Abstract
Standard treatment for advanced non-small cell lung cancer (NSCLC) historically consisted of systemic cytotoxic chemotherapy until the early 2000s, when precision medicine led to a revolutionary change in the therapeutic scenario. The identification of oncogenic driver mutations in EGFR, ALK and ROS1 rearrangements identified a subset of patients who largely benefit from targeted agents. However, since the proportion of patients with druggable alterations represents a minority, the discovery of new potential driver mutations is still an urgent clinical need. We provide a comprehensive review of the emerging molecular targets in NSCLC and their applications in the advanced setting.
Keywords: NSCLC, MET, RET, NTRK, KRAS, HER2, oncogene-addiction, new targets, precision medicine
1. Introduction: Driver Alterations beyond EGFR, ALK and ROS1: What Do We Know So Far?
Lung cancer represents the leading cause of cancer death, with an estimate of 2.2 million new cases and 1.8 million deaths in 2020 (18% of the total) [1]. The 5-year survival rate from diagnosis is modest, ranging from 10 to 20% globally [2]. Non-small cell lung cancer (NSCLC) accounts for 85% of cancer diagnoses and includes adenocarcinoma (ADC) and squamous cell carcinoma (SCC) as the most frequent histological subtypes [3].
Over the past two decades, following the identification of new druggable targets, the treatment management of NSCLC has considerably changed. The improved understanding of the molecular pathways driving malignancy led to the development of new agents selectively directed to inhibit the signal transduction pathways of protein kinases.
To date, molecular characterization of tumor tissue is essential in defining the treatment strategy of advanced disease and could soon also become a standard of care in early stage, following recent evidence of a survival benefit of targeted agents also in the adjuvant setting [4].
The majority of lung cancer patients presents with advanced disease at diagnosis and oncogene-addiction is found by molecular characterization in less than 25% of cases [5]. Well-established actionable targets in adenocarcinoma include mutations in the gene encoding epidermal growth factor receptor (EGFR, 9%) [6,7], anaplastic lymphoma kinase (ALK, 3.9%) [8], c-ros oncogene 1 (ROS1, 1%) [9] and B-type Raf proto-oncogene (BRAFV600E, 1%) [10]. In NSCLC harboring an EGFR mutation sensitive to tyrosine kinase inhibitors (TKIs), namely exon 19 deletions or L858R point mutation, first line treatment with osimertinib, a third-generation TKI, improved OS by nearly seven months [11]. The preferred option for ALK-rearranged tumors is a next generation ALK inhibitor, such as alectinib, brigatinib or lorlatinib. No comparisons have been made between second-generation ALK-inhibitors [12,13,14]. ROS1 translocation is highly sensitive to both crizotinib and entrectinib, preferring the latter in case of brain metastasis due to a better intracranial penetration [15,16]. For NSCLC carrying a BRAFV600E mutation, current guidelines recommend a combination of BRAF and MEK inhibitors, namely dabrafenib plus trametinib [17].
Most of the alterations mentioned above are extremely rare in SCC, for which, depending on the levels of PD-L1 expression, immunotherapy or immunochemotherapy represent the main options [5].
Among lung cancer patients, those carrying an oncogenic driver mutation and who received a targeted agent, present an increased median overall survival (OS) compared to patients with non-oncogene addicted disease, providing further evidence for an extensive molecular profiling in order to identify new molecular biomarkers [18]. Moreover, matching a specific target tends to reduce treatment-related adverse events, improving patients’ quality of life and treatment compliance.
Novel potential predictive biomarkers are currently emerging, expanding the landscape of targetable driver mutations.
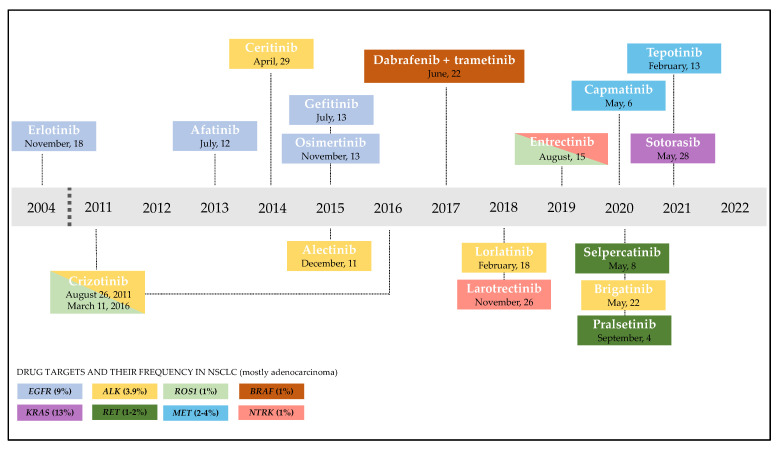
Currently FDA approved targeted agents are depicted in Figure 1.
Figure 1.
Timeline of FDA approval of targeted therapies for NSCLC (the colors are matched between driver alteration and targeted agent). The identification of actionable biomarkers led to significant progress in the treatment of NSCLC. EGFR alterations are detected in approximately 9% of NSCLC patients [6,7] and in the last two decades several agents targeting sensitive mutations received approval from the FDA. The first–generation EGFR–TKI inhibitors, erlotinib and gefitinib, received FDA approval for the treatment of advanced NSCLC in November 2004 and July 2015, respectively. Afatinib is the most studied second–generation inhibitor and received approval in July 2013. Two years later, the third–generation TKI-inhibitor, osimertinib, was initially approved for the treatment of EGFR–T790M mutation positive NSCLC, then in April 2018 it received approval as a first–line treatment for EGFR mutated NSCLC. ALK fusion–positive tumors account for 3.9% of NSCLC adenocarcinomas [8]. Several targeted drugs are available for this subset of patients: the first–generation drug crizotinib was approved in August 2011 and then the FDA expanded its use to treat ROS1–positive patients, a rare subgroup accounting for approximately 1% [9]. Second–generation ALK inhibitors, ceritinib, alectinib and brigatinib, were approved by the FDA between April 2014 and May 2020. The third–generation inhibitor lorlatinib received approval in 2018 for pretreated ALK–positive patients, and later in 2021 for the first–line setting. In June 2017, the FDA approved a combination therapy of dabrafenib and trametinib for BRAFV600E mutation–positive metastatic NSCLC, accounting for 1% of lung cancer patients [10]. NTRK is found in 1% of NSCLC [19,20]. Larotrectinib is a specific NTRK inhibitor approved in 2018 and represents the second tissue–agnostic FDA approval for the treatment of cancer Entrectinib received approval in August 2019 for both treatment of NTRK and ROS1- positive NSCLC. In the last two years, major progress has been made: in 2020 the FDA approved the targeted agents selpercatinib and pralsetinib for RET fusion–positive NSCLC (1–2%) [5,21]; capmatinib and tepotinib received FDA approval for NSCLC harboring a METex14 skipping mutation (2–4%) [22,23] in May 2020 and February 2021, respectively; sotorasib was approved in May 2021 for the treatment of KRASG12C mutated NSCLC (approximately 13%) [24] in patients who have received at least one prior systemic therapy.
Data regarding clinical outcomes of interest achieved with novel agents are summarized in Table 1.
Table 1.
Clinical activity of currently FDA approved targeted agents.
| Clinical Trial | Trial Type | Driver Mutation | Treatment Arms | Clinical Outcomes | Most Frequent AEs (All Grades) |
|---|---|---|---|---|---|
| GEOMETRY mono-1 | Phase 2, multicenter, multi-cohort, single-arm, non-randomized, open-label study | METex14 skipping mutation | Capmatinib | Pretreated pts: ORR 41%, DoR 9.7, mPFS 5.4 m. Treatment naïve: ORR 68%, DoR 12.6, mPFS 12.4 m. |
Peripheral edema, nausea, vomiting, creatinine increase. |
| VISION | Phase 2, multicenter, multi-cohort, single-arm, non-randomized, open-label study | METex14 skipping mutation | Tepotinib | Combined biopsy: ORR by ICR 46%, DoR 11.1 m, mPFS 8.5 m. Liquid biopsy: ORR by ICR 48%, DoR 9.9 m, mPFS 8.5 m. Tissue biopsy: ORR by ICR 50%, DoR 15.7 m, mPFS 11 m. |
Peripheral edema, nausea, diarrhea, creatinine increase. |
| LIBRETTO-001 | Phase 1/2 trial, international, open-label study | RET fusion | Selpercatinib | ORR by ICR 64%, DoR 17.5 m, mPFS 18.4 m. | Diarrhea, AST increase, dry mouth, hypertension, fatigue. |
| ARROW | Phase 1/2, multi-cohort, international, open-label study | RET fusion | Pralsetinib | Pretreated: ORR 61%, DoR NR, mPFS 17.1 m. Treatment naïve: ORR 70%, DoR 9.0 m, 9.1 m. |
AST/ALT increase, anemia, leucopenia, fatigue, constipation. |
| LOXO-TRK-14001 | Phase 1/2, multi-cohort, international, open-label study | NTRK gene fusion | Larotrectinib * | Overall population: ORR 75%, DoR NR, mPFS NR. (7 lung cancer pts enrolled) |
AST/ALT increase, anemia, neutropenia, weight increase. |
|
SCOUT/
NAVIGATE |
Phase 1/2, multicenter, multi-cohort, single-arm, non-randomized, open-label study | NTRK gene fusion | Larotrectinib * | ORR 30%, DCR 70%, mPFS 18.3 m, mOS NR. | AST/ALT increase, leucopenia, neutropenia, vomiting. |
|
STARTRK-1, STARTRK-2, ALKA-372-001
(Pooled analysis) |
Phase 1/2, multicenter, single-arm, non-randomized, open-label study | NTRK gene fusion, ROS1 rearrangement | Entrectinib | Overall population: ORR 59.3%, DoR 12.9, mPFS 12.9 m, mOS 23.9 m. | Fatigue, dysgeusia, paresthesia, nausea, myalgia. |
| CodeBreak 100 | Phase 2, multicenter, international, single arm, open-label study | KRASG12C mutation | Sotorasib | ORR by ICR 37.4%, DCR 80.5%, mPFS 6.7 m. | AST/ALT increase, leucopenia, anemia, diarrhea, myalgia, nausea, fatigue, hepatotoxicity, cough. |
Abbreviations: ORR, objective response rate; pts, patients; mPFS, median progression-free survival; mOS, median overall survival; m, months; AEs, adverse events; DoR, duration of response; ICR, independent central review; AST, aspartate aminotransferase; ALT, alanine transferase; NR, not reached; DCR, disease control rate. * Larotrectinib received agnostic approval for NTRK fusion-positive tumors.
Given the growing body of evidence regarding this topic, the present review mainly focuses on emerging driver alterations for which solid data of clinical relevance deriving from phase 2 or 3 clinical trials are currently available: MET alterations, RET rearrangements, NTRK1/2/3 fusions, KRAS mutations and HER2 amplifications or mutations. A literature search using the PubMed Advanced Search tool was conducted including original papers published between January 2010 and April 2022 regarding the above-mentioned alterations of interest. A clinical trials search was conducted using https://www.clinicaltrials.gov/ (accessed on 1 May 2022).
The purpose of this review is to provide a comprehensive overview of the main promising therapeutic targets beyond EGFR, ALK, ROS1 and BRAF and to discuss their potential implications in reshaping the current treatment algorithm for stage advanced NSCLC.
2. An Overview of the Main Rising Driver Alterations and Their Clinical Implications
2.1. Deregulation of MET Signalling Pathway
Mesenchymal Epithelial Transition (MET) is a proto-oncogene encoding for a tyrosine kinase receptor that binds hepatocyte growth factor (HGF), a protein involved in many crucial processes, including cell survival, migration and invasion [25]. MET alterations are reported in several solid tumors and are considered driver mutations in the carcinogenic process [26]. Three different genomic states can lead to the deregulation of this pathway: gene amplification, mutations and fusions [25]. These alterations result in tyrosine kinase activation and ligand-independent downstream signaling.
MET amplification is found in 1–5% of untreated NSCLC [27,28], and in 5–20% of EGFR mutated tumors with acquired resistance to EGFR-TKIs [29]. Gene amplification can be detected by FISH or NGS panels, however, to date there is no consensus on the actual definition of MET amplification. Studies have tested several cut-offs for FISH positivity: MET– to–chromosome 7 centromere ratio (MET/CEP7) values of 1.8 or higher, 2.0 or higher, 2.2 or higher and 5 or higher [30,31]. Literature data seem to suggest that a FISH MET/CEP7 ratio of 5 or higher could be the optimal cut-off for defining positivity, as a high-level MET amplification strictly correlates with oncogenic-dependance and there is no overlap with other oncogene drivers, and therefore treatment sensitivity [32].
Capmatinib is a highly potent and selective inhibitor of MET receptor that showed in vitro and in vivo activity in preclinical cancer models with diverse types of MET aberrations [33]. Its clinical efficacy and safety were analyzed in the prospective, international, multicohort, open-label, phase 2 trial, GEOMETRY mono-1. The trial included naïve or pretreated patients with stage IIIB and IV NSCLC with no EGFR mutation or ALK fusion, tested positive for MET amplification or MET exon 14 skipping mutation. MET amplified NSCLCs were classified according to gene copy number (GCN) in tumor tissue as follows: GCN less than 4, 4–5, 6–9 and 10 or higher. Notably, limited efficacy was registered in patients with a GCN less than 10, with an overall response rate (ORR) ranging from 7–12% and a median progression-free survival (PFS) from 2.7–3.6 months. Among tumors with a GCN of 10 or higher, an objective response was registered in 29% of pretreated, and in 40% of treatment naïve patients, although the overall response was lower than the prespecified threshold set for a clinically relevant activity [34].
Treatment options for advanced NSCLC with MET amplification also include the MET inhibitor, crizotinib. Efficacy data is derived from small cohorts of phase 1 and 2 trials. The phase 1 PROFILE 1001 trial enrolled 38 MET positive (with a MET/CEP7 ratio ≥ 1.8) NSCLC patients. Consistent with the findings of the GEOMETRY mono-1 trial, response rates were greater in patients with high MET amplification: ORR of 38% in MET/CEP7 ratio ≥4.0, 14.3% in MET/CEP7 ratio 2–4 and 33.3% in MET/CEP7 ratio >1.8, with a median PFS of 6.7, 1.9 and 1.8 months, respectively [35]. Similarly, the phase 2 METROS trial enrolled a cohort of 26 MET deregulated NSCLC patients, 16 of them with a MET amplification, 9 with a MET exon 14 (METex14) skipping mutation and 1 patient with cooccurrence of the two alterations. The ORR was 27% (all partial responses), stable disease was registered in 42% of cases, with a global disease control rate (DCR) of 69%. Although treatment response rates were promising for a pretreated population, survival outcomes were poor: at a follow up of 21 months, median PFS and OS were 4.4 and 5.4 months, respectively [36].
To date, neither capmatinib nor crizotinib have been approved by the FDA or EMA for pretreated NSCLC with high-level MET amplification.
Exon 14 encodes the 47-amino acid juxtamembrane domain of the MET receptor, a key regulatory region preventing MET overexpression and thus oversignalling [37]. The genomic events underlying the mis-splicing of MET exon 14 are complex and include several types of alterations, such as point mutations, insertions or deletions. The specific mechanism of carcinogenesis has not been fully elucidated yet, however, the loss of this region results in an impaired MET receptor degradation and aberrant activation of the signaling pathway [38].
In NSCLC, METex14 is observed in approximately 2–4% of cases [22,39] as tested by DNA or RNA NGS. Given the diversity of alterations that may lead to MET exon 14 skipping and the potential location of these alterations in the MET gene, the optimal testing technique is still matter of debate [23]. The phase 2 trial GEOMETRY mono-1, included 97 patients tested positive for a MET exon 14 skipping mutation. In this subset of patients, capmatinib showed substantial antitumor activity. An overall response was observed in 41% of patients who had received one or two prior lines of therapy and in 68% of treatment-naïve patients; the median duration of response (DoR) was 9.7 months and 12.6 months, respectively. The median PFS was 5.4 months in pretreated and 12.4 months in untreated patients [34]. Notably, responses to capmatinib were rapid, with the majority of patients showing response at the first radiological evaluation. No difference in response to the study drug was observed according to the specific genetic alteration causing METex14 skipping mutation.
Tepotinib activity was assessed in the multi-cohort, phase 2 VISION trial, conducted on advanced NSCLC patients with evidence of METex14 skipping mutation detected on tissue or liquid biopsy, who received up to two courses of previous therapy for metastatic disease. Globally, the response rate confirmed by independent central review (ICR) (primary study endpoint) was 46%, with a median duration of response of 11 months. The investigator-assessed response rate was 56%, similar to previous lines of therapy [40].
Following the results of the GEOMETRY mono-1 and VISION trials, the FDA approved capmatinib and tepotinib for adult patients with advanced NSCLC harboring a METex14 skipping mutation on 6 May 2020, and 13 February 2021, respectively [41]. On 16 February 2022, tepotinib received marketing authorization valid throughout the European Union [42]. On 22 April 2022, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of capmatinib authorization, however European Medical Agency (EMA) final approval is still awaited [43].
The safety profile of capmatinib and tepotinib was similar, and the most common adverse events (AEs) recurring in ≥10% of patients reported in clinical trials included peripheral edema, nausea, vomiting and increased blood creatinine level [34,40].
A better understanding of MET deregulation mechanisms contributed in recent years to the development of additional strategies: highly selective oral/intravenous MET inhibitors, combination therapies, humanized antibodies and antibody drug conjugates (ADC). Savolitinib is an oral selective MET inhibitor that showed promising results in a phase 2 trial on METex14 mutated lung sarcomatoid carcinomas with an ORR of 49% by ICR [44] and is currently under evaluation in combination with osimertinib in patients affected by MET-altered NSCLC with acquired resistance to EGFR-TKIs (NCT05015608, NCT05163249). Other molecules for MET-altered NSCLC are under investigation in early phase clinical trials (Table 2) including: glumetinib and APL-101 (oral MET inhibitors); SAR125844, a MET inhibitor administered intravenously; glesatinib (MGCD265) with activity against MET; VEGFR1/2/3; RON; TIE-2; and elzovantinib (TPX-022), a potent MET/CSF1R/SRC inhibitor. Current research is also directed to test the efficacy of biopharmaceutical drugs, such as Sym015, a mixture of two humanized IgG1 antibodies, directed against nonoverlapping epitopes of the MET ectodomain [45] and ADC telisotuzumab vedotin [46].
Table 2.
A selection of clinical trials testing novel strategies to target MET altered NSCLC.
| NCT Number | Gene Alteration | Experimental Drug | Phase | Study Design | Current Status |
|---|---|---|---|---|---|
| NCT05015608 | MET amplification | Savolitinib | 3 | Randomized: savolitinib + osimertinib vs. pemetrexed + cisplatin/carboplatin | Recruiting |
| NCT04338243 | MET amplification | Glumetinib | 1/2 | Single group assignment | Unknown |
| NCT02435121 | MET amplification | SAR125844 | 2 | Single group assignment | Completed |
| NCT02544633 | MET mutation, amplification | MGCD265 | 2 | Single group assignment | Completed |
| NCT03175224 | METex14, amplification, fusion | APL-101 | 1/2 | Single group assignment | Recruiting |
| NCT04270591 | MET mutation, amplification | Glumetinib | 1/2 | Single group assignment | Active, not recruiting |
| NCT02648724 | MET amplification | Sym015 | 1/2 | Single group assignment | Completed |
| NCT03539536 | MET amplification | Telisotuzumab vedotin | 2 | Single group assignment | Recruiting |
| NCT05163249 | MET amplification | Savolitinib | 2 | Randomized: osimertinib ± savolitinib | Not yet recruiting |
| NCT03993873 | MET mutation, amplification | TPX-0022 | 1/2 | Single group assignment | Recruiting |
2.2. RET Rearrangements
Rearranged during transfection (RET) gene encodes for a transmembrane receptor of the tyrosine protein kinase family that binds the glial cell-line derived neurotrophic factor [47]. In human cancer, oncogenic activation is mainly a consequence of cytogenetic rearrangements, and the derived chimeric genes determine the transcription of an aberrant receptor with dysregulation of downstream processes, such as cell proliferation, migration and survival [47]. RET fusion positive NSCLCs are an extremely rare subgroup of patients (1–2%) first described in 2012 [5,21]. Clinicians must be aware of the importance of the adoption of adequate molecular testing techniques and the use of NGS should be preferred over FISH or real-time PCR due to its higher sensitivity [48].
First attempts to target RET fusions included the use of several oral multikinase inhibitors with proven clinical efficacy in other solid tumors (e.g., cabozantinib, vandetanib and lenvatinib). The retrospective international registry GLORY represents the largest database of RET-rearranged lung cancers and data were collected with the aim to document outcomes of patients treated with these molecules. Globally, response rates reported ranged from 18–37%, with a median PFS of 2.3 months and a median OS of 6.8 months [49]. Outcomes are disappointing compared with the activity of targeted therapy in other genomic subsets of lung cancer, and, as consequence of the off-target side-effects, treatment is burdened by excessive toxicities.
The development of RET selective inhibitors represented an effective strategy to potentially overcome the poor results obtained with multikinase inhibitors while also reducing treatment-related adverse events (AEs). In this setting, selpercatinib is a novel agent that selectively binds to and targets various RET mutants and RET-containing fusion products. The phase 1/2 trial LIBRETTO-001, designed to test the safety and activity of selpercatinib, enrolled 105 patients with RET fusion-positive advanced NSCLC who had previously received at least a platinum-based chemotherapy. In 36% of cases, brain metastasis were present at baseline. Moreover, the study population was heavily pretreated with a median of three previous systemic lines of therapy and almost half of patients already exposed to a multitargeted kinase inhibitors with anti-RET activity. The study met its primary endpoint registering a 64% of ORR confirmed by ICR, mostly partial responses [50]. Among patients with measurable brain involvement, the objective intracranial response by ICR was 91%. Selpercatinib was effective regardless of previous therapy or specific RET fusion partner.
The ARROW trial is a multi-cohort, international, open-label, phase 1/2 study designed to define the maximum tolerated and recommended dose of the oral selective RET inhibitor, pralsetinib, and to test its clinical activity and safety. Overall, 92 patients treated with a median of two previous lines and 29 treatment naïve patients who were not candidates for standard platinum-therapies, were included in the phase 2 study. Notably, in 41% of cases, baseline central nervous system (CNS) involvement was documented. Response rates were remarkable in both the pretreated and the treatment naïve group, with an ORR of 61% and 70%, respectively. Shrinkage of intracranial metastases was seen in all patients with measurable intracranial metastases at baseline. At a median follow up of 14.7 months, in previously treated patients median PFS was 17.1 months and median OS not reached. At a median follow-up of 11.6 months, in untreated patients median PFS and median OS were 9.1 and not reached, respectively [51]. As seen for selpercatinib, pralsetinib activity was not affected by previous treatments received, including anti-PD1, anti-PDL1 or multikinase inhibitors, or by RET diverse fusion partners.
Overall, 93% of patients had treatment-related AEs, most common G ≥ 3 AEs were neutropenia (18%), anemia (10%), hypertension (11%) and pneumonia (10%). Most common G1-2 AEs (reported in ≥10% of patients) included hematological toxicity (neutropenia, anemia, leucopenia), AST/ALT increase, asthenia, constipation, hypertension, dysgeusia and increased blood creatinine [51]. Despite the limitation of cross-trial comparisons, the overall frequency of adverse events with pralsetinib was comparable to selpercatinib [50].
Following the results of LIBRETTO-001 and ARROW trials, the FDA granted approval of pralsetinib and selpercatinib for the treatment of RET fusion-positive NSCLC [52,53]. Selpercatinib and pralsetinib received a conditional marketing authorization from the EMA in February and November 2021, respectively [54,55]. Therefore, in the European Union these two targeted agents are still under additional monitoring.
Currently, the next generation of RET-TKIs is under exploration in early phase clinical trials (Table 3). Of particular interest is TPX-0046, a third generation orally bioavailable RET/SRC kinase inhibitor, with preliminary evidence of activity against a range of RET fusions and resistance mutations in tumors models [56]. BOS172738 is an investigational, potent, next generation selective oral RET kinase inhibitor with reported clinical activity and a good safety profile in a phase 1 trial [57]. As resistance is a major challenge for RET fusion-positive NSCLC [58], the development of next generation RET inhibitors with activity against acquired mutations could represent an effective treatment option after progression.
Table 3.
Trials testing novel agents targeting RET fusions in NSCLC.
| NCT Number | Gene Alteration | Experimental Drug | Phase | Study Design | Current Status |
|---|---|---|---|---|---|
| NCT04683250 | RET alterations | TAS0953/HM06 | 1/2 | Sequential assignment | Recruiting |
| NCT03784378 | RET mutation | RXDX-105 | 1 | Single group assignment | Completed |
| NCT03780517 | RET alterations | BOS172738 | 1 | Sequential assignment | Active, not recruiting |
| NCT04161391 | RET fusion, mutation | TPX-0046 | 1/2 | Single group assignment | Recruiting |
| NCT01877811 | RET rearrangement, fusion | RXDX-105 | 1 | Single group assignment | Completed |
2.3. NTRK1, NTRK2, NTRK3 Fusions
The Neurotrophic Tropomyosin Receptor Kinase (NTRK1, NTRK2, NTRK3) gene family encodes Tropomyosin Receptor Kinases (TRKA, TRKB, TRKC, respectively) [59,60,61,62].
Physiologically expressed in neuronal cells, these three transmembrane proteins, binding neurotrophic factors, are fundamental to the development and function of the nervous system. Ligand binding causes the oligomerization of these receptors’ kinases, leading to final activation of intracytoplasmic pathways (MAPK, PI3K and PLC-γ) involved in cell proliferation, differentiation and survival [61,62]. Alterations in TRK pathways are involved both in nervous system diseases (depression, epilepsy, or neuropathic pain, etc.) [63] and cancers [59,60,61,62]. The main oncogenic gene alteration in cancer is NTRK gene fusion, producing overexpressed or constitutively activated fusion receptor kinases [64,65]. Alternative oncogenic mechanisms include TrkA alternative splicing, implicated in neuroblastoma, and in-frame deletion of NTRK1, related to acute myeloid leukemia [61]. NTRK fusions represent a rare therapeutic target in solid neoplasms, in NSCLC their frequency is reported from 0.1% up to 1% [19,20] and generally are mutually exclusive with other oncogene alterations. NTRK fusions have also been described as a mechanism of acquired resistance to EGFR TKIs in patients with EGFR mutated NSCLC [66].
NTRK1/2/3 fusion gene detection is independent of tumor type and follows the European Society for Medical Oncology (ESMO) recommendations [67]. Molecular testing includes two alternative methods: screening with immunohistochemistry (IHC), followed by next generation sequency (NGS), if possible, RNA-based NGS; or NGS techniques confirmed by IHC in positive cases [67].
Several targeted drugs for NRTK rearrangements are under current development and some of these have been introduced in clinical practice thanks to different basket trials. Among TRK inhibitors, multi-kinase inhibitors also present anti-TRK activity (entrectinib, repotrectinib, selirectinib, taletrectinib, etc.), while larotrectinib is a member of selective inhibitors [62,67].
Larotrectinib is characterized by high selectivity for TRKA, TRKB and TRKC and it is the first oral pan-TRK inhibitor to receive tissue-agnostic FDA approval (November 2018) for advanced NTRK fusion-positive solid tumors, based on the results on 55 patients of three multicenter, open-label, single-arm clinical trials (LOXO-TRK-14001, SCOUT and NAVIGATE trials) [68]. Larotrectinib also received a conditional marketing authorization by the EMA in September 2019 with the same therapeutic indications [69].
In April 2020, Hong and colleagues [70] presented updated results of these three trials: among 159 patients, 12 patients had NSCLC with an ORR of 75%, consistent with ORR in the overall population (79%). Considering survival in all patients, a median OS of 44.4 months and a median PFS of 28.3 months were reported. In the safety analysis, larotrectinib was well tolerated with grade 4 adverse events in 1% and grade 3 in 13% of patients (grade 3–4: neutropenia in 2%, anemia in 2% and elevation of aspartate aminotransferase, AST, or alanine aminotransferase, ALT, in 3%), without treatment-related deaths. Among AEs of all grades, the most frequent ones were fatigue in 30% of patients, increase of liver aminotransferase in 28% and cough in 27%.
Entrectinib, as a member of the oral multi-kinase inhibitors, also inhibits (in addition to TRKA, TRKB, TRKC) ROS1 and ALK. Its peculiarity is its high ability to cross the blood–brain barrier, showing activity in patients with CNS disease [62,67]. In August 2019, Entrectinib reached FDA accelerated approval for advanced solid tumors with NTKR fusions and for metastatic ROS1-positive NSCLC based on the results of three multicenter, single-arm, clinical trials (STARTRK-1, STARTRK-2, ALKA-372-001) [71]. In July 2020, entrectinib received a conditional marketing authorization for both NTRK gene fusion solid tumors and ROS1-positive advanced NSCLC, addressing a major unmet medical need in this subset of patients [72].
Recently, an updated integrated analysis of three studies focused on ROS1 fusion-positive NSCLC showed an ORR of 67%, a median DoR of 15.7 months, a median PFS of 15.7 months and a median OS was not estimable [73]. Considering patients with CNS metastases, the intracranial ORR was 79% (95% CI, 58–93%), intracranial DoR was 12.9 months and median intracranial PFS was 12.0 months. The updated safety analysis was consistent with the primary one: most adverse events were grade 1–2 (dysgeusia 43%, dizziness 34%, constipation 31%), while grade 3 adverse events were weight increase (8%), ALT elevation (3%) and diarrhea (3%). Grade 4 adverse events were reported in 3% of patients (hyperuricemia, limbic encephalitis, anorectal disorder, hypertriglyceridemia, myocarditis, blood creatine phosphokinase myocardial band increase, anorectal disorder). Both for larotrectinib and entrectinib, dose modifications were able to control treatment-related AEs.
Another oral multi-kinase inhibitor is taletrectinib (DS-6051b/AB-106), which presents a high selectivity for ROS1/NTRK fusion genes. The efficacy of this TRK inhibitor in ROS1+ NSCLC emerged in two phase 1 studies (U101, conducted in United States, and J102, in Japan) [74]. With a median follow up of 14.9 months, in ROS1 TKI-naive patients an ORR of 67% was detected, while in crizotinib pretreated patients ORR was 33%. Considering survival, in the first group PFS was 29.1 months, and it was 14.2 months in the second group. Taletrectinib presented a manageable safety profile: most reported AEs were ALT and AST increase (both 73%), and nausea and diarrhea (both 50%), of which grade ≥3 were ALT and AST increase (18% and 9%, respectively) and diarrhea (5%). A multicenter, phase 2 clinical trial (TRUST, NCT04395677) is currently ongoing to evaluate the efficacy of taletrectinib in Chinese ROS1-positive NSCLC patients, while the TRUST-II trial is the ongoing global study (NCT04919811). At ASCO 2021, Zhou et al. [75] showed that all enrolled Chinese patients at the data cutoff presented a response to the treatment with an ORR of 100% (95% CI, 72–100%) and a safety profile consistent with phase 1 data.
Acquired resistance is still an inevitable circumstance in patients treated with TRK inhibitors, despite the durable and terrific duration of response, regardless of tumor type [67,76]. This acquired resistance is often due to the appearance of new NTRK mutations [67]. Repotrectinib (TPX-0005) and selirectinib (LOXO-195) are next generation TRK inhibitors designed to overcome resistance to first-generation TRK inhibitors. Repotrectinib is highly selective and active for ALK, ROS1 and NTRK, thus potentially overcoming acquired resistance [77]. The TRIDENT-1 trial is the ongoing phase 1/2 study of repotrectinib for ALK/ROS1/NTRK fusion gene-positive NSCLC (NCT03093116). Selirectinib’s chemical structure is similar to larotrectinib, apart from the more compact form. In a preclinical study, selirectinib showed resistance to secondary resistance mutations in the TRK kinase domain [76]. In 31 patients who received selirectinib after progression to a TRK inhibitor (mainly larotrectinib), the ORR was 34%, while it was 45% in patients with secondary resistance mutations [78]. An ongoing phase 1/2 study to test efficacy and safety of selirectinib is active, not recruiting (NCT03215511).
2.4. KRAS Mutations
The family of rat sarcoma oncogenes (RAS) includes the isoforms Kirsten rat sarcoma (KRAS), neuroblastoma rat sarcoma (NRAS) and the Harvey rat sarcoma (HRAS). Ras proteins activate signaling pathways controlling cell proliferation, differentiation and survival [79]. KRAS accounts for 85% of RAS mutations observed in human cancer and, given that RAS is the most frequently mutated oncogene, it is the most prevalent genomic driver event in NSCLC, present in up to 35% of lung cancers [80,81]. Notably, KRAS mutations are more common in the adenocarcinoma histotype than in squamous NSCLC (20–40% and 5%, respectively) and most frequently found in smokers (30%) vs. non-smokers (11%) and in the Caucasian vs. Asian population (26% and 11%, respectively) [82,83]. The KRAS p.G12C single-nucleotide variant, with glycine replaced by cysteine at codon 12, is the most recurrent variant in NSCLC, with a prevalence of nearly 13% in adenocarcinoma histotype [24]. It represents 39% of KRAS mutations, followed by G12V (21%) and G12D (17%) [84].
Sotorasib is a small molecule that specifically and irreversibly inhibits KRASG12C from binding covalently to a pocket present only in the inactive GDP-bound conformation, trapping KRASG12C in the inactive state and hindering KRAS oncogenic signaling [85]. In the phase 1/2 CodeBreak 100 trial, sotorasib monotherapy was evaluated in patients with locally advanced or metastatic KRASG12C mutated NSCLC. At a median follow-up of 15.3 months, ORR (primary endpoint) was 37.1% and DCR 80.6%, with a median time to response of 1.4 months (range, 1.2–10.1), a median duration of response of 11.1 months and a PFS of 6.8 months. The most common adverse events were diarrhea, nausea, fatigue, arthralgia and increase in the transaminases [86]. In May 2021, the FDA approved sotorasib as the first targeted agent for KRASG12C mutated NSCLC, pretreated with at least one prior systemic therapy. Sotorasib has also been given conditional authorization by the EMA in January 2022 in the same therapeutic setting [87]. This is the first authorized targeted therapy for tumors with KRAS mutation. Currently, the phase 3 trial, CodeBreak 200 is comparing sotorasib with docetaxel in patients with KRASG12C mutated NSCLC in progression to a platinum-based doublet chemotherapy and a checkpoint inhibitor (NCT04303780).
Adagrasib is another highly selective, small-molecule, covalent inhibitor of KRASG12C, with a longer half-life than sotorasib [88]. In the phase 1 trial, KRYSTAL-1, adagrasib was well tolerated and exhibited antitumor activity with 53.3% of partial responses, a median DoR of 16.4 months and a median PFS of 11.1 months [89]. Recently published data on the registrational phase 2 cohort of the KRYSTAL-1 trial, shows that heavily pretreated patients achieved an ORR of 42.9% with adagrasib, with a median DoR of 8.5 months, median PFS and OS of 6.5 months and 12.6 months, respectively [90]. Similarly to sotorasib, most common treatment-related adverse events (of any grade) were nausea, diarrhea, vomiting and fatigue [89]. Based on the findings from the phase 2 KRYSTAL-1 trial, adagrasib received breakthrough therapy designation from the FDA for patients with advanced NSCLC harboring the KRASG12C mutation, and a new drug application (NDA) was filed in February 2022. A marketing authorization application has also been submitted to the EMA seeking adagrasib approval in May 2022 [91]. The ongoing confirmatory phase 3 KRYSTAL-12 trial is evaluating the use of adagrasib compared with docetaxel in patients with KRASG12C-mutated NSCLC in a second line setting (NCT04685135).
Various KRAS inhibitors are currently under investigation (e.g., GDC-6036/RG6330; NCT04449874; JDQ443; NCT04699188; D-1553; NCT04585035; JAB-21822; NCT05276726; RMC-6236, NCT05379985; LY3537982; NCT04956640), as well as combination therapy. (Table 4). Other strategies under evaluation for KRAS mutant NSCLC include the inhibition of downstream signaling pathways with MEK inhibitors (NCT04967079, NCT03170206), either as monotherapy or combined with other molecules (NCT03170206, NCT04735068).
Table 4.
Trials testing novel agents or combination strategies for KRAS mutant NSCLC.
| NCT Number | Gene Alteration | Experimental Drug | Phase | Study Design | Current Status |
|---|---|---|---|---|---|
| NCT03170206 | KRAS mutation | Binimetinib plus palbociclib | 1/2 | Single group assignment | Recruiting |
| NCT05374538 | KRASG12C mutation | VIC-1911 plus sotorasib | 1 | Single group assignment | Not yet recruiting |
| NCT04685135 | KRASG12C mutation | Adagrasib | 3 | Randomized: adagrasib vs. docetaxel | Recruiting |
| NCT04735068 | KRAS mutation | Binimetinib | 2 | Single group assignment | Recruiting |
| NCT05132075 | KRASG12C mutation | JDQ443 | 3 | Randomized: JDQ443 vs. docetaxel |
Recruiting |
| NCT04967079 | KRAS mutation | Trametinib plus anlotinib | 1 | Single group assignment | Recruiting |
| NCT04620330 | KRAS mutation | VS-6766 | 2 | Randomized: VS-6766 ± defactinib |
Recruiting |
| NCT01933932 | KRAS mutation | Selumetinib | 3 | Randomized: docetaxel + selumetinib/placebo | Active, not recruiting |
| NCT04613596 | KRASG12C mutation | Adagrasib | 2 | Randomized: adagrasib ± pembrolizumab | Recruiting |
| NCT03299088 | KRAS mutation | Trametinib plus pembrolizumab | 1 | Single group assignment | Active, not recruiting |
| NCT03520842 | KRAS mutation | Regorafenib plus methotrexate | 1 | Single group assignment | Active, not recruiting |
| NCT05276726 | KRASG12C mutation | JAB 21822 | 1 | Sequential assignment | Not yet recruiting |
| NCT03681483 | KRAS mutation | RO5126766 | 1 | Single group assignment | Active, not recruiting |
| NCT02642042 | KRAS mutation | Trametinib plus docetaxel | 2 | Single group assignment | Active, not recruiting |
| NCT03808558 | KRAS mutation | TVB-2640 | 2 | Single group assignment | Recruiting |
| NCT05375994 | KRASG12C mutation | Defactinib plus adagrasib | 1/2 | Sequential assignment | Not yet recruiting |
| NCT04449874 | KRASG12C mutation | GDC-6036 | 2 | Single group assignment | Recruiting |
| NCT05379985 | KRASG12 mutation | RMC-6236 | 1 | Single group assignment | Not yet recruiting |
| NCT04965818 | KRAS mutation | Futibatinib and binimetinib | 1/2 | Single group assignment | Recruiting |
| NCT04263090 | KRAS mutation | Rigosetib | 1/2 | Sequential assignment | Recruiting |
| NCT04699188 | KRASG12C mutation | JDQ443, TNO155, tislelizumab | 1/2 | Sequential assignment | Recruiting |
| NCT05288205 | KRASG12C mutation | JAB-21822, JAB-3312 | 1/2 | Sequential assignment | Not yet recruiting |
| NCT05358249 | KRASG12C mutation | JDQ443 | 1/2 | Sequential assignment | Not yet recruiting |
| NCT05054725 | KRASG12C mutation | RMC-4630 plus sotorasib | 2 | Sequential assignment | Recruiting |
| NCT04956640 | KRASG12C mutation | LY3537982 | 1 | Single group assignment | Recruiting |
2.5. HER2 Mutations and Amplifications
Human epidermal growth factor receptor 2 (HER2, also known as ERBB2) is an EGFR family receptor tyrosine kinase that binds tightly with other EGFR family members forming a heterodimer and activating several downstream signaling pathways supporting cell proliferation and survival [92]. Since its discovery, HER2 has promptly emerged as a relevant oncogenic target in several solid tumors [93,94,95]. HER2 deregulation can occur through various mechanisms, mainly gene amplification that leads to receptor overexpression, as well as activating kinase domain mutations. No clear association has been reported between HER2 mutations and amplification.
Different therapeutic strategies are available for HER2-directed therapy: monoclonal antibody trastuzumab in combination with cytotoxic regimens; antibody drug conjugates (ADCs), such as ado-trastuzumab emtansine (T-DM1) and fam-trastuzumab deruxtecan (T-DXd, DS-8201a); panHER inhibitors (afatinib, neratinib, dacomitinib); and novel TKIs (poziotinib, pyrotinib and mobocertinib).
Following the success of anti-HER2 directed therapy in breast and gastroesophageal cancers, a growing interest emerged in a potential clinical application in NSCLC. However, HER2 amplification and overexpression has been studied as a predictive biomarker of response to targeted therapy in several trials with modest results.
Trastuzumab, in combination with taxanes or platinum-based chemotherapy, showed limited activity in phase 2 trials conducted on HER2 positive pretreated advanced NSCLC. Response rates were similar to those expected with chemotherapy alone, ranging from 8% to 25% across studies with a lack of substantial improvement in survival outcomes [96,97,98]. Likewise, phase 2 trials testing the ADC T-DM1 failed their primary endpoint, with ORRs <6% in the global study populations, signals of minimal activity were restricted to the HER2 amplified IHC 3+ subgroup [99,100].
Limited clinical benefit observed in HER2 amplified NSCLC patients led to the conclusion that overexpression and amplification seem to be a suboptimal biomarker for patients’ stratification, shifting the attention to HER2 mutations as valuable targets.
In NSCLC, the presence of HER2 mutations, specifically exon 20 in-frame insertions, identifies a small subset of patients accounting for 1% to 3% of cases [5]. HER2 gene mutations were first described in a cohort of 120 lung cancer patients in 2004 and seem to be mutually exclusive of other driver mutations, thus confirming their oncogene-addiction potential [101]. The hypothesis of HER2 mutations being a more reliable biomarker to drive therapy seems to find confirmation in the clinical outcomes of patients treated with ADCs. In a phased 2 basket trial, including a heavily pretreated cohort of HER2 mutant advanced NSCLC, T-DM1 produced an ORR of 44% with a median PFS of 5 months. 39% of patients had stable disease and durable disease control for up to 11 months. Regarding molecular characterization, responders were seen across all HER2 mutation subtypes, including exon 20 insertions and transmembrane and extracellular domain point mutations. The concurrent HER2 amplification observed in 11% of patients did not affect response to treatment [102]. Targeting HER2 mutations with the next generation ADC trastuzumab, deruxtecan, resulted in even higher response rates, with a confirmed ORR of 55%, a median PFS of 8.2 months and a median OS of 17.8 months [103]. Consistent with previous data, in the DESTINY-Lung01 trial, responses were documented across all types of HER2 mutations included in the study. A randomized, open-label, phase 3 trial (DESTINY-Lung04, NCT05048797) is currently evaluating T-Dxd compared to standard of care in NSCLC patients harboring HER2 exon 19 or 20 mutations in a first line setting.
Of note, at the interim analysis of the HER2 overexpressing cohort of the DESTINY-Lung01 trial, T-DXd demonstrated preliminary evidence of activity with 25% of response rate and a median PFS of 5.4 months [104]. Final analyses are awaited.
Case reports, retrospective and early-phase prospective clinical trials registered modest response rates in pretreated HER2 mutant tumors receiving afatinib, dacomitinib, or neratinib monotherapy. Reported ORRs varied from 0–19% [105,106,107,108,109,110].
While pan-HER2 inhibitors did not confirm the expected potential for disease control, novel and more selective HER2 TKIs showed a promising activity in HER2 exon20 insertion mutant pre-treated NSCLC patients. Poziotinib, pyrotinib and mobocertinib could become valid options after progression to standard treatments, pending further confirmatory data [111,112,113,114,115,116]. Two ongoing, randomized, phase 3 trials are testing the safety and efficacy of pyrotinib and poziotinib compared to docetaxel in HER2 mutated advanced NSCLC in progression to first line platinum-based chemotherapy (NCT04447118, NCT05378763).
Molecules under investigation in ongoing phase 1 clinical trials include: HER2 exon 20 inhibitors BAY2927088 (NCT05099172) and BI 1,810,631 (NCT04886804); ADCs, such as SBT6050 (NCT05091528, NCT04460456) and A166 (NCT03602079); BDC-1001 a HER2-targeting immune-stimulating antibody conjugate (ISAC) (NCT04278144); and ZW25, a HER2-targeted bispecific humanized antibody (NCT02892123).
To date, none of the above mentioned anti-HER2 agents have received approval from regulatory agencies (nor the FDA or EMA). Patients with a documented HER2 mutation should be enrolled in clinical trials, especially in case of good performance status and in absence of other available targeted therapy options.
2.6. Current Evidence and Ongoing Trials Testing Combination Strategies
The potential benefit of combining new pharmacological agents in oncogene-driven NSCLC patients is still a subject of study. Several clinical trials have started to associate both novel targeted therapies and immune checkpoint inhibitors (ICIs). Little data is available yet, and clinical studies have mainly focused on EGFR and ALK-TKIs.
Regarding possible associations, including drugs specific to new driver alterations, a combination therapy may also represent a mechanism to overcome pharmacological resistance. For example, MET amplification is reported in 5–26% of EGFR mutated NSCLC with acquired resistance to EGFR-TKI treatment, representing a potential actionable target after progression to first line treatment [117,118]. Dual targeting EGFR-MET with osimertinib in combination with savolitinib in MET-amplified, EGFR mutation-positive NSCLC progressed to first line EGFR-TKI showed promising antitumor activity at the interim analysis of the phase 1b SAVANNAH trial [119]. In the same setting, the efficacy of the association of tepotinib and gefitinib was tested in the phase 1b/2 INSIGHT trial, registering an improved PFS and OS in patients with high MET overexpression or amplification [120]. Moreover, cohort A of a phase 1/2 trial enrolled a small group of 12 patients with EGFR mutated, MET-amplified or with METex14 skipping mutation tumors, treated with capmatinib plus erlotinib; four out of nine patients displayed a durable benefit with the combination therapy [121]. Another phase Ib/II trial assessed promising results with the combination of capmatinib and gefitinib, with an ORR of 47% in patients with MET amplification [122]. Capmatinib is being tested in association with osimertinib in a phase III trial (NCT04816214). The combination of capmatinib with ICIs is also being currently investigated in several clinical trials. In a phase II trial, capmatinib plus pembrolizumab did not show a benefit in comparison to pembrolizumab alone as first line treatment in MET-unselected NSCLC patients with PD-L1 ≥ 50% [123]. The combination of capmatinib with ICIs is also being studied after first line failure (NCT03647488).
Of interest, KRAS inhibition combined with SHP2 (Src homology phosphatase 2), on which mutated KRASG12C cells are dependent [88], is a strategy currently in study (NCT04330664, NCT04185883), as well as dual inhibition of EGFR and KRAS. Both KRYSTAL-1 and CodeBreak 101 trials included an arm combining respectively adagrasib and sotorasib with afatinib (EGFR TKI) or with a pan-ErbB inhibitor (NCT01859026, NCT04185883). Moreover, preclinical data suggest a synergistic effect of KRAS with ICIs [85] [124]. Several trials are evaluating these combinations (e.g., NCT03785249, NCT04185883, NCT04613596).
Other clinical trials are currently exploring the association of new targeted therapies either with other targeted agents (NCT02258607) or chemotherapy (NCT05364645, NCT02642042).
3. Discussion
Over the last two decades, substantial advances have been made in the discovery of critical genetic alterations driving the carcinogenic process in NSCLC.
Given the costs in terms of toxicity and quality of life impairment for patients, to determine if there is an actual benefit from therapeutic advances represents a crucial point. Major evidence supporting a survival benefit of targeted therapies on lung cancer patients derives from a large study conducted in the United States (US) [18]. In the overall population, a decrease in lung cancer-related mortality during the study timeframe (2001–2016) has been registered. Looking closely at histological subtypes, for NSCLC, mortality declined from 2006 to 2013, when it underwent an acceleration, shortly after the introduction of routine molecular testing for EGFR and ALK and targeted therapies became available. Moreover, mortality decreased faster than it did for the NSCLC subtype, while for SCLC appeared to be steady from 2006–2016 and shows a similar decline in incidence [18]. These observations seem to suggest that a reduction in incidence coupled with treatment advances, in particular the rise of targeted therapies, is likely responsible for the reduced mortality in NSCLC subgroup. Despite strong evidence, this topic is still a matter of debate [125,126] and further confirmatory studies evaluating the effect of novel targeted therapies, subtype-specific mortality trends and screening, are needed.
It is known that oncogene-addicted disease is more frequent in, but not exclusive to, non-smokers, except for the mutation of KRAS. An area of interest is whether the target therapy has the same efficacy in smokers as non-smokers. Concerning FDA approved new targeted molecules, data are scarce. In the Geometry-mono1 trial, 3–4% and 32–39% of patients with MET Exon 14 Skipping Mutation patients and 53–78% and 10–30% with MET amplification were former active smokers or smokers, respectively [34]. In the post-hoc subgroup analysis of ORR, in METex14 patients, the ORR was 45% vs. 34.5% in the pretreated cohort and 72.2% vs. 60% in the naïve one in never smoker and smoker, respectively; in the MET amplification GCN10 pretreated cohort, ORR was 0% vs. 31% and in the naïve cohort 50% vs. 38.5% in never smoker and smoker patients [34]. A consistency of treatment effect achieved with capmatinib, despite a small sample size, was seen in all prespecified subgroups, including smoking status [34]. In the efficacy population of the VISION trial, 46% of patients were current or former smokers [40]. In the LIBRETTO-001 study testing selpercatinib and ARROW trial of pralsetinib, the percentage of patients with smoking history was 35% and 45% [50], 29% and 26% [51] in pretreated and treatment naïve cohorts, respectively. Sotorasib efficacy was tested in a phase 2 trial including 92.2% active or former smokers [86]. However, in none of the above-mentioned trials, except Geometry-mono1, was a subgroup analysis according to smoking status planned. In the pooled analysis of trials testing larotrectinib and entrectinib, data regarding smoking history of patients were not available [16,70]. This area is therefore of interest for future research.
To date, the standard approach for patients with advanced disease is based on tumor molecular characterization to select patients with an oncogene-addicted disease. The role of molecular pathology has therefore evolved from a merely diagnostic purpose to an essential tool to drive therapeutic decisions though the integration of morphologic and molecular information. Due to the increasing complexity of the therapeutic scenario and drug approval criteria, defining the proper method and platform for biomarker testing should be a priority to limit the probability of missing the detection of a driver alteration, thus potentially depriving patients of a highly effective treatment [127]. While testing for gene mutations or rearrangements has a binary outcome (detected/not detected), for gene copy number alterations the definition of the appropriate technique and threshold for positivity is challenging and several cut-offs, as for MET amplification, have been proposed. The success of drug testing in clinical trials strictly depends on the identification of an adequate molecular biomarker for patients’ selection. This can explain more than a decade of negative clinical trials testing anti-HER2 targeted agents in HER2 positive NSCLC. As for breast and gastroesophageal cancers, first studies were conducted on advanced lung cancers with HER2 amplification or overexpression determined by immunohistochemistry (IHC) with discouraging results. Further research then identified HER2 activating mutations as promising biomarkers to drive patients’ eligibility with improved outcomes in clinical trials [103].
While molecular characterization represents the current standard of care for NSCLC adenocarcinoma, squamous cells lung cancer genetic profiling, according to current guidelines, should be limited to patients with clinical features associated with a higher prevalence of mutations, such as a lack of smoking history [128]. The reported frequency of EGFR mutation in SCC varies from 4.5–9%, however these high rates could be due to the presence of a mixed adenosquamous histology [129,130]. Genomic data analyses evidenced that EGFR and KRAS mutations, the most common aberrations in lung adenocarcinoma, are extremely rare in pure SCC, while alterations in the FGFR kinase family, PIK3CA, CDKN2A and RB1 pathways are common [131,132]. ALK, ROS1 and RET gene rearrangements were not detected in a cohort of more than 200 SCC patients [133], similarly, the estimated prevalence of BRAF mutation is <1% [134]. MET amplification or exon 14 skipping have a reported frequency of approximately 5% in SCC [135]. As for other well-established driver alterations, HER2 mutation and amplification are less common in SCC compared to lung adenocarcinoma [136], while the prevalence of NTRK1/2/3 gene fusions do not vary across histologies [19].
Oncogenic driver alterations tend to present a peculiar molecular epidemiology and gender prevalence. The presence of MET exon14 skipping mutation and MET amplification can be typically found in elderly female patients with non-smoking history and are more frequent in adenocarcinoma or sarcomatoid histology [39,137,138,139]. Similarly, HER2 aberrations, particularly HER2 mutations, are more frequent in never smoker women, affected by NSCLC of adenocarcinoma histology [140,141,142,143]. Taken together, KRAS mutations recur more often in smokers versus nonsmokers and in western patients [83]. However, the clinically relevant mutation, KRASG12C, displays a higher frequency in women of younger age and lesser smoking history [84]. RET fusion gene occurs predominantly in young (<60 years), non-smoker patients and gender prevalence seems to have a geographic correlation: more frequent in European males and in Asian female patients [144,145]. An exception is represented by NTRK fusions that occur in NSCLC across sexes, ages, smoking histories and histologies [19].
The identification of driver alterations is clinically relevant since in oncogene-addicted subgroups immune checkpoint inhibitors (ICIs), even in presence of high levels of PD-L1 expression, are associated with poor outcomes [146,147,148,149,150]. Mechanisms underlying this phenomenon remain unclear, a possible explanation recognizes the lower tumor mutation burden (TMB) as responsible for ICIs’ modest response rates in this setting.
Globally, biomarker-driven therapy with innovative drugs produces response rates comparable to those seen with other targeted therapies, including osimertinib in EGFR-mutant NSCLC (80%), alectinib in ALK-positive NSCLC (83%), and entrectinib (77%) and crizotinib (72%) in ROS1 fusion-positive NSCLC [16,151,152]. These novel targeted agents are also characterized by intracranial activity, allowing disease control in patients with CNS metastases, a well-known poor prognostic factor. Finally, recent evidence suggests that MET amplification, NTRK rearrangements and HER2 mutations might have a role in acquired resistance to EGFR-TKI [29,153]. Targeting these alterations could be an effective strategy to overcome resistance, increasing the number of therapeutic options after progression, and possibly delaying chemotherapy initiation.
4. Conclusions
NSCLC represents the paradigm of how biomarker-driven therapy can change disease natural history. Unfortunately, the percentage of patients carrying a potentially targetable genetic alteration is low. An extensive understanding of disease biology and broad tumor molecular profiling are essential steps in order to discover novel druggable targets, thus expanding the proportion of patients eligible for targeted agents. Future research should also focus on unraveling the mechanisms of acquired resistance, defining the optimal therapeutic sequence and exploring the potential efficacy of combination treatments.
Acknowledgments
This work was supported by the Italian Ministry of Health (Ricerca Corrente) [no grant number provided].
Author Contributions
Conceptualization, A.M., M.d.S., E.D.C., A.D.C. and A.B.; data curation, A.M., E.B. and M.d.S.; writing—original draft preparation, A.M., E.B. and M.d.S.; writing—review and editing, A.M., M.d.S., E.B., E.D.C., A.D.C. and A.B.; supervision, E.D.C. and A.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J., et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inamura K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017;7:193. doi: 10.3389/fonc.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y.-L., Tsuboi M., He J., John T., Grohe C., Majem M., Goldman J.W., Laktionov K., Kim S.-W., Kato T., et al. Osimertinib in Resected EGFR -Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 5.Wang M., Herbst R.S., Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021;27:1345–1356. doi: 10.1038/s41591-021-01450-2. [DOI] [PubMed] [Google Scholar]
- 6.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 7.Pao W., Miller V., Zakowski M., Doherty J., Politi K., Sarkaria I., Singh B., Heelan R., Rusch V., Fulton L., et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cona M.S., Indini A., Testi A., Cresta S., Signorelli D., Garassino M.C., Sinno V., Sesana S., Pelosi G., de Braud F.G., et al. Druggable aberrations in solid tumors: An overview on ALK and ROS-1 status. Ann. Oncol. 2015;26:vi143. doi: 10.1093/annonc/mdv348.32. [DOI] [Google Scholar]
- 9.Bergethon K., Shaw A.T., Ou S.H.I., Katayama R., Lovly C.M., McDonald N.T., Massion P.P., Siwak-Tapp C., Gonzalez A., Fang R., et al. ROS1 rearrangements define a unique molecular class of lung cancers. J. Clin. Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinno T., Tsuta K., Shiraishi K., Mizukami T., Suzuki M., Yoshida A., Suzuki K., Asamura H., Furuta K., Kohno T., et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014;25:138–142. doi: 10.1093/annonc/mdt495. [DOI] [PubMed] [Google Scholar]
- 11.Ramalingam S.S., Vansteenkiste J., Planchard D., Cho B.C., Gray J.E., Ohe Y., Zhou C., Reungwetwattana T., Cheng Y., Chewaskulyong B., et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 12.Mok T., Camidge D.R., Gadgeel S.M., Rosell R., Dziadziuszko R., Kim D.W., Pérol M., Ou S.H.I., Ahn J.S., Shaw A.T., et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020;31:1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 13.Camidge D.R., Kim H.R., Ahn M.J., Yang J.C.H., Han J.Y., Hochmair M.J., Lee K.H., Delmonte A., García Campelo M.R., Kim D.W., et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J. Clin. Oncol. 2020;38:3592–3603. doi: 10.1200/JCO.20.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw A.T., Bauer T.M., de Marinis F., Felip E., Goto Y., Liu G., Mazieres J., Kim D.-W., Mok T., Polli A., et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 15.Shaw A.T., Ou S.-H.I., Bang Y.-J., Camidge D.R., Solomon B.J., Salgia R., Riely G.J., Varella-Garcia M., Shapiro G.I., Costa D.B., et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drilon A., Siena S., Dziadziuszko R., Barlesi F., Krebs M.G., Shaw A.T., de Braud F., Rolfo C., Ahn M.J., Wolf J., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Planchard D., Besse B., Groen H.J.M., Hashemi S.M.S., Mazieres J., Kim T.M., Quoix E., Souquet P.J., Barlesi F., Baik C., et al. Phase 2 Study of Dabrafenib Plus Trametinib in Patients With BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. J. Thorac. Oncol. 2022;17:103–115. doi: 10.1016/j.jtho.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Howlader N., Forjaz G., Mooradian M.J., Meza R., Kong C.Y., Cronin K.A., Mariotto A.B., Lowy D.R., Feuer E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farago A.F., Taylor M.S., Doebele R.C., Zhu V.W., Kummar S., Spira A.I., Boyle T.A., Haura E.B., Arcila M.E., Benayed R., et al. Clinicopathologic Features of Non–Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis. Oncol. 2018;2:1–12. doi: 10.1200/PO.18.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolfo C. NTRK gene fusions: A rough diamond ready to sparkle. Lancet Oncol. 2020;21:472–474. doi: 10.1016/S1470-2045(20)30026-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang R., Hu H., Pan Y., Li Y., Ye T., Li C., Luo X., Wang L., Li H., Zhang Y., et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 2012;30:4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 22.Schrock A.B., Frampton G.M., Suh J., Chalmers Z.R., Rosenzweig M., Erlich R.L., Halmos B., Goldman J., Forde P., Leuenberger K., et al. Characterization of 298 patients with lung cancer harboring MET Exon 14 skipping alterations. J. Thorac. Oncol. 2016;11:1493–1502. doi: 10.1016/j.jtho.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Socinski M.A., Pennell N.A., Davies K.D. MET Exon 14 Skipping Mutations in Non–Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations. JCO Precis. Oncol. 2021;5:653–663. doi: 10.1200/PO.20.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biernacka A., Tsongalis P.D., Peterson J.D., de Abreu F.B., Black C.C., Gutmann E.J., Liu X., Tafe L.J., Amos C.I., Tsongalis G.J. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet. 2016;209:195–198. doi: 10.1016/j.cancergen.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Library of Medicine MET MET Proto-Oncogene, Receptor Tyrosine Kinase [Homo Sapiens (Human)]—Gene—NCBI. [(accessed on 7 June 2022)]; Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=4233.
- 26.Guo R., Luo J., Chang J., Rekhtman N., Arcila M., Drilon A. MET-Dependent Solid Tumors: Molecular Diagnosis and Targeted Therapy. Nat. Rev. Clin. Oncol. 2020;17:569. doi: 10.1038/s41571-020-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Go H., Jeon Y.K., Park H.J., Sung S.W., Seo J.W., Chung D.H. High MET Gene Copy Number Leads to Shorter Survival in Patients with Non-small Cell Lung Cancer. J. Thorac. Oncol. 2010;5:305–313. doi: 10.1097/JTO.0b013e3181ce3d1d. [DOI] [PubMed] [Google Scholar]
- 28.Bubendorf L., Dafni U., Schöbel M., Finn S.P., Tischler V., Sejda A., Marchetti A., Thunnissen E., Verbeken E.K., Warth A., et al. Prevalence and clinical association of MET gene overexpression and amplification in patients with NSCLC: Results from the European Thoracic Oncology Platform (ETOP) Lungscape project. Lung Cancer. 2017;111:143–149. doi: 10.1016/j.lungcan.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Bean J., Brennan C., Shih J.Y., Riely G., Viale A., Wang L., Chitale D., Motoi N., Szoke J., Broderick S., et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casadevall D., Gimeno J., Clavé S., Taus Á., Pijuan L., Arumí M., Lorenzo M., Menéndez S., Cañadas I., Albanell J., et al. MET expression and copy number heterogeneity in nonsquamous non-small cell lung cancer (nsNSCLC) Oncotarget. 2015;6:16215. doi: 10.18632/oncotarget.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camidge D.R., Otterson G.A., Clark J.W., Ignatius Ou S.H., Weiss J., Ades S., Shapiro G.I., Socinski M.A., Murphy D.A., Conte U., et al. Crizotinib in Patients With MET-Amplified NSCLC. J. Thorac. Oncol. 2021;16:1017–1029. doi: 10.1016/j.jtho.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Noonan S.A., Berry L., Lu X., Gao D., Barón A.E., Chesnut P., Sheren J., Aisner D.L., Merrick D., Doebele R.C., et al. Identifying the Appropriate FISH Criteria for Defining MET Copy Number–Driven Lung Adenocarcinoma through Oncogene Overlap Analysis. J. Thorac. Oncol. 2016;11:1293–1304. doi: 10.1016/j.jtho.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baltschukat S., Engstler B.S., Huang A., Hao H.X., Tam A., Wang H.Q., Liang J., DiMare M.T., Bhang H.E.C., Wang Y., et al. Capmatinib (INC280) is active against models of non–small cell lung cancer and other cancer types with defined mechanisms of MET activation. Clin. Cancer Res. 2019;25:3164–3175. doi: 10.1158/1078-0432.CCR-18-2814. [DOI] [PubMed] [Google Scholar]
- 34.Wolf J., Seto T., Han J.-Y., Reguart N., Garon E.B., Groen H.J.M., Tan D.S.W., Hida T., de Jonge M., Orlov S.V., et al. Capmatinib in MET Exon 14–Mutated or MET -Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 35.Camidge D.R., Otterson G.A., Clark J.W., Ou S.-H.I., Weiss J., Ades S., Conte U., Tang Y., Wang S.C.-E., Murphy D., et al. Crizotinib in patients (pts) with MET-amplified non-small cell lung cancer (NSCLC): Updated safety and efficacy findings from a phase 1 trial. J. Clin. Oncol. 2018;36:9062. doi: 10.1200/JCO.2018.36.15_suppl.9062. [DOI] [Google Scholar]
- 36.Landi L., Chiari R., Tiseo M., D’Inca F., Dazzi C., Chella A., Delmonte A., Bonanno L., Giannarelli D., Cortinovis D.L., et al. Crizotinib in MET-deregulated or ROS1-rearranged pretreated non–small cell lung cancer (METROS): A phase II, prospective, multicenter, two-arms trial. Clin. Cancer Res. 2019;25:7312–7319. doi: 10.1158/1078-0432.CCR-19-0994. [DOI] [PubMed] [Google Scholar]
- 37.Ma P.C. MET receptor juxtamembrane exon 14 alternative spliced variant: Novel cancer genomic predictive biomarker. Cancer Discov. 2015;5:802. doi: 10.1158/2159-8290.CD-15-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peschard P., Fournier T.M., Lamorte L., Naujokas M.A., Band H., Langdon W.Y., Park M. Mutation of the c-Cbl TKB Domain Binding Site on the Met Receptor Tyrosine Kinase Converts It into a Transforming Protein. Mol. Cell. 2001;8:995–1004. doi: 10.1016/S1097-2765(01)00378-1. [DOI] [PubMed] [Google Scholar]
- 39.Awad M.M., Oxnard G.R., Jackman D.M., Savukoski D.O., Hall D., Shivdasani P., Heng J.C., Dahlberg S.E., Jänne P.A., Verma S., et al. MET exon 14 mutations in Non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J. Clin. Oncol. 2016;34:721–730. doi: 10.1200/JCO.2015.63.4600. [DOI] [PubMed] [Google Scholar]
- 40.Paik P.K., Felip E., Veillon R., Sakai H., Cortot A.B., Garassino M.C., Mazieres J., Viteri S., Senellart H., Van Meerbeeck J., et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathieu L.N., Larkins E., Akinboro O., Roy P., Amatya A.K., Fiero M.H., Mishra-Kalyani P.S., Helms W.S., Myers C.E., Skinner A.M., et al. FDA Approval Summary: Capmatinib and Tepotinib for the Treatment of Metastatic NSCLC Harboring MET Exon 14 Skipping Mutations or Alterations. Clin. Cancer Res. 2022;28:249–254. doi: 10.1158/1078-0432.CCR-21-1566. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency Tepmetko. [(accessed on 4 June 2022)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tepmetko#authorisation-details-section.
- 43.European Medicines Agency Tabrecta: Pending EC Decision. [(accessed on 4 June 2022)]; Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/tabrecta#key-facts-section.
- 44.Lu S., Fang J., Li X., Cao L., Zhou J., Guo Q., Liang Z., Cheng Y., Jiang L., Yang N., et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: A multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med. 2021;9:1154–1164. doi: 10.1016/S2213-2600(21)00084-9. [DOI] [PubMed] [Google Scholar]
- 45.Camidge D.R., Janku F., Martinez-Bueno A., Catenacci D.V.T., Lee J., Lee S.-H., Dowlati A., Rohrberg K.S., Navarro A., Moon Y.W., et al. Safety and preliminary clinical activity of the MET antibody mixture, Sym015 in advanced non-small cell lung cancer (NSCLC) patients with MET amplification/exon 14 deletion (METAmp/Ex14∆) J. Clin. Oncol. 2020;38:9510. doi: 10.1200/JCO.2020.38.15_suppl.9510. [DOI] [Google Scholar]
- 46.Camidge D.R., Moiseenko F., Cicin I., Horinouchi H., Filippova E., Bar J., Lu S., Tomasini P., Ocampo C., Sullivan D., et al. Abstract CT179: Telisotuzumab vedotin (teliso-v) monotherapy in patients with previously treated c-Met+ advanced non-small cell lung cancer. Cancer Res. 2021;81:CT179. doi: 10.1158/1538-7445.AM2021-CT179. [DOI] [Google Scholar]
- 47.National Library of Medicine RET Ret Proto-Oncogene [Homo Sapiens (Human)]—Gene—NCBI. [(accessed on 7 June 2022)]; Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=5979.
- 48.Tan A.C., Lai G.G.Y., Tan G.S., Poon S.Y., Doble B., Lim T.H., Aung Z.W., Takano A., Tan W.L., Ang M.K., et al. Utility of incorporating next-generation sequencing (NGS) in an Asian non-small cell lung cancer (NSCLC) population: Incremental yield of actionable alterations and cost-effectiveness analysis. Lung Cancer. 2020;139:207–215. doi: 10.1016/j.lungcan.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 49.Gautschi O., Milia J., Filleron T., Wolf J., Carbone D.P., Owen D., Camidge R., Narayanan V., Doebele R.C., Besse B., et al. Targeting RET in patients with RET-rearranged lung cancers: Results from the global, multicenter RET registry. J. Clin. Oncol. 2017;35:1403–1410. doi: 10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drilon A., Oxnard G.R., Tan D.S.W., Loong H.H.F., Johnson M., Gainor J., McCoach C.E., Gautschi O., Besse B., Cho B.C., et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020;383:813–824. doi: 10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gainor J.F., Curigliano G., Kim D.W., Lee D.H., Besse B., Baik C.S., Doebele R.C., Cassier P.A., Lopes G., Tan D.S.W., et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22:959–969. doi: 10.1016/S1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]
- 52.U.S. Food & Drug Administration (FDA) [(accessed on 8 May 2022)];FDA Approves Selpercatinib for Lung and Thyroid Cancers with RET Gene Mutations or Fusions. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-lung-and-thyroid-cancers-ret-gene-mutations-or-fusions.
- 53.U.S. Food & Drug Administration (FDA) [(accessed on 8 May 2022)];FDA Approves Pralsetinib for Lung Cancer with RET Gene Fusions. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pralsetinib-lung-cancer-ret-gene-fusions.
- 54.European Medicines Agency Gavreto. [(accessed on 4 June 2022)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/gavreto.
- 55.European Medicines Agency Retsevmo. [(accessed on 4 June 2022)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/retsevmo.
- 56.Drilon A.E., Zhai D., Rogers E., Deng W., Zhang X., Ung J., Lee D., Rodon L., Graber A., Zimmerman Z.F., et al. The next-generation RET inhibitor TPX-0046 is active in drug-resistant and naïve RET-driven cancer models. J. Clin. Oncol. 2020;38:3616. doi: 10.1200/JCO.2020.38.15_suppl.3616. [DOI] [Google Scholar]
- 57.Schoffski P., Cho B.C., Italiano A., Loong H.H.F., Massard C., Rodriguez L.M., Shih J.-Y., Subbiah V., Verlingue L., Andreas K., et al. BOS172738, a highly potent and selective RET inhibitor, for the treatment of RET-altered tumors including RET-fusion+ NSCLC and RET-mutant MTC: Phase 1 study results. J. Clin. Oncol. 2021;39:3008. doi: 10.1200/JCO.2021.39.15_suppl.3008. [DOI] [Google Scholar]
- 58.Lin J.J., Liu S.V., McCoach C.E., Zhu V.W., Tan A.C., Yoda S., Peterson J., Do A., Prutisto-Chang K., Dagogo-Jack I., et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann. Oncol. 2020;31:1725–1733. doi: 10.1016/j.annonc.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khotskaya Y.B., Holla V.R., Farago A.F., Mills Shaw K.R., Meric-Bernstam F., Hong D.S. Targeting TRK family proteins in cancer. Pharmacol. Ther. 2017;173:58–66. doi: 10.1016/j.pharmthera.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Vaishnavi A., Le A.T., Doebele R.C. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5:25. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amatu A., Sartore-Bianchi A., Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1:e000023. doi: 10.1136/esmoopen-2015-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haratake N., Seto T. NTRK Fusion-positive Non–small-cell Lung Cancer: The Diagnosis and Targeted Therapy. Clin. Lung Cancer. 2021;22:1–5. doi: 10.1016/j.cllc.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Boulle F., Kenis G., Cazorla M., Hamon M., Steinbusch H.W.M., Lanfumey L., van den Hove D.L.A. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog. Neurobiol. 2012;98:197–206. doi: 10.1016/j.pneurobio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Cocco E., Scaltriti M., Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Passiglia F., Caparica R., Giovannetti E., Giallombardo M., Listi A., Diana P., Cirrincione G., Caglevic C., Raez L.E., Russo A., et al. The potential of neurotrophic tyrosine kinase (NTRK) inhibitors for treating lung cancer. Expert Opin. Investig. Drugs. 2016;25:385–392. doi: 10.1517/13543784.2016.1152261. [DOI] [PubMed] [Google Scholar]
- 66.Russo A., Lopes A.R., McCusker M.G., Garrigues S.G., Ricciardi G.R., Arensmeyer K.E., Scilla K.A., Mehra R., Rolfo C. New Targets in Lung Cancer (Excluding EGFR, ALK, ROS1) Curr. Oncol. Rep. 2020;22:48. doi: 10.1007/s11912-020-00909-8. [DOI] [PubMed] [Google Scholar]
- 67.Marchiò C., Scaltriti M., Ladanyi M., Iafrate A.J., Bibeau F., Dietel M., Hechtman J.F., Troiani T., López-Rios F., Douillard J.Y., et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann. Oncol. 2019;30:1417–1427. doi: 10.1093/annonc/mdz204. [DOI] [PubMed] [Google Scholar]
- 68.Drilon A., Laetsch T.W., Kummar S., DuBois S.G., Lassen U.N., Demetri G.D., Nathenson M., Doebele R.C., Farago A.F., Pappo A.S., et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018;378:731. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.European Medicines Agency Vitrakvi. [(accessed on 4 June 2022)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vitrakvi.
- 70.Hong D.S., DuBois S.G., Kummar S., Farago A.F., Albert C.M., Rohrberg K.S., van Tilburg C.M., Nagasubramanian R., Berlin J.D., Federman N., et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doebele R.C., Drilon A., Paz-Ares L., Siena S., Shaw A.T., Farago A.F., Blakely C.M., Seto T., Cho B.C., Tosi D., et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.European Medicines Agency Rozlytrek. [(accessed on 4 June 2022)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rozlytrek.
- 73.Dziadziuszko R., Krebs M.G., de Braud F., Siena S., Drilon A., Doebele R.C., Patel M.R., Chul Cho B., Liu S.V., Ahn M.J., et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion–Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021;39:1253–1263. doi: 10.1200/JCO.20.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ou S.H.I., Fujiwara Y., Shaw A.T., Yamamoto N., Nakagawa K., Fan F., Hao Y., Gao Y., Jänne P.A., Seto T. Efficacy of Taletrectinib (AB-106/DS-6051b) in ROS1+ NSCLC: An Updated Pooled Analysis of U.S. and Japan Phase 1 Studies. JTO Clin. Res. Rep. 2021;2:100108. doi: 10.1016/j.jtocrr.2020.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou C., Fan H., Wang Y., Wu H., Yang N., Li K., Wang X., Qin X., Yu Q., Fang Y., et al. Taletrectinib (AB-106; DS-6051b) in metastatic non-small cell lung cancer (NSCLC) patients with ROS1 fusion: Preliminary results of TRUST. J. Clin. Oncol. 2021;39:9066. doi: 10.1200/JCO.2021.39.15_suppl.9066. [DOI] [Google Scholar]
- 76.Drilon A., Nagasubramanian R., Blake J.F., Ku N., Tuch B.B., Ebata K., Smith S., Lauriault V., Kolakowski G.R., Brandhuber B.J., et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior trk kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 2017;7:963–972. doi: 10.1158/2159-8290.CD-17-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drilon A., Ou S.H.I., Cho B.C., Kim D.W., Lee J., Lin J.J., Zhu V.W., Ahn M.J., Camidge D.R., Nguyen J., et al. Repotrectinib (Tpx-0005) is a next-generation ros1/trk/alk inhibitor that potently inhibits ros1/trk/alk solvent-front mutations. Cancer Discov. 2018;8:1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]
- 78.Anonymous. Combating Acquired TRK Inhibitor Resistance. Cancer Discov. 2019;9:684–685. doi: 10.1158/2159-8290.CD-NB2019-047. [DOI] [PubMed] [Google Scholar]
- 79.Simanshu D.K., Nissley D.V., McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prior I.A., Hood F.E., Hartley J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020;80:2669–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boch C., Kollmeier J., Roth A., Stephan-Falkenau S., Misch D., Grüning W., Bauer T.T., Mairinger T. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): Routine screening data for central Europe from a cohort study. BMJ Open. 2013;3:e002560. doi: 10.1136/bmjopen-2013-002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin P., Leighl N.B., Tsao M.S., Shepherd F.A. KRAS mutations as prognostic and predictive markers in non-small cell lung cancer. J. Thorac. Oncol. 2013;8:530–542. doi: 10.1097/JTO.0b013e318283d958. [DOI] [PubMed] [Google Scholar]
- 83.Adderley H., Blackhall F.H., Lindsay C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine. 2019;41:711–716. doi: 10.1016/j.ebiom.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dogan S., Shen R., Ang D.C., Johnson M.L., D’Angelo S.P., Paik P.K., Brzostowski E.B., Riely G.J., Kris M.G., Zakowski M.F., et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: Higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin. Cancer Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 86.Skoulidis F., Li B.T., Dy G.K., Price T.J., Falchook G.S., Wolf J., Italiano A., Schuler M., Borghaei H., Barlesi F., et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.European Medicines Agency Lumykras. [(accessed on 4 June 2022)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lumykras.
- 88.Hallin J., Engstrom L.D., Hargi L., Calinisan A., Aranda R., Briere D.M., Sudhakar N., Bowcut V., Baer B.R., Ballard J.A., et al. The KRAS G12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ou S.-H.I., Jänne P.A., Leal T.A., Rybkin I.I., Sabari J.K., Barve M.A., Bazhenova L., Johnson M.L., Velastegui K.L., Cilliers C., et al. First-in-Human Phase I/IB Dose-Finding Study of Adagrasib (MRTX849) in Patients With Advanced KRASG12C Solid Tumors (KRYSTAL-1) J. Clin. Oncol. 2022:JCO-21. doi: 10.1200/JCO.21.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jänne P.A., Riely G.J., Gadgeel S.M., Heist R.S., Ou S.-H.I., Pacheco J.M., Johnson M.L., Sabari J.K., Leventakos K., Yau E., et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 91.Cision PR Newswire Mirati Therapeutics Submits Marketing Authorization Application to the European Medicines Agency for Investigational Adagrasib as a Treatment for Previously-Treated KRASG12C-mutated Non-Small Cell Lung Cancer. [(accessed on 4 June 2022)]. Available online: https://www.prnewswire.com/news-releases/mirati-therapeutics-submits-marketing-authorization-application-to-the-european-medicines-agency-for-investigational-adagrasib-as-a-treatment-for-previously-treated-krasg12c-mutated-non-small-cell-lung-cancer-301551654.html.
- 92.National Library of Medicine ERBB2 erb-b2 Receptor Tyrosine Kinase 2 [Homo Sapiens (Human)]—Gene—NCBI. [(accessed on 7 May 2022)]; Available online: https://www.ncbi.nlm.nih.gov/gene/2064#gene-expression.
- 93.Wang J., Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019;4:34. doi: 10.1038/s41392-019-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gambardella V., Fleitas T., Tarazona N., Cejalvo J.M., Gimeno-Valiente F., Martinez-Ciarpaglini C., Huerta M., Roselló S., Castillo J., Roda D., et al. Towards precision oncology for HER2 blockade in gastroesophageal adenocarcinoma. Ann. Oncol. 2019;30:1254–1264. doi: 10.1093/annonc/mdz143. [DOI] [PubMed] [Google Scholar]
- 95.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 96.Lara P.N., Laptalo L., Longmate J., Lau D.H.M., Gandour-Edwards R., Gumerlock P.H., Doroshow J.H., Gandara D.R. Trastuzumab plus Docetaxel in HER2/neu–Positive Non–Small-Cell Lung Cancer: A California Cancer Consortium Screening and Phase II Trial. Clin. Lung Cancer. 2004;5:231–236. doi: 10.3816/CLC.2004.n.004. [DOI] [PubMed] [Google Scholar]
- 97.Zinner R.G., Glisson B.S., Fossella F.V., Pisters K.M.W., Kies M.S., Lee P.M., Massarelli E., Sabloff B., Fritsche H.A., Ro J.Y., et al. Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2-overexpressing, untreated, advanced non-small cell lung cancer: Report of a phase II trial and findings regarding optimal identification of patients with Her2-overexpressing disease. Lung Cancer. 2004;44:99–110. doi: 10.1016/J.LUNGCAN.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 98.Krug L.M., Miller V.A., Patel J., Crapanzano J., Azzoli C.G., Gomez J., Kris M.G., Heelan R.T., Pizzo B., Tyson L., et al. Randomized phase II study of weekly docetaxel plus trastuzumab versus weekly paclitaxel plus trastuzumab in patients with previously untreated advanced nonsmall cell lung carcinoma. Cancer. 2005;104:2149–2155. doi: 10.1002/cncr.21428. [DOI] [PubMed] [Google Scholar]
- 99.Hotta K., Aoe K., Kozuki T., Ohashi K., Ninomiya K., Ichihara E., Kubo T., Ninomiya T., Chikamori K., Harada D., et al. A Phase II Study of Trastuzumab Emtansine in HER2-Positive Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018;13:273–279. doi: 10.1016/j.jtho.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 100.Peters S., Stahel R., Bubendorf L., Bonomi P., Villegas A., Kowalski D.M., Baik C.S., Isla D., De Castro Carpeno J., Garrido P., et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019;25:64–72. doi: 10.1158/1078-0432.CCR-18-1590. [DOI] [PubMed] [Google Scholar]
- 101.Stephens P., Hunter C., Bignell G., Edkins S., Davies H., Teague J., Stevens C., O’Meara S., Smith R., Parker A., et al. Intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 102.Li B.T., Shen R., Buonocore D., Olah Z.T., Ni A., Ginsberg M.S., Ulaner G.A., Offin M., Feldman D., Hembrough T., et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results from a phase II basket trial. J. Clin. Oncol. 2018;36:2532–2537. doi: 10.1200/JCO.2018.77.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li B.T., Smit E.F., Goto Y., Nakagawa K., Udagawa H., Mazières J., Nagasaka M., Bazhenova L., Saltos A.N., Felip E., et al. Trastuzumab Deruxtecan in HER2 -Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2022;386:241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakagawa K., Nagasaka M., Felip E., Pacheco J., Baik C., Goto Y., Saltos A., Li B., Udagawa H., Gadgeel S., et al. OA04.05 Trastuzumab Deruxtecan in HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Interim Results of DESTINY-Lung01. J. Thorac. Oncol. 2021;16:S109–S110. doi: 10.1016/j.jtho.2021.01.285. [DOI] [Google Scholar]
- 105.Lai W.V., Lebas L., Barnes T.A., Milia J., Ni A., Gautschi O., Peters S., Ferrara R., Plodkowski A.J., Kavanagh J., et al. Afatinib in patients with metastatic or recurrent HER2-mutant lung cancers: A retrospective international multicentre study. Eur. J. Cancer. 2019;109:28–35. doi: 10.1016/j.ejca.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peters S., Curioni-Fontecedro A., Nechushtan H., Shih J.Y., Liao W.Y., Gautschi O., Spataro V., Unk M., Chih-Hsin Yang J., Lorence R.M., et al. Activity of Afatinib in Heavily Pretreated Patients With ERBB2 Mutation-Positive Advanced NSCLC: Findings From a Global Named Patient Use Program. J. Thorac. Oncol. 2018;13:1897–1905. doi: 10.1016/j.jtho.2018.07.093. [DOI] [PubMed] [Google Scholar]
- 107.De Grève J., Moran T., Graas M.P., Galdermans D., Vuylsteke P., Canon J.L., Schallier D., Decoster L., Teugels E., Massey D., et al. Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer. 2015;88:63–69. doi: 10.1016/j.lungcan.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 108.Dziadziuszko R., Smit E.F., Dafni U., Wolf J., Wasąg B., Biernat W., Finn S.P., Kammler R., Tsourti Z., Rabaglio M., et al. Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP) J. Thorac. Oncol. 2019;14:1086–1094. doi: 10.1016/j.jtho.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 109.Kris M.G., Camidge D.R., Giaccone G., Hida T., Li B.T., O’Connell J., Taylor I., Zhang H., Arcila M.E., Goldberg Z., et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann. Oncol. 2015;26:1421–1427. doi: 10.1093/annonc/mdv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mazéres J., Barlesi F., Filleron T., Besse B., Monnet I., Beau-Faller M., Peters S., Dansin E., Früh M., Pless M., et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: Results from the European EUHER2 cohort. Ann. Oncol. 2016;27:281–286. doi: 10.1093/annonc/mdv573. [DOI] [PubMed] [Google Scholar]
- 111.Elamin Y.Y., Robichaux J.P., Carter B.W., Altan M., Gibbons D.L., Fossella F.V., Lam V.K., Patel A.B., Negrao M.V., Le X., et al. Poziotinib for Patients With HER2 Exon 20 Mutant Non–Small-Cell Lung Cancer: Results From a Phase II Trial. J. Clin. Oncol. 2022;40:702–709. doi: 10.1200/JCO.21.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Le X., Cornelissen R., Garassino M., Clarke J.M., Tchekmedyian N., Goldman J.W., Leu S.Y., Bhat G., Lebel F., Heymach J.V., et al. Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial. J. Clin. Oncol. 2022;40:710–718. doi: 10.1200/JCO.21.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou C., Ramalingam S.S., Kim T.M., Kim S.W., Yang J.C., Riely G.J., Mekhail T., Nguyen D., Garcia Campelo M.R., Felip E., et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol. 2021;7:e214761. doi: 10.1001/jamaoncol.2021.4761. Erratum in JAMA Oncol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y., Jiang T., Qin Z., Jiang J., Wang Q., Yang S., Rivard C., Gao G., Ng T.L., Tu M.M., et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019;30:447–455. doi: 10.1093/annonc/mdy542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou C., Li X., Wang Q., Gao G., Zhang Y., Chen J., Shu Y., Hu Y., Fan Y., Fang J., et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2020;38:2753–2761. doi: 10.1200/JCO.20.00297. [DOI] [PubMed] [Google Scholar]
- 116.Li B., Offin M., Hembrough T., Cecchi F., Shen R., Olah Z., Panora E., Myers M., Brzostowski E., Buonocore D., et al. P1.13-44 Safety, PK, and Preliminary Antitumor Activity of the Oral EGFR/HER2 Exon 20 Inhibitor TAK-788 in NSCLC. J. Thorac. Oncol. 2018;13:S599. doi: 10.1016/j.jtho.2018.08.900. [DOI] [Google Scholar]
- 117.Sequist L.V., Waltman B.A., Dias-Santagata D., Digumarthy S., Turke A.B., Fidias P., Bergethon K., Shaw A.T., Gettinger S., Cosper A.K., et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Joon O.P., Lindeman N., Gale C.M., Zhao X., Christensen J., et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 119.Sequist L.V., Han J.Y., Ahn M.J., Cho B.C., Yu H., Kim S.W., Yang J.C.H., Lee J.S., Su W.C., Kowalski D., et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21:373–386. doi: 10.1016/S1470-2045(19)30785-5. [DOI] [PubMed] [Google Scholar]
- 120.Wu Y.L., Cheng Y., Zhou J., Lu S., Zhang Y., Zhao J., Kim D.W., Soo R.A., Kim S.W., Pan H., et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): An open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir. Med. 2020;8:1132–1143. doi: 10.1016/S2213-2600(20)30154-5. [DOI] [PubMed] [Google Scholar]
- 121.McCoach C.E., Yu A., Gandara D.R., Riess J.W., Vang D.P., Li T., Lara P.N., Gubens M., Lara F., Mack P.C., et al. Phase I/II Study of Capmatinib Plus Erlotinib in Patients With MET-Positive Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2021;1:177–190. doi: 10.1200/PO.20.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu Y.L., Zhang L., Kim D.W., Liu X., Lee D.H., Yang J.C.H., Ahn M.J., Vansteenkiste J.F., Su W.C., Felip E., et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018;36:3101–3109. doi: 10.1200/JCO.2018.77.7326. [DOI] [PubMed] [Google Scholar]
- 123.Mok T.S.K., Cortinovis D.L., Majem M., Johnson M.L., Mardjuadi F.I., Zhao X., Siripurapu S.V., Jiang Z., Wolf J. Efficacy and safety of capmatinib plus pembrolizumab in treatment (tx)-naïve patients with advanced non–small cell lung cancer (NSCLC) with high tumor PD-L1 expression: Results of a randomized, open-label, multicenter, phase 2 study. J. Clin. Oncol. 2022;40:9118. doi: 10.1200/JCO.2022.40.16_suppl.9118. [DOI] [Google Scholar]
- 124.Briere D.M., Li S., Calinisan A., Sudhakar N., Aranda R., Hargis L., Peng D.H., Deng J., Engstrom L.D., Hallin J., et al. The KRAS G12C Inhibitor MRTX849 Reconditions the Tumor Immune Microenvironment and Sensitizes Tumors to Checkpoint Inhibitor Therapy. Mol. Cancer Ther. 2021;20:975–985. doi: 10.1158/1535-7163.MCT-20-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wallrabenstein T., Del Rio J., Templeton A.J., Buess M. Much has changed in the last decade except overall survival: A Swiss single center analysis of treatment and survival in patients with stage IV non-small cell lung cancer. PLoS ONE. 2020;15:e0233768. doi: 10.1371/journal.pone.0233768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spini A., Gini R., Rosellini P., Singier A., Bellan C., Pascucci A., Leoncini L., Mathieu C., Martellucci I., Furiesi F., et al. First-Line Pharmacotherapies and Survival among Patients Diagnosed with Non-Resectable NSCLC: A Real-Life Setting Study with Gender Prospective. Cancers. 2021;13:6129. doi: 10.3390/cancers13236129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Malone E.R., Oliva M., Sabatini P.J.B., Stockley T.L., Siu L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020;12:8. doi: 10.1186/s13073-019-0703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kerr K.M., Bubendorf L., Edelman M.J., Marchetti A., Mok T., Novello S., O’Byrne K., Stahel R., Peters S., Felip E., et al. Second ESMO consensus conference on lung cancer: Pathology and molecular biomarkers for non-small-cell lung cancer. Ann. Oncol. 2014;25:1681–1690. doi: 10.1093/annonc/mdu145. [DOI] [PubMed] [Google Scholar]
- 129.Joshi A., Zanwar S., Noronha V., Patil V.M., Chougule A., Kumar R., Janu A., Mahajan A., Kapoor A., Prabhash K. EGFR mutation in squamous cell carcinoma of the lung: Does it carry the same connotation as in adenocarcinomas? Onco. Targets. Ther. 2017;10:1859. doi: 10.2147/OTT.S125397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hammerman P.S., Voet D., Lawrence M.S., Voet D., Jing R., Cibulskis K., Sivachenko A., Stojanov P., McKenna A., Lander E.S., et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rekhtman N., Paik P.K., Arcila M.E., Tafe L.J., Oxnard G.R., Moreira A.L., Travis W.D., Zakowski M.F., Kris M.G., Ladanyi M. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: Lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin. Cancer Res. 2012;18:1167–1176. doi: 10.1158/1078-0432.CCR-11-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kenmotsu H., Serizawa M., Koh Y., Isaka M., Takahashi T., Taira T., Ono A., Maniwa T., Takahashi S., Mori K., et al. Prospective genetic profiling of squamous cell lung cancer and adenosquamous carcinoma in Japanese patients by multitarget assays. BMC Cancer. 2014;14:786. doi: 10.1186/1471-2407-14-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao W., Choi Y.L., Song J.Y., Zhu Y., Xu Q., Zhang F., Jiang L., Cheng J., Zheng G., Mao M. ALK, ROS1 and RET rearrangements in lung squamous cell carcinoma are very rare. Lung Cancer. 2016;94:22–27. doi: 10.1016/j.lungcan.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 134.Sheikine Y., Pavlick D., Klempner S.J., Trabucco S.E., Chung J.H., Rosenzweig M., Wang K., Velcheti V., Frampton G.M., Peled N., et al. BRAF in Lung Cancers: Analysis of Patient Cases Reveals Recurrent BRAF Mutations, Fusions, Kinase Duplications, and Concurrent Alterations. JCO Precis. Oncol. 2018;2:1–15. doi: 10.1200/PO.17.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lam V.K., Tran H.T., Banks K.C., Lanman R.B., Rinsurongkawong W., Peled N., Lewis J., Lee J.J., Roth J., Roarty E.B., et al. Targeted Tissue and Cell-Free Tumor DNA Sequencing of Advanced Lung Squamous-Cell Carcinoma Reveals Clinically Significant Prevalence of Actionable Alterations. Clin. Lung Cancer. 2019;20:30–36.e3. doi: 10.1016/j.cllc.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 136.Campbell J.D., Alexandrov A., Kim J., Wala J., Berger A.H., Pedamallu C.S., Shukla S.A., Guo G., Brooks A.N., Murray B.A., et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vuong H.G., Ho A.T.N., Altibi A.M.A., Nakazawa T., Katoh R., Kondo T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer—A systematic review and meta-analysis. Lung Cancer. 2018;123:76–82. doi: 10.1016/j.lungcan.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 138.Lee G.D., Lee S.E., Oh D.Y., Yu D.B., Jeong H.M., Kim J., Hong S., Jung H.S., Oh E., Song J.Y., et al. MET Exon 14 Skipping Mutations in Lung Adenocarcinoma: Clinicopathologic Implications and Prognostic Values. J. Thorac. Oncol. 2017;12:1233–1246. doi: 10.1016/j.jtho.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 139.Cappuzzo F., Jänne P., Skokan M., Finocchiaro G., Rossi E., Ligorio C., Zucali P., Terracciano L., Toschi L., Roncalli M., et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann. Oncol. 2008;20:298–304. doi: 10.1093/annonc/mdn635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pillai R.N., Behera M., Berry L.D., Rossi M.R., Kris M.G., Johnson B.E., Bunn P.A., Ramalingam S.S., Khuri F.R. HER2 Mutations in Lung Adenocarcinomas: A Report from the Lung Cancer Mutation Consortium. Cancer. 2017;123:4099. doi: 10.1002/cncr.30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ricciardi G.R.R., Russo A., Franchina T., Ferraro G., Zanghì M., Picone A., Scimone A., Adamo V. NSCLC and HER2: Between Lights and Shadows. J. Thorac. Oncol. 2014;9:1750–1762. doi: 10.1097/JTO.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 142.Yu X., Ji X., Su C. HER2-Altered Non-Small Cell Lung Cancer: Biology, Clinicopathologic Features, and Emerging Therapies. Front. Oncol. 2022;12:860313. doi: 10.3389/fonc.2022.860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ninomiya K., Hata T., Yoshioka H., Ohashi K., Bessho A., Hosokawa S., Ishikawa N., Yamasaki M., Shibayama T., Aoe K., et al. A Prospective Cohort Study to Define the Clinical Features and Outcome of Lung Cancers Harboring HER2 Aberration in Japan (HER2-CS STUDY) Chest. 2019;156:357–366. doi: 10.1016/j.chest.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 144.Lin C., Wang S., Xie W., Chang J., Gan Y. The RET fusion gene and its correlation with demographic and clinicopathological features of non-small cell lung cancer: A meta-analysis. Cancer Biol. Ther. 2015;16:1019–1028. doi: 10.1080/15384047.2015.1046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Michels S., Scheel A.H., Scheffler M., Schultheis A.M., Gautschi O., Aebersold F., Diebold J., Pall G., Rothschild S., Bubendorf L., et al. Clinicopathological Characteristics of RET Rearranged Lung Cancer in European Patients. J. Thorac. Oncol. 2016;11:122–127. doi: 10.1016/j.jtho.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 146.Sabari J.K., Leonardi G.C., Shu C.A., Umeton R., Montecalvo J., Ni A., Chen R., Dienstag J., Mrad C., Bergagnini I., et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018;29:2085–2091. doi: 10.1093/annonc/mdy334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reis H., Metzenmacher M., Goetz M., Savvidou N., Darwiche K., Aigner C., Herold T., Eberhardt W.E., Skiba C., Hense J., et al. MET Expression in Advanced Non-Small-Cell Lung Cancer: Effect on Clinical Outcomes of Chemotherapy, Targeted Therapy, and Immunotherapy. Clin. Lung Cancer. 2018;19:e441–e463. doi: 10.1016/j.cllc.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 148.Hegde A., Andreev-Drakhlin A.Y., Roszik J., Huang L., Liu S., Hess K., Cabanillas M., Hu M.I., Busaidy N.L., Sherman S.I., et al. Responsiveness to immune checkpoint inhibitors versus other systemic therapies in RET-aberrant malignancies. ESMO Open. 2020;5:e000799. doi: 10.1136/esmoopen-2020-000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mazieres J., Drilon A., Lusque A., Mhanna L., Cortot A.B., Mezquita L., Thai A.A., Mascaux C., Couraud S., Veillon R., et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Skoulidis F., Goldberg M.E., Greenawalt D.M., Hellmann M.D., Awad M.M., Gainor J.F., Schrock A.B., Hartmaier R.J., Trabucco S.E., Gay L., et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018;8:822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Soria J.-C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 152.Peters S., Camidge D.R., Shaw A.T., Gadgeel S., Ahn J.S., Kim D.-W., Ou S.-H.I., Pérol M., Dziadziuszko R., Rosell R., et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 153.Ramalingam S.S., Cheng Y., Zhou Z., Ohe Y., Imamura F., Cho B.C., Lin M.-C., Majem M., Shah R., Rukazenkov Y., et al. Mechanisms of Acquired Resistance to First-Line Osimertinib: Preliminary Data from the Phase III FLAURA Study. [(accessed on 8 May 2022)]. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-2018-congress/Mechanisms-of-acquired-resistance-to-first-line-osimertinib-preliminary-data-from-the-phase-III-FLAURA-study.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.