Abstract
Plant laccase genes belong to a multigene family, play key roles in lignin polymerization, and participate in the resistance of plants to biotic and abiotic stresses. Switchgrass is an important resource for forage and bioenergy production, yet information about the switchgrass laccase gene family is scarce. Using bioinformatic approaches, a genome-wide analysis of the laccase multigene family in switchgrass was carried out in this study. In total, 49 laccase genes (PvLac1 to PvLac49) were identified; these can be divided into five subclades, and 20 of them were identified as targets of miR397. The tandem and segmental duplication of laccase genes on Chr05 and Chr08 contributed to the expansion of the laccase family. The laccase proteins shared conserved signature sequences but displayed relatively low sequence similarity, indicating the potential functional diversity of switchgrass laccases. Switchgrass laccases exhibited distinct tissue/organ expression patterns, revealing that some laccases might be involved in the lignification process during stem development. All five of the laccase isoforms selected from different subclades responded to heavy metal. The immediate response of lignin-related laccases, as well as the delayed response of low-abundance laccases, to heavy-metal treatment shed light on the multiple roles of laccase isoforms in response to heavy-metal stress.
Keywords: laccase, switchgrass, gene structure, lignification, heavy-metal stress
1. Introduction
Laccase (EC 1.10.3.2) belongs to the multicopper oxidase family, catalyzing the oxidation of a broad range of substrates using molecular oxygen as the final electron acceptor [1]. The catalytic center of laccase shares two conserved copper centers, including the T1 copper site and the T2/T3 trinuclear copper cluster. The reduction of T1 copper simultaneously couples the oxidation of a substrate and the reduction of dioxygen to water at the T2/T3 cluster. The oxidized substrate forms a free radical and undergoes nonenzymatic oxidation or reduction reactions [2]. This unique catalytic process grants laccases a nonspecific catalytic ability, allowing laccases to play multiple functions in living organisms, and to be utilized for biotechnological and environmental applications. Laccase is widely distributed in plants, fungi, bacteria, and insects. Although laccase was first discovered in the Japanese lacquer tree, the characterization and application of plant laccases is still limited compared with the research on microbe laccases [3]. Plant laccases belong to large, multigene families with high redundancy, which has restricted the understanding of their functions in vivo. In Arabidopsis, 17 laccase isoforms were annotated [4], while 24, 30, 52, and even 93 laccase sequences were identified in Citrus sinensis [5], Oryza sativa [6], Setaria viridis [7], and Glycine max [8]. Because of the functional redundancy of laccase genes, most of the laccase mutants identified in Arabidopsis exhibited no phenotype [4], and the genetic manipulation of a single laccase gene showed little influence on the phenotypes [7]. Consequently, recent approaches have proven that the comprehensive analysis of the laccase gene family in plants will accommodate the understanding of their properties and physiological functions.
Lignin is a complex phenolic polymer deposited in the secondary cell walls of all vesicular plants. It consists of canonical hydroxycinnamyl alcohol monomers (known as monolignols) including p-coumaryl (H-type), coniferyl (G-type), and sinapyl (S-type) alcohols. Laccase and peroxidase are the main enzymes catalyzing the polymerization of monolignols. Recent studies have revealed that both the content and composition of lignin are influenced by laccases. The irregular xylem phenotype was observed in the stem of an Arabidopsis lac4 lac17 double mutant with 40% less lignin content [9]. The phylogenetically related laccase genes in different plant species, including Miscanthus sinensis [10], Brachypodium distachyon [11], and Populus tomentosa [12], were also found to be involved in lignification. More recent research identified two laccase genes from Japanese cypress (Chamaecyparis obtusa) with different oxidation activities towards H-type and G-type monolignols, contributing to the precise localization of H-type and G-type lignin in distinct cell-wall layers [13]. Notably, a novel laccase (ChLac8) with caffeyl alcohol (C-type) oxidation activity was identified in Cleome hassleriana. The expression of ChLac8 in the Arabidopsis caffeic acid o-methyltransferase mutant resulted in the formation of C-lignin in the stems of transgenic plants [14]. These recent advances further highlight the importance of laccases in lignin biosynthesis, demanding more genetic evidence to characterize the function of laccase in lignin polymerization.
Many heavy metals are considered essential for plant growth, but toxic to all life forms at high concentrations [15]. For example, copper (Cu) serves as a cofactor and as an activator of enzyme reactions in plants, and plays key roles in photosynthetic and respiratory electron transport [16]. However, excess Cu in the environment, caused by human activities, can be toxic to plants, inhibiting the growth of plants and impairing the photosynthesis process. As a barrier for plant defense against both abiotic and biotic stresses, lignin also plays an important role in the resistance of plants to excess heavy metals. It is speculated that the toxicity of Cu could be alleviated by promoting lignin biosynthesis [17]. Furthermore, excess Cu increased laccase activity in rice, and the expression of Cu-induced OsLAC10 in Arabidopsis enhanced the tolerance of transgenic plants to Cu stress [6]. Plants also responded to other heavy metals including aluminum (Al), cadmium (Cd), and zinc (Zn), and accumulated lignin with different levels of sensitivity [18,19,20]. The process of lignin accumulation in the presence of heavy metals is correlated with induced laccase gene expression levels and enhanced activity of laccases in plant cell walls, but little is known about the functions of specific laccases in the process.
Switchgrass (Panicum virgatum) is a perennial grass that can be used for bioenergy and forage production [21]. In previous studies, we found that lignin modification through the manipulation of monolignol biosynthesis genes significantly enhanced the enzymatic saccharification yield of switchgrass biomass [22]. As a key player in monolignol polymerization, laccases provided promising targets for the genetic modification of the lignin biosynthesis pathway. Furthermore, it is indicated that switchgrass is also a promising platform resource for the bioremediation of contaminated soil. The overexpression of the switchgrass Cd-responsive gene PvBip1 in Arabidopsis significantly improved the plant’s Cd tolerance [23]. Additionally, the engineered switchgrass expressing xplA and xplB efficiently removed hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in a 3-year field trial [24].
Although laccase-directed lignification has been reported to be associated with the defense response against biotic and abiotic stresses, the function of switchgrass laccases in lignin biosynthesis, as well as their response to heavy-metal treatment, is still unknown. Thus, the aim of this study was to predict the PvLac candidates directly involved in lignification, and estimate their response to heavy metals in comparison with other laccase isoforms. In this study, we identified switchgrass laccase genes and analyzed their basic characteristics using bioinformatic methods. We then investigated the expression patterns of laccase candidates from each subclade upon heavy-metal treatment, and evaluated the laccase activity and lignin content in an excess copper environment. Our study highlighted the immediate response of lignin-related laccases, as well as the delayed response of low-abundance laccases to heavy-metal treatment, providing potential laccase candidates that respond to heavy-metal stress and regulate lignin biosynthesis.
2. Materials and Methods
2.1. Identification and Characterization of the Laccase Gene Family in Switchgrass
The laccase amino acid sequences from Oryza sativa [6] and Arabidopsis, obtained from the Arabidopsis information resource (TAIR, www.Arabidopsis.org/index.jsp, 5 October 2021), were used as queries for the BLAST (Basic Local Alignment Search Tool) searches against the switchgrass genome database in Phytozome v12.0 (Panicum virgatum v4.0, DOE-JGI, phytozome.jgi.doe.gov/, 6 October 2021). Using the HAMMER databases (www.ebi.ac.uk/Tools/hmmer/search/hmmscan, 10 October 2021), we further verified all candidate sequences according to the conserved plant laccase domains (Cu-oxidase: PF00394, Cu-oxidase_2: PF07731 and Cu-oxidase_3: PF07732). A total of 49 protein sequences were retrieved and numbered sequentially according to their position on the chromosomes. The chromosomal location information was download from Phytozome databases (phytozome-next.jgi.doe.gov/, 25 October 2021) and drawn using TBtools software [25]. Putative signal peptide length and cleavage site were predicted using SignalP (www.cbs.dtu.dk/services/SignalP/, 2 November 2021). The theoretical isoelectric point (pI) and molecular weight (MW) of the laccase proteins were calculated using the ExPASy website (web.expasy.org/compute_pi/, 2 November 2021).
2.2. Phylogenetic and Gene Duplication Analysis of the Laccase Proteins
The alignment of 17 AtLac, 29 OsLAC, and 49 PvLac peptides was performed using ClustalW in Mega 7.0 [26]. The phylogenetic tree was then constructed using MEGA 7.0 with the neighbor-joining (NJ) method (1000 bootstrap replicates), and then modified in iTOL (itol.embl.de/) [27]. Intra-species synteny blocks were analyzed using one-step MCScanX in TBtools [25]. The Arabidopsis and rice genome sequences and annotation information were downloaded from EnsemblPlants (http://plants.ensembl.org, 18 February 2022). Additionally, the collinearity between switchgrass and rice, and switchgrass and Arabidopsis, was analyzed and visualized using the one-step MScanX function in TBtools.
2.3. Gene Structure Analysis and Identification of Conserved Motifs as Well as Cis-Acting Elements
The exon–intron structures of PvLac genes were determined by comparing the coding sequence of each PvLac gene and its genomic sequence using Gene Structure Display Server (GSDS v2.0, http://gsds.cbi.pku.edu.cn/, 7 November 2021). The conserved domains of PvLACs were identified using the MEME server v5.4.1 (http://meme-suite.org/tools/meme, 7 November 2021). The promoter sequences of PvLac genes (2 kb upstream region) were retrieved from switchgrass genome database in Phytozome v12.0, and the cis-acting element analysis was performed using the online program PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, 17 November 2021). The miR397 target analysis of switchgrass laccases were conducted using psRNATarget (http://www.zhaolab.org/psRNATarget, 12 November 2021).
2.4. PvLac Expression Profiles in Various Switchgrass Tissues
The RNA-seq data on switchgrass laccase genes were obtained from JGI Plant Gene Atlas (JGI Plant Gene Atlas: An updateable transcriptome resource for improving structural annotations and functional descriptions across the plant kingdom, Sreedasyam et al., unpublished). The heatmap was constructed using the OmcStudio tools (https://www.omicstudio.cn, 17 December 2021). The expression data are shown in Table S1.
To elucidate the expression of PvLacs in different organs/tissues of switchgrass, the internodes, nodes, leaf blades, leaf sheaths, roots, and crowns were separately sampled with E4-stage switchgrass plants (growth stages determined using the criteria described by Moore et al. [28]. The relative expression level of selected PvLacs, as well as monolignol biosynthesis genes (COMT and CCoAOMT) in those samples, were then determined using qRT-PCR for collinear analysis.
2.5. Plant Materials and Treatments
The lowland switchgrass cultivar Alamo was used for all the experiments in this study. Seeds were spread on wet filter paper and incubated at room temperature for one week. The germinated seedlings were then transferred to 1/4 Murashige and Skoog (MS) solution to grow for another week. The growth solution was supplemented with different heavy-metal salts (CuSO4, NiCl2, FeSO4 and CdCl2) with concentrations of 100 μM and 500 μM for treatment. Samples were taken at 0 h, 6 h, and 24 h after treatment, immediately frozen in liquid nitrogen, and stored at −80 °C. E1-stage switchgrass plants were grown under the same conditions for 4 weeks, and different tissues of treated plants were collected for lignin analysis. Additionally, the seedlings were grown in 1/4 Murashige and Skoog (MS) under 4 °C for cold treatment, and 250 mM mannitol was supplemented for drought treatment. Samples were taken 1 h and 4 h after treatment.
2.6. RNA Extraction and qRT-PCR Analysis
Total RNA of samples was extracted using the Omega E.Z.N.A Plant RNA Kit (Omega Bio-Tek, Norcross, Georgia, USA) and reverse-transcribed into cDNA using Easyscript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech Co., Ltd., Beijing, China). To quantify the relative expression level of laccases in response to heavy-metal stress, qRT-PCR was performed using 2 × SYBR Green qPCR Mix (Shandong Sparkjade Biotechnology Co., Ltd., Qingdao, China). PvUbq2 (GenBank accession NO: HM209468) was used as reference to normalize the expression data. The primers used in this work are listed in Table S2.
2.7. Protein Extraction and Enzyme Activity Assay
The switchgrass plant materials were ground well in liquid nitrogen and the soluble protein was extracted as described before [29]. Laccase activity was determined using 2, 2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as the substrate. The laccase activity was calculated as described by measuring the increase in absorbance at 420 nm [10].
2.8. Lignin Analysis
Different tissues (leaf, root, and stem) of plants after heavy-metal treatment were harvested and oven-dried for 2 days at 55 °C. The dried samples were then ground using a CryoMill (Retsch GmbH, Haan, German) and the soluble component was removed via reflux extraction with acetone for 12 h. An acetyl bromide assay was performed to determine lignin content [22]. The cross sections of switchgrass internodes, as well as the root samples after heavy-metal treatment, were stained using the HCl-phloroglucinol method, as previously described [10].
3. Results
3.1. Identification and Characterization of LACs in the Switchgrass Genome
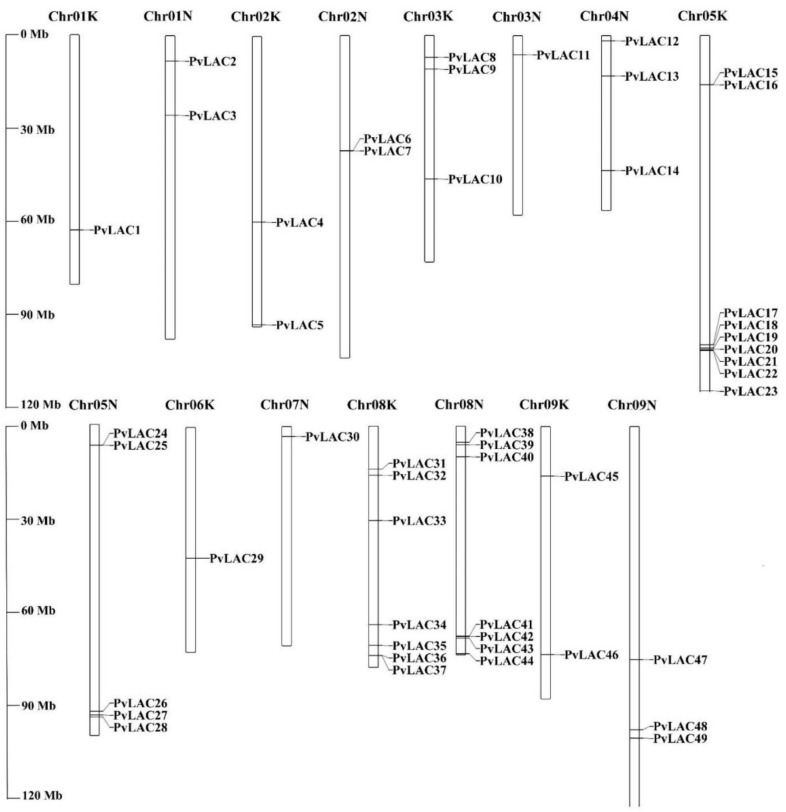
To identify the laccase genes in the switchgrass genome, AtLAC and OsLAC amino acid sequences were used as queries to BLAST against the switchgrass genome database in Phytozome. The putative candidates were then verified according to the conserved plant laccase domains (PF07731, PF07732, and PF00394) (Figure S1) [30]. In total, 49 laccase gene sequences were identified and named PvLac1 to PvLac49, according to their location on the chromosomes (Figure 1). The proteins encoded by the 49 identified PvLacs had 516 to 649 amino acid residues, and their molecular weights ranged from 57.95 kDa to 68.84 kDa. The theoretical isoelectric point (pI) analysis showed that the pI of switchgrass laccase proteins ranged from 5.19 (PvLAC10) to 9.78 (PvLAC14) (Table 1). More than 80% of switchgrass laccase had a signal peptide, which allows laccase to be secreted extracellularly (Table 1).
Figure 1.
Chromosomal localization of switchgrass laccase gene family. The chromosomal localization of PvLac genes was based on the information provided in Phytozome v12.0. ChrK and ChrN are two sets of subgenomes of switchgrass (2n = 4x = 36). The scale bar is shown on the left.
Table 1.
Characterization of putative switchgrass laccase proteins. ORF, open reading frame; pI, isoelectric point.
| Gene Name | Gene ID | Number of Amino Acids (aa) | Molecular Weight (kDa) | Signal Peptide Cleavage Site | Signal Peptide Length | ORF Length | pI |
|---|---|---|---|---|---|---|---|
| PvLAC1 | Pavir.1KG385700 | 581 | 63.6 | VAA-TP | 24 | 1746 | 6.76 |
| PvLAC2 | Pavir.1NG538200 | 581 | 64.0 | VAA-SP | 24 | 1746 | 6.39 |
| PvLAC3 | Pavir.1NG327600 | 575 | 63.0 | AQA-LR | 24 | 1728 | 6.62 |
| PvLAC4 | Pavir.2KG328400 | 570 | 62.4 | ARA-AT | 25 | 1713 | 6.95 |
| PvLAC5 | Pavir.2KG017500 | 569 | 62.1 | ADA-AV | 24 | 1710 | 8.55 |
| PvLAC6 | Pavir.2NG308200 | 566 | 62.4 | ARA-AT | 24 | 1701 | 6.81 |
| PvLAC7 | Pavir.2NG307700 | 568 | 62.4 | ARA-AT | 20 | 1706 | 8.84 |
| PvLAC8 | Pavir.3KG084800 | 619 | 68.5 | TMA-LP | 25 | 1860 | 7.09 |
| PvLAC9 | Pavir.3KG123800 | 560 | 61.2 | AEA-KV | 19 | 1683 | 6.51 |
| PvLAC10 | Pavir.3KG266100 | 530 | 58.2 | / | / | 1593 | 5.19 |
| PvLAC11 | Pavir.3NG041300 | 563 | 62.1 | TMA-LP | 22 | 1692 | 6.44 |
| PvLAC12 | Pavir.4NG327800 | 619 | 67.1 | / | / | 1860 | 8.98 |
| PvLAC13 | Pavir.4NG191400 | 585 | 63.0 | AEA-ET | 32 | 1758 | 6.92 |
| PvLAC14 | Pavir.4NG191000 | 579 | 62.6 | AAA-RT | 29 | 1740 | 9.78 |
| PvLAC15 | Pavir.5KG622300 | 578 | 62.9 | AEG-AI | 24 | 1737 | 7.64 |
| PvLAC16 | Pavir.5KG622200 | 582 | 63.3 | VQG-IT | 28 | 1749 | 8.83 |
| PvLAC17 | Pavir.5KG610700 | 569 | 62.7 | AGA-EV | 22 | 1710 | 5.90 |
| PvLAC18 | Pavir.5KG623300 | 580 | 63.4 | AAG-DT | 34 | 1743 | 9.05 |
| PvLAC19 | Pavir.5KG629400 | 649 | 67.6 | ADA-AT | 16 | 1950 | 5.81 |
| PvLAC20 | Pavir.5KG629500 | 535 | 58.0 | / | / | 1608 | 5.95 |
| PvLAC21 | Pavir.5KG634700 | 570 | 60.7 | AHA-AT | 18 | 1713 | 5.82 |
| PvLAC22 | Pavir.5KG634800 | 647 | 67.2 | ADA-AT | 16 | 1944 | 6.10 |
| PvLAC23 | Pavir.5KG007300 | 549 | 60.6 | VSS-AE | 22 | 1650 | 9.63 |
| PvLAC24 | Pavir.5NG584800 | 578 | 63.0 | TEG-AI | 24 | 1737 | 7.21 |
| PvLAC25 | Pavir.5NG584700 | 584 | 63.4 | VQG-IT | 29 | 1755 | 8.73 |
| PvLAC26 | Pavir.5NG569300 | 569 | 62.8 | AGA-EV | 22 | 1710 | 5.97 |
| PvLAC27 | Pavir.5NG585100 | 573 | 62.8 | AAG-DT | 27 | 1722 | 8.87 |
| PvLAC28 | Pavir.5NG053100 | 589 | 65.5 | / | / | 1770 | 6.60 |
| PvLAC29 | Pavir.6KG211600 | 516 | 55.8 | / | / | 1551 | 6.24 |
| PvLAC30 | Pavir.7NG016200 | 564 | 62.2 | GHA-AT | 18 | 1695 | 8.76 |
| PvLAC31 | Pavir.8KG104100 | 637 | 68.7 | / | / | 1914 | 9.77 |
| PvLAC32 | Pavir.8KG112100 | 567 | 62.3 | GRA-AI | 19 | 1704 | 9.05 |
| PvLAC33 | Pavir.8KG177500 | 573 | 61.9 | VVA-KE | 25 | 1722 | 6.43 |
| PvLAC34 | Pavir.8KG103400 | 583 | 62.8 | AEA-ET | 29 | 1752 | 7.67 |
| PvLAC35 | Pavir.8KG057100 | 563 | 61.5 | / | / | 1692 | 5.72 |
| PvLAC36 | Pavir.8KG368500 | 576 | 63.3 | AMA-AV | 28 | 1731 | 5.86 |
| PvLAC37 | Pavir.8KG368600 | 587 | 64.7 | GEA-AV | 22 | 1764 | 6.04 |
| PvLAC38 | Pavir.8NG320100 | 589 | 65.0 | GEA-AV | 24 | 1770 | 6.33 |
| PvLAC39 | Pavir.8NG328000 | 596 | 65.2 | AAG-AV | 28 | 1791 | 6.43 |
| PvLAC40 | Pavir.8NG353900 | 565 | 61.4 | VVA-KE | 22 | 1698 | 6.62 |
| PvLAC41 | Pavir.8NG327600 | 621 | 69.3 | AVA-AS | 24 | 1866 | 6.13 |
| PvLAC42 | Pavir.8NG327500 | 589 | 65.3 | GEA-AV | 24 | 1770 | 6.11 |
| PvLAC43 | Pavir.8NG321100 | 617 | 68.8 | AVA-AS | 23 | 1854 | 6.10 |
| PvLAC44 | Pavir.8NG003600 | 565 | 61.4 | VVA-KE | 22 | 1698 | 6.62 |
| PvLAC45 | Pavir.9KG472600 | 607 | 66.0 | ALA-EE | 28 | 1824 | 8.58 |
| PvLAC46 | Pavir.9KG493900 | 571 | 62.3 | SHG-AT | 22 | 1716 | 8.64 |
| PvLAC47 | Pavir.9NG438800 | 585 | 63.0 | AEA-ET | 32 | 1758 | 6.92 |
| PvLAC48 | Pavir.9NG711200 | 601 | 65.3 | ALA-EE | 26 | 1806 | 8.43 |
| PvLAC49 | Pavir.9NG700300 | 571 | 62.3 | SHG-AT | 22 | 1716 | 8.74 |
3.2. Phylogenetic Analysis of PvLac Family
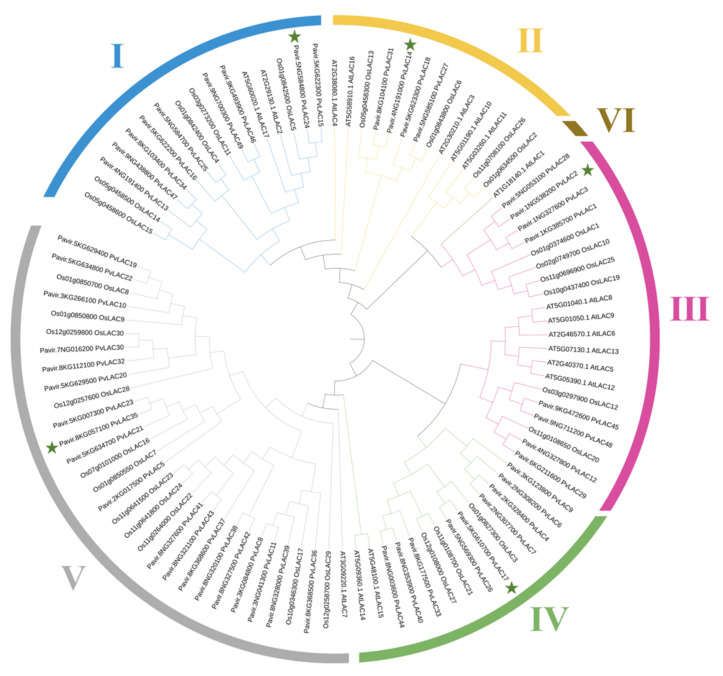
To further understand the phylogenetic relationships among PvLAC proteins, we constructed a neighbor-joining (NJ) phylogenetic tree using 17 AtLAC, 29 OsLAC, and 49 PvLAC amino acid sequences. As shown in Figure 2, 49 switchgrass laccases can be divided into five subclades, based on the previously reported studies on Arabidopsis [31]; this indicates an evolutionary conservation between these two species. However, there are also distinct differences: switchgrass and rice laccase seem to lack the Arabidopsis homologous gene in subclade VI, while the members in the subclade V are largely expanded (11 OsLACs and 22 PvLACs). In detail, nine PvLACs are clustered in subclade I with AtLAC17, and four PvLACs are clustered in subclade II with AtLAC4. Both AtLAC17 and AtLAC4 were reported to be necessary for lignin biosynthesis [9].
Figure 2.
Phylogenetic analysis of PvLACs, OsLACs, and AtLACs. In total, 49 switchgrass, 29 rice, and 17 Arabidopsis laccase proteins were aligned using the ClustalW program, and the phylogenetic tree was constructed with MEGA 7.0 software using the neighbor-joining method with 1000 bootstrap replicates. The asterisks represent the five switchgrass genes selected from each subclade in this study.
3.3. Chromosomal Locations and Duplication Events in PvLac Gene Family
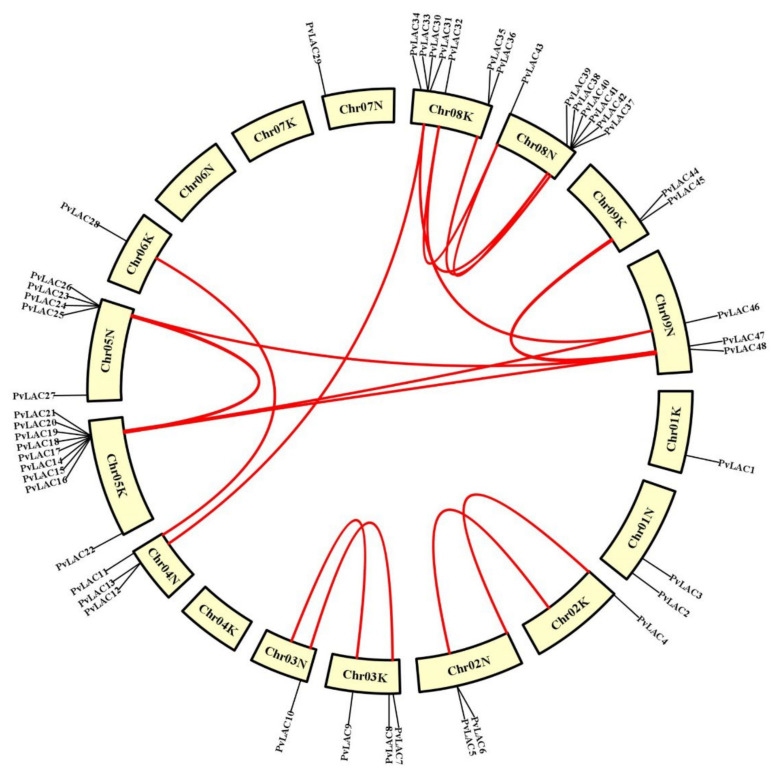
Since switchgrass is an autotetraploid with two subgenomes (2n = 4x = 36), the localization of laccases on the chromosomes was analyzed. As shown in Figure 1, the laccase genes were unevenly distributed across 15 chromosomes. Most of the PvLac genes were found on Chromosome 05 K/N and Chromosome 08 K/N (14 on Chr05K/N and Chr08K/N, respectively), yet only one laccase gene was identified on Chr06K and Chr07N. Three PvLac genes were localized on Chr01K/N and Chr04K/N, while four were found on Chr02K/N and Chr03K/N. The PvLac genes were gathered irregularly on each chromosome; they localized next to each other close to the two ends of Chr05K/N and Chr08K/N due to tandem duplication (Figure 1, Table S2), while the distribution was relatively uniform on other chromosomes. Segmental and tandem duplication play important roles in the evolution of a large gene family. In total, three paralogous gene pairs of PvLac genes, in tandem duplication, were found in the genome (Figure 3, Table S3). More than 19 PvLac gene pairs were confirmed as segmental duplications localizing on different chromosomes, including PvLac4/PvLac7, PvLac8/PvLac11, PvLac16/PvLac25, PvLac33/PvLac40, and PvLac45/PvLac48 on Chr02K/Chr02N, Chr03K/Chr03N, Chr05K/Chr05N, Chr08K/Chr08N, and Chr09K/Chr09N, respectively. The synteny analysis of laccase genes between switchgrass and rice, and switchgrass and Arabidopsis, revealed a higher collinearity between the PvLac and OsLac genes. In detail, 30 pairs with collinearity were found between the 22 PvLac genes and 12 OsLac genes, while only 13 pairs of collinearity were observed between the 8 PvLac genes and 4 AtLac genes (Figure S2).
Figure 3.
Colinear analysis of PvLac gene family. Red lines indicate duplicated PvLac gene pairs. The red line represented collinearity relationship between PvLac genes. Gene names are listed outside the circle, according to their chromosomal location.
3.4. Gene Structure, miRNA Target Site Prediction and Conserved Motif Analysis of PvLac Family
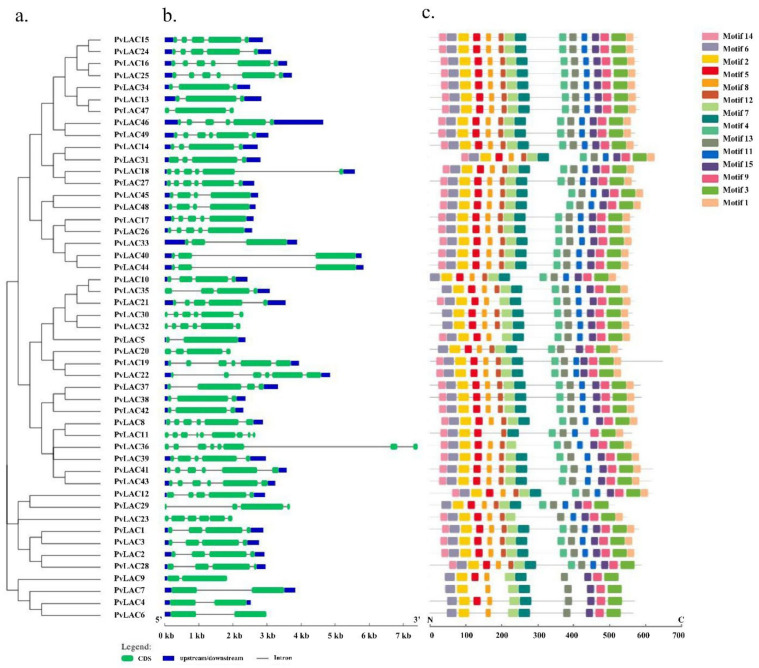
Another NJ phylogenetic tree was constructed to align the 49 PvLac genes (Figure 4). PvLac genes with a closer genetic relationship tend to share a similar gene structure. The exon number of PvLacs varied from two to nine. PvLac36 and PvLac11 contained eight and nine exons, respectively, while PvLac4, PvLac 5, PvLac 6, PvLac 7, and PvLac 9 exhibited only two. Most of PvLacs (36/49) contained three to five introns, among which long introns with a length greater than 2000 bp were found within PvLac18, PvLac36, PvLac40, and PvLac44.
Figure 4.
Structural analysis of laccases from switchgrass: (a) A neighbor-joining tree of 49 PvLACs constructed using MEGA 7.0; (b) the structure of 49 PvLAC genes. Green squares represent exons (coding sequences, CDS) and gray lines indicate introns, while the blue squares refer to the 5’ untranslated region (UTR) and 3’ UTR sequences; (c) conserved motifs of laccase genes in 49 switchgrasses. The colored square represents 15 conserved motifs, shown in supplementary table (Table S4).
To further elucidate the structure and function divergence of PvLAC proteins, we annotated 15 conserved motifs predicted by MEME on the full-length PvLAC sequences (Table S4, Figure 4c). In general, PvLACs shared similar motif profiles, especially members of the same subclade. Members of subclades I, III, and IV contained all 15 motifs, except PvLAC23 and PvLAC29; this indicates a possible functional redundancy among those members. Some motifs (motifs 1, 2, 6, 8, 11, and 14) were highly conserved and distributed across all PvLAC sequences, suggesting a potentially important role in the function of laccases. In subclade V, which is the most expanded subclade, nine laccases (PvLAC4, PvLAC6, PvLAC7, PvLAC9, PvLAC10, PvLAC20, PvLAC30, PvLAC32, and PvLAC35) lack motif 3. The distinct structural difference within PvLACs in the expanded subgroup may indicate functional divergence among proteins.
The expression of plant laccases was tightly regulated by miRNAs, including miR397, miR408, miR528, and miR857. The miRNA397 family in plants mainly targets the laccase genes involved in the lignin biosynthesis process, providing reliable evidence to predict the function of laccases. The sequences of the 49 PvLacs were analyzed for the presence of miRNA target sites, and 20 of them were identified as the targets of miR397; however, only 4 and 2 PvLacs can be targeted by miR408 and miR857, respectively (Table S5). The results revealed complex post-transcriptional regulation of PvLacs by miRNAs, indicating the functional diversity of laccases in the development of switchgrass.
3.5. Cis-Elements Analysis of PvLac Promoters
In order to investigate the regulation mechanism of laccase expression in the presence of abiotic and biotic stresses, the cis-acting elements of laccase promoters were predicted using PlantCARE. In the promoters, hormone-responsive, stress-responsive, flavonoid biosynthetic, circadian-control and light-response elements were identified (Table 2, Figure S3). To be more specific, the 49 PvLac promoters contain hormone-responsive elements related to (methyl jasmonate) MeJA (41/49), auxin (25/49), gibberellin (33/49), and abscisic acid (47/49). All 49 of the PvLac promoters contained light-response elements, and 10 of them exhibited circadian-rhythm-control elements, which indicates a potential role of laccases in cell growth. Only promoters of PvLac3, PvLac11, and PvLac48 share the flavonoid biosynthetic element, pointing to the involvement of these laccases in flavonoid biosynthesis. In addition, there were also abiotic stress response elements found across the 49 promoters, including elements responsive to drought, low temperature, and wounds. Based on the cis-element analysis results, the expression levels of PvLac2, PvLac14, and PvLac17 were evaluated after treatments under low temperature (4 °C) and high osmotic pressure (250 mM mannitol). The cold treatment induced the expression level of PvLac17 slightly, while both PvLac2 and PvLac17 responded to drought treatment significantly (Figure S4). The various regulatory elements predicted on the promoters of PvLacs confirmed the functional divergence of the laccase family, both in the stress response and plant growth in switchgrass.
Table 2.
The cis-element analysis of PvLAC promoters. MeJA, methyl jasmonate. The plus signs indicate the existence of a corresponding element. The detailed cis-element information is shown in Figure S3.
| Gene Name | Hormones-Responsive | Stress-Responsive | Flavonoid Biosynthetic | Circadian Control | Light Response | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MeJA | Auxin | Abscisic Acid | Gibberellin | Salicylic Acid | Low Temperature | Stress and Defense | Drought-Inducibility | ||||
| PvLAC1 | + | + | + | + | + | ||||||
| PvLAC2 | + | + | + | + | + | ||||||
| PvLAC3 | + | + | + | + | + | + | |||||
| PvLAC4 | + | + | + | + | + | ||||||
| PvLAC5 | + | + | + | + | + | + | + | + | + | + | |
| PvLAC6 | + | + | + | + | + | ||||||
| PvLAC7 | + | + | + | + | + | + | + | + | |||
| PvLAC8 | + | + | + | + | + | ||||||
| PvLAC9 | + | + | + | + | + | + | + | ||||
| PvLAC10 | + | + | + | + | + | + | + | ||||
| PvLAC11 | + | + | + | + | + | + | |||||
| PvLAC12 | + | + | + | + | + | + | + | + | + | + | |
| PvLAC13 | + | + | + | + | + | + | + | + | |||
| PvLAC14 | + | + | + | + | + | ||||||
| PvLAC15 | + | + | + | + | + | + | + | ||||
| PvLAC16 | + | + | + | + | + | ||||||
| PvLAC17 | + | + | + | + | + | + | + | + | |||
| PvLAC18 | + | + | + | + | + | ||||||
| PvLAC19 | + | + | + | + | + | + | + | + | |||
| PvLAC20 | + | + | + | + | + | + | + | ||||
| PvLAC21 | + | + | + | + | + | ||||||
| PvLAC22 | + | + | + | + | + | + | + | + | + | ||
| PvLAC23 | + | + | + | + | + | ||||||
| PvLAC24 | + | + | + | + | + | + | + | ||||
| PvLAC25 | + | + | + | + | + | + | |||||
| PvLAC26 | + | + | + | + | + | + | + | ||||
| PvLAC27 | + | + | + | + | + | + | |||||
| PvLAC28 | + | + | + | + | + | ||||||
| PvLAC29 | + | + | + | + | + | ||||||
| PvLAC30 | + | + | + | + | + | + | + | ||||
| PvLAC31 | + | + | + | + | + | + | + | ||||
| PvLAC32 | + | + | + | + | + | + | |||||
| PvLAC33 | + | + | + | + | + | + | |||||
| PvLAC34 | + | + | + | + | + | + | + | ||||
| PvLAC35 | + | + | + | + | + | + | + | + | + | ||
| PvLAC36 | + | + | + | + | + | + | |||||
| PvLAC37 | + | + | + | + | + | + | + | ||||
| PvLAC38 | + | + | + | + | + | + | + | ||||
| PvLAC39 | + | + | + | + | + | ||||||
| PvLAC40 | + | + | + | + | + | ||||||
| PvLAC41 | + | + | + | + | + | + | |||||
| PvLAC42 | + | + | + | + | + | ||||||
| PvLAC43 | + | + | + | + | + | + | |||||
| PvLAC44 | + | + | + | + | + | ||||||
| PvLAC45 | + | + | + | + | + | ||||||
| PvLAC46 | + | + | + | + | + | + | |||||
| PvLAC47 | + | + | + | + | + | + | + | ||||
| PvLAC48 | + | + | + | + | + | + | |||||
| PvLAC49 | + | + | + | + | + | + | |||||
3.6. Expression Patterns of PvLac Genes in Different Tissues
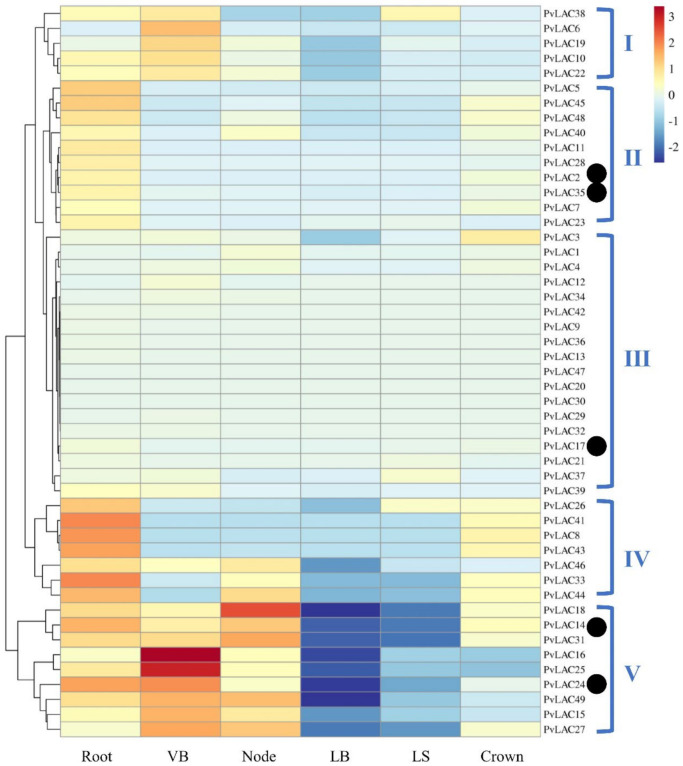
The expression pattern of plant laccases was tightly connected with their functions (He et al., 2019). To reveal the expression profiles of PvLacs in different tissues, a clustering heatmap was deduced based on the available expression data from Phytozome (https://phytozome-next.jgi.doe.gov/, 2021.12.09). Accordingly, the 49 switchgrass laccases can be classified into five subgroups according to their tissue-specific expression profiles (Table S1, Figure 5), suggesting various transcriptional regulation mechanisms for PvLacs along the switchgrass tissue. The members of subgroups IV and V showed the highest expression abundance among all the laccases, with an expression specificity in vascular bundles, nodes, and roots that were highly lignified. Conversely, the members of subgroup III were very poorly expressed. For example, PvLac20, PvLac30, and PvLac47 were not active in any tissues. In addition, the expression of genes in subgroup II was restricted to roots and crowns with only trace expression in leaf blades and sheaths. The remaining laccase genes in subgroup I experienced a relatively mild expression profile, with moderate abundance in all the tissues except leaf blades.
Figure 5.
Expression profile of the PvLacs in E4 stage switchgrass. The detailed expression data were obtained from JGI Plant Gene Atlas (https://phytozome-next.jgi.doe.gov/geneatlas/, 9 December 2021) and are listed in Table S1. The selected ones in this study are marked with black dots. VB, vesicular bundle of the third internode; LB, leaf blade; LS, leaf sheath. The numbers listed in the right indicate the classification of PvLacs based on the tissue-specific expression data.
To further evaluate the expression specificity of PvLacs, all the internodes, leaf sheathes, and leaf blades of E4-stage switchgrass plants were sampled for detailed expression analysis. Based on the heatmap, as well as the phylogenetic analysis, five genes from different subclades (PvLac2, PvLac14, PvLac17, PvLac24, and PvLac35) with high expression levels were selected (Figure 2) for expression analysis using qRT-PCR. Consistent with the mRNA-seq data, all selected laccases showed the highest expression abundance in internodes, except PvLac17 (Figure S5a). Notably, PvLac24 was expressed collinearly with two monolignol biosynthesis genes (COMT and CCoAOMT). The expression levels of PvLac24 and PvLac2 decreased along the stem and were positively correlated with lignin content (Figure S5b), potentially indicating a function in the lignification process.
3.7. Expression Patterns of Five PvLac Genes in Response to Various Heavy-Mental Treatments
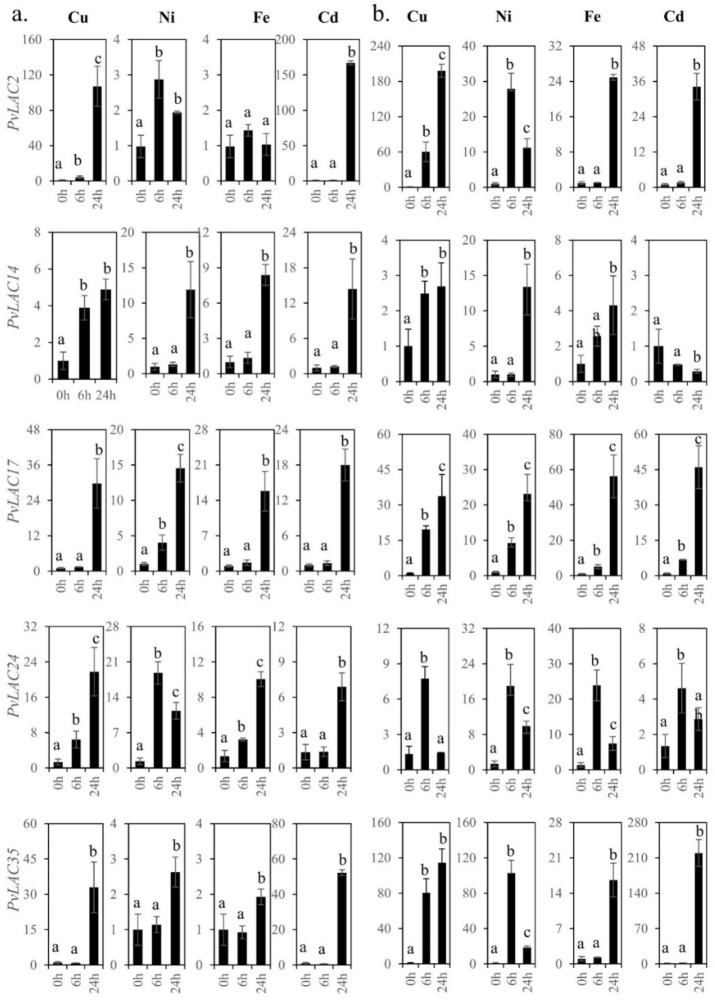
Previous studies have shown that laccase genes responded to abiotic stresses and played a key role in plant defense (Song et al., 2018). The analysis of the cis-elements on PvLac promoters indicated that the PvLac genes may respond to multiple stresses. The expression level was induced for all the selected genes in response Cu, Ni, Fe, and Cd stress, either at a concentration of 100 μM or 500 μM, compared to the mock treatment control (Figure 6). Among the laccases selected, the expression levels of PvLac2 and PvLac35, which were low in switchgrass (Figure 5 and Figure S5), were most significantly upregulated upon heavy-metal stress. After Cu and Cd treatment for 24 h, both genes experienced an over 100-fold induction of expression. All four of the heavy metals remarkably increased PvLac17 expression level, even at a low concentration. Additionally, the two remaining laccases (PvLac14 and PvLac24) were moderately induced by Cd and Cu, but appeared to be more sensitive to Ni.
Figure 6.
Response of PvLacs to heavy-metal treatment at concentrations of (a) 100 μM and (b) 500 μM. Significant differences are indicated by different letters (a, b, and c) after ANOVA analysis (p < 0.05). Error bars represent SD (n = 3).
Under low-concentration treatment, the five laccases shared a similar induction pattern to Cd, while clear differences were observed in their response to Cu, Ni, and Fe (Figure 6a). Fe treatment did not alter the expression of PvLac2. However, only PvLac14 and PvLac24 expression levels were significantly upregulated after 6 h of Cu treatment at a 100 μM concentration. Conversely, most of the genes responded to heavy-metal stress quickly at 6 h, but underwent upregulation or downregulation at 24 h under high-concentration treatment (Figure 6b). In particular, the expression level of PvLac24 significantly increased within 6h, followed by a sharp decrease at 24 h for all treatments at a 500 μM concentration.
To confirm the response of laccases to heavy-metal treatment, based on the RNAseq data (Table S1), two highly expressed laccase genes (PvLac16 and PvLac25), as well as two laccases with no expression determined in any of the samples (PvLac20 and PvLac30), were also selected for analysis. The expression levels of all the selected laccases were affected by heavy metals. However, the highly expressed laccases were only moderately upregulated, or even repressed, after heavy-metal treatment, while the laccases with low expression levels were significantly induced (Figure S4b).
3.8. Heavy-Metal Treatment Induced Both Lignin Content and Laccase Activity in Switchgrass
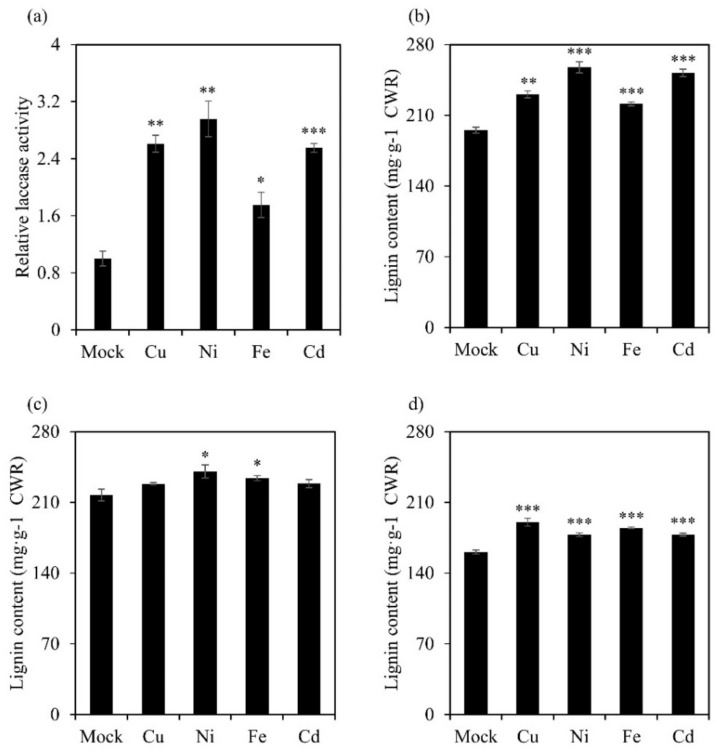
After a high concentration of heavy-metal treatment, the roots of switchgrass seedlings were stained red, suggesting an accumulation of lignin (Figure S6). The excess heavy metal also elevated the laccase activity in switchgrass. After treatment with Cu for one day, the laccase activity was induced 2.5-fold compared with the mock control. Nevertheless, treatment with Ni, Fe, and Cd also led to a 1.6- to 3-fold increase (Figure 7a), accompanied by the increased lignin staining.
Figure 7.
Lignin analysis and laccase activity analysis of switchgrass after heavy-metal treatments: (a) laccase activity of switchgrass after heavy-metal treatments. The E1-stage switchgrass was grown in 1/4 Murashige and Skoog (MS) solution supplemented with different heavy-metal salts (CuSO4, NiCl2, FeSO4, and CdCl2; 500 μM) for 4 weeks. Lignin content of switchgrass (b) root, (c) stem, and (d) leaf were measured after the treatment. Values are means ± SE (n = 3). The asterisks indicate significant differences calculated using t-test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
To evaluate the impact of heavy-metal stress on lignin content and deposition, different samples (root, stem, and leaf) were collected for lignin analysis. The existence of heavy metals significantly induced the lignin content in the root and leaf. More specifically, excess Ni and Cd resulted in 32% and 29% more lignin in the roots, while the plant treated with Cu and Fe accumulated 18% and 15% more lignin in the leaves (Figure 7b,d). However, the degree of lignification in the stems of the treated plants was only slightly affected. Fe and Ni treatment increased the lignin content by 8% and 11%, respectively (Figure 7c).
4. Discussion
Laccases are ubiquitous copper oxidases widely distributed in fungi, bacteria, insects, and plants. The plant laccase multigene family is quite extensive, and because of the functional redundancy of plant laccase genes, the specific function of individual isoforms remains to be elucidated. Recent studies revealed that plant laccases were essential for secondary cell-wall biosynthesis through monolignol oxidation [10,13], pigment formation via flavonoid polymerization [32], cell morphology [9], and resistance to biotic and abiotic stresses [6]. Switchgrass is an important perennial crop that produces both forage and biofuel feedstock. However, knowledge about the function of switchgrass laccases is very limited. In this study, the laccase gene family in switchgrass was intensively studied, and the basic characteristics were investigated using bioinformatics.
The laccase multigene family varies in isoform numbers among different plant species, with 17 in Arabidopsis [4], 23 in moso bamboo [33], 29 in sugarcane [34] and Brachypodium [11], 49 in poplar [35], and even 93 in soybean [36]. The genome of Panicum virgatum harbors an expanded laccase gene family with 49 members, which is comparable to the number observed in Setaria viridis [7]. The switchgrass laccases contained conserved copper-binding domains, and most of the putative proteins, including a secretion-signal peptide, were predicted to be localized extracellularly. However, the overall similarity among switchgrass laccase sequences was relatively low (Table S6), and the PvLac genes were different in structure, which indicates a functional diversity of laccases in switchgrass.
Similar to other monocot plants including rice [6] and maize [37], the switchgrass laccase family can be divided into five subclades, whereas dicot plants including the Arabidopsis [4] and citrus [5] laccase families had six subclades. Tandem and segmental duplication of laccases localized on Chr05 and Chr08 contributed to the expansion of laccase isoforms in switchgrass, leading to the expansion of subclade V laccase members. The absence of subclade VI members in grass genomes was reported, as well as the duplication of members in subclade V, specifically in species within the Paniceae tribe [6,8]. To elucidate the evolutionary significance of the loss and duplication of specific laccase subclade members, the functional characterization of individual members is required. However, the biological function of Arabidopsis subclade laccases (AtLac1 and AtLac6) remains unknown. Recent studies revealed the neofunctionalization of grass subclade V laccase. The Miscanthus subclade V laccase MsLac3 is mostly expressed in leaves. Stem-specific expression of MsLac3 in the Arabidopsis lac4 lac17 double mutant restored the lignin content, but failed to complement the growth phenotype [38]. The expression level of subclade V laccase PtLac110 in poplar was significantly upregulated in tension wood, which suggests a potential role in “stress” lignin formation [39]. Additionally, a special subclade V laccase from Cleome hassleriana, ChLAC8, facilitated the polymerization of C-lignin both in vitro and in vivo [14]. It is very likely that the laccase genes in different subclades shared a redundant function in lignification based on the catalytic ability of the monolignols. The specialized functions of subclade V laccases indicate the importance of laccase in plant growth and development, suggesting the potential neofunctionalization of expanded subclade V laccase genes in switchgrass.
To identify switchgrass laccases that potentially participated in lignification, a combination of phylogenetic analysis, tissue-specific expression pattern analysis, and collinear expression analysis with monolignol biosynthesis genes was carried out. It is a proven efficient way to predict laccase function via the clustering of laccase genes to a given group related to lignification. Laccases identified as orthologues of AtLAC17 in Brachypodium (BdLAC5) and Miscanthus (MsLAC1) were confirmed to play an important role in lignification [10,11]. In our study, nine switchgrass laccases were clustered with AtLac17, and six of them (PvLac16, PvLac17, PvLac24, PvLac25, PvLac27, and PvLac49) were dominantly expressed in the vascular bundle of mature stems and clustered into the same subgroup. Among those laccase candidates, PvLac24 was predicted to be the best target of miR397, with the highest expectation (Figure S5). All five of the selected laccases from each subclade showed the highest expression in switchgrass internodes, in line with typical lignin biosynthesis genes COMT and CCoAOMT; this suggests a potential role for these PvLACs in lignification. Among them, only PvLac14 and PvLac24 were expressed (Figure S5) exclusively in the stem, suggesting the involvement of PvLac14 and PvLac24 in stem developmental lignification. However, the spatial expression profile is not sufficient to envision a major role of laccase in lignification. In Arabidopsis, decreased lignin content was observed in the seed coat of the lac15 mutant as well as the stem of the lac4 lac17 double mutant [9,40]; conversely, the lac2 mutant significantly accumulated lignin in roots [41]. Since the majority of PvLacs were highly expressed in either stems or roots containing a significant number of lignified sclerenchyma cells, the major role of individual laccases clustered in different subclades in lignification still requires further determination. Taken together, our results provide genetic evidence that strongly suggests the involvement of PvLac24 in stem developmental lignification, but an in vivo study of gain or loss of function is still required to understand the specific function of individual laccase isoforms in switchgrass.
The analysis of all PvLac promoters indicated the presence of various cis-elements, including certain elements related to abiotic stress, such as TC-rich repeats (defense and stress responsiveness), MYB-binding sites (drought-inducibility), and LTR elements (low-temperature responsiveness). Light-response elements including G-box, GT1-motif, GATA-motif, ACE, Sp1, and 3-AF1-binding site were intensively distributed in all PvLac promoters, suggesting a role of PvLAC proteins in morphogenesis and development [5]. Despite a generally equal distribution of cis-elements on the promoters of five selected laccases, the MeJA response element was particularly abundant within a 100 bp region on PvLac2 and PvLac17 promoters. We further confirmed that laccase isoforms respond differently to environmental stresses, including low temperature and high osmotic pressure. The apparent differences in abundance and distribution of cis-regulatory elements on promoters of PvLac isoforms indicate the functional diversity of PvLACs in multiple physiological processes.
In plants, the lignification process is related to heavy-metal absorption and transportation. As a major component of the cell wall, lignin contains multiple functional groups which bind heavy-metal ions and, thus, protect the plant cells by preventing the absorption of heavy metal into the cytoplasm [42]. The increased degree of lignification in roots, induced by excess Cd and Cu, may play an important role in inhibiting the transport heavy metals from root to shoot [6,43]. A high concentration of heavy metals affects plants’ photosynthesis by reducing the chlorophyll content (Pb) or altering the photosynthetic electron-transport chain (Cu) in leaves; thus, it is toxic to plants [44]. The accumulated lignin induced by heavy-metal stress in switchgrass roots and leaves may, in turn, improve tolerance to heavy-metal-stress conditions. The expression of laccases also responds to heavy metals, regulating plant growth and stress tolerance by affecting lignin biosynthesis [45]. Excess Cu and Cd significantly induced the expression of laccase genes, resulting in enhanced laccase activity, as well as increased lignin content [6,46], while the expression level of all the selected switchgrass laccases increased significantly after Cu and Cd treatment.
Based on the phylogenetic analysis, the laccase with the highest expression level in each of the four subclades (PvLac24 from subclade I, PvLac14 from subclade II, PvLac2 from subclade III, and PvLac35 from subclade V), as well as PvLac17 from subclade IV, which is predicted to be a target of miR408, were selected to investigate their responses to heavy-metal treatment. Upon Fe stress, PvLac17 showed the strongest response, whereas its Arabidopsis orthologue AtLac12 played an important role in plant Fe homeostasis [47]. The overexpression of subclade III Cu-responsive laccase gene OsLac10 in Arabidopsis significantly promoted root elongation under Cu stress [6]. Nevertheless, the expression level of switchgrass subclade III laccase PvLac2 was upregulated 100 to 200 times after Cu treatment in this study, indicating a potential function of PvLac2 under Cu stress. Interestingly, although PvLac24, which was presumed to affect developmental lignification, responded rapidly after low concentration treatment, the induction fold was relatively limited. Switchgrass laccases exhibited various tissue expression specificities with distinct background expression levels; one hypothesis is that some of them are not involved in developmental lignification, but rather, function in lignin deposition under stress [7]. The genetic evidence in our study identified PvLac24 as a promising candidate involved in the lignification process during stem development. The differential responses of PvLac14 and PvLac17 to abiotic stresses suggest the functional diversity of individual laccase genes in the family. Our study revealed a primary response of the laccases that facilitate developmental lignification to heavy-metal stress, as well as a delayed induction of low-expression-level laccases; this highlights the potential complementary roles of switchgrass laccases in lignification and heavy-metal-stress tolerance.
Abbreviations
| Lac | Laccases |
| COMT | Caffeic acid 3-O-methyltransferase |
| CCoAOMT | Caffeyl CoA 3-O-methyltransferase |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23126530/s1.
Author Contributions
F.H., R.L., Y.Z., H.Z. and C.F. designed the experiments; F.H., R.L., Y.Z., Z.S. and Z.W. performed the experiments; F.H., R.L., Y.Z., H.W., H.Z. and C.F. analyzed the data; F.H., R.L., Y.Z., H.Z. and C.F. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its supplementary materials published online.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding Statement
This work was funded by the Agricultural Variety Improvement Project of Shandong Province (No. 2019LZGC010); the Natural Science Foundation of Shandong Province, China (ZR2021QC009 and ZR2021QC070); the Youth Innovation Promotion Association of CAS (2019213); the Shandong Energy Institute (SEI I202142); the Chinesisch-Deutschen Zentrum für Wissenschaftsförderung (No. M-0010); the Qingdao Postdoctoral Applied Research Project (20191226); and the National Natural Science Foundation of China (32160326).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giardina P., Faraco V., Pezzella C., Piscitelli A., Vanhulle S., Sannia G. Laccases: A never-ending story. Cell Mol. Life Sci. 2010;67:369–385. doi: 10.1007/s00018-009-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awasthi M., Jaiswal N., Singh S., Pandey V.P., Dwivedi U.N. Molecular docking and dynamics simulation analyses unraveling the differential enzymatic catalysis by plant and fungal laccases with respect to lignin biosynthesis and degradation. J. Biomol. Struct. Dyn. 2015;33:1835–1849. doi: 10.1080/07391102.2014.975282. [DOI] [PubMed] [Google Scholar]
- 3.Janusz G., Pawlik A., Swiderska-Burek U., Polak J., Sulej J., Jarosz-Wilkolazka A., Paszczynski A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020;21:966. doi: 10.3390/ijms21030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai X., Davis E.J., Ballif J., Liang M., Bushman E., Haroldsen V., Torabinejad J., Wu Y. Mutant identification and characterization of the laccase gene family in Arabidopsis. J. Exp. Bot. 2006;57:2563–2569. doi: 10.1093/jxb/erl022. [DOI] [PubMed] [Google Scholar]
- 5.Xu X., Zhou Y., Wang B., Ding L., Wang Y., Luo L., Zhang Y., Kong W. Genome-wide identification and characterization of laccase gene family in Citrus sinensis. Gene. 2019;689:114–123. doi: 10.1016/j.gene.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q., Luo L., Wang X., Shen Z., Zheng L. Comprehensive Analysis of Rice Laccase Gene (OsLAC) Family and Ectopic Expression of OsLAC10 Enhances Tolerance to Copper Stress in Arabidopsis. Int. J. Mol. Sci. 2017;18:209. doi: 10.3390/ijms18020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simoes M.S., Carvalho G.G., Ferreira S.S., Hernandes-Lopes J., de Setta N., Cesarino I. Genome-wide characterization of the laccase gene family in Setaria viridis reveals members potentially involved in lignification. Planta. 2020;251:46. doi: 10.1007/s00425-020-03337-x. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Feng J., Jia W., Fan P., Bao H., Li S., Li Y. Genome-Wide Identification of Sorghum bicolor Laccases Reveals Potential Targets for Lignin Modification. Front. Plant Sci. 2017;8:714. doi: 10.3389/fpls.2017.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Q., Nakashima J., Chen F., Yin Y., Fu C., Yun J., Shao H., Wang X., Wang Z.Y., Dixon R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell. 2013;25:3976–3987. doi: 10.1105/tpc.113.117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He F., Machemer-Noonan K., Golfier P., Unda F., Dechert J., Zhang W., Hoffmann N., Samuels L., Mansfield S.D., Rausch T., et al. The in vivo impact of MsLAC1, a Miscanthus laccase isoform, on lignification and lignin composition contrasts with its in vitro substrate preference. BMC Plant Biol. 2019;19:552. doi: 10.1186/s12870-019-2174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Bris P., Wang Y., Barbereau C., Antelme S., Cezard L., Legee F., D’Orlando A., Dalmais M., Bendahmane A., Schuetz M., et al. Inactivation of LACCASE8 and LACCASE5 genes in Brachypodium distachyon leads to severe decrease in lignin content and high increase in saccharification yield without impacting plant integrity. Biotechnol. Biofuels. 2019;12:181. doi: 10.1186/s13068-019-1525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin S., Fan C., Li X., Li Y., Hu J., Li C., Luo K. LACCASE14 is required for the deposition of guaiacyl lignin and affects cell wall digestibility in poplar. Biotechnol. Biofuels. 2020;13:197. doi: 10.1186/s13068-020-01843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiraide H., Tobimatsu Y., Yoshinaga A., Lam P.Y., Kobayashi M., Matsushita Y., Fukushima K., Takabe K. Localised laccase activity modulates distribution of lignin polymers in gymnosperm compression wood. New Phytol. 2021;230:2186–2199. doi: 10.1111/nph.17264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Zhuo C., Xiao X., Wang X., Docampo-Palacios M., Chen F., Dixon R.A. Substrate Specificity of LACCASE8 Facilitates Polymerization of Caffeyl Alcohol for C-Lignin Biosynthesis in the Seed Coat of Cleome hassleriana. Plant Cell. 2020;32:3825–3845. doi: 10.1105/tpc.20.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal D., Yadav A., Prasad M., Singh T.B., Shrivastav P., Ali A., Dantu P.K., Mishra S. Effect of Heavy Metals on Plant Growth: An Overview. In: Naeem M., Ansari A.A., Gill S.S., editors. Contaminants in Agriculture: Sources, Impacts and Management. Springer International Publishing; Cham, Switzerland: 2020. pp. 79–101. [Google Scholar]
- 16.Yruela I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009;36:409–430. doi: 10.1071/FP08288. [DOI] [PubMed] [Google Scholar]
- 17.Su N., Ling F., Xing A., Zhao H., Zhu Y., Wang Y., Deng X., Wang C., Xu X., Hu Z., et al. Lignin synthesis mediated by CCoAOMT enzymes is required for the tolerance against excess Cu in Oryza sativa. Environ. Exp. Bot. 2020;175:104059. doi: 10.1016/j.envexpbot.2020.104059. [DOI] [Google Scholar]
- 18.Kováčik J., Bačkor M. Phenylalanine Ammonia-Lyase and Phenolic Compounds in Chamomile Tolerance to Cadmium and Copper Excess. Water Air Soil Pollut. 2007;185:185–193. doi: 10.1007/s11270-007-9441-x. [DOI] [Google Scholar]
- 19.Tahara K., Norisada M., Hogetsu T., Kojima K. Aluminum tolerance and aluminum-induced deposition of callose and lignin in the root tips of Melaleuca and Eucalyptus species. J. For. Res.-Jpn. 2005;10:325–333. doi: 10.1007/s10310-005-0153-z. [DOI] [Google Scholar]
- 20.Van de Mortel J.E., Almar Villanueva L., Schat H., Kwekkeboom J., Coughlan S., Moerland P.D., Ver Loren Van Themaat E., Koornneef M., Aarts M.G.M. Large Expression Differences in Genes for Iron and Zinc Homeostasis, Stress Response, and Lignin Biosynthesis Distinguish Roots of Arabidopsis thaliana and the Related Metal Hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006;142:1127–1147. doi: 10.1104/pp.106.082073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu C., Mielenz J.R., Xiao X., Ge Y., Hamilton C.Y., Rodriguez M., Chen F., Foston M., Ragauskas A., Bouton J., et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA. 2011;108:3803–3808. doi: 10.1073/pnas.1100310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z., Wang N., Hisano H., Cao Y., Wu F., Liu W., Bao Y., Wang Z.Y., Fu C. Simultaneous regulation of F5H in COMT-RNAi transgenic switchgrass alters effects of COMT suppression on syringyl lignin biosynthesis. Plant Biotechnol. J. 2019;17:836–845. doi: 10.1111/pbi.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song G., Yuan S., Wen X., Xie Z., Lou L., Hu B., Cai Q., Xu B. Transcriptome analysis of Cd-treated switchgrass root revealed novel transcripts and the importance of HSF/HSP network in switchgrass Cd tolerance. Plant Cell Rep. 2018;37:1485–1497. doi: 10.1007/s00299-018-2318-1. [DOI] [PubMed] [Google Scholar]
- 24.Cary T.J., Rylott E.L., Zhang L., Routsong R.M., Palazzo A.J., Strand S.E., Bruce N.C. Field trial demonstrating phytoremediation of the military explosive RDX by XplA/XplB-expressing switchgrass. Nat. Biotechnol. 2021;39:1216–1219. doi: 10.1038/s41587-021-00909-4. [DOI] [PubMed] [Google Scholar]
- 25.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letunic I., Bork P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore K.J., Moser L.E., Vogel K.P., Waller S.S., Johnson B.E., Pedersen J.F. Describing and quantifying growth stages of perennial forage grasses. Agron. J. 1991;83:1073–1077. doi: 10.2134/agronj1991.00021962008300060027x. [DOI] [Google Scholar]
- 29.Liu Q., Zheng L., He F., Zhao F., Shen Z., Zheng L. Transcriptional and physiological analyses identify a regulatory role for hydrogen peroxide in the lignin biosynthesis of copper-stressed rice roots. Plant Soil. 2015;387:323–336. doi: 10.1007/s11104-014-2290-7. [DOI] [Google Scholar]
- 30.Lu S., Wang J., Chitsaz F., Derbyshire M.K., Geer R.C., Gonzales N.R., Gwadz M., Hurwitz D.I., Marchler G.H., Song J.S., et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turlapati P.V., Kim K.W., Davin L.B., Lewis N.G. The laccase multigene family in Arabidopsis thaliana: Towards addressing the mystery of their gene function(s) Planta. 2011;233:439–470. doi: 10.1007/s00425-010-1298-3. [DOI] [PubMed] [Google Scholar]
- 32.Wei J., Zhang X., Zhong R., Liu B., Zhang X., Fang F., Zhang Z., Pang X. Laccase-Mediated Flavonoid Polymerization Leads to the Pericarp Browning of Litchi Fruit. J. Agric. Food Chem. 2021;69:15218–15230. doi: 10.1021/acs.jafc.1c06043. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Yang K., Wang S., Lou Y., Zhu C., Gao Z. Genome-wide analysis of laccase genes in moso bamboo highlights PeLAC10 involved in lignin biosynthesis and in response to abiotic stresses. Plant Cell Rep. 2020;39:751–763. doi: 10.1007/s00299-020-02528-w. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W., Lin J., Dong F., Ma Q., Wu S., Ma X., Fatima M., Jia H., Ming R. Genomic and Allelic Analyses of Laccase Genes in Sugarcane (Saccharum spontaneum L.) Trop. Plant Biol. 2019;12:219–229. doi: 10.1007/s12042-019-09239-x. [DOI] [Google Scholar]
- 35.Lu S., Li Q., Wei H., Chang M., Tunlaya-Anukit S., Kim H., Liu J., Song J., Sun Y., Yuan L., et al. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc. Natl. Acad. Sci. USA. 2013;110:10848–10853. doi: 10.1073/pnas.1308936110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q., Li G., Zheng K., Zhu X., Ma J., Wang D., Tang K., Feng X., Leng J., Yu H., et al. The Soybean Laccase Gene Family: Evolution and Possible Roles in Plant Defense and Stem Strength Selection. Genes. 2019;10:701. doi: 10.3390/genes10090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caparrós-Ruiz D., Fornalé S., Civardi L., Puigdomènech P., Rigau J. Isolation and characterisation of a family of laccases in maize. Plant Sci. 2006;171:217–225. doi: 10.1016/j.plantsci.2006.03.007. [DOI] [Google Scholar]
- 38.He F. Ph.D. Thesis. Heidelberg University; Heidelberg, Germany: 2019. Exploring the Function of Laccases from Miscanthus Sinensis in Lignin Biosynthesis. [Google Scholar]
- 39.Ranocha P., Chabannes M., Chamayou S., Danoun S., Jauneau A., Boudet A.M., Goffner D. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol. 2002;129:145–155. doi: 10.1104/pp.010988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang M., Davis E., Gardner D., Cai X., Wu Y. Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta. 2006;224:1185–1196. doi: 10.1007/s00425-006-0300-6. [DOI] [PubMed] [Google Scholar]
- 41.Khandal H., Singh A.P., Chattopadhyay D. The MicroRNA397b -LACCASE2 Module Regulates Root Lignification under Water and Phosphate Deficiency. Plant Physiol. 2020;182:1387–1403. doi: 10.1104/pp.19.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo X., Zhang S., Shan X. Adsorption of metal ions on lignin. J. Hazard. Mater. 2008;151:134–142. doi: 10.1016/j.jhazmat.2007.05.065. [DOI] [PubMed] [Google Scholar]
- 43.Ederli L., Reale L., Ferranti F., Pasqualini S. Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol. Plantarum. 2004;121:66–74. doi: 10.1111/j.0031-9317.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- 44.Mustafa G., Komatsu S. Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016;1864:932–944. doi: 10.1016/j.bbapap.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Ghori N.H., Ghori T., Hayat M.Q., Imadi S.R., Gul A., Altay V., Ozturk M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019;16:1807–1828. doi: 10.1007/s13762-019-02215-8. [DOI] [Google Scholar]
- 46.Yang Y., Cheng L., Liu Z. Rapid effect of cadmium on lignin biosynthesis in soybean roots. Plant Sci. 2007;172:632–639. doi: 10.1016/j.plantsci.2006.11.018. [DOI] [Google Scholar]
- 47.Bernal M., Kramer U. Involvement of Arabidopsis Multi-Copper Oxidase-Encoding LACCASE12 in Root-to-Shoot Iron Partitioning: A Novel Example of Copper-Iron Crosstalk. Front. Plant Sci. 2021;12:688318. doi: 10.3389/fpls.2021.688318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its supplementary materials published online.