Abstract
The objective of this research is to assess the effects of seven different exercise therapies (aquatic exercise, aerobic exercise, yoga, Pilates, virtual reality exercise, whole-body vibration exercise, and resistance exercise) on the balance function and functional walking ability of multiple sclerosis disease patients. Materials and Methods: The effects of different exercise interventions on the balance function and functional walking ability in people with multiple sclerosis were assessed by searching five databases: PubMed, Embase, Cochrane Library, Web of Science, and CNKI; only randomized controlled trials were included. The included studies were assessed for risk of bias using the Cochrane assessment tool. Results: The RCTs were collected between the initial date of the electronic databases’ creation and May 2022. We included 31 RCTs with 904 patients. The results of the collected data analysis showed that yoga can significantly improve patients’ BBS scores (SUCRA = 79.7%) and that aquatic exercise can significantly decrease patients’ TUG scores (SUCRA = 78.8%). Conclusion: Based on the network meta-analysis, we suggest that although each type of exercise is useful, yoga, virtual reality training, and aerobic training are more effective in improving the balance function of people with MS; aquatic exercise, virtual reality training, and aerobic training are more effective in improving the functional walking ability of people with MS.
Keywords: rehabilitation, yoga, aquatic exercise, multiple sclerosis disease, network meta-analysis
1. Introduction
Multiple sclerosis is one of the most common disabling neurological diseases worldwide and has an average age of onset of 29 years. As of 2017, there were 2.5 million people with multiple sclerosis worldwide, and this number is increasing [1,2]. The disease has many adverse effects on patients, including, but not limited to, physical symptoms such as muscle weakness and reduced mobility and balance, as well as mental symptoms such as fatigue and cognitive decline [3,4]. This has a considerable impact not only on the patients themselves and their families, but also on public health and safety [5].
Due to the combination of reduced physical and mental function, approximately 75% of people with MS experience balance and walking-related impairments in the early and later stages of the disease [6], which increases their risk of falls and injuries [1]. The physical injuries and psychological fears associated with falls may further affect patients’ physical and mental health, creating a vicious cycle that further affects patients’ quality of life [7,8].
More and more research is focusing on the rehabilitation of people with MS, and in addition to traditional measures such as daily care and rehabilitation, different forms of exercise are increasingly being used in clinical non-pharmacological treatment and rehabilitation [9]. A number of randomized controlled trial studies show that exercise produces beneficial effects on mental aspects such as fatigue and cognitive performance in patients that exceed those of traditional rehabilitation measures. In addition, there are meta-analyses comparing one exercise intervention versus traditional rehabilitation measures on the physical and mental functional abilities of MS patients [10,11], as well as network meta-analyses comparing multiple exercise interventions versus traditional rehabilitation measures on the mental function of MS patients [12,13]; both provide considerable clinical evidence-based recommendations.
In addition to traditional meta-analysis, researchers invented a new evidence-based medical technique, network meta-analysis (NMA), which, in contrast to the original technique, allows one to compare and rank the effects of multiple interventions for a disease at the same time [14]. Therefore, in this study, we use network meta-analysis to compare different exercise programs (aquatic exercise, aerobic exercise, yoga, Pilates, virtual reality exercise, whole-body vibration exercise, and resistance exercise) in order to assess the effect of these programs on the physical function of people with MS, and to provide patients and clinicians with appropriate evidence-based recommendations.
2. Materials and Methods
2.1. Search Strategy
The authors in this paper searched five electronic databases (Pubmed, EMBASE, the Cochrane Central Register of Controlled Trials, Web of Science, and CNKI) from their inception to May 2022. The search strategy was constructed using the PICOS tool: (P) Population; people with multiple sclerosis, (I) Intervention; exercise, (C) Comparator; control group with usual care and usual rehabilitation measures only, (O) Outcomes; motor function tests of people with multiple sclerosis, and (S) Study type; RCTs [15]. The detailed search strategy is shown in Table 1 (using Pubmed as an example).
Table 1.
Search strategy on Pubmed.
| #1 | Search “Multiple Sclerosis” [MeSh] |
| #2 | Search (Multiple Sclerosis [Title/Abstract]) OR (MS [Title/Abstract]) OR (Relapsing-remitting Multiple Sclerosis [Title/Abstract]) OR (RRMS [Title/Abstract]) OR (Multilocular Sclerosis [Title/Abstract]) |
| #3 | Search #1 OR #2 |
| #4 | Search “Exercise” [MeSh] |
| #5 | Search (exercise [Title/Abstract]) OR (exercise intervention [Title/Abstract]) OR (exercise training [Title/Abstract]) OR (training [Title/Abstract]) OR (physical training [Title/Abstract]) OR (physical exercise [Title/Abstract]) OR (sports training [Title/Abstract]) OR (nurse intervention [Title/Abstract] |
| #6 | Search #4 OR #5 |
| #7 | Search #3 AND #6 |
2.2. Inclusion Criteria
The inclusion criteria includes: (1) experimental group; use of an exercise as an intervention to treat multiple sclerosis disease, (2) control group; treatment of multiple sclerosis disease using only daily care and conventional rehabilitation (no other types of exercise interventions, just the more popular and commonly used balance rehabilitation exercises; no training, just daily living care), (3) clinical randomized controlled trial, and (4) outcome indicators including at least one of either the Berg Balance Scale (BBS) score or the Timed-Up-and-Go (TUG) score.
2.3. Exclusion Criteria
The exclusion criteria includes: (1) studies with incomplete or unreported data, and (2) studies from non-randomized controlled trials (including quasi-randomized controlled trials, animal studies, protocols, meeting abstracts, case report correspondence).
2.4. Outcomes
The primary outcome is the Berg Balance Scale (BBS), and the secondary outcome is the Timed-Up-and-Go (TUG) score. The BBS and TUG are popular with clinical practitioners due to their comprehensiveness, sensitivity, and simplicity [16,17].
2.5. Study Selection
Two authors independently screened the papers using Zotero software to eliminate duplicate papers and complete the primary screening. The titles and abstracts of the remaining papers were then read to eliminate those that did not meet the inclusion criteria, completing the re-screening process. Finally, the remaining papers were read in full to complete the final screening process, and the results were compared. Articles were included when two authors agreed that the inclusion criteria were met; if there was a disagreement between the authors regarding the inclusion of a paper, a third author was consulted to determine whether or not it should be included in the analysis.
2.6. Data Extraction
A table with seven sections [18] was used to extract detailed data from the included papers: (1) author (abbreviations), (2) year of publication, (3) country in which each study was conducted, (4) sample size in each study, (5) details of the experimental group, (6) details of the control group, and (7) outcome indicators.
2.7. Risk of Bias of Individual Studies
Two authors independently assessed the risk of bias (ROB) in accordance with the Cochrane Handbook version 5.1.0 tool (Cochrane, London, UK) for assessing ROB in RCTs. The following seven domains were considered: (1) randomized sequence generation, (2) treatment allocation concealment, the blinding of (3) participants and (4) personnel, (5) incomplete outcome data, (6) selective reporting, and (7) other sources of bias. Trials were categorized into three levels of ROB according to the number of components for which high ROB potentially existed: high risk (five or more), moderate risk (three or four), and low risk (two or less). All of the studies are, by default, classified as having a high ROB with respect to the category “blinding of participants” because it is impossible to blind participants to group assignments in exercise intervention protocols [19,20].
2.8. Data Analysis
First, because we are studying the efficacy of exercise for a particular disease, we chose to use continuous variables for statistical analysis. To calculate the results more conservatively, we used the immediate post-intervention value minus the baseline value to express the size of the intervention effect. As the results we analyzed were all in uniform units, we chose to use standard difference (SD) rather than standardized mean difference (SMD) for our calculations. There is bound to be variation between the original studies, and to make the results more scientific, we chose to calculate a random effects model rather than a fixed effects model [14].
Secondly, Stata software was used to present the network graphs, which are important in NMA. In a network diagram, different graphs have different meanings: (1) each node represents an exercise intervention; (2) the size of the node indicates the sample size of the subjects who performed this intervention; (3) if there are no line segments between each node, it means that indirect comparisons will be made between the nodes, and if there are line segments, it means that direct comparisons will be made between the nodes; (4) the thickness of the line segments between the nodes indicates the original study sample size; and (5) the size of the nodes and the thickness of the line segments are positively correlated with the number [21].
Again, we used Stata software to summarize and analyze the NMA using Markov chain Monte Carlo simulation chains in a Bayesian-based framework. Thus, in a ranking table, treatments were ranked from best to worst along the leading diagonal. Above the leading diagonal are estimates from pairwise meta-analyses, and below the leading diagonal are estimates from network meta-analyses [22,23].
Finally, we calculated the SUCRA ranking in Stata software and used it as a criterion for evaluating the effect of the exercise interventions, which is a percentage with a maximum value of 1 and a minimum value of 0. The closer to 1, the better the intervention effect; the closer to 0, the worse the intervention effect. A funnel plot will also be generated to examine possible publication bias [21].
3. Results
3.1. Study Identification and Selection
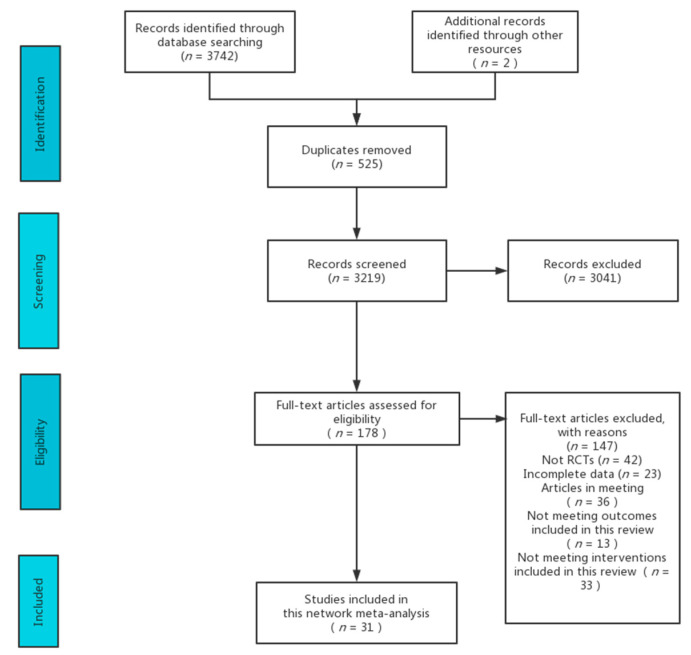
A total of 3744 articles were retrieved. A total of 525 articles were duplicates, and after eliminating them, 3219 articles remained. After screening the abstracts and titles of these 3210 articles, it was determined that 3041 articles were not relevant to the study. After reading the full texts of the 178 articles that remained after screening, 31 articles were finally included in the study. Refer to Figure 1 for specific details.
Figure 1.
Flow diagram of literature selection.
3.2. Characteristics of the Included Studies
We included 31 RCTs, with a total of 904 subjects in all of the studies combined. Interventions in the control group included Pilates training (seven studies) [24,25,26,27,28,29,30], whole-body vibration training (three studies) [31,32,33], aquatic training (two studies) [34,35], yoga training (two studies) [36,37], aerobic training (eight studies) [38,39,40,41,42,43,44,45], resistance training (three studies) [46,47,48], and virtual reality training (five studies) [43,49,50,51,52,53]. Sixteen studies were from Asia, four studies were from the Americas, and eleven studies were from Europe. The details of included studies are shown in Table 2.
Table 2.
Details of the studies included in the network meta-analysis.
| Author | Country | Year | Age (Mean + SD) | Total/Man/Woman | Intervention 1/Length (Weeks)/Frequency (Weeks)/Duration (Minutes) | Intervention 2/Length (Weeks)/Frequency (Weeks)/Duration (Minutes) | Control Group | Outcome |
|---|---|---|---|---|---|---|---|---|
| Ahmadi | Iran | 2010 | T: 36.8 (9.17) C: 36.7 (9.32) |
T: 10/NA/NA C: 10/NA/NA |
Aerobic training 8 weeks 3 times a week 30 min |
NA | No exercise |
BBS |
| Ahmadi | Iran | 2013 | T1: 36.8 (9.17) T2: 32.27 (8.68) C: 36.7 (9.32) |
T1: 10/NA/NA T2: 11/NA/NA C: 10/NA/NA |
Aerobic training 8 weeks 3 times a week 30 min |
Yoga training 8 weeks 3 times a week 60 min |
No exercise |
BBS |
| Gervasoni | Italy | 2014 | T: 49.6 (9.4) C: 45.7 (8.9) |
T: 15/NA/NA C: 15/NA/NA |
Aerobic training 2 weeks 6 times a week 45 min |
NA | No exercise |
BBS |
| Straudi | Italy | 2015 | T: 52.26 (11.11) C: 54.12 (11.44) |
T: 27/10/17 C: 25/8/17 |
Aerobic training 2 weeks 2 times a week 30 min |
NA | Usual care | BBS, TUG |
| Tollar | Hungary | 2019 | T: 48.1 (5.65) C: 44.4 (6.76) |
T: 14/1/13 C: 12/1/11 |
Aerobic training 5 weeks 5 times a week 40 min |
No exercise |
BBS | |
| Cakt | Turkey | 2010 | T1: 36.4 (10.5) T2: 35.5 (10.9) C: 43 (10.2) |
T1: 14/5/9 T2: 9/3/6 C: 10/2/8 |
Aerobic training 8 weeks 2 times a week 20 min |
Resistance training 8 weeks 2 times a week 20 min |
No exercise |
TUG |
| Orban | USA | 2019 | T: 44.7 (9.4) C: 48.7 (8.4) |
T: 10/1/9 C: 7/2/5 |
Aerobic training 8 weeks 4 times a week 30 min |
No exercise |
TUG | |
| Straudi | Italy | 2013 | T: 49.92 (7.51) C: 55.25 (13.82) |
T: 12/5/7 C: 12/2/10 |
Aerobic training 6 weeks 2 times a week 30 min |
No exercise |
TUG | |
| Asvar | Iran | 2020 | T: 32.1 (13) C: 33.9 (6) |
T: 15/0/15 C: 15/0/15 |
Pilates training 8 weeks 3 times a week 60 min |
No exercise |
BBS | |
| Gheitasi | Iran | 2020 | T: 30.6 (5.27) C: 32.1 (6.3) |
T: 15/15/0 C: 15/15/0 |
Pilates training 12 weeks 3 times a week 50 min |
No exercise |
BBS, TUG | |
| Gunduz | Turkey | 2014 | T: 36 (29-40) C:36 (27-45) |
T: 18/NA/NA C: 8/NA/NA |
Pilates training 8 weeks 2 times a week 60 min |
Usual care | BBS, TUG | |
| Karlon | Israel | 2016 | T: 42.9 (7.2) C: 44.3 (6.6) |
T: 23/8/14 C: 22/8/15 |
Pilates training 12 weeks 1 time a week 30 min |
Usual care | BBS, TUG | |
| Kara | Turkey | 2017 | T1: 49.77 (8.95) T2: 43.03 (10.26) C: 44.42 (5.98) |
T1: 9/NA/NA T2: 26/NA/NA C: 21/NA/NA |
Pilates training 8 weeks 2 times a week 45 min |
Aerobic training 8 weeks 2 times a week 45 min |
No exercise |
BBS |
| Kucuk | Turkey | 2016 | T: 47.2 (9.5) C: 49.7 (8.9) |
T: 11/NA/NA C: 9/NA/NA |
Pilates training 8 weeks 2 times a week 60 min |
Usual care | BBS | |
| Zuhal | Turkey | 2019 | T: 42.5 (6.76) C: 48.24 (11.79) |
T: 16/NA/NA C: 17/NA/NA |
Pilates training 8 weeks 3 times a week 60 min |
Usual care | TUG | |
| Gerson | Brazil | 2016 | T: 46 (8) C: 45 (9) |
T: 6/NA/NA C: 6/NA/NA |
Yoga training 24 weeks 2 times a week 60 min |
No exercise |
BBS | |
| Yazgan | Turkey | 2020 | T: 47.76 (10.53) C: 40.66 (8.82) |
T: 15/2/13 C: 15/2/13 |
VR training (Nintendo® Wii®) 8 weeks 2 times a week 60 min |
No exercise |
BBS, TUG | |
| Khalil | Jordan | 2019 | T: 39.88 (12.75) C: 34.87 (8.98) |
T: 16/4/12 C: 16/6/10 |
VR training (VR scenarios) 6 weeks 3 times a week 30 min |
Usual care | BBS, TUG | |
| Brichetto | Italy | 2013 | NA | T: 18/NA/NA C: 18/NA/NA |
VR training (Nintendo® Wii®) 4 weeks 3 times a week 60 min |
Usual care | BBS | |
| Lozana | Spain | 2014 | T: 48.33 (10.82) C: 40.6 (9.24) |
T: 6/3/3 C: 5/4/1 |
VR training (Kinect games) 10 weeks 1 time a week 60 min |
No exercise |
BBS, TUG | |
| Molhemi | Iran | 2020 | T: 36.8 (8.4) C: 41.6 (8.4) |
T: 19/7/12 C: 20/8/12 |
VR training (Kinect games) 6 weeks 3 times a week 35 min |
Usual care | BBS, TUG | |
| Tollar | Hungary | 2019 | T: 48.2 (5.48) C: 44.4 (6.76) |
T: 14/2/12 C: 12/1/11 |
VR training (Nintendo® Wii®) 5 weeks 5 times a week 40 min |
No exercise |
BBS | |
| Aidar | Brazil | 2018 | T: 41.3 (7.3) C: 43.6 (7.6) |
T: 13/4/9 C: 13/5/8 |
Aquatic training 12 weeks 3 times a week 45–60 min |
No exercise |
BBS, TUG | |
| Kargarfard | Iran | 2017 | T: 36.5 (9) C: 36.2 (7.4) |
T: 17/NA/NA C: 15/NA/NA |
Aquatic training 8 weeks 3 times a week 60 min |
No exercise |
BBS | |
| Aidar | Brazil | 2018 | T: 42.8 (8) C: 43.6 (7.7) |
T: 11/4/7 C: 12/4/8 |
Resistance training 12 weeks 3 times a week 45–60 min |
No exercise |
BBS, TUG | |
| Moradi | Iran | 2015 | T: 34.38 (11.07) C: 33.13 (7.08) |
T: 8/NA/NA C: 10/NA/NA |
Resistance training 8 weeks 6 times a week 30 min |
No exercise |
TUG | |
| Moghadasi | Iran | 2020 | T: 37.62 (4.58) C: 34.72 (5.01) |
T: 16/NA/NA C: 11/NA/NA |
Resistance training 8 weeks 3 times a week 30 min |
No exercise |
TUG | |
| Alguacil | Spain | 2012 | T: 44 (20) C: 43 (17) |
T: 15/7/8 C: 17/9/8 |
Whole-body vibration training 5 days 1 time a day 10 min |
No exercise |
BBS, TUG | |
| Broekmans | Belgium | 2010 | T: 46.1 (2.1) C: 49.7 (3.3) |
T: 11/7/4 C: 14//11/3 |
Whole-body vibration training 20 weeks 2 times a week 10 min |
No exercise |
TUG | |
| Schuhfried | Austria | 2005 | T: 49.3 (13.3) C: 46 (12.7) |
T: 6/1/5 C: 6/2/4 |
Whole-body vibration training 2 weeks 4 times a week 15 min |
Usual care | TUG | |
| Young | USA | 2018 | T: 48.35 (9.95) C: 47.29 (10.33) |
T: 26/6/20 C: 29/4/24 |
Yoga training 8 weeks 3 times a week 40 min |
No exercise |
TUG |
T: experimental group; C: control group; BBS: Berg Balance Scale; TUG: Timed-Up-and-Go score; Freq: frequency; NA: no mention in the original article.
3.3. Quality Assessment of the Included Studies
Only 13% of the studies had a high risk of bias. A total of 13 studies had a moderate risk of bias, and 12 studies had a low risk of bias. The overall risk of bias was acceptable, but it is worth noting that exercise as an intervention has the inherent disadvantage of it being difficult to implement a double-blind approach in an experiment. The specific risk of bias assessment scores for each study are presented in Supplementary Table S1.
3.4. Network Meta-Analysis
3.4.1. Berg Balance Scale (BBS)
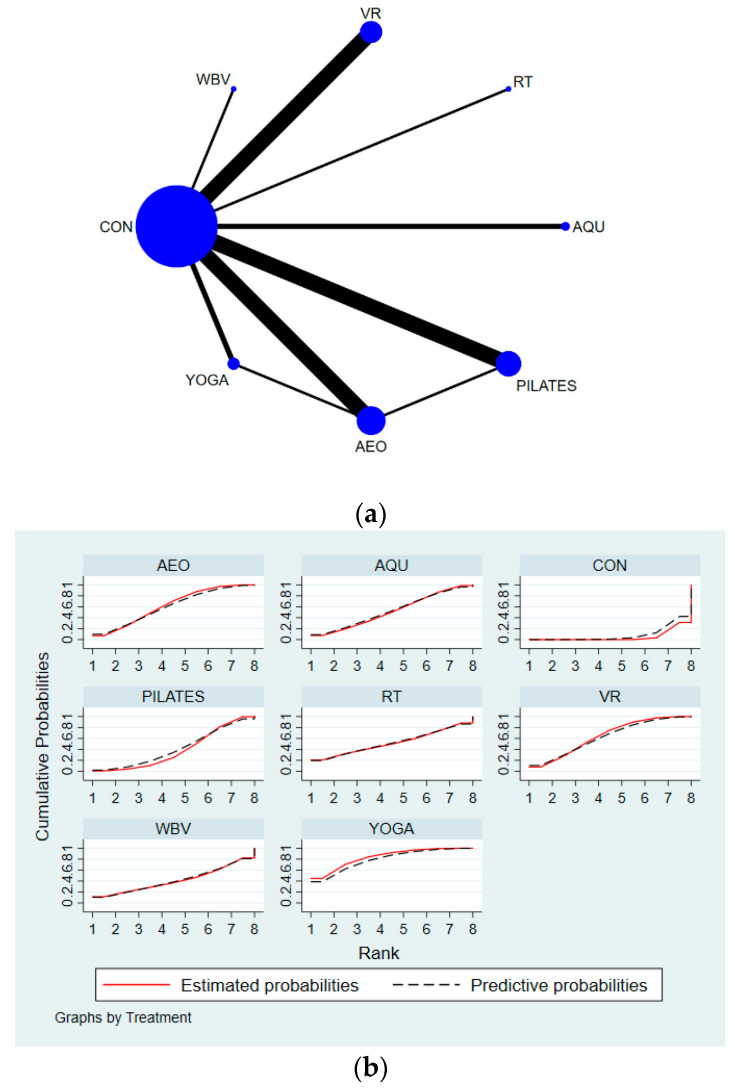
The results of the network meta-analysis showed that relative to the control group’s conventional rehabilitation measures, yoga (MD = 5.50, 95% CI = (2.55, 8.45)); virtual reality technology exercise (MD = 4.12, 95% CI = (2.15, 6.09)); aerobic exercise (MD = 4.01, 95% CI = (1.81, 6.20)); aquatic exercise (MD = 3.43, 95% CI = (0.23, 6.63)), and Pilates (MD = 2.70, 95% CI = (0.71, 4.69)) were superior compared to the control group in terms of increasing BBS scores (Table 3). Yoga achieved the number one SUCRA probability ranking in terms of increasing BBS scores (SUCRA: 79.7%). Details are shown in Figure 2. The p-values for the heterogeneity tests can be found in Supplementary Table S2.
Table 3.
League table for BBS.
| YOGA | VR | AEO | AQU | RT | PILATES | WBV | CON |
|---|---|---|---|---|---|---|---|
| YOGA | −1.38 (−4.92, 2.16) | −1.49 (−4.92, 1.93) | −2.07 (−6.42, 2.28) | −2.06 (−8.83, 4.70) | −2.80 (−6.35, 0.76) | −3.05 (−9.42, 3.31) | −5.50 (−8.45, −2.55) |
| 1.38 (−2.16, 4.92) | VR | −0.11 (−3.09, 2.86) | −0.69 (−4.44, 3.07) | −0.68 (−7.08, 5.72) | −1.42 (−4.22, 1.39) | −1.67 (−7.65, 4.30) | −4.12 (−6.09, −2.15) |
| 1.49 (−1.93, 4.92) | 0.11 (−2.86, 3.09) | AEO | −0.58 (−4.46, 3.31) | −0.57 (−7.04, 5.90) | −1.30 (−4.24, 1.63) | −1.56 (−7.61, 4.49) | −4.01 (−6.20, −1.81) |
| 2.07 (−2.28, 6.42) | 0.69 (−3.07, 4.44) | 0.58 (−3.31, 4.46) | AQU | 0.01 (−6.87, 6.89) | −0.73 (−4.50, 3.04) | −0.98 (−7.47, 5.50) | −3.43 (−6.63, −0.23) |
| 2.06 (−4.70, 8.83) | 0.68 (−5.72, 7.08) | 0.57 (−5.90, 7.04) | −0.01 (−6.89, 6.87) | RT | −0.74 (−7.14, 5.67) | −0.99 (−9.29, 7.31) | −3.44 (−9.53, 2.65) |
| 2.80 (−0.76, 6.35) | 1.42 (−1.39, 4.22) | 1.30 (−1.63, 4.24) | 0.73 (−3.04, 4.50) | 0.74 (−5.67, 7.14) | PILATES | −0.25 (−6.24, 5.73) | −2.70 (−4.69, −0.71) |
| 3.05 (−3.31, 9.42) | 1.67 (−4.30, 7.65) | 1.56 (−4.49, 7.61) | 0.98 (−5.50, 7.47) | 0.99 (−7.31, 9.29) | 0.25 (−5.73, 6.24) | WBV | −2.45 (−8.09, 3.19) |
| 5.50 (2.55, 8.45) | 4.12 (2.15, 6.09) | 4.01 (1.81, 6.20) | 3.43 (0.23, 6.63) | 3.44 (−2.65, 9.53) | 2.70 (0.71, 4.69) | 2.45 (−3.19, 8.09) | CON |
AQU: aquatic exercise; AEO: aerobic exercise; YOGA: yoga; PILATES: Pilates; VR: virtual reality exercise; WBV: whole-body vibration exercise; RT: resistance exercise; CON: control group.
Figure 2.
Specific details regarding the BBS’ network meta-analysis: (a) NMA plot for BBS and (b) SUCRA plot for BBS. AQU: aquatic exercise; AEO: aerobic exercise; YOGA: yoga; PILATES: Pilates; VR: virtual reality exercise; WBV: whole-body vibration exercise; RT: resistance exercise.
3.4.2. Timed-Up-and-Go Score (TUG)
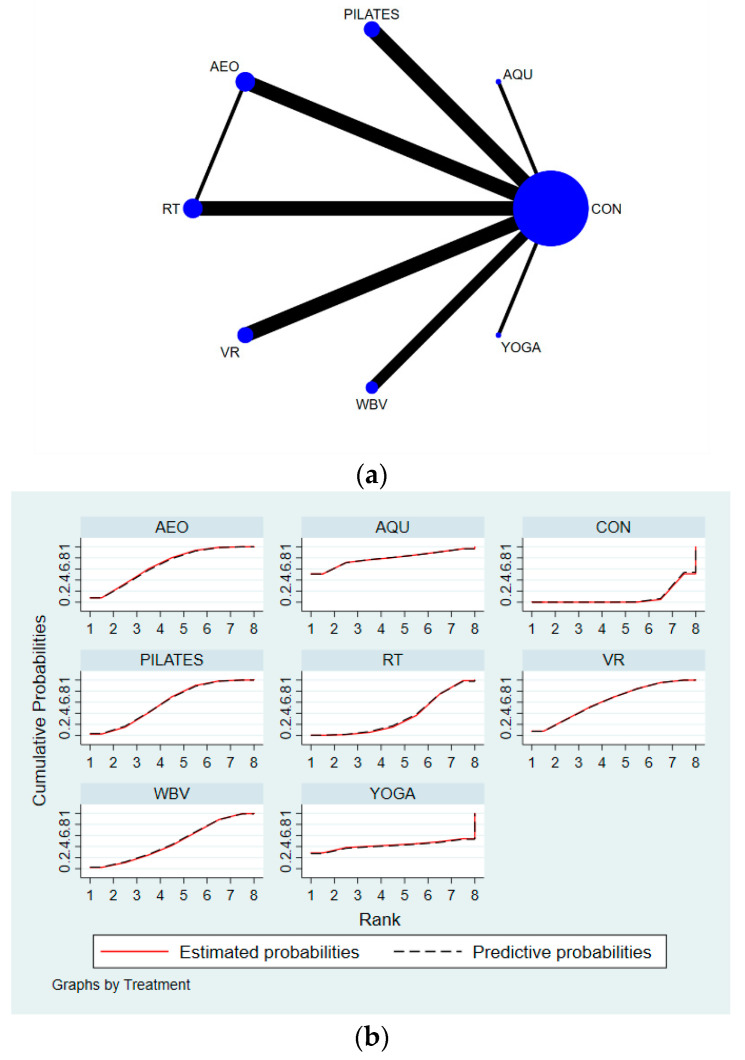
The results of the network meta-analysis showed that relative to the control group’s conventional measures, aquatic exercise (MD = −2.58, 95% CI = (−5.88, −0.72)); aerobic exercise (MD = −1.53, 95% CI = (−2.31, −0.75)); virtual reality exercise (MD = −1.45, 95% CI = (−2.45, −0.45)); Pilates (MD = −1.36, 95% CI = (−1.83, −0.88)); and resistance exercise (MD = −0.79, 95% CI = (−1.55, −0.02)) were superior to the control group in terms of reducing TUG time (Table 4). Aquatic exercise achieved the number one SUCRA probability ranking in terms of reducing the TUG time (SUCRA: 78.8%). Details are shown in Figure 3. The p-values for the heterogeneity tests can be found in Supplementary Table S3.
Table 4.
League table for TUG.
| AQU | AEO | VR | PILATES | WBV | YOGA | RT | CON |
|---|---|---|---|---|---|---|---|
| AQU | 1.05 (−2.34, 4.44) | 1.13 (−2.32, 4.58) | 1.22 (−2.11, 4.56) | 1.48 (−1.95, 4.92) | 2.28 (−6.86, 11.42) | 1.79 (−1.59, 5.18) | 2.58 (−0.72, 5.88) |
| −1.05 (−4.44, 2.34) | AEO | 0.08 (−1.20, 1.36) | 0.17 (−0.76, 1.10) | 0.43 (−0.80, 1.66) | 1.23 (−7.33, 9.79) | 0.74 (−0.19, 1.67) | 1.53 (0.75, 2.31) |
| −1.13 (−4.58, 2.32) | −0.08 (−1.36, 1.20) | VR | 0.09 (−1.00, 1.19) | 0.35 (−1.04, 1.74) | 1.15 (−7.43, 9.73) | 0.66 (−0.63, 1.95) | 1.45 (0.45, 2.45) |
| −1.22 (−4.56, 2.11) | −0.17 (−1.10, 0.76) | −0.09 (−1.19, 1.00) | PILATES | 0.26 (−0.82, 1.34) | 1.06 (−7.48, 9.59) | 0.57 (−0.41, 1.55) | 1.36 (0.88, 1.83) |
| −1.48 (−4.92, 1.95) | −0.43 (−1.66, 0.80) | −0.35 (−1.74, 1.04) | −0.26 (−1.34, 0.82) | WBV | 0.80 (−7.78, 9.38) | 0.31 (−0.88, 1.51) | 1.10 (0.15, 2.05) |
| −2.28 (−11.42, 6.86) | −1.23 (−9.79, 7.33) | −1.15 (−9.73, 7.43) | −1.06 (−9.59, 7.48) | −0.80 (−9.38, 7.78) | YOGA | −0.49 (−9.05, 8.07) | 0.30 (−8.22, 8.82) |
| −1.79 (−5.18, 1.59) | −0.74 (−1.67, 0.19) | −0.66 (−1.95, 0.63) | −0.57 (−1.55, 0.41) | −0.31 (−1.51, 0.88) | 0.49 (−8.07, 9.05) | RT | 0.79 (0.02, 1.55) |
| −2.58 (−5.88, −0.72) | −1.53 (−2.31, −0.75) | −1.45 (−2.45, −0.45) | −1.36 (−1.83, −0.88) | −1.10 (−2.05, −0.15) | −0.30 (−8.82, 8.22) | −0.79 (−1.55, −0.02) | CON |
AQU: aquatic exercise; AEO: aerobic exercise; YOGA: yoga; PILATES: Pilates; VR: virtual reality exercise; WBV: whole-body vibration exercise; RT: resistance exercise; CON: control group.
Figure 3.
Specific details regarding the network meta-analysis for TUG: (a) NMA plot for TUG and (b) SUCRA plot for TUG.
3.5. Publication Bias
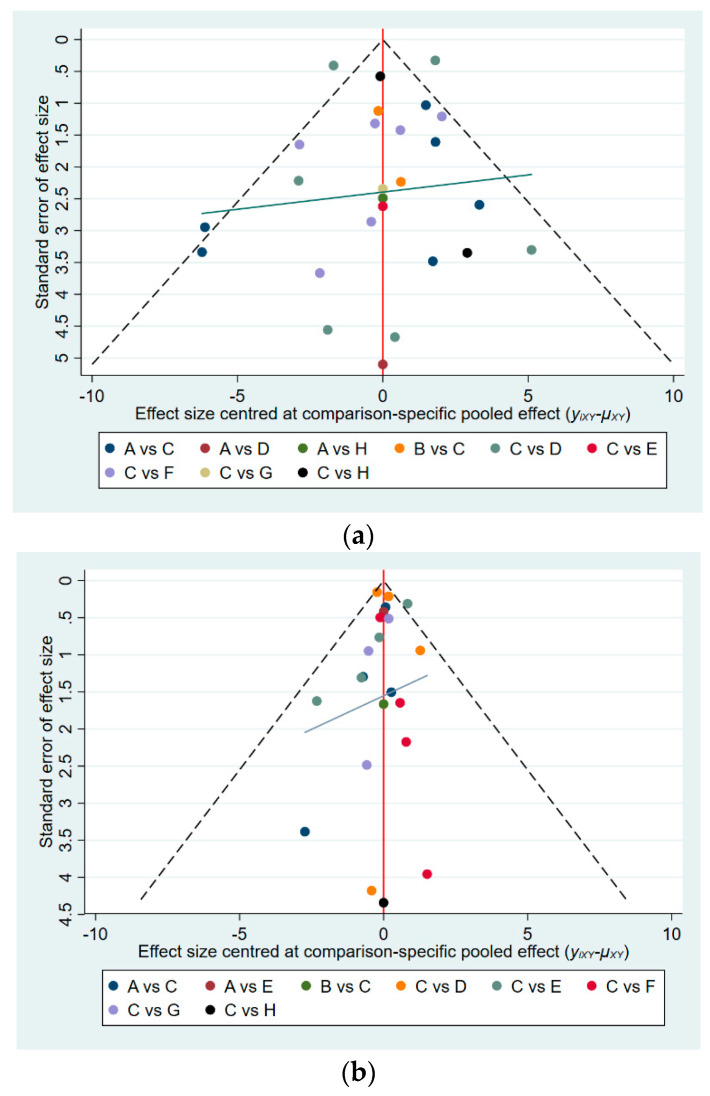
Looking at the parallelism of the horizontal line in the funnel plot to the x-axis, we concluded that there was no publication bias among the original studies that affected the NMA (Figure 4) [54].
Figure 4.
Funnel plot to test for publication bias: (a) funnel plot for BBS and (b) funnel plot for TUG. A: aerobic training, B: aquatic training, C: control group, D: Pilates, E: resistance training, F: virtual reality training, G: whole-body vibration training, H: yoga.
4. Discussion
Exercise therapy is shown to be effective as a rehabilitation measure to improve physical function and to promote neuroplasticity [9]. The combined effect of improved physical function and mental rehabilitation helps to reduce the risk of falls for people with MS. The results of previous meta-analyses show that different types of exercise therapy result in good improvements in the physical and mental functional abilities of people with MS compared to traditional rehabilitation measures. Harrison et al. ranked the different exercise interventions in their study to find the best exercise intervention for improving the mental health of people with MS [13]. However, controversy remains in the review regarding which exercises are most the effective in improving the physical function of people with MS. Our study explored the effects of different exercises on the physical functional abilities of people with MS for the first time. The results of the meta-analysis show that yoga is the best intervention to improve dynamic and static balance for people with MS and that aquatic exercise is the best intervention to improve the functional walking ability of people with MS, based on improvements in the BBS Balance Scale and the TUG test, respectively.
The BBS test is a comprehensive functional test that reflects the ability of MS patients to actively shift their center of gravity by examining their dynamic and static balance in a sitting or standing position; its results are accurate and acceptable [55]. The results of this study show that yoga is superior to other interventions in terms of improving patients’ dynamic and static balance and postural control (MD = 5.50, 95% CI = (2.55, 8.45)). The mechanism by which yoga improves BBS can be explained in several ways: (1) Yoga practice emphasizes the control of the nervous system over the muscles through less intense stretching-type activities, increasing the unconscious specific response of the muscles to dynamic joint stability signals and emphasizing the control of the core muscles throughout the whole trunk during the activity [56]. (2) Yoga training involves the training of joint function, which allows for the normal degree of motion in the joints to be maintained and deformed postures to be corrected through active or passive stretching to relieve abnormal tension in the joint capsule [57]. (3) Some yoga postures are performed with the eyes closed, emphasizing increased attention to the other sensory organs, particularly the vestibular organs [37].
In addition to dynamic static balance, improvements in physical function are also associated with increased functional walking ability, and the TUG test is often used to test the functional walking ability of people with MS due to its ease of administration and the sensitivity of the test [58]. Our findings suggest that aquatic exercise is superior to other interventions in improving functional walking ability. The mechanism by which aquatic exercise improves TUG can be explained in several ways: (1) The presence of water resistance allows the patient to perform exercises more slowly than land-based exercises, resulting in an increased weight-bearing time on the lower limbs; in addition, the patient’s torso is subjected to a certain amount of water pressure in the aquatic environment [59] which has a similar effect on the skeletal muscles as blood flow restriction training [60] (a training modality that was shown to have a significant effect on increasing muscle strength) [61]. (2) When exercising in the water, hydrostatic force causes the blood and lymphatic fluid to move up the torso, and combined with the gravitational offload and hydrostatic effect of the water, it increases the amount of blood circulating from the periphery to the center, resulting in an increase in the end-diastolic volume of the heart and thus an increase in cardiac output [62]. This increase in blood volume first reaches the brain and muscle tissue and is accompanied by an increase in serum brain-derived neurotrophic factor (BDNF), an anti-inflammatory factor that is particularly important for brain and muscle recovery [63]. (3) The period during and after aquatic exercise causes the body to reduce sympathetic activity and improves sympathetic–parasympathetic balance by increasing vagal tone [64].
The rehabilitative effects of both exercises in their respective domains are very useful, and we therefore recommend that MS patients prioritize both exercises when rehabilitating their physical function. In addition, we hypothesize that yoga and aquatic training can be alternated during a full rehabilitation cycle when conditions permit, but due to the lack of direct clinical evidence, we maintain a wait-and-see attitude towards this combined intervention in hopes that further experiments will prove or disprove our assumptions.
5. Strengths and Limitations
Our study has several strengths and weaknesses. Firstly, our study focused on finding the most effective exercise intervention for treating the physical function of people with MS among a variety of exercise interventions; this was not addressed in previous studies. Secondly, our study only included randomized controlled trials, which is the “gold standard” in the field of clinical research.
Admittedly, there are certain limitations in both our study and the original studies included. Heterogeneity between each of the original studies is inevitable (e.g., the ratio between male and female participants; the original studies are from different regions), and this heterogeneity can affect the scientific validity of the network meta-analysis to some extent. In addition, we did not include tests used to evaluate the physical function of MS patients in this study because there are too few original studies regarding these tests. However, we remain hopeful that more original studies will expand on the results of this study in the future in order to update and provide more solid clinical evidence-based recommendations.
In our study, readers should interpret the results with caution because of the small number of studies included and the limited head-to-head direct comparative evidence for some interventions. Our study highlights the need for the further expansion of relevant studies and their timeliness.
6. Conclusions
Based on studies using the Berg Balance Scale and the Timed-Up-and-Go test, we suggest that the exercise interventions discussed in this paper, compared with conventional care, all had an effect on improving the dynamic and static balance and the functional walking ability of patients with MS. However, yoga training, virtual reality training and aerobic training were more effective in improving dynamic and static balance; aquatic exercise, aerobic training and virtual reality training were more effective in improving functional walking ability.
Acknowledgments
We thank all of the reviewers for their assistance and support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph19127175/s1, Table S1: Risk of bias for each included studies. Table S2: Consistency test for BBS. Table S3: Consistency test for TUG.
Author Contributions
Z.H. interpreted the data, wrote the initial manuscript, and was involved in the data analysis; X.Z. was responsible for the collection of all of the relevant papers; P.C. was responsible for the supervision of the study. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of the study are available from the first author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reich D.S., Lucchinetti C.F., Calabresi P.A. Multiple Sclerosis. N. Engl. J. Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correale J., Gaitan M.I., Ysrraelit M.C., Fiol M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain. 2017;140:527–546. doi: 10.1093/brain/aww258. [DOI] [PubMed] [Google Scholar]
- 3.Ontaneda D., Thompson A.J., Fox R.J., Cohen J.A. Progressive multiple sclerosis: Prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–1366. doi: 10.1016/S0140-6736(16)31320-4. [DOI] [PubMed] [Google Scholar]
- 4.Freiha J., Riachi N., Chalah M.A., Zoghaib R., Ayache S.S., Ahdab R. Paroxysmal Symptoms in Multiple Sclerosis—A Review of the Literature. J. Clin. Med. 2020;9:3100. doi: 10.3390/jcm9103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee W.J., Hardy T.A., Fazekas F., Miller D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet. 2017;389:1336–1346. doi: 10.1016/S0140-6736(16)30959-X. [DOI] [PubMed] [Google Scholar]
- 6.Walton C., King R., Rechtman L., Kaye W., Leray E., Marrie R.A., Robertson N., La Rocca N., Uitdehaag B., van der Mei I., et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020;26:1816–1821. doi: 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dymecka J., Gerymski R., Tataruch R., Bidzan M. Sense of Coherence and Health-Related Quality of Life in Patients with Multiple Sclerosis: The Role of Physical and Neurological Disability. J. Clin. Med. 2022;11:1716. doi: 10.3390/jcm11061716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comber L., Coote S., Finlayson M., Galvin R., Quinn G., Peterson E. An exploration of fall-related, psychosocial variables in people with multiple sclerosis who have fallen. Br. J. Occup. Ther. 2017;80:587–595. doi: 10.1177/0308022617725492. [DOI] [Google Scholar]
- 9.Motl R.W., Sandroff B.M., Kwakkel G., Dalgas U., Feinstein A., Heesen C., Feys P., Thompson A.J. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017;16:848–856. doi: 10.1016/S1474-4422(17)30281-8. [DOI] [PubMed] [Google Scholar]
- 10.Akkan H., Kallem Seyyar G., Aslan B., Karabulut E. The effect of virtual reality-based therapy on fear of falling in multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022;63:103791. doi: 10.1016/j.msard.2022.103791. [DOI] [PubMed] [Google Scholar]
- 11.Abou L., Qin K., Alluri A., Du Y., Rice L.A. The effectiveness of physical therapy interventions in reducing falls among people with multiple sclerosis: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2022;29:74–85. doi: 10.1016/j.jbmt.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Torres-Costoso A., Martinez-Vizcaino V., Reina-Gutierrez S., Alvarez-Bueno C., Guzman-Pavon M.J., Pozuelo-Carrascosa D.P., Fernandez-Rodriguez R., Sanchez-Lopez M., Cavero-Redondo I. Effect of Exercise on Fatigue in Multiple Sclerosis: A Network Meta-analysis Comparing Different Types of Exercise. Arch. Phys. Med. Rehabil. 2022;103:970. doi: 10.1016/j.apmr.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Harrison A.M., Safari R., Mercer T., Picariello F., van der Linden M.L., White C., Moss-Morris R., Norton S. Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis. Mult. Scler. J. 2021;27:1657–1678. doi: 10.1177/1352458521996002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouse B., Chaimani A., Li T. Network meta-analysis: An introduction for clinicians. Intern. Emerg. Med. 2017;12:103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P.A., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 16.Podsiadlo D., Richardson S. The Timed up and Go—A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 17.Park S.-H. Tools for assessing fall risk in the elderly: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2018;30:1–16. doi: 10.1007/s40520-017-0749-0. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento A.S., Fagundes C.V., Mendes F.A.D.S., Leal J.C. Effectiveness of Virtual Reality Rehabilitation in Persons with Multiple Sclerosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Mult. Scler. Relat. Disord. 2021;54:103128. doi: 10.1016/j.msard.2021.103128. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P.T., Altman D.G., Gotzsche P.C., Jueni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ-Br. Med. J. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopp L. Risk of bias reporting in Cochrane systematic reviews. Int. J. Nurs. Pract. 2015;21:683–686. doi: 10.1111/ijn.12252. [DOI] [PubMed] [Google Scholar]
- 21.Chaimani A., Higgins J.P.T., Mavridis D., Spyridonos P., Salanti G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vats D., Flegal J.M., Jones G.L. Multivariate output analysis for Markov chain Monte Carlo. Biometrika. 2019;106:321–337. doi: 10.1093/biomet/asz002. [DOI] [Google Scholar]
- 23.Marotta N., Demeco A., Moggio L., Marinaro C., Pino I., Barletta M., Petraroli A., Pepe D., Lavano F., Ammendolia A. Comparative effectiveness of breathing exercises in patients with chronic obstructive pulmonary disease. Complement. Ther. Clin. Pract. 2020;41:101260. doi: 10.1016/j.ctcp.2020.101260. [DOI] [PubMed] [Google Scholar]
- 24.Kara B., Küçük F., Poyraz E.C., Tomruk M.S., İdıman E. Different types of exercise in Multiple Sclerosis: Aerobic exercise or Pilates, a single-blind clinical study. J. Back Musculoskelet. Rehabil. 2017;30:565–573. doi: 10.3233/BMR-150515. [DOI] [PubMed] [Google Scholar]
- 25.Gheitasi M., Bayattork M., Andersen L.L., Imani S., Daneshfar A. Effect of twelve weeks pilates training on functional balance of male patients with multiple sclerosis: Randomized controlled trial. J. Bodyw. Mov. Ther. 2021;25:41–45. doi: 10.1016/j.jbmt.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Küçük F., Kara B., Poyraz E.Ç., İdiman E. Improvements in cognition, quality of life, and physical performance with clinical Pilates in multiple sclerosis: A randomized controlled trial. J. Phys. Ther. Sci. 2016;28:761–768. doi: 10.1589/jpts.28.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalron A., Rosenblum U., Frid L., Achiron A. Pilates exercise training vs. physical therapy for improving walking and balance in people with multiple sclerosis: A randomized controlled trial. Clin. Rehabil. 2017;31:319–328. doi: 10.1177/0269215516637202. [DOI] [PubMed] [Google Scholar]
- 28.Asvar S., Taghian F. The Effect of an Eight-week Pilates Training on Interleukine-18 Level, Fatigue, and Balance in Women With Multiple Sclerosis. J. Res. Health. 2020;10:383–392. doi: 10.32598/JRH.10.6.552.4. [DOI] [Google Scholar]
- 29.Abasıyanık Z., Ertekin Ö., Kahraman T., Yigit P., Özakbaş S. The effects of Clinical Pilates training on walking, balance, fall risk, respiratory, and cognitive functions in persons with multiple sclerosis: A randomized controlled trial. EXPLORE. 2020;16:12–20. doi: 10.1016/j.explore.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Guclu-Gunduz A., Citaker S., Irkec C., Nazliel B., Batur-Caglayan H.Z. The effects of pilates on balance, mobility and strength in patients with multiple sclerosis. NeuroRehabilitation. 2014;34:337–342. doi: 10.3233/NRE-130957. [DOI] [PubMed] [Google Scholar]
- 31.Alguacil Diego I.M., Pedrero Hernández C., Molina Rueda F., Cano de la Cuerda R. Effects of vibrotherapy on postural control, functionality and fatigue in multiple sclerosis patients: A randomised clinical trial. Neurology. 2012;27:143–153. doi: 10.1016/j.nrleng.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Schuhfried O., Mittermaier C., Jovanovic T., Pieber K., Paternostro-Sluga T. Effects of whole-body vibration in patients with multiple sclerosis: A pilot study. Clin. Rehabil. 2005;19:834–842. doi: 10.1191/0269215505cr919oa. [DOI] [PubMed] [Google Scholar]
- 33.Broekmans T., Roelants M., Alders G., Feys P., Thijs H., Eijnde B.O. Exploring the effects of a 20-week whole-body vibration training programme on leg muscle performance and function in persons with multiple sclerosis. J. Rehabil. Med. 2010;42:866–872. doi: 10.2340/16501977-0609. [DOI] [PubMed] [Google Scholar]
- 34.Aidar F.J., Gama de Matos D., de Souza R.F., Gomes A.B., Saavedra F., Garrido N., Carneiro A.L., Reis V. Influence of aquatic exercises in physical condition in patients with multiple sclerosis. J. Sports Med. Phys. Fit. 2018;58:684–689. doi: 10.23736/S0022-4707.17.07151-1. [DOI] [PubMed] [Google Scholar]
- 35.Kargarfard M., Shariat A., Ingle L., Cleland J.A., Kargarfard M. Randomized Controlled Trial to Examine the Impact of Aquatic Exercise Training on Functional Capacity, Balance, and Perceptions of Fatigue in Female Patients With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2018;99:234–241. doi: 10.1016/j.apmr.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Young H.J., Mehta T.S., Herman C., Wang F., Rimmer J.H. The Effects of M2M and Adapted Yoga on Physical and Psychosocial Outcomes in People With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2019;100:391–400. doi: 10.1016/j.apmr.2018.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Oliveira G., Tavares M.D.C.C.G.F., de Faria Oliveira J.D., Rodrigues M.R., Santaella D.F. Yoga Training Has Positive Effects on Postural Balance and Its Influence on Activities of Daily Living in People with Multiple Sclerosis: A Pilot Study. EXPLORE. 2016;12:325–332. doi: 10.1016/j.explore.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Straudi S., Martinuzzi C., Pavarelli C., Sabbagh Charabati A., Benedetti M.G., Foti C., Bonato M., Zancato E., Basaglia N. A task-oriented circuit training in multiple sclerosis: A feasibility study. BMC Neurol. 2014;14:124. doi: 10.1186/1471-2377-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadi A., Arastoo A.A., Nikbakht M., Zahednejad S., Rajabpour M. Comparison of the Effect of 8 weeks Aerobic and Yoga Training on Ambulatory Function, Fatigue and Mood Status in MS Patients. Iran. Red Crescent Med. J. 2013;15:449–454. doi: 10.5812/ircmj.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cakit B.D., Nacir B., Genç H., Saraçoglu M., Karagöz A., Erdem H.R., Ergün U. Cycling Progressive Resistance Training for People with Multiple Sclerosis: A Randomized Controlled Study. Am. J. Phys. Med. Rehabil. 2010;89:446–457. doi: 10.1097/PHM.0b013e3181d3e71f. [DOI] [PubMed] [Google Scholar]
- 41.Orban A., Garg B., Sammi M.K., Bourdette D.N., Rooney W.D., Kuehl K., Spain R.I. Effect of High-Intensity Exercise on Multiple Sclerosis Function and Phosphorous Magnetic Resonance Spectroscopy Outcomes. Med. Sci. Sports Exerc. 2019;51:1380–1386. doi: 10.1249/MSS.0000000000001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gervasoni E., Cattaneo D., Jonsdottir J. Effect of treadmill training on fatigue in multiple sclerosis: A pilot study. Int. J. Rehabil. Res. Int. Z. Rehabil. Rev. Int. Rech. Readapt. 2013;37 doi: 10.1097/MRR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 43.Tollár J., Nagy F., Tóth B.E., Török K., Szita K., Csutorás B., Moizs M., Hortobágyi T. Exercise Effects on Multiple Sclerosis Quality of Life and Clinical-Motor Symptoms. Med. Sci. Sports Exerc. 2020;52:1007–1014. doi: 10.1249/MSS.0000000000002228. [DOI] [PubMed] [Google Scholar]
- 44.Ahmadi A., Arastoo A., Nikbakht M. The effects of a treadmill training programme on balance, speed and endurance walking, fatigue and quality of life in people with multiple sclerosis. Int. SportMed J. 2010;11:389–397. [Google Scholar]
- 45.Straudi S., Fanciullacci C., Martinuzzi C., Pavarelli C., Rossi B., Chisari C., Basaglia N. The effects of robot-assisted gait training in progressive multiple sclerosis: A randomized controlled trial. Mult. Scler. 2016;22:373–384. doi: 10.1177/1352458515620933. [DOI] [PubMed] [Google Scholar]
- 46.Moradi M., Sahraian M.A., Aghsaie A., Kordi M.R., Meysamie A., Abolhasani M., Sobhani V. Effects of Eight-week Resistance Training Program in Men With Multiple Sclerosis. Asian J. Sports Med. 2015;6 doi: 10.5812/asjsm.6(2)2015.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aidar F.J., Carneiro A.L., Costa Moreira O., Patrocínio de Oliveira C.E., Garrido N.D., Machado Reis V., Raineh I., Vilaça J.M., Gama de Matos D. Effects of resistance training on the physical condition of people with multiple sclerosis. J. Sports Med. Phys. Fit. 2018;58:1127–1134. doi: 10.23736/S0022-4707.17.07621-6. [DOI] [PubMed] [Google Scholar]
- 48.Moghadasi A., Ghasemi G., Sadeghi-Demneh E., Etemadifar M. The Effect of Total Body Resistance Exercise on Mobility, Proprioception, and Muscle Strength of the Knee in People With Multiple Sclerosis. J. Sport Rehabil. 2020;29:192–199. doi: 10.1123/jsr.2018-0303. [DOI] [PubMed] [Google Scholar]
- 49.Yazgan Y.Z., Tarakci E., Tarakci D., Ozdincler A.R., Kurtuncu M. Comparison of the effects of two different exergaming systems on balance, functionality, fatigue, and quality of life in people with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2020;39:101902. doi: 10.1016/j.msard.2019.101902. [DOI] [PubMed] [Google Scholar]
- 50.Molhemi F., Monjezi S., Mehravar M., Shaterzadeh-Yazdi M.-J., Salehi R., Hesam S., Mohammadianinejad E. Effects of Virtual Reality vs Conventional Balance Training on Balance and Falls in People With Multiple Sclerosis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021;102:290–299. doi: 10.1016/j.apmr.2020.09.395. [DOI] [PubMed] [Google Scholar]
- 51.Khalil H., Al-Sharman A., El-Salem K., Alghwiri A.A., Al-Shorafat D., Khazaaleh S., Abu Foul L. The development and pilot evaluation of virtual reality balance scenarios in people with multiple sclerosis (MS): A feasibility study. NeuroRehabilitation. 2018;43:473–482. doi: 10.3233/NRE-182471. [DOI] [PubMed] [Google Scholar]
- 52.Brichetto G., Spallarossa P., de Carvalho M.L.L., Battaglia M.A. The effect of Nintendo® Wii® on balance in people with multiple sclerosis: A pilot randomized control study. Mult. Scler. 2013;19:1219–1221. doi: 10.1177/1352458512472747. [DOI] [PubMed] [Google Scholar]
- 53.Lozano-Quilis J.-A., Gil-Gómez H., Gil-Gómez J.-A., Albiol-Pérez S., Palacios-Navarro G., Fardoun H.M., Mashat A.S. Virtual Rehabilitation for Multiple Sclerosis Using a Kinect-Based System: Randomized Controlled Trial. JMIR Serious Games. 2014;2:e2933. doi: 10.2196/games.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Aert R.C.M., Wicherts J.M., van Assen M.A.L.M. Publication bias examined in meta-analyses from psychology and medicine: A meta-meta-analysis. PLoS ONE. 2019;14:e0215052. doi: 10.1371/journal.pone.0215052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Momsen A.-M.H., Ortenblad L., Maribo T. Effective rehabilitation interventions and participation among people with multiple sclerosis: An overview of reviews. Ann. Phys. Rehabil. Med. 2022;65:101529. doi: 10.1016/j.rehab.2021.101529. [DOI] [PubMed] [Google Scholar]
- 56.Legault Z., Znaty A., Smith S., Boudrias M.-H. Yoga Interventions Used for the Rehabilitation of Stroke, Parkinson’s Disease, and Multiple Sclerosis: A Scoping Review of Clinical Research. J. Altern. Complement. Med. 2021;27:1023–1057. doi: 10.1089/acm.2021.0003. [DOI] [PubMed] [Google Scholar]
- 57.Luu K., Hall P.A. Hatha Yoga and Executive Function: A Systematic Review. J. Altern. Complement. Med. 2016;22:125–133. doi: 10.1089/acm.2014.0091. [DOI] [PubMed] [Google Scholar]
- 58.Quinn G., Comber L., McGuigan C., Galvin R., Coote S. Discriminative ability and clinical utility of the Timed Up and Go (TUG) in identifying falls risk in people with multiple sclerosis: A prospective cohort study. Clin. Rehabil. 2019;33:317–326. doi: 10.1177/0269215518793481. [DOI] [PubMed] [Google Scholar]
- 59.Corvillo I., Varela E., Armijo F., Alvarez-Badillo A., Armijo O., Maraver F. Efficacy of aquatic therapy for multiple sclerosis: A systematic review. Eur. J. Phys. Rehabil. Med. 2017;53:944–952. doi: 10.23736/S1973-9087.17.04570-1. [DOI] [PubMed] [Google Scholar]
- 60.Cohen E.T., Cleffi N., Ingersoll M., Karpatkin H. Blood-Flow Restriction Training for a Person With Primary Progressive Multiple Sclerosis: A Case Report. Phys. Ther. 2021;101:pzaa224. doi: 10.1093/ptj/pzaa224. [DOI] [PubMed] [Google Scholar]
- 61.Amedoro A., Berardi A., Conte A., Pelosin E., Valente D., Maggi G., Tofani M., Galeoto G. The effect of aquatic physical therapy on patients with multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2020;41:102022. doi: 10.1016/j.msard.2020.102022. [DOI] [PubMed] [Google Scholar]
- 62.Salem Y., Scott A.H., Karpatkin H., Concert G., Haller L., Kaminsky E., Weisbrot R., Spatz E. Community-based group aquatic programme for individuals with multiple sclerosis: A pilot study. Disabil. Rehabil. 2011;33:720–728. doi: 10.3109/09638288.2010.507855. [DOI] [PubMed] [Google Scholar]
- 63.Becker B.E. Aquatic Therapy in Contemporary Neurorehabilitation: An Update. PM&R. 2020;12:1251–1259. doi: 10.1002/pmrj.12435. [DOI] [PubMed] [Google Scholar]
- 64.Plecash A.R., Leavitt B.R. Aquatherapy for neurodegenerative disorders. J. Huntingt. Dis. 2014;3:5–11. doi: 10.3233/JHD-140010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of the study are available from the first author upon reasonable request.