Abstract
Long periods of immobilization, among other etiologies, would result is muscle atrophy. Exercise is the best approach to reverse this atrophy. However, the limited or the non-ability to perform the required physical activity for such patients and the limited pharmacological options make developing novel therapeutic approaches a necessity. Within this context, secreted protein acidic and rich in cysteine (SPARC) has been characterized as an exercise-induced gene. Whereas the knock-out of this gene leads to a phenotype that mimics number of the ageing-induced and sarcopenia-related changes including muscle atrophy, overexpressing SPARC in mice or adding it to muscular cell culture produces similar effects as exercise including enhanced muscle mass, strength and metabolism. Therefore, this piece of writing aims to provide evidence supporting the potential use of SPARC/SPARC as a molecular therapy for muscle atrophy in the context of immobilization especially for elderly patients.
Keywords: SPARC, muscle atrophy, immobilization, ageing
The increased number of hospitalized individuals lead to the development of various fields aiming to improve and optimize the healthcare within hospitals [1,2,3,4,5]. Patients admitted to hospitals have, beside treating the reasons of their admission, also to face other challenges such as possible nosocomial infections [6], bedsores [7,8] and musculoskeletal atrophy. Furthermore, post-hospitalization recovery of the mobility remains a challenge due to the immobilization (bed rest)-induced muscle atrophy. Such bed resting (immobilization) does not only lead to muscle atrophy, but also reduces both muscle strength as well as key regulators of mitochondrial biogenesis/remodeling and activity; it also alters genes expression and leads to metabolic decline including insulin resistance [9,10,11,12]. Bed resting also impacts bones and reduces their mineral density [13]. Cardiovascular complications and cardiac atrophy have also been reported following bed rest [14,15]. The consequences on the locomotor system impact the mass, the strength and the metabolism. Thus, patients, especially elderly people, have a difficulty to return to normal life after a certain period of bed rest caused by hospitalization or immobilization mainly because of muscle atrophy. In addition, ageing reduces both myogenesis [16] and skeletal muscle stem cells regenerative capacity [17]. Ageing also has specific genes expression signature [18,19] and shares numerous patterns with obesity such as epigenetic changes, inflammation and metabolic impairments [20]. These elements show the seriousness of the clinical outcomes of combining immobilization and ageing. The increased hospitalization rate represents one of the features of the current ongoing COVID-19 pandemic especially among the elderly patients who are already vulnerable. Intensive care unit patients (also increased with COVID-19) have more muscle loss especially with long hospitalization periods [21]. Furthermore, the elderly population has a limited physical activity within their lifestyle. Indeed, many of them spend long periods of immobilization due to some diseases or accidents requiring bed rest or hospitalizations. Ageing is another factor which, either independently or combined to immobilization, significantly contributes to the muscle and bone loss. Sarcopenia is an age-related decline in muscles mass and strength [22]. Age-related comorbidities such as chronic heart failure [23] and chronic obstructive pulmonary diseases [24] accelerate sarcopenia [25]. Clinically, sarcopenia epidemiological profile is increasing and enhances mortality [26] especially with the increasing number of elderly people who develop a poor lifestyle (reduced activity, unhealthy diet, etc.).
Muscle atrophy includes protein degradation, mitochondrial dysregulation and inflammation among its key biological features [27,28,29]. Biological markers suggested for sarcopenia [25,30] would represent significant diagnosis tools for muscle atrophy as well. Both muscle atrophy and bone loss (key tissues of the locomotor system) can be reversed by physical activity [31,32]. Exercise is known for its benefits in respect to muscle function and metabolism including as sarcopenia treatment [33,34,35,36]. The effects of exercise, including pre-training, on muscle atrophy and recovery has also been highlighted [37,38,39]. Indeed, muscle atrophy could be prevented by exercise [40], including a pretraining as suggested by electrical stimulation studies [41,42]. Exercise represents the main treatment approach and electrical stimulation and “cytoprotective” dietary interventions are also used against muscle atrophy [43,44]. Other therapeutic options represent potential approaches such as gene therapy and epigenetic drugs [45,46,47]. Pharmacological therapies, however, remain limited to some growth factors among which we cite insulin, ghrelin/IGF-1 analogues, testosterone and growth hormone [45,47]. The limitation in therapeutic options is in part due to the limited knowledge on the underlying molecular pathways and physiopathological processes.
To reveal such mechanism and deepen our understating of these immobilization-induced atrophy, animal models of immobilization-induced muscle atrophy (rats, mice, rabbit) [26,48,49,50] have been developed. Mice remain the best choice due to their affordable cost, genetic manipulation possibilities and short lifespan; in addition to the ageing process similarities, they share with humans [51,52,53,54,55]. Cast immobilization is the most used because it mimics prolonged immobilization in terms of muscle atrophy [56,57]. The immobilization also induces bone loss in both growing and adult mice [58]. Thus, such immobilization alters the two main parts of the locomotor system, muscles and bones. Bone and muscle mass are reduced with immobilization in which various biological changes such as inflammation, increased muscle RING finger 1 and mRNA contents of polyubiquitin and the ubiquitin ligases muscle atrophy F-box along with reduced rapamycin complex 1 signaling and reducing the myofiber size were reported [49,57,59,60,61]. Immobilization-induced muscle loss depends on factors such as age and sex. For instance, unilateral hindlimb immobilization in rats of different ages leads to a muscle mass loss inversely proportional to age [61]. The difference between male and female in muscle atrophy depends on whether it is aging-induced or inflammation-based [21]. In addition, hindlimb unloading induced more muscle loss in female rats than in males [62]. This could indicate that females would be more impacted by bed resting. Such age and sex differences suggest the need to adapt the treatment (nature and intensity) based on these two factors as well.
Functional genomics and genes expression patterns can lead to the identification of potential novel therapies for the atrophy resulting from the immobilization including during bed rest. Herein, we focus on the gene secreted protein acidic and rich in cysteine (SPARC/Sparc). SPARC is a non-collagenous protein that is abundant in mineralized tissues [63]. It is expressed in various situations in which tissues renewal and cell remodeling occur (exercise, regeneration, obesity, cancer, inflammation, etc.) [64]. It is also associated with cell turnover, remodeling and tissue repair [65]. Based on this expression pattern, we and others previously suggested using SPARC as a molecular physiological and pathological biomarker [64,66]. SPARC, also known as osteonectin or basement membrane-40 (BM-40) [67], has a calcium and collagen binding property [68]. It is a secreted protein that comprises three distinct structural domains [69] and its biosynthesis is regulated by various growth factors and cytokines [70,71,72]. As exemplified below, SPARC plays important roles in muscles biology. This gene was initially characterized as induced by exercise [73,74], potentially mediating exercise-induced muscle phenotype changes [75] and as up-regulated during skeletal muscle regeneration [76]. Sparc overexpression mimics exercise, including enhancing muscle mass, strength, metabolism as well as ameliorating glycemia [77]. SPARC is expressed both in fetal and neonatal muscle and following muscle damage as well [78]. Adding SPARC to muscle C2C12 (myoblast cell) culture increased myoblasts differentiation in addition to myogenic and mitochondrial proteins expression [79]. Moreover, SPARC plays roles in muscle stiffness maintenance [80], muscle morphological change [81] and promotes muscle progenitor cells myogenic differentiation in vitro [80]. On the other hand, Sparc expression [82] and muscle mass [83] decline with ageing. Such age-related decline in SPARC expression would explain why SPARC downregulation using siRNA reduced myogenesis in young rats skeletal muscle progenitor cells (SMPCs) but had little effect in SMPCs from old rats [84] since old rats would already have low SPARC levels. A resistance to SPARC with age is suggested by the fact that exogenous SPARC improved differentiation in young SMPCs, but exogenous SPARC did not affect old SMPCs [84]. This indicate that SPARC would be combined to other therapies which require further investigation especially with the other effects SPARC has on muscles as we detail below.
Furthermore, Sparc KO leads to a phenotype that mimics number of the ageing-induced and sarcopenia-related changes including muscle atrophy with a decrease in muscle mass, strength and metabolism [77]. Small interfering RNA (siRNA)-mediated transient suppression of SPARC leads to muscle atrophy [59] and myofibers atrophic changes [80]. Anti-SPARC antibodies reduced C2C12 differentiation and decreased myogenin expression [79,81]. These suggest that the muscle atrophy could have the decline of SPARC expression as one of its key underlying pathways. Thus, SPARC decline would be implicated within both sarcopenia as well as ageing process that impacts muscles as well.
Such similarities between SPARC impacts on muscles (enhanced functional, structural and metabolic properties) and the exercise-induced muscle changes hypothesize that exercise effects are mediated, at least in part, by SPARC. Therefore, increasing SPARC expression (gene therapy) or administering SPARC protein would possibly lead to exercise-like effects similarly to those seen in mice overexpressing Sparc [77]. This would result in increasing muscles mass, strength and metabolism and counteract the atrophy resulting from hospitalization (immobilization), ageing, or more importantly hospitalization of elderly patients (combines ageing and immobilization). Indeed, hospitalized patients have long periods of immobilization during which they are not able to perform physical activity. Similarly, elderly individuals usually have a limited ability to perform high amounts of exercise. Therefore, administering SPARC or inducing its expression could be an option to overcome these struggles by generating some of the exercise-induced effects without in fact performing exercise. As muscle atrophy is among the most important health problems for these patients (immobilized and/or aged), SPARC comes as a potential therapy as its specific impacts on muscles are well documents. Importantly, the literature also shows the divers beneficial properties and implications of SPARC including metabolic properties [85,86], anticancer [87], anti-inflammatory [88], collagen regulation in the heart [89], tissue repair and regeneration [90,91]. These SPARC properties allowed us to classify it as a regeneration factor [90] that would create a biological environment with optimum conditions for regeneration, muscle differentiation and growth properties.
The importance of SPARC in bones increases the potential of SPARC in managing the bed rest-induced atrophy since immobilization also leads to bone loss. Indeed, SPARC is important for bone formation, remodeling and regeneration [90]. Sparc KO mice develop osteopenia [92], decreased bone formation [93]. SPARC deficiency also affects bone marrow stromal function [94]. In addition, SPARC also plays roles in bone remodeling [95] and osteoblast maturation [67]. It also regulates hydroxyapatite crystals formation and growth [96] and influence osteogenic differentiation [97]. Furthermore, the implication of SPARC in other locomotor system constituents (such as ligaments [98,99] and tendons [100,101]) would make that treating with SPARC would not only improve muscle phenotype but could also have positive effects on the whole locomotion system. Therefore, SPARC administration might contribute to the maintenance of the musculoskeletal system responsible for the individual mobility during hospitalization and recovery periods. It is worth highlighting that increased SPARC expression has been reported in negative biological status such as metabolic disorders [102], rheumatoid arthritis [70], cancer [103], coronary artery disease [104] and intracranial aneurysms [105]. We have hypothesized that such expression would not indicate the involvement of SPARC in the pathogenesis or prognosis but rather represents an attempt to counteract the effects generated by such pathologies or disorders via the beneficial SPARC-mediated effects. Examples of SPARC counteracting inflammation [88] and cancer [87,106] would be two illustrations of such “regulatory feedback”.
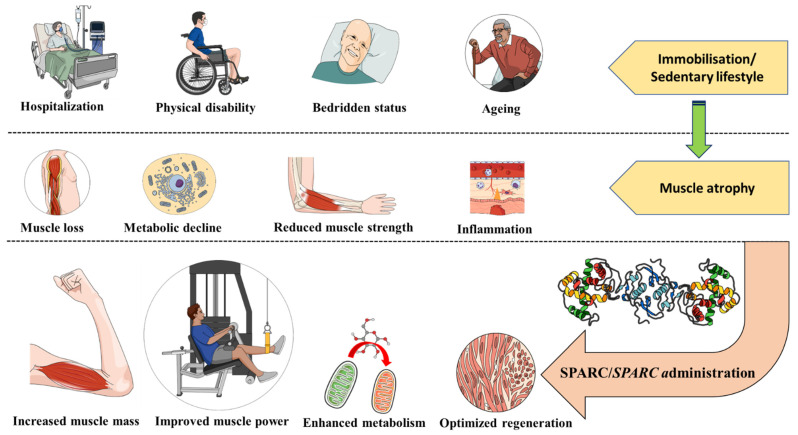
Such approach can also be extended to those chronically bedridden, with physical disability or even space missions (microgravity environment) [107] as summarized in Figure 1. Evidence suggests that Sparc decline contributes to the muscle atrophy, ageing and the resulting phenotypes, whereas its overexpression induced by exercise would be a mechanism via which exercise corrects and improves muscle atrophy and ageing. Therefore, we suggested measuring exercise-induced SPARC/SPARC/Sparc expression as a molecular tool to optimize exercise therapy towards a personalized medicine [108] and also using SPARC as a potential “exercise substitute” [109]. Such measure could be applied to immobilized patients during a potential pre-training session aiming to counteract muscle atrophy. We believe that further animal and clinical studies could lead to a new generation of molecular therapies for muscle atrophy based on SPARC and permit the overcoming of this challenging atrophy resulting from hospitalization, immobility and ageing. The best option, when available, is to rather focus on exercise-induced SPARC as a possible treatment and we emphasize that further studies are needed to further map the mechanistic links between exercise, the exercise-induced myokines (including SPARC) and the exercise induced effects.
Figure 1.
Secreted Protein Acidic and Rich in Cysteine (SPARC/SPARC) as a muscle atrophy therapy. Situations such as hospitalization, physical disability or being bedridden represent an immobilization that might lead to muscle atrophy. Ageing (usually accompanied with a sedentary lifestyle) is another risk factor for the muscle atrophy. SPARC properties of enhancing muscles mass, strength and metabolism are towards counteracting muscle atrophy and highlight SPARC/SPARC (protein administration or gene therapy) as a molecular therapy for muscle atrophy.
Acknowledgments
Abdelaziz Ghanemi received a scholarship under the Merit Scholarship Program for foreign students from the Ministry of Education and Higher Education of Quebec, Canada. The Fonds de recherche du Québec-Nature et technologies (FRQNT) is responsible for managing the program (Bourses d’excellence pour étudiants étrangers du ministère de l’Éducation et de l’Enseignement supérieur du Québec, Le Fonds de recherche du Québec-Nature et technologies (FRQNT) est responsable de la gestion du programme). Abdelaziz Ghanemi received the scholarship « Bourse Tremplin -Stage en milieu de pratique » (Internship scholarship) from the Fonds de recherche du Québec-Sante (FRQS), Quebec, Canada. Figure 1 was created using images from https://mindthegraph.com/ (accessed on 17 February 2022).
Author Contributions
A.G. designed the manuscript structure and wrote it. A.G., M.Y. and J.S.-A. discussed the content and exchanged ideas and suggestions (concepts to add, the figures, references selection, etc.) throughout the writing process, edited and critically revised the paper. J.S.-A. gave the final approval for the version to be published. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong H.J., Morra D. Excellent hospital care for all: Open and operating 24/7. J. Gen. Intern. Med. 2011;26:1050–1052. doi: 10.1007/s11606-011-1715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez M.A., Hall M., Auger K.A., Bettenhausen J.L., Colvin J.D., Cutler G.J., Fieldston E., Macy M.L., Morse R., Raphael J.L., et al. Care of Pediatric High-Cost Hospitalizations Across Hospital Types. Hosp. Pediatr. 2020;10:206–213. doi: 10.1542/hpeds.2019-0258. [DOI] [PubMed] [Google Scholar]
- 3.Copnell B., Hagger V., Wilson S.G., Evans S.M., Sprivulis P.C., Cameron P.A. Measuring the quality of hospital care: An inventory of indicators. Intern. Med. J. 2009;39:352–360. doi: 10.1111/j.1445-5994.2009.01961.x. [DOI] [PubMed] [Google Scholar]
- 4.Buttigieg S.C., Abela L., Pace A. Variables affecting hospital length of stay: A scoping review. J. Health Organ. Manag. 2018;32:463–493. doi: 10.1108/JHOM-10-2017-0275. [DOI] [PubMed] [Google Scholar]
- 5.Hua M., Lu Y., Ma X., Morrison R.S., Li G., Wunsch H. Association Between the Implementation of Hospital-Based Palliative Care and Use of Intensive Care During Terminal Hospitalizations. JAMA Netw. Open. 2020;3:e1918675. doi: 10.1001/jamanetworkopen.2019.18675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J.Y., Dickter J.K. Nosocomial Infections: A History of Hospital-Acquired Infections. Gastrointest. Endosc. N. Am. 2020;30:637–652. doi: 10.1016/j.giec.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Alwasel A., Alossimi B., Alsadun M., Alhussaini K. Bedsores Management: Efficiency Simulation of a New Mattress Design. Healthcare. 2021;9:1701. doi: 10.3390/healthcare9121701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bluestein D., Javaheri A. Pressure ulcers: Prevention, evaluation, and management. Am. Fam. Physician. 2008;78:1186–1194. [PubMed] [Google Scholar]
- 9.Buso A., Comelli M., Picco R., Isola M., Magnesa B., Pišot R., Rittweger J., Salvadego D., Šimunič B., Grassi B., et al. Mitochondrial Adaptations in Elderly and Young Men Skeletal Muscle Following 2 Weeks of Bed Rest and Rehabilitation. Front. Physiol. 2019;10:474. doi: 10.3389/fphys.2019.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeBlanc A., Gogia P., Schneider V., Krebs J., Schonfeld E., Evans H. Calf muscle area and strength changes after five weeks of horizontal bed rest. Am. J. Sports Med. 1988;16:624–629. doi: 10.1177/036354658801600612. [DOI] [PubMed] [Google Scholar]
- 11.Mahmassani Z.S., Reidy P.T., McKenzie A.I., Stubben C., Howard M.T., Drummond M.J. Age-dependent skeletal muscle transcriptome response to bed rest-induced atrophy. J. Appl. Physiol. 2019;126:894–902. doi: 10.1152/japplphysiol.00811.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirks M.L., Smeets J.S.J., Holwerda A.M., Kouw I.W.K., Marzuca-Nassr G.N., Gijsen A.P., Holloway G.P., Verdijk L.B., van Loon L.J.C. Dietary feeding pattern does not modulate the loss of muscle mass or the decline in metabolic health during short-term bed rest. Am. J. Physiol. Endocrinol. Metab. 2019;316:E536–E545. doi: 10.1152/ajpendo.00378.2018. [DOI] [PubMed] [Google Scholar]
- 13.Bloomfield S.A. Changes in musculoskeletal structure and function with prolonged bed rest. Med. Sci. Sports Exerc. 1997;29:197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Perhonen M.A., Franco F., Lane L.D., Buckey J.C., Blomqvist C.G., Zerwekh J.E., Peshock R.M., Weatherall P.T., Levine B.D. Cardiac atrophy after bed rest and spaceflight. J. Appl. Physiol. 2001;91:645–653. doi: 10.1152/jappl.2001.91.2.645. [DOI] [PubMed] [Google Scholar]
- 15.Dittmer D.K., Teasell R. Complications of immobilization and bed rest. Part 1: Musculoskeletal and cardiovascular complications. Can. Fam. Physician. 1993;39 [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura K., Yamanouchi K., Nishihara M. Secreted protein acidic and rich in cysteine internalization and its age-related alterations in skeletal muscle progenitor cells. Aging Cell. 2014;13:175–184. doi: 10.1111/acel.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sousa-Victor P., Muñoz-Cánoves P. Regenerative decline of stem cells in sarcopenia. Mol. Asp. Med. 2016;50:109–117. doi: 10.1016/j.mam.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Melouane A., Ghanemi A., Aubé S., Yoshioka M., St-Amand J. Differential gene expression analysis in ageing muscle and drug discovery perspectives. Ageing Res. Rev. 2018;41:53–63. doi: 10.1016/j.arr.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Melouane A., Ghanemi A., Yoshioka M., St-Amand J. Functional genomics applications and therapeutic implications in sarcopenia. Mutat. Res. Rev. Mutat. Res. 2019;781:175–185. doi: 10.1016/j.mrrev.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Ghanemi A., Yoshioka M., St-Amand J. Ageing and Obesity Shared Patterns: From Molecular Pathogenesis to Epigenetics. Diseases. 2021;9:87. doi: 10.3390/diseases9040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosa-Caldwell M.E., Greene N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. 2019;10:43. doi: 10.1186/s13293-019-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K., Yamanouchi K., Nishihara M. Transdisciplinary Approach for Sarcopenia. Molecular mechanism of sarcopenia: The role of skeletal muscle niche component SPARC in the regulation of myogenesis and adipogenesis and its alteration with age. Clin. Calcium. 2014;24:1471–1478. [PubMed] [Google Scholar]
- 23.Li H., Hastings M.H., Rhee J., Trager L.E., Roh J.D., Rosenzweig A. Targeting Age-Related Pathways in Heart Failure. Circ. Res. 2020;126:533–551. doi: 10.1161/CIRCRESAHA.119.315889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Easter M., Bollenbecker S., Barnes J.W., Krick S. Targeting Aging Pathways in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020;21:6924. doi: 10.3390/ijms21186924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qaisar R., Karim A., Muhammad T., Shah I., Khan J. Prediction of sarcopenia using a battery of circulating biomarkers. Sci. Rep. 2021;11:8632. doi: 10.1038/s41598-021-87974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek K.W., Jung Y.K., Kim J.S., Park J.S., Hah Y.S., Kim S.J., Yoo J.I. Rodent Model of Muscular Atrophy for Sarcopenia Study. J. Bone Metab. 2020;27:97–110. doi: 10.11005/jbm.2020.27.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonaldo P., Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis. Mech. 2013;6:25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji L.L., Yeo D. Mitochondrial dysregulation and muscle disuse atrophy. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.19139.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuttle C.S.L., Thang L.A.N., Maier A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020;64:101185. doi: 10.1016/j.arr.2020.101185. [DOI] [PubMed] [Google Scholar]
- 30.Kwak J.Y., Hwang H., Kim S.K., Choi J.Y., Lee S.M., Bang H., Kwon E.S., Lee K.P., Chung S.G., Kwon K.S. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci. Rep. 2018;8:8574. doi: 10.1038/s41598-018-26617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miokovic T., Armbrecht G., Gast U., Rawer R., Roth H.J., Runge M., Felsenberg D., Belavý D.L. Muscle atrophy, pain, and damage in bed rest reduced by resistive (vibration) exercise. Med. Sci. Sports Exerc. 2014;46:1506–1516. doi: 10.1249/MSS.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 32.Leblanc A.D., Schneider V.S., Evans H.J., Engelbretson D.A., Krebs J.M. Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Min. Res. 1990;5:843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 33.Landi F., Marzetti E., Martone A.M., Bernabei R., Onder G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:25–31. doi: 10.1097/MCO.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 34.Spaulding H.R., Selsby J.T. Is Exercise the Right Medicine for Dystrophic Muscle? Med. Sci. Sports Exerc. 2018;50:1723–1732. doi: 10.1249/MSS.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 35.Phu S., Boersma D., Duque G. Exercise and Sarcopenia. J. Clin. Densitom. 2015;18:488–492. doi: 10.1016/j.jocd.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Marzetti E., Calvani R., Tosato M., Cesari M., Di Bari M., Cherubini A., Broccatelli M., Savera G., D’Elia M., Pahor M., et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017;29:35–42. doi: 10.1007/s40520-016-0705-4. [DOI] [PubMed] [Google Scholar]
- 37.He N., Ye H. Exercise and Muscle Atrophy. Adv. Exp. Med. Biol. 2020;1228:255–267. doi: 10.1007/978-981-15-1792-1_17. [DOI] [PubMed] [Google Scholar]
- 38.Theilen N.T., Kunkel G.H., Tyagi S.C. The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J. Cell Physiol. 2017;232:2348–2358. doi: 10.1002/jcp.25737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salles J.I., Guimarães J.M., Filho G.M., Morrissey D. Effect of a specific exercise strategy on strength and proprioception in volleyball players with infraspinatus muscle atrophy. Scand. J. Med. Sci. Sports. 2018;28:2093–2099. doi: 10.1111/sms.13216. [DOI] [PubMed] [Google Scholar]
- 40.Czerwinski S.M., Kurowski T.G., O’Neill T.M., Hickson R.C. Initiating regular exercise protects against muscle atrophy from glucocorticoids. J. Appl. Physiol. 1987;63:1504–1510. doi: 10.1152/jappl.1987.63.4.1504. [DOI] [PubMed] [Google Scholar]
- 41.Baldi J.C., Jackson R.D., Moraille R., Mysiw W.J. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36:463–469. doi: 10.1038/sj.sc.3100679. [DOI] [PubMed] [Google Scholar]
- 42.Lake D.A. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med. 1992;13:320–336. doi: 10.2165/00007256-199213050-00003. [DOI] [PubMed] [Google Scholar]
- 43.Granic A., Sayer A.A., Robinson S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients. 2019;11:745. doi: 10.3390/nu11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anton S.D., Hida A., Mankowski R., Layne A., Solberg L.M., Mainous A.G., Buford T. Nutrition and Exercise in Sarcopenia. Curr. Protein. Pept. Sci. 2018;19:649–667. doi: 10.2174/1389203717666161227144349. [DOI] [PubMed] [Google Scholar]
- 45.Urso M.L. Disuse atrophy of human skeletal muscle: Cell signaling and potential interventions. Med. Sci. Sports Exerc. 2009;41:1860–1868. doi: 10.1249/MSS.0b013e3181a6458a. [DOI] [PubMed] [Google Scholar]
- 46.Guasconi V., Puri P.L. Epigenetic drugs in the treatment of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:233–241. doi: 10.1097/MCO.0b013e3282fa1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding S., Dai Q., Huang H., Xu Y., Zhong C. An Overview of Muscle Atrophy. Adv. Exp. Med. Biol. 2018;1088:3–19. doi: 10.1007/978-981-13-1435-3_1. [DOI] [PubMed] [Google Scholar]
- 48.Son J.S., Kim J.H., Kim H.J., Yoon D.H., Kim J.S., Song H.S., Song W. Effect of resistance ladder training on sparc expression in skeletal muscle of hindlimb immobilized rats. Muscle Nerve. 2016;53:951–957. doi: 10.1002/mus.24940. [DOI] [PubMed] [Google Scholar]
- 49.Caron A.Z., Drouin G., Desrosiers J., Trensz F., Grenier G. A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J. Appl. Physiol. 2009;106:2049–2059. doi: 10.1152/japplphysiol.91505.2008. [DOI] [PubMed] [Google Scholar]
- 50.Herbert R.D., Balnave R.J. The effect of position of immobilisation on resting length, resting stiffness, and weight of the soleus muscle of the rabbit. J. Orthop. Res. 1993;11:358–366. doi: 10.1002/jor.1100110307. [DOI] [PubMed] [Google Scholar]
- 51.Yuan R., Peters L.L., Paigen B. Mice as a mammalian model for research on the genetics of aging. Ilar. J. 2011;52:4–15. doi: 10.1093/ilar.52.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan R., Tsaih S.W., Petkova S.B., Marin de Evsikova C., Xing S., Marion M.A., Bogue M.A., Mills K.D., Peters L.L., Bult C.J., et al. Aging in inbred strains of mice: Study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barreto G., Huang T.T., Giffard R.G. Age-related defects in sensorimotor activity, spatial learning, and memory in C57BL/6 mice. J. Neurosurg. Anesth. 2010;22:214–219. doi: 10.1097/ANA.0b013e3181d56c98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graber T.G., Ferguson-Stegall L., Kim J.H., Thompson L.V. C57BL/6 neuromuscular healthspan scoring system. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1326–1336. doi: 10.1093/gerona/glt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parks R.J., Fares E., Macdonald J.K., Ernst M.C., Sinal C.J., Rockwood K., Howlett S.E. A procedure for creating a frailty index based on deficit accumulation in aging mice. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:217–227. doi: 10.1093/gerona/glr193. [DOI] [PubMed] [Google Scholar]
- 56.Palus S., Springer J.I., Doehner W., von Haehling S., Anker M., Anker S.D., Springer J. Models of sarcopenia: Short review. Int. J. Cardiol. 2017;238:19–21. doi: 10.1016/j.ijcard.2017.03.152. [DOI] [PubMed] [Google Scholar]
- 57.St-Amand J., Okamura K., Matsumoto K., Shimizu S., Sogawa Y. Characterization of control and immobilized skeletal muscle: An overview from genetic engineering. Faseb J. 2001;15:684–692. doi: 10.1096/fj.00-0150com. [DOI] [PubMed] [Google Scholar]
- 58.Friedman M.A., Zhang Y., Wayne J.S., Farber C.R., Donahue H.J. Single limb immobilization model for bone loss from unloading. J. Biomech. 2019;83:181–189. doi: 10.1016/j.jbiomech.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura K., Nakano S., Miyoshi T., Yamanouchi K., Nishihara M. Loss of SPARC in mouse skeletal muscle causes myofiber atrophy. Muscle Nerve. 2013;48:791–799. doi: 10.1002/mus.23822. [DOI] [PubMed] [Google Scholar]
- 60.Krawiec B.J., Frost R.A., Vary T.C., Jefferson L.S., Lang C.H. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 2005;289:E969–E980. doi: 10.1152/ajpendo.00126.2005. [DOI] [PubMed] [Google Scholar]
- 61.Kelleher A.R., Pereira S.L., Jefferson L.S., Kimball S.R. REDD2 expression in rat skeletal muscle correlates with nutrient-induced activation of mTORC1: Responses to aging, immobilization, and remobilization. Am. J. Physiol. Endocrinol. Metab. 2015;308:E122–E129. doi: 10.1152/ajpendo.00341.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshihara T., Natsume T., Tsuzuki T., Chang S.W., Kakigi R., Sugiura T., Naito H. Sex differences in forkhead box O3a signaling response to hindlimb unloading in rat soleus muscle. J. Physiol. Sci. 2019;69:235–244. doi: 10.1007/s12576-018-0640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosset E.M., Bradshaw A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016;52–54:78–87. doi: 10.1016/j.matbio.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghanemi A., Yoshioka M., St-Amand J. Secreted Protein Acidic and Rich in Cysteine as a Molecular Physiological and Pathological Biomarker. Biomolecules. 2021;11:1689. doi: 10.3390/biom11111689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan Q., Sage E.H. SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. 1999;47:1495–1506. doi: 10.1177/002215549904701201. [DOI] [PubMed] [Google Scholar]
- 66.Kao S.C., Kirschner M.B., Cooper W.A., Tran T., Burgers S., Wright C., Korse T., van den Broek D., Edelman J., Vallely M., et al. A proteomics-based approach identifies secreted protein acidic and rich in cysteine as a prognostic biomarker in malignant pleural mesothelioma. Br. J. Cancer. 2016;114:524–531. doi: 10.1038/bjc.2015.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delany A.M., Kalajzic I., Bradshaw A.D., Sage E.H., Canalis E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology. 2003;144:2588–2596. doi: 10.1210/en.2002-221044. [DOI] [PubMed] [Google Scholar]
- 68.Sage H., Johnson C., Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J. Biol. Chem. 1984;259:3993–4007. doi: 10.1016/S0021-9258(17)43194-2. [DOI] [PubMed] [Google Scholar]
- 69.Scavelli K., Chatterjee A., Rhee D.J. Secreted Protein Acidic and Rich in Cysteine in Ocular Tissue. J. Ocul. Pharm. 2015;31:396–405. doi: 10.1089/jop.2015.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamura S., Kamihagi K., Satakeda H., Katayama M., Pan H., Okamoto H., Noshiro M., Takahashi K., Yoshihara Y., Shimmei M., et al. Enhancement of SPARC (osteonectin) synthesis in arthritic cartilage. Increased levels in synovial fluids from patients with rheumatoid arthritis and regulation by growth factors and cytokines in chondrocyte cultures. Arthritis Rheum. 1996;39:539–551. doi: 10.1002/art.1780390402. [DOI] [PubMed] [Google Scholar]
- 71.Chandrasekhar S., Harvey A.K., Johnson M.G., Becker G.W. Osteonectin/SPARC is a product of articular chondrocytes/cartilage and is regulated by cytokines and growth factors. Biochim. Biophys. Acta. 1994;1221:7–14. doi: 10.1016/0167-4889(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 72.Fujita T., Shiba H., Van Dyke T.E., Kurihara H. Differential effects of growth factors and cytokines on the synthesis of SPARC DNA fibronectin and alkaline phosphatase activity in human periodontal ligament cells. Cell Biol. Int. 2004;28:281–286. doi: 10.1016/j.cellbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 73.Riedl I., Yoshioka M., Nishida Y., Tobina T., Paradis R., Shono N., Tanaka H., St-Amand J. Regulation of skeletal muscle transcriptome in elderly men after 6 weeks of endurance training at lactate threshold intensity. Exp. Gerontol. 2010;45:896–903. doi: 10.1016/j.exger.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 74.Ghanemi A., Melouane A., Yoshioka M., St-Amand J. Exercise and High-Fat Diet in Obesity: Functional Genomics Perspectives of Two Energy Homeostasis Pillars. Genes. 2020;11:875. doi: 10.3390/genes11080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghanemi A., Melouane A., Yoshioka M., St-Amand J. Exercise Training of Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Mice Suggests That Exercise-Induced Muscle Phenotype Changes Are SPARC-Dependent. Appl. Sci. 2020;10:9108. doi: 10.3390/app10249108. [DOI] [Google Scholar]
- 76.Petersson S.J., Jørgensen L.H., Andersen D.C., Nørgaard R.C., Jensen C.H., Schrøder H.D. SPARC is up-regulated during skeletal muscle regeneration and inhibits myoblast differentiation. Histol. Histopathol. 2013;28:1451–1460. doi: 10.14670/HH-28.1451. [DOI] [PubMed] [Google Scholar]
- 77.Ghanemi A., Melouane A., Yoshioka M., St-Amand J. Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice. Metabolites. 2022;12:125. doi: 10.3390/metabo12020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jørgensen L.H., Petersson S.J., Sellathurai J., Andersen D.C., Thayssen S., Sant D.J., Jensen C.H., Schrøder H.D. Secreted protein acidic and rich in cysteine (SPARC) in human skeletal muscle. J. Histochem. Cytochem. 2009;57:29–39. doi: 10.1369/jhc.2008.951954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Melouane A., Carbonell A., Yoshioka M., Puymirat J., St-Amand J. Implication of SPARC in the modulation of the extracellular matrix and mitochondrial function in muscle cells. PLoS ONE. 2018;13:e0192714. doi: 10.1371/journal.pone.0192714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omi S., Yamanouchi K., Nakamura K., Matsuwaki T., Nishihara M. Reduced fibrillar collagen accumulation in skeletal muscle of secreted protein acidic and rich in cysteine (SPARC)-null mice. J. Vet. Med. Sci. 2019;81:1649–1654. doi: 10.1292/jvms.19-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho W.J., Kim E.J., Lee S.J., Kim H.D., Shin H.J., Lim W.K. Involvement of SPARC in in vitro differentiation of skeletal myoblasts. Biochem. Biophys. Res. Commun. 2000;271:630–634. doi: 10.1006/bbrc.2000.2682. [DOI] [PubMed] [Google Scholar]
- 82.Aoi W., Naito Y., Takagi T., Tanimura Y., Takanami Y., Kawai Y., Sakuma K., Hang L.P., Mizushima K., Hirai Y., et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 83.Balachandran A., Krawczyk S.N., Potiaumpai M., Signorile J.F. High-speed circuit training vs hypertrophy training to improve physical function in sarcopenic obese adults: A randomized controlled trial. Exp. Gerontol. 2014;60:64–71. doi: 10.1016/j.exger.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 84.Nakamura K., Nakano S., Miyoshi T., Yamanouchi K., Matsuwaki T., Nishihara M. Age-related resistance of skeletal muscle-derived progenitor cells to SPARC may explain a shift from myogenesis to adipogenesis. Aging. 2012;4:40–48. doi: 10.18632/aging.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghanemi A., Yoshioka M., St-Amand J. Secreted Protein Acidic and Rich in Cysteine: Metabolic and Homeostatic Properties beyond the Extracellular Matrix Structure. Appl. Sci. 2020;10:2388. doi: 10.3390/app10072388. [DOI] [Google Scholar]
- 86.Ghanemi A., Melouane A., Yoshioka M., St-Amand J. Secreted protein acidic and rich in cysteine and bioenergetics: Extracellular matrix, adipocytes remodeling and skeletal muscle metabolism. Int. J. Biochem. Cell Biol. 2019;117:105627. doi: 10.1016/j.biocel.2019.105627. [DOI] [PubMed] [Google Scholar]
- 87.Ghanemi A., Yoshioka M., St-Amand J. Secreted protein acidic and rich in cysteine and cancer: A homeostatic hormone? Cytokine. 2020;127:154996. doi: 10.1016/j.cyto.2020.154996. [DOI] [PubMed] [Google Scholar]
- 88.Ghanemi A., Yoshioka M., St-Amand J. Secreted protein acidic and rich in cysteine and inflammation: Another homeostatic property? Cytokine. 2020;133:155179. doi: 10.1016/j.cyto.2020.155179. [DOI] [PubMed] [Google Scholar]
- 89.Harris B.S., Zhang Y., Card L., Rivera L.B., Brekken R.A., Bradshaw A.D. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H841–H847. doi: 10.1152/ajpheart.01247.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghanemi A., Yoshioka M., St-Amand J. Secreted Protein Acidic and Rich in Cysteine as A Regeneration Factor: Beyond the Tissue Repair. Life. 2021;11:38. doi: 10.3390/life11010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghanemi A., Yoshioka M., St-Amand J. Exercise, Diet and Sleeping as Regenerative Medicine Adjuvants: Obesity and Ageing as Illustrations. Medicines. 2022;9:7. doi: 10.3390/medicines9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mansergh F.C., Wells T., Elford C., Evans S.L., Perry M.J., Evans M.J., Evans B.A. Osteopenia in Sparc (osteonectin)-deficient mice: Characterization of phenotypic determinants of femoral strength and changes in gene expression. Physiol. Genom. 2007;32:64–73. doi: 10.1152/physiolgenomics.00151.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delany A.M., Amling M., Priemel M., Howe C., Baron R., Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Investig. 2000;105:915–923. doi: 10.1172/JCI7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo Z., Zhou Y., Luo P., Zhao Q., Xiao N., Yu Y., Yan Q., Lu G., Cheng L. SPARC deficiency affects bone marrow stromal function, resulting in impaired B lymphopoiesis. J. Leukoc. Biol. 2014;96:73–82. doi: 10.1189/jlb.1A0713-415RR. [DOI] [PubMed] [Google Scholar]
- 95.Ribeiro N., Sousa S.R., Brekken R.A., Monteiro F.J. Role of SPARC in bone remodeling and cancer-related bone metastasis. J. Cell Biochem. 2014;115:17–26. doi: 10.1002/jcb.24649. [DOI] [PubMed] [Google Scholar]
- 96.Sodek J., Zhu B., Huynh M.H., Brown T.J., Ringuette M. Novel functions of the matricellular proteins osteopontin and osteonectin/SPARC. Connect. Tissue Res. 2002;43:308–319. doi: 10.1080/03008200290001050. [DOI] [PubMed] [Google Scholar]
- 97.Rowe D.W. Chapter 61—Osteogenesis imperfecta. In: Bilezikian J.P., Martin T.J., Clemens T.L., Rosen C.J., editors. Principles of Bone Biology. 4th ed. Academic Press; Cambridge, MA, USA: 2020. pp. 1489–1505. [Google Scholar]
- 98.Trombetta J.M., Bradshaw A.D. SPARC/osteonectin functions to maintain homeostasis of the collagenous extracellular matrix in the periodontal ligament. J. Histochem. Cytochem. 2010;58:871–879. doi: 10.1369/jhc.2010.956144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rosset E.M., Trombetta-eSilva J., Hepfer G., Yao H., Bradshaw A.D. SPARC and the N-propeptide of collagen I influence fibroblast proliferation and collagen assembly in the periodontal ligament. PLoS ONE. 2017;12:e0173209. doi: 10.1371/journal.pone.0173209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gehwolf R., Wagner A., Lehner C., Bradshaw A.D., Scharler C., Niestrawska J.A., Holzapfel G.A., Bauer H.C., Tempfer H., Traweger A. Pleiotropic roles of the matricellular protein Sparc in tendon maturation and ageing. Sci. Rep. 2016;6:32635. doi: 10.1038/srep32635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang T., Wagner A., Gehwolf R., Yan W., Passini F.S., Thien C., Weissenbacher N., Lin Z., Lehner C., Teng H., et al. Load-induced regulation of tendon homeostasis by SPARC, a genetic predisposition factor for tendon and ligament injuries. Sci. Transl. Med. 2021;13:eabe5738. doi: 10.1126/scitranslmed.abe5738. [DOI] [PubMed] [Google Scholar]
- 102.Kos K., Wilding J.P. SPARC: A key player in the pathologies associated with obesity and diabetes. Nat. Rev. Endocrinol. 2010;6:225–235. doi: 10.1038/nrendo.2010.18. [DOI] [PubMed] [Google Scholar]
- 103.Chang C.-H., Yen M.-C., Liao S.-H., Hsu Y.-L., Lai C.-S., Chang K.-P., Hsu Y.-L. Secreted Protein Acidic and Rich in Cysteine (SPARC) Enhances Cell Proliferation, Migration, and Epithelial Mesenchymal Transition, and SPARC Expression is Associated with Tumor Grade in Head and Neck Cancer. Int. J. Mol. Sci. 2017;18:1556. doi: 10.3390/ijms18071556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takahashi M., Nagaretani H., Funahashi T., Nishizawa H., Maeda N., Kishida K., Kuriyama H., Shimomura I., Maeda K., Hotta K., et al. The expression of SPARC in adipose tissue and its increased plasma concentration in patients with coronary artery disease. Obes. Res. 2001;9:388–393. doi: 10.1038/oby.2001.50. [DOI] [PubMed] [Google Scholar]
- 105.Tan X., Li T., Zhu S., Zhong W., Li F., Wang Y. Induction of SPARC on Oxidative Stress, Inflammatory Phenotype Transformation, and Apoptosis of Human Brain Smooth Muscle Cells Via TGF-β1-NOX4 Pathway. J. Mol. Neurosci. 2020;70:1728–1741. doi: 10.1007/s12031-020-01566-z. [DOI] [PubMed] [Google Scholar]
- 106.Ma J., Gao S., Xie X., Sun E., Zhang M., Zhou Q., Lu C. SPARC inhibits breast cancer bone metastasis and may be a clinical therapeutic target. Oncol. Lett. 2017;14:5876–5882. doi: 10.3892/ol.2017.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Droppert P.M. A review of muscle atrophy in microgravity and during prolonged bed rest. J. Br. Interplanet. Soc. 1993;46:83–86. [PubMed] [Google Scholar]
- 108.Ghanemi A., Yoshioka M., St-Amand J. Measuring Exercise-Induced Secreted Protein Acidic and Rich in Cysteine Expression as a Molecular Tool to Optimize Personalized Medicine. Genes. 2021;12:1832. doi: 10.3390/genes12111832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghanemi A, Yoshioka M, St-Amand J: Genetic Expression between Ageing and Exercise: Secreted Protein Acidic and Rich in Cysteine as a Potential “Exercise Substitute” Antiageing Therapy. Genes. 2022;13:950. doi: 10.3390/genes13060950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.