Abstract
It is increasingly known that Parkinson’s (PD) and Alzheimer’s (AD) diseases occur more frequently in patients with inflammatory gastrointestinal diseases including inflammatory bowel (IBD) or celiac disease, indicating a pathological link between them. Although epidemiological observations suggest the existence of the gut-brain axis (GBA) involving systemic inflammatory and neural pathways, little is known about the exact molecular mechanisms. Parkinson’s disease 7 (PARK7/DJ-1) is a multifunctional protein whose protective role has been widely demonstrated in neurodegenerative diseases, including PD, AD, or ischemic stroke. Recent studies also revealed the importance of PARK7/DJ-1 in the maintenance of the gut microbiome and also in the regulation of intestinal inflammation. All these findings suggest that PARK7/DJ-1 may be a link and also a potential therapeutic target in gut and brain diseases. In this review, therefore, we discuss our current knowledge about PARK7/DJ-1 in the context of GBA diseases.
Keywords: PARK7/DJ-1, gut-brain axis, inflammatory bowel diseases, Crohn’s disease, ulcerative colitis, neurodegenerative disorders, Parkinson’s disease, Alzheimer’s disease, blood-brain barrier, oxidative stress
1. Introduction
There is a growing awareness within the medical and scientific communities about the frequent co-occurrence of gastrointestinal and neurodegenerative disorders.
Indeed, over the past few years, numerous epidemiological and experimental studies have demonstrated that inflammatory bowel diseases (IBD) or celiac disease (CeD) increase the risk of neurodegenerative disorders, including Parkinson’s (PD) or Alzheimer’s (AD) diseases [1]. This pathologic crosstalk between the two organs was described as the "gut-brain axis” (GBA) and is suggested to be regulated through systemic inflammatory and neuronal pathways [2]. IBD, the chronic inflammation of the small and/or large intestine, is characterized by inappropriate activation of the immune response against environmental factors and gastrointestinal dysbiosis resulting in local and systemic inflammation in genetically susceptible individuals [3]. As a result of the intestinal inflammation, plenty of intestine-derived inflammatory mediators, including cytokines and bacterial products, spread via the circulation [2]. It has been suggested that some of these factors can disrupt the blood-brain barrier (BBB), which is a selectively permeable border of the brain microvascular endothelial cells that protect the central nervous system (CNS) against the circulating toxins and pathogens [4]. The impaired BBB then allows the passage of intestine-derived factors to enter the brain thus inducing inflammation and neurodegenerative changes. This is what we call the systemic inflammatory pathway of GBA.
Besides the systemic pathway, however, the existence of a neural pathway is also suggested. Recent experimental results demonstrated that intestinal inflammation leads to increased local alpha-synuclein (α-syn) expression that can be transported retrogradely through the nervus vagus from the gut to the brain thereby facilitating the onset of PD [5]. However, our knowledge about the role of nervus vagus in GBA crosstalk is sparse.
In the past decade, the role of Parkinson’s disease 7 (PARK7/DJ-1) has been demonstrated in neurodegenerative diseases [6]. Indeed, the mutation altering the amount or function of PARK7/DJ-1 leads to the rare, autosomal recessive juvenile form of PD [7]. It has been shown that neuronal cells are more vulnerable to oxidative stress in the absence of PARK7/DJ-1 and the decreased expression of PARK7/DJ-1 leads to a toxic accumulation of misfolded proteins, including α-syn leading to neuronal apoptosis [6]. In addition, pharmacological activation of PARK7/DJ-1 was neuroprotective in animal models of PD, AD, and ischemic stroke, as well [8,9].
Besides the beneficial functions of PARK7/DJ-1 in the CNS, it has recently come into the focus of interest also in connection with gastrointestinal disorders. Our and other studies have demonstrated that intestinal expression of PARK7/DJ-1 is altered in the mucosa of patients with celiac disease or IBD [10]. Furthermore, experimental results revealed that PARK7/DJ-1 affects intestinal inflammation and protects the integrity of the mucosal epithelial barrier as well [11,12,13]. In a recent study, Singh et al. demonstrated that genetic deletion of PARK7/DJ-1 leads to dysbiosis and increased expression of pro-inflammatory molecules in the intestine, and also that of PD-related genes in the midbrain of mice [14].
Based on the increasing number of data demonstrating the protective role of PARK7/DJ-1 in both the gut and brain diseases, our review aims to point out how PARK7/DJ-1 may connect the pathologies of the two organs.
2. Gut-Brain Axis
2.1. Epidemiological Evidence of a Gut-Brain Axis
IBD, including Crohn’s disease (CD) and ulcerative colitis (UC), affects more than 6.8 million people worldwide with a constantly growing prevalence [15]. It is well known that besides the gastrointestinal manifestations of IBD it can also affect the musculoskeletal system and the skin [16]. More recent epidemiological data also suggest a pathological crosstalk between intestinal inflammation and the development of neurodegenerative disorders, including PD and AD. Indeed, Lin et al. were the first who demonstrated the associations between the development of PD and IBD in a retrospective cohort study [17]. They found that IBD was associated with a 35% increased risk of PD. Within a few years, numerous independent large cohort studies confirmed their original observation. A Danish study demonstrated that IBD patients have a 22% increased risk of PD as compared with non-IBD individuals [18]. Similarly, a Swedish and an American study also found an increased PD hazard ratio in IBD patients compared to controls [19,20]. Also, the meta-analysis of Zhu et al. showed that CD and UC increase the risk of PD by nearly 30% compared to controls [21]. In accordance with the above studies, a South Korean cohort study showed that IBD patients were at a 1.87 times higher risk for PD than controls, respectively [22].
Similar to PD, epidemiological studies also revealed an increased risk of AD and dementia in patients with IBD. A meta-analysis by Fu et al. demonstrated that the risk of AD was increased by 52% in patients with gastrointestinal pathologies [23]. In addition, a recently published population-based cohort study demonstrated that the overall incidence of dementia among patients with IBD was significantly elevated (5.5% vs. 1.4% among controls) [24].
Although it is still not clear how intestinal diseases affect the development of CNS diseases, some previous epidemiological observations suggest that the systemic spreading of intestinal inflammation may be involved in the pathological crosstalk between the two organs. Indeed, a study of Peter et al. demonstrated that anti-tumor necrosis factor (TNF-α) therapy of the patient with IBD reduced the incidence of PD by 78%, as well [19]. Moreover, a South Korean study found that in IBD patients receiving anti-TNF therapy did not develop PD, and corticosteroid therapy also reduced the risk of PD by 92% among CD patients [22]. Moreover, a lower risk of PD was demonstrated in IBD patients treated with anti-inflammatory mesalazine or its derivative sulfasalazine [25].
Besides IBD, the role of CeD and gluten sensitivity has been suggested in the development of neurological and psychiatric disorders, including epilepsy, anxiety, depression, autism, and schizophrenia, as well [26]. Although our knowledge is sparse it is easy to accept that, similarly to IBD, the systemic spreading of inflammation may contribute to the development of CNS diseases.
All of these epidemiological observations support the theory of the so-called "gut-brain axis”, in which the gastrointestinal alterations contribute to the development of CNS diseases, possibly through inflammatory mechanisms.
2.2. Experimental Evidences of Gut Brain-Axis
Besides the epidemiological observations, numerous experimental studies, using animal models of UC and CD, have demonstrated that gastrointestinal inflammation may affect the brain. In these experimental models, the chemical agent 2,4,6- trinitrobenzene sulfonic acid (TNBS) or dextran sodium sulfate (DSS) were used to induce local, human IBD-like inflammation in the intestine of the mice [27,28]. These data demonstrated that intestinal inflammatory processes induce PD and AD-related pathological changes in the brain, including increased BBB permeability, neuro-inflammation, α-syn aggregation, and dopaminergic loss [29,30,31].
These experimental results are in accordance with the epidemiological observations suggesting that intestinal inflammation can induce PD and AD-associated pathological alterations in the CNS. The relevant experimental evidences demonstrating the GBA crosstalk were summarized in Table 1.
Table 1.
Experimental results demonstrating the pathological crosstalk between the gut and brain.
| Animal Model of IBD | Effect on the CNS | Refs. |
|---|---|---|
| TNBS-induced colitis in rabbit | Increased blood-brain barrier permeability | Hathaway et al. [32] |
| TNBS-induced colitis in rat | Elevated blood-brain barrier permeability and reduced endothelial barrier antigen expression | Natah et al. [33] |
| Increased interleukin IL-6 expression in the hypothalamus and cerebral cortex | Wang et al. [34] | |
| DSS-induced colitis in mouse | Elevated TNF-α, IL-1ß, and IL-6 expression in the substantia nigra | Villarán et al. [35] |
| Increased TNF-α and IL-6 expression in the cortex and decreased TJ protein occludin and claudin-5 in the brain | Han et al. [36] | |
| Increased nigral level of IL-1ß and dopaminergic neuron death | Garrido Gil et al. [37] | |
| Increased COX-2 expression in the hippocampus and hypothalamus | Do et al. [38] | |
| α-syn aggregation in the midbrain | Grathwohl et al. [39] | |
| Microglial polarization into M1 and M2 phenotype in the medial prefrontal cortex | Sroor et al. [40] | |
| Increased IL-1ß, IL-6, TNF-α and IL-10 expression in the hippocampus | Gampierakis et al. [41] | |
| NLRP3 activation, amyloid plaque accumulation, and apoptosis in hippocampus, Cortex | He et al. [42] | |
| Elevated IL-1ß and TNF-α expression in the brain | Talley et al. [43] | |
| Increased microglia and astrocyte activation and loss of dopaminergic neurons in the substantia nigra pars compacta after PD inducing MPTP treatment | Gil-Martínez et al. [44] | |
| Increased neurotoxic effect of MPTP treatment | Houser et al. [45] |
3. Role of Intestinal Dysbiosis and Inflammation in CNS Diseases
In the healthy intestine, the mucosal barrier formed by epithelial cells and the junctional complexes, including tight junctions (TJ), adherent junctions, desmosomes, and gap junctions between the epithelial cells, play a pivotal role in the separation of the host’s luminal microbes from the gastrointestinal immune system [46]. In IBD, the composition of the microbiome and their metabolite production significantly changes and has been suggested as a causative factor that induces pathological alterations in the intestine [47].
The gut microbiome in healthy adults is mainly composed by two dominant bacterial phyla, Firmicutes (F) and Bacteroidetes (B), that represent more than 90% of the intestinal community, and also by other less dominant phyla, including Proteobacteria, Actinobacteria, and Verrucomicrobia [48]. The F/B ratio is considered as an important index of the health of the gut microbiome [49]. An imbalance in the composition of the microbiome is commonly found in IBD patients, with a trend toward the reduction of beneficial bacteria and an increase in invasive, toxin-producing, mucosal adherent bacterial species such as Escherichia coli (E. coli) [50,51]. E. coli strains impair the intestinal epithelial barrier in different ways, through a direct effect of their toxic factors that damage the intestinal actin cytoskeleton and via disruption of the epithelial barrier. They also impair it by adhering to the epithelial layer, thus stimulating the production of pro-inflammatory cytokines and disrupting the epithelial tight junctions and the intestinal barrier [51].
Dysbiosis of the gut can also lead to changes in the production of microbial metabolites. Indeed, it has been demonstrated that the metabolic profile is different between healthy and IBD patients [52]. The metabolites in the gut are actively absorbed and affect the integrity of the intestinal epithelial layer. Indeed, short-chain fatty acids such as acetate, propionate, and butyrate were demonstrated to facilitate the turnover of intestinal epithelial cells, thereby protecting intestinal integrity [53]. In addition, IBD patients tend to have lower fecal levels of short-chain fatty acids [53,54]. On the other hand, there are metabolites, such as the sulfur-containing metabolites hydrogen sulfide or bile acid chenodeoxycholic acid, that damage intestinal epithelial cells and induce mucosal inflammation [52,55].
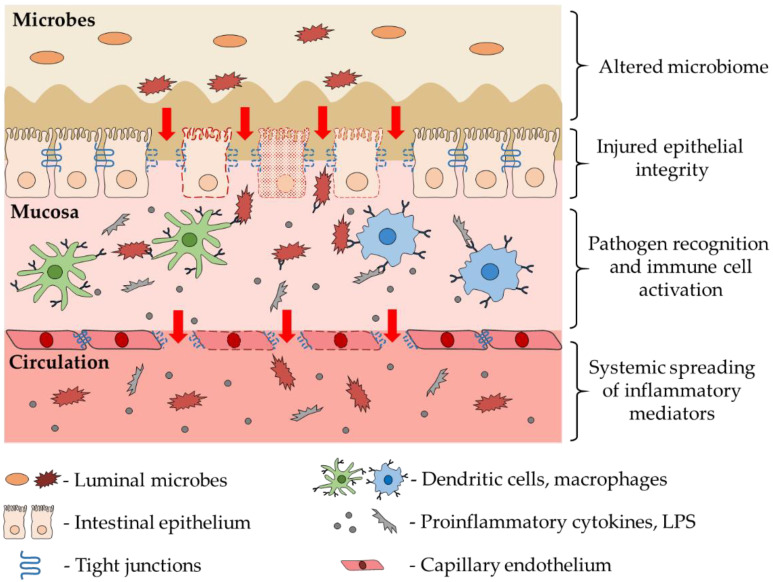
The injured epithelial cell layer allows the bacteria to penetrate into the mucosal layer and activate the local immune cells, including dendritic cells and macrophages, the frontline cells of innate immunity. These immune cells express a wide range of pattern recognition receptors, including Toll-like receptors (TLR), allowing them to recognize and respond to a wide range of endogenous substances and exogenous pathogens [46,56]. The activation of TLRs initiate signal transduction pathways that culminate in the activation of nuclear factor kappa B (NF-κB), interferon regulatory factors, or mitogen-activated protein kinases to regulate the expression of cytokines, chemokines, and type I interferons, reactive oxygen species that ultimately protect the host from microbial infection [57]. In IBD the immune cell activation in the intestinal wall contributes to the tissue damage by enhancing the production of matrix metalloproteinases (MMP) responsible for intestinal ulceration [58,59]. In the injured intestine the above-mentioned inflammatory mediators can enter into the microcirculation of the gastrointestinal wall, thereby spreading throughout the body and making the inflammation systemic (Figure 1).
Figure 1.
The role of intestinal alterations in the development of systemic inflammation. Disturbance of the gut microbiome leads to impairment of the epithelial barrier integrity and penetration of the luminal bacteria into the mucosal layer, thereby activating the resident immune cells. In the injured intestine the inflammatory factors, bacteria, and their parts can enter the circulation extending the intestinal inflammation to systemic inflammation.
Systemic inflammation has been demonstrated to induce pathological changes in the BBB integrity. Indeed, increased permeability of BBB has been described in various human diseases associated with systemic inflammation, including sepsis, diabetes, multiple sclerosis, or obesity [60,61,62,63]. The pro-inflammatory factors in the circulation have been suggested to play a key role in the disruption of the molecular connection between the capillary endothelial cells of BBB [62]. Indeed, bacterial lipopolysaccharide (LPS), IL-1β, IL-6, and TNF-α inhibit the transcription of junction proteins and induce the cytoskeleton-mediated redistribution of TJs from the cell membrane to the cytoplasm, resulting in junctional disorganization and the separation of the cells from each other [62,64]. Both experimental and human studies demonstrated that intestinal pathologies are associated with increased levels of inflammatory mediators, including IL-6, TNF-α, bacteria, and LPS in the circulation. [36,43,65,66,67,68,69,70,71]. Moreover, the above-mentioned data experiments using TNBS or DSS induced mice model of IBD demonstrated that intestinal inflammation leads to decreased expression of the TJ protein occludin and claudin-5 in the brain and increases the permeability of the BBB (Table 1). Therefore, it can be reasonably assumed that systemic inflammation in IBD may contribute to BBB impairment.
The role of the tight junction protein zonulin was also suggested in the impairment of BBB integrity [72]. Indeed, as a result of intestinal inflammation triggered by gluten or microbial imbalance, epithelial zonulin is released from its junctional complexes into the circulation [73]. Circulatory zonulin can bind to epidermal growth factor receptors and protease-activated receptor 2 receptors on the endothelial cells of BBB, resulting in dysfunctional reorganization of endothelial TJs [74]. Indeed, the study of Rahman et al. demonstrated that zonulin can modify the cellular localization of zonula occludens 1 (ZO-1), claudin-5, and occludin, thereby influencing the permeability of BBB [72].
Injury of the BBB is of great importance in neuroinflammation since it paves the way for intestine-derived exogenous and endogenous substances to enter into the brain and bind to their receptors on glial cells (astrocyte and microglia), thereby triggering their activation. Activated glial cells are the major source of those inflammatory cytokines, chemokines, and reactive oxygen species (ROS) which play a central role in the development of neurodegenerative disorders [75,76,77].
It is important to note that the vagus nerve-mediated crosstalk between gut and brain may also play a role in GBA, however, its role is still controversial. Previously, Holmqvist et al. found that the pathologic form of α-syn from a PD patient’s brain lysate injected into the gastric wall of rodents is taken up and transported from the gut to the dorsal motor nucleus via the VN [5]. However, on the other hand, stimulation of VN was demonstrated to inhibit cytokine production of the gut, including that of TNF-α, and improve intestinal integrity [78].
Taken together, an increasing number of experiments suggest that the crosstalk between gut and brain can be mediated by intestine-derived systemic inflammation that disrupts the BBB, allowing the spreading of harmful molecules into the brain, thereby facilitating the development of neurodegenerative diseases.
4. PARK7/DJ-1
PARK7/DJ-1 is an evolutionarily conserved 20 kDa protein that forms homodimers and is composed of 189 amino acids. PARK7/DJ-1 has 3 cysteine residues, of which Cys106 is perhaps the most studied, with four possible oxidative statuses (Cys106-SH/-SOH/-SO2H/-SO3H) that affects its three-dimensional structure and thereby its functions [79,80,81]. Excessive oxidation of PARK7/DJ-1 is characterized by the formation of Cys106-SO3H and results in the inactivation and degradation of the protein [80,81]. The relevance of this property of PARK7/DJ-1 is well-demonstrated by the studies of Kitamura et al., which prove that pharmacological prevention of the excessive oxidation of Cys106 of PARK7/DJ-1 is protective against oxidative stress-induced neuronal cell death [8,82].
PARK7/DJ-1 is expressed in almost all, if not all, human cells [83]; it is primarily localized in the cytoplasm, however, its occurrence in the mitochondria [84] and nucleus [85] and also its secretion into the extracellular space was reported [86]. Previously, the expression of PARK7/DJ-1 was mainly investigated regarding malignant tumors and neurodegenerative diseases. In malignant diseases, the increased expression of PARK7/DJ-1 was demonstrated in glioblastoma, non-small cell lung, thyroid, breast, hepatocellular, and colorectal carcinoma [87]. In addition, the elevated PARK7/DJ-1 expression was closely correlated with the poor survival of patients with colorectal and pancreas cancers [87,88,89]. On the contrary, the reduced expression and/or the increased amount of dysfunctional overoxidized form of PARK7/DJ-1 was found in the brain of patients with various neurodegenerative disorders, including PD, AD, Huntington’s disease, and ischemic stroke [90].
4.1. Regulation of PARK7/DJ-1
Most of the studies suggest that the main determinant of the proteomic degradation of PARK7/DJ-1 is oxidative stress [80,81]. However, an increasing amount of data shows that regulatory mechanisms leading to decreased PARK7/DJ-1 expression or function are more complex [87]. Indeed, it has been shown that the cellular stress-activated tumor suppressor p53 pathway leads to decreased expression of PARK7/DJ-1 in mouse embryonic fibroblasts cells [91]. The results of Qin et al. demonstrated that the Bcl-2 associated athanogene 5 (BAG5) chaperon interacts with PARK7/DJ-1 and negatively regulates its dimerization, thereby facilitating its degradation under oxidative stress in human embryonal kidney (HEK)-293 cells [92]. The role of MMP-3 was also suggested in the proteomic cleavage and degradation of PARK7/DJ-1 in CATH neuronal cells [93]. Our very recent results showed that inflammatory mediators of IBD, including LPS, TNF-α, and TGF-β, decreased the expression of PARK7/DJ-1 in HT-29 colonic adenocarcinoma cells [11].
The micro RNA (miR) mediated regulation of PARK7/DJ-1 expression has also been shown. Indeed, it was recently demonstrated that the miR-128-3p directly reduces the expression of PARK7/DJ-1 in hepatocellular carcinoma cells by binding to the 3′UTR region of PARK7/DJ-1 [94]. Similarly, miR-203 was shown to reduce PARK7/DJ-1 expression in pancreatic cancer cells [95]. Furthermore, the overexpression of miR-494 decreased the PARK7/DJ-1 level of adipocytes and neuroblastoma cells resulting in increased vulnerability of cells to oxidative stress. In line with this data, in an MPTP-induced mouse model of PD, the overexpression of miR-494 negatively regulated PARK7/DJ-1 levels and exacerbated neurodegeneration [96].
Besides the negative regulators of PARK7/DJ-1, recent studies identified molecules that can increase PARK7DJ-1 expression and functions. Indeed, it was shown that the signal transducer and activator of transcription factor (STAT5A) increases the expression of PARK7/DJ-1 in leukemic pre-B cells, thereby preventing their apoptotic cell death [97]. The role of cellular homeostasis regulator cell cycle autoantigen (SG2NA) has also been demonstrated to increase the expression of PARK7/DJ-1 by inhibiting its proteasome-mediated degradation in Neuro2a neuroblastoma cells [98,99,100]. Finally, our recent study showed that IL-17 and hydrogen peroxide increases the expression of PARK7/DJ-1 [11]. Interestingly, while increased oxidative stress is one of the main mediators of proteomic degradation of PARK7/DJ-1, our results showed that as a positive feedback mechanism it can facilitate its synthesis [11].
Based on the currently available data, the PARK7/DJ-1 level is negatively regulated by processes associated with inflammation and oxidative stress. However, at the same time, harmful effectors can increase PARK7 expression, which may help the survival of the cells (Table 2).
Table 2.
Molecular regulation of PARK7/DJ-1.
| Molecule | Effect on PARK7/DJ-1 | Tissue or Cell Type | Refs. |
|---|---|---|---|
| Negative regulators of PARK7/DJ1 | |||
| H2O2 | Overoxidation | Human brain | [80,81] |
| p53 | Reduced expression | mouse embryonic fibroblasts | [91] |
| BAG5 | Decreased stability | HEK293 human embryonic kidney | [92] |
| MMP-3 | Proteomic fragmentation | CATH.a mouse neuronal | [93] |
| LPS | Reduced expression | HT-29 human colonic adenocarcinoma | [11] |
| TNF-α | Reduced expression | HT-29 human colonic adenocarcinoma | [11] |
| TGF-β | Reduced expression | HT-29 human colonic adenocarcinoma | [11] |
| miR-128-3p | Reduced expression | Human hepatocellular carcinoma | [94] |
| miR-494 | Reduced expression | 3T3-L1 mouse adipocytes and Neuro-2a neuroblastoma | [96] |
| miR-203 | Reduced expression | SW1990/DDP human pancreatic cancer cells | [95] |
| Positive regulators of PARK7/DJ1 | |||
| STAT5A | Increased expression | human leukemic pre-B | [97] |
| SG2NA | Protection from degradation | Neuro2a neuroblastoma | [99,100] |
| IL-17 | Increased expression | HT-29 human colonic adenocarcinoma | [11] |
| H2O2 | Increased expression | HT-29 human colonic adenocarcinoma | [11] |
4.2. Functions of PARK7/DJ-1
The importance of PARK7/DJ-1 in cellular stress response and survival was first demonstrated by the fact that its loss-of-function mutation results in increased neuronal cell death by reducing resistance against oxidative stress, causing early onset of neurodegenerative diseases, including juvenile PD [7,101,102]. Many functions of PARK7/DJ-1 play a key role in the protection of cells against oxidative stress. Indeed, PARK7/DJ-1 is a peroxiredoxin-like peroxidase, which can directly eliminate intracellular reactive oxygen species (ROS) through the oxidation of its Cys-106 [103]. Moreover, by binding to complex I of the mitochondrial oxidative phosphorylation system PARK7/DJ-1 may play a role in the maintenance of the mitochondrial respiratory chain, thereby controlling the generation of intracellular ROSs [104,105].
However, in terms of antioxidant protection, the most important function of PARK7/DJ-1 is the regulation of transcription factors that are crucial in the cellular defense mechanisms against oxidative stress. Indeed, PARK7/DJ-1 activates the nuclear factor erythroid 2–related factor 2 (NRF2), which is one of the main transcriptional regulators of antioxidant response genes, including thioredoxin (TRX1), glutamate-cysteine ligase catalytic subunit (GCLC), heme oxygenase-1 (HMOX1), NAD(P)H quinone dehydrogenase-1 (NQO1) [106,107].
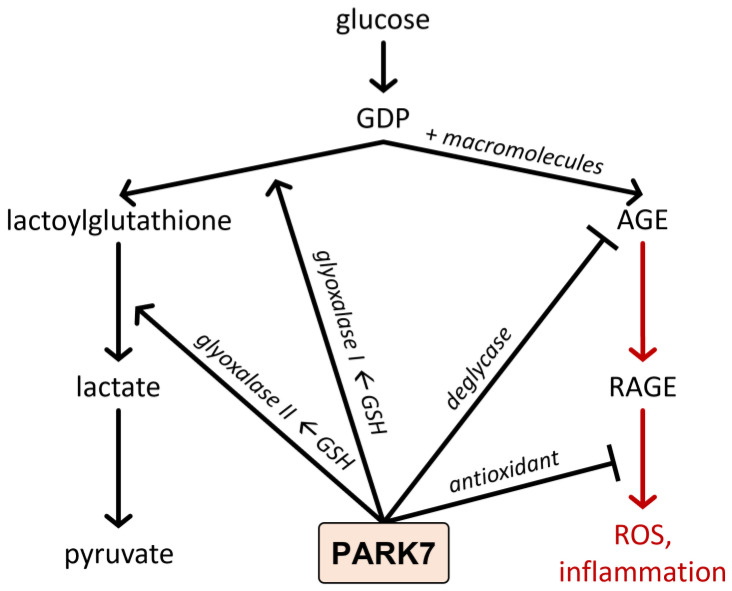
In addition to its direct antioxidant role, PARK7/DJ-1 has been demonstrated to influence the degradation of the advanced glycation end products (AGE) (Figure 2). AGE formation is facilitated by reactive glucose degradation products (GDP), such as 3-deoxyglucosone, 3,4-dideoxyglucosone-3-ene, glyoxal, or methylglyoxal, originated from the non-enzymatic, spontaneous decomposition of reducing monosaccharides [108]. AGEs then lead to the induction of oxidative stress and inflammation through the activation of their AGE receptors (RAGE). AGEs play a central role in the pathomechanism of several diseases, including IBD, AD, PD, stroke, alcoholic brain damage, or diabetes mellitus [109,110]. To eliminate toxic GDPs and AGEs, the glutathione system and different proteases degrade the GDPs leading to the generation of nontoxic lactate. PARK7/DJ-1 is involved in this process at several points. On the one hand, it was demonstrated that PARK7/DJ-1 upregulates the glutathione (GSH) synthesis, which is an essential cofactor of glyoxalase (Glo1)-1, and 2, which are responsible for the elimination of GDPs (Figure 1) [111,112,113]. During this enzymatic process, the deteriorative GDPs will be degraded to bioavailable pyruvate through lactoylglutathione and lactate intermediate products. Furthermore, PARK7/DJ-1 has its glyoxalase and deglycase activities [114,115]. PARK7/DJ-1, due to its glyoxalase activity, prevents the glycation of macromolecules, including the free amino residues of proteins, lipids, and DNA by cleaving the GDPs (Figure 1). In addition, as a deglycase, PARK7/DJ-1 repairs glycated proteins by releasing lactate from the damaged amino-acid residues (Figure 2) [116,117].
Figure 2.
Effect of PARK7/DJ-1 on the formation of AGEs. AGE formation is facilitated by reactive GDPs. AGEs activate their AGE receptors leading to oxidative stress and inflammation. To eliminate toxic GDPs, the glutathione system degrades them to nontoxic lactate. PARK7/DJ-1 is involved in this process at several points. Indeed, PARK7/DJ-1 has its own glyoxalase activity, and upregulates the glyoxalase-1 and 2 enzyme activity, which is responsible for the elimination of GDPs. In addition, PARK7/DJ-1 has deglycase activity, thereby preventing the glycation of macromolecules and AGE formation. Finally, as an antioxidant, PARK7/DJ-1 protects the cells against the harmful effect of oxidative stress.
Moreover, under oxidative stress, PARK7/DJ-1 mediates cell survival by activating Ras-dependent extracellular signal-regulated kinase (ERK1/2) and apoptosis signal-regulating kinase 1 (ASK-1) pathways [118,119].
As a molecular chaperone PARK7/DJ-1 has been demonstrated to prevent the aggregation of different macromolecules, including α-syn, which is a key factor in the development of PD [120,121].
Finally, the role of PARK7/DJ-1 in the inflammatory mechanism was also demonstrated; however, its anti- and pro-inflammatory role is still controversial and seems disease-specific. Indeed, anti-inflammatory properties of PARK7/DJ-1 have been suggested by Kim et al. who found that astrocytes and microglia originated from PARK7/DJ-1-knockout (KO) mice exhibited increased expression of inflammatory mediators, including TNF-α, Cyclooxygenase-2 (COX-2), and (inducible nitric oxide synthase) iNOS in response to interferon-gamma (IFN-γ) treatment [122]. Similarly, Peng et al. demonstrated that RNA interference-mediated PARK7/DJ-1 silencing resulted in increased IL-1ß, IL-6, and TNF-α expression in the cerebral cortex of rats after I/R injury and cultured brain astrocytes as well [123]. Moreover, our previous study investigating the role of PARK7/DJ-1 in DSS-induced colitis also demonstrated that pharmacological activation of PARK7/DJ-1 resulted in the decreased expression of pro-inflammatory cytokines, including IL-1β and IL-6, and decreased diseases activity index in the DSS treated mice [11]. However, other studies suggested the pro-inflammatory role of PARK7/DJ-1. Chen et al. demonstrated that RNA interference-mediated PARK7/DJ-1 silencing leads to decreased IL-6 and TNF-α expression in activated macrophages [124]. Similarly, Kim et al. found that PARK7/DJ-1 deficiency resulted in the reduced expression of IL-1β and IL-6 in adipocytes and decreased the activity of pro-inflammatory transcription factor NF-κB in macrophages [125].
Taken together, the most widely demonstrated function of PARK7/DJ-1 is its anti-oxidant functions. However, numerous additional functions of PARK7/DJ-1 were identified, including its enzyme activities, anti-apoptotic effect, and regulatory role on inflammation, respectively.
5. Role of PARK7/dj-1 in the Pathogenesis of Neurodegenerative Diseases
The connection between PARK7/DJ-1 and neurodegenerative diseases was first suggested nearly two decades ago when it was identified as a causative factor in rare inherited forms of PD [7]. Since then, the role of PARK7/DJ-1 in neurodegenerative diseases has been intensively studied, and it has become clear that PARK7/DJ-1 may play a role not only in PD but in almost all neurological diseases associated with oxidative stress, inflammation, and tissue damage [9,126,127].
5.1. Genetic Evidence for the Role of PARK7/DJ-1 in Parkinson’s Disease
The group of Parkinson’s disease molecules involves 23 genes located on different chromosomes and having different functions [128]. However, one thing that is common in the members of the PARK family is that they are all associated with the higher risk of PD [129]. This relationship was first suggested in 2003 when Bonifati and colleagues found a large (about 14 kb) deletion and a missense mutation (Leucine166Proline, L166P) in the PARK7/DJ-1 gene in a Dutch and an Italian family, leading to the identification of PARK7/DJ-1 as a causative gene for familial PD with recessive inheritance [7]. Since then, more than 20 PARK7/DJ-1 mutations have been associated with PD [130].
5.2. Role of PARK7/DJ-1 in Parkinson’s Disease
Experimental data suggest that PARK7/DJ-1 plays a protective role in neurodegenerative diseases via its antioxidant properties. Indeed, the lack of PARK7/DJ-1 in stem cell-derived neurons and SH-SY5Y cells resulted in increased vulnerability to oxidative stress [131,132,133]. In addition, the neuroprotective effect of the administration of recombinant PARK7/DJ-1 was demonstrated in the rodent model of 6-hydroxydopamine (6-OHDA) and MG-132 treatment-induced PD [134]. Furthermore, it has been demonstrated that pharmacological protection of PARK7/DJ-1 against overoxidation preserves its antioxidant properties. Indeed, Miyazaki et al. and Kitamura et al. identified small molecule compounds, including UCP0054277, UCP0054278, and Compound 23 (Comp23), that can bind to the C106 region of PARK7/DJ-1 and keep it in reduced, biologically active form [8,135]. The protective effects of these compounds against oxidative stress were confirmed in hydrogen peroxide- (H2O2) treated wild type and PARK7/DJ-1-knockdown SH-SY5Y neuronal cells [8,135,136]. In further experiments, they also demonstrated that administration of UCP0054278 and or Comp23 suppressed the loss of dopaminergic neurons and motor dysfunction in an animal model of 6-OHDA or rotenone-induced PD [8,126]. Glyoxalase activity of PARK7/DJ-1 in neuroprotection may also be of great importance since AGEs have been suggested to contribute to the development of neurodegenerative diseases. Indeed, glycation-mediated AGE formation has been reported in the Lewy bodies in PD patients [137]. The relationship between AGEs and PD could be due to the ability of AGEs to cross-link α-syn, as has been shown using in vitro studies [138].
5.3. Role of PARK7/DJ-1 in Alzheimer’s Disease
The role of PARK7/DJ-1 has also been suggested in AD. It was shown that the PARK7/DJ-1 binding compound UCP0054278 improved the AD-related cognitive deficits and prevented the degeneration of synaptic functions in AD modeling APdE9 transgenic mice [9]. Similarly, a recent study by Cheng et al. demonstrated that overexpression of DJ in the brain by lentiviral infection ameliorated β-amyloid protein (Aβ) deposition and the cognitive function of 5XFAD transgenic mice modeling AD [139]. The results also demonstrated that reactive oxygen species and oxidative stress marker malondialdehyde content were significantly decreased, while the antioxidant superoxide dismutase activity was significantly increased in the brain of 5XFAD mice overexpressing PARK7/DJ-1 [139]. In addition, AGEs are present in amyloid plaques in the brain of AD patients and have been suggested to promote the aggregation of Aβ and tau [140]. According to experimental data, the glyoxalase activity of PARK7/DJ-1 may reduce these deleterious effects of AGEs in neurons. Indeed, the protective role of PARK7/DJ-1 against dicarbonyl stress was demonstrated using mouse embryonic fibroblasts, human SH-SY5Y cells, and C. elegans, as well [114].
5.4. Role of PARK7/DJ-1 in Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant inherited disease associated with polyglutamine expansion in the huntingtin (Htt) protein, leading to its misfolding and toxic aggregation [141]. A recent study by Sajjad et al. demonstrated that the level of oxidized PARK7/DJ-1 Cys106 level was elevated in the frontal cortex of HD patients [127]. They demonstrated that overexpression of PARK7/DJ-1 ameliorated mutant Htt toxicity in a yeast and Drosophila model of HD, suggesting the importance of the chaperoning activity of PARK7/DJ-1 in vivo. Their results also demonstrated that mild oxidation of PARK7/DJ-1 at cysteine 106 is required for its chaperone function; however, the complete oxidation of cysteine 106 leads to impaired PARK7/DJ-1 and detrimental cellular outcomes [127].
5.5. Role of PARK7/DJ-1 in Ischemia-Reperfusion Induced Brain Injury
The importance of PARK7/DJ-1 has also been demonstrated in the ischemic-reperfusion injury of the brain. Indeed, intrastriatal injection of recombinant human PARK7/DJ-1 markedly reduced infarct size after middle cerebral artery occlusion of rats and protected SH-SY5Y against H2O2-induced apoptosis [133]. PARK7/DJ-1-deficient animals produced a significantly larger infarct size in the animal model of Endothelin-1 induced stroke compared to wild-type controls [142]. On the contrary, the administration of ND13 corresponding to the 13-N-terminal amino acids of PARK7/DJ-1 was shown to improve motor function after ischemic injury [143]. Similarly, PARK7/DJ-1 binding Comp23 reduced the infarct size of cerebral ischemia in rats [8,136,144].
6. Role of PARK7/DJ-1 in the Pathogenesis of Gastrointestinal Diseases
In addition to the previously discussed effects of PARK7/DJ-1 in the pathomechanism of CNS diseases, its role in the disease of other organs including the heart [145,146,147], lung [148,149] and intestine [10,12,13,14,114,150,151,152] was recently studied. However, in the present section, we focus on the recently emerged role of PARK7/DJ-1 in the pathomechanism of intestinal diseases.
6.1. Genetic Evidence of the Role of PARK7/DJ-1 in Gastrointestinal Diseases
First, the genome-wide association (GWA) study of Dubois et al. involving 4533 coeliac disease cases and 10750 controls suggested that the genomic region of the short arm of chromosome 1 containing the PARK7/DJ-1 gene and also that of TNF Receptor Superfamily Member 9 (TNFRSF9) is strongly associated with the risk of coeliac disease [153]. A year later, in 2011, another GWA study comprising 6687 cases with ulcerative colitis (UC) and 19718 controls prepared by Anderson et al. revealed that the 1p36 chromosomal region, containing TNFRSF9, ERFF11, UTS2, and PARK7/DJ-1 genes, is associated with a higher risk of UC [150].
Not long after this, Lee et al. demonstrated that cDJR-1.1, the C. elegans homolog of the human PARK7/DJ-1 is expressed in the intestine of the worms. In addition, their results showed that lack of cDJR-1.1 makes the worms vulnerable to glyoxal-induced intestinal toxicity, giving the first in vivo evidence suggesting the protective role of PARK7/DJ-1 in intestinal pathology [114].
6.2. Role of PARK7/DJ-1 in Coeliac Disease
The first direct human evidence suggesting the possible role of PARK7/DJ-1 in the pathomechanism of small intestinal diseases was the study of Vörös et al. [10]. In this study, our research group demonstrated the increased mRNA expression and protein level of PARK7/DJ-1 in the small intestinal mucosa of patients with untreated coeliac disease. In this study, we found that PARK7/DJ-1 immunopositivity is present in the epithelial cells of the duodenal crypt, and also in the lamina propria of duodenal biopsies derived from therapy-naive children with celiac disease. Moreover, we found that, following the introduction of a gluten-free diet, the amount of PARK7/DJ-1 normalizes, suggesting the possible role of PARK7/DJ-1 in the pathomechanism of celiac disease.
More recently, our research group examined the possible function of PARK7/DJ-1 on the pathomechanism of intestinal inflammation in more depth [12]. In this study, Comp-23, a PARK7/DJ-1 binding compound that protects PARK7/DJ-1 from overoxidation, was used to investigate the functional role of PARK7/DJ-1 in the pathomechanism of celiac disease [8].
We found that Comp23 decreased the intracellular accumulation of ROS in the H2O2 treated duodenal FHs74Int, epithelial cells. Our finding was in line with previous experiments demonstrating that lack of PARK7/DJ-1 is associated with increased accumulation of ROS in different cells, including dopaminergic cells, mast cells, regulatory T cells, or skeletal muscle cells, suggesting the role of PARK7/DJ-1 in antioxidant defense [154,155,156]. Investigating the underlying molecular mechanism, we found that Comp23 treatment increases the expression of NRF2 transcription factor—one of the major regulators of antioxidant defense—and also the expression of several NRF2 dependent antioxidant genes, including TRX1, GCLC, HMOX1, and NQO1, in the FHs74Int cells exposed to oxidative stress [12,157]. Indeed, our results are in line with the literature suggesting that PARK7/DJ-1 may influence the activation of the NRF2-related antioxidant genes and thereby oxidative stress [158]. Not surprisingly, by the increased antioxidant preparedness of the Comp23 treated cells, we demonstrated that Comp23 treatment improves the survival of H2O2-treated FHs74Int cells. Investigating the molecular mechanism of PARK7/DJ-1 on cell viability, we found that Comp23 treatment increased the expression of transcription factor tumor antigen P53 (TP53) and that of its target genes, including proliferating cell nuclear antigen (PCNA), cyclin-dependent kinase inhibitor 1-p21 (CDKN1A), apoptosis regulator Bcl-2 (BCL2), and apoptosis regulator BAX (BAX) in the H2O2-treated FHs74Int cells [12].
Finally, we have investigated the role of PARK7/DJ-1 in the maintenance of the small intestinal mucosa integrity. We demonstrated that Comp23 treatment restores the expression of cell adhesion molecules (CAM), including ZO-1, cadherin 1 (CDH1), vinculin (VCL), and integrin Subunit Beta 5 (ITGB5), and the healthy architecture of the actin cytoskeleton-CAM complexes in the FHs74Int cells exposed to oxidative stress (Figure 3). Not surprisingly, in our experiments, Comp-23 treatment also restored the oxidative stress-induced permeability of small intestinal sacs ex vivo, suggesting the role of PARK7/DJ-1 in the control of oxidative stress and the maintenance of mucosal integrity.
Figure 3.
Role of PARK7/DJ-1 against oxidative damage of duodenal epithelial cells in celiac disease. Our study demonstrated that PARK7/DJ-1-binding Comp23 altered the expression of stress-response elements, including antioxidant and cell-cycle regulator genes, and normalized the expression and localization of CAMs, contributing to the maintenance of mucosal integrity as demonstrated by our ex vivo experiment.
6.3. Role of PARK7/DJ-1 in Inflammatory Bowel Disease
Despite the growing interest, there are relatively little data about the role of PARK7/DJ-1 in the pathomechanism of IBD. Recently, Di Narzo et al. investigated the plasma proteome of adult patients with Crohn’s disease (CD; n = 126) and ulcerative colitis (UC; n = 46) compared to that of healthy subjects (n = 72) using a high-throughput SOMAmer-based capture array [152]. They found that a total of 493 proteins showed altered levels in the plasma of patients with IBD compared to healthy subjects; among them, 219 were up- and 274 were down-regulated. One identified protein with an increased presence in the plasma of UC patients compared to that of healthy subjects was PARK7/DJ-1.
More recently, Zhang et al. investigated the role of PARK7/DJ-1 in the pathomechanism of IBD [13]. They found decreased levels of PARK7/DJ-1 in the intestine of patients with active CD (n = 63) or UC (n = 23) compared to the healthy subjects (n = 15). They noted that the amount of PARK7/DJ-1 was even lower in the inflamed, compared to the non-inflamed mucosa of the individual patients. Interestingly, the results of our research group somehow differ from the study of Zhang et al. [11]. Indeed, we demonstrated an increased amount of PARK7/DJ-1 in the epithelial and lamina propria cells of macroscopically inflamed and non-inflamed mucosa of therapy-naive pediatric patients with CD compared to that of controls. However, in our study, the amount of PARK7/DJ-1 was at the level of control in the mucosa of children with UC.
In connection with the obvious contradictions of the aforementioned studies, it should be noted that these results are hardly comparable because of the different types of samples, patients, and methods. While Di Narzo et al. investigated the plasma proteome of adult IBD patients, our group determined the mucosal mRNA expression and protein level of PARK7/DJ-1 from fresh frozen biopsies of therapy-naive children. In fact, Di Narzo et al. also note that the plasma level of the investigated proteins had only little correlation with their expression in the intestine. Unfortunately, less is known about the paraffin-embedded colon sections used in the study of Zhang et al. Although they used a high number of samples, the detailed description of the samples and the patients remains unknown. Therefore, it is easy to suppose that the difference is mainly due to the origin of the samples, age, or medication of the patients.
Zhang et al. and our research group also investigated the expression of PARK7/DJ-1 in the colon of DSS-treated mice. According to these studies, the amount of PARK7/DJ-1 is increased on the 3rd day of DSS treatment, however later on the seventh day of the experiment it decreases to the level of the untreated control animals or below [11,13].
Zhang et al. demonstrated that following DSS treatment, more serious clinical symptoms, such as decreased body weight and higher disease activity index (DAI), develop in the PARK7/DJ-1 KO compared to the WT mice. Accordingly, they demonstrated the increased expression of TNF-α, IL-1β, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), and C-C Motif Chemokine Ligand 3 (CCL3) in the colon of DSS treated PARK7/DJ-1 KO, compared to that of WT mice, suggesting the anti-inflammatory role of PARK7/DJ-1 (Figure 3).
Similarly, using the same DSS induced mouse model of colitis, we demonstrated that treatment of the mice with the PARK7/DJ-1 binging Comp23 improves the clinical symptoms, decreases the histological lesions, spleen enlargement, and the mucosal expression of IL-1β, IL-6, and IL10, and TGF-β of DSS-treated mice, suggesting the strong anti-inflammatory role of PARK7/DJ-1 and Comp23 (Figure 4).
Figure 4.
Effect of PARK7/DJ-1 on DSS treatment-induced colitis. Comp23 treatment decreased the mucosal expression of IL-1β, IL-6, IL-10, and TGF-β in DSS-treated mice. Similarly, following DSS treatment, decreased body weight, a higher disease activity index, and increased expression of TNF-α, IL-1β, IL-6, IL-8, MCP-1, and CCL3 were observed in the colon of DSS treated PARK7/DJ-1 KO compared to that of WT mice.
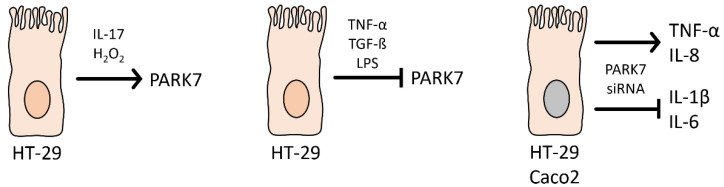
The above in vivo studies were confirmed by in vitro experiments demonstrating the strong, bidirectional interaction between the inflammatory factors and PARK7/DJ-1. Indeed, we showed that while TNF-α, TGF-β, or LPS treatment inhibits, IL-17 or H2O2 treatment increases the synthesis of PARK7/DJ-1 in vitro. Moreover, our group and Zhang et al. demonstrated that the gene silencing of PARK7/DJ-1 increases the expression of TNF-α and IL-8 of HT-29 or Caco2 colon epithelial cells and decreases the expression of IL-1β and IL-6 (Figure 5).
Figure 5.
Effect of inflammatory factors on the synthesis of PARK7/DJ-1 and that of gene silencing of PARK7/DJ-1 on the synthesis of different inflammatory factors. IL-17 and H2O2 treatment decrease while TNF-α, TGF-β, and LPS treatment increases the expression of PARK7/DJ-1 in HT-29 colonic epithelial cells. PARK7/DJ-1 RNA silencing facilitates TNF-α and IL-8 expression and decreases IL-1β and IL-6 expression in HT-29 or Caco2 colon epithelial cells.
To further specify the role of PARK7/DJ-1, Zhang et al. generated PARK7/DJ-1 bone marrow chimera mice. When PARK7/DJ-1 was disrupted only in the myeloid cells of the mice (KO to WT bone marrow chimera mice), the DSS treated animals had higher body weight and lower DAI compared to that control WT to WT chimera mice. Similarly, less pronounced activation of the NF-κβ signaling pathway and decreased expression of inflammatory cytokines, including IL-1β, IL-6, MCP1, and CCL3 were observed in the colon of KO to WT compared to WT chimera mice with DSS colitis (Figure 6). These observations suggest that the presence of PARK7/DJ-1 in immune cells may contribute to the exacerbation of DSS induced colitis, which is somehow in contradiction with the above experiment of the research group, demonstrating more serious symptoms and inflammation in the colon of PARK7/DJ-1 KO compared to WT mice.
Figure 6.
The role of PARK7/DJ-1 on immune cell activation in DSS-induced colitis. Symptoms of DSS-induced colitis were milder, and the expression of inflammatory cytokines was lower in the colon of KO to WT chimeric mice compared with WT to WT mice. Nevertheless, symptoms of DSS-induced colitis were more severe, and the expression of inflammatory cytokines was higher in the colon of WT to KO chimeric mice compared with KO to KO mice.
When only the myeloid cells of the mice expressed PARK7/DJ-1 (WT to KO bone marrow chimera mice), the symptoms of the mice were more severe compared to that of KO to KO chimera mice. Also, the expression of inflammatory cytokines, including IL-1β, IL-6, MCP1, and CCL3 were higher in the colon of DSS treated WT to KO compared to KO mice with colitis, revealing that the presence of PARK7/DJ-1 in the immune cells may contribute to DSS-induced inflammation, especially in a colon lacking PARK7/DJ-1 (Figure 6). Although the interpretation of the above in vivo experiments, using bone marrow chimera mice, is quite challenging because of the lack of a common group of animals, Zhang et al. suggest that the lack of PARK7/DJ-1 in the epithelium is critical to improving colitis whereas the role of PARK7/DJ-1 in immune cells may be the reverse.
Taken together, the above studies clearly demonstrate the role of PARK7/DJ-1 in coeliac disease and IBD-related intestinal inflammation, however, further studies are needed to resolve the contradictions and elucidate the precise role of PARK7/DJ-1 in IBD.
6.4. The Role of PARK7/DJ-1 in Intestinal Dysbiosis
A recent publication of Singh et al. investigated the effect of PARK7/DJ-1 deficiency on the intestinal microbiome of healthy mice [14]. They investigated the effect of PARK7/DJ-1 deletion on bacterial composition of the intestine by 16S rRNA sequencing. Analysis of fecal samples showed that overall composition of the microbiome did not differ between PARK7/DJ-1−/− and PARK7/DJ-1+/+ and mice at the phylum level. However, calculation of the F/B ratio showed that it decreased significantly in PARK7/DJ-1−/− mice compared to PARK7/DJ-1+/+ mice, suggesting the functional role of PARK7/DJ-1 on the composition of the intestinal microbiome. The deeper analysis of the data showed an increased presence of Alistipes and Rikenella species in PARK7/DJ-1−/− mice. The role of these species has been described regarding the pathomechanism of IBD [159].
Changes in the composition of intestinal microbiome affect its metabolite production. Accordingly, they demonstrated that the amount of fecal and also that of serum amino acids, including valine, leucine, phenylalanine, alanine, tyrosine and isoleucine, were downregulated, whereas SCFAs, including malonate, dimethylamine, trimethylamine and acetoin, were upregulated in PARK7/DJ-1−/− mice compared to that of PARK7/DJ-1+/+ mice. Although the metabolic changes of PARK7/DJ-1−/− mice were complex, Singh et al. suggested that it can lead to metabolic stress of the intestine, as demonstrated by the increased inflammation of the intestine. Indeed, they found increased levels of pro-inflammatory monocyte chemotactic protein-1 (MCP-1) and calprotectin in feces of PARK7/DJ-1−/− mice compared to that of PARK7/DJ-1+/+ mice. However, it must be also noted that the expression of other pro-inflammatories such as granulocyte monocyte colony stimulating factor IFN-β decreased, and that of many others, including IL-12p70, TNF-α, IL-17A, IFN-γ, IL-23 and IL-6 did not change.
Finally, since the association of the intestinal microbiome and also that of PARK7/DJ-1 with neurodegenerative diseases is well known, they investigated the molecular biological changes in the midbrain of PARK7/DJ-1−/− mice by RNA-sequencing. They found the upregulation of PD related inflammatory genes, including polymerase family member 1 (Parp1) and MMP-8 in the midbrain of PARK7/DJ-1−/− mice compared to that of PARK7/DJ-1+/+ mice, suggesting that changes in the intestinal microbiome and/or lack of PARK7/DJ-1 may alter the pathology of the CNS.
7. Role of PARK7/DJ-1 in GBA Diseases
Based on our current knowledge, it is assumed that PARK7/DJ-1 represents a molecular link between intestinal and brain diseases. Indeed, recent studies demonstrated that PARK7/DJ-1, through its antioxidant and anti-inflammatory properties, plays a role in the maintenance of healthy intestinal microbiome and mucosal integrity, thus influencing the local and systemic inflammation characteristic for IBD.
As intestine-derived inflammatory factors can reach the BBB and impair its integrity, the inflammation can also spread also to the brain. The increased presence of inflammatory mediators induces the inflammation of CNS and may also alter the synthesis and function of PARK7/DJ-1 itself in the brain (Figure 7). Indeed, it has been shown that TNF-α, TGF-β, or LPS reduces the expression of PARK7/DJ-1, and also that increased amounts of MMP-3 may induce its degradation (Table 2). Also, the oxidative stress is an important factor that regulates the synthesis and function of PARK7/DJ-1 in the brain. Moreover, oxidative stress has been demonstrated to play a key role in the inactivation and degradation of PARK7/DJ-1 (Table 2).
Figure 7.
The role of PARK7/DJ-1 in GBA diseases. PARK7/DJ-1, through its antioxidant, anti-inflammatory, glyoxalase, and chaperon activity properties, plays a role in the maintenance of intestinal integrity, thus diminishing the local and the systemic inflammation. The systemic inflammation of intestinal origin can be considered as a possible factor that negatively regulates PARK7/DJ-1 in the brain. Similarly to the gut, PARK7/DJ-1 also plays a protective role in the brain, therefore, its negative regulation enhances pathological GBA crosstalk and the development of neurodegenerative diseases.
PARK7/DJ-1 similarly to its effects in the gut has great importance in the protective mechanisms of the brain. Therefore, molecular mechanisms that alter PARK7/DJ-1 activity may contribute to the development of neurodegenerative disorders (Figure 7).
Although the function and regulation of PARK7/DJ-1 are still not completely understood, there is a growing body of evidence suggesting that it participates in the pathomechanism of diseases influenced by GBA.
8. Conclusions
There is a growing body of evidence about the connection between the development of gastrointestinal and neurodegenerative disorders. Epidemiological and experimental studies demonstrated that gastrointestinal diseases represent an increased risk of the development of neurodegenerative disorders, including Parkinson’s or Alzheimer’s diseases. Inflammatory and neuronal pathways may link the pathomechanism of intestinal and CNS diseases.
PARK7/DJ-1 plays a key role in maintaining the homeostasis of the intestine and brain via its antioxidant, anti-inflammatory, glyoxalase, and chaperone activity. Moreover, it can be suggested that intestine-derived systemic inflammation impairs the function of PARK7/DJ-1 in the CNS, thereby increasing the risk of the development of neurodegenerative diseases.
Although further studies are needed to accurately explore the role of PARK7/DJ-1 in the pathologic crosstalk between the gut and CNS, our existing knowledge draws our attention to PARK7/DJ-1 as a potential therapeutic target molecule for the treatment of GBA diseases.
Acknowledgments
We are grateful to Csenge Pajtók for her excellent technical assistance.
Author Contributions
Conceptualization, D.P. and Á.V.; Writing-original draft preparation, D.P., Á.V., A.V.-S. and B.S.; Review and editing, Á.V. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding Statement
This research was funded by National Research, Development and Innovation Office (NKFIH) TKP2020-NKA-09 and TKP2020-NKA-13, 20382-3/2018 FEKUTSTRAT, K124549, and K125470 grants; Semmelweis University, STIA-KFI-2020 and STIA-KFI-2021 (1492-15/IKP/2022) grants.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brudek T. Inflammatory Bowel Diseases and Parkinson’s Disease. J. Parkinson’s Dis. 2019;9:S331–S344. doi: 10.3233/JPD-191729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houser M.C., Tansey M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson’s Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y.-Z., Li Y.-Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galea I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021;18:2489–2501. doi: 10.1038/s41423-021-00757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Björklund T., Wang Z.-Y., Roybon L., Melki R., Li J.-Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 6.Huang M., Chen S. DJ-1 in neurodegenerative diseases: Pathogenesis and clinical application. Prog. Neurobiol. 2021;204:102114. doi: 10.1016/j.pneurobio.2021.102114. [DOI] [PubMed] [Google Scholar]
- 7.Bonifati V., Rizzu P., Van Baren M.J., Schaap O., Breedveld G.J., Krieger E., Dekker M.C., Squitieri F., Ibanez P., Joosse M., et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura Y., Watanabe S., Taguchi M., Takagi K., Kawata T., Takahashi-Niki K., Yasui H., Maita H., Iguchi-Ariga S.M., Ariga H. Neuroprotective effect of a new DJ-1-binding compound against neurodegeneration in Parkinson’s disease and stroke model rats. Mol. Neurodegener. 2011;6:48. doi: 10.1186/1750-1326-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura Y., Inden M., Kimoto Y., Takata K., Yanagisawa D., Hijioka M., Ashihara E., Tooyama I., Shimohama S., Ariga H. Effects of a DJ-1-Binding Compound on Spatial Learning and Memory Impairment in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;55:67–72. doi: 10.3233/JAD-160574. [DOI] [PubMed] [Google Scholar]
- 10.Vörös P., Sziksz E., Himer L., Ónody A., Pap D., Frivolt K., Szebeni B., Lippai R., Győrffy H., Fekete A., et al. Expression of PARK7 is increased in celiac disease. Virchows Arch. 2013;463:401–408. doi: 10.1007/s00428-013-1443-z. [DOI] [PubMed] [Google Scholar]
- 11.Lippai R., Veres-Székely A., Sziksz E., Iwakura Y., Pap D., Rokonay R., Szebeni B., Lotz G., Béres N.J., Cseh Á., et al. Immunomodulatory role of Parkinson’s disease 7 in inflammatory bowel disease. Sci. Rep. 2021;11:14582. doi: 10.1038/s41598-021-93671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veres-Székely A., Bernáth M., Pap D., Rokonay R., Szebeni B., Takács I.M., Lippai R., Cseh Á., Szabó A.J., Vannay Á. PARK7 Diminishes Oxidative Stress-Induced Mucosal Damage in Celiac Disease. Oxid. Med. Cell. Longev. 2020;2020:4787202. doi: 10.1155/2020/4787202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J., Xu M., Zhou W., Li D., Zhang H., Chen Y., Ning L., Zhang Y., Li S., Yu M., et al. Deficiency in the anti-apoptotic protein DJ-1 promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via p53. J. Biol. Chem. 2020;295:4237–4251. doi: 10.1074/jbc.RA119.010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh Y.A.-O., Trautwein C., Dhariwal A., Salker M.A.-O.X., Alauddin M., Zizmare L.A.-O., Pelzl L., Feger M., Admard J., Casadei N.A.-O., et al. DJ-1 (Park7) affects the gut microbiome, metabolites and the development of innate lymphoid cells (ILCs) Sci. Rep. 2020;10:16131. doi: 10.1038/s41598-020-72903-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alatab S., Sepanlou S.G., Ikuta K., Vahedi H., Bisignano C., Safiri S., Sadeghi A., Nixon M.R., Abdoli A., Abolhassani H., et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine J.S., Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Hepatol. 2011;7:235. [PMC free article] [PubMed] [Google Scholar]
- 17.Lin J.-C., Lin C.-S., Hsu C.-W., Lin C.-L., Kao C.-H. Association Between Parkinson’s Disease and Inflammatory Bowel Disease: A Nationwide Taiwanese Retrospective Cohort Study. Inflamm. Bowel Dis. 2016;22:1049–1055. doi: 10.1097/MIB.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 18.Villumsen M., Aznar S., Pakkenberg B., Jess T., Brudek T.A.-O. Inflammatory bowel disease increases the risk of Parkinson’s disease: A Danish nationwide cohort study 1977–2014. Gut. 2018;68:18–24. doi: 10.1136/gutjnl-2017-315666. [DOI] [PubMed] [Google Scholar]
- 19.Peter I., Dubinsky M., Bressman S., Park A., Lu C., Chen N., Wang A. Anti-Tumor Necrosis Factor Therapy and Incidence of Parkinson Disease Among Patients with Inflammatory Bowel Disease. JAMA Neurol. 2018;75:939–946. doi: 10.1001/jamaneurol.2018.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weimers P., Halfvarson J., Sachs M.C., Saunders-Pullman R., Ludvigsson J.F., Peter I., Burisch J., Olén O. Inflammatory Bowel Disease and Parkinson’s Disease: A Nationwide Swedish Cohort Study. Inflamm. Bowel Dis. 2019;25:111–123. doi: 10.1093/ibd/izy190. [DOI] [PubMed] [Google Scholar]
- 21.Zhu F., Li C., Gong J., Zhu W., Gu L., Li N. The risk of Parkinson’s disease in inflammatory bowel disease: A systematic review and meta-analysis. Dig. Liver Dis. 2019;51:38–42. doi: 10.1016/j.dld.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Park S.A.-O., Kim J., Chun J.A.-O., Han K., Soh H.A.-O., Kang E.A., Lee H.J., Im J.A.-O., Kim J.S. Patients with Inflammatory Bowel Disease Are at an Increased Risk of Parkinson’s Disease: A South Korean Nationwide Population-Based Study. J. Clin. Med. 2019;8:1191. doi: 10.3390/jcm8081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu P.A.-O., Gao M.A.-O., Yung K.A.-O. Association of Intestinal Disorders with Parkinson’s Disease and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. ACS Chem. Neurosci. 2019;11:395–405. doi: 10.1021/acschemneuro.9b00607. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B.A.-O., Wang H.A.-O., Bai Y.M., Tsai S.J., Su T.P., Chen T.J., Wang Y.A.-O., Chen M.A.-O. Inflammatory bowel disease is associated with higher dementia risk: A nationwide longitudinal study. Gut. 2021;70:85–91. doi: 10.1136/gutjnl-2020-320789. [DOI] [PubMed] [Google Scholar]
- 25.Ríos J.P., Navarro C.J.M., Navarro M.J.P., Tapia M.J.C., Vera M.J.P., Arillo V.C., García M.R.G., Castellanos A.M., Sevilla F.E. Association of Parkinson’s disease and treatment with aminosalicylates in inflammatory bowel disease: A cross-sectional study in a Spain drug dispensation records. BMJ Open. 2019;9:e025574. doi: 10.1136/bmjopen-2018-025574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerner A.A.-O., Benzvi C. “Let Food Be Thy Medicine”: Gluten and Potential Role in Neurodegeneration. Cells. 2021;10:756. doi: 10.3390/cells10040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antoniou E., Margonis G.A., Angelou A., Pikouli A., Argiri P., Karavokyros I., Papalois A., Pikoulis E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016;11:9–15. doi: 10.1016/j.amsu.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chassaing B., Aitken J.D., Malleshappa M., Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014;104:15–25. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kouli A., Torsney K.M., Kuan W.-L. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Codon Publications; Singapore: 2018. Parkinson’s disease: Etiology, neuropathology, and pathogenesis. [PubMed] [Google Scholar]
- 32.Hathaway C.A., Appleyard C.B., Percy W.H., Williams J.L. Experimental colitis increases blood-brain barrier permeability in rabbits. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999;276:G1174–G1180. doi: 10.1152/ajpgi.1999.276.5.G1174. [DOI] [PubMed] [Google Scholar]
- 33.Natah S.S., Mouihate A., Pittman Q.J., Sharkey K.A. Disruption of the blood-brain barrier during TNBS colitis. Neurogastroenterol. Motil. 2005;17:433–446. doi: 10.1111/j.1365-2982.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang K., Yuan C.-P., Wang W., Yang Z.-Q., Cui W., Mu L.-Z., Yue Z.-P., Yin X.-L., Hu Z.-M., Liu J.-X. Expression of interleukin 6 in brain and colon of rats with TNBS-induced colitis. World J. Gastroenterol. 2010;16:2252–2259. doi: 10.3748/wjg.v16.i18.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villarán R.F., Espinosa-Oliva A.M., Sarmiento M., De Pablos R.M., Argüelles S., Delgado-Cortés M.J., Sobrino V., Van Rooijen N., Venero J.L., Herrera A.J. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: Potential risk factor in Parkinsons disease. J. Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- 36.Han Y., Zhao T., Cheng X., Zhao M., Gong S.-H., Zhao Y.-Q., Wu H.-T., Fan M., Zhu L.-L. Cortical Inflammation is Increased in a DSS-Induced Colitis Mouse Model. Neurosci. Bull. 2018;34:1058–1066. doi: 10.1007/s12264-018-0288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrido-Gil P., Rodriguez-Perez A.I., Dominguez-Meijide A., Guerra M.J., Labandeira-Garcia J.L. Bidirectional Neural Interaction Between Central Dopaminergic and Gut Lesions in Parkinson’s Disease Models. Mol. Neurobiol. 2018;55:7297–7316. doi: 10.1007/s12035-018-0937-8. [DOI] [PubMed] [Google Scholar]
- 38.Do J., Woo J. From Gut to Brain: Alteration in Inflammation Markers in the Brain of Dextran Sodium Sulfate-induced Colitis Model Mice. Clin. Psychopharmacol. Neurosci. 2018;16:422. doi: 10.9758/cpn.2018.16.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grathwohl S., Quansah E., Maroof N., Steiner J.A., Spycher L., Benmansour F., Duran-Pacheco G., Siebourg-Polster J., Oroszlan-Szovik K., Remy H., et al. Experimental colitis drives enteric alpha-synuclein accumulation and Parkinson-like brain pathology. bioRxiv. 2018:505164. doi: 10.1101/505164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sroor H.M., Hassan A.M., Zenz G., Valadez-Cosmes P., Farzi A., Holzer P., El-Sharif A., Gomaa F.A.-Z.M., Kargl J., Reichmann F. Experimental colitis reduces microglial cell activation in the mouse brain without affecting microglial cell numbers. Sci. Rep. 2019;9:20217. doi: 10.1038/s41598-019-56859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gampierakis I.-A., Koutmani Y., Semitekolou M., Morianos I., Polissidis A., Katsouda A., Charalampopoulos I., Xanthou G., Gravanis A., Karalis K.P. Hippocampal neural stem cells and microglia response to experimental inflammatory bowel disease (IBD) Mol. Psychiatry. 2021;26:1248–1263. doi: 10.1038/s41380-020-0651-6. [DOI] [PubMed] [Google Scholar]
- 42.He X.F., Li L.L., Xian W.B., Li M.Y., Zhang L.Y., Xu J.H., Pei Z., Zheng H.Q., Hu X.Q. Chronic colitis exacerbates NLRP3-dependent neuroinflammation and cognitive impairment in middle-aged brain. J. Neuroinflamm. 2021;18:153. doi: 10.1186/s12974-021-02199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talley S., Valiauga R., Anderson L., Cannon A.R., Choudhry M.A., Campbell E.M. DSS-induced inflammation in the colon drives a proinflammatory signature in the brain that is ameliorated by prophylactic treatment with the S100A9 inhibitor paquinimod. J. Neuroinflamm. 2021;18:263. doi: 10.1186/s12974-021-02317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gil-Martínez A.-L., Estrada C., Cuenca L., Cano J.-A., Valiente M., Martínez-Cáceres C.-M., Fernández-Villalba E., Herrero M.-T. Local Gastrointestinal Injury Exacerbates Inflammation and Dopaminergic Cell Death in Parkinsonian Mice. Neurotox. Res. 2019;35:918–930. doi: 10.1007/s12640-019-0010-z. [DOI] [PubMed] [Google Scholar]
- 45.Houser M.C., Caudle W.M., Chang J., Kannarkat G.T., Yang Y., Kelly S.D., Oliver D., Joers V., Shannon K.M., Keshavarzian A., et al. Experimental colitis promotes sustained, sex-dependent, T-cell-associated neuroinflammation and parkinsonian neuropathology. Acta Neuropathol. Commun. 2021;9:139. doi: 10.1186/s40478-021-01240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chelakkot C., Ghim J., Ryu S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018;50:1–9. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stojanov S., Berlec A., Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu L.C.-H. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. J. Biomed. Sci. 2018;25:79. doi: 10.1186/s12929-018-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pawłowska B., Sobieszczańska B.M. Intestinal epithelial barrier: The target for pathogenic Escherichia coli. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2017;26:1437–1445. doi: 10.17219/acem/64883. [DOI] [PubMed] [Google Scholar]
- 52.Iyer N., Corr S.C. Gut Microbial Metabolite-Mediated Regulation of the Intestinal Barrier in the Pathogenesis of Inflammatory Bowel Disease. Nutrients. 2021;13:4259. doi: 10.3390/nu13124259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong L.-N., Wang M., Guo J., Wang J.-P. Role of intestinal microbiota and metabolites in inflammatory bowel disease. Chin. Med. J. 2019;132:1610–1614. doi: 10.1097/CM9.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atarashi K., Tanoue T., Oshima K., Suda W., Nagano Y., Nishikawa H., Fukuda S., Saito T., Narushima S., Hase K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 56.Johansson M.E., Gustafsson J.K., Holmén-Larsson J., Jabbar K.S., Xia L., Xu H., Ghishan F.K., Carvalho F.A., Gewirtz A.T., Sjövall H., et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawasaki T., Kawai T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pallone F., Monteleone G. Mechanisms of tissue damage in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2001;17:307–312. doi: 10.1097/00001574-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 59.O’Sullivan S., Gilmer J.F., Medina C. Matrix Metalloproteinases in Inflammatory Bowel Disease: An Update. Mediat. Inflamm. 2015;2015:964131. doi: 10.1155/2015/964131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barichello T., Generoso J.S., Collodel A., Petronilho F., Dal-Pizzol F. The blood-brain barrier dysfunction in sepsis. Tissue Barriers. 2021;9:1840912. doi: 10.1080/21688370.2020.1840912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Dyken P., Lacoste B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood-Brain Barrier. Front. Neurosci. 2018;12:930. doi: 10.3389/fnins.2018.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varatharaj A., Galea I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Varatharaj A., Liljeroth M., Cramer S., Stuart C., Zotova E., Darekar A., Larsson H., Galea I. Systemic inflammation and blood–brain barrier abnormality in relapsing–remitting multiple sclerosis. Lancet. 2017;389:S96. doi: 10.1016/S0140-6736(17)30492-0. [DOI] [Google Scholar]
- 64.Edelblum K.L., Turner J.R. The tight junction in inflammatory disease: Communication breakdown. Curr. Opin. Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergemalm D., Andersson E., Hultdin J., Eriksson C., Rush S.T., Kalla R., Adams A.T., Keita Å.V., D’Amato M., Gomollon F. Systemic inflammation in preclinical ulcerative colitis. Gastroenterology. 2021;161:1526–1539.e1529. doi: 10.1053/j.gastro.2021.07.026. [DOI] [PubMed] [Google Scholar]
- 66.Rojo Ó.P., Román A.L.S., Arbizu E.A., de la Hera Martínez A., Sevillano E.R., Martínez A.A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2007;13:269–277. doi: 10.1002/ibd.20019. [DOI] [PubMed] [Google Scholar]
- 67.Reichmann F., Hassan A.M., Farzi A., Jain P., Schuligoi R., Holzer P. Dextran sulfate sodium-induced colitis alters stress-associated behaviour and neuropeptide gene expression in the amygdala-hippocampus network of mice. Sci. Rep. 2015;5:9970. doi: 10.1038/srep09970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiely C.J., Pavli P., O’Brien C.L. The microbiome of translocated bacterial populations in patients with and without inflammatory bowel disease. Intern. Med. J. 2018;48:1346–1354. doi: 10.1111/imj.13998. [DOI] [PubMed] [Google Scholar]
- 69.Gutierrez A., Holler E., Zapater P., Sempere L., Jover R., Perez-Mateo M., Schoelmerich J., Such J., Wiest R., Frances R. Antimicrobial peptide response to blood translocation of bacterial DNA in Crohn’s disease is affected by NOD2/CARD15 genotype. Inflamm. Bowel Dis. 2011;17:1641–1650. doi: 10.1002/ibd.21537. [DOI] [PubMed] [Google Scholar]
- 70.Linares R., Francés R., Gutiérrez A., Juanola O. Bacterial Translocation as Inflammatory Driver in Crohn’s Disease. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.703310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar M., Leon Coria A., Cornick S., Petri B., Mayengbam S., Jijon H.B., Moreau F., Shearer J., Chadee K. Increased intestinal permeability exacerbates sepsis through reduced hepatic SCD-1 activity and dysregulated iron recycling. Nat. Commun. 2020;11:483. doi: 10.1038/s41467-019-14182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rahman M.T., Ghosh C., Hossain M., Linfield D., Rezaee F., Janigro D., Marchi N., van Boxel-Dezaire A.H.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018;507:274–279. doi: 10.1016/j.bbrc.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 73.Wood Heickman L.K., DeBoer M.D., Fasano A. Zonulin as a potential putative biomarker of risk for shared type 1 diabetes and celiac disease autoimmunity. Diabetes/Metab. Res. Rev. 2020;36:e3309. doi: 10.1002/dmrr.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fasano A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012;10:1096–1100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giovannoni F., Quintana F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020;41:805–819. doi: 10.1016/j.it.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matejuk A., Ransohoff R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020;11:1416. doi: 10.3389/fimmu.2020.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takata F., Nakagawa S., Matsumoto J., Dohgu S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021;15:344. doi: 10.3389/fncel.2021.661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonaz B., Sinniger V., Pellissier S. Vagus nerve stimulation: A new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 2017;282:46–63. doi: 10.1111/joim.12611. [DOI] [PubMed] [Google Scholar]
- 79.Ito G., Ariga H., Nakagawa Y., Iwatsubo T. Roles of distinct cysteine residues in S-nitrosylation and dimerization of DJ-1. Biochem. Biophys. Res. Commun. 2006;339:667–672. doi: 10.1016/j.bbrc.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 80.Kiss R., Zhu M., Jójárt B., Czajlik A., Solti K., Fórizs B., Nagy É., Zsila F., Beke-Somfai T., Tóth G. Structural features of human DJ-1 in distinct Cys106 oxidative states and their relevance to its loss of function in disease. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017;1861:2619–2629. doi: 10.1016/j.bbagen.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 81.Wilson M.A. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid. Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi-Niki K., Inafune A., Michitani N., Hatakeyama Y., Suzuki K., Sasaki M., Kitamura Y., Niki T., Iguchi-Ariga S.M.M., Ariga H. DJ-1-dependent protective activity of DJ-1-binding compound no. 23 against neuronal cell death in MPTP-treated mouse model of Parkinson’s disease. J. Pharmacol. Sci. 2015;127:305–310. doi: 10.1016/j.jphs.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L., Wang J., Wang J., Yang B., He Q., Weng Q. Role of DJ-1 in Immune and Inflammatory Diseases. Front. Immunol. 2020;11:994. doi: 10.3389/fimmu.2020.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Canet-Avilés R.M., Wilson M.A., Miller D.W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M.J., Ringe D., Petsko G.A., Cookson M.R. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagakubo D., Taira T., Kitaura H., Ikeda M., Tamai K., Iguchi-Ariga S.M.M., Ariga H. DJ-1, a Novel Oncogene Which Transforms Mouse NIH3T3 Cells in Cooperation withras. Biochem. Biophys. Res. Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 86.Maita C., Tsuji S., Yabe I., Hamada S., Ogata A., Maita H., Iguchi-Ariga S.M.M., Sasaki H., Ariga H. Secretion of DJ-1 into the serum of patients with Parkinson’s disease. Neurosci. Lett. 2008;431:86–89. doi: 10.1016/j.neulet.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 87.Jin W. Novel Insights into PARK7 (DJ-1), a Potential Anti-Cancer Therapeutic Target, and Implications for Cancer Progression. J. Clin. Med. 2020;9:1256. doi: 10.3390/jcm9051256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He X.Y., Liu B.Y., Yao W.Y., Zhao X.J., Zheng Z., Li J.F., Yu B.Q., Yuan Y.Z. Serum DJ-1 as a diagnostic marker and prognostic factor for pancreatic cancer. J. Dig. Dis. 2011;12:131–137. doi: 10.1111/j.1751-2980.2011.00488.x. [DOI] [PubMed] [Google Scholar]
- 89.Zhou J., Liu H., Zhang L., Liu X., Zhang C., Wang Y., He Q., Zhang Y., Li Y., Chen Q., et al. DJ-1 promotes colorectal cancer progression through activating PLAGL2/Wnt/BMP4 axis. Cell Death Dis. 2018;9:865. doi: 10.1038/s41419-018-0883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Antipova D., Bandopadhyay R. Expression of DJ-1 in Neurodegenerative Disorders. In: Ariga H., Iguchi-Ariga S., editors. DJ-1/PARK7 Protein. Springer; Berlin/Heidelberg, Germany: 2017. [DOI] [PubMed] [Google Scholar]
- 91.Vasseur S., Afzal S., Tomasini R., Guillaumond F., Tardivel-Lacombe J., Mak T.W., Iovanna J.L. Consequences of DJ-1 upregulation following p53 loss and cell transformation. Oncogene. 2012;31:664–670. doi: 10.1038/onc.2011.268. [DOI] [PubMed] [Google Scholar]
- 92.Qin L.-X., Tan J.-Q., Zhang H.-N., Rizwana K., Lu J.-H., Tang J.-G., Jiang B., Shen X.-M., Guo J.-F., Tang B.-S., et al. BAG5 Interacts with DJ-1 and Inhibits the Neuroprotective Effects of DJ-1 to Combat Mitochondrial Oxidative Damage. Oxid. Med. Cell. Longev. 2017;2017:5094934. doi: 10.1155/2017/5094934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi D.-H., Hwang O., Lee K.-H., Lee J., Beal M.F., Kim Y.-S. DJ-1 cleavage by matrix metalloproteinase 3 mediates oxidative stress-induced dopaminergic cell death. Antioxid. Redox Signal. 2011;14:2137–2150. doi: 10.1089/ars.2009.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo X.L., Wang H.B., Yong J.K., Zhong J., Li Q.H. MiR-128-3p overexpression sensitizes hepatocellular carcinoma cells to sorafenib induced apoptosis through regulating DJ-1. Eur. Rev. Med. Pharm. Sci. 2018;22:6667–6677. doi: 10.26355/eurrev_201810_16143. [DOI] [PubMed] [Google Scholar]
- 95.Du S.L., Xu L.Y., Gao P., Liu Q.S., Lu F.F., Mo Z.H., Fan Z.Z., Cheng X.L., Dong Z.H. MiR-203 regulates DJ-1 expression and affects proliferation, apoptosis and DDP resistance of pancreatic cancer cells. Eur. Rev. Med. Pharm. Sci. 2019;23:8833–8840. doi: 10.26355/eurrev_201910_19278. [DOI] [PubMed] [Google Scholar]
- 96.Xiong R., Wang Z., Zhao Z., Li H., Chen W., Zhang B., Wang L., Wu L., Li W., Ding J., et al. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol. Aging. 2014;35:705–714. doi: 10.1016/j.neurobiolaging.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 97.Cholez E., Debuysscher V., Bourgeais J., Boudot C., Leprince J., Tron F., Brassart B., Regnier A., Bissac E., Pecnard E., et al. Evidence for a protective role of the STAT5 transcription factor against oxidative stress in human leukemic pre-B cells. Leukemia. 2012;26:2390–2397. doi: 10.1038/leu.2012.112. [DOI] [PubMed] [Google Scholar]
- 98.Hwang J., Pallas D.C. STRIPAK complexes: Structure, biological function, and involvement in human diseases. Int. J. Biochem. Cell Biol. 2014;47:118–148. doi: 10.1016/j.biocel.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanti G.K., Goswami S.K. SG2NA recruits DJ-1 and Akt into the mitochondria and membrane to protect cells from oxidative damage. Free Radic. Biol. Med. 2014;75:1–13. doi: 10.1016/j.freeradbiomed.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Tanti G.K., Pandey S., Goswami S.K. SG2NA enhances cancer cell survival by stabilizing DJ-1 and thus activating Akt. Biochem. Biophys. Res. Commun. 2015;463:524–531. doi: 10.1016/j.bbrc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 101.Iguchi-Ariga H.A.M.M., editor. DJ-1/PARK7 Protein: Parkinson’s Disease, Cancer and Oxidative Stress-Induced Diseases. Springer; Berlin/Heidelberg, Germany: 2017. [Google Scholar]
- 102.Kahle P.J., Waak J., Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic. Biol. Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 103.Andres-Mateos E., Perier C., Zhang L., Blanchard-Fillion B., Greco T.M., Thomas B., Ko H.S., Sasaki M., Ischiropoulos H., Przedborski S., et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guo C., Sun L., Chen X., Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hayashi T., Ishimori C., Takahashi-Niki K., Taira T., Kim Y.-C., Maita H., Maita C., Ariga H., Iguchi-Ariga S.M.M. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem. Biophys. Res. Commun. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 106.Clements C.M., McNally R.S., Conti B.J., Mak T.W., Ting J.P.Y. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Im J.-Y., Lee K.-W., Woo J.-M., Junn E., Mouradian M.M. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum. Mol. Genet. 2012;21:3013–3024. doi: 10.1093/hmg/dds131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haybrard J., Simon N., Danel C., Pinçon C., Barthélémy C., Tessier F.J., Décaudin B., Boulanger E., Odou P. Factors Generating Glucose Degradation Products In Sterile Glucose Solutions For Infusion: Statistical Relevance Determination of Their Impacts. Sci. Rep. 2017;7:11932. doi: 10.1038/s41598-017-12296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Byun K., Yoo Y., Son M., Lee J., Jeong G.-B., Park Y.M., Salekdeh G.H., Lee B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017;177:44–55. doi: 10.1016/j.pharmthera.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 110.Prasad A., Bekker P., Tsimikas S. Advanced Glycation End Products and Diabetic Cardiovascular Disease. Cardiol. Rev. 2012;20:177–183. doi: 10.1097/CRD.0b013e318244e57c. [DOI] [PubMed] [Google Scholar]
- 111.He Y., Zhou C., Huang M., Tang C., Liu X., Yue Y., Diao Q., Zheng Z., Liu D. Glyoxalase system: A systematic review of its biological activity, related-diseases, screening methods and small molecule regulators. Biomed. Pharmacother. 2020;131:110663. doi: 10.1016/j.biopha.2020.110663. [DOI] [PubMed] [Google Scholar]
- 112.Wang Z., Zhao D., Chen L., Li J., Yuan G., Yang G., Zhang H., Guo X., Zhang J. Glycine increases glyoxalase-1 function by promoting nuclear factor erythroid 2-related factor 2 translocation into the nucleus of kidney cells of streptozotocin-induced diabetic rats. J. Diabetes Investig. 2019;10:1189–1198. doi: 10.1111/jdi.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou W., Freed C.R. DJ-1 Up-regulates Glutathione Synthesis during Oxidative Stress and Inhibits A53T α-Synuclein Toxicity*. J. Biol. Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 114.Lee J.-Y., Song J., Kwon K., Jang S., Kim C., Baek K., Kim J., Park C. Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 2012;21:3215–3225. doi: 10.1093/hmg/dds155. [DOI] [PubMed] [Google Scholar]
- 115.Sharma N., Rao S.P., Kalivendi S.V. The deglycase activity of DJ-1 mitigates α-synuclein glycation and aggregation in dopaminergic cells: Role of oxidative stress mediated downregulation of DJ-1 in Parkinson’s disease. Free Radic. Biol. Med. 2019;135:28–37. doi: 10.1016/j.freeradbiomed.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 116.Matsuda N., Kimura M., Queliconi B.B., Kojima W., Mishima M., Takagi K., Koyano F., Yamano K., Mizushima T., Ito Y., et al. Parkinson’s disease-related DJ-1 functions in thiol quality control against aldehyde attack in vitro. Sci. Rep. 2017;7:12816. doi: 10.1038/s41598-017-13146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mencke P., Boussaad I., Romano C.D., Kitami T., Linster C.L., Krüger R. The Role of DJ-1 in Cellular Metabolism and Pathophysiological Implications for Parkinson’s Disease. Cells. 2021;10:347. doi: 10.3390/cells10020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oh S.E., Mouradian M.M. Cytoprotective mechanisms of DJ-1 against oxidative stress through modulating ERK1/2 and ASK1 signal transduction. Redox Biol. 2018;14:211–217. doi: 10.1016/j.redox.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang F., Peng W., Zhang J., Wang L., Dong W., Zheng Y., Wang Z., Xie Z., Wang T., Wang C., et al. PARK7 enhances antioxidative-stress processes of BMSCs via the ERK1/2 pathway. J. Cell. Biochem. 2021;122:222–234. doi: 10.1002/jcb.29845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shendelman S., Jonason A., Martinat C., Leete T., Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou W., Zhu M., Wilson M.A., Petsko G.A., Fink A.L. The Oxidation State of DJ-1 Regulates its Chaperone Activity toward α-Synuclein. J. Mol. Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 122.Kim J.-H., Choi D.-J., Jeong H.-K., Kim J., Kim D.W., Choi S.Y., Park S.-M., Suh Y.H., Jou I., Joe E.-H. DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: A novel anti-inflammatory function of DJ-1. Neurobiol. Dis. 2013;60:1–10. doi: 10.1016/j.nbd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 123.Peng L., Zhou Y., Jiang N., Wang T., Zhu J., Chen Y., Li L., Zhang J., Yu S., Zhao Y. DJ-1 exerts anti-inflammatory effects and regulates NLRX1-TRAF6 via SHP-1 in stroke. J. Neuroinflamm. 2020;17:81. doi: 10.1186/s12974-020-01764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen L., Luo M., Sun X., Qin J., Yu C., Wen Y., Zhang Q., Gu J., Xia Q., Kong X. DJ-1 deficiency attenuates expansion of liver progenitor cells through modulating the inflammatory and fibrogenic niches. Cell Death Dis. 2016;7:e2257. doi: 10.1038/cddis.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim J.-M., Jang H.-J., Choi S.Y., Park S.-A., Kim I.S., Yang Y.R., Lee Y.H., Ryu S.H., Suh P.-G. DJ-1 contributes to adipogenesis and obesity-induced inflammation. Sci. Rep. 2014;4:4805. doi: 10.1038/srep04805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Inden M., Kitamura Y., Takahashi K., Takata K., Ito N., Niwa R., Funayama R., Nishimura K., Taniguchi T., Honda T., et al. Protection Against Dopaminergic Neurodegeneration in Parkinson’s Disease–Model Animals by a Modulator of the Oxidized Form of DJ-1, a Wild-type of Familial Parkinson’s Disease–Linked PARK7. J. Pharmacol. Sci. 2011;117:189–203. doi: 10.1254/jphs.11151FP. [DOI] [PubMed] [Google Scholar]
- 127.Sajjad M.U., Green E.W., Miller-Fleming L., Hands S., Herrera F., Campesan S., Khoshnan A., Outeiro T.F., Giorgini F., Wyttenbach A. DJ-1 modulates aggregation and pathogenesis in models of Huntington’s disease. Hum. Mol. Genet. 2014;23:755–766. doi: 10.1093/hmg/ddt466. [DOI] [PubMed] [Google Scholar]
- 128.Marras C., Lang A., van de Warrenburg B.P., Sue C.M., Tabrizi S.J., Bertram L., Mercimek-Mahmutoglu S., Ebrahimi-Fakhari D., Warner T.T., Durr A., et al. Nomenclature of genetic movement disorders: Recommendations of the international Parkinson and movement disorder society task force. Mov. Disord. 2016;31:436–457. doi: 10.1002/mds.26527. [DOI] [PubMed] [Google Scholar]
- 129.Li W., Fu Y., Halliday G.M., Sue C.M. PARK Genes Link Mitochondrial Dysfunction and Alpha-Synuclein Pathology in Sporadic Parkinson’s Disease. Front. Cell Dev. Biol. 2021;9:1755. doi: 10.3389/fcell.2021.612476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ariga H., Takahashi-Niki K., Kato I., Maita H., Niki T., Iguchi-Ariga S.M.M. Neuroprotective Function of DJ-1 in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2013;2013:683920. doi: 10.1155/2013/683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martinat C., Shendelman S., Jonason A., Leete T., Beal M.F., Yang L., Floss T., Abeliovich A. Sensitivity to Oxidative Stress in DJ-1-Deficient Dopamine Neurons: An ES- Derived Cell Model of Primary Parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taira T., Saito Y., Niki T., Iguchi-Ariga S.M.M., Takahashi K., Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yanagisawa D., Kitamura Y., Inden M., Takata K., Taniguchi T., Morikawa S., Morita M., Inubushi T., Tooyama I., Taira T., et al. DJ-1 Protects against Neurodegeneration Caused by Focal Cerebral Ischemia and Reperfusion in Rats. J. Cereb. Blood Flow Metab. 2007;28:563–578. doi: 10.1038/sj.jcbfm.9600553. [DOI] [PubMed] [Google Scholar]
- 134.Sun S.-Y., An C.-N., Pu X.-P. DJ-1 protein protects dopaminergic neurons against 6-OHDA/MG-132-induced neurotoxicity in rats. Brain Res. Bull. 2012;88:609–616. doi: 10.1016/j.brainresbull.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 135.Miyazaki S., Yanagida T., Nunome K., Ishikawa S., Inden M., Kitamura Y., Nakagawa S., Taira T., Hirota K., Niwa M., et al. DJ-1-binding compounds prevent oxidative stress-induced cell death and movement defect in Parkinson’s disease model rats. J. Neurochem. 2008;105:2418–2434. doi: 10.1111/j.1471-4159.2008.05327.x. [DOI] [PubMed] [Google Scholar]
- 136.Yanagida T., Kitamura Y., Yamane K., Takahashi K., Takata K., Yanagisawa D., Yasui H., Taniguchi T., Taira T., Honda T., et al. Protection Against Oxidative Stress-Induced Neurodegeneration by a Modulator for DJ-1, the Wild-Type of Familial Parkinson’s Disease-Linked PARK7. J. Pharmacol. Sci. 2009;109:463–468. doi: 10.1254/jphs.08323SC. [DOI] [PubMed] [Google Scholar]
- 137.Angeloni C., Zambonin L., Hrelia S. Role of Methylglyoxal in Alzheimer’s Disease. BioMed Res. Int. 2014;2014:238485. doi: 10.1155/2014/238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shaikh S., Nicholson L.F.B. Advanced glycation end products induce in vitro cross-linking of α-synuclein and accelerate the process of intracellular inclusion body formation. J. Neurosci. Res. 2008;86:2071–2082. doi: 10.1002/jnr.21644. [DOI] [PubMed] [Google Scholar]
- 139.Cheng L., Zhang W. DJ-1 affects oxidative stress and pyroptosis in hippocampal neurons of Alzheimer’s disease mouse model by regulating the Nrf2 pathway. Exp. Med. 2021;21:557. doi: 10.3892/etm.2021.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaudhuri J., Bains Y., Guha S., Kahn A., Hall D., Bose N., Gugliucci A., Kapahi P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018;28:337–352. doi: 10.1016/j.cmet.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Finkbeiner S. Huntington’s Disease. Cold Spring Harb. Perspect. Biol. 2011;3:a007476. doi: 10.1101/cshperspect.a007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aleyasin H., Rousseaux M.W., Phillips M., Kim R.H., Bland R.J., Callaghan S., Slack R.S., During M.J., Mak T.W., Park D.S. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc. Natl. Acad. Sci. USA. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Molcho L., Ben-Zur T., Barhum Y., Offen D. DJ-1 based peptide, ND-13, promote functional recovery in mouse model of focal ischemic injury. PLoS ONE. 2018;13:e0192954. doi: 10.1371/journal.pone.0192954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamane K., Kitamura Y., Yanagida T., Takata K., Yanagisawa D., Taniguchi T., Taira T., Ariga H. Oxidative Neurodegeneration Is Prevented by UCP0045037, an Allosteric Modulator for the Reduced Form of DJ-1, a Wild-Type of Familial Parkinson’s Disease-Linked PARK7. Int. J. Mol. Sci. 2009;10:4789–4804. doi: 10.3390/ijms10114789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mukherjee D., Chander V., Bandyopadhyay A. PARIS-DJ-1 Interaction Regulates Mitochondrial Functions in Cardiomyocytes, Which Is Critically Important in Cardiac Hypertrophy. Mol. Cell. Biol. 2020;41:e00106-20. doi: 10.1128/MCB.00106-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shimizu Y., Nicholson C.K., Polavarapu R., Pantner Y., Husain A., Naqvi N., Chin L.S., Li L., Calvert J.W. Role of DJ-1 in Modulating Glycative Stress in Heart Failure. J. Am. Heart Assoc. 2020;9:e014691. doi: 10.1161/JAHA.119.014691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang J., Zhang H., Du A., Li Y. DJ-1 alleviates anoxia and hypoglycemia injury in cardiac microvascular via AKT and GSH. Mol. Cell. Probes. 2020;53:101600. doi: 10.1016/j.mcp.2020.101600. [DOI] [PubMed] [Google Scholar]
- 148.Amatullah H., Maron-Gutierrez T., Shan Y., Gupta S., Tsoporis J.N., Varkouhi A.K., Teixeira Monteiro A.P., He X., Yin J., Marshall J.C., et al. Protective function of DJ-1/PARK7 in lipopolysaccharide and ventilator-induced acute lung injury. Redox Biol. 2021;38:101796. doi: 10.1016/j.redox.2020.101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu X.-W., Ma T., Cai Q., Wang L., Song H.-W., Liu Z. Elevation of Serum PARK7 and IL-8 Levels Is Associated With Acute Lung Injury in Patients With Severe Sepsis/Septic Shock. J. Intensive Care Med. 2017;34:662–668. doi: 10.1177/0885066617709689. [DOI] [PubMed] [Google Scholar]
- 150.Anderson C.A., Boucher G., Lees C.W., Franke A., D’Amato M., Taylor K.D., Lee J.C., Goyette P., Imielinski M., Latiano A., et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cheng Y.-T., Ho C.-Y., Jhang J.-J., Lu C.-C., Yen G.-C. DJ-1 plays an important role in caffeic acid-mediated protection of the gastrointestinal mucosa against ketoprofen-induced oxidative damage. J. Nutr. Biochem. 2014;25:1045–1057. doi: 10.1016/j.jnutbio.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 152.Di Narzo A.F., Brodmerkel C., Telesco S.E., Argmann C., Peters L.A., Li K., Kidd B., Dudley J., Cho J., Schadt E.E., et al. High-Throughput Identification of the Plasma Proteomic Signature of Inflammatory Bowel Disease. J. Crohns Colitis. 2019;13:462–471. doi: 10.1093/ecco-jcc/jjy190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dubois P.C.A., Trynka G., Franke L., Hunt K.A., Romanos J., Curtotti A., Zhernakova A., Heap G.A.R., Adány R., Aromaa A., et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi S.Y., Lu S.-Y., Sivasubramaniyam T., Revelo X.S., Cai E.P., Luk C.T., Schroer S.A., Patel P., Kim R.H., Bombardier E., et al. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat. Commun. 2015;6:7415. doi: 10.1038/ncomms8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Singh Y., Chen H., Zhou Y., Föller M., Mak T.W., Salker M.S., Lang F. Differential effect of DJ-1/PARK7 on development of natural and induced regulatory T cells. Sci. Rep. 2015;5:17723. doi: 10.1038/srep17723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang J., Kim M.J., Yoon W., Kim E.Y., Kim H., Lee Y., Min B., Kang K.S., Son J.H., Park H.T., et al. Isocitrate protects DJ-1 null dopaminergic cells from oxidative stress through NADP+-dependent isocitrate dehydrogenase (IDH) PLoS Genet. 2017;13:e1006975. doi: 10.1371/journal.pgen.1006975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharm. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Raninga P.V., Di Trapani G., Tonissen K.F. DJ-1/PARK7 Protein. Springer; Berlin/Heidelberg, Germany: 2017. The Multifaceted Roles of DJ-1 as an Antioxidant. [DOI] [PubMed] [Google Scholar]
- 159.Moschen A.R., Gerner R.R., Wang J., Klepsch V., Adolph T.E., Reider S.J., Hackl H., Pfister A., Schilling J., Moser P.L. Lipocalin 2 protects from inflammation and tumorigenesis associated with gut microbiota alterations. Cell Host Microbe. 2016;19:455–469. doi: 10.1016/j.chom.2016.03.007. [DOI] [PubMed] [Google Scholar]