Highlights
-
•
collaboration between opioid treatment programs (OTP) and community pharmacies would increase access to and satisfaction with methadone treatment.
-
•
the present study pilots the feasibility and outcomes of using a community pharmacy to dispense methadone prescribed by OTP physicians to stable OTP patients.
-
•
participants attended all pharmacy visits and OTP counseling sessions, submitted drug-negative urine specimens, and endorsed high satisfaction.
-
•
findings support conduct of full scale trials evaluating a broader array of OTPs, patients, and pharmacies.
Keywords: Community pharmacy methadone dispensing, Methadone, Opioid use disorder, Increasing access to treatment
Abstract
Access to methadone for opioid use disorder (OUD) in the United States remains limited to regulated and certified Opioid Treatment Programs (OTPs). Collaboration between OTPs and community pharmacies would increase access to and potentially satisfaction with methadone delivery. While it remains illegal for prescribers to write, and pharmacies to dispense, methadone when the indication is OUD, the present pilot study evaluates the feasibility, acceptability, and outcomes of using community pharmacies to dispense methadone prescribed by OTP physicians (in tablet formulation) to a subset of clinically stable OTP patients; all other treatment services were delivered within the OTP. Necessary Drug Enforcement Administration (DEA) exceptions for OTP prescribers and the pharmacies, along with required Substance Abuse and Mental Health Services Administration (SAMHSA) waiver for OTP participation were obtained. A final sample of 11 patients enrolled in the study and were followed for three months; one left treatment due to dissatisfaction with the tablet formulation. All remaining participants produced drug-negative urine specimens, attended all pharmacy visits and OTP counseling sessions, and completed the evaluation. Participant satisfaction was high. These findings clearly support the feasibility and acceptability of OTP physician prescribing and community pharmacy dispensing of methadone in a subset of abstinent OTP patients, and encourage full scale trials evaluating a broader array of OTPs, pharmacies and patients, in urban and, perhaps most importantly, rural settings.
1. Introduction
Prevalence of opioid use disorder (OUD) and overdose deaths continues to rise across most communities in the United States and remains sadly epidemic (Volkow and Blanco, 2021), consequences exacerbated by the COVID-19 pandemic on public health and everyday life (Volkow, 2020). One of the most studied and effective interventions for people with moderate to severe OUD is methadone maintenance (Gowing et al., 2011). Access to this treatment is restricted to Substance Abuse and Mental Health Service Administration (SAMHSA) certified opioid treatment programs (OTPs; SAMHSA, 2015), which are also monitored by the Drug Enforcement Administration (DEA). Patients in OTPs attend the clinic on varying schedules to receive counseling and medication, routinely provided in liquid formulation, though tablet and diskette formulations are used (Title 42, Part 8, Medication Assisted Treatment for OUD 2022).
Despite increases in the number of OTPs and other medication-based treatments for OUD (e.g., buprenorphine, office-based opioid treatment - OBOT), there remains a compelling need to scale-up access to methadone and other medication-based opioid treatments (Fiellin et al., 2013; Weinstein et al., 2017), particularly in rural areas (Jones et al., 2015). One opportunity to expand access to methadone involves OTP physicians and advanced practice providers prescribing methadone for patients, with community pharmacies administering the medication, at least in some patients. There are approximately 1700 OTPs and over 300,000 people receiving methadone for OUD across all 50 states, though eight states have fewer than five programs (SAMHSA, 2021). In contrast, the U.S. has over 60,000 pharmacies (Qato et al., 2017). Because people often live or work in closer proximity to retail pharmacies than OTPs (Qato et al., 2017), an OTP prescribing and pharmacy dispensing methadone for OUD enhances the treatment network and may increase patient satisfaction and retention by reducing travel burden and associated costs (Joudrey et al., 2019), an approach that might help reduce stigma in patients and others.
A major obstacle to using pharmacies to expand access to methadone is that it remains illegal to prescribe for OUD outside of an OTP regulated treatment setting. Federal regulations limit the use of methadone in the treatment of OUD to OTPs and their approved mobile services and organized formal medication units (Food and Drug Administration - FDA, 1974). This regulatory structure continues despite growing international experience (e.g., Canada, United Kingdom, Australia) using community pharmacies to increase access to methadone for OUD (Calcaterra et al., 2019; Look et al., 2019).
Only two studies of OTP prescribing and community pharmacy administration of methadone for OUD were conducted in the U.S. prior to publication of FDA's (1974, initially published in 1973) regulations for methadone treatment. Brill and Jaffe (1967) reported good outcomes in a sample of nine heroin users inducted into treatment with methadone and sent to a community pharmacy for methadone dispensing. Bowden et al. (1976) reported data from a pilot demonstration project of OTP prescribing and community pharmacy dispensing of methadone with new admissions (N = 96, 92% male, 96% Hispanic). Their results also documented the feasibility of OTPs working with community pharmacies to administer and dispense methadone, though limited reporting of findings and design problems confounded measures of effectiveness across both reports.
More than 30 years later, Tuchman et al. (2006) conducted a 2-group randomized trial (n = 26 women), comparing routine OTP to an office-based opioid treatment (OBOT) condition that included community pharmacy methadone dispensing. The pharmacy in this study was approved by DEA and SAMHSA as a Medication Unit of the OTP, which provided methadone to the pharmacy that was subsequently dispensed by written order (rather than by prescription) by an OTP prescriber. While group outcomes were comparable, methods deployed in this study (i.e., OTP Medication Unit and physician orders versus prescription-based) limit generalizability to the earlier studies evaluating methadone prescribed by OTP physicians and dispensed by pharmacies using their own medication stock.
Taken together, these studies provide initial evidence of the feasibility of community pharmacy dispensing of methadone for OUD when prescribed by OTP physicians. Outcomes reported in the earlier studies were restricted by limitations in methods and by partial reporting of findings. While outcomes were positive in the Tuchman et al. (1976) report, their use of an OTP established Medication Unit in the pharmacy substantially limits generalizability to the earlier work.
The present study is a 3-month, non-randomized pilot evaluation of the feasibility and acceptability of OTP physician prescribing and community pharmacy dispensing of methadone for OUD. All participants were receiving care at a single OTP in Baltimore Maryland (MD). Prescriptions for methadone were written by OTP physicians and electronically submitted to one pharmacy that administered and dispensed the medication; all other clinical services were provided within the OTP. Primary outcomes were measures of feasibility and acceptability that support proof-of-concept, medication and counseling adherence, participant satisfaction, and substance use.
2. Method
2.1. Federal and state approvals
DEA exceptions (Title 21 CFR 1306 and 1307) were required for each of the three prescribers and the two pharmacy locations, one in Baltimore MD and the other in Rosedale MD, along with a waiver of federal regulation (42 CFR 8.11 7 8.12) from SAMHSA, all were granted for a 2-year period and required extensions to complete the evaluation. A letter of support from the State of Maryland's Methadone Authority was also required by SAMHSA. The Johns Hopkins Institutional Review Board reviewed and approved the study, though COVID-19 university-wide restrictions on conducting human research further delayed recruitment for 5 months. The clinicaltrial.gov identifier is NCT02654366.
2.2. Participants
Patients were receiving routine care for OUD in the Addiction Treatment Services (ATS) program located on at the Johns Hopkins Bayview Medical Center campus in Baltimore Maryland. Eligibility criteria included: 1) having OUD and receiving a maintenance methadone dose between 20 mg and 90 mg; 2) testing drug-negative for illicit substances (testing included fentanyl) for at least 6 months; 3) meeting federal regulatory requirements (pre-COVID) for at least biweekly methadone take-home schedule; 4) no failed methadone take-home recalls during the past year; 5) no acutely debilitating medical problems, 6) absence of formal thought disorder, delusions, hallucinations, no assessed risk of harm to self or others, and 7) willingness to receive methadone in tablet formulation from a pharmacy outside of the OTP setting.
Many eligible patients were earning 27 methadone take-homes per visit, based on a SAMHSA's (2020) relaxation of methadone take-home guidelines, done in order to help patients adhere to social distancing guidelines during the COVID-19 pandemic. This exemption extended 27 take-homes to patients who submitted drug-negative urine samples, demonstrated full program adherence for at least 60 days that included at least monthly counseling attendance, and met other standard criteria indicating stability. To participate in the present study, eligible patients were asked to reduce their methadone take-home schedule to 13 days per visit. This schedule was selected in collaboration with the DEA and intended to reduce the risk level of take-home dose mismanagement.
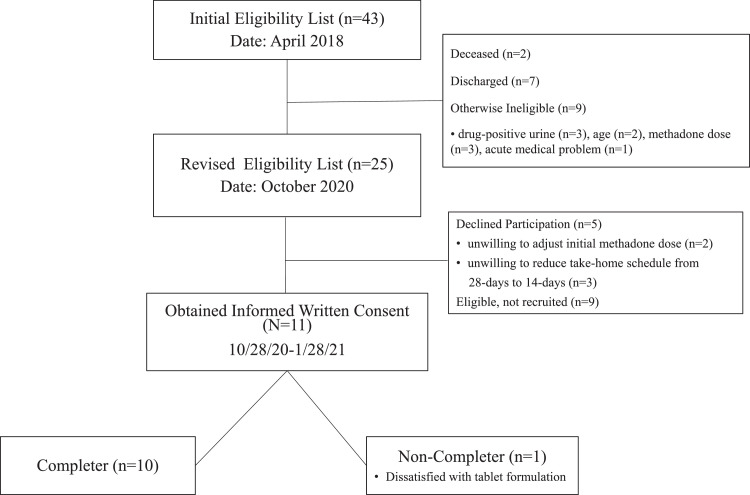
As shown in Fig. 1 (CONSORT diagram), a list of 43 patients were generated by the OTP's computerized methadone dosing software as receiving at least biweekly methadone take-home doses, living in one of the two largest residential zip codes of patients in the program (information used to select locations of the community pharmacies), and verified by research staff as satisfying remaining study inclusion criteria. The original planned sample of 20 enrollees was reduced to a final sample of 11 because of funding limitations related to unanticipated delays in the federal review and approval processes and the COVID-19 IRB delay in recruitment.
Fig. 1.
CONSORT diagram.
Participants (N = 11) completed the informed consent process either in person or via audiovisua technology or phone using HIPAA-compliant methods approved by the Johns Hopkins Health System. The consent form included consent to speak with pharmacy staff, consistent with routine clinic and provider practices for transmitting clinically relevant information with pharmacy and other health care providers.
2.3. Pharmacy setting
Three pharmacies located within the two most common residential zip codes of OTP patients were approached about the study and expressed an interest in participating, two of them submitted all of the required paperwork and received the necessary DEA exception to participate: 1) outpatient pharmacy owned by Johns Hopkins, and 2) private pharmacist-owned pharmacy. Only the Johns Hopkins outpatient pharmacy was assigned participants following the decision to reduce the final sample to 11 participants.
Pharmacy hours of operation were 8:00am to 7:00pm on weekdays, and 9:00am to 5:30pm on Saturdays (closed Sundays). The pharmacy is staffed by 3 licensed pharmacists and 6 pharmacy technicians, and offers medication administration and dispensing, patient counseling, and an array of disease management services. All HIPAA regulations were followed to safeguard protected health information (PHI), and due to the COVID-19 pandemic, the pharmacy strictly followed mask wearing and social distancing precautions. Methadone (10 mg tablet formulation) was dispensed from the pharmacy's stock supplies of methadone with restricted access.
2.4. Pharmacy training
Dr. Brooner developed a training protocol that focused on diagnosis and clinical course of OUD, methadone administration and safety, and review of the study protocol. One computer-assisted training and two additional in-person training sessions totaling approximately 4 h were completed with the pharmacy manager (P. Patel) and outpatient pharmacy staff. Pharmacists followed guidelines used in the OTP for observed methadone administration, including observed ingestion of one dose of methadone at each pharmacy visit. Methadone take-home dose schedule for all participants was adjusted prior to beginning the study so that their first dose in the pharmacy began on Mondays.
2.5. Methadone doses and electronic prescriptions
Participants were shifted from liquid to tablet formulations of methadone because pharmacies, including the ones selected for this evaluation, routinely use tablet formulations. We selected the 10 mg dose strength to both reduce the number of dispensed daily tablets and to avoid the pharmacy having to provide separate containers for different dose strengths (e.g., 10 mg and 5 mg tablets). For these reasons, participants were told that their usual daily methadone dose would likely be adjusted slightly to support use of the 10 mg tablets. For instance, patients receiving a maintenance dose of 81 mg to 89 mg were asked to choose either a decrease to 80 mg or an increase to 90 mg.
Prescriptions were submitted to the outpatient pharmacy electronically by one of the study authors (K. Stoller), a treating physician at the OTP, on or before Fridays preceding the Monday schedule for pharmacy administration to participants. All prescriptions included DEA registration number of the OTP, the prescriber's DEA number, and use indication (“for: opioid use disorder”). The use indication and OTP registration number on each prescription was required by DEA to facilitate the identification and differentiation of these methadone prescriptions from those written for pain.
2.6. Procedures
Following the consent process, research staff provided study instructions and a fact sheet to reinforce protocol procedures. Participants were informed that all methadone doses would be dispensed at the pharmacy, and that ATS would share information with pharmacy staff related to routine care. Participants were informed that they had to adhere to all COVID-19 safety requirements implemented by the pharmacy, and to bring personal identification and a container of water to ingest tablets. They were told that a pharmacist would observe on-site methadone ingestion on each visit, using methods consistent with those used in the OTP. A twice a month methadone take-home schedule was used, constituting one pharmacy visit every two weeks, receiving one dose at each visit administered by pharmacist and 13 take-home doses. Participants were scheduled to meet with their primary counselor once a month and submit a urine sample for drug testing.
Participants were told that they could return to the OTP for methadone dispensing temporarily at any point during the study, or permanently if they chose to discontinue their participation. They were also informed of situations that might lead the clinical team to modify counseling or medication take-home schedules or study participation, which included testing positive for illicit drugs or alcohol, presenting intoxicated or disruptive at the pharmacy or OTP clinic, or missing scheduled pharmacy pick-up days.
After obtaining written consent from participants, research staff faxed the pharmacy a participant identification form that included the participant's name, date of birth, address, and their start date (and completion date) in the evaluation, and first visit date to the pharmacy. The study prescriber was informed of these dates and prepared and submitted the prescription to the pharmacy at least three days prior to the pharmacy visit through an electronic health record.
Due to the COVID-19 pandemic, the OTP's routine methadone diversion policy of having patients call a dedicated methadone take-home recall line was temporarily suspended. Instead, research staff were directed to conduct methadone pill counts at each scheduled follow-up evaluation over the remaining course of the study. Given evolving changes to clinical and research practices related to the COVID-19 pandemic, methadone pill counts were obtained only on a subset of participants.
At the pharmacy, participants presented initially at the window used by all customers and were asked by the pharmacy technician to provide their name, date of birth and current address. Participants were flagged on the pharmacy computer as study participants, and asked to move to a more confidential area used for patient consultation services and medication administrations (e.g., vaccines). At this window, the pharmacist administered the methadone dose and offered counseling about the methadone or other prescriptions they might have at the pharmacy. Participants received the 13 days of methadone take-home doses in a standard pharmacy pill container placed within a tamper-resistant baggie. Pharmacists and technicians engaged in routine medication reconciliation for all administered and dispensed doses at each visit.
As per study protocol and agreement with Medicaid, pharmacy charges for uninsured participants and those with Medicaid were paid via interdepartmental transfers by the Department of Psychiatry; participants with commercial insurance coverage were billed by the pharmacy. Unpaid commercially billed claims were subsequently reimbursed via interdepartmental transfer by the Department of Psychiatry.
2.7. Assessments
Participants completed a Demographic Form at baseline, the AddictionSeverityIndex (ASI; McLellan et al., 2006) at baseline and monthly throughout the study, and the ClientSatisfactionQuestionnaire (CSQ; Larsen et al., 1979), adapted to the present study (see Attkisson & Greenfield, 2004), at baseline and monthly. Baseline assessment referred to routine OTP dispensing, while monthly assessments referred to the community pharmacy dispensing period. The CSQ (13 questions using a 1–4 Likert scale; higher scores indicate more satisfaction) yields an overall average score to assess satisfaction with medication delivery.
Participants were also asked at baseline and monthly their setting preference to receive methadone (OTP vs. pharmacy), and how much extra (if any) they would be willing to pay per week (0 to 50.00 dollars, intervals of $5.00) to ensure that medication delivery occur either at the pharmacy or the OTP setting. Assessments were administered in person or via telemedicine, using HIPAA-compliant Johns Hopkins University guidelines. Patients received a check for $30.00 for each completed assessment battery. Urinalysis testing (with ATS nursing staff observation) was conducted in the OTP, and all samples were tested for opioids, fentanyl, cocaine, benzodiazepines, alcohol (ethyl glucuronide, EtG), and cannabis. Testing was conducted monthly, rather than twice monthly as per usual treatment protocol, to limit contact with nursing staff during the pandemic.
2.8. Data analysis
Routine clinical data were abstracted from the medical record by research staff. Due to the small sample, descriptive data analyses were used to present counseling session attendance, urinalysis results, ASI, and CSQ data over the three follow-up assessment points.
3. Results
3.1. Feasibility and proof-of-concept
The first evidence of feasibility was observed in our success obtaining the necessary exceptions from DEA and waiver from SAMHSA, and the support of our state methadone authority and our final IRB approval to conduct the evaluation. Feasibility was further documented by the success in enrolling participants. Initial screening of OTP patients (April 2018) produced over 40 individuals expressing interest in participating, twice the anticipated sample size of 20 participants. Fig. 1 shows the reasons given by eligible patients for declining participation.
3.2. Baseline characteristics
Study demographics were as follows: M age = 58.2 (SD = 5.7); 82% female and 27% African American; M years of education = 12.1 (SD = 1.8); 45% married. Participants had been treated at ATS for M = 19.1 (SD = 8.8) years. It is noteworthy that 9 participants (82%) had been receiving 27 take-homes per clinic visit via the relaxed SAMHSA guidelines (2020), and for the study agreed to a 13-day take-home schedule.
3.3. Methadone dose, prescriber and pharmacy fidelity measures
Mean methadone dose of the sample at baseline was 54.6 mg (SD = 28.1), including any initial adjustments to achieve 10 mg dosing increments. Five participants received an initial dose adjustment at baseline. No dose changes were made over the course of the 3-month evaluation.
Methadone prescriptions were submitted electronically to the pharmacy. Seven separate prescriptions were written for each of the 10 participants who completed the evaluation, along with 2 prescriptions written for the single participant who left the study at the end of Month 1. As per study protocol, medication data collected by the pharmacy and the study team confirmed that pharmacy observed dosing was done for all participants at each visit to the pharmacy, and that methadone take-home doses were accurately dispensed.
3.4. Prescription drug monitoring program - PDMP
The PDMP was reviewed by the prescriber prior to electronic submission of each prescription; the pharmacy also reviewed the PDMP for every prescription they received. No participants had other prescriptions for opioids, two were prescribed a low dose of benzodiazepine for anxiety, one participant was prescribed zolpidem (10 mg) for sleep and one was prescribed pregabalin for pain. Program clinical staff was unaware of the prescribed benzodiazepine for one of the two participants identified by the PDMP. The counselor and participant worked together to coordinate this care with the prescribing psychiatrist. All remaining prescriptions were known to the medical director and prescribing psychiatrist, counseling and nursing staff, and study investigators.
3.5. Pharmacy billing
Participants had either commercial (n = 8) or Medicaid (n = 3) health insurance. A bill for service was submitted only to the commercial insurers in agreement with the Centers for Medicare and Medicaid Services (CMS). In all cases, commercial insurers requested pre-authorization for the medication, which was provided at least once for each case, and insurers denied the claims saying that methadone for OUD was not covered. All pharmacy charges were paid by agreement with the Johns Hopkins Department of Psychiatry (73 charges, totaling: $581.46).
3.6. Participant retention
One participant chose to leave the evaluation at the end of Month 1, reporting that the tablet formulation caused nausea. All of the remaining participants were retained throughout the 12-week evaluation (10/11 = 91%).
3.7. Medication schedule
With two exceptions, participants received their methadone dose (and take-home doses) in the pharmacy as scheduled (Table 1). One participant missed a scheduled Monday dispensing day, reporting that she thought the pharmacy was closed due to a federal holiday. She reported on Tuesday to the OTP and then the pharmacy to receive methadone and her remaining take-home doses. On the other occasion, seven participants were called by the OTP and pharmacy to come to the pharmacy two days earlier than scheduled in anticipation of an adverse weather event. Each participant presented at the pharmacy on Saturday with their take-home methadone dose for that day, ingested it in the presence of a pharmacist, and then received two take-home doses in order to remain on the Monday visit schedule.
Table 1.
Clinical outcomes across 3-month observation period.
| Outcome Measures | Baseline | Month 1 | Month 2 | Month 3 |
|---|---|---|---|---|
| M(SD) | M(SD) | M(SD) | M(SD) | |
| Number of counseling sessions | 0.73 (0.79) | 1.00 (1.18) | 0.90 (0.99) | 1.60 (1.40) |
| Urinalysis results (% positive, illicit drugs) 1 |
0.0 (0.0) |
0.0 (0.0) |
0.0 (0.0) |
0.0 (0.0) |
| Addiction Severity Index (ASI) composite scores | ||||
| Drug | 0.094 (0.046) | 0.086 (0.02) | 0.085 (0.019) | 0.086 (0.02) |
| Alcohol | 0.004 (0.011) | 0.012 (0.028) | 0.008 (0.021) | 0.002 (0.005) |
| Medical | 0.567 (0.387) | 0.419 (0.378) | 0.297 (0.364) | 0.392 (0.305) |
| Employment | 0.366 (0.169) | 0.372 (0.177) | 0.377 (0.173) | 0.371 (0.173) |
| Legal | 0.000 (0.0) | 0.000 (0.0) | 0.000 (0.0) | 0.000 (0.0) |
| Family/Social | 0.157 (0.185) | 0.170 (0.240) | 0.115 (0.194) | 0.164 (0.223) |
| Psychiatric | 0.185 (0.195) | 0.200 (0.251) | 0.207 (0.218) | 0.188 (0.253) |
One participant tested EtG-positive at baseline, another participant tested EtG-positive in month 3.
3.8. Counseling attendance
All counseling sessions were conducted via audiovisua or audio-only platforms due to COVID-19 precautions. Participants attended an average of about one session per month (Table 1), consistent with routine counseling schedules for stable abstinent patients.
3.9. Substance use and safety
As shown in Table 1, participants tested drug-negative on each urinalysis screening (33 tests). One patient tested EtG-positive at baseline, and another patient tested EtG-positive on one follow-up test. The ASI data suggested no changes in drug or alcohol use severity over time (Table 1). No safety issues were noted.
3.10. Participant satisfaction
Table 2 shows overall CSQ scores, and a sample of individual items, which remained relatively stable and high (> 3.8) from baseline (OTP delivery) throughout the 3 monthly follow-ups (pharmacy delivery), indicating good satisfaction for OTP and pharmacy administration and dispensing. When asked to choose between dispensing settings, between 80 and 100% of participants chose pharmacy dispensing at baseline and each follow-up assessment. Participants also showed preference for pharmacy dispensing by reporting a willingness to self-pay additional money (> $32 per month) to have access to pharmacy methadone dispensing (Table 2).
Table 2.
Patient Satisfaction.
| Satisfaction Measures | Baseline1 | Month 11 | Month 21 | Month 31 |
|---|---|---|---|---|
| M(SD) | M(SD) | M(SD) | M(SD) | |
| Client Satisfaction Questionnaire (CSQ)1 | ||||
| CSQ Overall Score | 3.84 (0.25) | 3.81 (0.29) | 3.85 (0.29) | 3.88 (0.18) |
| How would you rate the quality of services that you received at ATS / pharmacy? | 3.64 (0.67) | 3.70 (0.48) | 3.80 (0.42) | 3.80 (0.42) |
| How satisfied are you with the amount of help you received at ATS / pharmacy? | 3.73 (0.47) | 3.80 (0.42) | 3.80 (0.42) | 3.80 (0.42) |
| I am satisfied with the convenience of ATS/ pharmacy methadone dosing. | 3.90 (0.30) | 3.70 (1.68) | 3.80 (1.42) | 3.90 (0.32) |
| I was satisfied with the amount of privacy I received at ATS / pharmacy. | 3.90 (0.30) | 3.70 (0.48) | 3.90 (0.32) | 3.80 (0.42) |
| Additional Measures of Satisfaction | ||||
| ATS vs. Pharmacy choice 2 | Pharm: 91% | Pharm: 90% | Pharm: 80% | Pharm: 100% |
| Dollars willing to pay weekly to ensure pharmacy (vs. ATS) delivery 3 | 10.46 (15.89) | 8.50 (7.09) | 8.50 (6.26) | 9.00 (7.38) |
| Dollars willing to pay weekly to ensure ATS (vs. pharmacy) delivery 4 | 1.82 (4.10) | 0 (0) |
0 (0) |
0 (0) |
1–4 Likert scale, higher values indicate more satisfaction. Baseline CSQ references satisfaction with ATS; monthly follow-up CSQs reference satisfaction with the community pharmacy.
“I prefer to receive methadone dosing at: OTP or Pharmacy.”.
“How much extra money would you be willing to pay each week to receive your methadone at a community pharmacy, instead of at ATS?”.
“How much extra money would you be willing to pay each week to receive your methadone at ATS, instead of a community pharmacy?”.
4. Discussion
The present study is the first funded pilot demonstration project to evaluate the feasibility of OTP physician prescribing and pharmacy dispensing of methadone to be conducted in nearly 50 years. Study findings provide good support for the feasibility and acceptability of physician prescribing and pharmacy dispensing of methadone for OUD. It is noteworthy that the study was implemented year before a national Task Force was commissioned by NIDA's Center for Clinical Trials Network (CCTN) to develop a research agenda to increase access to methadone for OUD (Joudrey et al., 2021), which included recommendations for studies evaluating community pharmacy administration of methadone prescribed by OTP physicians and mid-level practitioners.
4.1. Feasibility and acceptability
Perhaps the strongest evidence of both feasibility and acceptability of this work was the approvals we received from DEA and SAMHSA to conduct the study, which took about 14 months to attain. A second NIDA-supported pilot of this work (Wu et al., 2021), utilizing the similar design of ePrescribing, obtained the same federal approvals in less than half the time. This development, occurring between the conduct of the two pilot studies, appeared to reflect DEAs increasing acceptance of this work and a clear streamlining of the approval process.
Feasibility was also evidenced by the initial interest in and preliminary eligibility of clinic patients, and the subsequent recruitment of the final sample within 3 months. The initial list of 43 study eligible patients created within 2-months reflected the high level of interest in clinic patients, screening was discontinued only because the list of 43 was twice the expected sample of 20 enrollees. This experience was replicated almost 2 years later in subsequent recruitment of the final sample of 11 participants. The parallel study reported by Wu et al. (2021) also recruited their final sample within 3 months. An unanticipated measure of both feasibility and acceptability was provided by 9 participants who agreed to switch from a monthly to biweekly take-home schedule to participate in the study. None of the participants expressed concern about the modest changes in daily methadone dose to accommodate the 10 mg tablets used in the study.
4.2. Satisfaction and clinical response
Participants reported strong satisfaction across several measures, including a behavioral choice paradigm showing that participants would self-pay a higher treatment fee if it included methadone dispensing at a community pharmacy. These findings are also consistent with the report by Wu et al. (2021) and may have positive implications on treatment retention.
The high study completer rate (91%) and high attendance rate to pharmacy visits (100%) are remarkable, and amplified by the very low rate of drug-positive urine specimens (1 alcohol positive) and self-reported drug use. These findings are consistent with those reported by Wu et al. (2021), but dissimilar from those reported in the two early demonstration projects conducted almost 50 years ago of physician prescribing and pharmacy administration of methadone in new admissions (Brill and Jaffee, 1967; Bowden et al., 1976). The Bowden et al. study, for example, reported that over 70% of participants had opioid positive urine specimens during the project.
4.3. Limitations and further development
The most significant limitation of this study is the small sample size (N = 11). The planned sample of 20 participants was reduced to 11 due to funding limitations caused by delays in federal approvals, extended five additional months by our IRB's COVID-19 delay in starting the recruitment phase. While eligibility criterion included being stable and abstinent for at least six months, the final sample had been in treatment at the program for almost two decades and abstinent considerable longer than 6 months. While this reflects both the possibility and benefits of long-term retention in opioid agonist treatment, it also constitutes a potential limitation to the generalizability of findings to less stable patients, and an urgent need for studies with larger and more clinically diverse samples and programs. While the failure to get pharmacy claims paid by any healthcare insurers was anticipated, it does highlight a significant but resolvable obstacle to expanding access to methadone for OUD using pharmacies.
Participants in the present study and the parallel one done by Wu et al. (2021) received counseling, medication prescribing, drug testing, and related services in the OTP setting; only administration of methadone was done in the pharmacy. While this practice in combination with methadone delivery in the OTP is wholly consistent with federal regulations for methadone treatment (SAMSHA, 2015), different approaches are used in other countries (e.g., Canada, U.K., Australia), where methadone is prescribed for OUD within addiction treatment programs and primary care practices, and administered in community pharmacies (e.g., Calcaterra et al., 2019; Look et al., 2019). Disconnecting the administration of methadone from the onsite provision of counseling and related services is certainly possible within the U.S., with changes to federal regulations, and might facilitate the uptake of methadone into more diverse care delivery systems.
Separating the delivery of methadone from comprehensive onsite services also conveys risks to patients, particularly new admissions and clinically unstable cases. One of these risks is a safety concern related to potential for methadone overdose. Methadone (full agonist) has a much greater risk of overdose compared to buprenorphine (mixed agonist/antagonist), which is increasingly available in the U.S. While the greater risk of overdose with methadone compared to buprenorphine is offset by the comprehensiveness of services usually provided within OTP settings, disconnecting these services from methadone delivery might elevate this risk in other settings. Unfortunately, the growing epidemic of fentanyl use in the U.S. has created substantial problems for buprenorphine treatment induction and stabilization efforts, and methadone has been suggested as a more effective option for fentanyl-using patients with moderate to severe OUD who do not respond well to buprenorphine (Mattick et al., 2014).
Expanding access to methadone for OTP patients to community pharmacies, along with other OUD patients in different health care settings that effectively manage the risks, would likely prove beneficial from clinical and public health perspectives. Using collaborative models of care that unify OTP and OBOT practice settings would absolutely grow the overall treatment network and increase access to methadone. Research that evaluates existing and alternative approaches for expanding access to methadone is important to advancing efforts to make methadone for OUD equitably available to more patients, while improving (and not hindering) quality of care.
Role of funding source
National Institute of Drug Abuse (NIDA) provided financial support for the conduct of this research (R34 DA040507; M. Kidorf, PI). NIDA had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the report; or the decision to submit this research for publication.
Contributors
All of the participating authors were meaningfully involved in the study and each of them made substantive contributions to the report.All authors approved of the final submission
Funding
This work was supported through NIH-NIDA supplemental funding to R34 DA040507 (M. Kidorf, PI). The sponsoring agency had no role in the design and analysis of the study, or review or writing of the report. The opinions expressed in this paper are those of the authors and do not represent the official position of the U.S. government.
Declaration of Competing Interest
No conflict declared.
Acknowledgments
This evaluation was otherwise possible only because of the assistance and support of other federal and state agencies and personnel. We specifically acknowledge the direction and support of staff from Drug Enforcement Agency (DEA), Substance Abuse and Mental Health Services Administration (SAMHSA), the State of Maryland's Methadone Authority. We gratefully acknowledge the two outpatient pharmacies that worked with us on the project and both applied for and received the required DEA exceptions (Johns Hopkins Outpatient Pharmacy in Baltimore, Maryland and the Professional Pharmacy in Rosedale, Maryland). Their support was crucial to the implementation of the project. We are likewise thankful for the patients who supported this work and participated in the study, and for the outstanding commitment, skill and integrity of our clinical research team, including Kori A. Kindbom, M.S., Michael Sklar, M.A., Jennifer Mucha, M.A., and Rachel Burns, B.A.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2022.100067.
Appendix. Supplementary materials
References
- Attkisson C.C., Greenfield T.K. In: The use of psychological testing for treatment planning and outcomes assessment: Instruments for adults. Maruish M.E., editor. Lawrence Erlbaum Associates Publishers; 2004. The UCSF Client Satisfaction Scales: I. The Client Satisfaction Questionnaire-8; pp. 799–811. [Google Scholar]
- Bowden C.L., Maddux J.F., Esquivel M. Methadone dispensing by community pharmacies. Am. J. Drug Alcohol Abuse. 1976;3:243–254. doi: 10.3109/00952997609077194. [DOI] [PubMed] [Google Scholar]
- Brill L., Jaffe J.H. The relevancy of some newer American treatment approaches to England. Br. J. Addict. 1967;62:375–386. [Google Scholar]
- Calcaterra S.L., Bach P., Chadi A., Chadi N., Kimmel S.D., Morford K.L., Roy P., Samet J.H. Methadone matters–What the United States can learn from the global effort to treatment opioid addiction. J. Gen. Intern. Med. 2019;34:1039–1042. doi: 10.1007/s11606-018-4801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin D.A., Barry D.T., Sullivan L.E., Cutter C.J., Moore B.A., O'Connor P.G., Schottenfeld R.S. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. Am. J. Med. 2013;126:74e1–74e17. doi: 10.1016/j.amjmed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L., Farrell M.F., Bornemann R., Sullivan L.E., Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst. Rev. 2011;(8) doi: 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- Jones C.M., Campopiano M., Baldwin G., McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am. J. Public Health. 2015;105:55–63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joudrey P.J., Edelman E.J., Wang E.A. Drive times to opioid treatment programs in urban and rural counties in 5 U.S. states. J. Am. Med. Assoc. 2019;322:1310–1312. doi: 10.1001/jama.2019.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joudrey P.J., Bart G., Brooner R.K., Brown L., Dickson-Gomez J., Gordon A., Kawasaki S.S., Liebschutz J.M., Nunes E., McCarty D., Schwartz R.P., Szapocnik J., Trivedi M., Tsui J.I., Williams A., Wu L.T., Fiellin D.A. Research priorities for expanding access to methadone treatment for opioid use disorder in the United States–A National Institute on Drug Abuse Clinical Trials network task force reports. Subst.Abuse. 2021;42:245–254. doi: 10.1080/08897077.2021.1975344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen D.L., Attkisson C.C., Hargreaves W.A., Nguyen T.D. Assessment of client/patient satisfaction–Development of a general scale. Eval. Progr. Plann. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- Look K.A., Kile M., Morgan K., Roberts A. Community pharmacies as access points for addiction treatment. Res. Soc. Adm. Pharm. 2019;15:404–409. doi: 10.1016/j.sapharm.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick R.P., Breen C., Kimber J., Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014;(2) doi: 10.1002/14651858.CD002207.pub4. IssueArt. No. CD002207. [DOI] [Google Scholar]
- McLellan A.T., Cacciola J.C., Alterman A.I., Rikoon S.H., Carise D. The Addiction Severity Index at 25–Origins, contributions and transitions. Am. J. Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- Qato D.M., Zenk S., Wilder J., Harrington R., Gaskin D., Alexander G.C. The availability of pharmacies in the United States–2007–2015. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Substance Abuse and Mental Health Services Administration; Rockville, MD: 2015. Federal Guidelines for Opioid Treatment Programs. HHS Publication No. (SMA) PEP15-FEDGUIDEOTP. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2020). Opioid treatment program guidance. Available at https://www.samhsa.gov/coronavirus.
- Title 42, Part 8. Medication Assisted Treatment for Opioid Use Disorder. 2022 Code of Federal Regulations. Available at https://www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8
- Substance Abuse and Mental Health Services Administration (SAMHSA) 2021. Opioid Treatment Program Directory. https:///dpt2.samhsa.gov/treatment/direcotry. aspxAccessed. [Google Scholar]
- Tuchman E., Gregory C., Simson M., Drucker E. Safety, efficacy, and feasibility of office-based prescribing and community pharmacy dispensing of Methadone–Results of a pilot study in New Mexico. Addict. Disord. Treat. 2006;5:43–51. doi: 10.1097/01.adt.0000210713.80198.d1. [DOI] [Google Scholar]
- Volkow N.D. Collison of the COVID-19 and addiction epidemics. Ann. Intern. Med. 2020;173:61–62. doi: 10.7326/M20-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Blanco C. The changing opioid crisis–Development, challenges, and opportunities. Mol. Psychiatry. 2021;26:218–233. doi: 10.1038/s41380-020-0661-4. 1038/s41380/s41380-020-0661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Z.M., Kim H.W., Cheng D.M., Quinn E., Hui D., Labelle C.T., Drainoni M.L., Bachman S.S., Samet J.H. Long-term retention in office based opioid treatment with buprenorphine. J. Subst. Abuse Treat. 2017;74:65–70. doi: 10.1016/j.jsat.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., John W.S., Morse E.D., Adkins S., Pippin J., Brooner R.K., Schwartz R.P. Opioid treatment program and community pharmacy collaboration for methadone maintenance treatment. Addiction. 2021 doi: 10.1111/add.15641. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.