Abstract
Resistance to protoporphyrinogen IX oxidase (PPO)-inhibitors in Amaranthus palmeri and Amaranthus tuberculatus is mainly contributed by mutations in the PPO enzyme, which renders herbicide molecules ineffective. The deletion of glycine210 (ΔG210) is the most predominant PPO mutation. ΔG210-ppo2 is overexpressed in rice (Oryza sativa c. ‘Nipponbare’) and Arabidopsis thaliana (Col-0). A foliar assay was conducted on transgenic T1 rice plants with 2× dose of fomesafen (780 g ha−1), showing less injury than the non-transgenic (WT) plants. A soil-based assay conducted with T2 rice seeds confirmed tolerance to fomesafen applied pre-emergence. In agar medium, root growth of WT rice seedlings was inhibited >90% at 5 µM fomesafen, while root growth of T2 seedlings was inhibited by 50% at 45 µM fomesafen. The presence and expression of the transgene were confirmed in the T2 rice survivors of soil-applied fomesafen. A soil-based assay was also conducted with transgenic A. thaliana expressing ΔG210-ppo2 which confirmed tolerance to the pre-emergence application of fomesafen and saflufenacil. The expression of A. palmeri ΔG210-ppo2 successfully conferred tolerance to soil-applied fomesafen in rice and Arabidopsis. This mutant also confers cross-tolerance to saflufenacil in Arabidopsis. This trait could be introduced into high-value crops that lack chemical options for weed management.
Keywords: transgenic rice, Amaranthus palmeri, fomesafen, protoporphyrinogen oxidase, herbicide resistance, root growth inhibition
1. Introduction
Palmer amaranth (A. palmeri L. Wats.) is a dioecious plant native to the desert region of the southwestern United States and northwest Mexico. Recent surveys denoted A. palmeri as one of the toughest weeds to manage in crop production in the southern US [1]. Since this weed has become resistant to several herbicide chemistries, its negative impact on crop production is high. Thus far, A. palmeri populations have evolved resistance to nine herbicide sites of action. These include inhibitors of acetolactate synthase, 5-enolpyruvyl-shikimate-3-phosphate synthase, microtubule assembly, photosystem II, 4-hydroxyphenylpyruvate dioxygenase, protoporphyrinogen oxidase, very-long-chain fatty acid synthesis, and glutamine synthetase as well as synthetic auxins [2,3]. Among the protoporphyrinogen oxidase (PPO, EC 1.3.3.4) inhibitors, fomesafen is highly used to control many dicot and monocot weeds in soybeans [4].
Herbicides that inhibit PPO control susceptible plants by stopping the oxidation of protoporphyrinogen IX into protoporphyrin [5]. This inhibition induces an excessive accumulation of the substrate (protoporphyrinogen IX), which leaks into the cytoplasm, where it reacts instantly with free oxygen. This oxidation process produces the highly photosensitive protoporphyrin IX, which generates singlet oxygen when exposed to light. Singlet oxygen molecules cause lipid peroxidation, cellular membrane disruption, the disintegration of cells, loss of carotenoids and chlorophyll (bleaching effect), and cell death [6,7,8,9,10]. In plants, PPO is nuclear-encoded in two forms: PPO1, which is compartmentalized in the chloroplast, and PPO2, which is compartmentalized in the mitochondria and, in a few species, also in the chloroplast [8,11,12]. In the Amaranthus genus, PPO2 is targeted to both mitochondria and plastids. The coding sequence of PPO2 has two in-frame start codons that result in two different lengths of PPO protein sequences. These two PPO proteins with different sequence lengths are thought to be targeted at the mitochondria and chloroplasts. Therefore, Amaranthus species are expected to have PPO2 activity in both compartments, which might be a requirement to confer herbicide resistance [13].
Fomesafen is one of the PPO-inhibiting herbicides with long residual soil activity, in addition to strong foliar activity. Consequently, it is applied preplant, pre-emergence, over-the-top, or postemergence-directed in various crops. Rice (O. sativa L.) does not have commercial tolerance to fomesafen, either applied pre-emergence or postemergence. In addition, rice is more sensitive to soil-applied than foliar-applied fomesafen. The half-life of fomesafen in soil varies from 8.5 to 100 days depending on the soil’s physical, chemical, and biological features and environmental conditions [3,14,15]. Evaluating the effect of residual fomesafen in soil on rotational crops, Cobucci et al. [16] recommended that rice not be planted for at least 95 days after fomesafen application. The label for fomesafen does not allow planting rice within 10 months from application; otherwise, the crop may be severely injured [17]. Resistance to fomesafen in A. palmeri is weaker when the herbicide is applied pre-emergence compared to postemergence, despite the presence of resistance-conferring ppo2 mutations. Soil-applied fomesafen reduces the germination of fomesafen-resistant A. palmeri by 64% [18]. Saflufenacil, flumioxazin, and sulfentrazone are other examples of soil-applied PPO-inhibiting herbicides commonly used in soybeans across the USA. Saflufenacil is the newest member of this group and is still effective on PPO-resistant populations.
In Amaranthaceae, resistance to PPO inhibitors is mainly attributed to mutations in PPO2. The three base pair deletion resulting in the disappearance of glycine at position 210 (ΔG210) of PPO2 is the first mutation reported to confer resistance to PPO herbicides in A. tuberculatus [19]. Later, this same mutation was identified as the resistance mechanism in a PPO-resistant A. palmeri population [20]. The substitution of arginine with glycine or methionine at position 128 (R128G or R128M) also confers resistance [21]. The latest resistance-conferring mutation discovered in A. palmeri is a glycine substitution with alanine at position 399 (G399A) [22]. Follow-up surveys on A. palmeri populations from the mid-Southern US showed ΔG210 as the predominant mutation along with the accumulation of other mutations in highly resistant populations [23,24]. The potential effect of these mutations on the functionality of PPO2, and hence resistance to herbicide, can be predicted using molecular modeling. A recent study by Noguera. et al., [23] using crystal structure molecular models of wild-type A. tuberculatus PPO2 and its ΔG210 mutant showed that the deletion of G210 in ppo2 causes the enlargement of the binding pocket or active site that allows water to enter more easily, which in turn interferes with the binding of fomesafen. This mutation causes minimum perturbation of substrate binding (or enzyme functionality), which explains the predominance of ΔG210 among resistant populations.
One way to determine the contribution of a particular mutation to whole-plant resistance is by expressing it in a heterologous system. Researchers have shown that the presence of the above-cited mutations reduces the binding affinity of PPO herbicides to PPO2, consequently decreasing the herbicide effect on resistant weeds [19,25,26]. In this study, we characterized ΔG210 mutation from A. palmeri using rice (O. sativa cv. ‘Nipponbare’) as a grass crop model and A. thaliana as a highly sensitive broadleaf plant model. A transgenic rice line was created using the A. palmeri ppo2 gene, and its response to fomesafen was evaluated. Additionally, we evaluated the response of the transgenic Arabidopsis line to soil-applied fomesafen and saflufenacil (Tables S3 and S4).
2. Materials and Methods
2.1. Generation of Transgenic Rice
A transgenic rice line was generated under controlled conditions at the University of Arkansas in Fayetteville, Arkansas, USA. The full-length cDNA (Genbank accession number MF583746) of fomesafen-resistant A. palmeri plant from field population 15CLA-A [20] was obtained using the ppo2 primer pair; kpnApxF: 5′-ggggtacccgggTAAACTGATCTTATGTTAATTC-3′ and sphApxR: 5′-ggaattcgagctcgcatgcTTACGCGGTCTTCTCATCCATC-3′ and RT-PCR procedure using 2 µL of the first-strand cDNA. This full-length cDNA also contained a dual organelle targeting signal. PCR amplification was performed using the following program: hot start at 95 °C for 2 min, denaturation at 95 °C for 1 min, annealing at 58 °C for 1 min, extension at 72 °C for 2 min for 39 cycles, followed by 72 °C for 10 min. The resulting PCR products were then gel-purified using the GeneClean kit (MP Biomedicals, Solon, OH, USA), digested with XmaI and SacI (New England Biolabs, Ipswich, MA, USA) and ligated with XmaI- and SacI-digested pRP7, resulting in the plasmids pACL1, which contained the mature protein coding region for A. palmeri ppo2 isolated from the fomesafen-resistant plant under the control of maize ubiquitin (ZmUbi) promoter (Figure S1). To ensure the accuracy of the cloned ppo2 sequence, PCR amplicon using pACL1 plasmid as a template was gel purified (GeneJET gel extraction kit, Thermo Fisher Scientific, Grand Island, NY, USA) and sequenced. The presence of intact ppo2 genes with G210 mutation, henceforth referred to as ΔG210-Apppo2, was confirmed using Sequencher 5.4.6 software (Gene Codes Corporation, Ann Arbor, MI, USA). Tissue culture media was prepared as described by Nishimura et al. [27]. Scutellar calluses of rice (‘Nipponbare’ background) were generated from mature seeds and maintained on N6D media in the dark at room temperature. Two- to three-week-old cultures were bombarded with 1-μm gold particles coated with an equimolar mixture of pACL1 and pHPT plasmid (~5 μg each) using a standard protocol with a PDS 1000/He gene gun (Bio-Rad Inc., Hercules, CA, USA). pHPT contains a 35S promoter-driven hygromycin phosphotransferase gene that serves as the selection marker gene. After bombardment, the calluses were kept in the dark for 24 h and transferred to selection media, N6D supplemented with hygromycin (50 mg L−1). The calluses were kept on selection media, 25 °C, 16 h light, for 4 to 6 weeks. The tolerant events were transferred to a new plate (2N6D + Hygromycin) and allowed to grow until they were ready to transfer to regeneration media, as described by Nishimura et al. [27]. A total of ~20 events were transferred to the regeneration medium. Two to three weeks later, only one event successfully regenerated into plantlets. When all plantlets reached approximately 1.0–2.0 cm in length, they were transferred to a rooting medium (MS1/2 supplemented with 50 mg L−1 hygromycin) under 16 h light duration. One to two weeks later, plantlets of approximately 8 cm in height, with well-developed roots, were transplanted into commercial soil (Sunshine® Premix No. 1; Sun Gro Horticulture, Bellevue, WA, USA) and grown to maturity. These primary transgenic plants were referred to as T0 plants. Subsequently, only one T0 plant survived to maturity. T1 seeds were harvested and used to investigate tolerance to herbicide.
2.2. Transgenic Rice Response to Foliar-Applied Fomesafen
The T1 rice seedlings were treated with fomesafen at the V3 stage (third-leaf collar visible [28]). The seedlings were sprayed with 780 g ha−1 (2×) fomesafen (Flexstar®, Syngenta Crop Protection, Greensboro, NC, USA) with 0.5% v/v non-ionic surfactant (Induce, Helena Chemical, Collierville, TN, USA). This dose corresponds to twice the maximum recommended dose in soybean. The herbicide was applied with a CO2-pressurized backpack sprayer attached to a handheld spray boom fitted with one 8002 XR even flat fan nozzle (Teejet, Wheaton, IL, USA) calibrated to deliver 187 L ha–1 at a pressure of 276 kPa. The plants were returned to the greenhouse after treatment and watered 48 h later and as needed. A total of 28 T1 plants (one plant pot−1) were sprayed. The experimental units were arranged in a completely randomized design. Wild-type (WT) rice plants were used as the susceptible reference. Plant injury (%) was evaluated at 2 weeks after treatment (WAT) on a rating scale of 0 to 100%, where 0 = no injury and 100 = dead plant without green tissue [29,30]. The respective nontreated checks of WT and T1 were used for comparison. Leaf tissues were collected from T1 plants. The presence of the transgene was verified by PCR as described in the cloning section. Eighteen healthy T1 plants, which were highly tolerant to foliar-applied fomesafen and showed the presence of transgene, were grown to generate T2 seeds. For further investigation, T2 seeds from 18 T1 plants were combined to create a bulk T2 population.
2.3. Transgenic Rice Response to Soil-Applied Fomesafen
Flats (12.2- by 9.5- by 5.7 cm) were filled with a 1:1 ratio of field soil and commercial potting soil. Before planting, the soil-filled flats were saturated and allowed to drain to pot water-holding capacity. Eight T2 (from the bulk population) or WT rice seeds were planted in each flat. Immediately after planting, the flats were sprayed with 390 g ha−1 fomesafen (1×). The herbicide was applied with a CO2-pressurized backpack sprayer attached to a handheld boom fitted with one 11002 XR even flat fan nozzle calibrated to deliver 187 L ha–1 at 276 kPa. The experiment was arranged in a completely randomized design with three replicates and two runs. Each flat was one replication. Nontreated checks were included. Seedling emergence count and visible injury of each emerged seedling (%) were evaluated at 3 WAT [29,30]. Height (cm), number of tillers, and number of panicles were recorded at the reproductive stage. Germination reduction (%) relative to nontreated checks was calculated using the formula:
Leaf tissues from nontreated T2 plants were also collected to verify the frequency of transgene presence (%) in the T2 generation and relate this to the frequency of survivors from the soil-based assay.
2.4. Molecular Analysis of Transgenic Rice Plants
Leaf tissues were collected from T0 and T1 plants, and genomic DNA was extracted following a modified version of the CTAB protocol [31]. The presence of ΔG210-Apppo2 transgene was confirmed using Apppo2 gene-specific primers and ZmUbi and nos primer (Figure S2a) using genomic DNA of the T0 transgenic rice line. Transgene expression was measured in selected 15 T2 rice plants using total RNA extracted from leaves following a modified extraction protocol developed by Hongbao et al. [32] using Trizol® Reagent (Invitrogen, Carlsbad, CA, USA). A total of 1 μg RNA was used for cDNA synthesis using the qScript® cDNA SuperMix kit (Quanta BioSciences, San Diego, CA, USA). The qPCR reaction was carried out using iTaq Universal SYBR® Green SuperMix (BioRad, Hercules, CA, USA) using primers listed in Table S1. The qPCR was conducted using a CFX96 Real-Time PCR machine (BioRad, Hercules, CA, USA) using the following conditions: 2 min at 95 °C, followed by 40 cycles of 30 s denaturation at 95 °C, 1 min annealing at 59 °C, 1 min extension at 65 °C, followed by melt curve analysis. Each sample was analyzed in two technical replicates. Primers were designed in such a way that they can distinguish between the target ΔG210-Apppo2 transgene and the native OsPPO2 gene. The Ct values were normalized against the native OsPPO2 and housekeeping gene ubiquitin (ubiQ). The fold-change in gene expression was calculated using the 2−ΔΔCt method [33].
2.5. Agar-Based Germination Assay with Transgenic Rice Line
Following the protocol by Nishimura et al. [27], rice seeds (WT and T2) were dehulled and then surface-sterilized. The sterilized seeds were placed in Petri plates containing 25 mL of half-strength MS medium with 0, 5, 10, 20, 40, 60, 80, and 100 μM of fomesafen. The stock solution (45,580 μM) was prepared by dissolving 50 mg technical grade fomesafen (Sigma Aldrich, St. Louis, MO, USA) in 250 μL acetone. The control plate was supplemented with just acetone to assess the potential toxicity of acetone. Each plate was divided in two; one half contained 5 seeds of T2, and the other half had 5 WT seeds. The plates were incubated at 26 °C. Root growth (%) was scored visually at 2 WAT relative to a nontreated check. Leaf tissues of T2 survivors from 20–100 μM treatments were collected for confirmation of transgene by PCR.
2.6. Generation of Transgenic A. thaliana Line
The transgenic Arabidopsis line was generated under controlled conditions at BASF in Ludwigshafen, Germany. To prepare the transgene, ppo2 containing ΔG210 was inserted into the RTP6557 transformation vector, which was then inserted into Agrobacterium tumefaciens strain C58C1pMP90. Acetolactate synthase–herbicide-resistance gene was used as a selectable marker to identify transformed Arabidopsis seedlings. Plant transformation was conducted using the floral-dip method previously described [34]. After dipping, plants were kept in a cabinet under high humidity and low light intensity for 24 h and then grown under long-day conditions until maturity. The T1 seeds were collected and stored at 4 °C. After 15 days, T1 seeds were sown into GS90 soil, and 5% sand was then treated with 20 ppm imazamox to select transformed plants. These were grown under short-day (8 h light/16 h dark) for 12–14 days. Resistant seedlings (4-leaf stage) were transplanted into 6 cm pots filled with GS90 soil and grown to maturity. In the following generation, homozygous T2 seeds were identified.
2.7. Transgenic A. thaliana Response to Soil-Applied Fomesafen and Saflufenacil
Arabidopsis is highly sensitive to herbicides in comparison to rice; therefore, it is an excellent plant model to test whether the mutant ppo2 transgene can confer resistance to PPO-inhibiting herbicides such as fomesafen and saflufenacil. For the soil-based assay, three laboratory spatula scoops of T2 Arabidopsis seeds were placed in falcon tubes containing 45 mL 0.1% agar and refrigerated for 3–4 days to stratify the seeds. For the dose–response assay, pots were filled with soil, watered, and seeds were sown. For sowing, 2 mL of the agar/seed mixture were used per pot which was around 50 seeds per pot. Nine rates of fomesafen (0, 0.08, 0.25, 0.74, 2.22, 6.67, 20, 60, and 120 g ha−1) and eight rates of saflufenacil (0, 0.25, 0.74, 2.22, 6.67, 20, 60, and 120 g ha−1) were applied. After application, the pots were placed into the Phytotrons and covered with hoods with 22 °C day/20 °C night—16 h day/8 h night conditions. After 5 days, the hoods were removed when seeds were germinated. The emerged seedlings were counted 20 days after planting.
2.8. Statistical Analysis
To obtain a clear distinction between T1 rice plants exhibiting high or low tolerance, injury data from foliar application of fomesafen to WT and T1 rice seedlings were subjected to hierarchical cluster analysis using Ward’s Minimum method in JMP Pro v.15 (SAS Institute, Cary, NC, USA). Constellation plots were produced to show the clustering patterns. Additionally, a scatter plot was prepared in SigmaPlot V.14.0 (Systat Software, San Jose, CA, USA) to visualize the correlation between transgene presence and plant injury level.
The germination reduction data obtained from the soil-based assay were subjected to analysis of variance (ANOVA) using the GLIMMIX function in SAS v. 9.4 (SAS Institute, Cary, NC, USA). The run x treatment effect was not significant; therefore, data from the two runs were combined. Since the germination reduction data did not fit a normal distribution via Shapiro–Wilk test, the β distribution was assumed for this response analysis [35]. The Student’s t-test (p < 0.05) was used to compare the means if the treatment effect was significant. Injury per survivor data were also subjected to hierarchical cluster analysis as described previously. Plant height, number of tillers, and number of panicles per plant within each injury-level cluster were subjected to ANOVA using the JMP Pro v.15 (SAS Institute, Cary, NC, USA). Phenotypic data from nontreated WT plants were used as references. When the treatment effect was significant, Fisher’s protected LSD (p < 0.05) was used to compare means. Gene expression was also quantified in 15 selected T2 survivors representing all injury level categories. The fold-change in gene expression and injury per plant were analyzed by Pearson’s correlation in JMP Pro v.15 (SAS Institute, Cary, NC, USA).
Root growth rating data from the agar-based assay were analyzed by regression using the “drc” package in R 3.5.1 [36]. A three-parameter log-logistic model was used as defined by the formula below, where Y is the root growth (%), d is the upper horizontal asymptote, x is the fomesafen dose, and b is the slope around ED50, which is the dose causing 50% response level of Y [37].
The herbicide dose that would inhibit root growth by 50% (ED50) was estimated using this equation.
3. Results
3.1. Transgenic Rice Response to Foliar-Applied Fomesafen
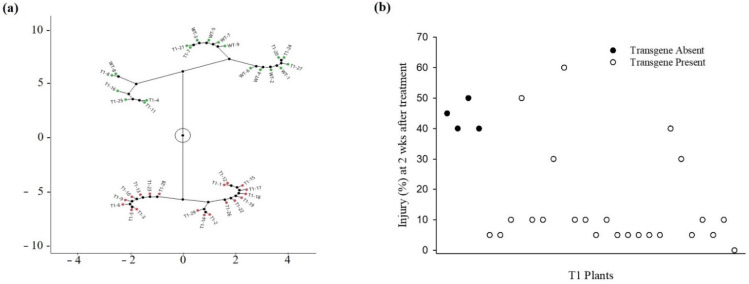
A foliar application assay conducted with plants derived from PCR-verified T0 plants (Figure S2b) showed that none of the T1 plants were controlled 100% with foliar-applied fomesafen. The foliar injury ranged from 0 to 60% among T1 and from 30 to 60% among WT plants. Thus, some transgenic plants were as sensitive as the WT plants. The hierarchical cluster analysis revealed two distinct groups of plants based on injury levels (Figure 1a). Cluster 1 was characterized as “highly tolerant” and cluster 2 as “minimally tolerant”. Cluster 1 consisted of plants with <10% injury and was comprised only of T1 plants. Sixty-eight percent (68%) of T1 plants were highly tolerant to fomesafen, showing low injury. All T1 plants with low injury harbored the transgene. Plants in cluster 2 exhibited 30 to 60% injury and included T1 and WT individuals. Out of 28 treated T1 plants, 4 tested negative for the transgene (Figure 1b). T1 plants without the transgene fell in cluster 2, among the highly injured individuals. Despite the strong correlation between transgene presence and low injury, five transgene-positive T1 plants showed high injury and grouped with cluster 2.
Figure 1.
Response of transgenic rice plants to foliar-applied fomesafen (780 g ha−1). (a) Constellation plot from the hierarchical clustering analysis of T1 and wild type (WT) injury data collected 2 weeks after foliar treatment. Cluster 1 (red) is composed of plants showing low injury. Only T1 plants were part of cluster 1. Cluster 2 (green) is composed of plants showing high injury. Both WT and T1 were present in cluster 2. (b) Scatter plot of foliar injury levels of T1 plants with (white circles) or without (black circles) the ΔG210-Apppo2 transgene.
3.2. Transgenic Rice Response to Soil-Applied Fomesafen
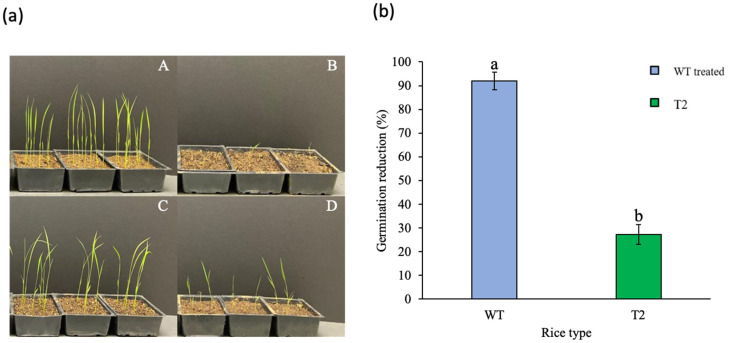
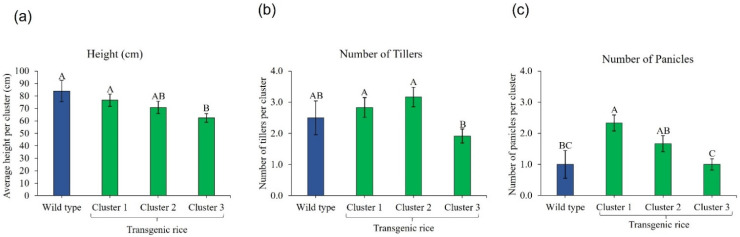
Soil-applied fomesafen delayed rice emergence for at least 5 days (up to one week) (Figure 2). The germination of WT seeds was reduced by 92% by fomesafen, and that of T2 seeds was reduced by 27% (Figure 2). The WT seedlings (8%) did not grow further and died, whereas T2 seedlings (73%) survived. All T2 survivors showed the presence of the transgene and were classified as tolerant. From 33 nontreated plants, 82% harbored the transgene [38]. Considering that 73% of T2 plants survived, the presence of the transgene can be correlated with tolerance to the pre-emergence application of fomesafen. Injury levels among T2 survivors varied widely (from 30 to 95%) and were grouped in three well-defined clusters. T2 survivors with 30–50%, 60–70%, and 80–95% injury were classified as highly tolerant, moderately tolerant, and slightly tolerant, respectively (Table S2). The rating of injury levels in T2 plants included delayed emergence, stunting, and foliar damage compared to nontreated plants. Although T2 plants in moderately tolerant and slightly tolerant clusters still incurred high injury, all T2 plants that survived fomesafen were transplanted and survived. The phenotypic traits (height, number of tillers, and number of panicles) of plants across clusters and WT plants were subjected to ANOVA. The plants in cluster 1 (<50% injury) were phenotypically different from those in cluster 3 (>80% injury) (Figure 3). Overall, plants in cluster 1 were the tallest across clusters, with the highest number of panicles. Plants in cluster 3 incurred substantial injury, which delayed phenological development. Despite some initial injury, plants in cluster 1 had significantly more panicles than the nontreated WT plants.
Figure 2.
Response of transgenic rice plants to soil-applied fomesafen (390 g ha−1). (a) The response of wild type and T2 rice plants 3 weeks after soil treatment. The photos show one run of the experiment; (A) WT nontreated, (B) WT treated, (C) T2 nontreated, and (D) T2 treated. (b) Wild-type and T2 germination reduction (%) calculated 3 weeks after soil-applied fomesafen treatment. Means were derived from a combined analysis of two runs since plant response to treatments did not vary across runs. Lowercase letters indicates significant difference (p < 0.05).
Figure 3.
Phenotypic data of T2 rice plants that survived 390 g ai ha−1 soil-applied fomesafen. (a) Height, (b) number of tillers, and (c) number of panicles of T2 survivors by phenotypic trait cluster. Data were collected when the majority of survivors transitioned to the reproductive stage. Significant means were separated using Fisher’s protected LSD (p < 0.05). Means with different letters above bars are different (p < 0.05).
3.3. Gene Expression Analysis of Fomesafen Tolerant Rice Plants
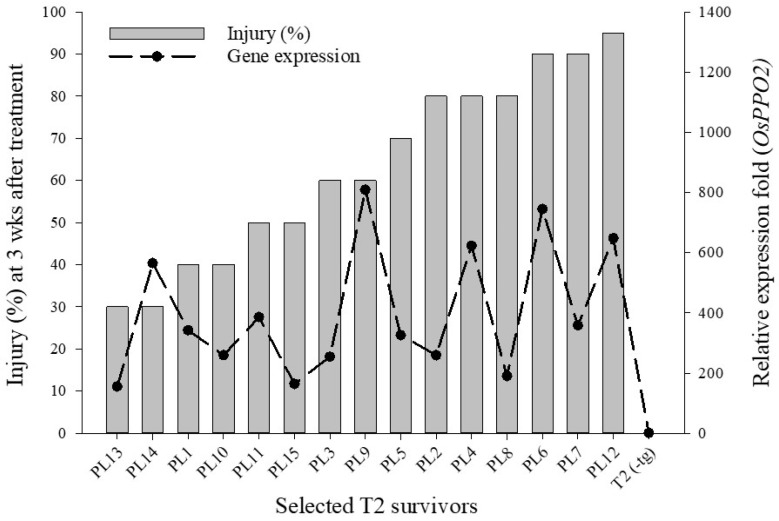
The T2 plants that survived soil-applied fomesafen showed a wide range of injuries. To understand this phenotypic variation, expression analysis of ΔG210-Apppo2 transgene was performed. Quantitative PCR analysis showed that all T2 survivors carrying the transgene had > 150-fold expression of the transgene compared to WT. The majority (10 of 15) of T2 plants showed transgene expression between 150 to 400-fold when calculated against native OsPPO2 (Figure 4). With one exception, individuals showing low injury had transgene expression values < 400-fold. When the transgene expression was calculated against ubiquitin, the majority of T2 survivors (12 out of 15) showed transgene expression < 800-fold (Figure S3). The injury per survivor did not correlate with fold-change in transgene expression calculated against O. sativa PPO2 (r = 0.3411, p = 0.2134) or rice ubiquitin (r = 0.1044, p = 0.7112).
Figure 4.
Visible injury (%) and transgene expression in T2 survivors in response to soil-applied fomesafen. Expression of transgene is calculated relative to native PPO2 from O. sativa. Nontreated T2(-tg) was used as a control for transgene expression.
3.4. Agar-Based Germination Assay with T2 Seeds of Transgenic Rice
To further characterize the pre-emergence tolerance conferred by the ΔG210-Apppo2 transgene, an agar-based germination assay was conducted using the T2 bulk population. All T2 and WT seeds germinated in agar media supplemented with up to 100 µM fomesafen. However, the root growth of WT seedlings was inhibited even at the lowest concentration (5 μM) of fomesafen (Figure 5). Therefore, only the T2 root growth rating data fitted the regression model (Figure S4). Root growth of the majority of T2 seedlings (88%) was not inhibited with up to 10 μM fomesafen but was reduced to 50% at 20 μM. Although reduced, roots continued to grow in all five T2 seedlings plated in MS media supplemented with 20, 40, and 60 μM fomesafen, whereas root growth of WT seedlings in the same treatments was negligible. Only one of five T2 seedlings had root growth on media supplemented with 80 and 100 μM fomesafen. The estimated fomesafen concentration that would result in a 50% growth reduction in the T2 population was 45 μM (Figure S4). Except for one seedling (that germinated on 60 μM fomesafen), ΔG210-Apppo2 transgene was present in all T2 seedlings that exhibited root growth.
Figure 5.
Agar-based assay showing root growth of rice seedlings in different concentrations of fomesafen. Root growth of wild type at 5 μM (A), 40 μM (C), and 100 μM (E), root growth of T2 plants at 5 μM (B), 40 μM (D), and 100 μM (F). White arrows show inhibited root growth in (A,C,E); and healthy root growth in (B,D,F).
3.5. Transgenic A. thaliana Response to Soil-Applied Fomesafen and Saflufenacil
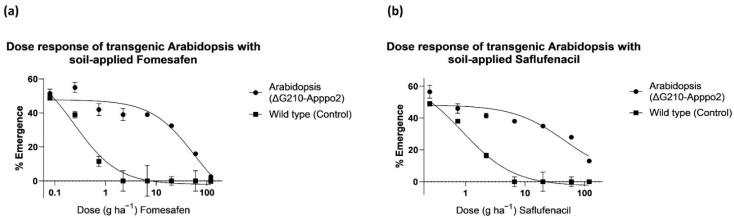
We generated a transgenic A. thaliana line using the ΔG210-Apppo2 gene to demonstrate whether ΔG210-Apppo2 can confer high tolerance to pre-emergence PPO inhibitors in the highly sensitive Arabidopsis. In the dose–response assay, the transgenic A. thaliana line was more tolerant to soil-applied herbicides than WT plants. WT plants were killed 100% at 2.22 and 6.67 g ha−1 fomesafen and saflufenacil, respectively (Figure 6 and Tables S3 and S4). On the other hand, the transgenic line was not completely controlled at the highest rate (120 g ha−1) of both herbicides. The dose–response experiment showed that the transgenic A. thaliana line was tolerant to both PPO herbicides tested, whereas WT plants were highly susceptible.
Figure 6.
The dose–response curves for percentages of germinated transgenic (ΔG210-Apppo2) Arabidopsis. (a) Response to increasing doses of fomesafen and (b) saflufenacil.
4. Discussion
Resistance to herbicides is one of the most important traits utilized in plant biotechnology, which is widely used to improve agricultural efficiency. There are more than 20 gene products targeted for herbicide action. The PPO is an ideal target as it is highly conserved and has a critical function within the plant. Field-evolved PPO mutations discovered in PPO-inhibitor-resistant weeds can be a tool for future crop engineering research. According to Kim et al. [39], G399A mutation is deemed the most appropriate or suitable site for gene-editing to make PPO-inhibitor resistant tomatoes. Recently, a mutant ppo2 gene having R128G mutation was isolated from PPO-resistant Amaranthus retroflexus L (Arppo2) and overexpressed in Arabidopsis. The presence of this mutation induced high tolerance to the foliar-applied PPO-herbicides (fomesafen, lactofen and carfentrazone-ethyl) tested [25].
In our study, we overexpressed the A. palmeri ppo2 gene containing field-evolved ΔG210 mutation in rice (as a crop model). Rice has some level of tolerance to foliar-applied PPO herbicides but not enough for commercial application of fomesafen. Our data on WT plants reflected this. In preliminary dose–response experiments conducted with the WT biotype, WT seedlings were not killed with rates up to eight times the maximum fomesafen dose [38]. Other diphenylether herbicides, which are analogs of fomesafen (chlomethoxyfen and oxyfluorfen), are safe to use on rice postemergence [40,41]. Even though none of the rice plants (WT or transgenic T1) were killed by foliar-applied fomesafen, all T1 plants that harbored the ΔG210-Apppo2 transgene showed low injury, indicating a reduced phytotoxic effect of fomesafen. To confirm the benefit of carrying ΔG210-Apppo2 transgene, it is necessary to have treatments that would obtain 100% control. This was achieved with the pre-emergence application of fomesafen to transgenic rice.
The soil-based assay confirmed that ΔG210-Apppo2 transgenic rice has a high tolerance to soil-applied fomesafen. The tolerance to soil-applied fomesafen in transgenic rice may be due to the constitutive transgene expression being driven by the maize ubiquitin promoter, which is active in germinating seedlings. Maize ubiquitin promoter directs high transgene expression in many young rice tissues [42,43], affecting the high expression of the transgene in rice seedlings. Tissues of germinating seeds and newly germinated seedlings are not equipped to counteract the strong oxidative effects of fomesafen action. By overexpressing a tolerant Apppo2, we hypothesize that the oxidative stress would be reduced, and sufficient functional protein would allow seedlings to continue growing.
Regarding the transgene effect on agronomic traits, the T2 survivors that had the lowest injury from the soil-based assay had a significantly higher number of panicles than the nontreated WT rice plants. This result suggests that overexpression of ΔG210-Apppo2 in rice may affect yield. To test this hypothesis, field experiments are needed to assess the transgene effect on yield and yield components, such as the number of seeds per panicle and the weight of 1000 seeds. Manipulation of PPO expression can produce divergent results. Jung et al. [44] attempted to increase the mitochondrial PPO activity by overexpressing human PPO in rice and instead increased the PPO activity in the chloroplast, which resulted in severe necrosis and growth inhibition. Yet, in another study, the advanced generation of the transgenic rice line (M4) expressing MxPPO produced a higher yield compared to WT when treated with PPO herbicides [45]. Lately, the oxyfluorfen-resistant rice line (M-206), which was developed in California, contains the mutant allele ROXY that confers non-transgenic resistance to this herbicide. Field testing with seeds from the ninth generation of this rice line showed a significantly higher yield than M-206 without the ROXY mutant allele after oxyfluorfen applications [46]. In these studies, the yield increase in comparison to WT is due to the combination of high WT injury and effective weed control by the PPO herbicides; there was no evidence that PPO manipulation directly contributed to the improvement of yield potential. Even though transgenic herbicide-resistant rice would be beneficial and profitable to rice farmers, it is unlikely that any transgenic rice varieties will be commercialized soon. Consumer acceptance is a major constraint to transgenic rice production and commercialization [47,48].
Initially, we hypothesized that the expression level of ΔG210-Apppo2 would be positively correlated with fomesafen tolerance. Our data did not support this hypothesis. Even though the transgene presence provided tolerance to foliar- and soil-applied fomesafen, plants displayed variable tolerance to fomesafen, which could be due to varying levels of transgene expression with the presence of hemizygous vs. homozygous states of the transgene. In PPO-resistant A. palmeri, Carvalho-Moore et al. [49] showed that among the resistant accessions tested, the accession with a high frequency of survivors that are homozygous for ΔG210 also had higher ED50 and less injury at a high dose of fomesafen. Variation in transgene expression is expected in the progeny since transgenic individuals generally differ in transgene expression, and full or partial levels of gene expression may be heritable [50]. Gene silencing factors and gene segregation across generations can also affect transgene expression in the offspring [51,52,53] until such a trait is fixed in subsequent generations.
An agar-based assay is an effective and quick technique to characterize plant response to herbicides. In our experiment, the root growth of WT rice seedlings was inhibited even at the lowest concentration of fomesafen. In a similar study, Lee et al. [54] showed that transgenic rice line overexpressing human PPO was tolerant to up to 5 μM of oxyfluorfen in agar-based seed germination assay, while the wild-type was sensitive to the lowest concentration of 2 μM. Like fomesafen, oxyfluorfen is also a diphenylether PPO herbicide. A transgenic rice line expressing Myxococcus xanthus PPO (MxPPO) showed high tolerance to oxyfluorfen, whereas the wild-type did not germinate at the lowest concentration tested [55].
Rice has natural tolerance to foliar-applied fomesafen due to higher antioxidative activities [56]; therefore, A. thaliana, which is a very sensitive species to PPO-inhibiting herbicides, was the perfect plant model to assess the effect of overexpressing ΔG210-Apppo2. Recently, Huang et al. [25] also used transgenic A. thaliana to evaluate the effect of a mutant Arppo2 enzyme on Arabidopsis response to foliar-applied fomesafen compared to a line overexpressing the wild type ArPPO2. The former showed tolerance to fomesafen, while the latter was as sensitive as the non-transgenic Arabidopsis line. Thus, our data suggest that the overproduction of the mutant Apppo2 protein, which is not inhibited by PPO herbicides, allows normal-rate conversion of the phototoxic protoporphyrinogen IX to porphyrin (in the presence of the inhibitor herbicide) enabling the plant to survive. It would be very interesting to know if transgenic ΔG210-Apppo2 protein was targeted towards both chloroplast and mitochondria, to overcome the soil-herbicide effect at the germinating stage. Transgenic expression of Apppo2 without the dual targeting signal peptide may show if simultaneous expression occurs in both organelles and if it is imperative for survival with soil-applied PPO herbicides.
In summary, the insertion of A. palmeri ppo2 containing ΔG210 mutation confers tolerance to fomesafen in rice and Arabidopsis. The majority of transgenic rice plants are able to fully recover from pre-emergence treatment with fomesafen, whereas WT plants are killed. Our research shows that if the increase in copy number or differential promotor activity of the PPO gene is selected under repetitive herbicide usage on key weed species such as A. tuberculatus and A. palmeri, the efficacy of pre-emergence PPO herbicide can be reduced. Additionally, this research presents an example of harnessing herbicide-resistant genes from weeds to develop herbicide-tolerant crops. Cross-tolerance to other soil-applied PPO-inhibiting herbicides will be determined for the ΔG210-Apppo2 transgenic rice in future work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13061044/s1, Figure S1: Schematic diagram showing construction of pACL1 used for rice transformation; Figure S2: Confirmation of presence of transgene carrying ΔG210-Apppo2 (a) Detection of ΔG210-Apppo2 transgene in rice genomic DNA by PCR amplification. (b) Nucleotide sequence alignment of protoporphyrinogen IX oxidase (PPO2) in wild type (Susceptible), resistant (Gly-210-deletion), and transformed survivor (T0 fragment 1 and 2); Figure S3: Visible injury (%) and transgene expression of T2 plants surviving 390 g ai ha−1 soil-applied fomesafen; Figure S4: Transformed rice (T2) seedling root growth assessment (%) relative to the nontreated check as affected by increasing concentration of fomesafen in agar medium; Table S1: Primer sequences used in the transgene expression analysis of rice transformants by qPCR; Table S2: Hierarchal clustering of T2 rice survivors based on injury levels (%) at 3 weeks after preemergence treatment with fomesafen at 390 g ha−1; Table S3: Dose response of transgenic Arabidopsis line to soil-applied fomesafen; Table S4: Dose response of transgenic Arabidopsis line to soil-applied saflufenacil.
Author Contributions
N.R.-B., V.S. and G.R.: Conceptualization; G.R. and N.R.-B.: Designed the experiments; G.R. and N.R.-B.: supervised all experiments; G.R., V.S. and A.C.L.: Designed and made the transgene construct; G.R. and A.C.L. generated the transgenic rice line; P.C.-M.: performed all experiments on transgenic rice line; P.C.-M. and G.R.: analyzed the data; A.P., S.J.B. and J.L.: Designed the experiments on Arabidopsis, generated and analyzed the transgenic Arabidopsis data; P.C.-M.: wrote the original draft of the manuscript; G.R., N.R.-B., P.C.-M., V.S., A.P., S.J.B. and J.L.: edited and reviewed the manuscript; N.R.-B. and V.S.: Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received funding from Arkansas Biosciences Institute Grant Program. The student sandwich doctoral fellowship of A.C.L. was financed by the CNPq—Proc.N. 88881.132552/2016-01 CNPq.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wychen V.L. 2019 Survey of the Most Common and Troublesome Weeds in Broadleaf Crops, Fruits & Vegetables in the United States and Canada. Weed Science Society of America National Weed Survey Dataset. [(accessed on 3 March 2022)]. Available online: https://wssanet/wp-content/uploads/2019-Weed-Survey_broadleaf-cropsxlsx.
- 2.Heap I. The International Survey of Herbicide Weeds. [(accessed on 1 February 2022)]. Available online: http://www.weedscience.org/
- 3.Ying Z.X.L., Jun X., Fengshou D., Xingang L., Yongquan Z. Determination of fomesafen residues in soybean and soil using QuEChERS and UPLC-MS/MS. Environ. Chem. 2012;31:1399–1404. [Google Scholar]
- 4.Hao G.F., Zuo Y., Yang S.G., Yang G.F. Protoporphyrinogen oxidase inhibitor: An ideal target for herbicide discovery. Chimia. 2011;65:961–969. doi: 10.2533/chimia.2011.961. [DOI] [PubMed] [Google Scholar]
- 5.Poulson R., Polglase W.J. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J. Biol. Chem. 1975;250:1269–1274. doi: 10.1016/S0021-9258(19)41809-7. [DOI] [PubMed] [Google Scholar]
- 6.Dayan F.E., Duke S.O. Porphyrin-generating herbicides. Pestic. Outlook. 1996;7:22–27. [Google Scholar]
- 7.Duke S.O., Lydon J., Becerril J.M., Sherman T.D., Lehnen Jr L.P., Matsumoto H. Protoporphyrinogen oxidase-inhibiting herbicides. Weed Sci. 1991;39:465–473. doi: 10.1017/S0043174500073239. [DOI] [Google Scholar]
- 8.Lermontova I., Kruse E., Mock H.P., Grimm B. Cloning and characterization of a plastidal and a mitochondrial isoform of tobacco protoporphyrinogen IX oxidase. Proc. Natl. Acad. Sci. USA. 1997;94:8895–8900. doi: 10.1073/pnas.94.16.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matringe M., Camadro J.M., Labbe P., Scalla R. Protoporphyrinogen oxidase as a molecular target for diphenyl ether herbicides. Biochem. J. 1989;260:231–235. doi: 10.1042/bj2600231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orr G.L., Hess F.D. Mechanism of Action of the Diphenyl Ether Herbicide Acifluorfen-Methyl in Excised Cucumber (Cucumis sativus L.) Cotyledons: Light activation and the subsequent formation of lipophilic free radicals. Plant Physiol. 1982;69:502–507. doi: 10.1104/pp.69.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patzoldt W.L., Tranel P.J., Hager A.G. A waterhemp (Amaranthus tuberculatus) biotype with multiple resistance across three herbicide sites of action. Weed Sci. 2005;53:30–36. doi: 10.1614/WS-04-087R. [DOI] [Google Scholar]
- 12.Watanabe N., Che F.S., Iwano M., Takayama S., Yoshida S., Isogai A. Dual targeting of spinach protoporphyrinogen oxidase II to mitochondria and chloroplasts by alternative use of two in-frame initiation codons. J. Biol. Chem. 2001;276:20474–20481. doi: 10.1074/jbc.M101140200. [DOI] [PubMed] [Google Scholar]
- 13.Dayan F.E., Barker A., Tranel P.J. Origins and structure of chloroplastic and mitochondrial plant protoporphyrinogen oxidases: Implications for the evolution of herbicide resistance. Pest. Manag. Sci. 2018;74:2226–2234. doi: 10.1002/ps.4744. [DOI] [PubMed] [Google Scholar]
- 14.Mueller T.C., Boswell B.W., Mueller S.S., Steckel L.E. Dissipation of fomesafen, saflufenacil, sulfentrazone, and flumioxazin from a Tennessee soil under field conditions. Weed Sci. 2014;62:664–671. doi: 10.1614/WS-D-13-00183.1. [DOI] [Google Scholar]
- 15.Shaner D.L.E. Herbicide Handbook. 10th ed. Weed Science Society of America; Westminster, CO, USA: 2014. [Google Scholar]
- 16.Cobucci T., Prates H.T., Falcão C.L., Rezende M.M. Effect of imazamox, fomesafen, and acifluorfen soil residue on rotational crops. Weed Sci. 1998;46:258–263. doi: 10.1017/S0043174500090500. [DOI] [Google Scholar]
- 17.Anonymous. Flexstar Herbicide Product Label. Syngenta Crop Protection, Inc.; Greensboro, NC, USA: 2022. [Google Scholar]
- 18.Umphres A.M., Steckel L.E., Mueller T.C. Control of protoporphyrinogen oxidase inhibiting herbicide resistant and susceptible Palmer amaranth (Amaranthus palmeri) with soil applied protoporphyrinogen oxi-dase-inhibiting herbicides. Weed Technol. 2018;32:95–100. doi: 10.1017/wet.2017.78. [DOI] [Google Scholar]
- 19.Patzoldt W.L., Hager A.G., McCormick J.S., Tranel P.J. A codon deletion confers resistance to herbicides inhibiting protoporphyrinogen oxidase. Proc. Natl. Acad. Sci. USA. 2006;103:12329–12334. doi: 10.1073/pnas.0603137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salas-Perez R.A., Burgos N.R., Rangani G., Singh S., Refatti J.P., Piveta L., Tranel P.J., Mauromoustakos A., Scott R.C. Frequency of Gly-210 deletion mutation among protoporphyrinogen oxidase inhibitor–resistant Palmer amaranth (Amaranthus palmeri) populations. Weed Sci. 2017;65:718–731. doi: 10.1017/wsc.2017.41. [DOI] [Google Scholar]
- 21.Giacomini D.A., Umphres A.M., Nie H., Mueller T.C., Steckel L.E., Young B.G., Scott R.C., Tranel P.J. Two new PPX2 mutations associated with resistance to PPO-inhibiting herbicides in Amaranthus palmeri. Pest. Manag. Sci. 2017;73:1559–1563. doi: 10.1002/ps.4581. [DOI] [PubMed] [Google Scholar]
- 22.Rangani G., Salas-Perez R.A., Aponte R.A., Knapp M., Craig I.R., Mietzner T., Langaro A.C., Noguera M.M., Porri A., Roma-Burgos N. A Novel Single-Site Mutation in the Catalytic Domain of Protoporphyrinogen Oxidase IX (PPO) Confers Resistance to PPO-Inhibiting Herbicides. Front. Plant Sci. 2019;10:568. doi: 10.3389/fpls.2019.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noguera M.M., Rangani G., Heiser J., Bararpour T., Steckel L.E., Betz M., Porri A., Lerchl J., Zimmermann S., Nichols R.L. Functional PPO2 mutations: Co-occurrence in one plant or the same ppo2 allele of herbicide-resistant Amaranthus palmeri in the US mid-south. Pest. Manag. Sci. 2021;77:1001–1012. doi: 10.1002/ps.6111. [DOI] [PubMed] [Google Scholar]
- 24.Varanasi V.K., Brabham C., Norsworthy J.K., Nie H., Young B.G., Houston M., Barber T., Scott R.C. A statewide survey of PPO-inhibitor resistance and the prevalent target-site mechanism in Palmer amaranth. Weed Sci. 2018;66:149–158. doi: 10.1017/wsc.2017.68. [DOI] [Google Scholar]
- 25.Huang Z., Cui H., Wang C., Wu T., Zhang C., Huang H., Wei S. Investigation of resistance mechanism to fomesafen in Amaranthus retroflexus L. Pestic. Biochem. Physiol. 2020;165:104560. doi: 10.1016/j.pestbp.2020.104560. [DOI] [PubMed] [Google Scholar]
- 26.Rousonelos S.L., Lee R.M., Moreira M.S., VanGessel M.J., Tranel P.J. Characterization of a common ragweed (Ambrosia artemisiifolia) population resistant to ALS- and PPO inhibiting herbicides. Weed Sci. 2012;60:335–344. doi: 10.1614/WS-D-11-00152.1. [DOI] [Google Scholar]
- 27.Nishimura A., Aichi I., Matsuoka M. A protocol for Agrobacterium-mediated transformation in rice. Nat. Protoc. 2006;1:2796–2802. doi: 10.1038/nprot.2006.469. [DOI] [PubMed] [Google Scholar]
- 28.Counce P.A., Keisling T.C., Mitchell A.J. A uniform, objective, and adaptative system for expressing rice development. Crop. Sci. 2000;40:436–443. doi: 10.2135/cropsci2000.402436x. [DOI] [Google Scholar]
- 29.Burgos N.R., Tranel P.J., Streibig J.C., Davis V.M., Shaner D., Norsworthy J.K., Ritz C. Confirmation of resistance to herbicides and evaluation of resistance levels. Weed Sci. 2013;61:4–20. doi: 10.1614/WS-D-12-00032.1. [DOI] [Google Scholar]
- 30.Frans R., Talbert R., Marx D., Crowley H. Experimental design and techniques for measuring and analyzing plant responses to weed control practices. In: Camper N.D., editor. Southern Weed Science Society, Research Methods in Weed Science. 1st ed. Southern Weed Science Society; Westminster, CO, USA: 1986. pp. 29–46. [Google Scholar]
- 31.Doyle J.J., Doyle J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- 32.Hongbao M., Young J., Shen C. RNA, DNA and protein isolation using TRIzol reagent. Nat. Sci. 2008;6:66–75. [Google Scholar]
- 33.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant. J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 35.Gbur E.E., Stroup W.W., McCarter K.S., Durham S., Young L.J., Christman M., West M., Kramer M. Analysis of Generalized Linear Mixed Models in the Agricultural and Natural Resources Sciences. American Society of Agronomy, Soil Science Society of America, Crop Science Society of America; Madison, WI, USA: 2012. [Google Scholar]
- 36.Ritz C., Baty F., Streibig J.C., Gerhard D. Dose-Response Analysis Using R. PLoS ONE. 2015;10:e0146021. doi: 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritz C. Toward a unified approach to dose–response modeling in ecotoxicology. Environ. Toxicol. Chem. 2010;29:220–229. doi: 10.1002/etc.7. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho de Lima P. Master Thesis. University of Arkansas; Fayetteville, AR, USA: 2020. Resistance to Herbicides Conferred by Amaranthus palmeri Protoporphyrinogen IX Oxidase Mutations. [Google Scholar]
- 39.Kim E., Park H., Yang S., Koo Y. Current status of herbicide-tolerant protein research and strategy for development of herbicide-tolerant tomato using CRISPR-Cas9 system. Trends Agric. Life Sci. 2019;57:1–8. doi: 10.29335/tals.2019.57.1. [DOI] [Google Scholar]
- 40.Ishizuka K., Matsumoto H., Hyakutake H. Selective inhibitory action of chlomethoxynil on rice and barnyardgrass [Echinochloa oryzicola] and its molecular fate in the light and dark. Weed Res. 1988;33:41–48. [Google Scholar]
- 41.Niki Y., Kuwatsuka S., Yokomichi I. Absorption, translocation and metabolism of chlomethoxynil (X-52) in plants. Agric. Biol. Chem. 1976;40:683–690. [Google Scholar]
- 42.Capell T., Bassie L., Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA. 2004;101:9909–9914. doi: 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toki S., Takamatsu S., Nojiri C., Ooba S., Anzai H., Iwata M., Christensen A.H., Quail P.H., Uchimiya H. Expression of a maize ubiquitin gene promoter-bar chimeric gene in transgenic rice plants. Plant Physiol. 1992;100:1503–1507. doi: 10.1104/pp.100.3.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung H.I., Kuk Y.I., Back K., Burgos N.R. Resistance pattern and antioxidant enzyme profiles of protoporphyrinogen oxidase (PROTOX) inhibitor-resistant transgenic rice. Pesti. Biochem. Pysiol. 2008;91:53–65. doi: 10.1016/j.pestbp.2008.01.005. [DOI] [Google Scholar]
- 45.Jung H.I., Kuk Y.I., Kim H.Y., Back K., Lee D.J., Lee S., Burgos N.R. Resistance levels and fitness of protoporphyrinogen oxidase (PROTOX) inhibitor-resistant transgenic rice in paddy fields. Field Crops Res. 2010;115:125–131. doi: 10.1016/j.fcr.2009.10.010. [DOI] [Google Scholar]
- 46.McKenzie K.S. Oxyfluorfen Resistant Rice Lines. No. 2018/0070548. U.S. Patent Application. 2018 March 15;
- 47.Bond C.A., Carter C.A., Farzin Y.H. Medium grains, high stakes: Economics of genetically modified rice in California. AgBioForum. 2003;6:146–154. [Google Scholar]
- 48.Mulvaney D.R., Krupnik T.J., Koffler K.B. Transgenic rice evaluated for risks to marketability. Calif. Agric. 2011;65:161–167. doi: 10.3733/ca.E.v065n03p161. [DOI] [Google Scholar]
- 49.Carvalho-Moore P., Rangani G., Heiser J., Findley D., Bowe S.J., Roma-Burgos N. PPO2 Mutations in Amaranthus palmeri: Implications on cross-resistance. Agriculture. 2021;11:760. doi: 10.3390/agriculture11080760. [DOI] [Google Scholar]
- 50.Vain P., James V., Worland B., Snape J. Transgene behavior across two generations in a large random population of transgenic rice plants produced by particle bombardment. Theor. Appl. Genet. 2002;105:878–889. doi: 10.1007/s00122-002-1039-5. [DOI] [PubMed] [Google Scholar]
- 51.Day C.D., Lee E., Kobayashi J., Holappa L.D., Albert H., Ow D.W. Transgene integration into the same chromosome location can produce alleles that express at a predictable level, or alleles that are differentially silenced. Genes Dev. 2000;14:2869–2880. doi: 10.1101/gad.849600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matzke A.J., Matzke M.A. Position effects and epigenetic silencing of plant transgenes. Curr. Opin. Plant Biol. 1998;1:142–148. doi: 10.1016/S1369-5266(98)80016-2. [DOI] [PubMed] [Google Scholar]
- 53.McCabe M.S., Mohapatra U.B., Debnath S.C., Power J.B., Davey M.R. Integration, expression, and inheritance of two linked T-DNA marker genes in transgenic lettuce. Mol. Breed. 1999;5:329–344. doi: 10.1023/A:1009681615365. [DOI] [Google Scholar]
- 54.Lee Y., Jung S., Back K. Expression of human protoporphyrinogen oxidase in transgenic rice induces both a photodynamic response and oxyfluorfen resistance. Pestic. Biochem. Physiol. 2004;80:65–74. doi: 10.1016/j.pestbp.2004.06.008. [DOI] [Google Scholar]
- 55.Jung S., Lee Y., Yang K., Lee S.B., Jang S.M., Ha S.B., Back K. Dual targeting of Myxococcus xanthus protoporphyrinogen oxidase into chloroplasts and mitochondria and high level oxyfluorfen resistance. Plant Cell Environ. 2004;27:1436–1446. doi: 10.1111/j.1365-3040.2004.01247.x. [DOI] [Google Scholar]
- 56.Matsumoto H., Lee J., Ishizuka K. Variation in crop response to protoporphyrinogen oxidase inhibitors. Porphyric Pesticides: Chemistry, Toxicology, and Pharmaceutical Applications. Am. Chem. Soc. Symp. Ser. 1995;559:120–132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.