Abstract
Background: One of the main challenges in the management of COVID-19 patients is to early assess and stratify them according to their risk of developing severe pneumonia. The alveolar–arterial oxygen gradient (D(A-a)O2) is defined as the difference between the alveolar and arteriolar concentration of oxygen, an accurate index of the ventilatory function. The aim of this study is to evaluate D(A-a)O2 as a marker for predicting severe pneumonia in COVID-19 patients, in comparison to the PaO2/FiO2. Methods: This retrospective, multicentric cohort study included COVID-19 patients admitted to two Italian hospitals between April and July 2020. Clinical and laboratory data were retrospectively collected at the time of hospital admission and during hospitalization. The presence of severe COVID-19 pneumonia was evaluated, as defined by the Infectious Diseases Society of America (IDSA) criteria for community-acquired pneumonia (CAP). Patients were divided in severe and non-severe groups. Results: Overall, 53 COVID-19 patients were included in the study: male were 30/53 (57%), and 10/53 (19%) had severe pneumonia. Patients with severe pneumonia reported dyspnea more often than non-severe patients (90% vs. 39.5%; p = 0.031). A history of chronic obstructive pulmonary disease (COPD) was recalled by 5/10 (50%) patients with severe pneumonia, and only in 6/43 (1.4%) of non-severe cases (p = 0.023). A ROC curve, for D(A-a)O2 >60 mmHg in detecting severe pneumonia, showed an area under the curve (AUC) of 0.877 (95% CI: 0.675–1), while the AUC of PaO2/FiO2 < 263 mmHg resulted 0.802 (95% CI: 0.544–1). D(A-a)O2 in comparison to PaO2/FiO2 had a higher sensibility (77.8% vs. 66.7%), positive predictive value (75% vs. 71.4%), negative predictive value (94% vs. 91%), and similar specificity (94.4% vs. 95.5%). Conclusions: Our study suggests that the D(A-a)O2 is more appropriate than PaO2/FiO2 to identify COVID-19 patients at risk of developing severe pneumonia early.
Keywords: COVID-19, severity marker, alveolar–arteriolar oxygen gradient
1. Introduction
Since 31 December 2019, when the World Health Organization (WHO) was informed of an outbreak of respiratory disease affecting the city of Wuhan, the world has been shaken by the most profound health crisis in several decades [1]. Coronavirus Disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has spread rapidly worldwide. COVID-19 patients often present a mild illness, but approximately 14% develop a severe disease which requires hospitalization and oxygen support. Moreover, 5% require admission to an intensive care unit [2]. The proper stratification of patients at hospital admission, according to their severity, is crucial for providing an early treatment, as well as for improving their management [3].
Therefore, one of the main challenges in the management of COVID-19 is to stratify patients early according to their risk of clinical deterioration. The alveolar–arteriolar oxygen gradient (D(A-a)O2) is defined as the difference between the alveolar and arteriolar concentration of oxygen, and it is a high accurate index of pulmonary function. Indeed, D(A-a)O2 describes all parameters involved in the phenomenon: (i) the amount of oxygen administered to the patient (FiO2), (ii) the atmospheric pressure (Patm), (iii) the partial oxygen pressure in arterial blood and the airway’s pressure of gaseous H2O (PH2O), (iv) the alveolar pressure of CO2 (PACO2), and (v) the respiratory quotient (R).
It is described by the equation: D(A-a)O2 = [FiO2 × (Patm − PH2O) − PACO2/R] − PaO2.
D(A-a)O2 is automatically calculated by a blood gas analyzer, and normally its value is between 5–10 mmHg.
It increases in the case of an alveolar–capillary membrane alteration (e.g., interstitial pneumonia) or ventilation/perfusion (V/Q) ratio impairment (e.g., pulmonary embolism, severe pneumonia etc.). As noted, all of the above are aspects of COVID-19 pneumonia [4].
D(A-a)O2 is automatically calculated in blood gas analysis (BGA), and has been proposed as an early marker of respiratory insufficiency.
The arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2) ratio (PaO2/FiO2) is currently used to evaluate the severity of hypoxia in patients who require oxygen supplementation. A PaO2/FiO2 > 300 mmHg identifies a normal lung function [5]. The PaO2/FiO2 ratio was included in the definition of acute respiratory distress syndrome (ARDS) according to the Berlin criteria, and is globally used to stratify the severity of respiratory insufficiency [6].
However, PaO2/FiO2 ratio has some limitations. Firstly, PaO2/FiO2 depends on the clinicians FiO2 setting, so, when the oxygen flow is increased without a clear clinical need, the PaO2/FiO2 ratio can dramatically decrease, even if the pulmonary ventilation is little impaired. Secondly, PaO2/FiO2 does not consider the alveolar pCO2 value, that is, an indirect measure of the patient’s respiratory effort (e.g., tachypnea that can cause hypocapnia), reflecting the subjective severity of the respiratory insufficiency.
Finally, PaO2/FiO2 cannot provide information on pulmonary V/Q. Therefore, PaO2/FiO2 are less performant than D(A-a)O2 in discriminating between types of respiratory insufficiency (e.g., pump vs. pulmonary insufficiency). Moreover, COVID-19 patients who are not responding to a gradual incrementation of the oxygen flow can benefit from the early use of continuous positive airway pressure (CPAP) or non-invasive ventilation (NIV) [7,8,9,10,11].
As noted, COVID-19 pneumonia is characterized by both an alveolar and vascular damage [3]. Even though, few studies have been performed to assess the accuracy of D(A-a)O2 in early identifying severe COVID-19 pneumonia, and, as far as we know, no previous studies have compared the performance of D(A-a)O2 and PaO2/FiO2 in this context [12,13,14,15,16,17].
The aim of our study is to evaluate the diagnostic appropriateness of D(A-a)O2 to early predict respiratory deterioration in COVID-19. In addition, we compared the performance of D(A-a)O2 and PaO2/FiO2 in predicting pneumonia severity.
2. Materials and Methods
A retrospective, multicentric, case-control study was performed in two acute-care Italian hospitals: Infectious Disease Unit, ARNAS Civico Hospital in Palermo, and Infectious Disease Unit, Vanvitelli Hospital in Naples. All COVID-19 patients (≥18 years old) admitted to hospital between April and July 2020 were enrolled in the study. Patients affected by SARS-CoV-2 pneumonia, described as the presence of infiltrates on a computer tomography (CT) scan plus the positivity of the nasopharyngeal swab for the virus, were enrolled. Demographical, epidemiological, clinical, and laboratory data were retrospectively collected from the clinical records of each patient. Risk factors for severe COVID-19, as well as signs and symptoms at onset, were also collected. D(A-a)O2 and PaO2/FiO2 were calculated on the first blood gas analysis (BGA) performed in the emergency department. Patients were divided in two groups, considering the development of (1) severe pneumonia or (2) non-severe pneumonia, according the 2019 Infectious Diseases Society of America (IDSA) criteria for community-acquired pneumonia [18] (Table 1). Quantitative variables are shown as median and interquartile ranges (IQR) 25–75%. Qualitative variables are shown as number and percentage. Quantitative variables were tested for normal distribution with a Shapiro–Wilk test, and a U Mann–Whitney test was used for non-normally distributed variables (α = 0.05) to compare severe and non-severe patients.
Table 1.
IDSA criteria for severity in community-acquired pneumonia (CAP), based on 2019 guidelines.
| IDSA Criteria for Severe CAP. One Major Criteria or Three or More Minor Criteria. |
|---|
| Minor criteria |
| Respiratory rate > 30 breaths/min PaO2/FIO2 ratio < 250 |
| Multilobar infiltrates |
| Confusion or disorientation |
| Uremia (blood urea nitrogen level >20 mg/dL) |
| Leukopenia (white blood cell count, 4.000 cells/μL due to infection alone |
| Thrombocytopenia (platelet count, 100.000/μL) |
| Hypothermia (core temperature, <36 °C) |
| Hypotension requiring aggressive fluid resuscitation |
| Major criteria |
| Septic shock with need for vasopressors |
| Respiratory failure requiring mechanical ventilation |
For categorical variables, a two-sided Fisher exact test (α = 0.05) was performed to compare the two groups. Odds ratio (OR) were calculated with Spearman’s rank correlation coefficient. receiver operating characteristic (ROC) curves with a 95% confidence interval (CI) were created, and an area under the curve (AUC) was calculated in order to evaluate the optimal cut-off of D(A-a)O2 and PaO2/FiO2 at admission to predict the development of severe pneumonia during hospitalization, as well as to confront the accuracy of the two tests. Post-hoc analyses were conducted to evaluate the statistical power of the difference between both D(A-a)O2 and PaO2/FiO2 values of the two groups (severe and non-severe), by using mean and standard deviation of continuous variables for two independent sample, α = 0.05 (Rosner B. Fundamentals of Biostatistics. 7th ed. Boston, MA: Brooks/Cole; 2011).
3. Results
Overall, the study included 53 COVID-19 patients, whose demographic and clinical characteristics are shown in Table 2; laboratory data are showed in Table 3.
Table 2.
Patients’ comorbidity, signs, and symptoms at presentation. Data are shown as median (interquartile range 25–75%), or number (percentage). Fisher exact tests were used (α < 0.05). COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; Data are shown as a number (percentage), or median and interquartile range (IQR) 25–75%. PaO2/FiO2: arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2) ratio. D(A-a)O2: alveolar–arteriolar oxygen gradient.
| Overall (n = 53) | Severe (n = 10) | Non Severe (n = 43) | p-Value | |
|---|---|---|---|---|
| Age in years (IQR 25–75) | 63 (49–75) | 66.5 (62.8–73.8) | 60 (47.5–74) | 0.294 |
| Male Sex | 30 (56.6%) | 9 (90%) | 21 (49%) | 0.031 |
| Caucasian | 51 (96.2%) | 9 (90%) | 42 (97.7%) | 0.254 |
| Comorbidity | ||||
| Hypertension | 35 (66%) | 7 (70%) | 28 (65.1%) | 0.719 |
| Cardiovascular Disease | 12 (22.6%) | 4 (40%) | 8 (18.6%) | 0.677 |
| COPD | 11 (20.8%) | 5 (50%) | 6 (14%) | 0.023 |
| CKD | 8 (15.1%) | 3 (20%) | 5 (14%) | 0.163 |
| Malignancy | 3 (5.7%) | 2 (20%) | 1 (2.3%) | 0.088 |
| Diabetes Mellitus (type II) | 6 (11.3%) | 1 (10%) | 5 (11.6%) | 1 |
| Signs and Symptom | ||||
| Fever | 43 (82.1%) | 8 (80%) | 35 (81.4%) | 1 |
| Dyspnea | 26 (49.1%) | 9 (90%) | 17 (39.5%) | 0.005 |
| Anosmia | 7 (13.2%) | 2 (20%) | 5 (11.6%) | 0.604 |
| Dysgeusia | 6 (11.3%) | 2 (20%) | 4 (9.3%) | 0.315 |
| Cough | 26 (49.1%) | 6 (60%) | 20 (46.5%) | 0.501 |
| Diarrhea | 4 (7.5%) | 0 (0%) | 4 (9.3%) | 1 |
| Arterial Blood Gas analysis, median (IQR) | ||||
| PaO2/FiO2 (mmHg) | 379.5 (303.1–426.8) | 246 (104.7–376.7) | 390.5 (321.6–432.1) | 0.157 |
| D(A-a)O2 (mmHg) | 33.6 (15.5–54.1) | 97.9 (49.9–241.7) | 28.6 (12.3–40.2) | <0.001 |
| Outcome | ||||
| Death n (%) | 3 (5.7%) | 3 (30%) | 0 (0%) | 0.0051 |
Table 3.
Laboratory data are showed as median (IQR 25–75), a Mann–Witney test was used (α < 0.05). WBC: white blood cells. CPR: C-reactive protein; AST: aspartate transaminases; ALT: alanine transaminases; LDH: lactate dehydrogenase; D(A-a)O2: alveolar–arteriolar gradient.
| Overall (n = 53) | Severe (n = 10) | Non-Severe (n = 43) | p-Value | |
|---|---|---|---|---|
| WBC (cell/ μ L) | 6.7 (5.27–9.02) | 6.9 (4.97–10.14) | 6.5 (5.43–7.75) | 0.869 |
| Neutrophils | 4.2 (3.09–6.06) | 5.5 (2.48–9.26) | 4.02 (3.3–5.12) | 0.592 |
| Lymphocites | 1.25 (0.91–1.93) | 0.76 (0.25–1.68) | 1.27 (0.99–2.09) | 0.432 |
| Platelets (cell/ μ L) | 209 (165–251) | 171 (109–250) | 210 (184–251) | 1 |
| D-Dimer (ng/mL) | 748.5 (402.2–1266) | 499 (328–1200) | 779 (442.5–1188) | 0.689 |
| Creatinine (mg/dL) | 0.82 (0.74–0.95) | 0.88 (0.75–1.46) | 0.81 (0.68–0.9) | 0.213 |
| CPR (mg/L) | 2.78 (0.95–8.12) | 8.48 (0.9–12.8) | 2.39 (0.59–5.43) | 0.056 |
| LDH (UI/L) | 229 (183–325) | 261 (235–527) | 205 (167–321) | 0.112 |
| AST (UI/L) | 29 (16–48) | 42 (29–52) | 28 (16–37) | 0.071 |
| ALT (UI/L) | 28 (17–44) | 40 (19–79) | 25 (16–39) | 0.334 |
| pO2 | 80 (69.6–95.4) | 69 (54.5–87) | 82 (71–98.2) | 0.204 |
| pCO2 | 33 (31–35.65) | 32.1 (31–43) | 33 (30.85–35.23) | 0.625 |
Among the patients, 10/53 (19%) developed severe pneumonia, and 43/53 (81%) non-severe pneumonia. One patient from each group had one episode of pulmonary thrombo-embolism during hospitalization.
Males were statistically prevalent among severe COVID-19 patients, compared to non-severe patients (90% vs. 49%, p = 0.031).
Comorbidities did not differ between the two study groups, with the exception of COPD, which was more prevalent in severe pneumonia patients than in non-severe patients (50% vs. 14%; p = 0.023), as well as malignancy (20% vs. 2.3%; p = 0.088). Table 2. The OR for severe pneumonia were calculated: for COPD, the OR was 6.167 (95% CI: 1.36–27.92, p = 0.011), and for male sex, the OR was 9.426 (95% CI: 0.940–50.644, p = 0.018).
At hospital admission in the emergency department, 42/53 (80%) patients presented fever, and 26/53 (50%) reported dyspnea. Dyspnea was diagnosed at admission in 9/10 (90%) severe and 17/43 (39.5%) non-severe patients (p = 0.005) Table 2.
A total of three patients died during the hospital stay (3/53, 5.6%), and all of deceased had severe COVID-19 pneumonia.
Median PaO2/FiO2 values at first BGA was lower in severe COVID-19 patients, as compared to non-severe patients, without reaching a statistical significance. For severe patients, the results were PaO2/FiO2 246 mmHg (IQR 104.7–376.7), and for non-severe patients, the results were 390 mmHg (IQR 321.6–432.1), p = 0.157. Median values of D(A-a)O2 at first BGA were significantly higher for severe patients compared to non-severe patients: 97.9 mmHg (IQR 49.9–241.7) and 28.6 mmHg (IQR 12.3–40.2), respectively, p < 0.001.
D(A-a)O2 and PaO2/FiO2 have been compared through the ROC curve analysis for the prediction of severe pneumonia development.
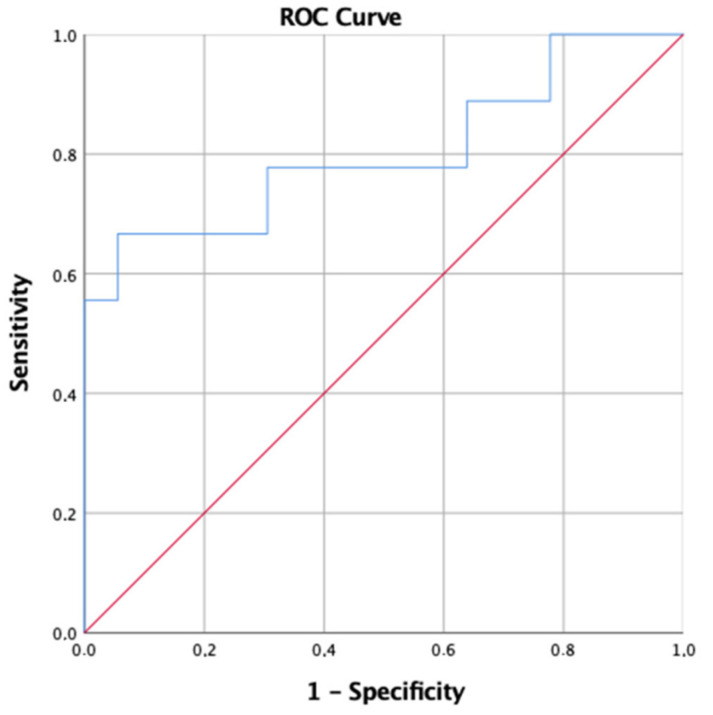
For PaO2/FiO2, the more performant cut-off value for determining the occurrence of severe pneumoniae was <263 mmHg. Accordingly, the area under curve (AUC) resulted in a value 0.802, with a sensibility of 66.7% and a specificity of 94.5% (p = 0.001); positive predictive value (PPV) 71.4% and a negative predictive value (NPV) 91% (Figure 1).
Figure 1.
Receiver operating characteristic (ROC) curve comparing accuracy of PaO2/FiO2 at first BGA in relation to the prediction of severe pneumonia. Area under the curve (AUC): 0.802 (95% CI: 0.544–1).
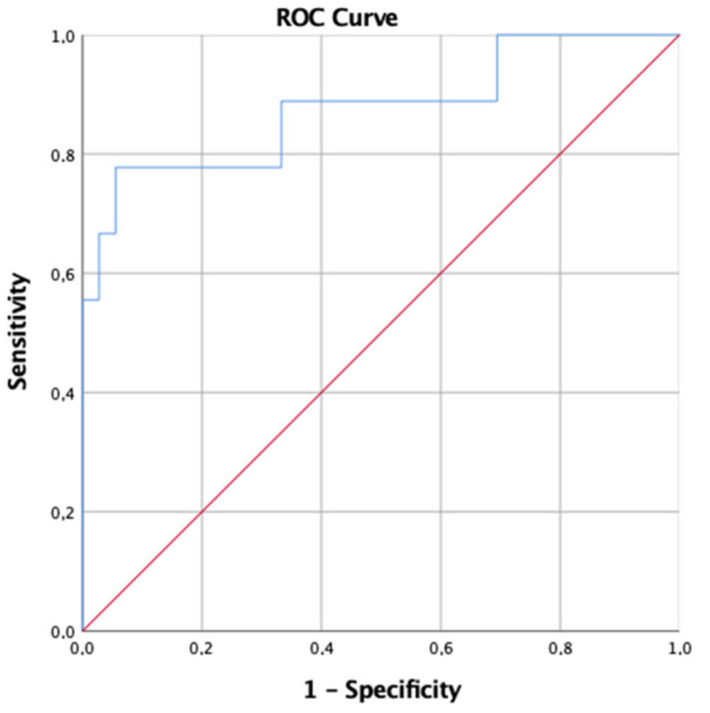
For D(A-a)O2, considering the cut-off value >60 mmHg for severe pneumonia, the AUC was 0.877, resulting in a sensibility of 77.8% and a specificity of 94.4%; PPV 75% and NPV 94% (Figure 2). Through a post-hoc analysis, the statistical power was calculated 87.7% for D(A-a)O2, and 88.2% for PaO2/FiO2.
Figure 2.
Receiver operating characteristic (ROC) curve comparing accuracy of D(A-a)O2 at first BGA in relation to the prediction of severe pneumonia. Area under the curve (AUC): 0.877 (95% CI: 0.675–1).
4. Discussion
During the COVID-19 pandemic, hospitals worldwide experienced overcrowding, leading to emergency and stressful situations [19]. A proportion of patients developed severe pneumonia, and one of the main challenges in their management was the early recognition of severe pneumonia itself, as well as the management of respiratory failure.
A large cohort study which enrolled 10,131 elderly patients with COVID-19 showed that dyspnea was associated with a higher risk of hospitalization (aHR: 2.18; 95% CI: 2.02–2.36), mechanical ventilation (aHR: 2.95; 95% CI: 2.49–3.49), and mortality (aHR: 1.78; 95% CI: 1.53–2.07) [20]. The data confirm that dyspnea is associated with severe COVID-19 [20,21,22,23,24,25], as well as the association between COPD [6,26,27,28,29,30] and male sex [28,31] with severe SARS-CoV-2 infection.
Recently, a study by de Roos et al. supported the use of D(A-a)O2 in association with chest computed tomography scanning to identify patients in need of hospitalization at an early stage [4]. Moreover, D(A-a)O2 at first blood gas analyses at arrival in hospital was identified as a predictive marker of intensive care unit (ICU) admission [12,14], and of mortality in COVID-19 patients [19]. In a retrospective cross-sectional study among 213 patients admitted to ICU, D(A-a)O2 values were not sensitive or specific in predicting mortality [32].
Thus, as documented in the literature, in a non-ICU setting, D(A-a)O2 values could be used to identify patients in need of hospitalization [14], to identify patients at risk for ICU admission [12,14], and to predict mortality as part of a score [13]. However, if patients are already admitted to the ICU, perhaps D(A-a)O2 loses its predictive value [32], and this could be explained with the alteration of blood gases values among these patients.
The results of the present study align with the data of the consulted literature, and suggests that a low PaO2/FiO2 is a severity marker of SARS-CoV-2 infection [12,28], as well as D(A-a)O2.
As far as is known, this study is the first that directly compared the performance of the PaO2/FiO2 ratio and D(A-a)O2 in predicting severe pneumoniae (defined as IDSA guidelines on CAP) in non-ICU COVID-19 patients. The results indicate that D(A-a)O2 is more effective than the commonly used PaO2/FiO2 method to identify COVID-19 patients with a high risk of developing severe pneumonia.
According to the data, D(A-a)O2 is a better predictor for severe COVID-19 pneumonia than the PaO2/FiO2 at admission to hospital. Interestingly, we identify the best cut-off value for D(A-a)O2 as >60 mmHg for predicting severe pneumonia, with a sensibility of 77.8%.
The study has several limitations. Firstly, it is a retrospective study involving only two national hospitals, and it may not identify the complex heterogeneity of SARS-CoV-2 patients. Secondly, the sample size is relatively small, even if it has a good statistical power. Finally, we did not stratify for a variant of concern that presents a different pneumonia picture, virulence, or mortality.
5. Conclusions
D(A-a)O2 is an appropriate and useful marker to identify the risk of developing severe pneumonia in COVID-19 patients at an early stage. D(A-a)O2 had a higher predictive value in diagnosing severe COVID-19 compared to PaO2/FiO2, with a higher sensibility and a similar specificity. As a consequence, we support the routine use of D(A-a)O2 in COVID-19 emergency departments, considering patients with D(A-a)O2 >60 mmHg at admission as having a high risk of developing severe COVID-19 pneumonia. Furthermore, we support the use of D(A-a)O2 as a severity marker of pulmonary disease in non-ICU daily ward routines, as well as its application in clinical studies. Further research is needed, particularly in the setting of new SARS-CoV-2 variants of concern with different virulence and mortality rates.
Author Contributions
Conceptualization, G.P.; methodology, G.P., M.C. and G.G.; writing—original draft preparation, G.P.; project administration, M.C.; writing—review and editing, M.C. and G.G.; visualization, A.S., F.D.L., C.B., A.F., D.S. and C.I. (Claudia Imburgia), I.A., F.O., C.S. and C.I. (Chiara Iaria); supervision, G.P.; conceptualization, C.S.; validation, C.I. (Chiara Iaria). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
An oral informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to privacy restriction.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. The World Bank Tracking Universal Health Coverage-2021 Global Monitoring Report, Geneva 2021. [(accessed on 15 April 2022)]. Available online: https://cdn.who.int/media/docs/default-source/world-health-data-platform/events/tracking-universal-health-coverage-2021-global-monitoring-report_uhc-day.pdf?sfvrsn=fd5c65c6_5&download=true.
- 2.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prediletto I., D’Antoni L., Carbonara P., Daniele F., Dongilli R., Flore R., Pacilli A.M.G., Pisani L., Tomsa C., Vega M.L., et al. Standardizing PaO2 for PaCO2 in P/F ratio predicts in-hospital mortality in acute respiratory failure due to COVID-19: A pilot prospective study. Eur. J. Intern. Med. 2021;92:48–54. doi: 10.1016/j.ejim.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu D.K., Kim L.H.-Y., Young P.J., Zamiri N., Almenawer S.A., Jaeschke R., Szczeklik W., Schünemann H.J., Neary J.D., Alhazzani W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet. 2018;391:1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 5.ARDS Definition of Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Living Guidance for Clinical Management of COVID-19. [(accessed on 13 April 2022)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-22021.
- 8.National Institutes of Health COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [(accessed on 30 July 2021)]; Available online: https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 9.Alhazzani W., Evans L., Alshamsi F., Møller M.H., Ostermann M., Prescott H.C., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving Sepsis Campaign Guidelines on the Management of Adults with Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021;49:e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 10.Damiani E., Adrario E., Girardis M., Romano R., Pelaia P., Singer M., Donati A. Arterial hyperoxia and mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care. 2014;18:711. doi: 10.1186/s13054-014-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni Y.-N., Wang Y.-M., Liang B.-M., Liang Z.-A. The effect of hyperoxia on mortality in critically ill patients: A systematic review and meta analysis. BMC Pulm. Med. 2019;19:53. doi: 10.1186/s12890-019-0810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlino M.V., Valenti N., Cesaro F., Costanzo A., Cristiano G., Guarino M., Sforza A. Predictors of Intensive Care Unit admission in patients with coronavirus disease 2019 (COVID-19) Monaldi Arch. Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1410. [DOI] [PubMed] [Google Scholar]
- 13.Kamran S.M., Mirza Z., Moeed H.A., Naseem A., Hussain M., Fazal I., Saeed F., Alamgir W., Saleem S., Riaz S. CALL Score and RAS Score as Predictive Models for Coronavirus Disease 2019. Cureus. 2019;12:e11368. doi: 10.7759/cureus.11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Roos M.P., Kilsdonk I.D., Hekking P.-P.W., Peringa J., Dijkstra N.G., Kunst P.W.A., Bresser P., Reesink H.J. Chest computed tomography and alveolar–arterial oxygen gradient as rapid tools to diagnose and triage mildly symptomatic COVID-19 pneumonia patients. ERJ Open Res. 2021;7:00737–2020. doi: 10.1183/23120541.00737-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. NEJM. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Secco G., Salinaro F., Bellazzi C., La Salvia M., Delorenzo M., Zattera C., Barcella B., Resta F., Vezzoni G., Bonzano M., et al. Can Alveolar-Arterial Difference and Lung Ultrasound Help the Clinical Decision Making in Patients with COVID-19? Diagnostics. 2021;11:761. doi: 10.3390/diagnostics11050761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farina G., Gianstefani A., Salvatore V., Anziati M., Baldassarri F., Beleffi M., Cannizzaro A.M., Casadei E., Fantini J., Tubertini E., et al. Alveolar-to-arterial oxygen gradient: Role in the management of COVID-19 infection mild population. ResearchSquare. 2020 doi: 10.21203/rs.3.rs-100668/v1. [DOI] [Google Scholar]
- 18.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., Cooley L.A., Dean N.C., Fine M.J., Flanders S.A., et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad K., McLoughlin C., Stillman M., Poplau S., Goelz E., Taylor S., Nankivil N., Brown R., Linzer M., Cappelucci K., et al. Prevalence and correlates of stress and burnout among U.S. healthcare workers during the COVID-19 pandemic: A national cross-sectional survey study. eClinicalMedicine. 2021;35:100879. doi: 10.1016/j.eclinm.2021.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K., Wu J., Wu F., Guo D., Chen L., Fang Z., Li C. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Investig. Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z., Chen H., Wang D., Liu N., Liu D., et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ioannou G.N., Locke E., Green P., Berry K., O’Hare A.M., Shah J.A., Crothers K., Eastment M.C., Dominitz J.A., Fan V.S. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US Veterans with SARS-CoV-2 Infection. JAMA Netw. Open. 2020;3:e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang N., Li D., Wang X., Sun Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rod J.E., Oviedo-Trespalacios O., Cortes-Ramirez J. A brief-review of the risk factors for COVID-19 severity. Rev. Saúde Pública. 2020;54:60. doi: 10.11606/s1518-8787.2020054002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y.-D., Ding M., Dong X., Zhang J.-J., Azkur A.K., Azkur D., Gan H., Sun Y.-L., Fu W., Li W., et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., Silva J., Mao T., Oh J.E., Tokuyama M., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camici M., Zuppi P., Lorenzini P., Scarnecchia L., Pinnetti C., Cicalini S., Nicastri E., Petrosillo N., Palmieri F., D’Offizi G., et al. Role of testosterone in SARS-CoV-2 infection: A key pathogenic factor and a biomarker for severe pneumonia. Int. J. Infect. Dis. 2021;108:244–251. doi: 10.1016/j.ijid.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A., Soni K.D., Singh Y., Aggarwal R., Venkateswaran V., Ashar M.S., Trikha A. Alveolar Arterial Gradient and Respiratory Index in Predicting the Outcome of COVID-19 Patients; A Retrospective Cross-Sectional Study. Arch. Acad. Emerg. Med. 2022;10:e28. doi: 10.22037/aaem.v10i1.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy restriction.