Abstract
The current literature demonstrates that not only is exercise during pregnancy safe, but it has substantial maternal and infant benefits and appears to influence infant growth/size throughout pregnancy and at birth. However, many existing studies have investigated only the effects of prenatal exercise on birth weight. The purpose of this review was to determine the impact or association of maternal physical activity during pregnancy on neonatal body composition assessed between birth and two weeks of age. Electronic database searches were conducted on 29 July 2019 for randomized control trials and cohort studies, with an updated search completed on 8 January 2021. A total of 32 articles that met eligibility criteria were selected for review. Overall, prenatal exercise was not associated with infant body composition at birth. Yet, five of the studies identified suggest that infant body composition could be influenced by higher volumes of mid-to-late term prenatal physical activity. This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered in PROSPERO (Registration No. CRD42020160138).
Keywords: exercise, infant anthropometrics, neonatal adiposity, maternal physical activity
1. Introduction
Scientific evidence has demonstrated the safety and efficacy of exercise during the perinatal period [1,2,3,4,5]. Physical activity (PA) during pregnancy provides many maternal health benefits, including improved glucose control, lower gestational weight gain, lower systemic inflammation, reduced risk for preeclampsia, reduced risk for operative deliveries, and faster postpartum recovery time [1,2,3,4,5]. In addition to improvements in maternal health, physical activity during pregnancy has also been shown to benefit the neonate, including healthy growth and improved cognition and intelligence [6,7]. Specifically, exercise during pregnancy has been linked to lower and healthier infant birth weight (without increasing risk for low birth weight) [8,9].
Birth weight has been used historically to indicate a healthy intrauterine growth environment; however, birthweight is not a strong predictor of important future health outcomes [10], particularly at the individual level [11]. Despite the lack of evidence to support its connection to downstream outcomes, it is widely utilized clinically and is typically one of the first assessments of a newborn. One reason why birth weight may be a poor predictor of outcomes is it cannot adequately estimate fat mass relative to fat-free mass in infants. It also does not account for how much an infant “should have weighed” based on their length and genetic potential [11]. Assessing body composition at birth (i.e., fat mass versus fat-free mass) may provide additional details indicating healthy fetal/neonatal growth/size. Further, adiposity levels at birth are a better predictor of metabolic syndrome and other non-communicable diseases later in life than birthweight alone [12].
Taken together, understanding the relationship between physical activity during pregnancy and infant body composition is essential. Exercise during pregnancy appears to influence infant growth/size [9,13]; however, many existing studies have investigated only the effects of prenatal exercise on birth weight and not body composition. Over the past 10 years, research investigating the role of exercise during pregnancy on infant body composition has gained momentum. However, the existing evidence on the impact physical activity during pregnancy has on infant body composition is unclear and oftentimes conflicting. The conflicting results may be due to how physical activity was assessed, what timepoint during pregnancy exercise was studied, and the frequency, intensity, time, and type of exercise performed. The purpose of this systematic review is to determine the association of maternal physical activity during pregnancy and neonatal body composition assessed between birth and two weeks of age.
2. Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review protocol was pre-registered with the International Prospective Registrar of Systematic Reviews (PROSPERO) registration number CRD42020160138. We followed the PRISMA reporting guidelines (see Appendix A).
2.1. Eligibility Criteria
2.1.1. Types of Study Designs
Eligible study designs included randomized controlled clinical trials (RCTs), prospective cohort studies, and retrospective cohort studies that assessed physical activity levels at any point during pregnancy and assessed infant body composition within two weeks of delivery. Studies were included regardless of intensity, duration, or mode of physical activity utilized. Only studies that were peer-reviewed with scientific credibility were included. The decision to include non-RCTs was based on several factors, including lack of existing RCTs assessing infant body composition as an outcome; poor adherence to interventions, making it difficult to truly evaluate the relationship between physical activity levels and infant outcomes; and the idea that other study designs may better allow assessment of physical activity as a continuous variable versus as a grouping variable (i.e., control group vs. exercise group).
2.1.2. Types of Participants
Women of all pre-pregnancy body mass indices (BMIs) were included. Studies including women with gestational diabetes and preeclampsia were excluded. These specific conditions have known and particular effects on infant body size/composition and would make it difficult to discern the impact of physical activity on infant outcomes. Studies including multiple gestation pregnancies were also excluded.
2.1.3. Types of Outcome Measurements
For the mode of exercise, studies including all modes of physical activity were included (aerobic training, resistance training, combination training). The physical activity assessment could be through self-reporting (e.g., the Pregnancy Physical Activity Questionnaire), compliance to an intervention, or objective assessments (i.e., accelerometer, pedometer, or doubly-labeled water). Exercise data could be collected during any timepoint of pregnancy (1st, 2nd, 3rd trimester, or throughout pregnancy). For the outcome of infant body composition, the study had to include an assessment or estimate of infant adiposity (and not just birth weight). These included ponderal index, BMI, abdominal circumference, or body fat percentage from bioelectrical impedance, dual X-ray absorptiometry (DXA), skinfold anthropometry, or air-displacement plethysmography. Any studies including an initial assessment of body composition on infants >2 weeks of age were excluded.
2.2. Search Strategy
Electronic searching of PubMed/MEDLINE, EMBASE (Elsevier), Web of Science Core Collection (Thomson Reuters), and CINAHL (EBSCO) databases took place on 29 July 2019. Phrases and controlled vocabulary headings for each component of the population and outcome framework (PICO) were combined using OR and then using AND (exercise/physical activity, pregnancy, neonate, and body composition). The search strategy was first created in PubMed (See Appendix B) and then translated for each database platform as appropriate. Manual searches of reference lists were conducted on all eligible articles following the screening. An updated search was completed on 8 January 2021.
2.3. Assessment of Bias
The risk of bias (RoB) was assessed on all studies selected for inclusion. Randomized control trials (RCTs) were evaluated using Version 2 of the Cochrane risk-of-bias tool (RoB 2) [14]. Each RCT was assessed using the study’s “per-protocol” effect by independent reviewers (CD, BM, EA). Discrepancies in RoB scoring were resolved by discussion between reviewers and an additional study team member (JM). Five bias domains were assessed (i.e., selection, performance, detection, attrition, and reporting), and an overall bias score was calculated.
RoB in case-control and single-arm cohort studies was evaluated using a modified Newcastle-Ottawa Scale (NOS) [15] (See Appendix C). Again, discrepancies in RoB scoring were resolved by discussion between reviewers and an additional study team member (JM). Three bias domains were assessed (i.e., selection, comparability, exposure/outcome), and an overall bias score was calculated (See Appendix D).
2.4. Data Management
All articles retrieved from the electronic databases were imported to EndNote, and duplicates were removed. After deduplication, titles and abstracts were uploaded to Rayyan, a web and mobile app for systematic reviews, for screening [16]. In stage one of screening, reviewers independently screened titles and abstracts of articles yielded by the search to identify potentially eligible studies based on the inclusion criteria (CD, BM). In stage two, if eligibility was unclear from the review of title and abstract, the full text was obtained for further assessment, and discrepancies were resolved by discussion between reviewers and consensus (CD, BM, JM, RT). In stage three of the screening process, full versions of relevant articles were obtained and carefully assessed to ensure they fit the predetermined study criteria. The full-text screening was conducted by two independent reviewers (CD, BM). Discrepancies were resolved by consensus among the study team (CD, BM, JM, RT).
2.5. Data Extraction
Extracted data included year published, study design, sample size, ethnicity, pre-pregnancy weight status, time point of pregnancy, physical activity assessment (frequency, intensity, time, type), infant body composition assessment, timepoint for infant body composition assessment, and main results found. Data from each study were extracted by one reviewer (BM) and checked by study team members (CD, RT, JM). All extracted data were organized by design type and risk of bias score, as shown in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9.
Table 1.
Characteristics of study populations.
| Randomized Control Trial | ||||
|---|---|---|---|---|
| Authors, Year, Ref. | Country | Sample Size | Ethnicity | Pre-Pregnancy Weight Status |
| Barakat et al., 2009 [17] | Spain | 160 | Caucasian | Training 24.3 ± 0.5 kg/m2
Control 23.4 ± 0.5 kg/m2 |
| Bisson et al., 2015 [18] | Canada | 50 | Caucasian (90%) Other |
Training 34.6 ± 5.4 kg/m2 Control 33.9 ± 4.5 kg/m2 |
| Clapp et al., 2000 [19] | USA | 46 | NA | Training 62.1 ± 1.1 kg Control 61.7 ± 1.3 kg |
| Clapp et al., 2002 [20] | USA | 75 | NA | Training * Lo–Hi 59.2 ± kg Mod–Mod 60.5 ± 1.1 kg Hi–Lo 58.9 ± 1.1 kg |
| Clark et al., 2019 [21] | USA | 36 | NA | Training 24.0 ± 5.2 kg/m2 Control 28.1 ± 8.0 kg/m2 |
| Garnaes et al., 2017 [22] | Norway | 74 | Caucasian Other |
Training 33.9 ± 3.8 kg/m2 Control 35.1 ± 4.6 kg/m2 |
| Hoffman et al., 2019 [23] | Germany | 2018 | German (88%) Other |
Training and Control 24.4 ± kg/m2 |
| Hopkins et al., 2010 [24] | New Zealand | 84 | European (94%) Other |
Training 25.5 ± 4.3 kg/m2 Control 25.4 ± 2.9 kg/m2 |
| Seneviratne et al., 2017 [25] | New Zealand | 72 | Maori, Pacific Islander, NZ/European, Other | Training and Control 33.25 ± 5.4 kg/m2 |
| Seneviratne et al., 2016 [26] | New Zealand | 75 | Maori, Pacific Islander, NZ/European, Other | Training and Control >25.0 kg/m2 |
| Sklempe Kokic et al., 2018 [27] | Croatia | 42 | NA | Training 24.39 ± 4.9 kg/m2 Control 25.29 ± 4.7 kg/m2 |
| Trak-Fellermeier et al., 2019 [28] | Puerto Rico | 31 | Black/AA (26%) Caucasian (22%) Other |
Training 34.6 ± 8.0 kg/m2 Control 36.0 ± 7.0 kg/m2 |
| Van Poppel, et al., 2019 [29] | Netherlands | 334 | Caucasian (65%) Other |
Training 33.8 ± 3.9 kg/m2 Control 33.7 ± 3.7 kg/m2 |
* Lo–Hi consisted of moderate intensity weight-bearing exercise for 20 min, 5 days/week, through week 20 gestational age (GA), gradually increasing to 60 min, 5 days/week, by week 24 and maintaining that regimen until delivery; Mod–Mod consisted of moderate-intensity weight-bearing exercise for 40 min, 5 days/week, from week 8 until delivery; Hi–Lo consisted of moderate-intensity weight-bearing exercise for 60 min, 5 days/week, through week 20, gradually decreasing to 20 min, 5 days/week, by week 24 and maintaining that regimen until delivery.
Table 2.
Characteristics of study populations.
| Cohort: Single-Arm Interventions | ||||
|---|---|---|---|---|
| Authors, Year, Ref. | Country | Sample Size | Ethnicity | Pre-Pregnancy Weight Status * |
| Badon et al., 2018 [30] | USA | 3687 | Caucasian (86%) Other |
Normal (73%) Overweight/Obese (26%) |
| Badon et al., 2016 [31] | USA | 3310 | Caucasian (86%) Other |
Normal (71%) Overweight/Obese (25%) |
| Bisson et al., 2017 [32] | Canada | 104 | Caucasian (96%) African American Other |
23.7 ± 0.4 kg/m2 |
| Collings et al., 2020 [33] | U.K. | 6921 | Caucasian (50%) Other |
Active 24.7 kg/m2 Inactive 25.6 kg/m2 |
| Dahly et al., 2018 [34] | Ireland | 1754 | Caucasian (98%) Other |
Normal (65%) Overweight/Obese (35%) |
| Diaz et al., 2020 [35] | USA | 209 | Caucasian (85%) Other |
26 kg/m2 |
| Harrod et al., 2014 [36] | USA | 826 | Caucasian (53.4%) Black (16.7%) Hispanic Other |
25.8 ± 0.4 kg/m2 |
| Jones et al., 2020 [37] | USA | 103 | Caucasian (76%) Black Other |
26.4 kg/m2 |
| Joshi et al., 2005 [38] | India | 770 | Other | 18.2 ± 0.3 kg/m2 |
| Juhl et al., 2010 [39] | Denmark | 79,692 | Caucasian Other |
Normal (68%) Overweight/Obese (27%) |
| Mudd et al., 2019 [40] | USA | 37 | Caucasian (81%) Other |
Normal (62%) Overweight/Obese (38%) |
| Nagpal et al., 2018 [41] | Canada | 61 | Caucasian (88%) Other |
Normal (69%) Overweight/Obese (31%) |
| Norris et al., 2017 [42] | Norway | 1200 | Caucasian (98%) Other |
24 ± 0.5 kg/m2 |
| Przybylowicz et al., 2014 [43] | Poland | 510 | NA | Normal (71%) Overweight/Obese (18.5%) |
| Rao et al., 2003 [44] | India | 797 | Indonesian Other |
41.6 ± 5.1 kg |
| Watson et al., 2018 [45] | South Africa | 130 | Black | 27.7 ± 5.2 kg/m2 |
* kilogram per meters squared (kg/m2).
Table 3.
Characteristics of study populations.
| Case Control Studies | ||||
|---|---|---|---|---|
| Authors, Year, Ref. | Country | Sample Size | Ethnicity | Pre-Pregnancy Weight Status * |
| Tinius, Cahill, Strand, and Cade, 2016 [5] | USA | 32 | Caucasian (44%) African American (50%) Other (6%) |
Active 34.0 ± 3.7 kg/m2 Inactive 36.3 ± 4.3 kg/m2 |
| Clapp et al., 1998 [6] | USA | 104 | Caucasian | Exercise 60.0 ± 1.1 kg Control 60.4 ± 1.6 kg |
| Clapp and Capeless, 1990 [46] | USA | 77 | Caucasian | Exercise 57.7 ± 5.2 kg Control 58.1 ± 5.9 kg |
* kilogram (kg); kilogram per meters squared (kg/m2).
Table 4.
Methods and timing of maternal physical activity data collection.
| Randomized Control Trial | |||||
|---|---|---|---|---|---|
| Authors, Year, Ref. | PA Assessment Time Points * | Self-Reported | Objective | Total PA ** | LTPA *** |
| Barakat et al., 2009 [17] | 12–39 w | - | X | X | - |
| Bisson et al., 2015 [18] | 14, 28, 36 w | X | X | X | - |
| Clapp et al., 2000 [19] | 8–40 w | - | X | X | - |
| Clapp et al., 2002 [20] | 8–40 w | - | X | X | - |
| Clark et al., 2019 [21] | 16–36 w | X | X | X | - |
| Garnaes et al., 2017 [22] | 12–40 w | X | X | X | - |
| Hoffman et al., 2019 [23] | <12, 29 w | X | - | X | - |
| Hopkins et al., 2010 [24] | 20 w–delivery | X | X | X | - |
| Seneviratne et al., 2017 [25] | 19–36 w | X | X | X | - |
| Seneviratne et al., 2016 [26] | 20–36 w | - | X | X | - |
| Sklempe Kokic et al., 2018 [27] | 30–40 w | X | X | - | X |
| Trak-Fellermeier et al., 2019 [28] | <16, 24–27, 35–36 w | - | X | X | - |
| Van Poppel, et al., 2019 [29] | <20–37 w | X | X | - | X |
* physical activity (PA) assessment time points; weeks gestational age (w); weeks gestational age until delivery (w–delivery); ** total physical activity (PA); *** leisure time physical activity (LTPA).
Table 5.
Methods and timing of maternal physical activity data collection.
| Cohort: Single-Arm Interventions | |||||
|---|---|---|---|---|---|
| Authors, Year, Ref. | PA Assessment Time Points * | Self-Reported | Objective | Total PA ** | LTPA *** |
| Badon et al., 2018 [30] | 15 w | X | - | - | X |
| Badon et al., 2016 [31] | 15 w | X | - | - | X |
| Bisson et al., 2017 [32] | 17, 36 w | X | X | X | - |
| Collings et al., 2020 [33] | 26–28 w | X | - | - | X |
| Dahly et al., 2018 [34] | <15, 20 w | X | - | - | X |
| Diaz et al., 2020 [35] | <10 w | - | X | X | - |
| Harrod et al., 2014 [36] | 17, 27 w, delivery | X | - | X | X |
| Jones et al., 2020 [37] | 8–14, 20–23, 32–35 w | - | X | X | X |
| Joshi et al., 2005 [38] | 18, 28 w | X | - | X | - |
| Juhl et al., 2010 [39] | 16, 31 w | X | - | - | X |
| Mudd et al., 2019 [40] | Follow up at 4 y | X | - | X | - |
| Nagpal et al., 2018 [41] | 24–28 w | - | X | X | X |
| Norris et al., 2017 [42] | <15, 20 w | X | - | - | X |
| Przybylowicz et al., 2014 [43] | Follow up at 1 month PP | X | - | - | X |
| Rao et al., 2003 [44] | 18, 28 w | X | - | - | X |
| Watson et al., 2018 [45] | 14–18, 29–33 w | - | X | X | - |
* physical activity (PA) assessment time points; weeks gestational age (w); year (y); postpartum (PP); ** total physical activity (PA); *** leisure time physical activity (LTPA).
Table 6.
Methods and timing of maternal physical activity data collection.
| Case Control Studies | |||||
|---|---|---|---|---|---|
| Authors, Year, Ref. | PA Assessment Time Points * | Self-Reported | Objective | Total PA ** | LTPA *** |
| Tinius, Cahill, Strand, and Cade, 2016 [5] | 32–37 w | X | X | X | X |
| Clapp et al., 1998 [6] | Follow up at 1 y | - | X | X | - |
| Clapp and Capeless, 1990 [46] | Preconception and throughout pregnancy | - | X | X | - |
* physical activity (PA) assessment time points; weeks gestational age (w); year (y); ** total physical activity (PA); *** leisure time physical activity (LTPA).
Table 7.
Description of maternal physical activity, newborn body composition assessment and timing, and summary of key findings.
| Randomized Control Trial | |||||
|---|---|---|---|---|---|
| Authors, Year, Ref. | Description of Maternal PA * | Newborn Body Comp. ASMT ** | ASMT Timing *** |
Key Findings | Body Comp.Diff. at Birth |
| Barakat et al., 2009 [17] | Resistance Training 3×/w | PI | At birth | No sig. assoc. between training women and infant outcomes. Control women’s pre-pregnancy weight positively assoc. with infant birthweight. | No |
| Bisson et al., 2015 [18] | Aerobics 3×/w; Resistance Training 3×/w; Accelerometer; PPAQ |
SF | w/n 72 h of birth | No sig. diff. in infant outcomes between the training and control group. | No |
| Clapp et al., 2000 [19] | Aerobics 3×/w | SF PI TBEC |
At birth TBEC at 5 days |
Infants were sig. heavier, longer, and had more lean mass in the exercise group compared to the control group. All other infant outcomes not sig. diff. | No |
| Clapp et al., 2002 [20] | Aerobics 3–5×/w | SF PI TBEC |
At birth TBEC at 5 days |
Infants with moms who slowly increased exercise volume from first trimester (low) to third (high) were sig. smaller (smaller birth weight, smaller PI, body fat percent, fat mass and lean mass) than those infants whose moms started the first trimester with a high volume and decreased volume throughout pregnancy (high–low). | Yes |
| Clark et al., 2019 [21] | Aerobics 3×/w; MPAQ; HR monitoring |
PI BMI Abd. Cir. |
At birth | Pre-pregnancy PA levels sig. assoc. with PI and BMI. Infant head circumference in the exercise group significantly larger than infants in the control group. | No |
| Garnaes et al., 2017 [22] | Aerobics 3×/w; Resistance Training 2×/w |
SF Abd. Cir. |
At birth | No sig. diff. in infant outcomes between exercise and control group. | No |
| Hoffman et al., 2019 [23] | PPAQ | BMI | At birth | Women who were more active in late pregnancy sig. assoc. with a larger infant birthweight. | No |
| Hopkins et al., 2010 [24] | Aerobics 5×/w; HR monitoring |
DXA PI BMI |
w/n 48 h of birth DXA at 17 days |
15 w exercise program during later pregnancy assoc. with reduced birth weights, but there were equal reductions in FM/FFM to account for the difference in weight, not just fat mass reductions, between the exercise and control groups. BMI sig. lower at birth in exercise vs. control; however, PI was not sig. diff. | No |
| Seneviratne et al., 2017 [25] | PPAQ; Aerobics 3–5×/w |
DXA PI |
w/n 2 w of birth | No sig. diff. in infant outcomes between the exercise and control group. | No |
| Seneviratne et al., 2016 [26] | Aerobics 3–5×/w | PI BMI |
At birth | No sig. diff. in infant outcomes between exercise and control groups. | No |
| Sklempe Kokic et al., 2018 [27] | PPAQ; Aerobics 2×/w; Resistance Training 2×/w |
PI | At birth | No sig. diff. in infant outcomes between the exercise and control group | No |
| Trak-Fellermeier et al., 2019 [28] | Accelerometer | SF PI Abd. Cir. |
w/n one week | No sig. diff. in infant outcomes between the exercise and control group. SF data not presented | No |
| Van Poppel, et al., 2019 [29] | Aerobics 2×/w; PPAQ; Interview |
SF Abd. Cir. |
w/n 48 h of birth | No sig. diff. in infant outcomes between exercise and control group. | No |
* Description of maternal physical activity (PA); times per week (x/w); pregnancy physical activity questionnaire (PPAQ); modifiable physical activity questionnaire (MPAQ); heart rate (HR); ** Newborn body composition (Comp.) assessment (ASMT); ponderal index (PI); skin fold (SF); total body electrical conductivity (TBEC); body mass index (BMI); dual energy X-ray absorptiometry (DXA) abdominal circumference (Abd. Cir.); *** Assessment (ASTM) timing; within (w/n); hours (h).
Table 8.
Description of maternal physical activity, newborn body composition assessment and timing, and summary of key findings.
| Cohort: Single-Arm Interventions | |||||
|---|---|---|---|---|---|
| Authors, Year, Ref. | Description of Maternal PA * | Newborn Body Comp. ASMT ** | ASMT Timing *** |
Key Findings | Body Comp. Diff. at Birth |
| Badon et al., 2018 [30] | Focus Group Interview |
PI | At birth | Yoga no assoc. with PI. Light to mod walking during pre-pregnancy and first trimester assoc. with infants’ greater PI. Longer durations/bouts of light to mod walking had larger increases of infant PI compared to women with no LTPA. | No |
| Badon et al., 2016 [31] | Interview | PI | At birth | Pre-pregnancy or first trimester LTPA not assoc. with PI. | No |
| Bisson et al., 2017 [32] | PPAQ Accelerometer |
DXA | ~12 days post birth | No sig. diff. in infant outcomes with any mod. PA across any trimester. Vig. PA in third trimester related to sig. lower infant BF and sig. smaller change in fat mass at 4 y. | No |
| Collings et al., 2020 [33] | GPPAQ | SF Abd. Cir. |
w/n 24–72 h of birth | Higher mid-pregnancy PA levels for white British women were assoc. with smaller infant SF tricep and subscapular sum. | Yes |
| Dahly et al., 2018 [34] | Interview | PeaPod | w/n 48 h of birth | Mod PA during first trimester assoc. with lower infant BF. | Yes |
| Diaz et al., 2020 [35] | Accelerometer | PeaPod | w/n 2 w of birth | No assoc. found between PA measured by accelerometer and infant %BF. Maternal %BF was positively assoc. with both male and female infant %BF. | No |
| Harrod et al., 2014 [36] | PPAQ | PeaPod SF Abd. Cir. |
w/n 72 h of birth | No sig. diff. for early and mid pregnancy total energy expenditure and infant fat mass/fat-free mass. Increasing late-pregnancy levels of TEE were sig. assoc. with decreased levels of infant adiposity; at extreme ends of total energy expenditure there was a sig. diff. in infant FM. | Yes |
| Jones et al., 2020 [37] | Accelerometer | PI Head Cir. |
At birth | High vs. Low SED was assoc. with larger HC, longer BL, and lower PI; High MVPA was assoc. with smaller HC but was not assoc. with PI. | No |
| Joshi et al., 2005 [38] | PA Survey | SF Abd. Cir. |
w/n 72 h of birth | No sig. diff. between PA levels and infant outcomes. | No |
| Juhl et al., 2010 [39] | Interview | PI Abd. Cir. |
At birth | Sig. negative trend of exercise time during second trimester and infant abd. cir. | No |
| Mudd et al., 2019 [40] | Recall Questionnaire | PeaPod | w/n 2 w to 3 months of birth | Any mod PA in any trimester not assoc. with infant body comp. Vig. PA in third trimester sig. assoc. with lower infant BF and sig. smaller change in FM at 4 y. | No |
| Nagpal et al., 2018 [41] | Accelerometer | SF | w/n 24–48 h of birth | Sedentary time and MVPA not assoc. with infant BW or %BF. | No |
| Norris et al., 2017 [42] | Interview | PeaPod | w/n 72 h of birth | Pre-pregnancy and first trimester PA levels not assoc. with infant adiposity. Women with no PA between 15–20 w gestation were twice as likely to give birth to infants with adiposity above the 90th percentile. | No |
| Przybylowicz et al., 2014 [43] | PA Survey | PI | At birth | No sig. diff. in PI with any PA levels. | No |
| Rao et al., 2003 [44] | Focus Group Interview |
SF Abd. Cir. |
At birth | Maternal PA level not assoc. with abd. cir. or infant FM/FFM. | No |
| Watson et al., 2018 [45] | Accelerometer | PI | w/n 48 h of birth | No maternal PA assoc. with PI. | No |
* Description of maternal physical activity (PA); pregnancy physical activity questionnaire (PPAQ); general practice physical activity questionnaire (GPPAQ); ** Newborn body composition (Comp.) assessment (ASMT); ponderal index (PI); skin fold (SF); head circumference (Cir.); dual energy X-ray absorptiometry (DXA); abdominal circumference (Abd. Cir.); *** Assessment (ASTM) timing; within (w/n); hours (h); weeks (w).
Table 9.
Description of maternal physical activity, newborn body composition assessment and timing, and summary of key findings.
| Case Control Studies | |||||
|---|---|---|---|---|---|
| Authors, Year, Ref | Description of Maternal PA * | Newborn Body Comp. ASMT. ** | ASMT Timing *** | Key Findings | Body Comp. Diff. @ Birth |
| Tinius, Cahill, Strand, & Cade, 2016 [5] | Health Survey Accelerometer |
PeaPod SF |
w/n 48 h of birth | No sig. diff. in infant outcomes assoc. with maternal PA levels | No |
| Clapp et al., 1998 [6] | Aerobics 3x/w HR monitoring |
SF Abd Cir. TBEC |
w/n 24 h of birth | Exercise group assoc. with sig. lower infant %BF at birth but not sig. at one year | No |
| Clapp & Capeless, 1990 [46] | Aerobics 3x/w HR monitoring |
SF PI |
<2 h of birth | Exercise group had infants with sig. smaller FM & %BF. Duration of exercise but not type of exercise assoc. with sig. smaller PI | Yes |
* Description of maternal physical activity (PA); heart rate (HR); times per week (x/w); ** Newborn body composition (Comp.) assessment (ASMT); ponderal index (PI); skin fold (SF); total body electrical conductivity (TBEC); abdominal circumference (Abd. Cir.); *** Assessment (ASTM) timing; within (w/n); hours (h).
3. Results
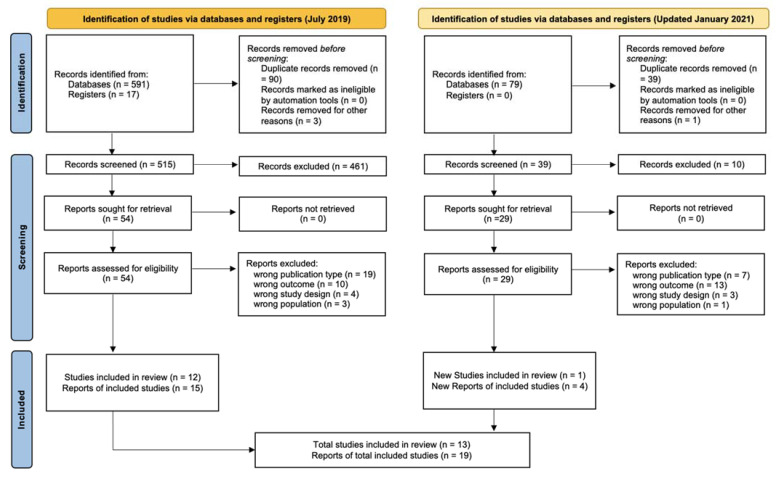
The PRISMA flow chart (Figure 1) shows the number of articles at each stage of the screening process. Database searches identified a total of 591 studies, and an additional 17 studies were identified through other sources. An updated search was completed on 8 January 2021, and a further 79 studies were identified. Duplicates were removed, and of the 554 studies screened, 471 were excluded based on title, abstract, or outcomes measured. The remaining 83 studies were further evaluated based on full text, and 51 articles were excluded for wrong publication type (n = 26) (i.e., systematic review, study protocol, cohort characteristics report, conference presentation, book), wrong study design (n = 7), and wrong outcome (n = 23) (i.e., ineligible infant measurements); 32 articles were included for evaluation. Of these, 13 were randomized controlled trials [17,18,19,20,21,22,23,24,25,26,27,28,29], 16 were single-arm cohort studies [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45], and 3 were case-control studies [5,6,46].
Figure 1.
PRISMA flow diagram. Modified from Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. doi:10.1136/bmj.n71 [47].
Overall, prenatal exercise was not associated with infant body composition at birth. Of the 32 articles included, only five suggested a difference in infant body composition could be influenced by prenatal physical activity [20,33,34,36,46]. Specifically, those five studies found that women with a greater total volume of mid-to-late term physical activity gave birth to infants with less fat mass than those who had little to no PA throughout pregnancy.
Risk of Bias Assessment
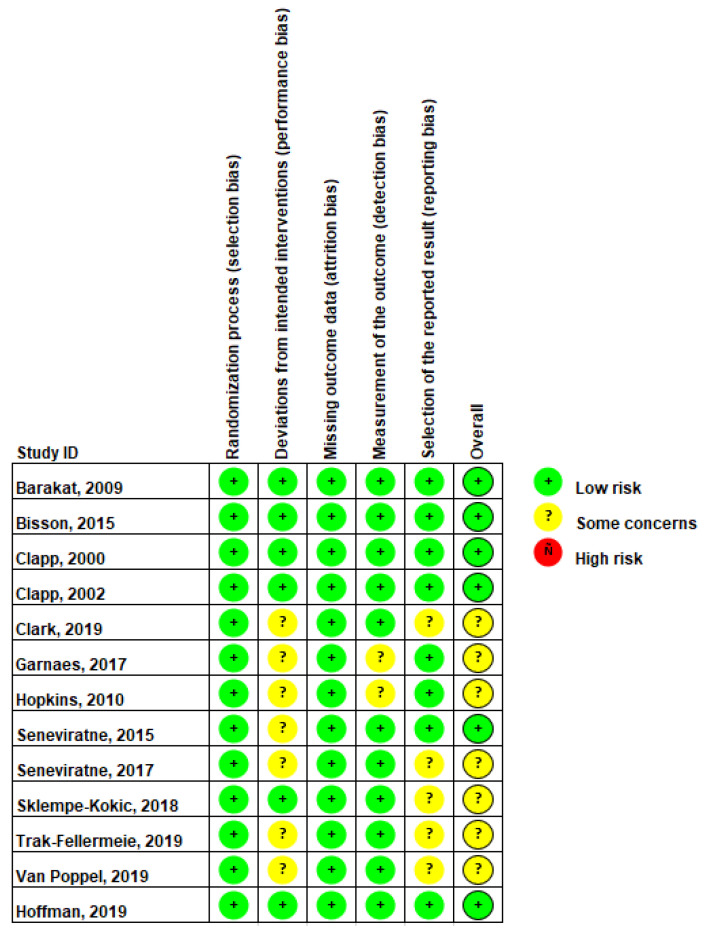
The overall risk of bias for randomized controlled trials was low to- moderate. Approximately 54% of the studies (7 of 13) were rated as having “some concerns” for the overall potential risk of bias [21,22,24,25,27,28,29] (Figure 2). This rating was primarily due to the seven studies being rated as having “some concerns” in the performance risk of bias domain, which was assessed by rating deviations from the intended intervention. There was a low risk of selection bias for all studies, as all studies reported a random allocation sequence. Only one study did not provide enough information regarding the allocation sequence being concealed until after participants were enrolled/assigned to the intervention [23]. A summary of the RCT RoB scoring is listed in Figure 2.
Figure 2.
Randomized controlled trial risk of bias summary. Baraket, 2009 [17], Bisson, 2015 [18], Clapp, 2000 [19], Clapp, 2002 [20], Clark, 2019 [21], Garnaes, 2017 [22], Hopkins, 2010 [24], Seneviratne, 2015 [25], Seneviratne, 2017 [26], Sklempe-Kokic, 2018 [27], Trak-Fellermeie, 2019 [28], Van Poppel, 2019 [29], Hoffman, 2019 [23].
For case-control and cohort studies, the risk of bias was generally low to moderate. We did not detect strong evidence of publication bias in these studies (See Appendix D).
4. Discussion
Overall, this systematic review suggests maternal PA during pregnancy has minimal implications on neonatal adiposity, except for extremely high volumes of PA. However, due to the variability in numerous elements of the study design (e.g., PA assessment method, neonatal adiposity assessment method, and timing of data collections), it remained a challenge to synthesize findings. These data suggest that maternal physical activity does not appear to have a negative or detrimental impact on the body composition of the neonate, which is an important conclusion given fear of harm to the unborn child is a factor that prevents many women from getting or staying active during pregnancy [48]. In this review, we report that five studies noted a relationship between maternal PA and infant body composition [20,33,34,36,46]; however, none of them reported utilizing rigorous methods for assessing both maternal PA and neonatal body composition.
4.1. Summary of Studies That Showed Increased Maternal PA Is Associated with Decreased Neonatal Adiposity
Only one of thirteen randomized clinical trials had findings indicating that maternal PA has an impact on neonatal anthropometrics [20]. Specifically, findings from this study indicate that previously active women that decrease their physical activity levels by 67% (below the recommended 150 min/week) starting at mid-pregnancy through delivery, had heavier babies at birth (3.9 kg) with a higher amount of body fat (12.1%) compared to women who maintained relatively moderate to high levels of physical activity (at least 150 min/week) from mid-pregnancy to delivery (3.4 kg, 7.9%). Given that RCTs are the highest level of evidence, and 12 of 13 did not detect significant differences in neonatal adiposity between active and inactive women (with a variety of activities and intervention protocols), these collective findings suggest physical activity has a minimal impact on offspring adiposity.
Out of the 16 single-arm cohort studies that were identified, three studies found that maternal physical activity levels were associated with smaller amounts of neonatal adiposity [33,34,36]. In the most recent study from Collings et al., 2020, the authors found that among British white women there was a negative dose-response relationship. Women with moderate to high levels of maternal physical activity during mid-pregnancy (150 min or more per week of moderate intensity exercise) were associated with smaller infant skinfold measurements. However, this relationship was not apparent in the portion of the cohort that identified as ethnic minorities.
The second cohort study by Dahly et al., 2018, determined that the prevalence of babies born with very high fat mass was lowest among women that engaged in frequent moderate-intensity exercise early in pregnancy [34]. While neonatal body composition was assessed with air displacement plethysmography, maternal physical activity was subjectively self-reported with a one-question survey at 15 weeks gestation. This is problematic, as oftentimes physical activity levels change throughout pregnancy [49]. In addition, the maternal PA data were collected with a non-validated single question with only three possible responses (none, some (1–3 times per week) or often (4+ times per week)), which may lead to a lack of sensitivity for detecting important relationships.
The third cohort study, by Harrod et al., 2014, found that increasing levels of maternal physical activity, particularly during late pregnancy, are negatively correlated to neonatal adiposity [36]. In this cohort, women with high levels of late-pregnancy activity had an increased likelihood of SGA compared to women with low levels of physical activity. The authors suggest that the effect could be attributed to a decrease in neonatal fat mass, because there were no differences in neonatal fat-free mass stratified by total energy expenditure quartiles.
Out of three case-control cohort studies included, one study found a relationship between maternal physical activity and neonatal body composition [46]. Clapp and Capeless, 1990, compared infant outcomes between conditioned recreational runners and aerobic dancers (who maintained activity at or above 50% of preconception levels) and matched inactive controls. Infants born to active women had lower birth weight, ponderal index, two-site skinfold thickness, and calculated percent body fat, leading to the conclusion that continuation of regular aerobic exercise results in a reduction in neonatal fat mass. Specifically, the authors reported that differences in neonatal fat mass explained over 70% of the difference in birth weight [46].
4.2. Accuracy of PA Assessment (i.e., Type of Assessment, Timing, and Intensity)
Although a cohort design allows for free-living PA to be reported and/or recorded in addition to sports and exercise leisure-time PA, a randomized control trial that implements an intervention may be able to pinpoint specific PA modifying factors (i.e., duration, frequency, type, and intensity) and determine a direct causal relationship between exercise and infant body composition. It is plausible that specific modes, intensities, and/or volumes can have differential effects on infant body composition. Once again, this poses significant challenges to drawing broad conclusions about the relationship between general physical activity and infant adiposity.
While the Pregnancy Physical Activity Questionnaire (PPAQ) is commonly used across the literature, it has been found to overestimate leisure-time PA across all activity categories [50]. The PPAQ also only provides subjective/self-reported exercise information that is subject to recall bias. The accelerometer is the gold standard for objectively measuring free-living PA [51] and is the most reliable and accurate method for assessing MVPA during pregnancy [52]. Because only eight studies utilized objective accelerometry, conclusions regarding the role of PA on infant outcomes must be drawn carefully. In addition to the measurement of PA, the studies included in the review contained a variety of exercise modes (e.g., running, walking, dancing, swimming, cycling, weightlifting), once again posing a challenge in drawing definitive conclusions across studies, as different modes and intensities may have different implications for fetal growth.
Another factor that warrants consideration is the timepoint in pregnancy at which maternal physical activity assessments are taken. Some studies focus on early pregnancy, others on late pregnancy, and some assess throughout (See Table 7, Table 8 and Table 9). Upon careful examination, it is clear that the gestation period in which exercise occurs can impact the interpretation of the relationship between maternal activity levels and neonatal adiposity. Several studies reported late pregnancy activity levels [36,46], while others measured activity early on [34]. Once again, these differences in study design make it challenging to synthesize results, as maternal–fetal physiology changes drastically throughout pregnancy. Therefore, it is feasible that physical activity can impact fetal growth differently depending on the timepoint of pregnancy. For example, pregnant women become more insulin resistant as pregnancy progresses [53]; thus, fuel and substrate utilization change dramatically throughout a pregnancy [54]. The timing of pregnancy in which exercise occurs most likely confounds the relationship between exercise and infant adiposity.
4.3. Accuracy of Infant Anthropometric Assessments (i.e., Type of Assessment and Timing)
Another aspect of study design that may play a role in interpreting the relationship between maternal PA and infant anthropometrics is the accuracy of the infant assessment. The majority of included studies utilized skinfolds and/or ponderal indices, both of which are considered poor and imprecise measures of assessing infant body composition [55]. They are often used due to low cost and feasibility; however, it is clear that more accurate assessments of infant body composition (e.g., air displacement plethysmography) are needed in order to design high-quality studies. In addition to the type of anthropometric assessment tool being used, studies often vary in the timepoint at which they assess infant anthropometrics. Varying the timepoint of assessment can be problematic when comparing across studies, as infant size changes rapidly during early development [56]. For example, once the infant starts receiving either breastmilk or formula, that difference in dietary composition alone can impact infant growth patterns [57]. In order to minimize the impact of the infant’s external environment and nutrient consumption (which can make it nearly impossible to draw any sort of conclusion about the direct impact of physical activity during pregnancy on infant adiposity), studies should attempt to take newborn assessments within the first 48 h of life. However, when observing the findings in Table 7, Table 8 and Table 9, many studies assessed infant adiposity around 2 weeks of age.
4.4. Is There an Optimal Amount of Physical Activity to Ensure Healthy Neonatal Adiposity?
Evidence suggests that only extreme levels of physical inactivity/activity seem to influence neonatal adiposity. Specifically, higher levels of maternal physical activity and vigorous maternal physical activity, particularly when performed throughout late pregnancy, are consistently associated with decreased neonatal fat mass. There may be a “threshold” point where moderate exercise is beneficial but heavy exercise is detrimental, at least with respect to birth weight [58]. It is unclear what the threshold is at this point. This does have some clinical utility in terms of being able to appropriately counsel the increasing number of women that wish to maintain high levels of physical activity during pregnancy. An increasing number of elite female athletes are choosing to enter motherhood during their competitive years. In fact, a recent consensus statement suggests that since the fertile age of many athletes overlaps with timing for peak performance, adequate information about the implications of exercise during pregnancy is needed so women can make informed decisions [59]. This systematic review adds to the body of literature that suggests pregnant women can feel reassured that they can continue exercise, but depending on the activity, may have to make small adjustments in intensity and volume in order to maintain appropriate fetal growth [59].
It is also possible that a minimal level of physical activity exists to prevent overgrowth. In fact, moderate maternal physical activity promotes appropriate fetal growth. For example, work from Barakat et al. suggests that light intensity resistance exercise training performed during the second and third trimester might attenuate the adverse consequences of maternal body weight before pregnancy on neonatal anthropometrics [17]. Specifically, Barakat et al. found that maternal body weight was positively and significantly associated with birthweight in the control group, but there was no association in the exercise training group. Others have reported that maternal pre-pregnancy BMI and gestational weight gain are positively and independently associated with neonatal adiposity [60]. It is possible that the impact of maternal physical activity on neonatal adiposity may differ according to maternal pre-pregnancy weight status and/or gestational weight gain status.
5. Conclusions
Despite the inherent limitations discussed above, which limit the ability to draw conclusions across studies, some important findings were noted. Collectively, the studies suggest that continuing a regular physical activity regimen during pregnancy does not compromise fetoplacental growth. This is critically important, as many women do not engage in appropriate levels of PA due to fear of harm to the unborn child [48]. Pregnant women should be counselled to continue or begin physical activity regimens, as it appears that physical activity (at reasonable intensities and volumes) has minimal effects on infant body composition.
Given the ongoing obesity epidemic, there is a need to identify children at risk of developing obesity as early in life as possible [48]. It is a challenge to identify neonates at risk as there are no published norms for “appropriate” or “ideal” levels of neonatal body fat percentage and/or fat mass (as exist for adults). Because of this, it is difficult to determine “ideal” amounts of PA as it relates to the impact of PA on infant body composition. However, given there were no clear trends of maternal PA on infant body fat percentage among the studies included in this review, it seems reasonable to conclude that PA during pregnancy, at most any level, is beneficial to the mother without unfavorably compromising fetal growth. With PA during pregnancy having substantial maternal and neonatal benefits [1], results from this review add to the consensus that health care providers should continue to encourage PA among patients while dispelling myths that PA could be harmful to the growth of the baby.
Acknowledgments
We would like to thank Rebecca Harrington, MSLIS, AHIP, for her initial help with the search strategy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19127127/s1.
Appendix A
Table A1.
PRISMA checklist.
| Section and Topic | Item # | Location Where Item Is Reported | Checklist Item |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | p. 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | p. 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | pp. 1–2 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | p. 2 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | p. 2–3 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | pp. 2–3 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | Appendix B |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | pp. 5–6 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | pp. 5–6 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | pp. 5–6 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | p. 4 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | p. 5 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | Supplemental Tables S1–S3 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | pp. 5–6 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics or data conversions. | NA | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | p. 3 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | p. 3 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | p. 3 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | NA |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | p. 5 |
| 16b | Cite studies that might appear to meet the inclusion criteria but which were excluded, and explain why they were excluded. | p. 5 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | p. 4 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | pp. 6, 21 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Supplemental Tables S1–S3 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | pp. 5–6 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | NA | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | NA | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | NA | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | p. 12 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | NA |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | p. 13 |
| 23b | Discuss any limitations of the evidence included in the review. | pp. 14–15 | |
| 23c | Discuss any limitations of the review processes used. | ||
| 23d | Discuss implications of the results for practice, policy, and future research. | p. 15 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | p. 2 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | ||
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | ||
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | |
| Competing interests | 26 | Declare any competing interests of review authors. | |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | |
Appendix B
This search strategy was developed with the assistance of an information specialist experienced with systematic reviews. The strategies combined medical subject headings (MeSH) and keywords for exercise/physical activity, pregnancy, neonate, and body composition.
PubMed Search Strategy:
(((((((“(“Birth Weight” [Title/Abstract] OR “Body Mass Index” [Title/Abstract] OR “Body Composition” [Title/Abstract] OR Anthropometr* [Title/Abstract] OR adipos* [Title/Abstract] OR peapod))) AND ((Infant, Newborn [Mesh] OR ((neonatal NOT (intensive OR ICU)))))) AND ((((Exercise [Title/Abstract] OR “physical activity” [Title/Abstract] OR aerobic [Title/Abstract]))) AND Pregnancy [Title/Abstract]))) NOT (((“Animals” [Mesh] NOT (“Animals” [Mesh] AND “Humans” [Mesh]))).
Appendix C. Newcastle–Ottawa Quality Assessment Scale
Note: This scale has been adapted from the Newcastle–Ottawa Quality Assessment Scale for cohort studies to perform a quality assessment of these studies for the systematic review.
Selection: Maximum of 7 stars.
-
(1)Representativeness of the exposed or high(er/est) PA cohort
-
(a)truly representative of all subjects from which they were recruited (1 star)
-
(b)somewhat representative (1 star)
-
(c)selected group (e.g., nurses, volunteers) or a subset of larger cohort study
-
(d)no description of the derivation of the cohort
-
(a)
-
(2)Selection of the non-exposed (control or sedentary or low PA) cohort
-
(a)drawn from the same community as the exposed cohort (1 star)
-
(b)drawn from a different source
-
(c)no description of the derivation of the non-exposed cohort
-
(a)
-
(3)Ascertainment of exposure (maternal PA)
-
(a)objective assessment (i.e., accelerometer), supervised exercise sessions, or validated measurement tool (PPAQ) (2 stars)
-
(b)non-validated PA measurement tool (but the tool is available or described), self-report, or self-reported or written exercise session attendance/recall (1 star)
-
(c)no description
-
(a)
-
(4)Timing/duration of exposure (maternal PA)
-
(a)maternal PA was assessed and reported at multiple time points during pregnancy (2 stars)
-
(b)maternal PA was assessed and reported in late pregnancy only (1 star)
-
(c)maternal PA was assessed and reported in early pregnancy or pre-conception only
-
(a)
-
(5)Sample size
-
(a)justified and satisfactory, including power analysis (1 star)
-
(b)not justified
-
(a)
Comparability: Maximum of 3 stars. If applicable, answer choices “a” AND “b” AND “c” can be selected.
-
(1)Comparability of cohorts/groups based on the design or analysis. The subjects in different exposure or outcome groups are comparable, based on the study design or analysis. Confounding factors are controlled.
-
(a)study controls for maternal weight status (1 star)
-
(b)study controls for neonatal sex and/or gestational age (1 star)
-
(c)study controls for any additional factors (i.e., maternal age, gestational weight gain, parity, other factors related to maternal metabolic health, etc.) (1 star)
-
(d)cohorts are not comparable based on the fact that the design or analysis failed to control for confounders
-
(a)
Outcome (neonatal adiposity): Maximum of 4 stars.
-
(1)Assessment of outcome (neonatal adiposity)
-
(a)gold standard assessment (e.g., ADP or DXA) and/or independent blind assessment (2 stars)
-
(b)record linkage (1 star)
-
(c)self-report
-
(d)no description or other
-
(a)
-
(2)Statistical test
-
(a)the statistical test used to analyze the data is clearly described and appropriate, and the measurement of the associated is presented, including confidence interval and the probability level (p-value) (1 star)
-
(b)the statistical test is not appropriate, not described, or incomplete
-
(a)
-
(3)Adequacy of follow-up of cohorts
-
(a)complete follow-up—all subjects accounted for (1 star)
-
(b)subjects lost to follow-up unlikely to introduce bias—small number lost ≥80% follow-up, or description provided of those lost (1 star)
-
(c)follow-up rate <80% and no description of those lost
-
(d)no statement.
-
(a)
Appendix D
Table A2.
Newcastle–Ottawa scale or non-randomized studies’ ROB rating.
| Study | Selection | Comparability | Exposure/Outcome | Overall Rating (More Stars = Lower Risk of Bias) |
|---|---|---|---|---|
| Case-Control | ||||
| Clapp-1990 | ★★★3 | ★★★3 | ★★2 | ★★★★★★★★8 |
| Clapp-1998 | ★★★★★5 | ★★★3 | ★★★★4 | ★★★★★★★★★★★★12 |
| Tinius-2016 | ★★★★★★6 | ★1 | ★★2 | ★★★★★★★★★9 |
| Single Arm Cohort | ||||
| Badon-2016 | ★★★3 | ★★★3 | ★★★3 | ★★★★★★★★★9 |
| Badon-2018 | ★★★3 | ★★★3 | ★★★3 | ★★★★★★★★★9 |
| Bisson-2017 | ★★★★★★★7 | ★★★3 | ★★★★4 | ★★★★★★★★★★★★★★14 |
| Collings-2020 | ★★★★★★6 | ★★★3 | ★★★3 | ★★★★★★★★★★★★12 |
| Dahly-2018 | ★★★3 | ★★2 | ★★2 | ★★★★★★★7 |
| Diaz-2020 | ★★★★4 | ★★★3 | ★★★★4 | ★★★★★★★★★★★11 |
| Harrod-2014 | ★★★★★★6 | ★★★3 | ★★★★4 | ★★★★★★★★★★★★★13 |
| Jones-2020 | ★★★★★★6 | ★★★3 | ★★★3 | ★★★★★★★★★★★★12 |
| Joshi-2005 | ★★★3 | −0 | ★1 | ★★★★4 |
| Juhl-2010 | ★★2 | ★★★3 | ★★★3 | ★★★★★★★★8 |
| Mudd-2019 | ★★★★★5 | ★★★3 | ★★★3 | ★★★★★★★★★★★11 |
| Nagpal-2020 | ★★★★★5 | ★★★3 | ★★★2 | ★★★★★★★★★★10 |
| Norris-2017 | ★★2 | ★★2 | ★★★★4 | ★★★★★★★★8 |
| Przybylowicz-2014 | ★★★3 | −0 | ★★2 | ★★★★★5 |
| Rao-2003 | ★★★★★5 | ★★★3 | ★★2 | ★★★★★★★★★★10 |
| Watson-2018 | ★★★★★5 | ★★★3 | ★★2 | ★★★★★★★★★★10 |
Author Contributions
Conceptualization, B.R.M., R.A.T. and J.M.M.; methodology, B.R.M., C.D., R.A.T., A.Q.W., E.A.A. and J.M.M.; validation, B.R.M., C.D., R.A.T., A.Q.W., E.A.A. and J.M.M.; formal analysis, B.R.M. and J.M.M.; writing—original draft preparation, B.R.M.; writing—review and editing, B.R.M., C.D., R.A.T., A.Q.W., E.A.A. and J.M.M.; visualization, B.R.M. and J.M.M.; supervision, R.A.T. and J.M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ACOG Committee Physical Activity and Exercise during Pregnancy and the Postpartum Period: ACOG Committee Opinion Summary, Number 804. Obstet. Gynecol. 2020;135:991–993. doi: 10.1097/AOG.0000000000003773. [DOI] [PubMed] [Google Scholar]
- 2.Lotgering F.K. 30(+) years of exercise in pregnancy. Adv. Exp. Med. Biol. 2014;814:109–116. doi: 10.1007/978-1-4939-1031-1_10. [DOI] [PubMed] [Google Scholar]
- 3.Mottola M.F. Physical activity and maternal obesity: Cardiovascular adaptations, exercise recommendations, and pregnancy outcomes. Nutr. Rev. 2013;71((Suppl. S1)):S31–S36. doi: 10.1111/nure.12064. [DOI] [PubMed] [Google Scholar]
- 4.Mudd L.M., Owe K.M., Mottola M.F., Pivarnik J.M. Health benefits of physical activity during pregnancy: An international perspective. Med. Sci. Sports Exerc. 2013;45:268–277. doi: 10.1249/MSS.0b013e31826cebcb. [DOI] [PubMed] [Google Scholar]
- 5.Tinius R.A., Cahill A.G., Strand E.A., Cade W.T. Maternal inflammation during late pregnancy is lower in physically active compared with inactive obese women. Appl. Physiol. Nutr. Metab. 2016;41:191–198. doi: 10.1139/apnm-2015-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapp J.F., 3rd, Lopez B., Harcar-Sevcik R. Neonatal behavioral profile of the offspring of women who continued to exercise regularly throughout pregnancy. Am. J. Obstet. Gynecol. 1999;180:91–94. doi: 10.1016/S0002-9378(99)70155-9. [DOI] [PubMed] [Google Scholar]
- 7.Moyer C., Reoyo O.R., May L. The Influence of Prenatal Exercise on Offspring Health: A Review. Clin. Med. Insights Womens Health. 2016;9:37–42. doi: 10.4137/CMWH.S34670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisson M., Croteau J., Guinhouya B.C., Bujold E., Audibert F., Fraser W.D., Marc I. Physical activity during pregnancy and infant’s birth weight: Results from the 3D Birth Cohort. BMJ Open Sport Exerc. Med. 2017;3:e000242. doi: 10.1136/bmjsem-2017-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastorino S., Bishop T., Crozier S.R., Granstrom C., Kordas K., Kupers L.K., O’Brien E.C., Polanska K., Sauder K.A., Zafarmand M.H., et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: Remote federated individual level meta-analysis from eight cohort studies. BJOG Int. J. Obstet. Gynaecol. 2019;126:459–470. doi: 10.1111/1471-0528.15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belbasis L., Savvidou M.D., Kanu C., Evangelou E., Tzoulaki I. Birth weight in relation to health and disease in later life: An umbrella review of systematic reviews and meta-analyses. BMC Med. 2016;14:147. doi: 10.1186/s12916-016-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law C.M. Significance of birth weight for the future. Arch. Dis. Child. Fetal Neonatal. Ed. 2002;86:F7–F8. doi: 10.1136/fn.86.1.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W., Riggs T., Koo W., Deter R.L., Yeo L., Romero R. The relationship of newborn adiposity to fetal growth outcome based on birth weight or the modified neonatal growth assessment score. J. Matern.-Fetal Neonatal Med. 2012;25:1933–1940. doi: 10.3109/14767058.2012.683084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owe K.M., Nystad W., Bo K. Association between regular exercise and excessive newborn birth weight. Obstet. Gynecol. 2009;114:770–776. doi: 10.1097/AOG.0b013e3181b6c105. [DOI] [PubMed] [Google Scholar]
- 14.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Wells G.A., Shea B., O’Connell D., Pereson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. [(accessed on 25 March 2020)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 16.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barakat R., Lucia A., Ruiz J.R. Resistance exercise training during pregnancy and newborn’s birth size: A randomised controlled trial. Int. J. Obes. 2009;33:1048–1057. doi: 10.1038/ijo.2009.150. [DOI] [PubMed] [Google Scholar]
- 18.Bisson M., Alméras N., Dufresne S.S., Robitaille J., Rhéaume C., Bujold E., Frenette J., Tremblay A., Marc I. A 12-Week Exercise Program for Pregnant Women with Obesity to Improve Physical Activity Levels: An Open Randomised Preliminary Study. PLoS ONE. 2015;10:e0137742. doi: 10.1371/journal.pone.0137742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clapp J.F., 3rd, Kim H., Burciu B., Lopez B. Beginning regular exercise in early pregnancy: Effect on fetoplacental growth. Am. J. Obstet. Gynecol. 2000;183:1484–1488. doi: 10.1067/mob.2000.107096. [DOI] [PubMed] [Google Scholar]
- 20.Clapp J.F., 3rd, Kim H., Burciu B., Schmidt S., Petry K., Lopez B. Continuing regular exercise during pregnancy: Effect of exercise volume on fetoplacental growth. Am. J. Obstet. Gynecol. 2002;186:142–147. doi: 10.1067/mob.2002.119109. [DOI] [PubMed] [Google Scholar]
- 21.Clark E., Isler C., Strickland D., McMillan A.G., Fang X., Kuehn D., Ravisankar S., Strom C., May L.E. Influence of aerobic exercise on maternal lipid levels and offspring morphometrics. Int. J. Obes. 2019;43:594–602. doi: 10.1038/s41366-018-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garnæs K.K., Nyrnes S.A., Salvesen K., Salvesen Ø., Mørkved S., Moholdt T. Effect of supervised exercise training during pregnancy on neonatal and maternal outcomes among overweight and obese women. Secondary analyses of the ETIP trial: A randomised controlled trial. PLoS ONE. 2017;12:e0173937. doi: 10.1371/journal.pone.0173937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann J., Günther J., Geyer K., Stecher L., Kunath J., Meyer D., Spies M., Rosenfeld E., Kick L., Rauh K., et al. Associations between Prenatal Physical Activity and Neonatal and Obstetric Outcomes-A Secondary Analysis of the Cluster-Randomized GeliS Trial. J. Clin. Med. 2019;8:1735. doi: 10.3390/jcm8101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopkins S.A., Baldi J.C., Cutfield W.S., McCowan L., Hofman P.L. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J. Clin. Endocrinol. Metab. 2010;95:2080–2088. doi: 10.1210/jc.2009-2255. [DOI] [PubMed] [Google Scholar]
- 25.Seneviratne S.N., Derraik J.G.B., Jiang Y., McCowan L.M.E., Gusso S., Biggs J.B., Parry G.K., Chiavaroli V., Cutfield W.S., Hofman P.L. Nulliparity is associated with subtle adverse metabolic outcomes in overweight/obese mothers and their offspring. Clin. Endocrinol. 2017;87:545–551. doi: 10.1111/cen.13426. [DOI] [PubMed] [Google Scholar]
- 26.Seneviratne S.N., Jiang Y., Derraik J., McCowan L., Parry G.K., Biggs J.B., Craigie S., Gusso S., Peres G., Rodrigues R.O., et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: A randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2016;123:588–597. doi: 10.1111/1471-0528.13738. [DOI] [PubMed] [Google Scholar]
- 27.Kokic I.S., Ivanisevic M., Biolo G., Simunic B., Kokic T., Pisot R. Combination of a structured aerobic and resistance exercise improves glycaemic control in pregnant women diagnosed with gestational diabetes mellitus. A randomised controlled trial. Women Birth J. Aust. Coll. Midwives. 2018;31:e232–e238. doi: 10.1016/j.wombi.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Trak-Fellermeier M.A., Campos M., Meléndez M., Pomeroy J., Palacios C., Rivera-Viñas J., Méndez K., Febo I., Willett W., Gillman M.W., et al. PEARLS randomized lifestyle trial in pregnant Hispanic women with overweight/obesity: Gestational weight gain and offspring birthweight. Diabetes Metab. Syndr. Obes. Targets Ther. 2019;12:225–238. doi: 10.2147/DMSO.S179009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Poppel M.N.M., Simmons D., Devlieger R., van Assche F.A., Jans G., Galjaard S., Corcoy R., Adelantado J.M., Dunne F., Harreiter J., et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: The DALI randomised controlled trial. Diabetologia. 2019;62:915–925. doi: 10.1007/s00125-019-4842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badon S.E., Littman A.J., Chan K.C.G., Williams M.A., Enquobahrie D.A. Associations of Maternal Light/Moderate Leisure-Time Walking and Yoga with Offspring Birth Size. J. Phys. Act. Health. 2018;15:430–439. doi: 10.1123/jpah.2017-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badon S.E., Wander P.L., Qiu C., Miller R.S., Williams M.A., Enquobahrie D.A. Maternal Leisure Time Physical Activity and Infant Birth Size. Epidemiology. 2016;27:74–81. doi: 10.1097/EDE.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 32.Bisson M., Tremblay F., St-Onge O., Robitaille J., Pronovost E., Simonyan D., Marc I. Influence of maternal physical activity on infant’s body composition. Pediatric Obes. 2017;12((Suppl. S1)):38–46. doi: 10.1111/ijpo.12174. [DOI] [PubMed] [Google Scholar]
- 33.Collings P.J., Farrar D., Gibson J., West J., Barber S.E., Wright J. Associations of Pregnancy Physical Activity with Maternal Cardiometabolic Health, Neonatal Delivery Outcomes and Body Composition in a Biethnic Cohort of 7305 Mother-Child Pairs: The Born in Bradford Study. Sports Med. 2020;50:615–628. doi: 10.1007/s40279-019-01193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahly D.L., Li X., Smith H.A., Khashan A.S., Murray D.M., Kiely M.E., Hourihane J.O.B., McCarthy F.P., Kenny L.C., Kearney P.M. Associations between maternal lifestyle factors and neonatal body composition in the Screening for Pregnancy Endpoints (Cork) cohort study. Int. J. Epidemiol. 2018;47:131–145. doi: 10.1093/ije/dyx221. [DOI] [PubMed] [Google Scholar]
- 35.Diaz E.C., Cleves M.A., DiCarlo M., Sobik S.R., Ruebel M.L., Thakali K.M., Sims C.R., Dajani N.K., Krukowski R.A., Børsheim E., et al. Parental adiposity differentially associates with newborn body composition. Pediatric Obes. 2020;15:e12596. doi: 10.1111/ijpo.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrod C.S., Chasan-Taber L., Reynolds R.M., Fingerlin T.E., Glueck D.H., Brinton J.T., Dabelea D. Physical activity in pregnancy and neonatal body composition: The Healthy Start study. Obstet. Gynecol. 2014;124:257–264. doi: 10.1097/AOG.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones M.A., Catov J.M., Jeyabalan A., Whitaker K.M., Gibbs B.B. Sedentary behaviour and physical activity across pregnancy and birth outcomes. Paediatr. Perinat. Epidemiol. 2021;35:341–349. doi: 10.1111/ppe.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi N.P., Kulkarni S.R., Yajnik C.S., Joglekar C.V., Rao S., Coyaji K.J., Lubree H.G., Rege S.S., Fall C.H. Increasing maternal parity predicts neonatal adiposity: Pune Maternal Nutrition Study. Am. J. Obstet. Gynecol. 2005;193:783–789. doi: 10.1016/j.ajog.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Juhl M., Olsen J., Andersen P.K., Nøhr E.A., Andersen A.M. Physical exercise during pregnancy and fetal growth measures: A study within the Danish National Birth Cohort. Am. J. Obstet. Gynecol. 2010;202:e61–e68. doi: 10.1016/j.ajog.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 40.Mudd L.M., Scheurer J.M., Pruett M., Demerath E.W., Kapur A., Ramel S.E. Relations among maternal physical activity during pregnancy and child body composition. Obes. Sci. Pract. 2019;5:246–250. doi: 10.1002/osp4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagpal T.S., Everest C., Souza S.C.S., da Silva D.F., Mohammad S., Bhattacharjee J., Adamo K.B. Does “Sitting” Stand Alone? A Brief Report Evaluating the Effects of Prenatal Sedentary Time on Maternal and Newborn Anthropometric Outcomes. J. Phys. Act. Health. 2020;17:915–919. doi: 10.1123/jpah.2020-0175. [DOI] [PubMed] [Google Scholar]
- 42.Norris T., McCarthy F.P., Khashan A.S., Murray D.M., Kiely M., Hourihane J.O., Baker P.N., Kenny L.C. Do changing levels of maternal exercise during pregnancy affect neonatal adiposity? Secondary analysis of the babies after SCOPE: Evaluating the longitudinal impact using neurological and nutritional endpoints (BASELINE) birth cohort (Cork, Ireland) BMJ Open. 2017;7:e017987. doi: 10.1136/bmjopen-2017-017987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Przybyłowicz K., Przybyłowicz M., Grzybiak M., Janiszewska K. Effects of physical activity during pregnancy and gestational weight gain on newborn weight and length at birth in Warmińsko-Mazurskie province. Acta Sci. Pol. Technol. Aliment. 2014;13:203–211. doi: 10.17306/J.AFS.2014.2.9. [DOI] [PubMed] [Google Scholar]
- 44.Rao S., Kanade A., Margetts B.M., Yajnik C.S., Lubree H., Rege S., Desai B., Jackson A., Fall C.H. Maternal activity in relation to birth size in rural India. The Pune Maternal Nutrition Study. Eur. J. Clin. Nutr. 2003;57:531–542. doi: 10.1038/sj.ejcn.1601582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson E.D., Brage S., White T., Westgate K., Norris S.A., Van Poppel M.N.M., Micklesfield L.K. The Influence of Objectively Measured Physical Activity during Pregnancy on Maternal and Birth Outcomes in Urban Black South African Women. Matern. Child Health J. 2018;22:1190–1199. doi: 10.1007/s10995-018-2504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clapp J.F., 3rd, Capeless E.L. Neonatal morphometrics after endurance exercise during pregnancy. Am. J. Obstet. Gynecol. 1990;163:1805–1811. doi: 10.1016/0002-9378(90)90754-U. [DOI] [PubMed] [Google Scholar]
- 47.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sytsma T.T., Zimmerman K.P., Manning J.B., Jenkins S.M., Nelson N.C., Clark M.M., Boldt K., Borowski K.S. Perceived Barriers to Exercise in the First Trimester of Pregnancy. J. Perinat. Educ. 2018;27:198–206. doi: 10.1891/1058-1243.27.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huberty J.L., Buman M.P., Leiferman J.A., Bushar J., Adams M.A. Trajectories of objectively-measured physical activity and sedentary time over the course of pregnancy in women self-identified as inactive. Prev. Med. Rep. 2016;3:353–360. doi: 10.1016/j.pmedr.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brett K.E., Wilson S., Ferraro Z.M., Adamo K.B. Self-report Pregnancy Physical Activity Questionnaire overestimates physical activity. Can. J. Public Health = Rev. Can. De Sante Publique. 2015;106:e297–e302. doi: 10.17269/cjph.106.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Troiano R.P., McClain J.J., Brychta R.J., Chen K.Y. Evolution of accelerometer methods for physical activity research. Br. J. Sports Med. 2014;48:1019–1023. doi: 10.1136/bjsports-2014-093546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.da Silva S.G., Evenson K.R., Ekelund U., da Silva I.C.M., Domingues M.R., da Silva B.G.C., Mendes M.A., Cruz G.I.N., Hallal P.C. How many days are needed to estimate wrist-worn accelerometry-assessed physical activity during the second trimester in pregnancy? PLoS ONE. 2019;14:e0211442. doi: 10.1371/journal.pone.0211442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonagra A.D., Biradar S.M., K D., Murthy D.S.J. Normal pregnancy-a state of insulin resistance. J. Clin. Diagn. Res. 2014;8:Cc01–Cc03. doi: 10.7860/JCDR/2014/10068.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butte N.F., Hopkinson J.M., Mehta N., Moon J.K., Smith E.O. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am. J. Clin. Nutr. 1999;69:299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- 55.Tennefors C., Forsum E. Assessment of body fatness in young children using the skinfold technique and BMI vs. body water dilution. Eur. J. Clin. Nutr. 2004;58:541–547. doi: 10.1038/sj.ejcn.1601842. [DOI] [PubMed] [Google Scholar]
- 56.Crossland D.S., Richmond S., Hudson M., Smith K., Abu-Harb M. Weight change in the term baby in the first 2 weeks of life. Acta Paediatr. 2008;97:425–429. doi: 10.1111/j.1651-2227.2008.00685.x. [DOI] [PubMed] [Google Scholar]
- 57.Ziegler E.E. Growth of breast-fed and formula-fed infants. Protein Energy Requir. Infancy Child. 2006;58:51–64. doi: 10.1159/000095010. discussion 59–63. [DOI] [PubMed] [Google Scholar]
- 58.Pivarnik J.M. Potential effects of maternal physical activity on birth weight: Brief review. Med. Sci. Sports Exerc. 1998;30:400–406. doi: 10.1097/00005768-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Bø K., Artal R., Barakat R., Brown W.J., Davies G.A.L., Dooley M., Evenson K.R., Haakstad L.A.H., Kayser B., Kinnunen T.I., et al. Exercise and pregnancy in recreational and elite athletes: 2016/2017 evidence summary from the IOC expert group meeting, Lausanne. Part 5. Recommendations for health professionals and active women. Br. J. Sports Med. 2018;52:1080–1085. doi: 10.1136/bjsports-2018-099351. [DOI] [PubMed] [Google Scholar]
- 60.Starling A.P., Brinton J.T., Glueck D.H., Shapiro A.L., Harrod C.S., Lynch A.M., Siega-Riz A.M., Dabelea D. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am. J. Clin. Nutr. 2015;101:302–309. doi: 10.3945/ajcn.114.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.