Abstract
The second Kidney Cancer Research Summit was held virtually in October 2020. The meeting gathered worldwide experts in the field of kidney cancer, including basic, translational, and clinical scientists as well as patient advocates. Novel studies were presented, addressing areas of unmet need related to different topics. These include novel metabolic targets, promising immunotherapeutic regimens, predictive genomic and transcriptomic biomarkers, and variant histologies of renal cell carcinoma (RCC). With the development of pioneering technologies, and an unprecedented commitment to kidney cancer research, the field has tremendously evolved. This perspective aims to summarize the different sessions of the conference, outline major advances in the understanding of RCC and discuss current challenges faced by the field.
Statement of translational relevance:
With multiple translational and clinical advances, the field of kidney cancer research is rapidly evolving. However, many significant challenges remain to be overcome in order to ensure better outcomes for patients with advanced and/or rarer forms of the disease. In this Perspective, we highlight the most relevant research presented at the second Kidney Cancer Research Summit with a focus on translational science and ensuing therapeutic implications. We explore novel targets and promising immunotherapeutic modalities in renal cell carcinoma, and analyze the role of different biomarkers and the biology of variant disease histologies. Additionally, future research directions in renal cell carcinoma are discussed in an effort to tackle areas of unmet need.
Kidney cancer is one of the most common cancers and it is estimated that in 2020, more than 75,000 cases were diagnosed, the majority being renal cell carcinomas (RCC), causing over 13,000 deaths in the United States (1). Recent years have seen remarkable advances in the understanding of genomic drivers and pathogenesis of RCC (2,3), especially within the most common histologic subtype, clear cell RCC (ccRCC). These insights paved the way for the introduction of many systemic therapies for advanced/metastatic disease, most importantly angiogenesis inhibitors, including tyrosine kinase inhibitors (TKIs) (4) and anti-VEGF monoclonal antibodies (5), as well as immune checkpoint inhibitors (ICIs) (6).
The standard of care for the first-line treatment of metastatic RCC is currently based on TKI-ICI or dual ICI combinations (7). While these combination regimens have yielded substantial survival benefits and durable responses, complete responses remain relatively rare and most patients eventually progress. Further improving the outcomes of patients with RCC will only be possible through novel target discovery, therapeutic development, and improved biomarker-based therapeutic allocation. These clinical and translational challenges require advances in the laboratory, where more representative RCC models are actively being developed. These challenges are even more pronounced for variant histologic subtypes of RCC, management of which lags that of ccRCC. Recently, an improved understanding of the molecular drivers of these diseases has begun to lead to improved treatment options for patients with these tumors (8-10).
In order to overcome the unique challenges posed by kidney cancer, the second annual Kidney Cancer Research Summit (KCRS) in October 2020 brought together researchers from the basic, translational, and clinical fields, along with patient advocates in order to stimulate interdisciplinary discussion on the most exciting and novel research for kidney cancer (Figure 1) and build on discussions from the preceding year’s meeting (11). While the COVID-19 pandemic prevented an in-person meeting, KCRS 2020 was attended virtually by more than 800 registrants from more than 40 countries and featured a keynote lecture by 2019 Nobel Laureate Dr. William Kaelin, Jr “Can Studies of the VHL Gene Get Us to Curative Combination Therapies for Kidney Cancer?”. This White Paper aims to summarize the lessons learned, outline the most exciting advances, and determine the outstanding challenges in the field of kidney cancer research, in light of new drug approvals and research advances that have occurred following the conclusion of this meeting (Table 1).
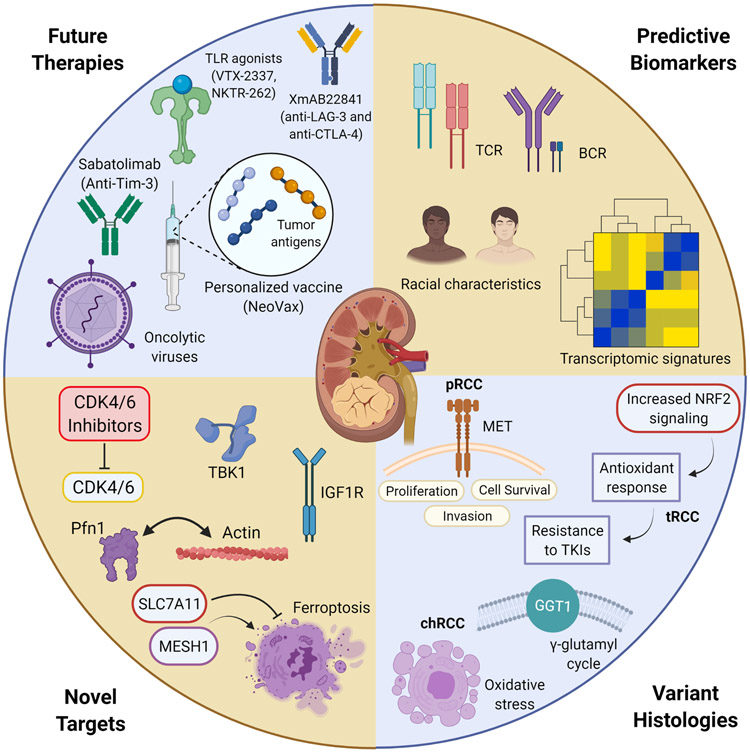
Figure 1: Subject areas covered in the different sessions at the Kidney Cancer Research Summit (KCRS) 2020.
Abbreviations: BCR: B-cell receptor ; chRCC: chromophobe renal cell carcinoma ; GGT1: gamma-glutamyl transferase 1 ; Pfn1: profilin 1 ; pRCC: papillary renal cell carcinoma ; TLR: toll-like receptor ; tRCC: translocation renal cell carcinoma
Table 1:
Summary of the progress, challenges and innovative strategies in the field of renal cell carcinoma (RCC) outlined during the Kidney Cancer Research Summit (KCRS) 2020.
| Progress (addressed at KCRS 2020) | Ongoing challenges | Innovative strategies |
|---|---|---|
|
|
|
Abbreviations: BCR: B-cell receptor ; ccRCC: clear cell renal cell carcinoma ; TCR: T-cell receptor ; scRNA-seq: single-cell RNA sequencing ; scTCR-seq: single-cell T-cell receptor sequencing
Promising Novel Targets and Drugs in Renal Cell Carcinoma
Therapies targeting VEGF, mTOR, PD-1, and CTLA-4 signaling have become part of the standard of care for RCC as either monotherapies or combinations, with clinical trials aiming to further refine regimens currently ongoing (e.g. COSMIC-313 [NCT03937219]; PDIGREE [NCT03793166]; [NCTO4736706]). While these therapies have yielded prolonged remissions in up to 30% of patients (12-14), it is likely that novel therapeutic targets need to be explored to achieve sustained remissions more frequently.
HIF-2α, a transcription factor that is a key driver of ccRCC, is one such novel target that is currently in clinical development (2). HIF-2α, a transcription factor, was long thought to be undruggable, but structure-based drug discovery efforts provided small molecule inhibitors that prevent its activity by blocking its dimerization with HIF-1β (2,15). HIF-2α inhibitors have demonstrated promising efficacy in both sporadic and Von Hippel-Lindau syndrome-associated ccRCC in clinical trials (16-19). The HIF-2α inhibitor belzutifan was recently approved for the treatment of malignancies associated with VHL syndrome and is currently being tested as a single agent in a phase III trial for the treatment of sporadic ccRCC [NCT04195750] (20) or in combination with immune checkpoint inhibitors, and/or TKIs ([NCT04736706], [NCT04586231]) (21).
Metabolic targets in RCC are in earlier stages of validation (Table 2). These include MESH1, a cytosolic NADPH phosphatase which has been shown to induce cystine deprivation leading to ferroptosis (i.e. iron-dependent cell death) (22), a cell state associated with programmed necrosis in VHL deficient RCC (23). Likewise, SLC7A11 encodes a cystine transporter that suppresses ferroptosis and is negatively regulated by BAP1 (24,25). As a result, BAP1 mutations lead to a downregulation of ferroptosis and consequent tumor growth (25). As BAP1 is mutated in 15% of ccRCC and is associated with poor prognosis (26), this finding is particularly relevant. Cystine homeostasis mediated through SLC7A11 may prove to be a targetable pathway in BAP1-deficient ccRCC (27).
Table 2:
Summary of novel targets in RCC, with the corresponding drugs and selected clinical trials.
| Molecular Targets | Drugs (when applicable) |
State of Development | Selected Clinical Trials (when applicable) |
|---|---|---|---|
| HIF-2α | Belzutifan (MK-6482) | Clinical* |
NCT04195750 (Phase III) NCT04736706 (Phase III) NCT04586231 (Phase III) |
| MESH1 | - | Preclinical | - |
| SLC7A11 | - | Preclinical | - |
| Profilin 1 | - | Preclinical | - |
| TBK1 | - | Preclinical | - |
| IGF1R | Linsitinib | Preclinical | - |
| CDK4/6 | Palbociclib, Abemaciclib | Clinical | NCT03905889 (Phase Ib) |
| TIM-3 | Sabatolimab (MBG453) | Clinical | NCT02608268 (Phase I/II) |
| LAG-3 | Eftilagimod alpha (IMP321) | Clinical | NCT00351949 (Phase I) |
| XmAB22841 | Clinical | NCT03849469 (Phase I) | |
| TLRs | VTX-2337 (TLR8 agonist) NKTR-262 (TLR7/8 agonist) |
Clinical |
NCT02650635 (Phase Ib) NCT03435640 (Phase I/II) |
| STING pathway | - | Preclinical | - |
Approved in patients with Von-Hippel Lindau (VHL)-associated ccRCC, in clinical development in patients with sporadic ccRCC
Profilin 1 (Pfn1), a protein that plays an important regulatory role in actin polymerization, is overexpressed in ccRCC and associated with more aggressive disease with poor prognosis (28,29). In vitro experiments have shown that Pfn1 is a major regulator of tumor cell proliferation and migration in ccRCC (30). Small molecule antagonists of the Pfn1-actin interaction appeared to significantly reduce tumor cell proliferation in ccRCC (30). TANK-binding kinase 1 (TBK1), a member of the IkB kinase family, is implicated in cell survival, autophagy, mTOR-AKT signaling, and KRAS-driven tumorigenesis (31,32). In ccRCC, VHL loss has been shown to increase TBK1 activity through a hypoxia-independent mechanism, thereby identifying a novel function of VHL not related to HIF (33,34). Pharmacologic inhibition of TBK1 resulted in substantially reduced proliferation of VHL-null RCC models, suggesting therapeutic vulnerability in kidney cancer (33,34). Another promising target in RCC is IGF1R. Decreased expression of methylthioadenosine phosphorylase (MTAP), a frequent metabolic aberration in ccRCC, leads to IGF1R upregulation and is associated with worse outcomes (35). Linsitinib, a selective IGF1R inhibitor, displayed potent inhibition of tumor cell migration and invasion in preclinical models (35), and hence the contribution of IGF1R signaling in RCC progression merits further exploration.
Cyclin-dependent kinases 4 and 6 (CDK4/6), which control progression through cell cycle from G1 to S (36), were recently identified in preclinical models as being synthetically lethal with VHL inactivation in a HIF-independent fashion (37,38). CDK4/6 inhibitors are being studied preclinically in combination regimens with either HIF-2α inhibitors or ICIs (37,39) [APART study, NCT pending] and in phase I/II clinical trials with VEGF inhibitors [NCT03905889].
As multiple cellular or metabolic pathways are explored, it will be crucial to find agents that do not potentiate each other’s toxicities while optimizing antitumor efficacy through synergy, additivity, or independent action (40).
Beyond PD-(L)1 and CTLA-4 inhibition – Translational advances in RCC immunology and preliminary therapeutic implications
RCC is among the most immune-responsive solid malignancies despite having only a moderate tumor mutational burden (TMB) (41). However, a large proportion of patients do not respond robustly to current immunotherapy-based regimens (6,12,14). The next breakthroughs in immunotherapy must address diverse tumor immune profiles and their dynamics to overcome intrinsic and acquired resistance to treatment and achieve better outcomes.
Current immunotherapies used in RCC target negative regulators PD-(L)1 and CTLA-4, and while combination regimens employing these agents have been studied, the search for new immune targets has been pursued in parallel. Other immune checkpoints under investigation include TIM-3, VISTA, LAG-3, PVRIG, and TIGIT (42-46). Recently, therapeutic agents directed against these molecules have been evaluated in phase I/II clinical trials with anti-PD-(L)1 inhibitors in different cancer types, yielding promising results (47-49). Some these agents are currently being investigated in patients with mRCC in early phase I/II clinical trials, such as eftilagimod alpha (IMP321) [NCT00351949], a soluble dimeric recombinant form of LAG-3, sabatolimab (MBG453) [NCT02608268], an anti-TIM-3 monoclonal antibody, and XmAb22841 [NCT03849469], a bispecific antibody targeting LAG-3 and CTLA-4 (Table 2).
Beyond immune checkpoints, another strategy is to inflame the RCC tumor microenvironment through the stimulation of innate immunity, such as by agonism of pattern recognition receptors such as Toll-like receptors and/or the STING pathway (50,51). This requires intratumoral injection of pro-inflammatory agonists, but could be avoided by developing tumor-targeting conjugates that can be delivered systemically in the form of prodrugs. TLR agonists are starting to be evaluated in RCC [NCT02650635, NCT03435640], among other solid malignancies, in phase I/II trials, while STING agonists in RCC remain to be tested in the clinical setting (Table 2). Oncolytic adenoviruses carrying immunostimulatory payloads honed to the kidney are also under preclinical investigation (52).
Personalized immunotherapy is likely to have an important place in future RCC treatment algorithms. Human endogenous retroviruses (hERVs) are transcriptionally silent remnants of past retroviral infections, many of which become aberrantly expressed under hypoxic conditions in RCC (53). hERVs are potentially actionable drug targets: hERV-E was found to be antigenic and to elicit reactive antitumor CD8+ T-cells in a patient with metastatic RCC following allogeneic stem cell transplantation (54), and an autologous T-cell therapy engineered with a T-cell receptor (TCR) targeting hERV-E is in phase I development [NCT03354390]. Additionally, the hypomethylating agent decitabine has been shown to increase hERV expression and activate immune signaling in ccRCC cells, and could also be used to indirectly increase immunogenicity of RCC (55). A more patient-specific approach is personalized cancer vaccines, which are composed of antigens identified from sequencing a patient’s TCR repertoire (56). Preliminary efficacy has been demonstrated in melanoma and glioblastoma (57,58) and studies are currently underway in RCC [NCT02950766].
While sequencing-based analyses of the immune microenvironment can describe the types and activation states of infiltrating immune cells, the spatial interplay between tumor, endothelial, and immune cells - immune infiltration phenotypes - can be evaluated by techniques that preserve the spatial architecture of tumor tissue, such as multiplex immunofluorescence. A potential method involves 3D reconstruction from multiplex immunofluorescence to elucidate the spatial distribution of different cell types in the TME (59). Spatial heterogeneity in tumor samples can be explored in relation to outcomes, and it has been shown that spatial clustering of CD68+ tumor associated macrophages (TAMs) was associated with worse overall survival in patients with metastatic RCC (60). Another possibility uses model systems: recently, a 3D vascularized flow-directed system was developed using cells obtained from ccRCC patients (61). This model is highly relevant as it recapitulates in vitro the major signaling interactions of RCC, providing a useful platform for investigating novel agents (61). Additionally, such models can help to map the spatial distribution of immune cells, and explore cytokines and chemokines involved in immune cell migration. While spatial exploration of RCC cannot be employed in clinical practice, it represents an important modality that could help better unravel the patterns of therapeutic resistance, tumor growth and tumor-immune interactions.
At this juncture, despite promising advances in immunotherapy of RCC, there are many unresolved questions. If TMB is low, which tumor antigens are driving response to immunotherapy? Why doesn’t CD8+ T-cell infiltration correlate with better outcomes? Emerging methods, such as single-cell RNA sequencing (scRNA-seq) and single-cell TCR sequencing (scTCR-seq), which help determine the molecular underpinnings of each specific cell type within the tumor, are starting to be applied to kidney cancer and could help answer some of these questions through the high-resolution offered (62-64), potentially unraveling determinants of response to PD-1 blockade (65).
Biomarkers and genomics
Mutations and copy number alterations associated with response or resistance to anti-PD-1 monotherapy in RCC have been recently described (66-68). In some reports, PBRM1 loss-of-function (LOF) mutations have been linked to response to PD-1 blockade in metastatic ccRCC resistant to VEGF TKIs (67,68). While other studies didn’t fully validate these findings (69,70), subsequent investigations demonstrated the same previously described association (71,72), arguing for the potential role of PBRM1 LOF mutations in predicting outcomes specifically and perhaps exclusively among VEGF-resistant ccRCC patients treated with single-agent ICIs. Focal loss of 10q23.31 also appeared to correlate with better survival outcomes with ICIs in ccRCC (66). Recent analyses of clinical trials investigating ICIs in combination with anti-angiogenesis agents in patients with mRCC (i.e. JAVELIN Renal 101, IMmotion150) have identified transcriptomic signatures associated with better responses to immunotherapy-based regimens (73,74). These signatures included multiple genes related to functions of the tumor immune microenvironment (TIME), such as TCR signaling, T-cell activation and proliferation, and myeloid inflammation, providing a clue for the development of biomarkers (73,74). Additionally, analysis of RNA-seq data from IMmotion151, the phase 3 trial of atezolizumab + bevacizumab versus sunitinib in advanced RCC, classified patients into biologically-relevant clusters in an effort to predict responses to immune checkpoint blockade (75). Certain hERVs have been also characterized and associated with response to checkpoint blockade (76). In a pan-cancer analysis, RCC displayed the highest hERV expression, which was further shown to be strongly associated with immune checkpoint activation (76). With many available systemic agents and multiple therapeutic combinations approved for patients with mRCC, the identification of predictive biomarkers to guide treatment selection remains a high priority for the field. More recently, scRNA-seq analyses in RCC have investigated cell-type specific patterns of response to immunotherapy, and characterized a pro-inflammatory phenotype of TAMs and T-cell subpopulations in responders (63). Additionally, tumor programs linked to better outcomes with ICIs were described using scRNA-seq and validated in bulk RNA-seq cohorts (63,64).
As shown in multiple studies, immunotherapy appears to modify the clonal dynamics of the adaptive immune system (77,78). Importantly, specific features of TCR and B-cell receptor (BCR) repertoires appear to correlate with response to ICIs across different cancer types. In melanoma, higher post-treatment clonality was associated with better survival outcomes (79). Additionally, the expansion of CD8+ memory effector cytotoxic T-cells was seen in responders during treatment (80). In non-small cell lung cancer, decreased TCR and BCR diversity after treatment with ICIs was seen among responders in the EGFR/ALK wild-type group (81). The relationship of TCR and BCR repertoires and response to ICIs in RCC remains to be further evaluated.
Among patients with ccRCC, African-Americans (AAs) have been shown to present with higher cancer-specific mortality (82). At the genomic level, these patients were less likely to harbor VHL mutations and exhibited lower expression of HIF- and VEGF-associated pathways as compared to Caucasians (83). Additionally, comprehensive genomic and transcriptomic analyses showed distinct DNA methylation and mRNA expression profiles in the AA population (84). More studies are still needed to confirm these findings and to analyze the mutational landscape of RCC across ethnicities. Moreover, as genomic racial differences in patients with lung cancer have been recently linked to immunotherapy response (85), the association between race and clinical benefit from VEGF-targeted therapies and ICIs in patients with RCC should be explored.
Cell-free tumor DNA (cfDNA) can help monitor RCC progression and detect minimal residual disease following surgery (86). In other cancer types, it has also been found to be associated with survival endpoints (87), and identify responders to specific therapies (88). Cell-free methylated DNA immunoprecipitation sequencing (cfMeDIP-seq) is a novel, non-invasive technology that was recently validated as a promising approach to detect RCC from blood and urine samples (89) with a much higher sensitivity than cfDNA variant analysis (90). Future efforts could evaluate a potential role of cfMeDIP-seq in predicting responses to commonly used therapeutic regimens in kidney cancer.
While some proposed biomarkers are promising, they will need to be evaluated and validated in prospectively designed clinical trials before they can be utilized in clinical practice (91). A pioneering example of such a study in RCC is the phase 2 BIONIKK prospective trial, which employed a 35-gene expression mRNA signature for treatment arm allocation (92) and found that such signatures may help guide treatment decision-making (93). However, owing to the number of patients required and the costs of sample collection and analysis, these trials are difficult, time-consuming, and expensive to run. Moreover, biomarkers specific to one systemic therapy regimen might not translate to another, which poses a particular challenge in RCC with so many approved agents. The main challenge for the field in the coming years will be to incorporate the most promising biomarkers (80,94,95) that are most likely to be incorporated into clinical practice due to ease of use, such as circulating biomarkers (cfDNA, cfMeDIP-seq, TCR/BCR repertoire), in the design of prospective kidney cancer trials, allowing for optimal selection of patients.
Variant Kidney Cancer Histologies – Characterization & Target Discovery
Variant RCC histologies (also called non-ccRCC) represent a heterogeneous group of cancers with unique genomic alterations and often dismal prognosis, including some variants with a higher proportion of pediatric cases than is seen in ccRCC (3). While discussion of each individual histologic variant is beyond the scope of this perspective, unifying features that limit research include pathologic misclassification at diagnosis, unclear disease etiologies, and a dearth of cell line and animal models. Recently, our understanding of the drivers in some of these diseases has improved.
Multi-region genomic analysis of papillary RCC (pRCC) recently revealed lower intratumor heterogeneity as compared with ccRCC (96). Moreover, many somatic copy number alterations were identified as clonal, helping to better delineate the genomic features of pRCC (96). MET, which encodes a receptor tyrosine kinase involved in proliferation and angiogenesis (97), is frequently activated (through mutation or copy number gain) in pRCC (98). Savolitinib, a selective MET inhibitor, was recently evaluated in MET-driven pRCC, and demonstrated encouraging efficacy (9), outlining the potential for molecularly targeted therapies based on genomic characterization of non-ccRCC tumors.
Chromophobe RCC (chRCC) represents less than 5% of all kidney tumors and appears to be resistant to ICIs (99). A known metabolic alteration underlying the pathogenesis of chRCC is the impairment of gamma-glutamyl transferase 1 (GGT1) (100). Defects in the gamma-glutamyl cycle might have an important role in chRCC pathogenesis, potentially caused by cells with accumulating dysfunctional mitochondria. Additionally, chRCC may be more sensitive to oxidative stress, a vulnerability that could represent a future avenue of investigation (100).
Renal medullary carcinoma (RMC) is a rare disease which disproportionately afflicts young patients of African descent often expressing sickle cell trait (101). Recent molecular characterization of this tumor has shown that it is driven by inactivation of the tumor suppressor gene SMARCB1 and subsequent gain of MYC during disease progression (102). Additionally, DNA damage repair pathways have been characterized as a therapeutic vulnerability with a high sensitivity to PARP inhibitors (i.e. olaparib) identified in preclinical models that should be further explored clinically (102).
Translocation RCC (tRCC) is a rare RCC subtype which nevertheless represents more than 50% of all pediatric RCCs, and is characterized by gene fusions involving the MiT/TFE gene family (TFE3, TFEB, or MITF) (103). tRCC tumors have a “quiet” mutational landscape as compared to other subtypes of RCC (104,105), and an increased expression of genes related to regulation of apoptosis, lysosomal function (mTORC1 signaling), and antioxidant stress response (105-107). tRCC display elevated NRF2 activity without harboring any somatic alterations in the associated pathway, which could be driving resistance to targeted therapies (106). These insights into the drivers of this disease could lead to novel molecularly-targeted therapies.
Recently, pembrolizumab monotherapy and combination therapy with nivolumab and cabozantinib demonstrated promising antitumor efficacy in patients with non-ccRCC (108,109). Other ICIs, as well as novel immunotherapy drugs, are currently under investigation in variant histologies of RCC [NCT03075423, NCT04413123, NCT04704219]. Similar to ccRCC, immune-based combinations with other targeted agents in these rarer subtypes of kidney cancer, such as MET inhibitors in MET-driven pRCC (110), are being evaluated [NCT02819596, NCT05043090] and could offer better outcomes for patients (111).
Conclusion
From basic to clinical research, KCRS 2020 represented a unique opportunity for researchers and physicians to exchange ideas and tackle the biggest challenges in kidney cancer research. While much progress has been made in recent years in the management of patients with RCC (Table 1) (11), much work remains to be done in order to obtain more durable remissions in a larger proportion of patients with metastatic RCC.
Acknowledgments:
The authors thank the speakers and attendees of the symposium; the Department of Defense Kidney Cancer Research Program, on which many of the projects presented at this conference were based; and KidneyCAN and their volunteers for organizational support.
References:
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. Wiley; 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK, Kaelin WG. Targeting the HIF2–VEGF axis in renal cell carcinoma. Nat. Med Nature Research; 2020. page 1519–30. [DOI] [PubMed] [Google Scholar]
- 3.Albiges L, Flippot R, Rioux-Leclercq N, Choueiri TK. Non–Clear Cell Renal Cell Carcinomas: From Shadow to Light. J Clin Oncol. 2018;36:3624–31. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczack P, Michaelson D, Bukowksi RM, Rixe O, et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2005;687–96. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A Randomized Trial of Bevacizumab, an Anti–Vascular Endothelial Growth Factor Antibody, for Metastatic Renal Cancer. N Engl J Med. 2003;687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Cancer - NCCN Clinical Practice Guidelines 2020. NCCN Evid. Blocks. 2020. [Google Scholar]
- 8.Bakouny Z, Braun DA, Shukla SA, Pan W, Gao X, Hou Y, et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun. Nature Publishing Group; 2021;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri TK, Heng DYC, Lee JL, Cancel M, Verheijen RB, Mellemgaard A, et al. Efficacy of Savolitinib vs Sunitinib in Patients With MET-Driven Papillary Renal Cell Carcinoma: The SAVOIR Phase 3 Randomized Clinical Trial. JAMA Oncol. American Medical Association; 2020;6:1247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan R, Gurram S, Al Harthy M, Singer EA, Sidana A, Shuch BM, et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J Clin Oncol. American Society of Clinical Oncology (ASCO); 2020;38:5004–5004. [Google Scholar]
- 11.Choueiri TK, Atkins MB, Bakouny Z, Carlo MI, Drake CG, Jonasch E, et al. Summary From the First Kidney Cancer Research Summit, September 12–13, 2019: A Focus on Translational Research. JNCI J Natl Cancer Inst. 2020;113:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, McDermott DF, Frontera OA, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. BMJ Specialist Journals; 2020;8:e000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BI Rini, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. Massachusetts Medical Society; 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 14.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. Massachusetts Medical Society; 2021;384:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheuermann TH, Li Q, Ma H-W, Key J, Zhang L, Chen R, et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. Nature Publishing Group; 2013;9:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan V, Maughan BL, et al. Phase II study of the oral HIF-2α inhibitor MK-6482 for Von Hippel-Lindau disease–associated renal cell carcinoma. J Clin Oncol. American Society of Clinical Oncology (ASCO); 2020;38:5003–5003. [Google Scholar]
- 17.Choueiri TK, Bauer TM, Papadopoulos KP, Plimack ER, Merchan JR, McDermott DF, et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med. Nature Publishing Group; 2021;27:802–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courtney K, Infante J, Lam E, Figlin R, Rini B, Brugarolas J, et al. Phase I Dose-Escalation Trial of PT2385, a First-in-Class Hypoxia-Inducible Factor-2α Antagonist in Patients With Previously Treated Advanced Clear Cell Renal Cell Carcinoma. J Clin Oncol. J Clin Oncol; 2018;36:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rini BI, Appleman LJ, Figlin RA, Plimack ER, Merchan JR, Wang K, et al. Results from a phase I expansion cohort of the first-in-class oral HIF-2α inhibitor PT2385 in combination with nivolumab in patients with previously treated advanced RCC. J Clin Oncol. American Society of Clinical Oncology; 2019;37:558–558. [Google Scholar]
- 20.Choueiri TK, Albiges L, Fan L, Perini RF, Zojwalla NJ, Powles T, et al. Phase III study of the hypoxia-inducible factor 2α (HIF-2α) inhibitor MK-6482 versus everolimus in previously treated patients with advanced clear cell renal cell carcinoma (ccRCC). J Clin Oncol. American Society of Clinical Oncology (ASCO); 2020;38:TPS5094–TPS5094. [Google Scholar]
- 21.Motzer RJ, Liu Y, Perini RF, Zhang Y, Heng DYC. Phase III study evaluating efficacy and safety of MK-6482 + lenvatinib versus cabozantinib for second- or third-line therapy in patients with advanced renal cell carcinoma (RCC) who progressed after prior anti-PD-1/L1 therapy. J Clin Oncol. American Society of Clinical Oncology; 2021;39:TPS372–TPS372. [Google Scholar]
- 22.Ding CKC, Rose J, Sun T, Wu J, Chen PH, Lin CC, et al. MESH1 is a cytosolic NADPH phosphatase that regulates ferroptosis. Nat Metab. Springer US; 2020;2:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang X, Wu J, Ding C, Lu M, Keenan M, Lin C, et al. Cystine Deprivation Triggers Programmed Necrosis in VHL-Deficient Renal Cell Carcinomas. Cancer Res. Cancer Res; 2016;76:1892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. Nature Publishing Group; 2018;20:1181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Zhuang L, Gan B. BAP1 suppresses tumor development by inducing ferroptosis upon SLC7A11 repression. Mol Cell Oncol. Taylor & Francis; 2019;6:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. Nature Publishing Group; 2012;44:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. Nature Publishing Group; 2020;31:107–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minamida S, Iwamura M, Kodera Y, Kawashima Y, Ikeda M, Okusa H, et al. Profilin 1 overexpression in renal cell carcinoma. Int J Urol. Int J Urol; 2011;18:63–71. [DOI] [PubMed] [Google Scholar]
- 29.Karamchandani J, Gabril M, Ibrahim R, Scorilas A, Filter E, Finelli A, et al. Profilin-1 expression is associated with high grade and stage and decreased disease-free survival in renal cell carcinoma. Hum Pathol. Hum Pathol; 2015;46:673–80. [DOI] [PubMed] [Google Scholar]
- 30.Allen A, Gau D, Francoeur P, Sturm J, Wang Y, Martin R, et al. Actin-binding protein profilin1 promotes aggressiveness of clear-cell renal cell carcinoma cells. J Biol Chem. 2020;295:15636–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu L, Li Y, Xie X, Zhou X, Gu M, Jie Z, et al. TBKBP1 and TBK1 form a growth factor signalling axis mediating immunosuppression and tumourigenesis. Nat Cell Biol. Nat Cell Biol; 2019;21:1604–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durand JK, Zhang Q, Baldwin AS. Roles for the IKK-Related Kinases TBK1 and IKKε in Cancer. Cells. Multidisciplinary Digital Publishing Institute; 2018;7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu L, Xie H, Liu X, Potjewyd F, James L, Wilkerson E, et al. TBK1 Is a Synthetic Lethal Target in Cancer with VHL Loss. Cancer Discov. Cancer Discov; 2020;10:460–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakouny Z, Barbie D. TBK1 Activation by VHL Loss in Renal Cell Carcinoma: A Novel HIF-Independent Vulnerability. Cancer Discov. Cancer Discov; 2020;10:348–50. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Chang WH, Fong LWR, Weiss RH, Yu SL, Chen CH. Targeting the insulin-like growth factor-1 receptor in MTAP-deficient renal cell carcinoma. Signal Transduct Target Ther. Springer US; 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. Nature Publishing Group; 2009;9:153–66. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson HE, Tariq Z, Housden BE, Jennings RB, Stransky LA, Perrimon N, et al. HIF-independent synthetic lethality between CDK4/6 inhibition and VHL loss across species. Sci Signal. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison SJ. Synthetic lethality between loss of CDK4/6 activity and VHL inactivation. Nat Rev Nephrol. Nature Publishing Group; 2019;16:2–2. [DOI] [PubMed] [Google Scholar]
- 39.Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell Rep. Cell Press; 2018;22:2978–94. [DOI] [PubMed] [Google Scholar]
- 40.Palmer AC, Sorger PK. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell. Cell Press; 2017;171:1678–1691.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377:2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lecocq Q, Keyaerts M, Devoogdt N, Breckpot K. The Next-Generation Immune Checkpoint LAG-3 and Its Therapeutic Potential in Oncology: Third Time’s a Charm. Int J Mol Sci. Int J Mol Sci; 2020;22:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alteber Z, Kotturi MF, Whelan S, Ganguly S, Weyl E, Pardoll DM, et al. Therapeutic Targeting of Checkpoint Receptors within the DNAM1 Axis. Cancer Discov. American Association for Cancer Research; 2021;11:1040–51. [DOI] [PubMed] [Google Scholar]
- 44.Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. BMJ Publishing Group; 2020;8:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X, Zhang X, Li E, Zhang G, Wang X, Tang T, et al. VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol 2020 131. BioMed Central; 2020;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braun DA, Bakouny Z, Hirsch L, Flippot R, Van Allen EM, Wu CJ, et al. Beyond conventional immune-checkpoint inhibition — novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol. Nature Publishing Group; 2021;18:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Abreu D, Johnson ML, Hussein MA, Cobo M, Patel AJ, Secen NM, et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol. American Society of Clinical Oncology (ASCO); 2020;38:9503–9503. [Google Scholar]
- 48.Clay TD, Majem M, Felip E, Doger B, Carcereny Costa E, Forster M, et al. Results from a phase II study of eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab in patients with PD-L1 unselected metastatic non-small cell lung carcinoma. J Clin Oncol. American Society of Clinical Oncology (ASCO); 2021;39:9046–9046. [Google Scholar]
- 49.Brana I, Forster M, Lopez-Pousa A, Doger B, Roxburgh P, Bajaj P, et al. Results from a phase II study of eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab in patients with PD-L1 unselected metastatic second-line squamous head and neck carcinoma. J Clin Oncol. American Society of Clinical Oncology (ASCO); 2021;39:6028–6028. [Google Scholar]
- 50.Heidegger I, Pircher A, Pichler R. Targeting the tumor microenvironment in renal cell cancer biology and therapy. Front Oncol. Frontiers Media S.A.; 2019;9:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li K, Qu S, Chen X, Wu Q, Shi M. Promising Targets for Cancer Immunotherapy: TLRs, RLRs, and STING-Mediated Innate Immune Pathways. Int J Mol Sci. Multidisciplinary Digital Publishing Institute; 2017;18:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin JD, Barry MA. Improving Molecular Therapy in the Kidney. Mol Diagnosis Ther. Adis; 2020;24:375–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siebenthall KT, Miller CP, Vierstra JD, Mathieu J, Tretiakova M, Reynolds A, et al. Integrated epigenomic profiling reveals endogenous retrovirus reactivation in renal cell carcinoma. EBioMedicine. Elsevier B.V.; 2019;41:427–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi Y, Harashima N, Kajigaya S, Yokoyama H, Cherkasova E, McCoy JP, et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest. The American Society for Clinical Investigation; 2008;118:1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Cubas AA, Dunker W, Zaninovich A, Hongo RA, Bhatia A, Panda A, et al. DNA hypomethylation promotes transposable element expression and activation of immune signaling in renal cell cancer. JCI Insight. American Society for Clinical Investigation; 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ott PA, Wu CJ. Cancer vaccines: Steering T-cells down the right path to eradicate tumors. Cancer Discov. American Association for Cancer Research Inc.; 2019;9:476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. Nature Publishing Group; 2019;565:240–5. [DOI] [PubMed] [Google Scholar]
- 58.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science (80-). American Association for the Advancement of Science; 2015;348:803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yagi Y, Aly RG, Tabata K, Barlas A, Rekhtman N, Eguchi T, et al. Three-Dimensional Histologic, Immunohistochemical, and Multiplex Immunofluorescence Analyses of Dynamic Vessel Co-Option of Spread Through Air Spaces in Lung Adenocarcinoma. J Thorac Oncol. Elsevier; 2020;15:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakiryan NH, Kimmel GJ, Kim Y, Hajiran A, Aydin AM, Zemp L, et al. Spatial clustering of CD68+ tumor associated macrophages with tumor cells is associated with worse overall survival in metastatic clear cell renal cell carcinoma. PLoS One. Public Library of Science; 2021;16:e0245415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller C, Tsuchida C, Zheng Y, Himmelfarb J, Akilesh S. A 3D Human Renal Cell Carcinoma-on-a-Chip for the Study of Tumor Angiogenesis. Neoplasia. Neoplasia; 2018;20:610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braun DA, Street K, Burke KP, Cookmeyer DL, Denize T, Pedersen CB, et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell. Elsevier BV; 2021;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bi K, He MX, Bakouny Z, Kanodia A, Napolitano S, Wu J, et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell. Cell Press; 2021;39:649–661.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krishna C, DiNatale RG, Kuo F, Srivastava RM, Vuong L, Chowell D, et al. Single-cell sequencing links multiregional immune landscapes and tissue-resident T cells in ccRCC to tumor topology and therapy efficacy. Cancer Cell. Elsevier; 2021;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gohil SH, Iorgulescu JB, Braun DA, Keskin DB, Livak KJ. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat Rev Clin Oncol. Nature Publishing Group; 2020;18:244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braun DA, Hou Y, Bakouny Z, Ficial M, Sant’ Angelo M, Forman J, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. Nature Research; 2020;26:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braun DA, Ishii Y, Walsh AM, Allen Van EM, Wu CJ, Shukla SA, et al. Clinical Validation of PBRM1 Alterations as a Marker of Immune Checkpoint Inhibitor Response in Renal Cell Carcinoma. JAMA Oncol. American Medical Association; 2019;5:1631–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science (80- ). American Association for the Advancement of Science; 2018;359:801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hakimi AA, Attalla K, DiNatale RG, Ostrovnaya I, Flynn J, Blum KA, et al. A pan-cancer analysis of PBAF complex mutations and their association with immunotherapy response. Nat Commun. Nature Publishing Group; 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X-D, Kong W, Peterson CB, McGrail DJ, Hoang A, Zhang X, et al. PBRM1 loss defines a nonimmunogenic tumor phenotype associated with checkpoint inhibitor resistance in renal carcinoma. Nat Commun. Nature Publishing Group; 2020;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Allen EM, Choueiri TK. Dissecting the immunogenomic biology of cancer for biomarker development. Nat Rev Clin Oncol. Nature Publishing Group; 2020;18:133–4. [DOI] [PubMed] [Google Scholar]
- 72.Conway J, Taylor-Weiner A, Braun D, Bakouny Z, Choueiri TK, Van Allen EM. PBRM1 loss-of-function mutations and response to immune checkpoint blockade in clear cell renal cell carcinoma. medRxiv. Cold Spring Harbor Laboratory Press; 2020; [Google Scholar]
- 73.Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med. Springer US; 2020;26:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Motzer RJ, Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, et al. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell. Cell Press; 2020;38:803–817.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Panda A, de Cubas AA, Stein M, Riedlinger G, Kra J, Mayer T, et al. Endogenous retrovirus expression is associated with response to immune checkpoint blockade in clear cell renal cell carcinoma. JCI insight. NLM (Medline); 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. Nature Publishing Group; 2019;25:1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, et al. Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1 – CD8 + Tumor-Infiltrating T Cells. Immunity. Cell Press; 2019;50:181–194.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valpione S, Galvani E, Tweedy J, Mundra PA, Banyard A, Middlehurst P, et al. Immune awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat Cancer. Springer Science and Business Media LLC; 2020;1:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu TD, Madireddi S, de Almeida PE, Banchereau R, Chen Y-JJ, Chitre AS, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. Nature Publishing Group; 2020;579:274–8. [DOI] [PubMed] [Google Scholar]
- 81.Nakahara Y, Matsutani T, Igarashi Y, Matsuo N, Himuro H, Saito H, et al. Clinical significance of peripheral TCR and BCR repertoire diversity in EGFR/ALK wild-type NSCLC treated with anti-PD-1 antibody. Cancer Immunol Immunother. Springer Science and Business Media Deutschland GmbH; 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marchioni M, Harmouch SS, Nazzani S, Bandini M, Preisser F, Tian Z, et al. Effect of African-American race on cancer specific mortality differs according to clear cell vs. non-clear cell histologic subtype in metastatic renal cell carcinoma. Cancer Epidemiol. Elsevier Ltd; 2018;54:112–8. [DOI] [PubMed] [Google Scholar]
- 83.Krishnan B, Rose TL, Kardos J, Milowsky MI, Kim WY. Intrinsic genomic differences between african American and white patients with clear cell renal cell carcinoma. JAMA Oncol. 2016;2:664–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams H, Mitchell KA. Abstract A112: Integrative epigenomic and transcriptomic analyses of kidney cancers from African Americans and European Americans. Cancer Epidemiol Prev Biomarkers. American Association for Cancer Research (AACR); 2020. page A112–A112. [Google Scholar]
- 85.McHayle A, Boles A, Diarra L, Mitchell KA. Abstract A33: Race, DNA methylation, and regulation of cancer immunotherapy response genes: A comparison of non-small cell lung cancers from African Americans and European Americans. Clin Cancer Res. American Association for Cancer Research (AACR); 2018. page A33–A33. [Google Scholar]
- 86.Smith CG, Moser T, Mouliere F, Field-Rayner J, Eldridge M, Riediger AL, et al. Comprehensive characterization of cell-free tumor DNA in plasma and urine of patients with renal tumors. Genome Med. BioMed Central Ltd.; 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye Z, Wang C, Wan S, Mu Z, Zhang Z, Abu-Khalaf MM, et al. Association of clinical outcomes in metastatic breast cancer patients with circulating tumour cell and circulating cell-free DNA. Eur J Cancer. Elsevier Ltd; 2019;106:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baird RD, van Rossum AGJ, Oliveira M, Beelen K, Gao M, Schrier M, et al. POSEIDON trial phase 1B results: Safety, efficacy and circulating tumor DNA response of the beta isoform-sparing PI3K inhibitor taselisib (GDC-0032) combined with tamoxifen in hormone receptor positive metastatic breast cancer patients. Clin Cancer Res. American Association for Cancer Research Inc.; 2019;25:6598–605. [DOI] [PubMed] [Google Scholar]
- 89.Nuzzo PV, Berchuck JE, Korthauer K, Spisak S, Nassar AH, Abou Alaiwi S, et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat Med [Internet]. Springer US; 2020;26:1041–3. Available from: 10.1038/s41591-020-0933-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lasseter K, Nassar AH, Hamieh L, Berchuck JE, Nuzzo PV, Korthauer K, et al. Plasma cell-free DNA variant analysis compared with methylated DNA analysis in renal cell carcinoma. Genet Med. Springer Nature; 2020;22:1366–73. [DOI] [PubMed] [Google Scholar]
- 91.Freidlin B, Korn EL. Biomarker enrichment strategies: matching trial design to biomarker credentials. Nat Rev Clin Oncol. Nature Publishing Group; 2013;11:81–90. [DOI] [PubMed] [Google Scholar]
- 92.Elaisdi RT, Oudard S, Braychenko E, Sun C-M, Sautès-Fridman C, Vano Y-A. A phase 2 BIOmarker driven trial with Nivolumab and Ipilimumab or VEGFR tKi in naïve metastatic Kidney cancer: the BIONIKK trial. Ann Oncol. 2017;28:v295. [DOI] [PubMed] [Google Scholar]
- 93.Vano Y, Elaidi RT, Bennamoun M, Chevreau CM, Borchiellini D, Pannier D, et al. LBA25 Results from the phase II biomarker driven trial with nivolumab (N) and ipilimumab or VEGFR tyrosine kinase inhibitor (TKI) in naïve metastatic kidney cancer (m-ccRCC) patients (pts): The BIONIKK trial. Ann Oncol. Elsevier; 2020;31:S1157. [Google Scholar]
- 94.Powles T, Assaf ZJ, Davarpanah N, Banchereau R, Szabados BE, Yuen KC, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. Nature Publishing Group; 2021;595:432–7. [DOI] [PubMed] [Google Scholar]
- 95.Bortone DS, Woodcock MG, Parker JS, Vincent BG. Improved T-cell Receptor Diversity Estimates Associate with Survival and Response to Anti–PD-1 Therapy. Cancer Immunol Res. American Association for Cancer Research; 2021;9:103–12. [DOI] [PubMed] [Google Scholar]
- 96.Zhu B, Poeta ML, Costantini M, Zhang T, Shi J, Sentinelli S, et al. The genomic and epigenomic evolutionary history of papillary renal cell carcinomas. Nat Commun. Nature Publishing Group; 2020;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. BioMed Central; 2018;17:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis C, et al. Comprehensive Molecular Characterization of Papillary Renal Cell Carcinoma. N Engl J Med. NIH Public Access; 2016;374:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McKay RR, Bosse D, Xie W, Wankowicz SAM, Flaifel A, Brandao R, et al. The clinical activity of PD-1/PD-L1 inhibitors in metastatic non–clear cell renal cell carcinoma. Cancer Immunol Res. 2018;6:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Priolo C, Khabibullin D, Reznik E, Filippakis H, Ogórek B, Kavanagh TR, et al. Impairment of gamma-glutamyl transferase 1 activity in the metabolic pathogenesis of chromophobe renal cell carcinoma. Proc Natl Acad Sci U S A. 2018;115:E6274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Msaouel P, Tannir NM, Walker CL. A model linking sickle cell hemoglobinopathies and smarcb1 loss in renal medullary carcinoma. Clin. Cancer Res American Association for Cancer Research Inc.; 2018. page 2044–9. [DOI] [PubMed] [Google Scholar]
- 102.Msaouel P, Malouf GG, Su X, Yao H, Tripathi DN, Soeung M, et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell. 2020;37:720–734.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kauffman EC, Ricketts CJ, Rais-Bahrami S, Yang Y, Merino MJ, Bottaro DP, et al. Molecular genetics and cellular features of TFE3 and TFEB fusion kidney cancers. Nat. Rev. Urol Nature Publishing Group; 2014. page 465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marcon J, DiNatale R, Sanchez A, Kotecha R, Gupta S, Kuo F, et al. Comprehensive Genomic Analysis of Translocation Renal Cell Carcinoma Reveals Copy-Number Variations as Drivers of Disease Progression. Clin Cancer Res. Clin Cancer Res; 2020;26:3629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malouf GG, Su X, Yao H, Gao J, Xiong L, He Q, et al. Next-Generation Sequencing of Translocation Renal Cell Carcinoma Reveals Novel RNA Splicing Partners and Frequent Mutations of Chromatin-Remodeling Genes. Clin Cancer Res. American Association for Cancer Research; 2014;20:4129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bakouny Z, Sadagopan A, Ravi P, Metaferia NY, Li J, Tang S, et al. Integrative Clinical and Molecular Characterization of Translocation Renal Cell Carcinoma 2. bioRxiv [Internet]. Cold Spring Harbor Laboratory; 2021;2021.04.14.439908. Available from: 10.1101/2021.04.14.439908 [DOI] [Google Scholar]
- 107.Durinck S, Stawiski E, Pavía-Jiménez A, Modrusan Z, Kapur P, Jaiswal B, et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat Genet. Nat Genet; 2015;47:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McDermott DF, Lee J-L, Ziobro M, Suarez C, Langiewicz P, Matveev VB, et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non–Clear Cell Renal Cell Carcinoma. J Clin Oncol. Wolters Kluwer Health; 2021;39:1029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee C-H, Voss MH, Carlo MI, Chen Y-B, Reznik E, Knezevic A, et al. Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: Results of a phase 2 trial. J Clin Oncol. Wolters Kluwer Health; 2021;39:4509–4509. [Google Scholar]
- 110.Rodriguez CS, Larkin J, Patel PM, Valderrama BP, Rodriguez-Vida A, Glen H, et al. Clinical activity of durvalumab and savolitinib in MET-driven, metastatic papillary renal cancer. J Clin Oncol. Wolters Kluwer Health; 2021;39:4511–4511. [Google Scholar]
- 111.de Vries-Brilland M, McDermott DF, Suárez C, Powles T, Gross-Goupil M, Ravaud A, et al. Checkpoint inhibitors in metastatic papillary renal cell carcinoma. Cancer Treat Rev. W.B. Saunders; 2021;99:102228. [DOI] [PubMed] [Google Scholar]