Abstract
The application of high pressure (HP) for food preservation requires insight into mechanisms of HP-mediated cell injury and death. The HP inactivation in model beer of Lactobacillus plantarum TMW1.460, a beer-spoiling organism, was investigated at pressures ranging from 200 to 600 MPa. Surviving cells were characterized by determination of (i) cell viability and sublethal injury, (ii) membrane permeability to the fluorescent dyes propidium iodide (PI) and ethidium bromide (EB), (iii) metabolic activity with tetrazolium salts, and (iv) the activity of HorA, an ATP binding cassette-type multidrug resistance transporter conferring resistance to hop compounds. HP inactivation curves exhibited a shoulder, an exponential inactivation phase, and pronounced tailing caused by a barotolerant fraction of the population, about 1 in 106 cells. During exponential inactivation, more than 99.99% of cells were sublethally injured; however, no sublethal injury was detected in the barotolerant fraction of the culture. Sublethally injured cells were metabolically active, and loss of metabolic activity corresponded to the decrease of cell viability. Membrane damage measured by PI uptake occurred later than cell death, indicating that dye exclusion may be used as a fail-safe method for preliminary characterization of HP inactivation. An increase of membrane permeability to EB and a reduction of HorA activity were observed prior to the loss of cell viability, indicating loss of hop resistance of pressurized cells. Even mild HP treatments thus abolished the ability of cells to survive under adverse conditions.
Treatment of food with a high pressure (HP) of 200 to 800 MPa is a novel process in food technology employed to change functional food properties, to selectively affect the activity of food enzymes, to improve food texture, and to eliminate microorganisms. The application of hydrostatic pressures is especially promising to achieve preservation of minimally processed foods, as a pressure treatment does not compromise the sensorial quality of food to the same extent as do thermal treatments with a comparable bactericidal effect. Nevertheless, it is advantageous to achieve food preservation by mild-pressure treatment in order to minimize quality deterioration and to reduce equipment and energy costs. Information on the mechanisms of HP-mediated inactivation of microorganisms will facilitate the deliberate choice of the parameters pressurization temperature, pH, pressure level, and holding time and allow the use of synergistic interactions between HP and other preservative principles. Proteins and membranes are considered to be the primary target for the pressure-induced inactivation of bacteria. Pressures of 150 to 250 MPa have been shown to induce dissociation of ribosomes in Escherichia coli (25). Wouters et al. (46) found no morphological changes in the cytoplasmic membrane upon lethal pressure treatment of Lactobacillus plantarum; however, the membrane permeability was increased, and the efflux of protons as well as the ability to maintain a ΔpH across the membrane was impaired. Pressure-induced leakage of sodium and calcium ions was observed for Saccharomyces cerevisiae (27). Effects of HP treatment on membrane potential and membrane-bound transport systems may result from phase transitions in the membrane (23). Adaptation of barophilic deep-sea bacteria to HP involved a shift of membrane lipid composition from saturated to unsaturated fatty acids (47). Both yeasts and bacteria have been found to exhibit a maximum of barotolerance at ambient temperature (12, 38). ter Steeg et al. (42) observed an increased efficacy of HP treatment if the pressurization temperature was reduced or if the incubation temperature of the preculture was increased, i.e., under conditions where the liquid crystalline state of the cytoplasmic membrane during growth of the organisms is altered to a more rigid, semicrystalline state during pressurization.
Comparison of cell counts of pressurized samples on selective and nonselective media shows that a large proportion of a given population is sublethally injured prior to cell death, i.e., pressure-treated cells fail to survive and multiply in harsh environments tolerated by untreated cells. The validity of this approach for gram-negative organisms was shown by use of selective media to probe the permeability barrier of the outer membrane with bile salts (14, 18, 19, 26). Many foods must be considered selective media for microorganisms where growth or survival requires specific resistance mechanisms, e.g., acid tolerance, osmotolerance, or resistance to inhibitory compounds. Sublethal injury of HP-treated cells may therefore indicate the inability to survive during food storage; however, mechanisms accounting for this effect have so far not been elucidated.
As a model system to study the kinetics and mechanisms of HP inactivation of lactic acid bacteria (LAB), we choose a beer-spoiling organism, L. plantarum TMW1.460. Beer is a highly selective medium for growth of microorganisms because of the content of hop bitter compounds, its low pH, and the high content of ethanol and carbon dioxide. The mechanisms that allow beer-spoiling bacteria to overcome these hurdles have been characterized in recent years. The major bactericidal components in beer are hop bitter compounds—colupulone, humulone, trans-isohumulone, and trans-humulinic acid—which dissipate the transmembrane pH gradient (35, 36). Beer-spoiling LAB have been shown to possess a plasmid-encoded hop resistance mechanism, HorA (32, 33). HorA mediates ATP-dependent transport of hop bitter compounds and has high homology to other bacterial ATP-binding cassette-type multidrug transporters as well as mammalian multidrug resistance proteins (33, 44). Sami et al. (31) screened 95 lactobacilli for the presence of the gene coding for the hop efflux pump, horA, and found that this resistance mechanism is a prerequisite for their growth in beer.
The objective of this study was to characterize the HP-treated cells not only to obtain information on cell viability but also to determine sublethal injury. A number of assays recently proposed for the rapid determination of cell viability (2, 5, 43) were adapted to L. plantarum TMW1.460 in order to determine whether the loss of metabolic activity or membrane integrity or the failure to maintain hop resistance accounts for sublethal injury of pressurized cells.
MATERIALS AND METHODS
Strains and culture conditions.
Saccharomyces cerevisiae subsp. uvarum TMW 3.001, a commercially available brewer's yeast (Technische Universität München, Lehrstuhl Technologie der Brauerei II, Freising, Germany), was cultured at ambient temperature on malt extract medium (12% [wt/wt] malt extract [Ireks, Kulmbach, Germany], sterilized at 121°C for 21 min). L. plantarum TMW1.460, an organism previously isolated from spoiled beer, was cultivated using model beer (MB), MRS agar (7), or MRS agar containing 4% NaCl (MRS-NaCl) at 30°C. Solid media contained 1.5% agar. MB was prepared by inoculating malt extract medium with S. cerevisiae TMW 3.001 to a cell count of about 5 × 106 cells ml−1. The mash was fermented for 140 h at 10°C and autoclaved (121°C, 20 min), and the yeast was removed by centrifugation (20 min, 20,000 × g, 0°C). The clear supernatant was collected, residual ethanol and CO2 were removed in a rotary evaporator under vacuum, and the weight loss was compensated for with demineralized water. The pH was adjusted to 4.0, and the medium was sterilized at 121°C for 21 min.
HP treatment.
An overnight culture of L. plantarum TMW1.460 in MB was subcultured in MB for 16 h with 1% inoculum to late stationary growth phase. Cells were harvested by centrifugation and resuspended in an equal volume of MB. This cell suspension was transferred to 2-ml Eppendorf reaction tubes, sealed with silicon stoppers avoiding enclosure of air, and stored on ice until pressurization. The HP inactivation kinetics of L. plantarum were investigated in HP autoclaves precooled to 15°C. Compression and decompression rates were 200 MPa min−1. The temperature increase in the HP autoclaves due to adiabatic compression was less than 4, 8, 10, and 12°C upon compression to 200, 400, 500, and 600 MPa, respectively, and the temperature reached 15°C after a 5-min pressure holding time or earlier. Upon pressurization, samples were stored on ice until further analysis as described below. For each HP inactivation curve, untreated cultures and cultures sterilized by treatment with 800 MPa for 10 min were used for preparation of calibration samples containing 100, 50, and 0% viable cells.
Determination of plate counts.
Cell counts were determined on MRS agar and MRS-NaCl agar for determination of viable and sublethally injured cells. Appropriate dilutions were plated using a spiral plater (IUL, Königswinter, Germany), and plates were incubated at 30°C for 2 days under a controlled atmosphere (76% N2, 20% CO2, and 4% O2). Cell counts of overnight cultures of L. plantarum in MB were (4.3 ± 2.1) × 108 CFU ml−1 on either MRS or MRS-NaCl agar (mean of 24 determinations). The cell counts on MRS are referred to as viable cells, and the difference between cell counts on MRS and on MRS-NaCl is referred to as sublethally injured cells.
Determination of metabolic activity.
Cells from a 100-μl sample were harvested by centrifugation at 0°C and 10,000 × g for 10 min, resuspended in 100 μl of phosphate buffer with glucose (PBG; 50 mM H2KPO4, 0.1 g of MgSO4 × 7H2O liter−1, 0.05 g of MnSO4 × H2O liter−1 and 4 g of glucose liter−1, pH 6.5), and transferred to microtiter plates. To this cell suspension was added a stock solution of 4 mmol of 2-(-iodophenyl)-3-(p-nitrophenyl)5-phenyltetrazolium chloride (INT; Molecular Probes, Eugene, Oreg.) liter−1 in PBG to a final concentration of 2 mmol of INT liter−1. The kinetics of reduction of the colorless INT to the red formazan dye was monitored by measuring absorption at 590 nm in a Spectrafluor microtiter plate reader (Tecan, Grödig, Austria) at 1-min intervals for 60 min at 30°C. The initial rate of INT reduction was used for calculation of the metabolic activity of the cells. A calibration curve was established for each inactivation curve using the calibration samples described above, and the results are reported as percent metabolic activity.
Determination of HorA activity.
Ethidium bromide (EB) is a substrate for HorA conferring hop resistance and related multidrug resistance transport systems of LAB (3, 33). Therefore, an assay for HorA activity was developed using EB as a substrate. EB stock solutions were prepared by dissolving 40 μmol of EB liter−1 in PBG and PB0 (50 mM H2KPO4, 0.1 g of MgSO4 · 7H2O liter−1, and 0.05 g of MnSO4 · H2O liter−1, pH 6.5). Cells were harvested from 1-ml samples by centrifugation (10 min at 15°C, 6,000 × g) and resuspended in 1 ml of PB0. Each sample was divided into two aliquots. One aliquot received 200 μl of cell suspension and 200 μl of EB stock solution in PB0 to obtain an EB concentration of 20 μmol liter−1. The other aliquot received 200 μl of EB stock solution in PBG to obtain the same buffer and EB concentrations and additionally 2 g of glucose liter−1 as an energy source. Samples were mixed and incubated at 30°C for 0, 20, 45, 100, and 140 min (assay validation) or 1.5 h (characterization of HP-treated samples) in the dark. After incubation, cells were harvested, resuspended in 200 μl of PB0, and transferred to black microtiter plates. The fluorescence of this cell suspension was measured using a Spectrafluor microtiter plate reader (λEx = 485 nm, λEm = 595 nm). The difference between EB fluorescence of starved cells and that of energized cells was considered to indicate HorA activity.
Determination of membrane integrity.
The integrity of the cytoplasmic membrane of HP-treated cells was determined using two different dye exclusion assays. (i) The BacLight Live/Dead Kit (Molecular Probes) was used according to the instructions of the supplier. In short, cells from 100-μl samples were harvested by centrifugation, resuspended in an equal volume of PB0, and transferred onto black microtiter plates. To each sample, 100 μl of dye solution (33.4 μmol of Syto9 liter−1 and 200 μmol of propidium iodide [PI] liter−1 in PB0) was added, and the plates were incubated for 15 min at ambient temperature. The ratio of Syto9 and PI fluorescence was used to calculate the percentage of intact membranes in the sample. The applicability of the BacLight kit to L. plantarum was verified with cells killed by severe heat (80°C for 10 min) or HP treatment (800 MPa, 10 min). A calibration curve was established for each inactivation curve using the calibration samples described above, and the results are reported as percent intact membranes. (ii) The determination of HorA activity described above required incubation of cells of L. plantarum with EB in the presence and absence of an energy source, glucose. In the absence of glucose (i.e., in the absence of a functional EB efflux system), EB uptake was considered to depend solely on the barrier properties of the cytoplasmic membrane. The fluorescence intensity of samples stained with EB in PB0 (see the HorA assay described above) thus provides further information on the membrane integrity of pressurized cultures of L. plantarum. The samples with known contents of viable and dead cells were stained with EB in the absence of glucose as described above, and the resulting calibration curve was used to calculate the percent intact membranes in the HP-treated samples.
RESULTS
Assay validation for determination of metabolic activity with tetrazolium salts.
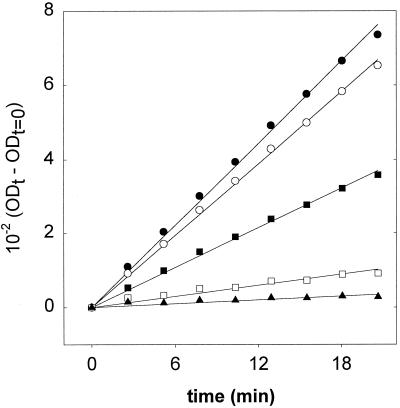
The use of tetrazolium salts has become a standard laboratory method to determine metabolic activity and viability of microorganisms. The assay is based on the ability of metabolically active cells to reduce INT to insoluble red formazan. However, the feasibility of this method to characterize metabolic activity of lactobacilli has so far not been demonstrated, and previous investigators have used the microscope to determine the deposition of formazan crystals in individual cells (43, 45). To develop a miniaturized assay for the rapid analysis of a large amount of samples, we evaluated whether the initial rate of formazan production by cell suspensions is a suitable means to estimate their metabolic activity. Mixtures of heat-killed, metabolically inactive cells of L. plantarum with metabolically active cells were incubated with INT, and the rate of formazan formation was determined. The increase of absorption at 595 nm is shown in Fig. 1. A linear rate of INT reduction was observed during the first 20 to 30 min of incubation, and the INT reduction rate was calculated from these data by linear regression. The INT reduction rate correlated well with the content of viable cells in the culture (r2 = 0.9985). This assay was therefore used for characterization of HP-treated cultures, and an r2 of 0.95 or greater was obtained for all calibration curves determined with HP experiments (n = 12).
FIG. 1.
Formazan formation by cultures of L. plantarum TMW1.460 containing 100% (●), 90% (○), 50% (■), 10% (□), and 0% (▴) viable cells. Lines represent curves obtained by linear regression. OD, optical density.
Assay validation for determination of HorA activity.
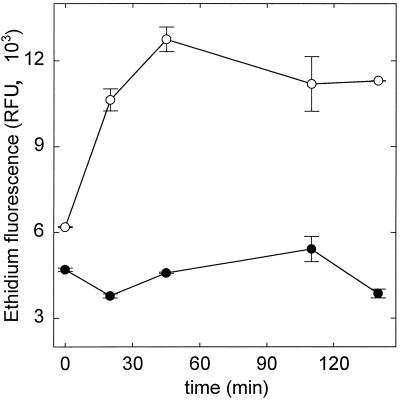
To demonstrate that L. plantarum TMW1.460, a highly hop-resistant beer isolate, has a functional HorA efflux system and to develop a HorA activity assay, the hop resistance was evaluated at the genetic and physiological level. Using horA-targeted primers and PCR conditions as described by Sami et al. (31), we obtained a PCR product of the expected size, 345 bp (data not shown), using chromosomal DNA from L. plantarum TMW1.460 as template. Sequencing of this PCR product revealed identity to the corresponding horA fragment of Lactobacillus brevis but for one conservative base pair exchange (references 31 and 32 and data not shown). This demonstrates that L. plantarum TMW1.460 carries a horA gene. HorA activity of L. plantarum TMW1.460 was investigated using EB as substrate. Energy-dependent EB efflux by L. plantarum was assessed by incubation of cells with EB in the presence or absence of an energy source, glucose, and monitoring of EB uptake over time. The results are shown in Fig. 2. In the presence of glucose, cells were able to maintain an internal low EB concentration, whereas starved cells accumulated EB. This points to the presence of an energy-dependent EB efflux system which was attributed to HorA activity. Based on the kinetics of EB accumulation, an incubation time of 1.5 h was chosen for determination of HorA activity in pressurized cells.
FIG. 2.
Kinetics of EB diffusion into starved (○) and energized (●) cells of L. plantarum TMW1.460. EB influx was determined by measuring the fluorescence of cells harvested after incubation times of 0 to 145 min. Symbols represent means ± standard deviations of two independent experiments.
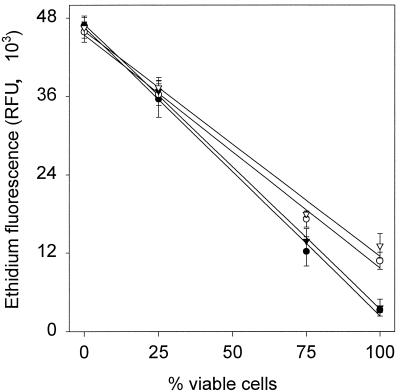
For assay calibration, mixtures of viable cells with cells killed by either HP treatment (800 MPa, 10 min) or heat (80°C, 10 min) were used. In Fig. 3 is shown the correlation of EB fluorescence in the presence and absence of glucose with the content of viable cells in the culture. Dead cells exhibited high EB fluorescence, and an increasing content of viable cells resulted in a decrease of EB fluorescence, as in viable cells EB influx occurs by diffusion through the lipid bilayer only. For samples containing viable cells, EB fluorescence in the presence of glucose was lower than that in the absence of glucose, in accordance with HorA activity of viable, energized cells. Cultures sterilized either by heat or by HP treatment exhibited the same behavior in the assay.
FIG. 3.
Correlation of EB fluorescence of starved (open symbols) and energized (black symbols) cells with the content of viable cells in the sample. Samples were prepared by mixing untreated cultures with cultures sterilized by heat (80°C, 10 min) (circles) or HP (800 MPa, 10 min) (inverted triangles). Symbols represent means ± standard deviations of three independent experiments.
It must be emphasized that the killing of cells by severe heat (80°C, 10 min) or HP (800 MPa, 10 min) treatments results in simultaneous disruption of membrane integrity, inactivation of glycolytic enzymes, and inactivation of HorA activity. However, during pressurization not all of these three components required for EB transport across the membrane are necessarily inactivated at the same time. Therefore, the interpretation of the assay results with respect to HorA activity requires additional information on metabolic activity and membrane integrity. Comparison of the EB fluorescence data presented in Fig. 2 and 3 shows that several mechanisms account for EB influx and efflux into L. plantarum. (i) Energized cells with HorA activity and an intact membrane (PI exclusion) are able to completely exclude EB. (ii) EB penetrates viable, deenergized cells by diffusion through the membrane. Within 1 h, a steady-state level is reached (Fig. 2). (iii) EB penetrates cells subjected to severe pressure or heat treatment by a mechanism comparable to that allowing PI influx into cells, probably through gaps in the cytoplasmic membrane. The steady-state EB fluorescence of viable, deenergized cells (diffusion through the cytoplasmic membrane only) is lower than the EB fluorescence of cells subjected to severe stress.
HP inactivation of L. plantarum: sublethal injury and cell death.
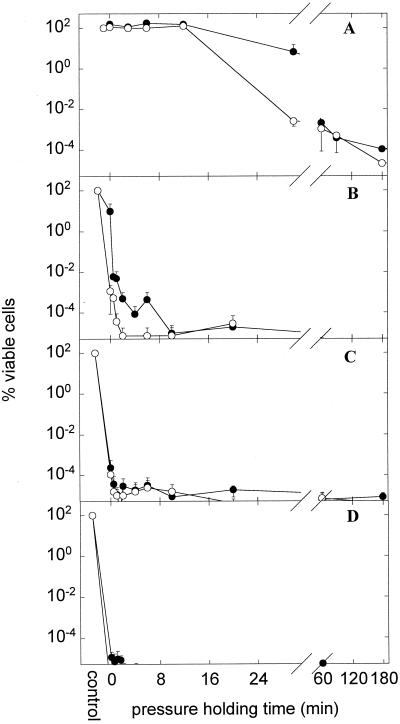
L. plantarum TMW1.460 was subjected to HP treatment at 200, 400, 500, and 600 MPa, and cell inactivation was monitored over time by plating on MRS and MRS-NaCl agar. The results are shown in Fig. 4. Cell counts of untreated cells on MRS and MRS-NaCl agars were not different (P < 0.001); therefore, failure of L. plantarum to grow on MRS-NaCl indicates sublethal injury. Because samples could not be taken during compression and decompression, the x axis in Fig. 4 indicates the pressure holding time.
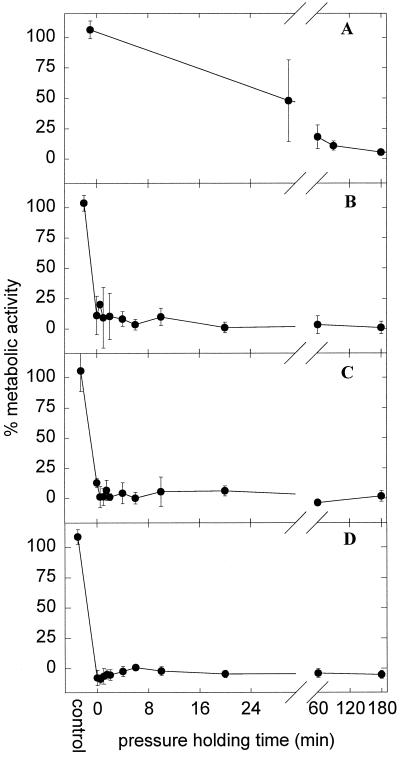
FIG. 4.
Kinetics of inactivation of L. plantarum TMW1.460 incubated at 200 (A) (n = 4), 400 (B) (n = 3), 500 (C) (n = 3), and 600 (D) (n = 2) MPa. Shown is the viable cell count on MRS (nonselective agar) (●) and MRS-NaCl (selective agar) (○) compared to that of untreated cultures. The cell count of control cultures was (4.27 ± 2.09) × 108 CFU ml−1 on either agar, and the detection limit was 120 CFU ml−1. Symbols represent means ± standard deviations of n independent experiments.
As shown in Fig. 4A, the inactivation curves of L. plantarum exhibited a sigmoid shape and were characterized by a shoulder, an exponential inactivation phase, and a pronounced tailing where no or little further inactivation took place. In the initial phase of pressurization, the shoulder, no loss of viability was observed. During the exponential inactivation phase, cells were sublethally injured prior to irreversible cell damage as demonstrated by the observation that more than 99.99% of viable cells lost their ability to grow on selective media. At longer pressure holding times (more than 60, 12, or 2 min at 200, 400, or 500 MPa, respectively), sublethal injury was no longer observed. Apparently, a fraction of the population, about 1 in 106 cells, was highly resistant to pressure, independent of whether the pressure treatment was performed at 200, 400, or 500 MPa. To evaluate whether this tailing of pressure inactivation curves stems from mutants or reflects phenotypic diversity of the population, survivors of HP treatments were isolated and subcultured once and their pressure tolerance was determined. If this selection cycle was carried out four times, no increase in barotolerance was observed (data not shown).
This characteristic shape of the inactivation curves was not observed at pressures of 400 and 500 MPa, as the shoulder and part of the exponential inactivation occurred already during the pressure ramps during which no samples were taken. However, a barotolerant fraction of the population exhibiting no sublethal injury was also observed. At 600 MPa, virtually all cells were killed during pressure ramps and the fraction of barotolerant cells was at or below the detection level (120 CFU ml−1).
HP inactivation of L. plantarum: loss of metabolic activity.
Cell counts demonstrated sublethal injury during HP treatment of L. plantarum. To evaluate whether this phenomenon is related to the loss of metabolic activity, the metabolic activity of pressurized cells was determined with tetrazolium chloride. The results are shown in Fig. 5. Whereas metabolic activity could be detected after a 60-min pressure holding time at 200 MPa, the pressure ramp to achieve 600 MPa sufficed to completely inactivate the cultures. Sublethally injured cells exhibited metabolic activity comparable to that of the untreated controls, but the loss of metabolic activity was highly correlated with cell death (r2 = 0.92). However, even upon pressure treatments killing more than 99.99% of the cells, metabolic activity above baseline level was observed ([18 ± 7]%, [9 ± 6]%, and [12 ± 4]% at 200 MPa, 60 min; 400 MPa, 1 min; and 500 MPa, 0 min, respectively). This indicates that the loss of metabolic activity is unlikely to be a primary cause for cell death.
FIG. 5.
Kinetics of inactivation of L. plantarum TMW1.460 incubated at 200 (A) (n = 4), 400 (B) (n = 3), 500 (C) (n = 3), and 600 (D) (n = 2) MPa. Shown is the INT reduction rate of treated cells compared to the INT reduction rate of untreated cells. Symbols represent means ± standard deviations of n independent experiments.
HP inactivation of L. plantarum: loss of membrane integrity.
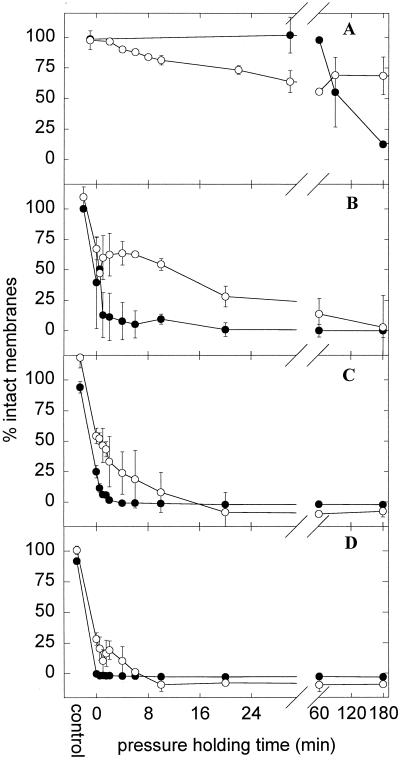
HP inactivation curves were further characterized by two different dye exclusion assays based on EB and PI permeation into cells. The results are shown in Fig. 6. Curves obtained with PI exhibited the same sigmoid shape as those observed with plate counts (Fig. 4) and staining with INT (Fig. 5); however, loss of PI exclusion was observed only after the cells were dead and lost any metabolic activity. This observation was highly significant at pressure levels of 200, 400, and 500 MPa, where 25 to 50% membrane integrity was observed after HP treatments resulting in reduction of viable cell counts by 4 to 6 orders of magnitude.
FIG. 6.
Kinetics of inactivation of L. plantarum TMW1.460 incubated at 200 (A), (n = 4), 400 (B) (n = 3), 500 (C) (n = 3), and 600 (D) (n = 2) MPa. Shown is the membrane permeability determined with the dye PI (●) or EB (○) and compared to the membrane permeability of untreated cells. Symbols represent means ± standard deviations of n independent experiments.
EB was used as a second probe for determination of membrane integrity. As opposed to PI, EB also penetrated intact membranes of viable cells to a steady-state level in between that of viable, energized cells and that of dead cells subjected to severe stress. Incubation of deenergized cells with EB excluded the possibility that ATP- or membrane potential-dependent transport mechanisms such as HorA affected EB uptake. In probing membrane integrity with EB during HP inactivation curves, biphasic kinetics were observed. EB diffusion into 200 MPa-treated cells was facilitated already after 4 to 20 min of pressure holding time, resulting in 50 to 75% membrane integrity, although cells were not stained with PI and neither sublethal injury nor loss of viability was detectable by plate counts. This facilitated diffusion of EB through membranes of pressurized cells indicates that membrane damage occurs prior to cell death. An incubation time greater than 3 h at 200 MPa was required for 0% EB membrane integrity (data not shown). Accordingly, pressurization at 400 and 500 MPa resulted in a rapid drop of EB membrane integrity to about 50% within 1 min followed by a much slower decrease to 0% within 3 h. Whereas EB appeared to be a more sensitive probe than PI for changes in membrane structure of viable, PI-excluding cells, longer pressurization times were required to obtain 0% membrane integrity as determined by EB.
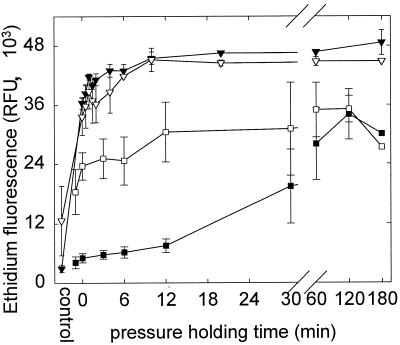
HorA activity of pressurized cells.
HorA activity is a prerequisite for growth of LAB in the presence of hop bitter compounds and is therefore crucial for the beer-spoiling capability of LAB. Therefore, in addition to membrane integrity and metabolic activity, the HorA activity of pressurized cells was estimated by determination of EB diffusion into starved and energized cells. The results for HP treatments at 200 and 600 MPa are shown in Fig. 7. As described above, control cultures (no HP treatment) exhibited a large difference in EB influx depending on the presence of glucose, indicating HorA activity (Fig. 2 and 3). Pressurization with 600 MPa resulted in complete loss of metabolic activity and membrane integrity (compared to Fig. 5 and 6); accordingly, EB uptake into cells was facilitated and reached the level of cells killed by heat (80°C, 10 min) after 6 to 10 min of pressurization time. A significant effect of glucose on EB uptake was not observed. Cells pressurized at 200 MPa for 0 to 12 min were able to maintain an internal low EB concentration although EB diffusion across the membrane was facilitated. During these first 12 min, no loss of cell viability or sublethal injury was observed by plate counts (Fig. 3). After a 30- and a 60-min pressure holding time, the cultures lost their ability to exclude EB even in the presence of glucose, indicating loss of hop resistance. This observation that viable but sublethally injured cells exhibited reduced HorA activity was more evident if data were corrected for the systematic difference (±2-log difference) between HP inactivation curves determined on different days.
FIG. 7.
HorA activity of L. plantarum TMW1.460 after pressure treatment at 200 (n = 4) (squares) and 600 (n = 2) (inverted triangles) MPa. Shown is the EB fluorescence of cells incubated in phosphate buffer with 20 μmol of EB liter−1 for 90 min in the presence (black symbols) and absence (open symbols) of glucose. Symbols represent means ± standard deviations of n independent experiments.
DISCUSSION
In this study, the pressure-mediated inactivation of L. plantarum was investigated and surviving cells were characterized with respect to viability, metabolic activity, membrane integrity, and functionality of hop resistance mechanisms. Based on this method, pressure inactivation of L. plantarum was found to involve a series of several inactivation steps. Mild-pressure treatment results in sublethal injury of cells. An increased permeability of the cytoplasmic membrane and reduced HorA activity were related to sublethal injury. Subsequently, loss of cell viability and concomitant loss of metabolic activity were observed. Membrane damage as determined with PI was observed later than cell death. These data provide a rationale for the observation that mild pressurization of microorganisms strongly reduces the ability of microorganisms to survive in adverse environments. Furthermore, assays developed for characterization of HP-treated cells have been found to be suitable methods for rapid determination of cell viability and activity.
First-order kinetics have been used to calculate pressure death-time data for bacteria and yeasts previously (10, 11, 48). However, for Bacillus species (16, 28) and for E. coli (15), it was shown that pressure inactivation curves may exhibit a pronounced shoulder and tailing. The shoulder of pressure inactivation curves was explained by phenotypic heterogeneity in the population, and a mathematical model that used a Weibull-distributed kinetics for the transition from a stable to a metastable state during pressure treatments fitted experimental death-time data well (16). If pressure treatment of bacteria indeed is a two-stage inactivation process leading to metastable or sublethally injured cells, it should be possible to identify physiological changes occurring during the shoulder of pressure death-time curves. A number of investigators have proposed selective media for determination of sublethally injured E. coli, Salmonella species, Staphylococcus aureus, and Listeria monocytogenes cells after pressure treatments (8, 14, 18, 19, 26). As a selective agent for lactobacilli, the use of 0.6% NaCl was proposed (41), and the failure of lactobacilli to grow on MRS-NaCl was thought to indicate membrane damage (40). We used 4% NaCl to inhibit growth of sublethally injured cells upon HP treatments and observed a shorter shoulder (200 MPa) or no shoulder at all (400 MPa) in pressure death-time curves characterized with MRS-NaCl agar. These data thus provide further physiological proof for a multistage inactivation process during pressurization of microorganisms. However, as shoulders were also observed if inactivation curves were characterized with cell counts on MRS-NaCl agar, this selective medium apparently failed to indicate cell damage occurring early during inactivation.
The determination of sublethal injury in HP-treated populations may be of major interest for food preservation, as sublethal injury may eliminate the ability of a culture to grow during food storage. Indeed, pressurized cells of E. coli failed to survive in fruit juices under conditions tolerated by untreated cells (8, 21), although only a minor part of the population was actually killed by the HP treatment.
Tailing of pressure inactivation curves obtained with spores of Bacillus subtilis was thought to reflect a heterogeneous distribution of barotolerance in the population (28) based on genotypic or phenotypic diversity. Hauben et al. (13) have isolated barotolerant mutants of E. coli. Eighteen selection cycles were required to obtain mutant strains that tolerated pressure treatments resulting in a greater than 8-log inactivation of the wild-type strain (13). We were unable to isolate baroresistant mutants after four selection cycles, indicating that tailing of pressure inactivation reflected phenotypic diversity within the population rather than the presence of baroresistant mutants.
The staining of cells with tetrazolium salts was proposed as a rapid method for determination of cell viability (9, 43, 45). Tetrazolium reduction to formazan by lactobacilli depends on the activity of NADH-dependent enzymes, such as NADH oxidase or peroxidase activities, or specific NADH dehydrogenases that are present in LAB (4, 20, 24, 30, 37, 39). Reduction of tetrazolium salts by lactobacilli thus requires NADH generation by an ongoing carbohydrate metabolism, and staining of cells with INT not only relies on the activity of a single enzyme system, i.e., NADH-reducing enzymes, but also may be considered an indicator of the overall metabolic activity of lactobacilli. Accordingly, we observed a loss of acidification capacity of pressurized cells of L. plantarum concomitant with loss of capability for INT reduction (data not shown). The observation that a loss of metabolic activity of HP-treated LAB is linked to cell death rather than to sublethal injury does conform with literature data obtained with different methods. Measuring the intracellular ATP pool and F0F1-ATPase activity of HP-treated L. plantarum revealed that ATP-generating glycolytic enzymes retained activity after treatments resulting in 60 to 95% reduction of viable cell counts (46) and that severe-pressure treatments (0.4% survival) were required to completely inhibit ATP-generating enzymes (46).
The assessment of membrane integrity by dye exclusion assays has been proposed by several authors to determine the efficacy of germ-killing processes, including HP treatments (1, 2, 5). Bunthof et al. (5) used PI staining for characterization of Lactococcus lactis stressed with freeze-thaw treatment, bile salts, low pH, and heat treatment. Whereas PI exclusion by L. lactis corresponded to plate counts for most stress treatments, membranes of heat-killed cells were impermeable to PI (5). The observation that HP-treated cells are not recoverable by plate counts but are not stained with PI was interpreted as an indication of the presence of living, but metabolically inactive, cells (1). Our data confirm that failure to grow on nonselective media may precede membrane permeability to PI. Membrane damage was observed with the PI assay only for treatments resulting in greater than 5-log reductions of viable cell counts. Thus, membrane impermeability to PI alone is an inappropriate indicator for cell viability as proposed by Arroyo et al. (1) but may serve as fail-safe method to evaluate the effect of pressure treatments.
EB penetrates cells with intact membranes and may therefore serve as an indicator of transient membrane perturbation. L. lactis and beer-spoiling LAB are known to possess transport systems mediating proton motive force- and ATP-dependent EB efflux, respectively, against a concentration gradient (3, 33). Therefore, to take into consideration both the changes in membrane permeability to EB and the inactivation of efflux systems, we performed EB transport assays with energized and starved cells. Membrane damage and inactivation of membrane-bound transport systems were identified as important events of pressure inactivation of L. plantarum, Salmonella enterica serovar Typhimurium, and S. cerevisiae (27, 29, 42, 46). Remarkably, EB uptake by starved cells revealed that the permeability of cells to EB was increased by pressurization. The kinetic resolution of HP inactivation of L. plantarum at 200 MPa indicates that membrane damage is an early event during pressurization of microorganisms, as it was observed even upon pressure treatments that did not affect viability or induce sublethal injury. We expect that membrane response to pressure may be nearly universal, as it was observed that pressures as low as 100 to 200 MPa result in destruction of lysosomal membranes in bovine muscle tissue (17).
In addition to increased permeability of HP-treated cells to EB, we observed a reduction of HorA activity prior to cell death. As the metabolic activity in these cells was largely unaffected by pressurization, this finding indicates inactivation of HorA. Modifications of lipid-enzyme interactions are considered to affect the activity of membrane-bound enzymes in eucaryotic cells. Membrane-bound Na+-Mg2+-ATPase of Acholeplasma laidlawii B is active only in association with liquid-crystalline lipids, and inactivation occurs when its boundary lipids undergo a phase transition (34). Phase transitions in the lipids associated with ATPase were considered to determine its activity at pressures ranging from 30 to 100 MPa (22, 23). Inhibition of Na-K-ATPase by pressure was directly correlated with increased membrane order (6). Extrapolating these findings with eucaryotic ATPase to corresponding bacterial enzymes suggests that phase transitions of membrane lipids may contribute to the pressure inactivation of membrane-bound transport systems such as HorA (this study) or F1F0-ATPase (46) in addition to pressure-mediated protein denaturation or subunit dissociation. Membrane damage and subsequent HorA inactivation thus result in sublethally injured, hop-sensitive cells that fail to survive or even grow during beer storage. We could thus demonstrate that relatively mild-pressure treatments are suitable in applications with the aim of preventing or delaying the growth of spoilage bacteria rather than the sterilization of foods.
ACKNOWLEDGMENT
This work was supported by the “Wissenschaftsförderung der Deutschen Brauwirtschaft,” grant no. B51.
REFERENCES
- 1.Arroyo G, Sanz P D, Prestamo G. Response to high-pressure, low-temperature treatment in vegetables: determination of survival rates of microbial populations using flow cytometry and detection of peroxidase activity using confocal microscopy. J Appl Microbiol. 1999;86:544–556. doi: 10.1046/j.1365-2672.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 2.Benito A, Ventoura G, Casadei M, Robinson T, Mackey B. Variation in resistance on natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl Environ Microbiol. 1999;65:1564–1569. doi: 10.1128/aem.65.4.1564-1569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolhuis H, Molenaar D, Poelarends G, van Veen H W, Poolman B, Driessen A J M, Konings W N. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug-resistant Lactococcus lactis. J Bacteriol. 1994;176:6957–6964. doi: 10.1128/jb.176.22.6957-6964.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolotin A, Mauger S, Malarme K, Ehrlich S D, Sorokin A. Low-redundancy sequencing of the entire Lactococcus lactis IL403 genome. Antonie Leeuwenhoek. 1999;76:27–76. [PubMed] [Google Scholar]
- 5.Bunthof C J, van den Braak S, Breeuwer P, Rombouts F M, Abee T. Rapid fluorescence assessment of the viability of stressed Lactococcus lactis. Appl Environ Microbiol. 1999;65:3681–3689. doi: 10.1128/aem.65.8.3681-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong P L-G, Fortes P A G, Jameson D M. Mechanisms of inhibition of (Na,K)-ATPase by hydrostatic pressure studied with fluorescent probes. J Biol Chem. 1988;260:14484–14490. [PubMed] [Google Scholar]
- 7.de Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 8.Garcia-Graells C, Hauben K J A, Michiels C W. High-pressure inactivation and sublethal injury of pressure-resistant Escherichia coli mutants in fruit juices. Appl Environ Microbiol. 1998;64:1566–1568. doi: 10.1128/aem.64.4.1566-1568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Lara J, Martinez J, Vilamu M, Vives-Rego J. Effect of previous growth conditions on the starvation-survival of Escherichia coli in seawater. J Gen Microbiol. 1993;139:1425–1431. doi: 10.1099/00221287-139-7-1425. [DOI] [PubMed] [Google Scholar]
- 10.Gervilla R, Sendra E, Ferragut V, Guamis B. Sensitivity of Staphylococcus aureus and Lactobacillus helveticus in ovine milk subjected to high hydrostatic pressure. J Dairy Sci. 1999;82:1099–1107. doi: 10.3168/jds.S0022-0302(99)75332-4. [DOI] [PubMed] [Google Scholar]
- 11.Gervilla R, Mor-Mur M, Ferragut V, Guamis B. Kinetics of destruction of Escherichia coli and Pseudomonas fluorescens inoculated in ewe's milk by high hydrostatic pressure. Food Microbiol. 1999;16:173–184. [Google Scholar]
- 12.Hashizume C, Kimura K, Hayashi R. Kinetic analysis of yeast inactivation by high pressure treatment at low temperatures. Biosci Biotechnol Biochem. 1995;59:1455–1458. doi: 10.1271/bbb.59.1455. [DOI] [PubMed] [Google Scholar]
- 13.Hauben K J A, Bartlett D H, Soontjens C C F, Cornelis K, Wuytack E Y, Michiels C W. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl Environ Microbiol. 1997;63:945–950. doi: 10.1128/aem.63.3.945-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauben K J A, Wuytack E Y, Soontjens C C F, Michiels C W. High-pressure transient sensitiation of Escherichia coli to lysozyme and nisin by disruption of outer-membrane permeability. J Food Prot. 1996;59:350–355. doi: 10.4315/0362-028X-59.4.350. [DOI] [PubMed] [Google Scholar]
- 15.Hauben K J A, Bernaerts K, Michiels C W. Protective effect of calcium on inactivation on Escherichia coli by high hydrostatic pressure. J Appl Microbiol. 1998;85:678–684. doi: 10.1111/j.1365-2672.1998.00577.x. [DOI] [PubMed] [Google Scholar]
- 16.Heinz V, Knorr D. High pressure inactivation kinetics of Bacillus subtilis cells by a three-state-model considering distributed resistance mechanisms. Food Biotechnol. 1996;10:149–161. [Google Scholar]
- 17.Jung S, de Lamballerie-Anton M, Courcoux P, Ghoul M. High pressure improvement of the meat aging enzymes activity. In: Isaacs N S, editor. High pressure food science, bioscience and chemistry. Cambridge, United Kingdom: Royal Society of Chemistry; 1998. pp. 295–303. [Google Scholar]
- 18.Kalchayanand N, Sikes A, Dunne C P, Ray B. Factors influencing death and injury of foodborne pathogens by hydrostatic pressure-pasteurization. Food Microbiol. 1998;15:207–214. [Google Scholar]
- 19.Kalchayanand N, Sikes T, Dunne C P, Ray B. Hydrostatic pressure and electroporation have increased bactericidal efficiency in combination with bacteriocins. Appl Environ Microbiol. 1994;60:4174–4177. doi: 10.1128/aem.60.11.4174-4177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanbe C, Uchida K. NADH dehydrogenase activity of Pediococcus halophilus as a factor determining its reducing force. Agric Biol Chem. 1987;51:507–514. [Google Scholar]
- 21.Linton M, McClements J M J, Patterson M F. Survival of Escherichia coli O157:H7 during storage in pressure-treated orange juice. J Food Prot. 1999;62:1038–1040. doi: 10.4315/0362-028x-62.9.1038. [DOI] [PubMed] [Google Scholar]
- 22.Macnaughtan W, Macdonald A G. Effects of pressure and pressure antagonists on the growth and membrane-bound ATP-ase of Acholeplasma laidlawii B. Comp Biochem Physiol A. 1982;72:405–414. doi: 10.1016/0300-9629(82)90238-9. [DOI] [PubMed] [Google Scholar]
- 23.Marquis R E. Reversible actions of hydrostatic pressure and compressed gases on microorganisms. In: Hurst A, Nasim A, editors. Repairable lesions in microorganisms. New York, N.Y: Academic Press, Inc.; 1984. pp. 273–302. [Google Scholar]
- 24.Marty-Teysset F, de la Torre F, Garel J R. Increased production of hydrogen peroxide by Lactobacillus delbrueckii subsp. bulgaricus upon aeration: involvement of an NADH oxidase in oxidative stress. Appl Environ Microbiol. 2000;66:262–267. doi: 10.1128/aem.66.1.262-267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niven G W, Miles C A, Mackey B M. The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: an in vivo study using differential scanning calorimetry. Microbiology. 1999;145:419–425. doi: 10.1099/13500872-145-2-419. [DOI] [PubMed] [Google Scholar]
- 26.Patterson M F, Quinn M, Simpson R, Gilmour A. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. J Food Prot. 1995;58:524–529. doi: 10.4315/0362-028X-58.5.524. [DOI] [PubMed] [Google Scholar]
- 27.Perrier-Cornet J-M, Hayert M, Gervais P. Yeast cell mortality related to a high-pressure shift: occurrence of cell membrane permeabilization. J Appl Microbiol. 1999;87:1–7. doi: 10.1046/j.1365-2672.1999.00779.x. [DOI] [PubMed] [Google Scholar]
- 28.Raso J, Barbosa-Canovas G, Swanson B G. Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus cereus. J Appl Microbiol. 1998;85:17–24. doi: 10.1046/j.1365-2672.1998.00460.x. [DOI] [PubMed] [Google Scholar]
- 29.Ritz M, Pilet M R P, Tholozan J L, Federighi M. High hydrostatic pressure effects on Salmonella typhimurium. Physiological and morphological damages. In: Tuijtelaars A C J, Samson R A, Rombouts F M, Notermans S, editors. Food microbiology and food safety into the next millennium. Zeist, The Netherlands: Foundation for Food Microbiology; 1999. pp. 295–298. [Google Scholar]
- 30.Sakamoto M, Komagata K. Aerobic growth of and activities of NADH oxidase and NADH peroxidase in lactic acid bacteria. J Ferment Bioeng. 1996;82:210–216. [Google Scholar]
- 31.Sami M, Yamashita H, Kadokura H, Kitamoto K, Yoda K, Yamasaki M. A new and rapid method for determination of beer-spoilage ability of lactobacilli. J Am Soc Brew Chem. 1997;55:137–140. [Google Scholar]
- 32.Sami M, Yamashita H, Hirono T, Kadokura H, Kitamoto K, Yoda K, Yamasaki M. Hop-resistant Lactobacillus brevis contains a novel plasmid harboring a multidrug resistance-like gene. J Ferment Bioeng. 1997;84:1–6. [Google Scholar]
- 33.Sami M, Suzuki K, Sakamoto K, Kadokura H, Kitamoto K, Yoda K. A plasmid pRH45 of Lactobacillus brevis confers hop resistance. J Gen Appl Microbiol. 1998;44:361–363. doi: 10.2323/jgam.44.361. [DOI] [PubMed] [Google Scholar]
- 34.Silvius J R, McElhaney R N. Membrane lipid physical state and modulation of the Na+, Mg2+-ATP-ase activity in Acholeplasma laidlawii B. Proc Natl Acad Sci USA. 1980;77:1255–1259. doi: 10.1073/pnas.77.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson W J, Smith A R W. Factors affecting antibacterial activity of hop compounds and their derivatives. J Appl Bacteriol. 1992;72:327–334. doi: 10.1111/j.1365-2672.1992.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 36.Simpson W J, Fernandez J L. Mechanism of resistance of lactic acid bacteria to trans-isohumulone. J Am Soc Brew Chem. 1994;52:9–11. [Google Scholar]
- 37.Smart J B, Thomas T D. Effect of oxygen on lactose metabolism in lactic streptococci. Appl Environ Microbiol. 1987;53:533–541. doi: 10.1128/aem.53.3.533-541.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonoike K, Setoyama T, Kuma Y, Kobayashi S. Effect of pressure and temperature on the death rates of Lactobacillus casei and Escherichia coli. In: Balny C, Hayashi R, Heremans K, Masson P, editors. High pressure and biotechnology. 1992 Colloque INSERM/John Libbey Eurotext. 1992. pp. 297–301. Montrouge, France. [Google Scholar]
- 39.Stolz P, Vogel R F, Hammes W P. Utilization of electron acceptors by lactobacilli isolated from sourdough. Z Lebensm-Unters-Forsch. 1995;201:402–410. [Google Scholar]
- 40.Teixeira P, Castro H, Kirby R. Spray drying as a method for preparing concentrated cultures of Lactobacillus bulgaricus. J Appl Bacteriol. 1995;78:456–462. [Google Scholar]
- 41.Teixeira P, Castro H, Mohacsi-Farkas C, Kirby R. Identification of sites of injury in Lactobacillus bulgaricus during heat stress. J Appl Microbiol. 1997;83:219–226. doi: 10.1046/j.1365-2672.1997.00221.x. [DOI] [PubMed] [Google Scholar]
- 42.ter Steeg P F, Hellemons J C, Kok A E. Synergistic actions of nisin, sublethal ultrahigh pressure, and reduced temperature on bacteria and yeast. Appl Environ Microbiol. 1999;65:4148–4154. doi: 10.1128/aem.65.9.4148-4154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thom S M, Horobin R W, Seidler E, Barer M R. Factors affecting the selection and use of tetrazolium salts as cytochemical indicators of microbial viability and activity. J Appl Bacteriol. 1993;74:433–443. doi: 10.1111/j.1365-2672.1993.tb05151.x. [DOI] [PubMed] [Google Scholar]
- 44.van Veen H W, Konings W N. The ABC family of multidrug transporters in microorganisms. Biochim Biophys Acta. 1998;1365:31–36. doi: 10.1016/s0005-2728(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 45.Walsh S, Lappin-Scott H M, Stockdale H, Herbert B N. An assessment of the metabolic activity of starved and vegetative bacteria using two redox dyes. J Microbiol Methods. 1995;24:1–9. [Google Scholar]
- 46.Wouters P C, Glaasker E, Smelt J P P M. Effects of high pressure on inactivation kinetics and events related to proton efflux in Lactobacillus plantarum. Appl Environ Microbiol. 1998;64:509–514. doi: 10.1128/aem.64.2.509-514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yano Y, Nakayama A, Ishihara K, Saito H. Adaptive changes in membrane lipids of barophilic bacteria in response to changes in growth pressure. Appl Environ Microbiol. 1998;64:479–485. doi: 10.1128/aem.64.2.479-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zook C D, Parish M E, Braddock R J, Balaban M O. High pressure inactivation kinetics of Saccharomyces cerevisiae ascospores in orange and apple juices. J Food Sci. 1999;64:533–535. [Google Scholar]