Abstract
Microbial transformation of the antimelanoma agent betulinic acid was studied. The main objective of this study was to utilize microorganisms as in vitro models to predict and prepare potential mammalian metabolites of this compound. Preparative-scale biotransformation with resting-cell suspensions of Bacillus megaterium ATCC 13368 resulted in the production of four metabolites, which were identified as 3-oxo-lup-20(29)-en-28-oic acid, 3-oxo-11α-hydroxy-lup-20(29)-en-28-oic acid, 1β-hydroxy-3-oxo-lup-20(29)-en-28-oic acid, and 3β,7β,15α-trihydroxy-lup-20(29)-en-28-oic acid based on nuclear magnetic resonance and high-resolution mass spectral analyses. In addition, the antimelanoma activities of these metabolites were evaluated with two human melanoma cell lines, Mel-1 (lymph node) and Mel-2 (pleural fluid).
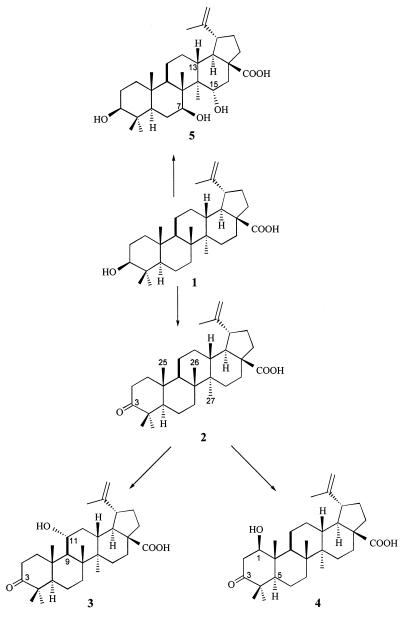
Betulinic acid (compound 1, Fig. 1), 3β-hydroxy-lup-20(29)-en-28-oic acid, is a pentacyclic lupane type of triterpene that is widely distributed in the plant kingdom (8, 19). Betulinic acid has been shown to exhibit a variety of biological activities, including inhibition of human immunodeficiency virus (HIV) replication in H9 lymphocyte cells (11), blockage of HIV type 1 entry into cells (20), and inhibition of DNA polymerase β (18). Synthetic derivatives of betulinic acid have also been investigated as specific inhibitors of HIV type 1 (11–13). In addition, betulinic acid has been reported to be a melanoma-specific cytotoxic agent in both in vitro cell culture and in vivo studies (24). The antitumor activity of this compound is mediated by the induction of apoptosis, as demonstrated by a variety of cellular responses (24). Due to its high level of antitumor activity and lack of toxicity, betulinic acid is an attractive and promising new lead compound for use against human melanoma and is currently undergoing preclinical development for treatment or prevention of malignant melanoma.
FIG. 1.
Proposed metabolic pathway for betulinic acid in B. megaterium ATCC 13368.
An essential part of the preclinical development of a drug is elucidation of its mammalian metabolism. Since there have been no reports on the mammalian metabolism of betulinic acid, a prospective approach was used to study its metabolism by utilizing microorganisms as in vitro model systems. Fungi, bacteria, and yeasts have been utilized successfully as in vitro models to mimic and predict the metabolic fate of drugs and other xenobiotics in mammalian systems (7, 9, 15, 16, 28). Studies have shown that many of the mammalian phase I and phase II biotransformation reactions, including hydroxylation, N oxidation, O and N dealkylation, dehydrogenation, and glucuronide and sulfate conjugation reactions, occur in microbial systems as well (27). The biochemical bases for this parallelism in metabolic reactions lie in the similarity between mammalian and microbial enzyme systems, such as cytochrome P450 monooxygenases and copper oxidases. These studies, which have been extensively reviewed in the literature, highlighted the potential value of microbial transformations as a powerful tool for mimicking and predicting mammalian biotransformations and for facilitating mammalian metabolism studies of drugs and other xenobiotics (7, 9). In addition, the attractiveness of microbial systems as predictive models for initial metabolism studies is attributed to the ease of scale-up, which provides quantities of metabolites that would be difficult to obtain from either animals, mammal-derived in vitro systems, or chemical synthesis. Preparative-scale bioconversions using microbial systems produce significant quantities of metabolites for structure elucidation and biological evaluation. The isolated microbial metabolites, which are either similar or identical to those formed in mammalian systems, can then be used to establish analytical procedures for the identification of mammalian metabolites. Other advantages of using microorganisms as models for drug metabolism studies include the low cost of carrying out microbial transformation studies compared to the cost of animal studies, the ease of experimental design, and a significant reduction in the number of research animals necessary for the evaluation of a drug's metabolic profile (9, 10, 15, 16).
Microbial cytochrome P450 systems display properties remarkably similar to those of known adrenal mitochondrial and hepatic microsomal systems (25, 28). Prokaryotic organisms appear to possess monooxygenase systems which are nearly identical to those found in adrenal mitochondria. In contrast, eukaryotic organisms, such as yeasts and fungi, possess monooxygenase systems that are much more similar to those found in mammalian hepatic microsomes. In microorganisms, monooxygenases play an important role in the intermediary metabolism of lipids, sterols, and alkanes and in such processes as nitrogen fixation, O and N dealkylations, and drug hydroxylations (27, 28). Due to their high similarity to mammalian cytochrome P450 systems, several microbial monooxygenase systems have been studied extensively as models of human and other mammalian P450 systems. Two of these models are CYP102 (BM-3) and CYPmeg, which are produced by Bacillus megaterium ATCC 14581 and ATCC 13368, respectively. CYP102, a barbiturate-inducible cytochrome P450, is a multifunctional enzyme which has been categorized as a class II (microsomal) cytochrome P450 system (21–23). In addition, the crystal structure of CYP102 has been utilized to construct templates for homology modeling of human and other mammalian microsomal P450 systems. CYPmeg, on the other hand, is a steroid-15β-monooxygenase which has been investigated as a bacterial model system for mammalian cytochrome P450-dependent hydroxylation reactions (2–4). These studies, which strongly indicated the potential for B. megaterium to serve as a prokaryotic model for mammalian drug metabolism, constituted the basis for our investigation of the biotransformation of betulinic acid by B. megaterium.
In the present report, we describe the isolation, structural elucidation, and in vitro antimelanoma activity of four metabolites of betulinic acid from resting-cell suspensions of B. megaterium ATCC 13368. The metabolites were identified as 3-oxo-lup-20(29)-en-28-oic acid (compound 2 [= metabolite 2], Fig. 1), 3-oxo-11α-hydroxy-lup-20(29)-en-28-oic acid (compound 3 [= metabolite 3]), 1β-hydroxy-3-oxo-lup-20(29)-en-28-oic acid (compound 4 [= metabolite 4]), and 3β,7β,15α-trihydroxy-lup-20(29)-en-28-oic acid (compound 5 [= metabolite 5]) based on one-dimensional and two-dimensional nuclear magnetic resonance (NMR) and high-resolution mass spectral analyses.
MATERIALS AND METHODS
General.
Melting points were determined in open capillary tubes with a Thomas-Hoover capillary melting point apparatus and are uncorrected. Optical rotations were measured with a Perkin-Elmer 241 digital polarimeter. Infrared (IR) spectra were recorded in KBr with a Nicolet Impact 400D IR spectrophotometer. The term in vacuo refers to removal of solvent with a rotary evaporator under a water aspirator vacuum (15 to 30 mm of Hg). Centrifugation was carried out with a Heraeus Megafuge 2.0R centrifuge at 4°C and 2,800 × g. Optical density of the culture medium was measured with a GBC Cintra 20 UV-visible spectrophotometer at 700 nm. 1H- and 13C-NMR spectra were obtained in C5D5N with a JEOL-Eclipse 400 FT-NMR spectrometer operating at 400 and 100 MHz, respectively. The chemical shifts are reported in parts per million, and the coupling constants (J values) are reported in hertz. Standard pulse sequences were used for attached proton test, distortionless enhanced polarization transfer (DEPT), long-range 1H-13C shift correlation sequence with three bilinear rotation decoupling pulses, heteronuclear chemical shift correlation, and nuclear Overhauser and exchange spectroscopy (NOESY) experiments. High-resolution electrospray ionization (ESI) mass spectra were obtained with a Mariner Electrospray TOF mass spectrometer (PE Biosystems, Foster City, Calif.) at the Mass Spectrometry Facility, Department of Medicinal Chemistry, University of Washington, Seattle.
Chromatographic conditions.
Thin-layer chromatography (TLC) analyses were carried out on precoated Silica UV254 plates (E. Merck, Darmstadt, Germany). The adsorbents used for column chromatography were Silica Gel 60 Å (70 to 230 mesh; Aldrich Chemical Co., Milwaukee, Wis.) and lipophilic Sephadex LH-20 (Sigma Chemical Co., St. Louis, Mo.). The solvent system used for TLC was CHCl3-methanol (9:1, vol/vol), and visualization of the spots on TLC plates was performed with anisaldehyde-H2SO4 spray reagent. The spots were visualized by spraying a plate and then heating it at 110°C for 3 min in an oven. Dimethyl formamide (DMF) and pyridine (C5H5N) were stored over 4-Å molecular sieves. All solvents were reagent grade quality or better.
Microorganism and media.
B. megaterium ATCC 13368 was obtained from the American Type Culture Collection, Manassas, Va. All preliminary screening experiments were carried out in a complex medium consisting of 20 g of dextrose, 5 g of yeast extract, 5 g of peptone, 5 g of NaCl, 5 g of K2HPO4, 3 g of beef extract, and 1,000 ml of distilled H2O. Stock cultures of B. megaterium were stored on slants of nutrient agar (Difco, Detroit, Mich.) at 4°C. The 0.1 M phosphate buffer (pH 7.2) used for resting-cell suspensions of B. megaterium consisted of 10.6 g of K2HPO4, 4.08 g of KH2PO4, and 1,000 ml of distilled H2O.
Fermentation procedures.
Microbial metabolism studies were carried out by incubating cultures on a Innova 5000 digital tier shaker (New Brunswick Scientific Co., Edison, N.J.) operating at 150 rpm and 25°C. Preliminary screening experiments were carried out in 125-ml foam-plugged culture flasks containing 25 ml of beef extract-enriched complex medium. The medium was sterilized at 121°C and 18 lb/in2 for 15 min. Fermentations were carried out according to a standard two-stage protocol (15, 25, 28). A B. megaterium stock inoculum was first prepared by suspending the cells from one agar slant in 1 ml of sterile distilled water. Submerged stage I cultures were then initiated by adding 0.1 ml of the B. megaterium stock inoculum to a 125-ml flask containing 25 ml of complex medium. Following incubation of stage I cultures for 3 days on the shaker, stage II cultures were initiated by inoculating 25 ml of fresh, sterile complex medium with 1 ml of stage I culture broth. Betulinic acid at a concentration of 0.1 mg/ml in DMF was added to the 26 ml of incubation medium 1 day after inoculation of stage II cultures. Cultures were sampled at 24-h intervals by extracting 3 ml of the broth with 3 ml of ethyl acetate. The extracts were concentrated and chromatographed on TLC plates. Substrate controls were composed of sterile medium to which betulinic acid was added and were incubated without the microorganism. Culture controls consisted of fermentation blanks in which B. megaterium was grown under identical conditions without betulinic acid. Substrate-autoclaved culture controls consisted of B. megaterium cultures that were grown for 3 days to maturity, autoclaved for 30 min, and then incubated after the addition of betulinic acid.
Biotransformation of betulinic acid to metabolites 2 to 5.
Betulinic acid was obtained from Indofine Chemical Company (Somerville, N.J.). Physical and spectral data for betulinic acid have been reported previously in the literature (1, 5, 29). A stock inoculum of B. megaterium ATCC 13368 was prepared by suspending the cells from one agar slant in 1 ml of sterile distilled H2O. The 1-ml stock inoculum was distributed equally among 10 stage I 125-ml flasks, each containing 25 ml of beef extract-enriched complex medium. Stage I cultures were then incubated on the shaker for 72 h at room temperature. The optical density at 700 nm of stage I cultures was recorded daily until a steady absorbance value of 1.4 was obtained. Following 72 h of incubation of stage I cultures, stage II cultures were initiated by inoculating 40 1-liter flasks, each containing 200 ml of fresh, sterile, beef extract-enriched complex medium, with stage I culture broth. Each stage II flask was inoculated with 5 ml of stage I culture broth. The stage II cultures were incubated on the shaker, and the optical density of the medium was monitored. Following a 24-h incubation period, the optical density at 700 nm of the stage II cultures reached 1.4, and the cells were harvested by centrifugation at 2,800 × g and 4°C. The cells were then washed with sterile distilled H2O and suspended in 65 1-liter flasks, each containing 200 ml of sterile 0.1 M phosphate buffer (pH 7.2), at a concentration of 2 g (wet mass) of cells/200 ml of buffer. Then 2.6 g of betulinic acid was dissolved in 5.2 ml of DMF and distributed equally among the 65 resting-cell-suspension flasks. After 6 days of incubation on the shaker, the suspensions were pooled and extracted three times with 5 liters of ethyl acetate. The organic layer was dried over anhydrous Na2SO4, filtered, and evaporated in vacuo, which yielded 3.63 g of a yellowish residue.
Isolation and purification of metabolites 2 to 5.
The yellowish residue (3. 63 g) was first chromatographed on a column (2.5 by 67 cm; 52 g of silica gel) by using 2 liters of CHCl3-methanol (90:10, vol/vol) as the eluent to obtain two fractions, fractions A (0.263 g) and B (62 mg). Fraction A was subjected to repeated silica gel column chromatography (1.8 by 31 cm; 10 g of silica gel) with an ethyl acetate-hexane gradient (0:100 to 15:85, vol/vol; total volume, 1 liter), which yielded fractions A1 (0.16 g) and A2 (25 mg). Fraction A1 was subjected to purification on a Sephadex LH-20 column (1.8 by 24 cm) with 500 ml of chloroform as the eluent at a flow rate of 5 ml/min, which resulted in homogeneous fractions (Rf 0.78). The homogeneous fractions were combined and evaporated in vacuo to obtain metabolite 2. Crystallization from ethyl acetate-methanol (90:10, vol/vol) produced white needles of metabolite 2 (0.11 g, 4.1% yield). The physical and spectral data for metabolite 2 were consistent with the reported data for betulonic acid (5).
Fraction A2 (25 mg) was subjected to silica gel column chromatography (1.8 by 31 cm; 10 g of silica gel) followed by crystallization from methanol to obtain white needles of metabolite 3 (5.2 mg, 0.19% yield) and fraction A3. Fraction A3 (7 mg) was subjected to a final purification step on a Sephadex LH-20 column (1.8 by 24 cm) with 200 ml of CHCl3 as the eluent, which yielded homogeneous fractions containing metabolite 4 (3.6 mg, 0.13% yield).
Fraction B (62 mg) was subjected to silica gel column chromatography (1.8 by 31 cm; 10 g of silica gel) with an ethyl acetate-hexane gradient (0:100 to 100:0, vol/vol; total volume, 500 ml) followed by an ethyl acetate-methanol gradient (100:0 to 100:10, vol/vol; 500 ml), which yielded homogeneous fractions containing metabolite 5. Purification on a Sephadex LH-20 column (1.8 by 24 cm) with 500 ml of CHCl3 yielded metabolite 5 (15.2 mg, 0.54% yield).
In vitro cytotoxicity assay.
The cytotoxicities of compounds 1 to 5 were determined with two cultured human melanoma cell lines, Mel-1 (lymph node) and Mel-2 (pleural fluid). The cells were cultured in appropriate media and under standard conditions as described previously (24). To maintain logarithmic growth, the media were changed 24 h before the cytotoxicity assays. On the day of the assay, the cells were harvested by trypsinization, counted, diluted in media, and added to 96-well plates containing test compounds dissolved in dimethyl sulfoxide (DMSO) in triplicate; the final DMSO concentration was 0.05%. The plates were incubated for 3 days. Following incubation, the cells were fixed with trichloroacetic acid and stained with 0.4% sulforhodamine B in 1% acetic acid. The bound dye was liberated with 0.1 M Tris base, and the A515 was measured with a microtiter plate reader. The growth of the compound-treated cells as judged by the A515 values was compared with the A515 values of a DMSO-treated control. Dose-response studies were performed to generate 50% effective dose (ED50) values (see Table 3).
TABLE 3.
Cytotoxicities of betulinic acid and compounds 2 to 5
| Compound | ED50s for human melanoma cell lines (μg/ml)
|
|
|---|---|---|
| Mel-1 | Mel-2 | |
| Betulinic acid | 3.3 | 1.0 |
| 2 | 16.0 | 0.1 |
| 3 | >20 | 0.2 |
| 4 | >20 | 0.3 |
| 5 | >20 | >20 |
RESULTS
B. megaterium ATCC 13368 was selected for preparative-scale transformation of betulinic acid due to its ability to reproducibly biotransform this compound. Prior to incubation of betulinic acid with B. megaterium, recovery studies were conducted to ensure that the compound was stable in the aqueous culture medium during incubation and that it and its metabolites could be recovered from the medium. Substrate control experiments, in which betulinic acid was added to either sterile culture medium or sterile phosphate buffer and incubated on the shaker, revealed that the compound is stable and easily recovered from aqueous media by extraction with ethyl acetate, with an average level of recovery of 98%. In addition, substrate-autoclaved culture control studies showed that the B. megaterium culture and its secondary products do not interfere with the stability or recovery of betulinic acid. Furthermore, a comparative study of B. megaterium incubated with betulinic acid and culture controls was carried out to discriminate between the metabolites of betulinic acid and the normal secondary products from B. megaterium which may be coextracted. The study confirmed that compounds 2 to 5 were true metabolites of betulinic acid. A preparative-scale biotransformation of betulinic acid with resting-cell suspensions of B. megaterium resulted in yields of compounds 2 to 5 of 4.1, 0.19, 0.13, and 0.54%, respectively. Betulinic acid was also recovered with an 88% yield. A time course study indicated that metabolite 2 was produced on the third day of incubation, whereas metabolites 3 to 5 were detected on the fifth day. In addition, incubation of B. megaterium with compound 2 resulted in production of metabolites 3 and 4 with yields of 0.2 and 0.1%, respectively.
Structural elucidation of compounds 2 to 5 was based on one-dimensional and two-dimensional NMR and high-resolution mass spectral analyses. 1H- and 13C-NMR chemical shift assignments for compounds 2 to 5 were based on two-dimensional one-bond (heteronuclear chemical shift correlation; J = 155 Hz) and long-range (two- and three-bond; J = 8 Hz) 1H-13C heteronuclear chemical shift correlation experiments and comparisons to the assignments for betulinic acid which have been reported in the literature (1, 5, 29). The multiplicities (CHn) of carbon signals in compounds 2 to 5 were determined based on the one-dimensional attached proton test and DEPT experiments. In these experiments, carbon resonances were sorted on the basis of the number of protons attached to each carbon signal. The relative stereochemistry at the new sites of hydroxylation in compounds 3 to 5 was established based on two-dimensional NOESY experiments. A NOESY experiment probes all possible 1H-1H polarization transfer interactions through space and provides crucial information regarding the spatial proximity of 1H resonances in molecules with regions having fixed geometry. Mass spectral analyses of compounds 2 to 5 were carried out by utilizing the ESI technique (positive ion). The physical and spectral data for metabolites 3 to 5 (1H-NMR, melting point, optical rotation, mass spectral, and IR data) are listed in Table 1. The 13C-NMR data for compounds 3 to 5 are listed in Table 2. The physical and spectral data for compounds 1 and 2 have previously been reported in the literature (1, 5, 29). The 13C-NMR assignments for betulinic acid are listed in Table 2 for comparative purposes.
TABLE 1.
Physical and spectral data for compounds 3 to 5
| Compound | Melting point (°C) | [α]25D in C5H5N | IR (KBr) νmax (cm−1) | ESI mass spectrum (m/z) | 1H-NMR (C5D5N) δ (ppm), J (Hz)a |
|---|---|---|---|---|---|
| 3 | 218-220 | +41.09° (c 0.58 g/100 ml) | 3,505; 2,954; 1,701; 1,686 | 493.3297 [M + Na]+ (C30H46O4Na, 493.3294) | H29 4.69 (s), 4.54 (s); H11 3.88 (dd, J = 4.4, 10.8); H18 3.54-3.30 (m); H12 3.12-3.05 (m); H13 2.81-2.75 (m); H2, 16 2.49-2.44 (m); H2 2.38-2.34 (m); H1 2.25-2.22 (m); H15, 22 2.11-2.09 (m); H12 1.83-1.79 (m); H5, 9, 19, 21 1.70-1.65 (m); Me30 1.60 (s); H1, 16, 22 1.49-1.41 (m); H6, 7 1.30-1.23 (m); H21 1.18-1.14 (m); Me25 1.06 (s); Me23 1.03 (s); Me24 or 27 1.00 (s); Me24 or 27 0.98 (s); Me26 0.97 (s) |
| 4 | 232-234 | +38.63° (c 0.23 g/100 ml) | 3,433; 2,953; 1,701; 1,684 | 493.3293 [M + Na]+ (C30H46O4Na, 493.3294) | H29 4.84 (s), 4.78 (s); H1 4.09-4.06 (m); H18 3.42-3.40 (m); H2 3.12-3.09 (m); H13 2.73-2.70 (m); H2, 16 2.60-2.56 (m); H21, 22 2.20-2.10 (m); H12 1.94-1.85 (m); H9, 19 1.77-1.72 (m); Me30 1.69 (s); H5, 7, 15, 16, 21, 22 1.48-1.32 (m); Me25 1.30 (s); H6, 11, 12 1.26-1.20 (m); Me23 1.09 (s), Me24, 26 or 27 1.03 (s); Me24, 26 or 27 0.97 (s); Me24, 26 or 27 0.85 (s) |
| 5 | 308-310 | −38.09° (c 0.21 g/100 ml) | 3,436; 2,944; 1,690 | 511.3396 [M + Na]+ (C30H48O5Na, 511.3399) | H29 4.94 (s), 4.78 (s); H15 4.67 (dd, J = 4.4, 10.8); H7 4.19 (dd, J = 4.4, 10.4); H3, 18 3.52-3.37 (m); H16 3.14 (dd, J = 4.4, 12.3); H13 2.68-2.65 (m); H6 2.37-2.27 (m); H22 2.24-2.20 (m); H2 2.10-2.06 (m); H12, 16 1.97-1.91 (m); H2, 19, 21 1.86-1.81 (m); Me30 1.81 (s); H1, 22 1.68-1.61 (m); H6, 11 1.54-1.51 (m); H9, 12 1.41-1.32 (m); Me26 or 27 1.25 (s); Me26 or 27 1.24 (s); Me23 1.10 (s); Me24 0.89 (s); H5 0.89-0.87 (m); Me25 0.77 (s) |
Abbreviations for NMR signals are as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad.
TABLE 2.
13C-NMR chemical shift assignments for betulinic acid and compounds 3 to 5 in C5D5N
| Carbon | Chemical shift assignments
|
|||
|---|---|---|---|---|
| Betulinic acid | Compound 3 | Compound 4 | Compound 5 | |
| 1 | 39.1 (2)a | 37.8 (2) | 79.4 (1) | 38.9 (2) |
| 2 | 28.1 (2) | 34.4 (2) | 46.1 (2) | 28.0 (2) |
| 3 | 78.1 (1) | 217.9 (0) | 215.7 (0) | 77.5 (1) |
| 4 | 39.4 (0) | 47.4 (0) | 47.1 (0) | 38.6 (0) |
| 5 | 55.7 (1) | 55.1 (1) | 51.4 (1) | 52.3 (1) |
| 6 | 18.6 (2) | 19.7 (2) | 19.7 (2) | 31.2 (2) |
| 7 | 34.7 (2) | 34.5 (2) | 33.5 (2) | 72.5 (1) |
| 8 | 40.9 (0) | 42.4 (0) | 41.3 (0) | 48.0 (0) |
| 9 | 50.8 (1) | 55.3 (1) | 51.2 (1) | 50.8 (1) |
| 10 | 37.3 (0) | 38.4 (0) | 43.2 (0) | 37.1 (0) |
| 11 | 21.1 (2) | 69.4 (1) | 23.4 (2) | 20.7 (2) |
| 12 | 25.9 (2) | 42.3 (2) | 26.2 (2) | 25.7 (2) |
| 13 | 38.4 (1) | 37.3 (1) | 38.7 (1) | 37.1 (1) |
| 14 | 42.4 (0) | 42.4 (0) | 42.9 (0) | 48.8 (0) |
| 15 | 31.1 (2) | 31.1 (2) | 31.1 (2) | 68.5 (1) |
| 16 | 32.7 (2) | 32.6 (2) | 32.6 (2) | 42.1 (2) |
| 17 | 56.3 (0) | 56.4 (0) | 56.6 (0) | 54.7 (0) |
| 18 | 47.6 (1) | 47.1 (1) | 47.5 (1) | 47.3 (1) |
| 19 | 49.5 (1) | 49.1 (1) | 49.7 (1) | 49.3 (1) |
| 20 | 150.7 (0) | 150.7 (0) | 150.6 (0) | 150.6 (0) |
| 21 | 30.1 (2) | 29.9 (2) | 30.2 (2) | 27.8 (2) |
| 22 | 37.5 (2) | 37.1 (2) | 37.4 (2) | 37.6 (2) |
| 23 | 28.5 (3) | 27.3 (3) | 27.8 (3) | 28.4 (3) |
| 24 | 16.3 (3)b | 21.1 (3) | 20.3 (3) | 15.7 (3)b |
| 25 | 16.3 (3)b | 16.5 (3)b | 12.2 (3) | 15.4 (3)b |
| 26 | 16.2 (3)b | 16.9 (3)b | 16.3 (3) | 11.1 (3)c |
| 27 | 14.8 (3) | 14.5 (3) | 14.6 (3) | 8.7 (3)c |
| 28 | 178.7 (0) | 178.6 (0) | 179.1 (0) | 178.6 (0) |
| 29 | 110.3 (2) | 110.2 (2) | 109.9 (2) | 109.3 (2) |
| 30 | 19.4 (3) | 19.3 (3) | 19.3 (3) | 19.1 (3) |
The number in parentheses is the number of hydrogens attached to the corresponding carbon and was determined from DEPT experiments.
Assignments may be reversed.
Assignments may be reversed.
Metabolite 2 was isolated as the major biotransformation product of betulinic acid in resting-cell suspensions of B. megaterium ATCC 13368. The physical and spectral (13C- and 1H-NMR) data for metabolite 2 were in agreement with the previously reported data for betulonic acid (5). Betulonic acid (metabolite 2) is an intermediate in the synthesis of betulinic acid from betulin (14) and has previously been isolated from a medicinal herb (17). The IR spectrum of metabolite 3 revealed absorption at 3,436 cm−1 (OH), 1,701 cm−1 (ketone), and 1,683 cm−1 (carboxylic acid). The high-resolution ESI mass spectrum of metabolite 3 showed an [M + Na]+ ion at m/z 493.3297. Similar to the 13C-NMR spectrum of metabolite 2, the 13C-NMR spectrum of metabolite 3 showed the presence of a new signal at 217.9 ppm and the disappearance of the signal at 78.1 ppm, which was assigned to C-3 in betulinic acid, indicating that the 3-hydroxyl group in betulinic acid was oxidized to a ketone in metabolite 3. In addition, the 1H-NMR spectrum of metabolite 3 exhibited a new proton signal at 3.88 ppm (1H, dt, J = 4.4, 10.8 Hz). Furthermore, the DEPT spectra of metabolite 3 showed the presence of a new CH signal at 69.4 ppm and the disappearance of a CH2 signal, which confirmed that metabolite 3 was a hydroxylated metabolite of betulinic acid. The new CH signal at 69.4 ppm in the metabolite 3 spectrum was assigned to C-11 based on one-bond and long-range 1H-13C correlation experiments. In these experiments, a one-bond correlation was observed between C-11 and the new proton signal at 3.88 ppm, which was assigned to H-11 based on NOESY data. A two-bond correlation between C-11 and H-9 was also observed.
The relative stereochemistry of the hydroxyl group at C-11 in metabolite 3 was established as alpha (equatorial) based on NOESY experiments. The NOESY spectrum of metabolite 3 revealed enhancements between H-11 and protons H-1equa, CH3-25, and CH3-26, indicating that H-11 must be beta (axial), as shown in Fig. 1. Based on all the evidence, metabolite 3 was identified as 3-oxo-11α-hydroxy-lup-20(29)-en-28-oic acid.
The high-resolution mass spectrum of metabolite 4 showed an [M + Na]+ ion at m/z 493.3294. The IR spectrum of metabolite 4 revealed absorption at 3,430 cm−1 (OH), 1,701 cm−1 (ketone), and 1,690 cm−1 (carboxylic acid). Similar to the 13C-NMR spectra of metabolites 2 and 3, the 13C-NMR spectrum of metabolite 4 showed the presence of a new signal at 215.7 ppm and the disappearance of the signal at 78.1 ppm, which was assigned to C-3 in betulinic acid, indicating the presence of a ketone at C-3 in metabolite 4. In addition, the DEPT spectra of metabolite 4 showed the presence of a new CH signal at 79.4 ppm and the disappearance of a CH2 signal. The new CH signal at 79.4 ppm was assigned to C-1 based on 1H-13C correlation experiments. A one-bond correlation was observed between C-1 and the new proton signal at 4.09 to 4.06 ppm, which was assigned to H-1 based on NOESY data.
The relative stereochemistry of the hydroxyl group at C-1 in metabolite 4 was established as beta (equatorial) based on NOESY measurements. The NOESY spectrum of metabolite 4 revealed enhancements between H-1 and H-2equa, H-5, and H-9, confirming that the stereochemistry of the hydroxyl group at C-1 is beta (equatorial), as shown in Fig. 1. Based on all these observations, metabolite 4 was identified as 1β-hydroxy-3-oxo-lup-20(29)-en-28-oic acid.
The high-resolution mass spectrum of metabolite 5 showed an [M + Na]+ ion at m/z 511.3396, indicating that two oxygen atoms were added to the structure of betulinic acid. Compared to the 1H-NMR spectrum of betulinic acid, the 1H-NMR spectrum of metabolite 5 exhibited two new proton signals at 4.67 ppm (1H, dd, J = 4.4, 10.8 Hz) and 4.19 ppm (1H, dd, J = 4.4, 10.4 Hz). In addition, the DEPT spectra of metabolite 5 revealed the presence of two new CH signals at 68.5 and 72.5 ppm and the disappearance of two CH2 signals, confirming that metabolite 5 was a dihydroxylated metabolite of betulinic acid. The new carbon signals at 72.5 and 68.5 ppm in metabolite 5 were assigned to C-7 and C-15, respectively, based on one-bond and long-range (two- and three-bond)1H-13C correlation experiments. In these experiments, a one-bond correlation was observed between C-7 and the new proton signal at 4.19 ppm, which was assigned to H-7 based on NOESY measurements. Two- and three-bond correlations were also observed between C-7 and protons H-9, CH3-26, and H-6. In addition, the new proton signal at 4.67 ppm, which was assigned to H-15 based on NOESY data, showed a one-bond correlation with C-15. Furthermore, two- and three-bond correlations were observed between C-15 and protons H-13, CH3-27, and H-16.
The relative stereochemistries of the hydroxyl groups at C-7 and C-15 in metabolite 5 were established as beta (equatorial) and alpha (equatorial), respectively, based on NOESY data. The NOESY spectrum of metabolite 5 showed enhancements between H-7 and protons H-5, H-9, and CH3-27, indicating that H-7 must be alpha (axial). In addition, enhancements were observed between H-15 and protons H-13, H-16equa, and CH3-26, confirming that H-15 is axial (beta). Based on all the evidence, metabolite 5 was identified as 3β,7β,15α-trihydroxy-lup-20(29)-en-28-oic acid, as shown in Fig. 1.
The in vitro cytotoxicities of betulinic acid and metabolites 2 to 5 were evaluated with two human melanoma cell lines, Mel-1 (lymph node) and Mel-2 (pleural fluid). The ED50s of betulinic acid and metabolites 2 to 5 for Mel-1 and Mel-2 are listed in Table 3. Compared to betulinic acid, metabolites 3 to 5 showed no activity against Mel-1. In addition, metabolite 5 was not active against Mel-2. Metabolites 2 to 4 were, however, more active than the parent compound (betulinic acid) against Mel-2. Furthermore, metabolite 2 was less active than betulinic acid against Mel-1. The cytotoxicity results indicate that the in vitro antimelanoma activity of betulinic acid is significantly affected by oxidation at different sites of the molecule. These results also demonstrate that the structural requirements for cytotoxicity in betulinic acid and its metabolites differ depending on the type of melanoma cell line used.
DISCUSSION
Betulinic acid is a promising new lead compound for use against human melanoma. The main objective of our research efforts is to study the mammalian metabolism of betulinic acid by utilizing microorganisms as in vitro model systems to predict and prepare potential mammalian metabolites of this compound. Using microorganisms as models to mimic mammalian metabolic processes provides insights into the metabolic pathways, mechanisms of action, toxicities, and pharmacological activities of drugs and other xenobiotics (9, 10, 25, 27, 28). The microbial metabolites of betulinic acid generated were evaluated for antimelanoma activity and will serve as reference standards to facilitate identification of the metabolites of betulinic acid in mammalian systems.
In the present study, we developed the bacterium B. megaterium ATCC 13368 as a microbial model to study the mammalian metabolism of betulinic acid. B. megaterium ATCC 13368 is known to produce the steroid-15β-monooxygenase cytochrome P450meg and has been studied extensively as a microbial model system for cytochrome P450-dependent hydroxylation reactions (2–4). Preparative-scale biotransformation of betulinic acid with resting-cell suspensions of B. megaterium ATCC 13368 resulted in isolation and structural elucidation of four metabolites, metabolites 2 to 5. A time course TLC analysis of the transformation of betulinic acid by B. megaterium revealed that metabolite 2 was the first metabolite to be formed and was detected on the third day following the addition of betulinic acid to B. megaterium resting-cell suspensions. Metabolites 3 to 5 were detected 5 days following the addition of the substrate. Furthermore, incubation of B. megaterium with metabolite 2 resulted in the production of metabolites 3 and 4, confirming that betulinic acid is biotransformed first to metabolite 2, which is then converted to metabolites 3 and 4. A proposed metabolic pathway for betulinic acid in B. megaterium ATCC 13368 is shown in Fig. 1.
A positive correlation between the enzymatic activity and the amount of cell mass in a B. megaterium culture has been reported in the literature (3). In an effort to maximize the enzymatic activity for biotransformation of betulinic acid, the growth of B. megaterium ATCC 13368 in several formulations of the culture medium was investigated. These studies indicated that a complex medium supplemented with 3 g of beef extract per 1,000 ml provided the best growth conditions for this organism and generated the largest amount of cell mass. Cell mass in the B. megaterium culture was monitored by determining the optical density at 700 nm of the culture broth over a period of 4 days, which reached a maximum of 1.3 to 1.4 after 50 h. Based on these observations, the two-stage fermentation protocol for B. megaterium was conducted in a beef extract-enriched complex medium. The cultures were incubated on the shaker until the optical density of the culture broth reached 1.4, which corresponds to the maximum amount of cell mass that can be produced under these conditions. The cells were then harvested and resuspended in buffer (2 g [wet mass]/200 ml) for preparative-scale bioconversion of betulinic acid.
The use of whole-cell (resting-cell) suspensions for preparative-scale bioconversion of betulinic acid by B. megaterium was advantageous. The biotransformation of betulinic acid by B. megaterium resulted in a low yield of metabolites, which necessitated the development and optimization of crisp and reproducible incubation, extraction, and chromatographic procedures for isolation and purification of metabolites 2 to 5. For resting-cell suspensions, phosphate buffer is a much cleaner incubation medium than the complex culture medium used for growing cultures, and it eliminates any interference of the incubation medium with the bioavailability of the substrate and/or extraction of metabolites. As a result, incubation of betulinic acid with resting-cell suspensions of B. megaterium in phosphate buffer resulted in cleaner reaction mixtures and made analysis and isolation of metabolites 2 to 5 much simpler.
Betulinic acid has been reported to be a selective inhibitor of human melanoma in cell culture and animal models (24). It has been shown that the cytotoxicity of betulinic acid is mediated by the induction of apoptosis and that the free carboxylic acid group in this compound is essential for its cytotoxic activity against melanoma (6, 14, 24, 26). Independent studies conducted by different research groups, including the National Cancer Institute, indicated that betulinic acid is not active against a variety of other human cancer models, such as COL-2 (colon), A431 (squamous), BC-1 (breast), LNCaP (prostate), Walker 256 murine carcinosarcoma, and L1210 murine lymphocytic leukemia models (24). The lack of activity against other cancer models further confirmed that betulinic acid is selectively toxic for human melanoma. Based on these findings, we elected to evaluate the cytotoxicities of metabolites 2 to 5 only with melanoma cell lines.
The in vitro cytotoxicities of betulinic acid and metabolites 2 to 5 were evaluated with two human melanoma cell lines, Mel-1 (lymph node) and Mel-2 (pleural fluid). The results of the cytotoxicity assay (Table 3) show that there were significant changes in the antimelanoma activity of betulinic acid as a result of metabolism at different sites of the molecule. In addition, metabolites which showed considerable activity against Mel-2 were not active against Mel-1, indicating that structure-activity relationships differ from one melanoma cell line to another. In both Mel-1 and Mel-2, hydroxylation of betulinic acid in the vicinity of the free carboxylic acid group, such as the hydroxylation of C-7 and C-15 in metabolite 5, resulted in a loss of activity, whereas a significant increase in activity against Mel-2 was observed as a result of oxidation at C-3, C-1, and C-11. For Mel-1, the cytotoxicity of betulinic acid was substantially attenuated following oxidation at C-1, C-3, and C-11. These findings highlight the importance of these regions of the molecule for antimelanoma activity.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant CA-74244 from the National Cancer Institute. Funding obtained from the Louisiana Education Quality Support Fund and the University of Louisiana at Monroe for the purchase of the 400-MHz NMR spectrometer is greatly appreciated.
REFERENCES
- 1.Ahmad V U, Rahman A U. Pentacyclic triterpenoids. New York, N.Y: Elsevier; 1994. [Google Scholar]
- 2.Berg A, Gustafsson J-A, Ingelman-Sundberg M. Characterization of cytochrome P-450-dependent steroid hydroxylase system present in Bacillus megaterium. J Biol Chem. 1976;251:2831–2838. [PubMed] [Google Scholar]
- 3.Berg A, Ingelman-Sundberg M, Gustafsson J-A. Characterization of cytochrome P-450meg. J Biol Chem. 1979;254:5264–5271. [PubMed] [Google Scholar]
- 4.Berg A, Rafter J J. Studies on the substrate specificity and inducibility of cytochrome P-450meg. Biochem J. 1981;196:781–786. doi: 10.1042/bj1960781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter R C, Sotheeswaran S, Sultanbawa M U S, Ternai B. 13C NMR studies of some lupane and taxarane triterpenes. Org Magn Reson. 1980;6:462–465. [Google Scholar]
- 6.Chatterjee P, Pezzuto J M, Kouzi S A. Glucosidation of betulinic acid by Cunninghamella species. J Nat Prod (Lloydia) 1999;62:761–763. doi: 10.1021/np980432b. [DOI] [PubMed] [Google Scholar]
- 7.Clark A M, Hufford C D. The use of microorganisms for the study of drug metabolism: an update. Med Res Rev. 1991;11:473–501. doi: 10.1002/med.2610110503. [DOI] [PubMed] [Google Scholar]
- 8.Cole B J W, Bentley M D. Triterpenoid extractives in the outer bark of Betula lenta (black birch) Holzforschung. 1991;45:265–268. [Google Scholar]
- 9.Davis P J. Microbial transformations: models of mammalian metabolism and catalysts in synthetic medicinal chemistry. In: Lamba S S, Walker C A, editors. Antibiotics and microbial transformations. Boca Raton, Fla: CRC Press; 1987. pp. 47–70. [Google Scholar]
- 10.Davis P J. Microbial models of mammalian drug metabolism. Dev Ind Microbiol. 1988;29:197–219. [Google Scholar]
- 11.Evers M, Poujade C, Solers F. Betulinic acid derivatives: a new class of human immunodeficiency virus type I specific inhibitors with a new mode of action. J Med Chem. 1996;39:1056–1068. doi: 10.1021/jm950670t. [DOI] [PubMed] [Google Scholar]
- 12.Fujioka T, Kashiwada Y, Kilkuskie R E, Cosentino L M, Ballas L M, Jiang J B, Janzen W P, Chen I S, Lee K H. Anti-AIDS agents. 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod (Lloydia) 1994;57:243–247. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 13.Kashiwada Y, Hashimoto F, Cosentino L M, Lee K H. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem. 1996;39:1016–1017. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 14.Kim D S H L, Pezzuto J M, Pisha E. Synthesis of betulinic acid derivatives with activity against human melanoma. Bioorg Med Chem Lett. 1998;8:1707–1712. doi: 10.1016/s0960-894x(98)00295-9. [DOI] [PubMed] [Google Scholar]
- 15.Kouzi S A, McChesney J D. Hydroxylation and glucoside conjugation in the microbial metabolism of the diterpene sclareol. Xenobiotica. 1991;10:1311–1323. doi: 10.3109/00498259109043206. [DOI] [PubMed] [Google Scholar]
- 16.Kouzi S A, McChesney J D, Walker L A. Identification of four biliary metabolites of the diterpene sclareol in the laboratory rat. Xenobiotica. 1993;6:621–632. doi: 10.3109/00498259309059400. [DOI] [PubMed] [Google Scholar]
- 17.Kurokawa M, Basnet P, Ohsugi M, Hozumi T, Kadota S, Namba T, Kawana T, Shiraki K. Anti-herpes simplex virus activity of moronic acid purified from Rhus javanica in vitro and in vivo. J Pharmacol Exp Ther. 1999;1:72–78. [PubMed] [Google Scholar]
- 18.Ma J, Starck S R, Hecht S M. DNA polymerase β inhibitors from Tetracera boiviniana. J Nat Prod (Lloydia) 1999;62:1660–1663. doi: 10.1021/np990326p. [DOI] [PubMed] [Google Scholar]
- 19.Maurya S K, Devi S, Pandey V B. Content of betulin and betulinic acid, antitumor agents of Zizyphus species. Fitoterapia. 1989;5:468–469. [Google Scholar]
- 20.Mayaux J F, Bousseau A, Panwels R, De Clerq E, Pecq J B. Triterpene derivatives that block the entry of human immunodeficiency virus type I into cells. Proc Natl Acad Sci USA. 1994;91:3564–3568. doi: 10.1073/pnas.91.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura Y, Fulco A J. ω-1, ω-2 and ω-3 hydroxylation of long-chain fatty acids, amides and alcohols by a soluble enzyme system from Bacillus megaterium. Biochim Biophys Acta. 1975;388:305–317. doi: 10.1016/0005-2760(75)90089-2. [DOI] [PubMed] [Google Scholar]
- 22.Narhi L O, Fulco A J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986;261:7160–7169. [PubMed] [Google Scholar]
- 23.Narhi L O, Fulco A J. Identification and characterization of two functional domains in cytochrome P-450BM-3, a catalytically self-sufficient monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1987;262:6683–6690. [PubMed] [Google Scholar]
- 24.Pisha E, Chai H, Lee I, Chagwedera T E, Farnsworth N R, Cordell G A, Beecher C W W, Fong H H S, Kinghorn A D, Brown D M, Wani M C, Wall M E, Hieken T J, Das Gupta T K, Pezzuto J M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 25.Reighard T B, Knapp J E. Microbial models of mammalian metabolism. Pharmacy Int. 1986;7:92–94. [Google Scholar]
- 26.Ryu S Y, Choi S U, Lee S H, Lee C O, No Z, Ahn J W. Antitumor triterpenes from medicinal plants. Arch Pharm Res (Seoul) 1994;17:375–377. [Google Scholar]
- 27.Smith R V, Acosta D, Rosazza J P. Cellular and microbial models of investigation of mammalian metabolism of xenobiotics. Adv Biochem Eng. 1977;5:70–100. [Google Scholar]
- 28.Smith R V, Rosazza J P. Microbial transformations as a means of preparing mammalian drug metabolites. In: Rosazza J P, editor. Microbial transformations of bioactive compounds. Vol. 2. Boca Raton, Fla: CRC Press; 1982. pp. 1–42. [Google Scholar]
- 29.Solichin Z M, Yamasaki K, Kasai R, Tanaka O. 13C nuclear magnetic resonance of lupane-type triterpenes, lupeol, betulin and betulinic acid. Chem Pharm Bull (Tokyo) 1980;28:1006–1008. [Google Scholar]