Abstract
Mitochondria, as the main site of cellular energy metabolism and the generation of oxygen free radicals, are the key switch for mitochondria-mediated endogenous apoptosis. Ca2+ is not only an important messenger for cell proliferation, but it is also an indispensable signal for cell death. Ca2+ participates in and plays a crucial role in the energy metabolism, physiology, and pathology of mitochondria. Mitochondria control the uptake and release of Ca2+ through channels/transporters, such as the mitochondrial calcium uniporter (MCU), and influence the concentration of Ca2+ in both mitochondria and cytoplasm, thereby regulating cellular Ca2+ homeostasis. Mitochondrial Ca2+ transport-related processes are involved in important biological processes of tumor cells including proliferation, metabolism, and apoptosis. In particular, MCU and its regulatory proteins represent a new era in the study of MCU-mediated mitochondrial Ca2+ homeostasis in tumors. Through an in-depth analysis of the close correlation between mitochondrial Ca2+ and energy metabolism, autophagy, and apoptosis of tumor cells, we can provide a valuable reference for further understanding of how mitochondrial Ca2+ regulation helps diagnosis and therapy.
Keywords: mitochondrial calcium, calcium homeostasis, calcium regulation, MCU, tumor
1. Introduction
Mitochondria are involved in a series of cellular biological processes such as adenosine triphosphate (ATP) generation, apoptosis, and cell cycle regulation to maintain the cell’s life activities [1]. Calcium ions (Ca2+) are distributed in the mitochondrial intermembrane gap and matrix [2]. Ca2+ shuttles between mitochondria and cytoplasm through different transport mechanisms, regulating the life activities of mitochondria and even the whole cell. Ca2+ is an indispensable messenger for many important physiologic processes, including metabolism, cell proliferation and death, protein phosphorylation, gene transcription, neurotransmission, contraction, and secretion [3]. The level of intracellular Ca2+ depends on the release of endoplasmic reticulum (ER) Ca2+ and the inflow of extracellular Ca2+ [4]. In animal body fluids and tissues, the concentration of Ca2+ varies between 2.1 and 2.6 mM [5] and the unit of total Ca2+ concentration in cells is also mM. However, in the cytoplasm of most cells, the concentration of free Ca2+ is about 10,000 times lower. In cells, inorganic compounds and low molecular weight organic molecules usually bind Ca2+ with low affinity and will not reduce their free concentration to nM, which is necessary for Ca2+ to effectively perform their signaling functions [6]. Abnormal Ca2+ homeostasis is one of the common pathological mechanisms of many diseases. Studies have shown that Ca2+ can not only be absorbed and released by mitochondria, but also the process of Ca2+ uptake and release by mitochondria plays an important role in maintaining cytoplasmic calcium homeostasis [7,8,9,10,11].
Ca2+ plays an indispensable role in signal transduction from cell surface receptors to the cytoplasm and from the cytoplasm to mitochondria, so as to jointly regulate cell metabolism [12]. Cytoplasmic calcium oscillation is the most prominent signal in cells, which refers to the transmission of a variety of regulatory information by cytosolic Ca2+ ([Ca2+]c) in the form of concentration oscillation [13]. Inositol 1,4,5-trisphosphate (IP3)-induced intracellular Ca2+ mobilization results in an increase in mitochondrial Ca2+ ([Ca2+]m) [14]. IP3-dependent hormone-induced [Ca2+]c oscillation is effectively transmitted to mitochondria in the form of [Ca2+]m oscillation [15]. Moreover, it has been reported that the concentration of free Ca2+ in mitochondria is closely related to the level of energy metabolism and the change in membrane permeability [16]. Mitochondrial Ca2+ accumulation triggers the activation of the mitochondrial metabolic mechanism, which increases ATP synthesis in the mitochondria and the ATP level in cytoplasm [17]. The uptake and release of mitochondrial Ca2+ also affects the intracellular calcium signal [18]. The abnormality in these calcium signaling-related activities is significantly related to the occurrence and development of heart disease, epilepsy, and neurodegenerative diseases [19].
At present, the incidence and mortality rate of malignant tumors is increasing year by year and it is the primary cause of death from all kinds of diseases. It is estimated that by 2040, 28.4 million new cases of cancer will be diagnosed worldwide, which represents an increase of 47% since 2020 [20]. There’s ample evidence that Ca2+ signaling is a key regulator in a series of tumor cell processes, including tumor growth, progression, and metastasis [21]. The alteration of Ca2+ is a hallmark of many tumors. For instance, Ca2+ is decreased in pancreatic cancer, colon cancer, and prostate cancer, while Ca2+ is increased in breast cancer and hepatocellular carcinoma (HCC) [22]. Altered Ca2+ signaling accelerates lipid accumulation and may promote HCC development [23]. Mitochondrial Ca2+ uptake is necessary for the progression of triple-negative breast cancer (TNBC) in vivo and it can also activate the hypoxia-inducible factor-1 alpha (HIF-1α) signal pathway, which contributes to tumor growth and metastasis [24]. In addition, intercellular Ca2+ signaling is altered in urinary bladder carcinoma cells [25]. Orai1-store-operated Ca2+ entry (SOCE) intracellular Ca2+ oscillation upregulation can activate downstream pathways, stimulate the proliferation and migration of esophageal squamous cell carcinoma (ESCC) cells, enhance their ability to invade other tissues, and promote the formation and growth of ESCC tumors in vitro and in vivo [26]. Meanwhile, SOCE also contributes to melanoma progression [27].
Regulated elevations in Ca2+ are required for the activity of several mitochondrial enzymes and this, in turn, regulates mitochondria-derived reactive oxygen species (ROS) generation; this is a known driver of pro-tumorigenic redox signaling, resulting in the activation of pathways implicated in cellular proliferation, metabolic alterations, stress adaptations and cell death [28,29,30,31,32]. Numerous studies have demonstrated that mitochondrial Ca2+ homoeostasis is involved in the metabolism, apoptosis, proliferation and other important processes of tumor cells [33,34]. In this review, we outline the role of mitochondrial Ca2+ in the regulation of tumor cell development and its molecular mechanisms, which is conducive to providing a basis for tumor therapy via targeting mitochondrial Ca2+ homoeostasis and regulation.
2. Regulation of Mitochondrial Ca2+
Due to the outer membrane of mitochondria possessing a high permeability for Ca2+, the concentration of Ca2+ in the membrane gap is equivalent to that in the cytoplasm [35]. In the resting state of cells, the concentration of Ca2+ in cytoplasm is about 100nM. When the cells are excited, the concentration of Ca2+ in the cytoplasm can rise to 1–3 µM. [36]. In fact, the uptake and release of Ca2+ by mitochondria can be regulated by the one-way transport mechanism or transporter [37]. The mitochondrial Ca2+ influx is mainly mediated by the mitochondrial calcium uniporter (MCU), voltage-dependent anion-selective channel (VDAC), and mitochondrial ryanodine receptor transporter. Furthermore, the mitochondrial Ca2+ efflux pathways mainly include leucine zipper/EF hand-containing transmembrane-1 (LETM1), mitochondrial Na+/Ca2+ exchanger (NCLX), and mitochondrial permeability transition pore (MPTP).
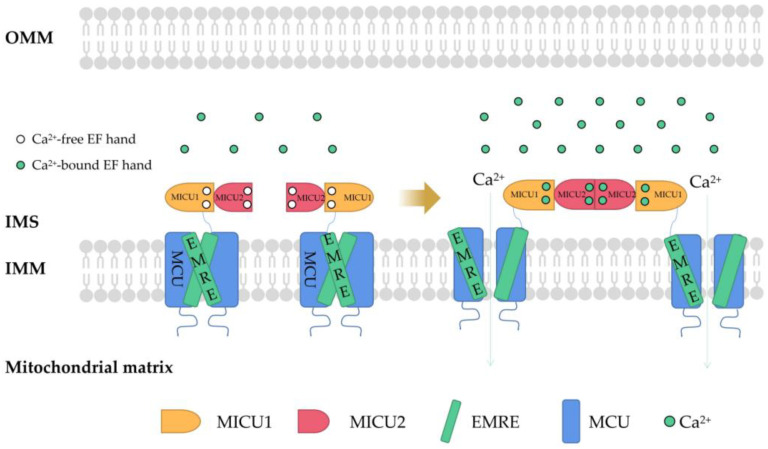
MCU is a Ca2+ channel ubiquitous in mitochondrial intima [38]. It is generally considered to be a key Ca2+ transporter [39] and silencing the MCU can severely abrogate mitochondrial Ca2+ uptake [40] (Figure 1). Knockout of the MCU completely inhibited mitochondrial Ca2+ uptake triggered by several stimuli in different cell types [41]. The MCU is an ion channel with electrophysiological characteristics. Ca2+ uptake through the MCU is driven by an electrochemical gradient. The MCU and related regulating molecules, including the essential MCU regulator (EMRE), mitochondrial calcium uptake (MICU)1, MICU2, MICU3, MCU-dominant negative beta subunit (MCUb), and MCU regulator 1 (MCUR1), form a large complex to manipulate the activities of the MCU [42]. The changes in the expression of these regulators are different in different cancer cells. For example, in pancreatic cancer cells, MICU1 and MICU2 are increased, while EMRE is decreased [43]. In breast cancer cells, MCU is elevated but MCUb is reduced [44]. In ovarian cancer cells, MICU1 mRNA is enhanced [45]. In HCC cells, the MCU, MCUR1, and MICU2 are elevated, while MICU1 is in decline [46].
Figure 1.
The structure of MCU and connections to its regulators. Mitochondrial Ca2+ uptake through MCU. In mammals, MCU contains four core components: pore-forming MCU protein, the gatekeepers MICU1 and MICU2, and an auxiliary subunit EMRE. MCU plays a vital role in Ca2+ transport. In order to prevent Ca2+ overload, the activity of MCU must be strictly regulated by MICUs, which can sense the change in cytosolic Ca2+ concentration to open and close the MCU. MCU, mitochondrial calcium uniporter; MICU, mitochondrial Ca2+ uptake; EMRE, essential MCU regulator; OMM, outer mitochondrial membrane; IMS, intermembrane space; IMM, inner mitochondrial membrane; EF hand, helix−loop−helix structure.
In higher eukaryotes, the EMRE mediates MICU1/MICU2 to regulate Ca2+ transport through a leverage mechanism. MICU1/MICU2 is associated with the MCU through the EMRE. Each MICU1 interacts with two EMRE subunits. The interaction sites are located at the N-terminal poly K, s339k340k341 domain of MICU1 and the C-terminal of the EMRE [47]. The regulation of MCU activity by MICU1 and MICU2 involves a gating mechanism: when cells in a resting state and the concentration of intracellular Ca2+ is low, MICU1−MICU2 inhibits Ca2+ from entering the mitochondria through the MCU. When cells are stimulated by signals and the concentration of Ca2+ in the cytoplasm increases and exceeds a certain threshold (more than about 1 mM), MICU1−MICU2 allows Ca2+ to enter the mitochondria through the MCU [48]. Down regulation of MICU1 can reduce Ca2+ flux, decrease mitochondrial oxidative phosphorylation (OXPHOS) and ATP production, and activate AMPK-dependent autophagy [49]. In parallel, MICU1 also regulates the cristae junction to maintain the structural mitochondrial membrane framework, and without the cristae junction, it can mediate uncoupling and increase ROS production [50,51].
MICU1 is upregulated in ovarian cancer cells and its expression is closely related to the survival of cancer cells and tumor growth [52]. In this pathway, MICU1 induces the accumulation of mitochondrial Ca2+ and the production of ROS, suggesting that the binding of MICU1 to the MCU is necessary for the function of the MCU complex and the entry of Ca2+ into mitochondria is a prerequisite survival factor of cancer cells. MICU1 has been shown to be methylated by protein arginine methyltransferase 1 (PRMT1) in cancer cells, yielding decreased Ca2+ sensitivity and reduced Ca2+ entry. UCP2/3 is fundamental for mitochondrial Ca2+ uptake in cancer cells [53]. When it binds to methylated MICU1, it can normalize the Ca2+ sensitivity of MICU1 and re-establishes Ca2+ entry into mitochondria [54]. This mechanism has also been found to be important in human cancer [55,56]. MICU2 can interact with MICU1 and elevate the Ca2+ threshold activated by the MCU. Therefore, MICU2 can inhibit MCU activity at low Ca2+ concentrations [57].
Although MICU1, MICU2, and MICU3 belong to the same family, they have different effects on the MCU. MICU2 is the gatekeeper of the MCU, while MICU3 is an MCU activation enhancer. Overexpression of MICU3 causes a 10-fold increase in transient Ca2+ [58]. MCUb directly interacts with the MCU and mainly performs negative regulation of the MCU [59]. At present, the research results on the effect of the MCUR1 on the MCU are still controversial. Some studies have pointed out that the MCUR1, as an essential scaffold factor of the MCU complex [60], is the key component of the MCU complex. It has also been reported that mitochondrial Ca2+ uptake does not depend on the MCUR1, which is only a regulator that sets the Ca2+ threshold of the transition in mitochondrial permeability. Inhibiting the expression of the MCUR1 increases the Ca2+ threshold for inducing MPTP conversion, which can reduce the mitochondrial cell death that is induced by an overload of Ca2+ [61].
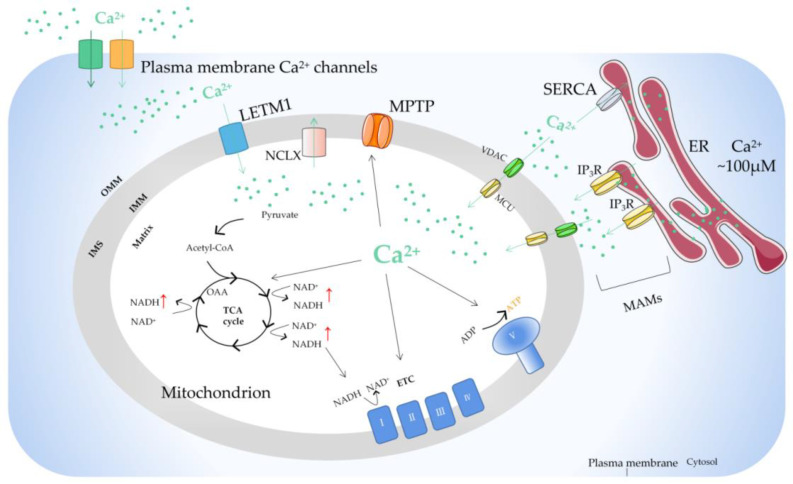
There is a sodium calcium transporter NCLX in the inner membrane of mitochondria, which is a sodium ion (Na+)-dependent Na+−Ca2+ reverse exchange channel and can positively regulate the outflow of Ca2+ in mitochondria [62]. When the concentration of Ca2+ in mitochondria is too high, it will enhance the activity of the NCLX and cause the opening of the MPTP on the inner membrane of mitochondria. Ca2+ is the center that regulates the MPTP. It can directly regulate the MPTP itself and indirectly affect the MPTP by regulating the adenosine diphosphate (ADP)/ATP balance, mitochondrial membrane potential, and ROS/reactive nitrogen level [63]. The study found that the MPTP has an important property: the increase in ADP and the recovery of Mg2+/Ca2+ caused by MPTP opening are reversible [64]. This reversibility makes MPTP opening have two modes: continuous opening and instantaneous opening, which can start the cell death signal pathway or maintain the normal physiological function of cells. In addition, there is LETM1 in the mitochondrial inner membrane [65]. When the concentration of Ca2+ in the mitochondrial matrix is low, LETM1 can transport Ca2+ into the matrix. On the contrary, Ca2+ is transported out of mitochondria. The study also found that silencing LETM1, despite the presence of the MCU, can still inhibit the influx of Ca2+ into mitochondria (Figure 2).
Figure 2.
The basic mechanism of mitochondrial Ca2+ regulation. Ca2+ transfer from ER to mitochondria occurs on the MAMs, where there are special Ca2+ channels. The opening of IP3R on the surface of ER results in the release of Ca2+ from the lumen of ER. Ca2+ passes through OMM via VDAC and traverses IMM via MCU. Stimulus acts by producing Ca2+ mobilization signals, triggering the increase of intracellular Ca2+ concentration. The function of mitochondrial Ca2+ uptake and release are mainly to regulate the matrix Ca2+ level, thus regulating the activity of mitochondrial dehydrogenase, resulting in increased NADH and ATP production. Ca2+ can also activate mitochondrial ETC complexes. In the steady state, Ca2+ entering mitochondria through MCU must exit through one of the mitochondrial Ca2+ efflux mechanisms. ER, endoplasmic reticulum; MAM, mitochondrial-associated ER membrane; IP3R, inositol triphosphate receptor; MCU, mitochondrial calcium uniporter; VDAC, voltage-dependent anion-selective channel; ETC, electron transport chain; OMM, outer mitochondrial membrane; IMS, intermembrane space; IMM, inner mitochondrial membrane; LETM1, leucine zipper/EF hand-containing transmembrane-1; MPTP, mitochondrial permeability transition pore; NCLX, mitochondrial Na+/Ca2+ exchanger; SERCA, sarco-endoplasmic reticulum Ca2+-ATPase; OAA, oxaloacetic acid; TCA cycle, tricarboxylic acid cycle.
Mitochondrial Ca2+ homeostasis is unbalanced in tumors because in tumor cells, the cellular microenvironment is remodeled and leads to further mitochondrial Ca2+ imbalance, which is an adaptive phenomenon of tumors, and the mitochondrial Ca2+ imbalance will further promote the development of tumors. Some studies have shown that cancer cells can change mitochondrial Ca2+ homeostasis mainly through the following methods: (1) Ca2+ exists in a domain formed between the ER and the mitochondria, which is called the mitochondrial-associated membrane (MAM) and controls mitochondrial Ca2+ homeostasis [66] (Figure 2). Cancer cells can remodel their MAMs to affect mitochondrial Ca2+ homeostasis and promote cell survival, migration, invasion, metastasis, autophagy, and inhibit apoptosis [67,68,69]. (2) Mechano- and proton-sensing proteins may cause an imbalance in Ca2+ levels in cancer cells [70]. (3) In cancer cells, the expression and function of the magnesium (Mg2+) transporter are abnormal. The imbalance of Mg2+ homeostasis may destroy Ca2+ homeostasis [71]. (4) Cancer cells modify the Ca2+ signaling network by changing the expression and function of cation channels, pumps, or transporters [72].
3. Mitochondrial Ca2+ and Energy Metabolism of Tumor Cells
Ca2+ participates in almost all physiological activities in cells. Mitochondria were originally considered to be a “Ca2+ pool” with the ability to absorb a large amount of Ca2+, and the uptake of Ca2+ by mitochondria increases significantly when the extramitochondrial Ca2+ is overloaded [73]. It has been found that Ca2+ can stimulate glycogen decomposition and glucose oxidation, resulting in an increase in ATP supply [74]. The increase in cytoplasmic Ca2+ concentration is transmitted to mitochondria and Ca2+-activated dehydrogenase is a key rate control enzyme in the tricarboxylic acid cycle (TAC) flux. Ca2+ activation will lead to the increase in pyridine nucleotide reduction and oxidative phosphorylation [75]. Mitochondrial Ca2+ uptake can activate matrix enzymes, stimulate ATP production, and regulate energy metabolism by activating pyruvate dehydrogenase, isocitrate dehydrogenase, and ketoglutarate dehydrogenase. This “parallel activation model” provides a mechanism in which Ca2+ stimulates the process of energy consumption caused by physiological activities such as various hormones, muscle contraction, or increased cardiac load [76]. It also provides a means for cells to upregulate ATP supply to keep up with this energy consumption.
Metabolic reprogramming in tumor cells is considered to be a sign of cancer and is involved in tumor growth and development. Compared to normal cells, under the condition of sufficient aerobic supply, tumor cells still obtain energy by aerobic glycolysis and produce a large amount of lactic acid and a small amount of ATP [77]. M2 isoform of pyruvate kinase (PKM2) is critical for the metabolic fate of the glycolytic intermediates [78,79,80]. During the course of the disease, tumor cells will develop overall metabolic adaptability so that they can survive in the tumor microenvironment with low oxygen and nutrient levels [81].
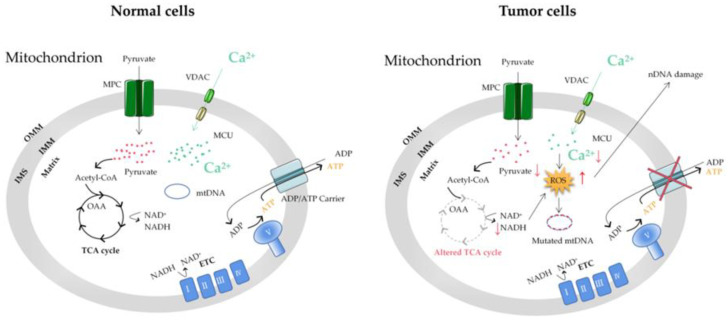
In summary, Ca2+ affects the functional changes of mitochondria (such as mitochondrial dysfunction, metabolic conversion to glycolysis, and mtDNA mutations) and thus, cell energy metabolism, which is closely related to the occurrence and development of tumors (Figure 3). At present, the adaptability of tumor cell metabolism is the main limitation of cancer treatment, which is highly related to the resistance to therapeutic drugs [82]. The unique metabolic pattern of tumor cells is both a challenge and an opportunity. Understanding the metabolic mechanism of tumor cells is greatly significant for the early diagnosis of a tumor’s metabolic phenotype and rational targeted therapy.
Figure 3.
The mitochondrial Ca2+ and energy metabolism in normal and tumor cells. The reprogramming of energy metabolism, including energy production disorders caused by cell respiratory defects, is the core symbol of cancer. The change in energy metabolism in cancer cells is related to the abnormal function of mitochondria. Accumulation of the ROS induced by mitochondrial Ca2+ dyshomeostasis and altered TCA in tumor cells can cause mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) mutations. MCU, mitochondrial calcium uniporter; VDAC, voltage-dependent anion-selective channel; ETC, electron transport chain; OMM, outer mitochondrial membrane; IMS, intermembrane space; IMM, inner mitochondrial membrane; MPC, mitochondrial pyruvate carrier; OAA, oxaloacetic acid; TCA cycle, tricarboxylic acid cycle.
4. Mitochondrial Ca2+ and the MCU in Autophagy/Mitophagy of Tumor Cells
Metabolic adaptations allow tumor cells to survive in the low oxygen and nutrient tumor microenvironment. Among these metabolic adaptations, tumor cells use glycolysis but also mitochondrial oxidation to generate ATP; another particular adaptation of tumor cell metabolism is the use of autophagy and mitophagy [83]. Autophagy plays a key role in maintaining cellular homeostasis [84]. Thus, autophagy disorders disrupt normal physiological processes and are implicated in the pathogenesis of various diseases, including tumors [85]. Autophagy is a highly conserved catabolic process that results in the degradation and recycling of proteins and organelles after the fusion of isolated vesicles, autophagosomes, and lysosomes that provide hydrolases [86]. The molecular process of autophagy is complex and involves sequential steps for nucleation, extension, and fusion of associated proteins, including autophagy-associated proteins [87]. Autophagy has two main physiological roles: the breakdown of dysfunctional proteins or organelles as a quality control mechanism and the recovery of biological macromolecules to maintain metabolic needs under nutritional stress [88]. Autophagy has been found to play two roles in a tumor: a protective role in the early stages of the tumor and the promotion of tumor growth in advanced stages [89].
Intracellular Ca2+ is considered a bidirectional regulator of autophagy [90,91], which may depend on the spatiotemporal parameters of Ca2+ signal transduction, nutrients, and the utilization of growth factors [92]. Ca2+ overload can affect autophagy, leading to normal cell carcinogenesis and the growth of tumor cells. It is demonstrated that Ca2+ agonists, such as vitamin D3 compounds, ionomycin, ATP, and thapsigargin, can stimulate the autophagy of MCF-7 breast tumor cells through Ca2+-activated kinase CaMKK [93]. Consistent with the activation of autophagy by Ca2+, researchers have found that mitochondrial fission-mediated Ca2+ signaling also significantly induces autophagy in HCC [94]. Conversely, some other research groups have found the inhibitory effect of Ca2+ on autophagy. At present, there are several ways for Ca2+ to inhibit autophagy: (1) the inositol 1,4,5-trisphosphate receptor (IP3R) mediates Ca2+ to reduce the release of Beclin1 so as to reduce autophagosome production and inhibit autophagy; (2) IP3R mediates Ca2+ activation of calpain, separates autophagy protein 5 from autophagy protein 12, reduces the level of their complex, and inhibits autophagy [95]; (3) The increase of Ca2+ released by the ER to the mitochondria enhances the TAC and ATP production and inhibits autophagy [96,97]; (4) IP3R mediates Ca2+ into mitochondria, resulting in increased ATP production and the inhibition of AMPK, thereby inhibiting autophagy. Therefore, Ca2+ may have different regulatory effects on autophagy.
As with non-selective autophagy, the role of mitophagy is complex and can depend on tumor type and stage. Since both autophagy and mitophagy are related to mitochondrial function, targeting mitochondrial ion channels may also be an interesting strategy to regulate autophagy or mitophagy in tumors. Ca2+ exchanges have been associated with autophagy and mitophagy regulation. Therefore, unsurprisingly, some mitochondrial calcium transporters, such as the MCU, have recently been found to be involved in autophagy and mitophagy regulation in tumor cells.
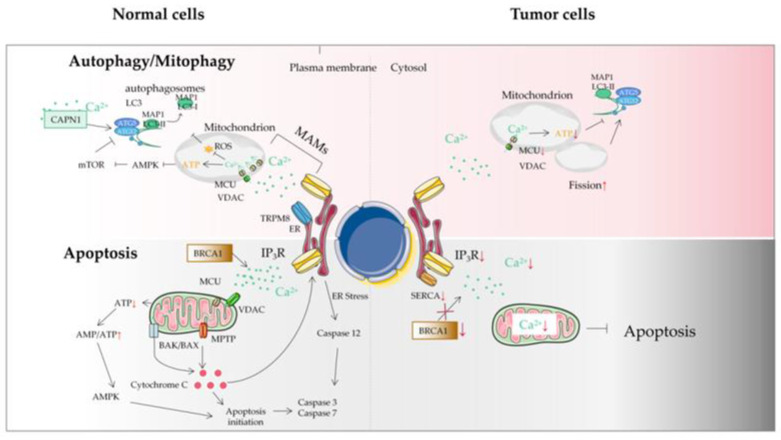
The MCU is generally considered to be the main Ca2+ transporter in the matrix, which is a major mediator of calcium influx into mitochondria. The MAM is an important part of Ca2+ transfer from the ER to the mitochondria to regulate mitochondrial enzymes. Ca2+ flow mainly occurs through IP3R and transient receptor potential cation channel subfamily M member 8 (TRPM8) in the ER membrane [98]. Sensitizing IP3Rs and the interruption of Ca2+ flow between the ER and the mitochondria break the calcium homeostasis and decrease mitochondrial bioenergetics, which subsequently decreases OXPHOS and activates autophagy [99,100]. However, unlike normal cells, autophagy activation caused by MAM destruction in tumor cells seems insufficient to maintain the required energy level, resulting in tumor cell death and reduced tumor growth [101] (Figure 4). Although the mechanisms linked with autophagy are not clearly understood, the MCU could be an interesting target to disrupt Ca2+ in the MAM in tumor cells, decreasing mitochondrial function and inducing cell death. The MCU has also been found to be altered in tumors from different tissues [102]. In particular, the expression of the MCU is associated with tumor progression and metastasis [103]. Therefore, mitochondrial Ca2+ and the MCU represent attractive antitumor targets for regulating mitochondrial dysfunction and autophagy/mitophagy in tumors.
Figure 4.
The autophagy/mitophagy and apoptosis of tumor cells. Autophagy plays a key role in maintaining cellular homeostasis. Autophagy disorder destroys normal physiological processes and can lead to cancer. Ca2+ can inhibit autophagy through an IP3R- or ER-mediated manner. Some mitochondrial Ca2+ transporters are also involved in autophagy and mitophagy regulation. Autophagy plays two roles in a tumor: a protective role in the early stages of tumor and the promotion of tumor growth in advanced stages. Tumor cells may avoid apoptosis by reducing Ca2+ influx into the cytoplasm. It can be achieved by downregulation of the expression of Ca2+ channels in the plasma membrane or by reducing the effectiveness of the signal pathways that activate these channels. This protective measure will greatly reduce the response of Ca2+ overload to pro-apoptotic stimulus, thus impairing the effectiveness of mitochondrial and cytoplasmic apoptotic pathways in tumor cells. Another mechanism is that tumor cells adapt to the reduction of Ca2+ in ER, without inducing the pro-apoptotic ER stress response usually accompanied by ER Ca2+ imbalance. ER, endoplasmic reticulum; MAM, mitochondrial-associated ER membrane; MCU, mitochondrial calcium uniporter; MPTP, mitochondrial permeability transition pore; ROS, reactive oxygen species; TRPM8, transient receptor potential cation channel subfamily M member 8; VDAC, voltage-dependent anion-selective channel; SERCA, sarco-endoplasmic reticulum Ca2+-ATPase; IP3R, inositol triphosphate receptor; BRCA1, breast cancer susceptibility gene.
5. Mitochondrial Ca2+ and Tumor Cell Apoptosis
Apoptosis involves the activation, expression, and regulation of a series of genes. It is not a phenomenon of autologous injury under pathological conditions, but a death process actively striving for better adaptation to the living environment [104]. The regulation of apoptosis is controlled by a very complex signal network system. There are three major signaling pathways: the mitochondrial pathway, the death receptor pathway, and the ER pathway [105]. These signal transduction pathways can eventually activate caspase-3, the executor of apoptosis, which hydrolyzes various cellular components and causes cell apoptosis [106]. In animal cells, the mitochondrial pathway is the most common apoptotic mechanism and the core of apoptosis [107,108]. In the early stage of apoptosis, mitochondria show changes such as increased permeability, Ca2+ uptake, decreased transmembrane potential, and the release of cytochrome C and apoptosis-inducing factors [109]. Changes in Ca2+ concentration may play a key role in the early apoptotic signal transduction pathway upstream of mitochondria [110]. However, this sensitive system can be affected to drive malignant transformation in cells.
In the process of apoptosis, intracellular Ca2+ overload can come from either extracellular Ca2+ influx or the release of the intracellular Ca2+ pool [111]. Some studies have suggested that the release of the intracellular Ca2+ pool can only cause a temporary increase in Ca2+, which is not enough to cause apoptosis. The triggering of apoptosis requires Ca2+ to reach a certain threshold and maintain this level for a long time [112]. With further research, there are many factors regulating Ca2+ levels in mitochondria, including intracellular regulation of the Bcl-2 family, the release of calcium pool ER and the participation of ROS [113]. At present, more than 20 members of the Bcl-2 family have been found. The proteins in the Bcl-2 family are widely distributed in the outer membrane of the mitochondria, nuclear membrane, and ER, regulating the activity of the caspases. Bcl-2 family members can be divided into three groups according to their structure and function. The first group includes Bcl-2, Bcl-XL, and Bcl-W, which have anti-apoptotic properties. The second group is a member of the Bcl-2 family with BH3-only proteins, which could increase the permeability of the mitochondrial outer membrane during cell apoptosis [114]. The third group, which contains all the domains except BH4, also increases membrane permeability and has pro-apoptotic activity [115].
The ER is an important Ca2+ reservoir in eukaryotic cells, so Ca2+ in the ER must maintain a stable level to ensure the accuracy of the Ca2+ signal [116]. Ca2+ released from the ER can directly flow into mitochondria and the uptake rate of mitochondrial Ca2+ depends on the concentration gradient of cytoplasmic Ca2+ at the IP3R opening on the ER. The opening of the ER InsP3/Ca2+ channel affects the Ca2+ balance in mitochondria and the InsP3/Ca2+ channel is one of the targets of caspase-3 [117]. Moreover, ER stress induced by the disturbance in the ER calcium state can activate caspase-12, a specific ER-localized protein, to trigger apoptosis in a mitochondria-independent way [118,119].
Mitochondria are the central link mediating apoptosis, as well as the main site of ROS generation [120]. With the discovery and further understanding of the role of mitochondrial Ca2+ in apoptosis, the research on the role of ROS in apoptosis is getting more and more in-depth. The regulation mechanisms of ROS on mitochondrial Ca2+ homeostasis are as follows: (1) After cells receive the pro-apoptotic signals, the increase of ROS promotes mitochondrial Ca2+ influx, which may be caused by affecting voltage-dependent Ca2+ channels, non-specific cell membrane Ca2+ permeability changes, and Na+/Ca2+ exchanges [121]; (2) Increased intracellular Ca2+ can activate other enzymes to further upregulate the level of oxygen free radicals, so ROS can indirectly produce more oxides and further promote the rise in mitochondrial Ca2+ level [122]. In addition, an overload of Ca2+ leading to oxidative metabolism impairment and ROS overproduction [123]. Previous studies have suggested that under oxygen stress, ROS produced by mitochondria will cause membrane lipid peroxidation and changes in mitochondrial function, resulting in the release of Ca2+ and apoptosis of mitochondria [124]; (3) ROS can regulate IP3R production and affect Ca2+ release from the ER into mitochondria [125]; (4) ROS can also affect the sarcoplasmic reticulum Ca2+ pump and inhibit intracellular or extracellular ER Ca2+ transfer by inhibiting the Ca2+-ATPase pump [126]; (5) Both ROS and Ca2+ can induce MPTP opening. On the other hand, MPTP opening leads to a large increase in ROS [127].
Several types of tumor cells have experienced extensive reorganization of their internal Ca2+ signal transduction mechanism, which promotes the occurrence of tumors [128]. Calcium ion exchange between mitochondria and the ER can be carried out through some Ca2+ signal proteins, including VDAC1, IP3R, and SERCA, which play vital roles in the processes of tumors. VDAC1 plays a significant role in cellular Ca2+ homeostasis and it has also been recognized as a key protein in mitochondria-mediated apoptosis [129]. For example, in several types of non-small cell lung cancer and cervical cancer, the expression level of VDAC1 is related to tumor growth and invasion [130]. The downregulation of IP3R1 in bladder cancer cells prevents mitochondrial Ca2+ overload by decreasing the uptake of ER−mitochondria Ca2+, thereby reducing cisplatin-mediated apoptosis [131]. The significant reduction or loss of SERCA3 subtypes in transformed colonic epithelial cells also proves that the Ca2+ signal is remodeled in tumorigenesis [132].
Recently, it has been found that in several cancer types, the imbalance of two new mechanisms will affect the renewal of the proteasome, so as to regulate the apoptosis sensitivity of tumor cells by affecting IP3R3 proteins and interfering with the Ca2+ exchange between the ER and mitochondria [133]. (1) The tumor suppressor protein PTEN and F-box/LRR repeat protein 2 (FBXL-2) compete for binding to IP3R3, which slows down FBXL-2-mediated IP3R3 proteasome degradation. This represents a new mechanism. The deletion of PTEN enables tumor cells to avoid apoptosis [134]. The downregulation of IP3R3 impairs the pro-apoptotic mitochondrial Ca2+ transfer. (2) The tumor suppressor protein BRCA1-associated protein 1 (BaP1) is a deubiquitinase that promotes the transfer of ER−mitochondria Ca2+ by stabilizing IP3R3. Under long-term environmental pressure, the function of BaP1 will be seriously disrupted, which is related to the acquired inactivating mutations of the BaP1 gene. The loss of BaP1 will lead to the downregulation of IP3R3, which hinders the effective apoptotic clearance of damaged cells and is conducive to the occurrence of tumors and the survival of malignant cells [135]. In addition, oncogenes and tumor suppressor proteins can play other roles in cancer development through Ca2+ signal regulation, such as resistance to apoptosis. Because mitochondrial Ca2+ overload is related to apoptosis and death, modifying ER−mitochondria Ca2+ transfer at the MAM will change the sensitivity of apoptosis, and tumor cells can acquire resistance to cell death accordingly [136]. For example, by inhibiting IP3R-mediated Ca2+ signaling or increasing the transmembrane distance at the MAM, the efficiency of ER−mitochondria Ca2+ transfer can be reduced, so as to decrease the sensitivity of tumor cells to apoptosis [137] (Figure 4).
6. The Relationship between the MCU and the Tumor
With the deepening of the research on the mechanism of cancer metastasis, the relationship between mitochondrial calcium homeostasis and the development of malignant tumors has attracted much attention [138,139]. The MCU is a major mediator of calcium influx into mitochondria and controls cellular energy metabolism, autophagy/mitophagy, and apoptosis. In most cancer tissues, the MCU showed moderate to strong immunostaining [140]. Increasingly, evidence shows that the MCU is closely related to multiple cancers, such as breast cancer, HCC, and colon cancer.
The MCU plays an important role in controlling the energy metabolism of tumor cells. The receptor-interacting protein kinase 1 (RIPK1) is an important signal molecule in the pathway of cell survival, apoptosis, and necrosis, which is significantly upregulated in colorectal cancer (CRC) cells. RIPK1 interacts with the MCU to promote CRC cell proliferation by increasing mitochondrial Ca2+ uptake and energy metabolism [141]. Compared with normal tissues, the MCU, MICU1, and MICU2 were overexpressed in oral squamous cell carcinoma (OSCC) tissues. The MCU is a new proto-oncogene of OSCC, which is regulated by nuclear factor erythroid 2-related factor 2 (Nrf2) transcription. The MCU can enhance the proliferation of OSCC cells and inhibit apoptosis [142]. Dihydroartemisinin can repress the proliferation and migration of OSCC cells by inhibiting the expression of the MCU [143]. MCU-mediated high mitochondrial Ca2+ can increase the proliferation of prostate cancer cells by inhibiting MPTP [144]. The MCU is involved in the autophagy of cancer cells. In kidney cancer cells, the upregulation of miR501-5p leads to the downregulation of the MCU, which leads to the activation of AMPK, thus promoting mTOR-independent autophagy [145]. The MCU also affects the apoptosis of cancer cells. Cathepsin S (CTSS) is overexpressed in glioblastomas (GBs). High levels of CTSS are associated with tumor progression and a poor prognosis of GB. Inhibiting the expression of CTSS in GB cells can increase the expression of MCUs. Enhanced mitochondrial Ca2+ uptake leads to mitochondrial Ca2+ overload, produces a large number of ROS, and, finally, causes apoptosis [146]. RY10-4 can induce the apoptosis of breast cancer cells by elevating Ca2+ through the MCU [147]. The MCUR1 is frequently upregulated in HCC cells, which enhances Ca2+ uptake into mitochondria in an MCU-dependent manner. The HCC cell survival rate is significantly improved by inhibiting mitochondrial-dependent apoptosis and promoting HCC cell proliferation, resulting in poor prognosis [148]. The data also show that miR-25 decreases mitochondrial Ca2+ uptake through selective MCU downregulation, thereby reducing apoptosis. The MCU seems to be downregulated in human colon cancer samples. Correspondingly, miR-25 is abnormally expressed, indicating that mitochondrial Ca2+ plays an important role in the survival of cancer cells [149].
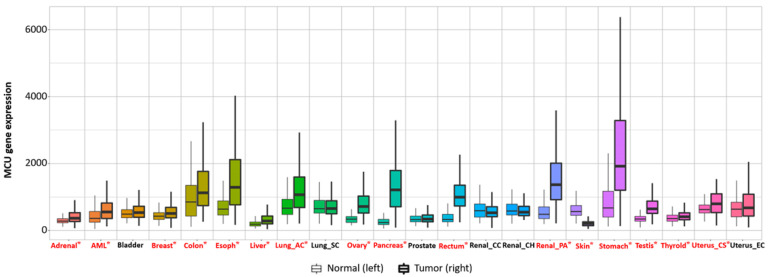
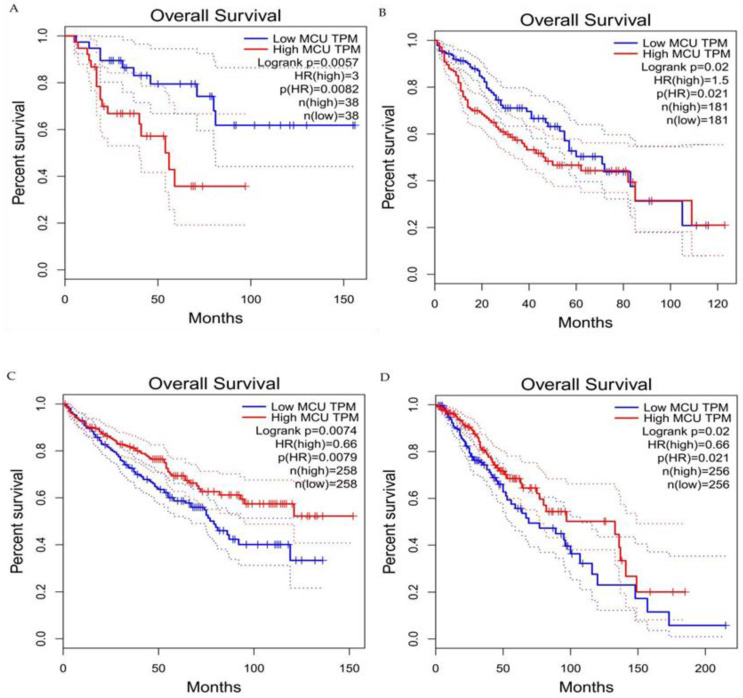
Current studies have suggested that the MCU is correlated with tumor size and lymphatic infiltration, which may contribute to tumor growth and metastasis [150,151]. It is speculated that the MCU affects the expression of VEGF through HIF-1α and the inhibition of MCU expression significantly reduces the invasion and migration ability of breast cancer cells [24,152]. In addition, the expression of the MCUR1 significantly affects the progression and prognosis of breast cancer [153,154]. However, the role of the MCU in cancer research remains controversial. Studies have shown that specific Ca2+ channels play different roles in some cancers due to different regulatory mechanisms. Previous studies have revealed that a highly expressed MCU promotes the metastasis of adrenocortical carcinoma breast cancer cells with poor prognosis. In hepatocellular carcinoma studies, MCU-dependent mitochondrial Ca2+ uptake promotes metastasis of HCC cells [155]. In fact, we analyzed the transcriptional expression levels of MCUs in different cancers through the relevant database (https://tnmplot.com/analysis/; accessed on 31 March 2022) [156] and demonstrated that the expression levels of MCUs in most tumors are not consistent with those in normal tissues (Figure 5). The majority of tumors have significantly elevated levels of MCU expression. However, high MCU expression in cancer patients may not always be beneficial. Coincidentally, we analyzed the survival curves between MCU expression levels and cancer patient survival through the GEPIA database (http://gepia.cancer-pku.cn/index.html; accessed on 31 March 2022) and found that in adrenocortical carcinoma and hepatocellular carcinoma, overall survival is significantly greater in low MCU expression than in high MCU expression (Figure 6A,B). On the contrary, in renal clear cell carcinoma and brain lower grade glioma, overall survival is significantly greater in high MCU expression than in low MCU expression (Figure 6C,D). To sum up, the relationship between the MCU and tumor is complex and needs more in-depth research.
Figure 5.
Transcriptional expression level of the MCU in various normal and cancerous organs. The MCU is closely related to multiple cancers; the expression levels of the MCU in most tumors are not consistent with those in normal tissues. The majority of tumors have significantly elevated levels of MCU expression. Significant differences by Mann−Whitney U test are marked with red and *. MCU, mitochondrial calcium uniporter.
Figure 6.
Survival curves for overall survival of high versus low expressing MCU. (A) Adrenocortical carcinoma. (B) Hepatocellular carcinoma. (C) Renal clear cell carcinoma. (D) Brain lower grade glioma. In adrenocortical carcinoma and hepatocellular carcinoma, overall survival is significantly greater in low MCU expression than in high MCU expression. In renal clear cell carcinoma and brain lower grade glioma, overall survival is significantly greater in high MCU expression than in low MCU expression. MCU, mitochondrial calcium uniporter; HR, hazard rate.
Mitochondria regulate Ca2+ homeostasis through the uptake of Ca2+ into the mitochondria via MCU and the release of Ca2+ from the mitochondria via NCLX, regulating intramitochondrial and intracytoplasmic Ca2+ concentrations. Therefore, the regulation of both is deeply intertwined. Since the reorganization of cytosolic calcium signaling commonly occurs in tumor cells, mitochondrial calcium imbalance causes alterations in cytosolic calcium signaling and thus, affects tumorigenesis and progression [157]. Given the important impact of mitochondrial calcium imbalance on tumors, a large number of studies have used mitochondrial calcium imbalance as a starting point to explore new diagnostic and therapeutic approaches to tumors. It is found that proteins associated with mitochondrial calcium uptake may serve as novel biomarkers for predicting poor prognosis in HCC. This study includes tumor specimens and adjacent normal liver tissue from 354 patients with confirmed HCC as study subjects and concluded that HCC patients with low MICU1 and high MCU/MICU2 expression exhibited poor survival rates, overall survival rates and disease-free survival rates [158]. Another study shows that the MCUR1 promotes in vitro invasion and in vivo metastasis of HCC cells by promoting epithelial−mesenchymal transition. This process is mainly done by the MCUR1 through the activation of the ROS/Nrf2/Notch1 pathway. It has also been found that treatment with the mitochondrial Ca2+-buffering protein parvalbumin significantly inhibits the ROS/Nrf2/Notch pathway, MCUR1-induced epithelial− mesenchymal transition and HCC metastasis [159]. In a study of CRC, the MCU is markedly increased in CRC tissues, and upregulated MCU is associated with poor prognosis in patients with CRC [160]. An upregulated MCU enhances mitochondrial Ca2+ uptake and causes mitochondrial Ca2+ imbalance, which, in turn, promotes CRC cell growth in vitro and in vivo. Ru360 is a highly potent and selective MCU inhibitor that can effectively block MCU-mediated mitochondrial Ca2+ uptake and, ultimately, slow CRC progress. These results may provide a potential pharmacological target for CRC treatment [161]. Saverio Marchi’s group demonstrated for the first time that MCUs are suitable targets for miRNA-25, which reduces prostate and colon cancer cells’ dependence on Ca2+ [162]. Therefore, we can induce the apoptosis in cancer cells by reducing MCU protein levels and mitochondrial Ca2+ uptake.
7. Conclusions
The occurrence and development of tumors is a complex process regulated by multiple signaling networks. In this paper, we summarize, analyze, and discuss that mitochondrial Ca2+ and the MCU play crucial roles in energy metabolism, autophagy/mitophagy, and apoptosis of tumor cells. The discovery of the MCU and its regulatory proteins represents a new era of research on MCU-mediated mitochondrial Ca2+ dyshomeostasis in cancer. Currently, drug candidates targeting the MCU or its regulatory factors are still emerging. Although a flurry of studies has confirmed the correlation between mitochondrial Ca2+ dyshomeostasis and the progression of a variety of tumors, the exact mechanism and targeted therapy remain to be further elucidated. The tumor diagnosis and treatment strategy for mitochondrial Ca2+ homeostasis will bring a new dawn to tumor risk prediction, precancerous lesion screening, clinical targeted therapy, and prognosis assessment.
Abbreviations
ATP, adenosine triphosphate; Ca2+, Calcium ion; [Ca2+]c, cytosolic Ca2+; [Ca2+]m, mitochondrial Ca2+; IP3, inositol 1,4,5-trisphosphate; ROS, reactive oxygen species; VDAC, voltage-dependent anion-selective channel; MCU, mitochondrial calcium uniporter; NCLX, mitochondrial Na+/Ca2+ exchanger; MPTP, mitochondrial permeability transition pore; ER, endoplasmic reticulum; EMRE, essential MCU regulator; MICU, mitochondrial calcium uptake; MCUb, MCU-dominant negative beta subunit; MCUR1, MCU regulator 1; OXPHOS, oxidative phosphorylation; MAM, mitochondrial associated ER membrane; IP3R, inositol 1,4,5-trisphosphate receptor; HCC, hepatocellular carcinoma cells; FBXL-2, F-box/LRR repeat protein 2; BaP1, BRCA1 associated protein 1; CRC, colorectal cancer.
Author Contributions
Conceptualization, L.Z. and J.L.; methodology, P.A., Y.L. and J.L.; software, X.Z. (Xu Zhang); validation, P.A., Y.L. and J.L.; formal analysis, L.Z., J.Q. and X.Z. (Xu Zhang); investigation, L.Z., X.Z. (Xu Zhang) and J.Q.; resources, L.Z., J.Q., X.Z. (Xu Zhang) and X.Z. (Xiya Zhao); data curation, L.Z., X.Z. (Xu Zhang) and J.Q.; writing—original draft preparation, L.Z.; writing—review and editing, P.A., Y.L. and J.L.; visualization, L.Z. and X.Z. (Xu Zhang); supervision, P.A., Y.L. and J.L.; project administration, P.A., Y.L. and J.L.; funding acquisition, P.A., Y.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Beijing Advanced Innovation Center for Food Nutrition and Human Health, the National Natural Science Foundation of China (31970717, 82170429), the Chinese Universities Scientific Fund (2020TC015), the Beijing Municipal Natural Science Foundation (7222111), and the China Postdoctoral Science Foundation (2021M703520).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nunnari J., Suomalainen A. Mitochondria: In sickness and in health. Chem. Commun. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bravo-Sagua R., Parra V., López-Crisosto C., Díaz P., Quest A.F., Lavandero S. Calcium Transport and Signaling in Mitochondria. Compr. Physiol. 2017;7:623–634. doi: 10.1002/cphy.c160013. [DOI] [PubMed] [Google Scholar]
- 3.Patergnani S., Danese A., Bouhamida E., Aguiari G., Previati M., Pinton P., Giorgi C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020;21:8323. doi: 10.3390/ijms21218323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giorgi C., Marchi S., Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018;19:713–730. doi: 10.1038/s41580-018-0052-8. [DOI] [PubMed] [Google Scholar]
- 5.Brini M., Calì T., Ottolini D., Carafoli E. Intracellular calcium homeostasis and signaling. Met. Ions Life Sci. 2013;12:119–168. doi: 10.1007/978-94-007-5561-1_5. [DOI] [PubMed] [Google Scholar]
- 6.Carafoli E., Krebs J. Why Calcium? How Calcium Became the Best Communicator. JBC. 2016;40:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zavodnik I.B. Mitochondria, calcium homeostasis and calcium signaling. Biomed. Khim. 2016;62:311–317. doi: 10.18097/PBMC20166203311. [DOI] [PubMed] [Google Scholar]
- 8.Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 9.Marchi S., Patergnani S., Missiroli S., Morciano G., Rimessi A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Magalhães P.J., Rizzuto R. Mitochondria and calcium homeostasis: A tale of three luminescent proteins. Luminescence. 2001;16:67–71. doi: 10.1002/bio.614. [DOI] [PubMed] [Google Scholar]
- 11.Godoy J.A., Rios J.A., Picón-Pagès P., Herrera-Fernández V., Swaby B., Crepin G., Vicente R., Fernández-Fernández J.M., Muñoz F.J. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules. 2021;11:1012. doi: 10.3390/biom11071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robb-Gaspers L., Burnett P., Rutter G., Denton R., Rizzuto R., Thomas A. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhlén P., Fritz N. Biochemistry of calcium oscillations. Biochem. Biophys. Res. Commun. 2010;396:28–32. doi: 10.1016/j.bbrc.2010.02.117. [DOI] [PubMed] [Google Scholar]
- 14.Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 15.Hajnóczky G., Robb-Gaspers L.D., Seitz M.B., Thomas A.P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 16.Sheu S.S., Jou M.J. Mitochondrial free Ca2+ concentration in living cells. J. Bioenerg. Biomembr. 1994;26:487–493. doi: 10.1007/BF00762733. [DOI] [PubMed] [Google Scholar]
- 17.Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 19.Paudel S., Sindelar R., Saha M. Calcium Signaling in Vertebrate Development and Its Role in Disease. Int. J. Mol. Sci. 2018;19:3390. doi: 10.3390/ijms19113390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung H., Ferlay J., Siegel R., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 21.Stewart T.A., Yapa K.T., Monteith G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta. 2015;1848:2502–2511. doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Cui C., Yang J., Fu L., Wang M., Wang X. Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: A potential target for cancer treatment. Br. J. Pharmacol. 2019;176:1190–1205. doi: 10.1111/bph.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali E.S., Rychkov G.Y., Barritt G.J. Deranged hepatocyte intracellular Ca2+ homeostasis and the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma. Cell Calcium. 2019;82:102057. doi: 10.1016/j.ceca.2019.102057. [DOI] [PubMed] [Google Scholar]
- 24.Tosatto A., Sommaggio R., Kummerow C., Bentham R.B., Blacker T.S., Berecz T., Duchen M.R., Rosato A., Bogeski I., Szabadkai G., et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Mol. Med. 2016;8:569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leinonen P., Aaltonen V., Koskela S., Lehenkari P., Korkiamäki T., Peltonen J. Impaired Gap Junction Formation and Intercellular Calcium Signaling in Urinary Bladder Cancer Cells can be Improved by Gö6976. Cell Commun. Adhes. 2007;14:125–136. doi: 10.1080/15419060701557065. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H., Zhang H., Jin F., Fang M., Huang M., Yang C.S., Chen T., Fu L., Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5:3455–3471. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umemura M., Baljinnyam E., Feske S., De Lorenzo M.S., Xie L.H., Feng X., Oda K., Makino A., Fujita T., Yokoyama U., et al. Store-Operated Ca2+ Entry (SOCE) Regulates Melanoma Proliferation and Cell Migration. PLoS ONE. 2014;9:e89292. doi: 10.1371/journal.pone.0089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tennakoon S., Aggarwal A., Kállay E. The calcium-sensing receptor and the hallmarks of cancer. Biochim. Biophys. Acta. 2016;1863:1398–1407. doi: 10.1016/j.bbamcr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Giampazolias E., Tait S. Mitochondria and the hallmarks of cancer. FEBS J. 2016;283:803–814. doi: 10.1111/febs.13603. [DOI] [PubMed] [Google Scholar]
- 30.Proietti S., Cucina A., Minini M., Bizzarri M. Melatonin, mitochondria, and the cancer cell. Cell Mol. Life Sci. 2017;74:4015–4025. doi: 10.1007/s00018-017-2612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tajada S., Villalobos C. Calcium Permeable Channels in Cancer Hallmarks. Front. Pharmacol. 2020;11:968. doi: 10.3389/fphar.2020.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteith G.R., Prevarskaya N., Roberts-Thomson S.J. The calcium-cancer signalling nexus. Nat. Rev. Cancer. 2017;17:367–380. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- 33.Marchi S., Corricelli M., Branchini A., Vitto V., Missiroli S., Morciano G., Perrone M., Ferrarese M., Giorgi C., Pinotti M., et al. Akt-mediated phosphorylation of MICU1 regulates mitochondrial Ca levels and tumor growth. EMBO J. 2019;38:e99435. doi: 10.15252/embj.201899435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modesti L., Danese A., Angela Maria Vitto V., Ramaccini D., Aguiari G., Gafà R., Lanza G., Giorgi C., Pinton P. Mitochondrial Ca Signaling in Health, Disease and Therapy. Cells. 2021;10:1317. doi: 10.3390/cells10061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pézier A., Acquistapace A., Renou M., Rospars J.P., Lucas P. Ca2+ stabilizes the membrane potential of moth olfactory receptor neurons at rest and is essential for their fast repolarization Chem. Senses. 2007;32:305–317. doi: 10.1093/chemse/bjl059. [DOI] [PubMed] [Google Scholar]
- 36.Pendin D., Greotti E., Filadi R., Pozzan T. Spying on organelle Ca2+ in living cells: The mitochondrial point of view. J. Endocrinol. Investig. 2015;38:39–45. doi: 10.1007/s40618-014-0178-2. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T. The Molecular Mechanisms of Mitochondrial Calcium Uptake by Calcium Uniporter. Yakugaku Zasshi J. Pharm. Jpn. 2021;141:491–499. doi: 10.1248/yakushi.20-00204-1. [DOI] [PubMed] [Google Scholar]
- 38.Stefani D.D., Raffaello A., Teardo E., Szabò I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belosludtsev K.N., Dubinin M.V., Belosludtseva N.V., Mironova G.D. Mitochondrial Ca2+ Transport: Mechanisms, Molecular Structures, and Role in Cells. Biochemistry. 2019;84:593–607. doi: 10.1134/S0006297919060026. [DOI] [PubMed] [Google Scholar]
- 40.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan X., Liu J., Nguyen T., Liu C., Sun J., Teng Y., Fergusson M.M., Rovira I.I., Allen M., Springer D.A., et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garbincius J., Elrod J. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022;102:893–992. doi: 10.1152/physrev.00041.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L., Sun Q., Zhou D., Song W., Yang Q., Ju B., Zhang L., Xie H., Zhou L., Hu Z., et al. HINT2 triggers mitochondrial Ca2+ influx by regulating the mitochondrial Ca2+ uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett. 2017;411:106–116. doi: 10.1016/j.canlet.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Curry M.C., Peters A.A., Kenny P.A., Roberts-Thomson S.J., Monteith G.R. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem. Biophys. Res. Commun. 2013;434:695–700. doi: 10.1016/j.bbrc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Sancak Y., Markhard A., Kitami T., Kovacs-Bogdan E., Kamer K., Udeshi N., Carr S.A., Chaudhuri D., Clapham D.E., Li A.A., et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W., Xie Q., Zhou X., Yao J., Zhu X., Huang P., Zhang L., Wei J., Xie H., Zhou L., et al. Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Lett. 2015;358:47–58. doi: 10.1016/j.canlet.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 47.Zhuo W., Zhou H., Guo R., Yi J., Zhang L., Yu L., Sui Y., Zeng W., Wang P., Yang M. Structure of intact human MCU supercomplex with the auxiliary MICU subunits. Protein Cell. 2020;12:220–229. doi: 10.1007/s13238-020-00776-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan M., Zhang J., Tsai C., Benjamin J., Rodriguez M., Xu Y., Liao M., Tsai M., Feng L. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature. 2020;582:129–133. doi: 10.1038/s41586-020-2309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomar D., Elrod J.W. Metabolite regulation of the mitochondrial calcium uniporter channel. Cell Calcium. 2020;92:102288. doi: 10.1016/j.ceca.2020.102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottschalk B., Madreiter-Sokolowski C.T., Graier W.F. Cristae junction as a fundamental switchboard for mitochondrial ion signaling and bioenergetics. Cell Calcium. 2022;101:102517. doi: 10.1016/j.ceca.2021.102517. [DOI] [PubMed] [Google Scholar]
- 51.Gottschalk B., Klec C., Leitinger G., Bernhart E., Rost R., Bischof H., Madreiter-Sokolowski C.T., Radulović S., Eroglu E., Sattler W., et al. MICU1 controls cristae junction and spatially anchors mitochondrial Ca2+ uniporter complex. Nat. Commun. 2019;10:3732. doi: 10.1038/s41467-019-11692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakraborty P.K., Mustafi S.B., Xiong X., Dwivedi S.K.D., Nesin V., Saha S., Zhang M., Dhanasekaran D., Jayaraman M., Mannel R., et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat. Commun. 2017;8:14634. doi: 10.1038/ncomms14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trenker M., Malli R., Fertschai I., Levak-Frank S., Graier W.F. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madreiter-Sokolowski C.T., Klec C., Parichatikanond W., Stryeck S., Gottschalk B., Pulido S., Rost R., Eroglu E., Hofmann N.A., Bondarenko A.I., et al. PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca(2+) uptake in immortalized cells. Nat. Commun. 2016;7:12897. doi: 10.1038/ncomms12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madreiter-Sokolowski C.T., Győrffy B., Klec C., Sokolowski A.A., Rost R., Waldeck-Weiermair M., Malli R., Graier W.F. UCP2 and PRMT1 are key prognostic markers for lung carcinoma patients. Oncotarget. 2017;8:80278–80285. doi: 10.18632/oncotarget.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jarrold J., Davies C.C. PRMTs and Arginine Methylation: Cancer’s Best-Kept Secret? Trends Mol. Med. 2019;25:993–1009. doi: 10.1016/j.molmed.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Payne R., Hoff H., Roskowski A., Foskett J. MICU2 restricts spatial crosstalk between InsP3R and MCU channels by regulating threshold and gain of MICU1-mediated inhibition and activation of MCU. Cell Reports. 2017;21:3141–3154. doi: 10.1016/j.celrep.2017.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patron M., Granatiero V., Espino J., Rizzuto R., De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019;26:179–195. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raffaello A., De Stefani D., Sabbadin D., Teardo E., Merli G., Picard A., Checchetto V., Moro S., Szabò I., Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomar D., Dong Z., Shanmughapriya S., Koch D., Thomas T., Hoffman N., Timbalia S., Goldman S., Breves S., Corbally D., et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep. 2016;15:1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaudhuri D., Artiga D.J., Abiria S.A., Clapham D.E. Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proc. Natl. Acad. Sci. USA. 2016;113:E1872–E1880. doi: 10.1073/pnas.1602264113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roy S., Dey K., Hershfinkel M., Ohana E., Sekler I. Identification of residues that control Li+ versus Na+ dependent Ca2+ exchange at the transport site of the mitochondrial NCLX. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:997–1008. doi: 10.1016/j.bbamcr.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Starkov A.A., Chinopoulos C., Starkova N.N., Konrad C., Kiss G., Stepanova A., Popov V.N. Divalent cation chelators citrate and EDTA unmask an intrinsic uncoupling pathway in isolated mitochondria. J. Bioenerg. Biomembr. 2017;49:3–11. doi: 10.1007/s10863-016-9656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson J.A., Lau Y.S., Gleeson J.G., Wilson J.S. The action of MPTP on synaptic transmission is affected by changes in Ca2+ concentrations. Brain Res. 1991;541:342–346. doi: 10.1016/0006-8993(91)91035-Y. [DOI] [PubMed] [Google Scholar]
- 65.Kolomiiets’ O., Danylovych I., Danylovych H. H+-Ca2+-exchanger in the myometrium mitochondria: Modulation of exogenous and endogenous compounds. Fiziol. Zh. 2014;60:33–42. doi: 10.15407/fz60.05.033. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Suaga P., Paillusson S., Stoica R., Noble W., Hanger D.P., Miller C.C. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr. Biol. 2017;27:371–385. doi: 10.1016/j.cub.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giorgi C., Bonora M., Sorrentino G., Missiroli S., Poletti F., Suski J.M., Galindo Ramirez F., Rizzuto R., Di Virgilio F., Zito E., et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA. 2015;112:1779–1784. doi: 10.1073/pnas.1410723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Betz C., Stracka D., Prescianotto-Baschong C., Frieden M., Demaurex N., Hall M.N. Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc. Natl. Acad. Sci. USA. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rimessi A., Marchi S., Patergnani S., Pinton P. H-Ras-driven tumoral maintenance is sustained through caveolin-1-dependent alterations in calcium signaling. Oncogene. 2014;33:2329–2340. doi: 10.1038/onc.2013.192. [DOI] [PubMed] [Google Scholar]
- 70.Glitsch M. Mechano- and pH-sensing convergence on Ca2+-mobilising proteins - A recipe for cancer? Cell Calcium. 2019;80:38–45. doi: 10.1016/j.ceca.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Trapani V., Wolf F.I. Dysregulation of Mg2+ homeostasis contributes to acquisition of cancer hallmarks. Cell Calcium. 2019;83:102078. doi: 10.1016/j.ceca.2019.102078. [DOI] [PubMed] [Google Scholar]
- 72.Santoni G., Morelli M.B., Marinelli O., Nabissi M., Santoni M., Amantini C. Calcium Signaling and the Regulation of Chemosensitivity in Cancer Cells: Role of the Transient Receptor Potential Channels. Adv. Exp. Med. Biol. 2020;1131:505–517. doi: 10.1007/978-3-030-12457-1_20. [DOI] [PubMed] [Google Scholar]
- 73.Denton R. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Schönekess B.O., Brindley P.G., Lopaschuk G.D. Calcium regulation of glycolysis, glucose oxidation, and fatty acid oxidation in the aerobic and ischemic heart. Can. J. Physiol. Pharmacol. 1995;73:1632–1640. doi: 10.1139/y95-725. [DOI] [PubMed] [Google Scholar]
- 75.McMillin J.B., Pauly D.F. Control of mitochondrial respiration in muscle. Mol. Cell. Biochem. 1988;81:121–129. doi: 10.1007/BF00219314. [DOI] [PubMed] [Google Scholar]
- 76.Konji V., Montag A., Sandri G., Nordenbrand K., Ernster L. Transport of Ca2+ and Mn2+ by mitochondria from rat liver, heart and brain. Biochimie. 1985;67:1241–1250. doi: 10.1016/S0300-9084(85)80133-4. [DOI] [PubMed] [Google Scholar]
- 77.Oliveira G.L., Coelho A.R., Marques R., Oliveira P.J. Cancer cell metabolism: Rewiring the mitochondrial hub. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867:166016. doi: 10.1016/j.bbadis.2020.166016. [DOI] [PubMed] [Google Scholar]
- 78.Iqbal M.A., Gupta V., Gopinath P., Mazurek S., Bamezai R.N. Pyruvate kinase M2 and cancer: An updated assessment. FEBS Lett. 2014;588:2685–2692. doi: 10.1016/j.febslet.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Li T., Han J., Jia L., Hu X., Chen L., Wang Y. PKM2 coordinates glycolysis with mitochondrial fusion and oxidative phosphorylation. Protein Cell. 2019;10:583–594. doi: 10.1007/s13238-019-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazurek S. Pyruvate kinase type M2: A key regulator of the metabolic budget system in tumor cells. Int. J. Biochem. Cell Biol. 2011;43:969–980. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Dey P., Kimmelman A.C., DePinho R.A. Metabolic Codependencies in the Tumor Microenvironment. Cancer Discov. 2021;11:1067–1081. doi: 10.1158/2159-8290.CD-20-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vasan N., Baselga J., Hyman D.M. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferro F., Servais S., Besson P., Roger S., Dumas J.F., Brisson L. Autophagy and mitophagy in cancer metabolic remodelling. Semin. Cell Dev. Biol. 2020;98:129–138. doi: 10.1016/j.semcdb.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 84.Kim K.H., Lee M.S. Autophagy--a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014;10:322–337. doi: 10.1038/nrendo.2014.35. [DOI] [PubMed] [Google Scholar]
- 85.Klionsky D.J., Petroni G., Amaravadi R.K., Baehrecke E.H., Ballabio A., Boya P., Bravo-San P.J.M., Cadwell K., Cecconi F., Choi A.M.K., et al. Autophagy in major human diseases. EMBO J. 2021;40:e108863. doi: 10.15252/embj.2021108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parzych K.R., Klionsky D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glick D., Barth S., Macleod K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doherty J., Baehrecke E.H. Life, death and autophagy. Nat. Cell Biol. 2018;20:1110–1117. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.White E., Mehnert J.M., Chan C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015;21:5037–5046. doi: 10.1158/1078-0432.CCR-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu Y.X., Han X.S., Jing Q. Ca(2+) Ion and Autophagy. Adv. Exp. Med. Biol. 2019;1206:151–166. doi: 10.1007/978-981-15-0602-4_7. [DOI] [PubMed] [Google Scholar]
- 91.Bootman M.D., Chehab T., Bultynck G., Parys J.B., Rietdorf K. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium. 2018;70:32–46. doi: 10.1016/j.ceca.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 92.Lam A.K., Galione A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim. Biophys Acta. 2013;1833:2542–2559. doi: 10.1016/j.bbamcr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 93.Høyer-Hansen M., Bastholm L., Szyniarowski P., Campanella M., Szabadkai G., Farkas T., Bianchi K., Fehrenbacher N., Elling F., Rizzuto R., et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y., Zhang J., Jiang P., Li K., Sun Y., Huang Y. ASIC1a promotes acidic microenvironment-induced HCC cells migration and invasion by inducing autophagy. Eur. J. Pharmacol. 2021;907:174252. doi: 10.1016/j.ejphar.2021.174252. [DOI] [PubMed] [Google Scholar]
- 95.Li M., Kondo T., Zhao Q.L., Li F.J., Tanabe K., Arai Y., Zhou Z.C., Kasuya M. Apoptosis induced by cadmium in human lymphoma U937 cells through Ca2+-calpain and caspase-mitochondria- dependent pathways. J. Biol. Chem. 2000;275:39702–39709. doi: 10.1074/jbc.M007369200. [DOI] [PubMed] [Google Scholar]
- 96.Cárdenas C., Miller R.A., Smith I., Bui T., Molgó J., Müller M., Vais H., Cheung K.H., Yang J., Parker I., et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Decuypere J.P., Paudel R.C., Parys J., Bultynck G. Intracellular Ca(2+) signaling: A novel player in the canonical mTOR-controlled autophagy pathway. Commun. Integr. Biol. 2013;6:e25429. doi: 10.4161/cib.25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bidaux G., Gordienko D., Shapovalov G., Farfariello V., Borowiec A., Iamshanova O., Lemonnier L., Gueguinou M., Guibon R., Fromont G., et al. 4TM-TRPM8 channels are new gatekeepers of the ER-mitochondria Ca2+ transfer. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:981–994. doi: 10.1016/j.bbamcr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 99.Cardenas C., Lovy A., Silva-Pavez E., Urra F., Mizzoni C., Ahumada-Castro U., Bustos G., Jaňa F., Cruz P., Farias P., et al. Cancer cells with defective oxidative phosphorylation require endoplasmic reticulum-to-mitochondria Ca2+ transfer for survival. Sci. Signal. 2020;13:eaay1212. doi: 10.1126/scisignal.aay1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kiviluoto S., Schneider L., Luyten T., Vervliet T., Missiaen L., De Smedt H., Parys J.B., Methner A., Bultynck G. Bax inhibitor-1 is a novel IP3 receptor-interacting and -sensitizing protein. Cell Death Dis. 2012;3:e367. doi: 10.1038/cddis.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doghman-Bouguerra M., Lalli E. ER-mitochondria interactions: Both strength and weakness within cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:650–662. doi: 10.1016/j.bbamcr.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 102.Vultur A., Gibhardt C.S., Stanisz H., Bogeski I. The role of the mitochondrial calcium uniporter (MCU) complex in cancer. Pflugers Arch. 2018;470:1149–1163. doi: 10.1007/s00424-018-2162-8. [DOI] [PubMed] [Google Scholar]
- 103.Yu C., Wang Y., Peng J., Shen Q., Chen M., Tang W., Li X., Cai C., Wang B., Cai S., et al. Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget. 2017;8:83831–83844. doi: 10.18632/oncotarget.19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fadeel B., Zhivotovsky B., Orrenius S. All along the watchtower: On the regulation of apoptosis regulators. FASEB J. 1999;13:1647–1657. doi: 10.1096/fasebj.13.13.1647. [DOI] [PubMed] [Google Scholar]
- 106.Allan L., Clarke P. Apoptosis and autophagy: Regulation of caspase-9 by phosphorylation. FEBS J. 2009;276:6063–6073. doi: 10.1111/j.1742-4658.2009.07330.x. [DOI] [PubMed] [Google Scholar]
- 107.Abate M., Festa A., Falco M., Lombardi A., Luce A., Grimaldi A., Zappavigna S., Sperlongano P., Irace C., Caraglia M., et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020;98:139–153. doi: 10.1016/j.semcdb.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 108.Sinha K., Das J., Pal P.B., Sil P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 109.Bock F.J., Tait S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 110.Jeong S., Seol D. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/BMBRep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- 111.Chen X., Zhang X., Kubo H., Harris D.M., Mills G.D., Moyer J., Berretta R., Potts S.T., Marsh J.D., Houser S.R. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ. Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 112.McConkey D.J. Biochemical determinants of apoptosis and necrosis. Toxicol. Lett. 1998;99:157–168. doi: 10.1016/S0378-4274(98)00155-6. [DOI] [PubMed] [Google Scholar]
- 113.Aharoni-Simon M., Shumiatcher R., Yeung A., Shih A.Z., Dolinsky V.W., Doucette C.A., Luciani D.S. Bcl-2 Regulates Reactive Oxygen Species Signaling and a Redox-Sensitive Mitochondrial Proton Leak in Mouse Pancreatic β-Cells. Endocrinology. 2016;157:2270–2281. doi: 10.1210/en.2015-1964. [DOI] [PubMed] [Google Scholar]
- 114.Warren C.F.A., Wong-Brown M.W., Bowden N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Selzer E., Schlagbauer-Wadl H., Okamoto I., Pehamberger H., Pötter R., Jansen B. Expression of Bcl-2 family members in human melanocytes, in melanoma metastases and in melanoma cell lines. Melanoma Res. 1998;8:197–203. doi: 10.1097/00008390-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 116.Berridge M.J. The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/S0143416002001823. [DOI] [PubMed] [Google Scholar]
- 117.Berridge M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 118.Kondratskyi A., Kondratska K., Skryma R., Prevarskaya N. Ion channels in the regulation of apoptosis. Biochim. Biophys. Acta. 2015;1848:2532–2546. doi: 10.1016/j.bbamem.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 119.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 120.Feissner R.F., Skalska J., Gaum W.E., Sheu S.S. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front. Biosci. 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yan X., Xun M., Li J., Wu L., Dou X., Zheng J. Activation of Na+/K+-ATPase attenuates high glucose-induced H9c2 cell apoptosis via suppressing ROS accumulation and MAPKs activities by DRm217. Acta. Biochim. Biophys. Sin. 2016;48:883–893. doi: 10.1093/abbs/gmw079. [DOI] [PubMed] [Google Scholar]
- 122.Madreiter-Sokolowski C.T., Thomas C., Ristow M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox. Biol. 2020;36:101678. doi: 10.1016/j.redox.2020.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Raimondi M., Fontana F., Marzagalli M., Audano M., Beretta G., Procacci P., Sartori P., Mitro N., Limonta P. Ca2+ overload- and ROS-associated mitochondrial dysfunction contributes to δ-tocotrienol-mediated paraptosis in melanoma cells. Apoptosis. 2021;26:277–292. doi: 10.1007/s10495-021-01668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ataizi Z.S., Ertilav K., Nazıroğlu M. Mitochondrial oxidative stress-induced brain and hippocampus apoptosis decrease through modulation of caspase activity, Ca2+ influx and inflammatory cytokine molecular pathways in the docetaxel-treated mice by melatonin and selenium treatments. Metab. Brain Dis. 2019;34:1077–1089. doi: 10.1007/s11011-019-00428-x. [DOI] [PubMed] [Google Scholar]
- 125.Li W., Liu B., Wang L., Liu J., Yang X., Zheng J. Melatonin Attenuates Cardiac Ischemia-Reperfusion Injury through Modulation of IP3R-Mediated Mitochondria-ER Contact. Oxid. Med. Cell. Longev. 2021:1370862. doi: 10.1155/2021/1370862. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Meis L. Role of the sarcoplasmic reticulum Ca2+-ATPase on heat production and thermogenesis. Biosci. Rep. 2001;21:113–137. doi: 10.1023/A:1013640006611. [DOI] [PubMed] [Google Scholar]
- 127.Zhou D., Shao L., Spitz D. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsu C.C., Tseng L.M., Lee H.C. Role of mitochondrial dysfunction in cancer progression. Exp. Biol. Med. 2016;241:1281–1295. doi: 10.1177/1535370216641787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shoshan-Barmatz V., Krelin Y., Shteinfer-Kuzmine A. VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease. Cell Calcium. 2018;69:81–100. doi: 10.1016/j.ceca.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 130.Szatrowski T., Nathan C. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 131.Tsunoda T., Koga H., Yokomizo A., Tatsugami K., Eto M., Inokuchi J., Hirata A., Masuda K., Okumura K., Naito S. Inositol 1,4,5-trisphosphate (IP3) receptor type1 (IP3R1) modulates the acquisition of cisplatin resistance in bladder cancer cell lines. Oncogene. 2005;24:1396–1402. doi: 10.1038/sj.onc.1208313. [DOI] [PubMed] [Google Scholar]
- 132.Brouland J.P., Valleur P., Papp B. Expression of SERCA pumps during cell differentiation and tumorigenesis: Application to colonic carcinogenesis. Ann. Pathol. 2006;26:159–172. doi: 10.1016/S0242-6498(06)70701-5. [DOI] [PubMed] [Google Scholar]
- 133.Kuchay S., Giorgi C., Simoneschi D., Pagan J., Missiroli S., Saraf A., Florens L., Washburn M.P., Collazo-Lorduy A., Castillo-Martin M., et al. PTEN counteracts FBXL2 to promote IP3R3- and Ca2+-mediated apoptosis limiting tumour growth. Nature. 2017;546:554–558. doi: 10.1038/nature22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bhagat S., Singh S. Co-delivery of AKT3 siRNA and PTEN Plasmid by Antioxidant Nanoliposomes for Enhanced Antiproliferation of Prostate Cancer Cells. ACS Appl. Bio. Mater. 2020;3:3999–4011. doi: 10.1021/acsabm.9b01016. [DOI] [PubMed] [Google Scholar]
- 135.Bononi A., Giorgi C., Patergnani S., Larson D., Verbruggen K., Tanji M., Pellegrini L., Signorato V., Olivetto F., Pastorino S., et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature. 2017;546:549–553. doi: 10.1038/nature22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jin C., Kumar P., Gracia-Sancho J., Dufour J.F. Calcium transfer between endoplasmic reticulum and mitochondria in liver diseases. FEBS Lett. 2021;595:1411–1421. doi: 10.1002/1873-3468.14078. [DOI] [PubMed] [Google Scholar]
- 137.Boutin B., Tajeddine N., Monaco G., Molgo J., Vertommen D., Rider M., Parys J.B., Bultynck G., Gailly P. Endoplasmic reticulum Ca(2+) content decrease by PKA-dependent hyperphosphorylation of type 1 IP3 receptor contributes to prostate cancer cell resistance to androgen deprivation. Cell Calcium. 2015;57:312–320. doi: 10.1016/j.ceca.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 138.Cui C., Merritt R., Fu L., Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Romero-Garcia S., Prado-Garcia H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review) Int. J. Oncol. 2019;54:1155–1167. doi: 10.3892/ijo.2019.4696. [DOI] [PubMed] [Google Scholar]
- 140.Colwill K., Gräslund S. A roadmap to generate renewable protein binders to the human proteome. Nat. Methods. 2011;8:551–558. doi: 10.1038/nmeth.1607. [DOI] [PubMed] [Google Scholar]
- 141.Zeng F., Chen X., Cui W., Wen W., Lu F., Sun X., Ma D., Yuan Y., Li Z., Hou N., et al. RIPK1 Binds MCU to Mediate Induction of Mitochondrial Ca2+ Uptake and Promotes Colorectal Oncogenesis. Cancer Res. 2018;78:2876–2885. doi: 10.1158/0008-5472.CAN-17-3082. [DOI] [PubMed] [Google Scholar]
- 142.Wu R., Zuo W., Xu X., Bi L., Zhang C., Chen H., Liu H. MCU That Is Transcriptionally Regulated by Nrf2 Augments Malignant Biological Behaviors in Oral Squamous Cell Carcinoma Cells. BioMed Res. Int. 2021:6650791. doi: 10.1155/2021/6650791. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143.Zheng S., Wu R., Deng Y., Zhang Q. Dihydroartemisinin represses oral squamous cell carcinoma progression through downregulating mitochondrial calcium uniporter. Bioengineered. 2022;13:227–241. doi: 10.1080/21655979.2021.2012951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marchi S., Vitto V.A.M., Patergnani S., Pinton P. High mitochondrial Ca2+ content increases cancer cell proliferation upon inhibition of mitochondrial permeability transition pore (mPTP) Cell Cycle. 2019;18:914–916. doi: 10.1080/15384101.2019.1598729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patergnani S., Guzzo S., Mangolini A., dell’Atti L., Pinton P., Aguiari G. The induction of AMPK-dependent autophagy leads to P53 degradation and affects cell growth and migration in kidney cancer cells. Exp. Cell Res. 2020;395:112190. doi: 10.1016/j.yexcr.2020.112190. [DOI] [PubMed] [Google Scholar]
- 146.Fei M., Zhang L., Wang H., Zhu Y., Niu W., Tang T., Han Y. Inhibition of Cathepsin S Induces Mitochondrial Apoptosis in Glioblastoma Cell Lines Through Mitochondrial Stress and Autophagosome Accumulation. Front. Oncol. 2020;10:516746. doi: 10.3389/fonc.2020.516746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xue P., Chen Q., Ren X., Liu D., Yang X. A novel protoapigenone analog RY10-4 induces apoptosis of breast cancer cells by exacerbating mitochondrial Ca2+ influx through mitochondrial calcium uniporter. Toxicol. Appl. Pharmacol. 2021;433:115776. doi: 10.1016/j.taap.2021.115776. [DOI] [PubMed] [Google Scholar]
- 148.Ren T., Wang J., Zhang H., Yuan P., Zhu J., Wu Y., Huang Q., Guo X., Zhang J., Ji L., et al. MCUR1-Mediated Mitochondrial Calcium Signaling Facilitates Cell Survival of Hepatocellular Carcinoma via Reactive Oxygen Species-Dependent P53 Degradation. Antioxid. Redox Signal. 2018;28:1120–1136. doi: 10.1089/ars.2017.6990. [DOI] [PubMed] [Google Scholar]
- 149.Marchi S., Pinton P. Mitochondrial calcium uniporter, MiRNA and cancer: Live and let die. Commun. Integr. Biol. 2013;6:e23818. doi: 10.4161/cib.23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun Y., Li M., Liu G., Zhang X., Zhi L., Zhao J., Wang G. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J. Cancer Res. Clin. Oncol. 2020;146:1139–1152. doi: 10.1007/s00432-020-03179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang X., Li Y., Li Z., Lin S., Wang H., Sun J., Lan C., Wu L., Sun D., Huang C., et al. Mitochondrial calcium uniporter drives metastasis and confers a targetable cystine dependency in pancreatic cancer. Cancer Res. 2022;3230:2021. doi: 10.1158/0008-5472.CAN-21-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang S., Wang X., Shen Q., Yang X., Yu C., Cai C., Cai G., Meng X., Zou F. Mitochondrial Ca2+ uniporter is critical for store-operated Ca2+ entry-dependent breast cancer cell migration. Biochem. Biophys. Res. Commun. 2015;458:186–193. doi: 10.1016/j.bbrc.2015.01.092. [DOI] [PubMed] [Google Scholar]
- 153.Gao P., Peng T., Lin S., Zhi W., Cao C., Wu P., Xi L., Wu P., Yang Q., Ding W. Key Role of MCUR1 in Malignant Progression of Breast Cancer. OncoTargets Ther. 2021;14:4163–4175. doi: 10.2147/OTT.S306854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Che X., Wang Q., Zhang B. MCUR1 is a marker for the progression and prognosis of breast cancer. Res. Square. 2022 preprint (Version 1) [Google Scholar]
- 155.Ren T., Zhang H., Wang J., Zhu J., Jin M., Wu Y., Guo X., Ji L., Huang Q., Zhang H., et al. MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene. 2017;36:5897–5909. doi: 10.1038/onc.2017.167. [DOI] [PubMed] [Google Scholar]
- 156.Bartha A., Győrffy B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021;22:2622. doi: 10.3390/ijms22052622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parekh A. Calcium signalling in health and disease. Semin Cell Dev Biol. 2019;94:1–2. doi: 10.1016/j.semcdb.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 158.Li C., Lin H., Ko C., Lai J., Chu P. A Novel Biomarker Driving Poor-Prognosis Liver Cancer: Overexpression of the Mitochondrial Calcium Gatekeepers. Biomedicines. 2020;8:451. doi: 10.3390/biomedicines8110451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jin M., Wang J., Ji X., Cao H., Zhu J., Chen Y., Yang J., Zhao Z., Ren T., Xing J. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019;38:136. doi: 10.1186/s13046-019-1135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao Y., Wang Y., Zhao J., Zhang Z., Jin M., Zhou F., Jin C., Zhang J., Xing J., Wang N., et al. PDE2 Inhibits PKA-Mediated Phosphorylation of TFAM to Promote Mitochondrial Ca2+-Induced Colorectal Cancer Growth. Front. Oncol. 2021;11:663778. doi: 10.3389/fonc.2021.663778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu Y., Jin M., Wang Y., Zhu J., Tan R., Zhao J., Ji X., Jin C., Jia Y., Ren T., et al. MCU-induced mitochondrial calcium uptake promotes mitochondrial biogenesis and colorectal cancer growth. Signal Transduct. Target Ther. 2020;5:59. doi: 10.1038/s41392-020-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marchi S., Vitto V.A.M., Danese A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle. 2019;18:1068–1083. doi: 10.1080/15384101.2019.1612698. [DOI] [PMC free article] [PubMed] [Google Scholar]