Abstract
This scoping review aims to explore the interplay between substance use (SU) and HIV in Latin America (LA). Database searches yielded 3481 references; 196 were included. HIV prevalence among people who used substances (PWUS) ranged from 2.8–15.2%. SU definitions were variable throughout studies, and thus data were not easily comparable. In 2019, only 2% of new HIV infections were attributed to injection drug use (IDU) in LA. Factors associated with HIV among PWUS included being female, IDU and homelessness, and PWUS were likely to engage in risky sexual behaviors, start antiretroviral treatment late, have poor adherence, have treatment failure, be lost to follow-up, have comorbidities, and experience higher mortality rates and lower quality of life, as has been reported in PLWH with SU in other regions. Five intervention studies were identified, and only one was effective at reducing HIV incidence in PWUS. Interventions in other regions have varying success depending on context-specific characteristics, highlighting the need to conduct more research in the LA region. Though progress has been made in establishing SU as a major concern in people living with HIV (PLWH), much more is yet to be done to reduce the burden of HIV and SU in LA.
Keywords: Latin America, HIV/AIDS, substance use
1. Introduction
Substance use disorders (SUD) are maladaptive patterns of use of alcohol; caffeine; cannabis; hallucinogens; inhalants; opioids; sedatives, hypnotics and anxiolytics; stimulants; tobacco; and other substances leading to social, legal and occupational consequences [1]. In 2019, approximately 17 million people had SUD in Latin America (LA) and the Caribbean, leading to almost 24,000 deaths and more than 3 million disability-adjusted life-years [2]. Though illegal drugs are used more frequently in high-income countries, the disease, disability, and death experienced in low- and middle-income countries (LMIC) is disproportionate. Substance use (SU) is an immense concern for most countries within LA and deserves significant public health focus [3].
It is estimated that one in eight injection drug users (IDU) lives with HIV, amounting to 1.4 million people in the world. IDU are 22 times more likely than the general population to have HIV [4]. Beyond IDU, SU in general increases the risk of HIV infection. As of 2020, approximately 2.1 million people live with HIV/AIDS (PLWH) in LA [5]. Throughout the years, significant progress has been made toward the UNAIDS 90-90-90 goals: 80% of PLWH in the region know their serostatus, 81% of these are on antiretroviral therapy (ART), and 92% of these have achieved viral suppression, which has led to a 21% decline in AIDS-related deaths in LA between 2010 and 2020. However, the number of new HIV infections per year in the last 10 years has not changed [5]. In LA, the epidemic mostly affects men who have sex with men (MSM) and transgender women (TGW), but people who use substances (PWUS) remain a crucial vulnerable group and an understudied one [6].
The interplay between HIV and SU is complex and bidirectional: PLWH may be more likely than HIV-negative people to suffer from SU [7], and SU places individuals at higher risk of bloodborne transmission due to sharing of injection equipment, and of sexual transmission due to more engagement in high-risk behaviors [8]. Additionally, PLWH with SU may be at higher risk for adverse health outcomes [9,10].
Although SU clearly contributes to the burden of HIV worldwide, it is not easy to elucidate how this relationship plays out in the context of LA, which is diverse both culturally and geographically. In this scoping review, we aim to explore the published literature regarding the interplay between SU and HIV in LA.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) 2020 statement [11] was used to guide this review.
2.1. Eligibility Criteria
Studies were eligible for inclusion if they met the following criteria: (1) study in LA; (2) described the association between HIV and SU; (3) published as a full-text, peer-reviewed paper in English, Portuguese, or Spanish; and (4) published since 2012. This timeframe was selected to provide an accurate image of the state of current research in the region. Studies were ineligible if they did not include either PLWH or PWUS, or were published as case reports, editorials, commentaries, conference briefs, or review articles.
Eligible LA countries were Argentina, Belize, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Uruguay, and Venezuela. We excluded studies from the Caribbean, Guyana, French Guiana, and Suriname.
We chose not to exclude studies based on their definition of SU. We use the term “substance” to refer to alcohol, tobacco, or recreational drugs, and “PWUS” to refer to those who use substances.
2.2. Information Sources and Search Strategy
The search strategy was developed in collaboration with librarians in the United States and Peru, including keywords and subject headings for three concepts: substance-related disorders, HIV, and LA. The complete, reproducible search strategy is available in Supplemental Materials.
2.3. Study Selection
Search results were exported to Endnote X8 (Philadelphia, PA, USA) and duplicates were removed. Remaining studies were placed into Covidence (Veritas Health Innovation, Melbourne, Australia, available online www.covidence.org, accessed on 4 February 2022). In the initial title and abstract screening stage, two researchers (HVH, PMC) independently screened each reference for eligibility criteria. Conflicts were resolved by discussion between reviewers. In the full text review stage, one reviewer screened each article for eligibility. When a reviewer deemed an article ineligible, the second reviewer screened it, and a final decision was made through discussion.
2.4. Data Extraction and Synthesis
For each study, one researcher independently extracted data. Unclear or missing information was discussed between two reviewers before making a final decision. The researchers synthesized data around themes identified during the extraction stage: prevalence of SU among PLWH, prevalence of HIV among PWUS, factors associated with HIV positivity among PWUS, risky sexual behaviors among PLWH and PWUS, health outcomes of HIV and SU, and risk reduction strategies for PLWH and PWUS.
3. Results
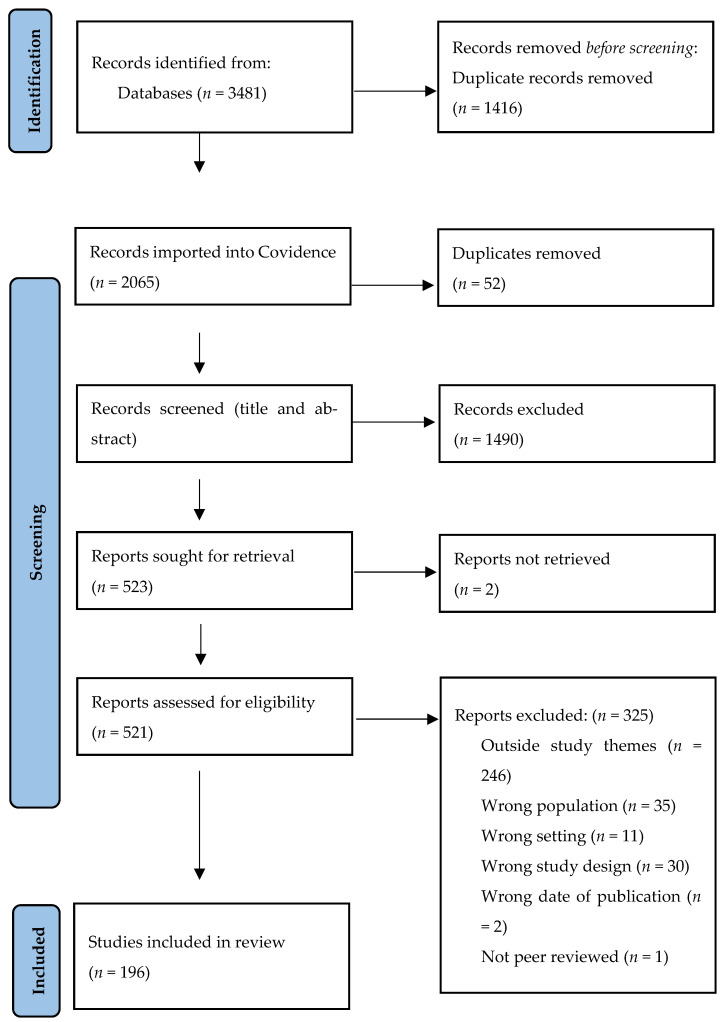
The PRISMA diagram is shown in Figure 1. Database searches yielded 3481 references, and 196 references were included. Table 1 shows the breakdown of studies by country.
Figure 1.
Prisma flowchart.
Table 1.
Number of papers per country.
| Country | # of Papers |
|---|---|
| Brazil | 127 |
| Mexico | 29 |
| Colombia | 17 |
| Peru | 16 |
| Argentina | 13 |
| El Salvador | 7 |
| Chile | 4 |
| Honduras | 3 |
| Ecuador | 3 |
| Guatemala | 3 |
| Costa Rica | 2 |
| Uruguay | 1 |
| Nicaragua | 1 |
| Venezuela | 1 |
| Panama | 1 |
| Belize | 1 |
Note: Sum of papers per country is not the same as the total number of papers included, because some papers were conducted in more than one country.
3.1. Prevalence of SU in PLWH
We identified 83 studies that reported estimates of the prevalence of SU in PLWH in Brazil (59), Argentina (5), Peru (5), Mexico (5), Colombia (2), Ecuador (1), Venezuela (1), Chile (1), Guatemala (1), and Uruguay (1), plus two multinational studies. Study populations and definitions and measurements of SU differed significantly between studies. Papers estimated SU based on lifetime use, current use or using special screening tools (Table 2).
Table 2.
Prevalence of substance use (SU) among people living with HIV (PLWH) in Latin America.
| Country | Population | Definition of SU | Prevalence of SU | Study |
|---|---|---|---|---|
| Brazil | PLWH (Bahia) | Alcohol use, current alcohol use | 42.4%, 38.5% | [99,100] |
| Illicit drug use | 4.9–11.5% | |||
| Alcohol and non-injectable illicit drug use | 11.8% | |||
| Smoking | 80.8% | |||
| PLWH with TB (Ceará) | Smoking | 33.5% | [182] | |
| History of alcoholism | 42.0% | |||
| History of illicit drug use | 26.5% | |||
| PLWH (Ceará) | Alcohol use disorder | 40.0–49.5% | [140,183] | |
| History of alcohol consumption | 44.8% | |||
| Alcohol use | 20.5% | |||
| Alcohol dependence | 19.0% | |||
| Risky alcohol consumption | 10.5% | |||
| Illicit drug use | 19.3% | |||
| Drug use | 12.7% | |||
| PLWH with HBV (Ceará) | IDU | 9.1% | [184] | |
| Inhaled cocaine use | 12.0% | |||
| Alcohol use | 36.0% | |||
| PLWH with HCV (Ceará) | IDU | 22.2% | ||
| Inhaled cocaine use | 16.7% | |||
| Alcohol use | 44.4% | |||
| PLWH (Espírito Santo) | Smoking | 22.6% | [21] | |
| Alcohol use | 32.8% | |||
| Illicit drug use | 4.5% | |||
| PLWH (Goiás) | Current, former smoking | 24.1%, 23.8% | [185] | |
| Risky consumption of alcohol | 71.4% | |||
| PLWH (Goiás) | Alcohol use | 41.8% | [114,186] | |
| Illicit drug use | 13.2–25.7% | |||
| PLWH (Minas Gerais) | Hazardous alcohol use (male, female) | 8.6%, 16.0% | [103] | |
| PLWH (Minas Gerais, Rio de Janeiro) | Previous smoking | 28.3% | [187] | |
| Current smoking | 37.6% | |||
| Past alcohol use | 35.0% | |||
| Current alcohol use | 57.7% | |||
| History of illicit drug use | 52.5% | |||
| Pregnant women with HIV with drug use history (Minas Gerais) | Drug use during pregnancy | 7.6% | [58] | |
| Smoking during pregnancy | 52.9% | |||
| Alcohol use during pregnancy | 30.6% | |||
| Smoking and alcohol use during pregnancy | 18.8% | |||
| Drug use prior to pregnancy | ||||
| Cocaine | 16.7% | |||
| Crack | 43.4% | |||
| Cocaine and crack | 8.3% | |||
| Marijuana | 15% | |||
| Cocaine, crack and marijuana | 8.3% | |||
| Other types of drugs | 8.4% | |||
| PLWH (Minas Gerais) | Alcohol use | 40.2–80.2% | [13,188,189] | |
| Smoking | 22.8–26.1% | |||
| Illicit drug use | 4.4–50.3% | |||
| PLWH with TB (Minas Gerais) | Alcoholism | 30.7% | [190] | |
| Illicit drug use | 23.5% | |||
| Smoking | 26.8% | |||
| PLWH (Pernambuco) | Smoking | 28.9–54.7% | [109,127,191] | |
| Alcohol use | 35.6% | |||
| Illicit drug use Marijuana Cocaine Crack Glue |
27.6% 26.6% 9.1–9.9% 6.5–6.7% 5.8% |
|||
| PLWH with pulmonary TB (Pernambuco) | Illicit drug use | 30.9% | [192] | |
| PLWH (Rio de Janeiro) | Smoking (lifetime, last 3 months) | 23.9–55.3%, 21–20.9% | [135,193,194] | |
| Alcohol use (lifetime, last 3 months) | 23.1%, 34.3% | |||
| Marijuana (lifetime) | 23.1% | |||
| Cocaine (lifetime) | 20.7% | |||
| Polysubstance (last 3 months) | 2.4% | |||
| PLWH with MDR TB (Rio de Janeiro) | Illicit drug use | 19.2% | [195] | |
| Women living with HIV (Rio de Janeiro) | Current or past smoking | 42.4% | [196] | |
| Lifetime illicit drug use | 16.6% | |||
| Men living with HIV (Rio de Janeiro) | Alcohol misuse | 34% | [113] | |
| Non-IDU | 76.7% | |||
| Pregnant women with HIV (Rio de Janeiro) | Illicit drug use (before, during pregnancy) | 18%, 6% | [95] | |
| Alcohol use (before, during pregnancy) | 51.3%, 14% | |||
| Smoking (before, during pregnancy) | 33%, 15% | |||
| PLWH (Rio Grande do Norte) | Smoking | 12% | [197] | |
| Alcohol use | 29% | |||
| Illicit drug use | 8% | |||
| Hospitalized PLWH (Rio Grande do Norte) | Smoking | 41% | [98] | |
| Alcohol use | 51% | |||
| Illicit drug use during week of admission | 31% | |||
| PLWH (Rio Grande do Sul) | Propensity for alcoholism | 37.5% | [12,94,123,198,199,200] | |
| Possible alcohol dependence | 5% | |||
| Alcohol (weekly use, use, harmful use, abuse) | 31.1%, 58.4%, 7.9%, 28.6% | |||
| Alcohol or drug use | 7.8% | |||
| Inhaled drug use | 33.1% | |||
| IDU | 13.9% | |||
| Smoking | 54.7% | |||
| People with AIDS (Rio Grande do Sul) | IDU | 12.2% | [39] | |
| PLWH attending Special Assistance Services (Rio Grande do Sul) | Smoking (abuse or addiction) | 45.6% | [134] | |
| Alcohol abuse or addiction | 32.7% | |||
| Other substance abuse or addiction | 15.7% | |||
| PLWH with TB (Rio Grande do Sul) | Alcoholism | 25.7–44.0% | [201,202] | |
| Smoking | 40% | |||
| Illicit drug use | 37.5% | |||
| Women living with HIV (Rio Grande do Sul) | History of drug use | 29.8% | [203] | |
| Mothers living with HIV (Rio Grande do Sul) | Drug use | 28.9% | [59] | |
| PLWH with oral lesions (Rio Grande do Sul) | Smoking | 30.7% | [204] | |
| Illicit drug use | 17.2% | |||
| Alcoholism | 14.4% | |||
| PLWH (Santa Catarina) | Smoking, current smoking | 45.9%, 32.1% | [131,205] | |
| Alcoholism, alcohol use | 13.3%, 31.1% | |||
| Illicit drug use | 10% | |||
| PLWH (São Paulo) | Alcohol use in last month (any amount, >1 time/week) | 50%, 16.9% | [73,120,126,206,207] | |
| Alcohol use, risky use, harmful use, abuse, dependence | 40.6%, 14%, 12.6%, 18.3%, 5.5% | |||
| Illicit drug use (in the last month) | 9.3–10% | |||
| SU (last month, last year) | 38%, 62% | |||
| Alcohol use during sex | 42.6% | |||
| Drug use during sex | 19.6% | |||
| PLWH without AIDS (São Paulo) | IDU (Pre-ART period, post-ART period) | 21%, 8% | [128] | |
| LGBT PLWH (São Paulo) | Drug use | 76.5% | [208] | |
| Women living with HIV (São Paulo) | Crack use | 3% | [22] | |
| Other drug use | 10% | |||
| TGW living with HIV (São Paulo) | Illicit or recreational drug use | 46% | [132] | |
| PLWH (multicentric) | Abusive use of alcohol (Recife, Goiania, Porto Alegre) | 22.8%, 26.3%, 5.6% | [115] | |
| Current smoking (Recife, Goiania, Porto Alegre) | 24.4%, 23.0%, 42.3% | |||
| Lifetime use of crack (Recife, Porto Alegre) | 6.0%, 8.9% | |||
| Lifetime use of cocaine (Recife, Goiania, Porto Alegre) | 9.0%, 10.7%, 29.6% | |||
| PLWH on ART (multicentric) | IDU (male, female, starting ART in 2006, starting ART in 2015) | 3%, 0.6%, 6.8%, 1,4% | [142] | |
| Non-IDU (female) | 23% | |||
| Women living with HIV (multicentric) | Smoking | 19.6% | [209] | |
| Illicit drug use | 18.7% | |||
| PLWH (nationwide) | Alcohol use | 49.5% | [210] | |
| Smoking | 45.3% | |||
| Amphetamines | 1.7% | |||
| Marijuana | 10.5% | |||
| Powder cocaine | 3.6% | |||
| Crack cocaine | 5.3% | |||
| Inhalants | 3.6% | |||
| Ketamine | 1.7% | |||
| Opioids | 1.7% | |||
| Ecuador | Newborns with HIV (Babahoyo) | Mothers using parenteral drugs | 38% | [23] |
| Peru | PLWH (Lima) | Current smoking | 16.6% | [14] |
| Current alcohol use, pathological use | 30.7%, 3.4% | |||
| Marijuana use (past, current) | 11.7%, 1% | |||
| Past cocaine paste use | 10.2% | |||
| Past cocaine use | 7.8% | |||
| Other past drug use (crack, poppers, terokal) | 1.4% | |||
| TGW living with HIV (Lima) | Hazardous alcohol use | 40% | [15] | |
| Harmful alcohol use | 2% | |||
| Alcohol dependency | 12% | |||
| Low drug use severity | 28% | |||
| Moderate drug use severity | 4% | |||
| Substantial or severe drug use severity | 6% | |||
| PLWH from tertiary care center (Lima) | Illicit drug use | 6.9% | [90] | |
| MSM and TGW living with HIV (Lima) | Alcohol use disorder | 28.0–43.2% | [24,211] | |
| Alcohol dependence | 3.9%–5.3% | |||
| Recent drug use | 6.0% | |||
| Low drug use severity | 20.2% | |||
| Moderate drug use severity | 5.3% | |||
| Substantial drug use severity | 1.7% | |||
| Mexico | PLWH (Jalisco) | Smoking | 60% | [18] |
| Alcohol use | 73% | |||
| Drug abuse | 59% | |||
| PLWH (San Luis Potosi) | Alcohol use (Male, Female) | 45%, 20% | [106] | |
| Drug use (Male, Female) | 21%, 3% | |||
| PLWH (Mexico City) | Illicit drug use | 20.7% | [25] | |
| Nicotine use | 24.3% | |||
| Recently pregnant women with HIV (Mexico City) | IDU, cocaine or heroin use | 0% | [17] | |
| Marijuana use | 2% | |||
| Inhaled solvent use | 26% | |||
| Current smoking | 20% | |||
| Alcohol use in last 6 months | 23% | |||
| PLWH (Puebla) | Drug or alcohol addiction | 30.6% | [112] | |
| Venezuela | PLWH (Valencia) | Inhaled drug use or IDU | 8.4% | [26] |
| Smoking 0.5 pack/year | 50% | |||
| Smoking 0.15 pack/year | 16% | |||
| Chile | PLWH (Araucanía, Metropolitan) | Drug use (Mapuche, other ethnicity) | 8.6%, 17.2% | [27] |
| Colombia | PLWH (Bogota) | Alcohol use | [28] | |
| ≤1 time/month | 55.8% | |||
| 2–4 times/month | 14.0% | |||
| 2–3 times/week | 2.3% | |||
| Smoking | 23.3% | |||
| Drug use | 7.0% | |||
| PLWH from a tertiary care center | Psychoactive substance use | 62% | [29] | |
| Argentina | Prisoners living with HIV (Buenos Aires) | Hazardous alcohol use | 23% | [20] |
| Alcohol dependence | 39% | |||
| SU in 30 days prior to incarceration | ||||
| Cocaine | 46% | |||
| Crack | 46% | |||
| Opioids | 2% | |||
| Benzodiazepines | 19% | |||
| TGW with HIV initiating ART (Buenos Aires) | Hazardous alcohol use | 52.5% | [19,139] | |
| Drug abuse | 13.1% | |||
| Cocaine use in past year | 52.5% | |||
| Marijuana use in past year | 54.1% | |||
| PLWH disengaged from HIV care (multicentric) | SU in last 6 months (TGW, cisgender) | 73.2%, 24.2% | [32] | |
| Cocaine in last 6 months (TGW, cisgender) | 76.7%, 24.2% | |||
| Substance-related problems (TGW, cisgender) | 39.0%, 10.3% | |||
| Hazardous alcohol use (TGW, cisgender) | 46.3%, 23.1% | |||
| PLWH reengaging in HIV care (multicentric) | Substance abuse | 19% | [31] | |
| Guatemala | PLWH attending an Infectious Diseases clinic (Guatemala City) | Smoking | 11.65 | [16] |
| Excessive alcohol consumption | 9.8% | |||
| Prior illicit drug use | 10.5% | |||
| Uruguay | PLWH who died from AIDS (nationwide) | History of drug use | 46.4% | [30] |
| History of IDU | 11.4% | |||
| Multi-country | PLWH (Buenos Aires, Argentina; Rio de Janeiro, Brazil; Santiago, Chile; Tegucigalpa, Honduras; Mexico City, Mexico; Lima, Peru) | >3 drinks of alcohol in last 7 days | 13.7% (4.9–23.9%) |
[118] |
| Marijuana use in last 7 days | 4.1% (1.9–11.1%) |
|||
| Cocaine use in last 7 days | 1.4% (0.7–3.5%) |
|||
| Crack use in last 7 days | 0.3% (0.0–3.3%) |
|||
| Heroin use in last 7 days | 0.1% (0.0–0.6%) |
|||
| Any illicit drug use in last 7 days | 5.0% (2.8–14.0%) |
|||
| PLWH (Buenos Aires, Argentina; Rio de Janeiro, Brazil; Santiago, Chile; Tegucigalpa, Honduras; Mexico City, Mexico; Lima, Peru) | Alcohol use only | 26.2% | [130] | |
| Non-IDU only | 2.1% | |||
| Alcohol + non-IDU | 3.5% |
Estimates of alcohol use (AU) prevalence varied within Brazil from 7.8 to 80.2%, with the majority between 30–50% [12,13]. In Peru, AU prevalence was 30.7–54% [14,15], and in Guatemala 9.8% [16]. In Mexico, it ranged from 23% in pregnant PLWH in Mexico City to 73% in Jalisco [17,18]; and in Argentina, from 52.5% in TGW to 23% in prisoners [19,20].
The prevalence of illicit drug use (DU) in PLWH also varied significantly between and within countries with ranges of 4.5–76.5% in Brazil [21,22], 38% in Ecuador [23], 6–38% in Peru [15,24], 20.7–59% in Mexico [18,25], 8.4% in Venezuela [26], 10.5% in Guatemala [16], 15.9% in Chile [27], 7–62% in Colombia [28,29], and 46.4% in Uruguay [30]. Engagement with HIV care affects DU in Argentina, where 19% of PLWH reengaging in care and 76.7% of PLWH disengaged from care reported DU [31,32]. For example, following HIV diagnosis, less individuals reported DU or AU prior to or during sex in Peru and Colombia [33,34]. Similarly, testing positive for HIV at baseline was associated with IDU cessation in Mexico [35], less patients consumed alcohol after HIV diagnosis in Ecuador [36], and drug and tobacco use decreased after initiating ART in Brazil [37]. The incidence of IDU among PLWH in LA has declined over the decades following the start of the HIV pandemic [38,39].
The prevalence of smoking tobacco was between 12–80.8% in Brazil 119,100], 16.6% in Peru [14], between 20–60% in Mexico [16,17], 23.3% in Colombia [28], 50% in Venezuela [26], and 11.65% in Guatemala [20].
3.2. Prevalence of HIV in PWUS
Twenty-six studies estimated the prevalence of HIV among PWUS, with most studies from Brazil (10). HIV rates varied widely depending on the population characteristics, methodology, and geographic location of the study (Table 3)
Table 3.
Prevalence of HIV among people who use substances in Latin America.
| Country | Population | Prevalence of HIV | Study |
|---|---|---|---|
| Brazil | Crack cocaine users in a referral hospital (Goiás) | 2.8% | [51] |
| Non-IDU (Goiás) | 3.2% | [81] | |
| Crack users (Rio/Salvador) | 3.7%/11.2% | [87] | |
| Illicit drug users (Northern Brazil) | 15.2% | [50] | |
| Drug users (Recife) | 5.3% | [47] | |
| Drug users (São Paulo) | 5.8% | [60] | |
| Migrant heroin users receiving treatment (São Paulo) | 12% | [82] | |
| Polydrug users (multicenter) | 5.8% | [49] | |
| Drug users (multicenter) | 6% | [212] | |
| Crack users (multicenter) | 4.3% | [67] | |
| Mexico | Drug treatment center clients (West Central Mexico) | [213] | |
| Community | 1.6% | ||
| In-prison | 6.7% | ||
| IDU (Tijuana) | 3–4.4% | [41,42,43,44] | |
| Low-risk non-IDU (Tijuana) | 3.7% | [40] | |
| IDU | [42] | ||
| Hermosillo | 5.2% | ||
| Ciudad Juárez | 7.7% | ||
| Colombia | IDU | [76] | |
| Pereira | 1.9% | ||
| Cali | 2.2% | ||
| Bogotá | 3.0% | ||
| Cúcuta | 6.7% | ||
| Heroin users (Pereira, Medellin) | 2.0% | [53] | |
| IDU (Medellin) | 3.6–3.8% | [55,76] | |
| IDU (Armenia) | 2.6–2.7% | [65,76] | |
| IDU (multicenter) | 4.8–6.5% | [52,214,215] | |
| Argentina | Current or former drug users from rehabilitation center and HIV clinic (Buenos Aires) | 34% | [216] |
| People who have sex under the influence of substances (multicenter) Chemsex users (multicenter) |
13.7% 23.3% |
[217] |
HIV prevalence among PWUS ranged from 2.8 to 15.2% in Brazil [40,41], 3–4.8% in Tijuana, Mexico, where female sex workers (FSW), IDUs and MSM are concentrated [40,41,42,43,44,45], 7.7% in Ciudad Juarez, where syringe sharing is common among IDU [44], 2.0% among heroin users and 6.5% among syringe sharers in Colombia [46,47,48], and 13.7–34% in Argentina [49,50].
3.3. Factors Associated with HIV Positivity among PWUS
Being female has been associated with higher risk of HIV among PWUS in Brazil, Mexico, and Colombia [45,46,47,51,52]. A study in Tijuana found that sex work mediated 84.3% of the association between female gender and HIV incidence [48]. In Brazil, HIV risk in females was mediated by high levels of unprotected sex, gender-based violence, and frequent unsafe injection habits [49]. However, several Colombian studies and one from Brazil failed to show significant differences in HIV rates between male and female IDU [50,53,54,55].
SU has also been associated with vertical transmission of HIV. A Brazilian study of pregnant PLWH showed a significant association between IDU and vertical transmission, increasing the odds of transmission by 11% [56]. Those who use substances were less likely to attend prenatal visits, use ART, and have an undetectable viral load (VL) close to birth and their HIV-exposed children were more likely to be lost to follow-up [57,58,59].
IDU seems to confer a higher HIV risk than non-IDU in Brazil [49,60]—for instance, a study conducted among DU in northern Brazil found an HIV prevalence of 27.8% among IDU and 13.1% among non-IDU [50]. This may be mediated by syringe sharing, an HIV risk factor reported in Ecuador, Colombia, and Mexico [43,55,61]. Among Colombian IDU, sharing needles and/or syringes led to an infection risk 5.07 times higher than for those who did not share [55]. Additionally, syringe confiscation by police was positively associated with testing positive for HIV in Mexico and similarly, difficult access to sterile needles was associated with elevated injection risk [62,63].
Other deleterious practices include not disinfecting syringes, having multiple injection partners, sharing drug mixtures, and being injected by a person who charges to inject [45,61,64]. More frequent DU and longer history, as well as having severe problems with DU, may also increase the risk of HIV [36,50,65,66].
Homelessness among PWUS has also been associated with higher HIV rates in Brazil [43,67]. A Mexican study found that more hours on the street per day was spatially correlated with HIV infection in these populations [45].
3.4. Risky Sexual Behaviors among PLWH and PWUS
Sexual behaviors that lead to higher HIV risk, such as engaging in condomless sex or having multiple sexual partners, are more frequently described among PWUS than in the general LA population [40,43,47,50,60,68,69]. A Brazilian study showed that PWUS had a probability of engaging in high-risk sexual behaviors 3.64 times greater than non-substance-users [66,70]. Inconsistent condom use has been associated with DU in Brazil [47,66,68,71,72,73,74], Costa Rica [75], Colombia [53,54,65,76], Mexico [40,77], Chile [69], El Salvador [78,79], and Guatemala [80]. In regions with high rates of sex work, these associations might explain the link between female sex, HIV and DU.
Some drugs may be associated with more risky sexual behaviors than others. In Colombia, cocaine use was associated with sharing drug mixtures and less condom usage [64]. In Brazil, high-risk sexual behaviors were more likely in users that snorted cocaine versus those than smoked cocaine [66,81]. More risky sexual behaviors were found in heroin-users in Brazil [82], heroin plus methamphetamine users in Mexico [83], and polydrug or poly route SU in Mexico [84].
PWUS are likely to use substances right before sex, thereby increasing risk of HIV infection and transmission: 62.1% and 59.1% of Brazilian crack users reported AU or DU, respectively, before sex [68]. Other studies report similar findings [71,73]. Using substances before sex may lead to reduced condom use by almost 68% [73], increased sexual promiscuity [85], and less likelihood of serostatus disclosure [86]. The correlation between SU before sex and inconsistent condom use was also described in serodiscordant couples [86], IDUs who share needles [54], and PLWH who use cocaine [64].
SU has also been associated with transactional sex in Chile [69], Mexico [41], Brazil [49,71,87], El Salvador [79] and Peru [88]. In a Brazilian study, 12.5% of crack users reported engaging in sex work, and 10% reported using sex to obtain drugs [87].
3.5. Health Outcomes of PLWH Who Use Substances
Late diagnosis and treatment initiation
A late HIV diagnosis can lead to worse response to treatment, higher mortality risk, and increased risk of onward transmission [89], and has been associated with DU in Peru, Uruguay and Argentina [30,90,91]. In Argentina, high levels of AU, but not DU, were significantly associated with delayed ART initiation [92]. Delays may be caused by providers’ belief that ART is contraindicated in DU, as was found in a qualitative study in El Salvador [79].
Treatment adherence (TA) and treatment failure (TF)
Several studies in LA have reported associations between SU and poor TA, and consequently TF [24,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118]. In Brazil, 29.3% of PLWH reported not taking ART because of SU [94]. Both Brazilian PLWH and their providers shared concerns about the interactions between drugs, alcohol and ART with some providers recommending stopping ART during SU [79,97]. Furthermore, AU has been found to increase the risk of hepatotoxicity associated with ART [13,119]. Nonetheless, several studies have found no association between SU and poor TA [12,29,101,102,113,118,120,121,122,123,124].
SU has also been associated with TF as evidenced by virologic failure (VF) in Brazil [37,99,113,119,120,125], Guatemala [16], Peru [15,121], and a multinational study [122]. In Brazil, PLWH with a detectable VL had 1.76 times higher chance of AU [120], while DU increased risk of a detectable VL by 3 times [119]. AU by caregivers was associated with detectable VL among Brazilian children and adolescents with HIV [125]. However, a few studies did not find an association between SU and viremia [123,124]. Other studies in Brazil found that SU was correlated with lower CD4-cell counts, another measure of TF [123,126,127], and shorter time to progression to AIDS [128].
Loss to follow-up (LTFU)
PLWH who use substances are also more likely to be LTFU. Argentinian providers reported that IDU had high rates of LTFU, missed appointments, and treatment nonadherence [129]. Additionally, DU among Brazilian HIV-positive mothers was associated with LTFU for their at-risk children [57]. AU also correlated with LTFU, though other SU did not [28,130].
Comorbidities, quality of life and mortality in PLWH with SU
SU can be considered a comorbidity in PLWH. Among Brazilian PLWH, smoking tobacco was the most common comorbidity [131]. SU in PLWH is also associated with several communicable and non-communicable comorbidities, as listed in Table 4.
Table 4.
Comorbidities associated with substance use among people living with HIV.
| Non-Infectious | Infectious |
|---|---|
| Cardiovascular | Bacterial |
| High blood pressure [194,218] | Pneumonia [197,219] |
| Strokes, cardiovascular accidents [194,197] | Tuberculosis [190,195,220] |
| Endocrine | Treatment default [190,221] |
| Diabetes mellitus [194] | Treatment delay [192] |
| Early natural menopause [222] | Treatment failure [195] |
| Nutritional | Loss to follow-up [195] |
| Malnutrition [200] | Mortality [221] |
| Low body mass index [200] | Multi drug resistance [221] |
| Low skeletal muscle mass index [200] | Chlamydia trachomatis urethritis [223] |
| Osteopenia and osteoporosis [188] | Syphilis [22] |
| Gastrointestinal symptoms [185] | Viral |
| Malignancies | HTLV-1/2 [224,225] |
| Lung cancer [197] | Hepatitis B [186,226] |
| Kaposi’s sarcoma [227] | Hepatitis C [215,216,228,229,230,231,232] |
| Anal intraepithelial neoplasia [187] | Hepatitis E [198] |
| Cervical cancer and HPV [209,233,234] | Epstein-Barr virus [39,235] |
| Psychiatric | Fungal |
| Depression [14,19,24,112,126,194,197] | Oral candidiasis [235,236] |
| Suicidal ideation [31,60] | |
| Anxiety [189] | |
| Bipolar disease [237] | |
| Manic symptoms [183] |
SU has also been linked to lower quality of life [21,132,133,134,135,136,137], poor sleep quality [25], discrimination [138] and gender identity stigma [139], although not with food insecurity [140].
Higher mortality rates among PLWH in Brazil [141,142,143,144,145,146,147] and Mexico [44] have been associated with SU. Brazilian PLWH using drugs had a relative mortality risk of 3.1 compared to non-SU [143]. However, history of DU in Uruguay was not associated with dying of AIDS [30]. Another study in Brazil found that although heavy AU and tobacco were significantly associated with death; light AU was protective [146].
3.6. Strategies for Reducing HIV-Risk in PWUS in LA
Educational interventions
We identified one randomized factorial trial conducted in Tijuana and Ciudad Juarez, Mexico, among FSW-IDUs, where participants underwent an interactive or didactic safe-sex educational intervention and an intervention to reduce needle sharing, after which they were interviewed and tested for HIV on a quarterly basis. One year following the intervention, the HIV/STI incidence decreased by more than 50% in groups that had received the interactive safe sex intervention in both cities. Additionally, when didactic injection risk interventions were coupled with expanded access to sterile injection equipment, there were significant reductions in needle sharing [148]. These findings underscore the importance of educational interventions that go beyond a standard lecture format, and the critical role of ensuring access to sterile syringes.
Peer-referral networks for HIV testing among PWUS
In El Salvador, an interrupted-time series study was conducted to assess the effect that peer-referral chains had on HIV testing among crack users. Participants compiled a list of crack users with HIV risk within their social network and were given coupons to refer them to HIV testing at a community site. This strategy was found to increase monthly numbers of HIV crack-using testers fourfold, but did not affect the HIV incidence [149], highlighting that although testing may be a critical component of HIV prevention, it is not sufficient. In the same study, a multi-level, community-based prevention intervention involving rapid HIV testing, social network referral systems, and peer small group educational sessions was carried out. Exposure to the interventions and increasing HIV testing led to reductions in condomless sex, but not in the full study sample, and changes in the HIV incidence were not mentioned [150].
3.7. Strategies for Reducing SU Risk in PLWH in LA
Medication-Assisted Therapy (MAT) for SU treatment
In Peru, a double-blind randomized controlled trial including 155 PLWH with alcohol use disorder (AUD) found that naltrexone, an effective aid in SUD treatment, was safe to use concurrently with ART, with no significant difference in adverse events in the treatment and placebo groups [151]. These findings are especially relevant in the context of LA, where, as previously described, some healthcare providers believe that ART is contraindicated in PWUS.
Trans-sensitive healthcare
A cohort study was conducted among TGW initiating ART in a trans-sensitive clinic in Argentina. The clinic had essential components including using patients’ preferred names and pronouns, having trans-competent trained providers, integrating multiple relevant services, adjusting to relevant social contexts, and including peer navigators that assist patients and link them with services. After 6 months of care, a significant reduction in SU was found [139]. Despite the lack of a control group in this study, these results highlight the potential that tailoring health services to at-risk populations may have on SU among PLWH.
4. Discussion
Through review of the included studies, several recurring themes became apparent regarding HIV and SU: unequal representation of countries in LA, heterogeneity in measurements of data, and a relative abundance of publications on prevalence, risk and outcomes as compared to interventions.
The prevalence of SU among PLWH worldwide is reported to be higher than in general populations. In this review, most papers describing this prevalence were from Brazil, with some papers from Peru, Mexico, Colombia, and Argentina, and many countries with one or zero papers. PLWH SU prevalence ranges were wide. The overall prevalence range for AU was 7.8–80.2%, for smoking 11.7–80.8%, and for DU 4.5–76.5%. In the United States, 27% of PLWH have AU, 33.6% smoke tobacco, and 36% report DU, with the most frequently used being marijuana, methamphetamines, and cocaine/crack [152,153,154]. The estimated average 1-year prevalence of AUD among PLWH in Africa was 20.03%, ranging from 16.6% in Uganda to 28.8% in South Africa [155]. Comparison of the ranges reported in our review with prevalence estimates from other regions is challenging, as the included studies lacked consistency in SU definitions. Furthermore, because not all studies identified the specific substance used, but rather categorized many as “illicit” or “recreational” drugs, establishing the most frequently used substances within LA is difficult. Additionally, the large variation in prevalences could also be attributed to the heterogeneity of cultures, geography, and economics of the various regions of LA.
The Pan American Health Organization (PAHO) and the World Health Organization have published recommendations for improving comparability of SU research studies including using standardized drug categories, using three time-periods to measure prevalence of use, asking about age at first use, routes of administration and consequences of use, and disaggregating data by sex [3,156]. Most reviewed studies did not follow these recommendations, despite being published after the recommendations were released. For instance, some studies did not disaggregate data by sex, or by type of drug use. Furthermore, a review in 123 LMIC intending to assess the availability and quality of HIV prevalence data among at risk groups (MSM, IDU, FSW and TGW) found that only 14.6% of countries had prevalence data on all four risk groups, yet it is estimated that 62% of new HIV infections occur among these populations and their partners [157,158]. Without quality data, it becomes difficult to understand the burden of the problem, develop interventions and measure their success.
Risk factors for HIV among PWUS in the LA literature included being female, IDU, unclean injection practices, and homelessness. Higher HIV risk among female IDU was explained by an overlap between DU and sex trade in one Mexican study [48] and partly by gender-based violence in a Brazilian study [49]. In contrast, stigma, physical and sexual violence, mental illness, social marginalization, and economic vulnerability have been identified in other regions as determinants of HIV infections among PWUS [159].
In 2019, globally, 10% of new adult HIV infections happened among IDU, though the percentage varied greatly by region. In eastern Europe and central Asia, 48% of new HIV infections were attributed to IDU as opposed to only 2% in LA [157]. In LA, the risk for new HIV infections is largely associated with risky sexual behaviors including condomless sex, having multiple partners, SU directly before sex and transactional sex. Vertical transmission of HIV has also been linked to maternal IDU, as previously reported [160].
Poor clinical outcomes are linked to SU among PLWH in LA, including late diagnosis and treatment initiation, poor TA, TF, LTFU, and numerous comorbidities. These findings mirror those from elsewhere in the world. In North America, PWUS are also less likely to be linked to HIV care, be retained, be adherent to ART, and achieve viral suppression [161,162,163,164]. Notably, we found that both PLWH and health providers in LA expressed concerns about interactions between ART and SU, and some providers even recommended stopping ART at times of SU. Despite a higher risk of toxicities and adverse events in PLWH with SU [165], ART should not be suspended even in the context of SU due to risks of viral rebound, immune decompensation or clinical progression [166]. Comorbidities among PLWH with SU in other regions have also been reported in the literature, including infectious [167,168,169] and noninfectious diseases [170,171,172].
In this review, only three papers described strategies to reduce HIV risk among PWUS in LA. One focused on educational interventions, the other two focused on increasing HIV testing through peer referral chains, but only the first was successful in reducing HIV incidence. The other two measured surrogate outcomes such as HIV testing and condomless sex, which did not translate into changes in HIV incidence. Studies in other regions have found Pre-Exposure Prophylaxis (PrEP) to significantly reduce HIV acquisition in IDU and to be well-accepted among PWUS [173,174]. Unfortunately, no studies were found that evaluate PrEP or Post-Exposure Prophylaxis (PEP) use in PWUS in LA.
HIV prevention studies among PWUS outside of LA include behavioral psychosocial interventions to reduce sex and drug risks, social service interventions, opioid antagonist therapy, financial and scheduling incentives, and syringe service programs to improve access to sterile injection equipment [175]. These interventions have varying degrees of success in reducing risky behaviors and HIV incidence depending on the populations and specific methodology [175], underlining the importance of researching their effectiveness in the context where they are to be applied.
Only two studies focused on strategies to reduce SU risk among PLWH in LA. One found trans-sensitive clinics successful, albeit using a cohort study design, and the other found concurrent ART-MAT use to be safe. Globally, interventions to reduce SU among PLWH include opioid antagonist therapy, behavioral or psychological interventions, and integrated HIV-SU services [176,177,178,179]. Once again, the effectiveness of these interventions is variable throughout studies, and generating strong evidence for the specific context of LA is fundamental.
Some themes, like pathophysiology or mechanisms of SUD in PLWH are well described in other regions, but are not present in the LA literature. For example, some US studies look at the long-term neurocognitive consequences of chronic cannabis use [180], while other studies even look into the beneficial properties of certain substance such as smoked medical cannabis for neuropathic pain [181]. The contexts as well as priorities for HIV research on SU therefore vary greatly depending on the region. Researchers in LA may face unique challenges such as population heterogeneity even within countries, competing public health priorities, and potential lack of funding and research infrastructure, which may explain the gaps in the literature.
PAHO identified three factors that lie at the heart of the problem of SU within LA: “inequities in development, lack of access to health services and the exclusion of some segments of the population” [3]. Collaborative efforts to bridge the gaps in knowledge and outcomes will lead to generation of better, meaningful data which can lead to policy changes and better patient outcomes. Despite gaps in information on certain countries and the challenge in comparability of other data, it is clear that SU plays a role in the HIV/AIDS pandemic as it affects LA and much more research needs to be conducted to provide equitable solutions.
5. Conclusions
The interplay between SU and HIV in LA is complex and bidirectional. Numerous studies present prevalence of SU within PLWH though with inconsistent definitions rendering the data difficult to analyze and compare with that of other regions. The data do, however, indicate that SU is a persistent problem of particular concern within PLWH in LA. The issue of SU within PLWH carries grave importance given numerous associated communicable and non-communicable comorbidities, as well as lower quality of life and higher mortality risk within this population. There are inequities in information with some countries such as Brazil having disproportionate representation in the literature. Additionally, certain themes such as prevalence, health outcomes and HIV risk factors are well represented, while important focuses such as interventions are neglected, with only three studies describing strategies to reduce HIV risk among PWUS in LA. The body of information presented here frames SUD as a major public health concern as it relates to PLWH; however, there is scant information regarding what does and does not work to fix the problem. Data assessing interventions are available for other regions; however, given the unique geographic and cultural contexts of LA, it is essential to perform similar research within this region. Though much progress has been made in highlighting that SU is a major concern as it relates to HIV, much more is yet to be done to work towards reducing HIV incidence in LA.
Abbreviations and Acronyms
| AIDS | Acquired immunodeficiency syndrome |
| ART | Antiretroviral therapy |
| AU | Alcohol Use |
| AUDIT | Alcohol Use Disorders Identification Test |
| AUD | Alcohol Use Disorder |
| DU | (Illicit) Drug Use |
| EBV | Epstein-Barr Virus |
| FSW | Female sex worker |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| HEV | Hepatitis E Virus |
| HIV | Human Immunodeficiency Virus |
| HPV | Human Papillomavirus |
| HTLV | Human T-Lymphotropic Virus |
| IDU | Injection drug use |
| LA | Latin America |
| LMIC | Low- and middle-income countries |
| LTFU | Loss to follow-up |
| MAT | Medication-Assisted Treatment |
| MDR | Multidrug resistant |
| MSM | Men who have sex with men |
| PLWH | People living with HIV or AIDS |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PWUS | People who use substances |
| SU | Substance Use |
| SUD | Substance Use Disorder |
| TA | Treatment adherence |
| TB | Tuberculosis |
| TF | Treatment Failure |
| TGW | Transgender women |
| VL | Viral load |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19127198/s1.
Author Contributions
Conceptualization, H.V.H., M.M.D. and P.J.G.; methodology, H.V.H. and P.M.C.; investigation, H.V.H. and P.M.C.; writing—original draft preparation, H.V.H., P.M.C. and J.L.C.; writing—review and editing, J.L.C., J.S. and R.A. assist with literature search strategy; H.V.H., P.M.C., P.J.G. and M.M.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Research reported in this publication was supported by the Fogarty International Center and National Institute of Mental Health, of the National Institutes of Health under Award Number D43 TW010543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; Washington, DC, USA: 2013. [Google Scholar]
- 2.Global Burden of Disease Collaborative Network . Global Burden of Disease Study 2019 (GBD 19) Results. Institute for Health Metrics and Evaluation (IHME); Seattle, DC, USA: 2020. [Google Scholar]
- 3.Pan American Health Organization . Drug Use Epidemiology in Latin America and the Caribbean: A Public Health Approach. Pan American Health Organization; Washington, DC, USA: 2009. [Google Scholar]
- 4.United Nations Office on Drugs and Crime . World Drug Report 2019. United Nations; Rome, Italy: 2019. [Google Scholar]
- 5.UNAIDS AIDSinfo. [(accessed on 16 April 2021)]. Available online: https://aidsinfo.unaids.org/
- 6.De Boni R., Veloso V.G., Grinsztejn B. Epidemiology of HIV in Latin America and the Caribbean. Curr. Opin. HIV AIDS. 2014;9:192–198. doi: 10.1097/COH.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 7.Hartzler B., Dombrowski J.C., Crane H.M., Eron J.J., Geng E.H., Mathews W.C., Mayer K.H., Moore R.D., Mugavero M.J., Napravnik S., et al. Prevalence and Predictors of Substance Use Disorders among HIV Care Enrollees in the United States. AIDS Behav. 2017;21:1138–1148. doi: 10.1007/s10461-016-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey M.P., Carey K.B., Maisto S.A., Schroder K.E.E., Vanable P.A., Gordon C.M. HIV Risk Behavior Among Psychiatric Outpatients: Association with Psychiatric Disorder, Substance Use Disorder, and Gender. J. Nerv. Ment. Dis. 2004;192:289–296. doi: 10.1097/01.nmd.0000120888.45094.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitsaz E., Meyer J.P., Krishnan A., Springer S.A., Marcus R., Zaller N., Jordan A.O., Lincoln T., Flanigan T.P., Porterfield J., et al. Contribution of Substance Use Disorders on HIV Treatment Outcomes and Antiretroviral Medication Adherence Among HIV-Infected Persons Entering Jail. AIDS Behav. 2013;17:118–127. doi: 10.1007/s10461-013-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klinkenberg W.D., Sacks S. Health Outcomes and Cost Study Group for the Hiv/aids Treatment Adherence Mental Disorders and Drug Abuse in Persons Living with HIV/AIDS. AIDS Care. 2004;16:22–42. doi: 10.1080/09540120412331315303. [DOI] [PubMed] [Google Scholar]
- 11.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 12.Zuge S.S., Primeira M.R., Remor E., de Souza Magnago T.S.B., de Paula C.C., de Mello Padoin S.M. Fatores Associados À AdesÃo Ao Tratamento Antirretroviral Em Adultos Infectados Pelo Hiv: Estudo Transversal. Rev. Enferm. UFSM. 2017;7:577–589. doi: 10.5902/2179769225657. [DOI] [Google Scholar]
- 13.Mendes J.C., Bonolo P.F., Ceccato M., Costa J.O., Reis A.M.M., Dos Santos H., Silveira M.R. Adverse Reactions Associated with First-Line Regimens in Patient Initiating Antiretroviral Therapy. Eur. J. Clin. Pharmacol. 2018;74:1077–1088. doi: 10.1007/s00228-018-2472-y. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz H.M., Olano R.F.P., Torvisco J.D.T. Frecuencia de Episodio Depresivo Mayor y Factores Relacionados En Pacientes Infectados Por El Virus de La Inmunodeficiencia Humana En Tratamiento Antirretroviral de Gran Actividad (TARGA) En Un Hospital Público de Lima. Rev. Neuro-Psiquiatr. 2015;78:3–13. doi: 10.20453/rnp.v78i1.2355. [DOI] [Google Scholar]
- 15.Rich K.M., Wickersham J.A., Huamaní J.V., Kiani S.N., Cabello R., Elish P., Arce J.F., Pizzicato L.N., Soria J., Sanchez J., et al. Factors Associated with HIV Viral Suppression Among Transgender Women in Lima, Peru. LGBT Health. 2018;5:477–483. doi: 10.1089/lgbt.2017.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortíz D.W., Roberts-Sano O., Marroquin H.E., Larson L., Franco K.B., Spec A., Melendez J.R., Pinzón R., Samayoa A.J., Mejia-Chew C., et al. Factors Associated with Viremia in People Living with HIV on Antiretroviral Therapy in Guatemala. AIDS Res. Ther. 2021;18:79. doi: 10.1186/s12981-021-00400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguilar-Zapata D., Piñeiruá-Menéndez A., Volkow-Fernández P., Rodríguez-Zulueta P., Ramos-Alamillo U., Cabrera-López T., Martin-Onraet A. Sociodemographic Differences among HIV-Positive and HIV-Negative Recently Pregnant Women in Mexico City. Medicine. 2017;96:e7305. doi: 10.1097/MD.0000000000007305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdivia-Cerda V., Alvarez-Zavala M., Sánchez-Reyes K., Cabrera-Silva R.I., Ruiz-Herrera V.V., Loza-Salazar A.D., Martínez-Ayala P., Vázquez-Limón J.C., García-García G., Andrade-Villanueva J.F., et al. Prevalence and Risk Factors of Chronic Kidney Disease in an HIV Positive Mexican Cohort. BMC Nephrol. 2021;22:317. doi: 10.1186/s12882-021-02526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aristegui I., Radusky P.D., Zalazar V., Cardozo N., Fabian S., Duarte M., Frola C., Cahn P., Sued O. Correlates of Depressive Symptoms in Transgender Women Initiating HIV Treatment in Argentina. J. Gay Lesbian Ment. Health. 2021;25:208–225. doi: 10.1080/19359705.2020.1868370. [DOI] [Google Scholar]
- 20.Alpert M., Wickersham J.A., Vázquez M., Altice F.L. Alcohol Use Disorders and Antiretroviral Therapy among Prisoners in Argentina. Int. J. Prison. Health. 2013;9:40–50. doi: 10.1108/17449201311310797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soares G.B., Garbin C.A., Rovida T.A., Garbin A.J. Quality of Life of People Living with HIV/AIDS Treated by the Specialized Service in Vitória-ES, Brazil. Cienc. Saude Coletiva. 2015;20:1075–1084. doi: 10.1590/1413-81232015204.00522014. [DOI] [PubMed] [Google Scholar]
- 22.Pinto V.M., Tancredi M.V., Buchalla C.M., Miranda A.E. History of Syphilis in Women Living with AIDS and Associated Risk Factors in São Paulo, Brazil. Rev. Assoc. Med. Bras. (1992) 2014;60:342–348. doi: 10.1590/1806-9282.60.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Zambrano A.M., Borja J.N., Shiguango N.S., Silva T.P., Andrade A.V., Guilcaso A.V., Cobeña K.F., Estrada A.M., Peralta C.S., Navas G.B. Incidencia y Características Clínicas de Neonatos Con Infección Por Virus de Inmunodeficiencia Humana Del Hospital Martín Icaza de Babahoyo, Ecuador. Arch. Venez. Farmacol. Ter. 2020;39:13–15. [Google Scholar]
- 24.Ferro E.G., Weikum D., Vagenas P., Copenhaver M.M., Gonzales P., Peinado J., Cabello R., Lama J.R., Sanchez J., Altice F.L. Alcohol Use Disorders Negatively Influence Antiretroviral Medication Adherence among Men Who Have Sex with Men in Peru. AIDS Care. 2015;27:93–104. doi: 10.1080/09540121.2014.963013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Estrada E., Iglesias-Chiesa M.C., Fresán-Orellana A., Reyes-Terán G. Factors Associated with Poor Sleep Quality among HIV-Positive Individuals in Mexico City. Salud Ment. 2018;41:123–129. doi: 10.17711/SM.0185-3325.2018.016. [DOI] [Google Scholar]
- 26.Ojeda M.F.C. Características Clínico-Epidemiológicas de Pacientes Con VIH y Neoplasias Atendidos En La Unidad de Infectología de La Ciudad Hospitalaria “Dr. Enrique Tejera” Valencia-Venezuela. 2001–2016. Bol. Venez. Infectol. 2018;29:5–10. [Google Scholar]
- 27.Alarcón A.M., Chahin C., Muñoz S., Wolff M., Northland R. Perfil de Personas Con Infección Por VIH/SIDA: Diferencial Étnico, Económico y Socio-Cultural En Chile. Rev. Chil. Infectol. 2018;35:276–282. doi: 10.4067/s0716-10182018000300276. [DOI] [PubMed] [Google Scholar]
- 28.Támara-Ramírez J.R., Álvarez C.A., Rodríguez J. Loss of Follow-up and Associated Factors in Patients Enrolled in the HIV/AIDS Program of the Hospital Universitario San Ignacio, Colombia, 2012–2013. Biomedica. 2016;36:265–275. doi: 10.7705/biomedica.v36i2.2939. [DOI] [PubMed] [Google Scholar]
- 29.Granada A.M., Vanegas C., Forero E., Silva C., Vergara E.P. Factores Asociados al Abandono de Terapia Antirretroviral de Alta Efectividad En Pacientes Con VIH SIDA En Un Hospital de Tercer Nivel. Acta Méd. Colomb. 2018;43:31–36. [Google Scholar]
- 30.Cabrera S., Pérez D., Meré J.J., Frantchez V., Iglesias C., Cabeza E. AIDS Mortality in Uruguay: Profile of Deceased People in 2014. Rev. Med. Del Urug. 2019;35:181–192. doi: 10.29193/RMU.35.3.2. [DOI] [Google Scholar]
- 31.Mandell L.N., Rodriguez V.J., De La Rosa A., Abbamonte J.M., Sued O., Cecchini D., Cassetti I., Cahn P., Weiss S.M., Jones D.L. Suicidal Ideation Among Adults Re-Engaging in HIV Care in Argentina. AIDS Behav. 2019;23:3427–3434. doi: 10.1007/s10461-019-02526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radusky P.D., Aristegui I., Mandell L.N., Dell’Isola E., Zalazar V., Cardozo N., Alcaide M.L., Weiss S.M., Jones D.L., Sued O. Examining Factors Associated with Gender Identity Among Individuals Disengaged from HIV Care in Argentina. Int. J. Behav. Med. 2022;29:69–77. doi: 10.1007/s12529-021-09998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montaño M.A., Alfaro R., Ness T., Ganoza C., Gonzales P., Sanchez J., Lama J.R., Duerr A.C. Sexual Behavior and Sexually Transmitted Infection Outcomes Among Men Who Have Sex With Men and Transgender Women Participating in a Study of the Timing of Antiretroviral Therapy in Lima, Peru. Sex. Transm. Dis. 2020;47:825–831. doi: 10.1097/OLQ.0000000000001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Arcila Rivera A.P., López L.Á.T., Orozco J.D.C., Lozano D.G., González L.D.H., Dussán L.C., Castaño M.R. Comportamientos Sexuales En Mayores de 18 Años Con Diagnóstico VIH/SIDA En Tres Ciudades de Colombia 2011. MedUNAB. 2016;19:95–102. doi: 10.29375/01237047.2187. [DOI] [Google Scholar]
- 35.Horyniak D., Strathdee S.A., West B.S., Meacham M., Rangel G., Gaines T.L. Predictors of Injecting Cessation among a Cohort of People Who Inject Drugs in Tijuana, Mexico. Drug Alcohol Depend. 2018;185:298–304. doi: 10.1016/j.drugalcdep.2017.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downen J.M., Swendener B., Bodlak A.A., Añazco D.F., Nicolalde B.I., Mhaskar R., Cevallos N., Castillo A., Larreategui D., Torres E., et al. Quantifying Alcohol Use among Ecuadorian Human Immunodeficiency Virus Positive Individuals and Assessing Alcohol as an Independent Risk Factor for Human Immunodeficiency Virus: A Case Control Study STROBE. Medicine. 2020;99:e23276. doi: 10.1097/MD.0000000000023276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa J.O., Ceccato M., Silveira M.R., Bonolo P.F., Reis E.A., Acurcio F.A. Effectiveness of Antiretroviral Therapy in the Single-Tablet Regimen Era. Rev. Saude Publica. 2018;52:87. doi: 10.11606/S1518-8787.2018052000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazarini F.M., Melchior R., González A.D., Matsuo T. Trends in the Epidemic of Aids Cases in Southern Brazil from 1986 to 2008. Rev. Saude Publica. 2012;46:960–968. doi: 10.1590/S0034-89102013005000003. [DOI] [PubMed] [Google Scholar]
- 39.Pereira L.M.S., Dos Santos França E., Costa I.B., Lima I.T., Freire A.B.C., de Paula Ramos F.L., Monteiro T.A.F., Macedo O., Sousa R.C.M., Freitas F.B., et al. Epidemiological Risk Factors Associated with Primary Infection by Epstein-Barr Virus in HIV-1-Positive Subjects in the Brazilian Amazon Region. Sci. Rep. 2021;11:18476. doi: 10.1038/s41598-021-97707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deiss R.G., Lozada R.M., Burgos J.L., Strathdee S.A., Gallardo M., Cuevas J., Garfein R.S. HIV Prevalence and Sexual Risk Behaviour among Non-Injection Drug Users in Tijuana, Mexico. Glob. Public Health. 2012;7:175–183. doi: 10.1080/17441692.2010.549141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armenta R.F., Abramovitz D., Lozada R., Vera A., Garfein R.S., Magis-Rodríguez C., Strathdee S.A. Correlates of Perceived Risk of HIV Infection among Persons Who Inject Drugs in Tijuana, Baja California, Mexico. Salud Publica Mex. 2015;57((Suppl. 2)):s107–s112. doi: 10.21149/spm.v57s2.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ospina-Escobar A., Magis-Rodríguez C., Juárez F., Werb D., Arredondo S.B., Carreón R., Ramos M.E., Strathdee S. Comparing Risk Environments for HIV among People Who Inject Drugs from Three Cities in Northern Mexico. Harm Reduct. J. 2018;15:27. doi: 10.1186/s12954-018-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kori N., Roth A.M., Lozada R., Vera A., Brouwer K.C. Correlates of Injecting in an HIV Incidence Hotspot among Substance Users in Tijuana, Mexico. Int. J. Drug Policy. 2014;25:525–532. doi: 10.1016/j.drugpo.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West B.S., Abramovitz D.A., Gonzalez-Zuniga P., Rangel G., Werb D., Cepeda J., Beletsky L., Strathdee S.A. Drugs, Discipline and Death: Causes and Predictors of Mortality among People Who Inject Drugs in Tijuana, 2011–2018. Int. J. Drug Policy. 2020;75:102601. doi: 10.1016/j.drugpo.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brouwer K., Rusch M.L., Weeks J.R., Lozada R., Vera A., Magis-Rodríguez C., Strathdee S. Spatial Epidemiology of HIV among Injection Drug Users in Tijuana, Mexico. Ann. Assoc. Am. Geogr. 2012;102:1190–1199. doi: 10.1080/00045608.2012.674896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vernaglia T.V.C., Leite T.H., Faller S., Pechansky F., Kessler F.H.P., Cruz M.S., Group B.C. The Female Crack Users: Higher Rates of Social Vulnerability in Brazil. Health Care Women Int. 2017;38:1170–1187. doi: 10.1080/07399332.2017.1367001. [DOI] [PubMed] [Google Scholar]
- 47.Santos N.T.V. Doctoral Dissertation. Centro de Pesquisas Aggeu Magalhães; Recife, Brazil: 2013. Vulnerabilidade e Prevalência de HIV e Sífilis Em Usuários de Drogas No Recife: Resultados de Um Estudo Respondent-Driven Sampling; p. 151. [Google Scholar]
- 48.Jain J.P., Abramovitz D., Strathdee S.A., Gonzalez-Zuniga P., Rangel G., West B.S., Pitpitan E.V. Sex Work as a Mediator Between Female Gender and Incident HIV Infection Among People Who Inject Drugs in Tijuana, Mexico. AIDS Behav. 2020;24:2720–2731. doi: 10.1007/s10461-020-02828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baptista C.J., Dourado I., de Andrade T.M., Brignol S., Bertoni N., Bastos F.I. HIV Prevalence, Knowledge, Attitudes, and Practices Among Polydrug Users in Brazil: A Biological Survey Using Respondent Driven Sampling. AIDS Behav. 2018;22:2089–2103. doi: 10.1007/s10461-017-1812-8. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira-Filho A.B., Silva F.Q., Santos F.J.A., Cardoso Y.M.N., Di Miceli J.F.F., Resque R.L., Silva-Oliveira G.C., Martins L.C., Pinheiro L.M.L., Machado L.F.A., et al. Prevalence and Risk Factors for HIV-1 Infection in People Who Use Illicit Drugs in Northern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2020;114:213–221. doi: 10.1093/trstmh/trz106. [DOI] [PubMed] [Google Scholar]
- 51.França D., Del-Rios N.H.A., Carneiro M., Guimarães R.A., Caetano K.A.A., Reis M., Martins R.M.B., Motta-Castro A.R.C., Stefani M.M.A., Teles S.A. HIV-1 Infection among Crack Cocaine Users in a Region Far from the Epicenter of the HIV Epidemic in Brazil: Prevalence and Molecular Characteristics. PLoS ONE. 2018;13:e0199606. doi: 10.1371/journal.pone.0199606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berbesi D., Segura A., Cardona D., Agudelo A. Factors Associated with Syringe Exchange among Injection Drug Users in Colombia. J. Subst. Use. 2017;22:365–371. doi: 10.1080/14659891.2016.1223763. [DOI] [Google Scholar]
- 53.Berbesi D., Segura-Cardona A., Montoya-Vélez L., Mateu-Gelabert P. Consumption of Injecting Heroin in Colombia and Risk Behaviors. Salud Ment. 2013;36:27–31. doi: 10.17711/SM.0185-3325.2013.004. [DOI] [Google Scholar]
- 54.Berbesi D., Segura A., Montoya L. Cross-Sectional Study of HIV Prevalence and the Characteristics of Injecting Drug Users in Colombia. J. Subst. Use. 2014;19:364–367. doi: 10.3109/14659891.2013.824037. [DOI] [Google Scholar]
- 55.Contreras H.J., Hoyos A.M., Gómez D.A., Roldán L.M., Huacuja S., Berbesí D.Y., Segura A.M., Castaño G.A., Mateu-Gelabert P. HIV Infection Prevalence and Associated Risk Factors in People Who Inject Drugs in Medellin. Infectio. 2020;24:88–91. doi: 10.22354/in.v24i2.838. [DOI] [Google Scholar]
- 56.Hoffmann I.C., dos Santos W.M., de Padoin S.M.M., de Barros S.M.O. A Five-Year Review of Vertical HIV Transmission in a Specialized Service: Cross-Sectional Study. Sao Paulo Med. J. 2016;134:508–512. doi: 10.1590/1516-3180.2016.0139140616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouveia P.A., da Silva G.A., de Albuquerque Mde F. Predictors of Loss to Follow-up among Children Registered in an HIV Prevention Mother-to-Child Transmission Cohort Study in Pernambuco, Brazil. BMC Public Health. 2014;14:1232. doi: 10.1186/1471-2458-14-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melo V.H., Botelho A.P.M., Maia M.M.M., Júnior M.D.C., Pinto J.A. Uso de Drogas Ilícitas Por Gestantes Infectadas Pelo HIV. Rev. Bras. Ginecol. Obstet. 2014;36:555–561. doi: 10.1590/So100-720320140005155. [DOI] [PubMed] [Google Scholar]
- 59.Burg M.R., Andreazza M., Silva S.R.E., de Oliveira M.M.T., dos Santos S., Raffo N.I. Acompanhamento De CrianÇas Expostas Ao Hiv Materno No MunicÍpio De Canoas/Rs. Rev. Enferm. UFSM. 2017;7:248–261. doi: 10.5902/2179769223608. [DOI] [Google Scholar]
- 60.Ribeiro A., Trevizol A., Oluwoye O., McPherson S., McDonell M.G., Briese V., Miguel A.C., Fratzinger R.C., Laranjeira R.R., Alonso A.L., et al. HIV and Syphilis Infections and Associated Factors among Patients in Treatment at a Specialist Alcohol, Tobacco, and Drugs Center in São Paulo’s “Cracolândia”. Trends Psychiatry Psychother. 2020;42:1–6. doi: 10.1590/2237-6089-2018-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rebolledo D.M., Martínez M.A.G., Orozco C.L.R., Granoble G.M., Gualpa J.O.M., Arias H.M. Caracterización de Pacientes VIH-SIDA y Hepatitis Consumidores de Drogas En Un Hospital General Ecuador 2019. Bol. Malariol. Salud Ambient. 2021;61:72–81. [Google Scholar]
- 62.Beletsky L., Lozada R., Gaines T., Abramovitz D., Staines H., Vera A., Rangel G., Arredondo J., Strathdee S.A. Syringe Confiscation as an HIV Risk Factor: The Public Health Implications of Arbitrary Policing in Tijuana and Ciudad Juarez, Mexico. J. Urban Health. 2013;90:284–298. doi: 10.1007/s11524-012-9741-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain J.P., Strathdee S.A., West B.S., Gonzalez-Zuniga P., Rangel G., Pitpitan E.V. Sex Differences in the Multilevel Determinants of Injection Risk Behaviours among People Who Inject Drugs in Tijuana, Mexico. Drug Alcohol Rev. 2020;39:898–907. doi: 10.1111/dar.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berbesi-Fernández D.Y., Segura-Cardona A., Montoya-Velez L., Medina A.F.R. Factores Asociados al Consumo de Cocaína En Usuarios de Heroína Inyectable En Colombia. Salud Ment. 2016;39:205–211. doi: 10.17711/SM.0185-3325.2016.024. [DOI] [Google Scholar]
- 65.Berbesi-Fernández D., Segura-Cardona Á., Montoya-Vélez L., Castaño-Perez G.A. Hepatitis C and HIV in Injecting Drug Users in Armenia, Colombia. Adicciones. 2015;27:246–252. doi: 10.20882/adicciones.749. [DOI] [PubMed] [Google Scholar]
- 66.Diehl A., Vieira D.L., Rassool G.H., Pillon S.C. Sexual Risk Behaviors in Non-Injecting Substance-Dependent Brazilian Patients. Adicciones. 2014;26:208–220. doi: 10.20882/adicciones.2. [DOI] [PubMed] [Google Scholar]
- 67.Halpern S.C., Scherer J.N., Roglio V., Faller S., Sordi A., Ornell F., Dalbosco C., Pechansky F., Kessler F., Diemen L.V. Clinical and Social Vulnerabilities in Crack Users According to Housing Status: A Multicenter Study in Six Brazilian State Capitals. Cad. Saude Publica. 2017;33:e00037517. doi: 10.1590/0102-311x00037517. [DOI] [PubMed] [Google Scholar]
- 68.De Araújo T.M.E., da Silva Sousa A., Soares T.R., Clementino R.A., de Sá L.C., de Lima S.M. Vulnerabilidade Dos Usuários de Crack à Infecção Pelo Vírus Da Imunodeficiência Humana. Enferm. Em Foco. 2014;5:45–48. doi: 10.21675/2357-707X.2014.v5.n1/2.605. [DOI] [Google Scholar]
- 69.Irarrázabal L.P., Ferrer L., Villegas N., Sanhueza S., Molina Y., Cianelli R. Mujeres Que Consumen Sustancias y Su Vulnerabilidad Frente al VIH En Santiago de Chile (Women Who Consume Substances and Their Vulnerability to HIV in Santiago of Chile) Hisp. Health Care Int. 2016;14:89–93. doi: 10.1177/1540415316647978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Julio R.S., Friedman R.K., Cunha C.B., De Boni R.B., Cardoso S.W., Torres T., Alves C.A., Castro C., Fernandes N.M., Veloso V.G., et al. Unprotected Sexual Practices among Men Who Have Sex with Women and Men Who Have Sex with Men Living with HIV/AIDS in Rio de Janeiro. Arch. Sex. Behav. 2015;44:357–365. doi: 10.1007/s10508-014-0357-4. [DOI] [PubMed] [Google Scholar]
- 71.Guimarães R.A., Rodovalho A.G., Fernandes I.L., Silva G.C., de Felipe R.L., Vera I., Gregório V.D., Lucchese R. Transactional Sex among Noninjecting Illicit Drug Users: Implications for HIV Transmission. Sci. World J. 2016;2016:4690628. doi: 10.1155/2016/4690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guimarães R.A., Rodovalho A.G., Fernandes I.L., Lucchese R., Vera I. Inconsistent Use of Condoms in Non-Injecting Illicit Drug Users. Braz. J. Infect. Dis. 2016;20:220–221. doi: 10.1016/j.bjid.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cardoso L.D., Malbergier A. Who is Not Using Condoms among HIV-Positive Patients in Treatment in the Largest City in Brazil? AIDS Care. 2015;27:629–636. doi: 10.1080/09540121.2014.986047. [DOI] [PubMed] [Google Scholar]
- 74.De Jacques I.J.A.A. Doctoral Dissertation. Instituto Aggeu Magalhãe; Recife, Brazil: 2016. Relações Sexuais Desprotegidas Entre Usuários de Crack No Estado de Pernambuco; p. 146. [Google Scholar]
- 75.Chacón M., Barrantes K., Achí R., Comerfold M., McCoy C. Sex Practices and Knowledge about Hiv/Aids among Drug Users in a Low-Income Urban Community of Costa Rica. Health Addict. Salud Drog. 2014;14:27–36. doi: 10.21134/haaj.v14i1.199. [DOI] [Google Scholar]
- 76.Berbesi-Fernández D., Segura-Cardona A.M., Montoya-Velez L., Lopez-Ramirez E. HIV among Intravenous Drug Users in Colombia. Infectio. 2016;20:70–76. doi: 10.1016/j.infect.2015.07.004. [DOI] [Google Scholar]
- 77.Villalobos-Gallegos L., Medina-Mora M.E., Benjet C., Ruiz-Velasco S., Magis-Rodriguez C., Marín-Navarrete R. Multidimensional Patterns of Sexual Risk Behavior and Psychiatric Disorders in Men with Substance Use Disorders. Arch. Sex. Behav. 2019;48:599–607. doi: 10.1007/s10508-018-1227-2. [DOI] [PubMed] [Google Scholar]
- 78.Salas-Wright C.P., Olate R., Vaughn M.G. Preliminary Findings on the Links between Violence, Crime, and HIV Risk among Young Adults with Substance Use Disorders in El Salvador. J. Subst. Use. 2016;21:35–40. doi: 10.3109/14659891.2014.949317. [DOI] [Google Scholar]
- 79.Dickson-Gomez J., McAuliffe T., de Mendoza L.R., Glasman L., Gaborit M. The Relationship between Community Structural Characteristics, the Context of Crack Use, and HIV Risk Behaviors in San Salvador, El Salvador. Subst. Use Misuse. 2012;47:265–277. doi: 10.3109/10826084.2011.635325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conners E.E., Swanson K., Morales-Miranda S., Casanueva C.F., Mercer V.J., Brouwer K.C. HIV Risk Behaviors and Correlates of Inconsistent Condom Use Among Substance Using Migrants at the Mexico/Guatemala Border. AIDS Behav. 2017;21:2033–2045. doi: 10.1007/s10461-017-1726-5. [DOI] [PubMed] [Google Scholar]
- 81.Guimarães R.A., Lucchese R., Fernandes I.L., Vera I., Rodovalho A.G., Guimarães V.A., Silva G.C., de Felipe R.L., de Castro P.A., Ferreira P.M. HIV Testing in Non-Injection Drug Users: Prevalence and Associated Factors. Jpn. J. Infect. Dis. 2017;70:340–346. doi: 10.7883/yoken.JJID.2015.490. [DOI] [PubMed] [Google Scholar]
- 82.Ribeiro M., Frajzinger R., Perrenoud L.O., Fischer B. Examining a Migration-Based Phenomenon of Heroin Use in an Urban Drug Scene in Sao Paulo, Brazil. Int. J. Migr. Health Soc. Care. 2021;17:274–285. doi: 10.1108/IJMHSC-06-2020-0065. [DOI] [Google Scholar]
- 83.Meacham M.C., Rudolph A.E., Strathdee S.A., Rusch M.L., Brouwer K.C., Patterson T.L., Vera A., Rangel G., Roesch S.C. Polydrug Use and HIV Risk Among People Who Inject Heroin in Tijuana, Mexico: A Latent Class Analysis. Subst. Use Misuse. 2015;50:1351–1359. doi: 10.3109/10826084.2015.1013132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meacham M.C., Roesch S.C., Strathdee S.A., Lindsay S., Gonzalez-Zuniga P., Gaines T.L. Latent Classes of Polydrug and Polyroute Use and Associations with Human Immunodeficiency Virus Risk Behaviours and Overdose among People Who Inject Drugs in Tijuana, Baja California, Mexico. Drug Alcohol Rev. 2018;37:128–136. doi: 10.1111/dar.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown S.E., Vagenas P., Konda K.A., Clark J.L., Lama J.R., Gonzales P., Sanchez J., Duerr A.C., Altice F.L. Men Who Have Sex With Men in Peru: Acceptability of Medication-Assisted Therapy for Treating Alcohol Use Disorders. Am. J. Mens Health. 2017;11:1269–1278. doi: 10.1177/1557988315576775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee L., Bastos F.I., Bertoni N., Malta M., Kerrigan D. The Role of HIV Serostatus Disclosure on Sexual Risk Behaviours among People Living with HIV in Steady Partnerships in Rio de Janeiro, Brazil. Glob. Public Health. 2014;9:1093–1106. doi: 10.1080/17441692.2014.952655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cruz M.S., Andrade T., Bastos F.I., Leal E., Bertoni N., Villar L.M., Tiesmaki M., Fischer B. Key Drug Use, Health and Socio-Economic Characteristics of Young Crack Users in Two Brazilian Cities. Int. J. Drug Policy. 2013;24:432–438. doi: 10.1016/j.drugpo.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 88.Yabar C.A., Vilcarino G.F., Espetia S., Lujan F., Vásquez-Domínguez A., Yaya M., Acuña M., Santos D., Mamani E., Rodriguez-Bayona R., et al. Social, Epidemiological, and Virological Characteristics from Peruvian Subjects Living with HIV-1/AIDS with Different Sexual Risk Behavior. AIDS Res. Hum. Retrovir. 2021;38:288–299. doi: 10.1089/aid.2021.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Girardi E.M., Sabin C.A.P., Monforte A., d’Arminio M. Late Diagnosis of HIV Infection: Epidemiological Features, Consequences and Strategies to Encourage Earlier Testing. JAIDS J. Acquir. Immune Defic. Syndr. 2007;46:S3. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- 90.Maquera-Afaray J., Cvetkovic-Vega A., Cárdenas M.M., Kälviäinen H., Mejia C.R. Late Diagnosis and Advanced Disease of HIV in Adult Patients from a Peruvian Social Security Hospital. Rev. Chil. Infectol. 2016;33:S20–S26. doi: 10.4067/S0716-10182016000700003. [DOI] [PubMed] [Google Scholar]
- 91.Kundro M.A., Terwel S.R., Toibaro J.J., Viloria G.A., Losso M.H. Late Diagnosis of HIV Infection in Asymptomatic Patients. Medicina. 2016;76:273–278. [PubMed] [Google Scholar]
- 92.Warley E., Galimberti G.F., Vieni M.I., Tavella S., Salas M., Desse J., D’Agostino G., Szyld E. Factors Associated to Late Clinical Stage at the Initiation of Antiretroviral Therapy. Medicina. 2012;72:367–370. [PubMed] [Google Scholar]
- 93.Santos M.A., Guimarães M.D.C., Helena E.T.S., Basso C.R., Vale F.C., Carvalho W., Alves A.M., Rocha G.M., Acurcio F.A., Ceccato M., et al. Monitoring Self-Reported Adherence to Antiretroviral Therapy in Public HIV Care Facilities in Brazil: A National Cross-Sectional Study. Medicine. 2018;97:S38–S45. doi: 10.1097/MD.0000000000009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Padoin S.M.M., Zuge S.S., dos Santos É.E.P., Primeira M.R., Aldrighi J.D., de Paula C.C. Adesão à Terapia Antirretroviral Para HIV/AIDS. Cogitare Enferm. 2013;18:446–451. doi: 10.5380/ce.v18i3.33553. [DOI] [Google Scholar]
- 95.Zonenschein A.C.C., Filho E.C.J., Cruz M.L.S., Gouvea M.I., Teixeira M.L.B., Fuller T., Dias M.A.B. Treatment Dropout after Pregnancy: A Study of Women Living with HIV in Rio de Janeiro. AIDS Care. 2020;32:1283–1289. doi: 10.1080/09540121.2020.1755011. [DOI] [PubMed] [Google Scholar]
- 96.Cruz M.L.S. Doctoral Dissertation. Escola Nacional de Saúde Pública Sergio Arouca; Rio de Janeiro, Brazil: 2015. Crianças e Adolescentes Vivendo Com HIV Em Acompanhamento Em Serviços Brasileiros: Análise Dos Fatores de Vulnerabilidade Na Adesão Ao Tratamento Antirretroviral; p. 128. [Google Scholar]
- 97.Biello K.B., Grinsztejn B., Fernandes N.M., Edeza A., Kamel L., Salhaney P., Veloso V., Mimiaga M.J. Development of a Social Network-Based Intervention to Overcome Multilevel Barriers to ART Adherence Among Adolescents in Brazil. AIDS Educ. Prev. 2019;31:111–126. doi: 10.1521/aeap.2019.31.2.111. [DOI] [PubMed] [Google Scholar]
- 98.Silva R., Costa R.H.S., Braz L., Lucena I.A., Ferreira K.D.S., Duarte F. People Living with AIDS: Association between Nursing Diagnoses and Sociodemographic/Clinical Characteristics. Rev. Bras. Enferm. 2018;71:2535–2542. doi: 10.1590/0034-7167-2017-0420. [DOI] [PubMed] [Google Scholar]
- 99.Teixeira C., Mde L.D., Santos M.P., Brites C. Impact of Use of Alcohol and Illicit Drugs by AIDS Patients on Adherence to Antiretroviral Therapy in Bahia, Brazil. AIDS Res. Hum. Retrovir. 2013;29:799–804. doi: 10.1089/aid.2012.0296. [DOI] [PubMed] [Google Scholar]
- 100.Oliveira L.Q., Campesatto E.A. Factors That Lead to Noncompliance in Patients Antiretroviral Therapy. Arq. Ciências Saúde UNIPAR. 2012;16:79–84. [Google Scholar]
- 101.Kreitchmann R., Coelho D.F., Kakehasi F.M., Hofer C.B., Read J.S., Losso M., Haberer J.E., Siberry G.K., Harris D.R., Yu Q. Long-Term Postpartum Adherence to Antiretroviral Drugs among Women in Latin America. Int. J. STD AIDS. 2016;27:377–386. doi: 10.1177/0956462415584483. [DOI] [PubMed] [Google Scholar]
- 102.Kreitchmann R., Harris D.R., Kakehasi F., Haberer J.E., Cahn P., Losso M., Teles E., Pilotto J.H., Hofer C.B., Read J.S. Antiretroviral Adherence during Pregnancy and Postpartum in Latin America. AIDS Patient Care STDS. 2012;26:486–495. doi: 10.1089/apc.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Oliveira L.C.M., dos Anjos M.G.T., Macedo Mustafé R., Sebastião Borges A. Alcohol Consumption and Associated Factors among HIV/AIDS Patients. Braz. J. Infect. Dis. 2016;20:320–321. doi: 10.1016/j.bjid.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Fatima Bonolo P., Ceccato M., Rocha G.M., de Assis Acúrcio F., Campos L.N., Guimarães M.D. Gender Differences in Non-Adherence among Brazilian Patients Initiating Antiretroviral Therapy. Clinics. 2013;68:612–620. doi: 10.6061/clinics/2013(05)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Demitto F.O., Schmaltz C.A.S., Sant’Anna F.M., Arriaga M.B., Andrade B.B., Rolla V.C. Predictors of Early Mortality and Effectiveness of Antiretroviral Therapy in TB-HIV Patients from Brazil. PLoS ONE. 2019;14:e0217014. doi: 10.1371/journal.pone.0217014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cedillo I.G., Delgado M.R. Situación En Que Viven y Adhesión al Tratamiento En Mujeres y Hombres de San Luis Potosí Con VIH/SIDA. Acta Univ. 2014;24:3–14. [Google Scholar]
- 107.Xochipa-García N., Mata-Marín J.A., Alcalá-Martínez E., Domínguez-Hermosillo J.C., Huerta-García G., Manjarrez-Tellez B., Gaytán-Martínez J. Prevalence and Risk Factors Associated with Adherence to Antiretroviral Therapy in HIV-Infected Adults in a Tertiary Care Hospital in Mexico. J. AIDS Clin. Res. 2015;6:518. [Google Scholar]
- 108.Ramírez S.A.M. Catacterísticas de Pacientes VIH/SIDA Con CD4+ Inferior a 200 Células/MM3 Atendidos En El Centro de Atención Integral (CAI) Hospital Escuela, Tegucigalpa, Honduras Durante El Período Septiembre 2012-Mayo 2013. [(accessed on 1 March 2022)];2013 Volume 92:iii. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/biblio-972246. [Google Scholar]
- 109.Batista J., Mde F.A., Santos M.L., Dde B.M.-F., Lacerda H.R., Maruza M., Moura L.V., Coimbra I., Ximenes R.A. Association between Smoking, Crack Cocaine Abuse and the Discontinuation of Combination Antiretroviral Therapy in Recife, Pernambuco, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2014;56:127–132. doi: 10.1590/S0036-46652014000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Camargo C.C., Cavassan N.R.V., Tasca K.I., Meneguin S., Miot H.A., Souza L.R. Depression and Coping Are Associated with Failure of Adherence to Antiretroviral Therapy among People Living with HIV/AIDS. AIDS Res. Hum. Retrovir. 2019;35:1181–1188. doi: 10.1089/aid.2019.0050. [DOI] [PubMed] [Google Scholar]
- 111.Soares R.C.A., de Brito A.M., Lima K., Lapa T.M. Adherence to Antiretroviral Therapy among People Living with HIV/AIDS in Northeastern Brazil: A Cross-Sectional Study. Sao Paulo Med. J. 2019;137:479–485. doi: 10.1590/1516-3180.2019.0212170919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gutiérrez-Gabriel I., Godoy-Guinto J., Lucas-Alvarado H., Pineda-Germán B., Vázquez-Cruz E., Hernández-De laRosa M., Sosa-Jurado F. Quality of Life and Psychological Variables Affecting Adherence to Antiretroviral Treatment in Mexican Patients with HIV/AIDS. Rev. Chil. Infectol. 2019;36:331–339. doi: 10.4067/S0716-10182019000300331. [DOI] [PubMed] [Google Scholar]
- 113.Tsuyuki K., Shoptaw S.J., Ransome Y., Chau G., Rodriguez-Diaz C.E., Friedman R.K., Srithanaviboonchai K., Li S., Mimiaga M.J., Mayer K.H., et al. The Longitudinal Effects of Non-Injection Substance Use on Sustained HIV Viral Load Undetectability Among MSM and Heterosexual Men in Brazil and Thailand: The Role of ART Adherence and Depressive Symptoms (HPTN 063) AIDS Behav. 2019;23:649–660. doi: 10.1007/s10461-019-02415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oliveira L.D.S., Caixeta L.M., Martins J.L.R., Segati K.D., Moura R.S., Daher M.C., Pinto E.M.H. Adherence to Antiretroviral Therapy and Correlation with Adverse Effects and Coinfections in People Living with HIV/AIDS in the Municipality of Goiás State. Rev. Soc. Bras. Med. Trop. 2018;51:436–444. doi: 10.1590/0037-8682-0467-2017. [DOI] [PubMed] [Google Scholar]
- 115.Ximenes R.A., Lacerda H.R., Miranda-Filho D.B., Mde F.A., Montarroyos U.R., Turchi M.D., Nery M.W., Martelli C.M., Alencastro P.R., Ikeda M.L., et al. Comparison between Potential Risk Factors for Cardiovascular Disease in People Living with HIV/AIDS in Areas of Brazil. J. Infect. Dev. Ctries. 2015;9:988–996. doi: 10.3855/jidc.5867. [DOI] [PubMed] [Google Scholar]
- 116.Zago A.M., Morelato P., Ede A.E., Dan Gde F., Ribeiro E.M., Miranda A.E. Abandonment of Antiretroviral Therapy among HIV-Positive Patients Attended at the Reference Center for HIV/AIDS in Vitória, Brazil. J. Int. Assoc. Physicians AIDS Care. 2012;11:5–8. doi: 10.1177/1545109711418363. [DOI] [PubMed] [Google Scholar]
- 117.Silva J.A., Dourado I., Brito A.M., Silva C.A. Factors Associated with Non-Adherence to Antiretroviral Therapy in Adults with AIDS in the First Six Months of Treatment in Salvador, Bahia State, Brazil. Cad. Saude Publica. 2015;31:1188–1198. doi: 10.1590/0102-311X00106914. [DOI] [PubMed] [Google Scholar]
- 118.De Boni R.B., Shepherd B.E., Grinsztejn B., Cesar C., Cortés C., Padgett D., Gotuzzo E., Belaunzarán-Zamudio P.F., Rebeiro P.F., Duda S.N., et al. Substance Use and Adherence Among People Living with HIV/AIDS Receiving CART in Latin America. AIDS Behav. 2016;20:2692–2699. doi: 10.1007/s10461-016-1398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pamplona L.F., Costa G.R.R., Silva B.M.E., de Araújo Pinto G., de Faria R.S., Cortez P.J.O. Factors Associated to the Control of Viral Load in HIV Positive Patients. Arch. Med. 2021;21:13–23. doi: 10.30554/archmed.21.1.3823.2021. [DOI] [Google Scholar]
- 120.Mesquita A.L., Melo E.S., Costa C.R.B., Pontes P.S., Gir E., Reis R.K. Consumo de Álcool de Pessoas Vivendo Com HIV e Suas Implicações Para Os Desfechos Clínicos. Rev. Eletrônica Enferm. 2020;22:1–8. doi: 10.5216/ree.v22.56418. [DOI] [Google Scholar]
- 121.Navarro R., Paredes J.L., Echevarria J., González-Lagos E., Graña A., Mejía F., Otero L. HIV and Antiretroviral Treatment Knowledge Gaps and Psychosocial Burden among Persons Living with HIV in Lima, Peru. PLoS ONE. 2021;16:e0256289. doi: 10.1371/journal.pone.0256289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cesar C., Jenkins C.A., Shepherd B.E., Padgett D., Mejía F., Ribeiro S.R., Cortes C.P., Pape J.W., Madero J.S., Fink V., et al. Incidence of Virological Failure and Major Regimen Change of Initial Combination Antiretroviral Therapy in the Latin America and the Caribbean: An Observational Cohort Study. Lancet HIV. 2015;2:e492–e500. doi: 10.1016/S2352-3018(15)00183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Da Silva C.M., Mendoza-Sassi R.A., da Mota L.D., Nader M.M., de Martinez A.M. Alcohol Use Disorders among People Living with HIV/AIDS in Southern Brazil: Prevalence, Risk Factors and Biological Markers Outcomes. BMC Infect. Dis. 2017;17:263. doi: 10.1186/s12879-017-2374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pereira-Morales A.J., Torres D.A., Zapata M.M., Sierra P.M., Hurtado J.A. Design and Development of a Risk Classification Instrument for Virological Failure in HIV, Using Psychosocial Determinants of Health: Preliminary Evidence from a South American Country. AIDS Behav. 2021;25:623–633. doi: 10.1007/s10461-020-03025-7. [DOI] [PubMed] [Google Scholar]
- 125.Cruz M.L., Cardoso C.A., Darmont M.Q., Souza E., Andrade S.D., D’Al Fabbro M.M., Fonseca R., Bellido J.G., Monteiro S.S., Bastos F.I. Viral Suppression and Adherence among HIV-Infected Children and Adolescents on Antiretroviral Therapy: Results of a Multicenter Study. J. Pediatr. 2014;90:563–571. doi: 10.1016/j.jped.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 126.Malbergier A., Amaral R.A., Cardoso L.D. Alcohol Dependence and CD4 Cell Count: Is There a Relationship? AIDS Care. 2015;27:54–58. doi: 10.1080/09540121.2014.947235. [DOI] [PubMed] [Google Scholar]
- 127.Montarroyos U.R., Miranda-Filho D.B., César C.C., Souza W.V., Lacerda H.R., de Albuquerque M.F.P.M., Aguiar M.F., Ximenes R.A.D.A. Factors Related to Changes in CD4+ T-Cell Counts over Time in Patients Living with HIV/AIDS: A Multilevel Analysis. PLoS ONE. 2014;9:e84276. doi: 10.1371/journal.pone.0084276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tancredi M.V., Waldman E.A. Predictors of Progression to AIDS after HIV Infection Diagnosis in the Pre- and Post-HAART Eras in a Brazilian AIDS-Free Cohort. Trans. R. Soc. Trop. Med. Hyg. 2014;108:408–414. doi: 10.1093/trstmh/tru078. [DOI] [PubMed] [Google Scholar]
- 129.Bofill L.M., Lopez M., Dorigo A., Bordato A., Lucas M., Cabanillas G.F., Sued O., Cahn P., Cassetti I., Weiss S., et al. Patient-Provider Perceptions on Engagement in HIV Care in Argentina. AIDS Care. 2014;26:602–607. doi: 10.1080/09540121.2013.844767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Boni R.B., Peratikos M.B., Shepherd B.E., Grinsztejn B., Cortés C., Padgett D., Gotuzzo E., Belaunzarán-Zamudio P.F., Rebeiro P.F., Duda S.N., et al. Is Substance Use Associated with HIV Cascade Outcomes in Latin America? PLoS ONE. 2018;13:e0194228. doi: 10.1371/journal.pone.0194228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Batista K.Z.S., Bogoni B., Müller R.C., Penedo C.C., Higino D.S.F.H. Influence of Comorbidities on CD4+/CD8+ Proportion in HIV-Positive Patients in Blumenau, State of Santa Catarina: A Retrospective Study. Rev. Soc. Bras. Med. Trop. 2017;50:666–669. doi: 10.1590/0037-8682-0523-2016. [DOI] [PubMed] [Google Scholar]
- 132.Sabino T.E., Avelino-Silva V.I., Cavalcantte C., Goulart S.P., Luiz O.C., Fonseca L.A.M., Casseb J.S. Adherence to Antiretroviral Treatment and Quality of Life among Transgender Women Living with HIV/AIDS in São Paulo, Brazil. AIDS Care. 2021;33:31–38. doi: 10.1080/09540121.2019.1710449. [DOI] [PubMed] [Google Scholar]
- 133.Da Frota Santos V., Galvão M.T.G., Da Cunha G.H., De Lima I.C.V., Gir E. Alcohol Effect on HIV-Positive Individuals: Treatment and Quality of Life. ACTA Paul. De Enferm. 2017;30:94–100. doi: 10.1590/1982-0194201700014. [DOI] [Google Scholar]
- 134.Passos S.M.K., de Souza L.D.M. An Evaluation of Quality of Life and Its Determinants among People Living with HIV/AIDS from Southern Brazil. Cad. Saude Publica. 2015;31:800–814. doi: 10.1590/0102-311X00000514. [DOI] [PubMed] [Google Scholar]
- 135.Castro R., De Boni R.B., Luz P.M., Velasque L., Lopes L.V., Medina-Lara A., Cardoso S.W., De Oliveira M.S., Friedman R.K., Grinsztejn B., et al. Health-Related Quality of Life Assessment among People Living with HIV in Rio de Janeiro, Brazil: A Cross-Sectional Study. Qual. Life Res. 2019;28:1035–1045. doi: 10.1007/s11136-018-2044-8. [DOI] [PubMed] [Google Scholar]
- 136.Machado I.K., Luz P.M., Lake J.E., Castro R., Velasque L., Clark J.L., Veloso V.G., Grinsztejn B., De Boni R.B. Self-Rated Health and Substance Use among Individuals in HIV Care in Rio de Janeiro, Brazil: A Cross-Sectional Study. Int. J. STD AIDS. 2017;28:1175–1183. doi: 10.1177/0956462417692278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Costa J.D.O., Pearson S.A., Acurcio F.D.A., Bonolo P.D.F., Silveira M.R., Ceccato M.D.G.B. Health-Related Quality of Life among HIV-Infected Patients Initiating Treatment in Brazil in the Single-Tablet Regimen Era. AIDS Care-Psychol. Socio-Med. Asp. AIDS HIV. 2019;31:572–581. doi: 10.1080/09540121.2019.1576841. [DOI] [PubMed] [Google Scholar]
- 138.Kerrigan D., Vazzano A., Bertoni N., Malta M., Bastos F.I. Stigma, Discrimination and HIV Outcomes among People Living with HIV in Rio de Janeiro, Brazil: The Intersection of Multiple Social Inequalities. Glob. Public Health. 2017;12:185–199. doi: 10.1080/17441692.2015.1064459. [DOI] [PubMed] [Google Scholar]
- 139.Radusky P.D., Zalazar V., Cardozo N., Fabian S., Duarte M., Frola C., Cahn P., Sued O., Aristegui I. Reduction of Gender Identity Stigma and Improvements in Mental Health Among Transgender Women Initiating HIV Treatment in a Trans-Sensitive Clinic in Argentina. Transgender Health. 2020;5:216–224. doi: 10.1089/trgh.2020.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Da Costa L.N.F., Braga M.M., da Rocha M., da Silveira Lima M., Campêlo W.F., de Vasconcelos C.M.C.S. Fatores Associados à Insegurança Alimentar Em Pessoas Que Vivem Com HIV/AIDS. Rev. Bras. Promoç. Saúde (Impr.) 2018;31:1–8. doi: 10.5020/18061230.2018.6884. [DOI] [Google Scholar]
- 141.Lopes L.A.B., da Silva E.M.K. Biological, Behavioral, and Socioeconomic Factors Associated with Death from AIDS in Brasília, Brazil, in 2007. Rev. Soc. Bras. Med. Trop. 2012;45:448–452. doi: 10.1590/S0037-86822012000400006. [DOI] [PubMed] [Google Scholar]
- 142.Mangal T.D., Meireles M.V., Pascom A.R.P., de Almeida Coelho R., Benzaken A.S., Hallett T.B. Determinants of Survival of People Living with HIV/AIDS on Antiretroviral Therapy in Brazil 2006–2015. BMC Infect. Dis. 2019;19:206. doi: 10.1186/s12879-019-3844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haguihara T., Silva M.D.O., Rebouças M.C., Netto E.M., Brites C. Factors Associated with Mortality in HIV Patients Failing Antiretroviral Therapy, in Salvador, Brazil. Braz. J. Infect. Dis. 2019;23:160–163. doi: 10.1016/j.bjid.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De Lima T.A., Beyrer C., Golub J.E., da Mota J.C., Malta M.S., da Silva C.M.F.P., Bastos F.I. Inequalities in HAART Uptake and Differential Survival According to Exposure Category in Rio de Janeiro, Brazil. Cad. Saude Publica. 2018;34:e00009617. doi: 10.1590/0102-311x00009617. [DOI] [PubMed] [Google Scholar]
- 145.Tancredi M.V., Waldman E.A. Survival of AIDS Patients in Sao Paulo-Brazil in the Pre- and Post-HAART Eras: A Cohort Study. BMC Infect. Dis. 2014;14:599. doi: 10.1186/s12879-014-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cunha R., Maruza M., Montarroyos U.R., Coimbra I., Miranda-Filho D.D.B., Albuquerque M.F., Lacerda H.R., Ximenes R. Survival of People Living with HIV Who Defaulted from Tuberculosis Treatment in a Cohort, Recife, Brazil. BMC Infect. Dis. 2017;17:137. doi: 10.1186/s12879-016-2127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Albuquerque M.F., Alves D.N., Salvi C.C.B., Batista J.D., Ximenes R.A., Miranda-Filho D.B., Melo H.R., Maruza M., Montarroyos U.R. Predictors of Immunodeficiency-Related Death in a Cohort of Low-Income People Living with HIV: A Competing Risks Survival Analysis. Epidemiol. Infect. 2017;145:914–924. doi: 10.1017/S0950268816003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Strathdee S.A., Abramovitz D., Lozada R., Martinez G., Rangel M.G., Vera A., Staines H., Magis-Rodriguez C., Patterson T.L. Reductions in HIV/STI Incidence and Sharing of Injection Equipment among Female Sex Workers Who Inject Drugs: Results from a Randomized Controlled Trial. PLoS ONE. 2013;8:e65812. doi: 10.1371/journal.pone.0065812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Glasman L.R., Dickson-Gomez J., Lechuga J., Tarima S., Bodnar G., de Mendoza L.R. Using Peer-Referral Chains with Incentives to Promote HIV Testing and Identify Undiagnosed HIV Infections Among Crack Users in San Salvador. AIDS Behav. 2016;20:1236–1243. doi: 10.1007/s10461-015-1267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dickson-Gomez J., Tarima S., Glasman L.R., Lechuga J., Bodnar G., de Mendoza L.R. Intervention Reach and Sexual Risk Reduction of a Multi-Level, Community-Based HIV Prevention Intervention for Crack Users in San Salvador, El Salvador. AIDS Behav. 2019;23:1147–1157. doi: 10.1007/s10461-018-2314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gonzales P., Grieco A., White E., Ding R., Ignacio R.B., Pinto-Santini D., Lama J.R., Altice F.L., Duerr A. Safety of Oral Naltrexone in HIV-Positive Men Who Have Sex with Men and Transgender Women with Alcohol Use Disorder and Initiating Antiretroviral Therapy. PLoS ONE. 2020;15:e0228433. doi: 10.1371/journal.pone.0228433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Crane H.M., McCaul M.E., Chander G., Hutton H., Nance R.M., Delaney J.A.C., Merrill J.O., Lau B., Mayer K.H., Mugavero M.J., et al. Prevalence and Factors Associated with Hazardous Alcohol Use Among Persons Living with HIV Across the US in the Current Era of Antiretroviral Treatment. AIDS Behav. 2017;21:1914–1925. doi: 10.1007/s10461-017-1740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frazier E.L., Sutton M.Y., Brooks J.T., Shouse R.L., Weiser J. Trends in Cigarette Smoking among Adults with HIV Compared with the General Adult Population, United States—2009–2014. Prev. Med. 2018;111:231–234. doi: 10.1016/j.ypmed.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 154.Crane H.M., Nance R.M., Whitney B.M., Ruderman S., Tsui J.I., Chander G., McCaul M.E., Lau B., Mayer K.H., Batey D.S., et al. Drug and Alcohol Use among People Living with HIV in Care in the United States by Geographic Region. AIDS Care. 2021;33:1569–1576. doi: 10.1080/09540121.2021.1874274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Necho M., Belete A., Getachew Y. The Prevalence and Factors Associated with Alcohol Use Disorder among People Living with HIV/AIDS in Africa: A Systematic Review and Meta-Analysis. Subst. Abus. Treat. Prev. Policy. 2020;15:63. doi: 10.1186/s13011-020-00301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Department of Mental Health and Substance Dependence Noncommunicable Diseases and Mental Health Cluster World Health Organization . Guide to Drug Abuse Epidemiology. Department of Mental Health and Substance Dependence Noncommunicable Diseases and Mental Health Cluster World Health Organization; Geneva, Switzerland: 2000. [Google Scholar]
- 157.UNAIDS . 2020 Global AIDS Update—Seizing the Moment—Tackling Entrenched Inequalities to End Epidemics. UNAIDS; Geneva, Switzerland: 2020. [Google Scholar]
- 158.Garcia S.A., Chen J., Calleja J.G., Sabin K., Ogbuanu C., Lowrance D., Zhao J. Availability and Quality of Surveillance and Survey Data on HIV Prevalence Among Sex Workers, Men Who Have Sex With Men, People Who Inject Drugs, and Transgender Women in Low- and Middle-Income Countries: Review of Available Data (2001–2017) JMIR Public Health Surveill. 2020;6:e21688. doi: 10.2196/21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stockman J.K., Strathdee S.A. HIV among People Who Use Drugs: A Global Perspective of Populations at Risk. J. Acquir. Immune Defic. Syndr. 2010;55:S17–S22. doi: 10.1097/QAI.0b013e3181f9c04c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Boldori H.M., dos Santos W.M., Hoffmann I.C., dos Santos C.M., Galarreta F.M.P., de Padoin S.M.M., de Barros S.M.O. Factors Associated with Miscarriage and Perinatal Mortality in Women with HIV Infection. Int. Res. J. Med. Med. Sci. 2014;2:73–78. [Google Scholar]
- 161.Centers for Disease Control and Prevention Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 Dependent Areas, 2014. HIV Surveill. Suppl. Rep. 2016;21:1–74. [Google Scholar]
- 162.Giordano T.P., Hartman C., Gifford A.L., Backus L.I., Morgan R.O. Predictors of Retention in HIV Care among a National Cohort of US Veterans. HIV Clin. Trials. 2009;10:299–305. doi: 10.1310/hct1005-299. [DOI] [PubMed] [Google Scholar]
- 163.Shuper P.A., Joharchi N., Irving H., Fletcher D., Kovacs C., Loutfy M., Walmsley S.L., Wong D.K.H., Rehm J. Differential Predictors of ART Adherence among HIV-Monoinfected versus HIV/HCV-Coinfected Individuals. AIDS Care. 2016;28:954–962. doi: 10.1080/09540121.2016.1158396. [DOI] [PubMed] [Google Scholar]
- 164.Tanner Z., Lachowsky N., Ding E., Samji H., Hull M., Cescon A., Patterson S., Chia J., Leslie A., Raboud J., et al. Predictors of Viral Suppression and Rebound among HIV-Positive Men Who Have Sex with Men in a Large Multi-Site Canadian Cohort. BMC Infect. Dis. 2016;16:590. doi: 10.1186/s12879-016-1926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Antoniou T., Tseng A.L.-I. Interactions between Recreational Drugs and Antiretroviral Agents. Ann. Pharmacother. 2002;36:1598–1613. doi: 10.1345/aph.1A447. [DOI] [PubMed] [Google Scholar]
- 166.U.S. Department of Health and Human Services . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. U.S. Department of Health and Human Services; Washington, DC, USA: 2021. Panel on Antiretroviral Guidelines for Adults and Adolescents. [Google Scholar]
- 167.Kim H.N., Newcomb C.W., Carbonari D.M., Roy J.A., Torgersen J., Althoff K.N., Kitahata M.M., Reddy K.R., Lim J.K., Silverberg M.J., et al. Risk of HCC With Hepatitis B Viremia Among HIV/HBV-Coinfected Persons in North America. Hepatology. 2021;74:1190–1202. doi: 10.1002/hep.31839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khatib A., Matiko E., Khalid F., Welty S., Ali A., Othman A., Haji S., Dahoma M., Rutherford G. HIV and Hepatitis B and C Co-Infection among People Who Inject Drugs in Zanzibar. BMC Public Health. 2017;17:917. doi: 10.1186/s12889-017-4933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Siza C., Bixler D., Davidson S. Proportion and Characterization of Co-Infections of HIV and Hepatitis C or Hepatitis B among People with HIV in Alabama, 2007–2016. South Med. J. 2020;113:298–304. doi: 10.14423/SMJ.0000000000001104. [DOI] [PubMed] [Google Scholar]
- 170.So-Armah K., Freiberg M.S. HIV and Cardiovascular Disease: Update on Clinical Events, Special Populations, and Novel Biomarkers. Curr. HIV AIDS Rep. 2018;15:233–244. doi: 10.1007/s11904-018-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lorenz D.R., Dutta A., Mukerji S.S., Holman A., Uno H., Gabuzda D. Marijuana Use Impacts Midlife Cardiovascular Events in HIV-Infected Men. Clin. Infect. Dis. 2017;65:626–635. doi: 10.1093/cid/cix391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Evangeli M. Mental Health and Substance Use in HIV-Infected Adolescents. Curr. Opin. HIV AIDS. 2018;13:204–211. doi: 10.1097/COH.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 173.Choopanya K., Martin M., Suntharasamai P., Sangkum U., Mock P.A., Leethochawalit M., Chiamwongpaet S., Kitisin P., Natrujirote P., Kittimunkong S., et al. Antiretroviral Prophylaxis for HIV Infection in Injecting Drug Users in Bangkok, Thailand (the Bangkok Tenofovir Study): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 174.Shrestha R., Karki P., Altice F.L., Dubov O., Fraenkel L., Huedo-Medina T., Copenhaver M. Measuring Acceptability and Preferences for Implementation of Pre-Exposure Prophylaxis (PrEP) Using Conjoint Analysis: An Application to Primary HIV Prevention Among High Risk Drug Users. AIDS Behav. 2018;22:1228–1238. doi: 10.1007/s10461-017-1851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Johnson W.D., Rivadeneira N., Adegbite A.H., Neumann M.S., Mullins M.M., Rooks-Peck C., Wichser M.E., McDonald C.M., Higa D.H., Sipe T.A. Human Immunodeficiency Virus Prevention for People Who Use Drugs: Overview of Reviews and the ICOS of PICOS. J. Infect. Dis. 2020;222:S278–S300. doi: 10.1093/infdis/jiaa008. [DOI] [PubMed] [Google Scholar]
- 176.Haldane V., Cervero-Liceras F., Chuah F.L., Ong S.E., Murphy G., Sigfrid L., Watt N., Balabanova D., Hogarth S., Maimaris W., et al. Integrating HIV and Substance Use Services: A Systematic Review. J. Int. AIDS Soc. 2017;20:21585. doi: 10.7448/IAS.20.1.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Madhombiro M., Musekiwa A., January J., Chingono A., Abas M., Seedat S. Psychological Interventions for Alcohol Use Disorders in People Living with HIV/AIDS: A Systematic Review. Syst. Rev. 2019;8:244. doi: 10.1186/s13643-019-1176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Scott-Sheldon L.A.J., Carey K.B., Johnson B.T., Carey M.P., MASH Research Team Behavioral Interventions Targeting Alcohol Use Among People Living with HIV/AIDS: A Systematic Review and Meta-Analysis. AIDS Behav. 2017;21:126–143. doi: 10.1007/s10461-017-1886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fanucchi L., Springer S.A., Korthuis P.T. Medications for Treatment of Opioid Use Disorder among Persons Living with HIV. Curr. HIV AIDS Rep. 2019;16:1–6. doi: 10.1007/s11904-019-00436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Towe S.L., Meade C.S., Cloak C.C., Bell R.P., Baptiste J., Chang L. Reciprocal Influences of HIV and Cannabinoids on the Brain and Cognitive Function. J. Neuroimmune Pharm. 2020;15:765–779. doi: 10.1007/s11481-020-09921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ellis R.J., Toperoff W., Vaida F., van den Brande G., Gonzales J., Gouaux B., Bentley H., Atkinson J.H. Smoked Medicinal Cannabis for Neuropathic Pain in HIV: A Randomized, Crossover Clinical Trial. Neuropsychopharmacology. 2009;34:672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Da Pires Neto R.J., Gadelha R.R.M., Herzer T.L., Peres D.A., do Leitão T.M.J.S., Façanha M.C., Holanda C.N., Girão E.S., Nogueira C.M.O., Alencar C.H. Características Clínico-Epidemiológicas de Pacientes Com Coinfecção HIV/Tuberculose Acompanhados Nos Serviços de Referência Para HIV/AIDS Em Fortaleza, Ceará, Entre 2004 e 2008. Cad. Saúde Colet. 2012;20:244–249. [Google Scholar]
- 183.Affective Disorders Study Group. de Luna J.R.G., de Gurgel W.S., de Matos K.J.N., Barros E.M., da Ribeiro C.M.F., de Matose Souza F.G. The Influence of Alcohol Use Disorders on Sex and Mood in an HIV-Infected Population in the State of Ceará, Northeastern Brazil. J. AIDS HIV Res. 2014;5:285. [Google Scholar]
- 184.Távora L.G.F., Hyppolito E.B., Da Cruz J.N.M., Portela N.M.B., Pereira S.M., Veras C.M. Hepatitis B, C and HIV Co-Infections Seroprevalence in a Northeast Brazilian Center. Arq. Gastroenterol. 2013;50:277–280. doi: 10.1590/S0004-28032013000400007. [DOI] [PubMed] [Google Scholar]
- 185.Santos A.S., Silveira E.A., Falco M.O. Gastrointestinal Symptoms in HIV-Infected Patients: Female Sex and Smoking as Risk Factors in an Outpatient Cohort in Brazil. PLoS ONE. 2016;11:e0164774. doi: 10.1371/journal.pone.0164774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oliveira M.P., Lemes P.S., Matos M.A., Del-Rios N.H., Carneiro M.A., Silva Á.M., Lopes C.L., Teles S.A., Aires R.S., Lago B.V., et al. Overt and Occult Hepatitis B Virus Infection among Treatment-Naïve HIV-Infected Patients in Brazil. J. Med. Virol. 2016;88:1222–1229. doi: 10.1002/jmv.24462. [DOI] [PubMed] [Google Scholar]
- 187.Melo V.H., Guimaraes M.D.C., Rocha G.M., Araujo A.C.L., Carmo R.A., Grinsztejn B., Pilotto J.H., Palefsky J.M. Prevalence and Risk Factors Associated with Anal Intraepithelial Neoplasia among HIV-Positive Men in Brazil. J. Low. Genit. Tract Dis. 2014;18:128–135. doi: 10.1097/LGT.0b013e31829ee855. [DOI] [PubMed] [Google Scholar]
- 188.Lara B.M.C., Pádua C.M., Mendicino C.C.P., Rocha G.M. Osteopenia and Osteoporosis among Treatment-Experienced People Living with HIV. Braz. J. Infect. Dis. 2020;24:288–295. doi: 10.1016/j.bjid.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Teixeira L.S.L., Ceccato M., Carvalho W.D.S., Costa J.O., Bonolo P.F., Mendes J.C., Silveira M.R. Prevalence of Smoking and Associated Factors in People Living with HIV Undergoing Treatment. Rev. Saude Publica. 2020;54:108. doi: 10.11606/s1518-8787.2020054001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Castro S.S., Scatena L.M., Miranzi A., Miranzi Neto A., Nunes A.A. Characteristics of Cases of Tuberculosis Coinfected with HIV in Minas Gerais State in 2016. Rev. Inst. Med. Trop. Sao Paulo. 2019;61:e21. doi: 10.1590/s1678-9946201961021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Batista J., Militão de Albuquerque Mde F., Ximenes R.A., Miranda-Filho Dde B., Lacerda de Melo H.R., Maruza M., Moura L.V., Pinto da Costa Ferraz E.J., Rodrigues L.C. Prevalence and Socioeconomic Factors Associated with Smoking in People Living with HIV by Sex, in Recife, Brazil. Rev. Bras. Epidemiol. 2013;16:432–443. doi: 10.1590/S1415-790X2013000200018. [DOI] [PubMed] [Google Scholar]
- 192.Coimbra I., Maruza M., Militão-Albuquerque Mde F., Moura L.V., Diniz G.T., Miranda-Filho Dde B., Lacerda H.R., Rodrigues L.C., Ximenes R.A. Associated Factors for Treatment Delay in Pulmonary Tuberculosis in HIV-Infected Individuals: A Nested Case-Control Study. BMC Infect. Dis. 2012;12:208. doi: 10.1186/1471-2334-12-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Machado I.K., Luz P.M., Lake J.E., Castro R., Velasque L., Clark J.L., Veloso V.G., Grinsztejn B., De Boni R.B. Substance Use among HIV-Infected Patients in Rio de Janeiro, Brazil: Agreement between Medical Records and the ASSIST Questionnaire. Drug Alcohol Depend. 2017;178:115–118. doi: 10.1016/j.drugalcdep.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Torres T.S., Luz P.M., Derrico M., Velasque L., Grinsztejn E., Veloso V.G., Cardoso S.W., Santini-Oliveira M., Grinsztejn B., De Boni R.B. Factors Associated with Tobacco Smoking and Cessation among HIV-Infected Individuals under Care in Rio de Janeiro, Brazil. PLoS ONE. 2014;9:e115900. doi: 10.1371/journal.pone.0115900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bhering M., Duarte R., Kritski A. Treatment Outcomes and Predictive Factors for Multidrug-Resistant TB and HIV Coinfection in Rio de Janeiro State, Brazil. Int. J. Tuberc. Lung Dis. 2021;25:292–298. doi: 10.5588/ijtld.20.0887. [DOI] [PubMed] [Google Scholar]
- 196.Domingues R., Silva C., Grinsztejn B.G.J., Moreira R.I., Derrico M., Andrade A.C., Friedman R.K., Luz P.M., Coelho L.E., Veloso V.G. Prevalence of Induced Abortion and Associated Factors in a Cohort of Women Living with HIV/AIDS, Rio de Janeiro, Brazil, 1996-2016. Cad. Saude Publica. 2020;36((Suppl. 1)):e00201318. doi: 10.1590/0102-311x00201318. [DOI] [PubMed] [Google Scholar]
- 197.Da Silva R.A.R., Silva R.T.S., do Nascimento E.G.C., Gonçalves O.P., Reis M.M., da Silva B.C.O. Perfil Clínico-Epidemiológico de Adultos HIV-Positivo Atendidos Emum Hospital de Natal/RN. Rev. Pesqui. (Univ. Fed. Estado Rio J.) 2016;8:4689–4696. doi: 10.9789/2175-5361.2016.v8i3.4689-4696. [DOI] [Google Scholar]
- 198.Da Silva C.M., Oliveira J.M., Mendoza-Sassi R.A., Figueiredo A.S., Mota L.D.D., Nader M.M., Gardinali N.R., Kevorkian Y.B., Salvador S.B.S., Pinto M.A., et al. Detection and Characterization of Hepatitis E Virus Genotype 3 in HIV-Infected Patients and Blood Donors from Southern Brazil. Int. J. Infect. Dis. 2019;86:114–121. doi: 10.1016/j.ijid.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 199.Ikeda M.L.R., Barcellos N.T., Alencastro P.R., Wolff F.H., Moreira L.B., Gus M., Brandão A.B.M., Fuchs F.D., Fuchs S.C. Alcohol Drinking Pattern: A Comparison between HIV-Infected Patients and Individuals from the General Population. PLoS ONE. 2016;11:e0158535. doi: 10.1371/journal.pone.0158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Real L.H.G., Moreira F.P., Gonzalez M.C., Jansen K. Tobacco Smoking and Body Composition in Persons Living with HIV/AIDS. Cienc. Saude Coletiva. 2021;26:1923–1930. doi: 10.1590/1413-81232021265.19532019. [DOI] [PubMed] [Google Scholar]
- 201.Da Marques C.C., de Medeiros E.R., Sousa M.E.S., Maia M.R., Da Silva R.A.R., Feijão A.R., Pinto E.S.G. Casos de Tuberculose Coinfectados Por HIV Em Um Estado Do Nordeste Brasileiro. Enferm. Actual Costa Rica. 2019:62–76. doi: 10.15517/revenf.v0i36.33583. [DOI] [Google Scholar]
- 202.Rossetto M., Brand É.M., Hahn G.V., Oliveira D., Teixeira L.B. Epidemiological Profile of Tuberculosis Cases with HIV Coinfection in Porto Alegre City, Brazil. Rev. Bras. Enferm. 2019;72:1211–1218. doi: 10.1590/0034-7167-2017-0613. [DOI] [PubMed] [Google Scholar]
- 203.Teixeira L.B., Pilecco F.B., Vigo A., Knauth D.R. Sexual and Reproductive Health of Women Living with HIV in Southern Brazil. Cad. Saude Publica. 2013;29:609–620. doi: 10.1590/S0102-311X2013000300018. [DOI] [PubMed] [Google Scholar]
- 204.Petruzzi M.N.M.R., Salum F.G., Cherubini K., De Figueiredo M.A.Z. Epidemiological Characteristics and HIV-Related Oral Lesions Observed in Patients from a Southern Brazilian City. Rev. Odonto Cienc. 2012;27:115–120. doi: 10.1590/S1980-65232012000200004. [DOI] [Google Scholar]
- 205.Justina L.B., Luiz M.C., Maurici R., Schuelter-Trevisol F. Prevalence and Factors Associated with Lipodystrophy in AIDS Patients. Rev. Soc. Bras. Med. Trop. 2014;47:30–37. doi: 10.1590/0037-8682-0240-2013. [DOI] [PubMed] [Google Scholar]
- 206.Sousa L.R.M., Elias H.C., Fernandes N.M., Gir E., Reis R.K. Knowledge of PEP and PrEP among People Living with HIV/Aids in Brazil. BMC Public Health. 2021;21:64. doi: 10.1186/s12889-020-10135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tufano C.S., Amaral R.A., Cardoso L.R., Malbergier A. The Influence of Depressive Symptoms and Substance Use on Adherence to Antiretroviral Therapy. A Cross-Sectional Prevalence Study. Sao Paulo Med. J. 2015;133:179–186. doi: 10.1590/1516-3180.2013.7450010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tauyr T.F.L., Lourenção L.G., Ponce M.A.Z., Neto F.R.G.X., de Lourdes Sperli Geraldes Santos M., dos Santos Sasaki N.S.G.M., Vendramini S.H.F. Vulnerability of the Brazilian LGBT Population in HIV Treatment. J. Infect. Dev. Ctries. 2021;15:1481–1488. doi: 10.3855/jidc.13707. [DOI] [PubMed] [Google Scholar]
- 209.Miranda A.E., Silveira M.F., Travassos A.G., Tenório T., Val I.C.C., Lannoy L., Junior H.S.M., Carvalho N.S. High-Risk Papillomavirus Infection among Women Living with Human Immunodeficiency Virus: Brazilian Multicentric Study. J. Med. Virol. 2017;89:2217–2223. doi: 10.1002/jmv.24906. [DOI] [PubMed] [Google Scholar]
- 210.De Boni R.B., de Vasconcellos M.T.L., Luis N.S.P., Silva K., Bertoni N., Coutinho C.F.S., Mota J.C., Bastos F.I. Substance Use, Self-Rated Health and HIV Status in Brazil. AIDS Care. 2021;33:1358–1362. doi: 10.1080/09540121.2020.1799923. [DOI] [PubMed] [Google Scholar]
- 211.Rich K.M., Huamaní J.V., Kiani S.N., Cabello R., Elish P., Arce J.F., Pizzicato L.N., Soria J., Wickersham J.A., Sanchez J., et al. Correlates of Viral Suppression among HIV-Infected Men Who Have Sex with Men and Transgender Women in Lima, Peru. AIDS Care. 2018;30:1341–1350. doi: 10.1080/09540121.2018.1476657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Guimarães M.L., Marques B.C., Bertoni N., Teixeira S.L., Morgado M.G., Bastos F.I. Assessing the HIV-1 Epidemic in Brazilian Drug Users: A Molecular Epidemiology Approach. PLoS ONE. 2015;10:e0141372. doi: 10.1371/journal.pone.0141372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Campollo O., Roman S., Panduro A., Hernandez G., Diaz-Barriga L., Balanzario M.C., Cunningham J.K. Non-Injection Drug Use and Hepatitis C among Drug Treatment Clients in West Central Mexico. Drug Alcohol Depend. 2012;123:269–272. doi: 10.1016/j.drugalcdep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 214.Fernández D.Y.B., Segura-Cardona Á., Montoya-Velez L., Hernández-Rendón M. Consumption of Crack Cocaine in Injection Drug Users in Colombia. Rev. Cuba. Salud Publica. 2016;42:276–283. [Google Scholar]
- 215.Toro-Tobón D., Berbesi-Fernández D. Prevalence of HIV/Hepatitis C Virus Co-Infection and Injection Risk Correlations in People Who Inject Drugs in Colombia: A Cross-Sectional Study Using Respondent Driven Sampling. Subst. Use Misuse. 2020;55:414–423. doi: 10.1080/10826084.2019.1683198. [DOI] [PubMed] [Google Scholar]
- 216.Sheehan H.B., Benetucci J., Muzzio E., Redini L., Naveira J., Segura M., Weissenbacher M., Tang A.M. High Rates of Serum Selenium Deficiency among HIV- and HCV-Infected and Uninfected Drug Users in Buenos Aires, Argentina. Public Health Nutr. 2012;15:538–545. doi: 10.1017/S1368980011001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Salusso D., Nuñez S., Cabrini M., Rolón M.J., Cahn P. Chemsex y Uso de Sustancias Durante Las Relaciones Sexuales: Resultados de Una Encuesta Realizada En Argentina. Actual SIDA. Infectol. 2020;28:40–50. doi: 10.52226/revista.v28i103.56. [DOI] [Google Scholar]
- 218.Ikeda M.L., Barcellos N.T., Alencastro P.R., Wolff F.H., Brandão A.B., Fuchs F.D., Fuchs S.C. Association of Blood Pressure and Hypertension with Alcohol Consumption in HIV-Infected White and Nonwhite Patients. Sci. World J. 2013;2013:169825. doi: 10.1155/2013/169825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lamas C.C., Coelho L.E., Grinsztejn B.J., Veloso V.G. Community-Acquired Lower Respiratory Tract Infections in HIV-Infected Patients on Antiretroviral Therapy: Predictors in a Contemporary Cohort Study. Infection. 2017;45:801–809. doi: 10.1007/s15010-017-1041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Espinoza-Chiong C., Quiñones-Laveriano D.M., Llanos-Tejada F., Patrón-Ordóñez G., Cárdenas M.M., Mejia C.R. Factores Asociados a La Coinfección Por Tuberculosis y Virus de Inmunodeficiencia Humana En Un Hospital Peruano. Rev. Cuba. Investig. Biomédicas. 2021;40:1–16. [Google Scholar]
- 221.Prado T.N., Rajan J.V., Miranda A.E., Dias E.D., Cosme L.B., Possuelo L.G., Sanchez M.N., Golub J.E., Riley L.W., Maciel E.L. Clinical and Epidemiological Characteristics Associated with Unfavorable Tuberculosis Treatment Outcomes in TB-HIV Co-Infected Patients in Brazil: A Hierarchical Polytomous Analysis. Braz. J. Infect. Dis. 2017;21:162–170. doi: 10.1016/j.bjid.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Calvet G.A., Grinsztejn B.G.J., Quintana M.D.S.B., Derrico M., Jalil E.M., Cytryn A., De Andrade A.C.V., Moreira R.I., Alves M.R., Dos Santos V.G.V., et al. Predictors of Early Menopause in HIV-Infected Women: A Prospective Cohort Study. Am. J. Obstet. Gynecol. 2015;212:765.e1–765.e13. doi: 10.1016/j.ajog.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 223.Silva G., Motta H., Souza E.F.A., Cardoso P., Pilotto J.H., Eyer-Silva W.A., Ribeiro L.C.P., Santos M.S.D., Azevedo M., Pinto J., et al. Chlamydia Trachomatis Asymptomatic Urethritis Recurrence among Males Living with HIV-1. Rev. Inst. Med. Trop. Sao Paulo. 2018;60:e65. doi: 10.1590/s1678-9946201860065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Galetto L.R., Lunge V.R., Béria J.U., Tietzmann D.C., Stein A.T., Simon D. Short Communication: Prevalence and Risk Factors for Human T Cell Lymphotropic Virus Infection in Southern Brazilian HIV-Positive Patients. AIDS Res. Hum. Retrovir. 2014;30:907–911. doi: 10.1089/aid.2013.0210. [DOI] [PubMed] [Google Scholar]
- 225.Group of Surveillance and Diagnosis of HTLV of São Paulo (GSuDiHTLV-SP) Caterino-de-Araujo A., Sacchi C.T., Gonçalves M.G., Campos K.R., Magri M.C., Alencar W.K. Current Prevalence and Risk Factors Associated with Human T Lymphotropic Virus Type 1 and Human T Lymphotropic Virus Type 2 Infections among HIV/AIDS Patients in São Paulo, Brazil. AIDS Res. Hum. Retrovir. 2015;31:543–549. doi: 10.1089/aid.2014.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vargas J.I., Jensen D., Sarmiento V., Peirano F., Acuña P., Fuster F., Soto S., Ahumada R., Huilcaman M., Bruna M., et al. Presence of Anti-HBc Is Associated to High Rates of HBV Resolved Infection and Low Threshold for Occult HBV Infection in HIV Patients with Negative HBsAg in Chile. J. Med. Virol. 2016;88:639–646. doi: 10.1002/jmv.24384. [DOI] [PubMed] [Google Scholar]
- 227.Saraceni V., Talhari C.C., Pereira G.F., Golub J.E., Talhari S., Miranda A.E. AIDS-Related Kaposi’s Sarcoma in Brazil: Trends and Geopolitical Distribution. Int. J. Dermatol. 2013;52:1525–1529. doi: 10.1111/ijd.12116. [DOI] [PubMed] [Google Scholar]
- 228.Kuehlkamp V.M., Schneider I.J.C., Biudes M.F., Galato D., da Silva J., Maurici R., Traebert J., Schuelter-Trevisol F. Factors Associated with Hepatitis C Seropositivity in People Living with HIV. Rev. Panam. Salud Publica. 2014;35:53–59. [PubMed] [Google Scholar]
- 229.Tizzot M.R., Grisbach C., Beltrame M.H., De Taborda Messias-Reason I.J. Seroprevalence of HCV Markers among HIV Infected Patients from Curitiba and Metropolitan Region. Rev. Assoc. Med. Bras. 2016;62:65–71. doi: 10.1590/1806-9282.62.01.65. [DOI] [PubMed] [Google Scholar]
- 230.Librelotto C.S., Simon D., Ikuta N., Lunge V.R. Low Prevalence of Human Immunodeficiency Virus and Hepatitis C Virus Co-Infection in a Medium Size City in Southern Brazil. Braz. J. Infect. Dis. 2014;18:689–690. doi: 10.1016/j.bjid.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Simon D., Michita R.T., Béria J.U., Tietzmann D.C., Stein A.T., Lunge V.R. Alcohol Misuse and Illicit Drug Use Are Associated with HCV/HIV Co-Infection. Epidemiol. Infect. 2014;142:2616–2623. doi: 10.1017/S0950268814000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Borda J.P., Friedman H.L., Castaño G.A., Rodríguez H.A., Muñoz C.F., Tofighi B. Barriers to HIV and Hepatitis C Care for People Who Inject Drugs in Colombia. AIDS Care. 2021;34:633–638. doi: 10.1080/09540121.2021.1889952. [DOI] [PubMed] [Google Scholar]
- 233.Lino O.J.C., Paredes N.O.C., Cuba F.S. Factors Associated with Normal or Abnormal Papanicolaou Smear among HIV-Infected Women at a National Hospital in Lima, Peru, 2012–2015. AIDS Care. 2021:1–4. doi: 10.1080/09540121.2021.2002254. [DOI] [PubMed] [Google Scholar]
- 234.Martins A.E., Lucena-Silva N., Garcia R.G., Welkovic S., Barboza A., Menezes M.L., Maruza M., Tenório T., Ximenes R.A. Prevalence of Human Papillomavirus Infection, Distribution of Viral Types and Risk Factors in Cervical Samples from Human Immunodeficiency Virus-Positive Women Attending Three Human Immunodeficiency Virus-Acquired Immune Deficiency Syndrome Reference Centres in Northeastern Brazil. Mem. Inst. Oswaldo Cruz. 2014;109:738–747. doi: 10.1590/0074-0276140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Petruzzi M.N., Cherubini K., Salum F.G., Figueiredo M.A. Risk Factors of HIV-Related Oral Lesions in Adults. Rev. Saude Publica. 2013;47:52–59. [PubMed] [Google Scholar]
- 236.Gonçalves L.S., Júnior A.S., Ferreira S.M., Sousa C.O., Fontes T.V., Vettore M.V., Torres S.R. Factors Associated with Specific Clinical Forms of Oral Candidiasis in HIV-Infected Brazilian Adults. Arch. Oral Biol. 2013;58:657–663. doi: 10.1016/j.archoralbio.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 237.De Sousa Gurgel W., da Silva Carneiro A.H., Rebouças D.B., de Matos K.J.N., do Menino Jesus Silva Leitão T., de Matos e Souza F.G. Prevalence of Bipolar Disorder in a HIV-Infected Outpatient Population. AIDS Care. 2013;25:1499–1503. doi: 10.1080/09540121.2013.779625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.