Abstract
Osteoarthritis (OA) is characterized by the infiltration and adhesion of monocytes into the inflamed joint synovium. Interleukin (IL)-17 is a critical inflammatory mediator that participates in the progression of OA, although the mechanisms linking IL-17 and monocyte infiltration are not well understood. Our analysis of synovial tissue samples retrieved from the Gene Expression Omnibus (GEO) dataset exhibited higher monocyte marker (CD11b) and vascular cell adhesion molecule 1 (VCAM-1) levels in OA samples than in normal, healthy samples. The stimulation of human OA synovial fibroblasts (OASFs) with IL-17 increased VCAM-1 production and subsequently enhanced monocyte adhesion. IL-17 affected VCAM-1-dependent monocyte adhesion by reducing miR-5701 expression through the protein kinase C (PKC)-α and c-Jun N-terminal kinase (JNK) signaling cascades. Our findings improve our understanding about the effect of IL-17 on OA progression and, in particular, VCAM-1 production and monocyte adhesion, which may help with the design of more effective OA treatments.
Keywords: IL-17, osteoarthritis, monocytes, VCAM-1, adhesion
1. Introduction
Osteoarthritis (OA) is a joint disorder that is accompanied by the migration and invasion of monocytes into the synovial membrane, leading to synovial inflammation, cartilage degradation, and bone breakdown [1,2], evoking pain and adversely affecting the patient’s quality of life. As the disease progresses, critical steps in the joint microenvironment regarding the synthesis of proinflammatory factors and chondrolytic mediators enhance the breakdown of cartilage and loss of bone [3,4]. Numerous OA synovial fibroblasts (OASFs) in the joint microenvironment control the development of OA by synthesizing proinflammatory factors and catabolic mediators [3,5,6], indicating that remedying the state of the synovium is appropriate for OA treatment [7,8].
Macrophage and monocyte infiltration into the joint microenvironment and their adhesion to the synovial membrane is an important mediator of OA development [9]. Several adhesion molecules regulate the infiltration and migration of macrophages and monocytes during OA development, including vascular cell adhesion molecule 1 (VCAM-1) [9,10]. Higher VCAM-1 expression is documented in human OA synovium than in normal synovium [10]. The inhibition of VCAM-1 levels in OA synovium reportedly lowers the inflammatory response during OA progression [11,12].
Accumulating evidence indicates that the interleukin (IL)-17 family of cytokines regulates the pathogenesis of OA [13]. A higher expression of IL-17 in OA serum and synovial fluid is reflected by radiographic OA severity scores [13,14]. IL-17 gene polymorphisms have been linked to the development of OA in several populations [15,16], while injections of IL-17 into the rabbit knee joint induces OA [17]. Moreover, IL-17 enhances osteoclastogenesis, and bone erosion plays an important role in arthritic diseases [18]. Thus, it is worth targeting IL-17 as a novel therapeutic for managing OA disease.
MicroRNAs (miRNAs, single-stranded noncoding RNAs) regulate the production of target genes at the post-transcriptional stage [19,20]. Several miRNAs mediate the development of OA by negatively or positively regulating synovial cell inflammation, differentiation, angiogenesis, and survival [21,22]. However, it is not clear as to whether IL-17 regulates miRNA-mediated VCAM-1 synthesis and the adhesion of monocytes during OA progression. Here, we found higher levels of the monocyte marker CD11b and VCAM-1 expression in OA synovial tissue than in normal, healthy tissue. Our results also indicate that IL-17 facilitates VCAM-1 production and promotes monocyte adhesion in human OASFs by decreasing miR-5701 expression in the protein kinase C (PKC)-α and c-Jun N-terminal kinase (JNK) signaling cascades, indicating that IL-17 may be worth targeting when treating OA.
2. Results
2.1. IL-17 Promotes Monocyte Adhesion in Human OASFs by Enhancing VCAM-1 Production
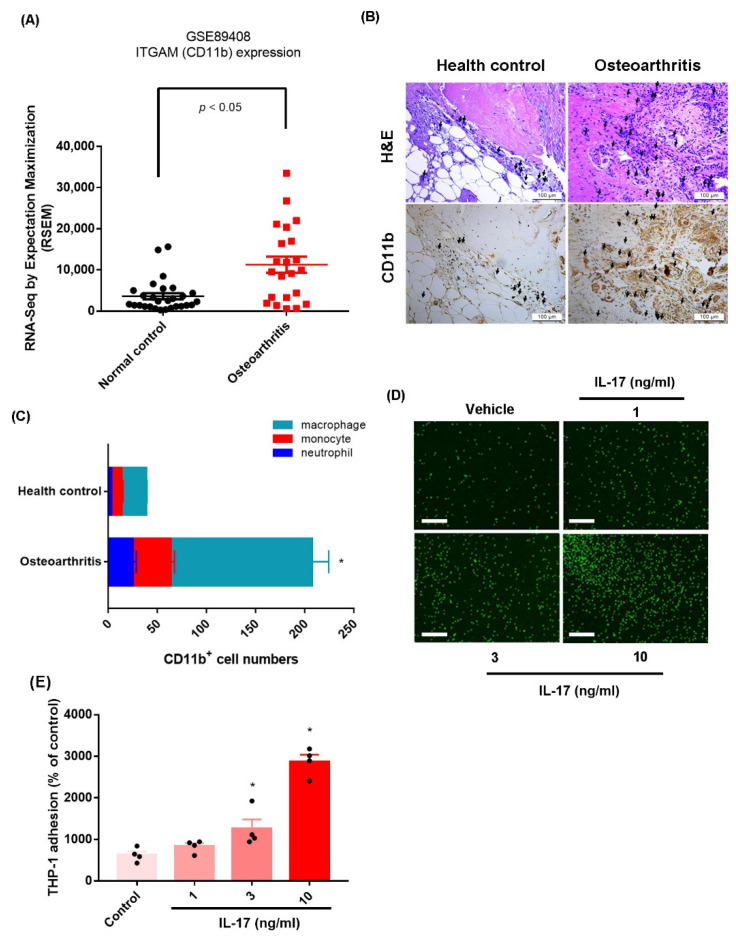
The infiltration of macrophages and monocytes and their adhesion to synovial membrane, where they promote a proinflammatory response, is a critical step in OA development [23]. Since CD11b is a major monocyte marker for OA disease, we examined the expression of CD11b in raw data from OA (n = 22) and healthy synovial tissue samples (n = 28) downloaded from the Gene Expression Omnibus (GEO) dataset (accession code: GSE89408). The analyses revealed significantly higher levels of the monocyte marker CD11b in OA synovial samples than in normal controls (Figure 1A), which was also the case in our histopathologic analysis of CD11b expression in our samples of human OA synovial tissue (n = 4) and healthy control samples (n = 4) (Figure 1B,C, Supplementary Figure S1A). OASFs were stimulated with IL-17 for 2 h, and then the culture was changed to a serum-free medium for the next 24 h (to exclude the direct effect of IL-17 on monocytes), and monocytes (THP-1 cells) were added to a monolayer of OASFs for 1 h. Monocyte adhesion analysis revealed that IL-17 treatment dose-dependently enhanced monocyte adhesion in OASFs (Figure 1D,E, Supplementary Figure S1B). Treatment of monocytes with IL-17 for 1 h did not increase their adherence to OASFs (Supplementary Figure S2). Stimulation of OASFs with TNF-α also promoted monocyte adherence (Supplementary Figure S3). Thus, IL-17 promotes monocyte adhesion in human OASFs; the other pathways, such as TNF-α, also have similar effects.
Figure 1.
IL-17 promotes monocyte adhesion in OASFs. (A) Levels of CD11b were investigated in OA and healthy control synovial tissue collected from the GEO database. (B,C) IHC staining for CD11b+ (neutrophil, monocyte, and macrophage) in synovium samples from OA patients and healthy individuals. (D,E) OASFs were stimulated with vehicle or IL-17 (1–10 ng/mL) for 24 h. Monocytes (THP-1 cells) were then applied to the OASFs. Adherent THP-1 cells were photographed and quantified under fluorescence microscopy. Size bar = 320 μm. * p < 0.05 compared with the control group.
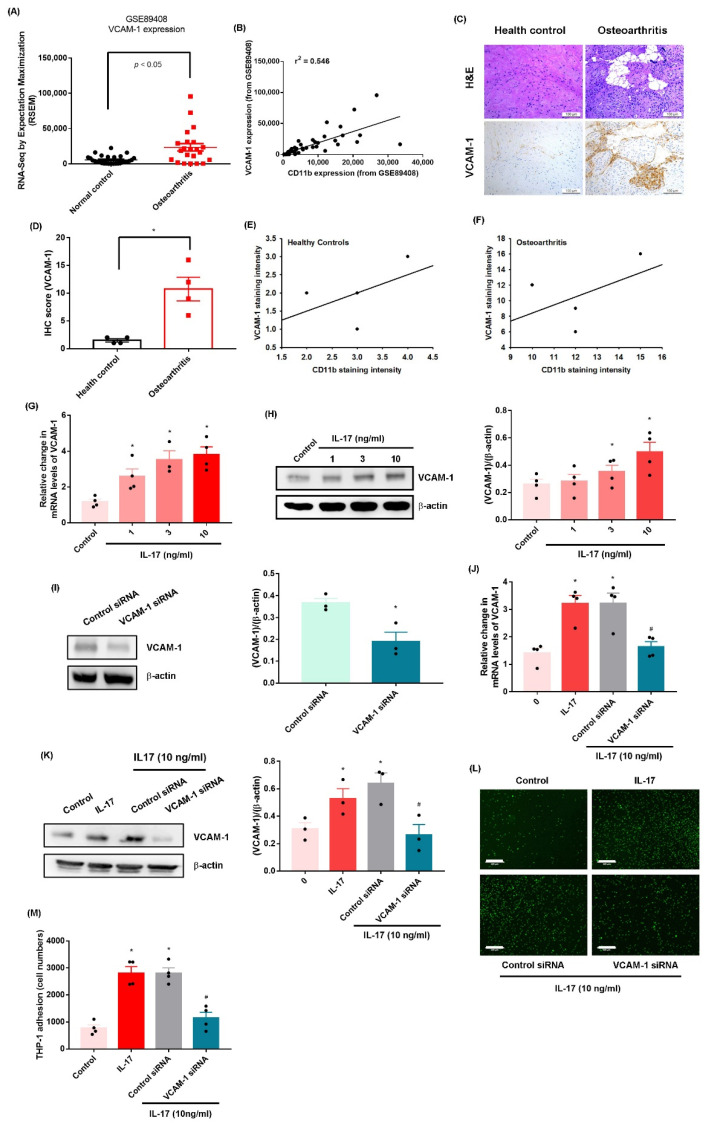
VCAM-1-regulated adhesion of macrophages and monocytes to the synovial membrane is crucial for OA [24]. Data from the GEO database and our clinical samples displayed higher expression of VCAM-1 in OA synovial tissue than in healthy tissue (Figure 2A–D, Supplementary Figure S4A), and we observed a significant positive correlation between CD11b and VCAM-1 levels (Figure 2E,F). Treatment of OASFs with IL-17 promoted the transcription of VCAM-1 mRNA and the translation of VCAM-1 protein (Figure 2G,H). Transfection of OASFs with VCAM-1 siRNA without IL-17 treatment inhibited VCAM-1 expression (Figure 2I). In addition, transfection of OASFs with VCAM-1 siRNA and IL-17 treatment abolished IL-17-induced promotion of VCAM-1 expression and monocyte adhesion (Figure 2J–M, Supplementary Figure S4B). VCAM-1 siRNA completely inhibited IL-17-induced promotion of VCAM-1 expression. Thus, IL-17 facilitates the adhesion of monocytes to human OASFs by facilitating VCAM-1 synthesis.
Figure 2.
IL-17 promotes monocyte adhesion in OASFs through upregulating VCAM-1 production. (A) Levels of VCAM-1 were investigated in synovial tissue collected from the GEO database containing OA patients and healthy controls. (B) Spearman’s rank correlation coefficient testing identified significant, positive correlations between VCAM-1 and CD11b. (C–F) IHC staining for VCAM-1 in synovium samples from OA patients and healthy individuals. (G,H) OASFs were stimulated with IL-17 for 24 h, and VCAM-1 synthesis was performed by qPCR and Western blot. (I–M) OASFs were transfected with a VCAM-1 siRNA for 24 h and then treated with or without IL-17 (10 ng/mL). VCAM-1 expression was examined by Western blot and qPCR. Adherent THP-1 cells were photographed and quantified under fluorescence microscopy. Size bar = 320 μm. * p < 0.05 compared with the control group; # p < 0.05 compared with the IL-17-stimulated group.
2.2. IL-17 Promotes VCAM-1-Dependent Monocyte Adhesion in OASFs via the PKC-α and JNK Signaling Pathways
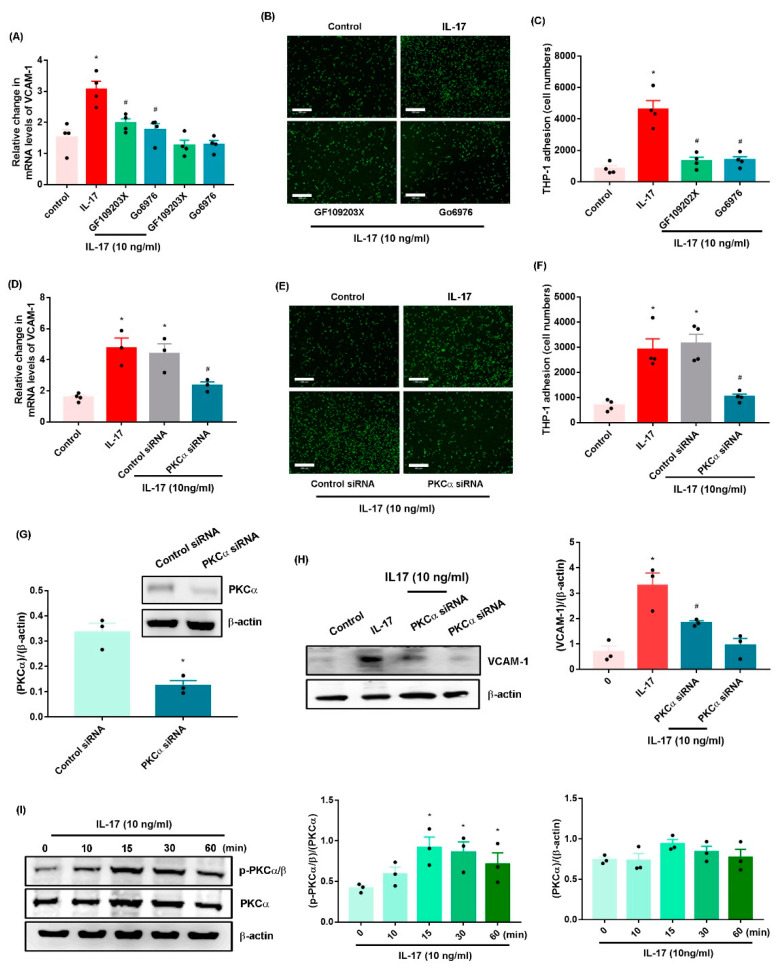
PKC activation is an important event in OA development [12,25]. Treatment of OASFs with the pan PKC inhibitor (GF109203X) and PKC-α/β inhibitor (Gö6976) or PKC-α siRNA antagonized IL-17-induced increases in VCAM-1 synthesis and monocyte adhesion (Figure 3A–F, Supplementary Figure S5A,B). The PKC inhibitors (GF109203X and Gö6976) did not affect the basal levels of VCAM-1 mRNA expression (Figure 3A). Transfection of OASFs with PKC-α siRNA inhibited PKC-α expression (Figure 3G); the knockdown of PKC-α produced similar effects (Figure 3H). In addition, Western blot analysis found that IL-17 facilitated PKC-α phosphorylation (Figure 3I).
Figure 3.
The PKC-α pathway mediates IL-17-induced promotion of VCAM-1 synthesis and monocyte adhesion in OASFs. (A,D,H) OASFs were stimulated with PKC inhibitors (GF109203X, 10 nM and Gö6976, 10 nM) for 30 min or transfected with a PKC-α siRNA for 24 h and then treated with or without IL-17 (10 ng/mL) for 24 h. Quantification of VCAM-1 expression was performed by qPCR and Western blot. (B,C,E,F) Adherent THP-1 cells were photographed and quantified under fluorescence microscopy. (G) PKC-α protein levels were measured by Western blot. (I) OASFs were stimulated with IL-17 for the indicated time intervals. PKC-α phosphorylation was performed by Western blot. Size bar = 320 μm. * p < 0.05 compared with the control group; # p < 0.05 compared with the IL-17-stimulated group.
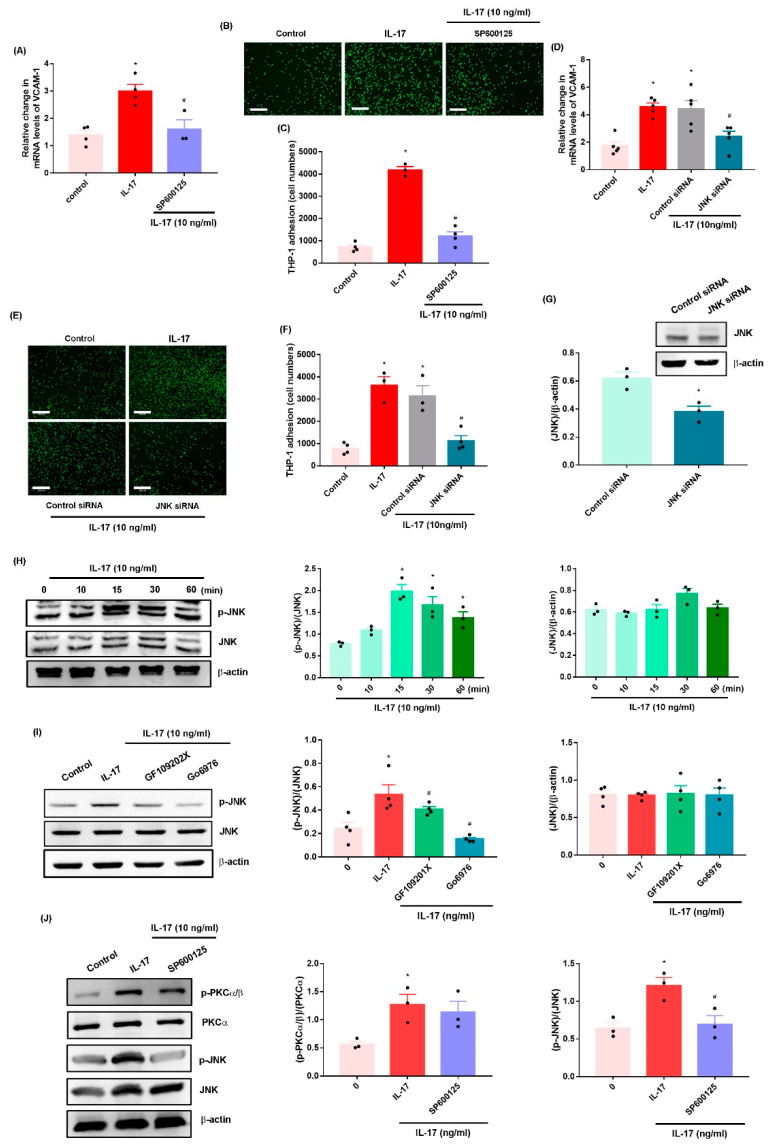
JNK is a downstream molecule of PKC in VCAM-1-mediated cell adhesion [12]. Both the JNK inhibitor (SP600125) and JNK siRNA reversed IL-17-induced promotion of VCAM-1 synthesis and monocyte adhesion (Figure 4A–F, Supplementary Figure S6A,B). Transfection of OASFs with JNK siRNA inhibited JNK expression (Figure 4G). IL-17 also enhanced the phosphorylation of JNK (Figure 4H), which was diminished by pretreatment with the PKC inhibitors (Figure 4I). In contrast, the JNK inhibitor inhibited IL-17-promoted JNK but not PKC-α phosphorylation (Figure 4J), suggesting that PKC-α-dependent JNK activation mediates IL-17-induced enhancement of VCAM-1 synthesis and monocyte adhesion in OASFs.
Figure 4.
The JNK pathway mediates IL-17-induced promotion of VCAM-1 synthesis and monocyte adhesion in OASFs. (A,D) OASFs were stimulated with a JNK inhibitor (SP600125, 10 nM) for 30 min or transfected with a JNK siRNA for 24 h and then treated with IL-17 (10 ng/mL) for 24 h. VCAM-1 levels were quantified by qPCR. (B,C,E,F) Adherent THP-1 cells were photographed and quantified under fluorescence microscopy. (G) JNK protein levels were measured by Western blot. (H–J) OASFs were stimulated with IL-17 for the indicated time intervals or pretreated with PKC or JNK inhibitors and then incubated with IL-17 (10 ng/mL). JNK or PKC phosphorylation was performed by Western blot. Size bar = 320 μm. * p < 0.05 compared with the control group; # p < 0.05 compared with the IL-17-stimulated group.
2.3. IL-17 Enhances VCAM-1 Synthesis and Monocyte Adhesion by Suppressing miR-5701 Expression
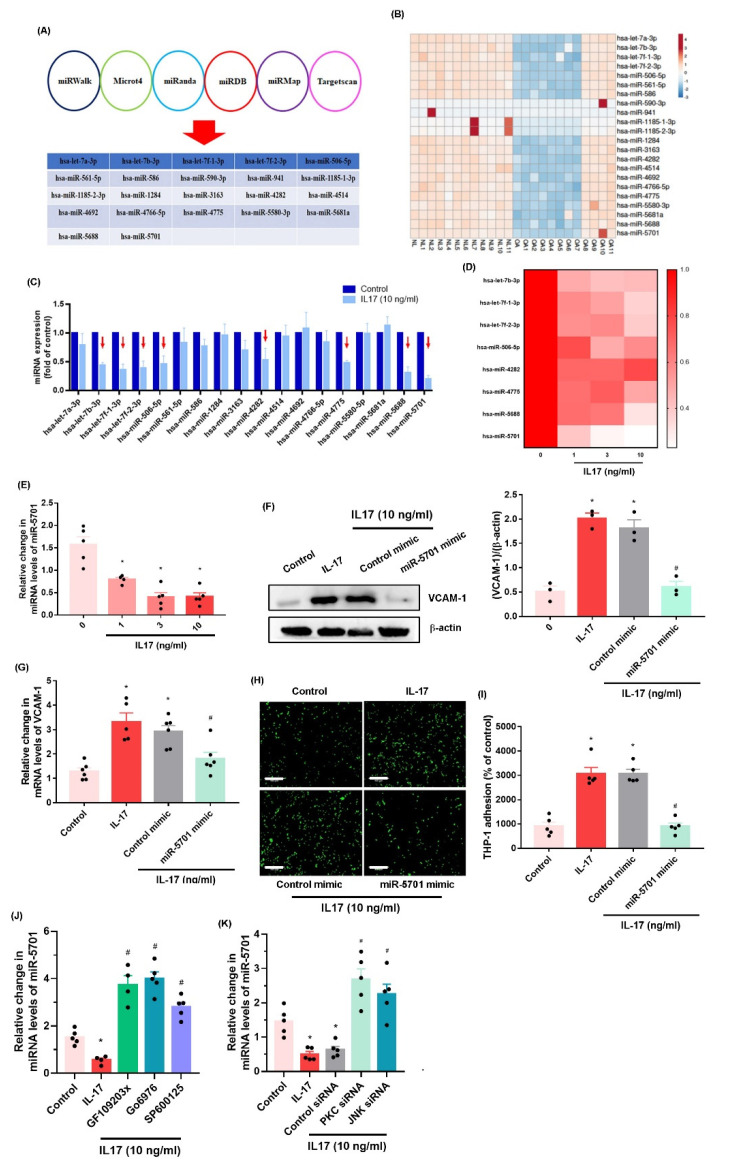
Numerous miRNAs are found at different levels of expression in normal and OA synovial tissue and regulate OA progression [26,27]. Analyses of six open-source software programs predicted that 21 miRNAs interfere with VCAM-1 mRNA transcription (Figure 5A). Our analysis of the GEO database found that among these miRNAs, eighteen (including miR-5701) exhibited lower levels of expression in OA patients than in normal, healthy individuals (Figure 5B). When OASFs were treated with IL-17, the expression of miR-5701 was suppressed by a markedly greater extent compared with the other miRNAs (Figure 5C). In addition, IL-17 concentration-dependently abolished miR-5701 synthesis (Figure 5D,E). Transfecting OASFs with miR-5701 mimic lowered VCAM-1 synthesis and monocyte adhesion (Figure 5F–I, Supplementary Figure S7). Treating OASFs with inhibitors and siRNAs of PKC-α and JNK antagonized IL-17-induced inhibition of miR-5701 synthesis (Figure 5J,K), indicating that IL-17 suppresses miR-5701 synthesis through the PKC-α and JNK signaling pathways.
Figure 5.
IL-17 enhances VCAM-1 production by inhibiting miR-5701 expression. (A) Six software databases were examined to predict which miRNAs interfere with VCAM-1 transcription. (B) Levels of miRNAs were investigated in OA and normal, healthy synovial tissue collected from the GEO database. (C–E) OASFs were treated with IL-17 (10 ng/mL). miRNA expression was examined by qPCR. (F,G) OASFs were transfected with miR-5701 mimic and then treated with IL-17 (10 ng/mL). VCAM-1 expression was examined by Western blot and qPCR. (H,I) Adherent THP-1 cells were photographed and quantified under fluorescence microscopy. (J,K) OASFs were stimulated with PKC and JNK inhibitors for 30 min or the respective siRNAs for 24 h and then incubated with IL-17 (10 ng/mL). miR-5701 expression was examined by qPCR. Size bar = 320 μm. * p < 0.05 compared with the control group; # p < 0.05 compared with the IL-17-stimulated group.
3. Discussion
OA causes much physical disability [1]. Much remains unknown about the pathogenesis of OA; however, synovial inflammation is well-recognized [28], so treatment targeting the synovium may inhibit disease progression [7,29]. Elevated levels of macrophage and monocyte expression are found in the OA joint [30]. In this study, an analysis of the GEO database and our clinical results found higher levels of the monocyte marker CD11b in synovial tissue from OA patients than in tissue from normal, healthy individuals. IL-17 appears to aggravate the symptoms of OA [13]. Higher IL-17 levels have been reported in the serum and synovial fluid of OA patients than in healthy controls [13,14]. In this study, we demonstrated that IL-17 enhanced monocyte adhesion in human OASFs by upregulating VCAM-1 production. Moreover, the suppression of miR-5701 expression via PKC-α and JNK signaling regulated the effects of IL-17. The anti-IL17 monoclonal antibody secukinumab is approved for the treatment of psoriasis [31]. Whether anti-IL-17 agents are appropriate in OA treatment needs to be clarified.
VCAM-1-dependent mononuclear cell infiltration and adhesion in synovial tissue influences arthritic progression [24,32]. In this study, the GEO database records and our clinical samples displayed higher VCAM-1 expression in synovial tissue from OA patients compared with that from normal, healthy controls. A positive correlation between CD11b and VCAM-1 levels indicates that these cytokines contribute to OA disease progression. Here, we found that VCAM-1 is a response mediator in IL-17 stimulation, promoting monocyte adhesion. This effect was antagonized when OASFs were transfected with VCAM-1 siRNA, which suggests that IL-17 facilitates VCAM-1-induced monocyte adhesion in human OASFs.
PKC activation is crucial for regulating different cellular events [33], such as the promotion of cell motility and adhesion [12,34]. Our data found that IL-17 enhances PKC-α phosphorylation, while the PKC inhibitor or siRNA diminished IL-17-facilitated promotion of VCAM-1 synthesis and monocyte adhesion in OASFs. JNK is essential for regulating the inflammatory process during OA disease [35,36]. Our data showed that a JNK inhibitor or siRNA antagonized IL-17-facilitated VCAM-1-dependent monocyte adhesion. Our findings also reveal that IL-17 enhances JNK phosphorylation, which was reversed by the PKC inhibitor, indicating that PKC-α-dependent JNK activation regulates IL-17-induced synthesis of VCAM-1 and monocyte adhesion in human OASFs.
miRNAs are critical post-transcriptional mediators of gene production and are found in several diseases, including OA [37,38]. It has been proposed that pharmacotherapy capable of controlling miRNA levels would inhibit OA inflammatory progression and thus be an appropriate therapeutic approach for this disease [37,39]. Here, our analysis of six miRNA software databases predicted that miR-5701 interferes with VCAM-1 transcription. This was supported by the study data showing lower miR-5701 expression in OA synovial tissue than in normal, healthy tissue. We found that IL-17 treatment inhibits miR-5701 expression, and treatment of OASFs with miR-5701 mimic antagonizes IL-17-induced promotion of VCAM-1 synthesis and monocyte adhesion. PKC-α and JNK inhibitors, as well as their respective siRNAs, antagonized IL-17-enhanced inhibition of miR-5701 synthesis, suggesting that IL-17 promotes VCAM-1 synthesis and monocyte adhesion in human OASFs by reducing miR-5701 production through the PKC-α and JNK pathways. Our results also suggest that the development of IL-17, PKC, JNK, and VCAM-1 antagonists inhibit OA progression.
4. Materials and Methods
Antibodies against PKC-α, JNK, VCAM-1, CD11b, and β-actin were purchased from GeneTex (Hsinchu, Taiwan). Antibodies against p-PKCα and p-JNK were purchased from Cell Signaling Technology (Danvers, MA, USA). 2′,7′-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein tetrakis(acetoxymethyl) ester (BCECF-AM) and GF109203X, Gö6976 and SP600125 inhibitors were obtained from Sigma-Aldrich (St. Louis, MO, USA).
4.1. Cell Culture
Synovial tissues freshly obtained from OA patients were washed with phosphate-buffered saline (PBS), minced thoroughly using a scalpel, and then subjected to 4 h of enzymatic digestion in serum-free DMEM medium with 2 mg/mL type I collagenase in an incubator at 37 °C, before removal of collagenase by centrifugation. OASFs were cultured in DMEM containing 10% fetal bovine serum (FBS), penicillin, and streptomycin (Invitrogen; Carlsbad, CA, USA) [25]. A total of 2 passages were performed of culture when the adherent cells approached 70% confluence, and experiments were performed using cells grown in vitro for 3–6 passages.
THP-1 cells (human monocytes) were purchased from the Bioresource Collection and Research Center (Hsinchu, Taiwan) and maintained in RPMI-1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 0.05 mM β-mercaptoethanol, 10 mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified 5% CO2 atmosphere. THP-1 cells were kept at a minimum density of 3 × 105 cells and were passaged when the density reached 8 × 105 cells.
4.2. Monocyte Adhesion Analysis
Human OASFs (1 × 105 cells) were cultured in 24-well culture plates and treated with IL-17 for 2 h, before changing the culture medium to serum-free conditions for 24 h. THP-1 cells were treated with BCECF-AM (10 μM; a pH-sensitive fluorescent dye used for determining living cells) at 37 °C for 1 h and subsequently washed twice with PBS by centrifugation. BCECF-AM-labeled THP-1 cells (2.5 × 105 cells/mL) were added to a monolayer of OASFs at 37 °C for 1 h. Nonadherent THP-1 cells were cleaned off using PBS. Adherent THP-1 cells were quantified under fluorescence microscopy. We counted the number of cells in three random fields under each condition.
4.3. Human Clinical Samples
The collection of synovial samples from patients with OA and those with trauma/joint injuries (serving as normal, healthy controls) was approved by the Institutional Review Board of China Medical University Hospital. Informed written consent was obtained from all patients.
4.4. Real-Time Quantitative PCR Analysis of mRNA and miRNA
Total RNA was isolated from OASFs using TRIzol reagent (MDBio; Taipei, Taiwan). RNA (1 μg) was reverse-transcribed into cDNA with oligo-DT primer, according to the manufacturer’s procedure (Invitrogen; Carlsbad, CA, USA). qPCR was performed using SYBR Green with sequence-specific primers (Invitrogen; Carlsbad, CA, USA) (Supplementary Table S1). Levels of GAPDH or U6 snRNA expression served as the endogenous controls for normalization purposes. qPCR assays were performed with StepOnePlus (Applied Biosystems; Foster City, CA, USA) [11,40].
4.5. Western Blot
OASFs were treated with RIPA buffer. Isolated proteins were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes (Merck; Darmstadt, Germany) [41,42]. The membranes were blocked with 5% nonfat milk and then treated with primary antibodies. The membranes were then washed and treated with secondary antibodies and then visualized using the ImageQuant™ LAS 4000 biomolecular imager [43,44,45].
4.6. Measurement of Data from the Gene Expression Omnibus (GEO) Database
Data on CD11b and VCAM-1 expression from normal, healthy control samples and OA samples were obtained from the GEO database, as according to our previous studies [46,47].
4.7. Immunohistochemistry (IHC)
Human synovial tissues were stained with anti-CD11b or VCAM-1 antibody and quantified according to the protocol described in our previous work [48,49]. The sum of the intensity and percentage scores was used as the final staining score [47].
4.8. Small Interfering RNA (siRNA) Transfection
ON-TARGETplus siRNAs of PKC (L-003523-00), JNK (L00351400), VCAM-1 (L-013351-00), and control (D0018101005) were purchased from Dharmacon Research (Lafayette, CO, USA). Transient transfection of siRNAs was carried out using DharmaFECT1 transfection reagent (T-2001-01). All siRNAs (100 nM) were formulated with DharmaFECT1 transfection reagent, according to the manufacturer’s instructions.
4.9. Statistical Analysis
All values are given as the mean ± standard deviation (S.D.). The Student’s t-test assessed between-group differences. A p value of < 0.05 was considered to be statistically significant.
5. Conclusions
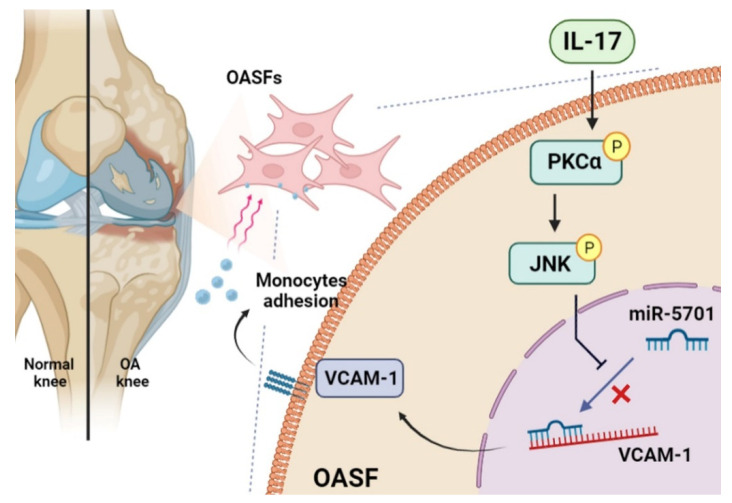
Our study indicates that IL-17 promotes VCAM-1 production in OASFs and facilitates monocyte adhesion by suppressing miR-5701 production via the PKC-α and JNK signaling cascades (Figure 6). We now have a better understanding about how IL-17-induced monocyte adhesion contributes to OA pathogenesis, which may help scientists design more effective therapy for OA.
Figure 6.
Schematic diagram summarizes the mechanisms by which IL-17 facilitates VCAM-1 production and monocyte adhesion in human OASFs. IL-17 promotes VCAM-1 synthesis and enhances monocyte adhesion in human OASFs by suppressing miR-5701 production in the PKC-α and JNK signaling cascades.
Acknowledgments
We would like to thank Iona J. MacDonald from China Medical University for her English language revision of this manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23126804/s1.
Author Contributions
Conceptualization, T.-J.W. and S.L.-Y.C.; methodology, C.-Y.L. (Chih-Yang Lin); validation, C.-Y.L. (Chih-Yang Lin) and C.-Y.L. (Chao-Yang Lai); formal analysis, X.-Y.H.; investigation, C.-H.T. (Chun-Hao Tsai) and Y.-C.F.; resources, C.-H.T. (Chih-Hsin Tang); data curation, C.-H.T. (Chun-Hao Tsai); writing—original draft preparation, C.-Y.K.; writing—review and editing, C.-M.S.; visualization, T.-J.W. and S.L.-Y.C.; funding acquisition, C.-H.T. (Chih-Hsin Tang) All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was approved by the Institutional Review Board (IRB) of China Medical University Hospital (CMUH108-REC3-039).
Informed Consent Statement
None of the authors of this paper has any financial or personal relationships with other people or organizations that could inappropriately influence this work.
Data Availability Statement
The original data to this present study are available from the corresponding authors.
Conflicts of Interest
None of the authors of this paper has any financial or personal relationships with other people or organizations that could inappropriately influence this work.
Funding Statement
This work was supported by a grant from the Ministry of Science and Technology of Taiwan (MOST 110-2314-B-195-003-; MOST 110-2314-B-039-008-), China Medical University (CMU110-ASIA-08), and China Medical University Hospital (DMR-110-176; DMR-111-108; DMR-111-229; DMR-111-165).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yuan X., Meng H., Wang Y., Peng J., Guo Q., Wang A., Lu S. Bone–cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014;22:1077–1089. doi: 10.1016/j.joca.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald I.J., Liu S.C., Su C.M., Wang Y.H., Tsai C.H., Tang C.H. Implications of angiogenesis involvement in arthritis. Int. J. Mol. Sci. 2018;19:2012. doi: 10.3390/ijms19072012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo S.J., Yang W.H., Liu S.C., Tsai C.H., Hsu H.C., Tang C.H. Transforming growth factor beta1 enhances heme oxygenase 1 expression in human synovial fibroblasts by inhibiting microrna 519b synthesis. PLoS ONE. 2017;12:e0176052. doi: 10.1371/journal.pone.0176052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S.S., Tang C.H., Chie M.J., Tsai C.H., Fong Y.C., Lu Y.C., Chen W.C., Lai C.T., Wei C.Y., Tai H.C., et al. Resistin facilitates vegf-a-dependent angiogenesis by inhibiting mir-16-5p in human chondrosarcoma cells. Cell Death Dis. 2019;10:31. doi: 10.1038/s41419-018-1241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benito M.J., Veale D.J., FitzGerald O., van den Berg W.B., Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehghan M., Asgharian S., Khalesi E., Ahmadi A., Lorigooini Z. Comparative study of the effect of thymus daenensis gel 5% and diclofenac in patients with knee osteoarthritis. Biomedicine. 2019;9:9. doi: 10.1051/bmdcn/2019090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z.Y., Liu Y.L., Lin J.B., Cheng K.L., Wang Y.G., Yao H.L., Wei P., Wu H.Y., Su W.W., Shaw P.C., et al. Preparative expression and purification of a nacreous protein n16 and testing its effect on osteoporosis rat model. Int. J. Biol. Macromol. 2018;111:440–445. doi: 10.1016/j.ijbiomac.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H., Cai D., Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020;28:555–561. doi: 10.1016/j.joca.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Schett G., Kiechl S., Bonora E., Zwerina J., Mayr A., Axmann R., Weger S., Oberhollenzer F., Lorenzini R., Willeit J. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum. 2009;60:2381–2389. doi: 10.1002/art.24757. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.-P., Liu S.-C., Wang Y.-H., Chen B.-C., Chen H.-T., Li T.-M., Huang W.-C., Hsu C.-J., Wu Y.-C., Tang C.-H. Cordycerebroside a suppresses vcam-dependent monocyte adhesion in osteoarthritis synovial fibroblasts by inhibiting mek/erk/ap-1 signaling. J. Funct. Foods. 2021;86:104712. doi: 10.1016/j.jff.2021.104712. [DOI] [Google Scholar]
- 12.Chen W.C., Lin C.Y., Kuo S.J., Liu S.C., Lu Y.C., Chen Y.L., Wang S.W., Tang C.H. Resistin enhances vcam-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by inhibiting mir-381 expression through the pkc, p38, and jnk signaling pathways. Cells. 2020;9:1369. doi: 10.3390/cells9061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Peng H., Meng Z., Wei M. Correlation of il-17 level in synovia and severity of knee osteoarthritis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015;21:1732–1736. doi: 10.12659/MSM.893771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y., Chen X., Gao Z., Liu K., Hou Y., Zheng J. Expression levels of il-15 and il-17 in synovial fluid of rheumatoid arthritis animal model. Exp. Ther. Med. 2018;16:3377–3382. doi: 10.3892/etm.2018.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y., Gao S., Liu Y., Jin S., Zhang H., Su K. Correlation between interleukin-17 gene polymorphism and osteoarthritis susceptibility in han chinese population. BMC Med. Genet. 2019;20:20. doi: 10.1186/s12881-018-0736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eftedal R., Vrgoc G., Jotanovic Z., Dembic Z. Alternative interleukin 17a/f locus haplotypes are associated with increased risk to hip and knee osteoarthritis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019;37:1972–1978. doi: 10.1002/jor.24334. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Zheng C., Zhong Y., He J., Cao X., Xia H., Ba H., Li P., Wu S., Peng C. Interleukin-17 can induce osteoarthritis in rabbit knee joints similar to hulth’s method. BioMed Res. Int. 2017;2017:2091325. doi: 10.1155/2017/2091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon Y.M., Yoon B.Y., Her Y.M., Oh H.J., Lee J.S., Kim K.W., Lee S.Y., Woo Y.J., Park K.S., Park S.H., et al. Il-32 and il-17 interact and have the potential to aggravate osteoclastogenesis in rheumatoid arthritis. Arthritis Res. Ther. 2012;14:R246. doi: 10.1186/ar4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee R.C., Feinbaum R.L., Ambros V. The c. Elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 20.Tehrani S.S., Zaboli E., Sadeghi F., Khafri S., Karimian A., Rafie M., Parsian H. Microrna-26a-5p as a potential predictive factor for determining the effectiveness of trastuzumab therapy in her-2 positive breast cancer patients. BioMedicine. 2021;11:30–39. doi: 10.37796/2211-8039.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahara H. Current status and strategy of microrna research for cartilage development and osteoarthritis pathogenesis. J. Bone Metab. 2016;23:121–127. doi: 10.11005/jbm.2016.23.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y.Y., Ko C.Y., Liu S.C., Wang Y.H., Hsu C.J., Tsai C.H., Wu T.J., Tang C.H. Mir-144-3p ameliorates the progression of osteoarthritis by targeting il-1beta: Potential therapeutic implications. J. Cell. Physiol. 2021;236:6988–7000. doi: 10.1002/jcp.30361. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., Lin C., Zeng C., Wang Z., Wang H., Lu J., Liu X., Shao Y., Zhao C., Pan J., et al. Synovial macrophage m1 polarisation exacerbates experimental osteoarthritis partially through r-spondin-2. Ann. Rheum. Dis. 2018;77:1524–1534. doi: 10.1136/annrheumdis-2018-213450. [DOI] [PubMed] [Google Scholar]
- 24.Liu J.F., Hou S.M., Tsai C.H., Huang C.Y., Hsu C.J., Tang C.H. Ccn4 induces vascular cell adhesion molecule-1 expression in human synovial fibroblasts and promotes monocyte adhesion. Biochim. Biophys. Acta. 2013;1833:966–975. doi: 10.1016/j.bbamcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Kuo S.J., Liu S.C., Huang Y.L., Tsai C.H., Fong Y.C., Hsu H.C., Tang C.H. Tgf-beta1 enhances foxo3 expression in human synovial fibroblasts by inhibiting mir-92a through ampk and p38 pathways. Aging. 2019;11:4075–4089. doi: 10.18632/aging.102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T.J., Lin C.Y., Tsai C.H., Huang Y.L., Tang C.H. Glucose suppresses il-1beta-induced mmp-1 expression through the fak, mek, erk, and ap-1 signaling pathways. Environ. Toxicol. 2018;33:1061–1068. doi: 10.1002/tox.22618. [DOI] [PubMed] [Google Scholar]
- 27.Taipaleenmaki H. Regulation of bone metabolism by micrornas. Curr. Osteoporos. Rep. 2018;16:1–12. doi: 10.1007/s11914-018-0417-0. [DOI] [PubMed] [Google Scholar]
- 28.Gao B., Gao W., Wu Z., Zhou T., Qiu X., Wang X., Lian C., Peng Y., Liang A., Qiu J., et al. Melatonin rescued interleukin 1beta-impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res. Ther. 2018;9:162. doi: 10.1186/s13287-018-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathiessen A., Conaghan P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017;19:18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X., Lee C.W., Xu H., Wang Y.F., Yung P.S.H., Jiang Y., Lee O.K. Phenotypic alteration of macrophages during osteoarthritis: A systematic review. Arthritis Res. Ther. 2021;23:110. doi: 10.1186/s13075-021-02457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasilewska A., Winiarska M., Olszewska M., Rudnicka L. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin diseases. Postepy Dermatol. Alergol. 2016;33:247–252. doi: 10.5114/ada.2016.61599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y.M., Hsu C.J., Liao Y.Y., Chou M.C., Tang C.H. The ccl2/ccr2 axis enhances vascular cell adhesion molecule-1 expression in human synovial fibroblasts. PLoS ONE. 2012;7:e49999. doi: 10.1371/journal.pone.0049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C.Y., Su C.M., Hsu C.J., Huang C.C., Wang S.W., Liu S.C., Chen W.C., Fuh L.J., Tang C.H. Ccn1 promotes vegf production in osteoblasts and induces endothelial progenitor cell angiogenesis by inhibiting mir-126 expression in rheumatoid arthritis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017;32:34–45. doi: 10.1002/jbmr.2926. [DOI] [PubMed] [Google Scholar]
- 34.Horng C.T., Shieh P.C., Tan T.W., Yang W.H., Tang C.H. Paeonol suppresses chondrosarcoma metastasis through up-regulation of mir-141 by modulating pkcdelta and c-src signaling pathway. Int. J. Mol. Sci. 2014;15:11760–11772. doi: 10.3390/ijms150711760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J.F., Chi M.C., Lin C.Y., Lee C.W., Chang T.M., Han C.K., Huang Y.L., Fong Y.C., Chen H.T., Tang C.H. PM2.5 facilitates il-6 production in human osteoarthritis synovial fibroblasts via ask1 activation. J. Cell. Physiol. 2021;236:2205–2213. doi: 10.1002/jcp.30009. [DOI] [PubMed] [Google Scholar]
- 36.Wu M.H., Tsai C.H., Huang Y.L., Fong Y.C., Tang C.H. Visfatin promotes il-6 and tnf-alpha production in human synovial fibroblasts by repressing mir-199a-5p through erk, p38 and jnk signaling pathways. Int. J. Mol. Sci. 2018;19:190. doi: 10.3390/ijms19010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nugent M. Micrornas: Exploring new horizons in osteoarthritis. Osteoarthr. Cartil. 2016;24:573–580. doi: 10.1016/j.joca.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Law Y.Y., Lin Y.M., Liu S.C., Wu M.H., Chung W.H., Tsai C.H., Fong Y.C., Tang C.H., Wang C.K. Visfatin increases icam-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by reducing mir-320a expression. Aging. 2020;12:18635–18648. doi: 10.18632/aging.103889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Modawi R.N., Brinchmann J.E., Karlsen T.A. Multi-pathway protective effects of micrornas on human chondrocytes in an in vitro model of osteoarthritis. Mol. Ther.-Nucleic Acids. 2019;17:776–790. doi: 10.1016/j.omtn.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H.-P., Wu Y.-C., Chen B.-C., Liu S.-C., Li T.-M., Huang W.-C., Hsu C.-J., Tang C.-H. Soya-cerebroside reduces interleukin production in human rheumatoid arthritis synovial fibroblasts by inhibiting the erk, nf-κb and ap-1 signalling pathways. Food Agric. Immunol. 2020;31:740–750. doi: 10.1080/09540105.2020.1766426. [DOI] [Google Scholar]
- 41.Lee H.P., Wang S.W., Wu Y.C., Lin L.W., Tsai F.J., Yang J.S., Li T.M., Tang C.H. Soya-cerebroside inhibits vegf-facilitated angiogenesis in endothelial progenitor cells. Food Agric. Immunol. 2020;31:193–204. doi: 10.1080/09540105.2020.1713055. [DOI] [Google Scholar]
- 42.Cheng F.J., Huynh T.K., Yang C.S., Hu D.W., Shen Y.C., Tu C.Y., Wu Y.C., Tang C.H., Huang W.C., Chen Y., et al. Hesperidin is a potential inhibitor against sars-cov-2 infection. Nutrients. 2021;13:2800. doi: 10.3390/nu13082800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H.P., Wang S.W., Wu Y.C., Tsai C.H., Tsai F.J., Chung J.G., Huang C.Y., Yang J.S., Hsu Y.M., Yin M.C., et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric. Immunol. 2019;30:1033–1045. doi: 10.1080/09540105.2019.1660623. [DOI] [Google Scholar]
- 44.Su C.M., Tang C.H., Chi M.J., Lin C.Y., Fong Y.C., Liu Y.C., Chen W.C., Wang S.W. Resistin facilitates vegf-c-associated lymphangiogenesis by inhibiting mir-186 in human chondrosarcoma cells. Biochem. Pharmacol. 2018;154:234–242. doi: 10.1016/j.bcp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Wu K.M., Hsu Y.M., Ying M.C., Tsai F.J., Tsai C.H., Chung J.G., Yang J.S., Tang C.H., Cheng L.Y., Su P.H., et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in h9c2 cardiomyoblast cells via ros suppression. Nutr. Metab. 2019;16:36. doi: 10.1186/s12986-019-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S.C., Tsai C.H., Wu T.Y., Tsai C.H., Tsai F.J., Chung J.G., Huang C.Y., Yang J.S., Hsu Y.M., Yin M.C., et al. Soya-cerebroside reduces il-1 beta-induced mmp-1 production in chondrocytes and inhibits cartilage degradation: Implications for the treatment of osteoarthritis. Food Agric. Immunol. 2019;30:620–632. doi: 10.1080/09540105.2019.1611745. [DOI] [Google Scholar]
- 47.Achudhan D., Liu S.C., Lin Y.Y., Lee H.P., Wang S.W., Huang W.C., Wu Y.C., Kuo Y.H., Tang C.H. Antcin k inhibits vegf-dependent angiogenesis in human rheumatoid arthritis synovial fibroblasts. J. Food Biochem. 2022;46:e14022. doi: 10.1111/jfbc.14022. [DOI] [PubMed] [Google Scholar]
- 48.Su C.-H., Lin C.-Y., Tsai C.-H., Lee H.-P., Lo L.-C., Huang W.-C., Wu Y.-C., Hsieh C.-L., Tang C.-H. Betulin suppresses tnf-α and il-1β production in osteoarthritis synovial fibroblasts by inhibiting the mek/erk/nf-κb pathway. J. Funct. Foods. 2021;86:104729. doi: 10.1016/j.jff.2021.104729. [DOI] [Google Scholar]
- 49.Lee K.T., Su C.H., Liu S.C., Chen B.C., Chang J.W., Tsai C.H., Huang W.C., Hsu C.J., Chen W.C., Wu Y.C., et al. Cordycerebroside a inhibits icam-1-dependent m1 monocyte adhesion to osteoarthritis synovial fibroblasts. J. Food Biochem. 2022:e14108. doi: 10.1111/jfbc.14108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data to this present study are available from the corresponding authors.