Abstract
With aging, sarcopenia and the associated locomotor disorders, have become serious problems. The roots of maca contain active ingredients (triterpenes) that have a preventive effect on sarcopenia. However, the effect of maca on muscle hypertrophy has not yet been investigated. The aim of this study was to examine the effects and mechanism of maca on muscle hypertrophy by adding different concentrations of yellow maca (0.1 mg/mL and 0.2 mg/mL) to C2C12 skeletal muscle cell culture. Two days after differentiation, maca was added for two days of incubation. The muscle diameter, area, differentiation index, and multinucleation, were assessed by immunostaining, and the expression levels of the proteins related to muscle protein synthesis/degradation were examined by Western blotting. Compared with the control group, the muscle diameter and area of the myotubes in the maca groups were significantly increased, and the cell differentiation index and multinucleation were significantly higher in the maca groups. Phosphorylation of Akt and mTOR was elevated in the maca groups. Maca also promoted the phosphorylation of AMPK. These results suggest that maca may promote muscle hypertrophy, differentiation, and maturation, potentially via the muscle hypertrophic signaling pathways such as Akt and mTOR, while exploring other pathways are needed.
Keywords: maca, sarcopenia, muscle hypertrophy, aging, protein
1. Introduction
Sarcopenia is a progressive and age-related decline in skeletal muscle mass and is associated with falls, physical disability, and mortality [1]. Healthy adults lose approximately 8% of their muscle mass every 10 years after the age of 40, and this rate will accelerate to 15% every 10 years after the age of 70 [2]. From a historical point of view, the aging population is a new problem, but projections indicate that by 2050, there will be more people aged 60 or older than adolescents aged 10–24 [3]. The muscles of elderly people have major defects in their regulatory and maintenance abilities during feeding and exercise [4]. In particular, age-related anabolic resistance may show up after prandial in the elderly [5], suggesting the importance of effective nutritional strategies against muscle wasting. Therefore, it is valuable to find a way to enhance the effects of exercise through nutrition or by simply relying on the nutrient intake to slow sarcopenia in elderly people.
Lepidium meyenii Walp (maca) grows at altitudes of 2800–5000 m above sea level and was probably domesticated between the years 4000–1200 BCE in Peru [6]. In human studies, it is already known that maca improves menopausal symptoms [7]. In other adult human subjects, maca improves mood, energy, and health status and reduces the chronic mountain sickness score (CMS), a kind of pathology that is observed in only people living at high altitudes [8]. In addition, as a typical lipid-soluble ingredient for maca, macamide extends the weight-loaded swimming time to exhaustion and improves the swimming endurance capacity by reducing the amounts of lactate, lactate dehydrogenase and blood ammonia, such that its antifatigue property has been proven [9]. Furthermore, in the forced swimming test, reduced immobility time indicated that maca has antidepressant activity [10].
Triterpenoid saponins have been suggested to have a muscle hypertrophy effect, as they may contribute to maintaining skeletal muscle mass and decreasing diet-induced obesity and muscle wasting [11,12,13]. Ursolic acid is a triterpenoid and is considered a treatment for skeletal muscle disorders [14]. Previous research has shown that triterpenoid saponins are found in maca [15], such as panaxytriol which enhances muscle protein synthesis [11]. In addition to triterpenoid saponins, maca contains abundant nutrients and various amino acids, such as leucine and arginine [16], which are also related to an increase in muscle mass [17,18,19]. Therefore, we hypothesized that maca may have a muscle hypertrophy-promoting effect.
The aim of this study was to clarify the effect of maca extract on the growth of skeletal muscle cells and its underlying mechanisms.
2. Results
2.1. Adding Maca Promoted Skeletal Muscle Hypertrophy, Differentiation, and Maturation
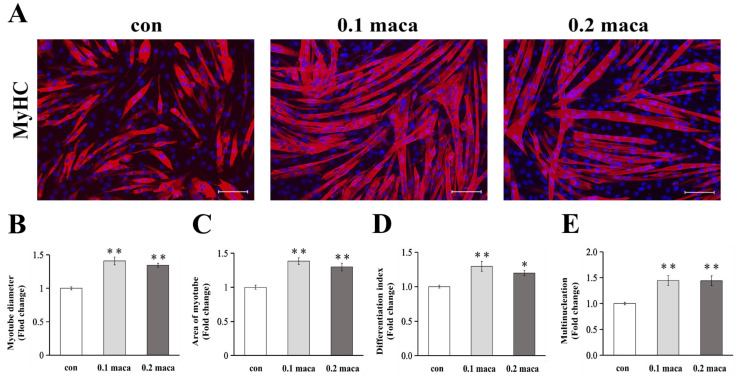
After adding maca to the C2C12 skeletal muscle cell culture for 2 days, we found that the maca groups had a better growth trend (Figure 1A). The myotube diameters in the 0.1 and 0.2 maca groups were significantly greater than those in the control (con) group (p < 0.01) (Figure 1B). We quantified the myotube areas to ascertain the effect of maca on the C2C12 skeletal muscle morphology, and the myotube areas in the 0.1 and 0.2 maca groups were significantly greater than that in the con group (p < 0.01) (Figure 1C). The differentiation index was significantly higher in the 0.1 and 0.2 maca groups than in the con group (p < 0.01 and p < 0.05, respectively) (Figure 1D). The multinucleation was significantly higher in the 0.1 and 0.2 maca groups than in the con group (p < 0.01) (Figure 1E).
Figure 1.
C2C12 myotubes were immunostained with myosin heavy chain (MyHC; red), and the nuclei were immunostained with DAPI (blue). Scale bar: 100 μm. Representative images were taken (A). Two independent experiments (n = 7) showed the same trend in myotube diameter (B), myotube area (C), differentiation index (D), and Multinucleation (E). * p < 0.05 vs. con; ** p < 0.01 vs. con.
2.2. Effect of Maca on Protein Expression Associated with Muscle Hypertrophy, Differentiation, and Maturation
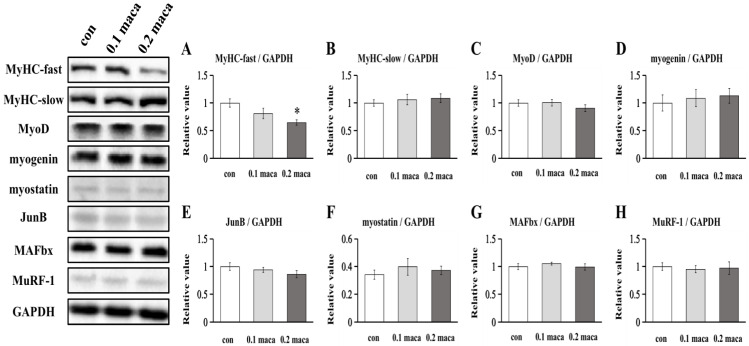
There was a significant difference in the expression of MyHC-fast between the con group and the 0.2 maca group but there was no significant difference between the con group and 0.1maca group (Figure 2A). There was no significant difference in the expression of MyHC-slow between the con group and the maca groups (Figure 2B). No significant difference was found regarding the expression of MyoD and myogenin, which are related to muscle cell differentiation (Figure 2C,D). The expression of JunB which promotes muscle hypertrophy was not significantly different. The expression of myostatin, MAFbx, and MuRF-1 associated with muscle atrophy was also not significantly different.
Figure 2.
Expression of proteins in C2C12 myotubes. Representative immunoblots are shown on the left side, and the quantification of MyHC-fast (A), MyHC-slow (B), myogenin (C), MyoD (D), JunB (E), myostatin (F), MAFbx (G), and MuRF-1 (H) was determined after normalizing to GAPDH. * p < 0.05 vs. con.
2.3. Effect of Maca on Protein Expression Related to Muscle Protein Synthesis and Metabolism
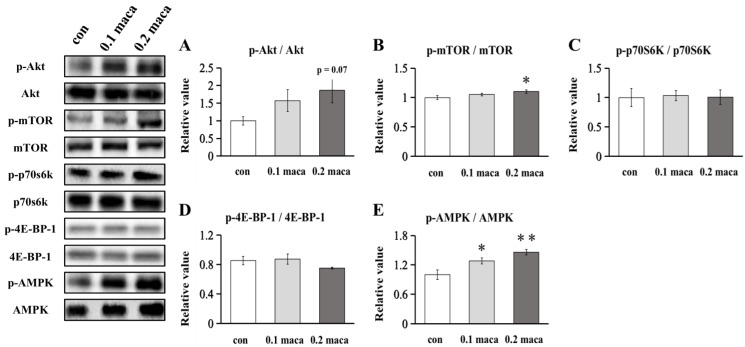
The phosphorylation of Akt in the 0.2 maca group tended to be higher than that in the con group (p = 0.07) (Figure 3A). The phosphorylation of mTOR in the 0.2 maca group was significantly higher than that in the con group (p < 0.05) (Figure 3B). There was no significant difference between the con group and the maca group regarding the phosphorylation of p70S6K (Figure 3C). There was no significant difference between the con group and the maca group in the phosphorylation of 4E-BP-1 (Figure 3D). The phosphorylation of AMPK in the 0.1 maca group (p < 0.05) and 0.2 maca group (p < 0.01) was significantly higher than that in the con group (Figure 3E).
Figure 3.
Protein expression in C2C12 myotubes. Representative immunoblots are shown on the left side, and the quantification of Akt (A), mTOR (B), p70S6K (C), 4E-BP-1 (D) and AMPK (E) phosphorylation was compared between the con group and maca groups. * p < 0.05 vs. con; ** p < 0.01 vs. con.
3. Discussion
In this study, we hypothesized that the addition of maca would enhance muscle synthesis, prevent muscle degradation and hence lead to muscle hypertrophy. As a result, after the addition of maca to myotubes for 2 days, the immunohistochemical results showed that adding maca to C2C12 skeletal muscle cell culture enhanced the muscle diameter, myotube area, differentiation index, and multinucleation, suggesting that maca has the potential to promote muscle cell hypertrophy, differentiation, and maturation.
Previous studies have shown that ursolic acid, a kind of triterpene, combined with leucine potentiates the differentiation of C2C12 skeletal muscle cells through the mTOR pathway [20]. Plant-derived ursolic acid can also prevent muscle wasting stimulated by excessive dexamethasone in C2C12 skeletal muscle cell culture [13]. In animal studies, ursolic acid and low-intensity treadmill exercise significantly decreased the expression of MuRF-1 and atrogin-1, thereby attenuating muscle atrophy [21]. Similarly, maslinic acid, pentacyclic triterpene found in olives, also attenuated denervation-induced muscle atrophy via the suppression of MuRF-1 and atrogin-1 [22]. Furthermore, maslinic acid promoted muscle hypertrophy via mTOR signaling [23]. Since maca also contains triterpenes, we predicted that the addition of maca would increase the mTOR pathway, such as the phosphorylation of Akt, mTOR, and p70S6K, in C2C12 skeletal muscle cell culture and lead to muscle hypertrophy. We found that the phosphorylation of Akt in the 0.2 maca group tended to increase, and the phosphorylation of mTOR in the 0.2 maca group was significantly higher than that in the con group, although the Akt-mTOR pathway’s downstream target, p70S6K (Figure 3C) and 4E-BP-1 (Figure 3C) [24] were not changed. Then, we further explored the possibility that there might be a pathway independent of Akt-mTOR that promoted muscle hypertrophy, such as JunB [25]. As a result, there was no significant difference in JunB expression (Figure 2E) and owing to no further research regarding the connection between JunB and muscle hypertrophy, we will not elaborate on it here. Of course, there may also be some other signaling pathways that influence muscle hypertrophy to maca treatment, such as decreased myostatin expression, as shown in ursolic acid treatment [13]. Unfortunately, there was no significant difference in myostatin expression (Figure 2F). In this regard, further studies such as omics (e.g., transcriptome and/or proteome) studies are needed to explore underlying mechanisms for maca treatment.
Interestingly, in this study, the addition of maca regulated skeletal muscle fiber type, especially MyHC-fast (Figure 2A). Previous studies showed that quercetin significantly increased the protein expression of MyHC-slow and significantly decreased MyHC-fast protein expression [26]. Since quercetin is also found in maca [27], we considered that maca may also have affected MyHC-slow, but no significant difference was detected in this study. Overexpression of the muscle-specific E3 ubiquitin-ligase enzymes MuRF-1 and MAFbx lead to skeletal muscle atrophy [28]. Since several previous animal studies have shown that ursolic acid, a kind of triterpene, inhibits muscle mass atrophy [12,29,30,31], we considered that maca also has an inhibitory effect on muscle atrophy, but the results here so no effect on the expression of MuRF-1 and MAFbx (Figure 2E,F). In summary, we suggest that the addition of maca promotes muscle synthesis at least partly via upregulating the Akt-mTOR pathway but the effect on the inhibition of muscle atrophy needs to be verified in future studies.
AMPK greatly affects skeletal muscle development and growth [32]. In this study, we found that the two different concentrations of maca promoted an increase in AMPK phosphorylation in skeletal muscle cell culture (Figure 3D). Basically, activated AMPK inhibits protein synthesis in skeletal muscle by downregulating the mTOR pathway [33] and negatively regulates myotube hypertrophy [34]. On the other hand, our recent studies showed that AMPK phosphorylation is accompanied by muscle hypertrophy in moderate hypoxia [35] and promotes attenuation of myotube atrophy by adding fucoxanthinol [36]. As repeated muscle contractions promote the activation of AMPK [37], activated AMPK stimulates glucose uptake and increases fatty acid oxidation [38]. Therefore, we suggest that the addition of maca promoted AMPK phosphorylation, which in turn stimulated cell metabolism without interfering with the muscle hypertrophic effect of maca.
The results of this study showed that the addition of maca promotes skeletal muscle hypertrophy and differentiation. Studies thus far have shown that maca promotes reproductive health, neuroprotection, antioxidants, antifatigue, antitumoral, liver protection, anti-memory impairment, and immune regulation [39,40,41,42,43,44,45,46]. The results of our study corroborate multiple antiaging effects of the natural food maca, which may be helpful to prevent sarcopenia and improve the quality of life of aging people. Of course, in future studies, more in vivo and in vitro studies are needed to demonstrate maca’s functions, such as testing maca in human muscle cell cultures or animal studies. Furthermore, we may need to examine the direct effects of maca extracts (e.g., triterpenoid saponins, amino acid) on skeletal muscle cell hypertrophy, differentiation, and maturation for further studies.
4. Materials and Methods
4.1. Cell Culture
C2C12 myoblasts (American Type Culture Collection, Manassas, VA, USA; ECACC, Salisbury, UK) were seeded in a 12-well plate at a density of 1 × 10⁵ cells per culture dish and grown in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% antibiotic antimycotic solution (Sigma–Aldrich, St. Louis, MO, USA). When the cells approached 80%–100% confluence, the medium was changed to DMEM with 2% horse serum (Thermo Fisher Scientific), and then the myoblasts were differentiated into myotubes. The medium was changed every two days, and all experimental cultures were carried out in a 37 °C humid air incubator containing 5% CO2.
4.2. Experimental Design
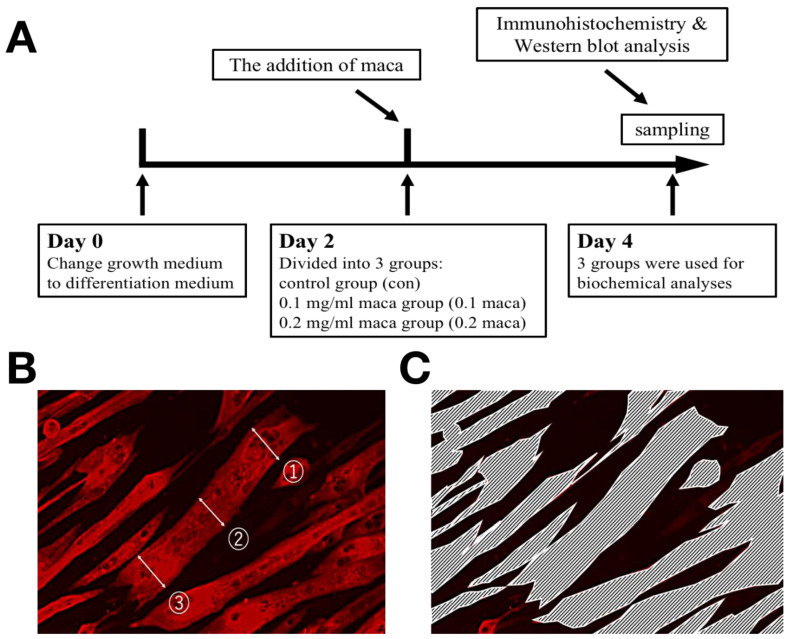
The experimental design is summarized in Figure 4A. On day 2 of the induction of differentiation, the myotubes were divided into three groups. The control (con) group was incubated normally, and the maca groups were treated with two different concentrations of maca: the “0.1 maca” group was treated with 0.1 mg/mL of maca and the “0.2 maca” group was treated with 0.2 mg/mL of maca for 48 h. On day 4, the three groups were used for biochemical analyses. The maca extract (lot. no. MCP-001) was provided by Bizen Chemical Co., Ltd. (Okayama, Japan). The extracts were obtained by ethanol extraction and spray drying. The maca extract, containing triterpenoid saponins [15], was dissolved in dimethyl sulfoxide (DMSO; Wako, Japan) and the solution mixed with a differentiation medium was passed through a syringe filter (Sartorius, Germany); DMSO without maca was added to the con group.
Figure 4.
Experimental scheme (A) and methods for measuring myotube cell diameter (B) and area (C) in the experiments.
4.3. Immunohistochemistry
All groups of C2C12 myotubes were cultured on coverslips and fixed with phosphate-buffered saline (PBS) containing 3.7% paraformaldehyde for 15 min at room temperature. Fixed myotubes were permeabilized in 0.2% Triton X-100/PBS for 10 min and blocked with 1% albumin from bovine serum, Fraction V, pH 7.0 (BSA) (FUJIFILM Wako Pure Chemical Corporation, Osaka, Osaka, Japan)/PBS. The cells were incubated with a primary antibody against myosin heavy chain (MyHC; 1:250, Sigma–Aldrich; M4276) overnight, washed with PBS, and incubated for 1 h with a secondary antibody conjugated with Alexa 647 (1:100, Molecular Probes, Grand Island, NY, USA; A31571) at 37 °C [47]. The differentiation index (%) was defined as the MyHC-positive nuclei divided by the total nuclei, and DAPI (Thermo Fisher Scientific, Waltham, MA, USA) was used for nucleic acid staining [35,47]. The multinucleation was defined as the nuclei (at least 2) on a myotube being counted and the percentage of myotubes with multi nuclei in each area was calculated [48]. The myotubes on the coverslips were observed and photographed (3 random areas) under a ×20 objective by using a Biorevo BZ-9000 fluorescence microscope (Keyence, Japan) [35,36,47], in which at least 200 myotubes per area, with a total of over 2400 myotubes were measured. The images of the myotubes were analyzed by BZ-II image analysis software (Keyence, Japan). In addition, we performed three independent experiments (n = 3–4 in each group), and the quantitative measurements were used as reported in previous studies. Briefly, in the randomly selected microscope fields, the diameters of at least 200 myotubes in each area were measured at randomly selected 3 locations (e.g., ①, ②, and ③) taken along the length of the myotubes (Figure 4B) [49]. Then, the average diameter of a myotube was considered as the mean of 3 measurements. The enclosed area in the picture (Figure 4C) was considered the area of myotubes [35,36].
4.4. Western Blot Analysis
The cells were rinsed with PBS and lysed in RIPA buffer containing 10 mM Tris–HCl pH 7.4, 1% Na deoxycholate, 1% Triton, 150 mM NaCl, 5 mM EDTA, and 0.1% SDS supplemented with phosphatase inhibitor cocktail (Nacalai Tesque, Japan), phenylmethanesulfonylfluoride solution, protease inhibitor cocktail and PhosSTOP phosphatase inhibitor cocktail (Sigma–Aldrich, St. Louis, MO, USA). Samples were incubated on ice for 1 h and centrifuged at 15,000× g for 15 min at 4 °C. To confirm the concentration, the supernatant was collected and analyzed with a Wako protein assay kit (FUJIFILM Wako Pure Chemical Corporation, Japan). Equal amounts of proteins were separated on 8% SDS-polyacrylamide gels at 40 mA for 1 h and transferred to polyvinylidene fluoride membranes at 60 V for 2 h. Blocking One P and Blocking One (Nacalai Tesque, Japan) were used for blocking at room temperature for 30 min. After blocking, the membranes were incubated with primary antibodies against MyHC-fast (Sigma–Aldrich; 1:1000; M4276), MyHC-slow (Abcam, Cambridge, MA, USA; 1:1000; ab11083), myogenin (Santa Cruz; 1:200; sc-12732), MyoD (Santa Cruz; 1:200; sc-32758), TRIM63 (MuRF-1) (Santa Cruz; 1:100; sc-398608), MAFbx (Santa Cruz; 1:100; sc-166806), phosphorylated-mTOR (Ser2448, Cell Signaling Technology; 1:1000; #5536), mTOR (Cell Signaling Technology; 1:1000; #2983), phosphorylated-Akt (Ser473, Cell Signaling Technology; 1:1000; #9271), Akt (Cell Signaling Technology, Danvers, MA, USA; 1:1000; #9272), phosphorylated-p70S6K (Thr389, Cell Signaling Technology; 1:1000; #9205), p70S6K (Cell Signaling Technology; 1:1000; #2708), phosphorylated-4E-BP-1 (Thr37/46, Cell Signaling Technology; 1:1000; #9459), 4E-BP-1 (Cell Signaling Technology; 1:1000; #9644), phosphorylated-AMPK (Thr172, Cell Signaling Technology; 1:1000; #2535), AMPK (Cell Signaling Technology; 1:1000; #2793), myostatin (Santa Cruz; 1:200; sc-34781), JunB (Abcam; 1:200; ab128878), and GAPDH (Sigma–Aldrich; 1:10,000; G9545). Immunoreactive proteins were incubated with anti-mouse IgG (1:10,000) and anti-rabbit IgG (1:10,000) to detect primary antibody binding. The membranes were washed three times with TBST for 10 min each time and treated with the Luminata Forte Western HRP substrate (Millipore) to visualize the bands under the FUSION FX7 EDGE (Vilber Lourmat, Collégien, France), and ImageJ software was used for quantification of the band intensities [50]. A minimum of three independent experiments (n = 4) were performed. As supplementary experiments, we used an independent experiment (n = 3) to detect the protein expression of myostatin and 4E-BP-1.
4.5. Statistical Analysis
The data are presented as the means ± S.D. Data were assessed by one-way analysis of variance (ANOVA) with Tukey’s or Games-Howell tests. A value of p < 0.05 was considered statistically significant.
5. Conclusions
The addition of maca promoted muscle hypertrophy, differentiation, and maturation in skeletal muscle cell culture. Adding maca to skeletal muscle cell culture regulated the expression of MyHC and promoted the phosphorylation of muscle hypertrophic proteins such as Akt and mTOR and the metabolic signal AMPK. Based on the results of immunohistochemistry and increased phosphorylation of Akt and mTOR, we suggest that the addition of maca appears to be effective in the treatment of sarcopenia. Yet, the limitation of the study is that it did not find sufficient molecular mechanisms to explain the effect of maca on skeletal muscle cell hypertrophy, differentiation, and maturation, so we suggest that further research is needed to explore other signaling pathways that influence muscle hypertrophy, differentiation, and maturation to maca treatment by means of omics studies.
Author Contributions
D.Y. performed experiments, analyzed data, prepared figures, and drafted the manuscript; D.Y., M.Y., T.S., K.T., Y.O. and T.H. approved the final version of the manuscript; D.Y., M.Y., T.S., K.T., Y.O. and T.H. interpreted the results of experiments; D.Y., M.Y., T.S., K.T. and T.H. edited and revised the manuscript; D.Y., and T.H. conceived and designed the study. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
Funding Statement
This research was funded by Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (18K19762 and 21H03384 to T.H.) and supported by JST, the establishment of university fellowships towards the creation of science technology innovation (JPMJFS2146 to D.Y.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimby G., Saltin B. The Ageing Muscle. Clin. Physiol. 1983;3:209–218. doi: 10.1111/j.1475-097X.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 3.Rudnicka E., Napierała P., Podfigurna A., Męczekalski B., Smolarczyk R., Grymowicz M. The World Health Organization (WHO) Approach to Healthy Ageing. Maturitas. 2020;139:6–11. doi: 10.1016/j.maturitas.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rennie M.J.R.J. Anabolic Resistance: The Effects of Aging, Sexual Dimorphism, and Immobilization on Human Muscle Protein Turnover This Paper Is One of a Selection of Papers Published in This Special Issue, Entitled 14th International Biochemistry of Exercise Conference—Muscles as Molecular and Metabolic Machines, and Has Undergone the Journal’s Usual Peer Review Process. Appl. Physiol. Nutr. Metab. 2009;34:377–381. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- 5.Wall B.T., Gorissen S.H., Pennings B., Koopman R., Groen B.B.L., Verdijk L.B., van Loon L.J.C. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE. 2015;10:e0140903. doi: 10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S., Zhu F. Chemical Composition and Health Effects of Maca (Lepidium meyenii) Food Chem. 2019;288:422–443. doi: 10.1016/j.foodchem.2019.02.071. [DOI] [PubMed] [Google Scholar]
- 7.Lee M.S., Shin B.-C., Yang E.J., Lim H.-J., Ernst E. Maca (Lepidium meyenii) for Treatment of Menopausal Symptoms: A Systematic Review. Maturitas. 2011;70:227–233. doi: 10.1016/j.maturitas.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales-Arimborgo C., Yupanqui I., Montero E., Alarcón-Yaquetto D.E., Zevallos-Concha A., Caballero L., Gasco M., Zhao J., Khan I.A., Gonzales G.F. Acceptability, Safety, and Efficacy of Oral Administration of Extracts of Black or Red Maca (Lepidium meyenii) in Adult Human Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceuticals. 2016;9:49. doi: 10.3390/ph9030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Q., Jin W., Lv X., Dai P., Ao Y., Wu M., Deng W., Yu L. Effects of Macamides on Endurance Capacity and Anti-Fatigue Property in Prolonged Swimming Mice. Pharm. Biol. 2016;54:827–834. doi: 10.3109/13880209.2015.1087036. [DOI] [PubMed] [Google Scholar]
- 10.Rubio J., Caldas M., Dávila S., Gasco M., Gonzales G.F. Effect of Three Different Cultivars of Lepidium meyenii (Maca) on Learning and Depression in Ovariectomized Mice. BMC Complement. Altern. Med. 2006;6:23. doi: 10.1186/1472-6882-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takamura Y., Nomura M., Uchiyama A., Fujita S. Effects of Aerobic Exercise Combined with Panaxatriol Derived from Ginseng on Insulin Resistance and Skeletal Muscle Mass in Type 2 Diabetic Mice. J. Nutr. Sci. Vitaminol. 2017;63:339–348. doi: 10.3177/jnsv.63.339. [DOI] [PubMed] [Google Scholar]
- 12.Kunkel S.D., Elmore C.J., Bongers K.S., Ebert S.M., Fox D.K., Dyle M.C., Bullard S.A., Adams C.M. Ursolic Acid Increases Skeletal Muscle and Brown Fat and Decreases Diet-Induced Obesity, Glucose Intolerance and Fatty Liver Disease. PLoS ONE. 2012;7:e39332. doi: 10.1371/journal.pone.0039332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu R., Chen J., Xu J., Cao J., Wang Y., Thomas S.S., Hu Z. Suppression of Muscle Wasting by the Plant-derived Compound Ursolic Acid in a Model of Chronic Kidney Disease. J Cachexia Sarcopenia Muscle. 2017;8:327–341. doi: 10.1002/jcsm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakhtiari N., Moslemee-Jalalvand E., Kazemi J. Ursolic Acid: A Versatile Triterpenoid Compound in Regulating the Aging. Physiol. Pharmacol. 2017;21:15–24. [Google Scholar]
- 15.Lee Y.-K., Chang Y.H. Physicochemical and Antioxidant Properties of Methanol Extract from Maca (Lepidium meyenii Walp.) Leaves and Roots. Food Sci. Technol. 2019;39:278–286. doi: 10.1590/fst.03818. [DOI] [Google Scholar]
- 16.Chen L., Li J., Fan L. The Nutritional Composition of Maca in Hypocotyls (Lepidium meyenii Walp.) Cultivated in Different Regions of China. J. Food Qual. 2017;2017:e3749627. doi: 10.1155/2017/3749627. [DOI] [Google Scholar]
- 17.Lim C.H., Gil J.H., Quan H., Viet D.H., Kim C.K. Effect of 8-week Leucine Supplementation and Resistance Exercise Training on Muscle Hypertrophy and Satellite Cell Activation in Rats. Physiol. Rep. 2018;6:e13725. doi: 10.14814/phy2.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasiakos S.M., McClung J.P. Supplemental Dietary Leucine and the Skeletal Muscle Anabolic Response to Essential Amino Acids. Nutr. Rev. 2011;69:550–557. doi: 10.1111/j.1753-4887.2011.00420.x. [DOI] [PubMed] [Google Scholar]
- 19.Tan B., Yin Y., Liu Z., Li X., Xu H., Kong X., Huang R., Tang W., Shinzato I., Smith S.B., et al. Dietary L-Arginine Supplementation Increases Muscle Gain and Reduces Body Fat Mass in Growing-Finishing Pigs. Amino Acids. 2009;37:169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim M., Sung B., Kang Y.J., Kim D.H., Lee Y., Hwang S.Y., Yoon J.-H., Yoo M.-A., Kim C.M., Chung H.Y., et al. The Combination of Ursolic Acid and Leucine Potentiates the Differentiation of C2C12 Murine Myoblasts through the MTOR Signaling Pathway. Int. J. Mol. Med. 2015;35:755–762. doi: 10.3892/ijmm.2014.2046. [DOI] [PubMed] [Google Scholar]
- 21.Kim J.C., Kang Y.S., Noh E.B., Seo B.W., Seo D.Y., Park G.D., Kim S.H. Concurrent Treatment with Ursolic Acid and Low-Intensity Treadmill Exercise Improves Muscle Atrophy and Related Outcomes in Rats. Korean J. Physiol. Pharm. 2018;22:427–436. doi: 10.4196/kjpp.2018.22.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi Y., Ferdousi F., Fukumitsu S., Isoda H. Maslinic Acid Attenuates Denervation-Induced Loss of Skeletal Muscle Mass and Strength. Nutrients. 2021;13:2950. doi: 10.3390/nu13092950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirai T., Uemichi K., Kubota K., Yamauchi Y., Takemasa T. Maslinic Acid Promotes Hypertrophy Induced by Functional Overload in Mouse Skeletal Muscle. J. Nutr. Sci. Vitaminol. 2021;67:317–322. doi: 10.3177/jnsv.67.317. [DOI] [PubMed] [Google Scholar]
- 24.Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. Mediation of IGF-1-Induced Skeletal Myotube Hypertrophy by PI(3)K/Akt/MTOR and PI(3)K/Akt/GSK3 Pathways. Nat. Cell. Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 25.Raffaello A., Milan G., Masiero E., Carnio S., Lee D., Lanfranchi G., Goldberg A.L., Sandri M., Jun B. Transcription Factor Maintains Skeletal Muscle Mass and Promotes Hypertrophy. J. Cell. Biol. 2010;191:101–113. doi: 10.1083/jcb.201001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X., Liang D., Huang Z., Jia G., Zhao H., Liu G. Quercetin Regulates Skeletal Muscle Fiber Type Switching via Adiponectin Signaling. Food Funct. 2021;12:2693–2702. doi: 10.1039/D1FO00031D. [DOI] [PubMed] [Google Scholar]
- 27.Lee K.-J., Dabrowski K., Sandoval M., Miller M.J.S. Activity-Guided Fractionation of Phytochemicals of Maca Meal, Their Antioxidant Activities and Effects on Growth, Feed Utilization, and Survival in Rainbow Trout (Oncorhynchus Mykiss) Juveniles. Aquaculture. 2005;244:293–301. doi: 10.1016/j.aquaculture.2004.12.006. [DOI] [Google Scholar]
- 28.Rom O., Reznick A.Z. The Role of E3 Ubiquitin-Ligases MuRF-1 and MAFbx in Loss of Skeletal Muscle Mass. Free. Radic. Biol. Med. 2016;98:218–230. doi: 10.1016/j.freeradbiomed.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Chu X., He X., Shi Z., Li C., Guo F., Li S., Li Y., Na L., Sun C. Ursolic Acid Increases Energy Expenditure through Enhancing Free Fatty Acid Uptake and β-Oxidation via an UCP3/AMPK-Dependent Pathway in Skeletal Muscle. Mol. Nutr. Food Res. 2015;59:1491–1503. doi: 10.1002/mnfr.201400670. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel S.D., Suneja M., Ebert S.M., Bongers K.S., Fox D.K., Malmberg S.E., Alipour F., Shields R.K., Adams C.M. MRNA Expression Signatures of Human Skeletal Muscle Atrophy Identify a Natural Compound That Increases Muscle Mass. Cell Metab. 2011;13:627–638. doi: 10.1016/j.cmet.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakhtiari N., Hosseinkhani S., Tashakor A., Hemmati R. Ursolic Acid Ameliorates Aging-Metabolic Phenotype through Promoting of Skeletal Muscle Rejuvenation. Med. Hypotheses. 2015;85:1–6. doi: 10.1016/j.mehy.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Thomson D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018;19:3125. doi: 10.3390/ijms19103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolster D.R., Crozier S.J., Kimball S.R., Jefferson L.S. AMP-Activated Protein Kinase Suppresses Protein Synthesis in Rat Skeletal Muscle through Down-Regulated Mammalian Target of Rapamycin (MTOR) Signaling. J. Biol. Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 34.Egawa T., Ohno Y., Goto A., Ikuta A., Suzuki M., Ohira T., Yokoyama S., Sugiura T., Ohira Y., Yoshioka T., et al. AICAR-Induced Activation of AMPK Negatively Regulates Myotube Hypertrophy through the HSP72-Mediated Pathway in C2C12 Skeletal Muscle Cells. Am. J. Physiol. Endocrinol. Metab. 2014;306:E344–E354. doi: 10.1152/ajpendo.00495.2013. [DOI] [PubMed] [Google Scholar]
- 35.Sakushima K., Yoshikawa M., Osaki T., Miyamoto N., Hashimoto T. Moderate Hypoxia Promotes Skeletal Muscle Cell Growth and Hypertrophy in C2C12 Cells. Biochem. Biophys. Res. Commun. 2020;525:921–927. doi: 10.1016/j.bbrc.2020.02.152. [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa M., Hosokawa M., Miyashita K., Fujita T., Nishino H., Hashimoto T. Fucoxanthinol Attenuates Oxidative Stress-Induced Atrophy and Loss in Myotubes and Reduces the Triacylglycerol Content in Mature Adipocytes. Mol. Biol. Rep. 2020;47:2703–2711. doi: 10.1007/s11033-020-05369-8. [DOI] [PubMed] [Google Scholar]
- 37.Vavvas D., Apazidis A., Saha A.K., Gamble J., Patel A., Kemp B.E., Witters L.A., Ruderman N.B. Contraction-Induced Changes in Acetyl-CoA Carboxylase and 5′-AMP-Activated Kinase in Skeletal Muscle. J. Biol. Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- 38.Lai Y.-C., Kviklyte S., Vertommen D., Lantier L., Foretz M., Viollet B., Hallén S., Rider M.H. A Small-Molecule Benzimidazole Derivative That Potently Activates AMPK to Increase Glucose Transport in Skeletal Muscle: Comparison with Effects of Contraction and Other AMPK Activators. Biochem. J. 2014;460:363–375. doi: 10.1042/BJ20131673. [DOI] [PubMed] [Google Scholar]
- 39.Gonzales C., Rubio J., Gasco M., Nieto J., Yucra S., Gonzales G.F. Effect of Short-Term and Long-Term Treatments with Three Ecotypes of Lepidium meyenii (MACA) on Spermatogenesis in Rats. J. Ethnopharmacol. 2006;103:448–454. doi: 10.1016/j.jep.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Yu Z., Jin W., Dong X., Ao M., Liu H., Yu L. Safety Evaluation and Protective Effects of Ethanolic Extract from Maca (Lepidium meyenii Walp.) against Corticosterone and H2O2 Induced Neurotoxicity. Regul. Toxicol. Pharmacol. 2020;111:104570. doi: 10.1016/j.yrtph.2019.104570. [DOI] [PubMed] [Google Scholar]
- 41.Zevallos-Concha A., Nuñez D., Gasco M., Vasquez C., Quispe M., Gonzales G.F. Effect of Gamma Irradiation on Phenol Content, Antioxidant Activity and Biological Activity of Black Maca and Red Maca Extracts (Lepidium meyenii Walp) Toxicol. Mech. Methods. 2016;26:67–73. doi: 10.3109/15376516.2015.1090512. [DOI] [PubMed] [Google Scholar]
- 42.Tang W., Jin L., Xie L., Huang J., Wang N., Chu B., Dai X., Liu Y., Wang R., Zhang Y. Structural Characterization and Antifatigue Effect In Vivo of Maca (Lepidium meyenii Walp) Polysaccharide. J. Food Sci. 2017;82:757–764. doi: 10.1111/1750-3841.13619. [DOI] [PubMed] [Google Scholar]
- 43.Fu L., Wei J., Gao Y., Chen R. Antioxidant and Antitumoral Activities of Isolated Macamide and Macaene Fractions from Lepidium meyenii (Maca) Talanta. 2021;221:121635. doi: 10.1016/j.talanta.2020.121635. [DOI] [PubMed] [Google Scholar]
- 44.Gencoglu H. Maca Modulates Fat and Liver Energy Metabolism Markers Insulin, IRS1, Leptin, and SIRT1 in Rats Fed Normal and High-Fat Diets. Arch. Physiol. Biochem. 2020:1–7. doi: 10.1080/13813455.2020.1821064. [DOI] [PubMed] [Google Scholar]
- 45.Rubio J., Qiong W., Liu X., Jiang Z., Dang H., Chen S.-L., Gonzales G.F. Aqueous Extract of Black Maca (Lepidium meyenii) on Memory Impairment Induced by Ovariectomy in Mice. Evid. Based Complementary Altern. Med. 2011;2011:enen063. doi: 10.1093/ecam/nen063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fei W., Hou Y., Yue N., Zhou X., Wang Y., Wang L., Li A., Zhang J. The Effects of Aqueous Extract of Maca on Energy Metabolism and Immunoregulation. Eur. J. Med. Res. 2020;25:24. doi: 10.1186/s40001-020-00420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oishi Y., Tsukamoto H., Yokokawa T., Hirotsu K., Shimazu M., Uchida K., Tomi H., Higashida K., Iwanaka N., Hashimoto T. Mixed Lactate and Caffeine Compound Increases Satellite Cell Activity and Anabolic Signals for Muscle Hypertrophy. J. Appl. Physiol. 2015;118:742–749. doi: 10.1152/japplphysiol.00054.2014. [DOI] [PubMed] [Google Scholar]
- 48.Horsley V., Friday B.B., Matteson S., Kegley K.M., Gephart J., Pavlath G.K. Regulation of the Growth of Multinucleated Muscle Cells by an Nfatc2-Dependent Pathway. J. Cell. Biol. 2001;153:329–338. doi: 10.1083/jcb.153.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohno Y., Oyama A., Kaneko H., Egawa T., Yokoyama S., Sugiura T., Ohira Y., Yoshioka T., Goto K. Lactate Increases Myotube Diameter via Activation of MEK/ERK Pathway in C2C12 Cells. Acta Physiol. 2018;223:e13042. doi: 10.1111/apha.13042. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto T., Okada Y., Yamanaka A., Ono N., Uryu K., Maru I. The Effect of Eleutherococcus Senticosus on Metabolism-Associated Protein Expression in 3T3-L1 and C2C12 Cells. Phys. Act. Nutr. 2020;24:13–18. doi: 10.20463/pan.2020.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.