Abstract
Leptin resistance is a hallmark of obesity. Treatments aiming to improve leptin sensitivity are considered a promising therapeutical approach against obesity. However, leptin receptor (LepR) signaling also modulates several neurovegetative aspects, such as the cardiovascular system and hepatic gluconeogenesis. Thus, we investigated the long-term consequences of increased leptin sensitivity, considering the potential beneficial and deleterious effects. To generate a mouse model with increased leptin sensitivity, the suppressor of cytokine signaling 3 (SOCS3) was ablated in LepR-expressing cells (LepR∆SOCS3 mice). LepR∆SOCS3 mice displayed reduced food intake, body adiposity and weight gain, as well as improved glucose tolerance and insulin sensitivity, and were protected against aging-induced leptin resistance. Surprisingly, a very high mortality rate was observed in aging LepR∆SOCS3 mice. LepR∆SOCS3 mice showed cardiomyocyte hypertrophy, increased myocardial fibrosis and reduced cardiovascular capacity. LepR∆SOCS3 mice exhibited impaired post-ischemic cardiac functional recovery and middle-aged LepR∆SOCS3 mice showed substantial arhythmic events during the post-ischemic reperfusion period. Finally, LepR∆SOCS3 mice exhibited fasting-induced hypoglycemia and impaired counterregulatory response to glucopenia associated with reduced gluconeogenesis. In conclusion, although increased sensitivity to leptin improved the energy and glucose homeostasis of aging LepR∆SOCS3 mice, major autonomic/neurovegetative dysfunctions compromised the health and longevity of these animals. Consequently, these potentially negative aspects need to be considered in the therapies that increase leptin sensitivity chronically.
Keywords: aging, cardiovascular system, heart, hypothalamus, leptin, obesity
1. Introduction
Obesity is a complex and multifactorial disease caused by a chronic imbalance between food intake and energy expenditure [1,2]. Obesity is considered a pandemic since a high percentage of people in numerous developed and developing countries present excessive accumulation of body fat [3,4]. Obesity increases the risk of multiple health problems, which ultimately lead to a significant decrease in quality of life, productivity, and life expectancy [1,2]. Thus, the development of efficient and safe approaches that can prevent and treat this metabolic disease are urgently needed.
Leptin resistance is a hallmark of obesity [5,6,7]. Obese individuals exhibit high leptin concentrations, which do not result in the expected suppression of food intake and increased energy expenditure [5,6,7]. Additionally, obese subjects in general do not respond to exogenous leptin treatment [8,9]. Consequently, leptin administration is inefficient for the treatment of obesity. In contrast, drugs aiming to increase the sensitivity to leptin are among the most promising pharmacological alternatives to treat obesity [6,7,10,11,12,13,14]. For example, previous studies have shown a synergistic interaction between amylin and leptin to reduce body weight [12,13]. Other studies have demonstrated that either celastrol or withaferin A are leptin sensitizers that are able to induce weight loss [10,11]. Furthermore, several plant-derived natural products may present leptin sensitizing properties by inhibiting molecules that induce leptin resistance, such as protein-tyrosine phosphatase 1B (PTP1B) [15]. Thus, drugs that increase leptin sensitivity in theory could take advantage of the anorexigenic action of the endogenously produced hormone to cause persistent weight loss.
Central leptin action not only regulates food intake and energy expenditure but also modulates several neurovegetative and autonomic functions, including the cardiovascular system and hepatic gluconeogenesis [7]. In this regard, central leptin administration or leptin action on pro-opiomelanocortin (POMC)-expressing neurons strongly modulates hepatic insulin sensitivity and suppresses glucose production [16,17,18]. Conversely, leptin action on neurons of the dorsomedial nucleus of the hypothalamus (DMH) regulates the sympathetic tone, heart rate (HR) and blood pressure [19,20]. Therefore, leptin receptor (LepR) signaling in DMH neurons is likely involved in the pathophysiology of hypertension that is associated with obesity.
Importantly, different neuronal populations exhibit a distinct predisposition to develop leptin resistance. While neurons of the arcuate nucleus of the hypothalamus frequently develop insensitivity to leptin in obese animals, other neuronal populations, including DMH neurons, remain responsive to leptin even in hyperleptinemic obese individuals [20,21]. The hyperactivity of leptin in the neurons that retain leptin sensitivity may produce long-term deleterious effects, such as favoring an increased sympathetic tone that leads to hypertension and other cardiovascular problems [19,20]. Thus, although therapies that increase leptin sensitivity may present clear beneficial effects in the regulation of energy balance, the potential negative aspects of such approaches have been poorly examined. In the present study, we investigated the long-term consequences of increased leptin sensitivity in mice. For this purpose, we used a mouse model carrying ablation of the gene encoding the suppressor of cytokine signaling 3 (SOCS3), which is a well-known protein that inhibits LepR signaling, its expression is increased in the hypothalamus of obese animals, and whose genetic deletion leads to increased leptin sensitivity and metabolic improvements [5,6,7,22,23,24,25,26,27,28,29,30,31,32]. The potential beneficial and deleterious consequences of SOCS3 ablation in LepR-expressing cells were investigated in young adult and middle-aged male mice.
2. Results
2.1. SOCS3 Ablation in LepR-Expressing Cells Improves Energy and Glucose Homeostasis in Aging Mice
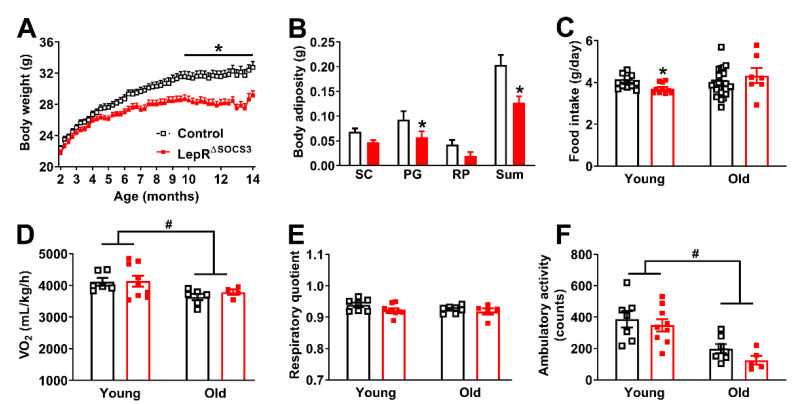
Considering the well-established role of the SOCS3 protein in inhibiting LepR signaling [5,6,7,22,23,24,25,26], we produced mice carrying SOCS3 deletion in LepR-expressing cells to investigate the long-term consequences of increased leptin sensitivity. This mouse model was characterized and validated in several previous studies [27,28,29,30,31,32]. For example, former studies have shown that SOCS3 deletion in LepR-expressing cells increases leptin sensitivity in pubertal and pregnant female mice [28,29,31], as well as in male mice consuming either chow or a high-fat diet [27,30]. Additionally, the ability of leptin, a high-fat diet or pregnancy to upregulate Socs3 mRNA levels in the hypothalamus is completely prevented in LepR∆SOCS3 mice [27,28]. Initially, the changes in body weight over time were determined. Although the body weight of LepR∆SOCS3 mice showed no changes in the first months of life, these conditional knockout mice exhibited a lower weight gain over time that led to a decreased body weight in comparison with control littermate mice (Figure 1A). LepR∆SOCS3 mice also showed reduced body adiposity, which was confirmed by a decrease in the perigonadal fat pad and in the sum of the fat deposits (Figure 1B). To determine the possible causes of these metabolic differences, food intake and energy expenditure (VO2) were determined in young adult (3-month-old) or middle-aged (approximately 15-month-old) mice. LepR∆SOCS3 mice exhibited reduced food intake at a young age, but not in middle-aged animals (Figure 1C). No significant changes in energy expenditure were observed in LepR∆SOCS3 mice, although middle-aged animals showed reduced energy expenditure, compared to young adult mice (Figure 1D). No significant changes between the groups were observed in the respiratory quotient (Figure 1E). The ambulatory activity of middle-aged mice was reduced when compared to young adult animals. However, LepR∆SOCS3 mice did not exhibit differences as compared to control animals (Figure 1F). Therefore, SOCS3 ablation in LepR-expressing cells prevents weight gain during aging, likely by reducing food intake.
Figure 1.
Reduced aging-induced weight gain in LepR∆SOCS3 mice. (A) Changes in body weight along time in control (n = 26–27) and LepR∆SOCS3 (n = 12–24) mice. (B) Masses of the subcutaneous (SC), perigonadal (PG) and retroperitoneal (RP) fat pads and the sum of these deposits in control (black column; n = 11) and LepR∆SOCS3 (red column; n = 10) mice. (C) Food intake in young adult (3-month-old; n = 10–11/group) and middle-aged (15-month-old; n = 7–26/group) mice. (D–F) Oxygen consumption (VO2), respiratory quotient and ambulatory activity in young adult (n = 7–9/group) and middle-aged (n = 5–7/group) mice. Mean ± SEM. * p < 0.05 control vs. LepR∆SOCS3 (post-hoc test two-way ANOVA). # p < 0.05 age effect (two-way ANOVA).
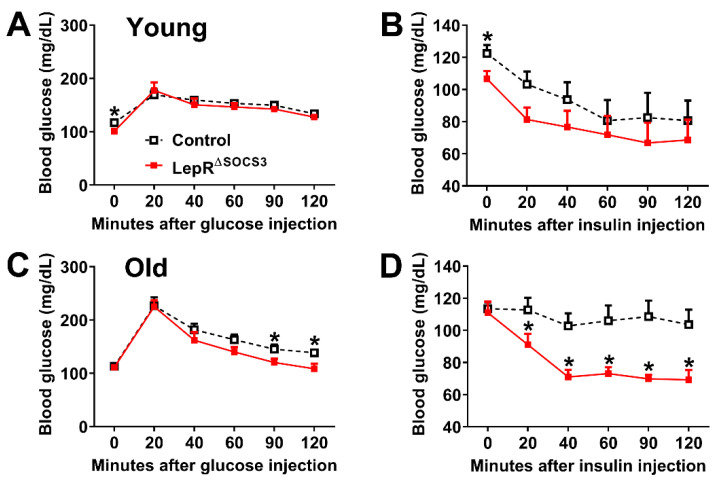
Then, possible changes in glucose homeostasis were evaluated. Although young adult LepR∆SOCS3 mice consistently showed reduced basal blood glucose concentrations, their glucose tolerance and insulin sensitivity were relatively similar to the control mice (Figure 2A,B). In contrast, middle-aged LepR∆SOCS3 mice displayed improved glucose tolerance and insulin sensitivity (Figure 2C,D). Thus, the absence of SOCS3 in LepR-expressing cells improves glucose homeostasis in aging mice.
Figure 2.
Improved glucose homeostasis in middle-aged LepR∆SOCS3 mice. (A,B) Changes in blood glucose concentrations during a glucose tolerance test and an insulin tolerance test in young adult (3-month-old) control (n = 11) and LepR∆SOCS3 (n = 10) mice. (C,D) Changes in blood glucose concentrations during a glucose tolerance test and an insulin tolerance test in middle-aged (15-month-old) control (n = 21–26) and LepR∆SOCS3 (n = 8–9) mice. Mean ± SEM. * p < 0.05 control vs. LepR∆SOCS3 (post-hoc test repeated measures two-way ANOVA).
2.2. Increased Leptin Sensitivity in Middle-Aged LepR∆SOCS3 Mice
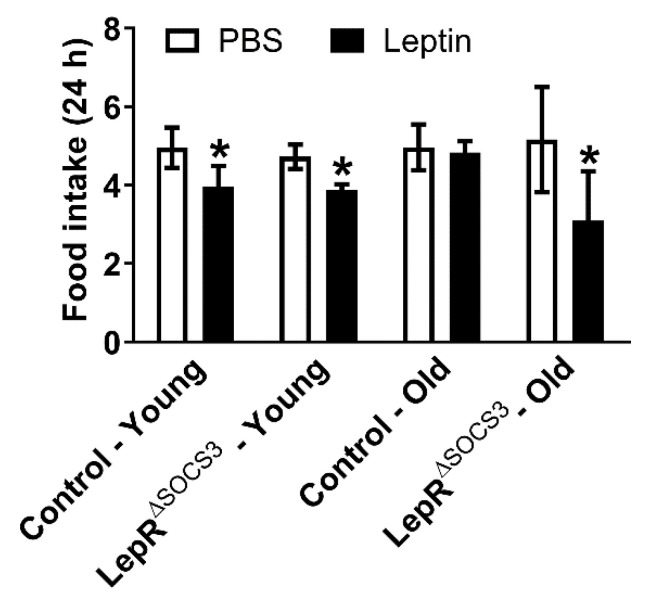
Aging induces leptin resistance and increases hypothalamic SOCS3 expression [33,34,35,36,37,38,39,40,41]. Thus, we determined whether SOCS3 ablation in LepR-expressing cells is sufficient to prevent aging-induced leptin resistance. As expected, an acute systemic leptin injection (2.5 µg/g body weight) reduced the food intake of young adult control mice (Figure 3). A similar reduction in food intake was observed in young adult LepR∆SOCS3 mice (Figure 3). In contrast, middle-aged control mice did not exhibit the anorexigenic response to leptin, suggesting leptin resistance. Importantly, middle-aged LepR∆SOCS3 mice remained responsive to leptin (Figure 3). Thus, SOCS3 ablation in LepR-expressing neurons is sufficient to prevent aging-induced leptin resistance.
Figure 3.
LepR∆SOCS3 mice are protected against aging-induced leptin resistance. Twenty-four hour food intake after an i.p. injection of PBS or leptin (2.5 µg/g body weight) in young adult (3-month-old) control (n = 6) and LepR∆SOCS3 (n = 7) mice and in middle-aged (15-month-old) control (n = 7) and LepR∆SOCS3 (n = 4) mice. Mean ± SEM. * p < 0.05 control vs. LepR∆SOCS3 (post-hoc test repeated measures two-way ANOVA).
2.3. Cardiac Abnormalities and Decreased Survival Rate in Aging LepR∆SOCS3 Mice
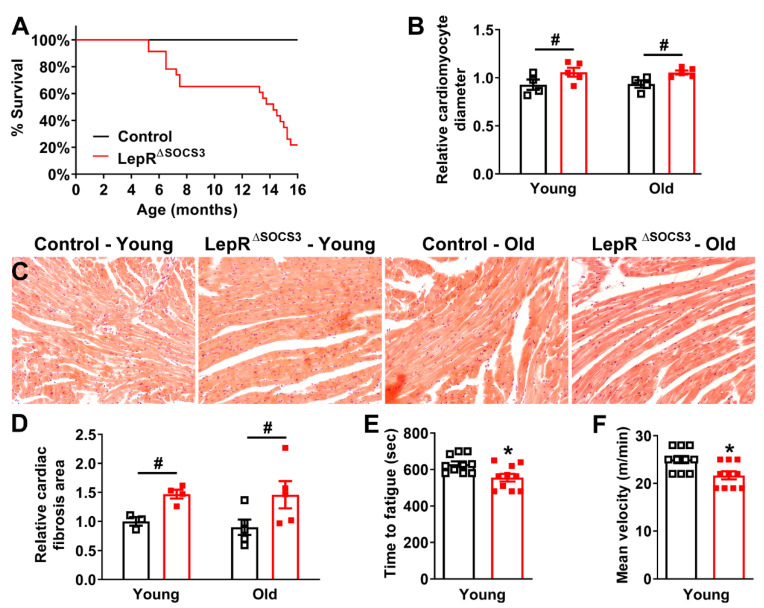
Although middle-aged LepR∆SOCS3 mice exhibited improvements in several metabolic parameters (e.g., reduced body weight and improved glucose homeostasis), we observed frequent deaths in LepR∆SOCS3 mice over time. Thus, a survival analysis was performed and, remarkably, a very high mortality rate was observed in aging LepR∆SOCS3 mice (p < 0.0001; Log-rank test). For example, only approximately 20% of the conditional knockout mice reached more than 16 months of life (Figure 4A). To identify the causes of this unexpected mortality, we investigated the presence of possible cardiac abnormalities in the mutant mice. The relative cardiomyocyte diameter was significantly increased in both young adult and middle-aged LepR∆SOCS3 mice (Figure 4B,C), indicating cardiomyocyte hypertrophy. Furthermore, SOCS3 ablation in LepR-expressing cells caused an increase in the relative collagen area of the heart in both young adult and middle-aged mice (Figure 4D), which indicates the development of myocardial fibrosis.
Figure 4.
Cardiac abnormalities and decreased survival rate in aging LepR∆SOCS3 mice. (A) Survival curve of control (black square; n = 27) and LepR∆SOCS3 (red square; n = 23) mice. (B) Relative cardiomyocyte transverse diameter in young adult (3-month-old; n = 4–5/group) and middle-aged (15-month-old; n = 4–5/group) mice. (C) Representative transverse heart sections stained with hematoxylin and eosin of young adult or middle-aged control and LepR∆SOCS3 mice. (D) Relative cardiac collagen area in young adult (n = 3–4/group) and middle-aged (n = 5/group) mice. (E,F) Time required until fatigue and mean velocity during an incremental treadmill maximal running test in young adult control (n = 10) and LepR∆SOCS3 (n = 10) mice. Mean ± SEM. * p < 0.05 control vs. LepR∆SOCS3 (t-test). # p < 0.05 SOCS3 ablation effect (two-way ANOVA).
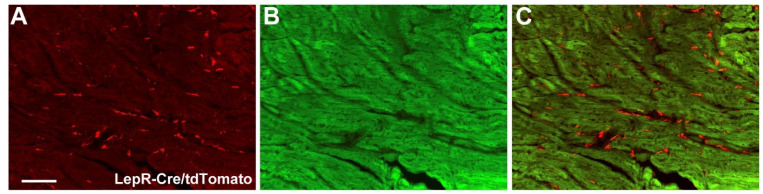
To determine whether cardiomyocytes express the long-isoform LepR (LepRb), we used a well-established LepRb-reporter mouse [42,43]. Although the tdTomato reporter protein was observed in the extracellular matrix, LepRb expression was practically absent in cardiomyocytes (Figure 5A,C).
Figure 5.
LepRb is not expressed in cardiomyocytes. (A–C) Epifluorescence photomicrographs showing the expression of tdTomato protein (red straining) in the heart of a LepR-reporter mouse. Note that tdTomato is expressed in the tissue matrix, but not in cardiomyocytes. Scale bar = 100 µm.
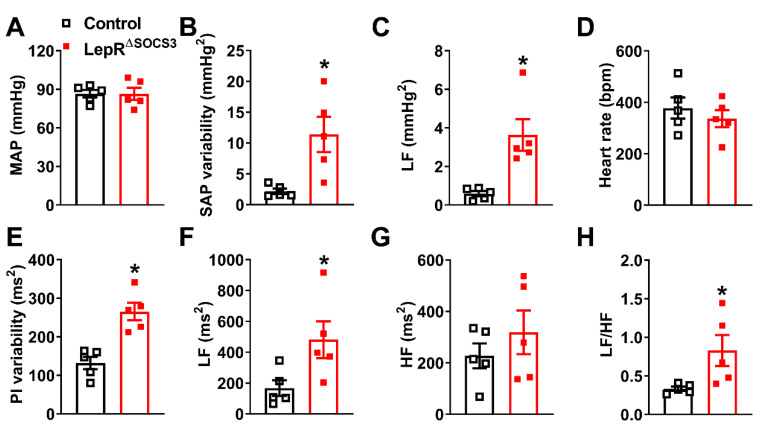
Subsequently, mean arterial pressure (MAP) and HR were determined. Although LepRΔSOCS3 mice exhibited similar MAP (Figure 6A) and HR (Figure 6D) as compared to the control mice, the systolic arterial pressure (SAP) and pulse interval (PI) variabilities were significantly increased in LepRΔSOCS3 mice (Figure 6B,E). Spectral analyses indicated an increase in the low-frequency (LF) SAP and PI variabilities in LepRΔSOCS3 mice (Figure 6C,F), whereas the high-frequency (HF) PI variability remained unchanged (Figure 6G). Consequently, the sympathovagal balance was significantly increased in LepRΔSOCS3 mice (Figure 6H). Considering the role played by the sympathetic nervous system regulating the LF variability of SAP and PI, and the increased LF/HF ratio [44,45,46,47], our findings suggest an increased sympathetic activity to the vasculature and heart in LepRΔSOCS3 mice.
Figure 6.
Increased sympathetic activity to the vasculature and heart in LepR∆SOCS3 mice. (A–C) Mean arterial pressure (MAP), systolic arterial pressure (SAP) variability and low-frequency (LF) variability of the SAP in approximately 8-month-old control (n = 5) and LepR∆SOCS3 (n = 5) mice. (D–G) Heart rate (HR), pulse interval (PI) variability and LF and high-frequency (HF) variability of the PI. (H) Sympathovagal balance (LF/HF ratio) of PI variability. Mean ± SEM. * p < 0.05 control vs. LepR∆SOCS3 (t-test).
To evaluate how SOCS3 ablation in LepR-expressing cells may affect functional aspects of the cardiovascular system, mice were subjected to an incremental treadmill maximal running test. Only young adult animals were used in this test since similar histological alterations were observed between young adult and middle-aged LepR∆SOCS3 mice. We observed that LepR∆SOCS3 mice fatigued faster (Figure 4E) and presented reduced mean velocity during the maximal test (Figure 4F) compared to control animals. Therefore, SOCS3 ablation in LepR-expressing cells leads to a lower survival rate, cardiac remodeling and reduced cardiovascular capacity that are apparent even in young adult animals.
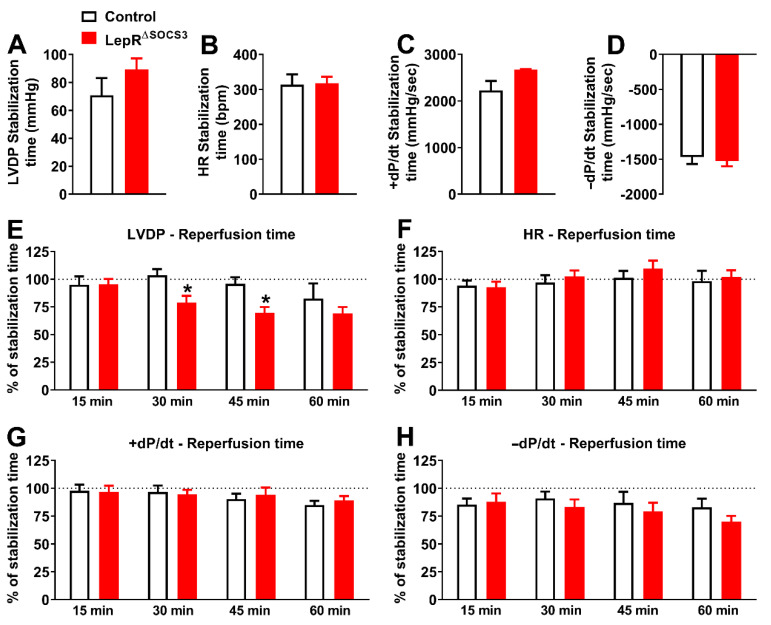
To further evaluate the cardiac function of LepR∆SOCS3 mice, the Langendorff technique of isolated heart perfusion was used [48]. Baseline cardiac function was assessed by measuring four different parameters during the stabilization time: left ventricular developed pressure (LVDP; mmHg), HR (bpm), the first derivative of the positive (+dP/dt; mmHg/s) and negative (−dP/dt; mmHg/s). LepRΔSOCS3 mice did not present alterations in LVDP, HR, +dP/dt, and −dP/dt relative to the respective control groups in both young adult (Figure 7A–D) and middle-aged animals (Figure 8A–D). After the ischemia period, a temporal analysis of cardiac parameters at 15, 30, 45 and 60 min of reperfusion was evaluated. Functional cardiac recovery values were expressed as a percentage of baseline values (during stabilization) in each group. In two different times of reperfusion period (30 and 45 min), the LVDP percentage of recovery significantly decreased in young adult LepRΔSOCS3 mice (Figure 7E), suggesting impairment of post-ischemic cardiac functional recovery. The HR, +dP/dt and −dP/dt did not differ between groups of young adult mice (Figure 7F–H).
Figure 7.
Impairment of post-ischemic cardiac functional recovery in young adult LepRΔSOCS3 mice. (A–D) Baseline cardiac function including left ventricular developed pressure (LVDP), heart rate (HR), first derivative of the positive (+dP/dt) and negative (−dP/dt) ventricular pressure in young adult control (n = 6) and LepR∆SOCS3 (n = 5) mice. (E–H) Temporal analysis of cardiac parameters at 15, 30, 45 and 60 min of reperfusion in young adult mice. Mean ± SEM. * p < 0.05 control vs. LepR∆SOCS3 (post-hoc test repeated measures two-way ANOVA).
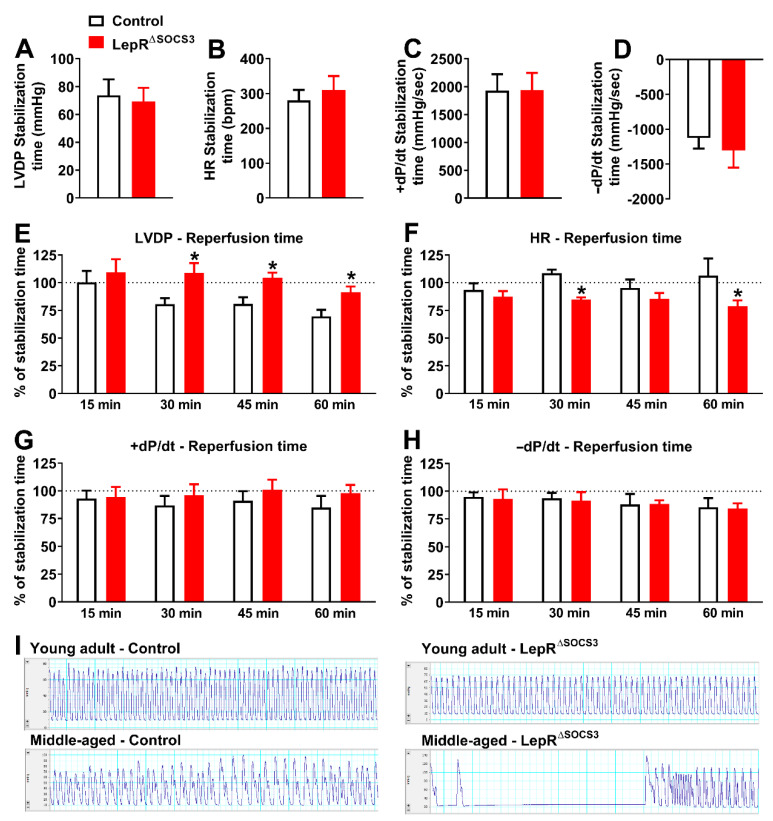
Figure 8.
Substantial arhythmic events during the reperfusion period in middle-aged LepRΔSOCS3 mice despite an apparent improvement in the post-ischemic cardiac functional recovery. (A–D) Baseline cardiac functions including left ventricular developed pressure (LVDP), heart rate (HR), first derivative of the positive (+dP/dt) and negative (−dP/dt) ventricular pressure in middle-aged control (n = 7) and LepR∆SOCS3 (n = 6) mice. (E–H) Temporal analysis of cardiac parameters at 15, 30, 45 and 60 min of reperfusion in middle-aged mice. (I) Representative recordings during the reperfusion period showing a robust arhythmic event in middle-aged LepRΔSOCS3 mice. Mean ± SEM. * p < 0.05 control vs. LepR∆SOCS3 (post-hoc test repeated measures two-way ANOVA).
When middle-aged animals were evaluated, LepRΔSOCS3 mice presented an increase in LVDP at 30, 45 and 60 min of the reperfusion, when compared to the control group (Figure 8E). HR was reduced in middle-aged LepRΔSOCS3 mice at 30 and 60 min of the reperfusion (Figure 8F). No differences between groups were found in the cardiac inotropism (+dP/dt) and lusiotropism (−dP/dt) during the reperfusion period (Figure 8G–H). Thus, in contrast to young adult LepRΔSOCS3 mice, middle-aged LepRΔSOCS3 mice exhibited an apparent improvement in the post-ischemic cardiac functional recovery. However, substantial arhythmic events were consistently observed in middle-aged LepRΔSOCS3 mice during the reperfusion period (Figure 8I). Therefore, SOCS3 ablation in LepR-expressing cells causes several cardiac abnormalities, which can compromise the cardiovascular capacity and possibly the survival of aging mice.
2.4. LepR∆SOCS3 Mice Exhibit Fasting-Induced Hypoglycemia That Is Associated with Reduced Gluconeogenesis
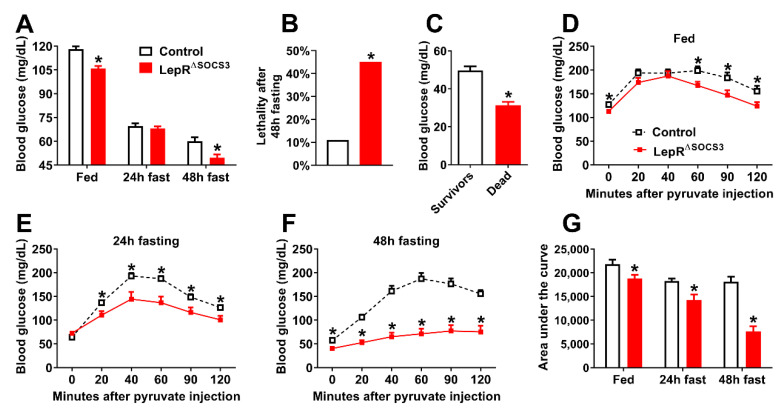
To investigate whether LepR∆SOCS3 mice may present defects in the control of other neurovegetative functions, mice were subjected to 24 h or 48 h fasting to evaluate their ability to maintain glycemia, which is highly dependent on brain centers influenced by leptin action [49,50,51,52,53,54,55]. LepR∆SOCS3 mice showed lower blood glucose concentrations at the fed state (Figure 9A). Although 24 h fasting reduced the glycemia similarly in the control and the LepR∆SOCS3 mice, the conditional knockout mice exhibited hypoglycemia when fasting was maintained for 48 h (Figure 9A). After providing food to the 48 h fasted mice, approximately 10% of the control animals were unable to recover and died (Figure 9B). However, a higher percentage of the LepR∆SOCS3 mice (~45%) were unable to recover from the fasting (Figure 9B). Comparing the mice that survived and those that died, we observed reduced glycemia in the mice that died after prolonged fasting (Figure 9C). Thus, the ability to sustain glycemia during fasting was probably decisive in increasing their chances of survival. To maintain glycemia during prolonged fasting, it is imperative that hepatic gluconeogenesis works properly [52,56]. Pyruvate tolerance tests were performed, and LepR∆SOCS3 mice exhibited impaired hepatic gluconeogenesis in the fed state and after 24 h or 48 h of fasting (Figure 9D–F). Of note, the longer the fasting time, the worse the gluconeogenesis capacity of LepR∆SOCS3 mice (Figure 9G). Thus, the inability to perform gluconeogenesis, when necessary, explains the intolerance to fasting and the high mortality rate of the LepR∆SOCS3 mice during prolonged food deprivation.
Figure 9.
LepR∆SOCS3 mice exhibit fasting-induced hypoglycemia that is associated with reduced gluconeogenesis. (A) Blood glucose concentrations at the fed state or after 24 h or 48 h fasting in young adult control (n = 40) and LepR∆SOCS3 (n = 32) mice. (B) Percentage of mice that died after a 48 h fasting period in control and LepR∆SOCS3 mice. (C) Blood glucose concentrations in mice that died (n = 20) or survived (n = 27) the 48 h fasting period (including the refeeding period). (D–F) Blood glucose concentrations during pyruvate tolerance tests in young adult control (n = 20–21) and LepR∆SOCS3 (n = 8–11) mice at the fed state or during 24 h or 48 h fasting. (G) Area under the curve of the pyruvate tolerance tests in control and LepR∆SOCS3 mice at the fed state or during 24 h or 48 h fasting. Mean ± SEM. * p < 0.05 control vs. LepR∆SOCS3 (post-hoc test two-way ANOVA or t-test).
2.5. Possible Alterations in the Sympathetic Nervous System Explain the Impaired Gluconeogenesis and Counterregulatory Response of LepR∆SOCS3 Mice
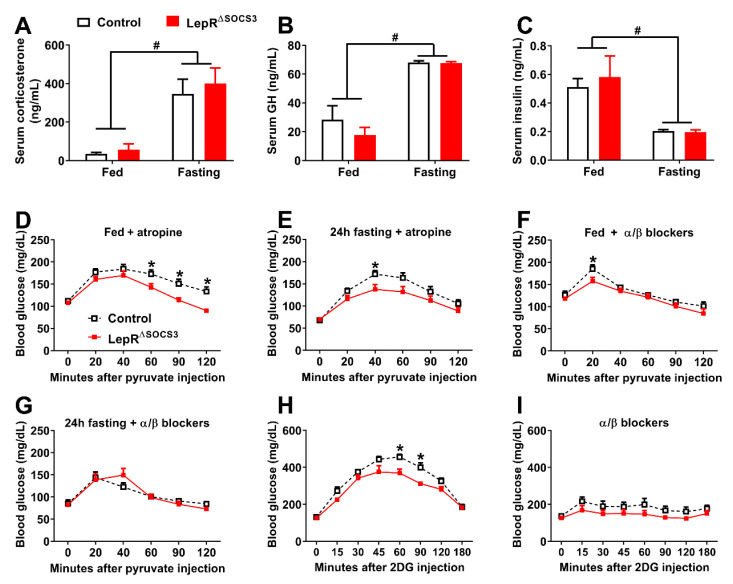
During fasting or hypoglycemia, several counterregulatory hormones are secreted, whereas a decrease in plasma insulin concentrations is observed [49,52,53,55]. To determine the physiological mechanisms behind the impaired gluconeogenesis of LepR∆SOCS3 mice during fasting, the concentrations of several counterregulatory hormones were assessed. Fasting led to an increase in corticosterone and growth hormone (GH) concentrations (Figure 10A,B). However, no differences between the control and the LepR∆SOCS3 mice were observed either in the fed state or during fasting (Figure 10A,B). In contrast, serum insulin concentrations decreased during fasting without differences between the groups (Figure 10C). Since similar hormonal responses were observed, we investigated the role of the autonomic nervous system in controlling hepatic gluconeogenesis in LepR∆SOCS3 mice. To block the parasympathetic nervous system, pyruvate was co-infused with atropine, a muscarinic receptor antagonist. Similar to our previous results, LepR∆SOCS3 mice showed a lower gluconeogenic capacity either in the fed state or in fasted animals, despite the blockade of the parasympathetic nervous system (Figure 10D,E). Conversely, the blockade of the sympathetic nervous system by the co-infusion of α and β antagonists made the differences between control and LepR∆SOCS3 mice disappear, since a similar area under the curve in the pyruvate tolerance tests was observed at the fed state or during fasting (Figure 10F,G).
Figure 10.
Possible alterations in the sympathetic nervous system explain the impaired gluconeogenesis and counterregulatory response of LepR∆SOCS3 mice. (A–C) Serum corticosterone, GH and insulin concentrations in the fed state or after 24 h fasting in young adult control (n = 5–8) and LepR∆SOCS3 (n = 4–8) mice. (D–G) Blood glucose levels during pyruvate tolerance tests in young adult control (n = 6–14) and LepR∆SOCS3 (n = 5–13) mice at the fed state or during 24 h fasting in combination with the co-infusion of atropine (D,E) or α/β blockers (F,G). (H,I) Blood glucose levels show the counterregulatory response induced by 2DG injection in young adult control (n = 7–9) and LepR∆SOCS3 (n = 7–9) mice. Mean ± SEM. # p < 0.05 fasting effect (two-way ANOVA). * p < 0.05 control vs. LepR∆SOCS3 (post-hoc test repeated measures two-way ANOVA).
To additionally determine the capacity of LepR∆SOCS3 mice to prevent glucopenia, the counterregulatory response to the infusion of 2-deoxy-D-glucose (2DG) was evaluated. Confirming the reduced capacity to prevent glucopenia, LepR∆SOCS3 mice showed a blunted counterregulatory response to 2DG (Figure 10H). Furthermore, the difference in the counterregulatory response between the control and the LepR∆SOCS3 mice was no longer observed when the sympathetic nervous system was pharmacologically blocked (Figure 10I). Gut motility is mainly regulated by the parasympathetic nervous system [57,58]. Gut motility was evaluated in the experimental animals and no differences between control (0.72 ± 0.04 arbitrary units) and LepR∆SOCS3 (0.73 ± 0.03 arbitrary units; p = 0.8694) mice were observed. Taken together, LepR∆SOCS3 mice probably exhibit alterations in the activity of the sympathetic nervous system leading to defects in their capacity to maintain glycemia during conditions of glucopenia. In contrast, no evidence of alterations in the activity of the parasympathetic nervous system was observed in LepR∆SOCS3 mice.
3. Discussion
Some degree of leptin resistance is frequently observed in obese individuals [5,6]. Considering that the activation of LepR in hypothalamic neurons reduces food intake and may increase energy expenditure and fat oxidation [2,7], leptin sensitizing therapies will likely produce robust beneficial effects in the prevention and treatment of obesity [6,7,26]. However, several neurocircuits activated by leptin lead to increased activity of the sympathetic system [20,59,60,61] and inhibition of hepatic glucose production [16,49,52,53]. Therefore, leptin sensitizing treatments may cause side effects, especially regarding the regulation of these autonomic functions. Accordingly, central leptin action increases the release of endogenous agonists of the melanocortin-4 receptor (MC4R), such as the α-melanocyte-stimulating hormone [7,62]. Although the activation of MC4R produces satiety, which is interesting from the point of view of treating obesity, it also increases sympathetic activity and blood pressure, which limits the clinical use of MC4R agonists [63,64]. In the present study, we investigated whether increased leptin sensitivity produces beneficial or deleterious effects in aging mice. As expected, increased leptin sensitivity improved energy and glucose metabolism in middle-aged animals. However, these beneficial consequences were insufficient to improve health and longevity, since LepR∆SOCS3 mice exhibited a high mortality rate, morphofunctional abnormalities in the cardiovascular system and impaired ability to maintain blood glucose concentrations in situations of glucopenia.
LepR∆SOCS3 mice were well-characterized in previous studies and showed increased leptin sensitivity, resistance against diet-induced leptin resistance and improved energy and glucose metabolism in different situations [27,28,29,30,31,32]. Thus, the reduced body weight and improved insulin sensitivity observed in middle-aged LepR∆SOCS3 mice were expected results. Aging increases SOCS3 expression in the hypothalamus [34] and aged animals exhibit central leptin resistance [33,35,36,37,38,39]. Since middle-aged LepR∆SOCS3 mice remained responsive to the anorexigenic effect of leptin, our findings suggest that the SOCS3 protein plays a key role in the development of aging-induced leptin resistance. However, it is important to mention that SOCS3 also modulates the activity of other cytokines besides leptin [65]. Consequently, we cannot rule out the possibility that part of the phenotype exhibited by LepR∆SOCS3 mice is not necessarily associated with leptin action but is caused by changes in the sensitivity to other hormones. This confounding factor should be considered when analyzing our findings.
Surprisingly, LepR∆SOCS3 mice showed increased mortality, precluding us to study animals older than 16 months of life. The causes of death were undetermined, but our general impression was that LepR∆SOCS3 mice were more fragile and appeared to be in worse physical condition than age-matched control animals. Since the improved metabolic parameters of LepR∆SOCS3 mice could not explain their increased mortality, we investigated possible alterations in the cardiovascular system, considering the well-established role of leptin in regulating cardiovascular functions via the sympathetic nervous system [19,20,61,63,66]. Using different cohorts of mice and after analyzing several morphofunctional parameters, both young adult and middle-aged LepR∆SOCS3 mice exhibit key alterations in the cardiovascular system. The cardiomyocyte hypertrophy associated with increased myocardial fibrosis help to explain the lower cardiovascular capacity of LepR∆SOCS3 mice during an incremental treadmill maximal running test, as compared to control mice. Importantly, these alterations were already detected in young adult mice, indicating that they were independent of aging. Additionally, there is evidence showing that leptin’s effects on blood pressure are also independent of changes in body weight [19,67].
Previous studies have shown that leptin can regulate cardiac functions either indirectly via the sympathetic nervous system [19,20,59,61,63,64,68] or by acting directly in the heart [69,70,71,72,73,74]. Lepr mRNA expression is detected in the heart [69,70,72]. However, immunohistological analysis shows controversial results, in which some studies demonstrate Lepr expression in cardiomyocytes [71], while others detect this receptor only in pathological conditions, such as after ischemia and reperfusion [69]. Since our conditional gene deletion was driven by the LepRb-Cre mice [43], we crossed this mouse model with a Cre-dependent tdTomato reporter mice to visualize LepRb-expressing cells in the heart. Using this approach, we only observed LepRb-expressing cells appear in the extracellular matrix and not in the cardiomyocytes. Thus, these findings suggest that SOCS3 deletion did not seem to directly affect cardiomyocytes, therefore, the cardiovascular dysfunctions observed in LepR∆SOCS3 mice were likely mediated by the central effects of leptin, which mainly involves the regulation of the activity of the sympathetic nervous system. Although we did not observe significant changes in the basal HR and MAP between the groups, spectral analysis of the SAP and PI waveforms identified increased LF variability and sympathovagal balance in LepR∆SOCS3 mice. These alterations can be explained by a suggested higher sympathetic activity [44,45,46,47] and they are in accordance with the well-established stimulatory effect of leptin, as well as the melanocortin system, on the sympathetic nervous system [19,20,59,61,63,64,68].
Using the Langendorff technique of isolated heart perfusion, we observed that young adult LepR∆SOCS3 mice exhibit impairment of post-ischemic cardiac functional recovery. In contrast, middle-aged LepR∆SOCS3 mice showed increased LVDP during the reperfusion. Although speculative, it is possible that the improved metabolic status of middle-aged LepR∆SOCS3 mice, as compared to control aged mice, has contributed to better cardiac function and recovery after ischemia. Nonetheless, severe arrhythmic events were frequently observed in the hearts of middle-aged LepRΔSOCS3 mice during the reperfusion period. Leptin can increase ischemia-related ventricular arrhythmias via sympathetic nerve activation [75]. Considering that obesity, which is a hyperleptinemia condition, represents an important risk factor for heart failure [76], more studies are necessary to investigate the possible association between increased leptin sensitivity and the occurrence of arhythmic events, especially in aged individuals.
Besides the role of leptin in regulating the cardiovascular system, hepatic glucose production is also under the control of leptin-responsive neurons [16,17,18]. Central leptin infusion improves hepatic insulin resistance and markedly inhibits glucose production [17,18]. These effects likely depend on POMC neurons since LepR expression only in POMC-expressing cells is sufficient to suppress hepatic glucose production and normalize blood glucose levels of otherwise LepR-deficient mice [16]. Agouti-related peptide-expressing neurons are also able to regulate systemic insulin resistance and they mediate the glucose-lowering action of leptin [77,78]. Thus, several LepR-expressing neuronal populations in the hypothalamus are able to regulate systemic glucose homeostasis.
Although the inhibition of hepatic glucose production is beneficial for the prevention of type 2 diabetes mellitus, there are situations, such as prolonged fasting, in which the activation of endogenous glucose production is necessary. We observed that LepR∆SOCS3 mice are not tolerant to prolonged (48 h) fasting because these animals develop hypoglycemia leading to a high mortality rate. Using pyruvate tolerance tests, we observed that LepR∆SOCS3 mice exhibit suppressed hepatic gluconeogenesis. Furthermore, the ability of LepR∆SOCS3 mice to convert pyruvate to glucose decreased over the fasting period, which helps to explain their fasting-induced hypoglycemia. The impaired capacity of LepR∆SOCS3 mice to recover from glucoprivic situations was further confirmed by the decreased counterregulatory response exhibited after 2DG injection. The concentrations of some counterregulatory hormones were determined in fed and fasted animals and no significant differences were observed. In addition, the co-infusion of atropine, a parasympathetic blocker, did not prevent the differences between the control and the LepR∆SOCS3 mice in blood glucose levels during the pyruvate tolerance tests. Thus, neither neuroendocrine defects nor the parasympathetic nervous system seems to account for the defects in glucose production exhibited by LepR∆SOCS3 mice. In contrast, co-infusion of α and β blockers with either pyruvate or 2DG was sufficient to abolish the differences between the groups. Therefore, the suppressed glucose production of LepR∆SOCS3 mice likely relies on alterations in the activity of the sympathetic nervous system.
Interestingly, SOCS3 ablation in steroidogenic factor-1 (SF1)-expressing cells, which includes the ventromedial nucleus of the hypothalamus (VMH), reduces blood glucose levels in fed and fasted mice, even though the animals do not develop hypoglycemia [79]. The prevention of glutamatergic neurotransmission from VMH neurons also reduces fasting glycemia and the counterregulatory response to hypoglycemia [80]. Thus, changes in leptin sensitivity in VMH neurons are possibly underlying the reduced capacity of LepR∆SOCS3 mice to recover from glucoprivic situations. Furthermore, since the blockade of the sympathetic nervous system prevents the defects exhibited by LepR∆SOCS3 mice, our findings are in accordance with the literature indicating that VMH neurons regulate glucose homeostasis predominantly via the sympathetic nervous system [52,68].
In conclusion, we provided evidence that mice carrying SOCS3 ablation in LepR-expressing cells retain leptin sensitivity and present improved energy and glucose homeostasis during aging. However, these animals display a high mortality rate, and they develop cardiomyocyte hypertrophy, increased myocardial fibrosis, reduced cardiovascular capacity and impaired cardiac functional recovery after ischemia. LepR∆SOCS3 mice also show fasting-induced hypoglycemia, which is caused by a profound reduction in hepatic glucose production. These neurovegetative dysfunctions are likely mediated by alterations in the sympathetic nervous system, compromising their health and longevity. In the context of therapies that seek to chronically increase leptin sensitivity for the treatment of obesity, these potentially negative aspects need to be considered and carefully evaluated in pre-clinical and clinical studies.
4. Materials and Methods
4.1. Animals
To induce SOCS3 ablation in cells that express the LepRb, the LepRb-IRES-Cre strain (Strain #008320, The Jackson Laboratory, Bar Harbor, ME, USA) was initially bred with SOCS3flox/flox mice (Strain #010944, The Jackson Laboratory). After successive generations, mice homozygous for the LepRb-Cre allele and heterozygous for the loxP-flanked Socs3 allele were continuously bred to generate the experimental animals. Mice carrying SOCS3 ablation in LepR-expressing cells were homozygous for both loxP-flanked Socs3 and LepRb-Cre alleles (hereafter named LepR∆SOCS3), whereas the control group was composed of littermate mice carrying only the LepRb-Cre allele. LepRb-reporter mouse was produced as previously described [42]. In the experiments, we used either young adult (approximately 3-month-old) or middle-aged (approximately 15-month-old) mice. Only male mice were used in the experiments. Animals were maintained in standard conditions of light (12 h light/dark cycle; lights on at 8:00) and temperature (22 ± 2 °C). Mice received a regular rodent chow (2.99 kcal/g; 9.4% kcal derived from fat; Nuvilab CR-1, Quimtia, Brazil) and filtered water ad libitum, except when indicated. The animal procedures were previously approved by the Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences at the University of São Paulo (protocol number 12/2013).
4.2. Energy and Glucose Homeostasis
The body weight of control and LepR∆SOCS3 mice were determined weekly from two months of age to approximately 15 months of life. After that, mice were single-housed for acclimation, followed by determination of food intake for 4 consecutive days. VO2, CO2 production, ambulatory activity (by infrared sensors) and respiratory quotient (CO2 production/O2 consumption) were determined using the Oxymax/Comprehensive Lab Animal Monitoring System (Columbus Instruments, Athens, OH, USA) for approximately 7 days. The data from the first 3 days of analysis were discarded because we considered the acclimatization period. The results used for each animal were the average of the analyzed days. Subsequently, glucose tolerance tests and insulin tolerance tests were performed. Initially, food was removed from cage 4 h before each test. After the evaluation of basal glucose level (time 0), mice received an intraperitoneal (i.p.) injection of 1 IU insulin/kg or 1 g glucose/kg in middle-aged mice or 2 g glucose/kg in young adult mice, followed by serial determinations of glycemia. During the entire follow-up period, any death of the animals was recorded in order to determine the survival curve.
4.3. Tissue Collection and Processing
Mice were euthanized by decapitation after 4 h of food deprivation. The masses of the subcutaneous, perigonadal and retroperitoneal fat pads were determined. Transverse heart sections were fixed in 4% paraformaldehyde overnight and stored in 70% ethanol. After dehydration, samples were embedded in paraffin and sectioned into 5 μm-thick slices. Transverse heart sections were stained with picrosirius red to determine myocardial collagen deposition (n = 4–5 hearts per group). The cardiac fibrosis area was measured, and the results are presented as relative fibrosis area in relation to total cardiac area. Transverse heart sections were stained with hematoxylin and eosin to quantify the relative cardiomyocyte transverse diameter (n = 50 cardiomyocytes per heart containing visible cell limits and central nuclei, n = 4–5 hearts per group). Images were obtained using a light microscope (Nikon Eclipse E600 microscope, Tokyo, Japan) and Nikon Software Nis-Elements AR (Hlinsko, Czech Republic) and measured using ImageJ software. Gastrointestinal motility was assessed by using the charcoal method as described previously [58].
4.4. Incremental Treadmill Maximal Running Test
Mice were adapted on the treadmill for 3 consecutive days at 5 m/min speed for 10 min. This speed was maintained to avoid a training effect. Then, mice performed an incremental treadmill maximal running test. Starting at 10 m/min, the speed was increased by 3 m/min every 3 min. The test stopped when the animal was incapable to maintain the running speed for more than 5 s. The time required to reach fatigue and the mean velocity of the test were determined.
4.5. Leptin Sensitivity
The feeding response to leptin was determined in mice that received an i.p. injection of phosphate-buffered saline (PBS) or mouse recombinant leptin (2.5 µg/g body weight; from Dr. A.F. Parlow, NHPP, Torrance, CA, USA) 2 h before the dark phase, and their food intake was recorded 24 h later. All animals received both injections, so each mouse acted as its own control, and we compared the food intake after PBS and leptin administration.
4.6. Constant Flow Langendorff Preparation
Young adult and middle-aged mice were euthanized by decapitation 10 min after an i.p. heparin sodium injection (150 IU). Immediately after emergency thoracotomy and rapid cardiac arrest by superfusion with ice-cold isotonic saline, the hearts were rapidly attached to the Langendorff apparatus (ADInstruments, Castle Hill, NSW, Australia) via aortic cannulation (21-gauge needles) and then were retrogradely perfused under a constant perfusion flow (3 mL/min). The composition of Krebs-Henseleit (KH) perfusate was as follows (mM): NaCl (118), KCl (4.7), CaCl2 (1.75), MgSO4 (1.66), NaHCO3 (24.88), KH2PO4 (1.18), dextrose 2 g/L, bi-distilled water and equilibrated with 95% O2 and 5% CO2, warmed at 37 ± 1 °C and pH of 7.4.
The KH buffer was prepared at the time of the experiment and filtered (47 mm Swinnex®, Merck KGaA, Darmstadt, Germany; membrane pore 0.22 mM) immediately before infusion [48]. The hearts from all groups were made to undergo a stabilization period of 30 min and then were subjected to global ischemia by stopping the perfusion pump. The flow was returned after 20 min, and the hearts were reperfused for 60 min. A short cannula was passed via the mitral valve and pierced through the apex of the left ventricle (LV) to vent Thebesian drainage. Shortly thereafter, a water-filled polyvinyl chloride film balloon was inserted into the left ventricle through an incision in the left atrial appendage, via the mitral valve, and secured by a ligature [48]. The ventricular balloon was connected via fluid-filled tubing to a pressure transducer coupled to software (LabChart 8.0) for assessment of ventricular performance during full experiment. The balloon was filled to yield a left ventricular end-diastolic pressure of 5–10 mmHg during the initial 10 min of stabilization; after which, the pressure was not further adjusted. To assess left ventricular systolic function, LVDP, +dP/dt, −dP/dt and HR were measured. All these parameters were monitored continuously by a recording system (PowerLab Chart 8-Lab, ADInstruments, Castle Hill, NSW, Australia).
4.7. Hemodynamic Recordings and Spectral Analysis
The animals were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (7 mg/kg; i.p.) and were implanted with a Micro-Renathane tubing catheter into the femoral artery for direct AP measurement. The muscle and skin layers were separately sutured, and the animals were treated with antibiotic protection (160,000 U kg−1 benzylpenicillin, intramuscular). On the next day, MAP and HR were acquired in non-anesthetized unrestrained mice in a time frame of 3 h. Baseline MAP, SAP and PI signals were recorded by connecting the arterial catheter to a pressure transducer (MLT0699, ADInstruments), which was coupled to a preamplifier (FE221 Bridge Amp, ADInstruments), and to a computer data acquisition system (model PowerLab 8SP, ADInstruments).
Spectral analysis of the SAP and PI waveforms was performed using the software CardioSeries (https://www.danielpenteado.com/; CardioSeries V2.4). A stable 10 min of SAP and PI records without artifacts or large sudden blood pressure changes of each animal were processed with computer software (LabChart Pro, ADInstruments) that employs an algorithm to detect beat-to-beat inflection points in the pulsatile arterial pressure signal. Beat-by-beat series with SAP and PI values were generated and loaded into the CardioSeries V2.4 software in order to perform cardiovascular variability within time and frequency domain analysis [81].
SAP and PI power spectral density were estimated by a fast Fourier transform algorithm for time series. Using 10 Hz of interpolation rate, beat-by-beat series were divided into half-overlapping sequential sets with 512 points. The spectra of SAP and PI were integrated into LF (0.2–0.75 Hz) and HF (0.75–3 Hz) bands. The results were expressed in absolute (ms2) and normalized units, obtained by calculating the percentage of LF and HF power with regard to the total power of the spectrum minus the very low-frequency band (<0.2 Hz) [82]. Sympatho-vagal balance, the LF/HF ratio of PI variability, was also calculated [44,81].
4.8. Glucose Responses to Fasting, Pyruvate and 2-Deoxy-D-Glucose
Blood glucose levels were determined after 24 h and 48 h of fasting using a glucometer (One Touch Ultra; Johnson & Johnson, New Brunswick, NJ, USA). Glycemia at the fed state was also determined in mice without access to food for 4 h to standardize the postprandial state. Enzyme-linked immunosorbent assays were used to determine the serum concentrations of corticosterone (Arbor Assays, Ann Arbor, MI, USA), GH (Millipore, Billerica, MA, USA) and insulin (Crystal Chem, Downers Grove, IL, USA). These assays have a limit of detection determined as 20.9 pg/mL, 0.07 ng/mL and 0.1 ng/mL, respectively, and intra- and inter-assay coefficients of variability ≤ 10%. Pyruvate tolerance tests (0.5 g pyruvate/kg; Sigma-Aldrich, St. Louis, MO, USA) were performed in mice at the fed state (4 h of food removal) or after 24 h and 48 h of fasting. The changes in blood glucose levels induced by an i.p. injection of 2DG (0.5 g/kg; Sigma-Aldrich) were also determined. The participation of the autonomic nervous system in the responses to these tests was determined by the co-infused of pyruvate or 2DG with either a muscarinic receptor blocker (3 mg/kg atropine; Sigma-Aldrich) or a combination of α and β receptor blockers (3 mg/kg phentolamine and 0.5 mg/kg propranolol, respectively; Sigma-Aldrich).
4.9. Statistical Analysis
The unpaired two-tailed Student’s t-test was used to compare data between two groups. Repeated measures two-way ANOVA, followed by Fisher’s least significant difference post-hoc test were used to compare changes along time or in the same animal after different treatments. Log-rank test was used to compare the survival between control and LepR∆SOCS3 mice. The GraphPad Prism software (GraphPad, San Diego, CA, USA) was used for the statistical analyses and generation of the graphs. Data were expressed as mean ± standard error of the mean (SEM).
Acknowledgments
We thank Ana Maria P. Campos for the technical assistance.
Author Contributions
Conceptualization, J.D.J.; methodology, T.S.M., G.P.D. and M.L.M.B.-C.; formal analysis, J.A.B.P., I.B.d.S., T.T.Z., T.S.M., G.P.D. and M.L.M.B.-C.; investigation, J.A.B.P., I.B.d.S., T.T.Z., L.T.T. and A.P.T.T.; data curation, J.D.J.; writing—original draft preparation, J.D.J.; supervision, J.D.J.; project administration, J.D.J.; funding acquisition, J.D.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences at the University of São Paulo (protocol number 12/2013).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP/Brazil; grant numbers: 2013/25032-2 to J.A.B.P.; 2011/12893-4 and 2013/04703-6 to I.B.d.S.; 2012/15517-6 to T.T.Z.; 2010/18086-0 to J.D.J.). The APC was funded by FAPESP (grant number: 2020/01318/8 to J.D.J.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gadde K.M., Martin C.K., Berthoud H.R., Heymsfield S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018;71:69–84. doi: 10.1016/j.jacc.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymsfield S.B., Wadden T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 3.Abarca-Gómez L., Abdeen Z.A., Hamid Z.A., Abu-Rmeileh N.M., Acosta-Cazares B., Acuin C., Adams R.J., Aekplakorn W., Afsana K., Aguilar-Salinas C.A., et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Balland E., Cowley M.A. New insights in leptin resistance mechanisms in mice. Front. Neuroendocr. 2015;39:59–65. doi: 10.1016/j.yfrne.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Andreoli M.F., Donato J., Cakir I., Perello M. Leptin resensitisation: A reversion of leptin-resistant states. J. Endocrinol. 2019;241:R81–R96. doi: 10.1530/JOE-18-0606. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Lobo A.M., Donato J., Jr. The role of leptin in health and disease. Temperature. 2017;4:258–291. doi: 10.1080/23328940.2017.1327003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hukshorn C.J., Saris W.H., Westerterp-Plantenga M.S., Farid A.R., Smith F.J., Campfield L.A. Weekly subcutaneous pegylated recombinant native human leptin (peg-ob) administration in obese men. J. Clin. Endocrinol. Metab. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 9.Heymsfield S.B., Greenberg A.S., Fujioka K., Dixon R.M., Kushner R., Hunt T., Lubina J.A., Patane J., Self B., Hunt P., et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. Jama. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 10.Lee J., Liu J., Feng X., Salazar Hernandez M.A., Mucka P., Ibi D., Choi J.W., Ozcan U. Withaferin a is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 2016;22:1023–1032. doi: 10.1038/nm.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J., Lee J., Salazar Hernandez M.A., Mazitschek R., Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevaskis J.L., Coffey T., Cole R., Lei C., Wittmer C., Walsh B., Weyer C., Koda J., Baron A.D., Parkes D.G., et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: Magnitude and mechanisms. Endocrinology. 2008;149:5679–5687. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- 13.Tam C.S., Lecoultre V., Ravussin E. Novel strategy for the use of leptin for obesity therapy. Expert Opin. Biol. Ther. 2011;11:1677–1685. doi: 10.1517/14712598.2011.619974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quarta C., Sanchez-Garrido M.A., Tschop M.H., Clemmensen C. Renaissance of leptin for obesity therapy. Diabetologia. 2016;59:920–927. doi: 10.1007/s00125-016-3906-7. [DOI] [PubMed] [Google Scholar]
- 15.Hung H.Y., Qian K., Morris-Natschke S.L., Hsu C.S., Lee K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012;29:580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- 16.Berglund E.D., Vianna C.R., Donato J., Jr., Kim M.H., Chuang J.C., Lee C.E., Lauzon D.A., Lin P., Brule L.J., Scott M.M., et al. Direct leptin action on pomc neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Investig. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pocai A., Morgan K., Buettner C., Gutierrez-Juarez R., Obici S., Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–3189. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- 18.Van den Hoek A.M., Teusink B., Voshol P.J., Havekes L.M., Romijn J.A., Pijl H. Leptin deficiency per se dictates body composition and insulin action in ob/ob mice. J. Neuroendocr. 2008;20:120–127. doi: 10.1111/j.1365-2826.2007.01626.x. [DOI] [PubMed] [Google Scholar]
- 19.Simonds S.E., Pryor J.T., Ravussin E., Greenway F.L., Dileone R., Allen A.M., Bassi J., Elmquist J.K., Keogh J.M., Henning E., et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enriori P.J., Sinnayah P., Simonds S.E., Garcia Rudaz C., Cowley M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Münzberg H., Flier J.S., Bjørbæk C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 22.Bjorbaek C., Elmquist J.K., Frantz J.D., Shoelson S.E., Flier J.S. Identification of socs-3 as a potential mediator of central leptin resistance. Mol. Cell. 1998;1:619–625. doi: 10.1016/S1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 23.Briancon N., McNay D.E., Maratos-Flier E., Flier J.S. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1b reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes. 2010;59:3074–3084. doi: 10.2337/db10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori H., Hanada R., Hanada T., Aki D., Mashima R., Nishinakamura H., Torisu T., Chien K.R., Yasukawa H., Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 25.Howard J.K., Flier J.S. Attenuation of leptin and insulin signaling by socs proteins. Trends Endocrinol. Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Pedroso J.A.B., Ramos-Lobo A.M., Donato J., Jr. Socs3 as a future target to treat metabolic disorders. Hormones. 2019;18:127–136. doi: 10.1007/s42000-018-0078-5. [DOI] [PubMed] [Google Scholar]
- 27.Pedroso J.A., Buonfiglio D.C., Cardinali L.I., Furigo I.C., Ramos-Lobo A.M., Tirapegui J., Elias C.F., Donato J., Jr. Inactivation of socs3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol. Metab. 2014;3:608–618. doi: 10.1016/j.molmet.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zampieri T.T., Ramos-Lobo A.M., Furigo I.C., Pedroso J.A., Buonfiglio D.C., Donato J., Jr. Socs3 deficiency in leptin receptor-expressing cells mitigates the development of pregnancy-induced metabolic changes. Mol. Metab. 2015;4:237–245. doi: 10.1016/j.molmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohlen T.M., Silveira M.A., Zampieri T.T., Frazao R., Donato J., Jr. Fatness rather than leptin sensitivity determines the timing of puberty in female mice. Mol. Cell Endocrinol. 2016;423:11–21. doi: 10.1016/j.mce.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Pedroso J.A., Silveira M.A., Lima L.B., Furigo I.C., Zampieri T.T., Ramos-Lobo A.M., Buonfiglio D.C., Teixeira P.D., Frazao R., Donato J., Jr. Changes in leptin signaling by socs3 modulate fasting-induced hyperphagia and weight regain in mice. Endocrinology. 2016;157:3901–3914. doi: 10.1210/en.2016-1038. [DOI] [PubMed] [Google Scholar]
- 31.Zampieri T.T., da Silva T.E., de Paula Romeu D., da Silva Torrao A., Donato J., Jr. Socs3 expression within leptin receptor-expressing cells regulates food intake and leptin sensitivity but does not affect weight gain in pregnant mice consuming a high-fat diet. Physiol. Behav. 2016;157:109–115. doi: 10.1016/j.physbeh.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Ramos-Lobo A.M., Furigo I.C., Teixeira P.D.S., Zampieri T.T., Wasinski F., Buonfiglio D.C., Donato J., Jr. Maternal metabolic adaptations are necessary for normal offspring growth and brain development. Physiol. Rep. 2018;6:e13643. doi: 10.14814/phy2.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Galaz C., Fernandez-Agullo T., Perez C., Peralta S., Arribas C., Andres A., Carrascosa J.M., Ros M. Long-term food restriction prevents ageing-associated central leptin resistance in wistar rats. Diabetologia. 2002;45:997–1003. doi: 10.1007/s00125-002-0851-4. [DOI] [PubMed] [Google Scholar]
- 34.Peralta S., Carrascosa J.M., Gallardo N., Ros M., Arribas C. Ageing increases socs-3 expression in rat hypothalamus: Effects of food restriction. Biochem. Biophys. Res. Commun. 2002;296:425–428. doi: 10.1016/S0006-291X(02)00906-3. [DOI] [PubMed] [Google Scholar]
- 35.Qian H., Azain M.J., Hartzell D.L., Baile C.A. Increased leptin resistance as rats grow to maturity. Proc. Soc. Exp. Biol. Med. 1998;219:160–165. doi: 10.3181/00379727-219-44330. [DOI] [PubMed] [Google Scholar]
- 36.Scarpace P.J., Tumer N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol. Behav. 2001;74:721–727. doi: 10.1016/S0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y., Matheny M., Tümer N., Scarpace P.J. Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance. Neurobiol. Aging. 2004;25:1349–1360. doi: 10.1016/j.neurobiolaging.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Radhika H.M., Xiaohui M., Xiaoman Y., Gil A., Nir B. Central resistance to the inhibitory effects of leptin on stimulated insulin secretion with aging. Neurobiol. Aging. 2006;27:1308–1314. doi: 10.1016/j.neurobiolaging.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Morrison C.D., White C.L., Wang Z., Lee S.Y., Lawrence D.S., Cefalu W.T., Zhang Z.Y., Gettys T.W. Increased hypothalamic protein tyrosine phosphatase 1b contributes to leptin resistance with age. Endocrinology. 2007;148:433–440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scarpace P.J., Matheny M., Moore R.L., Tümer N. Impaired leptin responsiveness in aged rats. Diabetes. 2000;49:431–435. doi: 10.2337/diabetes.49.3.431. [DOI] [PubMed] [Google Scholar]
- 41.Scarpace P.J., Matheny M., Tümer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111–1117. doi: 10.1016/S0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 42.Nagaishi V.S., Cardinali L.I., Zampieri T.T., Furigo I.C., Metzger M., Donato J., Jr. Possible crosstalk between leptin and prolactin during pregnancy. Neuroscience. 2014;259:71–83. doi: 10.1016/j.neuroscience.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 43.Scott M.M., Lachey J.L., Sternson S.M., Lee C.E., Elias C.F., Friedman J.M., Elmquist J.K. Leptin targets in the mouse brain. J. Comp. Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akselrod S., Gordon D., Ubel F.A., Shannon D.C., Berger A.C., Cohen R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 45.Parati G., Saul J.P., Di Rienzo M., Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25:1276–1286. doi: 10.1161/01.HYP.25.6.1276. [DOI] [PubMed] [Google Scholar]
- 46.Saul J. Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. Physiology. 1990;5:32–37. doi: 10.1152/physiologyonline.1990.5.1.32. [DOI] [Google Scholar]
- 47.Malliani A., Pagani M., Lombardi F., Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.CIR.84.2.482. [DOI] [PubMed] [Google Scholar]
- 48.Da Silva I.B., Gomes D.A., Alenina N., Bader M., Dos Santos R.A., Barreto-Chaves M.L.M. Cardioprotective effect of thyroid hormone is mediated by at2 receptor and involves nitric oxide production via akt activation in mice. Hear. Vessel. 2018;33:671–681. doi: 10.1007/s00380-017-1101-5. [DOI] [PubMed] [Google Scholar]
- 49.Garfield A.S., Shah B.P., Madara J.C., Burke L.K., Patterson C.M., Flak J., Neve R.L., Evans M.L., Lowell B.B., Myers M.G., Jr., et al. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab. 2014;20:1030–1037. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faber C.L., Matsen M.E., Velasco K.R., Damian V., Phan B.A., Adam D., Therattil A., Schwartz M.W., Morton G.J. Distinct neuronal projections from the hypothalamic ventromedial nucleus mediate glycemic and behavioral effects. Diabetes. 2018;67:2518–2529. doi: 10.2337/db18-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flak J.N., Goforth P.B., Dell’Orco J., Sabatini P.V., Li C., Bozadjieva N., Sorensen M., Valenta A., Rupp A., Affinati A.H., et al. Ventromedial hypothalamic nucleus neuronal subset regulates blood glucose independently of insulin. J. Clin. Investig. 2020;130:2943–2952. doi: 10.1172/JCI134135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verberne A.J., Sabetghadam A., Korim W.S. Neural pathways that control the glucose counterregulatory response. Front. Neurosci. 2014;8:38. doi: 10.3389/fnins.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flak J.N., Patterson C.M., Garfield A.S., D’Agostino G., Goforth P.B., Sutton A.K., Malec P.A., Wong J.M., Germani M., Jones J.C., et al. Leptin-inhibited pbn neurons enhance responses to hypoglycemia in negative energy balance. Nat. Neurosci. 2014;17:1744–1750. doi: 10.1038/nn.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedroso J.A.B., de Mendonca P.O.R., Fortes M.A.S., Tomaz I., Pecorali V.L., Auricino T.B., Costa I.C., Lima L.B., Furigo I.C., Bueno D.N., et al. Socs3 expression in sf1 cells regulates adrenal differentiation and exercise performance. J. Endocrinol. 2017;235:207–222. doi: 10.1530/JOE-17-0255. [DOI] [PubMed] [Google Scholar]
- 55.Furigo I.C., de Souza G.O., Teixeira P.D.S., Guadagnini D., Frazao R., List E.O., Kopchick J.J., Prada P.O., Donato J., Jr. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J. 2019;33:11909–11924. doi: 10.1096/fj.201901315R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li R.L., Sherbet D.P., Elsbernd B.L., Goldstein J.L., Brown M.S., Zhao T.J. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J. Biol. Chem. 2012;287:17942–17950. doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Browning K.N., Travagli R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014;4:1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vianna C.R., Donato J., Jr., Rossi J., Scott M., Economides K., Gautron L., Pierpont S., Elias C.F., Elmquist J.K. Cannabinoid receptor 1 in the vagus nerve is dispensable for body weight homeostasis but required for normal gastrointestinal motility. J. Neurosci. 2012;32:10331–10337. doi: 10.1523/JNEUROSCI.4507-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenfield J.R., Miller J.W., Keogh J.M., Henning E., Satterwhite J.H., Cameron G.S., Astruc B., Mayer J.P., Brage S., See T.C., et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 60.Mizuno A., Murakami T., Otani S., Kuwajima M., Shima K. Leptin affects pancreatic endocrine functions through the sympathetic nervous system. Endocrinology. 1998;139:3863–3870. doi: 10.1210/endo.139.9.6201. [DOI] [PubMed] [Google Scholar]
- 61.Carlyle M., Jones O.B., Kuo J.J., Hall J.E. Chronic cardiovascular and renal actions of leptin: Role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 62.Mizuno T.M., Kleopoulos S.P., Bergen H.T., Roberts J.L., Priest C.A., Mobbs C.V. Hypothalamic pro-opiomelanocortin mrna is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- 63.Greenfield J.R. Melanocortin signalling and the regulation of blood pressure in human obesity. J. Neuroendocr. 2011;23:186–193. doi: 10.1111/j.1365-2826.2010.02088.x. [DOI] [PubMed] [Google Scholar]
- 64.Copperi F., Kim J.D., Diano S. Role of the melanocortin system in the central regulation of cardiovascular functions. Front. Physiol. 2021;12:725709. doi: 10.3389/fphys.2021.725709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krebs D.L., Hilton D.J. Socs proteins: Negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 66.Da Silva A.A., Kuo J.J., Hall J.E. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43:1312–1317. doi: 10.1161/01.HYP.0000128421.23499.b9. [DOI] [PubMed] [Google Scholar]
- 67.Do Carmo J.M., da Silva A.A., Freeman J.N., Wang Z., Moak S.P., Hankins M.W., Drummond H.A., Hall J.E. Neuronal suppressor of cytokine signaling 3: Role in modulating chronic metabolic and cardiovascular effects of leptin. Hypertension. 2018;71:1248–1257. doi: 10.1161/HYPERTENSIONAHA.118.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seoane-Collazo P., Ferno J., Gonzalez F., Dieguez C., Leis R., Nogueiras R., Lopez M. Hypothalamic-autonomic control of energy homeostasis. Endocrine. 2015;50:276–291. doi: 10.1007/s12020-015-0658-y. [DOI] [PubMed] [Google Scholar]
- 69.Matsui H., Motooka M., Koike H., Inoue M., Iwasaki T., Suzuki T., Kurabayashi M., Yokoyama T. Ischemia/reperfusion in rat heart induces leptin and leptin receptor gene expression. Life Sci. 2007;80:672–680. doi: 10.1016/j.lfs.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 70.McGaffin K.R., Sun C.K., Rager J.J., Romano L.C., Zou B., Mathier M.A., O’Doherty R.M., McTiernan C.F., O’Donnell C.P. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc. Res. 2008;77:54–63. doi: 10.1093/cvr/cvm023. [DOI] [PubMed] [Google Scholar]
- 71.McGaffin K.R., Moravec C.S., McTiernan C.F. Leptin signaling in the failing and mechanically unloaded human heart. Circ. Hear. Fail. 2009;2:676–683. doi: 10.1161/CIRCHEARTFAILURE.109.869909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGaffin K.R., Witham W.G., Yester K.A., Romano L.C., O’Doherty R.M., McTiernan C.F., O’Donnell C.P. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc. Res. 2011;89:60–71. doi: 10.1093/cvr/cvq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hall M.E., Smith G., Hall J.E., Stec D.E. Cardiomyocyte-specific deletion of leptin receptors causes lethal heart failure in cre-recombinase-mediated cardiotoxicity. Am. J. Physiol. Integr. Comp. Physiol. 2012;303:R1241–R1250. doi: 10.1152/ajpregu.00292.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leifheit-Nestler M., Wagner N.M., Gogiraju R., Didie M., Konstantinides S., Hasenfuss G., Schafer K. Importance of leptin signaling and signal transducer and activator of transcription-3 activation in mediating the cardiac hypertrophy associated with obesity. J. Transl. Med. 2013;11:170. doi: 10.1186/1479-5876-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu L., Wang Y., Zhou X., Huang B., Wang M., Li X., Meng G., Yuan S., Xia H., Jiang H. Leptin injection into the left stellate ganglion augments ischemia-related ventricular arrhythmias via sympathetic nerve activation. Hear. Rhythm. 2018;15:597–606. doi: 10.1016/j.hrthm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Kenchaiah S., Evans J.C., Levy D., Wilson P.W., Benjamin E.J., Larson M.G., Kannel W.B., Vasan R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 77.Steculorum S.M., Ruud J., Karakasilioti I., Backes H., Engstrom Ruud L., Timper K., Hess M.E., Tsaousidou E., Mauer J., Vogt M.C., et al. Agrp neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell. 2016;165:125–138. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goncalves G.H., Li W., Garcia A.V., Figueiredo M.S., Bjorbaek C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. Cell Rep. 2014;7:1093–1103. doi: 10.1016/j.celrep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang R., Dhillon H., Yin H., Yoshimura A., Lowell B.B., Maratos-Flier E., Flier J.S. Selective inactivation of socs3 in sf1 neurons improves glucose homeostasis without affecting body weight. Endocrinology. 2008;149:5654–5661. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tong Q., Ye C., McCrimmon R.J., Dhillon H., Choi B., Kramer M.D., Yu J., Yang Z., Christiansen L.M., Lee C.E., et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dias D.P., Silva L.E., Katayama P.L., Silva C.A., Salgado H.C., Fazan R. Correlation between rr, inter-systolic and inter-diastolic intervals and their differences for the analysis of spontaneous heart rate variability. Physiol. Meas. 2016;37:1120–1128. doi: 10.1088/0967-3334/37/7/1120. [DOI] [PubMed] [Google Scholar]
- 82.Billman G.E. Heart rate variability—A historical perspective. Front. Physiol. 2011;2:86. doi: 10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.