on chromosome 22, one SNP located on chromosome 11, and one SNP located on chromosome 9 were candidates associated with collagen content and were located nearest to the genes calmodulin binding transcription activator 1 (CAMTA1, APL22), fyve, rhogef, and ph domain containing 5 (FGD5, APL11), glutamate metabotropic receptor 7 (GRM7), and rap guanine nucleotide exchange factor 5 (RAPGEF5, APL22), respectively (Figure 3c, Table S1). In addition, six candidate SNPs located on chromosomes 2, 3 and 34 were associated with the protein content of breast muscle. The protein content-associated SNPs were nearest to the genes of regulating synaptic membrane exocytosis 2 (RIMS2, APL2), heterogeneous nuclear ribonucleoprotein u (HNRNPU, APL3), and spectrin β, non-erythrocytic 1 (SPTBN1, APL34) (Figure 3d, Table S1).
Figure 3.
The Manhattan plots of meat quality traits. GWAS analysis for fat (a), water (b), collagen (c), and protein (d) content in breast muscle. The x-axis represents the chromosomes, and the y-axis represents the −log10 (p-value).
3.4. GWAS for Four Meat Quality Traits Based on CNVs
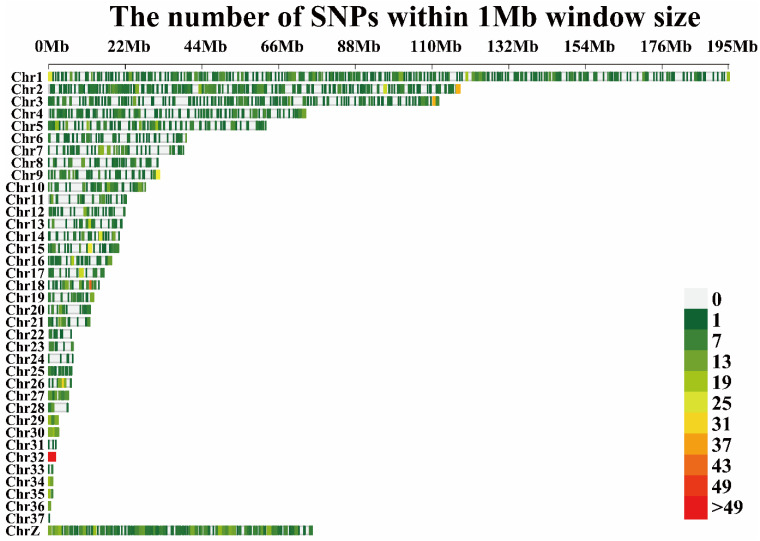
In addition to SNPs, CNVs are another important type of genetic variation. A total of 4163 CNVs were extracted using CNVnator (Figure 4). In the present study, all CNVs and four meat quality traits were used for GWAS. Three CNVs nearest to the genes, WASH complex subunit 7 (WASH7), GRB2 associated binding protein 1 (GAB1), Transporter 1, ATP binding cassette subfamily b member (TAP1), and major histocompatibility complex, class II, dm β (DMB) were associated with fat content in the breast muscle (Figure 5a, Table S2). Seven CNVs associated with water content were neatest to the following genes: collagen type XI α 1 chain (COL11A1), transporter 1, ATP binding cassette subfamily b member (TAP1), bromodomain containing 2 (BRD2), major histocompatibility complex, class II, dr α (DRA), complement component 4 (C4), lysine methyltransferase 2e (KMT2E), MLX Interacting Protein (MLXIP), and rb transcriptional corepressor 1 (RB1) (Figure 5b, Table S2). A CNV nearest to coiled-coil serine rich protein 1 (CCSER1) was associated with collagen content (Figure 5c, Table S2). Furthermore, three CNVs closest to the genes dedicator of cytokinesis 2 (DOCK2), inhibitory synaptic factor family member 2b (INSYN2B), PTTG1 Regulator of Sister Chromatid Separation, Securin (PTTG1), and Acyl-CoA Binding Domain Containing 6 (ACBD6) were associated with protein content (Figure 5d, Table S2).
Figure 4.
Distribution of CNVs on chromosomes.
Figure 5.
The Manhattan plots of meat quality traits. CNV-based GWAS analysis for fat (a), water (b), collagen (c), and protein (d) content in breast muscle. The x-axis represents the chromosomes, and the y-axis represents the −log10 (p-value).
4. Discussion
In recent years, the demand for poultry has continuously increased. In addition, with changes in consumer demand, poultry and livestock farmers want to obtain high-quality meat individuals to adapt to changes in the consumer market. Meat quality is an important quality trait of livestock and poultry meat products that directly influences the production performance and economic situation of farms [42,43]. Thus, exploring and finding candidate association variants and genes for meat quality may improve breeding efficiency and the consumer’s desire to buy meat [12]. As suggested by several studies, the accuracy of GWAS and the heritability (h2) of traits have a close relationship. In this study, the h2 of fat, water, collagen, and protein content in duck breast muscle was found to be 0.531, 0.2454, 0.42, and 0.401, respectively, which was calculated based on the phenotype. Moreover, there was a significant negative correlation between fat, moisture, and protein content. A previous study of Australian lamb found that the h2 of intramuscular fat (IMF) content, pH24, and myoglobin content (Myo) were 0.44, 0.32, and 0.25, respectively [14]. Previous studies reported or indicated meat quality has important effects on the oxidative stability, tenderness, and juiciness of livestock and poultry meat. Some previous studies based on Illumina 60K also identified 2 SNPs in Beijing-You chicken, which were located nearest the Cholecystokinin (CCK) and toll interacting protein (TOLLIP) genes on chromosomes 2 and 5, respectively [44]. At the same time, in the GWAS analysis of Jinghai-Yellow chicken, it was found that cerebellin 2 precursor (CBLN2), LOC101747478, hematopoietic prostaglandin d synthase (HPGDS), SET Domain Containing 2, Histone Lysine Methyltransferase (SETD2), ankyrin repeat domain 46 (ankrd46), zinc finger protein, fog family member 2 (ZFPM2) and glutamate metabotropic receptor 4 (GRM4) genes were significantly correlated with IMF [45]. Furthermore, the ECM receptor interaction pathway was a significantly enriched IMF-related pathway, which was evidenced by the compared and analyzed transcriptome of Zhuanghe Dagu chicken and Arbor Acres chickens [46]. Meanwhile, some previous reports considered that IMF differs from other fats in three ways: metabolic activities, adipocyte size, and developmental timing. Moreover, non-muscular adipocytes are larger than intramuscular adipocytes in cattle and pigs [47,48]. In the present study, we detected 41 SNPs located on chromosomes 8 and 10 neatest to the BARHL2, COPS7B, and CCDC50 genes. Meanwhile, three CNVs that were nearest to the genes WASHC4, GAB1 and TAP1, were related to fat content. Of these genes, the expression pattern in humans demonstrates that CCDC50 was shown to be associated with body fat in humans and mice [49].
For collagen content in muscle, collagen is an important component of meat quality. Collagen is an abundant connective tissue protein and is a factor in changes in meat tenderness and texture [50,51,52]. However, the disclosure of candidate genes for collagen is conducive to the selection and breeding of collagen for ducks and provides consumers with higher-quality meat products. In the present study, three SNPs nearest to CAMTA1, FGD5, GRM7, and RAPGEF5 were identified as the candidate for collagen content in duck breast muscle. Moreover, a CNV nearest to CCSER1 was associated with collagen content. The research in humans and mouse reported that FGD5 regulated the collagen IV content [53].
To date, relatively few GWAS have been performed on water and protein content in meat. In general, meat composition consists of approximately 75% of water, 19% of protein, 2.5% of fat, 1.2% of carbohydrates and 1.65% of nitrogen compounds [54]. Therefore, water and protein are important components of meat products. At the same time, the content of water and protein is directly related to the tenderness and water retention of meat. Therefore, the selection of water and protein content in meat can greatly improve meat quality. In the present study, three and six candidate SNPs were associated with water and collagen content, respectively. In addition, seven and three CNVs were associated with water and protein content in duck breast muscle. The identification of associated SNPs represents a key step forward in dissecting the genetic basis of meat quality-related traits in ducks and is also helpful for further demonstrating molecular mechanisms of related traits and designing better selection methods for these traits.
5. Conclusions
The results of the current study show that 41 SNPs and 3 CNVs potentially with fat content, 3 SNPs and 7 CNVs are candidates associated with water content, 3 SNPs and 1 CNV are candidates associated with collagen content, and 6 SNPs and 3 CNVs are candidates associated with protein content. These findings improve our understanding of poultry genetics and provide a genetic basis for breeding programs aimed at maximizing the economic potential of duck breeding and farming.
Acknowledgments
The authors thank Zhenjiang Tiancheng Agricultural Technology Co., Ltd. for providing duck embryos. We acknowledge the support given by Postgraduate Research and Innovation in Jiangsu Province (grant: KYCX21_3258), the China Agriculture Research System of MOF and MARA (grant: CARS-42) and the Jiangsu Agricultural Industry Technology System (grant: JATS [2021] 326).
Abbreviations
| SNP | Single nucleotide polymorphisms |
| CNV | Copy Number Variation |
| GWAS | Genome-Wide Association Studies |
| PCA | Principal component analysis |
| MAF | Minor allele frequency |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13060986/s1, Table S1: Information of all significant SNP of all meat quality trait; Table S2: Information of all significant CNV of all meat quality trait.
Author Contributions
Q.G. and L.H. performed the data analysis; G.C. (Guohong Chen) conceived and designed the experiments; Q.G. contributed to feeding the duck; Y.J., H.B., Y.B., Z.W., G.C. (Guohong Chen), and G.C. (Guobin Chang) contributed to the interpretation of data and to the reviewing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All blood samples were collected and meat quality traits were measured strictly in accordance with the guidelines proposed by the China Council on Animal Care and Min-istry of Agriculture of the People’s Republic of China. The study was approved by the In-stitutional Animal Care and Use Committee and the School of Animal Experiments Ethics Committee (license number: SYXK (Su) IACUC 2012-0029), Yangzhou University.
Data Availability Statement
The genome assembly and all of the re-sequencing data used in this research are deposited in the Genome Sequence Archive (GSA) at National Genomics Data Center (http://bigd.big.ac.cn/, 16 March 2022) Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA005019).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Postgraduate Research and Innovation in Jiangsu Province (grant: KYCX21_3258), the China Agriculture Research System of MOF and MARA (grant: CARS-42) and the Jiangsu Agricultural Industry Technology System (grant: JATS [2021] 326).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Henchion M., Moloney A.P., Hyland J., Zimmermann J., McCarthy S. Review: Trends for meat, milk and egg consumption for the next decades and the role played by livestock systems in the global production of proteins. Animal. 2021;15((Suppl. S1)):100287. doi: 10.1016/j.animal.2021.100287. [DOI] [PubMed] [Google Scholar]
- 2.Thornton P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010;365:2853–2867. doi: 10.1098/rstb.2010.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant C., Szejda K., Parekh N., Deshpande V., Tse B. A Survey of Consumer Perceptions of Plant-Based and Clean Meat in the USA, India, and China. Front. Sustain. Food Syst. 2019;3:11. doi: 10.3389/fsufs.2019.00011. [DOI] [Google Scholar]
- 4.Bryant C., Barnett J. Consumer Acceptance of Cultured Meat: An Updated Review (2018–2020) Appl. Sci. 2020;10:5201. doi: 10.3390/app10155201. [DOI] [PubMed] [Google Scholar]
- 5.Leal-Gutierrez J.D., Elzo M.A., Johnson D.D., Hamblen H., Mateescu R.G. Genome wide association and gene enrichment analysis reveal membrane anchoring and structural proteins associated with meat quality in beef. BMC Genom. 2019;20:151. doi: 10.1186/s12864-019-5518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leal-Gutiérrez J.D., Rezende F.M., Reecy J.M., Kramer L.M., Peñagaricano F., Mateescu R.G. Whole Genome Sequence Data Provides Novel Insights Into the Genetic Architecture of Meat Quality Traits in Beef. Front. Genet. 2020;11:1046. doi: 10.3389/fgene.2020.538640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picard B., Lefèvre F., Lebret B. Meat and fish flesh quality improvement with proteomic applications. Anim. Front. 2012;2:18–25. doi: 10.2527/af.2012-0058. [DOI] [Google Scholar]
- 8.Starcevic M., Mahmutovic H., Glamoclija N., Basic M., Andjelkovic R., Mitrovic R., Markovic R., Janjic J., Boskovic M., Baltic M.Z. Growth performance, carcass characteristics, and selected meat quality traits of two strains of Pekin duck reared in intensive vs semi-intensive housing systems. Animal. 2021;15:100087. doi: 10.1016/j.animal.2020.100087. [DOI] [PubMed] [Google Scholar]
- 9.Kokoszyński D., Wilkanowska A., Arpášová H., Hrnčár C. Comparison of some meat quality and liver characteristics in Muscovy and mule ducks. Arch. Anim. Breed. 2020;63:137–144. doi: 10.5194/aab-63-137-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biesek J., Banaszak M., Adamski M. Ducks’ Growth, Meat Quality, Bone Strength, and Jejunum Strength Depend on Zeolite in Feed and Long-Term Factors. Animals. 2021;11:1015. doi: 10.3390/ani11041015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao G., Gao N., Li S., Kuang W., Zhu L., Jiang W., Yu W., Guo J., Li Z., Yang C., et al. Genome-Wide Association Study of Meat Quality Traits in a Three-Way Crossbred Commercial Pig Population. Front. Genet. 2021;12:614087. doi: 10.3389/fgene.2021.614087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalewska M., Puppel K., Sakowski T. Associations between gene polymorphisms and selected meat traits in cattle—A review. Anim. Biosci. 2021;34:1425–1438. doi: 10.5713/ab.20.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedhane M., van der Werf J., Gondro C., Duijvesteijn N., Lim D., Park B., Park M.N. Genome-Wide Association Study of Meat Quality Traits in Hanwoo Beef Cattle Using Imputed Whole-Genome Sequence Data. Front. Genet. 2019;10:1235. doi: 10.3389/fgene.2019.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortimer S.I., van der Werf J.H., Jacob R.H., Hopkins D.L., Pannier L., Pearce K.L., Gardner G.E., Warner R.D., Geesink G.H., Edwards J.E., et al. Genetic parameters for meat quality traits of Australian lamb meat. Meat Sci. 2013;96:1016–1024. doi: 10.1016/j.meatsci.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez M.P., Tribout T., Iannuccelli N., Bouffaud M., Servin B., Tenghe A., Dehais P., Muller N., del Schneider M.P., Mercat M.J., et al. A genome-wide association study of production traits in a commercial population of Large White pigs: Evidence of haplotypes affecting meat quality. Genet. Sel. Evol. 2014;46:12. doi: 10.1186/1297-9686-46-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stafuzza N.B., Silva R.M.O., Fragomeni B.O., Masuda Y., Huang Y., Gray K., Lourenco D.A.L. A genome-wide single nucleotide polymorphism and copy number variation analysis for number of piglets born alive. BMC Genom. 2019;20:321. doi: 10.1186/s12864-019-5687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G.E., Hou Y., Zhu B., Cardone M.F., Jiang L., Cellamare A., Mitra A., Alexander L.J., Coutinho L.L., Dell’Aquila M.E., et al. Analysis of copy number variations among diverse cattle breeds. Genome Res. 2010;20:693–703. doi: 10.1101/gr.105403.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z.K., Li M., Cheng H., Fan W.L., Yuan Z.R., Gao Q., Xu Y.X., Guo Z.B., Zhang Y.S., Hu J., et al. An intercross population study reveals genes associated with body size and plumage color in ducks. Nat. Commun. 2018;9:2648. doi: 10.1038/s41467-018-04868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simcoe M., Valdes A., Liu F., Furlotte N.A., Evans D.M., Hemani G., Ring S.M., Smith G.D., Duffy D.L., Zhu G., et al. Genome-wide association study in almost 195,000 individuals identifies 50 previously unidentified genetic loci for eye color. Sci. Adv. 2021;7:eabd1239. doi: 10.1126/sciadv.abd1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Fernandez C., Campa A., Garzon A.S., Miklas P., Ferreira J.J. GWAS of pod morphological and color characters in common bean. BMC Plant. Biol. 2021;21:1–13. doi: 10.1186/s12870-021-02967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo J.Y., You S.W., Shin J.G., Kim Y., Park S.G., Won H.H., Kang N.G. GWAS Identifies Multiple Genetic Loci for Skin Color in Korean Women. J. Investig. Dermatol. 2021;142:1077–1084. doi: 10.1016/j.jid.2021.08.440. [DOI] [PubMed] [Google Scholar]
- 22.Wightman D.P., Jansen I.E., Savage J.E., Shadrin A.A., Bahrami S., Holland D., Rongve A., Borte S., Winsvold B.S., Drange O.K., et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 2021;53:1276–1282. doi: 10.1038/s41588-021-00921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen I.E., Savage J.E., Watanabe K., Bryois J., Williams D.M., Steinberg S., Sealock J., Karlsson I.K., Hagg S., Athanasiu L., et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019;51:404–413. doi: 10.1038/s41588-018-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertram L., Tanzi R.E. Genome-wide association studies in Alzheimer’s disease. Hum. Mol. Genet. 2009;18:R137–R145. doi: 10.1093/hmg/ddp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y., Song X., Shan H., Jiang J., Xiong P., Wu J., Shi F., Jiang Y. Genome-Wide Association Study of Body Weights in Hu Sheep and Population Verification of Related Sin-gle-Nucleotide Polymorphisms. Front. Genet. 2020;11:588. doi: 10.3389/fgene.2020.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha J., Choo H., Srikanth K., Lee S.H., Son J.W., Park M.R., Kim N., Jang G.W., Park J.E. Genome-Wide Association Study Identifies 12 Loci Associated with Body Weight at Age 8 Weeks in Korean Native Chickens. Genes. 2021;12:1170. doi: 10.3390/genes12081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tizioto P.C., Decker J.E., Taylor J.F., Schnabel R.D., Mudadu M.A., Silva F.L., Mourão G.B., Coutinho L.L., Tholon P., Sonstegard T.S., et al. Genome scan for meat quality traits in Nelore beef cattle. Physiol. Genom. 2013;45:1012–1020. doi: 10.1152/physiolgenomics.00066.2013. [DOI] [PubMed] [Google Scholar]
- 28.Milan D., Jeon J.T., Looft C., Amarger V., Robic A., Thelander M., Rogel-Gaillard C., Paul S., Iannuccelli N., Rask L., et al. A Mutation in PRKAG3 Associated with Excess Glycogen Content in Pig Skeletal Muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- 29.Stinckens A., Mathur P., Janssens S., Bruggeman V., Onagbesan O.M., Schroyen M., Spincemaille G., Decuypere E., Georges M., Buys N. Indirect effect of IGF2 intron3 g.3072G>A mutation on prolificacy in sows. Anim. Genet. 2010;41:493–498. doi: 10.1111/j.1365-2052.2010.02040.x. [DOI] [PubMed] [Google Scholar]
- 30.Oczkowicz M., Mucha A., Tyra M., Ropka-Molik K., Piórkowska K. Lack of the associations of the polymorphisms in IGF2, MC4R and GNAS genes with reproduction traits in pigs and imprinting analysis of IGF2 gene in ovary and cornus uteri. Reprod. Domest. Anim. 2013;48:562–568. doi: 10.1111/rda.12125. [DOI] [PubMed] [Google Scholar]
- 31.Lu H., Yan H., Ward M.G., Stewart T., Adeola O., Ajuwon K.M. Effect on Rendement Napole genotype on metabolic markers in Ossabaw pigs fed different levels of fat. J. Anim. Physiol. Anim. Nutr. 2018;102:e132–e138. doi: 10.1111/jpn.12720. [DOI] [PubMed] [Google Scholar]
- 32.Liu X., Usman T., Wang Y., Wang Z., Xu X., Wu M., Zhang Y., Zhang X., Li Q., Liu L., et al. Polymorphisms in Epigenetic and Meat Quality Related Genes in Fourteen Cattle Breeds and Association with Beef Quality and Carcass Traits. Asian-Australasian J. Anim. Sci. 2015;28:467–475. doi: 10.5713/ajas.13.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y., Zhao G., Liu R., Zheng M., Hu Y., Wu D., Zhang L., Li P., Wen J. The identification of 14 new genes for meat quality traits in chicken using a genome-wide association study. BMC Genom. 2013;14:1–11. doi: 10.1186/1471-2164-14-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sainz-De-Abajo B., García-Alonso J.M., Berrocal-Olmeda J.J., Laso-Mangas S., Torre-Díez I.D.L. FoodScan: Food Monitoring App by Scanning the Groceries Receipts. IEEE Access. 2020;8:227915–227924. doi: 10.1109/ACCESS.2020.3046031. [DOI] [Google Scholar]
- 35.Friendly M. Corrgrams. Am. Stat. 2002;56:316–324. doi: 10.1198/000313002533. [DOI] [Google Scholar]
- 36.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv. 20131303.3997 [Google Scholar]
- 37.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abyzov A., Urban A.E., Snyder M., Gerstein M. CNVnator: An approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 2011;21:974–984. doi: 10.1101/gr.114876.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin L., Zhang H., Tang Z., Xu J., Yin D., Zhang Z., Yuan X., Zhu M., Zhao S., Li X., et al. rMVP: A Memory-efficient, Visualization-enhanced, and Parallel-accelerated Tool for Genome-wide Association Study. Genom. Proteom. Bioinform. 2021;19:619–628. doi: 10.1016/j.gpb.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X., Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012;44:821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner S.D. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. Biorxiv. 2018:005165. doi: 10.21105/joss.00731. [DOI] [Google Scholar]
- 42.Hafez H.M., Attia Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future after the COVID-19 Outbreak. Front. Vet. Sci. 2020;7:516. doi: 10.3389/fvets.2020.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ismail I., Joo S.-T. Poultry Meat Quality in Relation to Muscle Growth and Muscle Fiber Characteristics. Korean J. Food Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu R., Sun Y., Zhao G., Wang F., Wu D., Zheng M., Chen J., Zhang L., Hu Y., Wen J. Genome-wide association study identifies Loci and candidate genes for body composition and meat quality traits in Beijing-You chickens. PLoS ONE. 2013;8:e61172. doi: 10.1371/journal.pone.0061172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang T., Fan Q.C., Wang J.Y., Zhang G.X., Gu Y.P., Tang Y. Genome-wide association study of meat quality traits in chicken. Genet. Mol. Res. 2015;14:10452–10460. doi: 10.4238/2015.September.8.6. [DOI] [PubMed] [Google Scholar]
- 46.San J., Du Y., Wu G., Xu R., Yang J., Hu J. Transcriptome analysis identifies signaling pathways related to meat quality in broiler chickens—The extracellular matrix (ECM) receptor interaction signaling pathway. Poult. Sci. 2021;100:101135. doi: 10.1016/j.psj.2021.101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller M.F., Cross H.R., Lunt D.K., Smith B.S. Lipogenesis in acute and 48-h cultures of bovine intramuscular and subcutaneous adipose tissue explants. J. Anim. Sci. 1991;69:162–170. doi: 10.2527/1991.691162x. [DOI] [PubMed] [Google Scholar]
- 48.Gardan D., Gondret F., Louveau I. Lipid metabolism and secretory function of porcine intramuscular adipocytes compared with subcutaneous and perirenal adipocytes. Am. J. Physiol. Endocrinol. Metab. 2006;291:E372–E380. doi: 10.1152/ajpendo.00482.2005. [DOI] [PubMed] [Google Scholar]
- 49.He H., Sun D., Zeng Y., Wang R., Zhu W., Cao S., Bray G.A., Chen W., Shen H., Sacks F.M. A Systems Genetics Approach Identified GPD1L and its Molecular Mechanism for Obesity in Human Adipose Tissue. Sci. Rep. 2017;7:1–15. doi: 10.1038/s41598-017-01517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weston A.R., Rogers R.W., Althen T.G. Review: The Role of Collagen in Meat Tenderness. Prof. Anim. Sci. 2002;18:107–111. doi: 10.15232/S1080-7446(15)31497-2. [DOI] [Google Scholar]
- 51.Lepetit J. A theoretical approach of the relationships between collagen content, collagen cross-links and meat tenderness. Meat Sci. 2007;76:147–159. doi: 10.1016/j.meatsci.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 52.Rogov I.A., Tokaev E.S., Kovalev Y.I., Tolstoguzov V.B. Collagen and its rational content in meat products: Part 1. Analytical studies. Meat Sci. 1992;31:35–42. doi: 10.1016/0309-1740(92)90070-K. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y., Sun Z., Liu F., Bai Y., Wu F. FGD5-AS1 Inhibits Osteoarthritis Development by Modulating miR-302d-3p/TGFBR2 Axis. Cartilage. 2021;13:1412–1420. doi: 10.1177/19476035211003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gillespie S. Food Security, Nutrition and Health. Epidemiology. 2009;20:S235. doi: 10.1097/01.ede.0000362788.85185.1c. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome assembly and all of the re-sequencing data used in this research are deposited in the Genome Sequence Archive (GSA) at National Genomics Data Center (http://bigd.big.ac.cn/, 16 March 2022) Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA005019).