Abstract
Simple Summary
Pest monitoring using traps is a key component of integrated pest management. For several insects, trapping is achieved using visual or olfactory stimuli. Although the combination of both is supposed to provide higher efficacy, this has often been overlooked in trap design. Through laboratory bioassays and field experiments we evaluated the use of UV-A and visible light in combination with olfactory stimuli to improve trapping of the invasive brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Our results may be useful for the improvement of monitoring strategies for early pest detection. Additionally, the higher efficacy of the multimodal traps would allow their use in attract-and-kill or push–pull strategies within integrated pest management.
Abstract
Capture strategies for the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), are challenging. Here we developed and evaluated a multimodal trap which combines visual and olfactory stimuli. Visual stimuli consisted of LEDs emitting UV-A and visible light. Olfactory stimuli were comprised of the synthetic aggregation pheromone and odours from trapped H. halys individuals. Stink bug attraction at different wavelengths was evaluated in laboratory two-choice bioassays, and different prototypes of the trap were tested in 2021 in natural, agricultural, and urban settings. Traps with a combination of UV-A and blue or green visible wavelengths provided higher H. halys attraction (up to ~8-fold) compared to traditional sticky or small pyramidal traps. The concurrent presence of synthetic pheromone and LED had a synergistic effect on H. halys positive phototaxis. Further development and implementation of the multimodal trap is discussed for prospective use in attract-and-kill or push–pull strategies.
Keywords: attract-and-kill, Hemiptera, integrated pest management, invasive species, LED light, monitoring, Pentatomidae, pest surveillance, pheromone, ultraviolet (UV)
1. Introduction
Invasive invertebrates that establish in a new area may cause environmental and economic damage to agriculture and society [1]. From 1970 to 2017 the mean annual cost for damage and management of invasive invertebrates was USD 8.7 billion [2,3]. Management of invasive invertebrates using integrated pest management (IPM) strategies represents an economically effective approach [4,5]. The main strategies for management of invasive invertebrates are based on mechanical, chemical, and biological control tools [6,7,8]. Monitoring is a key element of IPM (Directive 2009/128/EC) and supports farmers in the selection of chemicals and timing of spray applications for managing pests [9]. For example, pheromone-based traps are frequently used and could play a pivotal role in detecting invasive insects as soon as they arrive [10].
The brown marmorated stink bug Halyomorpha halys Stål (Hemiptera: Pentatomidae) is native to Asia and is now widespread in each continent of the Northern Hemisphere [11]. Its host range includes more than 275 plant species [12], and, in the areas where it has established, it is causing significant agricultural losses [13,14,15]. The first detection in Italy occurred in 2012 in Emilia-Romagna (northern Italy) with more than 50% damage in early maturing pear cultivars being reported [16]. Additionally, during late summer and fall seasons, H. halys adults seek shelter within human-made structures to overwinter, thereby being a nuisance to citizens [13,14].
Assessing the density of H. halys populations is crucial for anticipating further invasion, allowing growers to determine the best control tools and to ensure timely chemical applications [17]. In recent years, insecticide use has greatly risen for stink bug management [18]. As a consequence, invasion of H. halys has resulted in the disruption of IPM programmes for several important crops, with possible non-target effects on beneficial insects, e.g., predators and egg parasitoids notoriously sensitive to lethal and sublethal insecticide doses [19,20,21]. Before becoming invasive, little information was available on the best monitoring tools to be used for H. halys [22]. Sweep netting and visual counts were usually employed [23] but often with uncertain results because of stink bugs’ strong dispersal ability [24]. The identification of the two-component aggregation pheromone of H. halys [25] and the demonstrated synergistic effect of the aggregation pheromone of another Asian stink bug, Plautia stali Scott [26], has provided important insight for pest monitoring. Various studies have assessed the effectiveness of different pheromone-based trap designs, the combination of the pheromones to be employed, and the optimal trap placement [19,27,28]. Noteworthy, is that individuals tend to remain in the proximity of the pheromone trap instead of entering inside it [29]. To overcome this issue, traps combining different stimuli have been developed for monitoring purposes, and, ideally for H. halys direct control, e.g., in attract-and-kill or push-pull strategies [30,31]. Vibrational-based signals play an important role in H. halys mating behaviour [32], and the addition of such cues to pheromone traps was proven to increase trap efficacy [33]. Rotating live traps captured, on average, 7-fold more H. halys adults compared to classic sticky panels [34]. In addition to diurnal feeding activity, stink bugs exhibit intensive nocturnal movement [35]. Hence, light traps, e.g., traps equipped with black light lamps, could be useful tools to investigate population density and seasonal phenology of H. halys [35,36]. Black light refers to type-A ultraviolet (UV-A) light with a peak at or near 365 nm and a well-established insect-attracting aptitude [37]. Therefore, to further explore trapping methods for H. halys, we tested traps with the combined effect of different stimuli, i.e., LED lights, synthetic pheromone, and natural odours emitted by trapped insects. Captures of insects other than H. halys were also recorded to possibly evaluate the effect of such traps on non-targets. Multimodal traps were assessed in agricultural, urban, and natural landscapes of northern and central Italy where H. halys is responsible for serious economic losses to crops and citizen nuisance.
2. Materials and Methods
2.1. Design of the Multimodal Trap
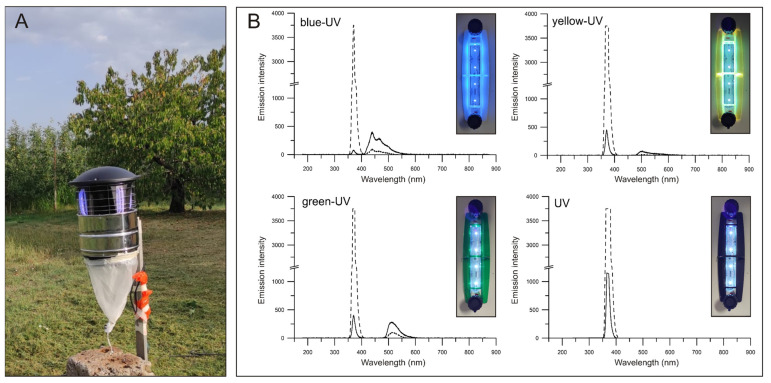
A trap prototype was developed by MO-EL S.p.A. (Reggio Emilia, Italy) based on their model “TurbiLED” (mod. 806, more details at https://www.mo-el.it/, accessed on 4 May 2022). The trap (Figure 1A and Figure S1) has a cylindrical shape (36 cm diameter, 84 cm height), and the main body is made of steel and black plastic and has four equidistantly spaced openings of approx. 400 cm2 each on the side. Between each opening, a digital UV-A LED lamp (10.5 cm length) is vertically fixed on the plastic body of the trap. Basically, each lamp consisted of four LEDs (peak emission at 365 nm) equidistantly positioned every 2.6 cm in a digital strip and is partially covered by a removable and interchangeable plastic grid (14 × 4.2 × 2.1 cm) made of translucid coloured polycarbonate. LED lamps are powered by an electronic converter for LEDs (220 V, 350 mA, Vossloh Schwabe, Sarsina, Italy). When a portion of the UV-A flux intercepts the plastic part of the grid it undergoes a wavelength change, i.e., from 360–380 nm to 380–780 nm, which corresponds to visible light (details in patent EP3909426A1). A second portion of the radiation emitted by LEDs does not intercept the plastic part of the grid and totally consists in UV-A radiation. A motorised fan (details in US20030131525A1) is horizontally placed inside the trap at a central position. By rotating, the fan generates an airstream that sucks the insects surrounding the LED lamp and eventually conveys them inside a polyamide funnel bag placed behind the trap. The trap is completed by a metal grill (approx. 2–4 × 8 cm rectangular holes) externally fixed to prevent the entrance of birds or small mammals into the openings (visible in Figure 1A and Figure S1).
Figure 1.
Overview of a multimodal electric trap used in field experiments (A). Emission intensity of single lamps emitting blue-UV, green-UV, yellow-UV, or UV only wavelengths were evaluated in laboratory conditions and are reported in arbitrary scale (B). For each combination of lamp and plastic grid, the emitted radiation flux was measured at close contact (continuous line) or at 2 cm distance (dashed line).
For our experiments, differently coloured plastic grids were evaluated (provided by MO-EL S.p.A.), allowing emission of wavelengths with a dominance of blue (hereafter “blue-UV”), green (“green-UV”), or yellow (“yellow-UV”). A black grid with no reflectance was also tested for emission of UV-only wavelengths. To increase stink bug captures, a dispenser of the aggregation pheromone of H. halys (Pherocon®, Trécé Inc., Adair, OK, USA) was positioned on the uppermost extremity of the trap. Dispensers comprised both the two-component H. halys aggregation pheromone and the synergistic MDT [26]. The novelty of the trap relies on its multimodal characteristics; including, the ability to collect stink bugs through visual stimuli (positive phototaxis toward the combination of UV-A and visible wavelengths) and olfactory stimuli (presence of the aggregation pheromone and odours from trapped individuals which are aerially distributed in the environment as a consequence of the effect of the fan rotation).
2.2. Insect Rearing for Laboratory Bioassays
Adults of H. halys were collected in northern and central Italy from overwintering sites (January-February 2021) or from agricultural and natural ecosystems (spring 2021) and served to establish a laboratory colony. Clear plastic food containers (30 × 20 × 15 cm) with 5-cm round holes covered by a mesh were used to rear adults in a laboratory under environmentally-controlled conditions (25 ± 1 °C; 16:8 h light/dark). A diet of apples, carrots, green beans, hazelnuts, and sunflower seeds was supplied. Diet was replaced twice a week. Vicia faba L. potted plants were placed inside the cages both as a supplementary food source and an oviposition substrate [38]. Eggs were collected daily and transferred to new cages for rearing of the offspring. Water was provided daily on wetted cotton in a Petri dish. Males and females in reproductive stages were used for experiments.
2.3. Measurement of the Radiation Flux and Laboratory Evaluation of H. halys Phototaxis
The radiation flux emitted by the different combinations of UV lamp and plastic grids was measured in the laboratory by positioning the sensor of a digital spectrophotometer (mod. USB2000, Ocean Optics, Ostfildern, Germany) at close contact or at 2 cm distance from the plastic grid covering the LED lamp. Emission intensity was measured up to 3750 photon count.
For the evaluation of H. halys phototactic behaviour, bioassays consisted of two-choice tests and were performed in a dark room maintained at a constant temperature of 25 ± 1 °C. Groups of 5 adult females or males were isolated in 50 mL Falcon tubes closed with a small net to allow aeration. Three hazelnut fruits were supplied as food, and insects were kept in the bioassay room for 1 h before being tested. One stink bug at time was assayed.
Bioassays were conducted on the surface of a black table (100 × 80 cm). Two visual stimuli were both provided at one of the longest sides of the table. The two stimuli were positioned at 60 cm distance and were separated by a black cardboard panel (length: 40 cm, height: 60 cm) to minimise interference between the two stimuli.
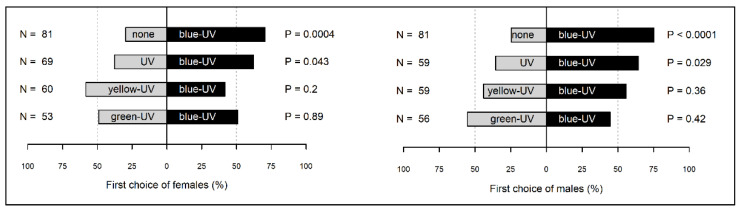
Treatments consisted of one UV lamp with four LEDs partially covered by a plastic grid (as described in Section 2.1) and vertically affixed on an electric box (10 × 16 × 7 cm), used as support. For all bioassays, one choice consisted of a blue-UV light. The other choice consisted in control stimulus (light off), or in UV, yellow-UV, or green-UV light.
A line was drawn on the table at 10 cm from each stimulus source. Each stink bug was released from the opposite side of the stimuli and allowed to freely move on the table surface and eventually choose between one of the two stimuli. A choice was considered when the insect passed the line and remained in the area close of the stimulus for at least 10 seconds. After that, the bioassay was ended. Each insect was observed for a maximum of 5 min. Stink bugs were assayed once and not re-used. The number of replications ranged from 53 to 81 for females and from 56 to 81 for males.
2.4. Field Experiments
Five experiments were conducted at locations in northern and central Italy with different habitat types (details of the experiments are presented in Table 1). Various multimodal trap settings were evaluated for attraction and trapping of H. halys and/or other insect groups. All multimodal and control traps, except for one in experiment 5, were provided with the aggregation pheromone lure (as in Section 2.1). Samplings were conducted daily at different times (details in Table 1). When one trap with differently coloured lights was used (experiments 1 and 2), the trap was rotated 90° after each sampling. In the other experiments, the position of all traps was changed after each sampling (experiments 3 and 5), or after two consecutive samplings (experiment 4).
Table 1.
Overview of the different field experiments (Exp).
| Habitat Type | Exp | Date (2021); Time of Samplings [Number of Samplings] |
Type of Trap | Type of Stimuli Evaluated | Phero-Mone | Number of Traps Used | Type of Data Collected |
|---|---|---|---|---|---|---|---|
| Conservation area | 1 | 27–28 July; 8:00–9:00 and 17:00–18:00 [4] | Multimodal trap with 4 lamps, each of a different colour. | Blue-UV, green-UV, yellow-UV, UV | Yes | 1 | arrestment of H. halys on trap surface during photophase |
| 2 | 29 July–01 August; 8:00–9:00 [4] | Multimodal trap with 2 lamps, each of a different colour. | Blue-UV, green-UV | Yes | 1 | ||
| Fruit orchard | 3 | 18–19 August, 23–24 August, 25–26 August; 8:00–9:00 [3] | Multimodal trap with 4 lamps of the same colour. A pyramidal trap (Rescue) was used as control. | Blue-UV, green-UV | Yes | 1 of each type | capture of H. halys in 24 h |
| 4 | 26–28 August, 02–04 September, 13–15 September; 8:00–9:00 [6] | Multimodal trap with 4 lamps of the same colour. Clear sticky traps were used as controls. | Blue-UV, green-UV | Yes | 2 of each type | capture of H. halys and other insect groups in 24 h |
|
| Urban park | 5 | 31 August–11 September; 17:00–18:00 [11] | Multimodal trap with 4 lamps of the same colour. | Blue-UV + pheromone, blue-UV only, pheromone only | Yes/no | 1 of each type | capture of H. halys and other insect groups in 24 h |
Experiments 1 and 2 were conducted in a conservation area in northern Italy near Montechiarugolo, Reggio Emilia (coordinates: 44.69330, 10.42654) between July and August 2021. A multimodal trap with 4 lamps emitting blue-UV, green-UV, yellow-UV, or UV-only wavelengths (experiment 1) and a trap with 2 lamps emitting blue-UV or green-UV (experiment 2) light were assessed for H. halys attraction. For experiment 1, two samplings were conducted over two consecutive days. For experiment 2, one sampling was conducted and repeated over four consecutive days. In these experiments the arrestment of H. halys, i.e., the number of insects that were present in each sector of the trap at each observation, was evaluated.
Experiments 3 and 4 were conducted in a fruit orchard in central Italy near Ponte Pattoli, Perugia (coordinates: 43.17663, 12.44938) between August and September 2021. The difference in daily capture among the multimodal trap and two different commercial traps as control was evaluated. The multimodal traps were equipped with 4 lamps emitting green-UV or blue-UV light. In experiment 3, a total of 3 samplings were conducted; only one trap for each type was positioned and only data on H. halys were collected. In experiment 4 (6 replicates in total), two traps per type were used and both H. halys and other insect groups were assessed. Controls were a pyramidal trap (Rescue, Sterling International Inc., Sokane, WA, USA) in experiment 3 and sticky traps (Certis, Milano, Italy) in experiment 4.
Experiment 5 was conducted in an urban park in northern Italy near Crevalcore, Bologna (coordinates: 44.71658, 11.14878) between August and September 2021. Three multimodal traps with different equipment were compared. These were: a multimodal trap with 4 blue-UV lamps and the pheromone lure, a trap with the pheromone lure but with the LED lamps turned off, a trap with the LED lamps turned on but without pheromone lure. Eleven samplings were conducted.
2.5. Statistical Analysis
For laboratory bioassays, generalised linear models (GLMs, logit link, Binomial error distribution) were fitted to test the first choice, i.e., the area close to the stimulus the stink bug entered first [39]. For all field experiments, treatment replications were split across different days. In addition, for experiment 4, true replications (2) were also conducted. Linear mixed-effects models (LMMs) were used to analyse the fixed effect of experimental treatments and the random effect of samplings on insect abundance (two factors complete-block design, [40]). The relevance of the random term was evaluated with a likelihood ratio test [41]. In those cases where its presence was not justified, only the fixed term was retained in a linear model (LM) [41]. Abundance data were box-cox transformed before analysis. Multiple comparisons were eventually conducted, adopting the Sidak correction. Analyses were conducted under R statistical environment, packages “MASS”, “nlme” and “emmeans” [42].
3. Results
3.1. Measurement of the Radiation Flux and Laboratory Evaluation of H. halys Phototaxis
The measurement of the radiation flux for blue-UV, green-UV, yellow-UV, or UV light revealed that the higher portion of the emission was represented by undisturbed UV-A wavelengths, and only a small fraction constituted visible light (Figure 1B).
In laboratory conditions, female H. halys were highly attracted to visual stimuli from blue-UV light compared to control (no light) (Binomial GLM, p = 0.0004, Figure 2) or to UV-only light (p = 0.043). No differences were detected between blue-UV and yellow-UV, or green-UV (p > 0.05 for both comparisons) light. Male H. halys where highly attracted towards visual stimuli from blue-UV compared to control (p < 0.0001) or to UV-only (p = 0.029). No differences were detected between blue-UV and yellow-UV or green-UV (p > 0.05 for both comparisons).
Figure 2.
First choice (%) of Halyomorpha halys females and males in a two-choice experimental setup. One choice (black bar) consisted of blue-UV lamp. The other choice (grey bar) consisted of the control stimulus (no light and no grid), or in a single lamp emitting UV, yellow-UV or green-UV light. For each comparison, H. halys preference was analysed by means of binomial GLM.
3.2. Field Experiments
Experiment 1: When the blue-UV, green-UV, yellow-UV, and UV light were compared in the same trap, the presence of the stink bugs (arrestment on the trap sector corresponding to a different light type) was higher in relation to green-UV light compared to all other treatments (results of LMM followed by multiple comparison procedure are reported in Table 2). Notably, the stink bug presence changed across the different observation periods (ΔAIC = 9.33, p = 0.0008). Experiment 2: When only blue-UV and green-UV light were compared in the same trap, the presence of the stink bugs was similar across treatments (ΔAIC = 1.93, p = 0.80) and sampling days (ΔAIC = 1.50, p = 0.48) (Table 2). Experiment 3: When blue-UV and green-UV light were compared in different traps, H. halys captures were similar across sampling days (ΔAIC = 2.00, p = 1.00) but higher for the blue and green traps compared to control trap (results of LM followed by multiple comparison procedure are reported in Table 2).
Table 2.
Arrestment/presence (mean ± SE) of H. halys in the different sectors of a single multimodal trap (Exp. 1 and 2) or total H. halys (mean ± SE) captured in multimodal or control traps (Exp. 3). For each experiment, means followed by different letters are significantly different according to linear mixed-effects model followed by multiple comparisons procedure (significance level α = 0.05).
| Exp | Treatment | H. halys |
|---|---|---|
| 1 | Blue-UV | 4.00 ± 2.04 b |
| Green-UV | 19.00 ± 7.12 a | |
| Yellow-UV | 2.25 ± 1.03 b | |
| UV | 3.00 ± 1.78 b | |
| 2 | Blue-UV | 14.00 ± 3.54 |
| Green-UV | 16.50 ± 5.92 | |
| 3 | Blue-UV | 34.33 ± 5.04 a |
| Green-UV | 33.00 ± 5.13 a | |
| Control (pyramidal trap) | 4.00 ± 2.08 b |
Experiment 4: Captures of H. halys were higher for both blue-UV and green-UV traps compared to sticky traps (Table 3). Similar patterns were detected for other Pentatomidae, Hemiptera, Lepidoptera, Coleoptera, Diptera, and Hymenoptera. Random term for sampling dates was never justified except for Coleoptera (ΔAIC = 2.60, p = 0.030).
Table 3.
Captures (mean ± SE) of H. halys and other main insect groups in multimodal and control traps (Exp. 4). Means in the same column followed by different letters are significantly different according to linear model or linear mixed-effects models followed by multiple comparisons procedure (significance level α = 0.05).
| Exp | Treatment | Hemiptera (H. halys) | Hemiptera (Other Pentatomidae) | Hemiptera (Other) | Lepidoptera | Coleoptera | Diptera | Hymenoptera |
|---|---|---|---|---|---|---|---|---|
| 4 | Blue-UV | 11.33 ± 2.93 a | 6.08 ± 1.33 a | 65.92 ± 21.52 a | 269.25 ± 38.47 a | 27.92 ± 5.74 a | 799.58 ± 121.41 a | 26.42 ± 7.42 a |
| Green-UV | 12.67 ± 3.07 a | 6.33 ± 1.14 a | 72.5 ± 26.42 a | 210.75 ± 32.77 a | 21.75 ± 4.78 a | 722.42 ± 162.29 a | 22.33 ± 4.53 a | |
| Control (sticky trap) | 2.83 ± 0.73 b | 0.92 ± 0.67 b | 0.17 ± 0.17 b | 1.33 ± 0.43 b | 0.00 ± 0.00 b | 10.25 ± 6.63 b | 3.42 ± 1.44 b |
Experiment 5: When captures were compared among a trap with blue-UV light and pheromone, a trap with pheromone only, and a trap with blue-UV light only, higher H. halys captures were detected for the complete multimodal trap compared to the other treatments (results of LM followed by multiple comparison procedure are reported in Table 4). Capture of noctuid moths (Lepidoptera: Noctuidae) changed across sampling days (ΔAIC = 2.28, p = 0.038) and was higher for the two treatments with light compared to the treatment with pheromone only. Captures of ladybird beetles (Coleoptera: Coccinellidae) were stable across sampling days (ΔAIC = 2.0, p = 1.00) and did not differ across treatments (ΔAIC = 1.30, p = 0.090). Captures of wasp-waisted wasps (Hymenoptera: Apocrita) were different across sampling days (ΔAIC = 0.97, p = 0.085) and were higher for the two treatments with lights compared to the treatments with only pheromone.
Table 4.
Captures (mean ± SE) of H. halys and other main groups in a standard or simplified multimodal trap (Exp. 5). Means in the same column followed by different letters are significantly different according to linear model or linear mixed-effects models followed by multiple comparisons procedure (significance level α = 0.05).
| Exp | Treatment | H. halys | Lepidoptera: Noctuidae |
Coleoptera: Coccinellidae |
Hymenoptera: Apocrita |
|---|---|---|---|---|---|
| 5 | Blue-UV + pheromone | 28.00 ± 5.09 a | 42.45 ± 5.04 a | 1.45 ± 0.47 | 19.00 ± 6.94 a |
| Blue-UV only | 9.00 ± 1.60 b | 45.45 ± 10.65 a | 1.27 ± 0.60 | 23.55 ± 9.78 a | |
| Pheromone only | 11.55 ± 1.36 b | 5.09 ± 0.81 b | 0.18 ± 0.12 | 2.18 ± 0.48 b |
4. Discussion
In agricultural ecosystems, early detection of a herbivorous insect population is crucial for effective pest management [43,44]. Hence, the improvement of insect trapping is a priority [34,45]. Our results revealed that, in laboratory and field conditions, H. halys males and females were attracted toward LED lights emitting both UV-A and visible wavelengths. In addition, the combination of such lights with odour stimuli resulted in higher captures compared to pheromone-based traps or light traps alone.
It is already known that H. halys, as well as herbivorous insects in general, including other stink bugs, are attracted by different light wavelengths [46]. In a free-flying experiment testing LEDs with emission of different wavelengths, both male and female Nezara viridula (L.) (Hemiptera: Pentatomidae) exhibited stronger attraction toward UV compared to visible wavelengths, and secondarily toward blue and green compared to orange light [47]. In dual-choice laboratory bioassays, H. halys showed positive phototaxis toward fluorescent black light (with a UV dominant output) or blue light (with a dominant output in the visible spectrum and no emission in the UV spectrum) [48]. Attraction to UV-dominant lamps was only marginally higher compared to the blue lamp. Interestingly, our results support laboratory results in [48], as it seems that the combination of UV and visible blue light provides higher attraction compared to UV alone. In another experiment, white, yellow, red, orange, and green source lights were attractive to H. halys [49]. Similarly, in our laboratory experiments, it seems that H. halys can detect and are attracted by either blue, green, or yellow light. Most insects have three types of pigments that ensure UV-blue-green trichromacy [50]. A recent genome analysis revealed the presence in H. halys of a singleton UV-opsin homolog and duplicates of long-wavelength opsin homologs but lacked the presence of blue-sensitive opsin ortholog [51]. In other insect orders, the expansion of long-wavelength sensitive opsins can restore trichromacy, but whether this is also likely for H. halys remains to be investigated [52,53]. It is possible that gene duplication may have occurred due to the need for wider colour vision. Apparently, from our field experiment 1, it could be expected that a multimodal trap with only green-UV light would capture more H. halys compared to other wavelengths, including blue-UV. However, subsequent field experiments revealed that green-UV or blue-UV multimodal traps captured similar numbers of H. halys. A speculative explanation is that because H. halys has a marked preference for immature and mature fruits, this species has evolved photoreceptors to better discriminate green and longer (yellow or red) wavelengths, and that such preference is only evident during the photophase (i.e., when experiment 1 was conducted). However, further experiments should aim at clarifying such findings.
Although light and pheromone alone were similarly attractive, the simultaneous presence of both stimuli synergistically improved the attractiveness. This result is consistent with a previous investigation where the combination of fluorescent blue light and aggregation pheromone increased mid-season H. halys trapping in mid-Atlantic sites of the US [24]. Modification of the trap design consisted of the addition of an electric fan. The light lamp attracted insects to the trap, but they tended to remain close to the light source [49] and were reluctant to enter inside the funnel bag [Authors personal observation]. The presence of the fan creates a suction vortex that possibly facilitated the movement of the stink bugs to the funnel net cage (similarly to [45]). The fan could help the dispersal of synthetic and natural aggregation pheromone. In Maryland, traps with live H. halys individuals successfully attracted conspecifics, possibly because of the production of natural aggregation pheromone from trapped individuals [29]. Similarly, in Italy, a prototype of a live trap ensured higher H. halys captures compared to a control trap [34]. In a soybean field in Miryang, Korea, the presence of a solar fan in pheromone baited traps improved captures of the stink bugs Riptortus pedestris, H. halys, and Piezodorus hybneri [54]. The addition of a blue LED lamp (no UV emission) to the traps further increased H. halys trapping [45]. In our experiment we left trapped adults for only one day before removal. Hence, further investigations would help clarify whether leaving trapped adults for a prolonged time would increase trap efficacy, or otherwise, decrease it due to possible emission by trapped individuals of defensive compounds (e.g., aldehydes, with demonstrated repellent activities on conspecifics) [55]. Another aspect is that insect populations of diverse geographical origins may exhibit different behaviour (e.g., the stem borers Sesamia nonagroides Lefèbvre [56,57] and Chilo partellus (Swinhoe) [58]). Hence, whether different populations of H. halys similarly respond to UV-A and visible wavelengths must be verified.
Non-target insect trapping is a side effect of traditional light traps [59,60]. In our multimodal traps we noticed generally low captures of Coccinellidae. This group was indeed abundant in the urban and agricultural systems under evaluation and included native and exotic species which provide effective control over herbivorous insect pests [61,62]. Previous studies investigated the phototactic behaviour of ladybirds to different wavelengths and have reported light response variability among different species and insect physiological status. In laboratory conditions, Coccinella septempunctata L. (Coleoptera: Coccinellidae) exhibited positive phototactic behaviour toward UV and red lights and much lower response to green or blue lights [63]. Notwithstanding, captures by blacklight UV traps in field trials were sporadic for several ladybird species, including C. septempunctata [64]. Conversely, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) was strongly attracted by UV and blue wavelengths [64,65]. Despite this species providing effective pest suppression [66], it is considered invasive in several parts of the world and puts native predators at risk due to competition for resources or intraguild predation [67,68,69]. Hence, an interesting opportunity would be the evaluation and definition of proper wavelengths that might reduce H. axyridis populations in those areas where this species has become a concern for threating insect biodiversity [70]. Notably, even if the multimodal trap captured some Hymenopterans, we counted very few honeybees and bumblebees. In fact, blue wavelengths are non-preferential for these beneficial insects [71,72]. Conversely, several Lepidoptera were found inside blue-UV and green-UV traps, and it is known that UV and blue wavelengths, are particularly attractive for them [73]. Because light attraction seems to be positively correlated with the size of the moth [74], the prototype of the multimodal traps could be improved. For instance, it is expected that the adoption of an external metal grill with smaller openings would likely prevent trapping of large Lepidoptera. The large number of Diptera captured in our multimodal traps can be explained by the peak sensitivity of their compound eye retinula cells to the UV, green, and blue wavelengths [75].
Concerning IPM, the multimodal trap that we evaluated could be used not only for pest monitoring, but also for directly controlling H. halys, e.g., through mass trapping [30]. Additionally, such traps could also be adopted in combination with repellent molecules (e.g., terpenes) in a push–pull strategy. Such IPM approaches would likely enhance H. halys control, also because it is expected to have low interference with complementary control practices, such as biocontrol with native and exotic parasitoids [76,77,78,79,80,81]. Concerning practicality of field use, our prototype is based on the TurbiLED trap which has a IPX4 waterproof level. That means the trap can be used externally and risk of water infiltration, e.g., by heavy rain, can be disregarded. A new prototype powered by a 12 V battery recharged by a solar panel is currently under development.
5. Conclusions
We have evaluated a multimodal trap that combines visual and olfactory stimuli and greatly enhanced H. halys captures. Other research would be needed to better understand the H. halys phototaxis behaviour in different environmental conditions. For example, the response at different light intensities [49], evaluation time (which could be related to diurnal and nocturnal circadian rhythm [60]), or physiological status [64]. Furthermore, phototaxis response of non-target insects should be properly evaluated in order to possibly reduce undesired biodiversity losses [50].
Acknowledgments
The authors are grateful to Carlo Bertani (MO-EL) for fruitful collaboration and to Franco Losi, Giuseppe Zandonai ("Sett’olmi” farm), and Andrea Luchetti for assistance with H. halys collection and field trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13060527/s1, Figure S1: Diagram of the multimodal trap.
Author Contributions
Conceptualisation, G.R., S.N., R.F. and E.C. (Eric Conti); methodology, G.R., S.N., R.F. and E.C. (Eric Conti); investigation, G.R., E.C. (Elena Chierici), E.M., S.N., R.F. and E.C. (Eric Conti); data analysis, G.R. and E.C. (Eric Conti); data interpretation, G.R., R.F. and E.C. (Eric Conti); writing—original draft preparation, G.R., E.C. (Elena Chierici) and E.C. (Eric Conti); writing—review and editing, G.R., E.C. (Elena Chierici), E.M., S.N., R.F. and E.C. (Eric Conti); supervision, G.R., R.F. and E.C. (Eric Conti); project administration, E.C. (Eric Conti); funding acquisition, G.R. and E.C. (Eric Conti). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The co-author S.N. is an employer of the company that produced the trap prototypes. Other authors declare no conflict of interest.
Funding Statement
Funding was partly provided by the Emilia-Romagna Region with the project “PSR 2014-2020, measure 16.1.01, Sistemi integrati sostenibili di comprensorio per il controllo della cimice asiatica (Halyomorpha halys), acronym S.I.S.C.C.C.A.”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bradshaw C.J.A., Leroy B., Bellard C., Roiz D., Albert C., Fournier A., Barbet-Massin M., Salles J.M., Simard F., Courchamp F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016;7:12986. doi: 10.1038/ncomms12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diagne C., Leroy B., Vaissière A.C., Gozlan R.E., Roiz D., Jarić I., Salles J.M., Bradshaw C.J.A., Courchamp F. High and rising economic costs of biological invasions worldwide. Nature. 2021;592:571–576. doi: 10.1038/s41586-021-03405-6. [DOI] [PubMed] [Google Scholar]
- 3.Dent D., Binks R.H. Insect Pest Management. CABI; Wallingford, UK: 2020. [Google Scholar]
- 4.Bueno A.F., Panizzi A.R., Hunt T.E., Dourado P.M., Pitta R.M., Gonçalves J. Challenges for adoption of integrated pest management (IPM): The soybean example. Neotrop. Entomol. 2021;50:5–20. doi: 10.1007/s13744-020-00792-9. [DOI] [PubMed] [Google Scholar]
- 5.Ehler L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006;62:787–789. doi: 10.1002/ps.1247. [DOI] [PubMed] [Google Scholar]
- 6.Koul O., Cuperus G., Elliott N. Areawide Pest Management: Theory and Implementation. CAB International; Wallingford, UK: 2008. [Google Scholar]
- 7.Haye T., Moraglio S.T., Stahl J., Visentin S., Gregorio T., Tavella L. Fundamental host range of Trissolcus japonicus in Europe. J. Pest Sci. 2020;93:171–182. doi: 10.1007/s10340-019-01127-3. [DOI] [Google Scholar]
- 8.Giovannini L., Sabbatini-Peverieri G., Marianelli L., Rondoni G., Conti E., Roversi P.F. Physiological host range of Trissolcus mitsukurii, a candidate biological control agent of Halyomorpha halys in Europe. J. Pest Sci. 2021;95:605–618. doi: 10.1007/s10340-021-01415-x. [DOI] [Google Scholar]
- 9.El-Ghany N.M.A. Semiochemicals for controlling insect pests. J. Plant Prot. Res. 2019;59:1–11. doi: 10.24425/jppr.2019.126036_rfseq1. [DOI] [Google Scholar]
- 10.Flaherty L., Gutowski J.M.G., Hughes C., Mayo P., Mokrzycki T., Pohl G., Silk P., Van Rooyen K., Sweeney J. Pheromone-enhanced lure blends and multiple trap heights improve detection of bark and wood-boring beetles potentially moved in solid wood packaging. J. Pest Sci. 2019;92:309–325. doi: 10.1007/s10340-018-1019-4. [DOI] [Google Scholar]
- 11.Kriticos D.J., Kean J.M., Phillips C.B., Senay S.D., Acosta H., Haye T. The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J. Pest Sci. 2017;90:1033–1043. doi: 10.1007/s10340-017-0869-5. [DOI] [Google Scholar]
- 12.Cianferoni F., Graziani F., Dioli P., Ceccolini F. Review of the occurrence of Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) in Italy, with an update of its European and World distribution. Biologia. 2018;73:599–607. doi: 10.2478/s11756-018-0067-9. [DOI] [Google Scholar]
- 13.Leskey T.C., Nielsen A.L. Impact of the invasive brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Annu. Rev. Entomol. 2018;63:599–618. doi: 10.1146/annurev-ento-020117-043226. [DOI] [PubMed] [Google Scholar]
- 14.Cullum J.P., Nixon L.J., Morrison W.R., Raupp M.J., Shrewsbury P.M., Venugopal P.D., Martinson H., Bergh J.C., Leskey T.C. Influence of landscape factors and abiotic conditions on dispersal behavior and overwintering site selection by Halyomorpha halys (Hemiptera: Pentatomidae) J. Econ. Entomol. 2020;113:2016–2021. doi: 10.1093/jee/toaa077. [DOI] [PubMed] [Google Scholar]
- 15.Francati S., Masetti A., Martinelli R., Mirandola D., Anteghini G., Busi R., Dalmonte F., Spinelli F., Burgio G., Dindo M.L. Halyomorpha halys (Hemiptera: Pentatomidae) on kiwifruit in northern Italy: Phenology, infestation, and natural enemies assessment. J. Econ. Entomol. 2021;114:1733–1742. doi: 10.1093/jee/toab126. [DOI] [PubMed] [Google Scholar]
- 16.Bariselli M., Bugiani R., Maistrello L. Distribution and damage caused by Halyomorpha halys in Italy. EPPO Bull. 2016;46:332–334. doi: 10.1111/epp.12289. [DOI] [Google Scholar]
- 17.Reay-Jones F., Greene J.K., Toews M.D., Reeves R.B. Sampling stink bugs (Hemiptera: Pentatomidae) for population estimation and pest management in southeastern cotton production. J. Econ. Entomol. 2009;102:2360–2370. doi: 10.1603/029.102.0643. [DOI] [PubMed] [Google Scholar]
- 18.Panizzi A.R. History and Contemporary Perspectives of the Integrated Pest Management of Soybean in Brazil. Neotrop. Entomol. 2013;42:119–127. doi: 10.1007/s13744-013-0111-y. [DOI] [PubMed] [Google Scholar]
- 19.Leskey T.C., Wright S.E., Short B.D., Khrimian A. Development of behaviorally-based monitoring tools for the brown marmorated stink bug (Heteroptera: Pentatomidae) in commercial tree fruit orchards. J. Entomol. Sci. 2012;47:76–85. doi: 10.18474/0749-8004-47.1.76. [DOI] [Google Scholar]
- 20.Rice K.B., Bergh C.J., Bergmann E.J., Biddinger D.J., Dieckhoff C., Dively G., Fraser H., Gariepy T., Hamilton G., Haye T., et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae) J. Integr. Pest Manag. 2014;5:1–13. doi: 10.1603/IPM14002. [DOI] [Google Scholar]
- 21.Bayram A., Salerno G., Onofri A., Conti E. Lethal and sublethal effects of preimaginal treatments with two pyrethroids on the life history of the egg parasitoid Telenomus busseolae. BioControl. 2010;55:697–710. doi: 10.1007/s10526-010-9288-8. [DOI] [Google Scholar]
- 22.Weber D.C., Morrison W.R., Khrimian A., Rice K.B., Leskey T.C., Rodriguez-Saona C., Nielsen A.L., Blaauw B.R. Chemical ecology of Halyomorpha halys: Discoveries and applications. J. Pest Sci. 2017;90:989–1008. doi: 10.1007/s10340-017-0876-6. [DOI] [Google Scholar]
- 23.Maistrello L., Vaccari G., Caruso S., Costi E., Bortolini S., Macavei L., Foca G., Ulrici A., Bortolotti P.P., Nannini R., et al. Monitoring of the invasive Halyomorpha halys, a new key pest of fruit orchards in northern Italy. J. Pest Sci. 2017;90:1231–1244. doi: 10.1007/s10340-017-0896-2. [DOI] [Google Scholar]
- 24.Rice K.B., Cullum J.P., Wiman N.G., Hilton R., Leskey T.C. Halyomorpha halys (Hemiptera: Pentatomidae) response to pyramid traps baited with attractive light and pheromonal stimuli. Fla. Entomol. 2017;100:449–453. doi: 10.1653/024.100.0207. [DOI] [Google Scholar]
- 25.Khrimian A., Zhang A., Weber D.C., Ho H.Y., Aldrich J.R., Vermillion K.E., Siegler M.A., Shirali S., Guzman F., Leskey T.C. Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J. Nat. Prod. 2014;77:1708–1717. doi: 10.1021/np5003753. [DOI] [PubMed] [Google Scholar]
- 26.Weber D.C., Leskey T.C., Walsh G.C., Khrimian A. Synergy of aggregation pheromone with methyl (E,E,Z)-2,4,6-decatrienoate in attraction of Halyomorpha halys (Hemiptera: Pentatomidae) J. Econ. Entomol. 2014;107:1061–1068. doi: 10.1603/EC13502. [DOI] [PubMed] [Google Scholar]
- 27.Morrison W.R., Cullum J.P., Leskey T.C. Evaluation of trap designs and deployment strategies for capturing Halyomorpha halys (Hemiptera: Pentatomidae) J. Econ. Entomol. 2015;108:1683–1692. doi: 10.1093/jee/tov159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice K.B., Morrison W.R., Short B.D., Acebes-Doria A., Bergh J.C., Leskey T.C. Improved trap designs and retention mechanisms for Halyomorpha halys (Hemiptera: Pentatomidae) J. Econ. Entomol. 2018;111:2136–2142. doi: 10.1093/jee/toy185. [DOI] [PubMed] [Google Scholar]
- 29.Aldrich J.R., Khrimian A., Chen X., Camp M.J. Semiochemically based monitoring of the invasion of the brown marmorated stink bug and unexpected attraction of the native green stink bug (Heteroptera: Pentatomidae) in Maryland. Fla. Entomol. 2009;92:483–491. doi: 10.1653/024.092.0310. [DOI] [Google Scholar]
- 30.Conti E., Avila G., Barratt B., Cingolani F., Colazza S., Guarino S., Hoelmer K., Laumann R.A., Maistrello L., Martel G., et al. Biological control of invasive stink bugs: Review of global state and future prospects. Entomol. Exp. Appl. 2021;169:28–51. doi: 10.1111/eea.12967. [DOI] [Google Scholar]
- 31.Zhang Q.H., Schneidmiller R.G., Hoover D.R., Zhou G., Margaryan A., Bryant P. Essential oils as spatial repellents for the brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) J. Appl. Entomol. 2014;138:490–499. doi: 10.1111/jen.12101. [DOI] [Google Scholar]
- 32.Polajnar J., Maistrello L., Bertarella A., Mazzoni V. Vibrational communication of the brown marmorated stink bug (Halyomorpha halys) Physiol. Entomol. 2016;41:249–259. doi: 10.1111/phen.12150. [DOI] [Google Scholar]
- 33.Mazzoni V., Polajnar J., Baldini M., Stacconi M.V.R., Anfora G., Guidetti R., Maistrello L. Use of substrate-borne vibrational signals to attract the Brown Marmorated Stink Bug, Halyomorpha halys. J. Pest Sci. 2017;90:1219–1229. doi: 10.1007/s10340-017-0862-z. [DOI] [Google Scholar]
- 34.Suckling D.M., Levy M.C., Roselli G., Mazzoni V., Ioriatti C., Deromedi M., Cristofaro M., Anfora G. Live traps for adult brown marmorated stink bugs. Insects. 2019;10:376. doi: 10.3390/insects10110376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen A.L., Holmstrom K., Hamilton G.C., Cambridge J., Ingerson-Mahar J. Use of black light traps to monitor the abundance, spread, and flight behavior of Halyomorpha halys (Hemiptera: Pentatomidae) J. Econ. Entomol. 2013;106:1495–1502. doi: 10.1603/EC12472. [DOI] [PubMed] [Google Scholar]
- 36.Lee D.H., Short B.D., Joseph S.V., Bergh J.C., Leskey T.C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013;42:627–641. doi: 10.1603/EN13006. [DOI] [PubMed] [Google Scholar]
- 37.Harris J.E. Insect Light Traps. In: Heaps J.W., editor. Insect Management for Food Storage and Processing. AACC International; St. Paul, MN, USA: 2006. pp. 55–66. [Google Scholar]
- 38.Rondoni G., Bertoldi V., Malek R., Djelouah K., Moretti C., Buonaurio R., Conti E. Vicia faba plants respond to oviposition by invasive Halyomorpha halys activating direct defences against offspring. J. Pest Sci. 2018;91:671–679. doi: 10.1007/s10340-018-0955-3. [DOI] [Google Scholar]
- 39.Rondoni G., Chierici E., Giovannini L., Sabbatini-Peverieri G., Roversi P.F., Conti E. Olfactory responses of Trissolcus mitsukurii to plants attacked by target and non-target stink bugs suggest low risk for biological control. Sci. Rep. 2022;12:1880. doi: 10.1038/s41598-022-05873-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinheiro J.C., Bates D.M. Mixed-Effects Models in S and S-PLUS. Springer; New York, NY, USA: 2000. [Google Scholar]
- 41.Zuur A.F., Ieno E.N., Walker N.J., Saveliev A.A., Smith G.M. Mixed Effects Models and Extensions in Ecology with R. Springer; New York, NY, USA: 2009. [Google Scholar]
- 42.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [(accessed on 4 April 2022)]. Available online: https://www.r-project.org/index.html. [Google Scholar]
- 43.Rondoni G., Ricci C., Conti E. Tracking seasonal emergence dynamics of an invasive gall wasp and its associated parasitoids with an open-source, microcontroller-based device. J. Pest Sci. 2019;92:361–369. doi: 10.1007/s10340-018-1037-2. [DOI] [Google Scholar]
- 44.Otuka A., Matsumura M., Tokuda M. Dispersal of the common cutworm, Spodoptera litura, monitored by searchlight trap and relationship with occurrence of soybean leaf damage. Insects. 2020;11:427. doi: 10.3390/insects11070427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae S., Yi H., Yoon Y., Jang Y., Kim Y., Maharjan R. Attraction of stink bugs to rocket traps with different combinations of wing and landing board color. J. Asia-Pac. Entomol. 2019;22:243–249. doi: 10.1016/j.aspen.2019.01.007. [DOI] [Google Scholar]
- 46.Chambers B.D., Leskey T.C., Pearce A.R., Kuhar T.P. Seasonal Response of Halyomorpha halys (Hemiptera: Pentatomidae) Adults to Light Bulbs. J. Agric. Urban Entomol. 2019;34:44. doi: 10.3954/1523-5475-34.1.44. [DOI] [Google Scholar]
- 47.Endo N., Wakakuwa M., Arikawa K., Hironaka M. Spectral preference in a free-flying condition of the southern green stink bug, Nezara viridula (Heteroptera: Pentatomidae) Jpn. J. Appl. Entomol. Zool. 2014;58:23–28. doi: 10.1303/jjaez.2014.23. [DOI] [Google Scholar]
- 48.Leskey T.C., Lee D.H., Glenn D.M., Morrison W.R., III. Behavioral responses of the invasive Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) to light-based stimuli in the laboratory and field. J. Insect Behav. 2015;28:674–692. doi: 10.1007/s10905-015-9535-z. [DOI] [Google Scholar]
- 49.Cambridge J.E., Francoeur L., Hamilton G.C. Brown marmorated stink bug (Hemiptera: Pentatomidae) attraction to various light stimuli. Fla. Entomol. 2017;100:583–588. doi: 10.1653/024.100.0315. [DOI] [Google Scholar]
- 50.Shimoda M., Honda K. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013;48:413–421. doi: 10.1007/s13355-013-0219-x. [DOI] [Google Scholar]
- 51.Sparks M.E., Bansal R., Benoit J.B., Blackburn M.B., Chao H., Chen M., Cheng S., Childers C., Dinh H., Doddapaneni H.V., et al. Brown marmorated stink bug, Halyomorpha halys (Stål), genome: Putative underpinnings of polyphagy, insecticide resistance potential and biology of a top worldwide pest. BMC Genom. 2020;21:227. doi: 10.1186/s12864-020-6510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharkey C.R., Fujimoto M.S., Lord N.P., Shin S., McKenna D.D., Suvorov A., Martin G.J., Bybee S.M. Overcoming the loss of blue sensitivity through opsin duplication in the largest animal group, beetles. Sci. Rep. 2017;7:8. doi: 10.1038/s41598-017-00061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lebhardt F., Desplan C. Retinal perception and ecological significance of color vision in insects. Curr. Opin. Insect Sci. 2017;24:75–83. doi: 10.1016/j.cois.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bae S., Yoon Y., Jang Y., Kang H., Maharjan R. Evaluation of an improved rocket traps, and baits combination for its attractiveness to hemipteran bugs in grass and soybean fields. J. Asia-Pac. Entomol. 2017;20:497–504. doi: 10.1016/j.aspen.2017.03.014. [DOI] [Google Scholar]
- 55.Zhong Y.Z., Tang R., Zhang J.P., Yang S.Y., Chen G.H., He K.L., Wang Z.Y., Zhang F. Behavioral evidence and olfactory reception of a single alarm pheromone component in Halyomorpha halys. Front. Physiol. 2018;9:1610. doi: 10.3389/fphys.2018.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moyal P., Tokro P., Bayram A., Savopoulou-Soultani M., Conti E., Eizaguirre M., Le Rü B., Avand-Faghih A., Frérot B., Andreadis S. Origin and taxonomic status of the Palearctic population of the stem borer Sesamia nonagrioides (Lefèbvre) (Lepidoptera: Noctuidae) Biol. J. Linn. Soc. 2011;103:904–922. doi: 10.1111/j.1095-8312.2011.01666.x. [DOI] [Google Scholar]
- 57.Glaser N., Gallot A., Legeai F., Harry M., Kaiser L., Le Ru B., Calatayud P.A., Jacquin-Joly E. Differential expression of the chemosensory transcriptome in two populations of the stemborer Sesamia nonagrioides. Insect Biochem. Mol. Biol. 2015;65:28–34. doi: 10.1016/j.ibmb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Dhillon M.K., Tanwar A.K., Kumar S., Hasan F., Sharma S., Jaba J., Sharma H.C. Biological and biochemical diversity in different biotypes of spotted stem borer, Chilo partellus (Swinhoe) in India. Sci. Rep. 2021;11:5735. doi: 10.1038/s41598-021-85457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma G., Ma C. Sen Differences in the nocturnal flight activity of insect pests and beneficial predatory insects recorded by light traps: Possible use of a beneficial-friendly trapping strategy for controlling insect pests. Eur. J. Entomol. 2012;109:395–401. doi: 10.14411/eje.2012.051. [DOI] [Google Scholar]
- 60.Kim K.N., Huang Q.Y., Lei C.L. Advances in insect phototaxis and application to pest management: A review. Pest Manag. Sci. 2019;75:3135–3143. doi: 10.1002/ps.5536. [DOI] [PubMed] [Google Scholar]
- 61.Rondoni G., Borges I., Collatz J., Conti E., Costamagna A.C., Dumont F., Evans E.W., Grez A.A., Howe A.G., Lucas E., et al. Exotic ladybirds for biological control of herbivorous insects—A review. Entomol. Exp. Appl. 2021;169:6–27. doi: 10.1111/eea.12963. [DOI] [Google Scholar]
- 62.Rondoni G., Fenjan S., Bertoldi V., Ielo F., Djelouah K., Moretti C., Buonaurio R., Ricci C., Conti E. Molecular detection of field predation among larvae of two ladybird beetles is partially predicted from laboratory experiments. Sci. Rep. 2018;8:2594. doi: 10.1038/s41598-018-20830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiuxuan Z., Rongping K., Zhen C., Siming W., Xia L. Phototactic behavior of Coccinella septempunctata L. (Coleoptera: Coccinellidae) Coleopt. Bull. 2013;67:33–39. doi: 10.1649/072.067.0108. [DOI] [Google Scholar]
- 64.Nalepa C.A. Coccinellidae captured in blacklight traps: Seasonal and diel pattern of the dominant species Harmonia axyridis (Coleoptera: Coccinellidae) Eur. J. Entomol. 2013;110:593–597. doi: 10.14411/eje.2013.080. [DOI] [Google Scholar]
- 65.Chen Z., Li H.-M., Zhou C.-L. Phototactic Behavior of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) Coleopt. Bull. 2020;74:513–522. doi: 10.1649/0010-065X-74.3.513. [DOI] [Google Scholar]
- 66.Koch R.L., Costamagna A.C. Reaping benefits from an invasive species: Role of Harmonia axyridis in natural biological control of Aphis glycines in North America. BioControl. 2017;62:331–340. doi: 10.1007/s10526-016-9749-9. [DOI] [Google Scholar]
- 67.Brown P.M.J., Zaviezo T., Grez A., Adriaens T., San Martin G., Roy H.E., Soares A.O. Invasive intraguild predators: Evidence of their effects, not assumptions. Ecol. Entomol. 2022;47:249–252. doi: 10.1111/een.13116. [DOI] [Google Scholar]
- 68.Rondoni G., Onofri A., Ricci C. Differential susceptibility in a specialised aphidophagous ladybird, Platynaspis luteorubra (Coleoptera: Coccinellidae), facing intraguild predation by exotic and native generalist predators. Biocontrol Sci. Technol. 2012;22:1334–1350. doi: 10.1080/09583157.2012.726607. [DOI] [Google Scholar]
- 69.Li H., Li B., Lövei G.L., Kring T.J., Obrycki J.J. Interactions among native and non-native predatory Coccinellidae influence biological control and biodiversity. Ann. Entomol. Soc. Am. 2020;114:119–136. doi: 10.1093/aesa/saaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu G., Arnaud P., Offmann B., Picimbon J.-F. Pheromone, Natural Odor and Odorant Reception Suppressing Agent (ORSA) for Insect Control. In: Picimbon J.F., editor. Olfactory Concepts of Insect Control—Alternative to Insecticides. Springer; Cham, Switzerland: 2019. [Google Scholar]
- 71.Han P., Niu C.Y., Lei C.L., Cui J.J., Desneux N. Use of an innovative T-tube maze assay and the proboscis extension response assay to assess sublethal effects of GM products and pesticides on learning capacity of the honey bee Apis mellifera L. Ecotoxicology. 2010;19:1612–1619. doi: 10.1007/s10646-010-0546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ludicke J.C., Nieh J.C. Thiamethoxam impairs honey bee visual learning, alters decision times, and increases abnormal behaviors. Ecotoxicol. Environ. Saf. 2020;193:110367. doi: 10.1016/j.ecoenv.2020.110367. [DOI] [PubMed] [Google Scholar]
- 73.Brehm G., Niermann J., Jaimes Nino L.M., Enseling D., Jüstel T., Axmacher J.C., Warrant E., Fiedler K. Moths are strongly attracted to ultraviolet and blue radiation. Insect Conserv. Divers. 2021;14:188–198. doi: 10.1111/icad.12476. [DOI] [Google Scholar]
- 74.van Langevelde F., Ettema J.A., Donners M., WallisDeVries M.F., Groenendijk D. Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 2011;144:2274–2281. doi: 10.1016/j.biocon.2011.06.004. [DOI] [Google Scholar]
- 75.Lunau K. Visual ecology of flies with particular reference to colour vision and colour preferences. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2014;200:497–512. doi: 10.1007/s00359-014-0895-1. [DOI] [PubMed] [Google Scholar]
- 76.Bertoldi V., Rondoni G., Brodeur J., Conti E. An egg parasitoid efficiently exploits cues from a coevolved host but not those from a novel host. Front. Physiol. 2019;10:746. doi: 10.3389/fphys.2019.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martorana L., Foti M.C., Rondoni G., Conti E., Colazza S., Peri E. An invasive insect herbivore disrupts plant volatile-mediated tritrophic signalling. J. Pest Sci. 2017;90:1079–1085. doi: 10.1007/s10340-017-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moraglio S.T., Tortorici F., Giromini D., Pansa M.G., Visentin S., Tavella L. Field collection of egg parasitoids of Pentatomidae and Scutelleridae in Northwest Italy and their efficacy in parasitizing Halyomorpha halys under laboratory conditions. Entomol. Exp. Appl. 2021;169:52–63. doi: 10.1111/eea.12966. [DOI] [Google Scholar]
- 79.Peverieri G.S., Talamas E., Bon M.C., Marianelli L., Bernardinelli I., Malossini G., Benvenuto L., Roversi P.F., Hoelmer K. Two Asian egg parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) emerge in northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) J. Hymenopt. Res. 2018;53:37–53. doi: 10.3897/jhr.67.30883. [DOI] [Google Scholar]
- 80.Scala M., Fouani J.M., Zapponi L., Mazzoni V., Wells K.E., Biondi A., Baser N., Verrastro V., Anfora G. Attraction of egg parasitoids Trissolcus mitsukurii and Trissolcus japonicus to the chemical cues of Halyomorpha halys and Nezara viridula. Insects. 2022;13:439. doi: 10.3390/insects13050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sabbatini Peverieri G., Bortolotti P.P., Nannini R., Marianelli L., Roversi P.F. Efficacy of long lasting insecticide nets in killing Halyomorpha halys in pear orchards. Outlooks Pest Manag. 2018;29:70–74. doi: 10.1564/v29_apr_05. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.