Abstract
Nuclear factor Y (NF-Y) is a heterotrimeric transcription factor that plays an important role in various biological processes in plants, such as flowering regulation, drought resistance, and salt stress. However, few in-depth studies investigated the alfalfa NF-Y gene family. In this study, in total, 60 MsNF-Y genes, including 9 MsNF-YAs, 26 MsNF-YBs, and 25 MsNF-YCs, were identified in the alfalfa genome. The genomic locations, gene structures, protein molecular weights, conserved domains, phylogenetic relationships, and gene expression patterns in different tissues and under different stresses (cold stress, drought stress, and salt stress) of these NF-Y genes were analyzed. The illustration of the conserved domains and specific domains of the different subfamilies of the MsNF-Y genes implicates the conservation and diversity of their functions in alfalfa growth, development, and stress resistance. The gene expression analysis showed that 48 MsNF-Y genes (7 MsNF-YAs, 22 MsNF-YBs, and 19 MsNF-YCs) were expressed in all tissues at different expression levels, indicating that these genes have tissue expression specificity and different biological functions. In total, seven, seven, six, and eight MsNF-Y genes responded to cold stress, the ABA treatment, drought stress, and salt stress in alfalfa, respectively. According to the WGCNA, molecular regulatory networks related to salt stress were constructed for MsNF-YB2, MsNF-YB5, MsNF-YB7, MsNF-YB15, MsNF-YC5, and MsNF-YC6. This study could provide valuable information for further elucidating the biological functions of MsNF-Ys and improving salt tolerance and other abiotic stress resistance in alfalfa.
Keywords: alfalfa (Medicago sativa L.), NF-Y gene family, abiotic stress, salt tolerance
1. Introduction
Alfalfa (Medicago sativa L.) is one of the top-quality forages for livestock, such as dairy cattle, because of its high nutrient content and crude protein content and has been hailed as the ‘king of forages’ [1,2]. However, alfalfa is frequently affected by various abiotic stresses, such as drought, cold, and salt, during alfalfa growth and development, which seriously affect the yield and quality of alfalfa [3]. Alfalfa has formed complex gene regulatory networks in response to increasingly deteriorating growth environments over a long period of evolution [3]. In addition, with the reporting of the alfalfa reference genome, several transcription factor families, such as WRKY, CBF, SPL, and MYB, have been identified to be involved in abiotic stress processes in alfalfa [3]. However, our knowledge of the complex transcriptional regulatory network is limited.
The nuclear factor Y (NF-Y) transcription factor, also known as the CCAAT-binding factor (CBF) or the heme activator protein (HAP), is widespread in eukaryotes. NF-Y consists of three distinct subunits, NF-YA, NF-YB, and NF-YC [4]. The subfamily (NF-YA, NF-YB, and NF-YC) members can be identified by the sequence length and conserved motifs or domains. In general, NF-YA subunits have a longer sequence than the NF-YB and NF-YC sequences [5]. The conserved region in the NF-YA subunits usually consists of two conserved domains, α1 and α2, which include 53 amino acids [5]. The NF-YB subunit is shorter in sequence length, and the conserved protein domains in the NF-YB and NF-YC subunits are extremely similar to the H2B and H2A histones, respectively [5]. NF-YB and NF-YC first form dimers in the cytoplasm and then they can be located into the nucleus and interact with NF-YA to form a heterotrimeric complex [5]. This typical NF-Y heterotrimeric complex activates or represses the expression of downstream genes by binding CCAAT sequences located in the promoters of target genes [5,6]. In addition, different NF-Y subunits can form complexes with other transcription factors, thereby regulating the expression of downstream genes [7,8].
The NF-Y transcription factor has been confirmed to be involved in multiple bio-logical processes, such as plant seed development, flowering, and fruit development [9,10,11,12]. AtNF-YB9, the first NF-Y gene to be cloned in plants, is involved as a key regulatory transcription factor in Arabidopsis seed development [13,14]. The mutation of OsNF-YB9, which is the homologous gene of AtNF-YB9 in rice, could cause abnormal seed development, such as seeds becoming longer, narrower, and thinner and exhibiting a higher chalkiness ratio [11]. AtNF-YB6 has also been found as a regulator of embryo development and specifically expressed in developing Arabidopsis embryos [15]. FtNF-YB7, which is the homologous gene of AtNF-YB9 in buckwheat, was also specifically expressed in fruit and indicated its important role in fruit development [16]. In recent years, the role of NF-Y gene family members during abiotic stress in plants has received much attention. AtNF-YA5 was highly expressed in vascular tissues and guard cells and was induced by drought stress [17]. The overexpression of AtNF-YA5 could reduce leaf water loss and increase the drought tolerance [17]. Overexpression of GmNF-YA5, which is the homologous gene of AtNF-YA5 in soybean, in Arabidopsis and soybean could enhance the drought tolerance in seedlings by decreasing the water loss from leaves [18]. AtNF-YB1 in Arabidopsis, ZmNF-YB2 in maize, and GmNF-YB01 in soybean are homologous and play an important role in drought stress [10,19]. The overexpression of AtNF-YB2 and AtNF-YB3 in Arabidopsis increased drought and heat tolerance, respectively [20]. The overexpression of the ZmNF-YB16 genes in maize improved drought resistance and yield [21]. GmNF-YC14, a member of the soybean NF-YC subunit family, was found to be significantly upregulated simultaneously in response to drought and salt stress and ABA treatment [22]. GmNF-YC14 was able to interact with GmNF-YB2 and GmNF-YA16 in soybean to form an intact and active NF-Y transcriptional complex [22]. This NF-Y complex could regulate the ABA signaling pathway mediated by ABA receptor PYR/PYL under drought stress to enhance drought and salt tolerance in soybean [22]. With the rapid development of genomics, an increasing number of NF-Y gene families have been identified and analyzed at the genomic level; for example, 38 NF-Y gene family members were identified in the bitter buckwheat genome [16], 34 NF-Y genes were identified in the grape genome [23], and 25 genes were identified in the castor bean genome, and their functions have been preliminarily analyzed [24]. However, the basic information and functions of NF-Y transcription factors in alfalfa remain poorly understood.
In this study, the role of MsNF-Ys in the regulation of multiple abiotic stresses of plants, especially salt stress in alfalfa, was investigated. In total, 60 MsNF-Y genes (9 MsNF-YAs, 26 MsNF-YBs, and 25 MsNF-YCs) were identified in the alfalfa reference genome based on bioinformatics methods. The gene structure, motif composition, conserved domains, and cis-acting elements in the promoter regions of the different NF-Y subfamilies in alfalfa were analyzed. In addition, a homology analysis and phylogenetic tree analysis of NF-Y genes from alfalfa, Arabidopsis, buckwheat, rice, and soybean were conducted to predict the function of MsNF-Ys. NF-Y gene members were identified to be involved in different biological processes by an expression pattern analysis in different tissues of alfalfa under different treatments (cold, ABA, drought, and salt). More importantly, the expression of MsNF-Y genes during salt stress was comprehensively analyzed by a WGCNA, indicating that some MsNF-Y genes are involved in the alfalfa salt stress process. This study may provide valuable information for the identification of candidate NF-Y genes involved in the regulation of abiotic stress (especially salt stress) in alfalfa.
2. Results
2.1. Identification of MsNF-Y Family Genes
In total, 60 MsNF-Y genes, including 9 MsNF-YAs, 26 MsNF-YBs, and 25 MsNF-YCs, were identified from the alfalfa reference genome (Xinjiangdaye). Then, we named these 60 MsNF-Y genes (the NF-YA subfamily was named MsNF-YA1 to MsNF-YA9, the NF-YB subfamily was named MsNF-YB1 to MsNF-YB26, and the NF-YC subfamily was named MsNF-YC1 to MsNF-YC25) according to their chromosomal locations and subfamilies (Table 1).
Table 1.
Information of the MsNF-Y genes in alfalfa.
| Name | Gene ID | CDs | Length (AA) | Chromosome Localization | Mw (kDa) | pI | Homologs in Arabidopsis |
|---|---|---|---|---|---|---|---|
| MsNF-YA1 | MS.gene84539 | 1002 | 333 | chr3.1:46159735:46162043 | 36.08 | 5.68 | AtNF-YA1/9 |
| MsNF-YA2 | MS.gene04098 | 990 | 329 | chr3.2:52938927:52941216 | 35.83 | 5.81 | AtNF-YA1/9 |
| MsNF-YA3 | MS.gene08079 | 990 | 329 | chr3.3:49398206:49400492 | 35.79 | 5.81 | AtNF-YA1/9 |
| MsNF-YA4 | MS.gene04396 | 990 | 329 | chr3.4:57338770:57341055 | 35.77 | 5.81 | AtNF-YA1/9 |
| MsNF-YA5 | MS.gene007115 | 1005 | 334 | chr7.1:6286954:6289818 | 36.28 | 9.48 | AtNF-YA10 |
| MsNF-YA6 | MS.gene23087 | 1005 | 334 | chr7.2:7507261:7510125 | 36.31 | 9.48 | AtNF-YA10 |
| MsNF-YA7 | MS.gene26493 | 1005 | 334 | chr7.3:7757499:7760361 | 36.28 | 9.48 | AtNF-YA10 |
| MsNF-YA8 | MS.gene011285 | 939 | 312 | chr8.2:75039696:75042162 | 33.23 | 7.70 | AtNF-YA6 |
| MsNF-YA9 | MS.gene53329 | 957 | 318 | chr8.4:73707595:73710074 | 35.03 | 8.48 | AtNF-YA6 |
| MsNF-YB1 | MS.gene004698 | 450 | 149 | chr1.1:54900382:54901482 | 16.34 | 5.32 | AtNF-YB1/8/10 |
| MsNF-YB2 | MS.gene32082 | 444 | 147 | chr1.2:55567170:55568359 | 16.09 | 5.54 | AtNF-YB8/10 |
| MsNF-YB3 | MS.gene036253 | 531 | 176 | chr1.3:52425710:52429054 | 19.47 | 5.56 | AtNF-YB1/8/10 |
| MsNF-YB4 | MS.gene40015 | 450 | 149 | chr1.4:59578396:59579496 | 16.34 | 5.32 | AtNF-YB1/8/10 |
| MsNF-YB5 | MS.gene045804 | 411 | 136 | chr2.1:36197735:36200699 | 15.28 | 4.61 | AtNF-YB12/13 |
| MsNF-YB6 | MS.gene048249 | 513 | 170 | chr2.3:62568258:62569485 | 18.58 | 5.48 | AtNF-YB2/3 |
| MsNF-YB7 | MS.gene83228 | 471 | 156 | chr2.4:34709719:34712823 | 17.42 | 4.63 | AtNF-YB12/13 |
| MsNF-YB8 | MS.gene02911 | 489 | 162 | chr2.4:62033643:62034131 | 17.67 | 5.78 | AtNF-YB3 |
| MsNF-YB9 | MS.gene94950 | 570 | 189 | chr3.1:44574260:44574829 | 20.12 | 6.21 | AtNF-YB2/3 |
| MsNF-YB10 | MS.gene05647 | 570 | 189 | chr3.2:51228991:51229560 | 20.12 | 6.21 | AtNF-YB2/3 |
| MsNF-YB11 | MS.gene04197 | 570 | 189 | chr3.3:48302965:48303534 | 20.06 | 6.21 | AtNF-YB2/3 |
| MsNF-YB12 | MS.gene04298 | 570 | 189 | chr3.4:55907374:55907943 | 20.12 | 6.21 | AtNF-YB2/3 |
| MsNF-YB13 | MS.gene37951 | 558 | 185 | chr5.1:76486331:76486888 | 20.21 | 5.87 | AtNF-YB2/3 |
| MsNF-YB14 | MS.gene81327 | 558 | 185 | chr5.2:80485723:80486280 | 20.32 | 6.23 | AtNF-YB2/3 |
| MsNF-YB15 | MS.gene020793 | 558 | 185 | chr5.3:77328038:77328595 | 20.20 | 5.75 | AtNF-YB2/3 |
| MsNF-YB16 | MS.gene22469 | 510 | 169 | chr7.1:8232375:8233608 | 18.62 | 8.49 | AtNF-YB1/8/10 |
| MsNF-YB17 | MS.gene20033 | 510 | 169 | chr7.2:11459416:11460648 | 18.62 | 8.49 | AtNF-YB1/8/10 |
| MsNF-YB18 | MS.gene007467 | 510 | 169 | chr7.2:11511734:11512962 | 18.62 | 8.49 | AtNF-YB1/8/10 |
| MsNF-YB19 | MS.gene09802 | 510 | 169 | chr7.3:12232958:12234190 | 18.62 | 8.49 | AtNF-YB1/8/10 |
| MsNF-YB20 | MS.gene012740 | 714 | 237 | chr8.1:730789:733261 | 26.28 | 6.26 | AtNF-YB6 |
| MsNF-YB21 | MS.gene044533 | 462 | 153 | chr8.1:14534380:14537789 | 17.15 | 4.72 | AtNF-YB12/13 |
| MsNF-YB22 | MS.gene95097 | 708 | 235 | chr8.2:735520:737984 | 26.14 | 6.26 | AtNF-YB6 |
| MsNF-YB23 | MS.gene042085 | 708 | 235 | chr8.3:558353:560819 | 26.14 | 6.26 | AtNF-YB6 |
| MsNF-YB24 | MS.gene038788 | 462 | 153 | chr8.3:14583864:14587257 | 17.15 | 4.72 | AtNF-YB12/13 |
| MsNF-YB25 | MS.gene43428 | 708 | 235 | chr8.4:784437:786904 | 26.14 | 6.26 | AtNF-YB6 |
| MsNF-YB26 | MS.gene033001 | 462 | 153 | chr8.4:17356790:17360381 | 17.15 | 4.72 | AtNF-YB12/13 |
| MsNF-YC1 | MS.gene96763 | 768 | 255 | chr1.1:61200434:61201201 | 28.41 | 5.62 | AtNF-YC1/2/3/9 |
| MsNF-YC2 | MS.gene055556 | 768 | 255 | chr1.2:60238503:60239270 | 28.40 | 5.62 | AtNF-YC1/2/3/9 |
| MsNF-YC3 | MS.gene44078 | 768 | 255 | chr1.3:58221159:58221926 | 28.40 | 5.62 | AtNF-YC1/2/3/9 |
| MsNF-YC4 | MS.gene005213 | 768 | 255 | chr1.4:66923142:66923909 | 28.40 | 5.62 | AtNF-YC1/2/3/9 |
| MsNF-YC5 | MS.gene073979 | 798 | 265 | chr2.1:30297070:30297867 | 29.76 | 5.79 | AtNF-YC1/2/3/4/9 |
| MsNF-YC6 | MS.gene039020 | 804 | 267 | chr2.2:24489042:24489845 | 30.13 | 5.97 | AtNF-YC1/2/3/4/9 |
| MsNF-YC7 | MS.gene77621 | 798 | 265 | chr2.3:30013127:30013924 | 29.85 | 5.97 | AtNF-YC1/2/3/4/9 |
| MsNF-YC8 | MS.gene92165 | 798 | 265 | chr2.4:27659056:27659853 | 29.82 | 5.79 | AtNF-YC1/2/3/4/9 |
| MsNF-YC9 | MS.gene49550 | 891 | 296 | chr3.1:8392449:8394316 | 32.70 | 5.66 | AtNF-YC11 |
| MsNF-YC10 | MS.gene039544 | 1140 | 379 | chr3.1:8402787:8408425 | 41.59 | 5.75 | AtNF-YC11 |
| MsNF-YC11 | MS.gene06223 | 678 | 225 | chr3.1:79056763:79057440 | 25.17 | 5.65 | AtNF-YC1/4 |
| MsNF-YC12 | MS.gene97173 | 1140 | 379 | chr3.2:6048908:6054534 | 41.60 | 5.75 | AtNF-YC11 |
| MsNF-YC13 | MS.gene055499 | 570 | 189 | chr3.2:82525648:82526217 | 20.97 | 5.71 | AtNF-YC1/4 |
| MsNF-YC14 | MS.gene88253 | 1203 | 400 | chr3.3:7831439:7839020 | 43.94 | 8.95 | AtNF-YC11 |
| MsNF-YC15 | MS.gene013147 | 483 | 160 | chr3.3:81706695:81707177 | 17.32 | 4.96 | AtNF-YC1/4 |
| MsNF-YC16 | MS.gene046309 | 1140 | 379 | chr3.4:8318736:8325208 | 41.60 | 5.75 | AtNF-YC11 |
| MsNF-YC17 | MS.gene050860 | 783 | 260 | chr7.1:2796742:2797524 | 29.04 | 6.03 | AtNF-YC1/2/3/4/9 |
| MsNF-YC18 | MS.gene29325 | 783 | 260 | chr7.2:3652860:3653642 | 29.04 | 6.03 | AtNF-YC1/2/3/4/9 |
| MsNF-YC19 | MS.gene67561 | 783 | 260 | chr7.3:3320464:3321246 | 29.04 | 6.03 | AtNF-YC1/2/3/4/9 |
| MsNF-YC20 | MS.gene050859 | 723 | 240 | chr7.3:3331958:3332680 | 26.95 | 6.04 | AtNF-YC1/2/3/4/9 |
| MsNF-YC21 | MS.gene072458 | 783 | 260 | chr7.3:3363915:3364697 | 29.04 | 6.03 | AtNF-YC1/2/3/4/9 |
| MsNF-YC22 | MS.gene072460 | 783 | 260 | chr7.3:3435457:3436239 | 29.04 | 6.03 | AtNF-YC1/2/3/4/9 |
| MsNF-YC23 | MS.gene063839 | 918 | 305 | chr8.1:62879212:62882462 | 33.26 | 5.08 | AtNF-YC11 |
| MsNF-YC24 | MS.gene88394 | 918 | 305 | chr8.3:56793662:56796860 | 33.37 | 5.13 | AtNF-YC11 |
| MsNF-YC25 | MS.gene78092 | 918 | 305 | chr8.4:58034595:58037795 | 33.39 | 5.02 | AtNF-YC11 |
The protein lengths of the 60 MsNF-Y genes showed a wide distribution, ranging from 136 AA (MsNF-YB5) to 400 AA (MsNF-YC14) (Table 1 and Table S1). The protein length of the MsNF-YA members ranged from 312 AA to 334 AA, while the protein length of the MsNF-YB members ranged from 136 AA to 237 AA, and the MsNF-YC members were distributed from 160 AA to 400 AA. Among the three MsNF-Y subfamilies, the MsNF-YA subfamily member proteins had the longest average length of 328 AA, whereas MsNF-YB had the shortest protein with an average length of 182.3 AA, and MsNF-YC had an intermediate average length of 240.9 AA. The predicted protein molecular weight (MW) results showed that the MWs of the 60 MsNF-Ys were widely distributed from 15.28 kDa to 43.94 kDa, with the MsNF-YA subfamily distribution ranging from 36.08 kDa to 36.31 kDa and a mean of 35.62 kDa, the MsNF-YB subfamily distribution ranging from 15.28 kDa to 26.28 kDa and a mean of 19.57 kDa, and the MsNF-YC subfamily distribution ranging from 17.32 kDa to 43.94 kDa and a mean of 30.81 kDa. The predicted pI of the 60 MsNF-Ys in alfalfa ranged from 4.61 (MsNF-YB5) to 8.48 (MsNF-YA9) (Table 1).
2.2. Analysis of the Motifs and Conserved Domains of MsNF-Y Family Members
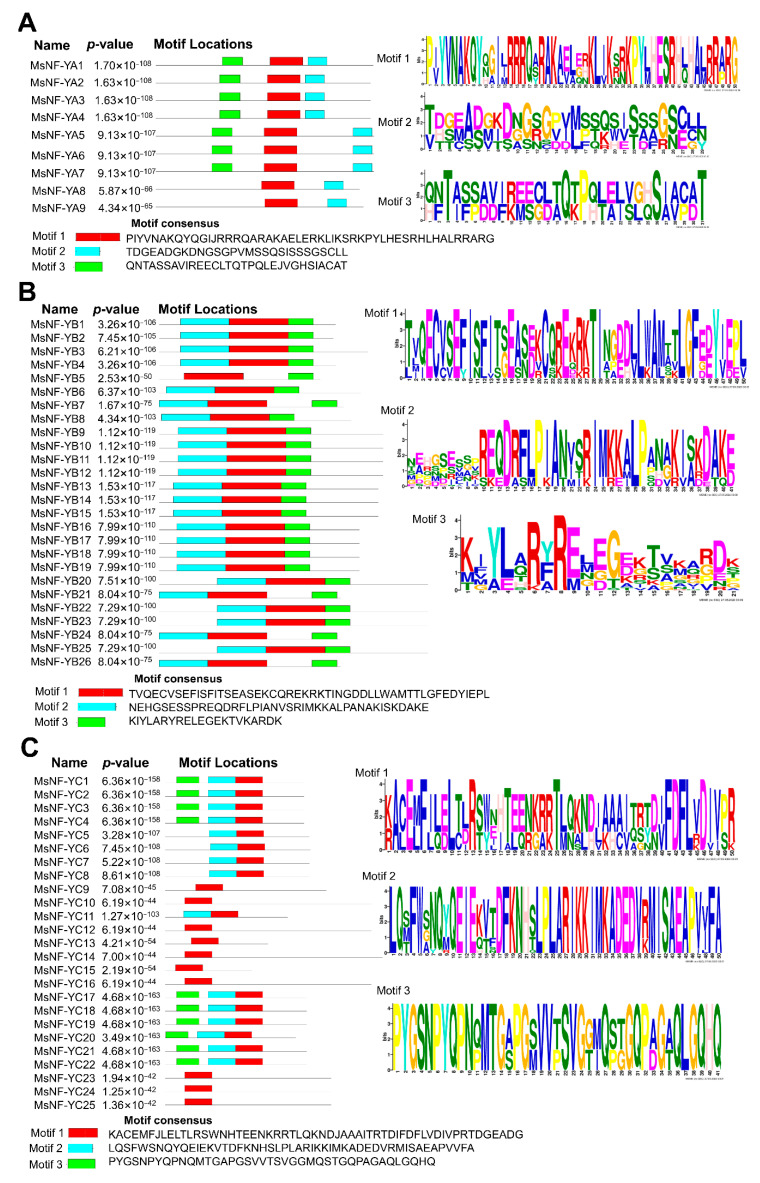
To analyze the motif composition of these MsNF-Y proteins, MEME software was used to identify the motifs. The results showed that different subfamilies had different motif compositions (Figure 1). In the MsNF-YA group, motif 1 and motif 2 were found in all members (Figure 1A). MsNF-YA8 and MsNF-YA9 did not have motif 3. In the MsNF-YB subfamily, all members, except for MsNF-YB5, contained the three motifs identified (Figure 1B). In the MsNF-YC subfamily, the composition of the motifs in these proteins was relatively diverse. There were only 10 MsNF-YC proteins with one motif and five MsNF-YC proteins with two motifs (Figure 1C).
Figure 1.
Motif analysis of MsNF-Y proteins. (A) MsNF-YA proteins; (B) MsNF-YB proteins; (C) MsNF-YC proteins.
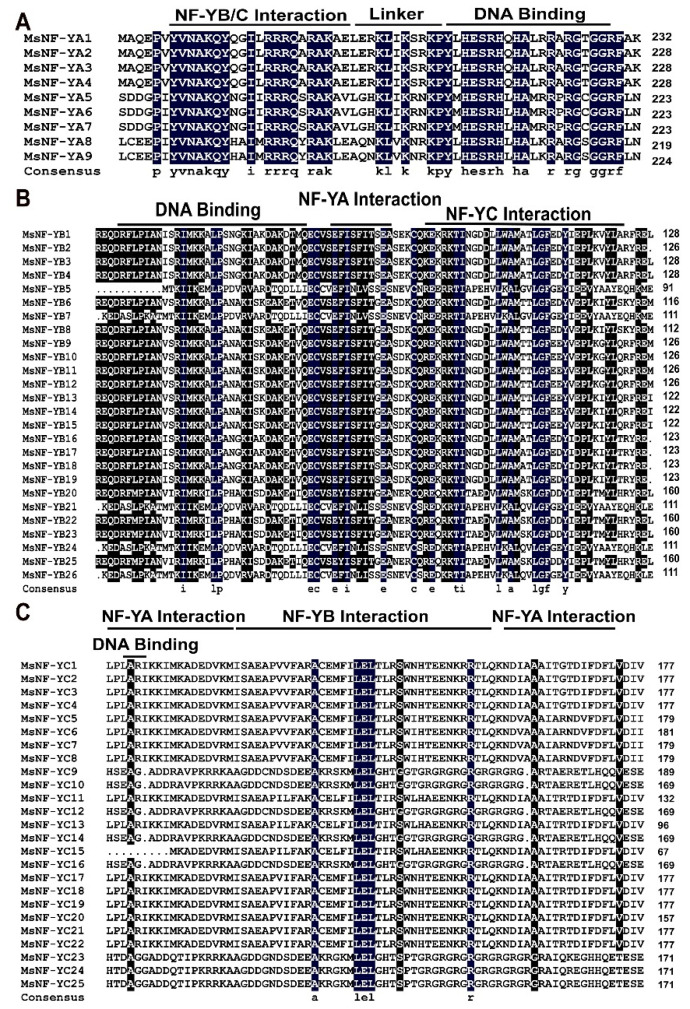
To analyze the conserved domains in the different NF-Y subfamilies, multiple alignments were conducted by using DNAMAN software. The results showed that there were a few conserved domains in the MsNF-Y proteins, indicating that these proteins belong to the same subgroups (Figure 2). For example, the MsNF-YA proteins have conserved domains, including one domain for the protein–protein interaction of NF-YB/C and another for the DNA-binding domain (Figure 2A). The MsNF-YB proteins have one domain for DNA binding and another domain for interacting with NF-YA and NF-YC proteins (Figure 2B). Among the MsNF-YC proteins, the results are the same (one domain for DNA binding and another domain for the interaction of NF-YA/B) but with lower conservation compared with that in the MsNF-YA and MsNF-YB subfamilies, implying a probably more abundant functional role in alfalfa (Figure 2C).
Figure 2.
Conserved domain alignments of MsNF-Y members. (A) MsNF-YA proteins; (B) MsNF-YB proteins; (C) MsNF-YC proteins.
2.3. Phylogenetic Relationships and Gene Structure of the MsNF-Y Family Members
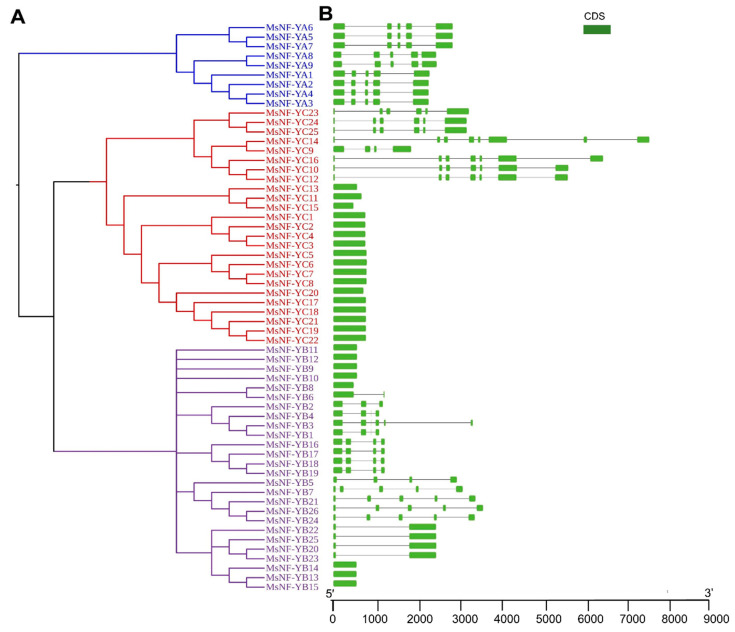
To understand the phylogenetic relationships among the MsNF-Ys, phylogenetic trees were constructed by using MEGA 5.0 software based on the protein sequences. The results showed that the neighbor-joining tree was divided into three branches corresponding to three different subfamilies (MsNF-YAs, MsNF-YBs, and MsNF-YCs) (Figure 3A). In the MsNF-YA subfamily, there are three major groups. One group contains MsNF-YA6, MsNF-YA5, and MsNF-YA7, another group contains MsNF-YA8 and MsNF-YA9, and the third group contains MsNF-YA1, MsNF-YA2, MsNF-YA3, and MsNF-YA4. In the MsNF-YB subfamily, we found that MsNF-YB11, MsNF-YB12, MsNF-YB9, and MsNF-YB10 were relatively independent of the five groups formed by the other MsNF-YBs. In the MsNF-YC subfamily, there are two major groups. The first group contains MsNF-YC23, MsNF-YC24, MsNF-YC25, MsNF-YC14, MsNF-YC9, MsNF-YC16, MsNF-YC10, and MsNF-YC12. The other MsNF-YCs formed the second group.
Figure 3.
Phylogenetic analysis and gene structure of MsNF-Y genes. (A) Phylogenetic tree of MsNF-Ys; (B) Gene structure of alfalfa NF-Y genes. The green box indicates exons, and the black lines indicate introns.
We used TBtools to analyze the gene structures of the MsNF-Ys. The results showed that all MsNF-YAs contained five exons and four introns (Figure 3B). Eight MsNF-YB genes contained only one exon, and five MsNF-YB genes contained two exons and one intron. The other MsNF-YBs contained three or more exons, and MsNF-YB3 harbored six exons and five introns (Figure 3B). Among the 25 MsNF-YC genes, 17 members contained only one exon, while MsNF-YC14, MsNF-YC16 (also including MsNF-YC10 and MsNF-YC12), MsNF-YC23 (also including MsNF-YC24 and MsNF-YC25), and MsNF-YC9 contained eight, seven, six, and four exons, respectively (Figure 3B). Based on the phylogenetic tree and gene structure, we found that the same tree groups had the same gene structure (Figure 3).
2.4. Genome Distribution and Gene Duplication Events of the MsNF-Ys in the Alfalfa Genome
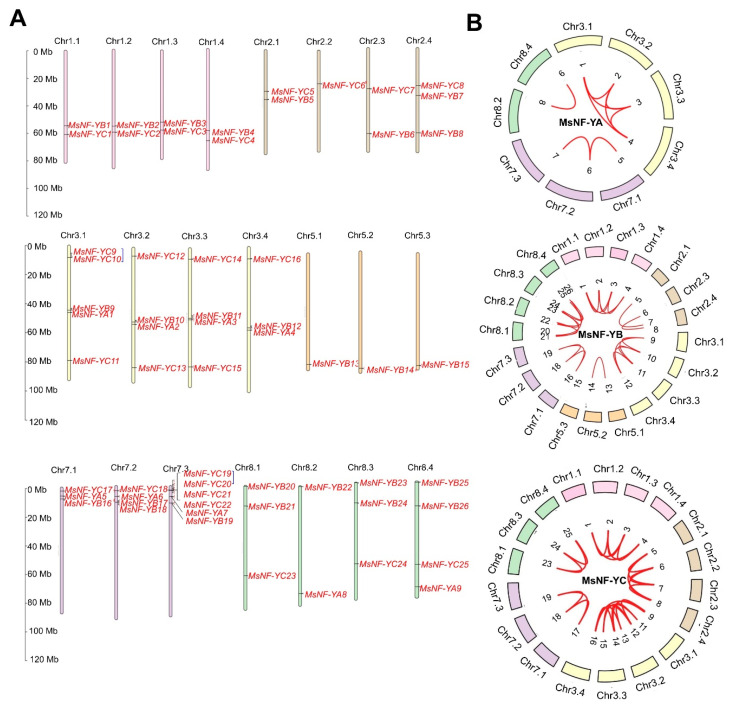
To show the genome distribution of the 60 MsNF-Y genes in the alfalfa genome, the TBtools program was used. The results showed that the 60 MsNF-Y genes were distributed unevenly on the 32 alfalfa chromosomes, except for chr4.1, chr4.2, chr4.3, chr4.4, chr5.4, chr6.1, chr6.2, chr6.3, chr6.4, and chr7.4 (Figure 4A). Six NF-Y genes were on chr7.3, while on the other chromosomes there were no more than five NF-Y genes.
Figure 4.
Genome locations and duplication analysis of 60 MsNF-Y genes in the alfalfa genome. (A) The physical locations of the 60 MsNF-Y genes. The blue lines connect the corresponding two pairs of paralogous genes in the same chromosome. (B) Duplication analysis of MsNF-Y genes on different chromosomes. From left to right are the NF-YA, NF-YB, and NF-YC subfamilies.
In total, 60 duplication events were found among these MsNF-Y genes in the alfalfa genome (Figure 4). Two duplication events (MsNF-YC9 and MsNF-YC10 on chr3.1 and MsNF-YC19 and MsNF-YC20 on chr7.3) were formed in the NF-YC genes on the same chromosome, and 58 duplication events were formed by different NF-Y genes on different chromosomes (Figure 4B and Table S2). Among the 58 duplication events, 9 duplication events were formed by the 9 MsNF-YA genes, 25 duplication events were formed by the 24 MsNF-YB genes, and 24 duplication events were formed by the 21 MsNF-YC genes. To understand the evolution type among these duplication events on different chromosomes, the Ka, Ks, and Ka/Ks values were calculated in this study. The results showed that among the 58 duplication events, 41 duplication events were estimated as purifying selection, 11 events were neutral evolution, and 6 events were positive selection (Table S2).
2.5. cis-Element Analysis in the Promoter Regions of MsNF-Y Genes
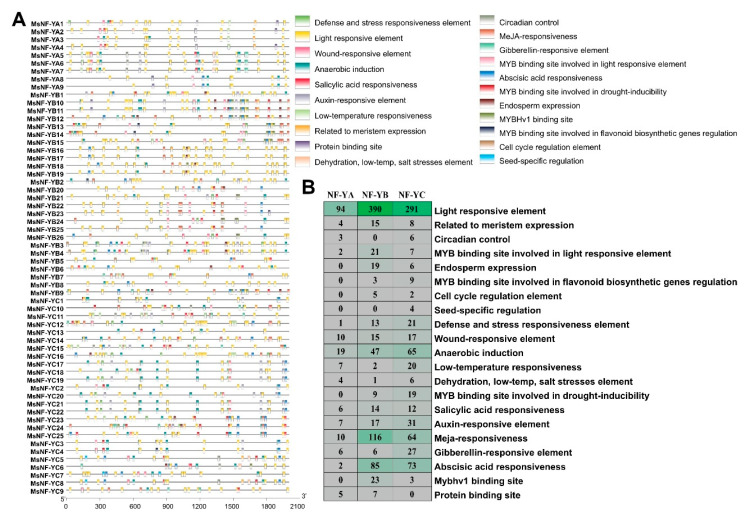
To clarify the transcriptional regulation of the MsNF-Y genes, the cis elements in the promoter regions were identified by using the PlantCare program. In total, 1679 cis elements belonging to 21 different categories were found in the promoter regions of the 60 MsNF-Y genes (Figure 5A). Light-responsive elements existed in the promoter regions of all MsNF-Ys, and there were 94, 390, and 291 light-responsive elements in MsNF-YA, MsNF-YB, and MsNF-YC, respectively (Figure 5B). In addition to light-responsive elements, MeJA-responsive elements are the second largest category in the promoter regions of MsNF-Ys. In total, 38 MsNF-Y genes (5 MsNF-YAs, 22 MsNF-YBs, and 11 MsNF-YCs) contained MeJA-responsive elements in the promoter regions (Figure 5B). In total, 160 abscisic acid response elements (ABREs) were found in the promoter regions of 51 MsNF-Y genes (2 MsNF-YAs, 25 MsNF-YBs, and 24 MsNF-YCs). There were 55 auxin-responsive elements and 39 gibberellin-responsive elements in the promoter regions of 29 and 27 MsNF-Y genes, respectively (Figure 5A). The promoter regions of 24, 11 and 21 MsNF-Y genes harbored MYB transcription-factor-binding sites related to drought inducibility, flavonoid biosynthetic gene regulation, and light-responsive elements, respectively. Many stress response elements were also found in the promoter regions of the MsNF-Ys (Figure 5B). The promoter regions of 19, 22, and 10 MsNF-Y genes contained 29 low temperature response elements (LTRs), 35 defense and stress responsiveness elements (TC-rich repeats), and 11 dehydration, low temperature, and salt stress elements (DREs), respectively. These results indicate that MsNF-Ys not only participate in the growth and development of alfalfa but also play a key role in various stresses.
Figure 5.
cis-element analysis of the 60 MsNF-Ys promoter regions. (A) The locations of these cis elements in the MsNF-Y promoter regions; the boxes with different colors indicate different cis elements. (B) Statistical analysis of different types of cis elements.
2.6. NF-Y Gene Evolutionary Analysis of Different Plant Species
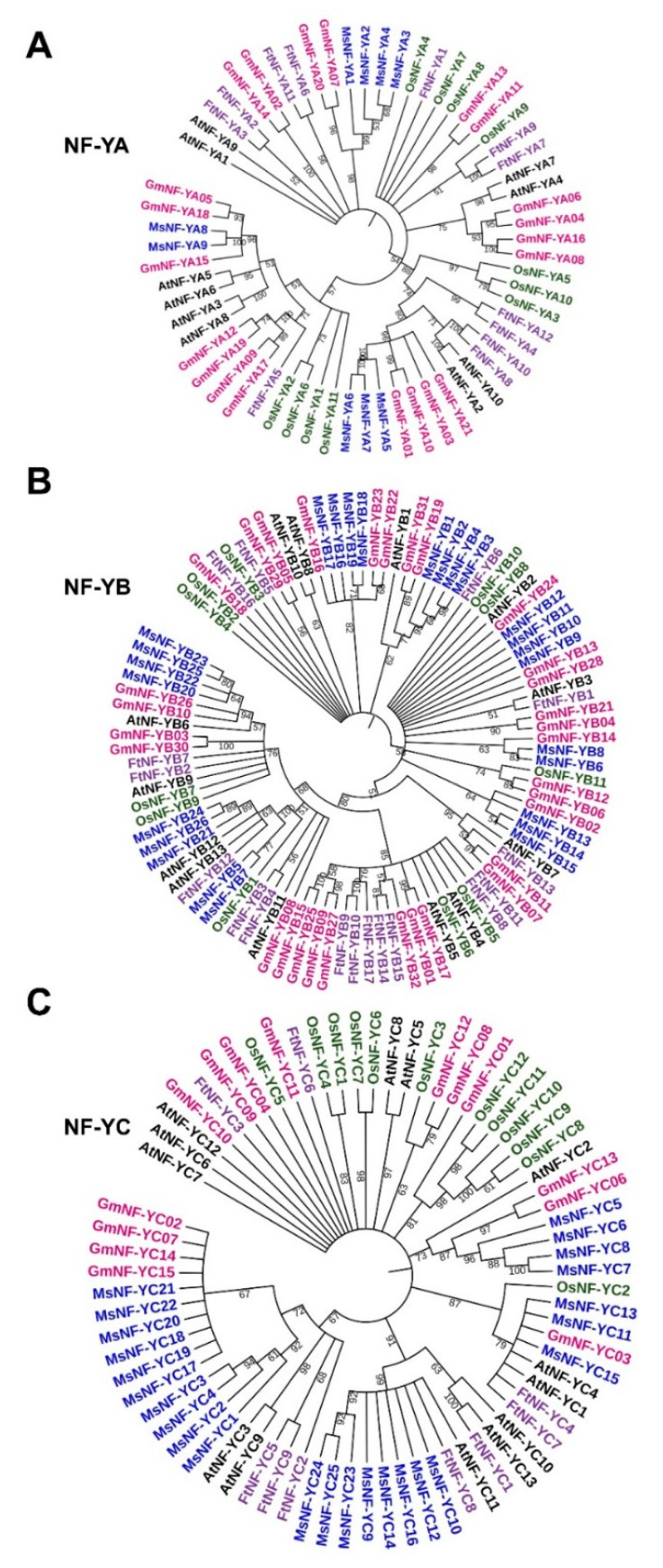
To understand the evolutionary relationships of the NF-Y gene in different plant species and predict the function of MsNF-Ys, the NF-Y protein sequences from rice, soybean, Arabidopsis, Tartary buckwheat, and alfalfa were collected (Table S1). Based on the phylogenetic analysis, the results showed that most MsNF-Y members were close to the homolog GmNF-Ys in the soybean genome (Figure 6). In the NF-YA subfamily, MsNF-YA1/2/3/4 were closer to GmNF-YA07/20, MsNF-YA8/9 were closer to GmNF-YA05/18, and MsNF-YA5/7/6 were closer to GmNF-YA01/10/03/21 (Figure 6A). In the NF-YB subfamily, MsNF-YB16/17/18/19 were closer to GmNF-YB16, MsNF-YB6/8 were closer to GmNF-YB14, and MsNF-YB20/22/23/25 were closer to GmNF-YB10/26 (Figure 6B). In the NF-YC subfamily, MsNF-YC5/6/7/8 were closer to GmNF-YC06/13. These results suggest that these alfalfa MsNF-Y genes may perform biological functions similar to those in soybean (Figure 6). In addition, some MsNF-Y genes were closer to homologs in other plant species. For example, MsNF-YB21/24/26 were closer to FtNF-YB12 from Tartary buckwheat and AtNF-YB12/13 from Arabidopsis. These results provide valuable information for further functional analysis of these MsNF-Ys.
Figure 6.
Phylogenetic relationship of NF-Y proteins from alfalfa, Arabidopsis, rice, soybean, and Tartary buckwheat. (A) NF-YA subfamily. (B) NF-YB subfamily. (C) NF-YC subfamily.
2.7. Expression Analysis of MsNF-Y Genes in Different Tissues from Alfalfa
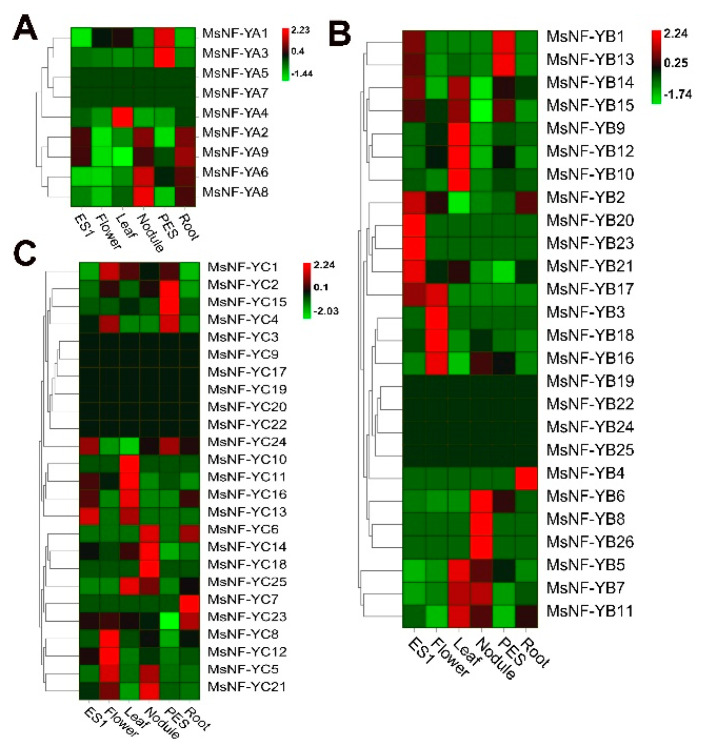
To investigate the expression pattern of these MsNF-Y genes, the global gene expression in different tissues (root, flower, nodule, leaf, ES1, and PES) of alfalfa was collected and analyzed. The results showed that the 60 MsNF-Y genes had significantly different expression patterns and indicated that these genes played different roles in alfalfa development (Figure 7). Among the nine MsNF-YA genes, two MsNF-YAs (MsNF-YA5 and MsNF-YA7) were not expressed in the six tissues in this study. MsNF-YA1 and MsNF-YA3 exhibited high expression levels in the PES, and MsNF-YA4 had a higher expression level in the leaf. MsNF-YA2/9/6/8 had a similar expression pattern and were mainly expressed in the roots and nodules (Figure 7A). Among the twenty-six MsNF-YB genes, four genes (MsNF-YB19/22/24/25) were not expressed in these tissues (Figure 7B). MsNF-YB4 was only expressed in the roots, and MsNF-YB6/8/26 were only expressed in the nodules. Based on the expression patterns, these MsNF-YB genes were clustered into different groups. MsNF-YB1/MsNF-YB13 were mainly expressed in ES1 and PES, and MsNF-YB14/15 were mainly expressed in ES1, PES, and leaves. MsNF-YB9/12/10 were mainly expressed in the leaf, while MsNF-YB17/3/18/16 were mainly expressed in the flower, and MsNF-YB6/8/26 were mainly expressed in the nodule (Figure 7B). Among the twenty-five MsNF-YC genes, six genes (MsNF-YC3, MsNF-YC9, MsNF-YC17, MsNF-YC19, MsNF-YC20, MsNF-YC22, and MsNF-YC24) were not expressed in these tissues (Figure 7C). MsNF-YC7 was only expressed in the root, while MsNF-YC10 is only expressed in the leaf. The other MsNF-YC genes were expressed in at least two different tissues (Figure 7C). These results indicate that these MsNF-Y genes perform different functions in alfalfa growth and development.
Figure 7.
Expression pattern analysis of the 60 MsNF-Y genes in various alfalfa tissues (ES1, elongated stem; flower; leaf; nodule; PES, preelongated stem; and root). (A) MsNF-YA genes. (B) MsNF-YB genes. (C) MsNF-YC genes.
2.8. Expression Analysis of MsNF-Y Genes in Alfalfa under Different Abiotic Stresses
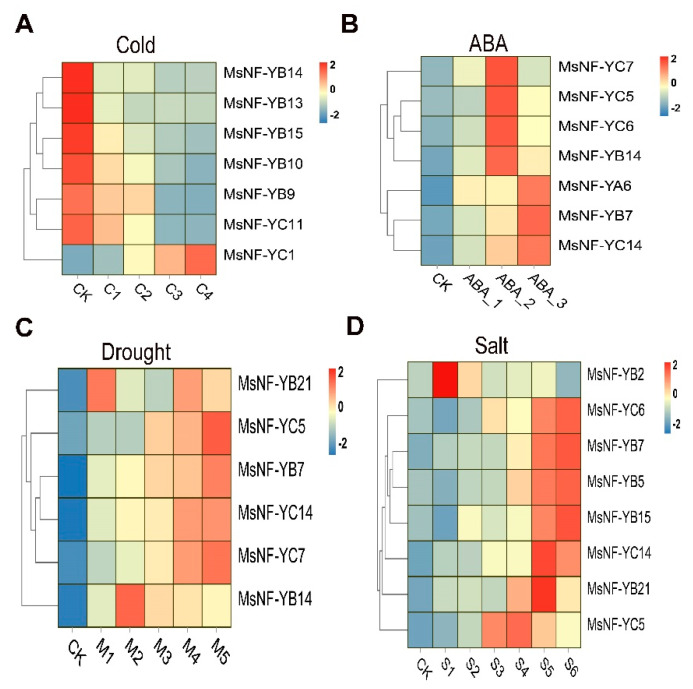
To clarify the role of the MsNF-Y genes under different abiotic stresses, we collected transcriptome data under cold, ABA, drought, and salt stress. The results showed that multiple MsNF-Y genes are involved in different abiotic stress processes (Figure 8). The expression levels of seven MsNF-Y genes (five MsNF-YB genes and two MsNF-YC genes) significantly changed under cold stress (Figure 8A). Among the seven genes, the expression levels of six genes decreased as the cold stress treatment time increased, and only one gene (MsNF-YC1) showed an increase as the cold stress treatment time increased (Figure 8A). Under the ABA treatment, the expression levels of seven genes (one MsNF-YA gene, two MsNF-YB genes, and four MsNF-YC genes) were significantly upregulated (Figure 8B). The expression levels of six MsNF-Y genes (three MsNF-YB genes and three MsNF-YC genes) were significantly upregulated under drought stress (Figure 8C). MsNF-YB21 and MsNF-YB14 expression abundance was significantly upregulated in the early stage of drought stress. The gene expression levels of the other four genes (MsNF-YC5, MsNF-YB7, MsNF-YC14, and MsNF-YC7) were significantly upregulated in the later stage of drought stress. Under salt stress, the expression levels of eight MsNF-Y genes (five MsNF-YB genes and three MsNF-YC genes) were significantly upregulated (Figure 8D). MsNF-YB2 gene expression was significantly upregulated in the early stage of salt stress. MsNF-YC5 and MsNF-YB21 gene expression first increased and then decreased as the salt treatment time increased. The expression levels of the other five genes were significantly upregulated in the later stage of salt stress (Figure 8D).
Figure 8.
Candidate MsNF-Y genes responsible for different abiotic stresses. (A) MsNF-Y genes involved in the cold stress response. (B) MsNF-Y genes involved in the ABA treatment response. (C) MsNF-Y genes involved in the drought response. (D) MsNF-Y genes involved in the salt stress response.
Combining these different abiotic stress results, we also found multiple MsNF-Y genes involved in two or more stresses. MsNF-YB14 responds to both cold and drought stresses, MsNF-YB15 responds to both cold and salt stresses, and MsNF-YB7, MsNF-YC5, and MsNF-YC14 respond to both drought and salt stresses.
2.9. Important Candidate MsNF-Y Genes in Salt Stress
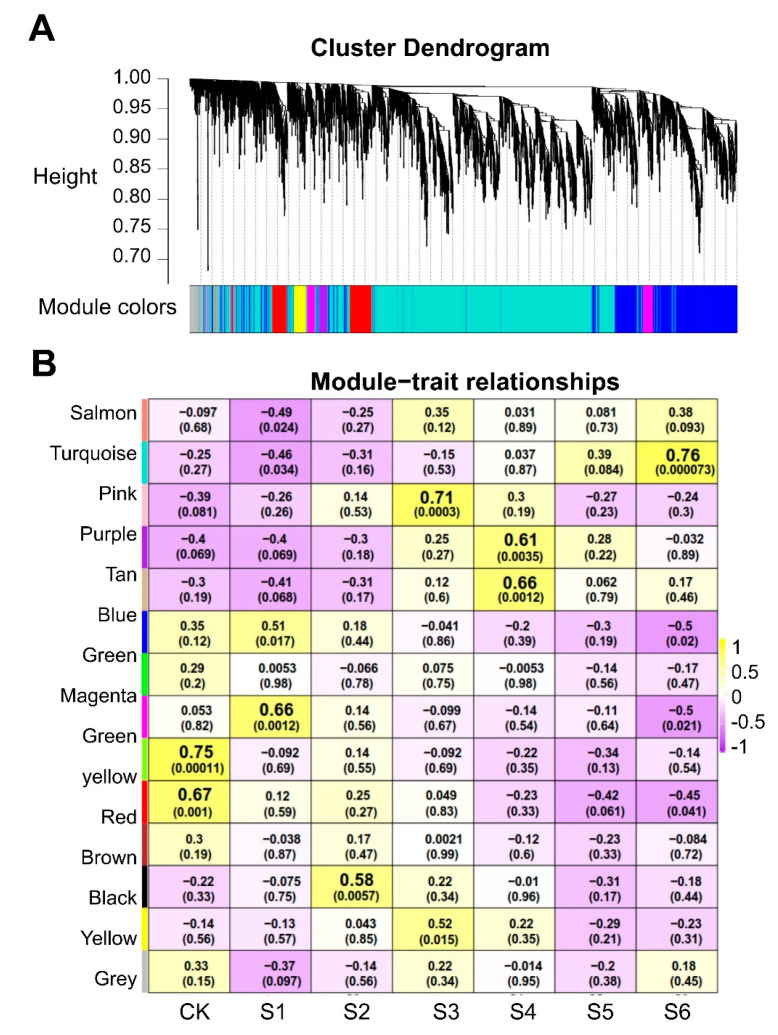
To further identify candidate genes among the eight differentially expressed MsNF-Y genes in salt stress, a weighted gene coexpression network analysis (WGCNA) was conducted in this study. The results showed that these genes were divided into 14 coexpression modules (Figure 9A). The correlation analysis between the modules and the salt stress treatment time revealed that a few modules were significantly correlated with salt stress (Figure 9B). The magenta and black modules were significantly correlated with the early stage (Tables S1 and S2) of salt stress (R = 0.66, p = 1.2 × 10−3; R = 0.58, p = 5.7 × 10−3). The pink, purple, and tan modules were significantly correlated with the middle stage (Tables S3 and S4) of salt stress (R = 0.71, p = 3.0 × 10−4; R = 0.61, p = 3.5 × 10−3; R = 0.66, p = 1.2 × 10−3). The turquoise module had a higher correlation with the later stage of salt stress (R = 0.76, p = 7.3 × 10−5).
Figure 9.
Weighted correlation network analysis (WGCNA) coexpression network and module-trait correlation analysis under salt stress. (A) Hierarchical cluster tree showing the coexpression modules. Different colors indicate different modules. (B) Correlation analysis between different modules and salt stress duration. The top number in the cell represents the correlation coefficient and the bottom number indicates the p value.
Among these significant modules, six MsNF-Y genes were included. MsNF-YB2, which responds to salt stress at the early stage, was found in the magenta module; MsNF-YC5, which responds to salt stress at the middle stage, was found in the purple module; and the other four MsNF-Y genes (MsNF-YB5, MsNF-YB7, MsNF-YB15, and MsNF-YC6) were located in the turquoise module.
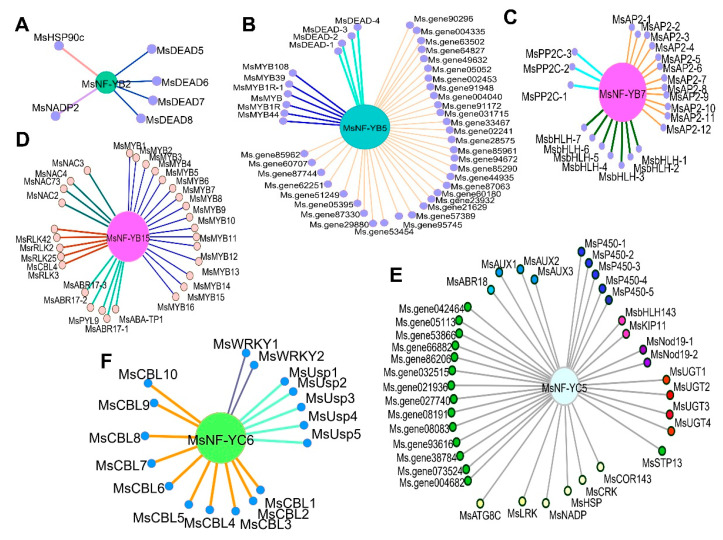
To further understand the coexpression network of the six MsNF-Y genes, the genes that were synergistically expressed under salt stress were identified. The results showed that MsNF-YB2 was mainly coexpressed with the DEAD genes under salt stress at the early stage (Figure 10A and Table S3). Regarding MsNF-YB5, there were 43 coexpressed genes, including 33 protein kinase genes, 6 MYB transcription factors and 4 DEAD genes (Figure 10B and Table S4). In total, 22 coexpressed genes containing 12 AP2 domain genes, 7 bHLH transcription factors, and 3 PP2C genes with MsNF-YB7 were found (Figure 10C and Table S5). Regarding MsNF-YB15, there were 30 coexpressed genes, which were mainly MYB transcription factors (Figure 10D and Table S6). In total, 38 genes were found to be coexpressed with MsNF-YC5 (Figure 10E and Table S7). Regarding MsNF-YC6, 17 genes were coexpressed. Among them, 10 genes encode CBL-interacting serine/threonine-protein kinases, indicating that MsNF-YC6 may be involved in the Ca2+ signaling process under salt stress (Figure 10F and Table S8). These results provide information for a regulatory network analysis of these MsNF-Y genes.
Figure 10.
Coexpression network of the six MsNF-Y genes under salt stress. (A) The network of MsNF-YB2, (B) MsNF-YB5, (C) MsNF-YB7, (D) MsNF-YB15, (E) MsNF-YC5, and (F) MsNF-YC6.
2.10. RT-qPCR Analysis of MsNF-Y Genes under Salt Conditions
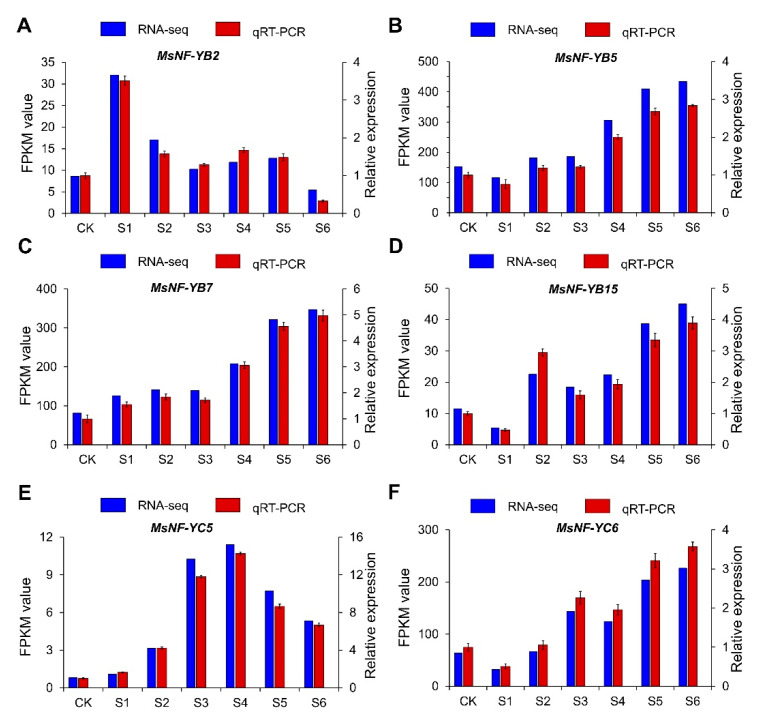
To confirm the salt-induced expression profiles of six MsNF-Y genes, a RT-qPCR was further applied. As shown in Figure 11, the expression profiles of the candidate genes were consistent with RNA-Seq results. The expression of MsNF-YB2 was significantly up-regulated at S1 stage and then decreased gradually. The expressions of MsNF-YB5, MsNF-YB7, MsNF-YB15, and MsNF-YC6 were increased gradually after salt treatment and reached the highest at S6 stage. The expression of MsNF-YC5 was comparatively high at S4 and S5 stage. The RT-qPCR results verified the results of RNA-Seq and indicated that these six MsNF-Y genes identified by our analyses were important candidate genes and may play roles in response to salt stress in alfalfa.
Figure 11.
qRT-PCR analysis for genes related salt stress. (A) MsNF-YB2, (B) MsNF-YB5, (C) MsNF-YB7, (D) MsNF-YB15, (E) MsNF-YC5, and (F) MsNF-YC6.
3. Discussion
As a gene family important for plant growth and development and abiotic stress resistance, NF-Y transcription factors have been identified and functionally analyzed genome-wide in many plant species. For example, 36 NF-Y gene members (10 NF-YAs, 13 NF-YBs, and 13 NF-YCs) were identified in Arabidopsis [25], 78 NF-Y gene members were identified in soybean (21 NF-Yas, 32 NF-Ybs, and 25 NF-YCs) [19], 34 NF-Y gene members were identified in rice (11 NF-YAs, 11 NF-YBs, and 12 NF-YCs) [26], and 50 NF-Y gene members were identified in maize (14 NF-Yas, 18 NF-Ybs, and 18 NF-YCs) [27]. However, because alfalfa is autotetraploid, genome assembly is difficult, and it was not until 2020 that the alfalfa reference genome was assembled successfully [1]. In this study, in total, 60 MsNF-Y members (9 NF-YAs, 26 NF-YBs, and 25 NF-YCs) were identified in the alfalfa reference genome, and their gene structures, sequence features, conserved domains, and expression patterns were systematically analyzed, particularly the expression changes of MsNF-Ys under different abiotic stresses. These findings provide valuable information for a subsequent functional analysis and molecular regulatory network construction of a single MsNF-Y gene.
Many studies provide evidence suggesting that NF-Y transcription factors are widely involved in plant flowering time regulation and seed development. The overexpression of AtNF-YB1 in Arabidopsis resulted in delayed flowering in plants [28], while a phylogenetic analysis found that MsNF-YB1/2/3/4 had high homology with AtNF-YB1 (Figure 6B). Furthermore, through an expression pattern analysis, MsNF-YB3 was found to be specifically expressed in flowers (Figure 7B); thus, we inferred that MsNF-YB3 might be an important candidate gene for flowering time regulation in alfalfa. AtNF-YB9 regulates seed development by integrating light signals with hormonal signals, and the homologous gene AtNF-YB6 is involved in the morphogenesis of seed embryos by mediating the ABA signaling pathway [28]. In the present study, we found that MsNF-YB20/22/23/25 and AtNF-YB6/9 were clustered in one evolutionary branch (Figure 6B); therefore, the four MsNF-YB genes might also be involved in the seed development process in alfalfa.
The NF-Y transcription factor is not only involved in plant growth and development but also plays an important role in plant abiotic stress [4]. The overexpression of AtNF-YB1 and ZmNF-YB2 in Arabidopsis and maize, respectively, significantly improved drought resistance in the transgenic lines [10,28]. Similarly, the overexpression of soybean GmNF-YA3 or poplar PdNF-YB7 in Arabidopsis can reduce leaf water loss and improve plant drought resistance [29,30]. The overexpression of AtNF-YA1 can significantly improve the resistance of Arabidopsis to salt stress [28]. GmNF-YA16, GmNF-YB2, GmNF-YC13, and GmNF-YC14 in soybean can significantly enhance the resistance of plants to drought and salt stress [19,22,29]. In this study, MsNF-YB14 and MsNF-YB15 were homologous genes of GmNF-YB2, while MsNF-YC5 and MsNF-YC6 were homologous genes of GmNF-YC13 (Figure 6). Their expression was induced by drought and salt stress (Figure 8). Therefore, MsNF-YB14/15 and MsNF-YC5/6 may also perform the function of regulating alfalfa resistance to drought and salt stress.
NF-Y transcription factors regulate drought and salt stress in plants by mediating multiple signaling and plant hormone pathways. The regulatory pathways mainly include two types. One type mediates the ABA signaling pathway to regulate expression changes in downstream genes. For example, PdNF-YB21 can interact with PdFUS3 to jointly activate the expression of downstream PdNCED3 genes, thereby promoting the synthesis of ABA and ultimately improving drought tolerance in poplars [31]. In soybean, GmNF-YC14 can interact with GmNF-YB2 and GmNF-YA16 to form a complete, active NF-Y transcriptional complex, and this complex can regulate the ABA signaling pathway mediated by the ABA receptor PYR/PYL to enhance drought resistance and salt tolerance in soybeans [22]. WGCNA, a method commonly used to identify coexpression regulatory networks, has been shown to be highly effective in many studies [32]. In this study, we used WGCNA and found that MsNF-YB2 was coexpressed with four DEAD genes in the ABA signaling pathway under salt stress, while MsNF-YB7 was coexpressed with three PP2C genes in the ABA pathway under salt stress (Figure 10). Another regulatory pathway of NF-Y genes alters the stress resistance ability of plants by mediating independent ABA pathway genes. After overexpressing AtNF-YB1 in Arabidopsis, although drought resistance was improved in the transgenic lines, there was no significant change in drought resistance genes related to the ABA pathway, suggesting that NF-Y family members exist in a drought resistance pathway independent of the ABA signaling pathway [10,28]. In this study, many genes independent of the ABA pathway were found to be coexpressed with the MsNF-Y genes under drought and salt stresses. For example, 6 MYB transcription factors were coexpressed with MsNF-YB5, 16 MYB transcription factors were coexpressed with MsNF-YB15, and 10 CBL genes were coexpressed with the MsNF-YC6 under salt stress (Figure 10). These results could greatly enhance our understanding of the molecular regulatory network of MsNF-Y genes in the future, especially under abiotic stress.
In summary, we provide genome-wide results of the alfalfa NF-Y genes and expression patterns and coexpression regulatory networks in different tissues under different abiotic stresses. The information described here could contribute to further studies investigating alfalfa NF-Y gene families, especially in the context of abiotic stress.
4. Materials and Methods
4.1. Identification of NF-Y Genes in the Alfalfa Genome
The reference genome of Xinjiangdaye was obtained from https://figshare.com/ (accessed on 14 November 2021). The HMMER3.0 program was used to identify the NF-Y gene family members in alfalfa based on e < 10−10 [33]. The protein sequences and the conserved domains of the NF-Y subfamilies were collected as described in Siefers et al. [25]. The candidate NF-Y gene family members in alfalfa were screened via CD-search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 22 December 2021) by default to analyze the presence of the conserved domains. The molecular weight (MW) and isoelectric point (pI) were analyzed by ExPASy (https://web.expasy.org, accessed on 29 December 2021).
4.2. Motif Analysis and Sequence Alignments
A motif analysis was conducted with the online program MEME (https://meme-suite.org/, accessed on 10 January 2022) by using the protein sequences of these NF-Y gene family members [34]. The gene structure was obtained by using TBtools software [35]. DNAMAN software was used to conduct the multiple sequence alignments.
4.3. Phylogenetic Tree Construction, Genome Localization and Gene Duplication
The NF-Y gene family sequences from Arabidopsis, rice, soybean, and Tartary buckwheat were collected from previous studies [21,24,25,26]. The neighbor-joining method with 1000 replicated bootstrap values was used to conduct a phylogenetic analysis by using MEGA 7.0 software based on the protein sequences. A duplication event analysis, including the parameters Ka and Ks, and a genome localization analysis were conducted by TBtools [35].
4.4. cis-Element Analysis
The 2 kb sequences upstream of ATG, which is the translation start codon of all NF-Y gene family members, were downloaded from the Xinjiangdaye reference genome by using TBtools [35]. Then, the cis elements of these sequences were analyzed using the online program PlantCARE, http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 8 February 2022) [36].
4.5. Transcription Data Analysis
Transcription data in different tissues (ES1, elongated stem; flower; leaf; nodule; PES, pre-elongated stem; root) were downloaded from the public NCBI database. The reads can be downloaded from the NCBI short read archive database under accession SRP055547 [37]. The RNA-Seq data of alfalfa under different abiotic stresses were available in the NCBI short read archive database (cold, accession SRR7091780-SRR7091794; drought, salt, and ABA, accession SRR7160313-SRR7160357) [38]. Here, the alfalfa accession Zhongmu No. 1 was planted in a plant growth chamber that was maintained at 16 h/8 h (light/dark) and 22 °C for 10 days. For cold stress, the seedlings were maintained at 4 °C for 0 h (CK), 2 h (C1), 6 h (C2), 24 (C3), and 48 h (C4) [38]. For the ABA treatment, 10 µΜ ABA in 1/2 MS nutrient solution were used. The root tips were collected at 0 h (CK), 1 h (ABA_1), 3 h (ABA_2), and 12 h (ABA_3) after the ABA treatment [38]. For drought stress, 400 mM mannitol were used in 1/2 MS nutrient solution. The root tips were collected at 0 h (CK), 1 h (M1), 3 h (M2), 6 h (M3), 12 h (M4), and 24 h (M5) [38]. For salt stress, 250 mM NaCl were used in a 1/2 MS nutrient solution. The root tips were collected at 0 h (CK), 0.5 h (S1), 1 h (S2), 3 h (S3), 6 h (S4), 12 h (S5), and 24 h (S6) [38]. The clean reads were mapped to the “Xinjiangdaye” genome by using TopHat2 software [39]. The gene expression level was estimated by using the FPKM value (fragments per kilobase of transcript per million reads mapped). The DEGs were obtained by using DESeq software based on padj < 0.05 and |log2FC| ≥ 1 [40].
4.6. Weighted Gene Coexpression Network Construction
We analyzed and constructed the weighted gene coexpression network (WGCNA) of alfalfa under salt stress using TBtools with the default parameters [32,35]. First, using transcriptome data (salt stress) collected at seven different time points at 0 h (CK), 0.5 h (S1), 1 h (S2), 3 h (S3), 6 h (S4), 12 h (S5), and 24 h (S6), all expressed genes were filtered based on FPKM values (more than 1) for the subsequent analysis. Then, the correlation between different modules and the salt stress treatment time was obtained using a cluster analysis and correlation analysis. Finally, coexpression network drawing was performed using Cytoscape software [41].
4.7. Expression Analysis by qRT-PCR
To further verify the expression of candidate genes under salt stress, we collected the root tips of Zhongmu No.1 under NaCl treatment at 0 h (CK), 0.5 h (S1), 1 h (S2), 3 h (S3), 6 h (S4), 12 h (S5), and 24 h (S6) according to a previous study [38]. All the samples were three biological repetitions. Total RNAs were extracted using an RNAprep Pure Plant Kit (Tiangen Biotech) and cDNAs were prepared using Tran-Script-Uni One-Step gDNA Removal and cDNA Synthesis SuperMix (TRAN). Then, we performed qPCR using a SYBR Green RT-PCR Kit (Takara). The primers were designed by Primer Premier 5 software, and the primer sequences were presented in Table S9. The qRT-PCR program was: 95 °C for 30 s; 40 cycles of 95 °C for 5 s; 58 °C for 34 s; and 95 °C for 15 s. MsActin was used to as the internal reference gene for data normalization analysis. For each analysis, three technical replicates from three biological replicates were conducted. Ultimately, we calculated the relative expression of MsNF-Ys by the 2−∆∆Ct method.
5. Conclusions
In our study, in total, 60 MsNF-Y genes, including 9 MsNF-YAs, 26 MsNF-YBs, and 25 MsNF-YCs, were identified in the alfalfa reference genome, and conserved domain, gene structure, genomic location, cis element, and expression pattern analyses were conducted. The expression analysis showed that 48 MsNF-Y genes were expressed in all tissues at different levels, showing that these MsNF-Y genes play an important role in alfalfa growth and development. In addition, we analyzed the expression of MsNF-Y genes under different abiotic stresses (cold, drought, and salt). The results indicated that a few MsNF-Y genes were involved in these abiotic stresses. Finally, the co-expression networks under salt stress of MsNF-YB2, MsNF-YB5, MsNF-YB7, MsNF-YB15, MsNF-YC5, and MsNF-YC6 were constructed by using a WGCNA. In future studies, these candidate genes should be over-expressed or gene knocked out in alfalfa to verify their biological functions and the yeast two hybrid system or Chip-seq should be used to identify the interaction network for these candidate genes. Overall, our results could provide valuable information for further elucidating the biological functions of MsNF-Ys and improving salt tolerance and other abiotic stress resistance in alfalfa.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms23126426/s1.
Author Contributions
Y.A.: data analysis and original draft preparation; X.S.: figures and tables preparation; Q.N.: data validation; S.Y.: data validation and manuscript review and editing; L.C.: software and data curation. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the China Postdoctoral Science Foundation (2021M700452) and the Joint Funds of the National Natural Science Foundation of China (Grant No. U20A2005).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen H., Zeng Y., Yang Y., Huang L., Tang B., Zhang H., Hao F., Liu W., Li Y., Liu Y., et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020;11:2494. doi: 10.1038/s41467-020-16338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L., He F., Long R., Zhang F., Li M., Wang Z., Kang J., Yang Q. A global alfalfa diversity panel reveals genomic selection signatures in Chinese varieties and genomic associations with root development. J. Integr. Plant Biol. 2021;63:1937–1951. doi: 10.1111/jipb.13172. [DOI] [PubMed] [Google Scholar]
- 3.He F., Wei C., Zhang Y., Long R., Li M., Wang Z., Yang Q., Kang J., Chen L. Genome-wide association analysis coupled with transcriptome analysis reveals candidate genes related to salt stress in alfalfa (Medicago sativa L.) Front. Plant Sci. 2022;12:826584. doi: 10.3389/fpls.2021.826584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers Z.A., Holt B.F. NUCLEAR FACTOR-Y: Still Complex after All These Years? Curr. Opin. Plant Biol. 2018;45:96–102. doi: 10.1016/j.pbi.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Nardone V., Chaves-Sanjuan A., Nardini M. Structural determinants for NF-Y/DNA interaction at the CCAAT box. Biochim. Biophys. Acta Gene Regul. Mech. 2017;1860:571–580. doi: 10.1016/j.bbagrm.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Kahle J., Baake M., Doenecke D., Albig W. Subunits of the heterotrimeric transcription factor NF-Y are imported into the nucleus by distinct pathways involving importin β and importin 13. Mol. Cell Biol. 2005;25:5339–5354. doi: 10.1128/MCB.25.13.5339-5354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang M., Hu Y., Liu X., Li Y., Hou X. Arabidopsis LEAFY COTYLEDON1 mediates postembryonic development via interacting with PHYTOCHROME-INTERACTING FACTOR4. Plant Cell. 2015;27:3099–3111. doi: 10.1105/tpc.15.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niu B.X., Deng H., Li T., Sharma S., Yun Q., Li Q., Zhiguo E., Chen C. OsbZIP76 interacts with OsNF-YBs and regulates endosperm cellularization in rice (Oryza sativa) J. Integr. Plant Biol. 2020;62:1983–1996. doi: 10.1111/jipb.12989. [DOI] [PubMed] [Google Scholar]
- 9.Das S., Parida S.K., Agarwal P., Tyagi A.K. Transcription factor OsNF-YB9 regulates reproductive growth and development in rice. Planta. 2019;250:1849–1865. doi: 10.1007/s00425-019-03268-2. [DOI] [PubMed] [Google Scholar]
- 10.Nelson D.E., Repetti P.P., Adams T.R., Creelman R.A., Wu J., Warner D.C., Anstrom D.C., Bensen R.J., Castiglioni P.P., Donnarummo M.G., et al. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. USA. 2007;104:16450–16455. doi: 10.1073/pnas.0707193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu B., Zhang Z., Zhang J., Zhou Y., Chen C. The rice LEC1-like transcription factor OsNF-YB9 interacts with SPK, an endosperm-specific sucrose synthase protein kinase, and functions in seed development. Plant J. 2021;106:1233–1246. doi: 10.1111/tpj.15230. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Li G., Li C., Zhang C., Cui L., Ai G., Wang X., Zheng F., Zhang D., Larkin R.M., et al. NF-Y plays essential roles in flavonoid biosynthesis by modulating histone modifications in tomato. New Phytol. 2021;229:3237–3252. doi: 10.1111/nph.17112. [DOI] [PubMed] [Google Scholar]
- 13.Braybrook S.A., Harada J.J. LECs go crazy in embryo development. Trends Plant Sci. 2008;13:624–630. doi: 10.1016/j.tplants.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Lotan T., Ohto M., Yee K.M., West M.A., Lo R., Kwong R.W., Yamagishi K., Fischer R.L., Goldberg R.B., Harada J.J. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93:1195–1205. doi: 10.1016/S0092-8674(00)81463-4. [DOI] [PubMed] [Google Scholar]
- 15.Kwong R.W., Bui A.Q., Lee B.H., Kwong L.W., Fischer R.L., Goldberg R.B., Harada J.J. LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell. 2003;15:5–18. doi: 10.1105/tpc.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan H., Liu C., Zhao J., Ye X., Wu Q., Yao T., Peng L., Zou L., Zhao G. Genome-wide analysis of the NF-Y gene family and their roles in relation to fruit development in Tartary buckwheat (Fagopyrum tataricum) Int. J. Biol. Macromol. 2021;190:487–498. doi: 10.1016/j.ijbiomac.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Li W.X., Oono Y., Zhu J., He X.J., Wu J.M., Iida K., Lu X.Y., Cui X., Jin H., Zhu J.K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and post-transcriptionally to promote drought resistance. Plant Cell. 2008;8:2238–2251. doi: 10.1105/tpc.108.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X.J., Yu T.F., Li X.H., Cao X.Y., Ma J., Chen J., Zhou Y.B., Chen M., Ma Y.Z., Zhang J.H., et al. Overexpression of GmNFYA5 confers drought tolerance to transgenic Arabidopsis and soybean plants. BMC Plant Biol. 2020;20:123. doi: 10.1186/s12870-020-02337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quach T.N., Nguyen H.T.M., Valliyodan B., Joshi T., Xu D., Nguyen H.T. Genome-wide expression analysis of soybean NF-Y genes reveals potential function in development and drought response. Mol. Genet. Genom. 2015;290:1095–1115. doi: 10.1007/s00438-014-0978-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato H., Mizoi J., Tanaka H., Maruyama K., Qin F., Osakabe Y., Morimoto K., Ohori T., Kusakabe K., Nagata M., et al. Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with NF-Y subunits. Plant Cell. 2014;26:4954–4973. doi: 10.1105/tpc.114.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B., Li Z., Ran Q., Li P., Peng Z., Zhang J. ZmNF-YB16 overexpression improves drought resistance and yield by enhancing photosynthesis and the antioxidant capacity of maize plants. Front. Plant Sci. 2018;9:709. doi: 10.3389/fpls.2018.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu T.F., Liu Y., Fu J.D., Ma J., Fang Z.W., Chen J., Zheng L., Lu Z.W., Zhou Y.B., Chen M., et al. The NF-Y-PYR module integrates the abscisic acid signal pathway to regulate plant stress tolerance. Plant Biotechnol. J. 2021;19:2589–2605. doi: 10.1111/pbi.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren C., Zhang Z., Wang Y., Li S., Liang Z. Genome-wide identification and characterization of the NF-Y gene family in grape (Vitis vinifera L.) BMC Genom. 2016;17:605. doi: 10.1186/s12864-016-2989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Xu W., Chen Z., Han B., Haque M.E., Liu A. Gene structure, expression pattern and interaction of Nuclear Factor-Y family in castor bean (Ricinus communis) Planta. 2018;247:559–572. doi: 10.1007/s00425-017-2809-2. [DOI] [PubMed] [Google Scholar]
- 25.Siefers N., Dang K.K., Kumimoto R.W., Bynum W.E., 4th, Tayrose G., Holt B.F., 3rd Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 2009;149:625–641. doi: 10.1104/pp.108.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thirumurugan T., Ito Y., Kubo T., Serizawa A., Kurata N. Identification, characterization and interaction of HAP family genes in rice. Mol. Genet. Genom. 2008;279:279–289. doi: 10.1007/s00438-007-0312-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Li X., Zhang C., Zou H., Wu Z. Isolation, structural analysis, and expression characteristics of the maize nuclear factor Y gene families. Biochem. Biophys. Res. Commun. 2016;478:752–758. doi: 10.1016/j.bbrc.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H., Wu D., Kong F., Lin K., Zhang H., Li G. The Arabidopsis thaliana nuclear factor Y transcription factors. Front. Plant Sci. 2017;7:2045. doi: 10.3389/fpls.2016.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni Z., Hu Z., Jiang Q., Zhang H. GmNFYA3, a target gene of miR169, is a positive regulator of plant tolerance to drought stress. Plant Mol. Biol. 2013;82:113–129. doi: 10.1007/s11103-013-0040-5. [DOI] [PubMed] [Google Scholar]
- 30.Han X., Tang S., An Y., Zheng D.C., Xia X.L., Yin W.L. Overexpression of the poplar NF-YB7 transcription factor confers drought tolerance and improves water-use efficiency in Arabidopsis. J. Exp. Bot. 2013;64:4589–4601. doi: 10.1093/jxb/ert262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y., Zhang Y., Wang X., Han X., An Y., Lin S., Shen C., Wen J., Liu C., Yin W., et al. Root-specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in Populus. New Phytol. 2020;227:407–426. doi: 10.1111/nph.16524. [DOI] [PubMed] [Google Scholar]
- 32.Zhao N., Cui S., Li X., Liu B., Deng H., Liu Y., Hou M., Yang X., Mu G., Liu L. Transcriptome and co-expression network analyses reveal differential gene expression and pathways in response to severe drought stress in peanut (Arachis hypogaea L.) Front. Genet. 2021;12:672884. doi: 10.3389/fgene.2021.672884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potter S.C., Luciani A., Eddy S.R., Park Y., Lopez R., Finn R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018;46:200–204. doi: 10.1093/nar/gky448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey T.L., Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994;2:28–36. [PubMed] [Google Scholar]
- 35.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y., Van de Peer Y., Rouzé P., Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Rourke J.A., Fu F., Bucciarelli B., Yang S.S., Samac D.A., Lamb J.A.F.S., Monteros M.J., Graham M.A., Gronwald J.W., Krom N., et al. The Medicago sativa gene index 1.2: A web-accessible gene expression atlas for investigating expression differences between Medicago sativa subspecies. BMC Genom. 2015;16:502–518. doi: 10.1186/s12864-015-1718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong X., Deng H., Ma W., Qiang Z., Liu Z. Genome-wide identification of the MADS-box transcription factor family in autotetraploid cultivated alfalfa (Medicago sativa L.) and expression analysis under abiotic stress. BMC Genom. 2021;22:603. doi: 10.1186/s12864-021-07911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.