Abstract
Aortic lymph node metastases are a relative common finding in locally advanced cervical cancer. Minimally invasive surgery is the preferred approach to perform para-aortic lymph nodal staging to reduce complications, hospital stay, and the time to primary treatment. This meta-analysis (CRD42022335095) aimed to compare the surgical outcomes of the two most advanced approaches for the aortic staging procedure: conventional laparoscopy (CL) versus robotic-assisted laparoscopy (RAL). The meta-analysis was conducted according to the PRISMA guideline. The search string included the following keywords: “Laparoscopy” (MeSH Unique ID: D010535), “Robotic Surgical Procedures” (MeSH Unique ID: D065287), “Lymph Node Excision” (MeSH Unique ID: D008197) and “Aorta” (MeSH Unique ID: D001011), and “Uterine Cervical Neoplasms” (MeSH Unique ID: D002583). A total of 1324 patients were included in the analysis. Overall, 1200 patients were included in the CL group and 124 patients in the RAL group. Estimated blood loss was significantly higher in CL compared with RAL (p = 0.02), whereas hospital stay was longer in RAL compared with CL (p = 0.02). We did not find significant difference for all the other parameters, including operative time, intra- and postoperative complication rate, and number of lymph nodes excised. Based on our data analysis, both CL and RAL are valid options for para-aortic staging lymphadenectomy in locally advanced cervical cancer.
Keywords: gynecological oncology, locally advanced cervical cancer, conventional laparoscopy, robotic-assisted laparoscopy
1. Introduction
Cervical cancer is one of the most common malignancies and is the most frequent cause of death from gynecological cancers worldwide [1]. Approximately more than one-third of patients present with locally advanced cervical cancer (LACC) at diagnosis, FIGO (International Federation of Gynecology and Obstetrics) stage IIB to IVA. This presentation is associated with 18–50% of lymph node metastases [2]. Para-aortic lymph-node status represents the most important prognostic factor in patients with LACC, with a severe negative impact on survival [3,4]. The recent FIGO 2018 classification of cervical cancer has included lymph node disease in the staging system to improve treatment allocation and inform about prognosis.
The current standard treatment in this population is concomitant chemoradiation, while the lymph node status assessment has an important staging role. The detection of para-aortic lymph node involvement is crucial to define the extent of the irradiation field and to personalize specific treatment protocols [5]. Para-aortic lymph nodal staging can be assessed using imaging, but the detection of lymph node metastases remains unsatisfactory. Even advanced imaging techniques, such as positron emission tomography-computed tomography (PET-CT), include a risk of 15% of false negative and 5–10% of false positive [6]. Computerized axial tomography (CT) and nuclear magnetic resonance (MRI) have shown lower sensitivity and specificity [7]. In this context, laparoscopic staging has been proposed as a valid alternative to the radiological nodal assessment, achieving more accurate results [8]. On the one hand, the survival benefit of surgical staging is still controversial due to conflicting results in available studies. On the other hand, the minimally invasive approach is known to improve peri- and postoperative outcomes, avoiding delay in the primary treatment. In a recent meta-analysis conducted by our group [9], we compared the two laparoscopic techniques for para-aortic surgical staging in cervical cancer: the transperitoneal laparoscopic lymphadenectomy (TLL) versus the extraperitoneal laparoscopic lymphadenectomy (ELL). Our results showed that TLL approach was associated with a higher rate of intraoperative complications, while no significant difference was found between the two techniques regarding postoperative complications. Since the introduction of the robotic surgery for gynecological oncological procedures, a significant increase of this approach has been reported following the hypothesis that the robotic route may represent an improvement compared with laparoscopic technique. Recently, the robotic approach has also been introduced for para-aortic staging in LACC patients, showing encouraging results [10].
In this scenario, the aim of our systematic review and meta-analysis was to compare the peri- and postoperative outcomes of conventional laparoscopy (CL; including either TLL and ELL) versus robotic-assisted laparoscopy (RAL) for surgical nodal assessment in LACC patients.
2. Materials and Methods
This systematic review was submitted in the PROSPERO international database (CRD42022335095) and conducted according to the PRISMA guideline (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [11]. We screened PubMed, Scopus, and Web of Science search engines from inception to March 2022. The search string included the following Medical Subject Headings (MeSH): “Laparoscopy” (MeSH Unique ID: D010535), “Robotic Surgical Procedures” (MeSH Unique ID: D065287), “Lymph Node Excision” (MeSH Unique ID: D008197) and “Aorta” (MeSH Unique ID: D001011), and “Uterine Cervical Neoplasms” (MeSH Unique ID: D002583).
Titles and/or abstracts of studies retrieved using the search strategy, and those from additional sources were screened independently by three review authors (M.C.D.D., V.G., G.L.B.) to identify studies that potentially met the aims of this systematic review. We excluded articles in languages other than English and reviews. The full text of these potentially eligible articles was retrieved and independently assessed for eligibility by other two review team members (G.S., A.A.). Any disagreement between them over the eligibility of particular articles was resolved through discussion with a third (external) collaborator. The references of the included studies were carefully evaluated to identify any potential additional source. A final review of the included articles was performed by the review supervisor (V.C.). In case of redundant articles or data used in previous studies, only the most recent articles with more comprehensive data were included in the analysis.
Prospective, retrospective, and pilot studies reporting surgical outcomes in women undergoing CL or RAL aortic lymph node dissection for surgical staging of LACC were considered and included. In case of studies reporting direct comparison between CL and RAL, each arm was separately included in the pooled analysis. Bibliographical and technical data extracted from the articles using a pre-piloted standardized form to collect the following elements: authors, publication year, the type of surgery, FIGO stage, median age and Body Mass Index (BMI), operative time (OT), estimated blood loss (EBL), hospitalization time (HT), intra- and postoperative complications, conversion to another technique, and the number of lymph nodes retrieved. The common terminology criteria for adverse events (CTCAE) grade >3 were considered for complications [12].
Statistical Analysis
Data were expressed as standard deviation (SD) or as number (percentage). Categorical variables were compared using the chi-square or Fisher exact test. Between-group comparison of continuous variables was undertaken using the t-test and the Mann–Whitney nonparametric equivalent test. Two-sided p-values were calculated, and p-values < 0.05 were considered as statistically significant. Meta-analyses of proportions were used to combine data. Between-study heterogeneity was explored using the I2 statistic, which indicates the percentage of between-study variation that is due to heterogeneity rather than chance. A value of I2 of 0% indicates no observed heterogeneity, whereas values > 50% indicate a substantial level of heterogeneity. Given the small sample size of the included studies, a random effect model was preferred regardless of I2. StatsDirect 3.0.17 (StatsDirect Ltd., Altrincham, UK) statistical software was used for all data analyses.
3. Results
Eight hundred and eighty-seven studies were identified through the database search. Duplicate articles were then eliminated. After selection criteria, twenty-seven studies were considered eligible for the analysis. Twenty studies were included in the CL group (group 1) [3,10,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] and seven studies in the RAL group (group 2) [10,27,28,29,30,31]. Studies involving different types of approaches were considered as separate studies. Specifically, two studies analyzed both robotic lymphadenectomy and laparoscopic lymphadenectomy approaches [10,27], with direct comparison between the two arms. In the study by Loverix et al. [10], patients who underwent RAL had a higher American Society of Anesthesiologists score (ASA2: 62% vs. 56%, ASA3: 20% vs. 2%, p < 0.001), more prior major abdominal surgery (18% vs. 6%, p = 0.016), less EBL (median, 25 mL vs. 62.5 mL, p < 0.001), more para-aortic lymph nodes removed (11 vs. 6, p < 0.001), shorter HT (1.8 vs. 2.3 days, p = 0.002), and a higher but non-significant rate of metastatic para-aortic lymph nodes (13% vs. 5%, p = 0.065) compared with the CL, respectively; in addition, authors did not find significant differences for complication rate as well as 2-year disease-free survival (p = 0.472) and overall survival (p = 0.749) between the two approaches. Similarly, Díaz-Feijoo et al. [27] found lower EBL (90 vs. 20 mL, p < 0.05), and more aortic nodes were removed (14 vs. 17 nodes, p < 0.05) in RAL compared with CL, with an almost overlapping rate of postoperative complications (17.6% vs. 8.4%).
Three studies described the two different laparoscopic approaches, TLL and ELL [14,19,26]. One study included robotic trans- and extra-peritoneal approach [29]. Furthermore, all studies were retrospective by design except one prospective randomized trial [18] and one prospective observational preliminary study [30]. The characteristics of the studies are showed in Appendix A; inclusion and exclusion criteria for each study are reported in Appendix B. The PRISMA flow chart is shown in Figure 1.
Figure 1.
PRISMA flow-chart of study selection and inclusion.
A total of 1324 patients were included in the analysis. Of these, 1200 patients were included in the CL group and 124 patients in the RAL group. The median age was 49.8 for CL and 51 for RAL. The median BMI was 25.5 and 25 for CL and RAL, respectively. The median of the OT was 129 min for patients who underwent CL aortic lymph node staging and 121.7 min for RAL aortic lymph node staging procedure. The median EBL was 81.1 mL and 26.9 mL in CL and RAL, respectively. The median length of HT was 1.9 and 3.3 days for CL and RAL, respectively. The median number of lymph nodes excised was 12.7 in the CL group and 15.7 in the RAL group. No significant differences were found between groups for BMI (p = 0.33), number of lymph node excised (p = 0.38), age (p = 0.62), and intraoperative time (p = 0.8). Conversely, EBL was significantly higher (p = 0.02) and HT significantly lower (p = 0.02) in the CL group compared with RAL group (Table 1).
Table 1.
Analysis of surgical outcomes between conventional laparoscopy and robotic groups.
| Laparoscopic | Robotic | p | |
|---|---|---|---|
| Number of studies | 20 | 7 | |
| Number of cases | 1200 | 124 | |
| Operative time (min) | 129 | 121.7 | 0.8 |
| Total complications (n) | 127 | 17 | 0.29 |
| Intraoperative complications (n) | 23 | 4 | 0.31 |
| Postoperative complications (n) | 104 | 13 | 0.5 |
| Number of lymph nodes excised | 12.7 | 15.7 | 0.38 |
| EBL | 81.1 | 26.9 | 0.02 |
| Hospital stay | 1.9 | 3.3 | 0.02 |
| Age | 49.8 | 51.0 | 0.62 |
| BMI | 25.5 | 25.0 | 0.33 |
Min, minutes; EBL, estimated blood loss; BMI, body mass index.
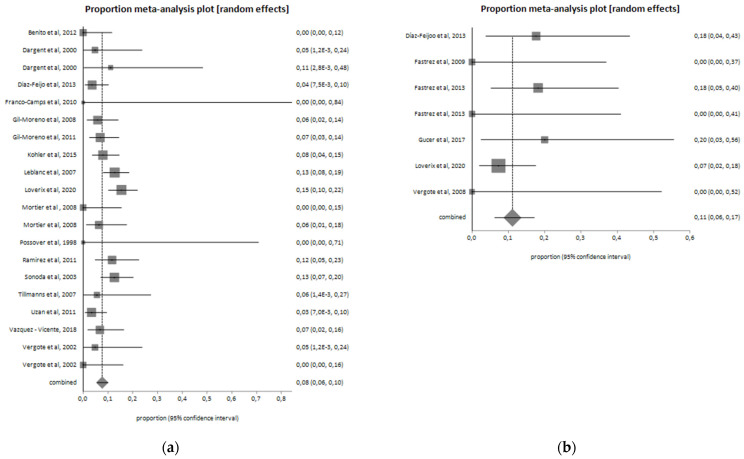
Intraoperative complications were reported in 23 patients (2%) in the CL group and in 4 patients (3.2%) in the RAL group. The most frequent intraoperative complications were vascular and urinary ones. The type of intraoperative complications occurred in the two groups are shown in Table 2. As shown in Figure 2, the intraoperative complications pooled proportion is 4.1% (I2 = 0%) for the RAL and 1.5% (I2 = 47.7%) for CL.
Table 2.
Analysis of intraoperative complications in conventional laparoscopy and robotic groups.
| Type of Intraoperative Complication | Laparoscopic (n = 1200) | Robotic (n = 124) | p |
|---|---|---|---|
| Vascular injuries | 18 (1.5%) | 2 (1.6%) | |
| Ureteric injuries | 3 (0.3%) | 2 (1.6%) | |
| Nerve injury | 1 (0.1%) | 0 (0%) | |
| Bowel injury total | 1 (0.1%) | 0 (0%) | |
| Total | 23 (2%) | 4 (3.2%) | 0.31 |
Figure 2.
Intraoperative complications in the (a) laparoscopic group (I2 = 47.7%; pooled proportion = 1.5%) and (b) robotic group (I2 = 0%; pooled proportion = 4.1%).
In total, 104 patients (8.6%) of the CL group developed postoperative complications, while 13 patients (9.7%) of the RAL group reported a complication after surgery. Postoperative complications are reported in Table 3. In the two groups, sixty-three lymphatic complications occurred, ten to urinary compartment, three trocar site hernia, and one bowel complication. The postoperative complications pooled proportion is 11.1% (I2 = 0%) for the RAL group and 7.7% (I2 = 43.9%) for CL. Pooled proportion of post-operative complications among the two groups are shown in Figure 3. In 14 (1.2%) cases of the CL group and in 2 (1.6%) of the RAL group, a conversion to laparotomy was required (Figure 4).
Table 3.
Analysis of postoperative complications in conventional laparoscopy and robotic groups.
| Type of Postoperative Complication | Laparoscopic (n = 1200) | Robotic (n = 124) | p |
|---|---|---|---|
| Lymphatic complication | 57 (4.7%) | 6 (4.8%) | |
| Vascular complication | 15 (1.2%) | 0 (0%) | |
| Urinary complication | 7 (0.6%) | 3 (2.4%) | |
| Bowel complication | 1 (0.1%) | 0 (0%) | |
| Trocar site hernia | 2 (0.2%) | 1 (0.8%) | |
| Others | 22 (1.8%) | 3 (2.4.%) | |
| Total | 104 (8.6%) | 13 (10.4%) | 0.5 |
Figure 3.
Postoperative complications in the (a) laparoscopic group (I2 = 43.9%; pooled proportion = 7.7%) and (b) robotic group (I2 = 0%; pooled proportion = 11.1%).
Figure 4.
Conversion from minimally invasive to open surgery (laparotomy) in the (a) laparoscopic group (I2 = 38.4%; pooled proportion = 1.2%) and (b) robotic group (I2 = 0%; pooled proportion = 2.2%).
4. Discussion
Aortic lymph-node metastases are common findings in LACC patients, reaching a rate of about 40–70% for stages III and IV, and the diagnostic value of surgical para-aortic lymph node dissection has been widely investigated [32]. Although the increased morbidity is associated with surgical procedures, minimally invasive surgery (MIS) for surgical staging in cervical cancer provides several advantages without compromising the course of the disease [3]. Reduction in peri- and postoperative complications and reduction of HT represent the main benefits of this surgical approach. Moreover, MIS allows to perform para-aortic lymphadenectomy even in the difficult case of abdominal vascular and urinary anomalies [33].
Considering the only staging intent, in order to avoid delay of the primary chemoradiation, it is essential to propose the most effective and technologically advanced surgical approach. Laparoscopic aortic staging is one of the most challenging procedures in gynecologic oncology: in this scenario, RAL can have advantages over CL with faster learning curve, technical improvements such as a 3D imaging, elimination of physiological tremor, and increased precision due to the seven-degree instrument’s articulation [34]. Accumulating evidence suggests the overall feasibility of robotic para-aortic lymph node staging for cervical cancer [10,27]; in addition, some authors have compared the RAL surgical staging to the CL and have found better perioperative outcomes and similar survival outcomes [10,27]. Our meta-analysis showed that RAL surgical staging in LACC patients is significantly associated with less EBL than conventional laparoscopy. Furthermore, OT and the number of lymph nodes excised are in favor of robotic approach although the difference is not statistically significant. Similar to previous series, our data analysis supports RAL as an appropriate alternative to CL for para-aortic lymph node dissection in LACC patients.
Although not significant, the higher number of lymph nodes excised using RAL compared with CL could be due, at least in part, to the greater precision of the robotic procedure and the possibility of being more radical with fine dissection in difficult anatomical spaces. Interestingly, we found shorter HT for the CL group compared with RAL: although we cannot explain with absolute certainty this data, this is probably due to the type of women who are addressed to RAL; indeed, patients undergoing robotic approach usually have more comorbidities (e.g., obese, more prior abdominal surgery, higher ASA score) [10], and this may be associated with longer HT.
Lymphatic complications represent the most frequent postoperative complication, especially for the robotic group. One possible and reliable explanation of this difference is related to using different instruments in the two surgical routes. During a laparoscopic lymphadenectomy, multifunction instruments seal and cut the lymphatic vessels, unlike the robotic approach, in which bipolar energy and scissors are commonly used. Moreover, some authors suggest systematically clipping any large lymphatic vessel to avoid lymphatic complications [3,16]. Vascular injuries and ureteral lesions were the most frequent intraoperative complications, especially in the robotic group. On the one hand, some authors suggested that during RAL, pneumoperitoneal pressure is lower than laparoscopy, and very often, the patients selected for RAL are already subjected to previous surgery, obese, and with comorbidities [35,36]: this challenging surgical scenario may limit the visualization of the ureter and the great vessels, increasing intraoperative complications [34,35]. On the other hand, the RAL approach allows a better exposition of the pre-cava and inter-aortocaval field than the CL. Indeed, robotic surgery is associated with a higher Trendelenburg inclination and a better range of motion of the instruments [10,27]. In addition, the learning curve for laparoscopic aortic lymphadenectomy [37] is longer than robotic procedure [38] due to limited rigid instruments and the 2-dimensional view of the laparoscope’s video camera, which requires greater surgeon skills to perform this procedure. Furthermore, aortic lymphadenectomy is a single-quadrant surgery and perfectly matches with the robotic approach, using its increasing precision that would otherwise suffer in case of re-docking [39]. However, the selection of the patients for lymph node staging surgery should be careful, considering that the benefits of diagnostic surgery should justify its possible morbidities.
Limits of the present meta-analysis are represented by the small sample size of robotic group, the heterogenicity of the studies included, the retrospective nature of most of the articles analyzed. In addition, most of the included studies reported insufficient information on the pre-operative characteristics of the patients, which did not allow us to perform a robust sub-analysis (meta-regression) of surgical outcomes based on these parameters. Finally, only one study reported either the transperitoneal or the extra peritoneal technique for the robotic aortic lymph node dissection. However, good heterogenicity of the studies is showed by the pooled analysis.
5. Conclusions
Based on our data analysis, both CL and RAL can be considered valid options for para-aortic staging lymphadenectomy in women with LACC, with comparable safety and surgical outcomes. In particular, the two techniques allowed similar operative time, intra- and postoperative complication rate, and number of lymph nodes excised.
Appendix A
Table A1.
Characteristics of the included studies.
| Authors, Years | Type of Study | Cases | Stage | OT (Minutes) |
EBL (mL) | Conversions | HT (Days) | Number of Lymph Nodes Excised | Intra-Operative Complications (n and Type) | Post-Operative Complications (n and Type) | BMI (Median) | Age (Median) | Technique |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Díaz-Feijoo et al., 2013 [27] | Retrospective study | 17 | IB2-IVA | 150 | 20 | 0 | 2 | 17 | 0 | 3 (not specified) | 23 | 49 | Robotic Retroperitoneal |
| Fastrez et al., 2009 [28] | Retrospective study | 8 | IB2-IVA | 137.5 | / | 1 | 4.5 | 14 | 0 | 0 | 24.3 | 58 | Robotic Transperitoneal |
| Fastrez et al., 2013 [29] | Retrospective study | 22 | IB2-IVA | 165 | / | 1 | 6 | 19.5 | 1 aortic injury | 4: 1 chylous ascites; 2 symptomatic lymphocele; 1 epiploic hernia through umbilical port |
27 | 55 | Robotic Transperitoneal |
| Fastrez et al., 2013 [29] | Retrospective study | 7 | IB2-IVA | 100 | / | 0 | 2.5 | 9.5 | 1 partial section of the right ureter | 0 | 24 | 50.5 | Robotic Retroperitoneal |
| Gucer et al., 2017 [30] | Prospective observational preliminary study | 10 | IIB-IVA | 141 | 12.5 | 0 | 4 | 25 | 0 | 2: 1 symptomatic lymphocyst; 1 local infection on assistant port site |
28.5 | 46 | Robotic Transperitoneal |
| Loverix et al., 2020 [10] | Retrospective study | 55 | IB1-IVA | 74.5 | 25 | 0 | 1.8 | 1 bleeding | 4: 3 urinary tract infection; 1 salpingitis |
24.7 | 49 | Robotic Transperitoneal and Retroperitoneal | |
| Vergote et al., 2008 [31] | Retrospective study | 5 | IIB-IIIB | 83.8 | 50 | 0 | 2.2 | 9.2 | 1 right ureter damage | 0 | 23.8 | 49.6 | Robotic Retroperitoneal |
| Benito et al., 2012 [13] | Retrospective study | 30 | IB2-IVA | 118.7 | 75 | 0 | 1.9 | 14.2 | 2: 1 lumbar artery injury; 1 bowel injury |
0 | 26.3 | 47.6 | Laparoscopic Retroperitoneal |
| Dargent et al., 2000 [14] | Retrospective study | 21 | IB1-IVA | 119 | / | 3 | / | 15 | 0 | 1 lymphocele | 23 | 50 | Laparoscopic Retroperitoneal |
| Dargent et al., 2000 [14] | Retrospective study | 9 | IB1-IVA | 160 | / | 0 | / | 19 | 0 | 1 phlebitis | 23 | 50 | Laparoscopic Transperitoneal |
| Diaz-Feijoo et al., 2013 [27] | Retrospective study | 83 | IB2-IVA | 150 | 20 | 0 | 2 | 17 | 0 | 3: 2 lymphocysts; 1 chylous ascites |
26.4 | 51 | Laparoscopic Retroperitoneal |
| Franco-Camps et al., 2010 [15] | Retrospective study | 2 | IIIB-IVA | 140 | 95 | 0 | 2 | 6 | 0 | 0 | 29 | 71 | Laparoscopic Retroperitoneal |
| Gil-Moreno et al., 2008 [17] | Retrospective study | 69 | 140 | 100 | 0 | 2 | 15.2 | 0 | 4: 2 retroperitoneal hematoma; 2 lymphocyst |
27 | 51 | Laparoscopic Retroperitoneal | |
| Gil-Moreno et al., 2011 [16] | Retrospective study | 87 | IB2-IVA | 150 | 0 | 2 | 0 | 6: 2 retroperitoneal hematoma; 3 lymphocysts;1 urinary tract infection |
26.5 | Laparoscopic Retroperitoneal | |||
| Köhler et al., 2015 [18] | Trial | 113 | IIB-IVA | / | / | 1 | / | 17 | 2 vascular injuries | 9: 1 thrombosis; 1 ileus; 4 symptomatic lymphoceles; 1 nerve irritation; and 2 others (not specified) |
26.2 | 47.2 | Laparoscopic Transperitoneal |
| Leblanc et al., 2007 [3] | Retrospective study | 173 | IB2-IVA | 155 | 100 | 2 | 1.4 | 20.8 | 4: 1 obturator nerve injury; 2 ureteric injuries; 1 vascular injury (vena cava) |
22: 17 symptomatic lymphocysts; 3 transient ascites; 1 retroperitoneal hematoma; 1 bowel obstruction resulting from herniating bowel through an umbilical port site |
27.1 | 45 | Laparoscopic Retroperitoneal |
| Loverix et al., 2020 [10] | Retrospective study | 162 | IIB-IVA | 75 | 62.5 | 2 | 2 | 14: 2 ureteral trauma;11 bleeding; 1 other |
25: 2 wound problem with conservative management; 1 retroperitoneal hematoma with conservative management; 1 severe pain; 4 urinary tract infection;8 blood transfusion; 1 vasovagal syncope; 1 severe vaginal blood; 1 iron supplements for anemia; 1 DVT with lung embolism treated with LMWH; 1 placement of ureteral stent for ureteral trauma; 1 retroperitoneal abscess with evacuation under anesthesia; 1 re-laparotomy for bleeding of the internal epigastric artery; 1 laparoscopic repair of ureteral trauma; 1 hospitalization in intensive care unit for hyponatremia |
24.4 | 48 | Laparoscopic Transperitoneal and Retroperitoneal | |
| Mortier et al., 2008 [19] | Retrospective study | 22 | IB2-IIIB | 68 | 90 | 0 | 2 | 5 | 0 | 0 | 24 | 48 | Laparoscopic Transperitoneal |
| Mortier et al., 2008 [19] | Retrospective study | 47 | IB2-IIIB | 62 | 90 | 1 | 2 | 8 | 0 | 3: 2 lymphocoeles; 1 retroperitoneal hematoma |
24 | 48 | Laparoscopic Retroperitoneal |
| Possover et al., 1998 [20] | Retrospective study | 3 | IIIB | 218 | 200 | 0 | 4 | 10 | 0 | 0 | / | 46.3 | Laparoscopic Retroperitoneal |
| Ramirez et al., 2011 [21] | Retrospective study | 60 | IB2-IVA | 140 | 22.5 | 0 | 1 | 11 | 1 bleeding from an ascending lumbar vein at the level of the left renal vein | 7 lymphocyst | 26,7 | 48 | Laparoscopic Retroperitoneal |
| Sonoda et al., 2003 [22] | Retrospective study | 111 | IB2-IVA | 157 | 100 | 0 | 2 | 19 | 0 | 14: 11 symptomatic lymphoceles; 2 retroperitoneal hematomas; 1 trocar-site hernia |
24 | 46 | Laparoscopic Retroperitoneal |
| Tillmanns et al., 2007 [23] | Retrospective study | 18 | IIB-IVA | 108 | 25 | 0 | 10 | 0 | 1 lymphocyst | 29 | 49 | Laparoscopic Retroperitoneal | |
| Uzan et al., 2011 [24] | Retrospective study | 89 | IB2-IVA | 185 | / | 0 | 3 | 13 | 0 | 3 lymphocysts | 23 | 45 | Laparoscopic Retroperitoneal |
| Vázquez-Vicente, 2018 [25] | Retrospective study | 59 | IB2-IVA | 180 | / | 0 | 1.7 | 16.4 | 0 | 4: 3 lymphoceles; 1 intrabdominal abscess |
24.6 | 52.3 | Laparoscopic Transperitoneal and Retroperitoneal |
| Vergote et al., 2002 [26] | Retrospective study | 21 | IB2-IIIB | 55 | 78 | 5 | 1 | 6 | 0 | 1 retroperitoneal hematoma | / | 51 | Laparoscopic Retroperitoneal |
| Vergote et al., 2002 [26] | Retrospective study | 21 | IB2-IIIB | 70 | 78 | 0 | 1 | 6 | 0 | 0 | / | 51 | Laparoscopic Transperitoneal |
OT, operative time; EBL, estimated blood loss; BMI, body mass index; HT, Hospitalization Time.
Appendix B
Table A2.
Inclusion and exclusion criteria of the studies.
| Author, Year | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Díaz-Feijoo et al., 2013 [27] |
|
|
| Fastrez et al., 2009 [28] |
|
/ |
| Fastrez et al., 2013 [29] |
|
/ |
| Gucer et al., 2017 [30] |
|
|
| Loverix et al., 2020 [10] |
|
|
| Vergote et al., 2008 [31] |
|
/ |
| Benito et al., 2012 [13] |
|
/ |
| Dargent et al., 2000 [14] |
|
/ |
| Franco-Camps et al., 2010 [15] |
|
/ |
| Gil-Moreno et al., 2008 [17] |
|
/ |
| Gil-Moreno et al., 2011 [16] |
|
|
| Köhler et al., 2015 [18] |
|
/ |
| Leblanc et al., 2007 [3] |
|
/ |
| Mortier et al., 2008 [19] |
|
|
| Possover et al., 1998 [20] |
|
/ |
| Ramirez et al., 2011 [21] |
|
|
| Sonoda et al., 2003 [22] |
|
|
| Tillmanns et al., 2007 [23] |
|
|
| Uzan et al., 2011 [24] |
|
|
| Vázquez-Vicente, 2018 [25] |
|
/ |
| Vergote et al., 2002 [26] |
|
|
FIGO, International Federation of Obstetrics and Gynecology; LN, lymph node; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Author Contributions
Conceptualization, M.C.D.D. and V.C.; methodology, G.L.B. and V.A.C.; validation, G.C., G.S. and A.A.; formal analysis, V.G.; data curation, G.L.B. and G.C.; writing—original draft preparation, M.C.D.D.; writing—review and editing, A.S.L.; visualization, S.G.; supervision, V.C.; project administration, V.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data analyzed in this systematic review and meta-analysis were already available in the single studies included.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Liu B., Gao S., Li S. A Comprehensive Comparison of CT, MRI, Positron Emission Tomography or Positron Emission Tomography/CT, and Diffusion Weighted Imaging-MRI for Detecting the Lymph Nodes Metastases in Patients with Cervical Cancer: A Meta-Analysis Based on 67 Studies. Gynecol. Obstet. Investig. 2017;82:209–222. doi: 10.1159/000456006. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc E., Narducci F., Frumovitz M., Lesoin A., Castelain B., Baranzelli M.C., Taieb S., Fournier C., Querleu D. Therapeutic Value of Pretherapeutic Extraperitoneal Laparoscopic Staging of Locally Advanced Cervical Carcinoma. Gynecol. Oncol. 2007;105:304–311. doi: 10.1016/j.ygyno.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Chantalat E., Vidal F., Leguevaque P., Lepage B., Mathevet P., Deslandres M., Motton S. Cervical Cancer with Paraaortic Involvement: Do Patients Truly Benefit from Tailored Chemoradiation Therapy? A Retrospective Study on 8 French Centers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;193:118–122. doi: 10.1016/j.ejogrb.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Rogers L., Siu S.S.N., Luesley D., Bryant A., Dickinson H.O. Radiotherapy and Chemoradiation after Surgery for Early Cervical Cancer. Cochrane Database Syst. Rev. 2012;5:CD007583. doi: 10.1002/14651858.CD007583.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi H.J., Ju W., Myung S.K., Kim Y. Diagnostic Performance of Computer Tomography, Magnetic Resonance Imaging, and Positron Emission Tomography or Positron Emission Tomography/Computer Tomography for Detection of Metastatic Lymph Nodes in Patients with Cervical Cancer: Meta-Analysis. Cancer Sci. 2010;101:1471–1479. doi: 10.1111/j.1349-7006.2010.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi H.J., Roh J.W., Seo S.-S., Lee S., Kim J.-Y., Kim S.-K., Kang K.W., Lee J.S., Jeong J.Y., Park S.-Y. Comparison of the Accuracy of Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Presurgical Detection of Lymph Node Metastases in Patients with Uterine Cervical Carcinoma: A Prospective Study. Cancer. 2006;106:914–922. doi: 10.1002/cncr.21641. [DOI] [PubMed] [Google Scholar]
- 8.Frumovitz M., Querleu D., Gil-Moreno A., Morice P., Jhingran A., Munsell M.F., Macapinlac H.A., Leblanc E., Martinez A., Ramirez P.T. Lymphadenectomy in Locally Advanced Cervical Cancer Study (LiLACS): Phase III Clinical Trial Comparing Surgical with Radiologic Staging in Patients with Stages IB2-IVA Cervical Cancer. J. Minim. Invasive Gynecol. 2014;21:3–8. doi: 10.1016/j.jmig.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capozzi V.A., Sozzi G., Monfardini L., Di Donna M.C., Giallombardo V., Lo Balbo G., Butera D., Berretta R., Chiantera V. Transperitoneal versus Extraperitoneal Laparoscopic Aortic Lymph Nodal Staging for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2021;47:2256–2264. doi: 10.1016/j.ejso.2021.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Loverix L., Salihi R.R., Van Nieuwenhuysen E., Concin N., Han S., van Gorp T., Vergote I. Para-Aortic Lymph Node Surgical Staging in Locally-Advanced Cervical Cancer: Comparison between Robotic versus Conventional Laparoscopy. Int. J. Gynecol. Cancer. 2020;30:466–472. doi: 10.1136/ijgc-2019-000961. [DOI] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basch E., Becker C., Rogak L.J., Schrag D., Reeve B.B., Spears P., Smith M.L., Gounder M.M., Mahoney M.R., Schwartz G.K., et al. Composite Grading Algorithm for the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) Clin. Trials. 2021;18:104–114. doi: 10.1177/1740774520975120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benito V., Lubrano A., Arencibia O., Andújar M., Pinar B., Medina N., Falcón J.M., Falcón O. Laparoscopic Extraperitoneal Para-Aortic Lymphadenectomy in the Staging of Locally Advanced Cervical Cancer: Is It a Feasible Procedure at a Peripheral Center? Int. J. Gynecol. Cancer. 2012;22:332–336. doi: 10.1097/IGC.0b013e31823c241b. [DOI] [PubMed] [Google Scholar]
- 14.Dargent D., Ansquer Y., Mathevet P. Technical Development and Results of Left Extraperitoneal Laparoscopic Paraaortic Lymphadenectomy for Cervical Cancer. Gynecol. Oncol. 2000;77:87–92. doi: 10.1006/gyno.1999.5585. [DOI] [PubMed] [Google Scholar]
- 15.Franco-Camps S., Cabrera S., Pérez-Benavente A., Díaz-Feijoo B., Bradbury M., Xercavins J., Gil-Moreno A. Extraperitoneal Laparoscopic Approach for Diagnosis and Treatment of Aortic Lymph Node Recurrence in Gynecologic Malignancy. J. Minim. Invasive Gynecol. 2010;17:570–575. doi: 10.1016/j.jmig.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Gil-Moreno A., Franco-Camps S., Cabrera S., Pérez-Benavente A., Martínez-Gómez X., Garcia A., Xercavins J. Pretherapeutic Extraperitoneal Laparoscopic Staging of Bulky or Locally Advanced Cervical Cancer. Ann. Surg. Oncol. 2011;18:482–489. doi: 10.1245/s10434-010-1320-9. [DOI] [PubMed] [Google Scholar]
- 17.Gil-Moreno A., Díaz-Feijoo B., Pérez-Benavente A., del Campo J.M., Xercavins J., Martínez-Palones J.M. Impact of Extraperitoneal Lymphadenectomy on Treatment and Survival in Patients with Locally Advanced Cervical Cancer. Gynecol. Oncol. 2008;110:S33–S35. doi: 10.1016/j.ygyno.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Köhler C., Mustea A., Marnitz S., Schneider A., Chiantera V., Ulrich U., Scharf J.-P., Martus P., Vieira M.A., Tsunoda A. Perioperative Morbidity and Rate of Upstaging after Laparoscopic Staging for Patients with Locally Advanced Cervical Cancer: Results of a Prospective Randomized Trial. Am. J. Obstet. Gynecol. 2015;213:503.e1–503.e7. doi: 10.1016/j.ajog.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Mortier D.G., Stroobants S., Amant F., Neven P., VAN Limbergen E., Vergote I. Laparoscopic Para-Aortic Lymphadenectomy and Positron Emission Tomography Scan as Staging Procedures in Patients with Cervical Carcinoma Stage IB2-IIIB. Int. J. Gynecol. Cancer. 2008;18:723–729. doi: 10.1111/j.1525-1438.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 20.Possover M., Krause N., Drahonovsky J., Schneider A. Left-Sided Suprarenal Retrocrural Para-Aortic Lymphadenectomy in Advanced Cervical Cancer by Laparoscopy. Gynecol. Oncol. 1998;71:219–222. doi: 10.1006/gyno.1998.5172. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez P.T., Jhingran A., Macapinlac H.A., Euscher E.D., Munsell M.F., Coleman R.L., Soliman P.T., Schmeler K.M., Frumovitz M., Ramondetta L.M. Laparoscopic Extraperitoneal Para-Aortic Lymphadenectomy in Locally Advanced Cervical Cancer: A Prospective Correlation of Surgical Findings with Positron Emission Tomography/Computed Tomography Findings. Cancer. 2011;117:1928–1934. doi: 10.1002/cncr.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonoda Y., Leblanc E., Querleu D., Castelain B., Papageorgiou T.H., Lambaudie E., Narducci F. Prospective Evaluation of Surgical Staging of Advanced Cervical Cancer via a Laparoscopic Extraperitoneal Approach. Gynecol. Oncol. 2003;91:326–331. doi: 10.1016/j.ygyno.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Tillmanns T., Lowe M.P. Safety, Feasibility, and Costs of Outpatient Laparoscopic Extraperitoneal Aortic Nodal Dissection for Locally Advanced Cervical Carcinoma. Gynecol. Oncol. 2007;106:370–374. doi: 10.1016/j.ygyno.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Uzan C., Souadka A., Gouy S., Debaere T., Duclos J., Lumbroso J., Haie-Meder C., Morice P. Analysis of Morbidity and Clinical Implications of Laparoscopic Para-Aortic Lymphadenectomy in a Continuous Series of 98 Patients with Advanced-Stage Cervical Cancer and Negative PET-CT Imaging in the Para-Aortic Area. Oncologist. 2011;16:1021–1027. doi: 10.1634/theoncologist.2011-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vázquez-Vicente D., Fernández Del Bas B., García Villayzán J., Di Fiore H.A., Luna Tirado J., Casado Echarren V., García-Foncillas J., Plaza Arranz J., Chiva L. Laparoscopic Paraaortic Surgical Staging in Locally Advanced Cervical Cancer: A Single-Center Experience. Clin. Transl. Oncol. 2018;20:1455–1459. doi: 10.1007/s12094-018-1878-4. [DOI] [PubMed] [Google Scholar]
- 26.Vergote I., Amant F., Berteloot P., Van Gramberen M. Laparoscopic Lower Para-Aortic Staging Lymphadenectomy in Stage IB2, II, and III Cervical Cancer. Int. J. Gynecol. Cancer. 2002;12:22–26. doi: 10.1136/ijgc-00009577-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Díaz-Feijoo B., Gil-Ibáñez B., Pérez-Benavente A., Martínez-Gómez X., Colás E., Sánchez-Iglesias J.L., Cabrera-Díaz S., Puig-Puig O., Magrina J.F., Gil-Moreno A. Comparison of Robotic-Assisted vs. Conventional Laparoscopy for Extraperitoneal Paraaortic Lymphadenectomy. Gynecol. Oncol. 2014;132:98–101. doi: 10.1016/j.ygyno.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Fastrez M., Vandromme J., George P., Rozenberg S., Degueldre M. Robot Assisted Laparoscopic Transperitoneal Para-Aortic Lymphadenectomy in the Management of Advanced Cervical Carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;147:226–229. doi: 10.1016/j.ejogrb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Fastrez M., Goffin F., Vergote I., Vandromme J., Petit P., Leunen K., Degueldre M. Multi-Center Experience of Robot-Assisted Laparoscopic Para-Aortic Lymphadenectomy for Staging of Locally Advanced Cervical Carcinoma. Acta Obstet. Gynecol. Scand. 2013;92:895–901. doi: 10.1111/aogs.12150. [DOI] [PubMed] [Google Scholar]
- 30.Gucer F., Misirlioglu S., Ceydeli N., Taskiran C. Robot-Assisted Laparoscopic Transperitoneal Infrarenal Lymphadenectomy in Patients with Locally Advanced Cervical Cancer by Single Docking: Do We Need a Backup Procedure? J. Robot. Surg. 2018;12:49–58. doi: 10.1007/s11701-017-0685-1. [DOI] [PubMed] [Google Scholar]
- 31.Vergote I., Pouseele B., Van Gorp T., Vanacker B., Leunen K., Cadron I., Neven P., Amant F. Robotic Retroperitoneal Lower Para-Aortic Lymphadenectomy in Cervical Carcinoma: First Report on the Technique Used in 5 Patients. Acta Obstet. Gynecol. Scand. 2008;87:783–787. doi: 10.1080/00016340802146946. [DOI] [PubMed] [Google Scholar]
- 32.Marnitz S., Köhler C., Roth C., Füller J., Hinkelbein W., Schneider A. Is There a Benefit of Pretreatment Laparoscopic Transperitoneal Surgical Staging in Patients with Advanced Cervical Cancer? Gynecol. Oncol. 2005;99:536–544. doi: 10.1016/j.ygyno.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Di Donna M.C., Cicero C., Sozzi G., Cucinella G., Scambia G., Chiantera V. Laparoscopic Aortic Lymphadenectomy in Left-Sided Inferior Vena Cava. Int. J. Gynecol. Cancer. 2020;30:1462–1463. doi: 10.1136/ijgc-2020-001469. [DOI] [PubMed] [Google Scholar]
- 34.Lim P.C., Kang E., Park D.H. Learning Curve and Surgical Outcome for Robotic-Assisted Hysterectomy with Lymphadenectomy: Case-Matched Controlled Comparison with Laparoscopy and Laparotomy for Treatment of Endometrial Cancer. J. Minim. Invasive Gynecol. 2010;17:739–748. doi: 10.1016/j.jmig.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Reyes Claret A., Martínez Canto M.C., Robles Gourley A., Llull Gomila M., Martín Jiménez Á. Transperitoneal Laparoscopic Para-Aortic Lymphadenectomy and Body Mass Index: Is It Really a Limiting Factor for the Procedure? J. Laparoendosc. Adv. Surg. Tech. A. 2020;30:416–422. doi: 10.1089/lap.2019.0529. [DOI] [PubMed] [Google Scholar]
- 36.Hong D.G., Park N.Y., Chong G.O., Cho Y.L., Park I.S., Lee Y.S., Lee D.H. Laparoscopic Transperitoneal Infrarenal Para-Aortic Lymphadenectomy in Patients with FIGO Stage IB1-II B Cervical Carcinoma. JSLS. 2012;16:229–235. doi: 10.4293/108680812X13427982376266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida H., Yamamoto M., Shigeta H. Learning Curve of Laparoscopic Extraperitoneal Para-Aortic Lymphadenectomy for Endometrial Carcinoma: A Cumulative Sum Analysis. Surg. Oncol. 2020;35:254–260. doi: 10.1016/j.suronc.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Ponce J., Barahona M., Pla M.J., Rovira J., Garcia-Tejedor A., Gil-Ibanez B., Gaspar H.M., Sabria E., Bartolomé C., Marti L. Robotic Transperitoneal Infrarenal Para-Aortic Lymphadenectomy with Double Docking: Technique, Learning Curve, and Perioperative Outcomes. J. Minim. Invasive Gynecol. 2016;23:622–627. doi: 10.1016/j.jmig.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Bentivegna E., Koual M., Nguyen-Xuan H.-T., Plait L., Seidler S., Achen G., Bats A.-S., Azaïs H. Docking for Robotic Extraperitoneal Para-Aortic Lymphadenectomy with Da Vinci Xi Surgical System. J. Gynecol. Obstet. Hum. Reprod. 2021;50:102131. doi: 10.1016/j.jogoh.2021.102131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed in this systematic review and meta-analysis were already available in the single studies included.