Abstract
Species in the genus Mucor have a worldwide distribution and are isolated from various substrata and hosts, including soil, dung, freshwater, and fruits. However, their diversity from insects is still much too little explored. The aim of this study was to characterize three new species of Mucor: Mucor grylli sp. nov., M. hyangburmii sp. nov., and M. kunryangriensis sp. nov., discovered in Kunryang-ri, Cheongyang in the Chungnam Province of Korea, during an investigation of Mucorales from cricket insects. The new species are described using morphological characters and molecular data including ITS and LSU rDNA regions. Mucor grylli is characterized by the highly variable shape of its columellae, which are subglobose to oblong, obovoid, strawberry-shaped, and sometimes slightly or strongly constricted in the center. Mucor hyangburmii is characterized by the production of azygospores and growth at 40 °C. Mucor kunryangriensis is characterized by the variable shape of its columellae, which are elongated-conical, obovoid, cylindrical ellipsoid, cylindrical, and production of abundant yeast-like cells on PDA, MEA, and SMA media. Based on the sequence analysis of two genetic markers, our phylogenic assessment strongly supported M. grylli, M. hyangburmii, and M. kunryangriensis as new species. Detailed descriptions, illustrations, and phylogenetic trees are provided.
Keywords: cricket insect, ITS, LSU, Mucorales, phylogeny, taxonomy
1. Introduction
The genus Mucor was described by Fresenius [1], and it is classified in the family Mucoraceae, order Mucorales, and phylum Mucoromycota, which belongs to the early diverging fungi [2]. With more than 90 currently accepted species [3,4,5,6,7,8], Mucor is the largest genus within the Mucorales. Species of Mucor are known to be saprotrophs that are usually isolated from dung, soil, freshwater, insects, or fruits [6,9,10,11,12,13,14]. Some species are human pathogens causing mucormycosis [15], and to date, 12 species are known to be involved in infections [3,16]. Mucor species have important industrial applications because of their ability to produce a wide range of metabolites [17,18]. Some Mucor species produce enzymes such as protease, phytase, cellulase, lipase, and uricase [18,19,20,21,22,23]. Moreover, some species are used to manufacture Asian fermented food products and beverages [24,25]. The study published by Walther et al. [10], which included more than 300 Mucor strains, placed species of Mucor in different groups. Those groups were intermingled in the LSU phylogenetic tree with species of other genera. The results were that species of Mucor divide into six groups, consisting of the M. mucedo group, the M. flavus group, the M. hiemalis group, the M. racemosus group, the M. amphibiorum group, and the M. recurvus group [10]. The M. amphibiorum group contains two species that are potentially involved in human infections: M. amphibiorum and M. ardhlaengiktus [3]. In recent years, the number of new species of Mucor has increased. However, there were only four species recorded in the M. amphibiorum group from 2015 to February 2022, including M. caatinguensis A.L. Santiago, C.A.F. de Souza & D.X. Lima [26], M. fluvii Hyang B. Lee, S.H. Lee & T.T.T. Nguyen [11], M. pernambucoensis C.L. Lima, D.X. Lima & A.L. Santiago [27], and M. chiangraiensis V.G. Hurdeal, E. Gentekaki, K.D. Hyde & H.B. Lee [5]. Most of those species were isolated from soil [5,26,27], except for M. fluvii, which was isolated from freshwater [11].
The purpose of this study was to expand the present knowledge of the fungal diversity found in poorly studied substrates or unexplored areas. Herein, we describe and illustrate three new species of Mucor isolated from insects in Korea.
2. Materials and Methods
2.1. Sampling and Isolation
Cricket insect (Gryllus sp.) samples were collected from Kunryang-ri, Cheongyang, Chungnam Province, Korea, between April 2020 and October 2021. The insects were collected in polyethylene bags, stored at ambient temperature, and transported to the laboratory. Fungal isolation from the insect samples was conducted following our previous methods [28]. Briefly, the samples were transferred to clean Petri dishes. The insect bodies were then broken up into small pieces and placed on PDA. The plates were then incubated at 25 °C for 2–5 days. Then, hyphal tips were transferred to fresh PDA. All isolates were purified by single spore isolation as previously described [28].
Ex-type living cultures were deposited at Environmental Microbiology Laboratory Fungarium, Chonnam National University (CNUFC), Gwangju, Korea and the Culture Collection of National Institute of Biological Resources (NIBR), Incheon, Korea. Dried cultures were deposited in the Herbarium Chonnam National University, Gwangju, Korea.
2.2. Morphological Studies
Pure cultures of Mucor spp. were cultured on potato dextrose agar (PDA), malt extract agar (MEA: 40 g malt extract, 4 g yeast extract, and 15 g agar in 1 L deionized water), and synthetic mucor agar (SMA: 40 g dextrose, 2 g asparagine, 0.5 g KH2PO4, 0.25 g MgSO4·7H2O, 0.5 mg thiamine hydrochloride, and 15 g agar in 1 L deionized water) [4,9]. The plates were incubated at 25, 30, 37, 39, 40, and 44 °C in the dark for 7 days. Fragments of mycelia were removed from the cultures and placed onto microscopy slides with 60% lactic acid. An Olympus BX53 microscope (Olympus, Tokyo, Japan) possessing differential interference contrast optics was used to obtain digital images.
2.3. DNA Extraction, PCR, Cloning, and Sequencing
Total genomic DNA was extracted from fresh fungal mycelia that were grown on cellophane at 25 °C after 4 days using the SolgTM Genomic DNA Preparation Kit (Solgent Co. Ltd., Daejeon, Korea) according to the manufacture’s protocol, and then stored at −20 °C. Two regions were amplified, including the internal transcribed spacer (ITS) region using primers ITS1 and ITS4 [29], and the large subunit rDNA region using primers LR0R and LR5 [30]. The PCR products were purified with the Accuprep PCR Purification Kit (Bioneer Corp., Daejeon, Korea) and sequenced at Macrogen (Daejeon, South Korea). Direct sequencing of the ITS PCR product failed; thus, we performed the cloning. PCR products after gel purification were ligated into the pGEM-T Easy Vector (Promega, Madison, WI, USA), following the manufacturer’s instructions. The ligation mixture was transformed into Escherichia coli DH5α by heat shock. The positive white colonies were grown in Luria broth (LB) media containing 100 μg of ampicillin per milliliter. The plasmids were purified using the Plasmid Purification Mini Kit (Nucleogen, Si-heung, South Korea). Then, purified plasmids of three clones were sequenced using the primers M13F forward (5′-GTAAAACGACGGCCAGT-3′) and M13R reverse (5′-GCGGATAACAATTTCACACAGG-3′).
2.4. Phylogenetic Analyses
DNA sequences were checked and were assembled by Seqman Pro 7.1.0 in Lasergene package (DNASTAR, Madison, WI, USA). All newly generated sequences were submitted to GenBank database under the accession numbers provided in Table 1.
Table 1.
Taxa, collection numbers, and GenBank accession numbers used in this study.
| Taxon Name | Strain Number | GenBank Accession Number | Host/Substrate | Country | Citation | |
|---|---|---|---|---|---|---|
| ITS | LSU | |||||
| Blakeslea trispora | CBS 564.91 | - | JN206515 | soil | China | [10] |
| Choanephora cucurbitarum | CBS 674.93 | - | JN206514 | n.a | China | [10] |
| C. infundibulifera | CBS 153.51 | - | JN206513 | n.a | n.a | [10] |
| Ellisomyces anomalus | CBS 243.57 (T) | JN205992 | JN206423 | dung of lizard | USA | [10] |
| Gilbertella persicaria | CBS 532.77 | - | JN206517 | dung of mouse | India | [10] |
| G. persicaria | CBS 190.32 | - | HM849691 | Prunus persica; fruit | USA | [10] |
| Hyphomucor assamensis | CBS 254.85 | JN206212 | JN206440 | Burmannia | Malaysia | [10] |
| H. assamensis | CBS 415.77 (T) | JN206211 | JN206439 | n.a | India | [10] |
| Mucor amphibiorum | CBS 763.74 (T) | HM999957 | HM849688 | amphibian | Germany | [10] |
| M. amphibiorum | CBS 185.77 | JN206170 | - | diseased Dendrobates sp. | Central America | [10] |
| M. azygosporus | CBS 292.63 (T) | JN206187 | JN206497 | soil | USA | [10] |
| M. ardhlaengiktus | CBS 210.80 (ET) | JN206172 | JN206504 | garden soil | India | [10] |
| M. ardhlaengiktus | CBS 650.78 | JN206174 | JN206499 | dung of lizard | India | [10] |
| M. caatinguensis | URM 7223 |
KT960375 KT960376 KT960377 |
KT960369 KT960370 KT960371 |
soil | Brazil | [26] |
| M. chiangraiensis | MFLUCC 21-0079 (T) | MZ433253 | MZ433250 | soil | Thailand | [5] |
| M. exponens | CBS 141.20 (NT) | JN206206 | JN206441 | n.a | Germany | [10] |
| M. falcatus | CBS 251.35 (HT) | JN206250 | JN206509 | honeycomb | Germany | [10] |
| M. falcatus | CBS 252.35 | JN206249 | - | dung of rabbit | Germany | [10] |
| M. fluvii | CNUFC-MSW21-2 | MF667991 | MF667996 | freshwater | Korea | [11] |
| M. fluvii | CNUFC-MSW21-1 (T) | MF667992 | MF667995 | freshwater | Korea | [11] |
| M. fuscus | CBS 282.78 | JN206201 | JN206442 | cheese | France | [10] |
| M. grylli | CNUFC CY102 (T) |
OM868230 (c1)
OM868231 (c2) |
OM843127 | Gryllus sp. | Korea | This study |
| M. grylli | CNUFC CY103 | - | OM843128 | Gryllus sp. | Korea | This study |
| M. hyangburmii | CNUFC CY22 (T) |
OM868232 (c1) OM868233 (c3) |
OM843129 | Gryllus sp. | Korea | This study |
| M. hyangburmii | CNUFC CY23 | - | OM843130 | Gryllus sp. | Korea | This study |
| M. inaequisporus | CBS 255.36 (T) | JN206177 | JN206502 | Spondias mombin; fruit | Ghana | [10] |
| M. inaequisporus | CBS 496.66 | JN206179 | JN206501 | Diospyros kaki; immature fruit | Japan | [10] |
| M. inaequisporus | CBS 351.50 | JN206178 | JN206500 | Musa sapientum; fruit | Indonesia | [10] |
| M. indicus | CBS 226.29 (ET) | HM999956 | HM849690 | n.a | Switzerland | [10] |
| M. indicus | CBS 671.79 | JN206183 | - | n.a | Indonesia | [10] |
| M. indicus | CBS 120585 | JN206180 | - | human; muscle | India | [10] |
| M. kunryangriensis | CNUFC CY223 (T) |
OM868234 (c1)
OM868235 (c2) OM868236 (c3) |
OM843131 | Gryllus sp. | Korea | This study |
| M. kunryangriensis | CNUFC CY224 | - | OM843132 | Gryllus sp. | Korea | This study |
| M. lanceolatus | CBS 638.74 | JN206205 | JN206443 | cheese | France | [10] |
| M. laxorrhizus | CBS 143.85 (NT) | JN206209 | JN206444 | lake mud | UK | [10] |
| M. nederlandicus | CBS 735.70 | JN206176 | JN206503 | n.a | n.a | [10] |
| M. nederlandicus | MFLU 21-0078 | MZ433254 | MZ433251 | soil | Thailand | [5] |
| M. pernambucoensis | URM 7640 (T) | MH155323 | MH155322 | soil | Brazil | [27] |
| M. prayagensis | CBS 652.78 |
JN206189 (c1) JN206190 (c3) |
JN206498 | dung of shrew | India | [10] |
| M. prayagensis | CBS 816.70 (T) | JN206188 | JN206496 | n.a | India | [10] |
| M. odoratus | CBS 130.41 (T) | JN206197 | JN206495 | laboratory air | Denmark | [10] |
| M. odoratus | CBS 201.71 | JN206198 | - | dung of horse | Netherlands | [10] |
| M. odoratus | CBS 120.71 | JN206195 | - | n.a | USA | [10] |
| M. ucrainicus | CBS 674.88 | JN206192 | JN206507 | soil of litter layer | Germany | [10] |
| M. ucrainicus | CBS 221.71 (T) | JN206191 | - | dung of mouse | Ukraine | [10] |
| M. zychae | CBS 416.67 (T) | JN206199 | JN206505 | manured soil | India | [10] |
| M. variisporus | CBS 837.70 (T) | JN206175 | JN206508 | n.a | India | [10] |
| Mycotypha microspora | CBS 230.32 (T) | - | JN206510 | Citrus aurantium; peel, contaminant | Netherlands | [10] |
| Mycotypha sp. | CBS 109960 | - | JN206511 | human; pus of wound | Thailand | [10] |
| Poitrasia circinans | CBS 153.58 (T) | JN206516 | soil | Trinidad and Tobago | [10] | |
Isolates and accession numbers determined in the current study are indicated in bold. CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CNUFC: Chonnam National University Fungal Collection, Gwangju, Korea; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; URM: Micoteca URM, Universidade Federal de Pernambuco, Recife, Brazil. Type, ex-neotype, ex-holotype, and ex-epitype strains are denoted by T, NT, HT, and ET, respectively.
Sequence data of closely related Mucor spp. were selected from data previously published by Walther et al. [10], Li et al. [26], Wanasinghe et al. [11], Lima et al. [27], and Hurdeal et al. [5], and downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 10 March 2022) [31] for molecular phylogenetic analyses (Table 1). Sequences of datasets ITS (44 taxa) and LSU (45 taxa) were aligned using MAFFT (http://mafft.cbrc.jp/alignment/server (accessed on 12 March 2022) with the algorithm L-INS-I [32], and the alignment was checked in MEGA7 [33]. Aligned sequences were automatically trimmed using trimAl with the gappyout method [34]. Data were converted from fasta format to nexus and phylip formats using the online tool Alignment Transformation Environment (https://sing.ei.uvigo.es/ALTER/ (accessed on 12 March 2022). Phylogenetic reconstructions by maximum likelihood (ML) and Bayesian inference (BI) were carried out using PhyML 3.0 [35], and MrBayes 3.2.2 [36], respectively. The most appropriate model was obtained using the software jModelTest v.2.1.10 [37,38]. We performed the ML analysis using 1000 bootstrap replicates under the best substitution model for the ITS (TPM2uf+I+G) and LSU (TIM3+I+G). BI analyses were performed using 5 million Markov chain Monte Carlo (MCMC) generations and the best substitution model HKY+G and HKY+I+G for ITS and LSU, respectively. The sample frequency was set to 100, the first 25% of trees were removed as burn-in, and the remaining trees were used to calculate the posterior probabilities. The resulting trees were viewed using FigTree v.1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 12 March 2022). The alignment files generated for phylogenetic analyses are provided in the Supplemental Materials Files S1 and S2.
3. Results
3.1. Molecular Phylogenetic Analysis
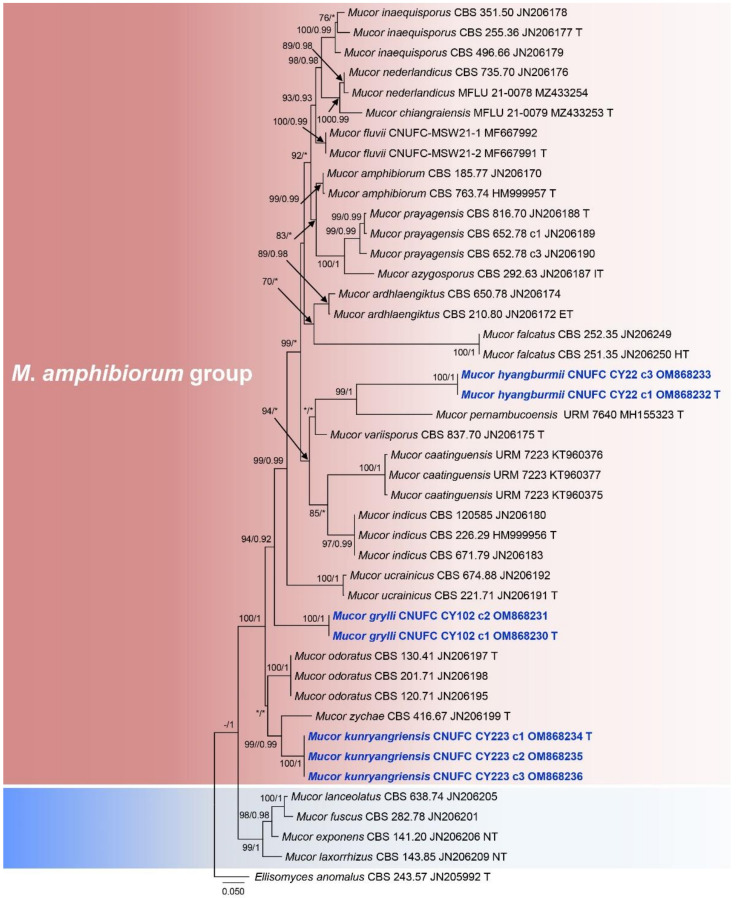
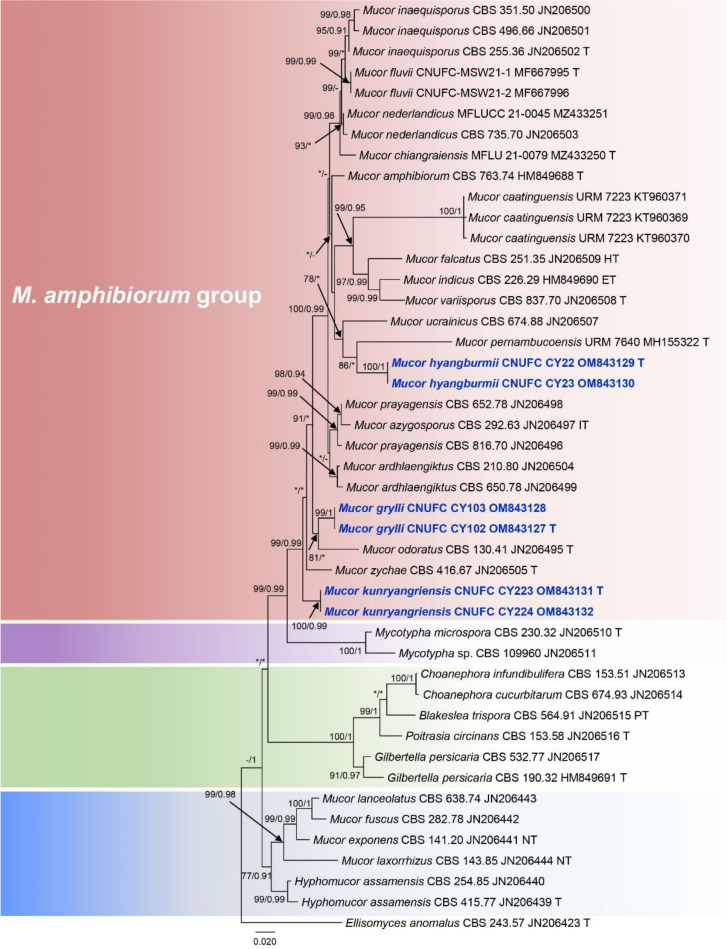
To understand the evolutionary relationship between isolated strains, sequences of ITS and LSU were used for the phylogenetic analysis (Figure 1 and Figure 2). The ITS and LSU phylogenetic analyses revealed that isolate CNUFC CY22 grouped with M. pernambucoensis, having strong statistical support in the ITS tree (ML/BI = 99/1) (Figure 1). However, this relationship was supported with a moderate bootstrap percentage (86%), but a low posterior probability value (<0.90) in the LSU tree (Figure 2). Isolate CNUFC CY223 was closely related to M. zachae in the ITS tree with high statistical support (ML/BI = 99/0.99) (Figure 1) but formed an independent clade with high statistical support (ML/BI = 99/0.99) in the LSU tree (Figure 2). In the ITS analysis (Figure 1), CNUFC CY102 was sister clade to M. ucrainicus with high statistical support (ML/BI = 94/0.92) and clustered with M. odoratus in our LSU (Figure 2) with a moderate posterior probability value (0.81) and a low bootstrap percentage (< 70%).
Figure 1.
Phylogenetic tree constructed by maximum likelihood analysis of the ITS. The numbers above branches represent maximum likelihood bootstrap percentages (left) and Bayesian posterior probabilities (right). Bootstrap values ≥70% and Bayesian posterior probabilities ≥0.90 are shown. Bootstrap values lower than 0.90 and 70% are marked with “*”, and absent bootstrap values are marked with “-”. The bar indicates the number of substitutions per position. Ellisomyces anomalus CBS 243.57 was used as outgroup. Type, ex-neotype, ex-holotype, and ex-epitype strains are marked with T, NT, HT, and ET, respectively. Newly generated sequences are in blue bold font.
Figure 2.
Phylogenetic tree constructed by maximum likelihood analysis of the LSU. The numbers above branches represent maximum likelihood bootstrap percentages (left) and Bayesian posterior probabilities (right). Bootstrap values ≥70% and Bayesian posterior probabilities ≥0.90 are shown. Bootstrap values lower than 0.90 and 70% are marked with “*”, and absent bootstrap values are marked with “-”. The bar indicates the number of substitutions per position. Ellisomyces anomalus CBS 243.57 was used as outgroup. Type, ex-neotype, ex-holotype, and ex-epitype strains are marked with T, NT, HT, and ET, respectively. Newly generated sequences are in blue bold font.
3.2. Taxonomy
Based on our phylogenies and morphological data, three new species of Mucor from cricket insects in Korea were described and illustrated here.
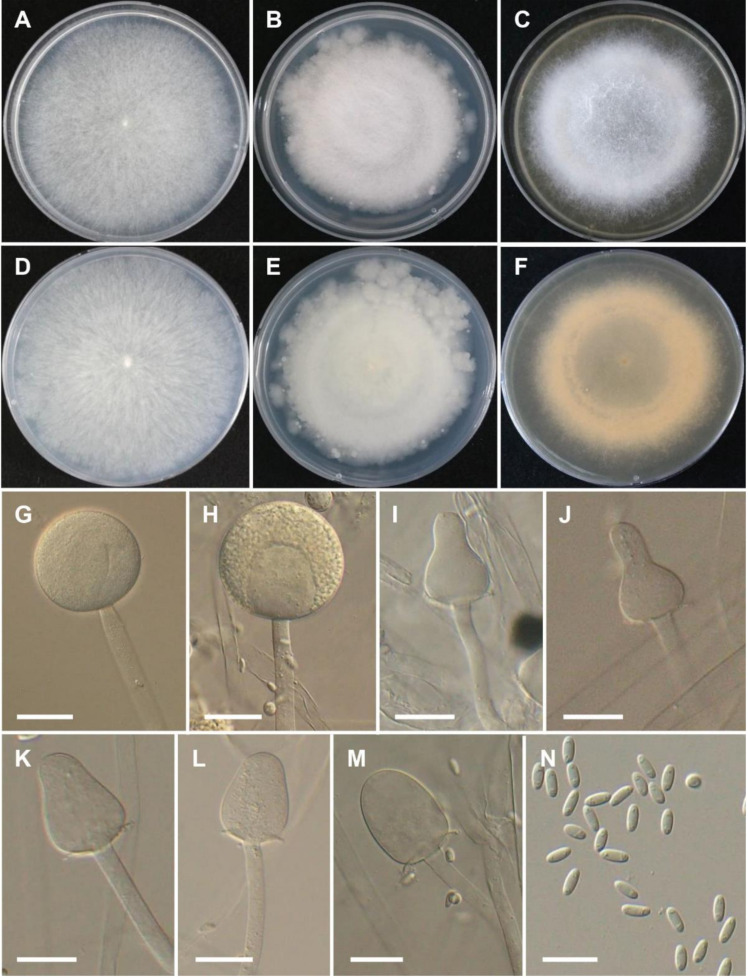
Mucor grylli Hyang B. Lee & T.T.T. Nguyen sp. nov. (Figure 3).
Figure 3.
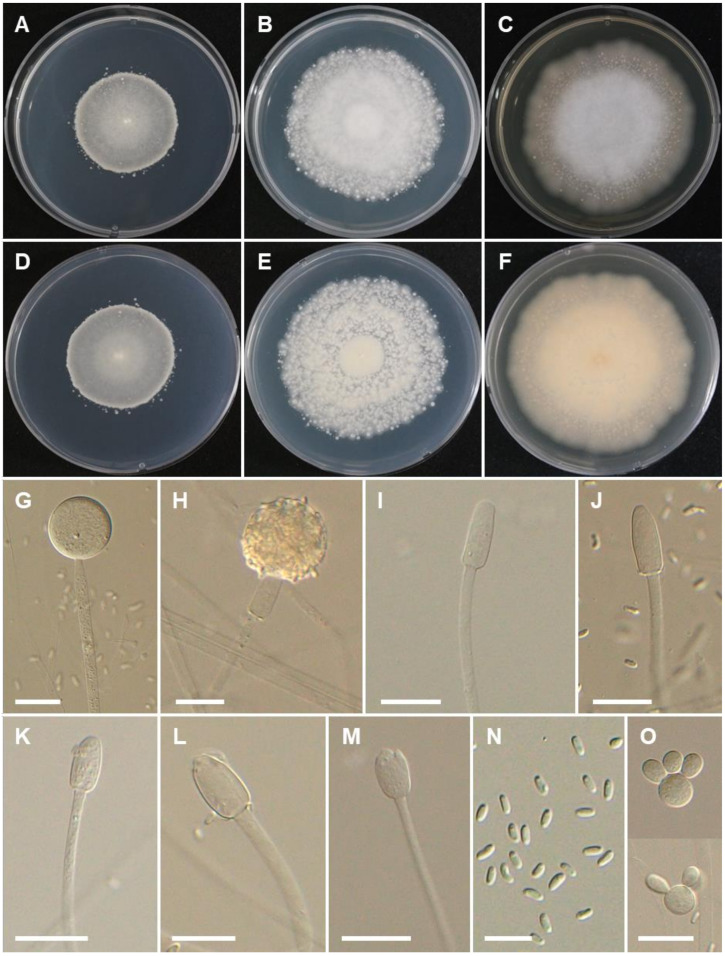
Morphology of Mucor grylli. (A,D) Colony on synthetic agar mucor (SMA). (B,E) Colony on potato dextrose agar (PDA). (C,F) Colony on malt extract agar (MEA). (G,H) Young and mature sporangia. (I–M) Typical columellae. (N) Sporangiospores. Scale bars = 20 µm.
Index Fungorum: 555247.
Etymology: Referring to the host, Gryllus sp., from which the species was first isolated.
Type: REPUBLIC OF KOREA: Kunryang-ri (36°26′16.2” N 126°46′04.6” E), Cheongyang-eup, Cheongyang, Chungnam Province, from Gryllus sp., 20th June 2021, J.S. Kim; holotype CNUFC HT2102; ex-type living culture, CNUFC CY102).
Description: Colonies on MEA at 25 °C at first white, becoming light gray, reaching 70–73 mm in diameter after 4 days of incubation; reverse uncolored. Sporangiophores erect, 6–14.5 µm diam., slightly sympodially branched with long branches. Sporangia globose, wall echinulate, (28–) 42–73.5 µm diam., slightly yellow, deliquescent in mature sporangia, and persistent in young sporangia. Columellae highly variable in shape, oblong (57.5–42.0 × 40–31.5 µm), subglobose, obovoid, or strawberry-shaped (25–44 × 18.5–35.5 µm), sometimes slightly or strongly constricted in the center (27.9–42.5 × 18.5–26.5 µm), with distinct collar. Sporangiospores mostly ellipsoid, 8.0–10.5 × 3–4 µm, usually with granules at each end. Chlamydospores formed on hyphae, terminal and intercalary, single, smooth and thick-walled, ellipsoidal. Zygospores not observed. On SMA and PDA, sporangia are larger (SMA: 42.5–105 µm diam.; PDA: 55.5–112 µm diam.) than on MEA. Columellae on SMA (up to 75 × 65.5 µm) are larger than PDA and MEA. Sporangiospores on SMA, MEA, and PDA are similar.
Culture characteristics: On PDA, the colonies attain a diameter of 61–63 mm after 4 days at 25 °C. On SMA, the colonies attain a diameter of 66–69 mm after 4 days at 25 °C. At 37 °C on SMA, PDA, and MEA, growth is observed but sporulation is lacking. The colony reaches a diameter of 19, 22, and 18 mm at 37 °C after 4 days on PDA, SMA, and MEA, respectively. No growth was observed at 39 °C.
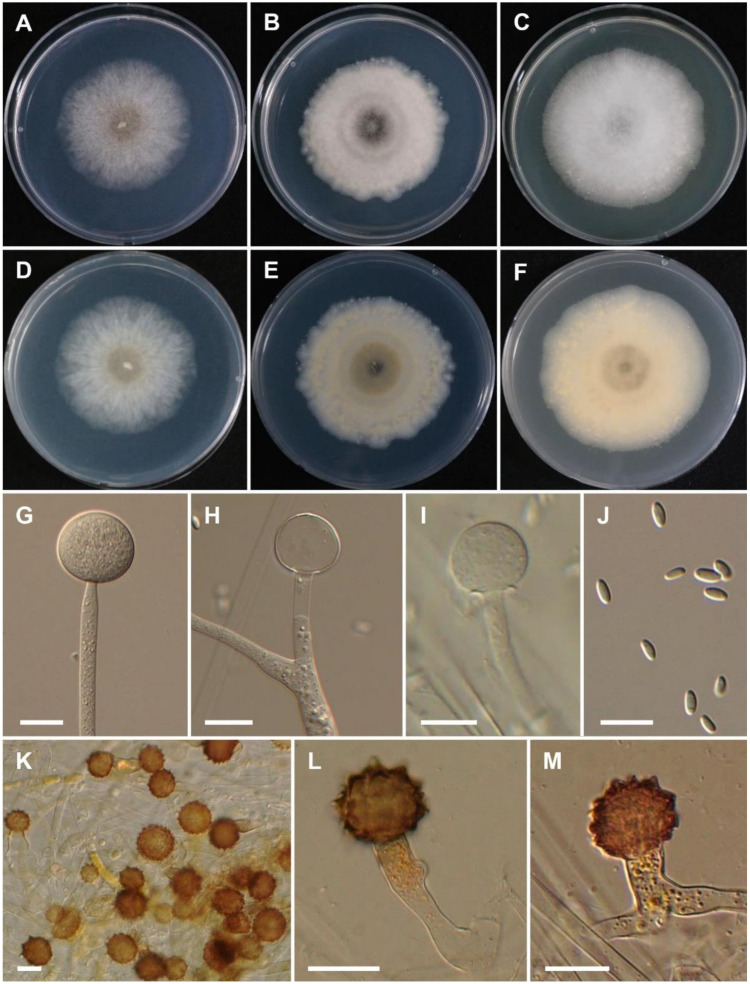
Mucor hyangburmii T.T.T Nguyen sp. nov. (Figure 4).
Figure 4.
Morphology of Mucor hyangburmii. (A,D) Colony on synthetic agar mucor (SMA). (B,E) Colony on potato dextrose agar (PDA). (C,F) Colony on malt extract agar (MEA). (G) Young sporangium. (H) Sterile sporangium. (I) Columella. (J) Sporangiospores. (K–M) Azygospores on MEA. Scale bars = 20 µm.
Index Fungorum: 555248.
Etymology: In honor of Dr. Hyang Burm Lee, a Korean mycologist who has studied basal fungal lineages in Korea and supervised the first author’s Ph.D. work.
Type: REPUBLIC OF KOREA: Kunryang-ri (36°26′16.2′′ N 126°46′04.6′′ E), Cheongyang-eup, Cheongyang, Chungnam Province, from the surface of leg of Gryllus sp., 28 July 2020, J.S. Kim; holotype CNUFC HT2021; ex-type living culture, CNUFC CY22.
Description: Colonies on MEA at 25 °C at first white, soon becoming pale, the central part with abundant azygospores, reaching 65–70 mm in diameter after 4 days of incubation; reverse moderate yellow. Sporangiophores erect, rare branched, 5–9 µm diam. The branches commonly bear a sterile sporangium that may form a new sporangium. Sporangia globose, yellow, (29–) 35.5–58.5 (–62) µm diam., rapidly deliquescent. Columellae globose, subglobose, (17–) 23.5–38.5 × (16–) 21.5–36 µm; collar present. Sporangiospores smooth, mostly ellipsoidal, (5.5–) 6.0–9.5 (–10.5) × (2.5–) 3.0–4.0 (–4.5) µm, sometime flattened at one side. Chlamydospores present in sporangiophores. Azygospores abundant, formed terminally on simple or branched azygophores, deep reddish brown subglobose, 19–45 µm diam. Zygospores were not observed. Colonies on SMA gray, chestnut in central part with abundant azygospores. Sporangiospores on SMA slightly smaller (5–8.5 × 2.5–4.0 µm) than on PDA and MEA. Chlamydospores are abundant and less sporangia on SMA media, but azygospores abundant and formed earlier than PDA and MEA. On PDA, sporangia slightly smaller (up to 56 µm diam.) than on MEA.
Culture characteristics: On PDA, the colonies attain a diameter of 55–57 mm after 4 days at 25 °C. On SMA, the colonies attain a diameter of 50–52 mm after 4 days at 25 °C. At 37 °C, colony reaches a diameter of 60, 67, and 61 mm after 4 days on PDA, SMA, and MEA, respectively. At 40 °C in SMA, PDA, and MEA, growth is observed but with no sporulation. The colony reaches a diameter of 31, 30, and 16 mm at 40 °C after 4 days on PDA, SMA, and MEA, respectively. No growth was observed at 44 °C.
Mucor kunryangriensis Hyang B. Lee & T.T.T. Nguyen sp. nov. (Figure 5).
Figure 5.
Morphology of Mucor kunryangriensis. (A,D) Colony on synthetic agar mucor (SMA). (B,E) Colony on potato dextrose agar (PDA). (C,F) Colony on malt extract agar (MEA). (G,H) Young and mature sporangia. (I–M) Typical columellae. (N) Sporangiospores. (O) Yeast-like cells. Scale bars = 20 µm.
Index Fungorum: 555249.
Etymology: Referring to the isolation location, Kunryang-ri from where the species was first isolated (Korea).
Type: REPUBLIC OF KOREA: Kunryang-ri (36°26′16.2′′ N 126°46′04.6′′ E), Cheongyang-eup, Cheongyang, Chungnam Province, from Gryllus sp., 9th August 2021, H.B. Lee and J.S. Kim; holotype CNUFC HT2105; ex-type living culture, CNUFC CY223).
Description: Colonies on MEA at 25 °C white to grayish white, reaching 62–65 mm in diameter after 4 days of incubation; reverse light yellowish brown. Sporangiophores erect, unbranched or once branched, 4–7 (–10) µm diam. Sporangia yellow to light brown, globose, (20.5–) 24–45.5 (–47.5) µm diam. Columellae elongated-conical, obovoid, cylindrical ellipsoid, cylindrical, (13–) 18–26.5 × (8.5–) 10.5–14.5 μm. Sporangiospores mostly ellipsoid, sometimes flattened at one side, containing granules at each end, 5.0–7.5 × 2–3 μm. Yeast-like cells were abundant on PDA, SMA, and MEA, globose, 12.5–20.5 μm diam. Zygospores not observed. On SMA, sporangia are smaller (17.5–39 µm diam.) than on MEA and PDA [(18–) 23.5–44.5 µm diam.]. Sporangiospores on SMA and MEA are similar, but slightly larger on PDA (5.5–8.0 × 2–3.5 µm).
Culture characteristics: On PDA, the colonies attain a diameter of 56–59 mm after 4 days at 25 °C. On SMA, the colonies attain a diameter of 29–32 mm after 4 days at 25 °C. At 37 °C on SMA, PDA, and MEA, growth is observed but with no sporulation. The colony reaches a diameter of 12, 13, and 21 mm at 37 °C after 4 days on PDA, SMA, and MEA, respectively. No growth was observed at 40 °C.
4. Discussion
This study reports on new Mucor species isolated from Gryllus insects collected from Kunryang-ri, Cheongyang, located in Chungnam Province, Korea. Three new Mucor species belonging to the M. amphibiorum group [10] are described. Members of the M. amphibiorum group are characterized by unbranched tall sporangiophores or sporangia with a maximum diameter of between 70–175 µm [10].
Phylogenetic analyses of ITS and LSU showed that M. grylli, M. ucrainicus, M. zychae, and M. odoratus are phylogenetically close species. Based on Blastn searches, the ITS and LSU sequences of M. grylli were most similar to M. zychae (GenBank NR_103641; 477/558 bp (85.5%), M. variisporus (GenBank NR_152951; 435/512 bp (84.9%), M. zychae (GenBank NR_057930; 652/675 bp (96.6%), and M. odoratus (GenBank NR_057927; 659/690 bp (95.5%), respectively. However, M. grylli differs from these species by production of strawberry-shaped columellae, sometimes slightly or strongly constricted in the center. Wagner et al. [4] reported the presence of strawberry-shaped columellae in M. variicolumellatus, but M. grylli produces larger sporangiospores and includes granules at the end. Moreover, M. grylli can grow at 37 °C, while M. variicolumellatus cannot [4]. Treschew [39] has mentioned that M. odoratus grew slowly at 40 °C, whereas M. grylli was not able to grow at 40 °C. Mucor ucrainicus differs from M. grylli in producing larger sporangia (up to 175 µm diam.) and smaller sporangiospores (4.4–8.1 × 2.7–4.7 µm) [40].
Mucor hyangburmii is phylogenetically related to M. pernambucoensis, M. ucrainicus, and M. variisporus in the ITS and LSU trees (Figure 1 and Figure 2). Based on Blastn search, the ITS and LSU sequences of M. hyangburmii were most similar to M. variisporus (GenBank NR_152951; 334/379 bp (88.1%), M. azygosporus (GenBank NR_103639; 332/378 bp (87.8%), M. ardhlaengiktus (GenBank NR_069778; 661/693 bp (95.4%), and M. azygosporus (GenBank NR_057928; 661/693 bp (95.4%), respectively. However, the production of azygospores can easily distinguish M. hyangburmii from these species, except for M. azygosporus. Unlike M. hyangburmii, colony color on SMA of M. azygosporus is orange to buff orange [41]. In addition, M. pernambucoensis, M. ucrainicus, and M. variisporus displayed limited growth at 35, 30, and 37 °C [27,40,42], respectively, but M. hyangburmii was able to grow even at 40 °C. Sporangiospores of M. variisporus are larger and variable in shape and size (5.5–13.5 × 3.5–8 μm) [42] than those of M. hyangburmii, which are mostly ellipsoidal [(5.5–) 6.0–9.5(–10.5) × (2.5–) 3.0–4.0 (–4.5) µm]. Mucor hyangburmii shared some similarities with M. pernambucoensis, such as ellipsoidal sporangiospores [27]. However, sporangiospores of M. pernambucoensis reported by Lima et al. [27] were slightly larger (4.5–12 (–14.5) × 2.5–5 µm) than those of the isolate obtained in this study. In addition, columellae of M. pernambucoensis are globose, obovoid, cylindrical, and pyriform, differing from those of M. hyangburmii that are globose and subglobose.
The phylogenetic analysis of ITS and LSU show that M. kunryangriensis is phylogenetically related to M. zychae, M. odoratus, and Mycotypha microspora (Figure 1 and Figure 2). Based on Blastn search, the ITS and LSU sequences of M. kunryangriensis were most similar to M. zychae (GenBank NR_103641; 485/539 bp (89.9%), M. odoratus (GenBank NR_145287; 520/590 bp (88.1%), M. zychae (GenBank NR_057930; 651/671 bp (97.1%), and M. ardhlaengiktus (GenBank NR_069778; 660/686 bp (96.2%), respectively. However, the new species can be easily distinguished from these species by the production of abundant yeast-like cells and columellae that are elongated-conical or cylindrical. In addition, sporangia and sporangiospores of M. kunryangriensis are smaller than those of M. odoratus [sporangia: up to 100 µm diam.; sporangiospores: (7.8–) 9.5–17.5 (–21.6) × (3.7–) 4–9.8 (–13.5) µm] [39]. Sporangia of M. zychae are larger (up to 70 µm diam.) [43] than those of M. kunryangriensis. As observed in M. kunryangriensis, M. guilliermondii, M. chuxiongensis, and M. gigasporus also produce elongated-conical and cylindrical columellae. However, M. chuxiongensis differs from M. kunryangriensis by the production of smaller columellae (12–15 × 5.0–8.5 µm) and sporangiospores (4.5–6.5 × 2.0–2.5 µm) [44]. Mucor guilliermondii and M. gigasporus differ from M. kunryangriensis by producing larger sporangia (up to 60 µm diam. for M. guilliermondii and (35.6–) 45.7–76.2 (–88.9) µm diam. for M. gigasporus) [45,46].
It is also noteworthy that M. grylli, M. hyangburmii, and M. kunryangriensis are the first species in the M. amphibiorum group collected on insects. Interestingly, the three new species can grow optimally at near-human-body temperature, which needs attention as a potential cause of diseases.
Mucor species are dimorphic fungi and exhibit either hyphal or yeast growth depending upon the conditions such as cultivation time, temperature, presence or absence of oxygen, and carbon and nitrogen sources [47,48]. Several studies reveal that M. indicus in different morphologies (filamentous and yeast-like forms) can produce ethanol with relatively high yields and productivity [49,50]. Interestingly, M. kunryangriensis also produces a yeast-like form, necessitating further studies.
Three new species described here were recovered from samples collected at Kunryang-ri, Cheongyang, located in Chungnam Province, Korea, an area recognized as a biodiversity hotspot and known as the “Alps of Chungnam” with significant mucoralean species richness [13,28]. With further investigations, we expect to discover additional unreported species in this genus. New species could be a source of novel drugs and other useful compounds.
Acknowledgments
The authors are grateful to Jeong Suk Kim, Lee’s mother, for kindly providing precious insect samples, and to Paul M. Kirk for kindly reviewing the manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8060601/s1, File S1: Sequence alignments of ITS; File S2: Sequence alignments of LSU.
Author Contributions
Conceptualization, H.B.L. and T.T.T.N.; methodology, T.T.T.N. and H.B.L.; software, T.T.T.N.; formal analysis, H.B.L. and T.T.T.N.; resources, H.B.L.; writing—original draft preparation, T.T.T.N.; writing—review and editing, T.T.T.N. and H.B.L.; supervision, H.B.L.; funding acquisition, H.B.L.; project administration, H.B.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequencing data were submitted to GenBank.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partly supported by the Graduate Program (NIBR202130202) for the Undiscovered Taxa of Korea and the Project (NIBR202102201) on Survey and Discovery of Indigenous Fungal Species of Korea funded by National Institute of Biological Resources (NIBR) of the Ministry of Environment (MOE), Korea.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fresenius G. Beiträge zur Mykologie. Bei Heinrich Ludwig Brönner; Frankfurt, Germany: 1850. pp. 1–38. [Google Scholar]
- 2.Voigt V., James T.Y., Kirk P.M., Santiago A.L.C.M., Waldman B., Griffith G.W., Fu M., Radek R., Strassert J.F.H., Wurzbacher C., et al. Early-diverging fungal phyla: Taxonomy, species concept, ecology, distribution, anthropogenic impact, and novel phylogenetic proposals. Fungal Divers. 2021;109:59–98. doi: 10.1007/s13225-021-00480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walther G., Wagner L., Kurzai O. Updates on the taxonomy of Mucorales with an emphasis on clinically important taxa. J. Fungi. 2019;5:106. doi: 10.3390/jof5040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner L., Stielow J.B., de Hoog G.S., Bensch K., Schwartze V.U., Voigt K., Alastruey-Izquierdo A., Kurzai O., Walther G. A new species concept for the clinically relevant Mucor circinelloides complex. Persoonia. 2020;44:67–97. doi: 10.3767/persoonia.2020.44.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurdeal V.G., Gentekaki E., Hyde K.D., Nguyen T.T.T., Lee H.B. Novel Mucor species (Mucoromycetes, Mucoraceae) from northern Thailand. Mycokeys. 2021;84:57–78. doi: 10.3897/mycokeys.84.71530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boonmee S., Wanasinghe D.N., Calabon M.S., Huanraluek N., Chandrasiri S.K., Jones G.E., Rossi W., Leonardi M., Singh S.K., Rana S., et al. Fungal diversity notes 1387–1511: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2021;111:1–335. doi: 10.1007/s13225-021-00489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai F., Yao S., Cai C., Zhang T., Wang Y., Liu W., Ma B., Rong C., Cheng C. Mucor rongii sp. nov., a new cold-tolerant species from China. Curr. Microbiol. 2021;78:2464–2469. doi: 10.1007/s00284-021-02494-w. [DOI] [PubMed] [Google Scholar]
- 8.Wijayawardene N.N., Hyde K.D., Al-Ani L.K.T., Dai D.Q., Sánchez-García M. Outline of fungi and fungus-like taxa—2021. Mycosphere. 2022;13:53–453. doi: 10.5943/mycosphere/13/1/2. [DOI] [Google Scholar]
- 9.Benny G.L. The methods used by Dr. R. K. Benjamin, and other mycologists, to isolate Zygomycetes. Aliso. 2008;26:37–61. doi: 10.5642/aliso.20082601.08. [DOI] [Google Scholar]
- 10.Walther G., Pawlowska J., Alastruey-Izquierdo A., Wrzosek M., Rodriguez-Tudela J.L., Dolatabadi S., Chakrabarti A., de Hoog G.S. DNA barcoding in Mucorales: An inventory of biodiversity. Persoonia. 2013;30:11–47. doi: 10.3767/003158513X665070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wanasinghe D.N., Phukhamsakda C., Hyde K.D., Jeewon R., Lee H.B., Jones E.G., Tibpromma S., Tennakoon D.S., Dissanayake A.J., Jayasiri S.C. Fungal diversity notes 709–839: Taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018;89:1–236. doi: 10.1007/s13225-018-0395-7. [DOI] [Google Scholar]
- 12.Nguyen T.T.T., Jeon Y.J., Mun H.Y., Goh J., Chung N., Lee H.B. Isolation and characterization of four unrecorded Mucor species in Korea. Mycobiology. 2020;48:29–36. doi: 10.1080/12298093.2019.1703373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen T.T.T., Lee H.B. Mucor cheongyangensis, a new species isolated from the surface of Lycorma delicatula in Korea. Phytotaxa. 2020;446:33–42. doi: 10.11646/phytotaxa.446.1.4. [DOI] [Google Scholar]
- 14.Lima D.X., Barreto R.W., Lee H.B., Cordeiro T.R.L., de Souza C.A.F., de Oliveira R.J.V., Santiago A.L.C.M. Novel Mucoralean fungus from a repugnant substrate: Mucor merdophylus sp. nov., isolated from dog excrement. Curr. Microbiol. 2020;77:2642–2649. doi: 10.1007/s00284-020-02038-8. [DOI] [PubMed] [Google Scholar]
- 15.Jeong W., Keighley C., Wolfe R., Lee W., Slavin M.A., Kong D.C.M., Chen C.A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Inf. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Wagner L., de Hoog S., Alastruey-Izquierdo A., Voigt K., Kurzai O., Walther G. A revised species concept for opportunistic Mucor species reveals species-specific antifungal susceptibility profiles. Antimicrob. Agents Chemother. 2019;63:e00653-19. doi: 10.1128/AAC.00653-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimi K., Zamani A. Mucor indicus: Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013;31:466–481. doi: 10.1016/j.biotechadv.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Souza P.M., Bittencourt M.L.A., Caprara C.C., Freitas M., Almeida R.P.C., Silveira D., Fonseca Y.M., Filho E.X.F., Junior A.P., Magalhães P.O. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015;46:337–346. doi: 10.1590/S1517-838246220140359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alves M.H., Campos-Takaki G.M.C., Okada K., Pessoa I.H.F., Milanez A.I. Detection of extracellular protease in Mucor species. Rev. Iberoam. Micol. 2005;22:114–117. doi: 10.1016/S1130-1406(05)70020-6. [DOI] [PubMed] [Google Scholar]
- 20.Roopesh K., Ramachandran S., Nampoothiri K.M., Szakacs G., Pandey A. Comparison of phytase production on wheat bran and oilcakes in solid-state fermentation by Mucor racemosus. Bioresour. Technol. 2006;97:506–511. doi: 10.1016/j.biortech.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 21.Voigt K., Wolf T., Ochsenreiter K., Nagy G., Kaerger K., Shelest E., Papp T. 15 Genetic and metabolic aspects of primary and secondary metabolism of the Zygomycetes. In: Hoffmeister D., editor. Biochemistry and Molecular Biology. 3rd ed. Volume III. Springer; Berlin, Germany: 2016. pp. 361–385. [Google Scholar]
- 22.Yazdi M.T., Zarrini G., Mohit E., Faramarzi M.A., Setayesh N., Sedighi N., Mohseni F.A. Mucor hiemalis: A new source for uricase production. World J. Microbiol. Biotechnol. 2006;22:325–330. doi: 10.1007/s11274-005-9030-3. [DOI] [Google Scholar]
- 23.Carvalho A.K.F., Faria E.L.P., Rivaldi J.D., Andrade G.S.S., de Oliveira P.C., de Castro H.F. Performance of whole-cells lipase derived from Mucor circinelloides as a catalyst in the ethanolysis of non-edible vegetable oils under batch and continuous run conditions. Ind. Crops Prod. 2015;67:287–294. doi: 10.1016/j.indcrop.2015.01.035. [DOI] [Google Scholar]
- 24.Batra L.R., Millner P.D. Some Asian fermented foods and beverages. Mycologia. 1974;66:942–950. doi: 10.1080/00275514.1974.12019699. [DOI] [Google Scholar]
- 25.Nout M.J.R., Aidoo K.E. Asian fungal fermented food. In: Hofrichter M., editor. Industrial Applications. Springer; Berlin/Heidelberg, Germany: 2010. pp. 29–58. [Google Scholar]
- 26.Li G.J., Hyde K.D., Zhao R.L., Hongsanan S., Abdel-Aziz F.A., Abdel-Wahab M.A., Alvarado P., Alves-Silva G., Ammirati J.F., Ariyawansa H.A., et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;78:1–237. doi: 10.1007/s13225-016-0366-9. [DOI] [Google Scholar]
- 27.Lima C.L.F., Lima D.X., De Souza C.A., De Oliveira R.J., Cavalcanti I.B., Gurgel L., Santiago A.L.C.M.A. Description of Mucor pernambucoensis (Mucorales, mucoromycota), a new species isolated from the Brazilian upland rainforest. Phytotaxa. 2018;350:274. doi: 10.11646/phytotaxa.350.3.6. [DOI] [Google Scholar]
- 28.Nguyen T.T.T., Voigt K., Santiago A.L.S., Kirk P.M., Lee H.B. Discovery of novel Backusella (Backusellaceae, Mucorales) isolated from invertebrates and toads in Cheongyang, Korea. J. Fungi. 2021;7:513. doi: 10.3390/jof7070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols. Academic Press; Cambridge, MA, USA: 1990. pp. 315–322. [Google Scholar]
- 30.Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several species of Cryptococus. J. Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayers E.W., Cavanaugh M., Clark K., Pruitt K.D., Schoch C.L., Stephen T., Sherry S.T., Karsch-Mizrachi I. GenBank. Nucleic Acids Res. 2022;50:D161–D164. doi: 10.1093/nar/gkab1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. TrimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 36.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guindon S., Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 38.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treschew Mucor odoratus. Bot. Tidskr. 1941;45:148. [Google Scholar]
- 40.Pidoplichko N.M., Mil’ko A.A. Atlas Mukoral’nykh Gribov [Atlas of the Mucorales]. Izdat. Naukova Dumka; Kiev, Ukraine: 1971. p. 115. [Google Scholar]
- 41.Benjamin R.K., Mehrotra B.S. Obligate azygospore formation in two species of Mucor (Mucorales) Aliso. 1963;5:240. doi: 10.5642/aliso.19630503.02. [DOI] [Google Scholar]
- 42.Schipper M.A.A.A. On certain species of Mucor with a key to all accepted species. Stud. Mycol. 1978;17:l–53. [Google Scholar]
- 43.Baijal U., Mehrotra B.S. Species of Mucor from India-II. Sydowia. 1965;19:204–212. [Google Scholar]
- 44.Chai C., Liu W., Cheng H., Hui F. Mucor chuxiongensis sp. nov., a novel fungal species isolated from rotten wood. Int. J. Syst. Evol. Microbiol. 2019;69:1881–1889. doi: 10.1099/ijsem.0.003166. [DOI] [PubMed] [Google Scholar]
- 45.Nadson M.M.G., Philippov G. Une nouvelle Mucorinée. Mucor guilliermondii nov. sp. et ses forms-levures. Rev. Gén. Bot. 1925;37:450–463. [Google Scholar]
- 46.Chen G.-Q., Zheng R.-Y. A new species of Mucor with giant spores. Acta. Mycol. Sin. Suppl. I. 1986;1:57–60. [Google Scholar]
- 47.Rogers P.J., Clark-Walker G.D., Stewart P.R. Effects of oxygen and glucose on energy metabolism and dimorphism of Mucor genevensis grown in continuous culture: Reversibility of yeast-mycelium conversion. J. Bacteriol. 1974;119:282–293. doi: 10.1128/jb.119.1.282-293.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orlowski M. Mucor dimorphism. Microbiol. Mol. Biol. Rev. 1991;55:234–258. doi: 10.1128/mr.55.2.234-258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharifia M., Karimi K., Taherzadeh M.J. Production of ethanol by filamentous and yeast-like forms of Mucor indicus from fructose, glucose, sucrose, and molasses. J. Ind. Microbiol. Biotechnol. 2008;35:1253–1259. doi: 10.1007/s10295-008-0422-x. [DOI] [PubMed] [Google Scholar]
- 50.Sharifyazs S., Karimi K. Effects of fermentation conditions on valuable products of ethanolic fungus Mucor indicus. Electron. J. Biotechnol. 2017;30:77–82. doi: 10.1016/j.ejbt.2017.09.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data were submitted to GenBank.