Abstract
Of 100 strains of iron-oxidizing bacteria isolated, Thiobacillus ferrooxidans SUG 2-2 was the most resistant to mercury toxicity and could grow in an Fe2+ medium (pH 2.5) supplemented with 6 μM Hg2+. In contrast, T. ferrooxidans AP19-3, a mercury-sensitive T. ferrooxidans strain, could not grow with 0.7 μM Hg2+. When incubated for 3 h in a salt solution (pH 2.5) with 0.7 μM Hg2+, resting cells of resistant and sensitive strains volatilized approximately 20 and 1.7%, respectively, of the total mercury added. The amount of mercury volatilized by resistant cells, but not by sensitive cells, increased to 62% when Fe2+ was added. The optimum pH and temperature for mercury volatilization activity were 2.3 and 30°C, respectively. Sodium cyanide, sodium molybdate, sodium tungstate, and silver nitrate strongly inhibited the Fe2+-dependent mercury volatilization activity of T. ferrooxidans. When incubated in a salt solution (pH 3.8) with 0.7 μM Hg2+ and 1 mM Fe2+, plasma membranes prepared from resistant cells volatilized 48% of the total mercury added after 5 days of incubation. However, the membrane did not have mercury reductase activity with NADPH as an electron donor. Fe2+-dependent mercury volatilization activity was not observed with plasma membranes pretreated with 2 mM sodium cyanide. Rusticyanin from resistant cells activated iron oxidation activity of the plasma membrane and activated the Fe2+-dependent mercury volatilization activity of the plasma membrane.
Thiobacillus ferrooxidans is an acidophilic, chemolithotrophic, iron-oxidizing bacterium that uses energy produced by oxidation of reduced sulfur compounds and ferrous iron. This bacterium is one of the most important bacteria for bacterial leaching of sulfide ores. To solubilize metal ions from sulfide ores much more efficiently, isolation of T. ferrooxidans strains which are resistant to heavy metal ions seems to be important. It has been reported that T. ferrooxidans is sensitive to Hg2+, Ag+, and MoO42− (5). The properties of mercuric reductase, a flavoenzyme that reduces Hg2+ to less toxic Hg0 with NADPH as an electron donor, have been studied actively with a wide range of gram-negative and gram-positive bacteria (1, 13–15). Mercuric reductase activity has also been found in T. ferrooxidans cells (3, 11, 12). The genes involved in volatilization of mercury have been cloned and characterized in detail (4, 6, 8, 11).
Recently, we partially characterized the difference between mercury-resistant and mercury-sensitive strains of T. ferrooxidans. The levels of NADPH-dependent mercuric reductase were not significantly different in these strains. Instead, purified cytochrome c oxidase from resistant strain Funis 2-1 was more resistant to Hg2+ than purified cytochrome c oxidase from a sensitive strain was (20). To explain the remarkable mercury resistance observed, we proposed that both a mercury-resistant cytochrome c oxidase and a cytosolic NADPH-dependent mercuric reductase, not the latter alone, function in resistant cells (20).
In this study, a more mercury-resistant strain (SUG 2-2) was obtained from 100 new strains of iron-oxidizing bacteria, and we show that the mercury volatilized by resting cells of T. ferrooxidans SUG 2-2 was activated in the presence of Fe2+. Although the plasma membrane of T. ferrooxidans SUG 2-2 did not have NADPH-dependent mercury reductase activity, the membrane had the ability to volatilize Hg0 from a salt solution containing Fe2+. Fe2+-dependent mercury volatilization activity with plasma membranes was inhibited by sodium cyanide and activated by the blue copper protein rusticyanin.
MATERIALS AND METHODS
Microorganisms, medium, and growth conditions.
Ninety-four strains of iron-oxidizing bacteria isolated from streams and soils in Japan and the United States and T. ferrooxidans ATCC 13661, ATCC 14119, ATCC 19859, ATCC 21834, ATCC 23270, and ATCC 33020 from the American Type Culture Collection were used in this study. To isolate iron-oxidizing bacteria, stream water or soil samples were incubated at 30°C under aerobic conditions in Fe2+ medium (pH 2.5) containing (per liter) 30 g of FeSO4 · 7H2O, 3 g of (NH4)2SO4, 0.5 g of K2HPO4, 0.5 g of MgSO4 · 7H2O, 0.1 g of KCl, and 0.01 g of Ca(NO3)2 (16). When the Fe2+ in the culture medium was oxidized, samples were plated on 1.0% gellan gum plates containing (per liter) 30 g of FeSO4 · 7H2O, 3 g of (NH4)2SO4, 0.5 g of K2HPO4, 0.5 g of MgSO4 · 7H2O, 0.1 g of KCl, 0.01 g of Ca(NO3)2, and 0.3 g of yeast extract. Rusty colonies appearing on the plate were picked. This process was repeated more than three times, and the final isolates were preserved on Fe2+ medium (pH 2.5) and used throughout this study. The method used for large-scale production of cells has been described previously (18).
Growth rate.
After cultivation in Fe2+ medium (pH 2.5) at 30°C, cells were separated from the particles of ferric hydroxide by filtering with a no. 5B Toyo paper filter. The numbers of cells in the filtrates were counted with a microscope and hemacytometer (Kayagaki Irika Kogyo Co., Ltd., Tokyo, Japan) after dilution with 0.1 N sulfuric acid when necessary.
Analysis of mercury volatilized from T. ferrooxidans culture medium.
A 50-ml culture flask with a screw cap contained 19 ml of Fe2+ medium (pH 2.5) supplemented with 0.7 M Hg2+ and 1 ml of an active seed culture of T. ferrooxidans. A small test tube containing 2 ml of a KMnO4 solution was inserted in the 50-ml culture flask to trap the Hg2+ volatilized from the culture medium. The KMnO4 solution used (100 ml) was composed of a 10-ml solution containing 0.6 g of KMnO4, 5 ml of concentrated H2SO4, and 85 ml of deionized water. After the culture medium was aerated by shaking at 30°C and 100 rpm, the concentration of Hg0 trapped in the KMnO4 solution was measured by cold-vapor atomic absorption spectroscopy.
Analysis of mercury volatilized by resting cells and the plasma membranes of T. ferrooxidans.
Each of several 50-ml flasks with screw caps contained a reaction mixture plus 2 ml of a KMnO4 solution as described above. The gas phase was air, and the reaction mixture was rotated at 100 rpm at 30°C. The reaction mixture used for the measurement of mercury volatilization with resting cells was composed of a salt solution (pH 2.5) (20 ml), resting cells of T. ferrooxidans (1 mg of protein), 0.7 μM HgCl2, and 25 mM ferrous sulfate. The reaction mixture used for the measurement of mercury volatilization with plasma membranes was composed of a salt solution (pH 3.8) (10 ml), plasma membranes of T. ferrooxidans SUG 2-2 (50 μg of protein), 0.7 μM HgCl2, and 1 mM ferrous sulfate. The salt solution used contained (per liter) 3 g of (NH4)2SO4, 0.5 g of K2HPO4, 0.5 g of MgSO4 · 7H2O, 0.1 g of KCl, and 0.01 g of Ca(NO3)2. The concentration of Hg0 trapped in the KMnO4 solution was measured by cold-vapor atomic absorption spectroscopy.
Preparation of plasma membranes and rusticyanin.
Cells of T. ferrooxidans SUG 2-2 grown in Fe2+ medium (pH 2.5) at 30°C for 1 week were washed three times with 0.1 M potassium phosphate buffer (pH 7.5), disrupted by sonication for 15 min with a sonicator (model INSONATOR 201M; Kubota Co., Tokyo, Japan), and centrifuged at 12,000 × g for 10 min to remove cell debris. The cell extract obtained was centrifuged at 105,000 × g for 60 min to obtain the plasma membrane fraction. Rusticyanin was prepared by the method described previously (2).
Protein content.
Protein content was determined by the method of Lowry et al. (9) with crystalline bovine serum albumin as the standard.
RESULTS
Volatilization of mercury from Fe2+ medium by mercury-resistant or -sensitive T. ferrooxidans strains.
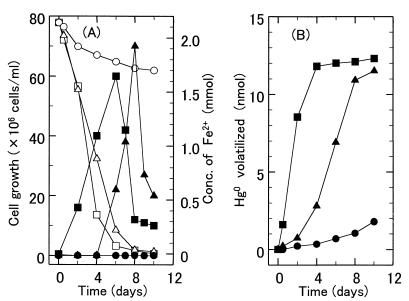
Screening 100 iron-oxidizing bacterial strains, including six T. ferrooxidans strains from the American Type Culture Collection, for resistance to Hg2+ was done. T. ferrooxidans SUG 2-2, isolated from hot spring water from Aomori Prefecture, Japan, was the strain most resistant to Hg2+ toxicity among the strains tested and could grow in Fe2+ medium supplemented with 6 μM Hg2+. This strain gave a cell yield of 6.0 × 107 cells/ml after 6 days of cultivation in Fe2+ medium (pH 2.5) supplemented with 0.7 μM Hg2+ (Fig. 1A). SUG 2-2 volatilized 86% of the total mercury (14 nmol) added to the culture medium after 4 days of cultivation (Fig. 1B). T. ferrooxidans Funis 2-1 (10, 20) volatilized 78% of the total mercury after 8 days of cultivation. In contrast, T. ferrooxidans AP19-3 (7, 17) could not grow in Fe2+ medium supplemented with 0.7 μM Hg2+ and volatilized only 10% of the total mercury added to the medium after 10 days of cultivation (Fig. 1). Neither Funis 2-1 nor AP19-3 could grow in Fe2+ medium supplemented with 6 μM HgCl2.
FIG. 1.
Volatilization of metal mercury from Fe2+ medium containing 0.7 M Hg2+ by T. ferrooxidans strains. (A) Cell growth and amount of Fe2+ remaining in Fe2+ medium (pH 2.5) containing 0.7 μM Hg2+. Symbols: ■, ▴, and ●, cell growth of T. ferrooxidans SUG 2-2, Funis 2-1, and AP19-3, respectively; □, ▵, and ○, amount of Fe2+ remaining with T. ferrooxidans SUG 2-2, Funis 2-1, and AP19-3, respectively. (B) Mercury volatilized in Fe2+ medium (pH 2.5) containing Hg2+ (0.7 μM). Cultures of T. ferrooxidans SUG 2-2 (■), Funis 2-1 (▴), and AP19-3 (●) were examined.
Effects of ferrous iron on the amount of mercury volatilized from resting cells.
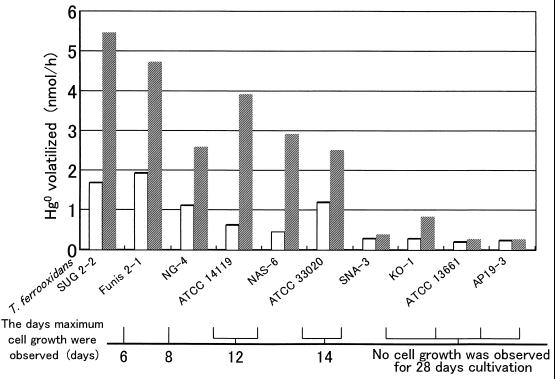
The mercury reductase activity of SUG 2-2 cells was determined with NADPH as an electron donor and cytosol prepared from the strain as an enzyme source. The NADPH-dependent mercury volatilization activity of the cytosol of strain SUG 2-2 was nearly the same as that of Funis 2-1 (data not shown). From our results, it seems that strain SUG 2-2 has another enzyme system to detoxify mercury. The role of the iron-oxidizing system of SUG 2-2 in mercury detoxification by the strain was studied. The amount of mercury volatilized from a 10-ml salt solution (pH 2.5) containing resting cells of SUG 2-2 and 0.7 μM Hg2+ was 20% of the total amount of mercury (14 nmol) added to the reaction mixture after 3 h of incubation. The amount of mercury volatilized by the resting cells increased approximately threefold when 25 mM Fe2+ was added, and approximately 62% of the total mercury added was volatilized. The amount of mercury volatilized by SUG 2-2 cells increased in proportion to the concentration of Fe2+ added to the reaction mixture and also in proportion to the cell concentration (data not shown). To clarify whether T. ferrooxidans strains other than SUG 2-2 also have Fe2+-dependent mercury volatilization activity, the same experiments were done with resting cells of nine additional iron-oxidizing bacterial strains with or without 25 mM Fe2+ (Fig. 2). After 60 min of incubation, resting cells of Funis 2-1 volatilized 34 and 14% of the total amount of mercury (14 nmol) added to the reaction mixture in the presence and in the absence of 25 mM Fe2+, respectively, indicating that Funis 2-1 as well as SUG 2-2 has Fe2+-dependent mercury volatilization activity. T. ferrooxidans ATCC 33020 from the American Type Culture Collection volatilized mercury in the presence of Fe2+. In comparison, the amounts of metal mercury volatilized by T. ferrooxidans AP19-3 and ATCC 13661 were less or the same with or without Fe2+. As shown in Fig. 2, the day after each of the strains exhibited the maximum cell number in Fe2+ medium containing 0.7 μM Hg2+, testing was carried out. The results indicate that the strains which had higher Fe2+-dependent mercury volatilization activities were more resistant to Hg2+ toxicity than the strains which had lower activities. The Fe2+-dependent mercury volatilization activities were completely inhibited by pretreating strain SUG 2-2 and Funis 2-1 cells with 5 mM NaCN for 15 min, suggesting that cytochrome c oxidase of T. ferrooxidans is involved in the Fe2+-dependent mercury volatilization reaction.
FIG. 2.
Fe2+-dependent mercury volatilization activities of resting cells of 10 T. ferrooxidans strains. The mercury volatilization activities of T. ferrooxidans strains were measured in 20 ml of salt solution (pH 2.5) containing resting cells (1 mg of protein), 0.7 μM Hg2+, and 25 mM Fe2+ (striped bars). The activities were also measured in 20 ml of salt solution (pH 2.5) containing resting cells and Hg2+ but no Fe2+ (open bars). The days on which maximum cell growth was observed in Fe2+ medium containing 0.7 μM Hg2+ are shown for the 10 strains of T. ferrooxidans.
Characteristics of Fe2+-dependent mercury volatilization activity.
The optimum pH and temperature for Fe2+-dependent mercury volatilization activity of strain SUG 2-2 cells were pH 2.3 and 30°C, respectively. These values are the same as the optimum pH and temperature values for the iron oxidase of T. ferrooxidans SUG 2-2 cells (data not shown). The effects of heavy metal ions on Fe2+-dependent mercury volatilization activity were studied with resting cells of SUG 2-2 (Table 1). Na2MO4 and Na2WO4 inhibited the activity more than 80%. Silver ions also markedly inhibited the activity at 5 mM. These compounds strongly inhibited both iron oxidase and cytochrome c oxidase activities of T. ferrooxidans (5, 10, 19).
TABLE 1.
Effects of heavy metal ions on the mercury volatilization activity of T. ferrooxidans SUG 2-2a
| Heavy metal | Concn (mM) | Remaining activity (%) |
|---|---|---|
| None | 100 | |
| NiSO4 | 10 | 72 |
| CuSO4 | 10 | 82 |
| AgNO3 | 10 | 0.0 |
| 5 | 0.1 | |
| 1 | 24 | |
| 0.1 | 63 | |
| 0.05 | 78 | |
| CdSO4 | 10 | 90 |
| (CH3COO)2Pb | 10 | 67 |
| ZnSO4 | 10 | 72 |
| MnSO4 | 10 | 69 |
| Na2MoO4 | 10 | 15 |
| Na2WO4 | 10 | 1 |
| SnCl2 | 10 | 104 |
| MgSO4 | 10 | 73 |
| Fe2(SO4)3 | 10 | 69 |
Volatilization activity was measured in a reaction mixture (20 ml) containing resting cells of strain SUG 2-2 (1 mg of protein), ferrous iron (25 mM), and heavy metal ions. One hundred percent of remaining activity was 5.4 nmol/mg of protein/h at 30°C.
Volatilization of mercury by the plasma membrane of T. ferrooxidans SUG 2-2.
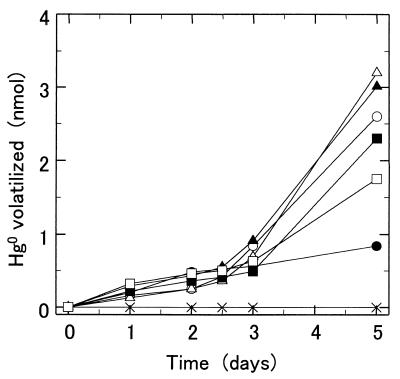
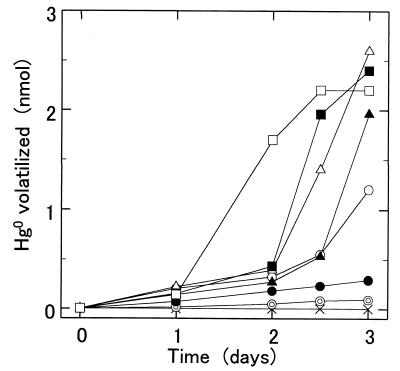
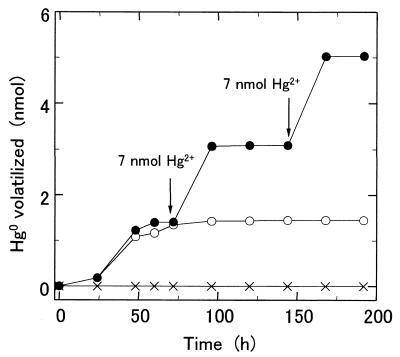
Fe2+-dependent mercury volatilization activity was measured with plasma membranes from strain SUG 2-2 cells. The plasma membranes did not have mercuric reductase activity when NADPH was used as an electron donor. To decrease the amount of reduced compounds in the plasma membrane, KMnO4 was added to the membrane until reduced type c and a cytochromes in the membrane were nearly completely oxidized. In the presence and absence of 1 mM Fe2+, 50 μg of plasma membranes prepared from SUG 2-2 cells volatilized 48 and 12%, respectively, of the total mercury (7 nmol) added to a 10-ml reaction mixture after 5 days of incubation at 30°C (Fig. 3). Mercury was not volatilized in a reaction mixture containing Hg2+, Fe2+, and plasma membranes boiled for 10 min. Since the plasma membranes prepared from T. ferrooxidans SUG 2-2 cells had low iron-oxidizing activity, we added the blue copper protein rusticyanin to the reaction mixture. Rusticyanin isolated from SUG 2-2 cells activated the iron-oxidizing activity of plasma membranes 4.5-fold (data not shown). Fe2+-dependent mercury volatilization activity markedly increased in the presence of 0.1 mg of rusticyanin (Fig. 4). In the presence of 0.1 mg of rusticyanin, plasma membranes of SUG 2-2 cells volatilized 1.7 and 0.2 nmol of mercury in 10-ml reaction mixtures containing 7 nmol of Hg2+ in the presence and in the absence of 1 mM Fe2+, respectively, after 2 days of incubation. However, since only 2.2 nmol of mercury was volatilized after 5 days of incubation, 7 nmol of HgCl2 and 10 μmol of Fe2+ were added to the reaction mixture every 3 days (Fig. 5). Further additions of HgCl2 and Fe2+ to the reaction mixture resulted in further volatilization of the mercury, suggesting that the membranes still had Fe2+-dependent mercury volatilization activity after 6 days of incubation. After 8 days of incubation and two more additions of mercury, the plasma membranes of SUG 2-2 cells volatilized 5 nmol of mercury in the reaction mixture. In contrast, only 1.5 nmol of mercury was volatilized when the membranes were incubated without a further addition of mercury. Mercury was not volatilized in the reaction mixture containing plasma membranes boiled for 10 min.
FIG. 3.
Effects of Fe2+ concentration on the mercury volatilization activity of plasma membranes of T. ferrooxidans SUG 2-2. The Fe2+-dependent mercury volatilization activity was measured in 10 ml of salt solution (pH 3.8) containing 50 μg of plasma membranes, 0.7 μM Hg2+ and Fe2+ (○, 0.01 mM; ▴, 0.2 mM; ▵, 1 mM; ■, 3 mM; and □, 5 mM). The activities were also measured in 10 ml of salt solution containing 50 μg of plasma membranes and 0.7 μM Hg2+ but no Fe2+ (●) and in 10 ml of salt solution containing 50 μg of plasma membranes boiled for 10 min, 0.7 μM Hg2+, and 1 mM Fe2+ (×).
FIG. 4.
Effects of rusticyanin on the Fe2+-dependent mercury volatilization activity of T. ferrooxidans SUG 2-2 cells. The Fe2+-dependent mercury volatilization activity was measured in 10 ml of salt solution (pH 3.8) containing 50 μg of plasma membranes, 1 mM Fe2+, 0.7 μM Hg2+, and rusticyanin (▴, 0.01 mg; ▵, 0.02 mg; ■, 0.05 mg; and □, 0.1 mg). The activities were also measured in 10 ml of salt solution containing 50 μg of plasma membranes, 1 mM Fe2+, and 0.7 μM Hg2+ (○), in a salt solution containing 50 μg of plasma membranes and 0.7 μM Hg2+ (●), in a salt solution containing 1 mM Fe2+, 0.7 μM Hg2+, rusticyanin (0.1 mg of protein), and boiled plasma membranes (×), and in a salt solution containing 1 mM Fe2+, 0.7 μM Hg2+, and rusticyanin (0.1 mg of protein) but no plasma membranes (◎).
FIG. 5.
Effects of further addition of HgCl2 to the reaction mixture on Fe2+-dependent mercury volatilization activity. The Fe2+-dependent mercury volatilization activity was measured in 10 ml of salt solution (pH 3.8) containing 50 μg of plasma membranes, 1 mM Fe2+, 0.7 μM Hg2+, and rusticyanin (0.1 mg of protein). Hg2+ (7 nmol) and Fe2+ (10 nmol) were added to the reaction mixture every 3 days (●). The Hg2+ volatilized was also measured in a 10-ml reaction mixture to which HgCl2 was not added (○). The arrows indicate the times at which HgCl2 (7 nmol) was added to the reaction mixture. The Fe2+-dependent mercury volatilization activity was also measured in 10 ml of salt solution (pH 3.8) containing 50 μg of plasma membranes boiled for 10 min, 1 mM Fe2+, 0.7 μM Hg2+, and rusticyanin (0.1 mg of protein) (×).
DISCUSSION
Bacteria resistant to mercury usually have a cytosolic NADPH-dependent mercuric reductase which catalyzes the reduction of soluble Hg2+ to volatile Hg0 with NADPH as an electron donor. Studies to clarify the characteristics of mercury reductase and its gene structure have been performed with various bacteria, including members of the genera Pseudomonas, Staphylococcus, Bacillus, and Serratia (1, 13, 15). The iron-oxidizing chemolithotrophic bacterium T. ferrooxidans also has mercuric reductase to detoxify Hg2+ (8, 11, 12). Recently, it was shown that both mercuric reductase and cytochrome c oxidase, but not mercuric reductase alone, function in mercury-resistant strain Funis 2-1 of T. ferrooxidans when the strain grows in Fe2+ medium containing Hg2+ (20).
In this report, it is shown that a newly isolated strain, T. ferrooxidans SUG 2-2, is much more resistant to mercury than the previously reported strain Funis 2-1 and that Fe2+-dependent mercury volatilization activity is present in six T. ferrooxidans strains, including strain SUG 2-2. Olson et al. (11, 12) reported that the rate of mercury volatilization by T. ferrooxidans BA-4 which was adapted to Hg2+ at concentrations up to 5 μM was slightly accelerated by the addition of 150 mM FeSO4. The level of Fe2+-dependent mercury volatilization activity found in the six strains of T. ferrooxidans corresponded well with the level of mercury resistance of these strains, which was estimated by the growth rate in Fe2+ medium (pH 2.5) containing 0.7 μM Hg2+. This strongly suggests the possibility that both Fe2+-dependent mercury volatilization and cytosolic NADPH-dependent mercury volatilization play a role in detoxification of Hg2+ in many strains of T. ferrooxidans. According to the level of Fe2+-dependent mercury volatilization activity, the T. ferrooxidans strains isolated can be categorized into two groups, one containing the strains possessing a high level of Fe2+-dependent mercury volatilization activity and the other containing the strains possessing a low level of activity.
Involvement of an iron oxidation enzyme system in the Fe2+-dependent mercury volatilization reaction of T. ferrooxidans SUG 2-2 cells is supported by the following findings. (i) Plasma membranes prepared from strain SUG 2-2 cells did not have NADPH-dependent mercuric reductase activity but had Fe2+-dependent mercury volatilization activity. Plasma membranes boiled for 10 min did not have mercury volatilization activity. (ii) Rusticyanin purified from strain SUG 2-2 cells enhanced both iron-oxidizing activity and the Fe2+-dependent mercury volatilization activity. (iii) NaCN, which strongly inhibits the iron oxidase activity of cells and the cytochrome c oxidase activity of plasma membranes, completely inhibited the Fe2+-dependent mercury volatilization activity. The Fe2+-dependent mercury volatilization activity measured with plasma membranes was very low compared with that of the resting cells. This is probably because the components needed for iron oxidation, for instance, rusticyanin and soluble cytochrome c oxidase, were lost during preparation of plasma membranes by disruption of the resting cells with a sonicator. The iron-oxidizing activities measured at pH 3.8 were 11.4 and 0.14 μl of O2 consumed/mg per min for the resting cells and the plasma membranes, respectively.
This paper shows that there is a novel Fe2+-dependent mercury volatilization activity in six strains of T. ferrooxidans, including strain SUG 2-2. To clarify the mechanism of the Fe2+-dependent mercury volatilization reaction more precisely, it is important to answer the following question precisely: is cytochrome c oxidase alone or cytochrome c oxidase plus other components of the iron oxidation enzyme system involved in the mercury volatilization reaction? Our preliminary experiments show that cytochrome c oxidase partially purified from T. ferrooxidans SUG 2-2 volatilizes mercury in the presence of Fe2+.
REFERENCES
- 1.Babich K, Engle M, Skinner J S, Laddaga R A. Deletion mutant analysis of the Staphylococcus aureus plasmid pI258 mercury resistance determinant. Can J Microbiol. 1991;37:624–631. doi: 10.1139/m91-106. [DOI] [PubMed] [Google Scholar]
- 2.Blake R C, II, Shute E. Respiratory enzymes of Thiobacillus ferrooxidans. J Biol Chem. 1987;262:14983–14989. [PubMed] [Google Scholar]
- 3.Booth J E, Williams J W. The isolation of a mercuric ion-reducing flavoprotein from Thiobacillus ferrooxidans. J Gen Microbiol. 1984;130:725–730. [Google Scholar]
- 4.Douglas E R, Kusano T. Molecular genetics of Thiobacillus ferrooxidans. Microbiol Rev. 1994;58:39–55. doi: 10.1128/mr.58.1.39-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai K, Sugio T, Tsuchida T, Tano T. Effect of heavy metal ions on growth and iron-oxidizing activity of Thiobacillus ferrooxidans. Agric Biol Chem. 1975;39:1349–1354. [Google Scholar]
- 6.Inoue T, Kusano T, Silver S. Mercuric ion uptake by Escherichia coli cells producing Thiobacillus ferrooxidans MerC. Biosci Biotechnol Biochem. 1996;60:1289–1292. doi: 10.1271/bbb.60.1289. [DOI] [PubMed] [Google Scholar]
- 7.Iwahori K, Kamimura K, Sugio T. Isolation and some properties of cytochrome c oxidase purified from a bisulfite ion resistant Thiobacillus ferrooxidans strain, OK1-50. Biosci Biotechnol Biochem. 1998;62:1081–1086. doi: 10.1271/bbb.62.1081. [DOI] [PubMed] [Google Scholar]
- 8.Kusano T, Ji G, Inoue C, Silver S. Constitutive synthesis of a transport function encoded by the Thiobacillus ferrooxidans merC gene cloned in Escherichia coli. J Bacteriol. 1990;172:2688–2692. doi: 10.1128/jb.172.5.2688-2692.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 10.Ng K Y, Oshima M, Blake R C, Sugio T. Isolation and some properties of an iron-oxidizing bacterium Thiobacillus ferrooxidans resistant to molybdenum ion. Biosci Biotechnol Biochem. 1997;61:1523–1526. [Google Scholar]
- 11.Olson G J, Iverson W P, Brinckman F E. Volatilization of mercury by Thiobacillus ferrooxidans. Curr Microbiol. 1981;5:115–118. [Google Scholar]
- 12.Olson G J, Porter F D, Rubinstein J, Silver S. Mercuric reductase enzyme from a mercury-volatilizing strain of Thiobacillus ferrooxidans. J Bacteriol. 1982;151:1230–1236. doi: 10.1128/jb.151.3.1230-1236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schottel J, Mandal A, Clark D, Silver S, Hedges R W. Volatilization of mercury and organomercurials determined by inducible R-factor systems in enteric bacteria. Nature (London) 1974;251:335–337. doi: 10.1038/251335a0. [DOI] [PubMed] [Google Scholar]
- 14.Schottel J L. The mercury and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem. 1978;253:4341–4349. [PubMed] [Google Scholar]
- 15.Silver S, Pheng L T. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 16.Sugio T, Tano T, Imai K. Isolation and some properties of silver ion-resistant iron-oxidizing bacterium Thiobacillus ferrooxidans. Agric Biol Chem. 1981;45:2037–2051. [Google Scholar]
- 17.Sugio T, Domatsu C, Munakata O, Tano T, Imai K. Role of a ferric ion-reducing system in sulfur oxidation of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1985;49:1401–1406. doi: 10.1128/aem.49.6.1401-1406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugio T, Katagiri T, Moriyama M, Zhen Y L, Inagaki K, Tano T. Existence of a new type of sulfite oxidase which utilizes ferric ion as an electron acceptor in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988;54:153–157. doi: 10.1128/aem.54.1.153-157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugio T, Hirayama K, Inagaki K, Tanaka H, Tano T. Molybdenum oxidation by Thiobacillus ferrooxidans. Appl Environ Microbiol. 1992;58:1768–1771. doi: 10.1128/aem.58.5.1768-1771.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi F, Iwahori K, Kamimura K, Sugio T. Isolation and some properties of Thiobacillus ferrooxidans strains with differing levels of mercury resistance from natural environments. J Biosci Bioeng. 1999;88:387–392. doi: 10.1016/s1389-1723(99)80215-1. [DOI] [PubMed] [Google Scholar]