Abstract
Two fermented milks containing angiotensin-I-converting-enzyme (ACE)-inhibitory peptides were produced by using selected Lactobacillus delbrueckii subsp. bulgaricus SS1 and L. lactis subsp. cremoris FT4. The pH 4.6-soluble nitrogen fraction of the two fermented milks was fractionated by reversed-phase fast-protein liquid chromatography. The fractions which showed the highest ACE-inhibitory indexes were further purified, and the related peptides were sequenced by tandem fast atom bombardment-mass spectrometry. The most inhibitory fractions of the milk fermented by L. delbrueckii subsp. bulgaricus SS1 contained the sequences of β-casein (β-CN) fragment 6-14 (f6-14), f7-14, f73-82, f74-82, and f75-82. Those from the milk fermented by L. lactis subsp. cremoris FT4 contained the sequences of β-CN f7-14, f47-52, and f169-175 and κ-CN f155-160 and f152-160. Most of these sequences had features in common with other ACE-inhibitory peptides reported in the literature. In particular, the β-CN f47-52 sequence had high homology with that of angiotensin-II. Some of these peptides were chemically synthesized. The 50% inhibitory concentrations (IC50s) of the crude purified fractions containing the peptide mixture were very low (8.0 to 11.2 mg/liter). When the synthesized peptides were used individually, the ACE-inhibitory activity was confirmed but the IC50s increased considerably. A strengthened inhibitory effect of the peptide mixtures with respect to the activity of individual peptides was presumed. Once generated, the inhibitory peptides were resistant to further proteolysis either during dairy processing or by trypsin and chymotrypsin.
Milk proteins are precursors of many different biologically active peptides. These peptides are inactive within the sequence of the precursor proteins but can be released by enzymatic proteolysis during intestinal digestion or during food processing. Milk protein-derived bioactive peptides may function as exogenous regulatory substances with hormone-like activity on the different intestinal and peripheral target sites of the mammalian organism. Opiate, antithrombotic, antihypertensive, immunomodulating, antibacterial, antigastric, human immunodeficiency virus type 1 proteinase inhibitory, and mineral carrying are some properties that have been attributed to several of the bioactive sequences identified (for reviews, see references 17–19 and 30).
Although a number of studies have indicated the need for further clarification, food hormones or “formones” such as bioactive peptides may be included in the formulas of physiologically functional foods and in industrial nutraceutical preparations. To date, antihypertensive peptides, together with phosphopeptides and immunomodulating peptides, are the favorite bioactive peptides for application to foodstuffs formulated to provide specific health benefits (18).
Angiotensin-I-converting enzyme (ACE; kininase II; EC 3.4.15.1) is a multifunctional ectoenzyme located in different tissues which plays a key physiological role in the regulation of local levels of several endogenous bioactive peptides (4, 25). ACE has been classically associated with the renin-angiotensin system which regulates peripheral blood pressure, where it catalyzes both the production of the vasoconstrictor angiotensin-II and the inactivation of the vasodilator bradykinin. ACE inhibition results mainly in an antihypertensive effect but may also influence different regulatory systems involved in modulating blood pressure, immune defense, and nervous system activity (16). Naturally occurring peptides in snake venom were the first reported competitive inhibitors of ACE (6, 26). Thereafter, many other ACE inhibitors were discovered from enzymatic hydrolysates or the related synthetic peptides of bovine and human caseins (CNs), as well as plant and other food proteins (29).
Although chemical and physical treatments may have some influence, proteolysis by naturally occurring enzymes in milk, exogenous enzymes, and enzymes from microbial starters such as lactic acid bacteria can potentially generate bioactive sequences from milk protein precursors during dairy processing. The formation of bioactive peptides by lactic acid bacteria in dairy products is currently being debated. There are only a few reports available, and some of the results are somewhat controversial. Biologically active peptides are generated after peptidase hydrolysis of long oligopeptides which are initially liberated by proteinase activity. Since peptidase activity is intracellular in lactic acid bacteria, it has been claimed that lactic acid bacteria probably contribute only after cell lysis, which is considered a rare event in fermented milk due to the short fermentation time (19). The formation of casomorphins in dairy products by lactic acid bacteria is considered particularly unlikely, since these bacteria have an X-prolyl-dipeptdyl-aminopeptidase which can easily alter the X-Pro sequence responsible for the bioactivity of this type of peptide (22). With regard to casokinins and lactokinins, some authors (27) have concluded that commercial lactic acid starter bacteria do not produce in vitro ACE-inhibitory peptides from either casein or whey. On the other hand, it has been shown that secondary proteolysis during cheese ripening generates various ACE-inhibitory peptides (20). Some ACE-inhibitory peptides have been isolated from several Italian cheeses, and in particular, the sequence of β-CN fragment 58-72 (f58-72) was found in Crescenza cheese (29). Several casokinins derived from β-CN have been liberated by a cell wall-associated serine-type proteinase of Lactobacillus helveticus CP790 (34), and milk fermentation with starter cultures containing L. helveticus CP790 and Saccharomyces cerevisiae produced two β-casokinins with elevated ACE-inhibitory activity (23). A fermented milk enriched with the opioid β-casomorphin 1-4 (f60-63) was produced by using a mutant strain of L. helveticus which lacks X-prolyl-dipeptidyl-aminopeptidase activity (15). Further studies using selected strains would help to determine the real contribution of lactic acid bacteria to the generation of bioactive peptides during dairy processing.
In this study, we used selected L. delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4 to produce fermented milk containing ACE-inhibitory peptides. The ACE-inhibitory peptides were isolated, sequenced, and chemically synthesized, and their bioactivity was characterized.
MATERIALS AND METHODS
Substrates and chemicals.
Bradykinin (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg), p-nitroanilides, hippuryl (Hip)-His-Leu, ACE (from rabbit lung; lyophilized powder, ca. 3 U/mg of protein), trypsin (from bovine pancreas; ca. 10,000 Nα-benzoyl-l-arginine ethyl ester [BAEE]/mg of protein), chymotrypsin (from bovine pancreas; 40 to 60 U/mg of protein), insulin chain A, and other chemicals, except acetonitrile, were from Sigma Chemical Co. (St. Louis, Mo.). High-pressure liquid chromatography grade acetonitrile was from BDH (Poole, England).
Microorganisms and culture conditions.
L. delbrueckii subsp. bulgaricus SS1 and L. lactis subsp. cremoris FT4 isolated from dairy products and belonging to the culture collection of the Institute of Dairy Microbiology, Agriculture University of Perugia, Perugia, Italy, were used. We routinely propagated lactobacilli in MRS broth (Oxoid, Basingstoke, Hampshire, England) and lactococci in M17 broth (Difco Laboratories, Detroit, Mich.) for 24 h at 37 and 30°C, respectively.
Twenty-four-hour-old cells of L. delbrueckii subsp. bulgaricus SS1 and L. lactis subsp. cremoris FT4 were used to inoculate (2%, vol/vol) 10 ml of ultra-high-temperature-treated (UHT) skim milk (protein, 3.15%; fat, <0.3%; lactose, 4.95%), which was incubated for 24 h at 37 and 30°C, respectively. These milk cultures were used to produce fermented milk.
Production of fermented milk.
UHT skim milk cultures of L. delbrueckii subsp. bulgaricus SS1 and L. lactis subsp. cremoris FT4 were used to inoculate (1%, vol/vol) 50 ml of fresh UHT skim milk. Incubation was carried out under sterile conditions several times at the temperatures previously indicated. Three batches of UHT skim milk were inoculated with each strain. Fermented milk was produced with UHT milk under sterile conditions in order to exclude enzyme interference by contaminant microorganisms. The extent of proteolysis in the fermented milk was monitored by urea-polyacrylamide gel electrophoresis (1) of pH 4.6-soluble and insoluble nitrogen fractions. The pH 4.6-soluble nitrogen fraction was also monitored by reversed-phase fast-protein liquid chromatography (RP-FPLC).
The number of lactic acid bacterial cells in the fermented milk was determined by plating on MRS agar (Oxoid) and M17 agar (Difco) for lactobacilli (72 h at 37°C) and lactococci (72 h at 30°C), respectively.
The protein concentration of the pH 4.6-soluble nitrogen fractions of the fermented milk was determined by the method of Bradford (3).
Isolation of peptides from fermented milk.
Peptides were separated from fermented milk by RP-FPLC using a PepRPC HR5/5 column and FPLC equipment with a UV detector operating at 214 nm (Pharmacia Biotech, Uppsala, Sweden). A 500-μl aliquot of the pH 4.6-soluble nitrogen fraction, diluted 1:1 with 0.2% trifluoroacetic acid (TFA), was loaded onto the column and eluted at a flow rate of 0.5 ml/min with a gradient (0 to 80%) of acetonitrile in 0.1% TFA. The concentration of CH3CN was increased linearly from 0 to 36% between 5 and 60 min, from 36 to 48% between 60 and 73 min, and from 48 to 80% between 73 and 78 min. Solvents were removed from the collected 1-ml peptide fractions by freeze drying. The peptide fractions were redissolved in 300 μl of water, and their effects on ACE were studied.
The protein concentration of the peptide fractions separated by RP-FPLC was determined by the method of Bradford (3).
ACE activity and inhibition.
ACE activity was determined by a modified version of the method of Nakamura et al. (23). Hip-His-Leu was dissolved (50 mM) in 100 mM Na-borate buffer (pH 8.3) containing 300 mM NaCl. Hip-His-Leu solution (200 μl) was mixed with 60 μl of a peptide fraction, a synthesized peptide, or water and with 40 μl of ACE (100 mU/ml); the mixture was incubated for 45 min at 37°C. The reaction was stopped with 250 μl of 1 N HCl; the hippuric acid liberated by ACE was extracted with 1.7 ml of ethyl acetate, and after the ethyl acetate was removed by vacuum evaporation, the hippuric acid was diluted in 1 ml of distilled water and determined spectrophotometrically at 228 nm. Percent inhibition was calculated as follows: (B − A) ÷ (B − C) × 100, where A is optical density in the presence of both ACE and the peptide fraction or synthesized peptide, B is optical density without the peptide fraction, and C is optical density without ACE. The inhibition values reported are the means of four determinations (29).
The concentration of an ACE inhibitor (crude fraction) needed to inhibit 50% of ACE activity is defined as the 50% inhibitory concentration (IC50). Kinetic constants (Ki) of the inhibition of ACE activity by synthesized peptides were calculated from Dixon plots (5) and used to determine the IC50s.
Purification, sequencing, and synthesis of inhibitory peptides.
The fractions of the pH 4.6-soluble nitrogen with the highest ACE-inhibitory activity were rechromatographed by RP-FPLC on the PepRPC HR5/5 column. The centers of the inhibitory peaks were then collected, freeze dried, and further purified by gel filtration on Superose 12 HR10/30 (Pharmacia Biotech). Finally, inhibitory fractions from Superose 12 were rechromatographed by RP-FPLC.
The peptides in the purified inhibitory fractions were sequenced by tandem fast atom bombardment-mass spectrometry (FAB-MS). High-energy collision-induced dissociation mass spectra were obtained on a ZAB-T four-sector (B1E1B2E2) mass spectrometer (Fisons Ltd., Manchester, England) under the control of an OPUS V3.1X data system and equipped with a focal plane array detector consisting of a 2,048-channel linear photodiode array detector. The sample was bombarded with a beam of Cs+ ions having an energy of 30 keV. Analyses were performed at an accelerating potential of 8 kV. For collision-induced dissociation experiments, argon collision gas was used until 50% attenuation of the parent ion beam. The collision cell was held at 50% of the accelerating potential. For each spectrum, 100 to 200 pmol of sample was dissolved in 1 μl of 5% acetic acid and the solution was placed on the glycerol-thioglycerol (1:1) matrix on the probe tip. Signals recorded in the spectra were associated to the corresponding peptides on the basis of expected molecular weights by using a suitable computer program (software Biolynx; Micromass, Altrincham, United Kingdom).
Some of the peptides identified by FAB-MS were chemically synthesized by NeoSystem Laboratoire (Strasbourg, France). The purity of the synthesized peptides was greater than 92% as determined by high-pressure liquid chromatography analysis and certified by the manufacturer.
Hydrolysis of synthesized peptides by trypsin and chymotrypsin.
Aliquots (10 μl) of the synthesized peptides (750 μM) were incubated with 10 μl of trypsin or chymotrypsyn (2 and 4 mg/ml, respectively) and 40 μl of Tris-HCl (0.25 M), pH 8.0, at 37°C for 50 min. The reaction was stopped with 100 μl of 0.1% TFA, and samples were analyzed by RP-FPLC as previously described. Insulin chain A (240 μg/ml, final concentration) was used as the control, and the trypsin and chymotrypsin concentrations used were standardized to have about 80% hydrolysis of insulin chain A.
RESULTS
Production of fermented milk.
L. delbrueckii subsp. bulgaricus SS1 and L. lactis subsp. cremoris FT4 were previously selected from among several strains belonging to the same species. The two selected strains were characterized by the highest proteinase and peptidase activities (data not shown).
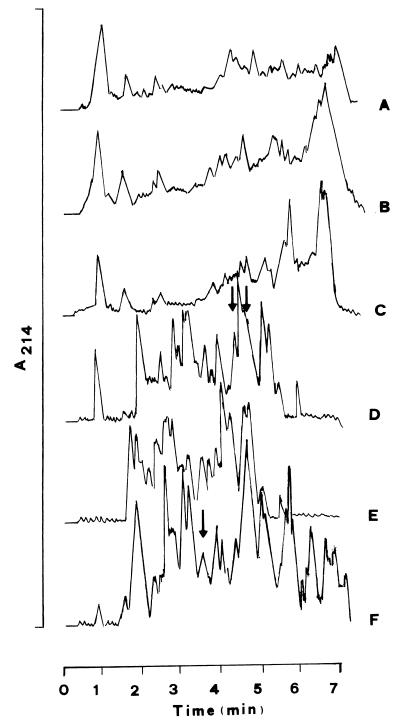
The kinetics of the degree of proteolysis of the UHT skim milk started with L. delbrueckii subsp. bulgaricus SS1 is reported in Fig. 1. A 72-h incubation time was selected to produce fermented milk because at that time the RP-FPLC analysis of the pH 4.6-soluble nitrogen fraction showed the most complex peptide profile (Fig. 1D). Urea-polyacrylamide gel electrophoresis analyses gave analogous information (data not shown). When the RP-FPLC analyses were compared, the peptide profiles of the three batches of UHT skim milk started with L. delbrueckii subsp. bulgaricus SS1 did not differ. The RP-FPLC peptide profile of the UHT skim milk started with L. lactis subsp. cremoris FT4 differed considerably from that of the milk started with L. delbrueckii subsp. bulgaricus SS1, particularly regarding the large amount of peptides contained in the hydrophobic zone of the acetonitrile gradient (Fig. 1F).
FIG. 1.
RP-FPLC chromatograms of the pH 4.6-soluble nitrogen fraction of fermented milk incubated with L. delbrueckii subsp. bulgaricus SS1 for 12 h (A), 24 h (B), 48 h (C), 72 h (D), and 192 h (E) and that incubated with L. lactis subsp. cremoris FT4 for 72 h (F). Arrows indicate peptide fractions inhibitory to ACE.
After 72 h of incubation, the two milk batches fermented with L. delbrueckii subsp. bulgaricus SS1 and L. lactis subsp. cremoris FT4 had pH values of 3.9 and 4.3, respectively. The number of lactobacilli and lactococci was ca. 109 CFU/g. The protein content of the pH 4.6-soluble nitrogen fractions ranged from 0.8 to 1.5 mg/ml.
Isolation of ACE-inhibitory peptides.
Thirty-four fractions from each fermented milk batch were collected by RP-FPLC. The ACE-inhibitory index of each fraction is shown in Table 1. Several fractions, variously distributed throughout the acetonitrile gradient, showed ACE-inhibitory indexes higher than 40%. In particular, fractions 15 and 16 of the UHT skim milk fermented by L. delbrueckii subsp. bulgaricus SS1 had ACE-inhibitory indexes of ca. 70%. Fraction 13 of the UHT skim milk started with L. lactis subsp. cremoris FT4 had a similar ACE-inhibitory index. All of these fractions were located in the 19.6 to 26.2% range of the acetonitrile gradient.
TABLE 1.
ACE inhibition indexes of peptide fractions derived from milk fermented by L. delbrueckii subsp. bulgaricus SS1 or L. lactis subsp. cremoris FT4
| Peptide fraction | ACE inhibition index (%)
|
|
|---|---|---|
| L. delbrueckii subsp. bulgaricus SS1 | L. lactis subsp. cremoris FT4 | |
| 1 | 0 | 2 |
| 2 | 1 | 4 |
| 3 | 2 | 3 |
| 4 | 0 | 1 |
| 5 | 4 | 1 |
| 6 | 5 | 29 |
| 7 | 4 | 40 |
| 8 | 6 | 2 |
| 9 | 56 | 29 |
| 10 | 54 | 40 |
| 11 | 19 | 23 |
| 12 | 57 | 40 |
| 13 | 37 | 69 |
| 14 | 46 | 13 |
| 15 | 70 | 6 |
| 16 | 69 | 34 |
| 17 | 57 | 26 |
| 18 | 44 | 42 |
| 19 | 32 | 3 |
| 20 | 22 | 2 |
| 21 | 27 | 1 |
| 22 | 49 | 0 |
| 23 | 31 | 2 |
| 24 | 22 | 0 |
| 25 | 19 | 1 |
| 26 | 29 | 19 |
| 27 | 18 | 25 |
| 28 | 23 | 32 |
| 29 | 33 | 1 |
| 30 | 36 | 2 |
| 31 | 28 | 1 |
| 32 | 24 | 3 |
| 33 | 33 | 29 |
| 34 | 40 | 40 |
After prolonged incubation, a moderate change in the overall peptide profile was evident (Fig. 1E); when the same fractions, 15 and 16, were collected from the UHT skim milk fermented by L. delbrueckii subsp. bulgaricus SS1 for 196 h, they still had an ACE-inhibitory index of ca. 70%. The same was found for the fermented milk produced by L. lactis subsp. cremoris FT4 (data not shown).
The above three fractions were subsequently purified by further RP-FPLC and gel filtration followed by RP-FPLC.
Sequencing and synthesis of peptides.
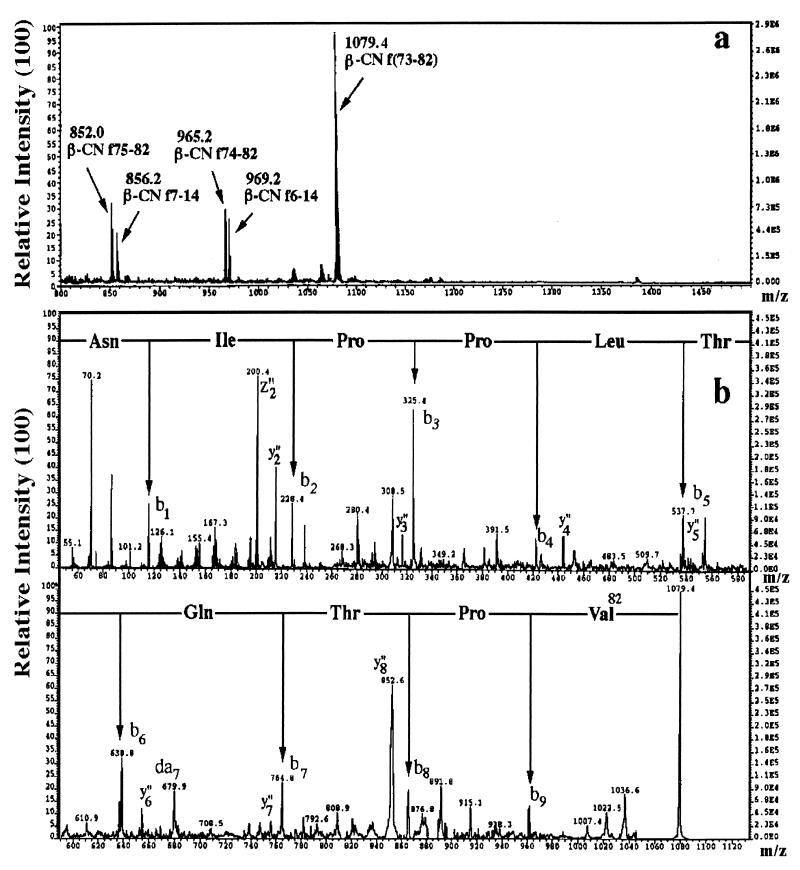
Peptides in the purified fractions were sequenced by FAB-MS. The profile obtained for the most complex fraction (fraction 16 from L. delbrueckii subsp. bulgaricus SS1) is shown in Fig. 2. All three fractions contained a mixture of peptides, and the respective sequences are reported in Table 2. Fractions 15 and 16 from the milk fermented by L. delbrueckii subsp. bulgaricus SS1 contained the sequences of β-CN f6-14 and f73-82 and f6-14, f7-14, f73-82, f74-82, and f75-82, respectively. Due to the very close positions within the acetonitrile gradient, an expected overlap of the sequences contained in the two fractions was found. All of the peptides contained in the two fractions originated from whole β-CN f73-82 and f6-14. Fraction 13 from the milk fermented by L. lactis subsp. cremoris FT4 contained β-CN f7-14, f47-52, and f169-175 and κ-CN f152-160 and f152-160. Also in this case, most of the peptides originated from β-CN (β-CN f7-14 was common to the other two fractions) and two peptides originated from κ-CN f155-160. It was interesting that the sequence of the hexapeptide Asp-Lys-Ile-His-Pro-Phe (β-CN f47-52) had the first N-terminal amino acid and the last four C-terminal amino acids in common with the octapeptide angiotensin-II physiologically generated by ACE hydrolysis of the decapeptide angiotensin-I.
FIG. 2.
FAB-MS spectrum of fraction 16 from L. delbrueckii subsp. bulgaricus SS1 recorded in the m/z region where peptides were detected (A). The peptide sequence (indicated for each peptide in the spectrum) was determined by tandem FAB-MS analysis of the single molecular ions, as shown in panel B for β-CN peptide f73-82 at m/z 1,079.4.
TABLE 2.
Sequences and corresponding CN fragments of peptides contained in crude fractions obtained from milk fermented by L. delbrueckii subsp. bulgaricus SS1 or L. lactis subsp. cremoris FT4
| Starter, fraction | Sequencea | CN fragment | Calculated massb | Expt 1 massb |
|---|---|---|---|---|
| L. delbrueckii subsp. bulgaricus SS1 | ||||
| 15 | LNVPGEIVE | β-CN f6-14 | 969.5 | 969.2 |
| NIPPLTQTPV | β-CN f73-82 | 1,079.6 | 1,079.4 | |
| 16 | NIPPLTQTPV | β-CN f73-82 | 1,079.6 | 1,079.4 |
| IPPLTQTPV | β-CN f74-82 | 965.5 | 965.3 | |
| PPLTQTPV | β-CN f75-82 | 852.4 | 852.0 | |
| LNVPGEIVE | β-CN f6-14 | 969.5 | 969.2 | |
| NVPGEIVE | β-CN f7-14 | 856.4 | 856.0 | |
| L. lactis subsp. cremoris FT4, 13 | NVPGEIVE | β-CN f7-14 | 856.4 | 856.0 |
| DKIHPF | β-CN f47-52 | 755.4 | 755.3 | |
| KVLPVPE | β-CN f169-175 | 780.4 | 780.1 | |
| VIGSPPEIN | κ-CN f152-160 | 996.5 | 997.1 | |
| SPPEIN | κ-CN f155-160 | 655.7 | 656.1 |
One-letter symbols are used as abbreviations for amino acids.
Monoisotopic masses are reported.
The peptides corresponding to the sequences of β-CN f6-14, f73-82, and f47-52 and κ-CN f155-160 were chemically synthesized because they were present in all three inhibitory fractions as a whole fragment or as an internal fragment (e.g., β-CN f6-14), were whole sequences which generated intermediate fragments (e.g., β-CN f6-14 and f73-82) and, in general, because these β-CN fragments were previously reported in the literature (19, 26) as sequences or parts of sequences of multifunctional bioactive peptides. The fragment from κ-CN was studied further because of the lack of information about the biological activity of peptides derived from this CN fraction.
ACE-inhibitory activities of purified fractions and synthesized peptides.
The IC50s of the crude peptide fractions and synthesized peptides are shown in Table 3. The crude fractions which contained the peptide mixtures (Table 2) had ACE-inhibitory activity characterized by very low IC50s, ranging from 8.0 to 11.2 mg/liter. When the peptides were used individually, the ACE-inhibitory activity was confirmed but the IC50s increased markedly, with the lowest (179.8 to 193.9 mg/liter) for β-CN f73-82, which was contained in both inhibitory crude fractions of L. delbrueckii subsp. bulgaricus SS1, and for β-CN f47-52, which was only contained in the inhibitory crude fraction of L. lactis subsp. cremoris FT4. All of the Dixon plots calculated for the synthesized peptides showed competitive inhibition (data not shown).
TABLE 3.
ACE-inhibitory activities of crude peptide fractions and synthesized peptides
| Crude peptide fraction or synthesized peptide | IC50
|
|
|---|---|---|
| mg/liter | μmol/liter | |
| Crude peptide fraction 15 from L. delbrueckii subsp. bulgaricus SS1 | 11.2 | NDa |
| Crude peptide fraction 16 from L. delbrueckii subsp. bulgaricus SS1 | 10.3 | ND |
| Crude peptide fraction 13 from L. lactis subsp. cremoris FT4 | 8.0 | ND |
| β-CN f6-14 from crude peptide fraction 15 | 290.7 | 300.1 |
| β-CN f73-82 from crude peptide fraction 16 | 179.8 | 173.3 |
| β-CN f47-52 from crude peptide fraction 13 | 193.9 | 256.8 |
| κ-CN f152-160 from crude peptide fraction 13 | >1,000.0 | >1,000.0 |
ND, not determined.
Hydrolysis of synthesized peptides by trypsin and chymotrypsin.
Trypsin and chymotrypsin were used to digest the synthesized peptides. Under assay conditions which caused about 80% hydrolysis of insulin chain A, the β-CN f6-14, f73-82, and f47-52 and κ-CN f155-160 produced by the two lactic acid bacteria in fermented milk were all completely resistant to hydrolysis by trypsin and chymotrypsin (data not shown).
DISCUSSION
L. delbrueckii subsp. bulgaricus and L. lactis subsp. cremoris, two of the most widely used industrial strains, are used as starters for fermented milks and several type of cheeses. In this study, we used two selected strains of these lactic acid bacteria to produce two types of fermented milk which contained ACE-inhibitory peptides.
Studies on the synthesis of bioactive peptides from food proteins are dated by several years, but only a few have considered the potential of lactic acid bacteria in dairy products. In some cases, the proteolytic activation of encrypted bioactive peptides from milk proteins was excluded due to some peculiarities (intracellular location and substrate specificity) of the peptidase system in lactic acid bacteria (19, 22, 27). It was also shown that during milk fermentation, probiotic strains (e.g., Lactobacillus sp. strain GG) may produce several oligopeptides which generate bioactive peptides only after subsequent digestion by pepsin and trypsin (28). Nevertheless, it must be borne in mind that proteinases of lactic acid bacteria can hydrolyze more than 40% of the peptide bonds of β-CN, resulting in the formation of more than 100 different oligopeptides, which are, in turn, actively degraded by the complex peptidase system (11, 21). More or less the same can be said for αsl-CN (12). Consequently, lactic acid bacteria could potentially generate a large variety of peptides, including bioactive sequences. Indeed, ACE-inhibitory peptides have been found in several types of cheese which differ with respect to the type of starter and the ripening conditions used (20, 29) and most of these cheeses, such as Gouda, Edam, cheddar, Crescenza, and Gorgonzola, used L. delbrueckii subsp. bulgaricus and/or L. lactis subsp. cremoris as a starter. Several studies (14, 24, 34) have reported the synthesis of ACE-inhibitory peptides in sour-milk Calpis fermented by an association of Lactobacillus helveticus and Saccharomyces cerevisiae, as well as the production of the same antihypertensive peptides by using the lactic acid bacterium alone or its extracellular proteinase. In a study conducted on the antihypertensive effects of different kinds of fermented milk in spontaneously hypertensive rats, it was shown that most of the whey fractions of milk fermented by L. helveticus and L. delbrueckii subsp. bulgaricus had high hemodynamic regulatory activity (33).
ACE is predominantly an ectoenzyme with two catalytic sites, one on each lobe of the extracellular portion (10). Structure-activity correlations among different peptide inhibitors of ACE indicate that binding to ACE is strongly influenced by the C-terminal tripeptide sequence of the substrate. ACE appears to prefer substrates or competitive inhibitors that contain mainly hydrophobic (aromatic or branched side chains) amino acid residues at the three C-terminal positions. However, the structure-activity relationship of ACE-inhibitory peptides has not yet been established and very different antihypertensive sequences have been derived from a large number of food proteins, such as β-, αsl-, and κ-CN, β-lactoglobulin, and α-lactalbumin, and plant and fish proteins (17). The purified crude fractions which showed the highest ACE-inhibitory activity in the milk fermented by L. delbrueckii subsp. bulgaricus SS1 contained a mixture of peptides such as β-CN f6-14, f7-14, f73-82, f74-82, and f75-82. Essentially two mother sequences were responsible for the bioactivity. The purified crude fraction from the milk fermented by L. lactis subsp. cremoris FT4 had β-CN f7-14 in common but differed in fragments such as β-CN f47-52 and f169-175 and κ-CN 152-160 and 155-160. All of the above CN fragments had a higher proportion of hydrophobic residues (>60%) within their entire sequences; in particular, β-CN f73-82 and related intermediates and β-CN f47-52 had the last two and three C-terminal amino acids which are hydrophobic (Table 2). Except for the N- and C-terminal amino acids, the hexapeptide β-CN f47-52 and the heptapeptide β-CN f169-175 contained only hydrophobic amino acids.
The genetic and biochemical properties of proteinases and peptidases of L. lactis strains have been studied in depth (11–13, 21). The proteolytic systems of lactobacilli are remarkably similar in their components and mode of action. Cleavage sites corresponding to the peptide bonds of residues 46 to 47, 52 to 53, 168 to 169, and 174 to 175 of β-CN and 160 to 161 of κ-CN are hydrolyzed by all of the lactococcal proteinases studied, while sites corresponding to residues 6 to 7, 72 to 73, and 82 to 83 of β-CN are cut by several L. lactis subsp. cremoris proteinases. As a consequence, most of the ACE-inhibitory peptides produced in the milks fermented by L. delbrueckii subsp. bulgaricus SS1 and L. lactis subsp. cremoris FT4 may result directly from these specific activities alone or together with the contribution of the broad-spectrum peptidase activities (11–13).
The peptides identified in this study have several other features in common with other reported ACE-inhibitory peptides. Concerning the ACE-inhibitory peptides contained in the milk fermented by L. delbrueckii subsp. bulgaricus SS1, β-CN f73-82 and the related intermediate β-CN f74-82 had, within their whole sequences, the tripeptide Ile-Pro-Pro (β-CN f74-76), which has been identified in sour-milk Calpis and showed an antihypertensive effect when orally administered to spontaneously hypertensive rats in a mixture with another tripeptide (Val-Pro-Pro) (33). The C-terminal sequence of β-CN f73-82 and related intermediates (Gln-Thr-Pro-Val) showed a high degree of homology with the C-terminal sequence Gln-Gln-Pro-Val of an antihypertensive heptapeptide which corresponded to β-CN f191-197 (33). β-CN f7-14 and a related intermediate contained the internal sequence Asn-Val-Pro-Gly, which also characterized part of the sequences of several antihypertensive peptides isolated from both β-CN and fish proteins (32, 34).
Concerning the peptides contained in the milk fermented by L. lactis subsp. cremoris FT4, β-CN f47-52 is a part of another ACE-inhibitory peptide corresponding to the longer β-CN f43-69 sequence (34) and the C-terminal His-Pro-Phe tripeptide had elevated homology with the C-terminal His-Thr-Phe sequence of several other ACE-inhibitory peptides derived from tuna muscle (32). It should be noted that the β-CN f47-52 sequence (Asp-Lys-Ile-His-Pro-Phe) has five residues (including the last four C-terminal residues) in common with the octapeptide angiotensin-II (product of the ACE activity) and that some drugs used in antihypertension therapy are based on compounds which may compete for the receptor sites of the vasoconstrictor angiotensin-II due to their partial homology with this product of ACE activity. Angiotensin-II receptor antagonists (such as losartan) competitively block angiotensin-II-induced vascular contraction (9). Moreover, especially in patients with diabetic nephropathy, the addition of an angiotensin-II receptor antagonist to the ACE inhibition therapy regimen attenuates angiotensin-II renal effects better than ACE inhibition therapy alone (8). The β-CN f169-175 sequence was also identified in CN hydrolysate produced by the purified extracellular proteinase of L. helveticus (14). This peptide did not show strong ACE-inhibitory activity (IC50, >1,000 μmol/liter). However, the corresponding hexapeptide, Lys-Val-Leu-Pro-Val-Pro, obtained after liberation of the C-terminal Gln residue by pancreatic digestion in vitro, had strong ACE-inhibitory activity (IC50, 5 μmol/liter), as well as a remarkable antihypertensive effect in vivo. Reports of ACE-inhibitory peptides derived from hydrolysis of κ-CN are rare and correspond to very short sequences, such as κ-CN f38-39, f25-34, and f24-26 (2, 17, 31). Most of the bioactive peptides from κ-CN have antithrombotic activity (19). The one characteristic common to κ-CN f152-160 and f155-160 in this study and other antihypertensive peptides derived from CNs is the elevated proportion of hydrophobic amino acids in the whole sequence. The synthesized peptide κ-CN f152-160 had the highest IC50 (>1,000.0 mg/liter).
The IC50s of the crude purified fractions containing the mixture of the identified peptides are very low and comparable to those of the most active ACE-inhibitory peptides reported in the literature (19, 26). When some of these peptides were chemically synthesized and individually used, the ACE-inhibitory activity was confirmed and, except for that of κ-CN f152-160, the IC50s were ca. 20 to 30 times higher but still within the range found for several other antihypertensive peptides.
Some regions in the primary structure of CNs have been considered to be strategic zones, since they are partially protected from proteolytic breakdown (7). On the other hand, bioactive peptides that have been produced by limited proteolysis during processing could be further digested by intestinal proteinases or brush border peptidases, which would decrease or eliminate their biological activity (19). The ACE-inhibitory fractions found in milk fermented by L. delbrueckii subsp. bulgaricus SS1 and that fermented by L. lactis subsp. cremoris FT4 for 96 h had the same ACE-inhibitory indexes after prolonged incubation for 196 h, thus excluding, under our conditions, further hydrolysis by microbial peptidases. All of the synthesized peptides identified in the crude inhibitory fractions were completely resistant to trypsin and chymotrypsin under the assay conditions used, which caused 80% hydrolysis of the insulin chain A used as a control. These findings may show that inhibitory peptide mixtures produced by the two selected lactic acid bacteria may withstand subsequent proteolysis during dairy processing and by intestinal proteinases.
To our knowledge, this is the first report which shows with certainty the production of CN-derived ACE-inhibitory peptides by L. delbrueckii subsp. bulgaricus and L. lactis subsp. cremoris. Further work will address the optimization of dairy processing conditions and the genetic manipulation of strains to favor the overproduction of ACE-inhibitory peptides.
REFERENCES
- 1.Andrews A T. Proteinases in normal bovine milk and their action on caseins. J Dairy Res. 1983;50:45–55. doi: 10.1017/s0022029900032519. [DOI] [PubMed] [Google Scholar]
- 2.Bottazzi V. Al di là della funzione nutrizionale. Mondo Latte. 1996;12:943–953. [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 4.Bruneval P, Hinglais N, Alhenc-Gelas F. Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization. Histochemistry. 1986;86:73–80. doi: 10.1007/BF00508656. [DOI] [PubMed] [Google Scholar]
- 5.Dixon M. The determination of enzyme inhibitor constants. Biochem J. 1953;55:170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira S H, Bartlet D C, Greene L J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9:2583–2592. doi: 10.1021/bi00815a005. [DOI] [PubMed] [Google Scholar]
- 7.Fiat A M, Jollès P. Caseins of various origins and biologically active casein peptides and olisaccharides: structural and physiological aspects. Mol Cell Biochem. 1989;87:5–30. doi: 10.1007/BF00421079. [DOI] [PubMed] [Google Scholar]
- 8.Hebert L A, Falkenhain M E, Nahman N S, Jr, Cosio F G, O'Dorisio T M. Combination ACE inhibitor and angiotensin II receptor antagonist therapy in diabetic nephropathy. Am J Nephrol. 1999;19:1–6. doi: 10.1159/000013417. [DOI] [PubMed] [Google Scholar]
- 9.Jagadeesh G. Angiotensin II receptors—antagonists, molecular biology, and signal transduction. Indian J Exp Biol. 1998;36:1171–1194. [PubMed] [Google Scholar]
- 10.Johnston C I. Renin-angiotensin system: a dual tissue and hormonal system for cardiovascular control. J Hypertension. 1992;10:S13–S26. [PubMed] [Google Scholar]
- 11.Juillard V, Laan H, Kunji E R S, Jeronimus-Stratingh C M, Bruins A P, Konings W N. The extracellular PI-type proteinase of Lactococcus lactis hydrolyzes β-casein into more than one hundred different oligopeptides. J Bacteriol. 1995;177:3472–3478. doi: 10.1128/jb.177.12.3472-3478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunji E R S, Mierau I, Hagting A, Poolman B, Konings W N. The proteolytic systems of lactic acid bacteria. Antonie van Leeuwenhoek. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 13.Kunji E R S, Fang G, Jeronimus-Stratingh C M, Bruins A P, Poolman B, Konings W N. Reconstruction of the proteolytic pathway for use of β-casein by Lactococcus lactis. Mol Microbiol. 1998;27:1107–1118. doi: 10.1046/j.1365-2958.1998.00769.x. [DOI] [PubMed] [Google Scholar]
- 14.Maeno M, Yamamoto N, Takano T. Identification of an antihypertensive peptide from casein hydrolysate by a proteinase from Lactobacillus helveticus CP790. J Dairy Sci. 1996;79:1316–1321. doi: 10.3168/jds.S0022-0302(96)76487-1. [DOI] [PubMed] [Google Scholar]
- 15.Matar C, Goulet J. β-Casomorphin 4 milk fermented by a mutant of Lactobacillus helveticus. Int Dairy J. 1996;6:383–397. [Google Scholar]
- 16.Meisel H. Casokinins as inhibitors of angiotensin-converting-enzyme. In: Sawatzki G, Renner B, editors. New perspectives in infant nutrition. Stuttgart, Germany: Thieme; 1993. pp. 153–159. [Google Scholar]
- 17.Meisel H. Biochemical properties of bioactive peptides derived from milk proteins: potential nutraceuticals for food and pharmaceutical applications. Livest Prod Sci. 1997;50:125–138. [Google Scholar]
- 18.Meisel H. Overview on milk protein-derived peptides. Int Dairy J. 1998;8:363–373. [Google Scholar]
- 19.Meisel H, Bockelmann W. Bioactive peptides encrypted in milks proteins: proteolytic activation and thropho-functional properties. Antonie van Leeuwenhoek. 1999;76:207–215. [PubMed] [Google Scholar]
- 20.Meisel H, Goepfert A, Günther S. ACE inhibitory activities in milk products. Milchwissenschaft. 1997;52:307–311. [Google Scholar]
- 21.Mierau I, Kunji E R S, Venema G, Kok J. Casein and peptide degradation in lactic acid bacteria. Biotech Genet Eng Rev. 1997;14:279–301. doi: 10.1080/02648725.1997.10647945. [DOI] [PubMed] [Google Scholar]
- 22.Muehlenkamp M R, Warthesen J J. β-Casomorphins: analysis in cheese and susceptibility to proteolytic enzymes from Lactobacillus lactis ssp. cremoris. J Dairy Sci. 1996;79:20–26. doi: 10.3168/jds.S0022-0302(96)76329-4. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y, Yamamoto N, Sakai K, Okubo A, Yamazaki S, Takano T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J Dairy Sci. 1995;78:777–783. doi: 10.3168/jds.S0022-0302(95)76689-9. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura Y, Yamamoto N, Sakai K, Takano T. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J Dairy Sci. 1994;78:1253–1257. doi: 10.3168/jds.S0022-0302(95)76745-5. [DOI] [PubMed] [Google Scholar]
- 25.Ondetti M A, Cushman D W. Enzymes of the renin-angiotensin system and their inhibitors. Annu Rev Biochem. 1982;51:283–308. doi: 10.1146/annurev.bi.51.070182.001435. [DOI] [PubMed] [Google Scholar]
- 26.Ondetti M A, Williams N J, Sabo E F, Pluvec J, Weaver E R, Kocy O. Angiotensin converting enzyme inhibitors from the venom of Bothrops jararaca, isolation, elucidation of structure and synthesis. Biochemistry. 1971;10:4033–4039. doi: 10.1021/bi00798a004. [DOI] [PubMed] [Google Scholar]
- 27.Pihlanto-Leppälä A, Rokka T, Korhonen H. Angiotensin I converting enzyme inhibitory peptides from bovine milk proteins. Int Dairy J. 1998;8:325–331. [Google Scholar]
- 28.Rokka T, Syväoja E L, Tuominen J, Korhonen H. Release of bioactive peptides by enzymatic proteolysis of Lactobacillus GG fermented UHT milk. Milchwissenschaft. 1997;52:675–678. [Google Scholar]
- 29.Smacchi E, Gobbetti M. Peptides from several Italian cheeses inhibitory to proteolytic enzymes of lactic acid bacteria, Pseudomonas fluorescens ATCC 948 and to the angiotensin I-converting enzyme. Enzyme Microb Technol. 1998;22:687–694. [Google Scholar]
- 30.Smacchi E, Gobbetti M. Bioactive peptides in dairy products: synthesis and interaction with proteolytic enzymes. Food Microbiol. 2000;17:129–141. [Google Scholar]
- 31.Takano T. Milk derived peptides and hypertension reduction. Int Dairy J. 1998;8:375–381. [Google Scholar]
- 32.Yamamoto N. Antihypertensive peptides derived from food proteins. Biopolymers. 1997;43:129–134. doi: 10.1002/(SICI)1097-0282(1997)43:2<129::AID-BIP5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto N, Akino A, Takano T. Antihypertensive effect of different kinds of fermented milk in spontaneously hypertensive rats. Biosci Biotech Biochem. 1994;58:776–778. [Google Scholar]
- 34.Yamamoto N, Akino A, Takano T. Antihypertensive effect of the peptides derived from casein by an extracellular proteinase from Lactobacillus helveticus CP790. J Dairy Sci. 1994;77:917–922. doi: 10.3168/jds.S0022-0302(94)77026-0. [DOI] [PubMed] [Google Scholar]